Abstract
Stem-acanthocephalans in the millimeter range might already have parasitized mandibulates in the Cambrian, while larger body sizes presumably evolved along with the upward-inclusion of gnathostome hosts. The characteristic morphology of modern acanthocephalans including the mostly hooked attachment organ (proboscis) should have emerged in the same context. Due to their rigidity, acanthocephalan hooks and copulatory caps are candidates for fossilization, but soft-tissue preservation might also have occurred under exceptional circumstances. Nonetheless, eggs represent the only ancient remains assigned to acanthocephalans to date. These were mostly retrieved from dried mammalian coprolites of up to ca. 12,000 years old. However, the recent discovery of eggs in a coprolite from the Upper Cretaceous illustrates that acanthocephalan eggs can also occur in fossilized remains. These and other aspects of acanthocephalan preservation, morphology, phylogeny, evolution, and pathogenicity are discussed in the present chapter that additionally includes a reflection of why Cambroclavida unlikely have an acanthocephalan origin.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Extant spiny- or thorny-worms (Acanthocephala) are endoparasites that occur worldwide where suitable hosts enable the establishment of their life cycle (Fig. 8.1). The intermediate host is recruited from Crustacea, Insecta, and Myriapoda and thus from Mandibulata (Arthropoda) (Schmidt 1971; Rota-Stabelli et al. 2013). Cartilaginous fishes (Chondrichthyes), teleost fishes (Teleostei), amphibians (Amphibia), sauropsids (Sauropsida), and mammals (Mammalia) and thus jawed vertebrates (Gnathostomata) serve as definitive hosts (see, e.g., Petrochenko 1956, 1958; Gibson et al. 2014). Thereby, the specificity of host usage seems generally to be weaker for definitive than intermediate hosts (Conway Morris and Crompton 1982; Parker et al. 2015).
Aquatic two-host life cycle of extant acanthocephalans. The palaeacanthocephalan genus Pomphorhynchus serves as an example. Presumably, the last common ancestor of crown-Acanthocephala already had an aquatic life cycle. Sketches of animals modified after Sielaff et al. (2016). A Acanthor inside egg, B blade of acanthor, C cystacanth, M metasoma, P presoma
Following mating in a definitive host, female acanthocephalans shed eggs into the intestine or cloaca from where they are released along with the excrements into the environment (Figs. 8.1 and 8.2). The ‘lucky’ egg is orally taken up by a suitable mandibulate whereupon the larva (acanthor) hatches inside the alimentary tract and then penetrates the intestinal wall, thus entering the host’s hemocoel. Thereafter, the larva experiences a drastic reorganization (catastrophic metamorphosis) to a growing juvenile (acanthella) which finally is encysted (cystacanth) (Fig. 8.1; Meyer 1932). The life cycle is closed when an intermediate host carrying one or more juveniles or cystacanths is ingested by a suitable definitive host (e.g., Conway Morris and Crompton 1982; Goater et al. 2014). Such an inclusion of a second host should increase the chance that a single male or female eventually meets a worm of the opposite sex so that they can mate and reproduce (Parker et al. 2015). However, the requirement for subsequent infection of two hosts lowers the chances of maintaining the life cycle. Acanthocephalans defy this adversity with two strategies. In particular, there is evidence that acanthocephalans manipulate the behavior of the intermediate host in such a way that the probability of host-transfer is increased (Bakker et al. 2017). Yet, while only some acanthocephalan species might be able to do so, the adults of all of them have increased fecundity (see Parker et al. 2015). In fact, males and females appear to be adapted to the production of high quantities of gametes and offspring (see below). In both respects, acanthocephalans resemble other endoparasites with complex life cycles such as tapeworms (Platyhelminthes, Cestoda) or trematodes (Platyhelminthes, Trematoda) (e.g., Goater et al. 2014).
Acanthocephalan eggs. (a) Drawing of an egg of Echinorhynchus coregoni (Palaeacanthocephala) (modified after Van Cleave 1921). (b) Sketch of an egg of Neoechinorhynchus dighaensis (Eoacanthocephala) (modified after Gautam et al. 2018). (c) Light micrograph of an egg of Macracanthorhynchus hirudinaceus (Archiacanthocephala: Oligacanthorhynchida) (modified after Kamimura et al. 2018). (a–c) The acanthella develops from the embryonic nuclei mass inside the acanthor
In extension of the obligatory two-host cycle, at least some of the extant acanthocephalan species can continue their life in a second definitive host. This can happen when a higher-level predator feeds on a first definitive host (post-cyclic transmission). It also occurs that acanthocephalans survive but do not mature in a first gnathostome host (paratenic host), and complete their development in a definitive host that had preyed on the paratenic host (e.g., Hernández-Orts et al. 2019). In addition, acanthocephalans can end up in an accidental host inside which the worms survive without having the ability to reproduce, but also without a (realistic) chance of transfer into a definitive host (e.g., Kennedy 2006). Taken together, every jawed vertebrate that at least occasionally feeds on mandibulates appears to be a potential host in the one way or another. Correspondingly, acanthocephalan diversity might be much higher than suggested by the figure of about 1,300 species currently known (Gibson et al. 2014). In support of this expectation, ongoing descriptions of species from previously under-investigated gnathostome vertebrates let the count of acanthocephalan species continuously grow (e.g., Gomes et al. 2015; Gautam et al. 2019).
Inside the alimentary tract of their definitive hosts , thorny-headed worms grow to body sizes of mostly several millimeters up to centimeters (Fig. 8.3), while only few of the extant species exceed body sizes of 10 cm or more. Whether a tiny creature or a considerable worm, males generally remain smaller than females (see, e.g., Petrochenko 1956, 1958). However, except for this inverse sexual dimorphism in body size, male and female acanthocephalans have pretty much the same outer appearance: The anterior-most section is occupied by the holdfast organ (proboscis) followed by the neck. Proboscis and neck together constitute a retractable functional unit named presoma (also praesoma). Farther posterior follows the trunk or metasoma, which mostly is more voluminous than the presoma in adult thorny-headed worms (Figs. 8.3 and 8.4a; Herlyn and Taraschewski 2017). This basic organization is widely conserved in three of the up to four acanthocephalan taxa with the traditional rank of a class, i.e. Palaeacanthocephala, Eoacanthocephala, and Polyacanthocephala. The same is true for Gigantorhynchida, Moniliformida, and Oligacanthorhynchida, which are ranked as orders within the fourth acanthocephalan class, Archiacanthocephala. In contrast, archiacanthocephalans belonging to the order Apororhynchida have an inflated proboscis and a comparably small metasoma (e.g., Petrochenko 1956, 1958; Herlyn 2017).
Drawings of the fish-parasite Neoechinorhynchus mexicoensis (Eoacanthocephala) . (a) Adult female with ovarian balls and eggs floating inside the metasomal body cavity. (b) Adult male with syncytial cement gland and two tandem-arranged testes. Except for the proboscis-receiving apparatus, which in eoacanthocephalans is comprised of receptacle and receptacle protrusor, the musculature is not shown (Modified after Pinacho-Pinacho et al. 2014)
Schematic depiction of the morphology of extant acanthocephalans (horizontal section plane). Not drawn to scale. (a) Palaeacanthocephala. (b) Eoacanthocephala. (c) Archiacanthocephala: Gigantorhynchida. (d) Archiacanthocephala: Oligacanthorhynchida. In acanthocephalans, hooks, copulatory cap, and eggs represent prime candidates for preservation but also the tegument is rather rigid and might occasionally have undergone fossilization. Internally, early crown-acanthocephalans should have possessed a two-layered muscular apparatus (light orange and lilac lines in (a–d)) suspending the cerebral ganglion and receiving the inverted proboscis (receptacle; see also Fig. 8.5a). Most likely, presomal sensory organs and tegument/epidermis cone evolved later on (b–d). Each presomal sensory organ consists of a bulbous swelling of the support cell (green), into which dendritic endings are imbedded (blue) which unite to nerves that extend to the cerebral ganglion (blue arrows). Successions of red lines and white dots underneath the basement membrane (also basal lamina) symbolize anastomosing strands of the circular musculature of the body wall. Light red solid lines highlight musculature with mainly longitudinally orientated anastomosing muscle strands. See main text for Apororhynchida (Archiacanthocephala), Moniliformida (Archiacanthocephala), and Polyacanthocephala. longit. longitudinal, musc. musculature
8.2 Acanthocephalans in Hominoids and Potential Reservoirs for Human Infections
The probably first case report of an infection of a human with an acanthocephalan was reported from Bohemia in the middle of the nineteenth century (Lambl 1859). The single female of Macracanthorhynchus hirudinaceus was excised from the intestine of a 9-year-old boy who had died of leukemia. How exactly the boy acquired the worm has to remain unanswered but one possible route of infection would be the intake of an infected grub of a scarabeid beetle (Coleoptera, Scarabeidae) (Pavlović et al. 2010). Indeed, people can generally acquire infections with acanthocephalans when they eat raw or insufficiently cooked intermediate hosts (e.g., Grassi and Calandruccio 1888; Zhong et al. 1983; Sahar et al. 2006; Mehlhorn 2016). Obviously, this happens quite regularly as demonstrated by Mathison et al. (2016). The study lists cases of infections with M. hirudinaceus and Macracanthorhynchus ingens from 11 countries in Asia, Australia, Europe, North America, and South America, and infections with Moniliformis moniliformis from 16 countries in the same continents plus Africa. In addition, humans may get infected when eating raw crayfish and fish, as discussed for acanthocephalans of the genera Bolbosoma and Corynosoma (e.g., Kaito et al. 2019; Sasaki et al. 2019).
The risk of human infections should be higher when mandibulates such as scarabeid grubs are infected at increased rate, and this should also be more likely to be the case when reservoir hosts live in vicinity of people. These can be omnivorous farm animals kept in the open. Outdoor management is still common in many parts of the world, and previously has been widespread in countries where it is now the exception. Thus, in the nineteenth century a veterinarian reported high morbidity in a herd of grazing piglets (Sus scrofa domestica, Suinae) in Hungary as a result of infection with M. hirudinaceus (Kocourek 1877). According to a note by the editorial office scarabeid beetles of the genus Melolontha represented the probable source of infection (see also Pavlović et al. 2010). Another report from the second half of the nineteenth century states that 40% of the swine slaughtered in Catania (Italy) were infected with M. hirudinaceus (Grassi and Calandruccio 1888). However, archiacanthocephalans have also found ways to exploit farm animals until more recently. Thus, M. hirudinaceus specimens could be collected for a study published in 2000 in the abattoir of La Paz (Bolivia), from butchered swine that were kept under outdoor management before. Yet macracanthorhynchiasis is not restricted to domestic swine as illustrated by an infected herd of white-lipped peccaries (Tayassu pecari, Suinae) kept in pasture in Brazil (de Almeida et al. 2006). Last but not least, archiacanthocephalans also occur in farm animals other than mammals. For example, chicken (Gallus gallus domesticus) can be parasitized by Mediorhynchus gallinorum, as has been observed in Indonesia (Amin et al. 2013). Accordingly, eggs of Archiacanthocephala might also be contained in old manure of human farm animals, although the preservation of pig and chicken manure might be a rarity per se.
Infections of farm animals and humans probably take place again and again somewhere in the world. However, it is also clear that the ongoing industrialization of animal production, in particular the separation of livestock from intermediate hosts, impairs the establishment of acanthocephalan life cycles with humans as hosts (compare Dunagan and Miller 1980). On the other hand, acanthocephalans do not depend on farm animals for getting into the vicinity of people since other mammals with a more or less close connection to humans can also be infected. In Tunisia, for instance, the occurence of M. hirudinaceus was shown for stray dogs (Canis familiaris, Carnivora) (Lahmar et al. 2017). This illustrates that the human companion can act as a reservoir for infections of people. But also mice and rats (Mus or Rattus, both Rodentia), raccoons (Procyon lotor, Carnivora), wild boar etc. can carry acanthocephalans, and due to their occurrence in or near settlements can be a reservoir for infections of humans and farm animals (Grassi and Calandruccio 1888; Dingley and Beaver 1985; Gassó et al. 2016; Kogi et al. 2016).
Raw animal food, including insects, should have contributed more to human nutrition in prehistoric times than today (Reinhard 1990; Gonçalves et al. 2003 Reinhard 2017). The mandibulates might have been collected in the surrounding landscape but also in human accomodations such as caves. In fact, the chance of ingesting an acanthella or cystacanth is not to be underestimated when eating a protein source like an insect in a raw or insufficiently cooked state. For example, the archiacanthocephalan Moniliformis dubius was found in up to 35% of the cockroaches (Periplaneta americana, Blattodea) collected from the lecture halls of a Nigerian university (Kogi et al. 2016). Thereby, the intensity of infection can reach considerable levels of more than 100 juvenile stages per individual intermediate host, as reported for M. moniliformis in beetles of the species Blaps mucronata (Coleoptera, Tenebrionidae) (Grassi and Calandruccio 1888). Accordingly, the uptake of a single intermediate host can be sufficient to enable acanthocephalan reproduction. In fact, Grassi and Calandruccio (1888) demonstrated that acanthocephalan eggs are contained in human stool upon infection with several cystacanths. Not least, eggs in human coprolites demonstrate that our species can serve as definitive host for archiacanthocephalans (see Table 8.1).
The anatomically modern Homo sapiens is not the only species within hominoids (Primates, Hominoidea) that can be parasitized by acanthocephalans. Also small apes (Hylobatidae), orangutans (Pongo), gorillas (Gorilla) and chimpanzees (Pan) sporadically take in insects, which can carry developmental stages of archiacanthocephalans (e.g., Van Thiel and Bruss 1945; Myers and Kuntz 1972). This phenomenon is actually not restricted to hominoids and rather affects other primates as well (Richart and Benirschke 1963; Solórzano-García and Pérez-Ponce de León 2018). Consequently, one should assume for the Last Common Ancestor (LCA) of diverse primate taxa that they were insectivorous to at least some extent. In the present context though, it is of particular interest that the LCAs of the crown-group taxa on the lineage to humans (Hominoidea, Hominidae, Homininae, Pan + Homo, and Hominini) should also have been insectivorous to some extent (for other character states of these LCAs, see Herlyn 2016). In expansion of this argument, insects are considered part of the diet of the Hominini genera Ardipithecus and Australopithecus. In the case of early members of Homo and prehistoric anatomical modern humans, there is even direct evidence for insectivory—and last but not least, in some human societies, insects continue to be part of the diet (Reinhard and Bryant 1992; Sayers and Lovejoy 2014; van Huis 2017). To put it the other way: There appears to be no principal reason why Ardipithecus ramidus, Australopithecus afarensis, Homo erectus etc. should not have been infected with (archi)acanthocephalans at least sporadically. Now only their eggs have to be discovered in coprolites of the corresponding taxa (compare Sistiaga et al. 2014; Chin 2021).
