Abstract
Electronic waste (e-waste) is termed as “urban mines” due to high metal content. Metals are major components of e-waste and have a share of 61 wt% of e-waste. E-waste contains various valuable metals such as gold, silver, platinum, palladium, copper, nickel, etc. Therefore, metal recovery is important to conserve the resources. Apart from this, the unregulated accumulation and improper recycling of e-waste have harmful effects on human health and environment. Therefore, environmentally friendly e-waste recycling is the need of the hour to mitigate the harmful effects. Currently, pyrometallurgy and hydrometallurgy are the conventional processes employed for recovery of metals from e-waste. However, these technologies are non-selective and energy-intensive, employ hazardous chemicals, and produce toxic gases. Biohydrometallurgy is a promising alternative and is an eco-friendly approach to recycle e-waste as it employs microorganisms for metal recovery. Biohydrometallurgy employs different approaches such as autotrophic bacteria bioleaching, heterotrophic bacteria bioleaching, and heterotrophic fungi bioleaching for leaching of metals and has been discussed in this chapter. In addition, the refining of metals from metal leached solution has also been discussed in this chapter. The development of continuous process for metal recovery is important, and we have discussed a coiled flow inverter (CFI) reactor as a promising option for the same.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
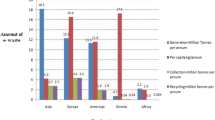
Electronic waste (e-waste) is rapidly growing, and 53.6 million tons (Mt) of e-waste was generated in 2019 [1]. It is expected that the e-waste generation will be around 74.7 Mt by 2030 [1, 2]. In 2019, Asia produced 24.9 Mt of e-waste and followed by America, Europe, Africa, and Oceania which have generated 13.1, 12, 2.9, and 0.7 Mt of e-waste, respectively [1]. The e-waste generation is increasing with an annual growth of 2 Mt from 2014 and is shown in Fig. 1 along with projected values till 2030 [1]. However, the recycling rate is not keeping pace with the e-waste generation. Europe is leading in the e-waste recycling with a recycling rate of 42.5%, while Asia is at the second position with an 11.7% recycling rate and followed by America, Oceania, and Africa which have a recycling rate of 9.4%, 8.8%, and 0.9%, respectively [1]. Only 17.4% of e-waste was properly recycled, while the fate of around 82.6% of e-waste generated in 2019 is not known [1].
The amount of e-waste which is not documented in 2019 contains 71 kilotons of brominated flame retardants (BFR) , 98 Mt of CO2 in the form of hydrochlorofluorocarbons (HCFCs) and chlorofluorocarbons (CFCs) , and 50 t of mercury [1]. Apart from this, e-waste contains various toxic substances such as Sb, As, Ba, Cd, Pb, polychlorinated biphenyls, etc. [3]. Unregulated accumulation of e-waste leads to the leaching of these substances into the soil and water and then containment the food chain. Table 1 shows the different harmful substances existing in e-waste and their hazardous effects. In addition, informal recycling, i.e., open burning of e-waste, leads to the formation of harmful chemicals such as dioxins and furans.
Although e-waste contains various toxic substances, it can also act as a secondary source of valuable metals owing to its high metal content. E-waste mainly consists of metals, plastics, rubber, ceramics, and glass [5]. Out of these, metals are the main components and have a share of 61% by wt. of e-waste [6]. E-waste contains different heavy metals, like Cu, Ni, Hg, Cd, and Pb, and precious metals like Au, Ag, Pt, and Pd [7]. Electrical and electronic equipment industry consumes over 50% of the ruthenium, antimony, and indium and more than 30% of silver, copper, and tin produced annually [8]. Hence, recovery of metals from e-waste is necessary to mitigate the scarcity of metals. The printed circuit board (PCB) of a personal computer contains 20% Cu and 250 g/ton of Au, whereas the concentrations of Cu and Au in ores are 0.5–1% and 1–10 g/ton, respectively [9, 10]. The metal recovery from e-waste will provide advantages such as the conservation of primary metal resources, energy-saving, and prevention of environmental pollution caused due to leaching of metals.
It is clear from the above discussion that it is very important to not dump the e-waste or treat it inappropriately. E-waste recycling is important from the aspect of recovery of metals and mitigating the environmental and human health hazards. Therefore, various technologies like pyrometallurgy [11,12,13], hydrometallurgy [14,15,16], and biohydrometallurgy [17,18,19] have been employed for the recovery of metals from e-waste. Out of these, pyrometallurgy and hydrometallurgy are conventional processes employed for the recovery of metals from e-waste. However, both these technologies have disadvantages associated with them. The use of pyrometallurgical approach leads to the generation of dioxins and furans due to the presence of halogenated flame retardants in e-waste [20]. In addition, pyrometallurgy uses very high temperature, i.e., above 1000 °C, for metal recovery and makes this process energy-intensive [21]. In the case of hydrometallurgy, there is no or less formation of toxic gases, but the hydrometallurgical process uses toxic solvents such as acid, cyanide, halide, thiosulfate, and thiourea for the recovery of metals [22, 23]. The use of these chemicals limits the industrial application of hydrometallurgy. Compared to pyrometallurgy and hydrometallurgy, biohydrometallurgy uses microorganisms for the recovery of metals which makes this process an eco-friendly approach. Biohydrometallurgy is proven efficient for the recovery of metal from primary ores and can play an important role in the efficient recovery of metal from e-waste [24, 25]. Biohydrometallurgy provides advantages such as selectivity toward valuable metals, cost-effectiveness, and lower environmental hazards [26, 27]. Bioleaching of e-waste is a new field compared to hydrometallurgy and pyroometallurgy, and it can be seen from Fig. 2. The numbers of publication in the field of e-waste are constantly increasing. The biohydrometallurgy is a new technique in the field of e-waste which can be seen from Fig. 2b.
This chapter focuses on the recent development in the recovery of valuable metals from e-waste using biohydrometallurgy. In this chapter, various biohydrometallurgical approaches, i.e., autotrophic bacteria bioleaching, heterotrophic bacteria bioleaching, and heterotrophic fungi bioleaching, have been discussed. In addition, this chapter also focuses on the different approaches such as biosorption and bioelectrochemical process for the recovery of metals from the metal leach solution.
2 Different Bioleaching Approaches for Metal Recovery
Bioleaching of metals has been investigated using various microorganisms, and these microorganisms have the natural capability to leach the metals into aqueous solution. Usually, autotrophic bacteria, heterotrophic bacteria, and heterotrophic fungi are the most frequently used microorganisms for the recovery of metals from different sources as shown in Fig. 3. The bioleaching using each of these is discussed in the following subsections.
2.1 Autotrophic Bacteria Bioleaching
The autotrophic bioleaching is mainly carried out using chemolithotrophic and acidophilic bacteria. These organisms use carbon dioxide from the atmosphere as a carbon source and ferrous ion (Fe2+), elemental sulfur (So), and/or reduced sulfur compounds as an energy source [28, 29]. Most chemolithotrophic bacteria have a high tolerance for heavy metal toxicity and therefore are the most widely used microorganisms to recover metals from polymetallic sources such as e-waste [30]. The microorganisms used in autotrophic bioleaching are sulfur-oxidizing bacteria, iron- and sulfur-oxidizing bacteria, and iron-oxidizing bacteria [19]. The above mentioned microorganisms lead to the sulfur and iron oxidation which causes metal sulfide solubilization and decreases the pH of the environment which ultimately causes the solubilization of other metal compounds. These microorganisms flourish on the iron- and sulfur-containing sources (e.g., pyrite, pentlandite, and chalcopyrite) at 45–75 °C [31]. However, in the case of e-waste, autotrophic bacteria cannot grow directly on the oxidation/dissolution of the e-waste matrix. Therefore, it is important to mixed e-waste with sulfur- or iron-containing sources such as pyrite, pentlandite, and chalcopyrite to provide energy for the growth of autotrophic bacteria [32]. As a result, the microbial oxidation of sulfur- and iron-containing sources will produce acidic environment and ferric ion, and this will help in the leaching of metals from e-waste. It is important to notice that the autotrophic bioleaching of sulfidic ores only leaches the metallic fraction of e-waste, while the non-metallic fraction remains as it is. The recovery of rare earth metals from other sources will also need to mix with the sulfur- and iron-containing sources for the growth of autotrophic bacteria. The research work carried for metal recovery from e-waste using autotrophic bacteria is mainly focused on transition metal and also rare earth element (REE) recovery.
