Abstract
Consuming gluten-containing foods derived from wheat, barley, rye and possibly oats can result in health problems for a significant proportion of people. However, in the case of several types of disorders related to consuming gluten-containing cereals, not only the gluten components are trigger compounds. According to medical experts the majority of people suffering from health problems because of gluten— except for celiac disease patients—instead of consuming gluten-free food have the option to choose food products containing healthier, low levels of fermentable oligosaccharides abbreviated FODMAP. In order to meet the health-related special needs of these particular consumer groups, cereal breeders aim to develop new germplasm, suitable for the food industry to produce healthier products. This chapter provides a summary of the latest developments in this booming research field, including: (i) describing the actual knowledge on cereal-related health problems, (ii) describing the current status of celiac-safe cereal breeding, (iii) enhancing the importance of developing healthier spelt-based cereal products through the advancement of an ongoing spelt breeding program and finally (iv) developing plant biotechnology improvements relative to special food quality parameters.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Celiac disease
- Irritable bowel syndrome (IBS)
- In vitro androgenesis
- Non-celiac gluten sensitivity
- Spelt
- Wheat allergy
14.1 Introduction
Islam et al. (2011), defined wheat gluten as a protein-lipid-carbohydrate complex formed through particular non-covalent and covalent correlations among flour constituents during dough making, as the constituents are hydrated and where the mixing process provides the mechanical energy input. Nevertheless, these days the use of technical terms such as gluten-free and gluten are more common (but inaccurate) among lay people. Gluten-free roughly refers to food products free from cereal prolamin proteins, i.e. wheat glutenin and gliadin, which are similar.
Consuming food with gluten content derived from wheat, barley, rye and possibly oats can result in health problems for a significant proportion of people. In this regard, considering oats as a harmless cereal is one of the oldest and open disputes among specialists. Some experts assert that a certain percentage of patients suffering from celiac disease are sensitive to epitopes located in the so-called avenins, the prolamin type storage proteins in oat (Comino et al. 2016; Pulido et al. 2009), while other publications explain its potential danger through contamination with other cereal.
However, in the case of several types of disorders related to consuming gluten-containing cereals, the gluten components are not the trigger compounds. We do not have enough specific knowledge about the reasons for different symptoms or diseases and the precise meaning of terms like gluten, prolamins, gliadin or glutenin, neither for average consumers nor for medical experts (Branchi et al. 2015).
Nowadays in most Western countries the general public has learned of the perceived dangers and adverse influence of cereals containing gluten, thanks to reports appearing in the lay press (Braly and Hoggan 2002; Ford 2008; Wangen 2009) recommending gluten-free diets. Many of these reports are not able to define the nature of the gluten intolerance an individual may have or enhance the significance of appropriate diagnosis. In this way they portray a severe public relations threat to the grain industry.
In Western countries the gluten-free food business has improved in the last 5 years. In 2013 the US retail trade in gluten-free products reached USD 10.5 billion, and up to USD 15 billion was predicted for 2016. It is forecast in Australia that in the following 5 years the gluten-free retail sales will exceed USD 100 million, where in 2014 already more than 20% of the newest baking products were launched in the market as being gluten free (Jargon 2014).
As information about mechanisms of certain disorders has been accumulating, it has been revealed that the causative components of cereal products are often the soluble proteins (albumins and globulins) or the soluble oligosaccharides instead of gluten proteins. This fact enhances the importance of research dealing with an appropriate diet for different types of gluten-sensitive people, instead of just suggesting a gluten-free diet.
Marketing activities associated with wheat allergy or gluten toxicity is frequently concentrated on gluten-free products. However, to meet the needs of different consumer groups, the medium or long-term solution based on more and more information collected in the medical research field is not simply having gluten free and traditional products on the shelves of the stores. A gluten-free diet is incomparably more expensive but not healthier than consuming gluten-containing foods (Missbach et al. 2015). Except for individuals who suffer from celiac disease (about 1% of the Caucasian population), people with some kind of cereal-related health problems can consume foods with lower levels and/or modified gluten content and decreased amounts of certain soluble carbohydrate (FODMAPs) components (Halmos et al. 2015).
To meet the special health related needs of certain consumer groups, cereal breeding has the duty to develop new germplasm, suitable for the food industry to produce new, healthier products. Setting achievable goals, comprehending the numerous health-related disorders, their presence and trigger composites, is essential not only in the current basic research but also in profit-based cereal breeding as well. In this chapter we present a summary of the latest developments in this booming research field, describing the actual knowledge on cereal-related health problems, describing the current status of celiac-safe cereal breeding and finally—through an example— enhancing the importance of developing healthier spelt-based cereal products through the advancement of an ongoing spelt breeding program.
14.2 Cereal-Related Health Disorders
Health problems caused by gluten can be classified basically into three types: allergic, autoimmune and nonallergic - not autoimmune disorders (Fig. 14.1, Sapone et al. 2012). The first two of these, are quite well researched; although, to reveal mechanisms related to various symptoms of allergic reactions, further studies are required. The allergic reactions comprise food allergy (Mills et al. 2004), respiratory allergy (Amano et al. 1998), contact urticaria (Lahti 1986) and wheat-dependent exercise-induced anaphylaxis (Armentia et al. 1990). The innate and adaptive pathways leading to celiac disease (CD)-specific symptoms have been described and studied extensively (Plugis and Khosla 2015). The autoimmune disorders include gluten ataxia, CD and dermatitis herpetiformis (Anderson and Wieser 2006; Lauriere et al. 2006).
The classification of wheat-related health disorders based on Sapone et al. (2012)
14.2.1 Celiac Disease
An abnormal response of autoantibodies causes the symptoms of celiac disease; for example, antibodies produced against glutamine- and proline-rich wheat gluten proteins, or their rye and barley homologues, or antibodies to tissue-transglutaminase (Green and Cellier 2007). There is a strong connection between celiac disease and one of the most essential genetic factors in developing detrimental symptoms, HLA-DQ alleles (Sollid et al. 2012). Heterodimers of these alleles, bound to the surface of antigen-presenting cells, function as surface-type receptor proteins. The presence of HLA-DQ compounds (DQ2.2, DQ2.5, DQ8), is a clear sign for the appearance of autoimmune symptoms, with modifying effects deriving from environmental and genetic factors (Anderson et al. 2000). The different peptides are recognized by different serotypes.
A set of criteria for the structure of an active CD epitope in a cereal protein can be described as follows: a) surrounded by amino acids with defined charge and hydrophobicity, b) the presence of a tissue transglutaminase 2 (tTG) enzyme-binding site and c) a size of nine amino acids fitting into the groove of the HLA-DQ heterodimers (Sollid et al. 2012). Patients suffering from celiac disease produce a range of autoimmune reactions to gliadins, LMW glutenins and some HMW glutenin subunits, just as to their analog polypeptides in the consumed cereal foods (Juhász et al. 2012).
14.2.2 Wheat Allergies
Due to specific IgE epitopes bound to mast cells, the development of wheat allergies is mediated more directly by the recognition of allergens (Catassi and Fasano 2008). Symptoms can be divided depending on the route of wheat allergen exposure into symptoms of classic food allergy: occupational asthma (bakers’ asthma), wheat-dependent exercise-induced anaphylaxis (WDEIA) and urticaria (Sapone et al. 2012). Common food allergy signs may affect the respiratory or gastrointestinal tract or the skin. One of the most typical respiratory allergies is bakers’ asthma, a serious symptom among adults who work with wheat flour.
Allergens presenting T-cells and B-cells have the same level of effects in a wheat allergy. Similar to other allergic reactions connected to food allergies, it is the result of cross-links between specific immunoglobulin E and short peptides that are rich in glutamine and proline and derive from the degradation of wheat grain proteins by endogenous proteases. This interaction stimulates mast cells and basophils to release chemical mediators, for instance, histamines resulting in different classes of inflammatory reactions. IgE-binding epitopes are the allergenic regions of proteins recognized by the binding sites of IgE molecules. These epitopes can be categorized into two groups: linear and conformational epitopes. Both types play a part in the development of allergic responses (Akagawa et al. 2007). In wheat, allergenic proteins are not just the prolamins (α, β, γ and ω gliadins, HMW and LMW glutenin subunits) but also proteins that are not elements of the gluten matrix {amylases, peroxidase, thioredoxin and serine proteinase inhibitors, the lipid transfer proteins (LTP)} (Tatham and Shewry 2008).
14.2.3 Non-Celiac Gluten Sensitivity
Non-celiac gluten sensitivity (NCGS) was first described about 40 years ago as mainly intestinal symptoms connected to consumption of gluten-containing cereals (Schuppan et al. 2015). There is significant proof that NCGS is induced by an innate immune response to wheat proteins (it is different from the adaptive, T cell-mediated response to gluten and non-gluten proteins in wheat allergy or to gluten in celiac disease) (Catassi et al. 2013; Sapone et al. 2012). A study by Junker et al. (2012) helped in the identification of the family of amylase-trypsin inhibitors (ATIs) as occasions of innate immunity in wheat. Nowadays NCGS has become a subject of growing interest for scientists. Research indicated a certain degree of awareness in connection with clinical relevance of gluten-related disorders among gastroenterologists (Zevallos et al. 2016).
