Abstract
The high worldwide prevalence of vitamin D deficiency is largely the result of low sunlight exposure with subsequently limited cutaneous vitamin D production. Classic manifestations of vitamin D deficiency are linked to disturbances in bone and mineral metabolism, but the identification of the vitamin D receptor in almost every human cell suggests a broader role of vitamin D for overall and cardiovascular health. The various cardiovascular protective actions of vitamin D such as anti-diabetic and anti-hypertensive effects including renin suppression as well as protection against atherosclerosis and heart diseases are well defined in previous experimental studies. In line with this, large epidemiological studies have highlighted vitamin D deficiency as a marker of cardiovascular risk. However, randomized controlled trials (RCTs) on vitamin D have largely failed to show its beneficial effects on cardiovascular diseases and its conventional risk factors. While most prior vitamin D RCTs were not designed to assess cardiovascular outcomes, some large RCTs have been initiated to evaluate the efficacy of vitamin D supplementation on cardiovascular events in the general population. When considering the history of previous disappointing vitamin RCTs in general populations, more emphasis should be placed on RCTs among severely vitamin D-deficient populations who would most likely benefit from vitamin D treatment. At present, vitamin D deficiency can only be considered a cardiovascular risk marker, as vitamin D supplementation with doses recommended for osteoporosis treatment is neither proven to be beneficial nor harmful in cardiovascular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D was initially described by McCollum in 1922 as a substance capable of curing rickets (Holick 1994). It was originally classified as a vitamin and given that it was the fourth known vitamin, it was named “vitamin D”. Based on our current knowledge, however, vitamin D should rather be re-classified as a pro-hormone since its biologically most active metabolite, i.e., 1,25-dihydroxyvitamin D (1,25[OH]2D), is per definition a steroid hormone (Holick 2007). The receptor required for its endocrine function, i.e., the vitamin D receptor (VDR), is expressed in almost all human cells and regulates hundreds of genes, i.e., approximately three percent of the human genome (Bouillon et al. 2008). It is therefore tempting to speculate that vitamin D deficiency may have widespread adverse health consequences.
While effects of vitamin D are historically known to be relevant to bone and mineral diseases, accumulating data suggest that vitamin D might also play a role in various extra-skeletal diseases including auto-immune and infectious diseases, cancer, neuropsychological and cardiovascular diseases (CVD) (Holick 2007; Pludowski et al. 2013; Souberbielle et al. 2010; Muscogiuri et al. 2012; Brouwer-Brolsma et al. 2013). In this narrative review, we aim to provide a general up-to-date overview of the role of vitamin D in CVD. Following a brief outline on basic vitamin D metabolism and classic vitamin D effects, we will cover the various study types relating vitamin D to CVD and its risk factors. While the general topic of our review, in our opinion, cannot be adequately addressed by a systematic approach in a single journal article, we put particular emphasis on recent findings, existing systematic reviews and meta-analyses reporting on specific research questions regarding vitamin D and CVD. In addition to the potential benefits of vitamin D in CVD, we also provide a summary of the existing literature on the possible adverse consequences of vitamin D. Finally, we discuss our personal expectations for the future development and finish this review with our conclusions on the current implications regarding vitamin D and CVD for research and clinical practice.
Basic vitamin D metabolism
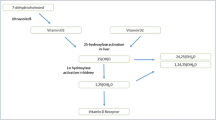
The chemical structure of vitamin D is a secosteroid. It has several isoforms with two major forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Its metabolism is unique since vitamin D can be derived from endogenous production in the skin as well as from dietary intake (Holick 2007). Cutaneous production requires ultraviolet-B (UV-B) radiation of the skin to convert the precursor 7-dehydrocholesterol, a liver-derived product, to previtamin D3 and finally to vitamin D3. Natural foods also contain vitamin D3 (e.g., fatty fish) or vitamin D2 (e.g., yeast or mushrooms). Additional sources for vitamin D3 or vitamin D2 are supplements and food fortification that has been introduced, for instance, in the US or in Finland (Black et al. 2012). The main source for vitamin D is sunlight (UV-B)-induced production in the skin that accounts for approximately 80% of vitamin D (MacDonald et al. 2011). This classic view of sunlight-induced vitamin D production as the major source of vitamin D has, however, been partially challenged by recent investigations suggesting that all source basal vitamin D inputs, in particular of undocumented food sources, might play a greater role than previously recognized (Heaney et al. 2013a). There is ongoing discussion on whether vitamin D3 supplementation is superior compared to vitamin D2 supplementation in raising the main circulating vitamin D metabolite 25-hydroxyvitamin D (25[OH]D) (Tripkovic et al. 2012). Some organizations and experts alike prefer the use of vitamin D3 rather than vitamin D2. Though since both isoforms are further converted to vitamin D metabolites that are considered to exert almost identical biological activities, the term vitamin D (meaning vitamin D3 and/or D2) will be used throughout the manuscript, unless otherwise stated, Vitamin D itself exerts no relevant effects unless it is further converted to its biologically active metabolites. Formation of the most active vitamin D metabolite 1,25-dihydroxyvitamin D (1,25[OH]2D), i.e., the “vitamin D hormone” calcitriol, requires two hydroxylation steps: the first one in the liver is catalyzed by 25-hydroxylase to form 25(OH)D, and the second one mainly takes place in the kidneys (proximal tubule cells) to form 1,25(OH)2D. This latter reaction is catalyzed by the enzyme 1-alpha-hydroxylase. The kidneys are the major site for the synthesis of circulating 1,25(OH)2D, but it has been demonstrated that several extra-renal tissues also express 1-alpha-hydroxylase (Höbaus et al. 2013). While kidney-derived 1,25(OH)2D circulates in the bloodstream to exert its endocrine functions, locally produced extra-renal 1,25(OH)2D does not significantly contribute to serum concentrations of 1,25(OH)2D. This in turn supports the concept that locally produced 1,25(OH)2D exerts its effects in an autocrine or paracrine manner (Peterlik and Cross 2009; Höbaus et al. 2013). A main difference between the renal and extra-renal 1-alpha-hydroxylases is that the activity of renal 1-alpha-hydroxylase is tightly controlled by parameters of mineral metabolism (e.g, stimulation by PTH or inhibition by fibroblast growth factor-23 [FGF-23]). Hence, circulating serum levels of 1,25(OH)2D are strongly influenced by renal 1-alpha-hydroxylase activity and are not a reliable indicator of whole body vitamin D status. In contrast, 25(OH)D levels are considered the most appropriate parameter for classification of vitamin D status, since the activity of 25-hydroxylase is primarily dependent on the supply of vitamin D. 25(OH)D is the major circulating vitamin D metabolite with serum levels up to ~1,000-fold higher than 1,25(OH)2D and has a significantly longer half-life (~3 weeks) compared to vitamin D or 1,25(OH)2D (a few hours for both). A general consensus has therefore been reached that 25(OH)D concentrations should be measured to assess vitamin D status (Holick 2007; Souberbielle et al. 2010; Ross et al. 2011). Similar to other steroid hormones, vitamin D metabolites are mainly transported in the circulation bound to its carrier, i.e, the vitamin D-binding protein (DBP). Only very small fractions of vitamin D metabolites circulate in completely unbound (free) forms or are bound to other carriers (e.g, lipoproteins). At present, laboratory methods measure and report the sum of all vitamin D metabolites, e.g, total 25(OH)D serum levels. It should, however, be noted that some, but not all, studies suggest that the free (unbound) fraction of 25(OH)D could be of high biological relevance and may be worth measuring or estimating using DBP concentrations (Powe et al. 2011; Dastani et al. 2013; Bhan et al. 2012). It must also be noted that the measurement of 25(OH)D is not straightforward and several studies have reported on significant assay and laboratory differences (Carter 2012). It is therefore important to standardize 25(OH)D measurements, which is the aim of the Vitamin D Standardization Program (VDSP) (Sempos et al. 2012; Schmid et al. 2013). Institutions working on the VDSP include the NIH Office of Dietary Supplements (ODS) in collaboration with the CDC National Center for Environmental Health (NCEH), the National Institute of Standards and Technology (NIST), and Ghent University (Sempos et al. 2012; Cashman et al. 2013).
While our understanding of vitamin D transport, cellular uptake or intracellular concentrations, and metabolism is still in its infancy, it is clear that VDR activation is required to exert vitamin D effects on gene regulation. The affinity of the VDR is much higher for 1,25(OH)2D than for 25(OH)D, which underscores why many authors consider 1,25(OH)2D as the active vitamin D hormone. As a steroid hormone receptor, the VDR is mainly located in an intracellular manner, but cell-membrane bound forms of the VDR have also been described suggesting some additional non-genomic effects (Bouillon et al. 2008). The classic pathway does, however, indicate that 1,25(OH)2D binds to the intracellular VDR and activates this receptor, followed by dimerization of the VDR and the retinoid X receptor (RXR). This VDR–RXR complex translocates to the nucleus where it binds to so-called vitamin D regulatory elements on the DNA. In concert with some interactions of the VDR/RXR complex with other regulatory factors, hundreds of genes are either up-regulated or down-regulated as a consequence of VDR activation (Bouillon et al. 2008). Degradation of vitamin D metabolites is initiated by 24-hydroxylation and results in the formation of, e.g, 24,25-dihydroxyvitamin D (24,25[OH]2D) or 1,24,25-dihydroxyvitamin D (1,24,25[OH]3D). These are known to be less active vitamin D metabolites that are further metabolized to calcitroic acid, which is then excreted via the urine (Reddy and Tserng 1989). While high levels of 1,25(OH)2D and subsequent VDR activation stimulate 24-hydroxylases in a regulatory loop, there remain some gaps in the literature regarding the biological meaning of many of the formed vitamin D metabolites (e.g, 24,25(OH)2D, 3-epimers and others) (Bailey et al. 2013; Jones et al. 2012).
Classic effects of vitamin D
Vitamin D has an established role in the regulation of bone and mineral metabolism (Bouillon et al. 2008; Holick 2007). Its effects on calcium metabolism by stimulation of calcium absorption in the gut ensure an adequate calcium supply required for physiologic bone mineralization. A hallmark of vitamin D deficiency is therefore an increase in parathyroid hormone (PTH) levels because PTH is synthesized in the parathyroid glands in response to low serum calcium. PTH effects on, for example, bone and kidneys are subsequently required to increase and maintain serum calcium concentrations within the normal range.
Overt vitamin D deficiency can therefore lead to rickets in children and osteomalacia in adults (Holick 2007; Lips 2001). These diseases are characterized by bone mineralization defects in addition to clinical symptoms such as proximal muscle weakness or bone pain (Lips 2001). Hence, vitamin D supplementation is recommended throughout the first year(s) of life in order to prevent rickets. Public health authorities base their dietary intake recommendations for vitamin D on doses that are considered to be sufficient to avoid osteomalacia or rickets (Ross et al. 2011). Beyond this, large RCTs and meta-analyses have shown that vitamin D supplementation reduce fractures and to some extent falls (Ross et al. 2011; Holick et al. 2011; Bischoff-Ferrari et al. 2012; Bischoff-Ferrari et al. 2009). Against this background, vitamin D supplementation is considered an integral part of the treatment of patients with osteoporosis. However, at present there is no consensus on the cutoff values for vitamin D deficiency. Suggested cutoffs for vitamin D deficiency according to 25(OH)D serum concentrations are <12 ng/mL (multiply by 2.496 to convert ng/mL to nmol/L), <20, and <30 ng/mL. A particularly hot debate is ongoing as to whether levels of 20 or 30 ng/mL can be considered sufficient (Holick et al. 2012; Rosen et al. 2012). In this context, it should be acknowledged that not all studies and meta-analyses have shown significant vitamin D effects on fractures and falls, so questions still remain regarding musculoskeletal vitamin D effects. These include, for example, the precise pathophysiological mechanisms, the optimal doses, and the impact of concomitant calcium supplementation (Ross et al. 2011; Holick et al. 2011; Brouwer-Brolsma et al. 2013). Important data for clinicians were derived from an individual patient data meta-analysis showing that vitamin D doses ranging from approximately 800–2,000 International Units (IU) per day (1 IU is equal to 0,025 μg vitamin D) are effective for the prevention of fractures (Bischoff-Ferrari et al. 2012).
