Abstract
Persistent hyperglycemia causes many microvascular and macrovascular complications in type 2 diabetics. Chronic wounds are one of the most common complications. Adipose tissue-derived mesenchymal stem cells (AT-MSCs) shows great potential for chronic wound healing because of their ability to secrete many factors to stimulate migration, growth, angiogenic. In previous research, we were determined that diabetic AT-MSCs (dAT-MSCs) impaired the ability of wound healing compared with non-diabetic AT-MSCs (nAT-MSCs). In this study, we aim to examine the effect of high D-glucose concentration on the mobility of nAT-MSCs and wound healing in vitro. The expression of migratory genes was determined by reverse transcription quantitative polymerase chain reaction (RT-qPCR). In vitro scratch assay was used to examine the healing ability of nAT-MSCs under the influence of high D-glucose concentration. The results show that high D-glucose treated-nAT-MSCs were reduced the expression of migration factors (SDF-1 and CXCR-4), which suggested the high D-glucose treated-nAT-MSCs may decrease their migration mobility compared with those of non-treated nAT-MSCs. Moreover, the wound healing ability in vitro was impaired high D-glucose treated-nAT-MSCs compared with those of non-treated nAT-MSCs. Interestingly, the migration mobility of nAT-MSCs under the effect of high D-glucose condition was similar to those of dAT-MSCs. Our study provides further insight into the impact of high D-glucose levels on reducing wound healing ability of nAT-MSCs in supporting of the improving AT-MSC function in the treatment of diabetic complications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Type 2 diabetes mellitus is a global health problem, characterized by insulin resistance and hyperglycemia [1]. The persistent hyperglycemia causes many complications in diabetic patients [1]. A chronic wound is a hard-to-heal wound, one of the most common complications, causing many difficulties in the lives of type 2 diabetes [2]. Nowadays, stem cell therapy shows great potential for chronic wound healing because of their ability to secrete many factors to stimulate migration, growth, angiogenic and immunomodulation [1, 3, 4].
However, our previous research showed that diabetic AT-MSCs (dAT-MSCs) were impaired wound healing ability compared with non-diabetic AT-MSCs (nAT-MSCs) in the flap mouse model and in vitro wound healing model [5, 6]. Stromal cell-derived factor-1 (SDF-1) and CXCR-4 (SDF-1 receptor) have been shown to direct the movement of stem cells related to repairing damage in many cells and tissues [7]. Besidesesides, the expression of migration factors (SDF-1, CXCR-4) in dAT-MSCs was lower than those of nAT-MSCs [6]. Furthermore, previous studies have shown that high D-glucose concentrations impaired proliferation and migration of human fibroblast, keratinocyte [8, 9]. In vitro scratch assay is an inexpensive and easy method to assess cell movement in vitro of many cells such as fibroblasts, keratinocytes, and mesenchymal stem cells in chronic wound healing [6, 10, 11].
Therefore, we examined the impact of high D-glucose concentration on the mobility and wound healing ability in vitro of nAT-MSCs compared with dAT-MSCs.
2 Material and Methods
2.1 Stem Cell Culture
Non-diabetic AT-MSCs (nAT-MSCs) and diabetic AT-MSCs (dAT-MSCs) are provided by Laboratory of Regenerative Medicine and Stem Cell Biology, University of Tsukuba, Japan. These cells were characterized in the previous report [6]. Briefly, AT-MSCs were cultured in Iscove’s Modified Dulbecco’s medium (Thermo, USA), with 10% fetal bovine serum (Thermo, USA), 1% antibiotics (Sigma, USA) and five ng/ml basic fibroblast growth factor (bFGF, Sigma, USA) at 37 ℃ and 5% CO2. The medium will be renewed every three days. Cells are frozen with cell banker solution (Sigma, USA) and preserved in liquid nitrogen for further experiments. The AT-MSCs at passage 5–8 were used for the experiments.
2.2 Cell Proliferation Assay
AT-MSCs were cultured on 24-well plate at a density of 1.5 × 104 cells/well and cultured under high D-glucose concentrations (100 and 200 mM). The cell culture medium was changed every three days. The morphology and growth of AT-MSCs will be monitored for 11 days after a supplement with D-glucose. Trypan blue staining method was used to determine cell proliferation every 48 h.