14.2.4 Irritable Bowel Syndrome
A common gastrointestinal condition is irritable bowel syndrome (IBS) that has an impact on quality of life and can lead to significant financial costs. Generally, the efficacy of the therapeutic strategies to treat IBS, is disappointing, but has been improved significantly by the research of Gibson et al. (2007, 2015). Numerous abdominal symptoms may originate from factors which alter bowel digestion. They contribute to luminal digestion, particularly the osmotic load within the lumen, and the fermentative gas content may offer symptomatic benefit. FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) is the collective term for candidate substrates that are highly fermentable causing an osmotic effect and are dietary, poorly absorbed and short-chain carbohydrates (Shepherd and Gibson 2006). Lactose and fructose are included in FODMAPs as there are patients in whom these are malabsorbed. Other FODMAP compounds are the polyols, for instance, sorbitol since they are poorly absorbed by humans, as well as galacto-oligosaccharides (such as raffinose) and fructo-oligosaccharides (fructans). The latter are always poorly absorbed since humans do not express suitable hydrolases (Shepherd et al. 2008). Fructans and fructose are included by major dietary FODMAPs. The most common food sources of fructans are cereals and onions; sources of fructose are fruits or honey (Biesiekierski et al. 2011; Muir et al. 2009).
A blinded placebo-controlled crossover study proved the efficiency of low FODMAP intake, confirming previous studies where about 75% of patients gain clinically-outstanding advantage (Halmos et al. 2014). As a first-line therapy, the low-FODMAP diet is progressively being applied by health specialists in patients with IBS (Halmos et al. 2014, 2015). Given the chronic nature and the high prevalence of IBS, many people may restrain the intake of FODMAPs over the short- or long-term (Catassi et al. 2017; Shepherd and Gibson 2006).
14.2.5 Prevalence of Different Disorders
In Western countries where the predisposing genes of celiac disease, HLA DQ8 and HLA DQ2 genes, can be found in approx. 30% of the population, celiac disease itself affect only 1% of these Western communities (Anderson and Wieser 2006).
Food allergy questionnaires were used with statistical analyses to establish relationships between individuals with clinical symptoms and immune responses (Vu 2014, Vu et al. 2014) in a study where a large number of blood samples was analyzed by the IgE RAST method against milk and wheat antigens from randomly-selected individuals (n = 1145) (Pasco et al. 2012). Outcomes indicated that the frequency of wheat allergy was 2.5% where both symptoms against wheat and a positive IgE immune response were observed. These results corroborate absolutely with similar wheat examinations where it was found that in European countries the prevalence of wheat IgE sensitization is 2.9% (Siles and Hsieh 2013; Zuidmeer et al. 2008). In summary it is assumed that those individuals (12.8%; n = 125) who exhibit increased IgE levels without any symptoms might have a latent wheat sensitivity. They can be potential patients sooner or later as they have the chance of developing the symptoms. In the investigated population there is a large proportion (12.9%) who have symptoms connected to a wheat-consuming diet but did not show increased IgE. They may suffer from other wheat-related disorders (i.e. not IgE mediated) such as reaction to fructans (FODMAPs) for those with IBS, celiac disease or non-celiac gluten sensitivity (NCGS).
14.3 Developing Celiac-Safe Wheat
Identification of amino acid sequences of gluten proteins which are the inducing factors in sensitive individuals have shown that their distribution varies between and among the different groups of gluten proteins (Shewry and Tatham 2016). Detecting and determining the quantity of gluten proteins are crucial for two reasons: due to its direct impact on end-use quality and also for food safety reasons. There is a difference between seed composition among cereal genotypes which creates methodological problems in food allergen research and genotype selection for quality in breeding. The multi-species origin of prolamins and high sequence analogy, coupled with restrictions in the available methodologies, reviewed by Haraszi et al. (2011), make the precise identification of proteins that generate health disorders and their genotypic stability, variability and frequency, difficult to define. An accurate quantitative relationship is required between the final gluten/prolamin content and the prolamin peptide biomarkers in high-resolution methods; for instance, in mass spectrometry (MS) to connect the detection of peptide mass to their protein bases. Due to environmental and genotypic variability, these quantitative interactions, however, are difficult to detect.
To assist in protein selection, epitope mapping, peptide biomarker searches and medical studies, a database (ProPepper, https://propepper.net) was created which contains linear epitopes responsible for wheat-related food disorders, peptides obtained with single and multi-enzyme in silico digestion and the members of the prolamin superfamily proteins identified from Poaceae species (Juhász et al. 2015).
Information about the amount and composition of allergen and toxic epitopes existing in a single wheat sample can represent a substantial gap. The very soon to be completed grain proteome datasets can ensure the essential information to perform an estimation of allergen/toxicity calculation for a single cultivar. The combined use of allergen/toxic databases and genome sequence, cereal chemistry and prediction methodology results in a better understanding of the level of toxicity present from wheat flour in the end-products. Information is available about the distribution and number of epitopes for a single protein or a protein fraction based on the workflow presented in the review of Juhász et al. (2012). The most detrimental proteins and epitopes occurring in the highest frequency can be identified as well. Researchers may obtain significant output from the analysis of large datasets such as the epitope toxicity value that can be well applied in food industry.
Two excellent reviews (Comino et al. 2013; Shewry and Tatham 2016) summarize recent research activities on the status of pseudo and alternative cereals and their derivatives, achieved by enzymatic or transgenic technology, breeding programs and natural selection, with the potential to develop products tolerated by celiac patients. Consequently, it is possible to use conventional breeding methods for selecting gluten protein fractions with lesser amounts of celiac epitopes. Molecular breeding methods can be applied to mutate celiac epitopes within individual proteins or to specifically downregulate celiac-toxic proteins. Gil-Humanes et al. (2008) provide well-known examples of this method, using RNA interference mediated gene silencing to downregulate the alpha and gamma gliadins in bread wheat (Triticum aestivum L.). A parallel method was applied by Altenbach et al. (2014a, b) to remove the omega-5 gliadins that are the most allergenic gluten proteins.
The combination of the above approaches may be used to develop celiac-safe wheat. However, due to the complex multigenic control of gluten protein composition, this remains a formidable challenge. Moreover, wheat must retain acceptable bread making or baking properties after any type of modification. It is not surprising that such celiac-safe wheat has not been developed, despite over a decade of research.
14.4 Gluten-Free Versus Low FODMAP Diet
According to a study of gluten-free foods (Market Research 2012), of individuals on a gluten-free diet, 65% consider it healthier, 36% do so for reasons other than sensitivity, 27% think it helps weight loss, 7% do so to lower inflammation and 4% to fight depression; only 5.7% claimed an certified medical diagnosis.
Due to the ubiquity of gluten in foods, a gluten-free diet (GFD) causes many social and economic repercussions (Comino et al. 2013). In the meantime, it cannot be considered as a healthy diet (Balakiereva and Zamyatin 2016), because gluten-free products usually are comprised of starches or refined flours containing a low amount of fiber. The fact is that consuming sufficient amounts of dietary fiber is associated with substantial health benefits, for instance, prevention of diabetes, colon cancer and cardiovascular disease. In consequence, GFD may cause different nutrient deficiencies in fiber which can lead to further subsequent health problems, as mentioned above. Numerous studies recommend using pseudo-cereal sources of fiber to maintain the required amount of fiber levels (Saturni et al. 2010). GFD can also induce a deficiency in folic acid and Vitamins B12, C and D, along with microelements, most importantly zinc, magnesium and calcium (Caruso et al. 2013; Hallert et al. 2002;). Besides, GFD comprises high amounts of hydrogenated fats and sugar, which can lead to an increased risk of obesity and hyperinsulinemia (Lamacchia et al. 2014). Hence, a GFD appears to be unbalanced and inadequate with regard to both micro- and macronutrients. According to medical experts the majority of people, who may feel better consuming gluten-free foods—except for celiac disease patients—are automatically choosing a low FODMAP diet by selecting gluten-free or wheat-free products (Halmos et al. 2014, 2015).
Active research work and development in analyzing FODMAP-content, together with using food ingredients with low FODMAP content, were initiated to determine the crucial significance of FODMAP constituents related to health disorders. Establishment of a low FODMAP diet includes a much wider range of plant-origin products in contrast with the gluten-free phenomena, where the problematic food sources are a relatively small group of certain cereals. Studies by Biesiekierski et al. (2011) and Muir et al. (2007, 2009), along with computer and mobile phone applications, have helped to improve custom-designed low FODMAP diets: (http://www.med.monash.edu.au/cecs/gastro/fodmap/iphone-app.html).
Products made from traditional cereals (wheat, barley and particularly rye) are not recommended in FODMAP diets since they contain relatively high FODMAPs (mostly fructans). However, the genotypic variation within certain cereal species (Table 14.1) opens new perspectives for reducing/increasing kernel fructan concentrations by breeding. In the case of wheat, it was shown that there is not a very strong genotype x environment (G x E) interaction, and that heritability is high, in a range of 0.64–0.94 (Huynh et al. 2008b).
Recent research studies to obtain information about gene regulation of fructan synthesis in cereals has primarily focused on barley and wheat. In the case of wheat, two major quantitative trait loci were identified so far on chromosomes 6D and 7A, and, in case of barley, several genes were mapped which encode fructan synthesizing enzymes (Huynh et al. 2008a, 2012; Wei et al. 2000).
A total of eight QTLs with two pairs of epistatic interactions were found for grain fructan concentration. Two QTLs on chromosomes 7A and 6D explained, respectively, 17 and 27% of the total phenotypic variation. Transgressive segregation was observed, and broad-sense heritability was estimated as 0.71 (Huynh et al. 2008b). Genes encoding the enzymes of fructan biosynthesis (1-SST, 1FFT, 6-SFT) form a functional cluster which was sequenced and mapped to the major QTL on chromosome 7A (Huynh et al. 2012).