Hong et al. investigated the bioleaching of copper using Acidithiobacillus thiooxidans bacteria and studied abiotic leaching and direct and indirect bioleaching [33]. The acidophilic bacteria were cultivated and during which the sulfuric acid was produced. This acid was used for indirect bioleaching. However, in the case of direct bioleaching, the sterilized e-waste was directly added during the growth phase of bacteria. The direct leaching was performed at 30 °C, and 10 g/dm3 e-waste were added to the microbe culture when pH was reached to 1. At the same process condition, there is not much difference between the Cu leaching in both direct and indirect bioleaching. Therefore, it can be concluded that the toxicity of e-waste at the concentration of 10 g/dm3 doesn’t have a significant effect on microorganism metabolism. The indirect leaching was also carried out at 90 °C, and the results were compared with abiotic leaching. It was observed that for 8 h of leaching time, the copper leaching is lower in the case of indirect leaching (60%) compared to abiotic leaching (98%). It was found that after 6 h of leaching, there is the formation of CuS on the surface which results in the passivation of Cu surface and decreases the leaching of Cu in biogenic acid. The formation of CuS can be attributed to the presence of incompletely oxidized sulfide and sulfates.
In a different study, Chen et al. studied the application of Acidithiobacillus ferrooxidans for copper leaching from WPCB [34]. Sulfuric acid and ferric ion play an important role during the bioleaching process. During the bioleaching, ferrous ions are oxidized to ferric ions as shown in Eq. (1), while Cu oxidizes to Cu2+ by Fe3+, and Fe3+ reduces to Fe2+ as shown in Eq. (2). This Fe cycle increases the rate of reaction significantly. The column bioleaching was employed for the copper recovery, and the temperature of the column reactor was maintained at 30 °C during the complete process. For a typical experiment, 250 g of washed sample was added to the column, and then 495 L of prepared 4.5 K medium along with 0.05 L of A. ferrooxidans culture was added into the column reactor. The pH of the solution in the column was maintained at 2.25 (±0.05) using 5 M of H2SO4. The maximum leaching of copper was measured to be 94.8% in 28 days. It was found that pH is an important factor and greatly influences the copper leaching. The kinetics of the bioleaching process does not change as the size and morphology of precipitates remain the same as the pH was maintained at 2.25. The addition of sulfuric acid and maintaining an acidic pH of solution aid in preventing the formation of jarosite precipitate, and this helps in the Fe2+–Fe3+ cycle to go on to create a favorable environment for copper bioleaching.
Isildar et al. studied the bioleaching of copper using Acidithiobacillus ferrivorans , Acidithiobacillus thiooxidans , and a mixture of both [35]. The leaching efficiencies of 94%, 89%, and 98% were reported using a pure culture of A. ferrivorans, A. thiooxidans, and a mixture of both, respectively, at a pulp density of 1%. It is reported that the pulp density below 2.5% is efficient for the bioleaching of copper. The hazardous components of PCB such as metals, phenols, and BFRs are harmful to the bacterial activity, and therefore, to prevent the toxic effect of these components on bacteria, a pre-growth method was applied. A pre-growth method was also helpful in producing favorable bioleaching conditions. The microorganisms were incubated in the bioleaching medium, and the culture was prepared in the absence of waste PCB. The waste PCBs were added after attaining the optimal bioleaching conditions. The measurement of pH and ORP helps in monitoring the bacterial activity, and these parameters also show the presence of both acidolysis and redoxolysis mechanisms. The increase in pulp density from 0% to 5% increases the pH due to the basic nature of waste material. When the pulp density is 2.5% or higher, then pH value does not drop below 2.5 where acidophiles thrive. The pulp density of 1% and lower is best suited for the growth of microorganisms. Acidophiles play an important role to catalyze the oxidation of Fe2+ to Fe3+ as shown in Eq. (1) and elemental sulfur (S0) to sulfuric acid as shown in Eq. (3):
The recovery of Cu takes places under low pH and high ORP conditions as shown in Eqs. (2) and (4):
The copper leaching is higher in the case of a co-culture of iron- and sulfur-oxidizing acidophiles. Therefore, it is reported that the involvement of redoxolysis and acidolysis is beneficial for metal recovery. Table 2 shows the summary of research works carried out for metal recovery using autotrophic bioleaching.
2.2 Heterotrophic Bioleaching
The scientific community has recently started to explore the various biotechnological approaches to recover REE from secondary metal sources [42,43,44]. Heterotrophic bioleaching is an encouraging technique for the recovery of metals from the sources which do not contain metal sulfides [44]. Microorganisms such as bacteria, fungi, and archaea are commonly employed during heterotrophic bioleaching [19, 45]. Heterotrophic bioleaching is microbial leaching where organisms get energy from organic carbon sources for growth during the leaching process [46]. The metabolic by-products of organic carbon such as acetic acid, citric acid, oxalic acid, and gluconic acid are responsible for the leaching of metals when the pH is between 4 and 6 [47]. Apart from this, the protein catabolism produces non-acidic complexion agents which can be employed during alkaline leaching [19]. The metal recovery using heterotrophic bioleaching mainly occurs via cyanide- and organic acid-producing organisms. The cyanogenic bioleaching is employed to recover precious and platinum group metals. However, chelation is used for the recovery of critical metals such as cobalt, gallium, germanium, lithium, antimony, and tungsten. The heterotrophic microorganism can be used for metal leaching in higher pH conditions and, therefore, can be employed to treat alkaline wastes compared to acidophiles [45].
2.2.1 Heterotrophic Bacterial Bioleaching
Pseudomonas aeruginosa , Pseudomonas fluorescens, and Pseudomonas putida are the Pseudomonas strains used for metal leaching. These microorganisms are omnipresent, and in soil, they solubilize metals due to various metabolic products.
Jujun et al. found a new strain of Pseudomonas which has the ability to produce CN− and can recover precious metals [48]. The ability to produce CN− of different strains such as Pseudomonas aeruginosa, Pseudomonas chlororaphis, Pseudomonas putida, Pseudomonas mosselii, Pseudomonas fluorescens, and Pseudomonas sp. was investigated. The experiments were performed to find out the concentration of produced CN− by the above mentioned strains. These strains were cultured for 24 h at 25 °C, and then the CN− concentration was tested. It was reported that the Pseudomonas chlororaphis has produced the highest concentration of CN− (7.11 mg/L). Afterward, Pseudomonas chlororaphis was selected to investigate the metal recovery, and the effect of various process variables like culture condition, pH, temperature, additive, and stirring speed on the ability to produce CN− was studied. The optimized conditions for producing maximum CN− were pH 7 and adding glycine (4.4 g/L) + methionine (2 g/L) into NB culture medium which is cultured for 72 h at 25 °C with a stirring speed of 60 rpm. At the optimized conditions, the recovery of gold, silver, and copper was 8.2%, 12.1%, and 52.3%, respectively, from the metallic particles obtained from crushed waste PCBs.