2.3 Quantitative Reverse Transcription-Polymerase Chain Reaction
Stem cell movement plays an important role in the healing of chronic wounds. Therefore, we cultured nAT-MSCs and dAT-MSCs in D-glucose (100 mM) supplementation medium to examine the influence of those on the expression of migratory genes. After adding D-glucose for three days, RNA was extracted with Sepasol-RNA I Super G (Nacalaitesque, Japan). Total RNA (300 ng) was reverse transcribed by transcription-polymerase chain reaction (RT-PCR) Kit (TOYOBO, Japan) to converse to cDNA. cDNA was analyzed using a LightCycler 96 System (Roche, Switzerland) using Maxima SYBR Green/ROX qPCR Master Mix (2X) Kit (Thermo, USA). The expression levels of migration genes were analyzed using the \({2}^{-\Delta \Delta {C}_{t}}\) methods. β-actin gene was used as an internal control. The primer sequences for the PCR reactions are shown in Table 1.
2.4 In Vitro Scratch Assay
In vitro, wound-healing assay as described previously [12, 13]. Briefly, nAT-MSCs and dAT-MSCs were cultured on 4-well plates with a density of 4 × 104 cells/well. After 24 h, AT-MSCs were treated with 100 mM D-glucose. When cells had reached confluent monolayers, a scratch was created on the surface of the culture dish by a p1000 pipette tip (width 1 mm). Images of wound areas were recorded and analyzed by Wimasis software (https://mywim.wimasis.com) at 0 h and after 24 h. Data have presented the average of five measurements from wound areas.
2.5 Statistical Analysis
The significant differences among many test groups have been used to identify one-way ANOVA (Tukey posthoc test; SPSS 20 software, IBM Corp.). P < 0.05 value is statistical significance. The data indicated the mean of the three independent experiments (mean ± SD).
3 Results
3.1 The Effect of High D-glucose Concentrations on Growth Ability of AT-MSCs
D-glucose is the main source of energy for many cellular activities [14]. Despite this, high D-glucose levels have been shown to increase free radicals and increase the level of ageing of many types of cells [15, 16]. Hence, we evaluated the impact of high D-glucose concentrations on the growth of nAT-MSCs by adding 100 mM and 200 mM D-glucose to the culture medium. Cell proliferation of nAT-MSCs was monitored for 11 days after D-glucose supplementation and compare with dAT-MSCs.
Our results showed that the doubling times of nAT-MSCs (35.67 ± 0.49 h) and dAT-MSCs (34.9 ± 1.26 h) were not statistically different. However, nAT-MSCs were cultured with high D-glucose concentrations had slower growth rates than the control sample and dAT-MSCs. Differences in growth rates are most evident since day 3 of the culture process. At the log phase, we found that the doubling time of nAT-MSCs treated with 100 mM D-glucose was 60.95 ± 4.18 h (1.70 fold decrease, p < 0.01, n = 3) compared to the control sample. It is worth noting that nAT-MSCs treated with 200 mM D-glucose hardly proliferated with the doubling time was 201.07 ± 34.92 h (Fig. 1). Therefore, this result proves that high D-glucose concentration inhibits the growth of nAT-MSCs, which can lead to reduced wound healing ability. According to the results of cell proliferation, we considered that the concentration of 200 mM D-glucose is not suitable for further experiments, so we only examined the effect of 100 mM D-glucose on nAT-MSCs for the expression of the migration factors (SDF-1 and CXCR-4) and wound healing in vitro.
Growth curve of AT-MSCs under the impact of high D-glucose concentrations. Several cells were determined every 48 h by using the trypan blue exclusion method for 11 days after D-glucose supplementation. High D-glucose concentrations inhibit the growth of nAT-MSCs. Data indicated the average values of three independent experiments (mean ± SD); Ctrl: control
3.2 The High D-glucose Concentration Decreased Migration Factors Expression in AT-MSCs
Chronic wounds are one of the common complications in diabetic patients [2]. SDF-1 and CXCR-4 factors are known to promote acute and chronic wound healings [7]. Previous studies have demonstrated that the wound healing ability of dAT-MSCs was impaired in vitro wound healing model and the flap mouse model [5, 6].