8.3 Solid-Parts and Their Preservation Potential
8.3.1 Acanthocephalan Propagules: Eggs in Space and Time
In acanthocephalans, the egg-producing units are not ovaries in the traditional sense. Instead, numerous so-called ovarian balls of unclear ontogenetic origin freely float in the fluid-filled metasoma, and release immature eggs into the body cavity wherein they mature (Figs. 8.3a and 8.4a; e.g., Dunagan and Miller 1991). The fluid remains in motion through movements of the body so that the eggs sporadically pass by an elaborated egg sorting apparatus. This so-called uterine bell is situated in the hind trunk and allows only mature eggs to enter the terminal genital tract from where the eggs are discharged through the genital pore into the alimentary tract or cloaca of the definitive host (Fig. 8.4a; Herlyn and Röhrig 2003 and references therein). The eggs are eventually released with the excrements into the environment (Fig. 8.1), a process that can take place at high rates upon mating. Gravid females of the larger species might even produce 82,000 eggs per day on average, and this for a patent period of 10 months (see Dunagan and Miller 1991). Consequently, acanthocephalan eggs should be quite common in the droppings of infected gnathostomes—and the hosts might not only have been taxa with extant members. As acanthocephalan life cycles can be extended by post-cyclic transmission in at least some extant species (Kennedy 1999, 2006), acanthocephalan eggs may also be contained in fossilized coprolites of higher-level predators belonging to Ichthyosauria and Pterosauria (both Sauropsida), to name just a few (compare Leung 2021). In addition, acanthocephalan eggs may be preserved in petrified sediments under favorable conditions.
The contour of acanthocephalan eggs enables a rough taxonomic diagnosis. Thus, eggs of archiacanthocephalans and polyacanthocephalans usually have a rounded-oval appearance. The same applies to at least some eoacanthocephalans and palaeacanthocephalans, while eggs of other palaeacanthocephalans are stronger elongated or spindle-shaped (Fig. 8.2; Uglem 1972; Schmidt 1985; Amin 1987; Taraschewski and Peters 1992; Golvan 1994; Amin and Heckmann 2017). Since also the next phylogenetic relatives of Acanthocephala as a whole, i.e., the different taxa traditionally regarded as wheel animals (Rotifera) (e.g., Verweyen et al. 2011; Weber et al. 2013), have rounded to oval eggs (see e.g., de Beauchamp 1909; Murray 1910; Edmondson 1965; Dartnall 1995; Habdija et al. 2011), this should be the plesiomorphic character state. Consequently, ancient acanthocephalans should have possessed rounded to oval eggs, too. These eggs presumably had thin shells because life cycles were most likely aquatic in early acanthocephalans (see below). Indeed, the shell is thinner in extant acanthocephalans with aquatic life cycles than in species exploiting terrestrial hosts. Such thicker eggshells are particularly well known from archiacanthocephalans (Fig. 8.2; e.g., Van Cleave 1921, 1947; Uglem 1972), and probably protect the acanthors from dehydration.
As far as the shells of eggs were investigated in more detail, most authors distinguished four envelopes or layers, of which the second-outermost one was found to contain keratin or a keratin-like substance. The same may be true for the second-innermost layer, as demonstrated for an archiacanthocephalan species. Lastly, a chitin-like constituent was reported for the innermost eggshell layer of palae- and archiacanthocephalans (compare Fig. 8.2a–c; Whitfield 1973; Peters et al. 1991; Taraschewski and Peters 1992; Taraschewski et al. 1992). The incorporation of keratin- and chitin-like compounds into the eggshell might account for its rigid nature and, in any case, should be advantageous for preservation. In fact, leaving aside a fossil from the Triassic of India with uncertain phylogenetic affiliation (see Kumar and Kumar 2001), eggs are the only known remains of higher age that clearly refer to acanthocephalans (Table 8.1 and references therein). With a single exception (next paragraph), the age of the finds ranges from at least several hundred up to about 12,000 years before present. The said eggs are from sites in North and South America, Asia and Africa, but there is no apparent reason why eggs that would testify to ancient infections with archiacanthocephalans should not also be found in Europe. Either way, most of the corresponding remains were obtained from dried feces, probably left by anteaters (Xenarthra), a dog (Carnivora, Canidae), a small felid (Carnivora, Felidae), a skunk (Carnivora, Mephtitidae), an unspecified carnivore, and humans (Table 8.1). Six of the eggs found in colon content of an Egyptian mummy from Roman times complete the list of ancient mammalian infections with acanthocephalans (Table 8.1: Horne 2002). In addition, acanthocephalan eggs retrieved from a preserved hairball could reflect an infection of one or more smaller mammals (Rodentia) which an owl (Aves, Strigiformes) preyed upon (Table 8.1: Beltrame et al. 2015).
According to the shape of the eggs as well as the thickness and structuring of the eggshell layers, these ancient acanthocephalan eggs belong to Archiacanthocephala. They were especially assigned to the genera Echinopardalis, Gigantorhynchus, Macracanthorhynchus, Moniliformis and Prosthenorchis (Table 8.1: Moore et al. 1969; Ferreira et al. 1989; Reinhard 1990; Noronha et al. 1994; Fugassa et al. 2011; Beltrame et al. 2015, 2018; Mowlavi et al. 2015), although the mostly poor preservation of the outer shell layer impairs comparisons with modern eggs (compare Table III in Fugassa et al. 2011; Beltrame et al. 2015). In other cases, the archiacanthocephalan genus remained unspecified (Table 8.1: Reinhard 1990; Horne 2002; Gonçalves et al. 2003 Hunt et al. 2012; Camacho et al. 2013; Nezamabadi 2014). A very recent paper additionally reports eggs of Oligacanthorhynchus, in addition to Macracanthorhynchus and Gigantorhynchus, from Tamandua tetradactyla coprolites which were collected from 3,190 to 8,870 year old layers at archeological sites in Piauí state, Brazil (de Souza et al. 2020). However, keratin- and chitin-like substances are not restricted to archiacanthocephalan eggs (see above). So the question arises why archiacanthocephalan eggs predominate and why are there so many human and carnivore coprolites among the egg-carrying samples. This could partly reflect sampling bias because terrestrial caves usually attract high research attention when they were human shelters in pre-historic times (e.g., Araújo et al. 2015). But the skew might have a natural background in addition: First, higher thickness of their shells should render archiacanthocephalan eggs particularly resistant to damaging effects (compare Camacho et al. 2013, 2018). Second, preservation through dehydration and undisturbed storage should be more likely to happen to droppings in terrestrial caves than to feces released in other environments. Yet, the gnathostomes that have left larger droppings in land caves in the relevant time were mostly members of Carnivora and humans (e.g., Camacho et al. 2018). In any case, these finds suggest that archiacanthocephalan eggs could be contained not only in preserved human stool from mummies and latrines but also in mummified cats etc., as has already been shown for eggs of endoparasitic nematodes (Nematoda) and flatworms (Platyhelminthes) (Ferreira et al. 1983; Novo and Ferreira 2016; Yeh and Mitchell 2016).
A recent report of ancient acanthocephalan eggs refers for the first time to fossilized remains. The corresponding four eggs were included in a phosphatized coprolite from Upper Cretaceous sediments in São Paulo State, Brazil (Cardia et al. 2019). The assignment of the four eggs to Acanthocephala and especially to Archiacanthocephala is supported by the thickness and multi-layered appearance of their eggshells, and also by the structures enclosed which in at least three cases are reminiscent of acanthors (see Fig. 6b–d in Cardia et al. 2019). The fact that these egg remains maximally show three eggshell layers does not preclude an archiacanthocephalan origin since the outermost egg shell layer generally tends to be poorly preserved (see above). However, the extant species of Archiacanthocephala are primarily known to use mammals and birds as definitive hosts (e.g., Near 2002), while the Cretaceous coprolite presumably has a crocodyliform origin (Cardia et al. 2019). Although a wider spectrum of taxa from Tetrapoda may serve as paratenic host (see e.g., Petrochenko 1958), there appears to be no indication that crocodiles belong to the usual hosts. It would therefore be conceivable that—comparable to the above example of a hairball—the Cretaceous animal, which left the dropping, devoured at least one paratenic or definitive host of an archiacanthocephalan. In support of such possibility, Cardia et al. (2019) state that the fluvial deposits of the respective Brazilian Lagerstätte contain fossilized bones of fishes, lizards (Lacertilia), turtles (Testudines), stem-group representatives of birds commonly referred to as “dinosaurs”, mammals and other taxa, besides remains of Crocodyliformes. Nonetheless, it may also be that the Cretaceous creature ingested one or more acanthocephalan developmental stages when preying on a crustacean or other intermediate host. Either way, the finding demonstrates that acanthocephalan eggs can fossilize, and that details of eggshell composition and acanthor morphology remain discernible under advantageous conditions. With such excellent preservation, the find reminds of putative hooklets inside the egg of a tapeworm (Platyhelminthes, Cestoda) discovered in a 270 million year old shark coprolite (Dentzien-Dias et al. 2013). Likewise, eggs of presumably cestode origin have been reported from rectum content of a Carboniferous shark fossil (Zangerl and Case 1976). Perhaps, acanthocephalan eggs will once be discovered in coprolites of similar or even higher age.
8.3.2 Hooks
While trunk and neck bear spines in some of the extant acanthocephalan species and not in others, the proboscis is almost always armed with recurved hooks (Figs. 8.4 and 8.5a, b). These hooks are extracellular differentiations consisting of protein (see Miller and Dunagan 1985), with roots resting in the basement membrane (basal lamina) underlying the tegument (also integument, epidermis, cutis) (Fig. 8.6a, b). However, this anchoring might actually be somewhat flexible due to the probable discontinuity of the fibers constituting hook roots and basement membrane (Fig. 8.6b; Taraschewski et al. 1989). In histological preparations, hook roots and shafts show a peripheral rind of condensed material and a core of less densely woven fibers (see labelled hook in Fig. 8.6a). This structuring seems to be reflected in a radial gradient of element abundances as revealed by X-ray analysis. Presumably, the incorporation of substances like calcium, sulfur, and phosphorus contributes to the stiffening of the hooks, which reportedly proceeds in anterio-posterior direction along the proboscis (Taraschewski 1989a, b; Amin and Heckmann 2017). In any case, the sclerotization should increase the chances that acanthocephalan hooks are contained in the fossil record (compare Littlewood and Donovan 2003).
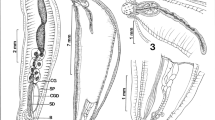
Comparison of hooks in the extant acanthocephalan species (a, b) and cambroclavid microfossils from the Cambrian (c, d). (a) Light micrograph of a mounted specimen of Acanthocephalus anguillae (Palaeacanthocephala): The proboscis bears recurved hooks. (b) Paratenuisentis ambiguus (Eoacanthocephala): Scanning electron micrograph of about the anterior two thirds of the proboscis. The tegument or epidermis cone is visible at the proboscis apex. (c, d) Scanning electron micrographs of Cambroclaves microfossils (Courtesy of Thomas Wotte, Geological Institute at TU Bergakademie Freiberg, Germany)
Paratenuisentis ambiguus (Eoacanthocephala): Light micrographs of consecutive transverse sections. (a) Proboscis at the level of the apical tegument or epidermis cone. (b) About halfway along the proboscis. (c, d) Foretrunk with the proboscis-receiving apparatus (receptacle protrusor plus receptacle) at its center. The receptacle has an anterior medullary (c) and a posterior contractile portion (d)
Although exceptional preservation conditions are required, fossilization of “invertebrate” hooks is possible. This is exemplified by fossil hooks in abdominal and gill regions of Devonian remains from Latvia assigned to Placodermi and Acanthodii (Upeniece 2001, 2011; De Baets et al. 2015). Their probable location on the surface of the fish fossils, their appearance and partially also their circular arrangement suggest that the hooks once belonged to ectoparasitic monogeneans (Platyhelminthes, Monogenea) (Upeniece 2011; Leung 2017; De Baets et al. 2021), and not acanthocephalans. Still, the size of the fossil hook-like remains (0.02–0.40 mm; Upeniece 2011) is in a range known from extant acanthocephalans (Figs. 8.5a and 8.6a). However, when likewise small monogenean hooks can fossilize, why have no fossil hooks of acanthocephalans been discovered so far? The most likely reason is that acanthocephalans live inside their gnathostome hosts—and even “worse”, inside the alimentary canal. Probably, acanthocephalans do so since they exploit gnathostomes as hosts (see evolutionary scenario below). For this reason, fossilization would require that acanthocephalan hooks first withstand autolysis of the bowels upon the death of a host, and then encounter excellent preservation conditions.
8.3.3 Copulatory Cap
Besides hooks and eggs , there is another structure, for which preservation seems to be in the range of the possible, and this structure has to do with acanthocephalan reproduction: In the course of mating, the male worm encloses the female hind end with the everted copulatory bursa (Fig. 8.3b), and introduces spermatozoa through a penile structure into the female genital tract. Before the male detaches from the female, it seals the female genital pore with the proteinaceous secretion from one or more so-called cement glands (Fig. 8.3b) (Dezfuli et al. 2001). Obviously, the female is hindered from additional copulations as long as the copulatory cap is present (Fig. 8.4a). Thus, cement and cement apparatus might reflect increased competition between males, as does the enlargement of the usually two testes (Fig. 8.3b; Poulin and Morand 2000). In any case, a capping structure at the posterior ending of a fossil intestinal parasite would be an indication of a female acanthocephalan.