Chi et al. studied the copper and gold recovery using Chromobacterium violaceum (C. violaceum) from waste mobile phone printed circuit boards [49]. The bioleaching was performed in the presence of YP medium (yeast extraction, polypeptone, and glycine), and the effect of pH and hydrogen peroxide on copper and gold recovery was investigated. It is reported that gold leaching increase from 7.78% to 10.9% when pH was increased from 8 to 11 in 8 days. Similarly, the copper leaching was also increased from 4.9% to 11.4% with an increase in pH from 8 to 10. Marsden and House reported that the Cu(CN)2− is formed when pH is less than 9, while at higher pH, more amounts of Cu(CN)3− and Cu(CN)43− will form [50]. Metal leaching is improved at higher pH due to the high stability of metal cyanide complexes and increases the stability of HCN at pH > 10 [51]. Similarly, Au(CN)2− forms at higher pH, and high dissolved oxygen favors gold leaching [52]. The hydrogen peroxide was employed to increase the dissolved oxygen to facilitate more metal leaching. The hydrogen peroxide concentration above 0.004% negatively hampers the bacteria, and therefore, 0.004% hydrogen peroxide was considered as the optimum for metal leaching. The increase in hydrogen peroxide concentration leads to an increase in the metal leaching from 7.23% to 24.6% with an increase in pH from 8.5 to 10 in 8 days. The recovery of gold is increased slightly from 8.1% to 11.32% when pH was increased from 8.5 to 11 in the presence of hydrogen peroxide. The higher copper recovery compared to gold can be attributed to the galvanic interaction and gold being nobler than copper. Therefore, it is important to leach copper prior to gold leaching to improve the gold bioleaching.
Cu is a major component of the e-waste, and as mentioned above, it is important to recover Cu before Au and Ag for their efficient recovery. Therefore, Isiladar et al. developed a two-stage bioleaching process for Cu and Au recovery. In the first step, 98% Cu was leached using a mixture of Acidithiobacillus ferrivorans and Acidithiobacillus thiooxidans [35]. In the next step, Au was recovered using Pseudomonas putida at 25 °C, and a 44% recovery of Au was reported. The low recovery of gold can be attributed to the lower amount of cyanide generation (21.4 mg/L) from Pseudomonas cultures , and this did not allow complete gold recovery. Marra et al. have also investigated metal bioleaching in two steps [53]. In the first step, rare earth metals such as cerium, europium, neodymium, lanthanum, and yttrium were recovered using Acidithiobacillus thiooxidans from e-waste dust. During the first step, recovery of cerium, europium, and neodymium was 99%, while that of lanthanum and yttrium is around 80%. In the second step, gold was recovered using the Pseudomonas putida and reported the 48% recovery of Au within 3 h.
Several researchers have tried to increase the production of biogenic cyanide using different approaches such as sequential nutrient addition, medium modification, and genetic modification. Natarajan et al. tried to improve the production of biogenic cyanide than Chromobacterium violaceum ; two metabolically engineered stains, pBAD hcn (induced by L-arabinose) and pTAC hcn (induced by IPTG), were prepared [54]. It is reported that the pBAD (induced with 0.002% L-arabinose) and pTAC (induced with 1 mM IPTG) have produced 34.5 and 31 mg/L of cyanide, respectively, whereas Chromobacterium violaceum has produced 20 mg/L cyanide. The increase in the cyanide concentration results in higher gold leaching. The pBAD and pTAC showed 30% and 27% gold leaching at 0.5% w/v pulp density compared to 11% gold leaching using wild-type bacteria. Similarly, Natarajan and Ting studied the effect of mutation of Chromobacterium violaceum bacteria to grow under alkaline environment on the production of biogenic cyanide for gold bioleaching [55]. The mutation of bacteria was performed by exposing the wild C. violaceum to 100 mM of the mutagen, i.e., N-nitroso-N-ethyl urea (ENU), at pH 9, 9.5, and 10. The gold recovery reported using the C. violaceum mutated at pH 9, 9.5, and 10 was 18%, 22.5%, and 19%, respectively. Under alkaline conditions, there are a growth of bacteria and production of cyanide which increases the availability of cyanide ion, thereby increasing the gold leaching. The lower gold leaching using the C. violaceum mutated at pH 10 as it has significantly lower growth compared to others. The wild strain of C. violaceum grown at pH 9 and 9.5 showed 14% and 16% gold leaching, respectively, whereas wild strain grown at pH 10 showed no gold leaching. Therefore, from these studies, it is clear that genetic modification or medium modification can lead to higher metal leaching. Table 3 shows the summary of research work on metal recovery using heterotrophic bacterial leaching.
2.2.2 Heterotrophic Fungi Bioleaching
Heterotrophic fungi bioleaching involves the metal leaching using the organically excreted acid (acidolysis and complexolysis) and changing the oxidation potential of the medium (redoxolysis) or a combination of acidolysis, complexolysis, and redoxolysis [31, 59]. The fungal redoxolysis leaching occurs at comparatively higher pH, i.e., near pH 7 or above [31]. Aspergillus niger and Penicillium simplicissimum are the most used microorganisms in the fungal leaching of metal from various waste sources [59]. Brandl et al. investigated the bioleaching of metals form electronic waste using Aspergillus niger and Penicillium simplicissimum [60]. During the growth, various organic acids such as citrate, gluconate, and oxalate were formed which are responsible for the metal leaching. It is reported that P. simplicissimum has been more efficient for the leaching of metals compared to A. niger under identical conditions. Both the fungal species were able to recover 65% of Cu and Sn, whereas the recovery of Al, Ni, Pb, and Zn was more than 95%. Authors have suggested a two-step approach for the bioleaching of metals as e-scrap has negative impact on growth of microorganisms. It is recommended that in the first step, growth of the microorganisms will take place without e-scrap and in the next step, the metabolites produced will be employed for metal leaching. It is previously recommended for the treatment of fly ash for metal leaching using bacteria and fungi leaching and has the following advantages [61, 62]:
-
1.
Microorganisms can be recycled.
-
2.
Optimization of acid formation due to absence of waste material.
-
3.
The high concentration of waste material can be used during the leaching step.
Desouky et al. studied the leaching of rare earth metals from the waste material using A. ficuum [63]. The metabolite containing organic acids was produced using A. ficuum at pH 3. The waste material (0.75 g) was then added to the metabolite, and then the mixture was stirred using rotary shaker at 175 rpm for 24 h. The leaching of uranium, thorium, lanthanum, cerium, and yttrium was reported as 30%, 29%, 20%, 33%, and 2.5%, respectively. It is also reported that thorium was precipitated from the leached solution as thorium oxalate using oxalic acid at pH 0.9 and uranium was precipitated as ammonium diuranate using ammonia solution at pH 5–6. In addition to this, rare earth metals were precipitated as rare earth oxalate using oxalic acid at pH 8–8.3.
Hassanien et al. compared the bioleaching efficiencies of one- and two-step bioleaching processes for rare earth metal leaching from Egyptian monazite and thorium-uranium concentrate using A. ficuum [64]. In one-step bioleaching, A. ficuum was grown in the media in the presence of the 1 g of monazite or thorium-uranium concentrate. However, in the indirect bioleaching process, the A. ficuum was grown in the media in the absence of monazite or thorium-uranium concentrate. The 1 g of monazite and thorium-uranium concentrate was added after the separation of microbial biomass. In the case of direct bioleaching, the leaching of rare earth elements using A. ficuum was reported as 60.6% and 50.3% within 10 days from monazite and thorium-uranium concentrate, respectively, whereas, in the case of indirect bioleaching, the leaching of rare earth metals was 55.0% and 47.7% from monazite and thorium-uranium concentrate, respectively. The pH was decreased from 3.9 to 3 due to the formation of amino acid and other metabolites during the growth of A. ficuum . These metabolites help in the leaching of metals using hydrogen ions or forming the metal complexes, whereas there is an increase in the pH during indirect bioleaching due to the consumption of proton to convert oxides present in a sample to soluble metal salts. The organic acid formed plays an important role and acts as leaching agents during the fungi bioleaching process. The organic acids such as citric, tartaric, oxalic, and gluconic acids were produced during the growth of A. ficuum. The complex formation reaction between rare earth element cations (Re2O3) and citric acid may take place as below:
The possible reactions between the oxalic acid and rare earth elements are as follows:
Similarly, the gluconic acid reacts with the rare earth elements as follows:
3 Metal Refining
In recent years, significant research for environmental protection is focused on the removal of metals from industrial wastewater, soil, and ground wastewater. The bio-based technologies are being employed for this purpose. Biosorption and bioprecipitation are some of the bio-based technologies employed for metal recovery which are low-cost, eco-friendly biological strategies with a low waste generation [65]. An integrated approach combining conventional metallurgical systems with bioelectrochemical and biosorption processes will be a great leap toward the development of green technology.