Experimental studies
Numerous experimental animal and cell culture studies have been performed on the involvement of vitamin D in CVD (Bouillon et al. 2008). The main findings of these investigations are summarized in this chapter. The starting point for these experimental studies was the identification of the VDR in cells of the vessel wall and the heart (Bouillon et al. 2008; Schnatz et al. 2012; Chen et al. 2008).
Knockout models
Knockout models for the VDR yield various pathologies, in particular regarding bone and mineral metabolism (Bouillon et al. 2008). With regard to the cardiovascular system, the VDR knockout mice develop a phenotype with arterial hypertension and myocardial hypertrophy (Bouillon et al. 2008). Additional features of these animals are increased thrombogenicity and overexpression of renin (Bouillon et al. 2008). Similar phenotypes were observed in knockout models for 1-alpha-hydroxylase (Zhou et al. 2008). Most notably, inhibition of the renin angiotensin aldosterone system (RAAS) prevented the cardiovascular damage in these experimental models suggesting that renin overexpression may play a crucial role. Considering that a general VDR knockout mouse may not be the best model to study specific effects of VDR on cardiovascular health, a cardiomyocyte selective knockout model was created (Chen et al. 2011). In this mouse model, bone and mineral metabolism were normal, but myocardial hypertrophy still occurred suggesting a specific effect of VDR activation on the heart (Chen et al. 2011).
Atherosclerosis
Several cell culture studies have shown that VDR activation may inhibit various steps in the pathogenesis of atherosclerosis. In detail, it has been shown that vitamin D is able to inhibit macrophage to foam cell formation, as well as suppressing the expression of endothelial adhesion molecules and smooth muscle cell proliferation (Bouillon et al. 2008; Riek et al. 2013; Weng et al. 2013). In animal studies, vitamin D deficiency was associated with accelerated atherosclerosis and lower VDR expression in coronary arteries correlated with a greater atherosclerotic plaque size (Schnatz et al. 2012; Weng et al. 2013). It has also been shown that VDR activation in macrophages inhibits atherosclerosis in mice by suppressing the local renin angiotensin aldosterone system (RAAS) (Szeto et al. 2012).
Heart diseases
Myocardial hypertrophy is accompanied by an increased expression of the VDR in the heart (Chen et al. 2008). This may be a beneficial adaptive mechanism when considering that VDR activation has been shown to exert antihypertrophic and antiproliferative effects on cardiomyocytes (Pilz et al. 2010a). In addition, vitamin D downregulates various genes that are involved in the development of myocardial hypertrophy (Pilz et al. 2010a). VDR effects are also involved in the regulation of calcium flux of cardiomyocytes and have been implicated in the maintenance of diastolic function (Green et al. 2006). Accumulating evidence also indicates that vitamin D may play a role in the regulation of myocardial extracellular matrix turnover and may protect against cardiac fibrosis (Gardner et al. 2013; Pilz et al. 2010a).
CVD risk factors
Numerous experimental studies have reported that vitamin D may exert beneficial effects on almost all cardiovascular risk factors.
VDR activation has been shown to suppress the expression of renin (Li 2011; Vaidya and Williams 2012). This is of particular interest since the RAAS is a major regulator of blood pressure, and plasma renin concentrations are a cardiovascular risk marker (Tomaschitz et al. 2010, 2011). Other proposed antihypertensive effects of vitamin D include anti-inflammatory properties such as suppression of nuclear-factor-κB (NF-κB), renoprotective actions, direct effects on the vasculature as well as regulation of calcium homeostasis including suppression of PTH, which may itself contribute to high blood pressure (Dusso and Tokumoto 2011; Kienreich et al. 2013).
Several but not all experimental studies have demonstrated that VDR activation stimulates insulin secretion and protects against beta-cell dysfunction (Pilz et al. 2013a). Vitamin D regulation of calcium homeostasis may be of relevance in beta-cell function as insulin secretion is a calcium-dependent process (Pilz et al. 2013a). Other suggested anti-diabetic effects include improved peripheral insulin resistance, anti-inflammatory actions, and stimulation of osteocalcin, a bone marker with putative effects on insulin secretion and insulin sensitivity (Pilz et al. 2013a). Various other effects of vitamin D on cardiovascular risk factors including blood lipids, inflammation, and oxidative stress have also been characterized in experimental studies as reviewed elsewhere (Bouillon et al. 2008; Muscogiuri et al. 2012; Beveridge and Witham 2013; Gardner et al. 2013; Pilz et al. 2013a; Kienreich et al. 2013).
Sunlight exposure and cardiovascular risk
In 1981, Robert Scragg reported on the seasonality of CVD with an increased incidence in winter and a lower incidence in summer. He hypothesized that more sunlight (UV-B) exposure with subsequent increased vitamin D synthesis in the skin might be a possible explanation for the seasonal differences in CVD incidence (Scragg 1981). Subsequent studies have confirmed that UV exposure is inversely associated with cardiovascular risk and mortality (Zittermann and Gummert 2010; Yang et al. 2011). Several reasons may account for these observations but these reports fit well to the hypothesis that sunlight-induced vitamin D production may protect against CVD. This is reinforced by data showing that the prevalence of arterial hypertension or type 1 diabetes is lowest in sunny countries, but gradually increases when moving away from the equator (Mohr et al. 2008; Rostand 1997). Similar data related to latitude have been reported for altitude showing that higher altitude with a more intense UV-B radiation is associated with a lower cardiovascular risk (Zittermann and Gummert 2010). A few clinical studies have evaluated effects of UV radiation on CVD risk factors with mixed results regarding blood pressure (Krause et al. 1998; Scragg et al. 2011). Interestingly, a high UV-B exposure has also been identified as a possible protective factor against developing heart failure (Zittermann et al. 2007). When discussing the aforementioned studies on UV exposure and cardiovascular risk, it should be acknowledged that sunlight exposure can raise 25(OH)D concentrations up to levels that are equivalent to a daily vitamin D supplementation of 10,000 IU per day (Holick 2007). Further, it should be noted that UV exposure exerts some direct, vitamin D independent effects that may be beneficial for cardiovascular health and include an increase in the vasodilator nitric oxide (NO) (Feelisch et al. 2010).
Vitamin D genetics and CVD
Several studies evaluated associations of the single-nucleotide polymorphisms (SNPs) of the VDR and of enzymes of the vitamin D metabolism with CVD and its risk factors. Despite some significant associations between certain SNPs and CVD and/or its risk factors, the majority of the studies reported a lack of significant findings (Shen et al. 2010; Jorde et al. 2012a; Kühn et al. 2013; Wilke et al. 2009; Li et al. 2013; Trummer et al. 2013). This is in line with data from patients with hereditary 1,25(OH)2D-resistant rickets (HVDRR) who did not exhibit major cardiovascular pathologies (Tiosano et al. 2011). Of note, however, is a large meta-analysis of prospective studies showing that the association between 25(OH)D and multiple outcomes (i.e, hip fracture, myocardial infarction, cancer or death) is modified by a certain SNP of the VDR (Levin et al. 2012).
Our understanding on vitamin D metabolism and its related genetic background was significantly improved by a large genome-wide association study (GWAS) (Wang et al. 2010a, b). That study aimed to identify genetic loci that are associated with 25(OH)D serum levels. Four genetic loci were identified in that study related to the enzyme for 7-dehydrocholesterol synthesis in the liver, the DBP, the 24-hydroxylase, and the 1-alpha-hydroxylase (Wang et al. 2010a, b). This GWAS was not only important for the characterization of a genetic predisposition for higher or lower 25(OH)D levels but also opened the door to perform so-called Mendelian Randomization (MR) studies. The MR approach uses the genetic determinants for 25(OH)D levels as an instrument to study associations with disease outcomes. Assuming that the inherited SNPs related to 25(OH)D levels are not significantly related to confounding factors, MR permits investigators to address research questions with an evidence level that is considered approximate to the results achieved by RCTs (Verduijn et al. 2010; Berry et al. 2012). One important question addressed by a MR study was the link between obesity and 25(OH)D. In a large meta-analysis, Vimaleswaran et al. (2013) demonstrated that the genetic determinants of 25(OH)D are not related to body mass index (BMI). By contrast, the genetic determinants for BMI were significantly associated with lower 25(OH)D concentrations. This so-called bi-directional MR study came therefore to the conclusion that a higher BMI seems to lower 25(OH)D levels but not vice versa (Vimaleswaran et al. 2013).
Accumulating evidence suggests that some of the proposed beneficial effects of vitamin D on common chronic diseases could be mediated by microRNAs (Salic and De Windt 2012; Ha 2011). MicroRNAs are small non-coding RNAs that can regulate gene expression on a post-transcriptional level by binding messengerRNAs (mRNAs) (Salic and De Windt 2012; Ha 2011). This in turn leads to mRNA degradation or translation inhibition. Apart from their role in regulating gene expression, microRNAs are also released into the circulation and have been found to be associated with various chronic diseases. At present, there is intensive research ongoing to explore the diagnostic and therapeutic potential of microRNAs. To this end, it should be noted that vitamin D effects seem to participate and interact with the function and regulation of microRNAs (Giangreco and Nonn 2013; Jorde et al. 2012b; Alvarez-Díaz et al. 2012). Currently, however, our knowledge on vitamin D and microRNAs is still in its infancy. There are only very limited data on alterations in circulating microRNAs that are induced by vitamin D supplementation as part of a large clinical RCT (Giangreco and Nonn 2013; Jorde et al. 2012b; Alvarez-Díaz et al. 2012).
Vitamin D and cardiovascular risk factors
Many observational studies have evaluated the relationship between vitamin D status and cardiovascular risk factors by utilizing serum levels of 25(OH)D. Most of these studies found that vitamin D deficiency was associated with an adverse cardiovascular risk profile. While this would support an involvement of vitamin D in CVD, such findings should be interpreted with caution: (1) observational studies cannot prove causality; (2) reverse causation may explain the observed associations (i.e, that an underlying disease may lead to vitamin D deficiency and not vice versa); (3) as sunlight-induced vitamin D production and nutrition are sources for vitamin D, it cannot be ruled out that residual confounding may exist (e.g, unmeasured or unknown lifestyle factors related to less sunlight exposure or unhealthy diet that may be the driving factors explaining the link between vitamin D deficiency and cardiovascular risk factors). Current available data from randomized controlled trials (RCTs) on vitamin D supplementation and cardiovascular risk factors are less in favor of significant effects of vitamin D on cardiovascular risk factors. This in turn may suggest that observational results in this field could be significantly influenced by unconsidered or unmeasured confounding factors. In this chapter, we briefly summarize the current literature on vitamin D and CVD risk factors by presenting observational and RCT data for each cardiovascular risk factor.
Obesity
It is well established that a higher BMI is associated with lower 25(OH)D levels (Earthman et al. 2012; Vanlint 2013). A biologically plausible and widely accepted explanation for this relationship is that vitamin D, as a fat-soluble vitamin, is trapped in adipose tissue (Earthman et al. 2012). Another factor suggested to contribute to this association is that obese individuals, due to socio-psychological factors (e.g. social physique anxiety), tend to avoid exposing their skin to sunlight in public areas. According to some experimental data suggesting VDR activation in adipose tissue may facilitate weight loss, it has been hypothesized that vitamin D deficiency could also partially contribute to obesity (Earthman et al. 2012; Vanlint 2013). Nevertheless, this hypothesis has not been consistently supported by epidemiological studies. The present available evidence is in clear favor of the concept that obesity contributes to vitamin D deficiency and not vice versa. This finding has clinical implications since the treatment and prevention of obesity has the potential to improve vitamin D status. In line with this, several studies have shown that weight loss is accompanied by an increase in 25(OH)D levels (Rock et al. 2012; Earthman et al. 2012; Vanlint 2013).
