Abstract
This paper provides an overview of the related scientific literature, with some of our own targeted research, to investigate the possible causes of the recently increased prevalence of various forms of dietary cereal sensitivities. Detailed scientific investigations do not support the controversial idea that human practices, particularly modern wheat breeding, may have contributed to the increase in celiac disease (CD) prevalence during the latter half of the twentieth century. Each of the primitive wheat relatives and each historic or modern bread and durum wheat variety contains more or less amounts of toxic/allergenic epitopes. In the last 120 years, health-related quality attributes have not been considered in pre-breeding or breeding, but the yield- and functional quality-oriented selection procedures have resulted in unintended spinoff effects on the amounts of harmful compounds in new lines. Because of the trend of decreases in overall protein content, as well as the alteration of the glutenin-to-gliadin content to improve dough strength, older varieties are higher in gliadin content with consequent higher CD antigenicity. Meanwhile practices, introduced during the last 50 years in utilizing wheat in the food industry, have significantly increased the consumption of untreated prolamin proteins, including gluten proteins. Other factors for consideration are the incorporation of vital gluten as a cheap protein supplement in some food products and the reduction of fermentation time during bread making. Beyond the obvious effects of improved and more widely used diagnostic tests in medical practice, the increased incorporation of untreated gluten proteins and residual FODMAPs might be major reasons for the increasing prevalence of wheat sensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 80% of the protein content of wheat has the essential biological role of storing nitrogen for use by the germinating seed. Meanwhile, this highly complex mixture of proteins is the major determinant of the processing (techno-functional) properties of the grain (Shewry 2019). Based on the generally accepted definition of Islam et al. (2011), wheat gluten is a protein–lipid–carbohydrate complex formed as a result of specific covalent and non-covalent interactions among flour components during dough making, during which the components are hydrated and energy from mechanical input from the mixing process is provided. Gluten proteins are encoded by multigene families at multiple loci on the three genomes of bread wheat, with a high degree of polymorphism between genotypes. The individual proteins have characteristic, unusual structure with glutamine- and proline-rich repetitive sequences.
Nowadays, the terms “gluten” and “gluten-free” are used more widely (and wrongly) in everyday practice, the latter loosely referring to food products not containing cereal prolamin proteins, i.e., wheat gliadin and glutenin analogues.
Although gluten was identified as the trigger for celiac disease (CD) almost 70 years ago, interest in this role of gluten has been limited outside of the scientific community. However, the last 20 years have seen an explosion of interest in gluten, particularly in the popular press and social media. Shewry (2019) reported that a “Google” search, carried out in December 2018, gave almost 400 million hits in less than a minute, all referring to the role of gluten in triggering a range of adverse reactions. Consequently, there have been increasing proportions of the population in many countries choosing to adopt a gluten-free, or low-gluten, diet.
Consumption of “gluten-containing” food is supposed to cause disease for a significant minority of people who consume foods derived from wheat, rye, and barley. Until a few years ago, celiac disease was the major (if not the only) well-known gluten-related disorder. However, in recent years it has become clear that gluten proteins may activate different pathological mechanisms, leading to a wide spectrum of human diseases, including non-celiac gluten sensitivity (NCGS), gluten ataxia, neuro-psychiatric disorders, and many others.
Cereal-related health disorders
The different gluten-related disorders share a trigger (namely gluten) and treatment (namely the gluten-free diet). However, these disorders show specific physiological mechanisms and clinical aspects. For a very long time, awareness of these disorders has been limited and, therefore, the epidemiology of gluten-related disorders is still a “work in progress.” Current research strives to clarify the boundaries between these entities, their disease mechanisms, and how a proper diagnosis can be implemented (Catassi and Fasano 2018). One of the most remarkable changes in our knowledge in this area is the realization that in several types of disorders related to the consumption of “gluten-containing” cereals, the trigger compounds are not components of the gluten but specific soluble proteins, such as amylase trypsin inhibitors (Kusaba-Nakayama et al. 2000; Junker et al. 2012; Zevallos et al. 2017; Bose et al. 2020) and fermentable oligosaccharides (FODMAPs), especially fructans (Hungin et al. 2003; Biesiekierski et al. 2013). Scientific literature published in the last 5 years differentiates non-celiac gluten sensitivity (NCGS) and non-celiac wheat sensitivity (NCWS) (Guandalini and Polanco 2015; Kucek et al. 2015; Molina-Infante and Carroccio 2017; Al-Toma et al. 2019). Furthermore, Uhde et al. (2016) reported that NCGS could be differentiated from a CD reaction if there were a reaction to native gliadin IgA but no reaction to deamidated gliadin. Another recently observed characteristic that seems to serve as a differential diagnostic tool is that the anti-gluten IgG antibody in NCGS is significantly different from that of CD in subclass distribution (Uhde et al. 2020).
Despite the numerous valuable recent research publications available in the area covering all aspects of the cereal-related health disorders (Kucek et al. 2015; Rybalka 2017; Brouns et al. 2019; Al-Toma et al. 2019; Rustgi et al. 2019; Caio et al. 2019; Tye-Din et al. 2018; Bose et al. 2020), there is some confusion and a lack of knowledge and understanding in the minds not only of consumers but also of medical practitioners (Branchi et al. 2015; Castillo et al. 2015).
To satisfy the specific health-related demands of specific consumer groups, the challenge is for cereal breeding and the food industry to develop new, “healthier” germplasm and/or to produce food products suitable for those with cereal sensitivities. To define these requirements, a better understanding of the different health-related disorders, their prevalence and the causal compounds is required, not only in the relevant basic research, but also in plant breeding and in the grain industry.
The general public in most Western countries is now aware of the potential adverse effects of cereals containing gluten with reports appearing in the lay press (Braly and Hogganm 2002; Ford 2008; Wangen 2009; Davis 2011) promoting gluten-free diets. Close to 10% of the population is electively following a gluten-free (GF) diet despite having no evidence of relevant disease (Mardini et al. 2015). As a result, the gluten-free market is expanding as GF foods are perceived as a therapeutic food as well as a valid lifestyle choice. The global market for gluten-free products was valued at 4.63 billion USD in 2015 and is projected to reach 7.59 billion USD by 2020, at a compound annual growth rate (CAGR) of 10.4% from 2015 to 2020. In Australia, where more than 23% of new bakery products were marketed as gluten-free in 2014, it is expected that gluten-free retail sales will exceed $100 million in the next 5 years (Jargon 2014). In 2017, Hester et al. (2017) reported that 20% of Australian consumers were avoiding gluten and purchasing gluten-free products, with long-lasting implications for commercial cereal breeding and the grain industry.
Many of these popular reports about the noxiousness of cereal-based food products fail to draw attention to the importance of appropriate diagnosis to define the nature of the presumed gluten “intolerance” that an individual may experience. This lack of appropriate advice poses a significant threat and challenge to the grain industry. Without any scientific or even practical evidence, the public draw the conclusion that the increasing prevalence of cereal-related health disorders are due to modern plant breeding, claiming that ancient relatives of wheat, such as spelt and heritage wheat cultivars, are harmless compared to modern wheats (Braly and Hogganm 2002; Ford 2008; Wangen 2009; Davis 2011; de Lorgeril and Salen 2014).
This report aims to give an overview of some aspects of recent developments in this booming area, providing information about the trigger compounds and prevalence of different cereal-related health disorders, about the scientific views concerning the overall healthiness of a gluten-free diet and of the status of celiac-safe cereal development. Based on the published scientific literature and some directly targeted aspects of our own research, we discuss the answer to the obvious question: What is behind the significantly larger number of cases of cereal-related health disorders in recent years; is it true that our breads today are different from those that our grandparents consumed?
Prevalence
Wheat-related sensitivities, prevalence, and wheat components responsible for these disease pathologies, based on the publication of Kucek et al. (2015), are shown in Table 1. Due to its rapidly increasing prevalence (Boukid et al. 2017; Manti et al. 2017), CD has gained much attention in recent years. Based on the report of Ludvigsson et al. (2013), the incidence of CD in the USA is about 1%. However, a survey conducted from 2009 to 2012 showed that potentially 0.79% of the general U.S. population demonstrates serologic evidence of CD autoimmunity (Mardini et al. 2015). Similar surveys report 2.4% in Finland, 0.3% in Germany, 0.7% in Italy (Mustalahti et al. 2010), and 0.76% north China. (Yuan et al. 2017). Based on the work of Catassi et al. (2014, 2015), the reports of low prevalence (or absence) in sub-Saharan Africa and in the Asia–Pacific region are related to the very low diagnostic rates, mostly due to lack of diagnostic facilities and poor disease reporting.
The most recently published data on the prevalence of CD is a systematic review and meta-analysis by Singh et al. (2018), carried out on global and regional data, underlining that a large proportion of patients with genetic disorders do not show physiological symptoms. The pooled global prevalence of CD was 1.4% in 275,818 individuals, based on positive results from tests for anti–tissue transglutaminase, while the biopsy-confirmed prevalence of CD was 0.7% (95% confidence interval in 138,792 individuals). The prevalence was higher in females vs male individuals (0.6% vs 0.4%; P < 0.001). The prevalence of celiac disease was significantly greater in children than adults (0.9% vs 0.5%; P < 0.001).
Results from a work carried out on a large set of blood samples from randomly selected individuals (n = 1145) (Pasco et al. 2012), analyzed by the IgE RAST method against wheat and milk antigens and food allergy questionnaires, indicated that the prevalence of wheat allergy was 2.5% where both a positive IgE immune response and symptoms against wheat were observed (Vu et al. 2014). It was postulated that the remaining 12.8% (n = 125) of individuals who showed raised IgE antibody levels without symptoms might have a latent wheat sensitivity with the potential of developing symptoms sooner or later. It was postulated that the large proportion (12.9%) of the investigated population, who have symptoms associated with the consumption of wheat products but who did not have raised IgE, may suffer from other wheat-related disorders (i.e., not IgE-mediated), such as celiac disease, non-celiac gluten sensitivity (NCGS), or a reaction to fructans (FODMAPs) for those with (irritable bowel syndrome IBS) (Vu et al. 2014). These results are in full agreement with similar investigations on wheat where the prevalence of wheat IgE sensitization in European countries is 2.9% (Zuidmeer et al. 2008; Siles and Hsieh 2013).
Gluten-free versus low-FODMAP diet
In a survey carried out in 2012 in the USA (Gluten-Free Foods in the U.S., 5th Edition, 2012) of those who eat gluten-free foods, only 5.7% claimed a formal medical diagnosis. From the rest of the consumers on a gluten-free diet, 36% do so for reasons other than sensitivity, 65% because they think it is healthier, 27% because they believe that it aids weight loss, 7% do so to help reduce inflammation, and 4% do so to combat depression (Digiacomo et al. 2013).
Consumers, food manufacturers, and health professionals are uniquely influenced by the growing popularity of the gluten-free diet. Consumer expectations have urged the food industry to continuously adjust and improve the formulations and processing techniques used in gluten-free product manufacture. Health experts have been interested in the nutritional adequacy of the GF diet, as well as its effectiveness in managing gluten-related disorders and other conditions. Several excellent text books and review articles (Arendt and Dal Bello 2008; El Khoury et al. 2018) provide a clear picture of the current motivations behind the use of gluten-free diets, as well as the technological and nutritional challenges of the diet as a whole. Alternative starches and flours, hydrocolloids, and fiber sources were found to play a complex role in mimicking the functional and sensory effects of gluten in gluten-free products. However, the quality of gluten-free alternatives is often still inferior to the respective gluten-containing products.
The gluten-free diet (GFD) has demonstrated benefits in managing some gluten-related disorders, though consequent nutritional imbalances have been reported (Di Nardo et al. 2019; Melini and Melini 2019).
Comino et al. (2013) provide a good overview on the social and economic repercussions generated by a GFD. Meanwhile, it cannot be regarded as a healthy diet (Balakireva and Zamyatnin 2016; Melini and Melin 2019). As is reviewed by Penagini et al. (2013), a GFD may lead to possible nutrient deficiencies in fiber as well as deficiencies in phytochemicals, trace elements, plus a high glycemic response resulting in consequent diseases. Alternatively, several studies suggest pseudo-cereal sources of fiber instead of gluten-free products to maintain the necessary fiber content levels (Saturni, et al. 2010; Békés et al. 2016). A GFD also leads to a deficiency in Vitamins C, B12, D, and folic acid (Hallert et al. 2002), as well as a lack of microelements, most importantly calcium, magnesium, and zinc (Caruso et al. 2013). At the same time, the gluten-free diet contains high amounts of sugar and hydrogenated fats, which could result in the occurrence of hyperinsulinemia and an increased obesity risk (Lamacchia et al. 2014). Thus, a GFD appears to be an unbalanced diet, being inadequate regarding both macro- and micronutrients. Recent intensive efforts of food manufacturers to improve the nutritional (and functional) characteristics of a GFD show good progress; however, these new-generation GFD products are significantly more expensive and therefore not available to a large proportion of customers.