3.1 Biosorption
Biosorption is a combination of adsorption and absorption which depends on the binding capacity of potential biological sorbents like algae, bacteria [66, 67], yeasts [68], and fungi with metal [69, 70]. Various living or dormant biological materials like fungus, bacteria, agriculture residue, and biomass residual of fermentation process have been studied for the biosorption process [71,72,73]. Generally, the biosorption process is an interaction of metal ions present in aqueous solution with the biological material surface, thereby reducing metal ion concentration in aqueous solution as shown in Fig. 4. There are several mechanisms of biosorption which include ion exchange, reduction, precipitation, etc., and due to the complex process and nature of biomaterials, there is a possibility of a combination of mechanism [66].
The cell structure of biosorbent plays a vital role in the sorption process. Bacteria are one of the widely used biosorbent and are classified based on the composition of the cell wall, which highly affects the efficiency of metal absorption [66, 70]. Teichoic acid is present in the cell membrane of gram-positive bacteria, and phosphodiester bonds between the teichoic acid impart negative charge which enhances the sorption of metal ions. Similarly, phospholipid and lipopolysaccharide layers are present in the outer layer of gram-negative bacteria which imparts negative charge and expedites metal biosorption [74]. Potential bacterial biosorbents are Bacillus [75, 76] and Streptomyces [77, 78] in gram-positive genus and Pseudomonas [76, 79] in gram-negative genus.
In addition to bacteria, fungal biosorbents have a huge potential in metal biosorption as it is easier to grow and can be modified genetically or chemically. Chitin, lipids, polyphosphates, and proteins are some species of fungi with metal binding groups such as amines, phosphates, carboxyl, hydroxyl, etc. Aspergillus [80], Rhizopus [81], and Penicillium [82, 83] are important fungal biosorbents. Cheap production and least sensitivity toward alteration in nutrients and process parameters (pH, temperature, aeration) make fungi fit for industrial use [84]. Yeast biomass is also used for specific metal biosorption. Saccharomyces cerevisiae is one of the widely used yeast biomass with high biosorption capacity [85]. Extensive use of microorganisms in food/pharmaceutical industries generates immense amounts of waste which can be reutilized in the metal sorption process [86, 87]. Functional groups of sorbent material play a major role in biosorption mechanism, and alteration of the functional group via physical or chemical means affects the sorption capacity [88]. Functional groups (mainly COOH, NH2, OH, and SH) present on biosorbent surface form complexes with metal ions in solution via chemical binding, microprecipitation, ion exchange, etc. [89]. Tuning of functional groups of biomaterials can be done by surface modification techniques like ultrasonication, heat treatment, or changing crosslinking by acid/alkali treatment [90]. The pre-treatment technique modifies surface groups by removing, masking, or exposing binding sites. It was observed that biosorption capacity is immensely affected by pretreatment technique and time [90].
Various researchers have studied the effect of process parameters such as pH, contact time, the concentration of feed, and amount of biosorbent on the biosorption [64, 65]. Kalak et al. studied the effect of process parameters like pH, biosorbent dosage, and initial concentration of metal ions which directly affect the efficiency of biosorption of Fe (III) onto elderberry [91]. The initial concentration of metal ions in aqueous solution affects the saturation of biosorbent surface, whereas considerable pH change leads to deprotonation of the acid group present on biomass, surging probability of adsorption of positive metal ions onto elderberry. Vendruscolo et al. discussed different microbial systems to recover Cr (VI) from industrial effluent and concluded that versatile biosorbents in their viable or non-viable forms can be used in the batch, fed-batch, or continuous reactors [92]. Further, it is also reported that microorganism’s isolation, novel selection, and genetic alterations are the key parameters for the technological advancement of biosorption in metal absorption. The summary of research work on the recovery of metals using biosorbent from aqueous solution is shown in Table 4.
Nicomal et al. recovered indium , a major component in the optical electronic industry, using microalgae biomass [94]. Microalgal biomass has shown high binding capacities for several metals as various functional groups, like carboxyl, amino, phosphate, etc., present in microalgal biomass act as binding sites for metals.
Immobilization of microbial biomass is an innovative way to improve biosorbent capacity along with enhancement in strength and durability. Various polymeric or biopolymeric materials are used for the immobilization process. Ahmed et al. investigated the biosorption of different metals on free and immobilized algae biomass. Free and immobilized biomass are equally effective for the metal aqueous solution in the batch system; however, biosorption via immobilized algae biomass shows better biosorption capacity and biosorbent reusability [71]. Chatterjee et al. recovered nickel from spent batteries using Aspergillus nominus (A. nominus) , a fungal strain [87]. Further, they have studied the effect of the production of enzymes and alcohol on the tolerance level of A. nominus . A phenomenon of a sudden rise in biosorption of heavy metal with an increase in pH was termed as absorption edge, and in this case, the pH was 5. Desorption capacity of fungal strain A. nominus was observed, and it is reported that fungal biosorbent could be reused multiple times. Sheel et al. used ammonium thiosulfate (AT) and Lactobacillus acidophilus for selective sorption of gold from the printed circuit board [99]. The combination of leaching and sorption method leads to a recovery of 85% gold. Several major challenges like metal binding capacity, renewability, stability, and cost efficiency should be addressed in the biosorption process [90].
To conclude, biosorption is a versatile process with certain advantages such as (a) selective absorption of metals at low concentration, (b) mild operating conditions like pH and temperature, (c) energy-efficient, and (d) regeneration of biosorbent [94]. One of the major challenges in biosorption is to find high biosorption capacity biosorbent for metal absorption. Even though it has several advantages, its limited commercial success and lack of understanding of its kinetics, mechanism, and thermodynamics limit its use. In the case of electronic waste, there are certain challenges, like multi-metal-rich feedstream, dissolution of metal ions into the aqueous solution, and chemical modifications for better selectivity, which need to be addressed. Till now, the recovery of metals from e-waste using biosorption is still at early stages; however, versatile biosorption process holds great potential in the near future [66, 99].
3.2 Bioelectrochemical Process (BES)
BES is a fusion of conventional electrochemical systems and microbial systems. It reduces organic matter and produces electrical energy from chemical energy via the metabolism of microorganisms [100]. BES is also referred to as microbial fuel cell (MFC) or microbial electrolytic cell and is a promising option because of its sustainable approach of harnessing energy and in the production of bioproducts and waste remedy. The microbial fuel cell has several advantages over conventional metallurgy techniques in terms of cost, energy, and environment hazard [101]. Low anode corrosion and recovery of material as well as harnessing electrical energy are the advantages of BES systems over conventional systems [101]. The general structure of BES reactors includes anode, cathode, and separator as shown in Fig. 5 [102]. An anode chamber is filled with oxidized biodegradable material. BES is a promising modern technology for metal recovery from electronic waste due to major improvements in electrode material and designs in the last decade [103]. In recent years, BES applications to remove metals from aqueous solutions are intensively studied with a hope that BES could be applied to metallurgical systems to recover base metals, precious metals, and rare earth metals.
