Abstract
Background:
Low serum vitamin D level is associated with both high blood pressure and incidence of primary hypertension. Experimental studies suggest that vitamin D supplements may reduce blood pressure.
Objective:
The aim of this study was to investigate whether vitamin D supplementation reduces systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) in Iranian patients with essential hypertension.
Method:
A total of 173 patients with essential hypertension participated in this open-label clinical trial. SBP, DBP, and serum vitamin D levels were measured at baseline and at the end of the study. Vitamin D was administered at a dose of 50,000 IU/week, and 1000 IU/day in patients with serum vitamin D levels <20 ng/mL and 20–30 ng/mL, respectively, for 8 weeks.
Results:
Based on serum vitamin D levels, 45.1%, 17.3%, and 29.5% of patients were deficient, insufficient, and sufficient for vitamin D intake, respectively. Baseline serum levels of vitamin D were not correlated with SBP, DBP, and MAP at the beginning of the study (p = ns). Multiple logistic regression analysis revealed that the risk of vitamin D deficiency was 2.5-fold times higher in women than in men (p = 0.03). After 8 weeks of supplementation with vitamin D, mean SBP and MAP were significantly reduced by 5.5 ± 16.16 (p = 0.01) and 3.7 ± 9.24 (p = 0.004) mmHg, respectively. Neither sex nor age could significantly predict BP response to vitamin D supplementation.
Conclusion:
Vitamin D supplementation may significantly reduce SBP and MAP but not DBP in patients with essential hypertension.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Vitamin D deficiency is a common problem with serious implications for human health [1,2,3]. Several studies revealed that low 25-hydroxy (25-OH) vitamin D levels are strongly associated with cardiovascular diseases including higher blood pressure and a higher rate of hypertension [4,5,6,7,8,9].
Previous observational studies and meta-analyses of vitamin D intervention suggest that vitamin D supplementation may decrease blood pressure in selected patient groups and populations. For instance, in a recent meta-analysis of observational studies, every 16 ng/mL reduction in serum vitamin D levels was associated with a 16% increase in the risk of hypertension [10]. Moreover, low serum vitamin D levels in normotensive individuals have been reported to predispose to future hypertension [4].
Vitamin D receptor (VDR) is a transcription factor belonging to the nuclear receptor family. Vitamin D receptor is highly expressed on the vascular smooth muscle endothelium and cardiomyocytes [11, 12]. Sufficient levels of vitamin D prevent contraction of venous smooth muscle cells and increase arterial compliance. Downregulation of vitamin D receptor in animal models led to elevation of blood pressure [13], which suggest that it can be amended with vitamin D oral supplementation.
Molecular events of vitamin D–vitamin D receptor ligation, including suppression of the renin–angiotensin–aldosterone system (RAAS), nephroprotective actions, or induction of endothelial/vascular function, suggest an antihypertensive properties of vitamin D [14, 15]. Vitamin D plays several roles in vascular structure and functions such as reducing the expression of thrombogenic genes, increasing vasodilation-related genes, and upregulation of prostacyclin, the latter being a vasodilator [16, 17].
However, several randomized controlled trials (RCTs) on vitamin D supplementation in hypertensive patients have shown disappointing results with most reports showing no beneficial effects [18,19,20,21,22,23,24,25]. For instance, Witham et al. reported that supplementation with 100,000 IU of oral vitamin D every 3 months for 1 year causes no appreciable change in blood pressure compared with placebo [26]. These findings have argued the value of vitamin D as a powerful treatment for hypertension.
Despite a wide range of antihypertensive drugs now being approved and available with various mechanism of actions, treatment of refractory hypertensive patients – the blood pressure remains above the goal despite use of three different classes of hypertensive agents – remains challenging, with numerous subjects experiencing treatment-limiting side effect [27]. Although recent strategies have focused on invasive approaches, such as renal denervation therapy with debatable result [28], the large burden of resistant hypertension at the population level means that low-cost, easy-to-apply interventions to mitigate the problem are still required.