The current view of medical experts is that, excluding people suffering from celiac disease, the majority of individuals who are feeling better on the “wheat-free” or “gluten-free” diet are automatically selecting a low-FODMAP diet, because by choosing “gluten-free” products they are generally also low in FODMAP (Halmos et al. 2014, 2015).
Realization of the crucial importance of certain fermentable carbohydrate components (FODMAPs, Gibson and Shepherd 2005) for food-related health disorders initiated active research and development in analyzing FODMAP contents, as well as recommending food materials with low-FODMAP content. Compared to the “gluten-free” phenomena, where the problematic food sources are a relatively small group of certain cereals, the establishment of a low-FODMAP diet involves considering a much wider range of plant-origin foodstuffs. Publications (Muir et al. 2007, 2009; Biesiekierski et al. 2011), and even computer and mobile phone applications (http://www.med.monash.edu.au/cecs/gastro/fodmap/iphone-app.html), are available to help to develop custom-designed low-FODMAP diets.
Traditional cereals (wheat, barley, and in particular rye) are relatively rich in FODMAPs, and therefore, food products made from them are not recommended for low-FODMAP diets. Compared to bread wheat, spelt (Triticum aestivum subsp. spelta) contains significantly lower amounts of fructose and fructans. But even more important is the finding that there is around a fivefold larger inter-varietal difference among spelt varieties compared to those found among bread-wheat genotypes (Pauk et al. 2019). This observation provides a basis for screening spelt cultivars for low-FODMAP content.
Consumers are urged to be mindful of the sensorial limitations and nutritional inadequacies of the GF diet, despite ongoing strategies to improve them.
The effect of breeding on the amount of harmful components in wheats
A large variation can be observed comparing the amounts of harmful compounds found in different cereal species and cultivars (Spaenij-Dekking et al. 2005; Carroccio et al. 2011). Variations in gliadin expression could contribute to the degree of CD antigenicity (Salentijn et al. 2009). Similarly, cereals containing diverse amounts of fructans (relevant to FODMAP) contribute differently to the development for IBS. If the cause of wheat-related health disorders is the result of spontaneous and later conscious selection activity in wheat breeding, there should be no harmful components (toxic/allergen epitopes) in the “old” wheat cultivars, landraces, even, those primitive wheat relatives from which evolution has built up the current hexaploid bread wheats. In the last 20 years, this working hypothesis was the basis for initiating intensive research activity to analyze the genetics/protein composition and health-related quality attributes of various primitive wheat species and also to re-investigate historic wheat cultivars, comparing them to modern varieties. The depth of this research was significantly improved recently by the availability of the complete wheat genome (Appels 2018), resulting in detailed and precise information on genes and products related to health-related issues (Juhász et al. 2018; Bose et al. 2020).
Comparison of primitive wheat relatives and present bread wheats
Gluten proteins
The triggering responses for wheat-sensitive individuals are specific amino acid sequences in proteins, toxic/allergenic epitopes. Intensive research on comparing amino acid sequences of proteins in wheat relatives (Molberg et al. 2005; Spaenij-Dekking et al. 2005; van Herpen et al. 2006) indicated large variations in the distribution among and between different groups of gluten proteins, resulting in variations in the epitope content in different species. Kucek et al. (2015) in their review explain the variation in celiac immune-reactivity among species of wheats, based on their genome composition. Species that lack the D genome of wheat, such as einkorn, emmer, and durum, appear to exhibit on average lower immunogenicity than common wheat, because the most harmful α-gliadins are coded by the D genome of wheat, which is present in common wheat, but absent in most of the “old” wheat species.
Since spelt is hexaploid, containing a D genome, its harmfulness was found to be similar to that of common wheat. In agreement with the data published by Vincentini et al. (2007), and van den Broeck et al. (2010b), Pauk et al. (2019) found similar levels of immune-reactivity when comparing spelt and common wheat. Einkorn, which has only the A genome of wheat, expressed the least number of celiac disease epitopes among cultivated species (Vincentini et al. 2007) with low immune-reactivity (Pizzuti et al. 2006; Zanini et al. 2009). Nevertheless, einkorn still expressed T cell immunogenic α- and γ-gliadin epitopes (Molberg et al. 2005; van Herpen et al. 2006). Although the average reactivity of emmer and durum (containing only the A and B genomes) is lower than that of common wheat (also having the D genome), there is a wide range of celiac responses depending on genotype (Auricchio et al. 1982; Molberg et al. 2005; Vincentini et al. 2009; van den Broeck et al. 2010a; Vader et al. 2002). The B genome of wheat encodes the smallest number of α-gliadin epitopes (van Herpen et al. 2006). Diploid species with genomes similar to the B genome of wheat are not cultivated or normally consumed by humans.
Relative quantitation of CD epitopes using immune techniques (Gell et al. 2015), mass spectroscopy, or proliferic assays with T cell lines from patients with CD when exposed to different diploid, tetraploid, and hexaploid wheats, showed (Suligoj et al. 2013) that all wheat species investigated contain varying levels of CD epitopes and that each of them is antigenic to some degree. So, while some diploid monococcum lines can be found with lower CD antigenicity (Pizzuti et al. 2006), neither diploid wheat relatives (Vaccino et al. 2009; Gianfrani et al. 2012) nor ancient durum relatives, such as Kamut (Gregorini et al. 2009; Colomba and Gregorini 2012), are safe for CD subjects.
FODMAP content
While there is a great amount of information about the FODMAP content and its nutritive importance in bread wheat, reviewed recently by Grausgruber et al. (2019), similar published data on wheat relatives is rather limited. FODMAP content in whole-grain flours and breads made of different varieties of bread wheat, spelt, durum, emmer, and einkorn were determined by Ziegler et al. (2016). Fructans and raffinose were the only FODMAP components detected in wheat flour. Total FODMAP contents ranged from 1.24 ± 0.38 to 2.01 ± 0.42 g/100 g DM in emmer and einkorn flours, respectively. By contrast, Brandolini et al. (2011) reported higher fructan concentrations in four einkorn accessions, compared to a check bread wheat variety; this conclusion was confirmed by Ziegler et al. (2016).
Gibson and Shepherd (2010) recommended consuming spelt products instead of bread wheat. Although the popular press (for example, Davis 2011) has indicated that consuming ancient or heritage wheat prevents sensitivity, the scientific literature does not support this claim. No wheat species or varieties are currently approved for diagnosed celiac or wheat-sensitive and allergic individuals to consume.
Compared to bread wheat, Escarnot et al. (2015) and Ziegler et al. (2016) reported lower fructan concentrations for spelt wheat, but the ranges of concentrations in spelt and bread wheat were largely overlapping. However, certain spelt lines show significantly lower fructan levels. For example, E3 spelt, grown in Australia, contains almost half of the usual levels of fructans in bread wheat and over 20% less than any other spelt wheat cultivar grown under the same conditions (Békés et al. 2016, 2017). Using E3 spelt as control, 105 spelt lines grown in Hungary have been screened for FODMAP content and seven lines showed even lower FODMAP levels (< 0.9%) than the control (Békés et al. 2016, 2017; Pauk et al. 2019). The FODMAP content of bread made from E3 spelt, using a typical no time dough (NTD) formulation and process, was considerably lower than the threshold defined for low-FODMAP products, showing significantly better responses to IBS patients than the control in a pilot scale single-blind, crossover intervention trial (Muir et al. 2014).
ATIs content
The genes coding for human α-amylase inhibitors (ATIs) are located on chromosomes 3BS and 3DS of wheat, while there is no evidence of their existence in the A genome. In previous studies, ATI sequences were identified in the A genome of wheat (both in bread and durum wheats), but these genes are silent or expressed at a low level (Zoccatelli et al. 2012; Capocchi et al. 2013). In addition, no activity against heterologous α-amylase has been found in extracts from diploid wheat species with the A genome (Garcia-Maroto et al. 1990, 1991; Dupont et al. 2011) and no inhibition of human α-amylases has been detected in einkorn wheat (Reig-Otero et al. 2017). Lower bioactivity was observed in older wheat variants, including spelt (a hexaploid wheat), emmer (tetraploid), and einkorn (diploid) (Zevallos et al. 2017).
In bread wheat, ATIs represent up to 4% of total wheat proteins and consist of at least 14 types of subunit proteins (Altenbach et al. 2011). As is discussed in depth by Huang et al. (2020), the nomenclature of ATI subunits is inconsistent, including the wheat ATI monomer with a molecular weight (MW) of 12 kDa, often referred to as the 0.28 inhibitor, based on its electrophoretic mobility; there is also the ATI homo-dimer with a MW 24 kDa, referred to as the 0.19 and 0.53 inhibitors, and the ATI hetero-tetramers with MW 60 kDa, also referred to as CM proteins. CM proteins, termed for their solubility in chloroform/methanol mixtures, include subunits CM1, CM2, CM3, CM16, and CM17. Each ATI subunit ranges from about 11 to 16 kDa in MW and contains 10 cysteine residues forming five intramolecular disulfide bonds while dimers and tetramers associate in a non-covalent manner (Altenbach et al. 2011). The multimeric forms of ATIs determine their biological activity; the ATI tetramer is five times more active than the monomer (Zevallos et al. 2017).
Until recently, contents of ATIs in different wheat species were not available. Geisslitz et al. (2018) quantitated the predominant ATI components (ATI 0.19 + 0.53, 0.28, CM2, CM3, and CM16) in eight cultivars, each identified from common wheat, durum wheat, spelt, emmer, and einkorn grown under the same environmental conditions by targeted liquid chromatography-tandem mass spectrometry (LC–MS/MS). The distribution of ATI types was characteristic for hexaploid, tetraploid, and diploid wheat species and suitable as species-specific fingerprints. Spelt and emmer had higher ATI contents than common wheat, with durum wheat in between. Only three of eight einkorn cultivars contained ATIs in very low concentrations.
Comparing historic and modern wheat varieties
Gluten proteins
The primary aim of wheat breeding since its beginning has been to develop new varieties with higher yields to satisfy the need of the growing population in the world. Modern agriculture (since the 1950s) has applied crossbreeding to different wheat and grass species, thus to generate new genetic varieties of increased yield. During the Green Revolution, the introduction of the reduced-height semi-dwarfing genes ((Rht)-B1b and Rht-D1b) led to impressive increases in wheat yields (Hedden 2003).
The impact of breeding on grain yields of wheat varieties released during the twentieth century has been extensively studied, and a general genetically determined relationship has been observed: There is a reciprocal relationship between protein concentration and grain yield (Mohler et al. 2011 and Sherman et al. 2014). Analyzing the results of the German official variety trials and on-farm during the 1983–2014 period, a yield increase of 1 dt/ha resulted in an absolute loss of − 0.071% protein concentration (Laidig et al. 2017). Similar results have been found by Simmonds (1995), Oury and Godin (2007), Oberforster and Werteker (2011), and Riaz et al. (2019b).
The lost protein content had to be compensated by altering protein composition to keep or even to improve the functional properties of new wheat varieties, while the yield–protein relationship is well documented, less information is available on the changes in gluten quality associated with effects on the amount and composition of glutenins and gliadins. Based on the data available, a general trend of two main alterations in protein composition can be observed: to improve dough strength and extensibility (a) glutenin-to-gliadin ratio increased and (b) lines containing superior HMW glutenin alleles have been selected in modern cultivars. Gliadin composition, the relative distribution of alpha-/beta-, gamma-, and omega-gliadins, plays a limited role in determining functional properties, and therefore, there is no consistent trend in the alteration in gliadin composition when comparing historic and modern varieties. In summary, the overall protein content and specifically the gliadin content of the flour have superior roles in determining the amounts of harmful epitopes in the flour.
Because of the considerable sequence variation in alpha-, gamma-, and omega-gliadins, both within wheat cultivars and among different cultivars, detailed knowledge about the composition of gliadin genes and proteins in individual wheat cultivars is critical for understanding how these proteins contribute to both the functional properties and the immunogenic potential of the flour (Wang et al. 2017; Cho et al. 2018).