The general methodology of the BES system is the biological conversion at the anode which releases electrons to the cathode and, therefore, reduces metals to its precipitate by considering metals as terminal electron acceptor (TEA). TEA is a compound that accepts electron during oxidation of organic source and plays a crucial role in BES if it is used for waste minimization. Several metal ions can substitute oxygen in a series of oxidation and reduction reactions and can act as an effective TEA [104].
Nachariah et al. discussed about several BES systems to remove metal ions such as Ag(I), Au(III), Co(II), etc. [105]. BES along with microbial electrolysis cell for individual metals recovery were discussed in detail. Biocathode usage was also discussed where cathodes use metal-reducing bacteria to enhance metal reduction on the cathode, with an additional separation method to recover metals.
Huang et al. recovered cobalt from lithium cobalt powder using a microbial electrolysis cell [106]. Overall cobalt recovery was 0.15 ± 0.01 g Co/g Co. This study could be linked to recover cobalt from spent lithium batteries with minimum energy consumption. Heijne et al. recovered copper using a MFC with the bipolar membrane [107]. Aerobic and anaerobic condition effect on copper and electricity generation was evaluated, and it was observed that there is improvement in power density of the MFC in aerobic conditions.
Hu et al. have recovered platinum group metals like palladium and rhodium on cathode from wastewater using microbial fuel cell [108]. The electromotive force was utilized to recover Pd, Pt, and Rh on the cathode with a removal efficiency of 99.2%, 99.5%, and 98.7%, respectively. The purity of metals was verified by SEM and EDS analysis. The bioelectrochemical system is a versatile process for the recovery of metals from waste or industrial effluent waste stream. A combination of cathodic reduction with organic component oxidation can be an effective tool to leach out several metals and remove metals. Configuration of BES in terms of cathode-anode along with applied potential, voltage, and initial metal concentration will vastly affect metal removal efficiency. The mechanism part of BES is still not explored well, and much research is needed to verify the mechanism. An integrated approach of combining hydrometallurgy with BES, along with its use in the leaching and extraction of metals from aqueous solution, still needs to be explored.
4 Future Perspective
4.1 Development of Continuous Process for Metal Extraction
The development of a continuous process is important from the aspect of the economic efficiency of the process. The continuous process will also help in mitigating the variation in the quality of the product compared to the batch process and provides more control over the quality of the product. Some recent studies showed that a novel coiled flow inverter (CFI) can be an attractive option for the development of a continuous process for metal extraction [109,110,111,112]. CFI was designed by Saxena and Nigam in 1984 [113]. CFI works on the principle of flow inversion, and it is the combination of coiling and 90° bends.
Due to superior efficiency, CFI has been used in various applications such as heat exchanger, gas-liquid-solid reactions, two-phase flow, food processing, mass transfer, pharmaceutical and biotechnology, micro-reactor for metal extraction, etc. as shown in Fig. 6 [114,115,116,117,118,119,120]. Different types of CFI designs such as standard CFI, symmetrical compact CFI, and asymmetrical compact CFI have been employed for process intensification as shown in Fig. 7 [114]. Very few studies have been carried out in the field of metal extraction using CFI. Zhang et al. employed CFI for the extraction of Co from Ni sulfate solution using Cyanex 272 [121]. The experimental setup employed in the study for the continuous extraction of metals is shown in Fig. 8. The two syringe pumps were employed to pump aqueous and organic solutions. A T-type joint was used to join the aqueous and organic phases. A CFI reactor of square shape with four 90° bends was employed in the study to facilitate more efficient mixing. Zhang et al. reported that CFI leads to the higher extraction of Co and separation factor between Co and Ni compared to batch operation. In addition, the optimized residence time in a continuous process using CFI was 60 s and it is very less compared to a batch process residence time of 450 s. The use of CFI provides advantages such as lower reagent consumption, higher productivity and recycle rate, and smaller plant footprint. The use of CFI leads to intensified liquid-liquid mass transfer and can provide a viable solution for the extraction and separation of metal ions at the industrial scale. A similar study was carried out by Gursel et al. and employed CFI to develop a continuous metal extraction process and efficiently recovered 99% Cu [122].
Application of CFI in different fields (Reproduced with permission from [114])
Schematic diagrams for CFI designs: (a) standard CFI, (b) symmetrical compact CFI, and (c) asymmetrical compact CFI (Reproduced with permission from [114])
(a) Schematic diagram for the setup of micro-flow extraction and separation system, (b) T-type joint, and (c) CFI structure (Reproduced with permission from [121])
The use of CFI for metal extraction offers a significant advantage for the development of a continuous process. However, the scale-up and industrial application of this process are still a concern, and there is a need to explore this technique in more detail for application in the field of metal extraction.
4.2 Synthesis of the Catalyst Using Recovered Metals from E-Waste
The real bottom profitability of any process depends on its ability to ensure the usability of the products obtained from it. In this regard, the treatment of e-waste for obtaining precious metals offers several advantages in terms of reusability of the metals for useful purposes, more specifically, the use of extracted metals such as Cu, Ag, Au, Pt, Zn, Ni, etc. in both homo- and heterogeneous catalysis. This can be easily defined in terms of the gap in meeting the demand for the extensive use of such metals in preparing efficient catalysts for a range of commercially relevant processes such as biomass valorization, CO2 conversion, and water splitting for H2 generation, among many others. Though the precious metals as mentioned above are used prevalently in preparing catalysts, still, there are several issues like low natural abundance, difficulty in availability, and high cost which are responsible for fluctuations in their continuous supply, hence raising concerns over the use of such strategically important metals in the preparation of valuable catalysts. Moreover, the incessant demand for precious metals creates economic concerns, and it also has substantial repercussions for the environment.
The underlying principles of “sustainable development” which stresses on meeting the present demands without compromising future needs can be truly replicated by the use of metals extracted from electronic waste. The sustainable use of extracted metals from e-waste can play a major role in subsidizing the heavy demand and scarcity of metals important for catalysis. As such, the overdependence of current industrial processes over precious metals can also be minimized through the sustainability of the overall electronic waste treatment process. Ultimately, such electronic waste derived from homo−/heterogeneous catalysts will go a long way in improving the energy efficiency of many current and neoteric chemical practices.
5 Summary
The global e-waste generation is increasing year by year, and it will reach to 74.7 Mt by 2030. E-waste is a rich source of metals and, therefore, can play an important role in mitigating the scarcity of metals. However, unregulated accumulation and improper recycling techniques lead to the loss of critical metals and also pose a threat to the environment and human health. Therefore, it is important to find a sustainable solution for the sound management of the e-waste and recovery of metals. Biohydrometallurgy is a promising option for the recovery of metals from e-waste in an environmentally friendly way. Biotechnology has been employed for the recovery of metals from ores, but e-waste is a different challenge due to the large number of metals and its complex structure. Biohydrometallurgy mainly uses autotrophic bacteria bioleaching, heterotrophic bacteria bioleaching, and heterotrophic fungi bioleaching. Out of these, autotrophic bacteria bioleaching is a conventional bioleaching process that uses sulfur-oxidizing bacteria, iron- and sulfur-oxidizing bacteria, and iron-oxidizing bacteria. These organisms use carbon dioxide from the atmosphere as a carbon source and ferrous ion (Fe2+), elemental sulfur (So), and/or reduced sulfur compounds as an energy source. However, e-waste includes metals in their metallic form, and therefore, there is a need to supply the microorganism with an additional energy source. Heterotrophic bioleaching uses microorganisms such as bacteria (e.g., Pseudomonas aeruginosa, Pseudomonas fluorescens, and Pseudomonas putida) and fungi (e.g., Aspergillus niger and Penicillium simplicissimum). Heterotrophic bioleaching is microbial leaching where organisms get energy from organic carbon sources for growth during the leaching process. The metabolic by-products of organic carbon such as acetic acid, citric acid, oxalic acid, and gluconic acid are responsible for the leaching of metals. Biosorption and bioelectrochemical processes are bio-based technologies used for the recovery of metals from aqueous solutions. Also, there is a need to explore technique to develop continuous process for metal recovery, and CFI can provide a viable solution for this. However, there is a need to extensively explore the application of CFI for metal recovery.