Arterial hypertension
Meta-analyses of cross-sectional and longitudinal studies have shown that vitamin D deficiency is a risk factor for higher blood pressure and arterial hypertension (Burgaz et al. 2011; Kunutsor et al. 2013). In 283,537 individuals from the general population, the relative risk (with 95% CIs) for incident arterial hypertension was 0.70 (0.58–0.86) in the highest compared with the lowest 25(OH)D tertile (Kunutsor et al. 2013). RCTs on vitamin D supplementation and blood pressure are already available, but these trials have produced mixed results, some showing a marginally significant blood pressure reduction following vitamin D supplementation and others reporting no relevant effect (Kienreich et al. 2013). Whereas various vitamin D RCTs reported on blood pressure as a secondary outcome, there are only a handful of trials that addressed the blood pressure effects of vitamin D as the primary study aim (Kienreich et al. 2013). In 102 hypertensive patients who were randomly allocated to either 3,000 IU vitamin D daily or placebo during winter, there was no significant effect on blood pressure in the entire cohort (Larsen et al. 2012). In a subgroup analysis of the same study restricted to patients with 25(OH)D less than 32 ng/mL, 24-h systolic and diastolic blood pressure decreased significantly by 3 and 4 mm Hg, respectively, in the intervention group compared with the placebo group (Larsen et al. 2012). In 159 older patients with isolated systolic blood pressure and 25(OH)D concentrations below 30 ng/mL, there was no significant blood pressure effect of 100,000 IU vitamin D every 3 months for 1 year (Witham et al. 2013). Strengths of this trial were the long treatment period and the availability of 24-h ambulatory blood pressure measurements, whereas a possible limitation might have been the relatively small increase in 25(OH)D (i.e, 8 ng/mL, in the treatment compared with the placebo group) (Witham et al. 2013). In the largest published study addressing the effects of vitamin D on blood pressure as a primary end point, 283 black participants were randomized in a 4-arm RCT to receive either placebo, 1,000, 2,000, or 4,000 IU vitamin D per day for 3 months (Forman et al. 2013). Main finding of this trial was that each additional vitamin D dose of 1,000 IU per day resulted in a decrease in systolic blood pressure of −1.4 mm Hg, while there was no relevant effect on diastolic blood pressure (Forman et al. 2013). It was further calculated that each increase in 25(OH)D concentrations of 1 ng/mL resulted in a decrease in systolic blood pressure of −0.2 mm Hg. Taken together, there appear to be no major effects, albeit vitamin D supplementation may have low-to-moderate effects on reducing systolic blood pressure. Further studies on the role of vitamin D toward arterial hypertension are warranted in particular as even minor reductions in systolic blood pressure (as shown by Forman et al. 2013) could have significant public health ramifications at a population level.
Diabetes mellitus
Cross-sectional and prospective studies have largely shown that low levels of 25(OH)D are associated with insulin resistance and type 2 diabetes mellitus (O’Hartaigh et al. 2013; Pilz et al. 2012a, 2013a). Meta-analyses of epidemiological studies indicate that vitamin D deficiency is an independent risk factor for type 2 diabetes mellitus (Song et al. 2013; Afzal et al. 2013). It is important to point out the finding of an epidemiological study showing that the association between 25(OH)D and insulin resistance (as well as blood pressure) is nonlinear with strong correlations at certain values within the vitamin D deficiency range but no association for 25(OH)D values above 32–36 ng/mL (Heaney et al. 2013b). Vitamin D RCTs have, with only rare exceptions, failed to show beneficial effects of vitamin D supplementation on glucose homeostasis or incident diabetes mellitus (Pilz et al. 2013a). Reviewing the current available literature, we propose that there is likewise no relevant effect of vitamin D on glucose metabolism in the general population, or among individuals at high risk of diabetes mellitus (Pilz et al. 2013a; Davidson et al. 2013). Of note is a RCT in 109 prediabetic individuals with 25(OH)D levels below 30 ng/mL (Davidson et al. 2013). That RCT failed to show any relevant benefits of high-dose vitamin D supplementation over 1 year on glucose metabolism (Davidson et al. 2013). Considering that some other RCTs reported an improved glucose metabolism after vitamin D supplementation in patients with diabetes mellitus, it remains unclear whether vitamin D can improve glucose control in diabetic patients (Pilz et al. 2013a). It must, however, be acknowledged that the majority of RCTs conducted in diabetic patients were unable to show beneficial effects of vitamin D supplementation on glycemic control (Pilz et al. 2013a; Breslavsky et al. 2013).
Meta-analyses of observational studies have shown that vitamin D deficiency during pregnancy is also an independent risk factor for gestational diabetes (Aghajafari et al. 2013; Poel et al. 2012). Some, but not all, studies in pregnant women have shown improved glycemic control with vitamin D supplementation but further studies are still needed (Soheilykhah et al. 2013; Wagner et al. 2013). One such promising study is the “DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention” study (Jelsma et al. 2013). A particular aim of that ongoing study in 880 pregnant women is to evaluate whether vitamin D supplementation is effective for the prevention of gestational diabetes mellitus (Jelsma et al. 2013).
Some, but not all studies, have shown that low 25(OH)D concentrations are a risk factor for type 1 diabetes mellitus (Munger et al. 2013; Thorsen et al. 2013; Chakhtoura and Azar 2013). RCTs with active or natural vitamin D on the preservation of beta-cell function in type 1 diabetic patients yielded inconsistent results, but it has been documented that vitamin D supplementation increases regulatory T cells, which are considered to protect against autoimmune diseases (Chakhtoura and Azar 2013; Bock et al. 2011; Prietl et al. 2010). While primary prevention studies are not available, it has been observed in Finland that vitamin D supplementation during childhood is associated with a significantly reduced risk to develop type 1 diabetes later in life (Hyppönen et al. 2001). This finding has been confirmed in a meta-analysis of existing studies on vitamin D intake during early life and subsequent risk of type 1 diabetes (Dong et al. 2013).
Lipids
Observational studies have, by the majority, revealed that low 25(OH)D concentrations are associated with higher triglycerides and total cholesterol but lower levels of HDL-cholesterol and apolipoprotein A-1 (Ponda et al. 2012; Jorde and Grimnes 2012). Data from RCTs showed mixed results but largely failed to demonstrate relevant effects of vitamin D supplementation on lipids (Zittermann et al. 2011a). A meta-analysis including 1,346 study participants derived from 12 clinical trials showed no significant effect of vitamin D on total cholesterol, HDL-cholesterol or triglycerides, but a marginally significant increase in LDL-cholesterol (Wang et al. 2012a, b). This modest increase in LDL-cholesterol post vitamin D supplementation could not be confirmed in a RCT of 305 postmenopausal women, and separately, in a retrospective cohort analysis when lipid profiles of patients with 25(OH)D levels below 20 ng/mL and an increase to ≥30 ng/mL during follow-up were analyzed (Wood et al. 2012; Ponda et al. 2012). Hence, the current literature does not support a relevant effect of vitamin D supplementation on blood lipids but further RCTs are required before drawing firm conclusions.
Parathyroid hormone (PTH)
PTH can also be considered a cardiovascular risk factor because PTH receptors are expressed in the cardiovascular system, and epidemiological studies have shown that higher PTH levels, even in the absence of chronic kidney disease (CKD), are a significant risk factor for CVD and mortality (van Ballegooijen et al. 2013a; van Ballegooijen et al. 2013b; Fitzpatrick et al. 2008; Pilz et al. 2010b; Hagström et al. 2009). Considering that high PTH levels are a biochemical sign of overt vitamin D deficiency, it is reasonable to speculate that PTH could partially mediate the effects of vitamin D deficiency on the cardiovascular system.
Inflammation and other cardiovascular risk factors
Vitamin D influences the innate and adaptive immune system and may play a role in autoimmunity and infectious diseases (Hewison 2012; Pludowski et al. 2013). Immune effects of vitamin D and their potential impact on inflammation are also relevant to the pathogenesis of CVD (Querfeld 2013). Associations between vitamin D deficiency and markers of inflammation (e.g, C-reactive protein) have been observed in some but not all epidemiological studies (Murr et al. 2012; Querfeld 2013). Results from vitamin D RCTs are also equivocal; some report vitamin D treatment exerts beneficial effects on inflammatory parameters, while others report no effect (Schleithoff et al. 2006; Alvarez et al. 2013; Abou-Raya et al. 2013; Grossmann et al. 2012; Beilfuss et al. 2012; Wood et al. 2012). Considering the accumulating trial reports on vitamin D effects on inflammation in combination with experimental data on this topic, it seems likely that vitamin D supplementation may exert some effects on inflammatory processes. Whether these anti-inflammatory effects of vitamin D are of clinical relevance, and in which populations, remains to be elucidated. Inflammation and/or acute stress per se may also have an impact on vitamin D status and could potentially contribute to lowering 25(OH)D levels, probably by modulating vitamin D metabolism (Quraishi and Camargo 2012; Reid et al. 2011). With reference to this, it has also been hypothesized that RAAS activity and VDR activation may counteract each other in a feedback relationship mediated in part by inflammatory processes (Ferder et al. 2013). Foremost, a single-center, open-label, blinded end point trial in 101 heart failure patients reported a significant reduction in renin levels after vitamin D supplementation at a daily dose of 2,000 IU for 6 weeks (Schroten et al. 2013).
Observational and some interventional studies suggest that vitamin D may exert anti-oxidative properties, but the overall evidence is still inconclusive (Nair-Shalliker et al. 2012; Nikooyeh et al. 2013; Asemi et al. 2013; Gradinaru et al. 2012). Low testosterone levels, which are considered a cardiovascular risk factor, have also been associated with a poor vitamin D status but further studies are needed to clarify this relationship (Wehr et al. 2010, 2013; Lerchbaum et al. 2012; Pilz et al. 2011a).
Vitamin D and CKD
Vitamin D treatment is widely used in CKD patients (Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group 2009). We therefore pay particular attention to clinical data and treatment modalities of vitamin D in these patients. Vitamin D deficiency is very common in patients suffering from CKD. In the German Diabetes Dialysis Study (4D Study), more than 70 percent of diabetes dialysis patients had 25(OH)D concentrations below 20 ng/mL (Drechsler et al. 2010). Factors contributing to this high prevalence of vitamin D deficiency in CKD include limited sunlight access and impaired vitamin D synthesis in the skin of uremic patients (Doorenbos et al. 2009), malnutrition and loss of vitamin D metabolites during dialysis as well as via urine (Chen et al. 1992). The latter process may be due to reduced tubular reabsorption of the 25(OH)D in patients with proteinuria because albumin and the DBP-25(OH)D complex compete for binding to the megalin receptor of the proximal tubular cells which is required for tubular reabsorption (Dusso and Tokumoto 2011). Low 25(OH)D levels in CKD are frequently accompanied by inadequate low 1,25(OH)2D levels. Reduced renal 1-alpha-hydroxylase activity may contribute to these relatively low 1,25(OH)2D levels, but some studies suggest that increased 24-hydroxylation may also significantly decrease 1,25(OH)2D levels in renal failure (Helvig et al. 2010). Apart from this, FGF-23 is secreted by osteocytes in response to high phosphate levels in CKD and reduces 1,25(OH)2D levels by inhibition of 1-alpha-hydroxylase activity (Quarles 2012).
As in the general population, low 25(OH)D levels are a risk marker for adverse health outcomes including mortality and CVD (Drechsler et al. 2011; Pilz et al. 2011b, c; Drechsler et al. 2010). Similar data were also obtained for low 1,25(OH)2D (Wolf et al. 2007). Considering that 1,25(OH)2D is usually not measured in clinical routine, there are only few data available on 1,25(OH)D and adverse outcomes, but accumulating evidence suggests that circulating 1,25(OH)2D might also be related to cardiovascular risk and mortality, even in the absence of significant CKD (Zittermann et al. 2009; Pilz et al. 2008). Future research should therefore put more emphasis on the relationship between circulating 1,25(OH)2D and cardiovascular risk (Zittermann et al. 2012a). Apart from this, it should be mentioned that several, but not all, studies reported that low 25(OH)D levels are also a risk factor for albuminuria, declining glomerular filtration rate (GFR) or CKD itself (de Boer et al. 2011, 2012; Damasiewicz et al. 2013). A role of vitamin D deficiency in the development of (renal) anemia has also been hypothesized (Icardi et al. 2013). Whether vitamin D is beneficial or harmful with regard to vascular calcification is at present unclear. It is also of interest to note that in 16,268 adults of the general population, there was no significant association between 25(OH)D levels and kidney stones (Tang et al. 2012). Considering that vitamin D supplementation did not significantly increase urinary calcium excretion in patients with a history of kidney stones, it has been suggested that even in these patients vitamin D supplementation is not contraindicated (Leaf et al. 2012).