However, it does not seem likely that vitamin D administration will have an effective and uniformly lowering effect on blood pressure across all populations and races. In this light, we conducted an open-label trial to investigate the effect of vitamin D on blood pressure in Iranian patients with essential hypertension.
2 Materials and Methods
2.1 Study Design
The present study was an open-label clinical trial. The study enrolled 173 patients, between 18 and 65 years old (mean age = 57.6 ± 9.3 years) who had an averaged SBP ≥ 140 or DBP ≥ 90 mm Hg based on Joint National Committee 7 (JNC7) criteria [29], or participants were eligible for inclusion if they had received ≥1 antihypertensive medication and recruited at endocrine disease clinic in Baqiyatallah Hospital, affiliated Tehran, Iran. Mean arterial pressure (MAP) was calculated based on systolic and diastolic blood pressure (SBP and DBP) using the formula below:
Enrollment began in October 2014, and the final follow-up visit was carried out in July 2015. Information regarding type and dose of antihypertensive drugs were documented. Exclusion criteria were as follows: patients with known cardiovascular disease (left ventricular ejection fraction (LVEF) ≤ 45%, as prior myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass, or stroke), renal disease (serum creatinine ≥1.5 mg/dl) and liver disorders (subjects with the liver enzyme alanine aminotransferase (ALT) ≥3 folds of upper limit of normal value range), secondary hypertension, and chronic obstructive pulmonary disorder. Individuals were also excluded if they had used any kind of vitamin D supplementation in the past 3 months. This study was performed in accordance with the Declaration of Helsinki guidelines, and the study protocol was approved by the Ethics Committee of the Baqiyatallah University of Medical Sciences, Tehran, Iran (IR.RMSU.REC.1394.31). Written informed consent was obtained from all participants. The trial was registered at IRCT.ir (IRCT20080901001165N57). At the first screening visit, the blood pressure was measured by a physician three times using standard sphygmomanometer [30].
2.2 Vitamin D Supplementation and Blood Pressure Monitoring
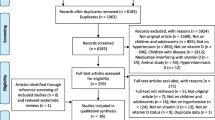
Following participants enrolling , vein blood was drawn from patients and serum level of 25-OH vitamin D was measured in the medical laboratory of Baqiyatallah Hospital. The patients were classified in three groups as vitamin D deficient (<20 ng/ml), insufficient (20–30 ng/ml), and sufficient (>30 ng/ml) (to convert to nanomoles per liter, multiply by 2.496) [31]. 50,000 U weekly and 1000 U daily vitamin D (pearl vitamin D3, Zahravi Pharma Co, Tehran, Iran) were administrated in vitamin D-deficient and vitamin D-insufficient patients, respectively. Vitamin D administration was continued for 8 weeks and endpoint blood pressure was measured. At week 8, serum level and relation of 25-OH vitamin D status to change in blood pressures were also determined. Outcomes, including 25-OH vitamin D serum levels and endpoint blood pressure, were all measured on the same day. Patients were not administrated with calcium supplementation; however, they were informed by lifestyle changes with advice on optimal calcium consumption . All patients were followed during the 8 weeks of intervention to ensure regular administration of vitamin D and to monitor suspected medication adverse effects (Fig. 1).
2.3 Statistical Methods
Sample size was calculated based on power (1-β probability error) = 80% and α = 0.05 using G Power version 3.1. We estimated the size of the sample for this study based on data from the previous study [32]. There was a significant inverse correlation between serum level of 25-OH vitamin D and blood pressure in our study. A power analysis using G*power software revealed that 52 subjects were required for our study to detect a significant difference between the groups.
