Abstract
Cassava (Manihot esculenta Crantz) is the most important crop among the tropical root and tuber crops (Pujol et al., 2002; Meireles da Silva et al., 2003). Along with maize (Zea mays L.), sugarcane (Saccharum spp.), and rice (Oryza sativa L.), cassava is among the most important sources of energy in the diet of most tropical countries of the world.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Cassava (Manihot esculenta Crantz) is the most important crop among the tropical root and tuber crops (Pujol et al., 2002; Meireles da Silva et al., 2003). Along with maize (Zea mays L.), sugarcane (Saccharum spp.), and rice (Oryza sativa L.), cassava is among the most important sources of energy in the diet of most tropical countries of the world.
Until four decades ago, with few exceptions, little scientific effort had been made to improve the crop in relation to the relevance of cassava for tropical and subtropical agriculture. However, with the creation of the International Institute of Tropical Agriculture (IITA) in Nigeria and the International Center of Tropical Agriculture (CIAT) in Colombia in the early 1970s, and rapid consolidation of several National Agriculture Research programs, a new era began for cassava with the implementation of successful breeding projects, modernization of cultural practices, and development of new processing methods (Cock, 1985; Jennings and Iglesias, 2002). National research centers in Brazil, Colombia, China, Cuba, India, Indonesia, Nigeria, Thailand, and Vietnam, among many other countries, have conducted successful research on cassava as well.
Currently cassava is a fundamental component in the diet of millions of people. Scott et al. (2000) estimated that for the year 1993, annual production of cassava was about 172.4 million tonnes, with a value of approximately US $ 9.31 billion. Between 1961–1963 and 1995–1997, cassava production increased at a rate of 2.35% per year (Scott et al., 2000), a trend comparable to that found in other crops, such as wheat (4.32%), potato (4.00%), maize (3.94%), yams (3.90%), rice (2.85%), and sweet potato (1.07%). Between 1994 and 2005, cassava productivity was expected to increase at 1.1% per year. In fact, worldwide productivity increased by about 18.4% in the last 10 years. Progress in increasing productivity, however, is not uniform. Vietnam doubled its productivity in a 10-year period from 8.5 tonnes/ha in 1997 and 1998 to 16.2 tonnes/ha in 2006 and 2007 (FAOSTAT). Thailand achieved remarkable progress in the last decade as well with average yields in 1997 and 1998 of 14.8 tonnes/ha up to 22.0 tonnes/ha in 2006 and 2007.
Cassava is a very rustic crop that grows well under marginal conditions where few other crops could survive. A large proportion of cassava varieties is drought tolerant, can produce in degraded soils, and is resistant to the most important diseases and pests. The crop is naturally tolerant to acidic soils and offers the convenient flexibility that it can be harvested when the farmers need it. Cassava is a perennial plant handled as annual. It does not have a pre-established development such as that of the cereals where the plants germinate, grow, flower, fill the grain, mature, and die. Cassava grows when conditions are favorable, and when they are not the plant drops its leaves and assumes dormancy until favorable conditions return. Farmers often abuse the advantages that the crop offers negating the minimum requirements for a sustainable and competitive production.
The most important commercial product of cassava is the storage root, full of starch. Roots are not tubers and, therefore, cannot be used for reproductive purpose. Cassava roots have a very short shelf life due to a process known as post-harvest physiological deterioration (PPD). PPD rapidly renders the roots unpalatable and unmarketable (Han et al., 2001; Reilly et al., 2003, 2007). Consequently, cassava roots need to be consumed or processed soon after harvesting (van Oirschot et al., 2000). The short shelf life of the roots severely limits the marketing options by increasing the likelihood of losses and the overall marketing costs. In addition, the access to urban markets and processing facilities is restricted to production sites that are relatively close to them. PPD begins with vascular streaking, which is a blue-black discoloration of the xylem parenchyma, followed by general discoloration of the storage parenchyma. Five to seven days later, microbial activity may cause further deterioration. The processes involved in PPD resemble typical changes associated with the plant’s response to wounding and trigger a cascade of biochemical reactions, in which reactive oxygen species are central. Specific genes involved in PPD have been identified and characterized and their expressions evaluated (Reilly et al., 2001).
Stem cuttings (stakes) are the most common source of planting material and are used for the commercial propagation of the crop. Cassava foliage is not widely exploited in spite of its high nutritive value, although consumption of leaves by human populations is relatively common in certain countries of Africa and Asia. Foliage is also used for animal feeding. Crude protein content in leaves typically ranges from 20 to 25% of dry weight (Gomez et al., 1983; Buitrago, 1990; Babu and Chatterjee, 1999), but levels as high as 30% have been identified (Buitrago, 1990). Exploitation of foliage in cassava is expected to increase because of the recent developments and testing of mechanical harvesters and alternative cultural practices to exploit it (Cadavid Lopez and Gil Llanos, 2003). Relevant traits for most cassava breeding projects include high and stable production of fresh roots and adequate levels of dry matter content. These are characteristics typically valued by the industry and farmers as well.
The inherent potential of cassava, its capacity to grow in marginal environments, the recent success in identifying high-value traits, and the incorporation of new tools for genetic enhancement, as described in several of the references provided in this review, offer bright prospects for the crop and the people that depend on it.
2 Origins and Domestication
All 98 species of the genus Manihot are native in the Neotropics from where it was introduced to other regions of the world (Rogers and Appan, 1973). The origin of cultivated cassava is still unclear. Three relevant questions were raised by Allem (2002) regarding the botanical origin (parental wild species that eventually lead to the emergence of M. esculenta); the geographic area where this emergence took place and the region where it was domesticated (agricultural origin). The prevailing hypothesis is that cultivated cassava originated in South America (Allem, 1990; 2002; Olsen and Schaal, 2001), but many of these questions remain open.
Although it is frequently considered a polyploid species, the analyses conducted during diakinesis and metaphase I indicate the presence of 18 small and similar bivalents in cassava (Hahn et al., 1990). In some cases occurrence of univalents/trivalents and late bivalent pairing has been observed. Cassava is therefore a functional diploid (2n = 2x = 36) (de Carvahlo and Guerra, 2002; Jennings, 1963; Nassar and Ortiz, 2008). It has been suggested that certain portions of the genome may be duplicated and, therefore, cassava may be a segmental allotetraploid (Maggon et al., 1969).
2.1 Species Involved
Rogers (1963) listed M. carthaginensis, M. aesculifolia, M. grahami, M. flabellifolia, and M. saxicola as the most closely related species to cultivated cassava based on morphological, ecological, and geographical evidence. Allem postulated in 1994 and 1999 that modern cultivated cassava originated directly from wild relatives of M. esculenta subsp. flabellifolia. This suggestion was further supported by Olsen and Schaal (2001). Nassar and Ortiz (2008), on the other hand, suggested that cultivated cassava arose as a result of hybridization of two species and proposed that M. pilosa would be one of them. The crop may have been domesticated more than once (Allem, 2002; Nassar and Ortiz, 2008).
2.2 Reproductive Biology
Commercial propagation of cassava is by stem cuttings (Fig. 2.1). However, sexual reproduction, a key element for conventional breeding, is common and relatively easy to achieve (Alves, 2002; Kawano, 1980). Most breeding programs generate new genetic variation through crossing. Controlled pollinations generate full-sib progenies. Alternatively, in polycross nurseries insects do the pollinations. In this case the exact origin of the pollen is not known, and half-sib families are produced. A certain proportion of seed from polycross nurseries may be the result of self-pollinations.
Occasionally botanical seed has been used for commercial propagation (Iglesias et al., 1994; Rajendran et al., 2000) but it is not a generalized practice. Propagation from true seed occurs occasionally in farmers’ fields and, as such, is also the starting point for the generation of useful genetic diversity (Eke-Okoro et al., 2001; Elias et al., 2001b; Pujol et al., 2002). Shamans or efficient farmers have been found to be key players in this informal genetic improvement process (Salik et al., 1997; Sambatti et al., 2001).
Cassava is a monoecious species, with female flowers opening 10–14 days before the male flowers on the same branch. Self-pollination can occur because male and female flowers on different branches or on different plants of the same genotypes can open simultaneously (Jennings and Iglesias, 2002). Flowering depends on the genotype and environmental conditions. Branching occurs when an inflorescence is formed (Fig. 2.2). Because erect, non-branching types are frequently preferred by farmers, the crossing of elite clones in certain regions may become more difficult because of the scarcity of their flowers. Synchronization of flowering remains a difficult issue in cassava breeding. Some clones flower relatively early at 4 or 5 months after planting (MAP), whereas others flower only at 8–10 MAP. Because of this and the time required for the seed to mature, it takes generally more than a year to obtain seeds of a planned cross. On average, between one and two seeds (out of the three possible formed in the trilocular fruit) per pollination are obtained. Several publications illustrate the procedures for controlled pollinations in cassava (Kawano 1980; Jennings and Iglesias 2002). Seeds often have a dormancy period of a few months after maturity, and they require relatively high temperatures (30–35°C) for optimum germination (Ellis et al., 1982).
Illustration of a cassava plant. The photograph on the left shows a plant close to harvesting time. The storage roots have swelled considerably and plant architecture is erect. The photograph on the right illustrates a plant that has been dissected slightly to expose how branching occurs in cassava. Four different flowering events along the life of this plant resulted in the respective four levels of branching
There is no evidence of incompatibility, so crosses can be done easily (except for the scarcity or absence of flowers in certain genotypes). There is no evidence of self-incompatibility either, so it is technically possible to make self-pollinations and obtain viable botanical seed. Male sterility is a frequent phenomenon and is currently being used to measure pollen flow.
2.3 History of Crop
Cassava originated in South America and was domesticated less than 10,000 years ago, with evidence of ancient cultivation in Brazil, Peru, Colombia, and Venezuela (Elias et al., 2001a; Allem, 2002; Nassar and Ortiz, 2008). Early European sailors and explorers soon recognized the advantages of the crop and carried it from Brazil to West Africa by the end of the sixteenth century. Cassava started to spread in East Africa later and one century on had reached the islands of Réunion, Madagascar, and Zanzibar (Janssens, 2001). From there, traders later introduced it to Asia. Cassava does not grow in temperate regions and therefore, until recently, its products were not well known outside the tropical and subtropical regions where it is grown and consumed. Recognizing the occurrence of many exceptions, cassava has become a major industrial crop in several countries of Asia, has a dual role in food security and as an industrial feedstock in Latin America and the Caribbean (LAC) and plays a key role in food security for sub-Saharan Africa.
3 Genetic Resources
Allem and co-workers suggested in 2001 that there are three M. esculenta subspecies: esculenta (cultivated cassava), flabellifolia, and peruviana. These three subspecies along with the closest wild relative (M. pruinosa) constitute the primary gene pool. The morphological characteristics of cultivated cassava are highly variable and there are numerous morphological descriptors that can be used for cultivar characterization (Alves 2002). However, within cultivated cassava the concept of varietal group has not been used.
The secondary gene pool includes M. triphylla, M. pilosa, M. brachyloba, M. anomala, M. pruinosa, M. gracilis, M. tripartita, M. leptophylla, M. pohlii, M. glaziovii, M. dichotoma, M. aesculifolia, and M. chlorosticta.
3.1 Germplasm
Early efforts were made to explore and assemble a large collection of cassava germplasm. CIAT holds in trust the worldwide cassava germplasm collection with more than 6,000 accessions. Three main types of accessions can be mentioned: (a) wild relatives of cassava within the Manihot genus; (b) traditional landraces grown by farmers in Africa, Asia, or LAC; and (c) improved cassava germplasm produced by breeding projects in the continents mentioned above. The collection is maintained in vitro at CIAT experimental station located in Palmira, Colombia.
It can be said that the domestication of cassava has been completed only recently. Therefore, many landraces can still be directly released as commercial varieties once they have proved to have stable and competitive productivity in a given environment. In this regard cassava differs from other crops such as the cereals where breeding has resulted in a large genetic distance between improved germplasm and landraces. Another feature of the germplasm collection is the limited knowledge that has so far been generated from it. Morphological descriptions of the accessions are now available. A large screening of starch quality traits from more than 4,000 genotypes was recently published (Sánchez et al., 2009). Still, a more aggressive approach to analyze self-pollinated progenies (in search of useful recessive characteristics) and further analysis of the collection in search of new sources of tolerance or resistance to abiotic and biotic stresses is required. Three pre-breeding activities conducted at CIAT in collaboration with IITA and research institutions in Thailand and Brazil will be described below.
Extensive exploration to increase the germplasm collections and to develop approaches that will allow for an efficient evaluation of such germplasm is also urgently needed. The lack of genetic variability in many breeding programs can be overcome through an enhanced exchange of germplasm. The availability of partially inbred genetic stocks that allows the exchange of source material using botanical seeds will facilitate germplasm exchange.
3.2 Pre-breeding
3.2.1 Inter-specific Crosses
Wild Manihot germplasm offers a wealth of useful genes for the cultivated M. esculenta species (Hahn et al., 1980b; Chavarriaga et al., 2004). Several accessions of M. esculenta subsp. flabellifolia, M. peruviana, and M. tristis have high levels of proteins (Asiedu et al., 1992). This trait was observed also in crosses with M. oligantha (Nassar and Ortiz, 2008). A source of tolerance to PPD has been identified in M. walkerae (Bertram, 1993) and introgressed into cassava (Cuambe, 2007). The only source of resistance to the cassava hornworm and a widely deployed source of resistance to cassava mosaic disease (CMD) were identified in fourth backcross derivatives of M. glaziovii (Jennings, 1976; Chavarriaga et al., 2004). Moderate to high levels of resistance to white flies have been found in inter-specific hybrids of M. esculenta subsp. flabellifolia. Resistance was recovered easily in F1 inter-specific hybrids, suggesting a simple inheritance of the trait. Accessions of M. crassisepala and M. chlorosticta are the only genotypes from the primary and secondary gene pool of the crop discovered to possess the waxy starch phenotype (Ceballos et al., 2006a). Apomixis has been observed in crosses with M. neusana (Nassar and Ortiz, 2008).