There is a need to carry out further investigation in the biohydrometallurgical approach for metal recovery from e-waste as some of the main leaching mechanisms are not clear. In addition, it is also important to carry out the pilot-scale studies to find out the feasibility of the biohydrometallurgical process for full-scale industrial applications.
References
Forti V, Blade CP, Kuehr R, Bel G (2020) The global e-waste monitor 2020: quantities, flows and the circular economy potential, no. July. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR) – Co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Geneva
Jadhao PR, Ahmad E, Pant KK, Nigam KDP (2020) Environmentally friendly approach for the recovery of metallic fraction from waste printed circuit boards using pyrolysis and ultrasonication. Waste Manag 118:150–160
Kiddee P, Naidu R, Wong MH (2013) Electronic waste management approaches : an overview. Waste Manag 33:1237–1250
Babu BR, Parande AK, Basha CA (2007) Electrical and electronic waste : a global environmental problem. Waste Manag Res 25:307–318
Ongondo FO, Williams ID, Cherrett TJ (2011) How are WEEE doing ? A global review of the management of electrical and electronic wastes. Waste Manag 31:714–730
Widmer R, Krapf H, Khetriwal D, Schnellmann M, Boni H (2005) Global perspectives on e-waste. Environ Impact Assess Rev 25:436–458
Das A, Vidyadhar A, Mehrotra SP (2009) A novel flowsheet for the recovery of metal values from waste printed circuit boards. Resour Conserv Recycl 53:464–469
Buekens A, Yang J (2014) Recycling of WEEE plastics: a review. J Mater Cycles Waste Manag 16:415–434
Tuncuk A, Stazi V, Akcil A, Yazici EY, Deveci H (2012) Aqueous metal recovery techniques from e-scrap : hydrometallurgy in recycling. Miner Eng 25:28–37
Kusch S, Hills CD (2017) The link between E-waste and GDP – new insights from data from the Pan-European region. Resources 6:1–10
Hsu E, Barmak K, West AC, Park A-H (2019) Advancements in the treatment and processing of electronic waste with sustainability : a review of metal extraction and recovery technologies. Green Chem 21:919–936
Khaliq A, Rhamdhani M, Brooks G, Masood S (2014) Metal extraction processes for electronic waste and existing industrial routes: a review and australian perspective. Resources 3:152–179
Hagelüken C (2007) Metals recovery from e-scrap in a global environment. In: 6th Session of OEWG Basel convention
Jadhao P, Chauhan G, Pant KK, Nigam KDP (2016) Greener approach for the extraction of copper metal from electronic waste. Waste Manag 57:102–112
Panda R, Jadhao PR, Pant KK, Naik SN, Bhaskar T (2020) Eco-friendly recovery of metals from waste mobile printed circuit boards using low temperature roasting. J Hazard Mater 395:122642
Chauhan G, Jadhao PR, Pant KK, Nigam KDP (2018) Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment : challenges & opportunities – a review. J Environ Chem Eng 6:1288–1304
Baniasadi M, Vakilchap F, Bahaloo-horeh N, Mousavi SM, Farnaud S (2019) Advances in bioleaching as a sustainable method for metal recovery from e-waste : a review. J Ind Eng Chem 76:75–90
Kumar A, Li J (2017) An overview of the potential of eco-friendly hybrid strategy for metal recycling from WEEE. Resour Conserv Recycl 126:228–239
Habibi A, Kourdestani SS, Hadadi M (2020) Biohydrometallurgy as an environmentally friendly approach in metals recovery from electrical waste : a review. Waste Manag Res 38:232–244
Cui J, Zhang L (2008) Metallurgical recovery of metals from electronic waste: a review. J Hazard Mater 158:228–256
Shuey SA, Taylor P (2005) Review of pyrometallurgical treatment of electronic scrap. In: SME annual meeting, pp 1–4
Zhang Y, Liu S, Xie H, Zeng X, Li J (2012) Current status on leaching precious metals from waste printed circuit boards. Proc Environ Sci 16:560–568
Akcil A, Erust C, Gahan CS, Ozgun M, Sahin M, Tuncuk A (2015) Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants – a review. Waste Manag 45:258–271
Morin D et al (2006) BioMinE - integrated project for the development of biotechnology for metal-bearing materials in Europe. Hydrometallurgy 83:69–76
Jain R et al (2016) Preferential adsorption of Cu in a multi-metal mixture onto biogenic elemental selenium nanoparticles. Chem Eng J 284:917–925
Muñoz AJ, Espínola F, Ruiz E (2017) Biosorption of Ag(I) from aqueous solutions by Klebsiella sp. 3S1. J Hazard Mater 329:166–177
Ilyas S, Lee J (2014) Biometallurgical recovery of metals from waste electrical and electronic equipment: a review. ChemBioEng Rev 1:148–169
Ilyas S, Anwar MA, Niazi SB, Afzal Ghauri M (2007) Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy 88:180–188
Beolchini F, Fonti V, Dell’Anno A, Rocchetti L, Vegliò F (2012) Assessment of biotechnological strategies for the valorization of metal bearing wastes. Waste Manag 32:949–956
Orell A, Navarro CA, Arancibia R, Mobarec JC, Jerez CA (2010) Life in blue: copper resistance mechanisms of bacteria and archaea used in industrial biomining of minerals. Biotechnol Adv 28:839–848
Işıldar A et al (2019) Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE) – a review. J Hazard Mater 362:467–481
Bas AD, Deveci H, Yazici EY (2013) Bioleaching of copper from low grade scrap TV circuit boards using mesophilic bacteria. Hydrometallurgy 138:65–70
Hong Y, Valix M (2014) Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J Clean Prod 65:465–472
Chen S, Yang Y, Liu C, Dong F, Liu B (2015) Chemosphere column bioleaching copper and its kinetics of waste printed circuit boards ( WPCBs ) by acidithiobacillus ferrooxidans. Chemosphere 141:162–168
Isildar A, Van De Vossenberg J, Rene ER, Van Hullebusch ED, Lens PNL (2016) Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag 57:149–157
Arshadi M, Mousavi SM (2015) Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep Purif Technol 147:210–219
Arshadi M, Mousavi SM (2014) Simultaneous recovery of Ni and Cu from computer-printed circuit boards using bioleaching: statistical evaluation and optimization. Bioresour Technol 174:233–242
Arshadi M, Mousavi SM (2015) Statistical evaluation of bioleaching of mobile phone and computer waste PCBs: a comparative study. Adv Mater Res 1104:87–92
Liang G, Li P, Liu W, Wang B (2016) Enhanced bioleaching efficiency of copper from waste printed circuit boards (PCBs) by dissolved oxygen-shifted strategy in Acidithiobacillus ferrooxidans. J Mater Cycles Waste Manag 18:742–751
Bajestani MI, Mousavi SM, Shojaosadati SA (2014) Bioleaching of heavy metals from spent household batteries using Acidithiobacillus ferrooxidans: statistical evaluation and optimization. Sep Purif Technol 132:309–316
Yang T, Xu Z, Wen J, Yang L (2009) Factors influencing bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 97:29–32
Muravyov MI, Bulaev AG, Melamud VS, Kondrat’eva TF (2015) Leaching of rare earth elements from coal ashes using acidophilic chemolithotrophic microbial communities. Microbiology 84:194–201