RP-HPLC-based quantitative analysis of gliadin types has been carried out on numerous modern bread wheat and durum varieties and landraces as well as spelt samples by Ribeiro et al. (2016). It was observed that the Triticum aestivum spp. and T. vulgare landraces, which have not been subject to breeding practices, presented greater amounts of potential celiac disease immuno-stimulatory epitopes when compared to modern varieties. The LC–MS-based comparison of thirty wheat cultivars released in North Dakota from 1910 to 2013 showed that immunogenic peptides are present in both historical and modern spring wheat cultivars, irrespective of year release (Malalgoda 2016).
The effects of breeding during the twentieth century on the gluten quality of durum wheat for processing and health were investigated by De Santis et al. (2017), comparing a set of old and modern Italian wheats. The better technological performance observed for the modern varieties was found to be due not only to the introgression of the superior alleles of high molecular weight (HMW GS) and low molecular weight (LMW GS) glutenin subunits encoded at the Glu-B1 and Glu-B3 loci, but also to differential expression of specific storage proteins. The higher gluten index observed in modern genotypes was correlated with an increased glutenin/gliadin ratio. Another marked difference between the old and modern cultivars was the significantly higher expression level of B-type LMW GS in the modern varieties. No significant differences were found among durum wheat genotypes in relation to the expression of the most harmful α-type and γ-type gliadins, while in the modern genotypes a significant decrease was observed in the expression of ω-5 gliadins, a major allergen in wheat-dependent exercise-induced anaphylaxis (WDEIA).
Comparing old and modern Iranian wheat cultivars, similar alterations in Glu/Gli ratio and the changes in the HMW GS alleles have been reported by Izadi-Darbandi et al. (2010).
A set of 78 varieties released between 1860 and 2015 were selected and studied by Riaz et al. (2019a, b) and Florides et al. (2019) in terms of grain quality, dough rheology plus protein composition and FODMAP content, as well as the extent of allergen epitopes in the gliadin proteins. Grain-protein contents have been found to be significantly lower in modern varieties compared to historical varieties and are negatively correlated to increased grain yield data. Over the years, significant changes in quality parameters of grains have been found. Compared to historical varieties, modern cultivars were found to provide stronger dough properties, as observed by micro-Doughlab and Mixograph results, including increases in dough development time, dough stability, peak mixing time, midline peak width and decreases in softening after 5 min of mixing, as well as a weakening slope. The improvement in protein quality, compensating for the decrease in protein content, was found to be associated with the increased glutenin-to-gliadin ratio and with a shift of size distribution for the polymeric proteins (%UPP). The higher %UPP is derived from the systematic alteration of HMW glutenin and LMW glutenin alleles in the modern cultivars. The two most significant examples in this alteration are the increases in the numbers of cultivars containing the Glu1Dd and Glu1Bal alleles (HMW glutenin subunits 5 + 10 and overexpressed 7) and also the effects of LMW-glutenin alleles determining functional properties.
Different separation methodologies, including reversed and size-exclusion high-performance liquid chromatography, MALDI-TOF, liquid chromatography, MS MS (qTOF), followed by the application of different bioinformatics procedures, and databases have all been used by Florides et al. (2019) to develop a breeder’s tool, to quantify the gliadin content and composition of wheat cultivars and to determine their immunoreactive epitope content. Using this tool, a small highly toxic ω-gliadin group was discovered, and the immunoreactive epitopes of its members were mapped. The allergenicity of 170 wheat cultivars was estimated, using this tool, and it was clearly shown that historic wheat varieties are potentially as immunoreactive as the more recently released cultivars.
Fructan content
No significant trend in the alteration of fructan content of wheat varieties released in Australia over the last 150 years has been observed by Riaz et al. (2019b). Fructan levels in flours varied between 1.01 to 2.27% on a dry basis, showing slight variations among the cultivars and significant effects of the harvest years (mean values for 2015 and 2016 samples are 1.38 and 1.74, respectively). Similar results have been reported on the fructan levels of historic and modern Austrian wheat varieties by Fretzdorff and Welge (2003a, b), while extremely high or extremely low fructan contents were not found, it was possible to select a few cultivars with consistently high or low fructan contents; thus, the latter offers the potential to re-utilize these historic wheats with low fructan content in present breeding programs (Riaz et al. 2019b).
ATIs content
The most detailed analysis on the ATI content and diversity across wheat cultivars has been published recently by Bose et al. (2020). The level of 18 ATI isoforms (63 peptides) grouped into four subtypes were monitored across 15 commercial wheat cultivars and the 8 parental lines from a multiparent advanced generation intercross (MAGIC) population (Cavanagh et al. 2008) using liquid chromatography–mass spectrometry (LC–MS) technique. Large variation of ATI content was observed among the of wheat samples, with significantly higher average ATI levels found in cultivars Baxter and Xiaoyan and significantly lower values in cultivars in Pastor and Longreach Scout. This work establishes a reference map for wheat ATIs that presents an opportunity for selecting low ATI wheat lines for use in breeding programs or the development of assays to monitor ATIs in clinical studies.
Breeding for “healthy” wheats
Values reported in the literature within and among wheat types for celiac reactivity, human α-amylase inhibitor (ATI) activity, allergenicity, and fructan content are shown in Fig. 1. These detailed scientific investigations do not support the controversial idea that human practices, i.e., modern wheat breeding may have contributed to the increase in celiac disease or NCWS prevalence during the latter half of the twentieth century. Each of the primitive wheat relatives and each historic or modern bread or durum wheat variety contain more or less amounts of toxic/allergenic epitopes with a declining level trend caused by the altered protein content and composition.
Values reported in the literature within and among wheat types for a celiac reactivity, b human α-amylase inhibitor (ATI) activity, c allergenicity, and d fructan content. Horizontal lines indicate the median value for each of the value ranges. Modern wheat includes varieties of common wheat that were developed after 1950, while heritage wheat includes varieties and landraces that were developed before 1950
In the light of these results, it can be concluded that while in the last 120 years, health-related quality attributes have not been considered in pre-breeding and breeding, selection has focused on yield and functional quality which has inadvertently resulted in a reduction of the amounts of harmful compounds in new lines. This is because of the decreasing trend in overall protein content as well as the alteration of glutenin to gliadin content to improve dough strength. Older varieties are higher in gliadin content and have higher CD antigenicity (Izadi-Darbandi et al. 2010; Prandi et al. 2014, 2017; Ribeiro et al. 2016; De Santis et al. 2017; Malalgoda 2016; Florides et al. 2019; Riaz et al. 2019a), while there is no alteration in the amounts of fructan (Fretzdorff and Welge 2003a, b; Riaz et al. 2019b).
Screening primitive wheat relatives and revisiting historic wheat cultivars in various studies have revealed some genotypes and old varieties containing low levels of certain gliadin classes and FODMAPs. These findings point to an important wheat genetic pool that can be further exploited for the development of celiac-safe wheat products, while also suggesting great potential in future conventional breeding practices. It would be difficult with this genetic diversity alone to obtain a wheat variety without toxicity while retaining the unique viscoelastic properties of gluten. However, this natural potential can be further exploited using cutting edge molecular breeding techniques encompassing mutagenesis, transgenesis, and genome editing (Shewry and Tatham 2016; Boukid et al. 2017; Jouanin et al. 2018a; Malalgoda et al. 2018; Rustgi et al. 2019).
The expression levels of compounds triggering wheat sensitivities are dependent of environmental factors and growing conditions. The related information, covering the effects of climate changes as well as the different forms of abiotic and biotic stresses on the allergen content of the wheat grain, is discussed in the overview of Juhász et al. (2020).
Aside from the development of such celiac-safe lines, there are several issues to be solved for the future commercial production of “safe” genotypes. As it is stated by García-Molina et al. (2019), low-gliadin wheat lines are being developed as an alternative cereal with a better nutritional profile and organoleptic properties, as well as improved bread making properties, when compared to current gluten-free products. On the other hand, the requirement of fertilization, especially nitrogen, for low-gliadin wheat is of concern for the farmers, as it is well known that protein accumulation during grain filling is strongly influenced by nitrogen fertilization, and it can modify grain–protein composition and yield. Therefore, fertilization strategies for such lines are undoubtedly important to keep gluten levels as low as possible with no compromise to yield and plant growth. Jouanin et al. (2018b) discuss political and economic issues which strongly define the commercial use of genetically modified wheats, including gene-edited genotypes in several countries.
Effects of processing on wheat sensitivity
If primitive wheats, as well as all historic and modern bread wheat cultivars, contain harmful amounts of toxic epitopes, why do we have increased numbers of reported cases of wheat sensitivity nowadays? Our grandparents produced breads from the same kind of “harmful” wheat as we have, today. Is the bread we consume today also the same as that of our grandparents?
To answer for this question, we need today’s processes of making bread to be investigated and compared with those in our grandparents’ era. This investigation must be extended beyond the baking industry to monitor the changes in processing and formulation across the entire food industry.
No epidemiological studies have evaluated the impact of wheat processing on the prevalence of wheat sensitivity over the last 50 years. Nevertheless, as it is stated by Kucek et al. (2015) in their review, increases in disease diagnoses correlate with food industry uses of compounds that have been reported to trigger sensitivity, such as gluten, inulin, and high-fructose corn syrup.
Industrial products supplied to the food industry
Since Basil Regan and Leonard Winch of Fielders Mills developed the first commercial method for drying unfermented gluten in Tamworth (NSW, Australia) in 1938 (Day et al. 2006), the by-product of wheat starch isolation, “vital wheat gluten,” is utilized all around the food industry (Hesser 1987). By the 1970s and 1980s, dried gluten as a cheap source of protein was readily included in many foodstuffs (Hesser 1987; Day 2011; Ortolan and Steel 2017).
Vital wheat gluten not only improves the structural integrity of industrial bakery products, but it costs less per ton of protein than soy, whey, or casein. Low-protein flours are often fortified with vital wheat gluten to improve baking characteristics (Day et al. 2006; Ortolan and Steel 2017). Vital wheat gluten is often used in multigrain bread formulations as a binding agent (Atchison et al. 2010).
Kasarda (2013) was the first to point out that, while the overall gluten content of modern wheats has declined, dietary exposure to gluten has indeed increased in the last 50 years caused by the increased application of vital gluten in many processed foods. Gluten is commonly used as thickeners, emulsifiers, and gelling agents. According to a survey by Atchison et al. (2010), an estimated 29.5% of supermarket food products contain wheat proteins (86% of packet soups, 65% of canned soups, 63% of candies, 61% of ice cream, 46% of marinades, 26% of vinegars and dressings, 23% of jams, and 21% of baby food). Beyond the baking industry, the largest amount of gluten is used in processed meat, reconstituted seafood, and vegetarian meat substitutes (Day et al. 2006).
It is important to note that isolated wheat gluten does not contain the endogenous wheat enzymes that assist in the degradation of gluten proteins. Leduc et al. (2003) documented the case of a patient who did not have an allergy to wheat/gluten, but experienced anaphylaxis after consuming a wheat isolate used by the meat industry. Isolated wheat proteins in hair and skin care products could also provoke contact urticaria in a small subset of patients who are not allergic to gluten (Lauriere et al. 2006).
Other potentially harmful, often-used food additives are high-fructose corn syrup and inulin, both implicated in fructose malabsorption, IBS, and NCWS. Fructose consumption has risen in the last 30 years, largely due to a 60.8% increase in sweetener availability since 1978 (Gibson et al. 2007; Marriott et al. 2009). Cereals, muffins, cake mixes, instant oatmeal, granola bars, cookies, and bread are often supplemented with inulin-type fructans for the purpose of fiber supplementation or fat replacement in low-fat products (Gibson et al. 2000; Kleessen et al. 2007; Grabitske and Slavin 2009). Inulin-type fructans are not derived from wheat, but rather are extracted from chicory root and Jerusalem artichoke (Kolida and Gibson 2007). Although inulin can benefit most consumers when eaten in moderate amounts, inulin may aggravate symptoms of fructose malabsorption for those with IBS, and NCWS. Of particular interest to individuals with wheat sensitivity, inulin is often used to improve structure, color, taste, and fiber content in gluten-free breads (Capriles and Areas 2014). Such food products highlight the need for patients with NCWS to understand the true causative agents of their symptoms. For individuals with fructose malabsorption, IBS, and certain cases of NCWS, gluten-free products with added inulin may be a poor dietary choice.