Moreover, TaMYB13, a transcriptional activator, was identified, which could control the expression of major fructosyltransferases that are involved in fructan synthesis (Xue et al. 2011). In transgenic wheat lines the overproduction of this transcriptional activator has raised the fructan concentrations in the top internode and flag leaf, but the result on grain fructans was not examined (Kooiker et al. 2013). In transgenic triticale lines, kernel fructan concentrations were increased up to 20 times by overexpressing a fructan synthesizing enzyme from wheat or rye behind an aleurone-layer specific promoter (Diedhiou et al. 2012). In addition, it is possible to enhance fructan concentrations by producing mutant lines lacking GBSSI, granule-bound starch synthase I and/or SSIIa, starch synthase IIa (Shimbata et al. 2011; Yasui and Ashida 2011). Shimbata et al. (2011) showed that in sweet wheat cultivars, where functional GBSSI and SSIIa are lacking, kernel fructan concentration are up to 6.5 times higher than in corresponding wild-type wheat lines. Due to a mutation in the SSIIa gene, Clarke et al. (2008) generated a 42-fold growth in grain fructan concentration of barley. Downregulation of FEH activity in cereal grains by RNA interference is the final strategy for increasing cereal grain fructan content. Definitely, FEH activity is high during the second phase of grain maturation (Verspreet et al. 2013) and it is essential for fructan degradation (Wardlaw and Willenbrink 2000; Yang et al. 2004). It is assumed that a reduction of FEH translation will cause higher cereal grain fructan levels.
Under stress conditions (drought, waterlogging and lack of nutrients), fructan and other water-soluble carbohydrates (WSC) accumulate temporarily—with a peak around late booting and emergence of the inflorescence—in vegetative plant tissues such as stems or roots, and can be remobilized during grain filling where fructan is again synthesized in the mature grain (Gebbing 2003). Genetic variation was reported not only for fructan contents in stems but also for its remobilization efficiency (Ehdaie et al. 2006). In the grain, net synthesis of fructan is most rapid, 5–9 days after anthesis, then reaches its maximum concentration (15–30%) and diminishes thereafter (Costantini et al. 2008).
A significant environmental influence was observed in most fructan studies (Costantini et al. 2008); however, with no evidence of strong G x E interactions (Huynh et al. 2008a). No difference was observed between fertilization levels and management system (organic vs. conventional), only unfertilized wheat showed a slightly higher fructan concentration indicating stress due to a lack of nutrients (Langenkämper et al. 2006).
Spelt, compared to bread wheat, is comprised of considerably lower amounts of fructans and fructose. More importantly, the intervarietal differences among spelt genotypes is about five-fold larger than among bread wheat cultivars (Fig. 14.2), (Békés 2015). This provides the basis for screening spelt cultivars for low FODMAP content.
Comparing the total fructan level found in the trade-marked cultivar, E3 spelt with those found in 12 wheat and 11 spelt samples grown at three locations in NSW, Australia. Error bars for wheat and spelt samples indicate the inter-variety variations. (Source: Békés 2015)
14.5 Spelt: A Nutritive Cereal
According to molecular evidence, spelt arose from hybridization between free-threshing hexaploid wheat and non-free threshing tetraploid wheat (2n = 4x = 28, genome = AABB). In the case of most hulled wild wheats and hexaploid varieties such as Triticum aestivum var. vavilovii (Tumanian) Sears, there is a recessive allele (q) at the Q locus that controls major domestication features in wheat (Faris 2014).
About 100 years ago, spelt was considered as the most important cereal in southern Germany, Austria, and Switzerland due to its hardiness and robustness concerning soil and climate, resistance to disease and nitrogen utilization efficiency. Bread wheat almost completely replaced spelt during the twentieth century because of higher yields, better baking performance and lower processing costs. However, spelt products have gained increasing popularity in the last 10 years. The major reasons are good digestibility, pleasant taste and aroma, and a better tolerance (in the case of wheat sensitivity) (Kling 1988). In addition, spelt is now being increasingly cultivated in water-protected areas and in organic farming as this cereal has fewer requirements with regard to use of herbicides or pesticides and fertilization compared to wheat (Kohajdova and Karovicova 2008).
Extensive systematic breeding and selection led to the creation of high-yielding and free-threshing cultivars of spelt, while retaining wide adaptability to different agro-climatic conditions. These new spelt cultivars have replaced most of the low-yielding traditional landraces and subspecies. However, in most parts of the world spelt has been superseded by bread wheat, leaving spelt to be grown as a marginal or niche crop with low inputs.
Recent interest in the use of spelt for ecologically-grown food has led to a revival in its cultivation after many years of marginal cultivation. As a matter of fact, in Europe spelt is used traditionally in the everyday diet, especially in Germany where numerous grain-based foods are produced using spelt (Cubadda and Marconi 2002). Several excellent works have been published in the last 10–15 years in connection with the evolution, physiology, genetics and the G x E effects on quality of spelt (Blatter et al. 2004; Förster et al. 2013, 2014).
The genetic diversity of major crops has suffered an overall reduction over time. Such genetic erosion can cause serious difficulties in the maintenance of the biodiversity of crop plants, as well as in crop improvement (Caballero et al. 2008). As a consequence of the reduced genetic variation, the stability of the crops in response to various stress factors has decreased in their local environments. In order to compensate for the loss of variation, breeders have turned to crops that were neglected in the recent past.
Spelt represents a useful gene reservoir for breeding programs, as it has the same ploidy level (2n = 6x = 42, AABBDD) as bread wheat. It has valuable nutritional quality due to its protein content and composition, which differ from those of wheat (Abdel-Aal and Huck 2002; Bonafaccia et al. 2002). The main difference is the variation in the amount and type of grain proteins, especially prolamins. Spelt dough is softer and stickier after kneading and is characterized by lower stability, less elasticity and higher extensibility than bread wheat dough (Schober et al. 2002).
In many modern breeding programs, spelt has intentionally been crossed with wheat cultivars for the improvement of yield, baking quality, and resistance to lodging and disease. It is essential to distinguish between spelt and bread wheat cultivars. The genetic diversity and chemical composition of spelt and modern wheat cultivars have been assessed and discussed from many perspectives. The gliadin protein patterns obtained with acidic polyacrylamide gel electrophoresis (A-PAGE) were reported to be an excellent tool for qualitative cultivar identification (Ng et al. 1988). Schober et al. (2006) used size-exclusion high-performance liquid chromatography to classify spelt cultivars by their gluten proteins. Furthermore, a spelt-specific gamma-gliadin gene was discovered (Büren and Lüthy 2000) and then utilized in a simple polymerase chain reaction (PCR) method to detect the wheat adulteration of spelt flour and products (Büren et al. 2001). In addition, capillary zone electrophoresis can detect not only differences between the gliadin patterns of closely related spelt cultivars, but also the presence of wheat elements in the gliadin patterns of wheat/spelt crosses. In a study by Schober and Kuhn (2003), the presence of bread wheat was revealed in a number of spelt cultivars using capillary zone electrophoresis (CZE). The genetic diversity of spelt has been extensively examined on the basis of prolamin composition using reversed-phase HPLC (Wieser 2000), acid polyacrylamide gel electrophoresis (A-PAGE) (Caballero et al. 2004), one- and two-dimensional polyacrylamide gel electrophoresis (An et al. 2005) and capillary zone electrophoresis (Schober and Kuhn 2003). Other methods were also used to characterize genetic variation in spelt, by analyzing the rheological and viscoelastic properties of spelt gluten (Pruska-Kedzior et al. 2008; Schober et al. 2002) or its fiber content (Escarnot et al. 2010).
The molecular characterization of cultivars is also a useful way of evaluating genetic diversity during the breeding process. The genetic diversity of the Triticeae has been explored using a range of molecular markers (Manifesto et al. 2001) and with diversity arrays technology (DArT) markers (White et al. 2008). A high level of genetic diversity was revealed in spelt germplasm by microsatellite markers (Bertin et al. 2004). Gulyás et al. (2012) reviewed the milling, technological, compositional and bread making characteristics of European spelt cultivars and breeding lines, and estimated the phylogenetic relationships among spelt accessions using AFLP markers and tried to establish relationships between the presence of wheat genes and the technological and nutritional quality characteristics. The extent of genetic diversity in spelt germplasm, characterized by polymorphism information content (PIC) (Anderson et al. 1993), was far greater than found by Martos et al. (2005), for durum, or by Lelley (2000), for bread wheat. It was concluded that the diversity could be due in part to the extent of crossing between pure spelt cultivars and bread wheat. This suggests that spelt cultivars cannot be characterized without considering their origin. In view of the fact that it is mostly genotypes with bread wheat in their pedigree which achieve the quality required for bread making, spelt could be used as a source of lodging and disease resistance (Campbell 1997) and a source of genetic diversity for grain protein and mineral nutrients (Gomez-Becerra et al. 2010). Both the quality assessment and molecular analysis revealed a great diversity in spelt accessions that can be further utilized by breeders to incorporate them into specific breeding programs aiming to improve not only resistance but quality traits.
Spelt can be cultivated in harsh ecological conditions, without the use of pesticides because it shows a higher tolerance of environmental factors than bread wheat (Raman et al. 2008). Furthermore, spelt is a proper crop for organic farming as it can grow on low-fertility and poorly drained soils (Bonafacci et al. 2002). Spelt was not among the targeted cereals in systematic breeding in the past; the objectives for improvement have only been identified recently (Neeson et al. 2008).