Qualitative and quantitative data are demonstrated by percentage and mean ± standard deviation of three independent measurements. The Kolmogorov–Smirnov test was used to assess the normal distribution of data. The statistical analysis was only performed on the treated group. The correlation between serum level of 25-OH vitamin D and SBP, DBP, and MAP was determined using Spearman correlation test. The number of the antihypertensive drug regimens was compared between normal and vitamin D-deficient groups using chi-square test (patients were divided into two: deficient and sufficient groups). Multivariate logistic regression method was applied to determine the potential effect of risk factors (sex, age, and body mass index (BMI)) on the goal of therapy, and in this regard, patients were classified in groups with or without age (≥55 for men, ≥65 for women) and body mass index (≥30 kg/m2) risk factors. The patients were classified as deficient and sufficient groups (because of low number of patients in insufficient group) for chi-square and logistic regression tests. SPSS 20 (SPSS Inc., Chicago, Illinois) software used to run statistical data analysis and P values of less than 0.05 were regarded as statistically significant.
3 Results
3.1 Demographic Data
In the present study, 173 patients with essential hypertension were evaluated. Representative data of sex, age, BMI, and clinical features of participants flow through the trial is shown in Table 1. The antihypertensive drug regimens were not changed during the present study. Hypertensive patients were classified in three groups of sufficient (51 patients), insufficient (36 patients), and deficient (86 patients) based on serum level of 25-OH vitamin D. No significant correlation was observed between baseline 25-OH vitamin D serum level and SBP, DBP, and MAP of 155 patients (Table 2). Fourteen patients were dropped out due to loss to follow up.
3.2 Effect of Intervention on 25-OH Vitamin D Serum Level
At the first visit screening, 86 (49.7%), 36 (20.8%), and 51 (29.5%) patients were characterized in deficient, insufficient, and sufficient groups regarding serum level of 25-OH vitamin D, respectively. After 8 weeks, mean 25-OH vitamin D serum levels significantly increased following supplementation from a baseline level in insufficient group from 25.3 ± 3.0 ng/ml to 35.1 ± 9.1 ng/mL and deficient group from 9.9 ± 5.0 ng/ml to 30.2 ± 10.6 ng/mL (P < 0.0001).
3.3 Effect of Vitamin D Supplementation on Systolic, Diastolic, and Mean Arterial Pressure
No significant correlation was observed between SBP, DBP, and MAP with 25-OH vitamin D serum level. However, vitamin D supplementation concomitant with conventional antihypertensive drug regimens caused a statistically significant 5.5 ± 16.2 mm Hg decrease of overall SBP (p = 0.01, 95% confidence interval = 1.3, 9.6). Vitamin D intervention decreased overall DBP by mean of 1.4 ± 12.4 mm Hg (p = 0.3, 95% confidence interval = −1.7, 4.6). Moreover, the intervention led to 3.7 ± 9.2 mmHg decrease of mean arterial pressure (MAP) (p = 0.004, 95% confidence interval = 1.2–6.2) (Table 3). In the present study, 58.4% of patients were receiving at least one RAAS blockers. The vitamin D supplementation in this group leads to a statistically significant reduction in SBP (p = 0.028, 95% confidence interval = 0.7–11.9) and MAP (p = 0.02, 95% confidence interval = −0.9-8.0) and a marginally nonsignificant reduction in DBP (p = 0.055, 95% confidence interval = 0.9–8.0).
3.4 The Relation of Antihypertensive Drug Regimens and 25-OH Vitamin D Serum Level
To investigate if 25-OH vitamin D serum level is in relation with the number of antihypertensive drug regimens, the number of administrated antihypertensive drugs was compared with vitamin D-sufficient and vitamin D-deficient patients using chi-square test. In the present set of analysis, the patients were categorized as vitamin D sufficient (≥30 ng/ml) and deficient (<30 ng/ml). There were no significant differences in the number of antihypertensive drugs that patients are taking in the vitamin D-sufficient and vitamin D-deficient groups according to the chi-square test (p > 0.05) (Table 4).
