Abstract
Diabetic patients display increased risk of diabetic foot ulcer and chronic wound, mainly caused by the persistence of hyperglycemia. Adipose tissue-derived mesenchymal stem cells (AT-MSCs) and platelet rich plasma (PRP) have shown relevant advantages for wound healing by their ability to stimulate cell proliferation, migration, secrete growth factors and cytokines to accelerate the resolution of wound. In this study, we investigated the effect of PRP on the proliferation and wound healing in-vitro of AT-MSCs under high D-glucose conditions. PRP was activated by Thrombin and Calcium chloride. The cell proliferation was determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay for 10 days. In-vitro scratch assay was used to examine the healing ability of AT-MSCs under the influence of high D-glucose concentrations. The results show that the doubling time of AT-MSCs cultured with PRP was significantly higher than those with FBS. Moreover, the wound healing ability in-vitro was induced significantly in PRP-treated AT-MSCs under high D-glucose conditions. Our research demonstrates the potential of PRP on the inducing wound-healing capacity of AT-MSCs under the impact of high D-glucose levels. This work gives a better knowledge of the influence of PRP on the activities of AT-MSCs under high D-glucose conditions for future applications of AT-MSCs in the treatment of diabetes complications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Diabetes is a serious, long-lasting disease that affects the lives and well-being of people, families, and communities all over the world [1]. Roughly 500 million people worldwide were affected by diabetes in 2019, and this number is estimated to increase by 10% by 2045. Diabetes affects the entire body and all wound sites as well. When your body suffers from diabetes, insulin is rarely produced or resisted by the body, so too much glucose is kept in the bloodstream without function. Elevated systemic glucose levels are the root cause of hyperglycemia. When hyperglycemia persists, it impairs the function of the vascular endothelium, which lowers oxygen delivery and restricts nutritional supplementation, delaying the wound healing process [2].
In-vivo and in-vitro research into diabetes are essential for understanding the disease’s pathology and pathophysiology, which are required for the development of more effective treatment alternatives [3]. In recent years, in-vitro studies have made it possible for scientists to look at multiple precise experimental settings at the same time using fewer resources than animal experiments. For disease research, cell lines, islets, human stem cells, and organoids serve as models for diabetes. Among these, the mesenchymal stem cell (MSC)-based model is highly regarded due to the fact that MSCs are a renewable source of target cells, genetically modified, and able to provide a suitable environment for the wound healing process [4].
MSCs are the origin of human life. They play a key role in regulating wound healing because of their ability to control cellular differentiation, immunological modulation, growth factor release, and angiogenesis [5]. Compared to other types of stem cells, adipose tissue-derived mesenchymal stem cells (AT-MSCs) have specific advantages and fewer limitations: they are abundant and easily harvested through a minimally invasive procedure; they are associated with fewer ethical concerns, and they have a lower risk of the host immune response [6]. In previous preclinical studies, AT-MSC treatment promoted wound healing in diabetic animal models. AT-MSCs work at the wound site by releasing growth factors, increasing the activity of existing cells at the wound site, and changing into different types of cells. Nonetheless, many studies demonstrate that AT-MSC function is impaired under conditions of high glucose. In a flap animal model and an in-vitro wound healing model [7, 8], Trinh T-T et al. (2016) found that diabetes AT-MSCs (dAT-MSCs) exhibited a lower capacity to repair wounds compared to non-diabetic AT-MSCs (nAT-MSCs). In 2010, “Camer et al.” reported that the proliferative ability of dAT-MSCs was lower than that of nAT-MSCs at high D-glucose concentrations (500 and 100 mg/dL) [9]. Yet, until 2017, Y. Li et al. showed that at 25 mM glucose concentration, the proliferation of primary MSCs was enhanced at under concentration of D-glucose levels [10].