The effect of wheat milling
The chemical composition of wheat is different in various layers of the wheat kernel (Jones et al. 2015). So, depending on the milling process, flours containing different amounts of harmful components are produced. Cysteine-type endoproteases are synthesized in the aleurone layer (Hammerton and Ho 1986), the removal of bran to produce white flour results in lower enzyme levels available for protein degradation (Hartmann et al. 2006; Schwalb et al. 2012).
The distribution of gliadin proteins from the three main protein classes shows characteristic differences among the morphological parts of the seed. Many of the celiac-reactive α-gliadins are located in the sub-aleurone layer of the wheat kernel, which can be partially removed by roller milling. However, the γ-gliadins and the HMW glutenins are concentrated in the endosperm, therefore appearing in high concentrations in white flour. Omega-gliadins are found throughout the grain (Tosi et al. 2011), so their amount seems not to be altered by the level of flour refinement.
ATIs surround starch molecules in the endosperm, protecting them from digestion by insects and mammals. Wheat bran elicited about twice the IgE activity for bakers’ asthma than white flour (Armentia et al. 2012).
Fructans, also, are not evenly distributed throughout the wheat grain. In terms of wheat-milling fractions, bran, and shorts contain more fructan than the related white flour (Knudsen 1997; Haska et al. 2008). The inclusion of bran in whole-wheat flour likely increases the total fructan content of whole-wheat flour, relative to white flour. Whole-wheat flour also contains fructans with a higher degree of polymerization than those in white flour. The lower degree of polymerization of fructans in white flour makes fructans more available for fermentation in the gut, which can aggravate symptoms in individuals with IBS. On the other hand, as fructans with lower degrees of polymerization are more easily degraded by yeast (Nilsson et al. 1987; Praznik et al. 2002), fructans in white bread are broken down more extensively than those in whole-wheat bread (Knez et al. 2014).
The use of microbial enzymes in baking formulation
Modern flour processing can also impact wheat sensitivity. Fungal enzymes are commonly added to wheat flour to improve baking properties. Various fungal enzymatic additives, including α-amylase derived from Aspergillus oryzae, xylanase, glucoamylase, cellulase, and β-xylosidase, have been associated with allergies, such as bakers’ asthma and contact dermatitis (Quirce et al. 1992; Morren et al. 1993; Baur et al. 1998; Sander et al. 1998; Quirce et al. 2002). These additives provide an additional exposure risk to bakers (Tatham and Shewry 2008). These enzymes do not represent any health-related risks for the consumer, while strict guidelines on the safe handling of enzymes in the bakery supply chain protect employees to avoid contamination (De Vos et al. 2018).
The effects of the dough-making process
Effects on gluten proteins
Yeast-mediated dough fermentation is a critical phase in the bread making process, determining the rheology of the dough and for the final bread product—its texture, volume, and taste. The production of CO2 and other metabolites by yeast cells involves a large number of reactions related to the enzymatic degradation of the carbohydrate and protein components of the dough. Different factors affect the fermentative performance of yeast cells during dough fermentation, including dough ingredients, fermentation conditions, the type of yeast strain used, and yeast pre-growth conditions. Traditionally most bread involving yeast leavening used to be made with long dough fermentation times of up to 6 h before baking (Kulp 1993; Struyf et al. 2017a, b; Parapouli et al. 2019).
Gluten degradation by proteolysis during dough making has been studied for more than 50 years (Frazer et al. 1959; Messer et al. 1964). Unlike human gastrointestinal proteases, microbial proteases can cleave the peptide bonds next to proline residues, which frequently occur in gluten proteins (10–15% proline). Based on the studies of Lyons (1982), Stepniak et al. (2006) and Walter et al. (2015), gluten proteins could be degraded to small peptides containing less than nine amino acid residues.
Sour dough fermentation for bread making plays a crucial role in the development of sensory properties such as taste, aroma, texture, and the overall quality of baked goods. This is due to the acidification, proteolysis, and activation of a number of enzymes (Melim-Miguel et al. 2013; Gobbetti et al. 2014). Sour dough is a mixture of flour and water that is fermented with lactic acid bacteria (LAB).
Typical sour dough bread is a staple food contributing to cultural identity in sundry diets, mostly in Central and Eastern Europe (Moroni et al. 2009). Beyond sour dough bread, traditionally, there are some sour dough sweet-leavened baked goods obtained by microbial protease fermentation from lactic acid bacteria, such as the Genoese dry biscuit, called lagaccio, and a soft cake from north Italy, panettone. All of them involve very long-fermentation processes, using lactic acid bacteria (LAB), which provide a sour taste to the product (De Vuyst and Neysens 2005). Sour dough bread, made by traditional techniques, is now becoming increasingly popular through artisan bakeries in many Western countries (Ross 2018).
In the last 20 years, attempts to reduce the harmful components of wheat have produced numerous strategies. The application of lactobacillus-based proteases during the sour dough process or supplementing these enzymes such as especially prolyl-oligopeptidases in yeasted-dough fermentation (Rizzello et al. 2007; Walter et al. 2015; Di Cagno et al. 2010; Greco et al. 2011) have been investigated in great detail (Rizzello et al. 2007, 2014; Ganzle et al. 2008; Gerez et al. 2006, 2012; Di Cagno et al. 2002, 2004, 2010; Rollan et al. 2005; Caputo et al. 2010; Walter et al. 2015; Rizzello et al. 2014; Deora et al. 2014; Guilliani 2012; Engstrom et al., 2015; Heredia-Sandoval et al. 2016; Kristensen 2016).
In most case, these treatments have been effective in reducing gluten immunogenicity, not only for making bread but in cases of other leavened products such as pizza (Pepe et al. 2003) and even pasta (De Angelis et al. 2010). It is also reported that these treatments have marginal effects on functional properties caused by partial depolymerization of polymeric glutenin and proteolysis of the HMW glutenin subunits (Loponen et al. 2003, 2007).
Importantly, several publications underline the significance of the fermentation time used either in cases of yeasted or sour dough fermentation (Thiele 2003; Thiele et al. 2004; Zotta et al. 2006, Siddiqi et al. 2016; Couch 2016; Sakandar et al. 2019): Significant proteolysis can be observed only after a minimum four hours of fermentation.
The effect on FODMAP content
Nilsson et al. (1987) were first to show that dough mixing can reduce the fructan concentration of various wheat flours from 40 to 75%, due to the flour-oxygenation action of the invertase enzyme in baker’s yeast (Saccharomyces cerevisiae). Fermentation without yeast did not affect the fructan concentration after mixing (Nilsson et al. 1987; Verspreet et al. 2013; Knez et al. 2014; Gelinas et al. 2016). Therefore, it was recommended that individuals sensitive to fructans should consume long-fermentation breads rather than products arising from short mixed and fermented industrial processes. A reduction or complete prevention of fructan degradation can be achieved also by using mutant yeast strains with lower sucrose degradation activity or lacking invertase (Verspreet et al. 2013). Yeast has been shown to have a preference for fructans with a low degree of polymerization (Nilsson et al. 1987; Rakha et al. 2011). Praznik et al. (2002) confirmed that fructan molecules with a higher degree of polymerization or higher average chain length are more resistant to degradation during the baking process. This might explain why fructan degradation during baking can be higher in wheat than in rye breads (Nilsson et al. 1987; Fretzdorff and Welge 2003b; Andersson et al. 2009).
The importance of proofing (fermentation) time, regarding the fructan content of baked products irrespective of the variety used, was demonstrated by Ziegler et al. (2016). Short proofing of only 1 h slightly decreased the raffinose and fructan level and significantly increased “excess fructose” due to the almost complete hydrolysis of sucrose. Longer proofing times of 2.5 h or 4.5 h also reduced the excess fructose levels significantly, resulting in a final FODMAP load of only 29–33% and 10–23% of the initial concentrations for bread and spelt wheats, respectively. Since spelt wheat is often processed by artisan bakeries using traditional recipes and long fermentation times, partly to improve the rather poor technological performance of spelt (Schober et al. 2002; Frakolaki et al. 2018), observations of individuals better tolerating spelt than bread wheat products might rather be related to different processing than to species-related causes. Similar results were obtained in Australia when commercial bread samples, made from wheat and spelt flour and baked with different baking technologies, have been collected and analyzed (Suter et al. 2018).
According to recently published scientific literature, simply monitoring the fructan levels in baked goods does not provide the full picture in relation to the physiological effects on IBS patients. Struyf et al. (2017a, b, 2018) described the alteration of FODMAP levels and the changes in the carbohydrate profile in whole-meal bread produced with yeast fermentation. The effect of yeast strain, fermentation parameters, such as yeast dosage and fermentation time, is discussed including the application of enzyme-based technologies. Recently, Benítez et al. (2018) demonstrated that dough preparation and baking significantly reduced fructan content, whereas no effect was observed on the concentrations of fructose, glucose, and raffinose, and a significant increase was recorded for sucrose and specifically for maltose.
Compared to the yeasted-dough process, sour dough technology results in an even greater drop in FODMAP content. Acidic conditions are advantageous for the invertase enzyme, playing a crucial role in fructan degradation (Nilsson et al. 1987, Kucek et al. 2015). Costabile et al. (2014) concluded that breads fermented by the traditional long-fermentation and sour dough processes are less likely to lead to IBS symptoms compared to bread made by short- or no-time fermentation processes. Loponen and Ganzle (2018) gave an overview of the biochemical processes during sour dough fermentation, providing details about the roles of different microorganisms in the culture and the enzymes involved. Yeast or sour dough culture plays a crucial role in fructan degradation. Selection of the correct strain and the optimal fermentation parameters are of major importance to steer fructan degradation. Conventional sour dough baking reduces and converts FODMAP in rye and wheat flour; however, the extent of FODMAP reduction is dependent on the fermentation organisms, the fermentation process, the grain raw material, and the sour dough starter dosage in the final bread dough. The production of low-FODMAP bread requires extracellular fructanase activity. Sour dough fermentation with lactobacilli expressing fructanases or the use of fructanase-positive yeasts provide breads with a low-FODMAP content.
Effects on ATI content
Food processing alters the conformation of allergenic wheat proteins and may abolish, and in rarer cases, increase their allergenicity (Pasini et al., 2001). IgE antibodies of wheat-allergic patients reacted with in vitro digested bread crumb and crust, but not with unheated bread dough (Loponen et al. 2007). The TLR4-stimulating bioactivity of ATI was reduced by 20–50% after bread and biscuit making, compared to unprocessed wheat flour (Zevallos et al. 2017).
The current data of Huang et al. (2020) suggest that sour dough fermentation can degrade ATI structure and bioactivity and point to strategies to improve product development for wheat-sensitive patients. ATI tetramers were isolated, fluorescein-labeled, and added to a mini-dough bread making system by Huang et al. (2020). In the case of sour dough fermentation, below pH 4, the ATI tetramers were degraded due to the activation of aspartic proteases, while in yeast fermentation, ATI tetramers remained intact. The amylase inhibitory activity after sour dough fermentation decreased significantly, while the concentration of free thiol groups increased. Compared to unfermented wheat, sour dough fermentation was able to decrease the release of pro-inflammatory cytokines monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor.
Comparing different mixing and fermentation procedures
Up until the early 1980s, most bread was made using yeast with a long dough fermentation of up to 6 h before the breads were baked. In 1961 in the UK, the British Bakers Industry Research Association (BBIRA) developed a new method of making bread called the Chorleywood Bread Process (Chanberlain et al. 1961). This process used high energy Tweedy mixers together with a range of bread improver agents; but in particular, fermentation time was reduced to only one hour (Cauvain and Young 2006). Obviously, this method of making bread was very fast and significantly cheaper than previous methods. This bread making method, also known as the “no-time dough” (NTD) process, was rapidly taken up in a lot of countries.