Spelt has been emerging as a worthy cereal for the past decade because of its repute as a healthy food not just for celiacs, but also for people suffering from wheat allergy (Elia et al. 2004; Galova and Knoblochova 2001). Spelt flour is also regarded to be more nutritious than bread wheat flour and to possess a unique flavor (Campbell 1997). Generally, spelt products, especially organic products, such as bread command a higher price in the marketplace.
In the last decade, the chemical, functional and nutritive properties of spelt compared to bread wheats have been described in numerous publications, recently reviewed by Escarnot et al. (2012). According to their results, on average, the spelt milling fractions and whole meal flour were higher in unsaturated fatty acids and lipids, but contained lower tocopherol content than in wheat samples. This suggests that there is no close relationship between high lipid content and high germ proportion in spelt. Although the proportion of bran and flour in milling fractionation is quite similar in wheat and spelt, there can be significant differences in mineral content. It was found that copper, zinc, phosphorus iron, magnesium and ash contents were higher in spelt samples, particularly in coarse bran and in aleurone-rich fine bran. Even spelt bran has the higher phosphorus content than wheat; the opposite trend was shown by phytic acid content, as it was 40% lower in spelt versus wheat fine bran. This suggest that spelt has either a lower phytic acid content or a higher endogenous phytase activity than wheat. Ruibal-Menieta et al. (2005) provide important suggestions about the true nutritional value of spelt compared to wheat. Furthermore, Gomez-Becerra et al. (2010) showed that the oleate/palmitate rate together with the Ca/Fe proportion provide a highly discriminating tool to face the growing issue of spelt-flour adulteration and to authenticate spelt from wheat flours. The results showed that spelt is a highly promising source of genetic diversity for mineral nutrients, particularly Zn and Fe, and for grain protein. Similar results have been obtained comparing wheat and spelt samples grown in Australia (Muir et al. 2014), (Table 14.2).
Spelt-based products have a slightly higher protein content and are more easily digestible than wheat. The variation in the amount and type of grain proteins, especially prolamins, is the main difference in the nutritive value of spelt flours (Galli et al. 2000; Shewry 2002).
Systematic crossbreeding of wheat and spelt was started at the beginning of the twentieth century to compensate, not for the detrimental properties of spelt in agriculture and processing, but to preserve its desirable properties. Not only pure spelt but also wheat/spelt crossbreeds have been cultivated and used in food processing since that time. The assignment of crossbreeds to sp. spelt is exclusively done based on morphological properties, for instance, shape of the ears and the fact that after threshing the husks are still attached to the seeds. Typical components of the seeds that assure the characteristics and quality of spelt are not accounted for at all and information on the rate of wheat crossing has not been obtainable to both consumers and producers of spelt products. To classify spelt cultivars, according to crossbreeding with bread wheat, is of special interest for persons tolerant towards spelt and sensitive to wheat (Catassi et al. 2013; Ludvigsson et al. 2013).
Several studies showed that reliable distinction between wheat and spelt can be provided by the differences in the protein patterns (Schober and Kuhn 2003; Wieser 2006); however, wheat and spelt seeds, due to their close botanical relationship, contain similar endosperm proteins.
Wheat proteins can be classified into the Osborne fractions of glutenins, GLUT; gliadins, GLIA and albumins/globulins ALGL. These can further be subgrouped into different types of gluten protein (α/β-,γ-, ω1,2-, ω5-, gliadins; low- and high-molecular-weight glutenin subunits, LMW-GS and HMW-GS (Wieser 2000). The main protein fractions of both cereals are the gliadins. Their patterns are determined by several components. For decades, the differentiation and identification of genotypes was based effectively on the variations of these patterns. SDS gel and acid gel electrophoresis, reversed-phase high-performance liquid chromatography (RP-HPLC) and gel isoelectric focusing have been applied, in particular, to the differentiation of wheat cultivars worldwide (Cornell and Hoveling 1998; Radic et al. 1997; Radic-Miehle et al. 1998; Wrigley and Bietz 1988).
Capillary zone electrophoresis (CE) of GLIA from 27 European spelt samples showed that differences were detected in the patterns of some wheat/spelt crossbreeds, even of closely-related cultivars and wheat elements (Schober and Kuhn 2003). Similarly, RPHPLC of GLIA allowed determination of the degree of wheat crossing in spelt and the differentiation of spelt and wheat cultivars (Wieser 2006). A total of 23 spelt cultivars were categorized into 5 groups ranging from pure spelts to spelts similar to wheat. Different degrees of wheat crossing and different GLIA patterns were detected in crossbreeds with identical parents (wheat and spelt). Therefore, no conclusion can be drawn from the pedigree on the real content of wheat elements. Until recently in spelt products the wheat contaminations could not be determined for authenticity and quality control of cereal grains and pure genotypes. It was demonstrated by Mayer et al. (2012) that a fast and simple observation of wheat in spelt flours can be accomplished by the combination of lab-on-a-chip capillary gel electrophoresis and the polymerase chain reaction-restriction fragment length (PCR-RFLP) analysis.
The quantitative protein compositions of the Osborne fractions of whole meal flours from 62 spelt and 13 wheat cultivars were determined by Koenig et al. (2015) using reversed-phase high-performance liquid chromatography. The result shows that the chromatograms of the reduced gliadin fractions could be applied best for spelt classification and to distinguish spelt from wheat. Composition of the reduced spelt gliadins revealed one to three markers that cannot be found in wheat. Spelt cultivars were categorized into three groups based on these markers ranging from typical spelt to similar to bread wheat. Marker 1 was referred as ω1,2-gliadin and markers 2, 3a and 3b were identified as γ-gliadins by the determination of the relative molecular mass by mass spectrometry and the means of N-terminal sequence analysis. Glutenin-bound ω-gliadins may be used to quantitate and detect even small amounts of wheat in spelt products because this type of protein is absent in spelt but present in wheat.
There have been anecdotal clinical observations for a long time that a high percentage of non-celiac disease patients who suffer from wheat-related health problems can consume goods made from some spelt cultivars. The first research paper with robust statistical and experimental results also proved this important discovery (Armentia et al. 2012).
Since the endosperm of the kernels contain the major amount of FODMAP components, producing less bran in the flour or reducing the milling yield do not increase the FODMAP level. Baking and milling technology has an important effect on FODMAP levels in the products because during fermentation a certain amount of soluble carbohydrates are metabolized by yeast. Thus, FODMAP content in the end-products can be altered both by the length of fermentation and/or by the amount of yeast applied in the bread formulation. Sourdough technology has an even more substantial effect compared to yeasted dough products in reducing the FODMAP level (Fig. 14.3).
FODMAP content of yeasted and sour dough breads made from wheat and spelt. (Source: based on the data of Muir et al. 2014)
14.6 Breeding less Allergenic Spelt with Low FODMAP Content
Australia grown spelt cultivar GWF was successfully selected with considerably lower FODMAP content. According to Muir et al. (2014), in the baked goods produced from this line, the FODMAP content—using an optimized technology and formulation—was significantly lower than the threshold defined for low FODMAP products.
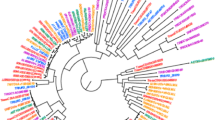
Intriguingly, this same line was observed to have considerably less immune reactivity than any other spelt and wheat cultivars to wheat sensitive individuals (Vu 2014, Vu et al. 2014). It was also observed that the albumin-globulin protein composition of GWF spelt is different from other wheat-related and wheat species (Fig. 14.4). One of these discrepancies has been identified as a mutation in a CACTA retrotransposon region tightly connected to a gene coding a beta-expansin protein in many spelt and wheat germplasm (Fig. 14.5) (Breen et al. 2010).
Principle component analysis based comparison of soluble protein composition (albumins and globulins) of 127 wheats and wheat relatives. (Source: Vu 2014)
Mutation in the expansin coding gene based on Breen et al. (2010)
A protein family closely connected to nonenzymatic proteins, the beta-expansins, located in plant cell walls, are known as pollen allergens. This alteration in the soluble protein composition was stated to be one of the reasons for the considerably lower immune reactivity of GWF spelt for wheat sensitive people (Vu 2014, Vu et al. 2014). The mutation in the expansin gene alone cannot account for the lower immune reactivity; however, it could be applied as an indicator for the uncommon soluble protein composition observed in GWF spelt. This modified protein configuration could be linked to the lower immune reactivity detected (Fig. 14.6).
Several bakeries in Australia have recognized the potential candidate source for producing cereal products with dual health benefits. A closed production line has been created, incorporating contract growing of GWF spelt with strict rules to avoid any contamination from other grains. A small milling capacity has been developed and special care has been devoted to clean the all milling equipment before GWF spelt processing. A PCR-based procedure has been established to reveal the abovementioned mutation in the expansin gene (Suter and Békés 2012) to screen for any contamination in the flour or grain samples induced by any other cereals with the wild type expansin protein (Fig. 14.7). This procedure is used for quality guarantee purposes. A dedicated laboratory monitors the FODMAP content of flour and their goods (sourdough bread with unique formulation). Flour from this unique spelt source, characterized by lower immune reactivity and low FODMAP, is currently being supplied to Australian artisan bakeries. The name of this special flour is Hildegard spelt flour, after St Hildegard von Bingen, an eleventh century nun who first observed the health advantages of spelt.
Monitoring the presence of mutation in the expansin gene, using a molecular marker, developed by Suter and Békés (2012). 1 - wheat; 2 - kamut; 3 - emmer; 4 - normal spelt; 5,6,7 - GWF spelt - 9 negative control. (Source: Békés et al. unpublished results)
In connection with this spelt line, there are prior experiences which provide some evidence to support the dual health benefits. The question is whether this observation is connected to the apparent lower allergenicity of spelt for those with wheat allergy. The answer needs a double-blind, placebo-controlled randomized clinical trial, compared to the allergic signs and IgE levels generated by consuming GWF spelt and wheat products, involving those who eat chiefly wheat and those who have replaced spelt for wheat in their diet.