8.4 Soft Tissue, Functional Morphology and the Ideal Fossil
Fossils from the lower Cambrian of China and Canada show that the preservation of worms can be so excellent that besides contour and appendages also internal structures like the digestive tract appear to be visible (Hu et al. 2008; Briggs and Caron 2017; Shu et al. 2017). The mentioned fossils are reminiscent of priapulids and might have dwelled the sediment, which buried them later on. Still, soft tissue preservation is not restricted to Cambrian sediment dwellers but also occurred in epibiotic or ectoparasitic helminths. A first example is Inquicus fellatus, a parasite from the Cambrian of China, which lived on priapulids or priapulid-like worms (Cong et al. 2017). Another example is a special find amongst the already-mentioned remains of fish ectoparasites from the Upper Devonian of Latvia: The corresponding fossil shows not only a circlet of six hooks reminiscent of a monogenean opisthaptor, but also the contour of the hook-bearing soft tissue (Upeniece 2001, 2011; De Baets et al. 2015). But when soft-tissue preservation is rare in ectoparasites, it should be even rarer in intestinal parasites.
8.4.1 Outer Contour and Tegument
Under the premise that preservation of intestinal parasites is unlikely, the tegument or epidermis should not be less suitable for fossilization in acanthocephalans than other helminths (compare Littlewood and Donovan 2003). Whether the tegument experiences sclerotization (Taraschewski et al. 1989) or not, it is very resistant to mechanical destruction and enzymatic decomposition (personal observation; but see Reinhard 1990). This toughness is probably due to the syncytial organization of the tegument and a presumably proteinaceous lamina underneath its distal plasma membrane, a character complex shared by acanthocephalans and their closer phylogenetic relatives (Ahlrichs 1997; Herlyn and Ehlers 2001; Near 2002). Provided that fossilization of the acanthocephalan tegument occurred, the outer contour of the hypothetical remains might show an increase in body diameter from neck to metasoma as it is common in the extant species (Figs. 8.3 and 8.4; e.g., Petrochenko 1956, 1958). Furthermore, if a larger bulbous differentiation shines through at the proboscis apex, this could be an intrusion of the tegument ((apical) tegument or epidermis cone), as it is characteristic of the monophylum including polyacanthocephalans and eoacanthocephalans (Figs. 8.4b and 8.5b; Herlyn 2001; Amin 2013; Gazi et al. 2016). However, if the contour of the hypothetical fossil is more reminiscent of a balloon with a shorter worm-like appendix , it could originate from an archiacanthocephalan, particularly a member of Apororhynchida (Herlyn 2017).
8.4.2 Presomal Musculature and Anchoring
Internally, the transition from presoma to metasoma is marked by a muscular apparatus which suspends the cerebral ganglion or brain and probably consists of two muscular layers in all extant species. The only complication in this general scheme is that each layer can be either a continuous muscular sheath or a muscular mesh of anastomosing strands. In Palaeacanthocephala, both muscular layers are continuous and thereby have such intimate contact that the entire apparatus has a double-walled appearance (Fig. 8.4a). Archiacanthocephalans belonging to Moniliformida show the same pattern. In archiacanthocephalans belonging to Gigantorhynchida and Oligacanthorhynchida as well as in all members of Eoacanthocephala, only the inner layer is a sheath-like muscle, whereas the outer layer forms a loose muscular mesh (Figs. 8.4b–d). Accordingly, the muscular apparatus suspending the cerebral ganglion looks single-walled in these species (Herlyn 2002; Herlyn and Taraschewski 2017). The same pattern seems to be realized in Polyacanthocephala (Amin 1987). Yet another way in which both muscle layers can be arranged is shown by the archiacanthocephalan taxon Apororhynchida: here both muscle layers consist of delicate vela-like muscular strands (Herlyn 2017).
Unfortunately, the established terminology does not reflect the homology of the two internal muscular layers at the presoma-metasoma transition. Thus, it is common practice to refer to a double-walled receptacle, when both muscular layers are sheath-like and have intimate contact (Palaeacanthocephala, Moniliformida). The layers themselves are then considered as inner and outer wall of the receptacle (Fig. 8.4a). Yet, the receptacle is commonly regarded as single-walled, when only the inner layer is sheath-like (Eoacanthocephala inclusively Polyacanthocephala, Gigantorhynchida, Oligacanthocephala). Then, this inner layer is termed just receptacle, while the established name for the strands of the receptacle-surrounding muscle is receptacle protrusor (Figs. 8.4b–d and 8.6c, d; Herlyn and Taraschewski 2017; also Amin 1987). The use of alternative names for the same muscles further complicates matters (for a survey, see Herlyn and Taraschewski 2017).
The alternative organization of the muscular apparatus carrying the cerebral ganglion has functional consequences: When both muscular layers are mesh-like (Apororhynchida), their contraction should not increase the pressure inside the presomal part of the body cavity relative to its metasomal portion (Herlyn 2017). However, as long as at least one of the muscular layers has a sheath-like organization (all other taxa of extant Acanthocephala; Figs. 8.4, 8.5a and 8.6c, d), presomal and metasomal body cavities are separated. Then, contraction of the either single- or double-walled muscular apparatus increases the hydrostatic pressure inside the presomal body cavity. This again leads to the eversion of neck and proboscis, which can be accompanied by partial rotation alongside the body axis and a bending relative to the trunk (Hammond 1966; Herlyn and Ehlers 2001). Reversal of the process results from relaxation of the “eversion muscles” and contraction of an retractor muscle. The presomal portion of the latter is termed proboscis retractor whereas the metasomal portion is commonly referred to as receptacle retractor (Fig. 8.4a). The proboscis retractor consists of circularly arranged longitudinal muscular strands (Figs. 8.4 and 8.6a, b) that anastomose and thus form a tube-like contractile mesh. The mesh can display a complicated folding in cross section depending on the taxon investigated (e.g., Dunagan and Miller 1991; Herlyn 2002). However, in all extant acanthocephalans the muscle inserts anteriorly at the inner site of the body wall just beneath the proboscis apex. After having extended through most of the presomal (part of the) body cavity, the muscle splits into two or three portions that separately pass through the bottom of the muscular apparatus suspending the cerebral ganglion (Fig. 8.4a). The separate portions continue through the metasomal (part of the) body cavity before they attach to the inner surface of the metasomal body wall (Fig. 8.4a) (Herlyn 2017; Herlyn and Taraschewski 2017 and references therein).
Eversion and inversion of the presoma are usually repeated until the worm is attached to the intestinal mucosa or deeper (Hammond 1966; Aguiar et al. 2018). Characteristic for the resting position is a subsequent slight withdrawal of the neck by contraction of the neck retractor, which also has a mesh-like organization (Figs. 8.4a and 8.6c, d). As some of its strands enclose the lemniscs, usually paired processes of the presomal tegument extending into the metasomal (part of the) body cavity (Figs. 8.4 and 8.6d), contraction of the neck retractor presumably presses fluid from the lemniscs into the presomal tegument, thus stiffening the everted proboscis. Thereby, the transportation of fluid takes place via a so-called lacunar system extending through tegument and lemniscs (e.g., Fig. 8.6c; Hammond 1966; Herlyn 2002, 2017; Herlyn and Taraschewski 2017). This may be the case or not, but it is almost certain that there is no connection between the lacunar system inside the tegument and lemniscs and any sub-tegumental structures including the body wall musculature (compare Nielsen 2012). In addition, the musculature is not hollow, although it may occasionally appear hollow due to preparation artifacts (for a discussion, see Herlyn and Taraschewski 2017; see also Nikishin 2004).
From a paleoparasitological point of view, the practical value of the above details on the functional morphology of acanthocephalan anchoring is currently limited. In principle, however, musculature and other decay-prone structures can fossilize under certain conditions (Parry et al. 2018). In addition, most of the aforementioned muscles are visible in total preparations of acanthocephalans (e.g., Fig. 5a, b in Herlyn and Taraschewski 2017). Accordingly, the one or other of the aformentioned muscles might shine through the body wall of the yet to be discovered fossilized thorny-headed worm, comparable to the presumed alimentary tract in I. fellatus (see Fig. 2a in Cong et al. 2017). Thus, if the fossil of a suspected endoparasite ever discloses details of its internal organization, then a broader longitudinal strand extending through the anterior body section (presoma) could correspond to the proboscis retractor of an acanthocephalan. Furthermore, strands with smaller diameter that extend through the foretrunk (anterior portion of metasoma) could represent the receptacle retractor and/or neck retractor of an acanthocephalan. In addition, a bag-like structure between a smaller (presomal) and a wider (metasomal) body cavity could testify to the muscular apparatus that once suspended the cerebral ganglion of a palaeacanthocephalan, eoacanthocephalan, gigantorhynchid, moniliformid, or oligacanthorhynchid. But if such a septum is not visible in the hypothetical fossil and the outer contour approximates a balloon with a worm-like appendix, the remain could originate from an apororhynchid.
8.4.3 Presomal Sensory Organs
Whether involved in the eversion of the proboscis or not, the muscular apparatus always carries the cerebral ganglion (Fig. 8.4a). Obviously, the acanthocephalan cerebral ganglion should not only steer muscular activity but also integrate incoming signals. In some acanthocephalans, such information will come from comparably prominent sense or sensory organs at the base of the neck (lateral sensory organs) and the proboscis apex (apical sensory organs). These organs reside just beneath the bottom of circular pits shaped by tapering tegument, which resemble pores (Gee 1987). Depending on the taxon, these pits can reside on the tips of conical elevations of the presomal tegument, comparable to the crater of a volcano (Fig. 8.4c, d). While this may sound quite complicated, things are getting easier with respect to the sensory organs themselves. In fact, lateral and apical sensory organs are identical in their general organization: They are essentially terminal bulbous swellings of the processes of a so-called support cell (which is a syncytium) into which dendritic endings are imbedded (Fig. 8.4b–d). Proximally, the dendritic differentiations leave the bulbs and unite to nerves that extend to the cerebral ganglion (e.g., Harada 1931; Gee 1987; Herlyn et al. 2001).
Although their occurrence is widespread, these sensory organs are not present in all extant acanthocephalan species. According to the available data, extant acanthocephalans possess either two apical sensory organs in addition to a pair of lateral sensory organs (Archiacanthocephala: Gigantorhynchida, Moniliformida) or one apical organ plus two lateral sensory organs (Archiacanthocephala: Oligacanthorhynchida) or two lateral sensory organs only (probably all eoacanthocephalans and some palaeacanthocephalans). The last alternative is the absence of any sensory organs of the described type (other palaeacanthocephalans) (e.g., Gee 1987; survey in Herlyn et al. 2001). How apororhynchids fit into the picture remains to be elucidated. Nonetheless, the available data suggest that a duplication of the presomal sensory apparatus occurred in archiacanthocephalans, namely from a state of only two lateral sensory organs (Fig. 8.4b) to the formation of two lateral plus two apical sensory organs (Fig. 8.4c). Once established, both apical sensory organs seem to have fused to an unpaired structure in the stem line of crown-Oligacanthorhynchida (Fig. 8.4d; Weber et al. 2013).
Here, too, the practical value of this morphological description can only arise if a fossil endoparasite with corresponding soft tissue preservation will ever be discovered. Nonetheless, the fossilization of decay-prone structures such as the nervous system is possible (Parry et al. 2018). Thus, if a fossil endoparasite would reveal subtegumental bulbous differentiations at the transition from a smaller body section (neck) to a broader body section (metasoma) and additionally at the presumed anterior tip, the structures could represent presomal sensory organs of an archiacanthocephalan (Moniliformida, Gigantorhynchida, Oligacanthorhynchida). However, if such bulbs would only be discernible at the base of the neck, the worm could rather have been an eoacanthocephalan or paleacanthocephalan.
8.4.4 Lack of an Intestinal Tract
In addition to the typical outer contour, a muscular septum between the presomal and metasomal body cavities (or between the corresponding parts of a singular body cavity) and sensory organs of the type described in the previous section, a fossilized crown-acanthocephalan should lack an intestinal tract. Indeed, the alimentary tract got reduced in the stem line of the taxon and acanthocephalans of all developmental stages take up nutrients exclusively via the surface (e.g., Near 2002), just as it is the case in tapeworms (compare Figs. 8.3 and 8.4; e.g., Goater et al. 2014). Obviously, the non-identification of a structure in a fossil does not necessarily imply that it was absent in the living animal. But if there should be evidence for a digestive tract in a fossil remain of a presumed endoparasitic worm, then the animal behind cannot have been a crown-acanthocephalan.
8.5 Pathological Manifestations of Infections with Acanthocephalans
The symptoms of humans suffering from infections with acanthocephalans have been studied in a self-experiment: Three weeks after swallowing M. moniliformis cystacanths, which before were excised from beetles (B. mucronata), increasing amounts of eggs appeared in the stool of S. Calandruccio. He presented with “severe abdominal pain, sometimes perceived as tearing and increased by pressure on the aching spot, and here and there some diarrhea, strong buzzing in the ears (later in the whole head), as well as great fatigue and flaccidity” (translated from German after Grassi and Calandruccio 1888). Additional symptoms reported for macracanthorhynchiasis and moniliformiasis are poor appetite, perianal itching, besides the appearance of worms in the stool. Consequently, the mostly young patients, who usually become infected when they take in an intermediate host, present to a doctor quite quickly (e.g., Sahar et al. 2006; Mathison et al. 2016).
Intensities of hundreds or even >1,500 thorny-headed worms per individual host, as reported for teleost fishes and birds, can cause life-threatening constipation (Wurmbach 1937; Perry 1942; Sanford 1978). The lives of gnathostome hosts may be additionally threatened if acanthocephalans penetrate the intestinal wall, enter the body cavity, other organs or mesenteries and thus elicit peritonitis (Choi et al. 2010). But even if death is not brought about, such high intensities can weaken gnathostome hosts, as primarily studied in fishes. In particular, the damaging of the intestinal wall by the activity of the proboscis (Hammond 1966; Herlyn and Taraschewski 2017 and references therein; also Aguiar et al. 2018) can cause inflammations, lesions and necroses and thus a decrease of the intact absorptive surface (Taraschewski et al. 1989; de Matos et al. 2017; Jerônimo et al. 2017). Additionally, acanthocephalans absorb components from disintegrating host tissue and infiltrating blood as well as nutrients from the intestinal contents, which are no longer available to the host (Taraschewski and Mackenstedt 1991a, b; Sures 2002; see also Sures et al. 2000). Depending on the acanthocephalan species, it also belongs to the normal attachment behavior that an acanthocephalan pierces the intestinal wall with the proboscis, whereupon a nodule forms toward the body cavity which encloses the anterior-most body section of the worm (Wurmbach 1937; Taraschewski 2000; Dezfuli et al. 2015).
