Abstract
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) of the immune system. They capture foreign antigens and can present them to lymphocytes, that is, T cells and B cells, to activate them. DCs are the most potent of all immune cells at inducing the adaptive immune system. Thus, the presence of DCs at the anatomical site of the immune challenge is imperative for the immune system to mount an effective immune response. From the anatomical site of the immune challenge, DCs cargo antigens to the draining lymph nodes, specialized immune organs where adaptive immunity is generated. DCs are heterogeneous as a type of immune cell, and various subsets of DCs have been reported and their functions described. In this chapter, we discuss various aspects of DC development and function. We further discuss how various tumor microenvironments can affect DC development, function, and migration, thus evading a strong adaptive immune response.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dendritic cells
- Antigen-presenting cells
- Lymph nodes
- Tumor microenvironment
- Anti-tumor immunity
- Danger signal
- Toll-like receptors
- Cytokines
- Migration
- Chemotaxis
- Development
- Longevity
- Subsets
- Immunosuppression
- Tertiary lymphoid organs
2.1 Introduction
Dendritic cells (DCs) play a crucial role in initiating and modulating adaptive immune responses during infections, allergies, autoimmune disorders as well as maintain T-cell homeostasis in steady-state conditions. Depending on the immune challenge, they can initiate or enhance an immune response. Moreover, they can tolerize or suppress the immune system toward innocuous antigens. DCs can infiltrate solid tumors, capture, and process tumor antigens and transport them to the draining lymph node (LN) to initiate an effective adaptive immune response against the tumor, by priming and expanding naïve T cells to become anti-tumor effector T cells. Although many other antigen-presenting cells (APCs) such as macrophages also contribute to this process, DCs are considered the most proficient of all APC types. Additionally, DCs can modulate the tumor microenvironment to influence the recruitment of other immune cell populations into the tumor. DCs are very heterogeneous and can be classified into many subsets depending on factors including their phenotype, specialized function, localization in peripheral tissue, lineage, etc. Even though the exact role of every DC subset in generating anti-tumor immunity has not yet been fully deciphered, DCs, in general, are an important arm in adaptive immune responses against tumors. However, a tumor environment can present many hurdles in the scheme of DC-mediated anti-tumor immunity. Here, we review different aspects of DC biology and how DC function can be influenced by tumors.
2.2 Tumor-Infiltrating Dendritic Cells Mediate Anti-tumor Immunity
For the generation of efficient anti-tumor immunity professional APCs need to capture tumor-derived antigens, process them to form a complex with major histocompatibility complex (MHC) molecules, migrate to the draining LN, and finally present them to cognate CD4 or CD8 T cells [1]. Presentation of antigen to T cells can lead to two possible outcomes. Either the T cells can be tolerized, that is, they become quiescent and/or get converted into an immune regulatory T cell, or they can be activated to mount a response against the immune threat [2]. The latter outcome is desired for an effective anti-tumor immune response and for that APCs, either alone or in co-operation with other APC types, need to provide three signals to the cognate T cells [3].
-
Signal one is the peptide:MHC complex. APCs capture exogenous antigens such as tumor-derived antigens and process them efficiently to eventually present them as a complex with MHCI or MHCII molecules to present them to CD8 T cells or CD4 T cells, respectively. The process of internalization of exogenous antigens to be processed and presented as a complex with MHCI complex to CD8 T cells is called “cross-presentation” and this process is important to generate effective anti-tumor immunity. Among all APC types, DCs are considered most proficient at antigen presentation, especially cross-presentation.
-
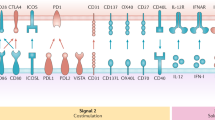
Signal two are co-stimulatory signals. APCs can present p:MHC complexes to T cells, but, in the absence of any inflammatory signal, it might not necessarily lead to T-cell activation. However, during an infection or other inflammatory conditions, APCs can sense the danger, as they express specific receptors which can bind to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and up-regulate the expression of co-stimulatory molecules such as CD40 and CD86. The ligands for these receptors are expressed on T cells eternally. An APC when presents p:MHC complexes in conjunction with co-stimulatory molecules leads to efficient activation of T cells. DCs express an array of receptors to detect PAMPs and DAMPs including toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectins, etc., which enable them to detect danger signals and over-express co-stimulatory molecules, making them capable of effectively activating T cells toward anti-tumor response [4].