Vitamin D intervention studies have a long-standing history in nephrology. It must be differentiated between “natural vitamin D therapy” (vitamin D3 or vitamin D2), which is usually used to treat vitamin D deficiency in non-CKD patients, and active vitamin D treatment with 1,25(OH)2D or its analogs. Evidently, the risk of side effects (i.e, hypercalcemia or hyperphosphatemia) is significantly higher with active vitamin D treatment, which is also more expensive than “natural” vitamin D.
In CKD, the best-known effect of vitamin D is the reduction in PTH, which is achieved by both active and “natural” vitamin D (Jean et al. 2009; Kandula et al. 2011). This has been demonstrated in a recent meta-analysis of RCTs (Kandula et al. 2011) and is remarkable because “natural” vitamin D has previously been considered ineffective in end-stage CKD due to reduced 1-alpha-hydroxylase activity. Consistently, in some RCTs, natural vitamin D significantly increased 1,25(OH)2D (Jean et al. 2009; Kandula et al. 2011). Interestingly, the vitamin D analog paricalcitol could significantly reduce albuminuria in patients with diabetic nephropathy who were already treated with renin angiotensin aldosterone system inhibitors (de Zeeuw et al. 2010). This has been confirmed in a meta-analysis of 9 paricalcitol intervention studies among 832 patients (Cheng et al. 2012). With reference to the heart, a recent multicenter RCT was unable to document any significant effect of paricalcitol on left ventricular mass or diastolic dysfunction (Thadhani et al. 2012). A post hoc analyses of that PRIMO study, however, showed that paricalcitol reduced the left atrial volume index significantly and attenuated an increase in brain natriuretic peptide (BNP), suggesting some beneficial effects on the heart (Tamez et al. 2012). Finally, in observational studies, the prescription of active vitamin D analogs (i.e. 1,25(OH)2D or its analogs such as paricalcitol) was associated with improved survival (Nigwekar and Thadhani 2013; Kalantar-Zadeh et al. 2010).
According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines 25(OH)D concentrations should be measured in CKD patients at stage 3 to 5D (i.e, GFR < 60 ml/min/m2) and be normalized by natural vitamin D treatment (Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group 2009). Active vitamin D treatment should only be used in the event that despite correction of vitamin D deficiency PTH is progressively rising and remains consistently above the normal range. In CKD patients stage 5D (dialysis patients), it is recommended to aim for PTH levels in the range of twofold to ninefold higher than the upper limit of the reference range. Dose titration for active vitamin D treatment is mainly done by measuring PTH concentrations.
Data on natural vitamin D supplementation and cardiovascular risk in CKD patients are limited so far and inconclusive (Alvarez et al. 2012, 2013). Accumulating evidence does however suggest that natural vitamin D supplementation in CKD may reduce albuminuria (Molina et al. 2013).
Vitamin D and atherosclerosis
Endothelial dysfunction
Observational studies have largely shown that vitamin D deficiency is associated with endothelial dysfunction. In 554 healthy adults, there was a significant inverse association between 25(OH)D, endothelial dysfunction, and arterial stiffness (Al Mheid et al. 2011). This is in line with data from 280 patients with type 2 diabetes mellitus showing that 25(OH)D concentrations are associated with brachial flow-mediated dilation (FMD) and circulating endothelial progenitor cell (EPC) numbers (Yiu et al. 2011).
RCTs of vitamin D supplementation for correcting endothelial dysfunction have produced mixed results. In 45 African-American adults, 60,000 IU vitamin D monthly for 16 weeks improved FMD (Harris et al. 2011). By contrast, in 100 patients with type 2 diabetes mellitus, there was no significant effect of a daily vitamin D supplementation of 5,000 IU over 12 weeks on brachial artery FMD, circulating levels of EPCs, and brachial-ankle pulse wave velocity (Yiu et al. 2013). In another RCT in 62 vitamin D-deficient patients with peripheral artery disease, there was also no significant effect of a single oral dose of 100,000 IU vitamin D on endothelial function and arterial stiffness (Stricker et al. 2012). In 58 stroke patients, a single dose of 100,000 IU vitamin D resulted in a significant improvement in FMD after 8 weeks that was no longer significant after 16 weeks (Witham et al. 2012). Hence, it is still unclear whether vitamin D exerts clinically relevant effects on endothelial function.
Carotid intima media thickness (IMT)
Observational studies on vitamin D status and carotid IMT showed controversial results. Some studies demonstrate that low 25(OH)D levels are associated with higher carotid IMT, whereas others did not report on any such association. In 654 community-dwelling older adults there was a significant inverse association between 25(OH)D and internal carotid IMT, but not with common carotid IMT (Reis et al. 2009). Further, carotid IMT and carotid plaques were measured at baseline and after a median follow-up of 9.4 years in 3,251 participants of the Multi-Ethnic Study of Atherosclerosis (Blondon et al. 2013). In that study, there was no significant association between 25(OH)D or PTH with carotid IMT or carotid plaques, neither in cross-sectional nor in prospective analyses (Blondon et al. 2013). Similarly, there was no significant association between 25(OH)D and carotid IMT in 1,193 participants with type 1 diabetes (Sachs et al. 2013). There are no relevant data on vitamin D RCTs and carotid IMT but the overall evidence argues against a significant relationship between vitamin D status and carotid IMT.
Peripheral artery disease
In 4,839 participants of the National Health and Nutrition Examination Survey (NHANES), the prevalence of patients with peripheral artery disease was significantly increased at lower 25(OH)D concentrations (Melamed et al. 2008a). Most of the studies in this field support a link between low vitamin D status and increased risk of peripheral artery disease, but there are no RCTs addressing whether vitamin D supplementation has significant effects on clinical endpoints related to peripheral artery disease (Chua et al. 2011).
Coronary artery disease (CAD)
Data on atherosclerosis of the coronary arteries (i.e, CAD) have partially but not consistently shown that low 25(OH)D levels are associated with coronary heart disease. In 130 patients with acute ST-segment elevation myocardial infarction (STEMI), there was no relationship between 25(OH)D and the angiographic severity of CAD (Goleniewska et al. 2013). There was also no significant and independent association between 25(OH)D and the presence of CAD in 2,015 patients referred for coronary angiography (Murr et al. 2012). By contrast, in 1,000 older men and women, vitamin D deficiency was identified as an independent marker for the presence of CAD (Lim et al. 2012). Another study in 100 patients undergoing coronary angiography revealed a significant relationship between vitamin D deficiency and both the presence and severity of CAD (Syal et al. 2012). Serum levels of 25(OH)D were also significantly lower in patients with type 2 diabetes mellitus and microalbuminuria who suffered from asymptomatic CAD when compared with controls without CAD (Joergensen et al. 2012). Interestingly, vitamin D deficiency in patients with type 1 diabetes was also a significant predictor of the prevalence and development of coronary artery calcification, a marker of coronary artery plaque burden (Young et al. 2011). These data are, however, in contrast to a study in 650 Amish adults which showed no association between 25(OH)D and coronary artery calcification (Michos et al. 2009). Data on coronary heart disease events, in particular myocardial infarction, are outlined further in the paragraph on vitamin D and cardiovascular events.
Vitamin D and heart failure
Numerous observational studies have shown that patients with heart failure frequently suffer from vitamin D deficiency (Zittermann et al. 2003; Pilz et al. 2010a; Gardner et al. 2013). Considering that heart failure leads to limited physical performance and probably low sunlight exposure, it is difficult to disentangle the true cause and effect relationship in observational studies. Echocardiographic examinations with prospective data have failed to show significant associations between 25(OH)D and left ventricular structure and function (van Ballegooijen et al. 2013c; Fall et al. 2012; van Ballegooijen et al. 2012). Only a few intervention studies have evaluated the effects of vitamin D on myocardial function. In a RCT in 123 patients with heart failure, there was no significant effect of vitamin D supplementation (2,000 IU per day over 9 months) on parameters of myocardial function, but there was a significant improvement in the cytokine profile (Schleithoff et al. 2006). In a RCT of 105 vitamin D-deficient patients with congestive heart failure, vitamin D supplementation with 100,000 IU vitamin D did not improve functional capacity or quality of life, but resulted in a significant decrease in B-type natriuretic peptide, a marker of heart failure and high cardiovascular risk (Witham et al. 2010a). In an uncontrolled trial in 100 heart failure patients, vitamin D supplementation for 4 months was associated with reduced levels of pro-brain natriuretic peptide and improvements in NYHA class as well as 6-min walking distance (Amin et al. 2013). B-type natriuretic peptide levels were also significantly lowered following vitamin D supplementation in a RCT in 61 patients with type 2 diabetes mellitus (Witham et al. 2010b). These findings are fitting with data from an observational study of 3,005 heart failure patients in whom vitamin D supplementation was associated with improved survival (Gotsman et al. 2012). In addition, there exist case reports on heart failure in vitamin D deficient rickets with significant improvement of myocardial function after calcium plus vitamin D supplementation in affected children (Maiya et al. 2008). Nevertheless, it remains to be determined, whether vitamin D supplementation has beneficial effects on the myocardium.
Vitamin D and cerebrovascular events
Meta-analyses of prospective studies have clearly shown that low 25(OH)D concentrations are a risk factor for stroke (Brøndum-Jacobsen et al. 2013; Chowdhury et al. 2012; Sun et al. 2012). In the largest meta-analysis including 58,384 participants and 2,644 events, the multivariate adjusted odds ratio (with 95% CIs) for ischemic stroke was 1.54 (1.43–1.65) in the lowest compared with the highest 25(OH)D quartile (Brøndum-Jacobsen et al. 2013). In sensitivity analyses of prospective general population studies, the hazard ratio for ischemic stroke remained significant with 1.46 (1.35–1.58). Apart from this, vitamin D deficiency is also an independent predictor of short-term mortality and poor functional outcome in stroke patients (Tu et al. 2013). While vitamin D deficiency is thus an established risk factor for cerebrovascular events, all of the previous vitamin D RCTs failed to show any significant reduction in stroke incidence (Kienreich et al. 2013). In light of this point, it must be taken into consideration that none of the published vitamin D RCTs was designed to evaluate vitamin D effects on stroke incidence so that final conclusions cannot be drawn (Hsia et al. 2007; Trivedi et al. 2003; Avenell et al. 2012). From a clinical perspective, it must be considered that stroke patients are at particularly high risk of osteoporosis, fractures and falls, thereby supporting the need for a sufficient vitamin D status among these individuals (Pilz et al. 2011d). Given that 25(OH)D crosses the blood–brain barrier, it has been suggested that VDR activation may also exert some neuroprotective effects that may be beneficial in stroke patients (Pilz et al. 2011d). It is therefore of interest to note that 25(OH)D levels are low in dementia (Annweiler et al. 2013; Llewellyn et al. 2010). Whether this reflects a causal relationship in the direction that vitamin D deficiency contributes to cognitive decline warrants large RCTs (Soni et al. 2012).
Vitamin D and cardiovascular events
Many prospective studies have largely but not consistently shown that low 25(OH)D levels are associated with an increased incidence rate of cardiovascular events (Pilz et al. 2011e; Karakas et al. 2013; Giovannucci et al. 2008; Wang et al. 2008; Schierbeck et al. 2012). Regarding specific cardiovascular events, vitamin D deficiency turned out as a risk factor for myocardial infarction and heart failure, but there are also data on a particular strong association with sudden cardiac death (SCD) (Pilz et al. 2008; Deo et al. 2011). In a meta-analysis including 65,994 study participants and 6,123 cardiovascular events, it was shown that there exists a generally linear and inverse association between 25(OH)D and cardiovascular risk for 25(OH)D levels ranging from 8 to 24 ng/mL (Wang et al. 2008). Insufficient data exist for the association between 25(OH)D and cardiovascular risk for 25(OH)D levels above 24 ng/mL (Wang et al. 2008). Further, vitamin D deficiency is also associated with poor outcomes of patients with acute coronary syndromes or myocardial infarction (Ng et al. 2013; Correia et al. 2013).
