Abstract
Lactose is the precursor for a number of compounds derived by chemical, physical or enzymatic conversion that have an established and expanding place in the health and food industries. These include lactulose and galacto-oligosaccharides both of which are manufactured in large tonnages worldwide. Lactitol and lactosucrose are produced commercially, but in much smaller amounts, while lactobionic acid is produced for limited industrial and medical applications and for research use. The only other lactose derivative of commercial interest is an isomer of galactose called tagatose in which there is an emerging interest as a sweetener.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
Lactose is the precursor for a number of compounds derived by chemical, physical or enzymatic conversion that have an established and expanding place in the health and food industries. These include lactulose and galacto-oligosaccharides both of which are manufactured in large tonnages worldwide. Lactitol and lactosucrose are produced commercially, but in much smaller amounts, while lactobionic acid is produced for limited industrial and medical applications and for research use. The only other lactose derivative of commercial interest is an isomer of galactose called tagatose in which there is an emerging interest as a sweetener.
None of these compounds occur naturally in cow’s milk except in trace amounts. Yet, lactose-derived oligosaccharides occur naturally in human milk at relatively high concentrations (5–12 g/L) and play a major role in directing the development of the intestinal microbiota of infants. One of the major and emerging uses of lactose derivatives has been to emulate the physiological effects of these natural oligosaccharides, and β-galacto-oligosaccharides (GOSs) and lactulose in particular have found applications as prebiotics in functional foods and beverages. βGOSs are also classified nowadays as dietary fibre. Prebiotic α-galacto-oligosaccharides are also available commercially, but since they are produced typically from soybean, they are not discussed here. Lactulose is an important pharmaceutical used in the treatment of constipation and hepatic encephalopathy, and lactitol is increasingly used in similar applications. Galactose, lactic acid and lactates are also produced from lactose, but are not reviewed here (refer to Chapters 1 and 4).
Extensive reviews on GOSs have been published in recent years (Matsumoto et al., 1993; Playne and Crittenden, 1996; Crittenden and Playne, 1996; Tanaka and Matsumoto, 1998; Sako et al., 1999; Schoterman, 2001; Playne, 2002a; Meyer and Tungland, 2001; Nakakuki, 2002). Readers may also like to compare the commercially produced GOSs to the complex oligosaccharides found in human milk, which have been reviewed thoroughly by Kunz et al. (2000) and Boehm and Stahl (2003, 2007). New methods for manufacturing lactose derivatives, as well as new applications, are emerging constantly. Patents on methods of the manufacture of oligosaccharides, lactulose, lactosucrose, lactitol, lactobionic acid and tagatose are extensive. The full patents provide a wealth of information on manufacturing procedures, the enzymes and microorganisms involved, analytical methods, separation and purification procedures and applications and uses of the products. Readers are advised to examine these resources and internet access is available free, for at least all US patents. In this chapter, we have drawn on this extensive body of scientific literature to provide an overview of the current state of the art in production, applications and physiological effects of lactose derivates.
5.1.1 Definitions
Oligosaccharides
-
are usually defined as glycosides composed of 2–10 covalently linked monomer sugar units. However, disaccharides, such as lactose and lactulose, are often not regarded as oligosaccharides and some saccharides of longer chain length than ten monomer units are called oligosaccharides, provided they are of defined length, composition and structure.
Non-digestible Oligosaccharides (NDOs)
-
not all oligosaccharides are NDOs. NDOs can be distinguished from other carbohydrates on the basis of being not digested in the stomach and small intestine, and therefore not digested under acid conditions and by pancreatic hydrolytic enzymes. NDOs include some larger saccharide molecules, such as inulin.
Polysaccharides
-
are high molecular weight polymers of one or more monosaccharides. They are of greater molecular size than oligosaccharides and usually of undefined length. Examples are starch and cellulose.
Galacto-oligosaccharides
-
are oligosaccharides composed primarily of galactose monomers linked together in a number of different structural configurations. They usually consist of a number of β(1→6) linked galactopyranosyl units linked to a terminal glucopyranosyl residue via an α(1→4) glycosidic bond. They are sometimes referred to as trans-galacto-oligosaccharides (TOSs).
-
abbreviations used are GOS and TOS.
Lactulose (4- O -β- d -Galactopyranosyl- d -fructofuranose; C 12 H 22 O 11 ; FW 342.30 Da)
-
is an isomer of lactose, wherein the glucose moiety of lactose is converted by alkaline isomerization to fructose. The disaccharide, lactulose, is therefore composed of β-d-galactose linked to β-d-fructose in a (1→ 4) configuration.
Lactosucrose ( β- d -Fructofuranosyl-4- O -β- d -galactopyranosyl- α- d -glucopyrano- side; C 18 H 34 O 17 ; FW 522 Da)
-
is a trisaccharide formed from lactose and sucrose by an enzymatic transglycosylation.
Lactitol (4- O -β- d -Galactopyranosyl- d -glucitol; C 12 H 24 O 11 ; FW 344.32 Da)
-
is a sugar alcohol derived from lactose by catalytic hydrogenation.
-
synonyms are lactit, lactositol, lactobiosit
Lactobionic acid (4- O -β- d -Galactopyranosyl- d -gluconic acid; C 12 H 22 O 12 ; FW 358.30 Da)
-
is an oxidation product of lactose.
Tagatose ( d -(–)-Tagatose; C 6 H 12 O 6 ; FW 180.16 Da)
-
is a d-lyxo-hexulose with a molecular weight of 180.16. It can occur naturally and is derived from galactose by alkaline isomerization.