CIAT and EMBRAPA-Brazil are actively searching for desirable traits in Manihot species other than M. esculenta with the support of the Generation Challenge Program.
3.2.2 Inbreeding to Unmask Useful Recessive Traits
Early attempts to identify useful starch quality traits in cassava germplasm (Sánchez et al., 2009) failed to achieve the objective because in most instances these traits are recessive in nature. Therefore, the cassava breeding project initiated a systematic effort to self-pollinate accessions from the germplasm collection and then patiently analyze the segregating progenies. Some important root quality discoveries have been made and are described in a separate section below. In addition, other traits such as male sterile genotypes or characters affecting plant morphology have gradually emerged.
A new generation of self-pollinated germplasm is evaluated in the field every year. S1 progenies from MVEN 331 segregated for a distinctive feature: leaves without petioles and a very erect plant type (Fig. 2.3) with absence of branching (at least for the first 6–8 months of age). This plant type offers interesting commercial applications. The most immediate one would be for the production of dried cassava foliage. One of the bottlenecks for this new market for cassava is the costs involved in the harvesting of the foliage. The only practical approach is a mechanical harvest that would also drag a considerable amount of young stems and petiole tissue. It should be easy to envisage a system where the kind of plant shown in Fig. 2.3 is harvested and, since the leaves have no petiole, and there is no branching (or few), leaves could be easily peeled off the stem. The result would be a reduced cost of harvest and, because of the reduced proportion of petiole and young stems, a better quality of foliage with reduced fiber content. This last characteristic would be fundamental for the use of dried foliage in the composition of diets for the poultry industry.
A second important potential application of this mutation would be the possibility of drastically increasing plant densities in commercial planting of cassava. It should also be easy to accept the idea that this new plant type could allow higher plant densities in cassava fields. Perhaps as many as 30,000 plants/ha could be used. This concept is important because most of the genetic gains achieved in different crops through the last century relates to modifications in plant architecture. The use of semi-dwarf wheat and rice varieties led to the highly successful green revolution. In the case of maize, if there is a single characteristic that can explain the consistent gains observed after the first introduction of commercial hybrids, it is reduced plant height with increased tolerance to higher plant densities (Duvick, 1999; Fehr, 1987; Troyer 2006). It should be mentioned that the petioles mutation was already known. What this research provides is evidence that it is simply inherited and has now allowed the segregation of several different phenotypes, a few of them also having the erect plant architecture.
There are many examples reported in the literature where tolerance to different herbicides has been found in different crops (canola, cotton, lentil, lettuce, maize, rice, sugar beet, sunflower, tobacco, tomato, and wheat), which led to the release of trade marks such as Clearfield, RoundUp Ready, and Liberty Link (Sherman et al., 1996; Tan et al., 2005; 2006; Tan and Bowe, 2008). In most of these cases, tolerance to herbicides was based on recessive or partially dominant genes and self-pollinations have facilitated their identification. During 2009 the cassava breeding project initiated activities to screen for natural tolerance to herbicides. A group of about 800 S1 genotypes were planted in six different blocks. Each genotype was represented by two plants in each block. Plants in each block will be treated with a different herbicide in search of sources of resistance. CIAT has had for many years transgenic cassava with resistance to BASTA, but restricted for research purposes only (Calderón-Urrea, 1988). This technology offers great potential once the intellectual property rights and biosafety issues are overcome.
3.2.3 Developing Partially Inbred Genetic Stocks
One of the most important uses of accessions from the germplasm collection is their role as source of useful traits. Currently the exchange of sources of specific traits is made through the shipment of in vitro accessions carrying the desired trait. Shipment of in vitro germplasm is expensive and troublesome. Therefore, exchange of germplasm is limited.
If a given genotype is to be used as source of a desirable trait, its value is in the trait itself, not in the whole genotype. CIAT and IITA have initiated a new approach by developing partially inbred genetic stocks. The source germplasm is self-pollinated to increase the degree of homozygosity (for the desirable trait). S1 genotypes (homozygous for simply inherited traits) could then be obtained. There are three different scenarios for identifying homozygous genotypes among the S1 (segregating) progeny: (a) if the trait is recessive (e.g., amylose-free starch) only homozygous recessive genotypes will express it and selection can be done using the phenotype of the S1 genotypes; (b) if the trait is dominant and molecular markers are available, co-dominant markers such as SSR can be used to identify homozygous S1 genotypes; and (c) if the trait is dominant and no molecular marker is available a second self-pollination would be necessary to identify S2 progenies that do not segregate for the trait (indicating that the progenitor S1 genotype was homozygous).
These partially inbred genotypes would become the backbone of these genetic stocks. A menu of options for tolerance/resistance to abiotic/biotic stresses, plant architecture, starch quality, nutritional quality, or other desirable traits will be gradually developed. The breeding value (for the trait) of these homozygous S1 genotypes doubles if the assumption of heterozygosity for the trait in the elite S0 genotype holds true. The selected S1 genotypes could then be registered as a source of the desirable trait. Also, the S1 genotype could be self-pollinated to produce S2 seed, which would also be homozygous for the desirable gene(s). The storage and exchange of these S2 botanical seeds would be considerably less expensive and faster than maintaining germplasm in vitro or in the field. Phytosanitary restrictions for the exchange of botanical seed are less limiting compared with the shipment of in vitro or vegetative cuttings. Finally, crosses of S1 genotypes homozygous for different desirable traits can be made to produce new S1 genotypes combining more than one desirable trait in a homozygous condition. Genetic stocks combining germplasm developed by IITA, CIAT, EMBRAPA, and other national programs in Africa, Asia, and Latin America could then contribute to a more dynamic exchange of germplasm and a more efficient exploitation of cassava genetic resources.
In the case of germplasm collections, 30–50 S1 genotypes could be used to represent accessions and kept as botanical seed as a backup (the original genotype would be lost but its genes would be maintained).
4 Major Breeding Achievements
Conventional plant breeding has one of the highest rates of return among the investments in agricultural research. The remarkable increase in the productivity of many crops during the twentieth century was reportedly due to genetic gains achieved through crop breeding (Fehr 1987). Cassava has also benefited from technological inputs in the area of breeding (Kawano, 2003). New varieties in Africa, Asia, and LAC have satisfied the needs of farmers, processors, and consumers, bringing millions of dollars in additional income to small farmers. The applications of tissue culture technologies have also made positive contributions (DeVries and Toenniessen, 2001) as well as the definition of adequate cultural practices, particularly in relation to fertilization protocols (Howeler, 2007). Genetic transformation and molecular biology (Blair et al., 2007; Calderón-Urrea, 1988; Fregene et al., 1997, 2000; Puonti-Kaerlas et al., 1997) offer great potential but have not had any measurable commercial impact yet.
During the past 30–40 years, significant progress has been achieved in the initial phase of the scientific genetic improvement of cassava. It can be said that with the turn of the millennium the adaptation of the crop to more intensive cultivation systems was completed. This process involved assembling major traits such as improved yield (mainly through a higher harvest index), low cyanogenic content (when desirable), improved plant architecture, and resistance/tolerance to the major diseases and pests. All these activities contributed to the general aim of increasing productivity and improving stability of production.
Two major factors influence the impact of plant breeding: the quality of the germplasm developed (i.e., its yield potential and stability) and the degree of adoption by farmers. Adoption of new varieties tends to be faster and more dynamic where there are markets for whatever the farmer produces. On the other hand, in subsistence farming where different crops play mostly a food security role, the adoption of new varieties is frequently slower and/or limited. Participatory plant breeding has a clear advantage for subsistence farming where many subtle criteria define the success of a given variety and its chances of adoption by farmers. The impact of conventional breeding in cassava tends to reach a maximum when and where the crop is largely used for processing and, therefore, there are strong markets for it. Figure 2.4 illustrates the increases in productivity of cassava in Thailand and Vietnam (strong markets for processed cassava) compared with the performance in LAC and sub-Saharan Africa. Yield productivity in Thailand and Vietnam increased an average of about 0.5 t/ha/year since 1990, whereas in South America and Africa the increase was much slower with 0.09 t/ha/year (FAOSTAT).
In addition to increases in productivity, Kawano (2003) reported a major improvement in dry matter content of cassava varieties released in SE Asia and also demonstrated the importance of selection for adequate levels of harvest index, particularly in early stages of the selection process. Jennings and Iglesias (2002) provided an assessment of the significant progress achieved to develop cassava cultivars tolerant to the main viral diseases (CMD and CBSD), bacterial blight, and super-elongation diseases. Resistance to CMD has been deployed and analyzed from the molecular point of view (Fregene et al., 2000, 2004; Egesi et al., 2007). Important progress in identifying and deploying tolerance/resistance to CBSD has also been achieved in recent years (McSween et al., 2006).
5 Current Goals of Breeding
High and stable productivity will also be key traits for the future but there will also be increasing opportunities and needs for developing cassava cultivars particularly suited to specific end uses. It is therefore envisaged that multipurpose varieties will gradually give way to specific varieties with special quality traits developed for different processing pathways. This situation will hopefully lead to a closer interaction between the processing and production sectors of the different value-added chains.
Climate change will most likely have an impact in regions where cassava is a key commodity. Currently cassava can be grown in very contrasting set of conditions from the Amazon basin to arid conditions in sub-Saharan Africa or the northeast of Brazil. The crop, therefore, has the capacity to adapt to the predicted changes in climate. Climate change will negatively affect cassava through changes in the occurrence of pests and diseases. There is likely to be a shift in these biotic problems. The other negative impact that climate change will have is around unpredictability of rains both in amount and timing. The problem with these changes is not the lack of adaptation of cassava to the new environmental conditions but the time required for farmers to change from one variety (adapted to the old conditions) to a new one (adapted to the new conditions).
5.1 Cassava Utilization
Cassava is a unique crop because every part of the cassava plant can be utilized. There are many different uses of cassava (Balagopalan, 2002; Ceballos et al., 2006a) which are described below. These alternative uses, in turn, create an array of requirements that can be satisfied by conventional breeding. The roots are by far the most important product of cassava. Table 2.1 provides a summary of the most important characteristics of cassava roots. There is variation in starch quality in relation to its amylose percentage with a mean around 21% (Sánchez et al., 2009; Wheatley et al., 1993). Cassava roots are low in protein and fat contents with an average below 2% (dry weight basis). There have been, however, some preliminary results suggesting that protein content in the roots can be considerably higher (6–8%) in some landraces, particularly from Central America (Ceballos et al., 2006b). Yellow cassava roots have considerable amounts of carotenoids (Chávez et al., 2000, 2005; Iglesias et al., 1997) and through conventional breeding the original levels have been increased threefold in a period of 4–6 years (CIAT, 2009).
Cyanogenic glucosides (CG) are found in every tissue of cassava, except in the seed. The most abundant CG is linamarin (about 85%), with lesser amounts of lotaustralin. The CG, synthesized in the leaves and transported to the roots, is broken down by the enzyme linamarase to produce hydrogen cyanide (HCN), a volatile poison (Andersen et al., 2000; Du et al., 1995; McMahon et al., 1995; Wheatley and Chuzel, 1995). Linamarin and linamarase accumulate in different parts of the cell, thus preventing the formation of free cyanide. However, most processing methods disrupt the tissues, allowing the enzyme to act on the substrate for a rapid release of cyanide. CG accumulation varies with genotypes, environments, agronomic practices, age of the plant and plant tissue, being highest in the leaves and peel of roots (Cock, 1985). Concentration of CG in the roots frequently defines the specific uses a given variety can have.
5.1.1 Starch
Cassava is one of the four most important sources of starch worldwide, together with maize, potato (Solanum tuberosum), and wheat (Triticum aestivum and T. durum) (Davis et al., 2003; Ellis et al., 1998). Because of the low levels of protein and fat in the roots, the starch from cassava roots has excellent characteristics and is relatively inexpensive to extract. Key traits for this industry include white parenchyma, high dry matter content, and variation in the starch quality properties and composition.
5.1.2 Animal Feeding
Cassava is a competitive source of calories for animal diets because of its efficiency converting light into chemical energy. However, cassava roots have reduced levels of fat and protein, and lack vitamins and minerals compared with maize. Roots can be processed into dried chips, meals, or pellets for animal feed. The price of dried cassava roots, when used for animal feeding, is lower than that of maize (typically around 70% the price of maize) because of its reduced nutritional value. Key traits for this industry are high dry matter content and if possible at all, increased nutritional value (particularly in relation to protein and vitamins content). This industry may also consume dried foliage which is an excellent source of proteins, minerals, and vitamins.
5.1.3 Bio-ethanol
This is a relatively new end use for cassava and is the result of increased prices of oil and technological developments for the hydrolysis of starch prior to the fermentation process. For this industry a key breeding objective would be to maximize the productivity of energy per hectare. In certain circumstances, therefore, when fresh roots are ground and used for fermentation without being first dried, it may be acceptable to release clones with high fresh root productivity per hectare, even if they do not possess a minimum of dry matter content. This kind of clone would not be acceptable for the starch or dried chip industries because of unacceptably higher prices of processing. Another characteristic that would be desirable for this industry is roots whose starch is easier to degrade into simple sugar syrups. Carvalho et al. (2004) described a “sugary” mutation in cassava collectively known in Brazil as “mandiocabas.”
5.1.4 Cassava for Processed Food
There are many different ethnic uses of cassava for processed food (gari, fufu, kokonte, farinha, casabe, gaplek, etc.) (Cock, 1985). Each of these products requires specific organoleptic and physico-chemical properties, which in turn imply that there is a large variation of requirements for these end uses.
In some cases, high CGs are preferred because they confer a particular taste to the product, or they prevent theft, or they protect from monkeys and other mammals, or simply because of cultural preferences. In others cases (e.g., pre-cooked frozen croquettes for the export markets) very low levels of CG are a critical requirement. Cultivars with less than 100 mg CG/kg fresh weight in the roots are considered “sweet.” Above this level cassava roots are considered “bitter.” In an extensive evaluation of a large sample of genotypes, Sánchez et al. (2009) found that breeding has tended to reduce the cyanogenic potential of cassava roots.