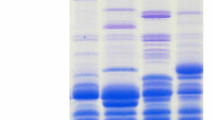
The consequent alterations in gliadin and fructan levels in bread wheat and spelt flour, in doughs during fermentation, and in the final products have been monitored, comparing the effects of the NTD process to long-fermentation yeasted doughs and sour dough methods (Suter et al. 2019a, b). With minor changes observed for the NTD process, long fermentation in both yeasted- and sour doughs dramatically changed the composition and structure of the gliadin proteins (Fig. 2a) and significantly reduced the fructan content of the baked bread (Fig. 2b). The relative amounts of total gliadin (protein extracted with 70% ethanol) expressed as the percentage of total gliadin in the flour was determined to be 87% and 85% in NTD breads made from wheat and spelt flours, respectively. Fructan levels in the same NTD breads (1.10% and 0.96%, for NTDW and NTDS, respectively) showed less than 5% decrease compared to those in the corresponding flours. These fructan content data are in a good agreement with those resulted in from a survey of commercial NTD bread samples carried out by Suter et al. (2018). Compared to these marginal changes, significant reductions have also been observed in the total gliadin and fructan levels in long-fermentation yeasted dough (LFYD) and sour dough (LFSD) breads made from wheat. Decreases in gliadin content were as follows: in wheat breads LFYD = 65.1%, LFSD = 88.6% and spelt LFYD = 59.4%, LFSD = 90.4%. Similar comparisons of fructan contents also showed significant decreases in wheat breads: LFYD = 85.1%, LFSD = 88.9% and in spelt breads: LFYD = 93.0%, LFSD = 98.4%.
The obvious conclusion from these data is that fermentation time is a major factor in reducing gliadin and fructan levels both in wheat and spelt breads, caused either by yeast or sour dough activities. The sour dough process seems to be more effective in reducing harmful component levels, but it does not produce gliadin-free bread.
Producing “healthy” bread
Numerous individuals have reported a series of health difficulties after consuming bread wheat, whereas they claimed spelt products to be easily digestible (Stallknecht et al. 1996; Vu et al. 2014). Biesiekierski et al. (2011) suggested that lower FODMAP contents in spelt products might be the reason for these observations. There has been anecdotal clinical evidence for a long time that a large proportion of non-celiac patients, suffering wheat-related health disorders, can tolerate products made from certain spelt varieties. The first research paper with robust experimental and statistical results also demonstrated this important observation (Armentia et al. 2012).
Bread wheat and spelt flours are often processed differently due to their differing techno-functional characteristics (Schober et al. 2002). Most spelt products in the market are made using artisan-type technologies (Ross 2018) with long yeasted or lactobacillus fermentation. These processing differences may be the main factor responsible for the acceptability by sensitive individuals rather than the grain species alone.
Detailed analysis on the individual peaks in the RP-HPLC analysis of gliadins extracted from long-fermentation yeasted and sour dough breads (Fig. 2a) showed that the proteases of the two types of microorganism have different preference among gliadin polypeptides, so the composition of the remaining gliadins in the breads are different. It suggests there is an opportunity to optimize the fermentation process with the combination of different yeast and lactobacillus strains or using different proteases of lactobacillus origin during yeasted fermentation which may produce products with significantly less harm for sensitive consumers.
Natural primitive wheat relatives or wheat cultivars with low gliadin and/or fructan content have a great potential in traditional breeding in combination with modern gene-modifying technologies to develop healthier wheat cultivars. The utilization of these grains, using proper processing technologies where the harmful components of wheat are partially and hopefully fully eliminated by enzymatic hydrolysis during fermentation, could produce harmless products for sensitive individuals. These breads could be even “healthier” than those that our grandparents consumed.
Abbreviations
- CD:
-
Coliac disease
- NCGS:
-
Non-coliac gluten sensitivity
- NCWS:
-
Non-coliac wheat sensitivity
- IBS:
-
Irritable bowel syndrome
- HMW GS:
-
High molecular weight glutenin subunits
- LMW GS:
-
Low molecular weight glutenin subunits
- ATIs:
-
Amylase-tripsin inhibitors
- FODMAP:
-
Fermentable oligo-, di-, mono-saccharides and polyols
- GFD:
-
Gluten-free diet
- LAB:
-
Lactic acid bacteria
- NTD:
-
No-time dough procedure
- LC–MS:
-
Liquid chromatography–mass spectrometry
References
Altenbach SB, Vensel WH, Dupont FM (2011) The spectrum of low molecular weight alpha-amylase/protease inhibitor genes expressed in the US bread wheat cultivar Butte 86. BMC Res Notes 4:242
Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders D, Cellier C, Mulder CJ, Lundin KAE (2019) European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur Gastroenterol J 7:583–613
Andersson R, Fransson G, Tietjen M, Åman P (2009) Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. J Agric Food Chem 57:2004–2008
Appels R (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. In: International wheat genome sequencing consortium, Science 361, eaar7191
Arendt EK, Dal Bello F (2008) Gluten-free cereal products and beverages. Academic Press, San Diego
Armentia A, Martín S, Diaz-Perales A, Palacín A, Tordesillas L, Herrero M, Martín-Armentia M (2012) A possible hypoallergenic cereal in wheat food allergy and Baker’s asthma. Am J Plant Sci 3:1779–1781
Atchison J, Head L, Gates A (2010) Wheat as food, wheat as industrial substance; comparative geographies of transformation and mobility. Geoforum 41:236–246
Auricchio S, De Ritis G, De Vincenzi M, Occorsio P, Silano V (1982) Effects of gliadin-derived peptides from bread and durum wheats on small intestine cultures from rat fetus and coeliac children. Pediatr Res 16:1004–1010
Balakireva A, Zamyatnin AA (2016) Properties of gluten intolerance: gluten structure, evolution, pathogenicity and detoxification capabilities. Nutrients 8:644
Battais F, Courcoux P, Popineau Y, Kanny G, Moneret-Vautrin DA, Denery-Papini S (2005a) Food allergy to wheat: differences in immunoglobulin E-binding proteins as a function of age or symptoms. J Cereal Sci 42:109–117
Battais F, Mothes T, Moneret-Vautrin DA, Pineau F, Kanny G, Popineau Y, Bodinier M, Denery-Papini S (2005b) Identification of IgE-binding epitopes on gliadins for patients with food allergy to wheat. Allergy 60:815–821
Battais F, Richard C, Jacquenet S, Denery-Papini S, Moneret-Vautrin DA (2008) Wheat grain allergies: an update on wheat allergens. Eur Ann Allergy Clin Immunol 40:67–76
Baur X, Sander I, Posch A, Raulf-Heimsoth M (1998) Baker’s asthma due to the enzyme xylanase—a new occupational allergen. Clin Exp Allergy 28:1591–1593
Békés F, Schoenlechner R, Tömösközi S (2016) Ancient wheats and pseudocereals for possible use in cereal-grain dietary intolerances. In: Wrigley CW, Batey I, Miskelly D (eds) Cereal grains assessing and managing quality, 2nd edn. Elsevier, Amsterdam, pp 353–389
Békés F, Ács K, Gell G, Lantos C, Kovács AM, Birinyi Z, Pauk J (2017) Towards breeding less allergenic spelt wheat with low FODMAP content. Acta Aliment 46:246–258
Benítez V, Esteban RM, Moniz E, Casado N, Aguilera Y, Mollá E (2018) Breads fortified with wholegrain cereals and seeds as source of antioxidant dietary fibre and other bioactive com-pounds. J Cereal Sci 82:113–120
Biesiekierski JR, Rosella O, Rose R, Liels K, Barrett JS, Shepherd SJ, Gibson PR, Muir JG (2011) Quantification of fructans galacto-oligosaccharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet 24:154–176
Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR (2013) No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 145:320–328
Bose U, Juhász A, Broadbent JA, Byrne K, Howitt CA, Colgrave ML (2020) Identification and quantitation of amylase trypsin inhibitors across cultivars representing the diversity of bread wheat. J Proteome Res 19:2136–2148
Boukid F, Mejri M, Pellegrini N, Sforza S, Prandi B (2017) How looking for celiac-safe wheat can influence its technological properties. Compr Rev Food Sci Food Saf 16:797–807
Braly J, Hogganm R (2002) Dangerous grains: Why gluten cereal grains may be hazardous to your health. Penguin Group, New York
Branchi F, Ferretti F, Norsa L, Roncoroni L, Conte D, Bardella MT, Elli L (2015) Management of nonceliac gluten sensitivity by gastroenterology specialists: data from an Italian survey. Biomed Res Int. https://doi.org/10.1155/2015/530136
Brandolini A, Hidalgo A, Plizzari L, Erba D (2011) Impact of genetic and environmental factors on einkorn wheat (Triticum monococcum L. subsp. monococcum) polysaccharides. J Cereal Sci 53:65–72
Brighenti F, Casiraghi MC, Pellegrini N, Riso P, Simonetti P, Testolin G (1995) Comparison of lactulose and inulin as reference standard for the study of resistant starch fermentation using hydrogen breath test. Ital J Gastroenterol 27:122–128
Brouns F, van Rooy G, Shewry PR, Rustgi S, Jonkers D (2019) Adverse reactions to wheat or wheat components. Compr Rev Food Sci Food Saf 18:1437–1452
Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, Fasano A (2019) Celiac disease: a comprehensive current review. BMC Med 17:142
Capocchi A, Muccilli V, Cunsolo V, Saletti R, Foti S, Fontanini D (2013) A heterotetrameric alpha-amylase inhibitor from emmer (Triticum dicoccon Schrank) seeds. Phytochemistry 88:6–14
Capriles VD, Areas JAG (2014) Novel approaches in gluten-free breadmaking: interface between food science, nutrition, and health. Compr Rev Food Sci Food Saf 13:871–890
Caputo I, Lepretti M, Martucciello S, Esposito C (2010) Enzymatic strategies to detoxify gluten: implications for CD. Enzyme Res 2010:174354
Carroccio A, Di Prima L, Noto D, Fayer F, Ambroiano G, Villanacci V, Cammers K, Lafiandra D, De Ambrogio E, Di Fede G, Iacono G, Pogna N (2011) Searching for wheat plants with low toxicity in celiac disease: between direct toxicity and immunologic activation. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver 43:34–39
Caruso R, Pallone F, Stasi E, Romeo S, Monteleone G (2013) Appropriate nutrient supplementation in celiac disease. Ann Med 45:522–531
Castillo NE, Theethira TG, Leffler DA (2015) The present and the future in the diagnosis and management of celiac disease. Gastroenterol Rep (Oxf) 3:3–11
Catassi C, Fasano A (2018) From ptolemaus to copernicus: the evolving system of gluten-related disorder. Preface. Nutrients special issues (ISSN 2072-6643) vii
Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J (2014) Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients 5:3839–3853
Cauvain SP, Young LS (2006) A brief history of the chorleywood bread process. In: Chapter 2. the chorleywood bread process, p 6–16. CRC Press, Washington, DC, USA, Woodhead Publishing Limited, Cambridge, England
Cavanagh C, Morell M, Macka I, Powell W (2008) From mutations to MAGIC: resources for gene discovery validation and delivery in crop plants. Curr Opin Plant Biol 11:215–221
Chanberlain N, Collins TH, Eltonm GAH (1961) The Chorleywood bread process. BIRS Report 59. Chorleywood, Herts, pp 1–30
Cho K, Beom H-R, Jang Y-R, Altenbach SB, Vensel WH, Simon-Buss A, Lim S-H, Kim MG, Lee J-Y (2018) Proteomic profiling and epitope analysis of the complex α-, γ -, and ω-Gliadin Families In A Commercial Bread Wheat. Front Plant Sci 9:818