A possible interaction of 25(OH)D and race has been suggested by data from 6,436 participants of the Multi-Ethnic Study of Atherosclerosis who were followed up for a median of 8.5 years (Robinson-Cohen et al. 2013). In that study low 25(OH)D was an independent risk factor for coronary heart disease events in participants who were white or Chinese, but not black or Hispanic. Similar data were obtained for the association between 25(OH)D and stroke risk (Michos et al. 2012). Hence, further studies are needed to evaluate whether there exists a significant racial difference for the relationship between 25(OH)D and cardiovascular risk.
Individual intervention studies and meta-analysis of RCTs failed to show significant effects of vitamin D supplementation on cardiovascular events (Mao et al. 2013; Wang et al. 2010a, b). These results argue against major beneficial, but also harmful effects of vitamin D supplementation on cardiovascular risk. However, several points need to be considered when interpreting these findings. First, none of the previous vitamin D RCTs was designed to evaluate cardiovascular events. Second, the vitamin D doses used were relatively low and frequently calcium was given at the same time. Third, study participants were included regardless of their vitamin D status.
Vitamin D and mortality
When reviewing data on vitamin D and CVD, it is also of interest to briefly describe the existing literature on vitamin D and mortality, as CVDs are the major cause of death. Observational studies have largely, but not always, shown that low 25(OH)D levels are an independent risk factor for mortality. Indeed, this has been documented in the general population (Schöttker et al. 2013; Melamed et al. 2008b), and also in different patient populations such as CKD patients (Pilz et al. 2011b), patients suffering from CVD (Zittermann et al. 2013a), patients with liver diseases (Putz-Bankuti et al. 2012), patients with type 2 diabetes mellitus and the metabolic syndrome (Thomas et al. 2012), cancer patients (Pilz et al. 2013b) or nursing home residents (Pilz et al. 2012b). The notion that low 25(OH)D is an independent risk factor for mortality has previously been confirmed in a large meta-analysis (Zittermann et al. 2012b). The optimal 25(OH)D concentrations associated with the lowest mortality risk in that study ranged from 30 to 35 ng/mL. While some studies showed that mortality risk is not only increased at very low 25(OH)D levels but also at high 25(OH)D levels in the sense of a U- or J-shaped curve, there was no significantly increased risk of mortality in the meta-analysis by Zittermann et al. It must, however, be noted that sufficient data are not available to draw firm conclusions on the relationship between 25(OH)D and mortality at concentrations above approximately 45–50 ng/mL. In line with data from the general population, it has also been demonstrated in a meta-analysis that low 25(OH)D is associated with increased mortality in patients with CKD (Pilz et al. 2011c). As magnesium is important for vitamin D metabolism and transport, it has been argued that the association between 25(OH)D and mortality may be modified by magnesium intake (Deng et al. 2013).
Meta-analyses of RCTs on vitamin D supplementation have established that vitamin D treatment is associated with a moderate but statistically significant reduction in overall mortality (Bjelakovic et al. 2011; Rejnmark et al. 2012). A Cochrane meta-analysis based on 74,789 study participants reported a 6% mortality reduction in vitamin D3 treatment (Bjelakovic et al. 2011). It was estimated that 161 individuals must be treated with vitamin D3 for approximately 2 years to prevent one premature death (Bjelakovic et al. 2011). Similar results were reported by another individual patient data meta-analysis (Rejnmark et al. 2012). That study showed, however, that mortality reduction was only achieved with combined calcium plus vitamin D supplementation. The interpretation of these findings is obviously not straightforward and there remain several open questions as to the optimal vitamin D dose and the combined supplementation of calcium.
Vitamin D and calcium
Clinical studies on bone health suggest interactions of vitamin D and calcium (Lips 2012). While calcium and vitamin D seem to act in concert to exert beneficial skeletal effects, there are accumulating data suggesting that calcium supplementation may slightly increase cardiovascular risk (Bolland et al. 2011; Reid 2013; Uusi-Rasi et al. 2013; Zittermann et al. 2011b; Heaney et al. 2012). In detail, it has been demonstrated in meta-analyses of RCTs that calcium supplementation is associated with a significantly elevated risk of myocardial infarction (Reid 2013; Bolland et al. 2011). This is of particular concern since many RCTs were performed with the combination of both vitamin D and calcium and are thus hard to interpret. Obviously, calcium supplementation may contribute to calcification of vascular smooth muscle cells and alters their gene expression toward a more osteoblastic phenotype (Reid 2013). There is currently an ongoing discussion on whether the effects of calcium supplementation related to improved bone health outweigh adverse cardiovascular effects or vice versa (Reid 2013; Heaney et al. 2012; Uusi-Rasi et al. 2013). In addressing this issue, it has been calculated that “treating 1,000 people with calcium supplements for 5 years would cause additional 14 myocardial infarctions, 10 strokes and 13 deaths, while preventing 26 fractures” (Reid 2013; Bolland et al. 2011). Finally, there is evidence that the combined intake of calcium plus vitamin D intake may decrease the drug compliance compared to intake of vitamin D alone (Autier et al. 2012).
Vitamin D toxicity
In humans, vitamin D doses up to 10,000 IU per day have not been associated with significant adverse effects (Hathcock et al. 2007; Zittermann et al. 2013b). This appears biologically plausible since the capacity of the body to produce vitamin D in the skin is approximately equivalent to an oral intake of 10,000 IU per day and there is no known case of acute vitamin D intoxication caused by sunlight exposure (Holick 2007). Some health authorities, in particular those who set vitamin D intake levels for the general population, are more conservative and consider 4,000 IU vitamin D per day as absolutely safe, i.e., as the tolerable upper intake level (EFSA 2012; Ross et al. 2011). Acute vitamin D intoxication, which is characterized by hypercalcemia, can, however, occur if 25(OH)D levels rise above approximately 150–200 ng/mL (Zittermann et al. 2013b). Clinical features of acute vitamin D intoxication include hypercalciuria, with its possible consequences of dehydration and acute renal failure as well as calcifications of the vessels or the kidneys. Such high levels of 25(OH)D can only be achieved by extreme overdosing of vitamin D, in particular if one considers that the relationship between vitamin D intake and the 25(OH)D concentration becomes flat at higher doses (Cashman et al. 2011). Hence, in the absence of extremely rare conditions such as granulomatous diseases (e.g, sarcoidosis) in which extrarenal 1-alpha-activity is increased, commonly used vitamin D doses are extremely unlikely to cause acute intoxication or hypercalcemia. We have, however, only sparse data available on long term safety with currently no compelling evidence that high-dose vitamin D supplementation exerts clinically relevant harmful or beneficial effects on cardiovascular risk factors (Jorde et al. 2013; Davidson et al. 2013).
Expectations for the future of vitamin D
Some large vitamin D RCTs in the general older population have just started within the last few years and will probably be finished in 2017–2020 (Kupferschmidt 2012). On the background of previous experience with other vitamins, it seems very unlikely that these vitamin D RCTs will reveal significant benefits of vitamin D supplementation (Pilz et al. 2012c). Such considerations are supported by the fact that hardly any drug reduces clinical endpoints when given to unselected individuals of the older population. Other, probably more serious issues with the ongoing large vitamin D RCTs are that they mainly use single vitamin D doses for everyone, allow vitamin D supplementation in addition to the study drugs in the placebo group, and do not select individuals on the basis of low 25(OH)D levels. This latter point is of particular concern because the results from epidemiological studies and meta-analyses draw a relatively clear picture on the association between 25(OH)D and outcomes such as mortality or CVD. Depending on the study and outcome of interest, the risk of adverse events associated with vitamin D deficiency was only visible at relatively low 25(OH)D levels and thus in only a certain proportion of the analyzed patient populations. On the other hand, several studies suggest a U-shaped association between 25(OH)D and outcomes with some indications for a possible risk increase at very high 25(OH)D levels. Thus, a logical design for a vitamin D RCT would be to include participants with very low 25(OH)D levels and with the intention to supplement vitamin D doses to achieve optimal levels without overtreatment to very high and putative harmful 25(OH)D concentrations. Such RCTs are therefore still necessary, but the time frame to obtain sufficient funding for vitamin D trials may close within the next few years if negative results of large ongoing vitamin D RCTs reduce the interest for research and clinical applications of vitamin D.
Conclusions
Experimental and cell culture studies have clearly shown that VDR activation exerts various beneficial effects on the cardiovascular system. This is in line with the findings from large epidemiological studies that highlight a significant association between vitamin D deficiency and various cardiovascular risk factors such as arterial hypertension and diabetes mellitus. Moreover, meta-analyses of prospective studies revealed that low 25(OH)D levels are an independent risk marker for future cardiovascular events and mortality. By contrast, the results from RCTs on vitamin D treatment and cardiovascular risk are inconsistent, and consequently, we are unable to draw firm conclusions regarding effects of vitamin D on cardiovascular risk. We therefore have to wait for results of large vitamin D RCTs to assess cardiovascular endpoints. Such studies are currently ongoing but they aim to evaluate whether vitamin D supplementation is effective among generally healthy older adults. Looking at the history of other studies in unselected individuals from general populations it is likely that these studies will not show significant effects. If this is the case, there only remains a small time frame within the next few years to perform additional RCTs to address the question whether severely vitamin D-deficient individuals benefit from vitamin D treatment.
In conclusion, vitamin D deficiency is indeed a cardiovascular risk factor, but vitamin D supplementation with commonly used doses as recommended for osteoporosis treatment is neither proven to be beneficial nor harmful in CVD.