Machado MD, Soares EV, Soares HMVM (2010) Removal of heavy metals using a Brewer’s yeast strain of Saccharomyces cerevisiae : chemical speciation as a tool in the prediction and improving of treatment efficiency of real electroplating effluents. J Hazard Mater 180:347–353
Das N, Das D (2013) Recovery of rare earth metals through biosorption : an overview. J Rare Earths 31:933–943
Burgstaller W, Schinner F (1993) Leaching of metals with fungi. J Biotechnol 27:91–116
Shabani MA, Irannajad M, Azadmehr AR, Meshkini M (2013) Bioleaching of copper oxide ore by Pseudomonas Aeruginosa. Int J Miner Metall Mater 20:1130–1133
Rezza I, Salinas E, Sanz de Tosetti M, Donati E (2001) Mechanisms involved in bioleaching of an aluminosilicate by heterotrophic microorganisms. Process Biochem 36:495–500
Jujun R, Xingjiong Z, Yiming Q, Jian H (2014) A new strain for recovering precious metals from waste printed circuit boards. Waste Manag 34:901–907
Chi TD, Lee JC, Pandey BD, Yoo K, Jeong J (2011) Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner Eng 24:1219–1222
Marsden JO, House CI (2006) The chemistry of gold extraction, second. Society of Mining, Metallurgy, and Exploration, Inc., Englewood, CO
Rees KL, Van Deventer JSJ (1999) The role of metal-cyanide species in leaching gold from a copper concentrate. Miner Eng 12:877–892
Kita Y, Nishikawa H, Ike M, Takemoto T (2009) Enhancement of Au dissolution by microorganisms using an accelerating cathode reaction. Metall Mater Trans B Process Metall Mater Process Sci 40B:39–44
Marra A, Cesaro A, Rene ER, Belgiorno V, Lens PNL (2018) Bioleaching of metals from WEEE shredding dust. J Environ Manag 210:180–190
Natarajan G, Tay SB, Yew WS, Ting YP (2015) Engineered strains enhance gold biorecovery from electronic scrap. Miner Eng 75:32–37
Natarajan G, Ting YP (2014) Pretreatment of e-waste and mutation of alkali-tolerant cyanogenic bacteria promote gold biorecovery. Bioresour Technol 152:80–85
Li J, Liang C, Ma C (2015) Bioleaching of gold from waste printed circuit boards by Chromobacterium violaceum. J Mater Cycles Waste Manag 17:529–539
Pradhan JK, Kumar S (2012) Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads sp. Waste Manag Res 30:1151–1159
Faramarzi MA, Brandl H (2006) Formation of water-soluble metal cyanide complexes from solid minerals by Pseudomonas plecoglossicida. FEMS Microbiol Lett 259:47–52
Lee J, Pandey BD (2012) Bio-processing of solid wastes and secondary resources for metal extraction – a review. Waste Manag 32:3–18
Brandl H, Bosshard R, Wegmann M (2001) Computer-munching microbes : metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 59:319–326
Brombacher C, Bachofen R, Brandl H (1998) Development of a laboratory-scale leaching plant for metal extraction from fly ash by Thiobacillus strains. Appl Environ Microbiol 64:1237–1241
Bosshar PP, Bachofen R, Brandl H (1996) Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environ Sci Technol 30:3066–3070
Desouky OA, El-Mougith AA, Hassanien WA, Awadalla GS, Hussien SS (2016) Extraction of some strategic elements from thorium – uranium concentrate using bioproducts of Aspergillus ficuum and Pseudomonas Aeruginosa. Arab J Chem 9:S795–S805
Hassanien WAG, Desouky OAN, Hussien SSE (2014) Bioleaching of some rare earth elements from Egyptian Monazite using Aspergillus ficuum and Pseudomonas Aeruginosa. Walailak J Sci Tech 11:809–823
Lu N, Hu T, Zhai Y, Qin H, Aliyeva J, Zhang H (2020) Fungal cell with artificial metal container for heavy metals biosorption: equilibrium, kinetics study and mechanisms analysis. Environ Res 182:109061
Arshadi M, Mousavi SM, Rasoulnia P (2016) Enhancement of simultaneous gold and copper recovery from discarded mobile phone PCBs using Bacillus megaterium: RSM based optimization of effective factors and evaluation of their interactions. Waste Manag 57:158–167
Huang H et al (2016) A novel Pseudomonas Gessardii strain LZ-E simultaneously degrades naphthalene and reduces hexavalent chromium. Bioresour Technol 207:370–378
Mahmoud A, Cezac P, Hoadley AFA, Contamine F, D’Hugues P (2017) A review of sulfide minerals microbially assisted leaching in stirred tank reactors. Int Biodeterior Biodegradation 119:118–146
Xia M et al (2018) Bioleaching of low-grade waste printed circuit boards by mixed fungal culture and its community structure analysis. Resour Conserv Recycl 136:267–275
Veglio F, Beolchini F (1997) Removal of metals by biosorption : a review. Hydrometallurgy 44:301–316
Ahmad A, Bhat AH, Buang A (2018) Biosorption of transition metals by freely suspended and Ca-alginate immobilised with chlorella vulgaris : kinetic and equilibrium modeling. J Clean Prod 171:1361–1375
Ilyas S, Lee J, Chi R (2013) Bioleaching of metals from electronic scrap and its potential for commercial exploitation. Hydrometallurgy 131–132:138–143
Salvadori MR, Ando RA, Nascimento CAO, Correa B (2017) Dead biomass of Amazon yeast : a new insight into bioremediation and recovery of silver by intracellular synthesis of nanoparticles. J Environ Sci Heal A 52:1–9
Paknikar KM, Pethkar AV, Puranik PR (2003) Bioremediation of metalliferous wastes and products using inactivated microbial biomass. Indian J Biotechnol 2:426–443
Baran MF (2019) Biosorption of Pb 2 + from aqueous solutions by Bacillus licheniformis Isolated from Tigris River with a comparative study. Int J Latest Eng Manag Res 4:108–121
Joo JH, Hassan SHA, Oh SE (2010) Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int Biodeterior Biodegrad 64:734–741
Sahmoune MN (2018) Performance of Streptomyces Rimosus biomass in biosorption of heavy metals from aqueous solutions. Microchem J 141:87–95
Selatnia A, Bakhti MZ, Madani A, Kertous L, Mansouri Y (2004) Biosorption of Cd2+ from aqueous solution by a NaOH-treated bacterial dead Streptomyces rimosus biomass. Hydrometallurgy 75:11–24
Ahmady-Asbchin S, Safari M, Tabaraki R (2015) Biosorption of Zn (II) by Pseudomonas aeruginosa isolated from a site contaminated with petroleum. Desalin Water Treat 54:3372–3379
Mulligan CN, Yong RN, Gibbs BF (2001) An evaluation of technologies for the heavy metal remediation of dredged sediments. J Hazard Mater 85:145–163
Park D, Yun YS, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15:86–102
Dursun AY, Uslu G, Tepe O, Cuci Y, Ekiz HI (2003) A comparative investigation on the bioaccumulation of heavy metal ions by growing Rhizopus arrhizus and Aspergillus niger. Biochem Eng J 15:87–92
Say R, Yilmaz N, Denizli A (2003) Removal of heavy metal ions using the fungus Penicillium canescens. Adsorpt Sci Technol 21:643–650
Leitão AL (2009) Potential of Penicillium species in the bioremediation field. Int J Environ Res Public Health 6:1393–1417
Ponce de León CA, Bayón MM, Paquin C, Caruso JA (2002) Selenium incorporation into Saccharomyces cerevisiae cells: a study of different incorporation methods. J Appl Microbiol 92:602–610