-
Signal three is activating cytokines. Along with receiving signal one and two con-currently from a single APC type, T cells need inflammatory cytokines such as IL-12 and IL-15 which boost and sustain their effector status and enable them to keep expanding [5]. Certain subsets of APCs, depending on the environment, can provide these signals along with signal 1 and signal 2. However, a new concept of APC co-operation is emerging which suggests that, while a single APC can provide signals 1 and 2 to the cognate T cells, another myeloid cell can serve as a source of signal 3 during an inflammatory challenge [6, 7]. Whether this co-operation between myeloid cells exists in the tumor microenvironment is a challenging research question.
DCs are considered as the most proficient APCs that provide signals 1, 2, and 3 to T cells and thus initiate adaptive immune responses against tumor antigens. However, to do so, differentiated DCs must be able to infiltrate the tumor microenvironment from neighboring tissues. Precursors of DCs which originate in the bone marrow (BM) can also enter the tumor parenchyma and differentiate in situ. While it is still unclear which path DCs take to infiltrate tumors, their presence in the tumor is beneficial toward anti-tumor immunity. In clinical samples, the presence of DCs in the primary tumor site has been correlated with increased survival in many tumor typesincluding ovarian carcinoma, head and neck tumors, pancreatic adenocarcinoma, lung, and breast cancer [8,9,10,11].
2.3 Migration of DCs to and from Tumor Parenchyma
Chemotaxis is a major mechanism utilized by immune cells for directional migration. DCs exhibit classic directional migration engaging their chemokine receptors to move toward gradients of the corresponding chemokine ligands. DCs are pliable in their expression of chemokine receptors and, depending on the subset, anatomical location and pathophysiological condition, they can express varying levels of multiple chemokine receptors [12]. DCs do not develop fully at their site of origin, that is, BM, and complete their differentiation program in peripheral tissues [13]. For instance, skin DCs develop from DC precursors traveling from BM into the skin via blood. In the tissue DCs capture antigens, follow the chemokine gradients to reach terminal lymphatics, and through the lymphatic vessels enter the nearest LN. Unlike other immune cell populations, DCs, except pDCs, migrate dominantly through the lymphatic system [12]. This scheme of DC development and migration raises an important question regarding the migration of DCs into solid tumors, that is, do terminally differentiated DCs migrate from surrounding tissue into the tumor? or if DC precursors migrate into the tumors and differentiate in situ. Assuming either possibility, another key aspect that needs investigation is what chemokine gradients do DC or DC precursors follow to reach into the tumor parenchyma. DC migration toward the LN has been well characterized. C–C Motif Chemokine Receptor (CCR)7 on DCs is the dominant chemokine receptor which guides DCs toward gradients of C–C Motif Chemokine Ligand (CCL)19/21 on the way to LN and also from the boundaries of the LN into the deep T-cell zone of the LN. However, steps involved in the migration of DCs/DC precursors toward non-lymphoid tissues have only been partly characterized [14]. Adoptive transfer experiments revealed that DCs can adhere to skin venules by attaching to certain selectins expressed on endothelial cells; however, attachment to selectins is only the start of a multistep adhesion cascade and subsequent intravasation [15]. All the steps entailed in DC migration from blood to tissue are not fully characterized for solid tumors. This is a challenge, as tumors are diverse and there might not be a common adhesion and chemotactic axes that guide DCs into solid tumors. Nonetheless, certain chemokine ligands that have been seen in certain tumors can attract DCs. These chemokines can be secreted either by the tumor themselves such as CCL4 or immune cells within the tumor [16]. For example, CD8 T cells or NK cells can secrete CCL5 and CXCL1 which can attract DCs expressing the corresponding chemokine receptor [17]. In various settings, it has been demonstrated that immune cells reaching a site can secrete chemokines to attract other types of immune cells as a part of the ongoing immune response. Thus, it seems likely that certain immune cells in tumors have the potential to attract other immune cells, and thus the role of other immune cells in attracting DCs into the tumor environment must be further investigated.