3.5 Effect of Sex, Age, and Body Mass Index on Clinical Response of Vitamin D Treatment in Patients with Hypertension
Multiple logistic regression analysis showed a significant association between sex but not age and BMI with 25-OH vitamin D serum level (p = 0.03) (Table 5). This test was also employed to analyze association of sex, age, and BMI risk factors and clinical response of vitamin D intervention (Table 6). In accordance with JNC7 guideline, the goal of therapy was determined as systolic/diastolic blood pressure <130/80 mmHg and <140/90 for diabetic and nondiabetic patient groups, respectively [29]. Comparison of risk factors between patients whose blood pressure reached the goal of therapy and not showed that neither sex nor age had significant association with goal of therapy in both groups of patients. However, a marginally significant (p value: 0.053) and negative correlation was seen between BMI and goal of therapy (Table 6).
4 Discussion
Hypertension has recently emerged as an important risk factor for the public health burden. Recently, several preventive and protective effects of vitamin D are found in a wide range of diseases including cancer, autoimmune disease, diabetes, infections, depression, osteoporosis, and cardiovascular diseases [33]. It is of interest to investigate if vitamin D supplementation has a uniformly lowering effect on blood pressure to clarify the beneficial effect of vitamin D for public health [34].
There are consistent epidemiological evidences linking low vitamin D status to a higher risk of hypertension. However, the findings of randomized controlled trials investigating the effects of vitamin D supplementation on blood pressure have not been fully conclusive, though a trend toward a modest reduction in blood pressure could be implied [35]. Several genetic and environmental factors can influence the consequence of vitamin D supplementation on blood pressure. These underlying factors include the vitamin D baseline status, the vitamin D dose, the dose–response relation between vitamin D and parathyroid hormone, calcium intake, and other factors, such as age, sex, BMI, genetics, and races [26]. It is important to put these variations in context of other evidence. One important aspect of the potential vitamin D–blood pressure relationship to consider is the population and biological variation [36].
In the present study, we conducted an open-label trial to investigate the effect of vitamin D on 173 Iranian patients with essential hypertension. Based on baseline 25-OH vitamin D serum level, 49.7%, 20.8%, and 29.5% of patients were classified as vitamin D deficient, insufficient, and normal, respectively. Mean 25-OH vitamin D serum level of patients was 24.0 ± 18.1 ng/ml. The data implies higher incidence (70.5%) of vitamin D shortage in the hypertensive patients in comparison to incidence of vitamin D deficiency of the general population in Iran (51%) [37]. In accordance with our finding, 81.3% and 72% of essential hypertensive patients were found with vitamin D deficiency in previous studies [38, 39].
No significant correlation was observed between SBP, MAP, and DBP with 25-OH vitamin D serum level. A similar study on 251 patients also showed no significant association of high blood pressure and 25-OH vitamin D serum level [40]. However, Vimaleswaran et al. showed a significant association between increased 25-OH vitamin D level and decreased SBP and odds of hypertension of 49,363 patients [41]. In a cross-sectional study, a higher vitamin D level was associated with lower blood pressure [42]. Several possibilities merit discussion to explain these controversial findings. The geographical and seasonal differences and smaller sample size of our study may explain the discrepancy. However, some aspect like the dose–response relation between 25-OH vitamin D and parathyroid hormone, calcium intake, and underlying population factors such as age, sex, BMI, genetics, and medications can be considered in the potential vitamin D–blood pressure relationship [26]. All participants were resident of Tehran and all examinations and measurements were carried out in spring.
Eight weeks of oral vitamin D supplementation concomitant with conventional antihypertensive drug regimens caused 5.5 ± 16.2 mm Hg, 1.4 ± 12.6 mm Hg, and 3.7 ± 9.2 mm Hg decrease of overall SBP, DBP, and MAP, respectively. Altogether, the present intervention significantly reduced SBP and MAP in patients with essential hypertension.