5.2 Breeding Objectives
Breeding objectives depend on the ultimate use of the crop. The processing industry has relatively few requirements which can be summarized as high and stable productivity and a minimum dry matter content of about 35%. However, the globalization of economies during the 1990s has opened up opportunities never available to cassava before because both governments and private sectors realized that the crop was a key but underutilized commodity (Ceballos et al., 2004). These changes made clear that, in addition to high and stable productivity, cassava breeding projects had the opportunity of expanding and exploiting genetic variability that would generate clones with increased value for the different industrial processes where cassava can be a strategic raw material. Examples of key traits for the different industries have been described in the section about cassava utilization.
When cassava is used as a food security crop, additional requirements need to be addressed for a variety to be adopted. Human consumption frequently emphasizes cooking quality or starch characteristics over productivity as a determining trait. Good cooking quality is often associated with other morphological traits, such as the color of the peel of the roots, the leaf petiole, or the shoot. Farmers frequently reject any change in such morphological traits, although they may have little or no correlation with actual cooking quality. Because of those types of farmers and consumers’ preferences, participatory research and breeding approaches have been incorporated in cassava breeding (DeVries and Toenniessen, 2001; Gonçalvez Fukuda et al., 2000; Gonçalvez Fukuda and Saad, 2001).
Other root quality traits relevant to different cassava breeding programs around the world are the cyanogenic potential in the roots (Dixon et al., 1994) and early bulking capacity. Unfortunately it is very difficult to monitor clones that have the early bulking trait because breeding programs need to standardize their harvesting time (typically at 11–12 MAP), particularly when genotypes are represented by only a few plants. Early bulking is typically assessed after several selections have been made and potentially useful genotypes may have unknowingly been discarded.
5.2.1 Abiotic Stresses
Stable production relies on tolerance to biotic and abiotic stresses, which vary with the environment. There are a variety of abiotic factors limiting cassava productivity which would probably accentuate as a result of changes in the climate generating wider fluctuations in relevant weather parameters. The crop is frequently grown in drought-prone regions and/or on low-fertility soils. It can also be found in alkaline or acidic soils, most frequently the latter. Some traits associated with adaptation to these conditions have been suggested (Jennings and Iglesias, 2002), such as leaf longevity (CIAT 2001; Fregene and Puonti-Kaerlas, 2002; Lenis et al., 2006), optimum leaf area index, and ideal plant architecture (Hanh et al., 1979; Kawano et al., 1998; Kawano 2003). The capacity of the stems to withstand long storage periods (sometimes up to 2 months) from harvest to planting is an important trait (Fig. 2.5). This characteristic affects final density of established plants and is fundamental for areas with relatively long dry spells or erratic rainfall, because the storage period may extend to the point that it compromises their viability. While there is known genetic variation for stem storability, it has not been a major breeding objective of any program so far.
Although it is a self-inflicted reaction, PPD and the resulting short shelf life of cassava roots after harvest is frequently grouped as an abiotic stress. Consequently, cassava roots need to be consumed or processed soon after harvesting (van Oirschot et al., 2000). The short shelf life of the roots severely limits the marketing options by increasing the likelihood of losses, the overall marketing costs, and by limiting the access to urban markets or processing centers to production sites close to them. Extending the shelf life by only 2–3 weeks would offer huge advantages to the cassava community.
5.2.2 Herbicide Tolerance
Herbicide tolerance in crops offers several advantages. The handling of herbicides can be made in a much more efficient way, applying them at the optimal timing when weeds are most vulnerable. This implies that there is a reduction in the amount of herbicides used, reducing costs of production on the one hand and having a positive impact on the environment on the other. Perhaps more important is the possibility that herbicide tolerance allows direct planting, which without proper technologies to handle the problem of weeds, is often unviable. Direct planting also offers several advantages: it allows the maintenance of a mulch of crop residues on the soil, thereby reducing soil erosion and maximizing the capture and conservation of water and soil nutrients. Direct planting reduces the operations of soil preparation at planting time, which offer the dual advantages of reducing costs and the negative impact on the environment. There are a few alternative approaches to develop cassava tolerant to herbicides, and one of them is inbreeding accessions from the germplasm collection.
5.2.3 Disease and Pest Resistance
The distribution of diseases is not uniform worldwide. In Africa, Cassava Mosaic Disease (CMD) and Cassava Brown Streak Disease (CBSD) are important constraints. A disease similar to CMD is also present in southern India, but they are fortunately neither present in the rest of Asia nor in the LAC. On the other hand frogskin disease causes roots to become “corky” and commercially unusable but it is only present in LAC. The causal agent has not yet been identified, although it has been suspected for many years that it may be a virus or a phytoplasm. Bacterial blight, induced by Xanthomonas axonopodis pv. manihotis (also known as X. campestris pv. manihotis), is found worldwide and can have devastating effects on yield and the availability of planting material, particularly in Africa and LAC (Hillocks and Wydra, 2002). Several fungal diseases may also affect cassava productivity. Super-elongation disease, induced by Sphaceloma manihoticola (Teleomorph: Elsinoe brasiliensis), is widespread in the Americas, from Mexico to Southern Brazil. In tropical lowlands with high rainfall, Cercospora, Cercosporidium, Phaeoramularia, or Colletotrichum species can affect cassava productivity (Jennings and Iglesias, 2002). Phoma species cause leaf and stem lesions in the tropical highlands. Several species of Phytophthora induce root rot. Root rots are also induced by different species of the genera Sclerotium, Armillaea, and Fusarium. Fortunately, there are sources of genetic resistance to most of these diseases (CIAT, 2001; Hilloocks and Wydra, 2002).
Pests that feed on cassava can reduce productivity through direct feeding damage or as vectors for diseases. The green mite (Mononychellus tanajoa) devastated cassava fields upon its introduction in Africa in the 1970s (Nyiira, 1975). Other mites important for cassava are Tetranychus urticae, T. cinnabarinus, Mononychellus caribbeanae, and Oligonychus peruvianus (Bellotti, 2002). The mealybugs Phenacoccus manihotis and P. herreri feed on cassava fields of Africa and LAC, respectively. IITA and CIAT collaborated in the introduction into Africa of agents for the biological control of mealybugs found in Brazil and Colombia. This is one of the most successful interventions in the deployment of biological control agents with huge economic and environmental benefits (Neuenschwander, 1994). Unfortunately P. manihotis has recently been introduced into Asia. Thrips (particularly Frankliniella williamsi and Scyrtotrips manihoti) considerably reduce yields of susceptible genotypes. Clones with pubescent leaves in their early stages of development offer excellent levels of resistance to these insects (Belloti, 2002), and this trait has been broadly incorporated into improved varieties.
Whiteflies are among the most widespread pests in cassava. Aleurotrachelus socialis is the predominant species in northern South America, where it causes considerable crop damage through direct feeding. Bemisia tabaci is widely distributed in tropical Africa and several Asian countries. Until 1990, B. tabaci biotypes found in the Americas did not feed on cassava. The major effect of B. tabaci is through its role as a vector of the devastating CMD disease in Africa. Several other species of whiteflies affect cassava in different regions. Genetic resistance to whiteflies in cassava has been found particularly for A. socialis in several germplasm accessions from the CIAT collection (Bellotti, 2002). Based on breeding work at CIAT, Colombia released the first whitefly-resistant variety of any crop in 2002, targeted toward the Tolima Valley, where whiteflies typically devastate plantations. There are several other arthropod pests affecting cassava roots, foliage, and/or stems, particularly Lepidoptera, Diptera, and Hemiptera. There is little or no genetic resistance to those pests and their management is commonly achieved through biological control measures. Attempts to produce transgenic resistance to the horn worm in cassava have succeeded with the introduction of cry genes encoding insect-specific endotoxins (Bt toxins) from Bacillus thuringiensis (Fregene and Puonti-Kaerlas, 2002; Ladino et al., 2001; Taylor et al., 2004).
6 Breeding Methods and Techniques
6.1 Evaluation and Selection Scheme Used in Cassava Breeding
Cassava genetic improvement starts with the assembly and evaluation of a broad germplasm base, followed by production of new recombinant genotypes derived from selected elite clones and careful evaluation in a set of representative environments. Directed and systematic cassava breeding began only a few decades ago and, therefore, the divergence between landraces and improved germplasm is not as wide as in crops with a more extensive breeding history. As a result, landrace accessions, as explained above, probably play a more relevant role in cassava than in other crops. Parental lines are selected based mainly on their per se performance and little progress has been made in using general combining ability (Hallauer and Miranda, 1988) as a criterion of parental selection.
The botanical seed obtained by the different crossing schemes (Kawano, 1980) may then be planted directly in the field or first germinated in greenhouse conditions and then transplanted to the field when they are about 20–25 cm tall (Jennings and Iglesias, 2002). Root systems in plants derived from botanical seed or vegetative cuttings may differ considerably. The taproots from seedlings tend to store fewer starches than roots from cuttings (Alves, 2002; Rajendran et al., 2000). Because of this, it is difficult to correlate the root yield of clones at later stages in the evaluation/selection process, with early results from the plants obtained from botanical seeds (Morante et al., 2005). However, when seeds are germinated in containers and later transplanted, the taproot often does not develop, and the seedling-derived plant may be more similar to subsequent stake-derived plants regarding the shape of their starchy roots.
For general purpose breeding, once a set of crosses is planned, it takes 2 years for the crosses to be ready. Certain crosses can be made (and the seed harvested) within a year, but crossing blocks are generally maintained for up to 2 years in the field thus allowing several flowering events per plant so numerous seeds per cross and many crosses per parental line can be obtained. Most of the seed is obtained from the second flowering event (6–8 MAP) through the third or fourth event (about 14–18 MAP). The last pollinations are then made early enough for the seed to mature and be harvested and processed for germination. Table 2.2 describes a typical recurrent selection cycle. The first stage of that cycle includes the 2 years for the recombinant seed to be produced, harvested, and processed.
The vegetative multiplication rate of cassava is low. From one plant, 5–10 cuttings typically can be obtained, although this figure varies widely by genotype. This situation implies a lengthy process to reach the point where replicated evaluations across several locations can be conducted, just because of the time required to produce enough planting material. It takes about 5–6 years from the time the botanical seed is germinated until the evaluation/selection cycle reaches the regional trial stage when several locations can be included (Table 2.2). One further complication in a cassava program is the number of factors that can affect quality of planting material. For example, the original positioning of the vegetative cutting along the stem affects considerably the performance of the plant it originates. Cuttings from the mid-section of the stems usually produce better performing plants than those at the top or the bottom. This variation in the performance of the plant, depending on the physiological status of the vegetative cutting, results in larger experimental errors and undesirable variation in the evaluation process.
There is some variation among different cassava breeding programs regarding the numbers of genotypes and plants representing them through the different stages of selection. However, the numbers presented in Table 2.2 are fairly common and illustrate the different stages required to complete a selection cycle and the kind of selection pressures that are generally applied (Ceballos et al., 2004, 2007a; CIAT, 2009). The first selection can be conducted in the third year on the nurseries with plants derived from botanical seed (F1 in Table 2.2). Because of the low correlations between the performance at this early stage of selection and when the genotypes reach replicated trials, the early selections are based on high heritability traits, such as plant type, branching habits, and, particularly, reaction to diseases (Hahn et al., 1980a, b; Hershey, 1984; Iglesias and Hershey, 1994; Morante et al., 2005). At IITA, combined selection for resistance to CMD and bacterial blight (X. axonopodis pv. manihotis) begins with about 100,000 seedlings and only about 3,000 genotypes survive this first stage of selection, which is based on single plant performance.
The second stage of selection is called the clonal evaluation trial (CET). The surviving genotypes from the single plant selection conducted during the F1 stage produce the 6–10 vegetative cuttings required for this second step. The capacity to produce this number of cuttings is in fact another selection criterion used at the F1 stage. CETs usually range from 2,000 to 3,000 clones. Within a given trial, however, the same number of plants is used to avoid the confounding effects between number of plants and genotypic differences. Because the competition between neighboring genotypes in the CET may favor more vigorous plant architectures, selection at this stage still relies heavily on high heritability traits, such as harvest index (Kawano et al., 1998; Kawano, 2003). Plant type is an important selection criterion at early stages of selection; plants whose main stem does not branch until it reaches atleast 1 m are preferred (Kawano et al., 1978; Hahn et al., 1979). Other selection criteria at this stage include high dry matter and cyanogenic potential (Iglesias and Hershey, 1994). Between 100 and 300 clones survives the CET. A common feature in the first two stages of selection for most programs is that selection is frequently visual with no data recording to manage a larger number of materials at lower costs. One important trait that makes the harvest of large trials, such as the CET, expensive and time demanding is the measurement of dry matter content (DMC) in the roots. The productivity of cassava depends ultimately on the amount of fresh roots produced and the DMC of those roots. Heritability for DMC is considered to be intermediate.
The following stage of selection is the preliminary yield trial (PYT). At CIAT, PYTs are currently based on the evaluation of 10 plants in three replications. The 10 plants in each replication are planted in two 5-plant rows. If possible, rows are spaced only 0.8 m apart (instead of the standard 1.0 m), and one empty row is left between plots to increase within-clone competition and reduce between-clone competition. Alternatively, row spacing can be maintained at 1.0 m but then the plant-to-plant distance within the row is reduced to 0.8 m. In this case also an empty row is left separating plots with different genotypes. Large genetic variability occurs among clones, even within the same family. Although poorly performing clones are mostly eliminated at the CET stage, there is still a considerable variation in the PYT trials. This highlights the need for a gradual process of selection and the need to avoid strong selection pressures in early stages.