References
Abou-Raya A, Abou-Raya S, Helmii M (2013) The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol 40:265–272
Afzal S, Bojesen SE, Nordestgaard BG (2013) Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem 59:381–391
Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM (2013) Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ 346:f1169
Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, Kavtaradze N, Uphoff I, Hooper C, Tangpricha V, Alexander RW, Brigham K, Quyyumi AA (2011) Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol 58:186–192
Alvarez J, Wasse H, Tangpricha V (2012) Vitamin D supplementation in pre-dialysis chronic kidney disease: a systematic review. Dermatoendocrinol 4:118–127
Alvarez JA, Zughaier SM, Law J, Hao L, Wasse H, Ziegler TR, Tangpricha V (2013) Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. Eur J Clin Nutr 67:264–269
Alvarez-Díaz S, Valle N, Ferrer-Mayorga G, Lombardía L, Herrera M, Domínguez O, Segura MF, Bonilla F, Hernando E, Muñoz A (2012) MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum Mol Genet 21:2157–2165
Amin A, Minaee S, Chitsazan M, Naderi N, Taghavi S, Ardeshiri M (2013) Can vitamin d supplementation improve the severity of congestive heart failure? Congest Heart Fail 19:E22–E28
Annweiler C, Llewellyn DJ, Beauchet O (2013) Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 33:659–674
Asemi Z, Samimi M, Tabassi Z, Shakeri H, Esmaillzadeh A (2013) Vitamin d supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. J Nutr 143:1432–1438
Autier P, Gandini S, Mullie P (2012) A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab 97:2606–2613
Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA; RECORD Trial Group (2012) Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab 97:614–622
Bailey D, Veljkovic K, Yazdanpanah M, Adeli K (2013) Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin Biochem 46:190–196
Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E (2012) Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine 60:870–874
Berry DJ, Vimaleswaran KS, Whittaker JC, Hingorani AD, Hyppönen E (2012) Evaluation of genetic markers as instruments for Mendelian randomization studies on vitamin D. PLoS ONE 7:e37465
Beveridge LA, Witham MD (2013) Vitamin D and the cardiovascular system. Osteoporos Int 24:2167–2180
Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI (2012) Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int 82:84–89
Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J (2009) Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 339:b3692
Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stähelin HB, Theiler R, Dawson-Hughes B (2012) A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 367:40–49
Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C (2011) Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev (7):CD007470
Black LJ, Seamans KM, Cashman KD, Kiely M (2012) An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr 142:1102–1108
Blondon M, Sachs M, Hoofnagle AN, Ix JH, Michos ED, Korcarz C, Gepner AD, Siscovick DS, Kaufman JD, Stein JH, Kestenbaum B, de Boer IH (2013) 25-Hydroxyvitamin D and parathyroid hormone are not associated with carotid intima-media thickness or plaque in the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 33(11):2639–2645. doi:10.1161/ATVBAHA.113.301781
Bock G, Prietl B, Mader JK, Höller E, Wolf M, Pilz S, Graninger WB, Obermayer-Pietsch BM, Pieber TR (2011) The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: a randomized controlled trial. Diabetes Metab Res Rev 27:942–945
Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR (2011) Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 342:d2040
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M (2008) Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29:726–776
Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M (2013) Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr. doi:10.1016/j.clnu.2013.01.020
Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M (2013) 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol 73:38–47
Brouwer-Brolsma EM, Bischoff-Ferrari HA, Bouillon R, Feskens EJ, Gallagher CJ, Hypponen E, Llewellyn DJ, Stoecklin E, Dierkes J, Kies AK, Kok FJ, Lamberg-Allardt C, Moser U, Pilz S, Saris WH, van Schoor NM, Weber P, Witkamp R, Zittermann A, de Groot LC (2013) Vitamin D: do we get enough? A discussion between vitamin D experts in order to make a step towards the harmonisation of dietary reference intakes for vitamin D across Europe. Osteoporos Int 24:1567–1577
Burgaz A, Orsini N, Larsson SC, Wolk A (2011) Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens 29:636–645
Carter GD (2012) 25-hydroxyvitamin D: a difficult analyte. Clin Chem 58:486–488
Cashman KD, Fitzgerald AP, Kiely M, Seamans KM (2011) A systematic review and meta-regression analysis of the vitamin D intake-serum 25-hydroxyvitamin D relationship to inform European recommendations. Br J Nutr 106:1638–1648
Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu RA, Tian L, Zhang Y, Lucey A, Flynn A, Gibney MJ, Vesper HW, Phinney KW, Coates PM, Picciano MF, Sempos CT (2013) Evaluation of vitamin D standardization program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program’s potential for national nutrition and health surveys. Am J Clin Nutr 97:1235–1242
Chakhtoura M, Azar ST (2013) The role of vitamin d deficiency in the incidence, progression, and complications of type 1 diabetes mellitus. Int J Endocrinol 2013:148673
Chen WT, Yamaoka K, Nakajima S, Tanaka Y, Yamamoto T, Satomura K, Okada S, Seino Y (1992) Evaluation of vitamin D-binding protein and vitamin D metabolite loss in children on continuous ambulatory peritoneal dialysis. Bone Miner 17:389–398
Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, Law CS, Gardner DG (2008) Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension 52:1106–1112
Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG (2011) Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 124:1838–1847
Cheng J, Zhang W, Zhang X, Li X, Chen J (2012) Efficacy and safety of paricalcitol therapy for chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol 7:391–400
Chowdhury R, Stevens S, Ward H, Chowdhury S, Sajjad A, Franco OH (2012) Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur J Epidemiol 27:581–591
Chua GT, Chan YC, Cheng SW (2011) Vitamin D status and peripheral arterial disease: evidence so far. Vasc Health Risk Manag 7:671–675
Correia LC, Sodré F, Garcia G, Sabino M, Brito M, Kalil F, Barreto B, Lima JC, Noya-Rabelo MM (2013) Relation of severe deficiency of vitamin D to cardiovascular mortality during acute coronary syndromes. Am J Cardiol 111:324–327
Damasiewicz MJ, Magliano DJ, Daly RM, Gagnon C, Lu ZX, Sikaris KA, Ebeling PR, Chadban SJ, Atkins RC, Kerr PG, Shaw JE, Polkinghorne KR (2013) Serum 25-hydroxyvitamin D deficiency and the 5-year incidence of CKD. Am J Kidney Dis 62:58–66
Dastani Z, Berger C, Langsetmo L, Fu L, Wong BY, Malik S, Goltzman D, Cole DE, Richards JB (2013) In healthy adults, biological activity of vitamin D, as assessed by serum PTH, is largely independent of DBP concentrations. J Bone Miner Res doi. doi:10.1002/jbmr.2042
Davidson MB, Duran P, Lee ML, Friedman TC (2013) High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 36:260–266
de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, Siscovick DS, Kestenbaum B (2011) Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol 6:2141–2149
de Boer IH, Sachs MC, Cleary PA, Hoofnagle AN, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Brunzell JD, Diabetes Control and Complication Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group (2012) Circulating vitamin D metabolites and kidney disease in type 1 diabetes. J Clin Endocrinol Metab 97:4780–4788
de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D (2010) Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376:1543–1551
Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, Ness RM, Seidner DL, Dai Q (2013) Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med 11:187
Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B (2011) Vitamin D, parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension 58:1021–1028
Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ (2013) Vitamin d intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients 5:3551–3562
Doorenbos CR, van den Born J, Navis G, de Borst MH (2009) Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol 5:691–700
Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, Wanner C, Boeschoten EW, Brandenburg V, NECOSAD Study Group (2011) Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transpl 26:1024–1032
Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, März W, Ritz E, Wanner C (2010) Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J 31:2253–2261
Dusso AS, Tokumoto M (2011) Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int 79:715–729
Earthman CP, Beckman LM, Masodkar K, Sibley SD (2012) The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 36:387–396
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2012) Scientific opinion on the tolerable upper intake level of vitamin D. EFSA J 10(2813):1–45
Fall T, Shiue I, Bergeåaf Geijerstam P, Sundström J, Ärnlöv J, Larsson A, Melhus H, Lind L, Ingelsson E (2012) Relations of circulating vitamin D concentrations with left ventricular geometry and function. Eur J Heart Fail 14:985–991
Feelisch M, Kolb-Bachofen V, Liu D, Lundberg JO, Revelo LP, Suschek CV, Weller RB (2010) Is sunlight good for our heart? Eur Heart J 31:1041–1045
Ferder M, Inserra F, Manucha W, Ferder L (2013) The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol 304:C1027–C1039
Fitzpatrick LA, Bilezikian JP, Silverberg SJ (2008) Parathyroid hormone and the cardiovascular system. Curr Osteoporos Rep 6:77–83
Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, Bennett GG, Chandler PD, Hollis BW, Emmons KM, Giovannucci EL, Fuchs CS, Chan AT (2013) Effect of vitamin D supplementation on blood pressure in blacks. Hypertension 61:779–785
Gardner DG, Chen S, Glenn DJ (2013) Vitamin D and the heart. Am J Physiol Regul Integr Comp Physiol. doi:10.1152/ajpregu.00322.2013
Giangreco AA, Nonn L (2013) The sum of many small changes: microRNAs are specifically and potentially globally altered by vitamin D(3) metabolites. J Steroid Biochem Mol Biol 136:86–93
Giovannucci E, Liu Y, Hollis BW, Rimm EB (2008) 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 168:1174–1180
Goleniewska BM, Kacprzak M, Zielinska M (2013) Vitamin D level and extent of coronary stenotic lesions in patients with first acute myocardial infarction. Cardiol J. doi:10.5603/CJ.a2013.0048
Gotsman I, Shauer A, Zwas DR, Hellman Y, Keren A, Lotan C, Admon D (2012) Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail 14:357–366
Gradinaru D, Borsa C, Ionescu C, Margina D, Prada GI, Jansen E (2012) Vitamin D status and oxidative stress markers in the elderly with impaired fasting glucose and type 2 diabetes mellitus. Aging Clin Exp Res 24:595–602
Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV (2006) Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 41:350–359
Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V (2012) Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr 66:1072–1074
Ha TY (2011) MicroRNAs in human diseases: from cancer to cardiovascular disease. Immune Netw 11:135–154
Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J (2009) Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 119:2765–2771
Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y (2011) Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens 24:557–562
Hathcock JN, Shao A, Vieth R, Heaney R (2007) Risk assessment for vitamin D. Am J Clin Nutr 85:6–18
Heaney RP, Kopecky S, Maki KC, Hathcock J, Mackay D, Wallace TC (2012) A review of calcium supplements and cardiovascular disease risk. Adv Nutr 3:763–771
Heaney RP, Armas LA, French C (2013a) All-source basal vitamin D inputs are greater than previously thought and cutaneous inputs are smaller. J Nutr 143:571–575
Heaney RP, French CB, Nguyen S, Ferreira M, Baggerly LL, Brunel L, Veugelers P (2013b) A novel approach localizes the association of vitamin D status with insulin resistance to one region of the 25-hydroxyvitamin D continuum. Adv Nutr 4:303–310
Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, Ramjit NJ, Ryder K, Tabash SP, Herzenberg AM, Epps TM, Petkovich M (2010) Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int 78:463–472
Hewison M (2012) Vitamin D and immune function: autocrine, paracrine or endocrine? Scand J Clin Lab Invest Suppl 243:92–102
Höbaus J, Thiem U, Hummel DM, Kallay E (2013) Role of calcium, vitamin D, and the extrarenal vitamin D hydroxylases in carcinogenesis. Anticancer Agents Med Chem 13:20–35
Holick MF (1994) McCollum Award Lecture, 1994: vitamin D–new horizons for the 21st century. Am J Clin Nutr 60:619–630
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2012) Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab 97:1153–1158
Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M, Women’s Health Initiative Investigators (2007) Calcium/vitamin D supplementation and cardiovascular events. Circulation 115:846–854
Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM (2001) Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358:1500–1503
Icardi A, Paoletti E, De Nicola L, Mazzaferro S, Russo R, Cozzolino M (2013) Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrol Dial Transpl 28:1672–1679
Jean G, Souberbielle JC, Chazot C (2009) Monthly cholecalciferol administration in haemodialysis patients: a simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transpl 24:3799–3805