The previous study using similar dose of vitamin D has shown significant reductions in blood pressure in patients with type 2 diabetes mellitus and low vitamin D levels [43]. However, in a recent study, a total of 100,000 U of oral cholecalciferol every 3 months for 1 year did not improve blood pressure or markers of vascular health in patients with hypertension [44]. 100,000 U of vitamin D every 2 months for 6 months did not reduce 24-hour ambulatory and office blood pressure in patients with resistant hypertensive patients [35]. Some possibilities merit discussion to enlighten these controversial findings. It is possible that the dose of vitamin D was inadequate to reach clinical response. Moreover, regional variation may influence mean of serum level of vitamin D and the therapeutic effect of vitamin D intervention [36]. Finally, a wide range of antihypertensive agents have been used by subjects; thus, many of the available molecular pathways of blood pressure may have already been engaged. Vitamin D exerts antihypertensive effects through renin–angiotensin–aldosterone system [13]. A high proportion of hypertensive patients are regularly taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists that may obviate any further therapeutic benefit of vitamin D intervention. In the present study, 58.4% of patients were taking RAAS blockers. In this group, vitamin D supplementation could lead to a significant decrease in SBP and MAP. This effect may be caused by a synergistic effect of vitamin D supplementation and RAAS blockers, which led to achieving the goal of therapy in hypertensive patients.
We hypothesized that 25-OH vitamin D serum level may be in relation with the number of antihypertensive drug regimens. So, in the next set of analysis, the number of administrated antihypertensive drugs was compared in vitamin D-sufficient and vitamin D-deficient groups. This study failed to prove the hypothesis, and no significant differences was found in the number of antihypertensive drugs that patients administrated in vitamin D-sufficient and vitamin D-deficient groups.
Multiple logistic regression analysis in our study showed that risk of vitamin D deficiency was 2.5-fold times higher in women subjects. Women dress code (Hijab) in Islamic regions as sunlight barrier may explain lower vitamin D in women participants [45]. This finding emphasizes the necessity of vitamin D fortification of food in countries where some people may not be exposed to sunlight because of cultural and religious dress styles. Moreover, neither sex nor age showed significant association with goal of therapy. However, a marginally nonsignificant and negative correlation was seen between BMI and goal of therapy. In Multi-Ethnic Study of Atherosclerosis, a cohort and prospective study, on 3002 subjects free of prevalent cardiovascular disease and hypertension, lower serum 25-OH vitamin D categories were associated with higher unadjusted incident hypertension, but after adjustment for potential confounders such as BMI (as continuous variable) and kidney function, the association was no longer significant [46]. On the other hand, in meta-analysis of 108,173 subjects of 35 studies, increased 25-OH levels were associated with reduced SBP but not DBP and this association was not altered after adjustment for age, sex, method of blood pressure measurement, geographical region, and BMI [41]. To the best of our knowledge, until now, no study was found that investigate the effect of age, sex, or BMI on the goal of therapy after vitamin D supplementation.
One limitation of the current study was lack of placebo control group in the trial, which might have caused an overestimation of the effects of vitamin D supplementation. Also the present study was not blinded, and therefore, the results, though objective in nature, might have been biased due to potential placebo effects.
In conclusion, the present results suggested that vitamin D supplementation decreases SBP and MAP but not DBP in patients with essential hypertension on antihypertensive treatment regimens. Further investigations could explore if higher doses of vitamin D at longer treatment durations may be more effective, or whether selected patients (e.g., those not taking renin–angiotensin–aldosterone system inhibitors) may elicit differential blood pressure response to vitamin D supplementation. Molecular experiments on vitamin D receptor expression levels and polymorphisms of patients who reached treatment goal would shed more light on the underlying mechanism action of vitamin D on blood pressure.