For enhancing the efficacy of AT-MSCs in wound healing under a high D-glucose environment, the autologous cellular treatments comprising platelet-rich plasma and MSC applications are offered as a more potential routine wound care therapy method for patients with chronic and refractory wounds. Fetal Bovine serum is widely used as a supplement to the standard stem cell in-vitro model by their ability to promote proliferation and matrix synthesis. However, the potential risk of bovine pathogens in FBS serum limits its use in clinical applications. An alternative for FBS is, therefore, urgently needed. Platelet-rich plasma (PRP) is a valuable source to replace FBS by their potential to promote wound healing, blood vessel reduction, and soft and hard tissue regeneration [11, 12]. PRP is an enhanced blood fraction containing platelets at a concentration higher than that in circulating blood [9]. With plasma products containing high levels of platelets, they have the potential to have an effect on wound healing and promotion, blood vessel reduction, and soft and hard tissue regeneration [11, 12]. When platelets in PRP are activated, they release granules containing growth factors and regulatory proteins such as PDGF, EGF, IGFs, TGF-, VEGF, and others [9, 11, 12] that aid in cell proliferation, migration, and differentiation.
To date, there has been relatively little study on the combination of AT-MSCs with PRP for the therapy of wound healing under the effect of high D-glucose. Previous studies reported at 10% PRP and AT-MSCs together [13, 14], which showed that the AT-MSCs’ ability to migrate and multiply was significantly increased. Because of this, we decided to compare the efficacy of serum FBS with that of supplementation of PRP since the risk of infection from animal origin makes it less useful as a therapy. As the effect of low to high D-glucose concentrations on AT-MSCs is still unknown, this work investigates the effect of 10% PRP on the proliferation and in-vitro wound healing of AT-MSCs under 3 concentrations of D-glucose (25, 50, and 100 mM).
2 Materials and Methods
Stem Cell Culture
AT-MSCs (AT-MSCs) are provided by the Laboratory of Regenerative Medicine and Stem Cell Biology, University of Tsukuba, Japan. These cells were characterized in the previous report [6]. Briefly, AT-MSCs were cultured in Iscove’s modified Dulbecco’s medium (Thermo, USA), with 10% fetal bovine serum (Thermo, USA), 1% antibiotics (Sigma, USA) and 5 ng/ml basic fibroblast growth factor (bFGF, Sigma, USA) at 37 °C and 5% CO2. The medium will be renewed every 3 days. Cells are frozen with cell banker solution (Sigma, USA) and preserved in liquid nitrogen for further experiments. The AT-MSCs in passages 5 and 9 were used for the experiments.
Preparation of Activated PRP
PRP, PPP (Platelet-poor plasma) and thrombin were provided from Miracle Plastic Surgery by the following procedure. Activated PRP was obtained using the method given by Natsuko. K from the Department of Plastic and Reconstructive Surgery, Kansai Medical University, Japan [19]. As an activator, a 1:1 (v/v) combination of 0.5 M CaCl2 and autologous thrombin was produced in advance. At room temperature, a 10:1 (v/v) combination of PRP and activator was centrifuged at 90 g and then 9000 g for 10 min each. The supernatant was filtered through a 0.22-m membrane and labeled as PRP-2 and PPP-2. Keep at −80 ℃ laboratory freezer until use.
Cell Proliferation by MTT Assay
AT-MSCs were seeded in IMDM medium with 10% FBS; after 24 h, the media was removed, and the cells were cultured in IMDM medium with 10% FBS (control), 10% PRP-1, 10% PPP-1, 10% PRP-2, and 10% PPP-2. The cell culture medium was replaced every three days. Five days following supplementation with PRP and PPP, the proliferation of AT-MSCs will be evaluated using an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) test. After 3, 6, 9 and 12 days of culture, all the medium was removed and added 50 μL of MTT reagent for each well. The plate was kept away from light and incubated for 4 h in incubator 37 °C, 5% CO2. After incubation, cells were treated with 100 μL DMSO solvent in each well for 15 min at room temperature. Absorbance was measured at OD = 540 nm.
In-Vitro Scratch Assay
Briefly, AT-MSCs were cultured on 24-well plates with a density of 5 × 104 cells/well and cultured in IMDM medium with 10% FBS. When cells had reached confluent monolayers (higher than 90%), aspirated 3 ml culture medium in each well then transferred into an eppendorf. Used a 1000 μL pipette tip (width 1 mm) to create on the surface of the culture. Gently shake the plate, then use the pipette to remove all the remaining medium in each well. Add 3 mL of media that has been aspirated into the eppendorf in the previous step and gently shake the plate to remove all the cell pieces. Add IMDM medium with 10% FBS (control), 10% PRP-1, 10% PPP-1, 10% PRP-2, and 10% PPP-2 into each well. Images of wound areas were recorded by inverted microscope and analyzed by Wimasis software (https://mywim.wimasis.com) at 0 h and after 24 h. Data are presented with the average of three measurements from wound areas.