Colomba MS, Gregorini A (2012) Are ancient durum wheats less toxic to celiac patients? A study of alpha-gliadin from Graziellara and Kamut. Sci World J 8:837416
Comino I, Moreno M, Real A, Rodríguez-Herrera A, Barro F, Sousa C (2013) The gluten-free diet: testing alternative cereals tolerated by celiac patients. Nutrients 5:4250–4268
Costabile A, Santarelli S, Claus S, Sanderson J, Hudspith BN, Brostoff J, Ward JL, Lovegrove A, Shewry PR, Jones HE, Gibson GR (2014) Effect of breadmaking process on in vitro gut microbiota parameters in irritable bowel syndrome. PLoS ONE 9:e111225
Couch GW (2016) Effect of sourdough fermentation parameters on bread properties. PhD Theses. 2581, Clemson University, Clemson
Davis W (2011) Wheat belly: lose the wheat, lose the weight, and find your path back to health. Rodale Press, Emmaus
Day L (2011) Wheat gluten: production, properties and application. In: Phillips GO, Williams PA (eds) Handbook of food proteins. Woodhead Publishing, Sawston, pp 267–288
Day L, Augustin MA, Batey IL, Wrigley CW (2006) Wheat-gluten uses and industry needs. Trends Food Sci Technol 17:82–90
De Angelis M, Cassone A, Rizzello CG, Gagliardi F, Minervini F, Calasso M, Di Cagno R, Francavilla R, Gobbetti M (2010) Mechanism of degradation of immunogenic gluten epitopes from Triticum turgidum L. var. durum by sourdough lactobacilli and fungal proteases. Appl Environ Microbiol 76:508–518
de Lorgeril M, Salen P (2014) Gluten and wheat intolerance today: Are modern wheat strains involved? Int J Food Sci Nutr 65(5):577–581
De Santis MA, Giuliani MM, Giuzio L, De Vita P, Lovegrove A, Shewry PR, Flagella Z (2017) Differences in gluten protein composition between old and modern durum wheat genotypes in relation to 20th century breeding in Italy. Eur J Agron 87:19–29
De Vos C, Simonsen M, van Oort M, Autton S, Alanen A, Van Caelenberg T (2018) Industry guidelines on the safe handling of enzymes in the bakery supply chain. Ampfep, Fedima, Brussels
De Vuyst L, Neysens P (2005) The sourdough microflora: biodiversity and metabolic interactions. Trends Food Sci Technol 16:43–56
Deora NS, Deswal A, Mishra HN (2014) Alternative approaches towards gluten-free dough development: recent trend. Food Eng Rev 6:89–104
Di Cagno R, De Angelis M, Lavermicocca P, De Vincenzi M, Giovannini C, Faccia M, Gobbetti M (2002) Proteolysis by sourdough lactic acid bacteria: effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl Environ Microbiol 68:623–633
Di Cagno R, De Angelis M, Auricchio S, Greco L, Clarke C, De Vincenzi M, Giovannini C, D’Archivio M, Landolfo F, Parrilli G (2004) Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl Environ Microbiol 70:1088–1096
Di Cagno R, Barbato M, Di Camillo C, Rizzello CG, De Angelis M, Giuliani G, De Vincenzi M, Gobbetti M, Cucchiara S (2010) Gluten-free sourdough wheat baked goods appear safe for young celiac patients: a pilot study. J Pediatric Gastroenterol Nutr 51:777–783
Di Nardo G, Villa MP, Conti L, Ranucci G, Pacchiarotti C, Principessa L, Raucci U, Paris P (2019) Nutritional Deficiencies in children with celiac disease resulting from a gluten-free diet: a systematic review. Nutrients 11:1588. https://doi.org/10.3390/nu11071588
Digiacomo D, Tennyson CA, Green PH, Demmer RT (2013) Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand J Gastroenterol 48:921–925
Dupont FM, Vensel WH, Tanaka CK, Hurkman WJ, Altenbach SB (2011) Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci 9:10. https://doi.org/10.1186/1477-5956-9-10
El Khoury D, Balfour-Ducharme S, Joye IJ (2018) A review on the gluten-free diet: technological and nutritional challenges. Nutrients 10:1410
Engstrom N, Sandberg AS, Scheers N (2015) Sourdough fermentation of wheat flour does not prevent the interaction of transglutaminase 2 with α2-gliadin or gluten. Nutrients 7:2136
Escarnot E, Dornez E, Verspreet J, Agneessens R, Courtin CM (2015) Quantification and visualization of dietary fibre components in spelt and wheat kernels. J Cereal Sci 62:124–133. https://doi.org/10.1016/j.jcs.2015.01.003
Fasano A, Sapone A, Zevallos V, Schuppan D (2015) Non-celiac gluten sensitivity. Gastroenterology 148:1195–1204
Florides C, Juhász A, Ma W, Vanniasinkam T, Eastwood R, Békés F, Blanchard CL (2019) A gluten protein allergenicity study in Australian wheat varieties from historic times to present. In: Cereals and grains conf of AACCI, London, 21–23 Oct
Ford R (2008) The gluten syndrome is wheat causing you harm?. RRS Global LT, Christchurch
Frakolaki G, Giannou V, Topakas E, Tzia C (2018) Chemical characterization and breadmaking potential of spelt versus wheat flour. J Cereal Sci 79:50–56
Frazer AC, Fletcher RF, Ross CAC, Shaw B, Sammons HG, Schneider R (1959) Gluten-induced enteropathy the effect of partially digested gluten. Lancet 2:252–255
Fretzdorff B, Welge N (2003a) Fructan- und Raffinosegehalte im Vollkorn einiger Getreidearten und Pseudo-Cerealien. Getreide Mehl Brot 57:3–8
Fretzdorff B, Welge N (2003b) Abbau von getreideeigenen Fructanen während der Herstellung von Roggenvollkornbrot. Getreide Mehl Brot 57:147–151
Ganzle MG, Loponen J, Gobbetti M (2008) Proteolysis in sourdough fermentations: mechanisms and potential for improved bread quality. Trends Food Sci Technol 19:513–521
Garcia-Maroto F, Marana C, Mena M, Garcia-Olmedo F, Carbonero P (1990) Cloning of cDNA and chromosomal location of genes encoding the three types of subunits of the wheat tetrameric inhibitor of insect α-amylase. Plant Mol Biol 14:845–853
Garcia-Maroto F, Carbonero P, Garcia-Olmedo F (1991) Site-directed mutagenesis and expression in Escherichia coli of WMAI-1, a wheat monomeric inhibitor of insect α-amylase. Plant Mol Biol 17:1005–1011
García-Molina MD, Giménez MJ, Sánchez-Leon S, Barro F (2019) Gluten free wheat: are we there? Nutrients 11:487
Geisslitz S, Ludwig C, Scherf KA, Koehler P (2018) Targeted LC − MS/MS reveals similar contents of α-amylase/trypsin-inhibitors as putative triggers of nonceliac gluten sensitivity in all wheat species except einkorn. J Agric Food Chem 66:12395–12403
Gelinas P, McKinnon C, Gagnon F (2016) Fructans, water-soluble fibre and fermentable sugars in bread and pasta made with ancient and modern wheat. Int J Food Sci Technol 51:555–564
Gell Gy, Kovács K, Molnár I, Zs Bugyi, Tömösközi S, Juhász A (2015) CD-specific prolamin peptide content of wheat relatives and wild species determined by ELISA assays and bioinformatics analyses. Cereal Res Commun 43:133–143
Gerez CL, Rollan GC, de Valdez G (2006) Gluten breakdown by lactobacilli and pediococci strains isolated from sourdough. Lett Appl Microbiol 42:459–464
Gerez CL, Dallagnol A, Rollan G, de Valdez GV (2012) A combination of two lactic acid bacteria improves the hydrolysis of gliadin during wheat dough fermentation. Food Microbiol 32:427–430
Gianfrani C, Maglio M, Aufiero VR, Camarca A, Vocca I, Iaquinto G, Giardullo N, Pogna N, Troncone R, Auricchio S, Mazzarella G (2012) Immunogenicity of monococcum wheat in celiac patients. Am J Clin Nutr 96:1339–1345
Gibson PR, Shepherd SJ (2005) Food for thought: western lifestyle and susceptibility to Crohn’s disease: the FODMAP hypothesis. Alim Pharm Ther 2:1399–1409
Gibson PR, Shepherd S (2010) Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol 25:252–258
Gibson GR, Ottaway PB, Rastall RA (2000) Prebiotics: new developments in functional foods. Chandos, Oxford
Gibson PR, Newnham E, Barrett JS, Shepherd SJ, Muir JG (2007) Review article: fructose malabsorption and the bigger picture. Aliment Pharmacol Ther 25:349–363
Gobbetti M, Rizzello CG, Di Cagno R, De Angelis M (2014) How the sourdough may affect the functional features of leavened baked goods. Food Microbiol 37:30–40
Grabitske HA, Slavin JL (2009) Gastrointestinal effects of low-digestible carbohydrates. Crit Rev Food Sci Nutr 49:327–369
Grausgruber H, Lovegrove A, Shewry PR, Békés F (2019) FODMAP in wheat. In: Igrejas G, Ikeda T, Guzman C (eds) Improving wheat quality for processing and health. Springer, Berlin, pp 515–532
Greco L, Gobbetti M, Auricchio R, Maglio M, Troncone R, Auricchio S (2011) Safety for celiac patients of baked goods made of wheat flour hydrolyzed during food processing. Clin Gastroenterol Hepatol 9:24–29
Gregorini A, Colomba M, Ellis HJ, Ciclitira PJ (2009) Immunogenicity characterization of two ancient wheat alpha-gliadin peptides related to coeliac disease. Nutrients 1:276–290
Guandalini S, Polanco I (2015) Nonceliac gluten sensitivity or wheat intolerance syndrome? J Pediatr 166:805–811
Guilliani G (2012) Method for partial degradation of gluten. Patent: WO 2014/033765 Al
Hallert C, Grant C, Grehn S, Granno C, Hulten S, Midhagen G, Strom M, Svensson H, Valdimarsson T (2002) Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther 16:1333–1339
Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG (2014) A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146:67–75
Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG (2015) Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 64:93–100
Hammerton RW, Ho T-HD (1986) Hormonal regulation of the development of protease and carboxypeptidase activities in barley aleurone layers. Plant Physiol 80:692–697
Hartmann G, Koehler P, Wieser H (2006) Rapid degradation of gliadin peptides toxic for coeliac disease patients by proteases from germinating cereals. J Cereal Sci 44:368–371
Haska L, Nyman M, Andersson R (2008) Distribution and characterisation of fructan in wheat milling fractions. J Cereal Sci 48:768–774
Hedden P (2003) The genes of the green revolution. Trends Genet 19:5–9
Heredia-Sandoval NG, Valencia-Tapia MY, Calderón de la Barca AM, Islas-Rubio AR (2016) Microbial proteases in baked goods: modification of gluten and effects on immunogenicity and product quality. Foods 5:59. https://doi.org/10.3390/foods5030059
Hesser JM (1987) Use and functionality of wheat gluten. In: Lásztity R, Békés F (eds) Proc. 3rd international gluten workshop, Budapest. Word Sci., Singapore, pp 441–455
Huang X, Schuppan D, Tovar LER, Zevallos VF, Loponen J, Ganzle M (2020) Sourdough fermentation degrades wheat alpha-amylase/trypsin inhibitor (ATI) and reduces pro-inflammatory activity. Foods 9:943
Hungin APS, Whorwell PJ, Tack J, Mearin F (2003) The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 17:643–650
Islam S, Ma W, Yan G, Békés F, Appels R (2011) Modifying processing and health attributes of wheat bread through changes in composition genetics and breeding. In: Cauvain SP, Tran B (eds) Bread making, improving quality, 2nd edn. Woodhead Publishing Limited, Cambridge, pp 259–296
Izadi-Darbandi A, Yazdi-Samadi B, Shanejat-boushehri AA (2010) Allelic variations in Glu-1 and Glu-3 loci of historical and modern Iranian bread wheat (Triticum aestivum L.) cultivars. J Genet 89:193–199
Jargon J (2014) The gluten-free craze: is it healthy?. Wall Street J. https://www.wsj.com/articles/how-we-eat-the-gluten-free-craze-is-it-healthy-1403491041
Jones JM, Adams J, Harriman C, Miller C, Van der Kamp JW (2015) Nutritional impacts of different whole grain milling techniques: a review of milling practices and existing data. Cereal Food World 60:130–139
Jouanin A, Boyd L, Visser RGF, Smulders MJM (2018a) Development of wheat with hypoimmunogenic gluten obstructed by the gene editing policy in Europe. Front Plant Sci 9:1523
Jouanin A, Gilissen LJWJ, Boyd LA, Cockram J, Leigh FJ, Wallington EJ, Smulders R (2018b) Food processing and breeding strategies for coeliac-safe and healthy wheat products. Food Res Int 110:11–21