With the initiation of replicated trials, the emphasis of selection shifts from high heritability traits to those of low heritability, such as yield. Starting with PYT and increasingly during the advanced yield trials (AYT) and the regional trials (RT), a greater weight is given to yield and its stability across locations. Cooking quality trials (relevant for the different ethnic ways cassava may be consumed) also begin at these stages, when the number of genotypes evaluated is more manageable. The AYTs are typically grown in 1–2 locations for 1 or 2 consecutive years. They have three replications per trial and plots are four (or five) rows with five plants per row. Yield data are taken from the six (nine) central plants of the plot and the remaining 14 (16) plants are used as source of planting material for the next season. The RTs are conducted for at least 2 years in 3–6 locations each year. Plots have five rows with five plants per row. Yield data are taken from the nine plants from the center. Clones that show an outstanding performance in the RT are released as new varieties after a few years of informal evaluation in semi-commercial evaluations with key farmers. They are also sooner rather than later incorporated into the crossing blocks as progenitors to initiate a new recurrent selection cycle.
The breeding scheme described above can be classified as mass phenotypic recurrent selection. No family data are used in the selection process and individual clones are evaluated and selected or discarded. It has been suggested that data are recorded in all entries at the CET level (Ceballos et al., 2004, 2007a), and estimates of general combining ability of the progenitors that generated the CET are obtained.
6.2 Strategies for Improving the Efficiency of Cassava Breeding
6.2.1 Rapid Cycling Recurrent Selection for High Heritability Traits
The HarvestPlus initiative aims at improving the nutritional quality of several crops (Pfeiffer and McClafferty, 2007). In the case of cassava the main focus is to increase the levels of pro-vitamin A carotenoids in the root. As explained above, the normal recurrent selection cycle in cassava requires about 8 years for completion (Morante et al., 2005, Table 2.2). Carotenoids content is a highly heritable characteristic and taking advantage of this feature, and in response to the need for rapid progress, a rapid cycling recurrent selection system was implemented.
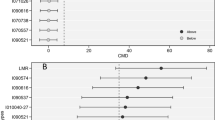
Basically root samples of each F1 plant are harvested 10–12 MAP, but leaving the plant still growing in the field. As results from the laboratory become available, the same plants could be used to generate new recombinant seed. Within 2–3 years new recombinant seed is produced from the genotypes with higher carotenoid content values. This system, unusual for cassava, produced outstanding results. Figure 2.6 illustrates the gains from selection for high carotenoids content. This figure presents the results of the seedling (F1 trials) nurseries during the harvest of nurseries in the last 4 years. Every year an average of almost 3 μg carotenoids/g of fresh root was added to the best genotypes. These are unprecedented gains for a crop like cassava. These results also highlight the importance of adapting the breeding schemes to the specific needs and characteristics of the trait to be improved.
6.2.2 Correcting Data for Missing Plants
A major problem in cassava breeding is the impact that the storage of stems has on their sprouting capacity. Evaluations with a uniform plant stand are fundamental for reliable results. However, it is very difficult to correct data for missing plants within an experimental plot. Linear covariance analysis of crop yield data using plot stand as the covariate is not a satisfactory approach especially when the plants are missed soon after sprouting or early in the growing season. The following formula to adjust for missing plants in AYT or RT has been developed (Pérez et al., 2010):
where y a is the adjusted plot yield, y o the observed plot yield, N a is the ideal plot stand (in the case of AYT and RT, nine plants), and N 0 is the number of plants actually harvested. This correction should be extremely useful in overcoming the problems of missing plants and lack of uniform plant densities illustrated in Fig. 2.5.
6.2.3 Inbreeding to Exploit Heterosis
Cassava, as a typical cross-pollinated species, shows good levels of heterosis (Cach et al., 2005a, b; Calle et al., 2005; Jaramillo et al., 2005; Pérez et al., 2005a, b) as well as severe inbreeding depression (Contreras Rojas et al., 2009). Given the length of each cycle of selection and the complexities derived from improving non-additive genetic effects such as dominance and epistasis, the availability of fully inbred progenitors would allow a gradual but consistent building up of heterosis and, eventually, the identification (or creation) of heterotic populations where reciprocal recurrent selection could be used. However, to produce fully inbred genotypes through successive self-pollinations is not practical in cassava for two major bottlenecks: (a) it would require 12–15 years to reach the S6 stage of inbreeding (six successive self-pollinations) and (b) based on CIAT experiences in attempting to produce self-pollinated generations beyond the S2 or S3 level, favored plants with profuse flowering result in the generally disliked early branching types. There is an ongoing effort to develop a protocol for the production of doubled haploids through microspore culture. This could be an ideal approach for producing highly homozygous clones in less than 2 years.
6.2.4 Inbreeding to Reduce Genetic Load
Inbreeding has been intentionally omitted in the breeding scheme. Therefore large genetic loads are likely to remain hidden in cassava populations and useful recessive traits are difficult to detect. Contreras Rojas et al. (2009) reported that on average S1 progenies produce only 30% of the root yield harvested in their respective progenitors. This degree of inbreeding depression highlights the size of the genetic load (undesirable alleles in breeding populations) still present in cassava. The introduction of inbreeding would allow (actually require) the selection of tolerance to inbreeding depression, which basically means breeding to eliminate the undesirable alleles collectively known as genetic load.
6.2.5 Inbreeding to Identify Useful Recessive Traits
An amylose-free mutation has been reported for many other crops (maize, potato, rice, wheat, barley, etc.). Amylose-free starch offers special functional properties that are beneficial for the starch industry (Davis et al., 2003; Ellis et al., 1998). In addition, it has been shown to reduce processing costs in the production of ethanol from starchy crops (Sharma et al., 2007). In 2006 a spontaneous amylose-free starch mutation was discovered in cassava (Ceballos et al., 2007b). ECOTILLING (see Section 7.3) can be used in the future for the identification of specific spontaneous mutations in different populations and will also benefit from the availability of a sequenced genome of cassava.
6.2.6 Inbreeding to Allow for the Backcross Scheme
In the sections on breeding objectives and development of high-value clones, several examples of useful traits (putatively) controlled by single genes have been provided. Their discovery will have undoubtedly a huge impact on cassava production and processing. However, since progenitors in cassava breeding are not homozygous it is impossible to introgress these single genes through sexual crosses and recover the original progenitor. The current strategy relies on making crosses with the source of the desirable trait and basically, breeding a new variety again. This is a very expensive and inefficient process. If progenitors were homozygous the backcross scheme could be easily implemented. Backcrossing has been one of the most successful breeding approaches used consistently in many different crops (Allard, 1960).
6.2.7 Improving Testing Methodologies
Several characteristics rely on expensive or time-consuming tests. This is particularly the case of breeding for improved nutritional quality. The standard methodology for estimating protein content, for example, has been through the indirect method of quantifying N by the Kjeldahl method and then multiplying it by a 6.25 constant. There is growing evidence that the N-to-protein conversion factor, in the case of cassava roots, may be considerably lower because of the presence of non-protein sources of N. A direct method for the quantification of total soluble proteins contents has recently been tested using the Bradford colorimetric method (BioRad dye reagent). Preliminary results confirmed a large variation in N as well as protein content where high levels are threefold higher than low levels of protein (Chávez et al., 2009). This system is considerably less expensive and faster than the traditional approach based on N quantification.
Another example is in breeding for increased levels of carotenoids content. The current system relies on a tandem selection based first on visual assessment of the intensity of root pigmentation, followed by total carotenoids content determination by spectrophotometry (on selected genotypes) and then by HPLC analysis to determine β-carotene content. Depending upon the equipment used, between 8 and 24 samples per day can be processed by HPLC. This means only about genotypes per harvesting season. An alternative approach would be the use of near-infrared reflectance spectroscopy (NIRS) which could allow (once the standard curves are developed) screening as many as 400–600 genotypes per day. An additional advantage is that predictions for several traits can be simultaneously obtained from a single NIRS reading (total carotenoids, β-carotene, protein, dry matter and protein content, cyanogenic potential, total and reducing sugars, amylose/amylopectin ratio, etc.).
6.2.8 Stratification of Large Trials
A major problem with the CET is its large size (easily 2 ha in size) and the unavoidable environmental effect in the selection. This problem is particularly relevant in the case of cassava, because the target environments for cassava are typically in “marginal” agriculture conditions and prone to large variation. Since CETs are frequently the first stage of evaluation, only a few stakes (<10) are available for trials. So the introduction of replications that could help overcome this problem is not practical.
The same simple principles as suggested by Gardner in 1961 were introduced for the evaluations of CETs several years ago. The field where the CET is going to be planted is divided into three “blocks” of about equal size trying to maximize differences among blocks and minimize variation within each block. Clones could actually be replicated (i.e., two replications of four plants rather than one replication with eight plants) but the logistical complexity and additional efforts to generate twice as much data proved to be too much compared with the advantages (CIAT, unpublished data). However, clones are grouped in either full- or half-sib families. Generally, since many clones are available from each family, they can be randomly allocated to one of these three “blocks.” In other words instead of planting all the clones from a given family together, one after the other, they are split into three groups. This approach provides two interesting advantages: (a) there is a replication effect for the families because all the clones from a given family are distributed in three “repetitions” in the field. The averages from all the clones are less affected by the environmental variation in such a large experiment and (b) selection is made within each block therefore reducing the environmental component affecting the selection. This is similar to the stratified mass selection suggested by Gardner in 1961. This approach effectively overcomes the environmental variation that can be measured by comparing the means of each block. In general, variation in the order of 10–20% has been observed among average performances of the three blocks. These are, in other words, the gains in the precision attained by introducing the stratification of the CETs.
6.2.9 Estimation of General Combining Ability of Progenitors
One of the major decisions taken by any breeder is the selection of parents used to produce a new generation of segregating progenies. In cassava, this decision has been mainly based on the per se performance of each clone. Nonetheless, some empirical knowledge of the quality of progenies produced by different parents could be developed. This lack of organized information on the breeding values of parental lines used in the breeding projects was partially due to the fact that no data were taken and recorded during the first stages of selection or else, they were incomplete. Therefore, it was not possible to generate a balanced set of data that would allow the breeder to have an idea of the relative performance of the progeny of each elite parental line. In other words, no formal process to assess the breeding values of the progenitors used in the cassava breeding projects was available (Allard, 1960; Simmonds and Smartt, 1999).
To overcome this problem, the decision was taken to record data and to introduce the use of selection indexes (Baker, 1986). Selection is made within each stratum as explained in the previous section. Data from each family are then pooled across the three blocks in which it was planted. The stratification means that, in a way, there is a replication effect at the family level. Since a given progenitor may be used more than once, data from all the families in which each progenitor participated are pooled to obtain an idea of the general performance of all the progenies from a given parental clone. The advantages of this approach have already been reported (Ceballos et al., 2004, 2007a).
7 Integration of New Biotechnologies in Breeding Programs
Biotechnology tools have been adapted to cassava and are currently incorporated in different projects for its genetic improvement. A molecular map has been developed (Fregene et al., 1997, 2000; Mba et al., 2001) and marker-assisted selection is currently used for key traits, such as resistance to CMD. The recent sequencing of the cassava genome will certainly contribute to a more efficient implementation of molecular markers, which will also benefit from the availability of homozygous germplasm (doubled haploids) developed through microspore or anther culture (CIAT, 2009). The efficiency of TILLING (induced mutations) and ECOTILLING (a similar tool applied to spontaneous mutations) will also be greatly facilitated by the sequencing of the cassava genome. Finally genetic improvement of cassava by genetic transformation is also now possible.
7.1 Use of Molecular Markers
Molecular markers represent a limitless source of neutral markers for the quantitative assessment of genetic diversity and “signposts” in gene and genetic diversity. They rely on differences in the nucleotide sequences (either nuclear or in organelles) that can be uncovered using diverse methods based upon PCR and DNA–DNA hybridization or both. Molecular markers are abundant and many of them show high level of polymorphism and the assays can be done at any stage in the development of the plant. They are not influenced by the environment but require that phenotypic data associated with them have properly taken into consideration genotype-by-environment interactions.
Markers have been used to generate several molecular genetic maps for cassava (Fregene et al., 1997; Mba et al., 2001; Okogbenin et al; 2006). In an attempt to make marker technology more widely applicable in breeding programs, highly polymorphic SSR markers were mainly used in the construction of subsequent genetic maps for cassava. Presently over 525 SSR markers have been used in the development of the new SSR-based maps which have yet to be published. An initiative toward completing the saturation of the cassava genetic map has also resulted in the generation of expressed sequence tags (ESTs) and SNPs. Several ESTs have been developed for cassava (Lopez et al., 2004; Lokko et al., 2007) with over 80,000 ESTs for cassava available.
The generation challenge program (GCP) is currently supporting an initiative to develop SNPs for drought tolerance. A physical map of the cassava genome was constructed by fingerprinting 70,000 BAC clones and sequencing the ends of 9,000 clones distributed throughout the genome. The availability of a physical map is an important tool for map-based cloning of agronomically relevant genes in cassava. The selected low-copy sequences spread throughout the genome will be re-sequenced in a panel of 10 cassava genotypes to identify SNPs that can be used for genetic mapping. Recently, a general genotyping array of 1,536 cassava SNPs has already been designed from cassava EST sequences. SNPs are ideal markers as they allow the use of genotyping platforms that can assay many individuals for thousands of SNP markers in parallel. The strategy for utilizing markers is primarily driven by their availability and cost of genotyping platforms.
One of the primary objectives of genetic mapping and gene tagging efforts in cassava is to provide tools that can increase the cost effectiveness and efficiency of cassava breeding. It includes pests and diseases, traits expressed only at the end of the crop’s growing cycle, and those for which phenotype is difficult to measure. Various markers have been used to tag several traits in cassava. Molecular markers have been used to tag three different sources of CMD resistance originally found in M. glaziovii, TME3, and TMS97/2205 (Fregene et al., 2000; Akano et al., 2002). Two SSR markers have been found associated with CGM (NS1009 and NS346). About six markers were found associated with CBB explaining 9–27% of the phenotypic variance of response to five Xam strains (Jorge et al., 2000). Early bulking is another trait evaluated in cassava and results from the analysis of this trait showed that it was mostly affected by harvest index and dry foliage. Three QTLs explaining 25–33% of phenotypic variance were found for dry foliage while five other QTLs associated with harvest index were identified with phenotypic variance in the range of 18–27% (Okogbenin and Fregene, 2002). Several other gene tagging projects have since been conducted or are ongoing in cassava at CIAT for other traits such as PPD, whiteflies, and β-carotene. Except for CMD and CGM, markers identified for several traits have yet to be validated and there is the need to test these markers and to further conduct fine mapping of the genomic regions for the markers with a view to developing better markers to enhance their application in marker-assisted selection (MAS). Generally molecular markers have been applied in cassava genetic improvement in the following areas.