Munoz AJ, Espínola F, Ruiz E (2017) Biosorption of Ag (I) from aqueous solutions by Klebsiella sp . 3S1. J Hazard Mater 329:166–177
Chatterjee A, Das R, Abraham J (2020) Bioleaching of heavy metals from spent batteries using Aspergillus nomius JAMK1. Int J Environ Sci Technol 17:49–66
Abdallah MAM, Mahmoud ME, Osman MM, Ahmed SB (2017) New Biosorbent in removing some metals from industrial wastewater in El Mex Bay, Egypt. Appl Water Sci 7:1931–1942
Escudero LB, Quintas PY, Wuilloud RG, Dotto GL (2019) Recent advances on elemental biosorption. Environ Chem Lett 17:409–427
Vieira RHSF, Volesky B (2000) Biosorption: a solution to pollution? Int Microbiol 3:17–24
Kalak T, Dudczak-Halabuda J, Tachibana Y, Cierpiszewski R (2020) Effective use of elderberry ( Sambucus nigra ) pomace in biosorption processes of Fe (III) Ions. Chemosphere 246:125744
Vendruscolo F, Ferreira GLR, Filho NRA (2017) Biosorption of hexavalent chromium by microorganisms. Int Biodeterior Biodegradation 119:87–95
Li L, Hu Q, Zeng J, Qi H, Zhuang G (2011) Resistance and biosorption mechanism of silver ions by Bacillus cereus biomass. J Environ Sci 23:108–111
Nicomel NR et al (2020) Microalgae : a sustainable adsorbent with high potential for upconcentration of indium (III) from liquid process and waste streams. Green Chem 22:1985–1995
Saranya K, Sundaramanickam A, Shekhar S, Meena M, Sathishkumar RS, Balasubramanian T (2018) Biosorption of multi-heavy metals by coral associated phosphate solubilising Bacteria Cronobacter Muytjensii KSCAS2. J Environ Manag 222:396–401
De Freitas F, Battirola LD, Arruda R, de Andrade RT (2019) Assessment of the Cu ( II ) and Pb ( II ) removal efficiency of aqueous solutions by the dry biomass Aguapé : kinetics of adsorption. Env Monit Assess 191:751
Cid H, Ortiz C, Pizarro J, Moreno-piraján JC (2020) Effect of copper (ii) biosorption over light metal cation desorption in the surface of Macrocystis Pyrifera Biomass. J Environ Chem Eng 8:103729
Moghaddam SAE, Harun R, Mokhtar MN, Zakaria R (2020) Kinetic and equilibrium modeling for the biosorption of metal ion by zeolite 13X-algal-alginate beads (ZABs). J Water Process Eng 33:101057
Sheel A, Pant D (2018) Recovery of Gold from Electronic Waste using Chemical Assisted Microbial Biosorption ( hybrid ) Technique. Bioresour Technol 247:1189–1192
Ai C et al (2020) Recovery of metals from acid mine drainage by bioelectrochemical system Inoculated with a Novel Exoelectrogen, Pseudomonas sp. E8. Microorganisms 8:1–16
Chaturvedi V, Verma P (2016) Microbial fuel cell: a green approach for the utilization of waste for the generation of bioelectricity. Bioresour Bioprocess 3:1–14
Tugtas AE, Calli B (2018) Removal and recovery of metals by using bio-electrochemical system. In: Das D (ed) Microbial fuel cell. New Delhi, Capital Publishing Company, pp 307–333
Huang T, Liu L, Zhang S (2019) Microbial fuel cells coupled with the bioleaching technique that enhances the recovery of cu from the secondary mine tailings in the bio-electrochemical system. Environ Prog Sustain Energy 38:1–9
Velvizhi G, Goud RK, Mohan SV (2014) Anoxic bio-electrochemical system for treatment of complex chemical wastewater with simultaneous bioelectricity generation. Bioresour Technol 151:214–220
Nancharaiah YV, Mohan SV, Lens PNL (2015) Metals removal and recovery in bioelectrochemical systems: a review. Bioresour Technol 195:102–114
Huang L, Yao B, Wu D, Quan X (2014) Complete cobalt recovery from lithium cobalt oxide in self-driven microbial fuel cell - microbial electrolysis cell systems. J Power Sources 259:54–64
Ter Heijne A, Liu F, Van Der Weijden R, Weijma J, Buisman CJN, Hamelers HVM (2010) Copper recovery combined with electricity production in a microbial fuel cell. Environ Sci Technol 44:4376–4381
Hu N, Cui Y, Choi C (2019) Recovery of platinum-group metals using a microbial fuel cell. Trends Diabetes Metab 2:1–9
Vural Gürsel I, Kockmann N, Hessel V (2017) Fluidic separation in microstructured devices – concepts and their integration into process flow networks. Chem Eng Sci 169:3–17
Kurt SK, Vural Gürsel I, Hessel V, Nigam KDP, Kockmann N (2016) Liquid-liquid extraction system with microstructured coiled flow inverter and other capillary setups for single-stage extraction applications. Chem Eng J 284:764–777
Kurt SK, Akhtar M, Nigam KDP, Kockmann N (2016) Modular concept of a smart scale helically coiled tubular reactor for continuous operation of multiphase reaction systems. In: Proceedings of the ASME 2016 14th international conference on nanochannels, microchannels, and minichannels, pp 1–12
Vural Gürsel I et al (2016) Utilization of milli-scale coiled flow inverter in combination with phase separator for continuous flow liquid-liquid extraction processes. Chem Eng J 283:855–868
Chauhan G, Kaur P, Pant KK, Nigam KDP (2020) Sustainable metal extraction from waste streams. WILEY-VCH Verlag GmbH, Berlin
Soni S, Sharma L, Meena P, Roy S, Nigam KDP (2019) Compact coiled flow inverter for process intensification. Chem Eng Sci 193:312–324
Mandal MM, Aggarwal P, Nigam KDP (2011) Liquid-liquid mixing in coiled flow inverter. Ind Eng Chem Res 50:13230–13235
Singh J, Nigam KDP (2016) Pilot plant study for effective heat transfer area of coiled flow inverter. Chem Eng Process Process Intensif 102:219–228
Singh J, Choudhary N, Nigam KDP (2014) The thermal and transport characteristics of nanofluids in a novel three-dimensional device. Can J Chem Eng 92:2185–2201
Kateja N, Agarwal H, Saraswat A, Bhat M, Rathore AS (2016) Continuous precipitation of process related impurities from clarified cell culture supernatant using a novel coiled flow inversion reactor (CFIR). Biotechnol J 11:1320–1331
Parida D et al (2014) Coil flow inversion as a route to control polymerization in microreactors. Macromolecules 47:3282–3287
Vashisth S, Nigam KDP (2008) Experimental investigation of void fraction and flow patterns in coiled flow inverter. Chem Eng Process Process Intensif 47:1281–1291
Zhang L, Hessel V, Peng J, Wang Q, Zhang L (2017) Co and Ni extraction and separation in segmented micro-flow using a coiled flow inverter. Chem Eng J 307:1–8
Gürsel IV, Aldiansyah F, Wang Q, Noël T, Hessel V (2015) Continuous metal scavenging and coupling to one-pot copper-catalyzed azide-alkyne cycloaddition click reaction in flow. Chem Eng J 270:468–475
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jadhao, P.R., Mishra, S., Pandey, A., Pant, K.K., Nigam, K.D.P. (2021). Biohydrometallurgy: A Sustainable Approach for Urban Mining of Metals and Metal Refining. In: Pant, K.K., Gupta, S.K., Ahmad, E. (eds) Catalysis for Clean Energy and Environmental Sustainability. Springer, Cham. https://doi.org/10.1007/978-3-030-65017-9_27
Download citation
DOI: https://doi.org/10.1007/978-3-030-65017-9_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65016-2
Online ISBN: 978-3-030-65017-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)