Juhász A, Belova T, Florides CG, Maulis C, Fischer I, Gell G, Birinyi Z, Ong J, Keeble-Gagnere G, Maharajan A (2018) Genome mapping of seed-borne allergens and immunoresponsive proteins in wheat. Sci Adv 4:eaar8602
Juhász A, Haraszi R, Békés F (2020) Effects of environmental changes on the allergen content of wheat grain. In: Igrejas G, Tatsuya Ikeda T, Guzman C (eds) Improving wheat quality for processing and health. Springer, Berlin, pp 451–468
Junker Y, Zeissig S, Kim S-J, Barisani D, Wieser H, Leffler DA, Zevallos V, Libermann TA, Dillon S, Freitag TL, Kelly CP, Schuppan D (2012) Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med 209:2395–2408
Kasarda DD (2013) Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J Agric Food Chem 61:1155–1159
Kleessen B, Schwarz S, Boehm A, Fuhrmann H, Richter A, Henle T, Krueger M (2007) Jerusalem artichoke and chicory inulin in bakery products affect faecal microbiota of healthy volunteers. Br J Nutr 98:540–549
Knez M, Abbott C, Stangoulis JCR (2014) Changes in the content of fructans and arabinoxylans during baking processes of leavened and unleavened breads. Eur Food Res Technol 239:803–811
Knudsen KEB (1997) Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Feed Sci Technol 67:319–338
Kolida S, Gibson GR (2007) Prebiotic capacity of inulin-type fructans. J Nutr 137:2503S–2506S
Kristensen MT (2016) Gluten degradation in long time fermented dough. MSc thesis, Univ Copenhagen, Denmark
Kucek LK, Veenstra LD, Amnuaycheewa P, Sorrells ME (2015) A grounded guide to gluten: how modern genotypes and processing impact wheat sensitivity. Compr Rev Food Sci Food Saf 14:285–302
Kulp K (1993) Enzymes as dough improvers. In: Kamel BS, Stauffer CE (eds) Advances in baking technology. Springer, New York, pp 152–165
Kusaba-Nakayama M, Ki M, Iwamoto M, Shibata R, Sato M, Imaizumi K (2000) CM3, one of the wheat alpha-amylase inhibitor subunits, and binding of IgE in sera from Japanese with atopic dermatitis related to wheat. Food Chem Toxicol 38:179–185
Laidig F, Piepho HP, Rentel D, Drobek T, Meyer U, Huesken A (2017) Breeding progress, environmental variation and correlation of winter wheat yield and quality traits in German official variety trials and on-farm during 1983–2014. Theor Appl Genet 130:223–245
Lamacchia C, Camarca A, Picascia S, Di Luccia A, Gianfrani C (2014) Cereal-based gluten-free food: how to reconcile nutritional and technological properties of wheat proteins with safety for celiac disease patients. Nutrients 6:575–590
Laurière M, Pecquet C, Bouchez-Mahiout I, Snégaroff J, Bayrou O, Raison-Peyron N, Vigan M (2006) Hydrolysed wheat proteins present in cosmetics can induce immediate hypersensitivities. Contact Dermat 54(5):283–289
Leduc V, Moneret-Vautrin D-A, Guerin L, Morisset M, Kanny G, Allerbio L, Argonne V (2003) Anaphylaxis to wheat isolates: immunochemical study of a case proved by means of double-blind, placebo-controlled food challenge. J Allergy Clin Immunol 111:897–899
Loponen J, Ganzle MG (2018) Use of sourdough in low FODMAP baking. Foods 7:96
Loponen J, Mikola M, Katina K, Sontag-Strohm T, Salovaara H (2003) Degradation of HMW glutenins during wheat sourdough fermentations. Cereal Chem 81:87–93
Loponen J, Sontag-Strohm T, Venalainen J, Salovaara H (2007) Prolamin hydrolysis in wheat sourdoughs with differing proteolytic activities. J Agric Food Chem 55:978–984
Ludvigsson JF, Rubio-Tapia A, van Dyke CT, Melton LJ, Zinsmeister AR, Lahr BD, Murray JA (2013) Increasing incidence of celiac disease in a North American population. Am J Gastroenterol 108:818–824
Lyons TP (1982) Proteinase enzymes relevant to the baking industry. Biochem Soc Trans 10(4):287–290
Malalgoda MMG (2016) Investigation of protein composition in historic and modern hard red spring wheat cultivars. PhD thesis.Nort Dakota Fargo
Malalgoda MMG, Manthey F, Simsek S (2018) Reducing the celiac disease antigenicity of wheat. Cereal Chem 95:49–58
Manti S, Cuppari C, Tardino L, Parisi G, Spina M, Salpietro C, Leonardi S (2017) Applied nutritional investigation: HMGB1 as a new biomarker of celiac disease in children: a multicenter study. Nutrition 37:18–21
Mardini HE, Westgate AP, Grigorian RY (2015) Differences in the prevalence of celiac disease in the US population: National Health and Nutrition Examination Survey (NHANES) 2009–2012. Dig Dis Sci 60:1738–1742
Marriott BP, Cole N, Lee E (2009) National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 139:1228S–1235S
Hester K, Saliba A, McIntyre,E (2017). A preliminary exploration of non-coeliac gluten avoidance behaviours in Australia. GRDC Updata Papers, 14 Feb 2017
Melim-Miguel AS, Martins-Meyer TS, da Costa Figueiredo EV, Paulo-Lobo PW, Dellamora-Ortiz GM (2013) Enzymes in bakery: current and future trends. In: Muzzalupo I (ed) Food industry. InTech, Rijeka
Melini V, Melin F (2019) Gluten-Free Diet: gaps And Needs For A Healthier Diet. Nutrients 11:170
Messer M, Anderson CM, Hubbard L (1964) Studies on the mechanism of destruction of toxic action of wheat gluten in celiac disease by crude papain. Gut 5:295–303
Mohler V, Schweizer G, Hartl L (2011) Bread-making quality and grain yield in German winter wheat. II. Marker-trait associations. 61. Tagung der Vereinigung der Pflanzenzuechter und Saatgutkaufleute Oesterreichs 2010:29–31
Molberg O, Uhlen AK, Jensen T, Flate NS, Fleckenstein B, Arentz-Hansen H, Raki M, Lundin KE, Sollid LM (2005) Mapping of gluten T-cell epitopes in the bread wheat ancestors: implications for celiac disease. Gastroenterology 128:393–401
Molina-Infante J, Carroccio A (2017) Suspected nonceliac gluten sensitivity confirmed in few patients after gluten challenge in double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol 15:339–348
Morita E, Matsuo H, Chinuki Y, Takahashi H, Dahlstrom J, Tanaka A (2009) Food-dependent exercise-induced anaphylaxis importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Allergol Int 58:493–498
Moroni AV, Dal Bello F, Arendt EK (2009) Sourdough in gluten-free bread-making: an ancient technology to solve a novel issue? Food Microbiol 26:676–684
Morren M-A, Janssens V, Dooms-Goossens A, Van Hoeyveld E, Cornelis A, De Wolf-Peeters C, Heremans A (1993) Alpha-Amylase, a flour additive: an important cause of protein contact dermatitis in bakers. J Am Acad Dermatol 29:723–728
Muir JG, Shepherd SJ, Rosella O, Rose R, Gibson PR (2007) Fructan and free fructose content of common Australian vegetables and fruit. J Agric Food Chem 55:6619–6627
Muir JG, Rose R, Rosella O, Liels K, Barrett JS, Shepherd SJ, Gibson PR (2009) Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography. J Agric Food Chem 57:554–565
Muir JG, Mills J, Suter D, Békés F, Liels K, Yao LK, Gibson PR (2014) FODMAP in gluten-free grains may explain improved gastrointestinal symptoms in IBS on a gluten-free diet. J Nutr Intermed Metab 1:14–15
Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, Murray L, Metzger MH, Gasparin M, Bravi E, Maki M (2010) Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 42:587–595
Nilsson U, Oste R, Jagerstad M (1987) Cereal fructans: hydrolysis by yeast invertase, in vitro and during fermentation. J Cereal Sci 6:53–60
Oberforster M, Werteker M (2011) Inverse and non-inverse relations between grain yield and quality in the Austrian cultivars of wheat, barley and rye. 61. Tagung der Vereinigung der Pflanzenzuechter und Saatgutkaufleute Oesterreichs 2010, pp 9–17
Ortolan F, Steel CJ (2017) Protein characteristics that affect the quality of vital wheat gluten to be used in baking: a review. Compr Rev Food Sci Food Saf 16:369–381
Oury FX, Godin C (2007) Yield and grain protein concentration in bread wheat: How to use the negative relationship between the two characters to identify favourable genotypes? Euphytica 157:45–57
Parapouli M, Vasileiadis A, Afendra AS, Hatziloukas E (2019) Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol 6:1–31
Pasco JA, Nicholson GC, Kotowicz MA (2012) Cohort profile: geelong osteoporosis study. Int J Epidemiol 41:1565–1575
Pasini G, Simonato B, Giannattasio M, Peruo ADB, Curioni A (2001) Modifications of wheat flour proteins during in vitro digestion of bread dough, crumb, and crust: an electrophoretic and immunological study. J Agric Food Chem 49:2254–2261
Pauk J, Cs Lantos, Ács K, Gy Gell, Tömösközi S, Békés F (2019) Chapter 18. Spelt breeding via in vitro androgenesis for special food quality parameters. In: Al-Khayri JM, Jain SM, Johnson DV (eds) Advances in plant breeding strategies. Volume 5. Cereals and legumes, vol 5. Springer, Berlin, pp 525–558
Penagini F, Dilillo D, Meneghin F, Mameli C, Fabiano V, Zuccotti GV (2013) Gluten-free diet in children: an approach to a nutritionally adequate and balanced diet. Nutrients 5:4553–4565
Pepe O, Villani F, Oliviero D, Greco T, Coppola S (2003) Effect of proteolytic starter cultures as leavening agents of pizza dough. Int J Food Microbiol 84:319–326
Pizzuti D, Buda A, D’Odorico A, D’Inca R, Chiarelli S, Curioni A, Martines D (2006) Lack of intestinal mucosal toxicity of Triticum monococcum in celiac disease patients. Scand J Gastroenterol 41:1305–1311
Prandi B, Faccini A, Tedeschi T, Cammerata A, Sgrulletta D, D’Egidio MG, Sforza S (2014) Qualitative and quantitative determination of peptides related to celiac disease in mixtures derived from different methods of simulated gastrointestinal digestion of wheat products. Anal Bioanal Chem 406:4765–4775
Prandi B, Tedeschi T, Folloni S, Galaverna G, Sforza S (2017) Peptides from gluten digestion: a comparison between old and modern wheat varieties. Food Res Int 91:92–102
Praznik W, Cieslik E, Filipiak-Florkiewicz A (2002) Soluble dietary fibres in Jerusalem artichoke powders: composition and application in bread. Nahrung 46:151–157
Quirce S, Cuevas M, Dıez-Gomez ML, Fernandez-Rivas M, Hinojosa M, Gonzalez R, Losada E (1992) Respiratory allergy to Aspergillus-derived enzymes in bakers’ asthma. J Allergy Clin Immunol 90:970–978
Quirce S, Fernandez-Nieto M, Bartolome B, Bombın C, Cuevas M, Sastre J (2002) Glucoamylase: another fungal enzyme associated with baker’s asthma. Ann Allergy Asthma Immunol 89:197–202
Rakha A, Åman P, Andersson R (2011) Dietary fiber in triticale grain: variation in content, com-position, and molecular weight distribution of extractable components. J Cereal Sci 54:324–331
Reig-Otero Y, Manes J, Manyes L (2017) Amylase-trypsin inhibitors in wheat and other cereals as potential activators of the effects of nonceliac gluten sensitivity. J Med Food 21:207–218
Rewers M (2005) Epidemiology of celiac disease: What are the prevalence, incidence, and progression of celiac disease? Gastroenterology 128:S47–S51
Riaz Q, Ács K, Békés F, Eastwood RF, Farahnaky A, Majzoobi M, Blanchard CL (2019a) Fructan contents in Australian wheat varieties released over the last 150 years. Cereal Res Commun 47:669–677
Riaz Q, Farahnaky A, Majzoobi M, Pleming D, Eastwood R, Békés F, Blanchard CL (2019b) Investigating the changes in quality of historical and modern Australian wheat varieties. In: Cereals and grains conf of AACCI, London, 21–23 Oct
Ribeiro M, Rodriguez-Quijano M, Nunes FM, Carrillo JM, Branlard G, Igrejas G (2016) New insights into wheat toxicity: breeding did not seem to contribute to a prevalence of potential celiac disease’s immunostimulatory epitopes. Food Chem 213:8–18
Rizzello CG, De Angelis M, Di Cagno R, Camarca A, Silano M, Losito I, De Vincenzi M, De Bari MD, Palmisano F, Maurano F (2007) Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: new perspectives for celiac disease. Appl Environ Microbiol 73:4499–4507