7.1.1 Genetic Diversity
Phylogenetic relationships of Manihot species were revealed through the use of RFLP markers, indicating little variation within species and a close relationship between M. chlorosticta and M. esculenta (Hayson et al., 1994). Olsen and Schaal (2001) used microsatellites to postulate possible ancestors of cultivated cassava. AFLP markers have been used for quantitative assessment of genetic relationships in a representative sample of the crop’s diversity and six wild taxa (Roa et al., 1997). In this study, Manihot species M. esculenta subsp. flabellifolia, M. trisitis, and M. esculenta were found to be more similar to cassava than its Mexican relative M. aesculifolia indicating that cassava might have its origin in these close relatives (Roa et al., 1997). Evidence of introgression into cassava from M. glaziovii was also observed in an AFLP evaluation of genetic diversity in a large collection of cassava from the South American center of diversity (Second et al., 1997). In other studies, markers have also been used to obtain a quantitative assessment of genetic similarity in cassava (Beeching et al., 1993; Second et al., 1997; Elias et al., 2000) and to study the genetic structure of germplasm resistant to disease (Sanchez et al., 1999; Fregene et al., 2000), including the genetic structure and the basis of genetic differentiation of cassava landraces in Africa (Mkumbira et al., 2003).
Germplasm studies with markers have also revealed intravarietal polymorphism, indicating that a variety could also be made up of more than one genotype (Elias et al., 2000). Markers have also been used to study the effect of disease on genetic diversity in cassava. Kizito et al. (2005) reported the loss of rare alleles in areas with high CMD incidence in Uganda. From Genetic diversity studies conducted at CIAT, an SSR diversity kit of 36 SSR markers has been developed (Fregene et al., 2004) and is presently being used in breeding programs for genetic diversity analysis. Other applications include the use of markers for the identification of duplicates in germplasm (Chavarriaga-Aguirre et al., 1999) and the analysis of germplasm from the littoral and Amazonian regions of Brazil.
7.1.2 Marker-Assisted Selection
MAS is best used to investigate traits that are difficult or expensive to evaluate. Markers can be particularly useful where pathogen or pest pressure is low, variable, erratic, or just absent. To be useful for MAS, markers must adequately account for a large proportion of the genetic variance. MAS can increase the efficiency of breeding schemes by identifying plants with the desired trait at a very large stage, enabling much smaller populations to be grown in the field. This in turn reduces the cost of breeding, but more importantly, enables a larger number of families to be produced and analyzed.
7.1.2.1 Cassava Mosaic Disease
Several variants of the disease exist in Africa and South Asia (Swanson and Harrison, 1994). CMD is the most important disease of Cassava in Africa considering the high evolutionary capacity of the virus and largely accounts for yield losses of over a billion US $ in Africa alone. The discovery of markers linked to a dominant resistance gene (CMD2) has allowed the selection of resistant CMD cassava genotypes in the absence of the pathogen (Akano et al., 2002; Okogbenin et al., 2007). Five markers were initially identified for this gene with the closest being RME1 (a SCAR marker) and NS 158 (a SSR marker) at distances of four and seven cM, respectively. MAS has been carried out using multiple flanking markers involving these two markers with 68% efficiency (Okogbenin et al., 2007). Recent fine mapping of the CMD2 region to identify closely linked markers and positional cloning of the gene have been done with the construction of BAC libraries and characterization and screening of the BAC clones. This resulted in the identification of two SSCP–SNP markers which have been identified between RME1 and CMD2. Current efforts are being directed toward screening for new sources of CMD resistance in African germplasm. Where different sources of CMD resistance exist, this is indistinguishable phenotypically, and markers are being used to screen identified genotypes in the breeding populations in Africa. Under the Genotyping Support Services activities of the GCP, crosses were carried out using six parents resulting in the development of nine F1 segregating populations. Four of the populations were selected and phenotypically evaluated and analyzed with 530 SSR markers to identify new sources of CMD resistance different from CMD2. IITA is currently conducting further research to improve the molecular markers currently available for resistance to CMD.
7.1.2.2 Cassava Green Mite
Two markers were found to be associated with the reaction to CGM (NS1009 and NS346) and have been used in MAS as part of validation studies. In preliminary evaluations, progenies selected with the markers showed good resistance to the pest in East Africa but not in Nigeria where moderate tolerance was observed for CGM based on the markers. The phenotypic differences between both African sub-regions might be due to variation in the CGM pressure which is higher in Umudike, Nigeria where a long dry season of 4–5 months in the humid transition ecology often results in high CGM incidence compared with Tanzania and Uganda where the pressure is less severe.
7.1.3 Introgression of Useful Traits from Wild Relatives
As stated above, wild Manihot germplasm offers a wide array of useful genes frequently related to tolerance to biotic and abiotic stresses. The use of wild relatives, however, implies the need to reduce or eliminate a large proportion of the undesirable donor genome. Linkage drag can lengthen the process, making it a daunting task for breeders. The desirable traits can frequently be observed in F1 inter-specific hybrids indicating dominant or additive gene action of the gene(s) involved. Simulation by Stam and Zeven (1981) indicated that markers could reduce linkage drag and the number of generations required in backcross scheme. Hospital et al. (1992) corroborated this in achieving a reduction of two backcross generations with the use of molecular marker selection. Frisch et al. (1999), through a simulation study, found that use of molecular markers for the introgression of a single target allele saved two to four backcross generations. They inferred that marker-assisted selection had the potential to reach the same level of recurrent parent genome in generation BC3 as is reached in BC7 without molecular markers. These studies, however, are based on schemes where the recurrent progenitor is homozygous, a condition yet to be satisfied in the case of cassava.
7.2 Genome Sequencing of Cassava
The Joint Department of Energy’s Joint Genome Institute (JGI-DOE) initiated a pilot cassava genome sequencing project under its Community Sequencing Program (CSP; http://www.jgi.doe.gov/CSP), using a whole genome shatgum (WGS) strategy. The draft genome sequence has just recently been completed in 2009 with the support of 454 Life Sciences using the Genome Sequencer FLX platform with long-read GS FLX Titanum chemistry to rapidly generate the DNA sequence data. The cassava sequencing project had other participating institutions such as the International Laboratory for Tropical Agricultural Biotechnology at the Danforth Plant Science Center in St. Louis; USDA laboratory in Fargo, ND; Washington University, St. Louis; University of Chicago; The Institute for Genomic Research (TIGR); Missouri Botanical Garden; the Broad Institute; and Ohio State University.
More than 61 million reads were generated and assembled in a draft genome that contains an estimated 95% of cassava genes. The annotated draft genome sequence is available at DOE JGI’s phytozome website (http://www.phytozome.net/cassava). It is one of the first large genome projects to primarily use 454 Life Sciences long-read sequencing platform which enabled both improved quality of the sequence and its rapid generation.
The new development in cassava of a genome sequence database will open a new vista to address intractable knowledge gaps in cassava genetics. Sequencing the cassava genome is expected to result in a better understanding of starch and protein biosynthesis, root storage, and stress controls, and enable crop improvements, while shedding light on such mechanisms shared by other important related plants, including the rubber tree and castor bean. The genome organization of cassava, its evolution and the mechanism involved, will also be properly elucidated. The sequencing information is expected to increase knowledge on the molecular interactions mediating growth and development processes in cassava. Functional genomics research will be further accelerated by the wealth of information to be made available. The first application will be in the development of SNPs for mapping with huge potential for the genetic analysis of complex traits. Current efforts are underway to identify a large collection of high-quality SNPs which will be integrated with genome sequence and annotation in order to make a comprehensive genomic resource available to the cassava community.
7.3 Mutation Breeding, TILLING, and ECOTILLING
In addition to the strategic pre-breeding work described above, there are other alternatives for the incorporation of high-value traits in commercial varieties of cassava. Breeders have used chemical products or irradiation such as gamma rays to induce mutations and generate genetic variability with relative success, particularly in the 1950s and 1960s (Maluszynski et al., 2001; Ahloowalia et al., 2004). Mutation breeding has a few drawbacks. Events are totally random, usually recessive in nature and usually appear as chimeras. Therefore, thousands of genotypes need to be evaluated before a useful mutation in the desired gene can be found. With the advent of molecular biology tools, an interesting system was developed to overcome some of the limitations of mutation breeding. DNA TILLING (for Targeting Induced Local Lesions in Genome) has been successfully used in different plant species (McCallum et al., 2000; Perry et al., 2003; Till et al., 2003). Sexual seeds are mutagenized and, to avoid ambiguities caused by chimeras in the first-generation plants (M 1 ), they are self-pollinated. The resulting plants (M 2 ) are then evaluated while DNA is extracted from them. For screening purposes, DNAs are pooled eightfold to maximize the efficiency of mutation detection (description of the TILLING method adapted from Till et al., 2003).
CIAT participated in a project led by Universidad Nacional de Colombia and supported by the IAEA (International Atomic Energy Agency). About 4,000 seeds from six different cassava clones were irradiated with gamma rays (using a Cobalt 60 source with a dosage level of 200 Gy) or with fast neutrons. As many as 5,000 M 2 seeds, from about 140 different M 1 plants have been obtained. Several mutations were identified in the M 2 generation but only the two most interesting will be described, together with a future use of ECOTILLING.
7.3.1 Small Granule – High Amylose Starch Mutation
This mutation has been reported and described already in the literature (Ceballos et al., 2008). The initial discovery was facilitated by the unusual starch granule size which is about one-third the normal size for cassava. Figure 2.7 illustrates how different the starch granules of this mutation are, not only in relation to size but also regarding their surface. Normal cassava starch granules have a very smooth surface. However, the surface of the granules in the mutated genotype is very irregular and rough (Fig. 2.7).
The small size and irregular surface of the starch granule would make this mutation ideal for ethanol production because it facilitates the activity of starch-degrading enzymes (Lehman and Robin, 2007; Thu et al., 2007). The production of bio-ethanol from starch requires its degradation (liquefaction and saccharification) prior to the initiation of fermentation. However, the mutation also has a biochemical abnormality, with almost twice the normal levels of amylose (Ceballos et al., 2008; Sánchez et al., 2009). Amylose is more difficult to degrade (Sharma et al., 2007). It is not possible at this time to say if the morphology of the starch granule will be more prevalent than the biochemical characteristics (high amylose) of the mutation in the process of starch hydrolysis. Only when enough roots can be produced will the analysis be made.
The higher-than-normal level of amylose in these mutations has important commercial implications. Increased amylose levels leads to slowly digestible and resistant starches (Jobling, 2004; Lehman and Robin, 2007), which have distinctive advantage in health, particularly in diabetes management. Slowly digestible starches may influence satiety and help control overweight problems and have also been linked to improved mental performances (Lehman and Robin, 2007). In addition, high amylose starches in different crops offer advantages in the production of sweets, adhesives, corrugated boards and in the paper industry, and reduces the uptake of fat in certain fried products (Jobling, 2004). Very high levels of amylose result in “resistant” starches. Maize starches with more than 50% and up to 90% amylose can be produced commercially. Resistant starches cannot be digested but they are fermented in the large intestine, resulting in the production of butyrate that has been found to be beneficial to colon health (Jobling, 2004).
7.3.2 Tolerance to PPD
A second high-value trait that was identified in the mutagenized population was tolerance to post-harvest physiological deterioration (PPD). Two genotypes were tentatively characterized as PPD tolerant and one of them (2G15-1) proved to have quite good levels of tolerance (Fig. 2.8). As quantification of the reaction to PPD requires many commercial-size roots, these genotypes were first multiplied to produce the number of roots required. In addition, several other genotypes considered as potential sources of tolerance to PPD were evaluated. Relevant results from an evaluation conducted in April–May 2009 are presented in Fig. 2.8. Three genotypes did not show any symptoms of PPD even after 40 days of storage (GM 905-66, AM 206-5, and WAXY 4). The tolerance to PPD in GM 905-66 can be explained by the antioxidant properties of the high carotenoids concentration in the roots of this genotype. AM 206-5 and Waxy-4 are partially inbred genotypes related to each other and have in common the waxy starch mutation. The tolerance to PPD in these two genotypes is not considered to be a pleiotropic effect of the waxy starch mutation but most likely it is due to a gene linked to the waxy starch locus. The mutagenized genotype (2G15-1), and one of the backcrosses from the inter-specific cross with M. walkerae (BC289-30), also showed low values of PPD. This is a remarkable finding where many different sources of tolerance to PPD seem to have been discovered and highlights the importance of aggressive and systematic screening of germplasm for different traits (Morante et al., 2010).
Reaction to PPD 20 and 40 days after harvest. Top photographs show reaction in the susceptible checks (CM 523-7 and MCOL 1505). Second row shows the reaction in high-carotene clones (GM 909-66 and CB 44-15). The third row shows photographs of two waxy starch genotypes (AM 206-5 and Waxy-4). Genotype BC289-30 (last row) is a backcross from an inter-specific cross with M. walerae. Finally genotype 2G15-1 is from the mutagenized population
7.4 Genetic Transformation
Genetic transformation protocols are available and have been used successfully for the incorporation of different genes but mostly in just one cultivar. Insect and disease resistance, tolerance to herbicides, manipulation of starch content and quality, enhanced nutritional quality, reduction of cyanogenic potential, and enhanced leaf retention are among the traits incorporated (Munyikwa et al., 1997; Taylor et al., 2004). Additional work needs to be done to increase the efficiency of genetic transformation to a wider range of elite germplasm, to overcome the regulatory issues (particularly where it is used for food), and to improve our capacity to regulate the expression of the transgene.