2.4 Longevity of DCs in the Tumor Microenvironment
A study, investigating the life of major immune cell populations revealed that while B cells and T cells can live up to 20 days after they leave their site of origin, DCs have a relatively short life of around 3 days in lymphoid and non-lymphoid tissues at steady state [18]. However, the ratio of DCs to other immune cells remains constant, which suggests that DCs have a high turnover. However, in many tumors, the decreased presence of DCs can be attributed to factors released in the tumor microenvironment, which can affect the lifespan of DCs in the tumor. These factors include mucins, gangliosides neuropeptides, and nitric oxide. A study showed that gangliosides including GM3 and GD3 induce apoptosis of DCs in vitro [19]. These gangliosides are found at high concentrations in melanoma patients. The presence of these gangliosides in melanoma explains the lower number of DCs in malignant melanoma as compared to that in benign skin lesions. MUC2 mucins derived from conditioned medium of LS180 cells, a colorectal cancer cell line, can cause apoptosis of mature DCs [20]. Overexpression of mucins, large extracellular proteins that are heavily glycosylated with complex oligosaccharides , is associated with many epithelial cancers. Thus, considering these unfavorable conditions, the life of DCs could be much different compared to healthy tissue.
2.5 Immunosuppression of DCs in Solid Tumors
Some tumors, such as Merkel Cell Carcinoma (MCC), are initiated by oncoviruses, and principally these viruses can provide danger signals to DCs which could trigger them to their path toward initiating anti-tumor immunity. However, most solid tumors do not provide any inflammatory or danger signals to infiltrating DCs, unless somehow the tumor microenvironment can derive inflammatory signals. Besides a lack of adequate inflammatory signals, a solid tumor environment can actively suppress DC-mediated anti-tumor immunity. Across many studies, various mechanisms have been postulated by which tumor or tumor components can suppress DC activity. For instance, in a mouse model of ovarian tumor, tumor cells induced the activation of transcription factor Xbox binding protein (XBP) which caused endoplasmic reticulum stress in DCs which impeded their ability to prime T cells [21]. Cancer cells by initiating the B-catenin signaling pathway can also limit DC recruitment into tumors [22]. Interleukin (IL)-10 is a widely known immunosuppressive cytokine, and tumor cells, as well as other components of the tumor environment such as tumor-associated macrophages (TAMs) , can secrete IL-10 [23]. CD103+ tumor-infiltrating DCs have a high expression of IL-10 receptor and, in response to sensing IL-10, they can downregulate the production of IL-12, a cytokine known to enhance CD8+ T-cell proliferation and effector function [24]. IL-10 can also skew the differentiation of monocytes toward immunosuppressive macrophages instead of monocyte-derived DCs [25]. The role of IL-10 in the suppression of anti-tumor immunity is a broad area of investigation and the studies describing its mechanisms have been covered in this review [26].