Statistical Analysis
The significant differences among many test groups have been used to identify one-way ANOVA (Tukey post-hoc test; SPSS 20 software, IBM Corp.). P < 0.05 value is statistical significance. The data indicated the mean of the three independent experiments (mean ± SD).
3 Results and Discussion
3.1 Activated PRP by Calcium Chloride and Autologous Thrombin
Activated PRP and PPP were obtained using the method given by Natsuko.K (Fig. 1). The interaction of CaCl2 and thrombin produces fibrin that is rich in platelets. CaCl2 inhibits citrate, allowing plasma to coagulate, while thrombin polymerizes fibrin, resulting in a coagulated gel. After the first centrifugation, the coagulates as a result of the fibrin polymerization process. At the second high-speed centrifugation, the fibrin fibers were completely removed from the plasma and the platelet was degranulated inside the soft gel to release growth factors. Finally, the plasma was filtered with 0.22 μm cell strainer to eliminate the gel formation and collect the growth factors-containing plasma.
Preparation of activated PRP and PPP from the whole blood procedure. Activated PRP and PPP were obtained in Plastic Surgery following the procedure described in the methodology section (Fig. 1 a–e). Collect thrombin without anticoagulant from whole blood (Fig. 1f). PRP (Fig. 1g) and PPP (Fig. 1h) were activated by the addition of 0.5 M CaCl2 and thrombin in a ratio of 1:1 (v/v). After 10 min of activation, the gel was formed in PRP (Fig. 1i) and PPP (Fig. 1k).
3.2 The effect of 10% PRP on Cell Viability of AT-MSCs Under High D-Glucose Levels
Platelet-Rich Plasma (PRP) is an autologous platelet plasma that includes many growth factors to accelerate wound healing and recovery after surgical repair. Hence, we evaluated the impact of 10% PRP to assess the influence of autologous platelet plasma on the growth of AT-MSCs in different high D-glucose levels (25 mM, 50 mM, 100 mM). Cell proliferation of AT-MSCs cultured with 10% PRP was monitored for 12 days after D-glucose supplementation and compared with AT-MSCs cultured with 10% FBS.
AT-MSCs proliferation under the impact of high D-glucose concentrations. Growth curve of AT-MSC under high D-glucose levels cultured with 0 mM D-glucose (A), 25 mM D-glucose (B), 50 mM D-glucose (C), 100 mM D-glucose (D). Cell viability graph of AT-MSCs in different groups after 12 days of culture (C). A number of cells were determined every 72 h by using MTT reagent for 12 days after D-glucose supplementation. Results are expressed as % of the control: AT-MSCs cultured with 10% FBS at day 03 as 100%. Data indicated the average values of three independent experiments (mean ± SD), **, P < 0.01.
Growth curve of AT-MSC under high D-glucose levels. AT-MSC cultured with 10% FBS and 10% PRP in control group (A) and various D-glucose concentrations: 25 mM (B), 50 mM (C) and 100 mM (D). A number of cells were determined every 72 h by using MTT reagent for 12 days after D-glucose supplementation. Results are expressed as % of the control: AT-MSCs cultured with 10% FBS at day 03 as 100%. Data indicated the average values of three independent experiments (mean ± SD), **, P < 0.01.
Our results showed that the statistical change in cell viability of AT-MSCs using the medium containing 10% FBS under the treatment of different D-glucose levels was not found during 12 days. Similarly, the results of AT-MSCs viability using 10% PRP did not receive any significant difference at the different D-glucose levels of treatment. Instead, the stimulatory effects on cell viability of PRP were stronger than those of FBS at the same different D-glucose levels. The doubling times of AT-MSCs cultured with 10% PRP (1,1574 ± 0,1332) were statistically different when compared with 10% FBS (0,2019 ± 0,0671). Differences in growth rates are most evident since day 6 of the culture process (Fig. 2).