A pre-breeding activity has been initiated in Hungary based on the positive experiences on evaluating GWF spelt to screen large spelt populations, selecting suitable processing quality and lines with low FODMAP content. The chosen lines are further examined by searching for genotypes containing the expansin-mutation, applying the ELISA based techniques (Bodinier et al. 2008) and PCR-based test of Suter and Békés (2012), respectively. As described in an initial survey carried out on 105 spelt lines (Pauk et al. unpublished results), the FODMAP content of spelt lines grown in the Carpathian Basin show large variation (0.7–1.8 g fructan in 100 g grain). More than 10% of the lines comprise lower or equal level of fructan than the Australian control, found to be helpful in low FODMAP diet (Fig. 14.8).
Variation of fructan levels in 105 spelt cultivars grown in Hungary at the same location. (Source: Ács et al. 2017)
Selection for quality in this program is based on three independent attributes: low FODMAP content, satisfactory bread making properties and the presence of the expansin mutation to be able to monitor the purity of the samples on grain, flour, and even the end-product stage.
In the early stages of a breeding program, when the sample size is extremely limited for traditional dough testing methodologies, a set of small-scale testing methods is applied. The FQC2000 micro mill (MeteFEM Budapest, Hungary) is used to mill flour for the functional studies (Békés et al. 2000; Tömösközi et al. 2001). This equipment is capable of making good quality flour from only 2–5 g of grain. Mixing properties of the breeders’ lines are monitored on a micro-doughLAB (Perten Instruments) mixer (Dang and Bason 2013; Haraszi et al. 2004). The Kieffer rig methodology and equipment (Stable MicroSystems) is applied for the determination of extensional parameters (Rmax and extensibility) (Kieffer et al. 1998), while a prototype SediCom System micro Zeleny equipment (Lab-Intern Ltd., Budapest, Hungary) was used for determination of Zeleny values. The measurements are carried out according to the modified ICC Standard No. 116/1. (Tömösközi et al. 2010).
14.7 Spelt Breeding Via in Vitro Androgenesis Using Anther and Isolated Microspore Culture Methods
Classical breeding is a time-consuming process. It takes about 10–12 years to release a new cultivar, because the selection of segregated population and growing and testing of different generations requires much time. To accelerate the generation changing in plant breeding and to achieve the homogeneity of selected lines within one generation, an efficient plant biotechnology method—based on in vitro androgenesis—was developed in spelt (Lantos et al. 2016, 2018).
14.7.1 Induction of in Vitro Androgenesis in Anther Culture
In spelt, in vitro anther culture was first tested with Hungarian spelt cv. GK Fehér. Later the protocol was also implemented with three other spelt cultivars. The number of produced embrioids and embryo-like structures (ELS) was 62/100 anthers. These structures regenerated dominantly green plantlets in vitro. The green plantlets production was 89.0% among the regenerated plantlets while the number of albino plantlets was low, 3.8/100 anthers. More than 1000 in vitro green plantlets were produced from anther culture of different spelt cultivars. After ploidy-level analyses (measurement of stomata length and flow cytometric analyses), using the haploid plants, doubled haploid plants were produced via colchicine treatment (Pauk et al. 2003) and produced seeds after rediploidization. The colchicine induced and spontaneous DH spelt plants were propagated in the greenhouse. The in vitro doubled haploid plant production method was integrated into our spelt breeding program in the similar way as was done with bread wheat (Pauk et al. 2003).
In our study, the first anther culture-derived ELS were observed after 3 weeks of androgenesis induction. The microspore-derived ELS with 1–2 mm size (Fig. 14.9a) were transferred to a Petri dish containing regeneration medium. The ELS produced significantly more green plantlets than albinos (Fig. 14.9b). Green plantlets were rooted in individual culture tubes (Fig. 14.9c) and regenerated plantlets acclimatized and grown in the greenhouse (Fig. 14.9d).
Important steps of anther culture in spelt: (a) Microspore-derived ELS, (b) Green plantlets in regeneration, (c) Rooting of individual plantlets, (d) Acclimatized plantlets in greenhouse. Red bars 5 mm for a; 10 mm for b and c; 50 mm for d. (Source: Lantos et al. 2018)
14.7.2 Effect of Genotype and Cold Pre-Treatment in Anther Culture
ELS could be produced with or without cold pre-treatment of the donor tillers (0 and 12 day cold pre-treatment) in anther culture. However, cold pre-treatment significantly improved the production of ELS and the number of regenerated green and albino plantlets in anther culture of each tested genotype (Table 14.3). The number of albinos was limited in anther culture (3.48 albinos/100 anthers), these values had a range of 0.5–7.47 albino plantlets/100 anthers depending on the cultivar. However, the production of green plantlets achieved high values, on average 41.45 in vitro green plantlets were regenerated from 100 anthers. The number of produced in vitro green plantlets had a range of 20.93–83.07 green plantlets/100 anthers. The most green plantlets (83.07/100 anthers) was regenerated from anther culture-derived ELS of cv. Franckenkorn. Altogether, more than 1000 green plantlets were produced from the anther culture of the 4 cultivars.
In a two-way analysis of variance (Lantos et al. 2018), the effect of pre-treatment and genotype was tested in anther culture of four spelt cultivars (data are not shown here). Based on the ANOVA test, genotype and cold pre-treatment of donor tillers significantly influenced the parameters of androgenesis (number of ELS, regenerated, green and albino plantlets). The effect of genotype × pre-treatment interaction was significant in the number of ELS, regenerated and green plantlets, too.
On the greenhouse grown plants, the fertile and partial fertile spikes were harvested. In the case of four tested spelt cvs. (Frankenkorn, Oberkulmer Rotkorn, GK Fehér, Mv Martongold) the values of seed setting were 11.8, 19.35, 44.44 and 21.47%, respectively. These data show that the anther culture method of spelt is ready for integration into the breeding process.
14.7.3 Induction of Androgenesis in Isolated Microspore Culture of Spelt
Because the anther culture technique needs significant manual work in isolation, to reduce this manual effort, we began to study the isolation microspore culture in spelt wheat as well. The microspore isolation was started, when in donor spikes, the stage of microspores was in uni- and binucleate stages (Fig. 14.10a, b). The ovary coculture was crucial in isolated microspore culture. Multicellular structures and ELS were not observed in isolated microspore culture without ovaries. The ovaries supported the development of multicellular structures and ELS (Fig. 14.10c, d). The transferred ELS produced both green and albino plantlets (Fig 14.10e) on the regeneration medium (Lantos et al. 2018).
Critical steps of isolated microspore culture: (a) Late uninucleate microspore, (b) Early binucleate microspore, (c) Multicellular structures (∗) and divided microspores, (d) Obtained ELS in ovary-supported (+) microspore culture, e Regenerated green and albino plantlets. Red bars 10 μm for (a) and (b); 50 μm for (c); 10 mm for (d) and (e). (Source: Lantos et al. 2018)
In isolated microspore culture, cv. Franckenkorn produced the most ELS and regenerated plantlets, while the production of ELS was the lowest in case of cv. Oberkulmer Rotkorn. In isolated microspore culture, the growth regulators were not essential in induction of androgenesis, but growth regulators increased the efficiency of the method (ELS, green and albino plantlets). The ELS production was high in isolated microspore culture. In practical terms, it was a big problem that most of the regenerants were albino plantlets. Altogether, three isolated microspore culture-derived in vitro green plantlets were regenerated. Two from cv. Franckenkorn and one from GK Fehér, respectively.
Androgenesis was induced in isolated microspore culture of each cultivar. The cultivars influenced the number of produced ELS, in vitro regenerated plantlets and albinos, while the exogenous growth regulators had significant effect on the tested parameter.
14.8 Conclusions and Prospects
The abovementioned method of producing a cereal line which is beneficial to human health can be used as a model, demonstrating how to protect baked goods from contamination by any other cereal material from the field to the bakery and how to accomplish (protect, monitor, check) the quality of the final product. These kinds of issues will hopefully emerge soon because of selecting and producing germplasm with proven reduced allergenicity and altered gluten and/or non-gluten protein composition.
Developing cereals with largely reduced allergenicity and with low FODMAP content is a much more realistic approach, compared to the ultimate task, developing celiac-safe cereals. Most wheat sensitive individuals—except for celiac patients—can consume these kinds of products.
Further detailed basic and applied research is required for the development, manufacturing and commercial availability of such products. This must include developing cost-effective, reliable and high-throughput analytical tools to monitor the quantity and the presence/absence of certain key components in the source material, the intermediates and the final product through the whole production chain, from the farm to the supermarket. Nevertheless, developing the necessary infrastructure for the production and commercialization of a multipurpose, healthier cereal product basis is only one of numerous requirements needing attention in the complex matter of providing appropriate food for people suffering from cereal-based health problems. It is essential to alter the legislative environment in order to permit the production/commercialization of products with altered/low gluten content, giving instructions on analytical procedures, defining thresholds of certain key components, determining food safety control systems, labelling, policing and regulating the very often misleading media-based marketing. However, the ultimate purpose is a more effective collaboration and communication among plant science, the grain industry as well as medical researchers and practitioners, together with up-to-date, accurate and permanent information of customers.