Tumor cells can also secrete thymic stromal lymphoprotein (TSLP) which induces OX40 OX40L expression in DCs [27]. OX40L-expressing DCs induce a type 2 immune response which is not as potent an anti-tumor response as a type 1 response. Plasmacytoid dendritic cells (pDCs), which are known to secrete high amounts of type 1 interferon during viral infections, can also limit their production of type 1 interferon when immunoglobulin-like transcript 7 (ILT7) on pDCs engages with bone marrow stromal antigen 2 (BST2) on tumor cells [28]. Tumors secrete growth factors and produce chemokines that help sustain the tumor and even metastasize. A prominent growth factor that is over-produced in many tumors is Vascular Endothelial Growth Factor (VEGFA). Tumors expressing VEGFA can cause “angiogenic switch” which means that new vasculature can develop which will support tumor growth and metastasis [29]. Besides supporting tumor growth, VEGF is an immunosuppressant for DCs [30]. Treatment of DCs with VEGF results in inhibition of their maturation [31, 32]. Some tumor-associated chemokines that can also cause similar inhibition of DC maturation include CCL2, C-X-C Motif Chemokine Ligand (CXCL)1, and CXCL5 [33]. In a mouse model of Ovarian cancer, Programmed cell death-1 (PD-1) was expressed on tumor-infiltrating DCs [34]. PD-1 is generally expressed on T cells which are exhausted in solid tumors or during chronic viral infections [35]. The same study further showed that these PD-1+ DCs could block T-cell proliferation. Factors that induce PD-1 expression on DCs are largely unknown. It is tempting to assume that the same factors that induce PD-1 expression on T cells would do the same for DCs. Nonetheless, anti-PD-1 therapy which is the most commonly applied immunotherapy for solid tumors could potentially work by blocking the immunosuppressive activity of PD-1-expressing DCs. Another marker of exhaustion expressed generally on T cells, that is, T-cell Ig and mucin domain 3 (Tim-3), was also expressed on tumor-infiltrating DCs in mouse models for Lewis lung cancer tumors and colorectal adenocarcinoma. Nucleic acids (NA) from dying tumor cells can lead to NA-mediated anti-tumor immunity. Tim-3-expressing DCs were shown to suppress NA-mediated anti-tumor immunity [36]. Thus, much like T cells, DCs can also become dormant or immunosuppressive in solid tumor microenvironments and this can be reflected by the expression of certain markers. Besides inducing immunity, DCs also play a pivotal role in maintaining immune tolerance to innocuous antigens. In the steady state, some subsets of DCs such as migratory DCs and pDCs have been shown to specialize in inducing immune tolerance, by transporting peripheral harmless antigens to secondary lymphoid organs such as lymph nodes and priming T cells to an anergic, that is, non-responsive state, or even convert naïve CD4+ T cells into regulatory T cells [36]. Many solid tumors have a relatively high frequency of Tregs, which adds to the immunosuppressive environment. In mice and rats bearing melanoma, it was shown that a fraction of DCs accumulated in the draining LNs and these DCs were proficient in inducing expansion of Treg cells.
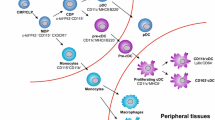
2.6 DC Subsets in Tumors
DCs are a heterogeneous group of cells and depending on the anatomical location and physiological condition many DC subsets can be identified [37]. However, recently a study that attempted to relate DCs between species and anatomical locations broadly grouped all DC subsets into five subsets, that is, cDC1s and cDC2s for conventional DCs, pDCs, Langerhans cells, and MoDCs [38]. These subsets express exclusive markers that can be used to identify them. For instance, in both mice and humans, cDC1s express C-type lectin endocytic receptor CLEC9A and chemokine receptor XCR1, whereas cDC2s express CD1c, a transmembrane glycoprotein [39]. Many studies have investigated the above subsets for specialized roles. While this remains a topic of intense investigation, some specialized roles have been specifically ascribed to individual subsets. For instance, in various settings including infection and tumor models, it has been shown that cDC1s are specialized at cross-presentation of exogenous antigens and crucial for inducing an effective CD8 T-cell response [40,41,42]. Since the cross-presentation of exogenous antigens in the context of MHC-I molecules to CD8 T cells is essential to generate effective anti-tumor immunity, the presence of cDC1s in tumors is believed to be beneficial to anti-tumor immunity [43]. Indeed, defect in cDC1s in the tumor microenvironment has been shown to negatively impact tumor immunosurveillance in many tumor models [44,45,46]. However, cDC1s are the minority DC type in tumors, as well as lymphoid and non-lymphoid tissues, making them a precious immune population. However, more studies across different cancer types looking at relative cDC1 frequencies in solid tumors and their impact on patient survival and tumor progression are warranted.