Jelsma JG, van Poppel MN, Galjaard S, Desoye G, Corcoy R, Devlieger R, van Assche A, Timmerman D, Jans G, Harreiter J, Kautzky-Willer A, Damm P, Mathiesen ER, Jensen DM, Andersen L, Dunne F, Lapolla A, Di Cianni G, Bertolotto A, Wender-Oegowska E, Zawiejska A, Blumska K, Hill D, Rebollo P, Snoek FJ, Simmons D (2013) DALI: vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: an European multicentre, randomised trial - study protocol. BMC Pregnancy Childbirth 13:142
Joergensen C, Reinhard H, Schmedes A, Hansen PR, Wiinberg N, Petersen CL, Winther K, Parving HH, Jacobsen PK, Rossing P (2012) Vitamin D levels and asymptomatic coronary artery disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Diabetes Care 35:168–172
Jones G, Prosser DE, Kaufmann M (2012) 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys 523:9–18
Jorde R, Grimnes G (2012) Vitamin D and lipids: do we really need more studies? Circulation 126:252–254
Jorde R, Schirmer H, Wilsgaard T, Joakimsen RM, Mathiesen EB, Njølstad I, Løchen ML, Figenschau Y, Berg JP, Svartberg J, Grimnes G (2012a) Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromsø study. PLoS ONE 7:e37295
Jorde R, Svartberg J, Joakimsen RM, Coucheron DH (2012b) Plasma profile of microRNA after supplementation with high doses of vitamin D3 for 12 months. BMC Res Notes 5:245
Jorde R, Strand Hutchinson M, Kjærgaard M, Sneve M, Grimnes G (2013) Supplementation with high doses of vitamin D to subjects without vitamin D deficiency may have negative effects: pooled data from four intervention trials in Tromsø. ISRN Endocrinol 348705
Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP (2010) Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int Suppl 117:S10–S21
Kandula P, Dobre M, Schold JD, Schreiber MJ Jr, Mehrotra R, Navaneethan SD (2011) Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 6:50–62
Karakas M, Thorand B, Zierer A, Huth C, Meisinger C, Roden M, Rottbauer W, Peters A, Koenig W, Herder C (2013) Low levels of serum 25-hydroxyvitamin D are associated with increased risk of myocardial infarction, especially in women: results from the MONICA/KORA Augsburg case-cohort study. J Clin Endocrinol Metab 98:272–280
Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130
Kienreich K, Grubler M, Tomaschitz A, Schmid J, Verheyen N, Rutters F, Dekker JM, Pilz S (2013) Vitamin D, arterial hypertension & cerebrovascular disease. Indian J Med Res 137:669–679
Krause R, Bühring M, Hopfenmüller W, Holick MF, Sharma AM (1998) Ultraviolet B and blood pressure. Lancet 352:709–710
Kühn T, Kaaks R, Teucher B, Hirche F, Dierkes J, Weikert C, Katzke V, Boeing H, Stangl GI, Buijsse B (2013) Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident myocardial infarction and stroke in the European prospective investigation into cancer and nutrition (EPIC)-Germany study. PLoS ONE 8:e69080
Kunutsor SK, Apekey TA, Steur M (2013) Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol 28:205–221
Kupferschmidt K (2012) Uncertain verdict as vitamin D goes on trial. Science 337:1476–1478
Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB (2012) Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. Am J Hypertens 25:1215–1222
Leaf DE, Korets R, Taylor EN, Tang J, Asplin JR, Goldfarb DS, Gupta M, Curhan GC (2012) Effect of vitamin D repletion on urinary calcium excretion among kidney stone formers. Clin J Am Soc Nephrol 7:829–834
Lerchbaum E, Pilz S, Boehm BO, Grammer TB, Obermayer-Pietsch B, März W (2012) Combination of low free testosterone and low vitamin D predicts mortality in older men referred for coronary angiography. Clin Endocrinol (Oxf) 77:475–483
Levin GP, Robinson-Cohen C, de Boer IH, Houston DK, Lohman K, Liu Y, Kritchevsky SB, Cauley JA, Tanaka T, Ferrucci L, Bandinelli S, Patel KV, Hagström E, Michaëlsson K, Melhus H, Wang T, Wolf M, Psaty BM, Siscovick D, Kestenbaum B (2012) Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 308:1898–1905
Li YC (2011) Molecular mechanism of vitamin D in the cardiovascular system. J Investig Med 59:868–871
Li L, Wu B, Liu JY, Yang LB (2013) Vitamin D receptor gene polymorphisms and type 2 diabetes: a meta-analysis. Arch Med Res 44:235–241
Lim S, Shin H, Kim MJ, Ahn HY, Kang SM, Yoon JW, Choi SH, Kim KW, Song JH, Choi SI, Chun EJ, Shin CS, Park KS, Jang HC (2012) Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: the Korean longitudinal study on health and aging. J Clin Endocrinol Metab 97:169–178
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Lips P (2012) Interaction between vitamin D and calcium. Scand J Clin Lab Invest Suppl 243:60–64
Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, Ferrucci L, Melzer D (2010) Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med 170:1135–1141
Macdonald HM, Mavroeidi A, Fraser WD, Darling AL, Black AJ, Aucott L, O’Neill F, Hart K, Berry JL, Lanham-New SA, Reid DM (2011) Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: a major cause for concern? Osteoporos Int 22:2461–2472
Maiya S, Sullivan I, Allgrove J, Yates R, Malone M, Brain C, Archer N, Mok Q, Daubeney P, Tulloh R, Burch M (2008) Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life-threatening infant heart failure. Heart 94(5):581–584
Mao PJ, Zhang C, Tang L, Xian YQ, Li YS, Wang WD, Zhu XH, Qiu HL, He J, Zhou YH (2013) Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol S0167–5273(13):01617
Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P (2008a) Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol 28:1179–1185
Melamed ML, Michos ED, Post W, Astor B (2008b) 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168:1629–1637
Michos ED, Streeten EA, Ryan KA, Rampersaud E, Peyser PA, Bielak LF, Shuldiner AR, Mitchell BD, Post W (2009) Serum 25-hydroxyvitamin d levels are not associated with subclinical vascular disease or C-reactive protein in the old order amish. Calcif Tissue Int 84:195–202
Michos ED, Reis JP, Post WS, Lutsey PL, Gottesman RF, Mosley TH, Sharrett AR, Melamed ML (2012) 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: the NHANES-III linked mortality files. Nutrition 28:367–371
Mohr SB, Garland CF, Gorham ED, Garland FC (2008) The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 51:1391–1398
Molina P, Górriz JL, Molina MD, Peris A, Beltrán S, Kanter J, Escudero V, Romero R, Pallardó LM (2013) The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrol Dial Transpl. doi:10.1093/ndt/gft360
Munger KL, Levin LI, Massa J, Horst R, Orban T, Ascherio A (2013) Preclinical serum 25-hydroxyvitamin D levels and risk of type 1 diabetes in a cohort of US military personnel. Am J Epidemiol 177:411–419
Murr C, Pilz S, Grammer TB, Kleber ME, Meinitzer A, Boehm BO, Marz W, Fuchs D (2012) Vitamin D deficiency parallels inflammation and immune activation, the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chem Lab Med 50:2205–2212
Muscogiuri G, Sorice GP, Ajjan R, Mezza T, Pilz S, Prioletta A, Scragg R, Volpe SL, Witham MD, Giaccari A (2012) Can vitamin D deficiency cause diabetes and cardiovascular diseases? Present evidence and future perspectives. Nutr Metab Cardiovasc Dis 22(2):81–87
Nair-Shalliker V, Armstrong BK, Fenech M (2012) Does vitamin D protect against DNA damage? Mutat Res 733:50–57
Ng LL, Sandhu JK, Squire IB, Davies JE, Jones DJ (2013) Vitamin D and prognosis in acute myocardial infarction. Int J Cardiol. 168(3):2341-2346. doi:10.1016/j.ijcard.2013.01.030
Nigwekar SU, Thadhani RI (2013) Shining light on vitamin D trials in chronic kidney disease. Kidney Int 83:198–200
Nikooyeh B, Neyestani TR, Tayebinejad N, Alavi-Majd H, Shariatzadeh N, Kalayi A, Zahedirad M, Heravifard S, Salekzamani S (2013) Daily intake of vitamin D- or calcium-vitamin D-fortified Persian yogurt drink (doogh) attenuates diabetes-induced oxidative stress: evidence for antioxidative properties of vitamin D. J Hum Nutr Diet. doi:10.1111/jhn.12142
O’Hartaigh B, Neil Thomas G, Silbernagel G, Bosch JA, Pilz S, Loerbroks A, Kleber ME, Grammer TB, Böhm BO, März W (2013) Association of 25-hydroxyvitamin D with type 2 diabetes among patients undergoing coronary angiography: cross-sectional findings from the LUdwigshafen Risk and Cardiovascular Health (LURIC) Study. Clin Endocrinol (Oxf) 79:192–198
Peterlik M, Cross HS (2009) Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology. Eur J Clin Nutr 63:1377–1386
Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H (2008) Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 93:3927–3935
Pilz S, Tomaschitz A, Drechsler C, Dekker JM, März W (2010a) Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res 54:1103–1113
Pilz S, Tomaschitz A, Drechsler C, Ritz E, Boehm BO, Grammer TB, März W (2010b) Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J 31:1591–1598
Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A (2011a) Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res 43:223–225
Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, Boehm BO, Grammer TB, März W (2011b) Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transpl 26:3603–3609
Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S (2011c) Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis 58:374–382
Pilz S, Tomaschitz A, Drechsler C, Zittermann A, Dekker JM, März W (2011d) Vitamin D supplementation: a promising approach for the prevention and treatment of strokes. Curr Drug Targets 12:88–96
Pilz S, Tomaschitz A, März W, Drechsler C, Ritz E, Zittermann A, Cavalier E, Pieber TR, Lappe JM, Grant WB, Holick MF, Dekker JM (2011e) Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 75:575–584
Pilz S, van den Hurk K, Nijpels G, Stehouwer CD, Van’t Riet E, Kienreich K, Tomaschitz A, Dekker JM (2012a) Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: the Hoorn study. Nutr Metab Cardiovasc Dis 22:883–889
Pilz S, Dobnig H, Tomaschitz A, Kienreich K, Meinitzer A, Friedl C, Wagner D, Piswanger-Sölkner C, März W, Fahrleitner-Pammer A (2012b) Low 25-hydroxyvitamin D is associated with increased mortality in female nursing home residents. J Clin Endocrinol Metab 97:E653–E657
Pilz S, Rutters F, Dekker JM (2012c) Disease prevention: vitamin D trials. Science 338:883
Pilz S, Kienreich K, Rutters F, de Jongh R, van Ballegooijen AJ, Grübler M, Tomaschitz A, Dekker JM (2013a) Role of vitamin D in the development of insulin resistance and type 2 diabetes. Curr Diab Rep 13:261–270
Pilz S, Kienreich K, Tomaschitz A, Ritz E, Lerchbaum E, Obermayer-Pietsch B, Matzi V, Lindenmann J, März W, Gandini S, Dekker JM (2013b) Vitamin D and cancer mortality: systematic review of prospective epidemiological studies. Anticancer Agents Med Chem. 13(1):107–117
Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, Shoenfeld Y, Lerchbaum E, Llewellyn DJ, Kienreich K, Soni M (2013) Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—a review of recent evidence. Autoimmun Rev 12:976–989
Poel YH, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S (2012) Vitamin D and gestational diabetes: a systematic review and meta-analysis. Eur J Intern Med 23:465–469
Ponda MP, Huang X, Odeh MA, Breslow JL, Kaufman HW (2012) Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation 126:270–277
Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I (2011) Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res 26:1609–1616
Prietl B, Pilz S, Wolf M, Tomaschitz A, Obermayer-Pietsch B, Graninger W, Pieber TR (2010) Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? Isr Med Assoc J 12:136–139
Putz-Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, Obermayer-Pietsch B, Stauber RE (2012) Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int 32:845–851
Quarles LD (2012) Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol 8:276–286
Querfeld U (2013) Vitamin D and inflammation. Pediatr Nephrol 28:605–610
Quraishi SA, Camargo CA Jr (2012) Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care 15:625–634
Reddy GS, Tserng KY (1989) Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry 28:1763–1769
Reid IR (2013) Cardiovascular effects of calcium supplements. Nutrients 5:2522–2529
Reid D, Toole BJ, Knox S, Talwar D, Harten J, O’Reilly DS, Blackwell S, Kinsella J, McMillan DC, Wallace AM (2011) The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr 93:1006–1011
Reis JP, von Mühlen D, Michos ED, Miller ER 3rd, Appel LJ, Araneta MR, Barrett-Connor E (2009) Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis 207:585–590
Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, Salovaara K, Cooper C, Smith HE, Jacobs ET, Torgerson D, Jackson RD, Manson JE, Brixen K, Mosekilde L, Robbins JA, Francis RM, Abrahamsen B (2012) Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab 97:2670–2681
Riek AE, Oh J, Bernal-Mizrachi C (2013) 1,25(OH)2 vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol 136:309–312
Robinson-Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH (2013) Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA 310:179–188
Rock CL, Emond JA, Flatt SW, Heath DD, Karanja N, Pakiz B, Sherwood NE, Thomson CA (2012) Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity (Silver Spring) 20:2296–2301
Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL (2012) IOM committee members respond to endocrine society vitamin D guideline. J Clin Endocrinol Metab 97:1146–1152
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Rostand SG (1997) Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension 30:150–156
Sachs MC, Brunzell JD, Cleary PA, Hoofnagle AN, Lachin JM, Molitch ME, Steffes MW, Zinman B, de Boer IH, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) Research Group (2013) Circulating vitamin d metabolites and subclinical atherosclerosis in type 1 diabetes. Diabetes Care 36:2423–2429