An effective in vitro androgenesis method was developed in spelt to develop the homogeneity of newly-selected spelt lines and to accelerate the pace of generation changes in breeding (Lantos et al. 2016). In this activity we were able to apply successfully those small and micro scale dough testing methods which have been developed earlier for characterizing bread wheat samples. With Hungarian cv. GK Fehér the anther culture method was carried out; however, in three other registered spelt cultivars tested using the protocol, 62 ELS/100 anthers produced embryo-like structures (ELS), from which we were able to regenerate green plantlets. A total of 89% of green plantlets production was achieved among the regenerated plantlets whereas the number of albinos was limited (3.8/100 anthers). All in all, from an anther culture of different spelt genotypes over 1000 in vitro green plantlets were created. The doubled haploid plantlets were produced via colchicine treatment and obtaining seed after spontaneous diploidization, based on ploidy-level analyses from the haploid plantlets (Pauk et al. 2003). In the greenhouse the colchicine treated and spontaneous DH plants were grown out. The in vitro haploid induction system was incorporated into the breeding program of spelt in the same way as was done with bread wheat (Pauk et al. 2003, 2004).
References
Abdel-Aal SM, Hucl P (2002) Amino acid composition and in vitro protein digestibility of selected ancient wheats and their end products. J Food Comp Anal 15:737–747
Ács K, Békés F, Lantos CS et al (2017) The role of carbohydrates in the development of food intolerance: is the low FODMAP diet the solution in the IBS treatment? GK Fórum 2017 02 01-02, GK. Szeged, Hungary. (in Hungarian)
Akagawa M, Handoyo T, Ishii T (2007) Proteomic analysis of wheat flour allergens. J Agr Food Chem 55:6863–6870
Altenbach SB, Tanaka CT, Allen PV (2014a) Quantitative proteomic analysis of wheat grain proteins reveals differential effects of silencing of omega-5 gliadin genes in transgenic lines. J Cereal Sci 59:118–125
Altenbach SB, Tanaka CT, Seabourn BW (2014b) Silencing of omega-5 gliadins in transgenic wheat eliminates a major source of environmental variability and improves dough mixing properties of flour. BMC Plant Biol 14(1):1–13
Aman P (1987) The variation in chemical composition of Swedish oats. Acta Agric Scand 37:347–352
Aman P, Hesselman K, Tilly AC (1985) The variation in chemical composition of Swedish barleys. J Cereal Sci 3:73–77
Amano MH, Ogawa K, Kojima T et al (1998) Identification of the major allergens in wheat flour, responsible for baker's asthma. Biochem J 330:1229–1234
An X, Li Q, Yan Y et al (2005) Genetic diversity of European spelt wheat (Triticum aestivum ssp. spelta L. em. Thell.) revealed by glutenin subunit variations at the Glu-1 and Glu-3 loci. Euphytica 146:193–201
Anderson RP, Wieser H (2006) Medical applications of gluten-composition knowledge. In: Wrigley CW, Békés F, Bushuk W (eds) Gliadin and glutenin: the unique balance of wheat quality. AACCI Press, St Paul MN, pp 387–409
Anderson JA, Churchilll GA, Autrique JE et al (1993) Optimizing parental selection for genetic linkage maps. Genome 36:181–186
Anderson RP, Degano P, Godkin AJ et al (2000) In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant a gliadin t-cell epitope. Nat Med 6:337–342
Andersson R, Fransson G, Tietjen M et al (2009) Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. J Agric Food Chem 57:2004–2008
Andersson AAM, Andersson R, Piironen V et al (2013) Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the HEALTHGRAIN diversity screen. Food Chem 136:1243–1248
Armentia A, Martin-Santos JM, Blanco M (1990) Exercise induced anaphylaxis reaction to grain flours. Ann Allerg 65:149–151
Armentia A, Martin-Santos JM, Diaz-Perales A et al (2012) A possible hypoallergenic cereal in wheat food allergy and baker’s asthma. Amer J Plant Sci 3:1779–1781
Balakireva A, Zamyatnin AA (2016) Properties of gluten intolerance: gluten structure, evolution, pathogenicity and detoxification capabilities. Nutrients 8:644
Békés F (2015) Health related quality attributes of wheat. In: Pauk J (ed) Proceedings 3rd conference of cereal biotechnology and breeding (cbb3) Aekadémiai Kiadó, Budapest, pp 1–3
Békés F, Southan MS, Tömösközi S, et al (2000) Comparative studies on a new micro scale laboratory mill. In: Panozzo JF, Ratcliffe M, Wootton M et al (eds) Proceedings 49th RACI Conference, RACI Melbourne Australia, pp 483–487
Bertin P, Gregoire D, Massart S, de Froidmont D (2004) High level of genetic diversity among spelt germplasm revealed by microsatellite markers. Genome 47:1043–1052
Biesiekierski JR, Rosella O, Rose R et al (2011) Quantification of fructans galacto-oligosaccharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet 24:154–176
Blatter RH, Jacomet S, Schlumbaum A (2004) About the origin of European spelt (Triticum spelta l.): allelic differentiation of the HMW glutenin B1-1 and A1-2 subunit genes. Theor Appl Genet 108:360–367
Bodinier M, Brossard C, Triballea S (2008) Evaluation of an in vitro mast cell degranu-lation test in the context of food allergy to wheat. Int Arch Allergy Imm 146:307–320
Bonafacci G, Galli V, Francisci R et al (2002) Characteristics of spelt wheat products and nutritional value of spelt wheat-based bread. Food Chem 68:437–441
Braly J, Hoggan R (2002) Dangerous grains. Why gluten cereal grains may be hazardous to your health. Penguin group, NY
Branchi F, Ferretti F, Norsa L et al (2015) Management of nonceliac gluten sensitivity by gastroenterology specialists: data from an Italian survey Biomed Res Int 2015. https://doi.org/101155/2015/530136
Brandolini A, Hidalgo A, Plizzari L et al (2011) Impact of genetic and environmental factors on einkorn wheat (Triticum monococcum L. subsp. monococcum) polysaccharides. J Cereal Sci 53:65–72
Breen M, Li D, Dunn DS et al (2010) Wheat beta-expansion (expb11) genes: identification of the expressed gene on chromosome 3bs carrying a pollen allergen domain. BMC Plant Biol 10:99
Büren M, Lüthy J, Hübner P (2000) A spelt-specific γ-gliadin gene: discovery and detection Theor Appl Genet 100:271–279
Büren M, Stadler M, Lüthy J (2001) Detection of wheat adulteration of spelt flour and products by PCR. Eur Food Res Technol 212:234–239
Caballero L, Martín LM, Alvarez BJ (2004) Variation and genetic diversity for gliadins in Spanish spelt wheat accessions. Genet Res Crop Evol 51:679–686
Caballero L, Martín LM, Alvarez JB (2008) Variation of high molecular weight glutenin subunits in two neglected tetraploid wheat subspecies. Czech J Genet Plant Breeding 44:140–146
Campbell KG (1997) Spelt: agronomy, genetics and breeding. Plant Breed Rev 15:187–213
Caruso R, Pallone F, Stasi E et al (2013) Appropriate nutrient supplementation in celiac disease. Ann Med 45:522–531
Catassi C, Fasano A (2008) Celiac disease. In: Arendt EK, DalBello F (eds) Gluten-free cereal products and beverages. Academic, San Diegos, pp 1–28
Catassi C, Bai JC, Bonaz B et al (2013) Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrient 5:3839–3853
Catassi G, Lionetti E, Gatti S et al (2017) The low FODMAP diet: many question marks for a catchy acronym. Nutrients 2017(9):292. https://doi.org/10.3390/nu9030292
Clarke B, Liang R, Morell MK et al (2008) Gene expression in a starch synthase IIa mutant of barley: changes in the level of gene transcription and grain composition. Funct Integr Genom 8:211–221
Comino I, Moreno M, Rodriguez-Herrera A et al (2013) The gluten-free diet: testing alternative cereals tolerated by celiac patients. Nutrients 5:4250–4268
Comino I, Bernardo D, Bancel E et al (2016) Identification and molecular characterization of oat peptides implicated on celiac immune response. Food Nutr Res 60:30324
Constantini A, Amoriello T, Cecchini C, D’Egibio MG (2008) Analysis of factors influencing fructans production in winter cereals. J Genet Breed 65(1–4):15–24
Cornell HJ, Hoveling AW (1998) Wheat – chemistry and utilization. Technomic Publishing, Landcaster-Basel, pp 327–373
Cubadda R, Marconi E (2002) Spelt wheat. In: Belton P, Taylor JRN (eds) Pseudocereals and less common cereals: grain properties and utilization potential. Springer, Berlin, pp 153–177
Dang JMC, Bason ML (2013) Determining elasticity of dough in the micro-doughlab. Perten Sci World 7:2–3
Diedhiou C, Gaudet D, Liang Y et al (2012) Carbohydrate profiling in seeds and seedlings of transgenic triticale modified in the expression of sucrose:sucrose-1- fructosyltransferase (1-SST) and sucrose:fructan-6-fructosyltransferase (6-SFT). J Biosci Bioeng 114:371–378
Ehdaie B, Alloush GA, Madore MA et al (2006) Genotypic variation for stem reserves and mobilization in wheat I. Postanthesis changes in internode dry matter. Crop Sci 46:735–746
Elia M, Moralejo M, Rodríguez-Quijano M et al (2004) Spanish spelt: a separate gene pool within the spelt germplasm. Plant Breed 123:297–299