In mouse tumor models, specific depletion of cDC1s impairs the CD8 T cell-mediated anti-tumor response and the ability to reject transplanted tumors [47]. Across many studies, much evidence suggests that cDC1s in tumors are the most proficient of all DC subsets at cross-presentation and some other key unique features of cDC1s make them the most prominent DC type for generating anti-tumor immunity. For instance, cDC1s produce CXCL9/10, chemokine ligands that can recruit effector and memory T cells expressing the corresponding chemokine receptor CXCR3 [48]. cDC1s have been shown to efficiently capture, process, and transport tumor-derived antigens to the draining LN, and ex vivo experiments showed that they were most proficient at stimulating and activating T cells [49]. cDC1s also produce high amounts of IL-12, an inflammatory cytokine that has been shown to enhance the CD8 T cell- and NK cell-mediated cytotoxicity [50]. cDC1s are also most sensitive and responsive to type I interferons, as they enhance their cross-presentation capacity in response to stimulation by type I interferons [51]. Due to the above features, cDC1s are regarded as the DC type essential for mounting effective anti-tumor immunity. However, it is important to realize that the anti-tumor functions of cDC1s have been ascribed to them based on mouse tumor models. Besides the obvious caveat of species-specific discrepancies, there are other issues with mouse models that could complicate the interpretation of results. For instance, none of the models could exclusively deplete cDC1s. The most common model uses knocking out of Basic Leucine Zipper ATF-Like Transcription Factor 3 (BATF3) , which is crucial for cDC1 development. However, the same transcription factor is also crucial in the development of other DC types. Similarly, some of the genetic ablation models have not been able to exclusively deplete cDC1s and thus better models are warranted to support the above findings [52]. In addition to caveats of the mice models, discrepancies between the profile and function of cDC1s in mice and humans still leaves some doubt about the exclusive specialty of cDC1s in generating anti-tumor immunity. For instance, a few studies showed that other DC subsets, for example, the cDC2 subset, produced more IL-12 than cDC1s in response to certain adjuvants [53]. Also, while in mouse models, cDC1s were shown to efficiently capture and transport tumor-derived antigens, cDC1s in humans were shown not to efficiently capture antigens from the parenchyma of non-lymphoid tissues to the draining LNs [54]. The study also shows that cDC1s express a lower level of CCR7, a receptor pivotal for the migration of DC from peripheral tissues to LNs. Thus, in summary, while experimental models have highlighted the importance of cDC1s in anti-tumor immunity, more data regarding the alignment of the phenotype and function of DC subsets across species, especially in different cancer types, are warranted to endorse the notion that cDC1s are the specialized DC subset in cross-presentation and generating anti-tumor immunity.
cDC2s are the more abundant population in lymphoid and non-lymphoid tissues [54]. Unlike cDC1s, their role in cancer is much less established, perhaps because they are considered less efficient at cross-presentation than cDC1s. However, studies have shown that cDC2s are present in solid tumors and can migrate to draining LNs [55]. The current belief is that while cDC1s are more potent at cross-presentation and priming CD8 T cells, cDC2s are better activators of CD4 T cells [54]. Effective priming of CD4 T cells for an anti-tumor response is crucial as a large body of literature emphasizes the role of CD4 T cells in helping CD8 T cells to infiltrate and kill tumor cells [56]. Thus, cDC2s, although not considered specialized at cross-presentation, serves as a crucial arm in anti-tumor immune responses by effectively priming CD4 T cells. This idea is corroborated by studies that show that the presence of gene signature of cDC2s directly correlates with better survival for cancer patients [56, 57].
pDCs were initially discovered as cells specialized to produce type-1 interferon in response to viral ligands [58]. However, afterward, many reports postulated a role of pDCs in driving both central and peripheral tolerance [59,60,61,62,63]. Currently, the consensus is that pDCs can be tolerogenic or immunogenic depending on their environmental stimuli. While type 1 interferons have a clear role in anti-viral immunity, they can be both anti-tumor and pro-tumor [64]. pDCs can contribute to anti-tumor immunity by serving as APCs and via type 1 interferon production, but there have been many mechanisms described through which various solid tumors can induce the tolerogenic or regulatory function of pDCs to promote tumor growth. Demoulin, S et al. in their review describe mechanisms by which various tumors can induce tolerogenic function and inhibit immunogenic functions of pDCs [65].