References
Akbas, E. M., Gungor, A., Ozcicek, A., Akbas, N., Askin, S., & Polat, M. (2016). Vitamin D and inflammation: Evaluation with neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Archives of Medical Science, 12(4), 721–727.
Capusa, C., Stefan, G., Stancu, S., Ilyes, A., Dorobantu, N., & Mircescu, G. (2016). Subclinical cardiovascular disease markers and vitamin D deficiency in non-dialysis chronic kidney disease patients. Archives of Medical Science, 12(5), 1015–1022.
Krela-Kazmierczak, I., Szymczak, A., Lykowska-Szuber, L., Eder, P., Stawczyk-Eder, K., Klimczak, K., et al. (2015). The importance of vitamin D in the pathology of bone metabolism in inflammatory bowel diseases. Archives of Medical Science, 11(5), 1028–1032.
Forman, J. P., Curhan, G. C., & Taylor, E. N. (2008). Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension, 52(5), 828–832.
Forman, J. P., Giovannucci, E., Holmes, M. D., Bischoff-Ferrari, H. A., Tworoger, S. S., Willett, W. C., et al. (2007). Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension, 49(5), 1063–1069.
Scragg, R., Sowers, M., & Bell, C. (2007). Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. American Journal of Hypertension, 20(7), 713–719.
Wang, T. J., Pencina, M. J., Booth, S. L., Jacques, P. F., Ingelsson, E., Lanier, K., et al. (2008). Vitamin D deficiency and risk of cardiovascular disease. Circulation, 117(4), 503–511.
Ilincic, B., Stokic, E., Stosic, Z., Kojic, N. E., Katsiki, N., Mikhailidis, D. P., et al. (2017). Vitamin D status and circulating biomarkers of endothelial dysfunction and inflammation in non-diabetic obese individuals: A pilot study. Archives of Medical Science, 13(1), 53–60.
Dziedzic, E. A., Przychodzen, S., & Dabrowski, M. (2016). The effects of vitamin D on severity of coronary artery atherosclerosis and lipid profile of cardiac patients. Archives of Medical Science, 12(6), 1199–1206.
Burgaz, A., Orsini, N., Larsson, S. C., & Wolk, A. (2011). Blood 25-hydroxyvitamin D concentration and hypertension: A meta-analysis. Journal of Hypertension, 29(4), 636–645.
Merke, J., Milde, P., Lewicka, S., Hugel, U., Klaus, G., Mangelsdorf, D. J., et al. (1989). Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. The Journal of Clinical Investigation, 83(6), 1903–1915.
Somjen, D., Weisman, Y., Kohen, F., Gayer, B., Limor, R., Sharon, O., et al. (2005). 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation, 111(13), 1666–1671.
Li, Y. C., Kong, J., Wei, M., Chen, Z. F., Liu, S. Q., & Cao, L. P. (2002). 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. The Journal of Clinical Investigation, 110(2), 229–238.
Kienreich, K., Grubler, M., Tomaschitz, A., Schmid, J., Verheyen, N., Rutters, F., et al. (2013). Vitamin D, arterial hypertension & cerebrovascular disease. The Indian Journal of Medical Research, 137(4), 669–679.
Pilz, S., Gaksch, M., O'Hartaigh, B., Tomaschitz, A., & Marz, W. (2013). The role of vitamin D deficiency in cardiovascular disease: Where do we stand in 2013? Archives of Toxicology, 87(12), 2083–2103.
Wakasugi, M., Noguchi, T., Inoue, M., Kazama, Y., Tawata, M., Kanemaru, Y., et al. (1991). Vitamin D3 stimulates the production of prostacyclin by vascular smooth muscle cells. Prostaglandins, 42(2), 127–136.
Wu-Wong, J. R., Nakane, M., Ma, J., Ruan, X., & Kroeger, P. E. (2006). Effects of vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis, 186(1), 20–28.