Figure 3 exhibits a clearer evaluation of different supplemented serums including 10% FBS and 10% PRP ability to stimulate proliferation of under various D-glucose concentrations. In general, 10% PRP promoted the highest proliferation rate when compared to that of 10% FBS after 12 days of culture period, yet has no statistical difference among various D-glucose concentrations. In addition of 10% FBS, control group showed a better proliferation rate than other groups (0,2019 ± 0,0671), however it still had less efficiency than group of 10% PRP (1,1574 ± 0,1332). In 25 mM and 50 mM D-glucose-treated groups, 10% PRP (10% PRP + 25, 0,7216 ± 0,0406 and 10% PRP + 50, 0,7073 ± 0,0764) shown statistically different from day 6 to day 12 of culture when compared with 10% FBS (10% FBS + 25 mM D-glucose, 0,3367 ± 0,0154 and 10% FBS + 50 mM D-glucose, 0,3471 ± 0,0308). The control and 100 mM group promote significant proliferation ability of AT-MSCs during 12 days. Under all D-glucose levels, 10% PRP shows the increased trend in cell proliferation; however, at day 12, the control group, 25 mM and 100 mM D-glucose group no longer support the cell growth; thus, they appear to have a slight decrease in cell’s number. Our investigation showed that 10% PRP had a higher potential for proliferation than 10% FBS. This result agrees with previous studies [13, 18,19,20] that PRP concentrations between 10 and 20% in the cell culture media had the greatest effect on cell growth. In addition, our previous publication showed that 100 mM and 200 mM D-glucose decreased the proliferation of AT-MSCs [17]. Furthermore, the high D-glucose concentrations also impaired proliferation and migration of human fibroblast, keratinocyte [9, 10]. In this present study, the results demonstrated that 10% PRP significantly increased the cell proliferation of AT-MSCs when exposed under 25 mM, 50 mM and 100 mM D-glucose. This evidence supports that 10% PRP brings valuable promise to improve the growth ability of AT-MSCs under exposure to high D-glucose concentrations.
3.3 10%PRP Induced the Ability of AT-MSCs to Heal Chronic Wounds In-Vitro
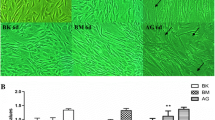
The results demonstrated that the wound areas at 24 h of AT-MSCs in 10% PRP medium were significantly recovered in comparison with those of 10% FBS (wound area: 10%PRP, 10.98 ± 0.79% vs. 10%FBS, 25.64 ± 1.98%; P < 0.01; n = 5) (Fig. 4). Moreover, at all three high D-glucose levels, 10% PRP also shown significantly higher the ability to heal the wound when compared with 10% FBS (wound area: 10%PRP + 25 mM D-glucose, 15.10 ± 1.32 vs. 10%FBS + 25 mM D-glucose, 2.27 ± 2.72 and 10%PRP + 50 mM D-glucose, 12.09 ± 0.45 vs. 10%FBS + 50 mM D-glucose, 44.79 ± 1.77 and 10%PRP + 100 mM D-glucose, 32.89 ± 1.31 vs. 10%FBS + 100 mM D-glucose, 52.24 ± 1.96, P < 0.01; n = 5). Notably, the ability to cover wounds of AT-MSCs has been reduced when exposed to high D-glucose at 100 mM in comparison with control group (wound area: 10%PRP, 10.98 ± 0.79% vs. 10%PRP + 100 mM D-glucose, 32.89 ± 1.31 and 10%FBS, 25.64 ± 1.98% vs. 10%FBS + 100 mM D-glucose, 52.24 ± 1.96; P < 0.01; n = 5) (Fig. 3). The wound areas in 10% FBS + 100 mM D-glucose were not statistical differences (wound area: 10%FBS + 100 mM D-glucose, 52.24 ± 1.96, P < 0.01; n = 5) (Fig. 3). These results indicate that 10% PRP enhances the ability of migration in AT-MSCs in comparison with 10% FBS when exposed to high D-glucose conditions (50 and 100 mM).