2.7 DCs Help in Anti-tumor Immunity: Beyond T-Cell Priming
Besides their classic function of antigen presentation , DCs also play a key role in the recruitment of other immune cells. In a landmark study, it was shown that LNs of mice lacking DCs are much smaller than those of wild-type (WT) mice [66]. Mechanistic experiments in the same study further showed that DCs modulate the phenotype of specialized endothelial venules of the LN, called high endothelial venules (HEVs) which are portals through which other immune cells including T cells and B cells can enter into the LN from the bloodstream. DCs induced the expression of multiple adhesion molecules on endothelial cells to which immune cells can adhere as the first step in their intravasation into the LNs. A similar phenomenon is observed in ectopic lymphoid structures that form in certain pathophysiological conditions, such as persistent pathogenic infections, or autoimmune diseases, such as rheumatoid arthritis, to generate local immunity [67]. The neogenesis of tertiary lymphoid structures (TLS) has been described in many models and across many of these studies; DCs have been shown to play a pivotal role in aiding genesis of TLS [68]. Even in cancer patients, a positive correlation between the presence of DCs and the presence of TLSs has been demonstrated [8, 10, 61,62,63]. While the role of TLS in cancer immunity is still an area of investigation, the majority of the correlation studies show that the presence of TLS in the tumor is positively correlated with patient survival for several different cancer types. After their genesis, TLS can play several roles including serving as a site for DC-T cell priming, and somatic hypermutation. Additionally, TLS provide the necessary adhesion molecules and chemokines to serve as a portal for the recruitment of immune cells into the tumor [72,73,74]. Thus, by aiding the formation of TLS in cancer, intra-tumoral DCs play a pivotal role in the recruitment of immune cells into the tumor microenvironment, akin to their role in LN.
Besides inducing the maturation of endothelial cells of TLS, DCs themselves can modulate the chemotactic environment of tumors to further assist in lymphocyte recruitment. For instance, in a mouse model of melanoma, it was shown that DCs are the chief source of the chemokine CXCL10 which is a ligand for CXCR3. CXCR3–CXCL10 chemokine axis plays a pivotal role in the migration of effector T cells into the tumor [75].
2.8 Summary
Dendritic cells are a heterogeneous population of cells and research into the specialized function of different DC subsets continues. Ultimately, as the development and function of each subset becomes more clear, specific DC subsets can be targeted to either enhance or suppress immunity. Additionally, a clear understanding of the development requirements of DC subsets will also enable us to skew in vitro generated DCs toward one type or other, which then can be used as therapeutics. Migration of DCs and DC precursors into tumors is not well studied. Unlike lymphoid organs, solid tumors might not have the necessary lymphatic structure and chemokine gradients to allow for the migration of DCs from the adjacent healthy tissue. Thus, even if appropriate DCs can be generated in vitro or identified in vivo, one has to think about their route to the tumor parenchyma. It is also crucial to understand better tumor-derived factors that can lead to suppression of DC activity or their death or cause aberrant development of DC precursors. Strategies to make DCs immunogenic and refractory to tumor-derived apoptotic factors can help in the effort to use DC-based therapeutics to treat cancer (Fig. 2.1).