8 Commercial Propagation and Production of Planting Material
Commercial propagation of cassava is by stem cuttings (Fig. 2.1). Different rapid multiplication methods have been developed ranging from the use of microstakes to tissue culture techniques. One-node microstakes have been used successfully for rapid multiplication schemes. For these conditions, irrigation is highly desirable. The size of the stake to be used depends on the moisture in the soil at planting time (adequate rains or access to irrigation would allow shorter stakes), storage period of the planting material (the shorter the period the shorter the stakes can be), varietal characteristics (some clones have better sprouting capacity than others), and overall physiological and nutritional quality of the planting material. Alternatively, 2-node microstakes can be grown at high density in moist chambers where they sprout. The resulting shoots (15–20 cm long) are harvested about every 3 weeks and their lower section immersed in water for them to produce roots and then transferred to soil. The facilities needed for an efficient hardening of these small plants are not sophisticated. Availability of a cool or fresh environment in shade (screen houses are ideal) where extreme temperature can be avoided and adequate humidity provided is required. When the plantlets are about 2-month old they can be transplanted to the field.
Tissue culture approaches (pre-existing meristems and somatic embryogenesis) have been used for rapid multiplication of cassava (Fregene et al., 2002). The common products of these two protocols are the small plants grown in vitro, which would require a hardening process described by Segovia et al. (2002). The critical period for the hardening process, after the plants are taken from the in vitro condition, lasts for 1 week. Hardening (using facilities described above) starts with the transfer of the small plant from the in vitro condition to a container (plastic bags or seedling trays) with a mixture of soil and sand that has hopefully been previously sterilized with high temperature (100°C). A high moisture condition is required for the first week after transplanting. Different alternatives have been proposed ranging from the use of moist chambers to the use of plastic disposable coffee cups with small holes at the base and placed inverted over the plant so it is completely covered (Fregene et al., 2002; Segovia et al., 2002). After the first week the plants are gradually exposed to conditions with lower humidity and higher temperature and in 2 months can be transplanted to the field.
8.1 Field Management Requirements
In general, there is no production of planting material for cassava independent from the commercial fields for root production. It is recommended that an area in the production field is assigned as source of new planting material for next cycle. This area (about 10% of the total area) is specially managed following the same criteria used for specially designed nurseries. From this point on, the distinction between nurseries or sections of commercial fields will not be made.
When a new variety is identified, or a clean planting material (through meristem culture) of an old variety is produced, specific multiplication nurseries are planted. In this case the primary product is the planting material rather than the roots. This would be particularly relevant for planting material that is certified to be clean of the viral diseases present in Africa or frogskin in the Americas. When planting material is certified to be disease free, special efforts are made to prevent their re-infection, avoiding the contact of the new, clean crop with the insect vectors of the diseases (e.g., white flies). Although the use of insecticides may be considered, it is not 100% effective. White flies are prevalent in lowland environments and are seldom present beyond 1,800 m above sea level. Crop rotations are important in the case of fields that have been affected by root rots because the inoculum would remain in the soil and infection of a new crop would be likely to occur.
The production of planting material needs to be properly managed to avoid lack or excess of water (irrigation, drainages, etc.), prevent attacks by pests and diseases, and provide adequate soil fertility. The ultimate objective is to have cassava plants (10–18 months of age) that have stems with optimum sanitary and physiological conditions, properly nourished and irrigated. Adequate soil fertility is important because it maximizes quick sprouting in the next generation, with vigorous and healthy plants and a uniform plant stand.
8.2 Monitoring Nurseries
Production of planting material begins with materials that are free from pests and diseases. The producer will first verify, before planting, the absence of contaminants and volunteers from previous seasons, as well as the availability of irrigation and drainages in the field. Other inspections would take place during the development of the crop to monitor crop establishment 1 month after planting (m.a.p.); then at least every other month until harvest (typically 10–12 m.a.p). In certain conditions, planting material is harvested from older plants (i.e., 18 months). This is the case when cassava is grown at high altitude (>1,500 m.a.s.l.), in conditions with short rainy period or cold winters (in latitudes >20°). In these conditions, the visits can be spread.
During the visits the whole nursery should be screened for potential sanitary problems. Plants attacked by diseases or pests should be eliminated. Proper availability of nutrients and water should be guaranteed. Weed control should be carefully done in the first 3 months of the crop. The varietal purity of the nursery can be checked 3–5 m.a.p. The most distinctive descriptors of cassava (color and length of the petiole, shape of leaf lobules, presence of pubescence in the shoot, and color of the stem) allow an easy identification of off-type plants that can then be eliminated. For some diseases such as CBSD or frogskin disease, it is necessary to inspect the roots since they may offer the only source of symptoms that allow the identification of infected plants (Calvert and Thresh, 2002).
It is desirable that 5–7 m.a.p. an official inspection of the plant multiplication nursery is made. Up to 1% of off-type plants can be accepted. For CBB and SED, up to 2% of plants with symptoms is acceptable, provided they are discarded. Depending on disease pressure and the variety being multiplied, CMD and CBSV acceptable levels at that time can range from 0 to 5%.
8.3 Harvest and Storage of Planting Material
Any part of the cassava stem can be used for propagation purposes. However, the thickness of the stem used for cuttings should not be less than one half the diameter of the thickest part of the stem of the particular variety being used. Cuttings from green stems (slightly lignified) will germinate, but they are susceptible to attack by pathogens and insects and tend to dehydrate rapidly. Cuttings from stems older than 18 months are too lignified, contain small amounts of food reserves, and have reduced viability, delayed and slow sprouting, and/or poor vigor. It is recommended that planting material be taken from stems ranging from 8 to 18 months of age. The younger the plant the more lignified should be the part of the stem selected for the cutting. One practical way of knowing whether a stem is sufficiently mature is to determine the relationship between the diameter of the pith and the stem cutting in a transversal cut. If the diameter of the pith is equal to or less than 50% of the diameter of the stem, it is sufficiently mature to be used for planting (Fig. 2.9).
It is preferable to maintain the planting materials standing in the nurseries, rather than harvest them too early and then stored for 2–3 months. Cassava can be kept in the field because there is no physiological maturity for the plant. The young branches are cut and discarded and the main stems, offering the quality standards described above, are cut and tied together in bunches of about 50 stems. On average, each stem yields 5–7 stakes. However, depending on age and varietal characteristics stems can yield from 3 to 12 stakes. There is no dormancy period and stakes can be planted immediately after harvest, when even thin (green) stems could sprout and produce a vigorous plant. Each bunch is identified with a plastic tag with the name of the variety, date, and location of harvest clearly written using permanent ink markers or graphite pencils. At harvest stems are screened for the presence of (damage by) insects, particularly stem borers. If relevant for the region, roots should be checked for FSD or CBSD symptoms.
Stems can be stored as they have been harvested from the field (long stems about 1–2 m long) or else, cut to the proper size for planting (about 20 cm long). To prevent dehydration during storage, however, it is recommended that the stems be cut into planting stakes just prior to their planting. Bunches of long stems are placed vertically on the ground, in shade (usually a tree, Fig. 2.1b) and in an upward position (the apical portion of the stem up). Sometimes farmers cover the stem bunches with remaining foliage of the crop to further reduce dehydration of the stems. The storage area should be shaded and offer high, but not excessive relative humidity (about 80%) and moderate temperatures (20–30°C).
It is recommended that stems are sprayed or submerged in a solution with an insecticide (dimetoathe or malathion) and a fungicide (copper oxychloride). The standard practice is to prepare a solution with 1 l of water, 5 g of Malathion W.P. (4%), and 2 g copper oxychloride (Vitigran 35%). Alternatively the insecticide can be applied as 1.5 cc Malathion (E.C. 57%) or 1 cc dimetoathe (E.C. 40%). This solution has proved to be useful, relatively inexpensive and its use is also suggested for the planting material just prior to planting. In the case of stems that are stored for long periods of time, therefore, they are treated twice with the same solution (immediately after the harvest of the stem and just before planting). Operators should wear protective gloves, aprons, glasses, and breathing equipment.
References
Ahloowalia BS, Maluszynski M, Nichterlein K (2004) Global impact of mutation-derived varieties. Euphytica 135: 187–204.
Akano AO, Dixon AGO, Mba C, Barrera E, Fregene M (2002) Genetic mapping of a dominant gene conferring resistance to cassava mosaic disease. Theor Appl Genet 105: 521–525.
Allard RW (1960) Principles of plant breeding. Wiley, New York, NY.
Allem AC (1994) Manihot germplasm collecting priorities. Report of the First Meeting of the International Network for Cassava Genetic Resources, International Plant Genetic Resources Institute, International Crop Network Series No. 10, Rome, pp. 87–110.
Allem AC (1990) The closest wild relatives of cassava (Manihot esculenta Crantz). Euphytica 107: 123–133.
Allem AC (2002) The origins and taxonomy of cassava. In: Hillocks RJ, Tresh JM, Bellotti AC (eds.) Cassava: biology, production and utilization. CABI Publishing, Wallingford, pp. 1–16.
Allem AC, Mendes RA, Salomão AN, Burle ML (2001) The primary gene pool of cassava (Manihot esculenta Crantz subspecies esculenta, Euphorbiaceae). Euphytica 120: 127–132.
Alves AAC (2002) Cassava botany and physiology. In: Hillocks RJ, Tresh JM, Bellotti AC (eds.) Cassava: biology, production and utilization. CABI Publishing, Wallingford, pp. 67–89.
Andersen MD, Busk PK, Svendsen I, Møller BL (2000) Cytochromes P-450 from cassava (Manihot esculenta Crantz) catalyzing the first steps in the biosynthesis of the cyanogenic glucosides linamarin and lotaustralin. J Biol Chem 275(3): 1966–1975.
Asiedu R, Hahn SK, Bai KV, Dixon AGO (1992) Introgression of genes from wild relatives into cassava. In: Akoroda MO, Arene OB (eds.) Proceedings of the 4th Triennial Symposium of the International Society for Tropical Root Crops – Africa Branch. ISTRC-AB/IDRC/IITA, Nigeria, pp. 89–91.
Balagopalan C (2002) Cassava utilization in food, feed and industry. In: Hillocks RJ, Tresh JM, Bellotti AC (eds.) Cassava: biology, production and utilization. CABI Publishing, Wallingford, pp. 301–318.
Babu L, Chatterjee SR (1999) Protein content and amino acid composition of cassava tubers and leaves. J Root Crops 25(20): 163–168.
Baker RJ (1986) Selection indices in plant breeding. CRC Press, Boca Raton, FL.
Beeching JR, Marmey P, Gavalda MC, Noirot M, Haysom HR, Hughes MA, Charrier A (1993) An assessment of genetic diversity within a collection of cassava (Manihot esculenta Cranz) germplasm using molecular markers. Ann Bot 72: 515–520.
Bellotti AC (2002) Arthropod pests. In: Hillocks RJ, Thresh JM, Bellotti AC (eds.) Cassava: biology, production and utilization. CABI Publishing, Wallingford, pp. 209–235.
Bertram RB (1993) Application of molecular techniques resources of cassava (Manihot esculenta Crantz, Euphorbiaceae) interspecific evolutionary relationships and intraspecific characterization. PhD. Thesis, University of Maryland.
Blair MW, Fregene MA, Beebe SE, Ceballos H (2007) Marker-assisted selection in common beans and cassava. In: Marker-Assisted Selection (MAS) in Crops, Livestock, Forestry and fish: current status and the way forward. Food and Agriculture Organization of the United Nations (FAO), Rome, Italy, pp. 81–115.
Buitrago AJ (1990) La yuca en la alimentación animal. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia, 446p.
Cadavid López LF, Gil Llanos L (2003) Investigación en producción de yuca forrajera en Colombia. Informe annual de Actividades CLAYUCA. Apdo Aéreo 6713, Cali, Colombia, pp. 266–275.
Cach NT, Perez JC, Lenis JI, Calle F, Morante N, Ceballos H (2005a) Epistasis in the expression of relevant traits in cassava (Manihot esculenta Crantz) for subhumid conditions. J Heredity 96(5): 586–592.
Cach TN, Lenis JI, Perez JC, Morante N, Calle F, Ceballos H (2005b) Inheritance of relevant traits in cassava (Manihot esculenta Crantz) for sub-humid conditions. Plant Breed 124:1–6.
Calderón-Urrea A (1988) Transformation of Manihot esculenta (cassava) using Agrobacterium tumefaciens and expression of the introduced foreing genes in transformed cell lines. M.Sc. Thesis, Vrije University, Brussels, Belgium.
Calle F, Perez JC, Gaitán W, Morante N, Ceballos H, Llano G, Alvarez E (2005) Diallel inheritance of relevant traits in cassava (Manihot esculenta Crantz) adapted to acid-soil savannas. Euphytica 144(1–2): 177–186.
Calvert LA, Thresh JM (2002) The viruses and virus diseases of cassava. In: Hillocks RJ, Thresh JM, Bellotti AC (eds.) Cassava: biology, production and utilization. CABI Publishing, Wallingford, pp. 237–260.
Carvalho LJCB, de Souza CRB, Cascardo JCM, Junior CB, Campos L (2004) Identification and characterization of a novel cassava (Manihot esculenta Crantz) clone with high free sugar content and novel starch. Plant Mol Biol 56: 643–659.
Ceballos H, Iglesias CA, Pérez JC, Dixon AGO (2004) Cassava breeding: opportunities and challenges. Plant Mol Biol 56: 503–515.
Ceballos H, Fregene M, Lentini Z, Sánchez T, Puentes YI, Pérez JC, Rosero A, Tofiño AP (2006a) Development and identification of high-value cassava clones. Acta Hortic 703:63–70.
Ceballos H, Sánchez T, Chávez AL, Iglesias C, Debouck D, Mafla G, Tohme J (2006b) Variation in crude protein content in cassava (Manihot esculenta Crantz) roots. J Food Comp Anal 19:589–593.