Arora, P., Song, Y., Dusek, J., Plotnikoff, G., Sabatine, M. S., Cheng, S., et al. (2015). Vitamin D therapy in individuals with prehypertension or hypertension: The DAYLIGHT trial. Circulation, 131(3), 254–262.
Dalbeni, A., Scaturro, G., Degan, M., Minuz, P., & Delva, P. (2014). Effects of six months of vitamin D supplementation in patients with heart failure: A randomized double-blind controlled trial. Nutrition, Metabolism, and Cardiovascular Diseases, 24(8), 861–868.
Jorde, R., Sneve, M., Torjesen, P., & Figenschau, Y. (2010). No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. Journal of Internal Medicine, 267(5), 462–472.
Kunutsor, S. K., Burgess, S., Munroe, P. B., & Khan, H. (2014). Vitamin D and high blood pressure: Causal association or epiphenomenon? European Journal of Epidemiology, 29(1), 1–14.
Larsen, T., Mose, F. H., Bech, J. N., Hansen, A. B., & Pedersen, E. B. (2012). Effect of cholecalciferol supplementation during winter months in patients with hypertension: A randomized, placebo-controlled trial. American Journal of Hypertension, 25(11), 1215–1222.
Sollid, S. T., Hutchinson, M. Y., Fuskevag, O. M., Figenschau, Y., Joakimsen, R. M., Schirmer, H., et al. (2014). No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care, 37(8), 2123–2131.
Witham, M. D., Dove, F. J., Khan, F., Lang, C. C., Belch, J. J., & Struthers, A. D. (2013). Effects of vitamin D supplementation on markers of vascular function after myocardial infarction – A randomised controlled trial. International Journal of Cardiology, 167(3), 745–749.
Wood, A. D., Secombes, K. R., Thies, F., Aucott, L., Black, A. J., Mavroeidi, A., et al. (2012). Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: A parallel-group, double-blind, placebo-controlled RCT. The Journal of Clinical Endocrinology and Metabolism, 97(10), 3557–3568.
Giovannucci, E. (2013). Cholecalciferol treatment in older patients with isolated systolic hypertension. JAMA Internal Medicine, 173(18), 1680–1681.
Pirmohamed, M., James, S., Meakin, S., Green, C., Scott, A. K., Walley, T. J., et al. (2004). Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ, 329(7456), 15–19.
Davis, M. I., Filion, K. B., Zhang, D., Eisenberg, M. J., Afilalo, J., Schiffrin, E. L., et al. (2013). Effectiveness of renal denervation therapy for resistant hypertension: A systematic review and meta-analysis. Journal of the American College of Cardiology, 62(3), 231–241.
Chobanian, A. V., Bakris, G. L., Black, H. R., Cushman, W. C., Green, L. A., Izzo, J. L., Jr., et al. (2003). Seventh report of the joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension, 42(6), 1206–1252.
Pickering, T. G., Hall, J. E., Appel, L. J., Falkner, B. E., Graves, J., Hill, M. N., et al. (2005). Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation, 111(5), 697–716.
Looker, A. C., Johnson, C. L., Lacher, D. A., Pfeiffer, C. M., Schleicher, R. L., & Sempos, C. T. (2011). Vitamin D status: United States, 2001–2006. NCHS Data Brief, 59, 1–8.
Lind, L., Hanni, A., Lithell, H., Hvarfner, A., Sorensen, O. H., & Ljunghall, S. (1995). Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. American Journal of Hypertension, 8(9), 894–901.
Pludowski, P., Holick, M. F., Pilz, S., Wagner, C. L., Hollis, B. W., Grant, W. B., et al. (2013). Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality – A review of recent evidence. Autoimmunity Reviews, 12(10), 976–989.
Lim, S. S., Vos, T., Flaxman, A. D., Danaei, G., Shibuya, K., Adair-Rohani, H., et al. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380(9859), 2224–2260.