Considering the different ways of how acanthocephalans can damage vertebrates it may surprise that fishes can tolerate high intensities of infection (e.g., Đikanović et al. 2010). Nonetheless, the pathological manifestations outlined above can negatively affect their growth rate, general condition, and survival rate (Martins et al. 2001; Malta et al. 2001; Jerônimo et al. 2017). In addition, growing fishes can develop spinal deformations due to the reduced mineral availability resulting from intense infections with acanthocephalans (Silva-Gomes et al. 2017). Consequently, acanthocephalans could have contributed to spinal deformations such as shown by some remains of Miocene killifish (Teleostei, Cyprinodontidae) from Kenya (Altner and Reichenbacher 2015). Another indirect indication of infection could be the presence of the aforementioned nodules on the outer surface of intestinal remains of fossil Teleostei, Elasmobranchii, Ichthyosauria etc.
8.6 Phylogenetic Relationships of Acanthocephala and Taxonomic Implications
Penis worms (Priapulida) long belonged to the circle of candidate taxa for the acanthocephalan sister-group due to similarities in body organization and other morphological features (Conway Morris and Crompton 1982). For priapulids share with acanthocephalans the presence of hooks or scalids, as they are called in priapulids, in the anterior body section (Habdija et al. 2011). However, it is meanwhile quite certain that Priapulida do not belong to the closer relatives of Acanthocephala, which rather have a nested position inside Gnathifera (Ahlrichs 1997; Witek et al. 2009; Fröbius and Funch 2016). The taxon name refers to the evolutionary novelty of jaw-like solid-parts inside the pharynx (Ahlrichs 1997) as they are present in Gnathostomulida, Micrognathozoa, and already-mentioned wheel animals or Rotifera. The jaw-like elements serve in food uptake, whereby rod-like elements with a characteristic substructure seem to provide flexibility to the “jaws” (Rieger and Tyler 1995; Herlyn and Ehlers 1997; Kristensen and Funch 2000). The rods again might be homologous to the grasping spines in arrow worms (Chaetognatha; Shu et al. 2017), and, indeed, arrow worms currently appear to be close relatives of Gnathifera (Fig. 8.7; Fröbius and Funch 2016). Alternatively, arrow worms may branch off within Gnathifera, then as a sister to a clade comprised of micrognathozoans, rotifers and acanthocephalans (Marlétaz et al. 2019). In any case, the naming after jaw-like elements (ἡ γνάθος) is a recurrent theme in the kinship circle of Gnathifera. Yet, the eponymous differentiations obviously got lost in the stem line of crown-Acanthocephala, along with the reduction of the alimentary tract (e.g., Conway Morris and Crompton 1982)—which has the noteworthy consequence that acanthocephalans are jaw-less members of a clade, which is named after jaw-like solid-parts (Gnathifera). To top it all, these jaw-less members of Gnathifera parasitize jawed arthropods (Mandibulata) and jawed vertebrates (Gnathostomata).
Evolution of the endoparasitic two-host cycle of crown-Acanthocephala via an epibiotic/ectoparasitic stage. Italics give evolutionary novelties. Changes in lifestyle and host usage are highlighted by orange and red labelling, respectively. Colored symbols at nodes and leaves of the tree give presumed lifestyles of the last common ancestor (LCA) of the corresponding taxa each. Time estimates in green refer to fossil members of arrow worms or Chaetognatha (according to Shu et al. 2017; Briggs and Caron 2017), and to the onset of the fossil record of potential hosts, i.e., mandibulate arthropods and gnathostome vertebrates (according to Sansom et al. 2015; Daley et al. 2018; see also Janvier 2003). Extant host species are boxed. The phylogenetic tree shown combines results from Ahlrichs (1997, 1998), Herlyn and Ehlers (1997), Ferraguti and Melone (1999), Herlyn et al. (2003), Witek et al. (2008, 2009), Wey-Fabrizius et al. (2014), Sielaff et al. (2016), and Fröbius and Funch (2016). Chaetognatha may alternatively be sister to a monophylum comprised of Micrognathozoa and Syndermata/Rotifera (Marlétaz et al. 2019) or to Syndermata/Rotifera alone (Vinther and Parry 2019). However, both alternatives would have no influence on the proposed stepwise establishment of an endoparasitic two-host cycle on the lineage to crown-acanthocephalans via an epibiotic or ectoparasitic stage (as retained in seisonids), invasion of mandibulates, and upward inclusion of gnathostome hosts. Sketches of animals after Ahlrichs (1997), Sielaff et al. (2016), and Kassatkina (2016)
Inside Gnathifera, the already-mentioned rotifers or wheel animals represent the next phylogenetic relatives of acanthocephalans amongst the extant species (Fig. 8.7). Such a relationship was already expected by von Haffner (1950) and others (Rieger and Tyler 1995; see also Conway Morris and Crompton 1982), and in the meantime gained support from molecular analyses (e.g., Mark Welch 2000; Herlyn et al. 2003; Struck et al. 2014). Inspired by the presumed evolutionary novelty of a syncytial organization of the tegument, the name Syndermata was introduced for the Rotifera-Acanthocephala clade (Ahlrichs 1997). However, while some authors use the newly introduced name, others preferentially regard acanthocephalans as highly derived rotifers (e.g., Mark Welch 2000; García-Varela and Nadler 2006). In fact, the naming of the taxon heated some authors in a surprising way although there is wide agreement on the decisive point, i.e., the monophyletic origin of Acanthocephala and the three traditional rotifer taxa Monogononta, Bdelloidea, and Seisonidea (also Seisonacea, Seisonidae) (e.g., Nielsen 2012). Apart from its syncytial organization, the tegument is special in all members of the Acanthocephala-Rotifera group, by having an intra-syncytial lamina—a fact mentioned above with respect to the preservation potential of the acanthocephalan tegument. Another evolutionary novelty of the Acanthocephala-Rotifera group seems to be that the distal plasma membrane of the tegument shapes crypt-like infoldings (Ahlrichs 1997; Near 2002). These infoldings increase the surface of the tegument, which should be of special relevance for nutrient uptake via surface in gut-less acanthocephalans (Graeber and Storch 1978). Members of the Rotifera-Acanthocephala clade are also specific with respect to sperm ultrastructure, as far as spermatozoa are produced at all (only females are known for bdelloid rotifers). In particular, the flagellum inserts at the anterior pole of the sperm head, instead of at its rear end (Ahlrichs 1997; Ferraguti and Melone 1999).
Molecular studies suggest that Rotifera in the traditional understanding (Monogononta, Bdelloidea, Seisonidea) represents a paraphyletic assemblage. In particular, bdelloids appear to be closer related to acanthocephalans than to monogononts (e.g., Near et al. 1998; Near 2002; García-Varela and Nadler 2006; Witek et al. 2008). Even closer related to acanthocephalans could be seisonids (Fig. 8.7). A seisonid-acanthocephalan sister-group relationship gains support from part of the molecular studies, whereby the choice of the substitution model seems to be crucial for its recognition (Wey-Fabrizius et al. 2014; Supplementary Figure S1 to Laumer et al. 2015; also Herlyn et al. 2003). Mitochondrial gene order additionally accords with a monophyletic origin of Seisonidea and Acanthocephala (Sielaff et al. 2016). A grouping of Seisonidea and Acanthocephala (Pararotatoria) is further in line with morphological data, whereby some evolutionary novelties refer to the tegument, again. In particular, the already-mentioned infoldings of the distal plasma membrane widen inside the tegument to larger caverns in seisonids and acanthocephalans. This characteristic has obviously undergone expansion in the stem line of crown-Acanthocephala, towards the already mentioned lacunar system (Fig. 8.6c). The tegument of seisonids and acanthocephalans is further distinguished by containing larger filament bundles (Ahlrichs 1997). The third evolutionary novelty in support of monophyletic Seisonidea-Acanthocephala relates to ultrastructural details of the spermatozoa again, which in both taxa have two rows of electron-dark bodies that accompany the anterior portion of the sperm flagellum (Ahlrichs 1997, 1998). Furthermore, there is considerable similarity in the cytomorphology of spermatogenesis states between seisonids and acanthocephalans (Marchand and Mattei 1976; Ferraguti and Melone 1999). In contrast, there seems to be no morphological feature that could represent an evolutionary novelty of a clade comprising acanthocephalans and bdelloids only (Ricci 1998), although such grouping re-occurs in part of the molecular analyses (e.g., García-Varela and Nadler 2006). Summing up all evidence, a plausible tree topology for Syndermata or Rotifera (inclusively Acanthocephala) appears to be: (Monogononta, (Bdelloidea, (Seisonidea, Acanthocephala))) (Fig. 8.7). This phylogenetic hypothesis provides the backbone for the inference of a scenario for the evolution of the endoparasitic two-host cycle in the stem line of crown-Acanthocephala in the next section.
8.7 Evolution of Acanthocephalan Endoparasitism: A Conditional Hypothesis
Given the life styles of extant species, the last common ancestors (LCAs) of crown-Gnathostomulida, crown-Micrognathozoa, crown-Monogononta, and crown-Bdelloidea were most probably free-living (e.g., Near et al. 1998; Kristensen and Funch 2000; Sterrer and Sørensen 2015). The same should apply to the LCAs of crown-Gnathifera and crown-Rotifera/Syndermata. The LCA of crown-Hemirotifera, from which Bdelloidea, Seisonidea, and Acanthocephala evolved, should also have been free-living. Lifestyles of monogonont, bdelloid and seisonid LCAs may be regarded as semi-sessile (compare Ahlrichs and Riemann 2019) but is herein referred to as free-living because the animals can easily detach from their substrates. However, while the substrate of monogononts and bdelloids is variable, the LCA of seisonids probably lived on mandibulates, possibly also from such hosts, just as the extant species of the group are doing or are assumed to do (e.g., Sørensen et al. 2005; see also Fontaneto and de Smet 2015). Lastly, the endoparasitic two-host cycle of all extant Acanthocephala should be a heritage of their LCA.
When matching the character states presumed for single LCAs with the phylogenetic tree in Fig. 8.7, it is the most parsimonious to postulate a shift from free-living to an epibiotic (epizoic) or ectoparasitic lifestyle on mandibulate arthropods for the branch uniting Seisonidea and Acanthocephala. Presumably, a second shift in the way of living followed on the acanthocephalan stem line, namely towards an endoparasitic lifestyle with mandibulates as hosts. In a third step, the acanthocephalan life cycle was expanded by upward-inclusion of gnathostomes as hosts (Conway Morris and Crompton 1982; Herlyn et al. 2003; Wey-Fabrizius et al. 2014; Sielaff et al. 2016). If the steps towards the two-host cycle of crown-Acanthocephala involved several host species or only a single one, each, has to remain unanswered. Nonetheless, several up to many individuals of at least one host species should have been the ground on which each of the evolutionary steps took place. In this sense, at least, the use of plural seems appropriate in respect to acanthocephalan hosts.
The stepwise establishment of a two-host cycle should have taken place in an aquatic environment. This is indeed very likely considering that extant chaetognaths, gnathostomulids, micrognathozoans, monogononts, bdelloids, and seisonids live in aquatic environments and many acanthocephalans use aquatic hosts. In turn, terrestrial life cycles as in extant archiacanthocephalans and some palaeacanthocephalan species should reflect secondary changes (Near et al. 1998). Yet, it is less clear whether the LCA of crown-acanthocephalans used marine or freshwater species as hosts since both alternatives occur in extant thorny-headed worms and their closer phylogenetic relatives (Ax 2001; Petrochenko 1956, 1958; Kristensen and Funch 2000; Fontaneto and de Smet 2015; Sterrer and Sørensen 2015). However, if seisonids retained not only an epibiotic/ectoparasitic lifestyle from their LCA with acanthocephalans but also the exploitation of marine crustaceans, acanthocephalan evolution should also have begun in a marine environment.
The establishment of the acanthocephalan two-host cycle was evidently accompanied by the evolution of several morphological novelties such as an invertible proboscis, a particular muscular apparatus suspending the cerebral ganglion, absence of an alimentary tract, a re-organized tegument and specialties in the reproductive systems of both sexes (Dezfuli et al. 2001; Near 2002; Herlyn and Röhrig 2003; Herlyn and Taraschewski 2017, etc.). Early stem-acanthocephalans, however, should have displayed the plesiomorphic alternatives. In particular, they presumably did not reach the body sizes known from extant species (see, e.g., compilation in Petrochenko 1956, 1958). According to body sizes in extant gnathostomulids, micrognathozoans, monogononts, bdelloids and seisonids, adults of ancient thorny-headed worms have presumably measured in the range of less than one to a few limiting their fossilisation potential. Subsequent increases of body size along with the evolution of gigantism in “invertebrate” host lineages can not be ruled out (Klug et al. 2015). However, if evolution took such a path it unlikely happened in the stem line of crown-acanthocephalans. With respect to this lineage, the presumed upward-inclusion of gnathostomes into the life cycle more likely paved the way for larger body sizes. In extension of this argument, it was found for extant acanthocephalans that size is positively correlated with body mass of their vertebrate hosts (Poulin et al. 2003). Another positive correlate was the temperature regime imposed by the host. In particular, adult acanthocephalans were found to grow to larger body sizes in endothermic than ectothermic hosts. It is to be said, however, that both effects largely disappeared when correlation analyses included a correction against possible phylogenetic bias.
8.8 Acanthocephala and Gnathifera: Fossil Report and Time Line
The following estimates of the time line of gnathiferan and especially acanthocephalan evolution have as a condition that the phylogenetic and temporal assignment of fossils is at least approximately correct. This especially applies to the dating of the earliest appearance of potential hosts (for a discussion, see De Baets and Littlewood 2015; Warnock and Engelstädter 2021). In Gnathifera , another obstacle is that fossil evidence is comparably sparse. In fact, there seems to be only one report on fossilized eggs attributed to Acanthocephala, from the Upper Cretaceous (see above). All other ancient acanthocephalan eggs are remains of several hundred to about 12,000 years (Table 8.1). In addition, only few fossil wheel animals have been found so far, i.e., a monogonont from Eocene North Maslin Sands in South Australia (Southcott and Lange 1971) and bdelloids in Dominican amber from the Miocene (Poinar and Ricci 1992; Waggoner and Poinar 1993; Iturralde-Vinent and MacPhee 1996). However, Eocene and Miocene fossils almost certainly do not shed light on the emergence of Rotifera-Acanthocephala. In fact, if the already-mentioned Cambrian species I. fellatus really belongs to Gnathifera, the stem line of Rotifera-Acanthocephala may go back to the Early Palaeozoic (Cong et al. 2017). An Early Paleozoic origin of Gnathifera and Rotifera-Acanthocephala would receive additional confirmation if arrow worms (Chaetognatha) really are sister to or occupy a nested position within Gnathifera (Fig. 8.7; Fröbius and Funch 2016; Marlétaz et al. 2019; Vinther and Parry 2019). Thus, well preserved fossils of arrow worms are documented from the Cambrian Chengjiang Lagerstätte in China and from the Burgess Shale in Canada (Shu et al. 2017; Briggs and Caron 2017), and a potential stem chaetognath, Amiskwia sagittiformis, also lived in the Cambrian (Vinther and Parry 2019).