Escarnot E, Ageessens R, Wathelet B, Paquot S (2010) Quantitative and qualitative study of spelt and wheat fibres in milling fractions. Food Chem 122:867–863
Escarnot E, Jacquemin JM, Agneessens R et al (2012) Comparative study of the content and profiles of macronutrients in spelt and wheat (a review). Biotech Agron Soc 16:243–256
Faris JD (2014) Wheat domestication: key to agricultural revolutions past and future. In: Tuberosa R (ed) Genomics of plant genetic resources. Springer, Dordrecht, pp 439–464
Ford R (2008) The gluten syndrome is wheat causing you harm? RRS Global Lt, Christchurch
Forster S, Schumann E, Baumann M et al (2013) Copy number variation of chromosome 5a and its association with q gene expression, morphological aberrations, and agronomic performance of winter wheat cultivars. Theor Appl Genet 126:3049–3063
Forster S, Schumann E, Pillen K et al (2014) Genetic and environmental effects on the occurrence of speltoids in winter wheat cultivars. Plant Breed 133:442–447
Fretzdorff B, Welge N (2003a) Abbau von getreideeigenen Fructanen wahrend der Herstellung von Roggenvollkornbrot. Getreide, Mehl und Brot 57:147–151
Fretzdorff B, Welge N (2003b) Fructan- und raffinosegehalte im vollkorn einiger getreidearten und pseudo-cerealien. Getreide Mehl Brot 57:3–8
Galli G, Francisci V, Mair R et al (2000) Characteristics of spelt wheat products and nutritional value of spelt wheat based bread. Food Chem 68:437–441
Galova Z, Knoblochova H (2001) Biochemical characteristics of five spelt wheat cultivars (Triticum spelta L). Acta Fytotechn Zootechn 4:85–86
Gebbing T (2003) The enclosed and exposed part of the peduncle of wheat (Triticum aestivum) – spatial separation of fructan storage. New Phytol 159:245–252
Gibson PR, Newnham R, Barrett JS et al (2007) Review article: fructose malabsorption and the bigger picture. Aliment Pharm Ther 25:349–363
Gibson PR, Varney J, Malakar S et al (2015) Food components and irritable bowel syndrome. Gastroent 148:1158–1174
Gil-Humanes J, Pistón F, Hernando A et al (2008) Silencing of γ-gliadins by RNA interference (RNAi) in bread wheat. J. Cereal Sci 48:565–568
Gomez-Becerra HF, Erdem H, Yazici A et al (2010) Grain concentrations of protein and mineral nutrients in a large collection of spelt wheat grown under different environments. J Cereal Sci 52:342–349
Green PH, Cellier C (2007) Celiac disease. New Eng J Med 357:1731–1743
Gulyás G, Rakszegi M, Bognár Z, Láng L, Bedő Z (2012) Evaluation of genetic diversity of spelt breeding materials based on AFLP and quality analyses. Cereal Res Comm 40:185–193
Hallert C, Grant C, Grehn S et al (2002) Evidence of poor vitamin status in celiac patients on a gluten-free diet for 10 years. Aliment Pharm Ther 16:1333–1339
Halmos EP, Power VA, Shepherd SJ et al (2014) A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroent 146:67–75
Halmos EP, Christophersen CT, Bird A et al (2015) Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 64:93–100
Hansen HB, Rasmussen CV, Bach-Knudsen KE et al (2003) Effects of genotype and harvest year on content and composition of dietary fibre in rye (Secale cereale L) grain. J Sci Food Agric 83:76–85
Haraszi R, Gras PW, Tömösközi S et al (2004) The application of a micro z-arm mixer to characterize mixing properties and water absorption of wheat flour. Cereal Chem 81:555–560
Haraszi R, Chassaigne H, Maquet A (2011) Analytical methods for detection of gluten in food - method developments in support to the legislations on labelling of foodstuffs. J AOAC Int 94:1006–1025
Huynh BL, Palmer L, Mather DE et al (2008a) Genotypic variation in wheat grain fructan content revealed by a simplified HPLC method. J Cereal Sci 48:369–378
Huynh BL, Wallwork H, Stangoulis JCR et al (2008b) Quantitative trait locifor grain fructan concentration in wheat (Triticum aestivum L.). Theor Appl Gen 117:701–709
Huynh BL, Mather D, Schreiber A et al (2012) Clusters of genes encoding fructan biosynthesizing enzymes in wheat and barley. Plant Mol Biol 80:299–314
Islam S, Ma W, Yan G et al (2011) Modifying processing and health attributes of wheat bread through changes in composition genetics and breeding. In: Cauvain SP, Tran B (eds) Bread making, improving quality, 2nd edn. Woodhead Publishing, Cambridge, UK, pp 259–296
Jargon J (2014) The gluten-free craze: is it healthy? The Wall Street Journal, 14 June 2014
Juhász A, Gy G, Békés F et al (2012) The epitopes in wheat proteins for defining toxic units relevant to human health. Funct Integr Genom 12:585–598
Juhász A, Haraszi R, Maulis CS (2015) ProPepper: a curated database for identification and analysis of peptide and immune-responsive epitope composition of cereal grain protein families. Database 2015:1–16. https://doi.org/101093/database/bav100
Junker Y, Zeissig S, Kim SJ et al (2012) Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of the toll-like receptor. J Exp Med 209:2395–2408
Karppinen S, Myllymaki O, Forssell P et al (2003) Fructan content of rye and rye products. Cereal Chem 80:168–171
Kieffer R, Wieser H, Henderson MH et al (1998) Correlations of the breadmaking performance of wheat flour with rheological measurements on a micro-scale. J Cereal Sci 27:53–60
Kling CI (1988) Dinkel - ein altes getreide tritt in den vordergrund. Dinkel-Ackerstiftung zur Förderung des Getreides Dinkel, Dinkelsymposium 1:31–47
Koenig A, Konitzer K, Wieser H et al (2015) Classification of spelt cultivars based on differences in storage protein compositions from wheat. Food Chem 168:176–182
Kohajdova Z, Karovicova J (2008) Nutritional value and baking applications of spelt wheat. Acta Sci Pol Technol Aliment 7:5–14
Kooiker M, Drenth J, Glassop D et al (2013) TaMYB13-1, a R2R3 MYB transcription factor, regulates the fructan synthetic pathway and contributes to enhanced fructan accumulation in bread wheat. J Exp Bot 64:3681–3696
Lahti A (1986) A contact urticaria to plants. Clin Dermatol 4:127–136
Lamacchia C, Camarca A, Picascia S et al (2014) Cereal-based gluten-free food: how to reconcile nutritional and technological properties of wheat proteins with safety for celiac disease patients. Nutrients 6:575–590
Langenkämper G, Zörb C, Seifert M et al (2006) Nutritional quality of organic and conventional wheat. J Appl Bot Food Qual 80:150–154
Lantos C, Jenes B, Bona L et al (2016) High frequency of doubled haploid plant production in spelt wheat. Acta Biol Cracov Ser Bot 58(2):35–40
Lantos C, Bona L, Nagy E et al (2018) Induction of in vitro androgenesis in anther and isolated microspore culture of different spelt wheat genotypes. Plant Cell Tissue Organ Cult 133:385–393
Lauriere M, Pecquet C, Bouchez-Mahiout I et al (2006) Hydrolyzed wheat proteins present in cosmetics can induce immediate hypersensitivities. Contact Dermatitis 54:283–289
Lelley T, Stachel M, Grausgruber H et al (2000) Analysis of relationships between Aegilops tauschii and the D genome of wheat utilizing microsatellites. Genome 43:661–668
Ludvigsson JF, Leffler DA, Bai JC et al (2013) The Oslo definitions for coeliac disease and related terms. Gut 62:43–52
Manifesto MM, Schlatter AR, Hopp HE et al (2001) Quantitative evaluation of genetic diversity in wheat germplasm using molecular markers. Crop Sci 41:682–690
Market Research (2012) Gluten-free foods in the U.S. 5th ed. Research and Markets, Dublin, Ireland. https://www.packagedfacts.com/gluten-free-foods-8108350/
Martos V, Royo C, Rharrabti Y et al (2005) Using AFLPs to determine phylogenetic relationships and genetic erosion in durum wheat cultivars released in Italy and Spain throughout the 20th century. Field Crops Res 91:107–116
Mayer F, Haase I, Graubner A et al (2012) Use of polymorphisms in the γ-gliadin gene of spelt and wheat as a tool for authenticity control. J Agric Food Chem 60:1350–1357
Mills ENC, Jenkins JA, Alcocer MJC et al (2004) Structural biological and evolutionary relationships of plant food allergens sensitizing via the gastrointestinal tract. Crit Rev Food Sci Nutr 44:379–407
Missbach B, Schwingshackl L, Billmann A et al (2015) Gluten-free food database: the nutritional quality and cost of packaged gluten-free foods. Peer J 3:e1337. https://doi.org/107717/peerj1337
Muir JG, Shepherd SJ, Rosella O et al (2007) Fructan and free fructose content of common Australian vegetables and fruit. J Agr Food Chem 55:6619–6627
Muir JG, Rose R, Rosela O et al (2009) Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography. J Agr Food Chem 57:554–565
Muir JG, Mills J, Suter DAI et al (2014) FODMAPs in gluten-free grains may explain improved gastrointestinal symptoms in IBS on a gluten-free diet. J Nutr Intermed Metab 1:14–15
Neeson R, Evans J, Burnett V et al (2008) Optimising the quality and yield of spelt under organic production in SE Australia. Proceedings of 14th Australian Society of Agronomy Conference ASAC, Adelaide, pp 24–31
Ng PKW, Scanlon MG, Bushuk W (1988) Electrophoretic and HPLC patterns of registered Canadian wheat cultivars. Cereal Res Comm 17:5–10