Ceballos H, Sánchez T, Morante N, Fregene M, Dufour D, Smith AM, Denyer K, Pérez JC, Calle F, Mestres C (2007a) Discovery of an Amylose-free Starch mutant in cassava (Manihot esculenta Crantz). J Agric Food Chem 55(18): 7469–7476.
Ceballos H, Fregene M, Pérez JC, Morante N, Calle F (2007b) Cassava genetic improvement. In: Kang MS, Priyadarshan PM (eds.) Breeding major food staples, Blackwell Publishing. Ames, IA, pp. 365–391.
Ceballos H, Sánchez T, Denyer K, Tofiño AP, Rosero EA, Dufour D, Smith A, Morante N, Pérez JC, Fahy B (2008) Induction and identification of a small-granule, high-amylose mutant in cassava (Manihot esculenta Crantz). J Agric Food Chem 56(16): 7215–7222.
Chavarriaga-Aguirre P, Maya MM, Tohme J, Duque MC, Iglesias C, Bonierbale MW, Kreovich S, Fand Kochert G (1999) Using microsatellite, isozymes and AFLPs to evaluate genetic diversity and redundancy in the cassava core collection and to assess the usefulness of DNA based markers to maintain germplasm collections. Mol Breed 5: 263–273.
Chavarriaga P, Prieto S, Herrera CJ, Lopez D, Bellotti A, Tohme J (2004) Screening transgenic unveils apparent resistance to hornworm (E. ello) in the non-transgenic, African cassava clone 60444. In: Alves A, Tohme J (eds.) Adding value to a small farmer crop. Proceedings of the 6th International Scientific Meeting. Cassava Biotech Network, Book of Abstracts, p. 4. CIAT, Cali, Colombia.
Chavez AL, Sánchez T, Jaramillo G, Bedoya JM, Echeverry J, Bolaños EA, Ceballos H, Iglesias CA (2005) Variation of quality traits in cassava roots evaluated in landraces and improved clones. Euphytica 143: 125–133.
Chavez AL, Bedoya JM, Sánchez T, Iglesias CA, Ceballos H, Roca W (2000) Iron, carotene, and ascorbic acid in cassava roots and leaves. Food Nutr Bull. 21: 410–413.
Chávez AL, Sánchez T, Morante N, Pérez JC, Calle F, Ceballos H (2009) Progress in developing a system for direct and simple measurement of protein content in cassava roots. Proceeding of the 15th Triennial Symposium of the International Society for Tropical Root Crops, Centro International de la Papa (CIP), Lima, Peru, 2–6 November.
CIAT (Centro Internacional de Agricultura Tropical) (2001) Project IP3, Improved cassava for the developing world, Annual Report 2001, Apdo Aéreo 6713, Cali, Colombia.
CIAT (Centro Internacional de Agricultura Tropical) (2002) Project IP3, Improved cassava for the developing world, Annual Report 2002, Apdo Aéreo 6713, Cali, Colombia.
CIAT (Centro Internacional de Agricultura Tropical) (2009) Project IP3, Improved cassava for the developing world, Annual Report 2008, Apdo Aéreo 6713, Cali, Colombia.
Cock J (1985) Cassava. New potential for a neglected crop. Westview Press. Boulder, CO, 240pp.
Contreras Rojas M, Pérez JC, Ceballos H, Baena D, Morante N, Calle F (2009) Introduction of inbreeding and analysis of inbreeding depression in eight S1 cassava families. Crop Sci 49: 543–548.
Cuambe CE (2007) Evaluación del deterioro fisiológico postcosecha y mapeo preliminar de QTLs en el primer retrocruzamiento derivado del híbrido inter-específico (CW429-1) entre Manihot esculenta Crantz y la especie silvestre Manihot walkerae Croizat, M.Sc. Degree Thesis, National University of Colombia, Palmira Campus, 74p, December 2007.
Davis JP, Supatcharee N, Khandelwal RL, Chibbar RN (2003) Synthesis of novel starches in planta: opportunities and challenges. Starch/Stärke 55: 107–120.
de Carvalho RD, Guerra M (2002) Cytogenetics of Manihot esculenta Crantz (cassava) and eight related species. Hereditas 136: 159–168.
DeVires J, Toenniessen G (2001) Securing the harvest: biotechnology, breeding and seed systems for African crops. Chapter 13: cassava. CABI Publishing, Oxon, UK and New York, NY. pp. 147–156.
Dixon AGO, Asiedu R, Bokanga M (1994) Breeding of cassava for low cyanogenic potential: problems, progress and perspectives. Acta Hortic 375: 153–161.
Du L, Bokanga M, Møller BL, Halkier BA (1995) The biosynthesis of cyanogenic glucosides in roots of cassava. Phytochemistry 39(2): 323–326.
Duvick DN (1999) Heterosis: feeding people and protecting natural resources. In: Coors JG, Pandey S (eds.) The genetic exploitation of heterosis in Crops. American Society of Agronomy, Madison, WI, pp. 19–29.
Egesi CN, Ogbe FO, Akoroda M, Ilona P, Dixon A (2007) Resistance profile of improved cassava germplasm to cassava mosaic disease in Nigeria. Euphytica 155: 215–224.
Eke-Okoro ON, Okereke OU, Okeke JE, (2001) Effect of stake sizes on some growth indices and yield of three cassava cultivars (Manihot esculenta). J Agric Sci 137: 419–426.
Elias M, Panaud O, Robert T (2000) Assessment of genetic variability in a traditional cassava (Manihot esculenta Crantz) farming system, using AFLP markers. Heredity 85: 219–230.
Elias M, McKey D, Panaud O, Anstett MC, Robert T, (2001a) Traditional management of cassava morphological and genetic diversity by the Makushi Amerindians (Guyana, South America): perspectives for on-farm conservation of crop genetic resources. Euphytica 120: 143–157.
Elias M, Penet L, Vindry P, McKey D, Panaud O, Robert T, (2001b) Unmanaged sexual reproduction and the dynamics of genetic diversity of a vegetatively propagated crop plant, cassava (Manihot esculenta Crantz) in a traditional farming system. Mol Ecol 10: 1895–1907.
Ellis RH, Hong TD, Roberts EH (1982) An investigation of the influence of constant and alternating temperature on the germination of cassava seed using a two-dimensional temperature gradient plate. Ann Bot 49: 241–246.
Ellis RP, Cochrane MP, Dale MFB, Duffus CM, Lynn A, Morrison IM, Prentice RDM, Swanston JS, Tiller SA (1998) Starch production and industrial uses. J Sci Food Agric 77: 289–311.
FAO. FAOSTAT database (various years). http://www.fao.org.
Fehr WR (ed.) (1987) Genetic contributions to yield gains of five major crop plants. Crop Science Society of America, Madison, WI, 101p.
Fregene M, Angel F, Gomez R, Rodríguez F, Chavarriaga P, Roca W, Tohme J (1997) A molecular genetic map of cassava (Manihot esculenta Crantz). Theor Appl Genet 95: 431–441.
Fregene M, Bernal A, Duque M, Dixon A, Tohme J (2000) AFLP analysis of African cassava (Manihot esculenta Crantz) germplasm resistant to the cassava mosaic disease (CMD). Theor Appl Genet 100: 678–685.
Fregene M, Puonti-Kaerlas J (2002) Cassava biotechnology. In: Hillocks RJ, Thresh JM, Bellotti AC (eds.) Cassava: biology, production and utilization. CABI Publishing, Wallingford, pp. 179–207.
Fregene M, Tohme J, Roca W, Chavarriaga P, Escobar R, Ceballos H (2002) Biotecnología de yuca. . In: Ceballos H, Ospina B (eds.) La Yuca en el Tercer Milenio. CIAT, Cali, Colombia. pp. 377–405.
Fregene MH, Matsumura A, Akano A, Dixon A, Terauchi R (2004) Serial analysis of gene expression (SAGE) of host-plant resistance to the cassava mosaic disease (CMD). Plant Mol Biol 56: 563–571.
Frisch M, Bohn M, Melchinger AE (1999) Comparison of selection strategies for marker-assisted backcrossing of a gene. Crop Sci 39: 1295–1301.
Gardner CO (1961) An evaluation of effects of mass selection and seed irradiation with thermal neutrons on yields of corn. Crop Sci 1: 241–245.
Gonçalvez Fukuda WM, Fukuda C, Leite Cardoso CE, Lima Vanconcelos O, Nunes LC (2000) Implantação e evolução dos trabalhos de pesquisa participativa em melhoramento de mandioca no nordeste Brasileiro, Documento CNPMF No. 92, EMBRAPA, Cruz das Almas, Bahia, Brazil.
Gonçalvez Fukuda WM, Saad N (2001) Participatory research in cassava breeding with farmers in Northeastern Brazil, Document CNPMF No. 99, EMBRAPA, Cruz das Almas, Bahia, Brazil.
Gomez G, Santos J, Valdivieso M (1983) Utilización de raíces y productos de yuca en alimentación animal. In: Domínguez CE (ed.) Yuca: investigación, producción y utilización. Working Document No. 50. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia.
Hahn SK, Bai KV, Asiedu R (1990) Tetraploids, triploids, and 2n pollen from diploid interspecific crosses with cassava. Theor Appl Genet 79: 433–439
Hahn SK, Terry ER, Leuschner K, Akobundu IO, Okali C, Lal R (1979) Cassava improvement in Africa. Field Crops Res 2: 193–226.
Hahn SK, Terry ER, Leuschner K (1980a) Breeding cassava for resistance to cassava mosaic disease. Euphytica 29: 673–683.
Hahn SK, Howland AK, Terry ER (1980b) Correlated resistance to cassava to mosaic and bacterial blight diseases. Euphytica 29: 305–311.
Hallauer AR, Miranda Fo JB (1988) Quantitative genetics in maize breeding. 2nd edn. Iowa State University Press. Ames, IA, pp. 45–114.
Han Y, Gómez-Vásquez R, Reilly K, Li H, Tohme J, Cooper RM, Beeching JR (2001) Hydroxyproline-rich glycoproteins expressed during stress responses in cassava. Euphytica 120: 59–70.
Haysom HR, Chan TLC, Hughes MA (1994) Phylogenetic relationships of Manihot species revealed by restriction fragment length polymorphism. Euphytica 76: 227–234.
Hershey CH (1984) Breeding cassava for adaptation to stress conditions: development of a methodology. In: Proceedings of the 6th Symposium of the International Society for Tropical Root Crops, Lima, Peru, pp. 20–25, February, 1983.
Hilloocks RJ, Wydra K (2002) Bacterial, fungal and nematode diseases. In: Hillocks RJ, Thresh JM, Bellotti AC (eds.) Cassava: biology, production and utilization. CABI Publishing, Wallingford, pp. 261–280.
Hospital F, Chevalet C, Mulsant P (1992) Using markers in gene introgression breeding programs. Genetics 132: 1119–1210.
Howeler R (2007) Agronomic practices for sustainable cassava production in Asia. In: Centro Internacional de Agricultura Tropical (CIAT). Cassava research and development in Asia: exploring new opportunities for an ancient crop. Proceedings of the Seventh Regional Workshop held in Bangkok, Thailand, pp. 288–314, October 28–November 1, 2002.
Iglesias CA, Hershey C (1994) Cassava breeding at CIAT: heritability estimates and genetic progress in the 1980s In: Ofori F, Hahn SK (eds.), Tropical Root Crops in a Developing Economy. ISTRC/ISHS, Wageningen, pp. 149–163.
Iglesias CA, Hershey C, Calle F, Bolaños A (1994) Propagating cassava (Manihot esculenta Crantz) by sexual seed. Exp Agric 30: 283–290.
Iglesias CA, Mayer J, Chávez AL, Calle F (1997) Genetic potential and stability of carotene content in cassava roots. Euphytica 94: 367–373.
Janssens M (2001) Cassava. In: Raemaekers RH, (ed.) Crop production in tropical Africa. Directorate General for International Co-operation (DGIC), Brussels, pp. 165–187.
Jaramillo G, Morante N, Pérez JC, Calle F, Ceballos H, Arias B, Bellotti AC (2005) Diallel analysis in cassava adapted to the midaltitude valleys environment. Crop Sci 45: 1058–1063.
Jennings DL (1963) Variation in pollen and ovule fertility in varieties of cassava, and the effect of interspecific crossing on fertility. Euphytica, 12: 69–76.
Jennings DL (1976) Breeding for resistance to African cassava mosaic. African cassava mosaic report of an interdisciplinary workshop held at Muguga, Kenya. IDRC071e, pp. 39–44
Jennings DL, Iglesias CA (2002) Breeding for crop improvement. In: Hillocks RJ, Thresh JM, Bellotti AC (eds.) Cassava: biology, production and utilization. CABI Publishing, Wallingford, pp. 149–166.
Jobling S (2004) Improving starch for food and industrial applications. Curr Opin Plant Biol 7: 210–218.
Jorge V, Fregene M, Duque MC, Bonierbale MW, Tohme J, Verdier V (2000) Genetic mapping of resistance to bacterial blight disease in cassava (Manihot esculenta Crantz). Theor Appl Genet 101: 865–872.
Kawano K (1980) Cassava. In: Fehr WR, Hadley, HH (eds.) Hybridization of crop plants. ASA, CSSA. Madison, WI, pp. 225–233.
Kawano K (2003) Thirty years of cassava breeding for productivity – biological and social factors for success. Crop Sci 43: 1325–1335.
Kawano K, Daza P, Amaya A, Ríos M, Gonçalvez MF (1978) Evaluation of cassava germplasm for productivity. Crop Sci 18: 377–380.
Kawano K, Narintaraporn K, Narintaraporn P, Sarakarn S, Limsila A, Limsila J, Suparhan D, Sarawat V, Watananonta W (1998) Yield improvement in a multistage breeding program for cassava. Crop Sci 38(2): 325–332.
Kizito EB, Bua A, Fregene M, Egwang T, Gullberg U, Westerbergh A (2005) The effect of cassava mosaic disease on the genetic diversity of cassava in Uganda. Euphytica 146: 45–54.