Witham, M. D., Ireland, S., Houston, J. G., Gandy, S. J., Waugh, S., Macdonald, T. M., et al. (2014). Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension: Randomized, controlled trial. Hypertension, 63(4), 706–712.
Mithal, A., Wahl, D. A., Bonjour, J. P., Burckhardt, P., Dawson-Hughes, B., Eisman, J. A., et al. (2009). Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis International, 20(11), 1807–1820.
Palacios, C., & Gonzalez, L. (2014). Is vitamin D deficiency a major global public health problem? The Journal of Steroid Biochemistry and Molecular Biology, 144, 138–45.
Hashemipour, S., Larijani, B., Adibi, H., Javadi, E., Sedaghat, M., Pajouhi, M., et al. (2004). Vitamin D deficiency and causative factors in the population of Tehran. BMC Public Health, 4, 38.
Mahdavi, K., Amirajam, Z., Yazdankhah, S., Majidi, S., Adel, M. H., Omidvar, B., et al. (2013). The prevalence and prognostic role of vitamin D deficiency in patients with acute coronary syndrome: A single centre study in south-west of Iran. Heart, Lung & Circulation, 22(5), 346–351.
Kashi, Z., Mirmiran, P., Mehrabi, Y., Hedayati, M., & Azizi, F. (2003). Association of blood pressure, serum vitamin D, calcium and PTH in individuals over 40 in East Tehran. Iranian Journal of Endocrinology and Metabolism, 5(4), 261–270.
Vimaleswaran KS, Cavadino A, Berry DJ, LifeLines Cohort Study i, Jorde R, Dieffenbach AK et al. (2014). Association of vitamin D status with arterial blood pressure and hypertension risk: A Mendelian randomisation study. The Lancet Diabetes and Endocrinology, 2(9), 719–729.
He, J. L., & Scragg, R. K. (2011). Vitamin D, parathyroid hormone, and blood pressure in the National Health and Nutrition Examination Surveys. American Journal of Hypertension, 24(8), 911–917.
Sugden, J. A., Davies, J. I., Witham, M. D., Morris, A. D., & Struthers, A. D. (2008). Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabetic Medicine, 25(3), 320–325.
Witham, M. D., Price, R. J., Struthers, A. D., Donnan, P. T., Messow, C. M., Ford, I., et al. (2013). Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: The VitDISH randomized controlled trial. JAMA Internal Medicine, 173(18), 1672–1679.
Alagol, F., Shihadeh, Y., Boztepe, H., Tanakol, R., Yarman, S., Azizlerli, H., et al. (2000). Sunlight exposure and vitamin D deficiency in Turkish women. Journal of Endocrinological Investigation, 23(3), 173–177.
van Ballegooijen, A. J., Kestenbaum, B., Sachs, M. C., de Boer, I. H., Siscovick, D. S., Hoofnagle, A. N., et al. (2014). Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology, 63(12), 1214–1222.
Acknowledgments
We thank for laboratory analyses and participating practices of the Baqiyatallah Hospital.
Conflict of Interest
None.
Funding Agency
This work was supported by Baqiyatallah University of Medical Sciences.
Ethics Approval and Consent to Participate
This study was performed in accordance with the Declaration of Helsinki guidelines, and the study protocol was approved by the Ethics Committee of the Baqiyatallah University of Medical Sciences, Tehran, Iran (IR.RMSU.REC.1394.31).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Panahi, Y. et al. (2021). Effect of Vitamin D Supplementation on the Regulation of Blood Pressure in Iranian Patients with Essential Hypertension: A Clinical Trial. In: Sahebkar, A., Sathyapalan, T. (eds) Natural Products and Human Diseases. Advances in Experimental Medicine and Biology(), vol 1328. Springer, Cham. https://doi.org/10.1007/978-3-030-73234-9_35
Download citation
DOI: https://doi.org/10.1007/978-3-030-73234-9_35
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73233-2
Online ISBN: 978-3-030-73234-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)