10% PRP enhances the ability of in vitro cell migration under high D-glucose conditions. (A) The image was taken using a microscope and analyzed by Wimasis software at 0 h and 24 h. (B) Percentage of wound areas of nAT-MSCs (control and 100 mM) and dAT-MSCs at 0 h and 24 h. Data were taken from the average of eight different wound areas and three replications (mean value ± SD); **, P < 0.01. Scale bar: 500 μm.
The ability of stem cells to migrate into the damaged area plays a very important role in wound healing [8, 14]. Our previous studies have shown that high D-glucoses concentrations is impaired the ability to heal wounds of AT-MSCs in-vitro model. Here, we aim to demonstrate that the PRP can induce the ability to heal wounds of AT-MSCs in-vitro under high D-glucose conditions. To study the influence of autologous platelet plasma on the wound healing capacity of AT-MSC under high D-glucose conditions, a scratch assay mimicking cell movement during in-vivo wound healing was conducted. Previous studies indicate that 10% PRP stimulates fibroblast migration within 24 h [16]. Similarly, our results show that the wound healing ability of AT-MSCs cultured with 10% PRP significantly enhanced the migration ability after 24 h in comparison with the control group. Our previous study demonstrated that the ability to cover wounds of AT-MSCs has been significantly reduced when exposed to 100 mM D-glucose [17]. In this present study, our data showed that 10% PRP can significantly enhance the migration ability of AT-MSC under exposure of 100 mM D-glucose. Nevertheless, in all groups treated with high D-glucose concentrations (25, 50 and 100 mM), 10% PRP also statistically promoted the migration ability of AT-MSCs when compared with 10% FBS media (Fig. 4). Thus, the result of this study shows that 10% PRP concentrations stimulate proliferation and migration of AT-MSC in-vitro.
4 Conclusion
Taken together, our study demonstrated that 10% PRP enhances the in-vitro wound healing ability of AT-MSCs in high D-glucose conditions compared with 10% FBS and 10% PPP. This work gives a better knowledge of the influence of PRP on the activities of AT-MSCs for future applications of AT-MSCs coupled with autologous platelet plasma products in the realms of wound healing and cosmetology.
References
Saeedi, P., et al.: Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diab. Res. Clin. Pract. 157, 107843 (2019). https://doi.org/10.1016/j.diabres.2019.10784
Okonkwo, U.A., DiPietro, L.A.: Diabetes and wound angiogenesis. Int. J. Mol. Sci. 18(7), 1419 (2017). https://doi.org/10.3390/ijms180714
Lilao-Garzón, J., Valverde-Tercedor, C., Muñoz-Descalzo, S., Brito-Casillas, Y., Wägner, A.M.: In vivo and in vitro models of diabetes: a focus on pregnancy. In: Islam, M.S. (ed.) Diabetes: from Research to Clinical Practice. AEMB, vol. 1307, pp. 553–576. Springer, Cham (2020). https://doi.org/10.1007/5584_2020_536
Dewangan, H., Tiwari, R.K., Sharma, V., Shukla, S.S., Satapathy, T., Pandey, R.: Past and future of in-vitro and in-vivo animal models for diabetes: a review. Indian J. Pharmaceut. Educ. Res. 51(4S), s522–s530 (2017). https://doi.org/10.5530/ijper.51.4s.79
Gadelkarim, M., Abushouk, A.I., Ghanem, E., Hamaad, A.M., Saad, A.M., Abdel-Daim, M.M.: Adipose-derived stem cells: effectiveness and advances in delivery in diabetic wound healing. Biomed. Pharmacother. 107, 625–633 (2018). https://doi.org/10.1016/j.biopha.2018.08.013
Mazini, L., Rochette, L., Amine, M., Malka, G.: Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int. J. Mol. Sci. 20(10), 2523 (2019). https://doi.org/10.3390/ijms20102523
Trinh, N.T., et al.: Increased expression of EGR-1 in diabetic human adipose tissue-derived mesenchymal stem cells reduces their wound healing capacity. Stem Cells Develop. 25(10), 760–773 (2016). https://doi.org/10.1089/scd.2015.0335