Due to the lack of direct evidence, time estimates regarding acanthocephalan evolution have to rely on the appearance of mandibulate and gnathostome hosts in the fossil record (compare De Baets et al. 2015). In particular, the presumed ancestors of Seisonidea and Acanthocephala should not have lived on mandibulates prior to the emergence of such hosts in the Cambrian (Daley et al. 2018). Probably, these hosts had a crustacean-like appearance or were crustaceans (see Zhang and Pratt 2012; Harvey et al. 2012). It might even be possible to narrow down the spectrum of first hosts to single taxa within Crustacea. Thus, extant seisonids live on Phyllocarida (Crustacea), especially on Leptostraca (Fontaneto and de Smet 2015; see also Sørensen et al. 2005), which emerged in the Permian according to the present knowledge. However, leptostracans very much resemble Cambrian-Carboniferous phyllocarids collectively called Archaeostraca (Collette and Hagadorn 2010). Consequently, ancient seisonids may already have lived on phyllocarids in the Paleozoic, and they might have retained this host usage from their LCA with acanthocephalans, just as they presumably kept the epibiotic/ectoparasitic lifestyle. If so, first stem-acanthocephalans should have exploited phyllocarids as well. But parasitization of other Paleozoic “invertebrates” by early acanthocephalans can also not be ruled out. Among them might have been species of Trilobita, the potential sister group of crown-Mandibulata (Scholtz and Edgecombe 2006).
Whether only phyllocarids or (also) other mandibulates or even trilobites were exploited in the beginning of acanthocephalan evolution will probably remain elusive. It will also be difficult if not impossible to assess more precisely when the presumed shift from an ecto- to an endoparasitic lifestyle might have taken place in acanthocephalan evolution. However, the establishment of a two-host cycle should post-date the emergence of fish-like gnathostomes in the Middle Ordovician (Sansom et al. 2015; also Janvier 2003) or later (Friedman and Sallan 2012; Brazeau and Friedman 2015; Klug et al. 2017). In case that early acanthocephalans also used fish-like vertebrates without jaws as definitive hosts, the two-host cycle could have been established even earlier. Such possibility can not be ruled out since extant acanthocephalans were occasionally reported from lampreys (Petromyzontida) (Petrochenko 1956; Conway Morris and Crompton 1982).
In any case, acanthocephalan diversity might subsequently have increased along with the diversification of crown-Gnathostomata upon extinction of placoderms in the Upper Devonian (Trinajstic et al. 2007; Sansom et al. 2015). When conquering new hosts, acanthocephalans may have benefited from generally less tight bonds to definitive than intermediate hosts (Conway Morris and Crompton 1982; Parker et al. 2015).
The emergence of the individual gnathostome taxa used as hosts provides an approximate orientation for the earliest possible origin of individual lineages within Acanthocephala. Thereby, life cycles involving tetrapods should have evolved from cycles with fish-like gnathostomes (Near 2002). Although not necessarily representing a suitable model for such transitions, it is worth noting that some of the extant acanthocephalan species exploit an aquatic intermediate host and a terrestrial definitive host (e.g., Dezfuli and Giari 1999). Either way, most of the extant species retained the ancestral condition of an aquatic cycle. Thus, life cycles in extant eoacanthocephalans involve sharks (Elasmobranchii, Selachii) and ray-finned fishes (Actinopterygii), in particular bowfin (Amiiformes) and teleost fishes, besides turtles (Petrochenko 1956; Near et al. 1998). Polyacanthocephalans also have aquatic life cycles, with teleost fishes and caimans (Crocodilia) serving as gnathostome hosts (Amin 1987; Echi et al. 2015). Since Polyacanthocephala either has a nested position inside Eoacanthocephala or is sister to Eoacanthocephala (Verweyen et al. 2011; Echi et al. 2015; Gazi et al. 2016), the LCAs of both taxa might already have used fish-like gnathostomes in the Middle Ordovician or later (see above, for references). Under the renewed assumption that the exploitation of fish-like gnathostomes represents the ancestral state, the origin of the palaecanthocephalan stem line could also go back to the Paleozoic. In fact, extant palaeacanthocephalans infect diverse fish-like gnathostomes, especially sharks and rays (Elasmobranchii, Batoidea), sturgeons (Acipenseriformes), and bowfin and teleost fishes in addition to Amphibia, Sauropsida and Mammalia (Petrochenko 1956, 1958; Near et al. 1998). As mentioned above, the taxon Archiacanthocephala is specific because the extant species have life cycles with terrestrial mammals and birds as definitive hosts (e.g., Near et al. 1998). If this definitive host spectrum reflects an association of early archiacanthocephalans with stem-Amniota, the transition to the usage of terrestrial definitive hosts should not have occurred much earlier than about 346–358 million years ago, which approximately marks the divergence of Amphibia and Amniota. However, if early archiacanthocephalans had originally exploited solely mammals and later on additionally conquered birds, the transition to a terrestrial cycle should postdate the divergence of Sauropsida and Mammalia 297–326 million years ago (split estimates according to timetree.org). The age of fossil archiacanthocephalan eggs obtained from an Upper Cretaceous coprolite (Cardia et al. 2019) is in line with both possibilities.
Above estimates on the emergence of individual taxa contrast to some degree with phylogenetic reconstructions suggesting a closer relationship of Eoacanthocephala (inclusively Polyacanthocephala) to Palaeacanthocephala than Archiacanthocephala (e.g., García-Varela and Nadler 2005; Verweyen et al. 2011), thus giving the following tree: (Archiacanthocephala, (Palaeacanthocephala, Eoacanthocephala)). In fact, such a phylogeny implicates that the stem line of archiacanthocephalans goes back further in time than the stem lines of palaeacanthocephalans and eoacanthocephalans. However, archiacanthocephalans could have obtained an aquatic life cycle long before a terrestrial cycle was established. In any case, some inconsistency between the distribution of traits and phylogenetic trees is not uncommon in acanthocephalan research: The presence or absence of lateral sensory organs (see above), for example, can only be aligned with the aforementioned phylogeny if one assumes their secondary loss within Palaeacanthocephala (Weber et al. 2013). The complex situation is also reflected in the naming of the three major acanthocephalan taxa: The prefixes palae- (old, ancient or primitive), eo- (earliest), and archi- (primary) all express the view that the respective taxon combines to a remarkable extent ancient characters (compare Meyer 1932; Van Cleave 1936).
8.9 Cambroclavida: Microfossils of Questionable Acanthocephalan Affiliation
Certain Cambrian sclerites were repeatedly regarded as acanthocephalan remains (Qian and Yin 1984; Amin 2013). These fossils are collectively referred to as Cambroclavida and actually are of unclear phylogenetic affiliation (Clausen and Álvaro 2006; Kouchinsky et al. 2012). The microfossils were discovered in peri-Gondwanan deposits of China and Europe, amongst others (e.g., Elicki and Wotte 2003). Part of them is remotely reminiscent of the hooks of extant acanthocephalans (Fig. 8.5): They show hook-like recesses that emerge from a basis that might appear similar to the root of an acanthocephalan hook. On the other hand, the basis of the cambroclavid sclerites is hollow (Fig. 8.5d), which is not the case in the roots of acanthocephalan hooks (Fig. 8.6a, b). Moreover, the hooks of extant acanthocephalans are imbedded into the basement membrane underlying the tegument (Fig. 8.6a, b). In addition, even if the fibers of basement membrane and hook roots should be discontinuous (c.f. Taraschewski et al. 1989), it remains uncertain whether fossil remains of acanthocephalan hooks would present as isolated units as it is usually the case in cambroclavid microfossils. Furthermore, hooks of extant acanthocephalan species are more or less recurved, while this is not the case in at least part of the cambroclavid microfossils (Figs. 8.5 and 8.6a). Most of all, the size of cambroclavid fossils does not accord with the expectation for early acanthocephalans, which should have remained much smaller than the extant species (see above). For example, the maximum extension of Cambroclaves fossils can easily reach >500 μm (Elicki and Wotte 2003). Recalling that early stem-acanthocephalans should have measured in the range of one or few millimeters, hooks of cambroclavid dimension would appear huge. Not least, the question arises why acanthocephalan hooks—if cambroclavid microfossils are such—occur in peri-Gondwanan deposits, whereas they have not been found in any other context so far. Thus, a closer affinity of cambroclavids to acanthocephalans seems unlikely at present.
8.10 Conclusions
Analyses of molecular and morphological data have shown that the taxon Acanthocephala (thorny-headed worms) has a nested position inside Gnathifera, a clade that also includes Gnathostomulida, Micrognathozoa and Rotifera. Especially, Rotifera appears to be a paraphyletic assemblage as long as Acanthocephala is excluded (Fig. 8.7). In addition, arrow worms (Chaetognatha) seem to belong to the kinship of the Gnathifera. In support of this possibility, recent studies suggest that arrow worms, for which Cambrian fossils are known (Shu et al. 2017; Briggs and Caron 2017), are either sister to Gnathifera or occupy a nested position within the gnathiferan clade (Fröbius and Funch 2016; Marlétaz et al. 2019; Vinther and Parry 2019). Beyond that, Cambrian fossils have been attributed to Gnathifera (Caron and Cheung 2019; Vinther and Parry 2019), some of which might even have been epibionts or ectoparasites (Cong et al. 2017). Accordingly, a Cambrian origin of Gnathifera is likely.
With regard to the Rotifera-Acanthocephala group, the temporal origin is less clear. In fact, the oldest known fossils of Rotifera (inclusively Acanthocephala) or Syndermata, as the group is also called, are acanthocephalan eggs from an Upper Cretaceous coprolite (Cardia et al. 2019). Before this recently published finding, only few remains of monogonont and bdelloid rotifers from the Eocene and Miocene were known (Southcott and Lange 1971; Poinar and Ricci 1992; Waggoner and Poinar 1993; Iturralde-Vinent and MacPhee 1996). On the other hand, estimates for the appearance of mandibulates and gnathostomes enable rough time constraints for the earliest possible associations with members of these taxa. In particular, the postulated first epibiotic or ectoparasitic association with mandibulates in the common stem line of seisonids and acanthocephalans can not have occurred prior to the emergence of jawed arthropods in the Cambrian (Daley et al. 2018). Such a one-host cycle was probably passed on to the seisonid and acanthocephalan lineages, followed by a change from living on to living in mandibulates in acanthocephalan evolution (Herlyn et al. 2003; Wey-Fabrizius et al. 2014; Sielaff et al. 2016). Likewise, the presumed upward-inclusion of gnathostomes into the acanthocephalan life cycle should not have occurred prior to the emergence of corresponding hosts in the Middle Ordovician (Sansom et al. 2015; also Janvier 2003) or later (Brazeau and Friedman 2015; Klug et al. 2017). Although we cannot be sure whether evolution has taken the path outlined, the following appears to be more certain: The LCA of crown-acanthocephalans probably showed an obligate two-host cycle involving mandibulates and gnathostomes as intermediate and definitive hosts, respectively (Fig. 8.1). Extensions of this two-host cycle by paratenic and second definitive hosts could have occurred subsequently.
The presumed one-host-cycle in early acanthocephalan evolution implicates that adult worms should have differed considerably with respect to morphology, when compared to the adults in extant species. In particular, early acanthocephalans should not have grown to body sizes as known from extant species. A marked increase in body size rather followed the upward-inclusion of gnathostomes as hosts. Several other evolutionary novelties should also have evolved along with the two-host cycle. Especially, metamorphosis of the larval stage inside the mandibulate intermediate host (acanthor) to a young adult (acanthella) is obviously a developmental correlate of the two-host cycle (compare Meyer 1932). A hooked proboscis and a muscular apparatus suspending the cerebral ganglion (receptacle and receptacle-surrounding muscle) likely evolved in the same context. Likewise, traits that are related to an increase in fecundity (large testes, fragmented ovaries, uterine bell, etc.) should have emerged in the stem line of crown-acanthocephalans, along with the establishment of a two-host cycle (Herlyn and Röhrig 2003; Poulin and Morand 2000; Parker et al. 2015). However, there might also be characters in extant acanthocephalans that already existed in the supposed one-host stage (Sielaff et al. 2016). In particular, a digestive tract might then already have been lacking as suggested by its absence in all developmental stages of the extant species (compare Near et al. 1998; Wey-Fabrizius et al. 2014). Correspondingly, morphological and physiological changes that enable nutrient uptake via the tegument at least in part occurred prior to the establishment of a two-host cycle (Mauer et al. 2020).
Eggs are the only free propagules in the life cycles of the extant acanthocephalan species (Figs. 8.1 and 8.2). They are also the sole ancient remains of acanthocephalans known to date. This probably reflects their enhanced preservability due to the incorporation of keratin and, depending on the taxon, chitin (Whitfield 1973; Peters et al. 1991; Taraschewski and Peters 1992; Taraschewski et al. 1992). The ancient eggs discovered so far have most likely an archiacanthocephalan origin, as suggested by their size and the increased thickness and structure of their shells (Table 8.1 and references therein). In most of the cases, the eggs were retrieved from human, carnivoran and xenarthran coprolites of several hundred to about 12,000 years. However, there seems to be no reason why gnathostome vertebrates feeding on intermediate, paratenic, or definitive hosts should not have been infected tens or hundreds of thousands or millions of years ago. In line with this, a coprolite from the Upper Cretaceous of Brazil was recently found to contain remains reminiscent of archiacanthocephalan eggs (Cardia et al. 2019). The defecating animal might have been a member of Crocodyliformes but various extinct predators such as ichthyosaurs as well as taxa with extant species like sharks might also have been infected by ancient acanthocephalans.