Pasco JA, Nicholson GC, Kotowicz MA (2012) Cohort profile: Geelong osteoporosis study. Int J Epidemiol 41:1565–1575
Pauk J, Mihály R, Puolimatka M (2003) Protocol for wheat (Triticum aestivum L.) anther culture. In: Kasha K, Maluszynski M (eds) Doubled haploid production in crop plants. Kluwer Academic Publisher, Dordrecht/Boston/London, pp 59–64
Pauk J, Hassan MS, Puolimatka M et al (2004) Microspore- and anther culture improvements for wheat breeding. In: Mujib A, Cho MJ, Predieri S et al (eds) In vitro application in crop improvement: recent progress. Science Publisher, Enfield, pp 131–151
Plugis MP, Khosla C (2015) Therapeutic approaches for celiac disease. Best Pract Res Cl Ga 29:503–521
Pruska-Kedzior A, Kędzior ZM, Kedzior ZM et al (2008) Comparison of viscoelastic properties of gluten from spelt and common wheat. Eur Food Res Technol 227:199–207
Pulido OM, Gillespie Z, Zarkadas M et al (2009) Introduction of oats in the diet of individuals with celiac disease: a systematic review. Adv Food Nutr Res 57:235–825
Radic H, Günther T, Kling CI et al (1997) Characterization of spelt (Triticum spelta L.) forms by gel-electrophoretic analyses of seed storage proteins. II. The glutenins. Theor Appl Gen 94:882–886
Radic-Miehle H, Saam C, Hüls R et al (1998) Characterization of spelt (Triticum spelta L.) forms by gel-electrophoretic analyses of seed storage proteins. III. Comparative analyses of spelt and central European winter wheat (Triticum aestivum L.) cultivars by SDS-PAGE and acid-PAGE. Theor Appl Gen 97:1340–1346
Rakha A, Aman P, Andersson R (2011) Dietary fiber in triticale grain: variation in content, composition, and molecular weight distribution of extractable components. J Cereal Sci 54:324–331
Raman H, Rahman R, Luckett D et al (2008) Characterisation of genetic variation for aluminium resistance and polyphenol oxidase activity in genebank accessions of spelt wheat. Breeding Sci 59:373–381
Ruibal-Menieta ML, Delacroix DL, Mignelot E et al (2005) Spelt (Triticum aestivum ssp spelta) as a source of breadmaking flours and bran naturally enriched in oleic acid and minerals but not phytic acid. J Agr Food Chem 53:2751–2759
Sapone A, Bai JC, Ciacci C et al (2012) Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med 10:13. https://doi.org/101186/1741-7015-10-13
Saturni L, Ferretti G, Bacchetti T (2010) The gluten-free diet: safety and nutritional quality. Nutrients 2:16–34
Schober TJ, Kuhn M (2003) Capillary zone electrophoresis for gliadin separation: application in a spelt breeding program. Eur Food Res Tech 217:350–359
Schober TJ, Clarke CI, Kuhn M (2002) Characterization of functional properties of gluten proteins in spelt cultivars using rheological and quality factor measurements. Cereal Chem 79:408–414
Schober TJ, Bean SR, Kuhn M (2006) Gluten proteins from spelt (Triticum aestivum ssp. spelta) cultivars: a rheological and size-exclusion high-performance liquid chromatography study. J Cereal Sci 44:161–167
Schuppan D, Pickert G, Ashfaq-Khan M et al (2015) Non-celiac wheat sensitivity: differential diagnosis triggers and implications. Best Pract Res Cl Ga 29:469–476
Shepherd SJ, Gibson PR (2006) Fructose malabsorption and symptoms of IBS: management. J Am Diet Assoc 106:1631–1639
Shepherd SJ, Parker FC, Muir JG et al (2008) Dietary triggers of abdominal symptoms in patients with IBS: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 6:765–771
Shewry PR (2002) The major seed storage proteins of spelt wheat, sorghum millets and pseudocereals. In: Belton P, Taylor J (eds) Pseudocereals and less common cereals: grain properties and utilization potential. Springer, Berlin, pp 1–24
Shewry PR, Tatham AS (2016) Improving wheat to remove celiac epitopes but retain functionality. J Cereal Sci 67:12–21
Shimbata T, Inokuma T, Sunohara A et al (2011) High levels of sugars and fructan in mature seed of sweet wheat lacking GBSSI and SSIIa enzymes. J Agric Food Chem 59:4794–4800
Siles RI, Hsieh FH (2013) Allergy blood testing: a practical guide for clinicians. Clev Clin J Med 78:585–592
Sollid LM, Qiao SW, Anderson RP et al (2012) Nomenclature and listing of celiac disease relevant gluten t-cell epitopes restricted by HLA-dq molecules. Immunogenetics 64:455–460
Suter DAI, Békés F (2012) Wheat immunoreactivity. Aust Patent http://v3 espacenetcom/ publicationdetails/ biblio?cc=hu&nr= au2011000468
Tatham AS, Shewry PR (2008) Allergy to wheat and related cereals. Clin Exp Allergy 38:1712–1726
Tömösközi S, Varga J, Fodor D et al (2001) Laboratory mill for small-scale testing. In: Shewry PR, Tatham AS (eds) Wheat gluten. Royal Society of Chemistry, Cambridge, pp 317–320
Tömösközi S, Nádosi M, Balázs G et al (2010) Revival of sedimentation value – method development, quality prediction and molecular background. In: Branlard G (ed) Gluten proteins 2009. Proceedings of 10th international gluten workshop. INRA Clermont-Ferrand, pp 104–108
Verspreet J, Cimini S, Vergauwen R et al (2013) Fructan metabolism in developing wheat (Triticum aestivum L.) kernels. Plant Cell Phys 54:2047–2057
Verspreet J, Dornez E, Van den Ende W et al (2015) Cereal grain fructans: structure, variability and potential health effects. Trends Food Sci Technol 43:32–42
Vu NT (2014) Comparative analysis of the soluble wheat proteins and human health. PhD thesis. Sydney University, Sydney Australia
Vu NT, Chin J, Pasco JA et al (2014) The prevalence of wheat and spelt sensitivity in a randomly selected Australian population. Cereal Res Comm 43:97–107
Wangen S (2009) Healthier without wheat. A new understanding of wheat allergies, celiac disease and non-celiac gluten intolerance. Innate Health Pub, Seattle
Wardlaw IF, Willenbrink J (2000) Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytol 148:413–422
Wei JZ, Chatterton NJ, Larson SR et al (2000) Linkage mapping and nucleotide polymorphisms of the 6-SFT gene of cool-season grasses. Genome 43:931–938
White J, Law JR, MacKay I et al (2008) The genetic diversity of UK, US and Australian cultivars of Triticum aestivum measured by DArT markers and considered by genome. Theor Appl Genet 116:439–453
Wieser H (2000) Comparative investigations of gluten proteins from different wheat species. I Qualitative and quantitative composition of gluten protein types. J Eur Food Res Tech 211:262–268
Wieser H (2006) Comparison of pure spelts and spelt/wheat crossbreeds. Getreide-technologie 60:223–231
Wrigley CW, Bietz JA (1988) Proteins and amino acids. In: Pomeranz Y (ed) Wheat – chemistry and technology, vol I. AACC, St Paul MN, pp 159–275
Xue GP, Kooiker M, Drenth J et al (2011) TaMYB13 is a transcriptional activator of fructosyltransferase genes involved in beta-2,6-linked fructan synthesis in wheat. Plant J 68:857–870
Yang JC, Zhang JH, Wang ZQ et al (2004) Activities of fructan- and sucrose-metabolizing enzymes in wheat stems subjected to water stress during grain filling. Planta 220:331–343
Yasui T, Ashida K (2011) Waxy endosperm accompanies increased fat and saccharide contents in bread wheat (Triticum aestivum L.) grain. J Cereal Sci 53:104–111
Zevallos VF, Raker V, Tenzer S et al (2016) Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017. https://doi.org/10.1053/j.gastro.2016.12.006
Zuidmeer L, Goldhahn K, Rona RJ et al (2008) The prevalence of plant food allergies: a systematic review. J Allergy Clin Immun 121:1210–1218
Acknowledgement
This study was supported within project OTKA-K_16-119835, funded by the National Research, Development and Innovation Office. The experiments were interlocked with GINOP project (GINOP-2.2.1.-15-2016-00026). This project was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the ÚNKP-18-4-BME-393 National Excellence Program of the Ministry of Human Capacities. The work is connected to fulfil of the scientific goals of BME-Biotechnology FIKP grant of EMMI (BME FIKP-BIO).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Appendices
14.1.1 Appendix I: Research Institutes Relevant to Spelt
Institution | Specialization and research activities | Contact information and website |
|---|---|---|
Cereal research non-profit ltd., Szeged, Hungary | Research and breeding | |
Centre for Agricultural Research, agricultural institute, Martonvásár, Hungary | Breeding and research (FODMAP) |
14.1.2 Appendix II: Spelt Genetic Resources
Cultivar | Important traits | Cultivation location |
|---|---|---|
Center for Plant Diversity, Tápiószele, Hungary | Gene bank, genetic resources, germplasm collection |
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pauk, J. et al. (2019). Spelt (Triticum spelta L.) In Vitro Androgenesis Breeding for Special Food Quality Parameters. In: Al-Khayri, J., Jain, S., Johnson, D. (eds) Advances in Plant Breeding Strategies: Cereals. Springer, Cham. https://doi.org/10.1007/978-3-030-23108-8_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-23108-8_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23107-1
Online ISBN: 978-3-030-23108-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)