References
Banchereau J, Steinman R (1998) M. Dendritic cells and the control of immunity. [Review] [103 refs]. Nature 392:245–252
Mueller DL (2010) Mechanisms maintaining peripheral tolerance. Nat Immunol 11:21–27
Corthay A (2006) A three-cell model for activation of naïve T helper cells. Scand J Immunol 64:93–96
Mogensen TH (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273
Curtsinger JM, Mescher MF (2010) Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22:333–340
Pulendran B (2006) Division of labor and cooperation between dendritic cells. Nat Immunol 7:699–700
De Koker S et al (2017) Inflammatory monocytes regulate Th1 oriented immunity to CpG adjuvanted protein vaccines through production of IL-12. Sci Rep 7:1–14
Truxova I et al (2018) Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer 6:1–13
Hirooka S et al (2011) The role of circulating dendritic cells in patients with unresectable pancreatic cancer. Anticancer Res 31:3827–3834
Goc J et al (2014) Dendritic cells in tumor-associated tertiary lymphoid structures signal a th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ t cells. Cancer Res 74:705–715
Lee H et al (2018) CD11c-positive dendritic cells in triple-negative breast cancer. In Vivo (Brooklyn) 32:1561–1569
Worbs T, Hammerschmidt SI, Förster R (2017) Dendritic cell migration in health and disease. Nat Rev Immunol 17:30–48
Liu K, Nussenzweig MC (2010) Origin and development of dendritic cells. Immunol Rev 234:45–54
Alvarez D, Vollmann EH, von Andrian UH (2008) Mechanisms and consequences of dendritic cell migration. Immunity 29:325–342
Ginhoux F et al (2007) Blood-derived dermal langerin + dendritic cells survey the skin in the steady state. J Exp Med 204:3133–3146
Spranger S, Bao R, Gajewski TF (2015) Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523:231–235
Böttcher JP et al (2018) NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell172:1022–1037.e14
Kamath AT et al (2000) The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol 165:6762–6770
Bennaceur K et al (2009) Different mechanisms are involved in apoptosis induced by melanoma gangliosides on human monocyte-derived dendritic cells. Glycobiology 19:576–582
Ishida A et al (2008) Mucin-induced apoptosis of monocyte-derived dendritic cells during maturation. Proteomics 8:3342–3349
Cubillos-Ruiz JR et al (2015) ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161:1527–1538
Xue J et al (2019) Intrinsic β-catenin signaling suppresses CD8 + T-cell infiltration in colorectal cancer. Biomed Pharmacother 115:108921
O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C (2008) Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev 223:114–131
Zitvogel L, Kroemer G (2014) CD103+ dendritic cells producing Interleukin-12 in anticancer immunosurveillance. Cancer Cell 26:591–593
Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A (1998) IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophag. Eur. J. Immunol 28:359–369
Mannino MH et al (2015) The paradoxical role of IL-10 in immunity and cancer. Cancer Lett 367:103–107
Ito T et al (2005) TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 202:1213–1223
Cao W et al (2009) Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med 206:1603–1614
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097–1105
Della Porta M et al (2005) Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology 68:276–284
Morrison SJ et al (1996) The aging of hematopoietic stem cells. Nat Med 2(9):1011–1016.