Though nothing corresponding has been found or recognized so far, acanthocephalan hooks should be the prime candidates for preservation, besides eggs (Figs. 8.4, 8.5a, b and 8.6a, b). They are rather rigid and undergo sclerotization, as reported for some of the extant species (Taraschewski 1989a, b). The hooks might still be in position in the hypothetical ideal fossil of an acanthocephalan, such that their arrangement gives the contour of the proboscis. However, disaggregated acanthocephalan hooks might also be contained in the fossil record. If so, it will be anything but trivial to distinguish them from fossil hooks of other endoparasites (see, e.g., Plate IV in Lambl 1859). Either way, acanthocephalan hooks might once be detected in the abdomen and especially in the intestine of fossilized gnathostomes. In addition, presence of a structure covering the hind end of a fossil endoparasite could be an indication of a female acanthocephalan (Fig. 8.4a): Such a structure would correspond to the copulatory cap, which males of extant acanthocephalans produce from the secretion of their cement gland(s) (Dezfuli et al. 2001). Obviously, such excellent preservation of an acanthocephalan is not too likely.
The chance for preservation of acanthocephalan soft-tissue is certainly smaller than for comparably rigid structures such as eggs, hooks and copulatory cap. Still, it can not be ruled out, not even for the tegument. Actually, the tegument (also integument or epidermis) of extant acanthocephalans is rather resistant to mechanical damage and enzymatic digestion, which probably is due to its syncytial organization and a presumably proteinaceous lamina inside (e.g., Díaz Cosín 1972; Graeber and Storch 1978; Ahlrichs 1997; Herlyn and Ehlers 2001). Provided that a corresponding remain will ever be found, an increase in body diameter behind the hooked attachment organ could indicate the presoma-metasoma transition. The ideal acanthocephalan fossil might also show a tegument cone (apical epidermis cone) in the anterior proboscis section as known from extant members of Eoacanthocephala and Polyacanthocephala (Figs. 8.4b, 8.5b and 8.6a). Subtegumental sensory structures of the presoma or the muscular apparatus suspending the cerebral ganglion (receptacle plus receptacle protrusor) might also be discernable in the ideal, though still hypothetical, fossil of an acanthocephalan worm (Figs. 8.4, 8.5a and 8.6c, d). All this may seem unlikely, but the preservation of fragile structures such as muscles and the nervous system is possible per se, even in worm-like organisms (Parry et al. 2018).
References
Aguiar LS, de Oliveira MIB, de Matos LV, Gomes ALS, da Costa JI, da Silva GS (2018) Distribution of the acanthocephalan Neoechinorhynchus buttnerae and semiquantitative analysis of histopathological damage in the intestine of tambaqui (Colossoma macropomum). Parasitol Res 117:1689–1698
Ahlrichs WH (1997) Epidermal ultrastructure of Seison nebaliae and Seison annulatus, and a comparison of epidermal structures within the Gnathifera. Zoomorphology 117:41–48
Ahlrichs WH (1998) Spermatogenesis and ultrastructure of the spermatozoa of Seison nebaliae (Syndermata). Zoomorphology 118:255–261
Ahlrichs WH, Riemann O (2019) Seisonidae. In: Schmidt-Rhaesa A (ed) Handbook of zoology, Gastrotricha and Gnathifera. De Gruyter, Berlin
Altner M, Reichenbacher B (2015) †Kenyaichthyidae fam. nov. and †Kenyaichthys gen. nov. - first record of a fossil aplocheiloid killifish (Teleostei, Cyprinodontiformes). PLoS One 29:e0123056
Amin OM (1987) Key to the families and subfamilies of Acanthocephala, with the erection of a new class (Polyacanthocephala) and a new order (Polyacanthorhynchida). J Parasitol 73:1216–1219
Amin OM (2013) Classification of the Acanthocephala. Folia Parasitol 60:273–305
Amin OM, Heckmann R (2017) Rhadinorhynchus oligospinosus n. sp. (Acanthocephala, Rhadinorhynchidae) from mackerels in the Pacific Ocean off Peru and related rhadinorhynchids in the Pacific, with notes on metal analysis. Parasite 24:19
Amin OM, Heckman RA, Yudhanto S (2013) The finding of Mediorhynchus gallinorum (Acanthocephala: Gigantorhynchidae) in chickens from Indonesia, with expanded description using SEM. Comp Parasitol 80:39–46
Araújo A, Reinhard K, Ferreira LF (2015) Palaeoparasitology – human parasites in ancient material. Adv Parasitol 90:349–387
Ax P (2001) Das System der Metazoa III. Spektrum Akademischer Verlag, Heidelberg
Bakker TCM, Frommen JG, Thünken T (2017) Adaptive parasitic manipulation as exemplified by acanthocephalans. Ethology 2017:1–6
Beltrame MO, Fernández FJ, Sardella NH (2015) First paleoparasitological record of acanthocephalan eggs from Northwestern Patagonia (Late Holocene, Argentina). Acta Trop 146:33–35
Beltrame MO, Bellusci A, Fernández FJ, Sardella NH (2018) Carnivores as zoonotic parasite reservoirs in ancient times: the case of the Epullán Chica archaeological cave (Late Holocene, Northwestern Patagonia, Argentina). Archaeol Anthropol Sci 10:795–804
Brazeau MD, Friedman M (2015) The origin and early phylogenetic history of jawed vertebrates. Nature 520:490–497
Briggs DEG, Caron J-B (2017) A large Cambrian chaetognath with supernumerary grasping spines. Curr Biol 27:2536–2543
Camacho M, Pessanha T, Leles D, Dutra JMF, Silva R, de Souza SC, Araújo A (2013) Lutz’s spontaneous sedimentation technique and the paleoparasitological analysis of sambaqui (shell mound) sediments. Mem Inst Oswaldo Cruz 108:155–159
Camacho M, Araújo A, Morrow J, Buikstra J, Reinhard K (2018) Recovering parasites from mummies and coprolites: an epidemiological approach. Parasit Vectors 11:248
Cardia DFF, Bertini RJ, Camossi LG, Letizio LA (2019) First record of Acanthocephala parasites eggs in coprolites preliminary assigned to Crocodyliformes from the Adamantina Formation (Bauru Group, Upper Cretaceous), São Paulo, Brazil. An Acad Bras Cienc 91(Suppl 2):e20170848
Caron J-B, Cheung B (2019) Amiskwia is a large Cambrian gnathiferan with complex gnathostomulid-like jaws. Commun Biol 2:164
Chin K (2021) Gastrointestinal parasites of ancient non-human vertebrates: evidence from coprolites and other materials. In: De Baets, K., Huntley, J. W. (eds). The evolution and fossil recordof Parasitism: Coevolution and paleoparasitological techniques. Topics in Geobiology 50
Choi C-J, Lee H-J, Go J-H, Park Y-K, Chai J-Y, Seo M (2010) Extraintestinal migration of Centrorhynchus sp. (Acanthocephala: Centrorhynchidae) in experimentally infected rats. Korean J Parasitol 48:139–143
Clausen S, Álvaro JJ (2006) Skeletonized microfossils from the Lower–Middle Cambrian transition of the Cantabrian Mountains, northern Spain. Acta Palaeontol Pol 51:223–238
Collette JH, Hagadorn JW (2010) Early evolution of phyllocarid arthropods: phylogeny and systematics of Cambrian–Devonian archaeostracans. J Paleontol 84:795–820
Cong P, Ma X, Williams M, Siveter DJ, Siveter DJ, Gabbot SE, Zhai D, Goral T, Edgecombe GD, Hou X (2017) Host-specific infestation in early Cambrian worms. Nat Ecol Evol 1:1465–1469
Conway Morris S, Crompton DWT (1982) The origins and evolution of the Acanthocephala. Biol Rev 57:85–115
Daley AC, Antcliffe JB, Drage HB, Pates S (2018) Early fossil record of Euarthropoda and the Cambrian explosion. Proc Natl Acad Sci U S A 115:5323–5331
Dartnall H (1995) The rotifers of Heard island: preliminary survey with notes on other freshwater groups. Pap Proc R Soc Tasmania 129:7–15
de Almeida Souza A, Sobestiansky J, Linhares GFC, de Oliveira WP, de Barros Araújo JL (2006) Surto de Macracantorrincose em queixada (Tayassu pecari) criado extensivamente nos arredores de Goiânia – Estado de Goiás. Patologia 34:213–220
De Baets K, Littlewood TJ (2015) The importance of fossils in understanding the evolution of parasites and their vectors. In: De Baets K, Littlewood TJ (eds) Advances in parasitology 90: fossil parasites. Academic, Heidelberg
De Baets K, Dentzien-Dias PC, Upeniece I, Verneau O, Donoghue PCJ (2015) Constraining the deep origin of parasitic flatworms and host-interactions with fossil evidence. Advances in Parasitology 90:93–135.
De Baets K, Dentzien-Dias P, Harrison GWM, Littlewood DTJ, Parry LA (2021) Fossil Constraints on the Timescale of Parasitic Helminth Evolution. In: De Baets K, Huntley JW (eds) The evolution and fossil Record of Parasitism: Identification and macroevolution of parasites. Topics in Geobiology 49
de Beauchamp P (1909) Recherches sur les Rotifères: les formations tégumentaires et l’appareil digestif. Arch Zool Exp Gén 10(4):1–410
de Matos LV, de Oliveira MIB, Gomes ALS, da Silva GS (2017) Morphological and histochemical changes associated with massive infection by Neoechinorhynchus buttnerae (Acanthocephala: Neoechinorhynchidae) in the farmed freshwater fish Colossoma macropomum Cuvier, 1818 from the Amazon State, Brazil. Parasitol Res 116:1029–1037
de Souza MV, Chame M, Mendonça de Souza SMF, Daltrini Felice G, Guidon N, Sianto L (2020) New parasite occurences in Tamandua tetradactyla (Pilosa: Myrmecophagidae) in the Northeast of Brazil: a paleoparasitological study. Oecologia autralis 24:141–153
Dentzien-Dias PC, Poinar G, de Figueiredo AE, Pacheco AC, Horn BL, Schultz CL (2013) Tapeworm eggs in a 270 million-year-old shark coprolite. PLoS One 8:e55007
Dezfuli BS, Giari L (1999) Amphipod intermediate host of Polymorphus minutus (Acanthocephala), parasite of water birds, with notes on ultrastructure of host-parasite interface. Folia Parasitol 46:117–122
Dezfuli BS, Simoni E, Mischiati C (2001) The cement apparatus of larval and adult Acanthocephalus anguillae (Acanthocephala), with notes on the copulatory cap and origin of gland secretion. Parasitol Res 87:299–305
Dezfuli BS, Bo T, Lorenzoni M, Shinn AP, Giari L (2015) Fine structure and cellular responses at the host–parasite interface in a range of fish–helminth systems. Vet Parasitol 208:272–279
Díaz Cosín DJ (1972) La pared del cuerpo de Macracanthorhynchus hirudinaceus. Bol R Soc Española Hist Nat 70:239–270
Đikanović V, Nikolić V, Simić V, Jakovčev-Todorović D, Cakić P (2010) The intestinal parasite Pomphorhynchus laevis Müller, 1776 (Acanthocephala) from Barbel Barbus Barbus L. from the Danube river in the area of Belgrade. BALWOIS 2010
Dingley D, Beaver PC (1985) Macracanthorhynchus ingens from a child in Texas. Am J Trop Med Hyg 34:918–920
Dunagan TT, Miller DM (1980) Macracanthorhynchus hirudinaceus from swine: an eighteen-year record of Acanthocephala from Southern Illinois. Proc Helminthol Soc Wash 47:33–36
Dunagan TT, Miller DM (1991) Acanthocephala. In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates 4: Aschelminthes. Wiley-Liss, New York
Echi PC, George S, Kumar S (2015) Differential size-biased parasitism between Polyacanthorhynchus nigerianus (nomen nudum) and Polyacanthorhynchus echiyensis (nomen nudum) (Acanthocephala: Polyacanthocephala). Braz J Biol Sci 3:113–119
Edmondson WT (1965) Reproductive rate of planktonic rotifers as related to food and temperature in nature. Ecol Monogr 35:61–111
Elicki O, Wotte T (2003) Cambroclaves from the Cambrian of Sardinia (Italy) and Germany: constraints for the architecture of western Gondwana and the palaeogeographical and palaeoecological potential of cambroclaves. Palaeogeogr Palaeoclimatol Palaeoecol 195:55–71
Ferraguti M, Melone G (1999) Spermiogenesis in Seison nebaliae (Rotifera, Seisonidea): further evidence of a rotifer-acanthocephalan relationship. Tiss Cell 31:428–440
Ferreira LF, de Araújo AJ, Confalonieri UE (1983) The finding of helminth eggs in a Brazilian mummy. Trans R Soc Trop Med Hyg 77:65–67
Ferreira LF, Araújo A, Confalonieri U, Chame M (1989) Acanthocefalan eggs in animal coprolites from archeological sites from Brazil. Mem Inst Oswaldo Cruz 84:201–203
Fontaneto D, de Smet W (2015) Rotifera. In: Schmidt-Rhaesa A (ed) Handbook of zoology, Gastrotricha and Gnathifera. De Gruyter, Berlin. (electronic resource)
Friedman M, Sallan LC (2012) Five hundred million years of extinction and recovery: a phanerozoic survey of large-scale diversity patterns in fishes. Palaeontology 55:707–742
Fröbius AC, Funch P (2016) Rotiferan Hox genes give new insights into the evolution of metazoan bodyplans. Nat Commun 8:9
Fugassa MH, Reinhard KJ, Johnson KL, Gardner SL, Vieira M, Araújo A (2011) Parasitism of prehistoric humans and companion animals from antelope cave, Mojave County, Northwest Arizona. J Parasitol 97:862–867
García-Varela M, Nadler SA (2005) Phylogenetic relationships of Palaeacanthocephala (Acanthocephala) inferred from SSU and LSU rDNA gene sequences. J Parasitol 91:1401–1409
García-Varela M, Nadler SA (2006) Phylogenetic relationships among Syndermata inferred from nuclear and mitochondrial gene sequences. Mol Phylogenet Evol 40:61–72
Gassó D, Serrano E, Castillo-Contreras R, Aguilar XF, Cadena AC, Velarde R, Mentaberre G, López-Olvera JR, Risco D, Gonçalves P, Lavín S, Fernandez-Llário P, Segalés J, Ferrer D (2016) Coprological tests underestimate Macracanthorhynchus hirudinaceus burden in wild boar. Parasitol Res 115:2103–2105
Gautam NK, Mohapatra A, Saxena AM (2018) On a new species of Neoechinorhynchus Hamann, 1892 (Neoechinorhynchinae Travassos, 1926) from Eleutheronema tetradactylum (Shaw, 1804) from Digha coast, West Bengal, India. J Parasit Dis 42:462–466
Gautam NK, Misra PK, Saxena AM (2019) Four new species of the genus Pallisentis (Quadrigyridae, Van Cleave, 1920) from freshwater fish in Uttar Pradesh, India. Acta Parasitol 15.