Ladino J, Mancilla LI, Chavarriaga P, Tohme J, Roca WM (2001) Transformation of cassava cv. TMS60444 with A. tumefaciens carrying a cry 1Ab gene for insect resistance. Proceeding of the Fifth International Scientific Meeting of the Cassava Biotechnology Network, Donald Danforth Plant Science Center, St. Louis, MO, 4–9 November, 2001.
Lehman U, Robin F (2007) Slowly digestible starch – its structure and health implications: a review. Trends Food Sci Technol 18: 346–355
Lenis JI, Calle F, Jaramillo G, Pérez JC, Ceballos H, Cock J (2006) Leaf retention and cassava productivity. Field Crops Res 95(2–3): 126–134.
Lokko Y, Anderson JV, Rudd S, Raji A, Horvath D, Mikel MA, Kim R, Liu L, Hernandez A, Dixon AGO, Igenbrecht IL (2007) Characterization of a 18166 EST dataset for cassava (Manihot esculenta Crantz) enriched for drought-response genes. Plant Cell Rep 26: 1605–1618.
Lopez C, Jorge V, Piegu B, Mba C, Cortes D, Restrepo S, Soto M, Laudie M, Berger C, Cooke R, Delseny M, Tohme J, Verdier V (2004) A unigene catalogue of 5700 expressed genes in cassava. Plant Mol Biol 56(4): 541–554.
Magoon ML, Krishnan R, Bai KV (1969) Morphology of the pachytene chromosomes and meiosis in Manihot esculenta Crantz. Cytologia 34: 612–626.
Maluszynski M, Szarejko I, Barriga P, Balcerzyk A (2001) Heterosis in crop mutant crosses and production of high yielding lines using double haploid systems. Euphytica 120: 387–398.
Mba REC, Stephenson P, Edwards K, Melzer S, Mkumbira J, Gullberg U, Apel K, Gale M, Tohme J, Fregene M (2001) Simple sequence repeat (SSR) markers survey of the cassava (Manihot esculenta Crantz) genome: towards an SSR-based molecular genetic map of cassava. Theor Appl Genet 102: 21–31.
McCallum CM, Comai L, Greene EA, Henikoff S (2000) Targeting induced local lesions in genomes (TILLING) for plant functional genomics. Plant Physiol 123: 439–442.
McMahon JM, White WLB, Sayre RT (1995) Cyanogenesis in cassava (Manihot esculenta Crantz). J Exp Bot 46: 731–741.
McSween S, Walker T, Salegua V, Pitoro R (2006) Economic impact on food security of varietal tolerance to cassava brown streak disease in coastal Mozambique, Research Report Series No. 1E, Institute of Agricultural Research of Mozambique, Maputo, Mozambique.
Mkumbira JL, Chiwona-Karltun U, Lagercrantz N, Meso Mahungu J, Saka A, Mhone M, Bokanga L, Brimer U, Gullberg U, Rosling H (2003) Classification of cassava into ‘bitter’ and ‘cool’ in Malawi: from farmers’ perception to characterisation by molecular markers. Euphytica 132:7–22.
Meireles da Silva R, Bandel G, Martins PS (2003) Mating system in an experimental garden composed of cassava (Manihot esculenta Crantz) ethnovarieties. Euphytica 134: 127–135.
Morante N, Moreno X, Pérez JC, Calle F, Lenis JI, Ortega E, Jaramillo G, Ceballos H (2005) Precision of selection in early stages of cassava genetic improvement. J Root Crops 31: 81–92.
Morante N, Sánchez T, Ortiz D, Chávez AL, Calle F, Ceballos H (2009) Progress increasing carotenoids content in cassava roots. Proceeding of the 15th Triennial Symposium of the International Society for Tropical Root Crops, Lima, Peru.
Morante N, Sánchez T, Ceballos H, Calle F, Pérez JC, Egesi C, Cuambe CE, Escobar AF, Ortiz D, Chávez AL (2010) Tolerance to post-harvest physiological deterioration in cassava roots. Crop Sci 50: 1333–1338.
Munyikwa TRI, Lageveld S, Salehuzzaman SNIM, Jacobsen E, Visser RGF (1997) Cassava starch biosynthesis: new avenues for modifying starch quantity and quality. Euphytica 96: 65–75.
Nassar NMA, Ortiz R (2008) Cassava genetic resources: manipulation for crop improvement. Plant Breed Rev 31: 247–275.
Neuenschwander P (1994) Control of cassava mealybug in Africa: lessons from a biological control project. Afr Crop Sci J 2: 369–383.
Nyiira ZM (1975) Advances in research on the economic significance of the green cassava mite Mononychellus tanajoa Bondar in Uganda. International exchange and testing of cassava germplasm in Africa. In: Terry ER, MacIntyre R (eds.) Proceedings of an Interdisciplinary Workshop, Ibadan, Nigeria, 17–21. November 1975. IDRC-063e, Ottawa, ON, pp. 22–29.
Okogbenin E, Fregene M (2002) Genetic analysis and QTL mapping of early root bulking in an F1 population of non-inbred parents in cassava (Manihot esculenta Crantz). Theor Appl Genet 106: 58–66.
Okogbenin E, Marin J, Fregene M (2006) An SSR-based molecular genetic map of cassava Euphytica 147: 433–440.
Okogbenin E, Porto MCM, Egesi C, Mba C, Ospinosa E, Guillermo Santos L, Ospina C, Marin J, Barera E, Gutierrez J, Ekanayake I, Iglesias C, Fregene M (2007) Marker aided introgression of CMD resistance in Latin American Germplasm for genetic improvement of cassava in Africa. Crop Sci 47: 1895–1904.
Olsen KM, Schaal BA (2001) Microsatellite variation in cassava (Manihot esculenta, Euphorbiaceae) and its wild relatives: further evidence for a southern Amazonian origin of domestication. Am J Bot 88(1): 131–142.
Perez JC, Ceballos H, Jaramillo G, Morante N, Calle F, Arias B, Bellotti AC (2005a) Epistasis in cassava adapted to mid-altitude valley environments. Crop Sci 45: 1491–1496.
Perez JC, Ceballos H, Calle F, Morante N, Gaitán W, Llano G, Alvarez E (2005b) Within-family genetic variation and epistasis in cassava (Manihot esculenta Crantz) adapted to the acid-soils environment. Euphytica 145(1–2): 77–85.
Pérez JC, Ceballos H, Ramirez IC, Lenis JI, Calle F, Morante N, Jaramillo G, Lentini D del C (2010) Adjustment for missing plants in cassava evaluation trials. Euphytica 172(1): 59–65.
Perry JA, Wang TL, Welham TJ, Gardner S, Pike JM, Yoshida S, Parniske M (2003) A TILLING reverse genetics tool and a web-accessible collection of mutants of the legume Lotus japonicus. Plant Physiol 131: 866–871.
Pfeiffer WH, McClafferty B (2007) HarvestPlus: breeding crops for better nutrition. In: IBPS Proceeding published by Crop Science Society of America. Crop Sci 47: 88–105.
Pujol B, Gigot G, Laurent G, Pinheiro-Kluppel M, Elias M, Hossaert-McKey M, McKey D (2002) Germination ecology of cassava (Manihot esculenta Crantz Euphorbiaceae) in traditional agroecosystems: seed and seedling biology of a vegetatively propagated domesticated plant. Econ Bot 56: 366–379.
Puonti-Kaerlas J, Frey P, Potrykus I (1997) Development of meristem gene transfer techniques for cassava. Afr J Root Tuber Crops 2: 175–180.
Rajendran PG, Ravindran CS, Nair SG, Nayar TVR (2000) True cassava seeds (TCS) for rapid spread of the crop in non-traditional areas. Central Tuber Crops Research Institute (Indian Council of Agricultural Research). Thiruvananthapuram, 695 017, Kerala, India.
Reilly K, Gomez-Vasquez R, Buschman H, Tohme J, Beeching JR (2003) Oxidative stress responses during cassava post-harvest physiological deterioration. Plant Mol Biol 53: 669–685.
Reilly K, Bernal D, Cortes DF, Gomez-Vasquez R, Tohme J, Beeching JR (2007) Towards identifying the full set of genes expressed during cassava post-harvest physiological deterioration. Plant Mol Biol 64: 187–203.
Reilly K, Han Y, Tohme J, Beeching JR (2001) Isolation and characterization of a cassava catalase expressed during post-harvest physiological deterioration. Biochim Biophys Acta 1518: 317–323.
Roa AC, Maya MM, Duque M, Allem C, Tohme J, Bonierbale MW (1997) AFLP analysis of relationships among cassava and other Manihot species. Theor Appl Genet 95: 741–750.
Rogers DJ (1963) Studies of Manihot esculenta Crantz and related species. Bull Torrey Bot Club 90: 43–54.
Rogers DJ, Appan SG (1973) Manihot and manihotoides (Euphorbiaceae). A computer-assisted study. Flora Neotropica. Monograph No. 13. Hafner Press, New York, NY. 272p.
Salik J, Cellinese N, Knapp S (1997) Indigenous diversity of cassava: generation, maintenance, use and loss among Amuesha, Peruvian upper Amazon. Econ Bot 51: 6–19.
Sambatti JBM, Martins PS, Ando A (2001) Folk taxonomy and evolutionary dynamics of cassava: a case study in Ubatuba, Brazil. Econ Bot 55: 93–105.
Sánchez T, Mafla G, Morante N, Ceballos H, Dufour D, Calle F, Moreno X, Pérez JC, Debouck D (2009) Screening of starch quality traits in cassava (Manihot esculenta Crantz). Starch/Stärke 61: 12–19.
Sánchez G, Restrepo S, Duque M, Fregene M, Bonierbale M, Verdier V (1999) AFLP assessment of genetic variability in cassava accessions (Manihot esculenta) resistant and susceptible to cassava bacterial blight (CBB). Genome 42: 163–172.
Scott GJ, Rosegrant MW, Ringler C (2000) Roots and tubers for the 21st century. Trends, projections, and policy options. International Food Policy Research Institute (IFPRI)/Centro Internacional de la papa (CIP). Washington, DC, 64p.
Second G, Allem A, Emperaire L, Ingram C, Colombo C, Mendes R, Carvalho L (1997) AFLP based Manihot and cassava numerical taxanomy and genetic structure analysis in progress: implications for dynamic conservation and genetic mapping. Afr J Root Tuber Crops 2: 140–147.
Segovia RJ, Bedoya A, Triviño W, Ceballos H, Gálvez G, Ospina PB (2002) Metodología para el Endurecimiento de ‘vitroplantas’ de yuca. In: Ceballos H, Ospina B (eds.) La Yuca en el Tercer Milenio. CIAT, Cali, Colombia, pp. 573–584.
Sharma V, Rausch KD, Tumbleson ME, Singh V (2007) Comparison between granular starch hydrolyzing enzyme and conventional enzymes for ethanol production form maize starch with different amylose:amylopectin ratios. Starch/ Stärke 59: 549–556.
Sherman TD, Vaughn KC, Duke SO (1996) Mechanisms of action and resistance to herbicides. In: Duke SO (ed.) Herbicide resistant crops. CRC Press, Boca Ratón, FL, pp. 13–35.
Simmonds NW, Smartt J (1999) Principles of crop improvement. Blackwell Science, London.
Stam P, Zeven AC (1981) The theoretical proportion of the donor genome in near-isogenic lines of self-fertilizers bred by backcrossing. Euphytica 30: 227–238.
Swanson MM, Harrison BD (1994) Properties, relationships and distribution of cassava germiviruses. Trop Sci 34: 15–25.
Tan SY, Bowe S (2008) Developing herbicide-tolerant crops from mutations. FAO/IAEA International Symposium on Induced Mutations in Plants, Vienna, Austria, p. 134, 12–15 August.
Tan S, Evans R, Singh B (2006) Herbicidal inhibitors of amino acid biosynthesis and herbicide-tolerant crops. Amino Acids 30: 195–204.
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005) Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci 61: 246–257.
Taylor N, Cavarriaga P, Raemakers K, Siritunga D, Zhang P (2004) Development and application of transgenic technologies in cassava. Plant Mol Biol 56: 671–688.
Thu LTN, Gheewala SH, Garvait S (2007) Full chain energy analysis of fuel ethanol from cassava in Thailand. Environ Sci Technol 41: 4135–4142.
Till BJ, Reynolds SH, Greene EA, Codomo CA, Enns LC, Johnson JE, Burtner C, Odden AR, Young K, Taylor NE, Henikoff JG, Comai L, Henikoff S (2003) Large-scale discovery of induced point mutations with high-throughput TILLING. Genome Res 13: 524–530.
Troyer AF (2006) Adaptedness and heterosis in corn and mule hybrids. Crop Sci 46: 528–543.
van Oirschot QEA, O’Brien GM, Dufour D, El-Sharkawy MA, Mesa E (2000) The effect of pre-harvest pruning of cassava upon root deterioration and quality characteristics. J Sci Food Agric 80: 1866–1873.
Wheatley CC, Sanchez T, Orrego JJ (1993) Quality evaluation of the core cassava collection at CIAT. In: Roca WM, Thro AM (eds.), Proceedings of the 1st International, Scientific Meeting of the Cassava Biotechnology Network, Cartagena, Colombia, August 1992. CIAT, Cali, Colombia, pp. 255–264.
Wheatley CC, Chuzel G (1995) Cassava: the nature of the tuber and use as a raw material. In: Macrae R, Robinson RK, Sadler MJ (eds.) Encyclopedia of food science, food technology and nutrition, Academic Press, London.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Ceballos, H., Okogbenin, E., Pérez, J.C., López-Valle, L.A.B., Debouck, D. (2010). Cassava. In: Bradshaw, J. (eds) Root and Tuber Crops. Handbook of Plant Breeding, vol 7. Springer, New York, NY. https://doi.org/10.1007/978-0-387-92765-7_2
Download citation
DOI: https://doi.org/10.1007/978-0-387-92765-7_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-92764-0
Online ISBN: 978-0-387-92765-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)