Oyama T et al (1998) Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 160:1224–1232
Michielsen AJ et al (2011) Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS One 6:e27944
Krempski J et al (2011) Tumor-infiltrating programmed death receptor-1 + dendritic cells mediate immune suppression in ovarian cancer. J Immunol 186:6905–6913
McDermott DF, Atkins MB (2013) PD-1 as a potential target in cancer therapy. Cancer Med 2:662–673
Chiba S et al (2012) Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol 13:832–842
Mildner A, Jung S (2014) Development and function of dendritic cell subsets. Immunity 40:642–656
Guilliams M et al (2016) Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45:669–684
Collin M, Mcgovern N, Haniffa M (2013) Human dendritic cell subsets. Immunology 140:22–30
Theisen D, Murphy K (2017) The role of cDC1s in vivo: CD8 T cell priming through cross-presentation. F1000 Res 6:98
Jongbloed SL et al (2010) Human CD141 + (BDCA-3) + dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 207:1247–1260
Bachem A et al (2010) Superior antigen cross-presentation and XCR1 expression define human CD11c + CD141 + cells as homologues of mouse CD8 + dendritic cells. J Exp Med 207:1273–1281
Böttcher JP, Reise Sousa C (2018) The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer 4:784–792
Salmon H et al (2016) Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44:924–938
Meyer MA et al (2018) Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat Commun 9:1–19
Barry KC et al (2018) A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat Med 24:1178–1191
Spranger S, Dai D, Horton B, Gajewski TF (2017) Tumor-residing Batf3 dendritic cells are required for effector T Cell trafficking and adoptive T Cell therapy. Cancer Cell 31:711–723.e4
Mikucki ME et al (2015) Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun 6:7458
Roberts EW et al (2016) Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30:324–336
Mittal D et al (2017) Interleukin-12 from CD103 + Batf3-dependent dendritic cells required for NK-cell suppression of metastasis. Cancer Immunol Res 5:1098–1108
Cauwels A et al (2018) Delivering type i interferon to dendritic cells empowers tumor eradication and immune combination treatments. Cancer Res 78:463–474
Cance JC, Crozat K, Dalod M, Mattiuz R (2019) Are conventional type 1 dendritic cells critical for protective antitumor immunity and how? Front Immunol 10:9
Nizzoli G et al (2013) Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 122:932–942
Granot T et al (2017) Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 46:504–515
Fu C, Jiang A (2018) Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol 9:3059
Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W (2018) CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 18:635–647
Michea P et al (2018) Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol 19:885–897
Cella M et al (1999) Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med 5:919–923
Ochando JC et al (2006) Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 7:652–662
Villard-truc F et al (2008) Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29:464–475
Chappell CP et al (2014) Targeting antigens through blood dendritic cell antigen 2 on plasmacytoid dendritic cells promotes immunologic tolerance. J Immunol 192:5789–5801
Hadeiba H et al (2012) Article plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity 36:438–450
Kohli K, Janssen A, Förster R (2016) Plasmacytoid dendritic cells induce tolerance predominantly by cargoing antigen to lymph nodes. Eur J Immunol 46:2659–2668
Musella M, Manic G, De Maria R, Vitale I, Sistigu A (2017) Type-I-interferons in infection and cancer: unanticipated dynamics with therapeutic implications. Onco Targets Ther 6:1–12
Demoulin S, Herfs M, Delvenne P, Hubert P (2013) Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol 93:343–352
Moussion C, Girard JP (2011) Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 479:542–546
Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH (2014) Tertiary lymphoid structures in cancer and beyond. Trends Immunol 35:571–580
Muniz LR, Pacer ME, Lira SA, Furtado GC (2011) A critical role for dendritic cells in the formation of lymphatic vessels within tertiary lymphoid structures. J Immunol 187:828–834
Martinet L et al (2013) High endothelial Venule blood vessels for tumor-infiltrating lymphocytes are associated with lymphotoxin β–producing dendritic cells in human breast cancer. J Immunol 191:2001–2008
Hiraoka N et al (2015) Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer 112:1782–1790
Martinet L et al (2012) High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 1:829–839
Pimenta EM, Barnes BJ (2014) Role of tertiary lymphoid structures (TLS) in anti-tumor immunity: potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancers (Basel) 6:969–997
Teillaud JL, Dieu-Nosjean MC (2017) Tertiary lymphoid structures: an anti-tumor school for adaptive immune cells and an antibody factory to fight cancer? Front Immunol 8:1–6
Engelhard VH et al (2018) Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity. J Immunol 200:432–442
Vilgelm AE, Richmond A (2019) Chemokins modulate immune surveillance in tumorigenesis, metastatsis, and response to immunotherapy. Front Immunol 10:6–8
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kohli, K., Pillarisetty, V.G. (2020). Dendritic Cells in the Tumor Microenvironment. In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1273. Springer, Cham. https://doi.org/10.1007/978-3-030-49270-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-49270-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49269-4
Online ISBN: 978-3-030-49270-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)