The results showed that nAT-MSCs have higher expression of migration factors than dAT-MSCs. Specifically, the expression of SDF-1 (5.32 ± 0.35 fold, P < 0.01, n = 3) and CXCR-4 (8.99 ± 0.60 fold, P < 0.01, n = 3) were higher than dAT-MSCs (Fig. 2). Notably, the expression of SDF-1 and CXCR-4 in 100 mM D-glucose treated-nAT-MSCs were reduced compared with control nAT-MSCs (SDF-1, 1.24 ± 0.08 fold decrease and CXCR-4, 1.94 ± 0.13 fold decrease, P < 0.01, n = 3). However, the expression of these factors in nAT-MSCs were still higher than dAT-MSCs (SDF-1, 4.30 ± 0.15 fold increase, P < 0.01, n = 3; CXCR-4, 4.63 ± 0.18 fold increase, P < 0.01, n = 3) (Fig. 2). Thus, the results suggest that high D-glucose concentration reducing the expression of migration factors in AT-MSCs. This may reduce the ability of nAT-MSCs to heal the wound under hyperglycemia or in diabetic patients.
The high D-glucose concentration decreased migration factors expression in nAT-MSCs. The mRNA expression level was examined by qRT-PCR and normalized to β-actin. The expression of SDF-1 and CXCR-4 were decreased in nAT-MSCs under the influence of 100 mM D-glucose. The data indicated the mean of the three independent experiments (mean ± SD); P < 0.01 (**); Ctrl: Control
3.3 The High D-glucose Concentration Reduced the Ability of AT-MSCs to Heal Chronic Wounds in Vitro
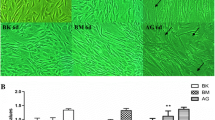
Previous results have elucidated that high D-glucose concentration altered the expression of genes involved in the ability of wound healing of AT-MSCs (Fig. 2). We performed the scratch assay to examine the effect of high D-glucose concentration on the ability of AT-MSCs to treat chronic wounds. This is an appropriate method to study the effect of cell–matrix and interaction, mimicking cell migration during wound healing in vivo [13].
The results demonstrated that the wound areas at 24 h of nAT-MSCs were significantly recovered in comparison with those of dAT-MSCs (wound area: nAT-MSCs, 12.80 ± 0.88% vs. dAT-MSCs, 22.95 ± 3.22%; P < 0.01; n = 5) (Fig. 3). Notably, the ability to cover wounds of nAT-MSCs has been significantly reduced when exposed to high D-glucose concentration (wound area: 100 mM D-glucose treated-nAT-MSCs, 23.48 ± 3.68% vs. nAT-MSCs, 12.80 ± 0.88%; P < 0.01; n = 5) (Fig. 3). The wound areas in 100 mM D-glucose treated-nAT-MSCs and dAT-MSCs were not statistical differences (wound area: 100 mM D-glucose treated-nAT-MSCs, 23.48 ± 3.68% vs. dAT-MSCs, 22.95 ± 3.22%, P > 0.05, n = 5) (Fig. 3). These results indicate that the ability of wound healing in nAT-MSCs is impaired by the effects of high D-glucose concentration, possibly due to the inhibition of factors that promote cell migration.

taken from the average of five different wound areas and three replications (mean value ± SD); **, P < 0.01. Scale bar 500 μm
High D-glucose concentration inhibits the ability of in vitro cell migration. a The image was taken using a microscope and analyzed by Wimasis software at 0 and 24 h. b Percentage of wound areas of nAT-MSCs (control and 100 mM) and dAT-MSCs at 0 and 24 h. Data were
4 Discussion
The stem cell migration to the damaged area plays a very important role in wound healing [7, 12]. Previous studies have shown that the wound healing ability of dAT-MSCs is impaired due to an increase in the number of adhesion molecules and a prolonged inflammation stage [5, 6]. Here, we demonstrated that the high D-glucose concentration reduced the expression of migration factors (SDF-1 and CXCR-4) and impaired the ability to heal wounds of AT-MSCs in vitro.