Trinh, N.T., et al.: Microvesicles enhance the mobility of human diabetic adipose tissue-derived mesenchymal stem cells in vitro and improve wound healing in vivo. Biochem. Biophys. Res. Commun. 473(4), 1111–1118 (2016). https://doi.org/10.1016/j.bbrc.2016.04.025
Aliakbari, S., Mohammadi, M., Rezaee, M.A., Amini, A.A., Fakhari, S., Rahmani, M.R.: Impaired immunomodulatory ability of type 2 diabetic adipose-derived mesenchymal stem cells in regulation of inflammatory condition in mixed leukocyte reaction. EXCLI J. 18, 852 (2019). https://doi.org/10.17179/excli2019-1575
Li, Y.M., et al.: Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem. Biophys. Res. Commun. 363(1), 209–215 (2007). https://doi.org/10.1016/j.bbrc.2007.08.161
Burnouf, T., Goubran, H.A., Chen, T.M., Ou, K.L., El-Ekiaby, M., Radosevic, M.: Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 27(2), 77–89 (2013). https://doi.org/10.1016/j.blre.2013.02.001
Cabaro, S., et al.: White cell and platelet content affects the release of bioactive factors in different blood-derived scaffolds. Platelets 29(5), 463–467 (2018). https://doi.org/10.1080/09537104.2017.1319046
Felthaus, O., et al.: Effects of different concentrations of Platelet-rich Plasma and Platelet-Poor Plasma on vitality and differentiation of autologous Adipose tissue-derived stem cells. Clin. Hemorheol. Microcirc. 66(1), 47–55 (2017). https://doi.org/10.3233/CH-160203
Xu, P., et al.: Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma (2020). https://doi.org/10.1093/burnst/tkaa028
Sun, Y., Cao, Y., Zhao, R., Xu, F., Wu, D., Wang, Y.: The role of autologous PRP on deep partial-thickness burn wound healing in Bama pigs. J. Burn Care Res. 41(3), 657–662 (2020). https://doi.org/10.1093/jbcr/iraa012
Park, H.B., Yang, J.H., Chung, K.H.: Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J Hematol. 46(4), 265–273 (2011). https://doi.org/10.5045/kjh.2011.46.4.265
Mai, H.-P., Trinh, N.-T., Long, V.B., Binh, N.T., Nguyen, D.-Q., Duong, H.-X.: The high D-glucose concentration reduces the ability of wound healing in vitro of human adipose tissue-derived mesenchymal stem cells. In: Van Toi, V., Nguyen, T.-H., Long, V.B., Huong, H.T.T. (eds.) BME 2020. IP, vol. 85, pp. 581–590. Springer, Cham (2022). https://doi.org/10.1007/978-3-030-75506-5_49
Cavallo, C., et al.: Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. BioMed Res. Int. 2016, 6591717 (2016). doi:https://doi.org/10.1155/2016/6591717
Kakudo, N., et al.: Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plastic Reconstruct. Surg. 122(5), 1352–1360 (2008). https://doi.org/10.1097/PRS.0b013e318188204
Stessuk, T., et al.: Platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells: stimulatory effects on proliferation and migration of fibroblasts and keratinocytes in vitro. Arch. Dermatol. Res. 308(7), 511–520 (2016). https://doi.org/10.1007/s00403-016-1676-1
Acknowledgement
This research is also funded by Vietnam National University-Ho Chi Minh city (VNU-HCM) under the grant number NCM2020–28-01. We thank Professor Osamu Ohneda for providing stem cells and Biotechnology Center for supporting some research materials and equipment.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
No conflict of interest regarding the publication of this paper.
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Trinh, C.NM., Ha, N.Ny., Vong, L.B., Nguyen, M.Nh., Trinh, T.N., Nguyen, T.Tk. (2024). The Effect of 10% Platelet-Rich Plasma on In-Vitro Wound Healing Ability of Adipose Tissue-Derived Mesenchymal Stem Cells Under High D-Glucose Conditions. In: Vo, V.T., Nguyen, TH., Vong, B.L., Le, N.B., Nguyen, T.Q. (eds) 9th International Conference on the Development of Biomedical Engineering in Vietnam. BME 2022. IFMBE Proceedings, vol 95. Springer, Cham. https://doi.org/10.1007/978-3-031-44630-6_19
Download citation
DOI: https://doi.org/10.1007/978-3-031-44630-6_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44629-0
Online ISBN: 978-3-031-44630-6
eBook Packages: EngineeringEngineering (R0)