Gazi M, García-Varela M, Park C, Littlewood DTJ, Park JK (2016) Mitogenomic phylogeny of Acanthocephala reveals novel class relationships. Zool Scripta 45:437–454
Gee RJ (1987) A comparative morphological study of the stutzzelle (support cell) in the phylum Acanthocephala. Can J Zool 65:660–668
Gibson DI, Bray RA, Hunt D, Georgiev BB, Scholz T, Harris PD, Bakke TA, Pojmanska T, Niewiadomska K, Kostadinova A, Tkach V, Bain O, Durette-Desset M-C, Gibbons L, Moravec F, Petter A, Dimitrova ZM, Buchmann K, Valtonen ET, de Jong Y (2014) Fauna europaea: helminths (animal parasitic). Biodivers Data J 2:e1060
Goater TG, Goater CP, Esch GW (2014) Parasitism – the diversity and ecology of animal parasites, 2nd edn. Cambridge University Press, Cambridge
Golvan YJ (1994) Nomenclature of the Acanthocephala. Res Rev Parasitol 54:135–205
Gomes AP, Olifiers N, Souza JG, Barbosa HS, D’Andrea PS, Maldonado A (2015) A new acanthocephalan species (Archiacanthocephala: Oligacanthorhynchidae) from the crab-eating fox (Cerdocyon thous) in the Brazilian pantanal wetlands. J Parasitol 101:74–79
Gonçalves ML, Araújo A, Ferreira LF (2003) Human intestinal parasites in the past: new findings and a review. Mem Inst Oswaldo Cruz 98(Suppl 1):103–118
Graeber K, Storch V (1978) Elektronenmikroskopische und morphometrische Untersuchungen am Integument der Acanthocephala (Aschelminthes). Z Parasitenkd 57:121–135
Grassi B, Calandruccio S (1888) Ueber einen Echinorhynchus, welcher auch in Menschen parasitirt und dessen Zwischenwirth ein Blaps ist. Centralbl Bakt u Parasitenk 3:521–525
Habdija I, Primc Habdija B, Radanović I, Špoljar M, Matoničkin Kepčija R, Vujčić Karlo S, Miliša M, Ostojić A, Sertić Perić M (2011) Protista - Protozoa; Metazoa – Invertebrata. Alfa d.d, Zagreb
Hammond RA (1966) The proboscis mechanism of Acanthocephalus ranae. J Exp Biol 45:203–213
Harada I (1931) Das Nervensystem von Bolbosoma turbinella (Dies.). Jpn J Zool 3:161–199
Harvey THP, Vélez MI, Butterfield NJ (2012) Exceptionally preserved crustaceans from western Canada reveal a cryptic Cambrian radiation. Proc Natl Acad Sci U S A 109:1589–1594
Herlyn H (2001) First description of an apical epidermis cone in Paratenuisentis ambiguus (Acanthocephala: Eoacanthocephala) and its phylogenetic implications. Parasitol Res 87:306–310
Herlyn H (2002) The musculature of the praesoma in Macracanthorhynchus hirudinaceus (Acanthocephala, Archiacanthocephala) – re-examination and phylogenetic significance. Zoomorphology 121:173–182
Herlyn H (2016) The phylogenetic system of primates – character evolution in the light of a consolidated tree. Org Divers Evol 16:689–713
Herlyn H (2017) Organization and evolution of the proboscis musculature in avian parasites of the genus Apororhynchus (Acanthocephala: Apororhynchida). Parasitol Res 116:1801–1810
Herlyn H, Ehlers U (1997) Ultrastructure and function of the pharynx of Gnathostomula paradoxa (Gnathostomulida). Zoomorphology 117:135–145
Herlyn H, Ehlers U (2001) Organisation of the praesoma in Acanthocephalus anguillae (Acanthocephala, Palaeacanthocephala) with special reference to the muscular system. Zoomorphology 121:13–18
Herlyn H, Röhrig H (2003) Ultrastructure and overall organization of ligament sac, uterine bell, uterus and vagina in Paratenuisentis ambiguus (Acanthocephala, Eoacanthocephala) – the character evolution within the Acanthocephala. Acta Zool 84:239–247
Herlyn H, Taraschewski H (2017) Evolutionary anatomy of the muscular apparatus involved in the anchoring of Acanthocephala to the intestinal wall of their vertebrate hosts. Parasitol Res 116:1207–1225
Herlyn H, Martini N, Ehlers U (2001) Organisation of the praesoma of Paratenuisentis ambiguus (Van Cleave, 1921) (Acanthocephala: Eoacanthocephala), with special reference to the lateral sense organs and musculature. Syst Parasitol 50:105–116
Herlyn H, Piskurek O, Schmitz J, Ehlers U, Zischler H (2003) The Syndermatan phylogeny and the evolution of acanthocephalan endoparasitism as inferred from 18S rDNA sequences. Mol Phylogenet Evol 26:155–164
Hernández-Orts JS, Montero FE, García NA, Crespo EA, Raga JA, García-Varela M, Aznar FJ (2019) Transmission of Corynosoma australe (Acanthocephala: Polymorphidae) from fishes to South American sea lions Otaria flavescens in Patagonia, Argentina. Parasitol Res 118:433–440
Horne PD (2002) First evidence of enterobiasis in ancient Egypt. J Parasitol 88:1019–1021
Hu S, Li Y, Luo H, Fu X, You T, Pang J, Liu Q, Steiner M (2008) New record of palaeoscolecids from the Early Cambrian of Yunnan, China. Acta Geol Sin 82:244–248
Hunt AP, Lucas SG, Milàn J, Spielmann JA (2012) Vertebrate coprolites. New Mex Mus Nat Hist Sci Bull 57:5–23
Iturralde-Vinent MA, MacPhee RDE (1996) Age and paleogeographical origin of Dominican amber. Science 273:1850–1852
Janvier P (2003) Vertebrate characters and the Cambrian vertebrates. C R Palevol 2:523–531
Jerônimo GT, de Pádua SB, de Andrade Belo MA et al (2017) Neoechinorhynchus buttnerae (Acanthocephala) infection in farmed Colossoma macropomum: a pathological approach. Aquaculture 469:124–127
Kaito S, Sasaki M, Goto K, Matsusue R, Koyama H, Nakao M, Hasegawa H (2019) A case of small bowel obstruction due to infection with Bolbosoma sp. (Acanthocephala: Polymorphidae). Parasitol Int 68:14–16
Kamimura K, Yonemitsu K, Maeda K, Sakaguchi S, Setsuda A, Varcasia A, Sato H (2018) An unexpected case of a Japanese wild boar (Sus scrofa leucomystax) infected with the giant thorny-headed worm (Macracanthorhynchus hirudinaceus) on the mainland of Japan (Honshu). Parasitol Res 117:2315–2322
Kassatkina P (2016) Arrow worms (Chaetognatha) from the Arctic Seas of Russia: five new species of the family Sagittidae from the Laptev Sea. Biol Bull 43:1184–1194
Kennedy CR (1999) Post-cyclic transmission in Pomphorhynchus laevis (Acanthocephala). Folia Parasitol 46:111–116
Kennedy CR (2006) Ecology of Acanthocephala. Cambridge University Press, Cambridge
Klug C, De Baets K, Kröger B, Bell MA, Korn D, Payne JL (2015) Normal giants? Temporal and latitudinal shifts of Palaeozoic marine invertebrate gigantism and global change. Lethaia 48:267–288
Klug C, Frey L, Pohle A, De Baets K, Korn D (2017) Palaeozoic evolution of animal mouthparts. Bull Geosci 92:511–524
Kocourek F (1877) Der Riesenkratzer (Echinorhynchus gigas) als Ursache einer seuchenartigen Sterblichkeit in einer Schweineherde. Oesterreichische Monatsschrift fuer Thierheilkunde mit Beruecksichtigung der Viehzucht und Landwirtschaft 2:89–93
Kogi E, Yaro CA, Mbah CE (2016) Occurrence of acanthocephalan, Moniliformis dubius in cockroach, Periplaneta americana and Blattella germanica in Zaria, Nigeria. World J Biol Med Sci 3:157–164
Kouchinsky A, Bengtson S, Runnegar B, Skovsted C, Steiner M, Vendrasco MJ (2012) Chronology of early Cambrian biomineralisation. Geol Mag 149:221–251
Kristensen RM, Funch P (2000) Micrognathozoa: a new class with complicated jaws like those of Rotifera and Gnathostomulida. J Morphol 246:1–49
Kumar P, Kumar P (2001) Phthirapteran insect and larval Acanthocephala from the late Triassic sediments of the Satpura basin, India. J Palaeontol Soc India 46:141–146
Lahmar S, Arfa I, Ben Othmen S, Jguirim W, Saïd Y, Dhibi A, Boufana B (2017) Intestinal helminths of stray dogs from Tunisia with special reference to zoonotic infections. Parasitol Open 3:1–9
Lambl VDF (1859) Mikroskopische Untersuchungen der Darm-Excrete. Beitrag zur Pathologie des Darms und Diagnostik am Krankenbette. Vrtljschr Prakt Heilk 16:1–58
Laumer CE, Bekkouche N, Kerbl A, Goetz F, Neves RC, Sørensen MV, Kristensen RM, Hejnol A, Dunn CW, Giribet G, Worsaae K (2015) Spiralian phylogeny informs the evolution of microscopic lineages. Curr Biol 25:2000–2006
Leung TLF (2017) Fossils of parasites: what can the fossil record tell us about the evolution of parasitism? Biol Rev 92:410–430
Leung TLF (2021) Parasites of fossil vertebrates: what we know and what can we expect from the fossil record? In: De Baets K, Huntley JW (eds) The evolution and fossil Record of Parasitism: Identification and macroevolution of parasites. Topics in Geobiology 49
Littlewood DTJ, Donovan SK (2003) Fossil parasites: a case of identity. Geol Today 19:136–142
Malta JDO, Gomes ALS, de Andrade SMS et al (2001) Massive infestation by Neoechinorhynchus buttnerae Golvan, 1956 (Eoacanthocephala: Neochinorhynchidae) in young “tambaquis” Colossoma macropomum (Cuvier, 1818) cultured in the Central Amazon. Acta Amazon 31:133–143
Marchand B, Mattei X (1976) La Spermatogenèse des Acanthocéphales. I. L’Appareil Centriolaire et Flagellaire au Cours de la Spermiogenèse d’Illiosentis furcatus var africana Golvan, 1956 (Palaeacanthocephala, Rhadinorhynchidae). J Ultrastruct Res 54:347–358
Mark Welch DB (2000) Evidence from a protein-coding gene that acanthocephalans are rotifers. Invert Biol 119:17–26
Marlétaz F, Peijnenburg KTCA, Goto T, Satoh N, Rokhsar DS (2019) A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans. Curr Biol 29:312–318.e3
Martins ML, de Moraes FR, Fujimoto RY et al (2001) Prevalence and histopathology of Neoechinorhynchus curemai Noronha, 1973 (Acanthocephala: Neoechinorhynchidae) in Prochilodus lineatus Valenciennes, 1836 from Volta Grande Reservoir, MG, Brazil. Braz J Biol 61:517–522
Mathison BA, Bishop HS, Sanborn CR, dos Santos Souza S, Bradbury R (2016) Macracanthorhynchus ingens infection in an 18-month-old child in Florida: a case report and review of Acanthocephaliasis in humans. Clin Infect Dis 63:1357–1359
Mauer K, Hellmann SL, Groth M et al (2020) The genome, transcriptome, and proteome of the fish parasite Pomphorhynchus laevis (Acanthocephala). PLoS One 15:e0232973
Mehlhorn H (2016) Human parasites – diagnosis, treatment, prevention. Springer, Heidelberg
Meyer A (1932) Acanthocephala. In: Bronn’s Klassen und Ordnungen des Tierreichs, vol 4. Akademische Verlagsgesellschaft, Leipzig
Miller DM, Dunagan TT (1985) Functional morphology. In: Crompton DWT, Nickol BB (eds) Biology of Acanthocephala. Cambridge University Press, Cambridge
Moore JG, Fry GF, Englert E (1969) Thorny-headed worm infection in North American prehistoric man. Science 163:1324–1325
Mowlavi G, Makki M, Heidari Z, Rezaeian M, Mohebali M, Araújo A, Boenke N, Abolfazl A, Stollner T, Mobedi I (2015) Macracanthorhynchus hirudinaceus eggs in canine coprolite from the Sasanian Era in Iran (4th/5th century CE). Iran J Parasitol 10:245–249
Murray J (1910) British Antarctic Expedition 1907–9, under the command of Sir E.H. Shackleton, c.v.o. Reports on the scientific investigations 1
Myers BJ, Kuntz RE (1972) A checklist of parasites and commensals reported for the chimpanzee (Pan). Primates 13:433–471
Near TJ (2002) Acanthocephalan phylogeny and the evolution of parasitism. Integr Comp Biol 41:668–677
Near TJ, Garey JR, Nadler SA (1998) Phylogenetic relationships of the Acanthocephala inferred from 18S ribosomal DNA sequences. Mol Phylogenet Evol 10:287–298
Nezamabadi M (2014) New contribution of paleoparasitology in the middle east and first data on the Iranian plateau adjacent area. Dissertation thesis, Université de Franche-Comté
Nielsen C (2012) Animal evolution – interrelationships of living phyla, 3rd edn. Oxford University Press, Oxford
Nikishin VP (2004) Subsurface musculature of spiny-headed worms (Acanthocephala) and its role in formation of intercellular matrix. Biol Bull 31:598–612
Noronha D, Ferreira LF, Rangel A, Araújo A, Gomes DC (1994) Echinopardalis (Acanthocephala, Oligacanthorhynchidae) eggs in felid coprolites dated from 9,000 years before present found in the Brazilian Northeast. Mem Inst Oswaldo Cruz 89:119–120
Novo SPC, Ferreira LF (2016) The paleoparasitology in Brazil and findings in human remains from South America: a review. Korean J Parasitol 54:573–583
Parker GA, Ball MA, Chubb JS (2015) Evolution of complex life cycles in trophically transmitted helminths. Host incorporation and trophic ascent. J Evol Biol 28:267–291
Parry LA, Smithwick F, Nordén KK, Saitta ET, Lozano-Fernandez J, Tanner AR, Caron JB, Edgecombe GD, Briggs DEG, Vinther J (2018) Soft-bodied fossils are not simply rotten carcasses – toward a holistic understanding of exceptional fossil preservation: exceptional fossil preservation is complex and involves the interplay of numerous biological and geological processes. BioEssays 40:1700167