Previous studies have elucidated that the high D-glucose concentrations increase free radicals and the ageing level of any types of cells [15, 16]. Our results show that 100 and 200 mM D-glucose decreased the proliferation of nAT-MSCs (Fig. 1). This result agrees with previous studies high D-glucose concentration inhibits the proliferation of mice bone stem cells, MG63 bone cells and some stem cell lines from other sources in human (bones, placenta, umbilical cord and chorion) [17,18,19]. Also, our previous publication showed that dAT-MSCs were impaired wound healing ability is compared with nAT-MSCs in the flap mouse model and in vitro wound healing model [5, 6]. Furthermore, the high D-glucose concentrations also impaired the proliferation and migration of human fibroblast, keratinocyte [8, 9].
The interaction between SDF-1 and CXCR-4 recruit stem cells into the wound area, promoting wound repair and neovascularization [2]. In vivo wound healing model, the concentration of SDF-1 remarkedly increased at the wound site, and it was manifested throughout the healing process. Blocking of SDF-1/CXCR4 leads to a significant reduction in epidermal stem cell movement and impairs wound healing in vivo [20]. Our results found that the expression of SDF-1 and CXCR-4 have significantly downregulated in dAT-MSCs than those in nAT-MSCs (Fig. 2). The reduction of these factors is expected to reduce the migration ability of nAT-MSCs during wound healing. Therefore, the scratch assay was performed to mimic cell movement during wound healing in vivo. The result of in vitro scratch assay shows that the wound healing ability of nAT-MSCs treated with high D-glucose concentration was similar as dAT-MSCs in wound healing in vitro (Fig. 3), indicating the reducing in cell proliferation, gene expression, and wound healing ability under high glucose concentration. Although we demonstrated the effect of high D-glucose concentration on impairing the wound healing ability of nAT-MSCs in vitro, the further study on the flap mouse model is needed to elucidate the effect of high D-glucose concentration on nAT-MSCs in wound healing in vivo.
Taken together, our study demonstrated that the expression of migration factors and the wound healing ability in high D-glucose treated-nAT-MSCs and dAT-MSCs were inhibited compared with non-treated nAT-MSCs. This study provides a better understating in the adverse effect of high D-glucose concentration on AT-MSC functions for future application of AT-MSCs under diabetic conditions.
Abbreviations
- SDF-1:
-
Stromal cell-derived factor-1
- CXCR-4:
-
SDF-1 receptor
References
Ralph EF, DeFronzo A, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Ronald Kahn C, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R (2015) Type 2 diabetes mellitus. Nat Rev 1:1–22. https://doi.org/10.1038/nrdp.2015.19
Jude E, Blakytny R (2006) The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 23:594–608. https://doi.org/10.1111/j.1464-5491.2006.01773.x
Kern HES, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301. https://doi.org/10.1634/stemcells.2005-0342
Seeberger KL,Yeung TY, Kin T, Adesida A, Jomha N, James Shapiro AM, Korbutt GS (2012) Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One 7. https://doi.org/10.1371/journal.pone.0038189
Trinh T-T, Ohneda K, Kimura K, Salazar GT, Sato F, Ohneda O (2016) Increased expression of EGR-1 in diabetic human adipose tissue-derived mesenchymal stem cells reduces their wound healing capacity. Stem Cells Dev 25:760–773. https://doi.org/10.1089/scd.2015.0335
Trinh TYNT, Tran Cam Tu, Kato T, Ohneda K, Sato F, Ohneda O (2016) Microvesicles enhance the mobility of human diabetic adipose tissuederived mesenchymal stem cells in vitro and improve wound healing in vivo. Biochem Biophys Res Commun 473:1111–1118. https://doi.org/10.1016/j.bbrc.2016.04.025