Rizzello CG, Curiel JA, Nionelli L, Vincentini O, Di Cagno R, Silano M, Gobbetti M, Coda R (2014) Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol 37:59–68
Roberfroid M (1993) Dietary fiber, inulin, and oligofructose: a review comparing their physiological effects. Crit Rev Food Sci Nutr 33:103–148
Rollan G, De Angelis M, Gobbetti M, de Valdez GF (2005) Proteolytic activity and reduction of gliadin-like fractions by sourdough lactobacilli. J Appl Microbiol 99:1495–1502
Ross AS (2018) Flour quality and artisan bread. Cereal Foods World 63:57–62
Rumessen JJ, Gudmand-Hoyer E (1998) Fructans of chicory: intestinal transport and fermentation of different chain lengths and relation to fructose and sorbitol malabsorption. Am J Clin Nutr 68:357–364
Rustgi S, Shewry PR, Brouns F, Deleu LJ, Delcour JA (2019) Wheat seed proteins: factors influencing their content, composition, and technological properties, and strategies to reduce adverse reactions. Compr Rev Food Sci Food Saf 18:1751–1769
Rybalka AI (2017) Is wheat indeed a destructive food product? Plant Physiol Genet 49:188–210
Sakandar HA, Kubow S, Azadi B, Faryal R, Ali B, Ghazanfar S, Quraishi UM, Imran M (2019) Wheat fermentation with enterococcus mundtii QAUSD01 and Wickerhamomyces anomalus QAUWA03 consortia induces concurrent gliadin and phytic acid degradation and inhibits gliadin toxicity in caco-2 monolayers. Front Microbiol 9:3312
Salentijn EMJ, Goryunova SV, Bas N, Meer IM, Broeck HC, Bastien T, Gilissen LJWJ, Smulders MJM (2009) Tetraploid and hexaploid wheat varieties reveal large differences in expression of alpha-gliadins from homoeologous Gli-2 loci. BMC Genom 10:1–14
Sanchez-Monge R, Garcia-Casado G, Lopez-Otin C, Armentia A, Salcedo G (1997) Wheat flour peroxidase is a prominent allergen associated with baker’s asthma. Clin Exp Allergy 27:1130–1137
Sander I, Raulf-Heimsoth M, Siethoff C, Lohaus C, Meyer HE, Baur X (1998) Allergy to Aspergillus-derived enzymes in the baking industry: identification of β-xylosidase from Aspergillus niger as a new allergen (Asp n 14). J Allergy Clin Immunol 102:256–264
Sandiford CP, Tatham AS, Fido R, Welch JA, Jones MG, Tee RD, Shewry PR, Newman Taylor AJ (1997) Identification of the major water/salt insoluble wheat proteins involved in cereal hypersensitivity. Clin Exp Allergy 27:1120–1129
Saturni L, Ferretti G, Bacchetti T (2010) The gluten-free diet: safety and nutritional quality. Nutrients 2:16–34
Schober TJ, Clarke CI, Kuhn M (2002) Characterization of functional properties of gluten proteins in spelt cultivars using rheological and quality factor measurements. Cereal Chem 79:408–441
Schwalb T, Wieser H, Koehler P (2012) Studies on the gluten-specific peptidase activity of germinated grains from different cereal species and cultivars. Eur Food Res Technol 235:1161–1170
Sherman JD, Nash D, Lanninng SP, Martin JM, Blake NK, Morris CF, Talbert LE (2014) Genetics and end-use quality differences between modern and historical spring wheat. Crop Sci 54:1972–1980
Shewry PR (2019) What is gluten—Why is it special? Front Nutr 6:101
Shewry PR, Tatham AS (2016) Improving wheat to remove coeliac epitopes but retain functionality. J Cereal Sci 67:12–21
Siddiqi RA, Sogi DS, Sehajpal PK (2016) Effect of short-term sourdough fermentation on wheat protein. Cogent Food Agric 2(1132983):1–10
Siles RI, Hsieh FH (2013) Allergy blood testing: a practical guide for clinicians. Clevel Clin J Med 78:585–592
Simmonds NW (1995) The relation between yield and protein in cereal grain. J Sci Food Agric 67:309–315
Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, Kelly CP, Ahuja V, Makharia GK (2018) Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 16:823–836
Spaenij-Dekking L, Kooy-Winkelaar Y, van Veelen P, Wouter D, Drijfheut J, Jonker H, van Soest L, Smulders MM, Bosch D, Gilissen LJWJ, Koning F (2005) Natural variation in toxicity of wheat: potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 129:797–806
Stallknecht G, Gilbertson K, Ranney J (1996) Alternative wheat cereals as food grains: einkorn, emmer, spelt, kamut, and triticale. In: Janick J (ed) Proc 3rd nat symp: new crops, new opportunities, new technologies, Indianapolis. ASHS Press, Alexandria, pp 156–170
Stepniak D, Spaenij-Dekking L, Mitea C, Moester M, de Ru A, Baak-Pablo R, Van Velen P, Edens L, Koning F (2006) Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol 291:G621–G629
Struyf N, Laurent J, Lefevere B, Verspreet J, Verstrepen KJ, Courtin CM (2017a) Establishing the relative importance of damaged starch and fructan as sources of fermentable sugars in wheat flour and whole meal bread dough fermentations. Food Chem 218:89–98
Struyf N, Van der Maelen E, Hemdane S, Verspreet J, Verstrepen KJ, Courtin CM (2017b) Bread dough and baker’s yeast: an uplifting synergy. Compr Rev Food Sci Food Saf 16:850–867
Struyf N, Verspreet J, Courtin CM (2018) FODMAP reduction in yeast-leavened whole wheat bread. Cereal Food World 63:152–154
Suligoj T, Gregorini A, Colomba M, Ellis HJ, Ciclitira PJ (2013) Evaluation of the safety of ancient strains of wheat in coeliac disease reveals heterogeneous small intestinal T cell responses suggestive of coeliac toxicity. Clin Nutr 32:1043–1049
Suter DAI, Brown G, Békés F (2018) Development of spelt wheat products with low FODMAP content and low allergenicity. In: 1st internat. conf. “Wheat Landraces for healthy food systems”, Bologna, 13–15 June 2018
Suter DAI, Békés F, Florides C, Ács K, Durant M, Brown G (2019a) Why do so many consumers purchase “gluten free” breads. In: Australian grains science conference, Melbourne, 27–29 Aug 2019
Suter DAI, Brown G, Békés F (2019b) A possible explanation for the gluten free phenomenon. In: AIFST conf. Sydney. 1 July 2019
Tatham AS, Shewry PR (2008) Allergens to wheat and related cereals. Clin Exp Allergy 38:1712–1726
Thiele C (2003) Hydrolysis of gluten and the formation of flavor precursors during sourdough fermentation. PhD thesis, Techn. Univ, Munich
Thiele C, Grassl S, Ganzle M (2004) Gluten hydrolysis and depolymerization during sourdough fermentation. J Agric Food Chem 52:1307–1314
Tosi P, Gritsch CS, He J, Shewry PR (2011) Distribution of gluten proteins in bread wheat (Triticum aestivum) grain. Ann Bot 108:23–35
Tye-Din JA, Galipeau HJ, Agardh D (2018) Celiac disease: a review of current concepts in pathogenesis, prevention, and novel therapies. Front Pediatr 21:350
Uhde M, Ajamian M, Caio G, De Giorgio R, Indart A, Green PH, Verna EC, Volta U, Alaedini A (2016) Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. BMJ J 65:1930–1937
Uhde M, Caio G, De Giorgio R, Green PH, Volta U, Alaedini A (2020) Subclass profile of IgG antibody response to gluten differentiates non-celiac gluten sensitivity from celiac disease. Gastroenterology S0016–5085(20):34992
Vaccino P, Becker HA, Brandolini A, Salamini F, Kilian B (2009) A catalogue of Triticum monococcum genes encoding toxic and immunogenic peptides for celiac disease patients. Mol Genet Genomics 281:289–300
Vader W, Kooy Y, van Veelen P, de Ru A, Harris D, Benckhuijsen W, Pena S, Mearin L, Drijfhout JW, Koning F (2002) The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122:1729–1737
van den Broeck HC, Hongbing C, Lacaze X, Dusautoir J-C, Gilissen LJ, Smulders MJ, van der Meer IM (2010a) In search of tetraploid wheat accessions reduced in celiac disease-related gluten epitopes. Mol BioSyst 6:2206–2213
van den Broeck HC, de Jong HC, Salentijn EMJ, Dekking L, Bosch D, Hamer RJ, Gilissen LJWJ, van der Meer IM, Smulders MJM (2010b) Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: wheat breeding may have contributed to increased prevalence of celiac disease. Theor Appl Genet 121:1527–1539
van Herpen TWJM, Goryunova SV, van der Schoot J, Mitreva M, Salentijn E, Vorst O, Schenk MF, van Veelen PA, Koning F, van Soest LJM (2006) Alpha-gliadin genes from the A, B, and D genomes of wheat contain different sets of celiac disease epitopes. BMC Genom 7:1. https://doi.org/10.1186/1471-2164-7-1
Verspreet J, Hemdane S, Dornez E, Cuyvers S, Delcour JA, Courtin CM (2013) Maximizing the concentrations of wheat grain fructans in bread by exploring strategies to prevent their yeast (Saccharomyces cerevisiae)–mediated degradation. J Agric Food Chem 61:1397–1404
Vincentini O, Maialetti F, Gazza L, Silano M, Dessi M, De Vincenzi M, Pogna NE (2007) Environmental factors of celiac disease: cytotoxicity of hulled wheat species Triticum monococcum, T. turgidum ssp. dicoccum and T. aestivum ssp. spelta. J Gastroenterol Hepatol 22:1816–1822
Vincentini O, Borrelli O, Silano M, Gazza L, Pogna N, Luchetti R, De Vincenzi M (2009) T-cell response to different cultivars of farro wheat, Triticum turgidum ssp. dicoccum, in celiac disease patients. Clin Nutr 28:272–277
Vu NT, Chin J, Pasco JA, Kovács A, Wing LW, Békés F, Suter DAI (2014) The prevalence of wheat and spelt sensitivity in a randomly selected Australian population. Cereal Res Commun 43:97–107
Walter T, Wieser H, Koehler P (2015) Degradation of gluten in rye sourdough products by means of a proline-specific peptidase. Eur Food Res Technol 240:517–524
Wang DW, Li D, Wang J, Zhao Y, Wang Z, Yue G, Liu X, Qin H, Zhang K, Dong L, Wang D (2017) Genome-wide analysis of complex wheat gliadins, the dominant carriers of celiac disease epitopes. Sci Rep 7:44609
Wangen S (2009) Healthier without wheat. A new understanding of wheat allergies, Celiac disease and non-celiac gluten intolerance. Innate Health Publ, Seattle
Yuan J, Zhou C, Gao J, Li J, Yu F, Lu J, Li X, Wang X, Tong P, Wu Z, Yang A, Yao Y, Nadif S, Shu H, Xu J, Wu Y, Gilissen L, Chen H (2017) Prevalence of celiac disease autoimmunity among adolescents and young adults in China. Clin Gastroenterol Hepatol S1542–3565(17):30468–8
Zanini B, Petroboni B, Not T, Pogna N, Lanzini A (2009) A phase II, single blind, cross-over study of acute administration of Triticum monococcum (cultivar Monlis) in patients with coeliac disease. AGA Abstr 140:S–444
Zevallos VF, Raker V, Tenzer S, Jimenez-Calvente C, Ashfaq-Khan M, Rüssel N, Pickert G, Schild H, Steinbrink K, Schuppan D (2017) Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 152:1100–1113
Ziegler JU, Steiner D, Longin CFH, Würschum T, Schweiggert RM, Carle R (2016) Wheat and the irritable bowel syndrome—FODMAP levels of modern and ancient species and their retention during bread making. J Funct Food 25:257–266. https://doi.org/10.1016/j.jff.2016.05.019
Zoccatelli G, Sega M, Bolla M, Cecconi D, Vaccino P, Rizzi C (2012) Expression of α-amylase inhibitors in diploid Triticum species. Food Chem 135:2643–2649
Zotta T, Piraino P, Ricciardi A, McSweeney PLH, Paente E (2006) Proteolysis in model sourdough fermentations. J Agric Food Chem 54:2567–2574
Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers C, Sodergren E, Dahlstrom J, Lindner T, Sigurdardottir ST, McBride D, Keil T (2008) The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol 121:1210–1218
Acknowledgements
We acknowledge Dr C.W. Wrigley for his valuable suggestions to improve this overview, Matt Durrant, Berkelo, for bakery trials, Brookvale, NSW 2100, Australia. This work was supported within project OTKA-K 16-119835, funded by the National Research, Development and Innovation Office, Hungary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suter, D.A.I., Békés, F. Who is to blame for the increasing prevalence of dietary sensitivity to wheat?. CEREAL RESEARCH COMMUNICATIONS 49, 1–19 (2021). https://doi.org/10.1007/s42976-020-00114-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-020-00114-0