Pavlović IN, Kulišić ZB, Tambur ZŽ, ProtiŽ NM (2010) Scarabidae – intermediate host for Macracanthorhynchus hirudinaceus. Zb Mat Srp Prir Nauke 119:89–95
Perry ML (1942) A new species of the acanthocephalan genus Filicollis. J Parasitol 28:385–388
Peters W, Taraschewski H, Latka I (1991) Comparative investigations of the morphology and chemical composition of the eggshells of Acanthocephala I. Macracanthorhynchus hirudinaceus (Archiacanthocephala). Parasitol Res 77:542–549
Petrochenko VI (1956) Acanthocephala of domestic and wild animals I. English translation from Russian published in 1971. Keter Press, Jerusalem
Petrochenko VI (1958) Acanthocephala of domestic and wild animals II. English translation from Russian published in 1971. Keter Press, Jerusalem
Pinacho-Pinacho CD, Sereno-Uribe AL, García-Varela M (2014) Morphological and molecular data reveal a new species of Neoechinorhynchus (Acanthocephala: Neoechinorhynchidae) from Dormitator maculatus in the Gulf of Mexico. Parasitol Int 63:763–771
Poinar GO, Ricci C (1992) Bdelloid rotifers in Dominican amber: evidence for parthenogenetic continuity. Experientia 48:408–410
Poulin R, Morand S (2000) Testes size, body size and male-male competition in acanthocephalan parasites. J Zool 250:551–558
Poulin R, Wise M, Moore J (2003) A comparative analysis of adult body size and its correlates in acanthocephalan parasites. Int J Parasitol 33:799–805
Qian Y, Yin G (1984) Zhijinitae and its stratigraphical significance. Acta Palaeontol Sin 23:216–223
Reinhard KJ (1990) Archaeoparasitology in North America. Am J Phys Anthropol 82:145–163
Reinhard KJ (2017) Reestablishing rigor in archaeological parasitology. Int J Paleopathol 19:124–134
Reinhard KJ, Bryant VM (1992) Coprolite analysis: a biological perspective on archaeology. Pap Nat Resour 46:245–288
Ricci C (1998) Are lemnisci and proboscis present in the Bdelloidea? Hydrobiologia 387/388:93–96
Richart R, Benirschke K (1963) Causes of death in a colony of marmoset monkeys. J Pathol Bacteriol 86:221–223
Rieger RM, Tyler S (1995) Sister-group relationship of Gnathostomulida and Rotifera-Acanthocephala. Invertebr Biol 114:186–188
Rota-Stabelli O, Daley AC, Pisani D (2013) Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr Biol 23:292–398
Sahar MA, Madani TA, Al Mohsen IZ, Almodovar EL (2006) A child with an acanthocephalan infection. Ann Saudi Med 26:321–324
Sanford SE (1978) Mortality in mute swans in Southern Ontario associated with infestation with the thorny-headed worm, Polymorphus boschadis. Can Vet J 19:234–236
Sansom RS, Randle E, Donoghue PCJ (2015) Discriminating signal from noise in the fossil record of early vertebrates reveals cryptic evolutionary history. Proc R Soc B 282:20142245
Sasaki M, Katahira H, Kobayashi M, Kuramochi T, Matsubara H, Nakao M (2019) Infection status of commercial fish with cystacanth larvae of the genus Corynosoma (Acanthocephala: Polymorphidae) in Hokkaido, Japan. Int J Food Microbiol 305:108256
Sayers K, Lovejoy CO (2014) Blood, bulbs, and bunodonts: on evolutionary ecology and the diets of Ardipithecus, Australopithecus, and early Homo. Q Rev Biol 89:319–357
Schmidt GD (1971) Acanthocephalan infections of man, with two new records. J Parasitol 57:582–584
Schmidt GD (1985) Development and life cycles. In: Crompton DWT, Nickol BB (eds) Biology of Acanthocephala. Cambridge University Press, Cambridge
Scholtz G, Edgecombe GD (2006) The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev Genes Evol 216:395–415
Shu D, Conway Morris S, Han J, Hoyal Cuthill JF, Zhang Z, Cheng M, Huang H (2017) Multi-jawed chaetognaths from the Chengjiang Lagerstätte (Cambrian, Series 2, Stage 3) of Yunnan, China. Palaeontology 60:763–772
Sianto L, Chame M, Silva CSP, Gonçalves MLC, Reinhard K, Fugassa M, Aráujo A (2009) Animal helminths in human archaeological remains: a review of zoonoses in the past. Rev Inst Med trop S Paulo 51:119–130
Sielaff M, Schmidt H, Struck TH, Rosenkranz D, Mark Welch DB, Hankeln T, Herlyn H (2016) Phylogeny of Syndermata (syn. Rotifera): mitochondrial gene order verifies epizoic Seisonidea as sister to endoparasitic Acanthocephala within monophyletic Hemirotifera. Mol Phylogenet Evol 96:79–92
Silva-Gomes AL, Gomes Coelho-Filho J, Viana-Silva W, Braga-Oliveira MI, Bernardino G, Costa JI (2017) The impact of Neoechinorhynchus buttnerae (Golvan, 1956) (Eoacanthocephala: Neochinorhynchidae) outbreaks on productive and economic performance of the tambaqui Colossoma macropomum (Cuvier, 1818), reared in ponds. Lat Am J Aquat Res 45:496–500
Sistiaga A, Mallol C, Galván B, Summons RE (2014) The Neanderthal meal: a new perspective using faecal biomarkers. PLoS One 9:e101045
Solórzano-García B, Pérez-Ponce de León G (2018) Parasites of Neotropical primates: a review. Int J Primatol 39:155–182
Sørensen MV, Segers H, Funch P (2005) On a new Seison Grube, 1861 from coastal waters of Kenya, with a reappraisal of the classification of the Seisonida (Rotifera). Zool Stud 44:34–43
Southcott RV, Lange RT (1971) Acarine and other microfossils from the Maslin Eocene, South Australia. Rec South Austr Mus 16:1–21
Sterrer W, Sørensen MV (2015) Gnathostomulida. In: Schmidt-Rhaesa A (ed) Handbook of zoology, Gastrotricha and Gnathifera. De Gruyter, Berlin
Struck TH, Wey-Fabrizius AR, Golombek A, Hering L, Weigert A, Bleidorn C, Klebow S, Iakovenko N, Hausdorf B, Petersen M, Kück P, Herlyn H, Hankeln T (2014) Platyzoan paraphyly based on phylogenomic data supports a noncoelomate ancestry of Spiralia. Mol Biol Evol 31:1833–1849
Sures B (2002) Competition for minerals between Acanthocephalus lucii and its definitive host perch (Perca fluviatilis). Int J Parasitol 32:1117–1122
Sures B, Franken M, Taraschewski H (2000) Element concentrations in the archiacanthocephalan Macracanthorhynchus hirudinaceus compared with those in the porcine definitive host from a slaughterhouse in La Paz, Bolivia. Int J Parasitol 30:1071–1076
Taraschewski H (1989a) Host-parasite interface of Paratenuisentis ambiguus (Eoacanthocephala) in naturally infected eel and in laboratory-infected sticklebacks and juvenile carp and rainbow trout. J Parasitol 75:911–919
Taraschewski H (1989b) Host-parasite interface of Neoechinorhynchus rutili (Eoacanthocephala) in naturally infected salmonids. J Fish Dis 12:39–48
Taraschewski H, Mackenstedt U (1991a) Autoradiographic and morphological studies on the uptake of the triglyceride [3H]-glyceroltrioleate by acanthocephalans. Parasitol Res 77:247–254
Taraschewski H, Mackenstedt U (1991b) Autoradiographic and morphological investigations on the uptake and incorporation of tritiated lysin by acanthocephalans. Parasitol Res 77:536–541
Taraschewski H (2000) Host-parasite interactions in Acanthocephala: a morphological approach. Adv Parasitol 46:1–179
Taraschewski H, Peters W (1992) Comparative investigations of the morphology and chemical composition of the eggshells of Acanthocephala II. Palaeacanthocephala. Parasitol Res 78:376–381
Taraschewski H, Sagani C, Mehlhorn H (1989) Ultrastructural study of the host-parasite interface of Moniliformis moniliformis (Archiacanthocephala) in laboratory-infected rats. J Parasitol 75:288–296
Taraschewski H, Peters W, Latka I (1992) Comparative investigations of the morphology and chemical composition of the eggshells of Acanthocephala. III. Eoacanthocephala. Parasitol Res 78:382–387
Trinajstic K, Marshall C, Long J, Bifield K (2007) Exceptional preservation of nerve and muscle tissues in Late Devonian placoderm fish and their evolutionary implications. Biol Lett 3:197–200
Uglem GL (1972) The life cycle of Neoechinorhynchus cristatus Lynch, 1936 (Acanthocephala) with notes on the hatching of eggs. J Parasitol 58:1071–1074
Upeniece I (2001) The unique fossil assemblage from the Lode Quarry (Upper Devonian, Latvia). Mitt Mus Natkd Berl Geowiss 4:101–119
Upeniece I (2011) Palaeoecology and juvenile individuals of the Devonian placoderm and acanthodian fishes from Lode site, Latvia. Dissertation thesis, Riga
Van Cleave HJ (1921) Acanthocephala from the eel. Trans Amer Microsc Soc 40:1–13
Van Cleave HJ (1936) The recognition of a new order in the Acanthocephala. J Parasitol 22:202–206
Van Cleave HJ (1947) A critical review of terminology for immature stages in acanthocephalan life histories. J Parasitol 33:118–125
van Huis A (2017) Did early humans consume insects. J Insects Food Feed 3:161–163
Van Thiel PH, Bruss W (1945) Presence of Prosthenorchis spirula chez les chimpanzes, son role pathogene et son developpement dans Blattella germanica. Ann Parasitol 20:304–320
Verweyen L, Klimpel S, Palm HW (2011) Molecular phylogeny of the Acanthocephala (class Palaeacanthocephala) with a paraphyletic assemblage of the orders Polymorphida and Echinorhynchida. PLoS One 6:e28285
Vinther J, Parry LA (2019) Bilateral jaw elements in Amiskwia sagittiformis bridge the morphological gap between gnathiferans and chaetognaths. Curr Biol 29(5):881–888.e1
von Haffner K (1950) Organisation und systematische Stellung der Acanthocephalen. Zool Anz 145:243–274
Waggoner BM, Poinar GO (1993) Fossil habrotrochid rotifers in Dominican amber. Experientia 49:354–357
Warnock RCM, Engelstädter J (2021) The molecular clock as tool for understanding host-parasite evolution. In: de Baets K, Huntley JW (eds) The Evolution and Fossil Record Of Parasitism: Coevolution and Paleoparasitological Techniques. Topics in Geobiology 50
Weber M, Wey-Fabrizius AR, Podsiadlowski L, Witek A, Schill RO, Sugár L, Herlyn H, Hankeln H (2013) Phylogenetic analysis of endoparasitic Acanthocephala based on mitochondrial genomes suggests secondary loss of sensory organs. Mol Phylogenet Evol 66:182–189
Wey-Fabrizius AR, Herlyn H, Rieger B, Rosenkranz D, Witek A, Mark Welch DB, Ebersberger I, Hankeln T (2014) Transcriptome data reveal syndermatan relationships and suggest the evolution of endoparasitism in Acanthocephala via an epizoic stage. PLoS One 9:e88618
Whitfield PJ (1973) The egg envelopes of Polymorphus minutus (Acanthocephala). Parasitology 66:387–403
Witek A, Herlyn H, Meyer A, Boell L, Bucher G, Hankeln T (2008) EST based phylogenomics of Syndermata questions monophyly of Eurotatoria. BMC Evol Biol 8:345
Witek A, Herlyn H, Ebersberger I, Mark Welch DB, Hankeln T (2009) Support for the monophyletic origin of Gnathifera from phylogenomics. Mol Phylogenet Evol 53:1037–1041
Wurmbach H (1937) Zur krankheitserregenden Wirkung der Acanthocephalen. Z f Fischerei u Hilfswiss 35:217–232
Yeh H-Y, Mitchell PD (2016) Ancient human parasites in ethnic Chinese populations. Korean J Parasitol 54:565–572
Zangerl R, Case GR (1976) Cobelodus aculeatus (Cope), an anacanthous shark from Pennsylvanian black shales of North America. Palaeontogr Abt A 154:107–157
Zhang X-g, Pratt BR (2012) The first stalk-eyed phosphatocopine crustacean from the Lower Cambrian of China. Curr Biol 22:2149–2154
Zhong HL, Feng LB, Wang CX, Kang B, Wang ZZ, Zhou GH, Zhao Y, Zhang YZ (1983) Human infection with Macracanthorhychus hirudinaceus causing serious complications in China. Chin Med J 96:661–668
Acknowledgements
My thanks go to Dr. Kenneth De Baets and Dr. John Warren Huntley for giving the opportunity to contribute this chapter and for useful comments on earlier drafts. I am also grateful to Prof. Thomas Wotte (Freiberg, Germany) who made available the scanning electron micrographs of Cambroclaves fossils shown in Fig. 8.5. Not least, I thank the holders of rights who have agreed to the usage of various previously published illustrations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Herlyn, H. (2021). Thorny-Headed Worms (Acanthocephala): Jaw-Less Members of Jaw-Bearing Worms That Parasitize Jawed Arthropods and Jawed Vertebrates. In: De Baets, K., Huntley, J.W. (eds) The Evolution and Fossil Record of Parasitism. Topics in Geobiology, vol 49. Springer, Cham. https://doi.org/10.1007/978-3-030-42484-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-42484-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42483-1
Online ISBN: 978-3-030-42484-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)