Salic K, De Windt LJ (2012) MicroRNAs as biomarkers for myocardial infarction. Curr Atheroscler Rep 14:193–200
Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, Køber L, Jensen JE (2012) Vitamin D deficiency in postmenopausal, healthy women predicts increased cardiovascular events: a 16-year follow-up study. Eur J Endocrinol 167:553–560
Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R (2006) Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83:754–759
Schmid J, Kienreich K, Gaksch M, Grübler M, Raggam R, Meinitzer A, Rutters F, Dekker JM, März W, Verheyen N, Tomaschitz A, Pilz S (2013) The importance of assays in vitamin D status classification: a comparison of four automated 25-hydroxyvitamin D immunoassays. LaboratoriumsMedizin 37:261–268
Schnatz PF, Nudy M, O’Sullivan DM, Jiang X, Cline JM, Kaplan JR, Clarkson TB, Appt SE (2012) The quantification of vitamin D receptors in coronary arteries and their association with atherosclerosis. Maturitas 73:143–147
Schöttker B, Haug U, Schomburg L, Köhrle J, Perna L, Müller H, Holleczek B, Brenner H (2013) Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr 97:782–793
Schroten NF, Ruifrok WP, Kleijn L, Dokter MM, Silljé HH, Lambers Heerspink HJ, Bakker SJ, Kema IP, van Gilst WH, van Veldhuisen DJ, Hillege HL, de Boer RA (2013) Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open-label, blinded end point, randomized prospective trial (VitD-CHF trial). Am Heart J 166:357–364
Scragg R (1981) Seasonality of cardiovascular disease mortality and the possible protective effect of ultra-violet radiation. Int J Epidemiol 10:337–341
Scragg R, Wishart J, Stewart A, Ofanoa M, Kerse N, Dyall L, Lawes CM (2011) No effect of ultraviolet radiation on blood pressure and other cardiovascular risk factors. J Hypertens 29:1749–1750
Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin D Standardization Program (VDSP) (2012) Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 243:32–40
Shen H, Bielak LF, Ferguson JF, Streeten EA, Yerges-Armstrong LM, Liu J, Post W, O’Connell JR, Hixson JE, Kardia SL, Sun YV, Jhun MA, Wang X, Mehta NN, Li M, Koller DL, Hakonarson H, Keating BJ, Rader DJ, Shuldiner AR, Peyser PA, Reilly MP, Mitchell BD (2010) Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler Thromb Vasc Biol 30:2648–2654
Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny-Ardekani A (2013) The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecol Endocrinol 29:396–399
Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB (2013) Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 36:1422–1428
Soni M, Kos K, Lang IA, Jones K, Melzer D, Llewellyn DJ (2012) Vitamin D and cognitive function. Scand J Clin Lab Invest Suppl 243:79–82
Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, Bischoff-Ferrari HA, Cavalier E, Ebeling PR, Fardellone P, Gandini S, Gruson D, Guérin AP, Heickendorff L, Hollis BW, Ish-Shalom S, Jean G, von Landenberg P, Largura A, Olsson T, Pierrot-Deseilligny C, Pilz S, Tincani A, Valcour A, Zittermann A (2010) Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 9:709–715
Stricker H, Tosi Bianda F, Guidicelli-Nicolosi S, Limoni C, Colucci G (2012) Effect of a single, oral, high-dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: a randomised controlled pilot study. Eur J Vasc Endovasc Surg 44:307–312
Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM (2012) 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke 43:1470–1477
Syal SK, Kapoor A, Bhatia E, Sinha A, Kumar S, Tewari S, Garg N, Goel PK (2012) Vitamin D deficiency, coronary artery disease, and endothelial dysfunction: observations from a coronary angiographic study in Indian patients. J Invasive Cardiol 24:385–389
Szeto FL, Reardon CA, Yoon D, Wang Y, Wong KE, Chen Y, Kong J, Liu SQ, Thadhani R, Getz GS, Li YC (2012) Vitamin D receptor signaling inhibits atherosclerosis in mice. Mol Endocrinol 26:1091–1101
Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, Pritchett Y, Chang Y, Agarwal R, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Singh B, Zehnder D, Pachika A, Manning WJ, Shah A, Solomon SD, Thadhani R (2012) Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J 164:902–909
Tang J, McFann KK, Chonchol MB (2012) Association between serum 25-hydroxyvitamin D and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988-94. Nephrol Dial Transpl 27:4385–4389
Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD (2012) Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 307:674–684
Thomas GN, O’Hartaigh B, Bosch JA, Pilz S, Loerbroks A, Kleber ME, Fischer JE, Grammer TB, Böhm BO, März W (2012) Vitamin D levels predict all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome: the Ludwigshafen risk and cardiovascular health (LURIC) study. Diabetes Care 35:1158–1164
Thorsen SU, Mortensen HB, Carstensen B, Fenger M, Thuesen BH, Husemoen LL, Bergholdt R, Brorsson C, Pociot F, Linneberg A, Svensson J (2013) No difference in vitamin d levels between children newly diagnosed with type 1 diabetes and their healthy siblings: a 13-year nationwide danish study. Diabetes Care 36:e157–e158
Tiosano D, Schwartz Y, Braver Y, Hadash A, Gepstein V, Weisman Y, Lorber A (2011) The renin-angiotensin system, blood pressure, and heart structure in patients with hereditary vitamin D-resistance rickets (HVDRR). J Bone Miner Res 26:2252–2260
Tomaschitz A, Pilz S, Ritz E, Obermayer-Pietsch B, Pieber TR (2010) Aldosterone and arterial hypertension. Nat Rev Endocrinol 6:83–93
Tomaschitz A, Pilz S, Ritz E, Morganti A, Grammer T, Amrein K, Boehm BO, März W (2011) Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J 32:2642–2649
Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, Lanham-New S (2012) Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 95:1357–1364
Trivedi DP, Doll R, Khaw KT (2003) Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326:469
Trummer O, Pilz S, Hoffmann MM, Winkelmann BR, Boehm BO, März W, Pieber TR, Obermayer-Pietsch B, Renner W (2013) Vitamin D and mortality: a Mendelian randomization study. Clin Chem 59:793–797
Tu WJ Sr, Zhao SJ, Xu DJ, Chen H (2013) Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischemic stroke. Clin Sci (Lond). doi:10.1042/CS20130284
Uusi-Rasi K, Kärkkäinen MU, Lamberg-Allardt CJ (2013) Calcium intake in health maintenance—a systematic review. Food Nutr Res 57:21082
Vaidya A, Williams JS (2012) The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism 61:450–458
van Ballegooijen AJ, Snijder MB, Visser M, van den Hurk K, Kamp O, Dekker JM, Nijpels G, Stehouwer CD, Henry RM, Paulus WJ, Brouwer IA (2012) Vitamin D in relation to myocardial structure and function after eight years of follow-up: the Hoorn study. Ann Nutr Metab 60:69–77
van Ballegooijen AJ, Reinders I, Visser M, Dekker JM, Nijpels G, Stehouwer CD, Pilz S, Brouwer IA (2013a) Serum parathyroid hormone in relation to all-cause and cardiovascular mortality: the Hoorn study. J Clin Endocrinol Metab 98:E638–E645
van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA (2013b) Parathyroid hormone and cardiovascular disease events: a systematic review and meta-analysis of prospective studies. Am Heart J 165:655–664
van Ballegooijen AJ, Visser M, Kestenbaum B, Siscovick DS, de Boer IH, Gottdiener JS, deFilippi CR, Brouwer IA (2013c) Relation of vitamin D and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the Cardiovascular Health Study). Am J Cardiol 111:418–424
Vanlint S (2013) Vitamin D and obesity. Nutrients 5:949–956
Verduijn M, Siegerink B, Jager KJ, Zoccali C, Dekker FW (2010) Mendelian randomization: use of genetics to enable causal inference in observational studies. Nephrol Dial Transplant 25:1394–1398
Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, Wood AR, Michaëlsson K, Vandenput L, Zgaga L, Yerges-Armstrong LM, McCarthy MI, Dupuis J, Kaakinen M, Kleber ME, Jameson K, Arden N, Raitakari O, Viikari J, Lohman KK, Ferrucci L, Melhus H, Ingelsson E, Byberg L, Lind L, Lorentzon M, Salomaa V, Campbell H, Dunlop M, Mitchell BD, Herzig KH, Pouta A, Hartikainen AL, Genetic Investigation of Anthropometric Traits-GIANT Consortium, Streeten EA, Theodoratou E, Jula A, Wareham NJ, Ohlsson C, Frayling TM, Kritchevsky SB, Spector TD, Richards JB, Lehtimäki T, Ouwehand WH, Kraft P, Cooper C, März W, Power C, Loos RJ, Wang TJ, Järvelin MR, Whittaker JC, Hingorani AD, Hyppönen E (2013) Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 10:e1001383
Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, Hamilton SA, Hollis BW (2013) Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. J Steroid Biochem Mol Biol 136:313–320
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS (2008) Vitamin D deficiency and risk of cardiovascular disease. Circulation 117:503–511
Wang L, Manson JE, Song Y, Sesso HD (2010a) Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 152:315–323
Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD (2010b) Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376:180–188
Wang H, Xia N, Yang Y, Peng DQ (2012a) Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis 11:42
Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD (2012b) Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 5:819–829
Wehr E, Pilz S, Boehm BO, März W, Obermayer-Pietsch B (2010) Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 73:243–248
Wehr E, Pilz S, Boehm BO, März W, Grammer T, Obermayer-Pietsch B (2013) Low free testosterone is associated with heart failure mortality in older men referred for coronary angiography. Eur J Heart Fail 13:482–488
Weng S, Sprague JE, Oh J, Riek AE, Chin K, Garcia M, Bernal-Mizrachi C (2013) Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS ONE 8:e54625
Wilke RA, Simpson RU, Mukesh BN, Bhupathi SV, Dart RA, Ghebranious NR, McCarty CA (2009) Genetic variation in CYP27B1 is associated with congestive heart failure in patients with hypertension. Pharmacogenomics 10:1789–1797
Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME (2010a) The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail 3:195–201
Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD (2010b) The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 53:2112–2119
Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD (2012) The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutr Metab Cardiovasc Dis 22:864–870
Witham MD, Price RJ, Struthers AD, Donnan PT, Messow CM, Ford I, McMurdo ME (2013) Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. doi:10.1001/jamainternmed.2013.9043
Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA Jr, Tonelli M, Thadhani R (2007) Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72:1004–1013
Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, Simpson WG, Fraser WD, Reid DM, Macdonald HM (2012) Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab 97:3557–3568
Yang L, Lof M, Veierød MB, Sandin S, Adami HO, Weiderpass E (2011) Ultraviolet exposure and mortality among women in Sweden. Cancer Epidemiol Biomarkers Prev 20:683–690
Yiu YF, Chan YH, Yiu KH, Siu CW, Li SW, Wong LY, Lee SW, Tam S, Wong EW, Cheung BM, Tse HF (2011) Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. J Clin Endocrinol Metab 96:E830–E835
Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, Lee SW, Tam S, Wong EW, Lau CP, Cheung BM, Tse HF (2013) Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis 227:140–146
Young KA, Snell-Bergeon JK, Naik RG, Hokanson JE, Tarullo D, Gottlieb PA, Garg SK, Rewers M (2011) Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care 34:454–458
Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D (2008) Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int 74:170–179
Zittermann A, Gummert JF (2010) Sun, vitamin D, and cardiovascular disease. J Photochem Photobiol, B 101:124–129
Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Körfer R, Stehle P (2003) Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol 41:105–112
Zittermann A, Fischer J, Schleithoff SS, Tenderich G, Fuchs U, Koerfer R (2007) Patients with congestive heart failure and healthy controls differ in vitamin D-associated lifestyle factors. Int J Vitam Nutr Res 77:280–288
Zittermann A, Schleithoff SS, Frisch S, Götting C, Kuhn J, Koertke H, Kleesiek K, Tenderich G, Koerfer R (2009) Circulating calcitriol concentrations and total mortality. Clin Chem 55:1163–1170
Zittermann A, Gummert JF, Börgermann J (2011a) The role of vitamin D in dyslipidemia and cardiovascular disease. Curr Pharm Des 17:933–942
Zittermann A, Pilz S, Börgermann J, Gummert JF (2011b) Calcium supplementation and vitamin D: a trigger for adverse cardiovascular events? Future Cardiol 7:725–727
Zittermann A, Börgermann J, Gummert JF, Pilz S (2012a) Future directions in vitamin D and cardiovascular research. Nutr Metab Cardiovasc Dis 22:541–546
Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S (2012b) Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 95:91–100
Zittermann A, Kuhn J, Dreier J, Knabbe C, Gummert JF, Börgermann J (2013a) Vitamin D status and the risk of major adverse cardiac and cerebrovascular events in cardiac surgery. Eur Heart J 34:1358–1364
Zittermann A, Prokop S, Gummert JF, Börgermann J (2013b) Safety issues of vitamin D supplementation. Anticancer Agents Med Chem 13:4–10
Acknowledgments
Martin Gaksch is supported by funding of the Austrian National Bank (Jubilaeumsfond: Project Numbers: 13878 and 13905).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilz, S., Gaksch, M., O’Hartaigh, B. et al. The role of vitamin D deficiency in cardiovascular disease: where do we stand in 2013?. Arch Toxicol 87, 2083–2103 (2013). https://doi.org/10.1007/s00204-013-1152-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1152-z