Chai L, Guo R, Chen L, Chen W, Ge L, Li X, Li H, Li S, Cao C (2015) Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells. In Vitro Cell Dev Biol Anim. https://doi.org/10.1007/s11626-014-9862-y
Mizutani K, Buranasin P, Iwasaki K, Pawaputanon Na Mahasarakham C, Kido D, Takeda K, Izumi Y (2018) High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS One 12. https://doi.org/10.1371/journal.pone.0201855
Zhang J, Li L, Zhang Q, Zhang D, Xiang F, Jia J, Wei P, Zhang J, Hu J, Huang Y (2019) High glucose suppresses keratinocyte migration through the inhibition of p38 MAPK/autophagy pathway. Front Physiol 10. https://doi.org/10.3389/fphys.2019.00024
Wright KT, Waltera MNM, Fuller HR, MacNeil S, Johnson WEB (2010) Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 316:1271–1281. https://doi.org/10.1016/j.yexcr.2010.02.026
Gilger BC, Sherman AB, Berglund AK, Schnabe LV (2017) Effect of bone marrow-derived mesenchymal stem cells and stem cell supernatant on equine corneal wound healing in vitro. Stem Cell Res Ther 8. https://doi.org/10.1186/s13287-017-0577-3
Babaheydari FM, Dehkordi AN, Chehelgerdi M, Dehkordi SR (2019) Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther 10. https://doi.org/10.1186/s13287-019-1212-2
Park AY, Liang C-C, Guan J-L (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333. https://doi.org/10.1038/nprot.2007.30
Aguiari BZSLP, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, Rizzuto R (2008) High glucose induces adipogenic differentiation of muscle-derived stem cells. PNAS 105:1226–1231. https://doi.org/10.1073/pnas.0711402105
Castoldi M, Horn P, Wagner W, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD (2008) Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE 3. https://doi.org/10.1371/journal.pone.0002213
Munsey TS, Abuarab N, Jiang L-H, Li J, Sivaprasadarao A (2017) High glucose-induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn2+-mediated mitochondrial fission. Sci Signal 10. https://doi.org/10.1126/scisignal.aal4161
Tantrawatpan C, Manochantr S, Hankamolsiri W, Tantikanlayaporn D, Tapanadechopone P, Kheolamai P (2016) The effects of high glucose on adipogenic and osteogenic differentiation of gestational tissue-derived MSCs. Stem Cells Int https://doi.org/10.1155/2016/9674614
Wang XZW, Zheng J, Yang J (2010) High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol Cell Biochem 338:115–122. https://doi.org/10.1007/s11010-009-0344-6
Song G, Cao X, Shao X, Zhao Y, Shi B (2014) Metformin rescues the MG63 osteoblasts against the effect of high glucose on proliferation. J Diab Res. https://doi.org/10.1155/2014/453940
Fangqiang Zhu Xiang Xu, Zhang M, Zeng D, Luo D, Liu G, Cui W, Wang S, Guo W, Xing W, Liang H, Li L, Xiaobing Fu, Jiang J, Huang H (2013) Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs 197:103–113. https://doi.org/10.1159/000342921
Acknowledgements
The funding for this research is from the Biotechnology Center of Ho Chi Minh City (DV01/17-19). We thank professor Osamu Ohneda (Head of Department of Regenerative Medicine and Stem Cell Biology at the University of Tsukuba, Japan) for providing the AT-MSC samples.
Conflict of Interest
The authors declare that there is no conflict of interest in this study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this paper
Cite this paper
Mai, HP., Trinh, NT., Long, V.B., Binh, N.T., Nguyen, DQ., Duong, HX. (2022). The High D-Glucose Concentration Reduces the Ability of Wound Healing in Vitro of Human Adipose Tissue-Derived Mesenchymal Stem Cells. In: Van Toi, V., Nguyen, TH., Long, V.B., Huong, H.T.T. (eds) 8th International Conference on the Development of Biomedical Engineering in Vietnam. BME 2020. IFMBE Proceedings, vol 85. Springer, Cham. https://doi.org/10.1007/978-3-030-75506-5_49
Download citation
DOI: https://doi.org/10.1007/978-3-030-75506-5_49
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-75505-8
Online ISBN: 978-3-030-75506-5
eBook Packages: EngineeringEngineering (R0)