Prebiotic
-
a prebiotic is a selectively fermented ingredient that allows specific changes, in both the composition and/or the activity in the gastrointestinal microflora, that confer benefits upon host well-being and health (Gibson et al., 2004; Roberfroid, 2007). These ingredients are normally restricted to certain carbohydrates (particularly oligosaccharides), but could include certain proteins, peptides and lipids. The concept of a prebiotic ingredient arose initially from the idea of compounds called “bifidus factors” which could enhance the growth of bifidobacteria within the intestinal microbiota.
Synbiotic
-
are foods containing both probiotic bacteria and prebiotic ingredients to provide a diet in which the intestinal growth and/or metabolic activity of the probiotic bacteria is selectively enhanced by the presence of the prebiotic, thus promoting the chance of the probiotic bacteria becoming established in the gut.
5.2 History of Prebiotics
5.2.1 The Genus Bifidobacterium
The concept of prebiotics arose from the realization that some compounds could enhance the growth of bifidobacteria in the intestinal tract of humans. These compounds were called the “bifidus factors”, and followed Tissier’s early work in France around 1900 on bifidobacteria and their presence in the gastrointestinal tract of babies. He realized that bifidobacteria could possibly control diarrhoea in infants. At that time, bifidobacteria were called Bacillus bifidus. Although the name Bifidobacterium was proposed as early as 1920, it did not gain official recognition as a separate genus until 1974. There are now 36 recognized species within the genus Bifidobacterium (German Culture Collection, 2007).
5.2.2 Bifidus Factors
In 1953, Gyorgy discovered a strain of Bifidobacterium bifidum which would grow only in the presence of human milk, or more specifically in the presence of derivatives of N-acetylglucosamine. Further specific requirements for the growth of different strains were recorded, e.g., human casein hydrolysates (Gyorgy et al., 1954a,b). In 1953, lactulose as a growth factor for bifidobacteria in infant milk was being studied, and infant milk containing lactulose was being sold by Morinaga in Japan as early as 1960. Lactulose was recognized as a “bifidus” factor by Petuely (1957). Bifidus factors have been summarized by Modler et al. (1990) and are shown in Table 5.1.The value of a range of oligosaccharides and polysaccharides as bifidus factors was recognized by Yazawa et al. (1978) and Yazawa and Tamura (1982). Ballongue (1998) and Tamura (1983), respectively, have described the historical developments of the genus Bifidobacterium and the recognition of bifidus factors summarized above.
5.2.3 Oligosaccharides as Prebiotics
The earliest scientific publications on oligosaccharides as prebiotics occurred in Japan (Yazawa et al., 1978) and were followed soon after by a number of papers by Japanese researchers examining, particularly, various galacto- and fructo-oligosaccharides (for example, see Minami et al., 1983). The earliest patents on methods for the production of oligosaccharides occurred in 1982, also in Japan. Most of the Japanese commercial development of various food-grade oligosaccharides occurred during that decade. However, the widespread use of prebiotics in products in Japan did not really start until 1990. By the end of 1999, prebiotics dominated the ingredients in FOSHU (Foods for Specified Health Use) – approved products in Japan (see Section 5.10.1).
The actual use of the word “prebiotic” is credited to Gibson and Roberfroid (1995), and this word is now widely used to include oligosaccharides, polysaccharides, inulin, lactulose, lactitol, glucans, resistant starches and many dietary fibres. The word “prebiotic” relates to “probiotics”, which are live microbial food ingredients, such as lactobacilli and bifidobacteria, that are consumed with the aim of supplementing the intestinal microbiota and improving health. The development of prebiotics in Europe did not occur until the early 1990s, with the start of inulin and fructo-oligosaccharide production in Belgium and later the development of galacto-oligosaccharide production in Holland and the research on gluco-oligosaccharides in Toulouse. Meiji (Japan) also formed alliances with Beghin Say in France and with Coors in the USA to produce fructo-oligosaccharides. However, European researchers have dominated the scientific literature on prebiotics since then, and the European food industry has very much claimed the “prebiotic concept” in recent years. Other than the development of fructo-oligosaccharide production in Korea and in Taiwan, there has been little development outside Europe and Japan. However, companies in a number of countries, including Australia, produce galacto-oligosaccharides in-house primarily for infant milk formula markets. The commercial development of the prebiotic carbohydrate market is shown in Table 5.2.
5.2.4 Use of β-Galactosidases for the Synthesis of Oligosaccharides
The ability of glycosidase enzymes to carry out synthetic reactions by reversing the equilibrium conditions has long been known (Croft-Hill, 1898). The enzymatic synthesis of galacto-oligosaccharides from lactose was first studied in detail by Pazur (1953, 1954) and Pazur et al. (1958). A number of papers published in the 1970s and 1980s examined the production of oligosaccharides from lactose using enzymes derived from a number of sources (for example, Toba et al., 1985). The mechanisms of the action of β-galactosidases on lactose were first described by Wallenfels and Malhotra in 1960 (see Prenosil et al., 1987a). Prenosil et al. (1987a,b) described the nature of the oligosaccharide products formed from lactose by β-galactosidases from a number of different microbial sources. However, Wallenfels (1951) first described enzymatic synthesis of oligosaccharides from disaccharides.
5.3 Chemistry – Structures and Reactivity
Glycans, with nucleic acids and proteins, are widely distributed in living organisms. All of these polymers are covalently linked moieties, but glycans have characteristics not found in the other two. Notably, the nature of the linkage between monomeric units in glycans is much more variable than those found in the other polymers, and this leads to a much greater variety in the sequence of the biopolymer. Thus, this leads to a huge structural diversity of oligosaccharides in glycosylated compounds (glycoproteins, glycolipids).
The number of combinations of structural linkages between monomers is high. For example, a galactose unit can be linked to a mannose unit at four positions (C2, C3, C4 and C6), and thus form four isomeric structures. Additionally, a galactose moiety can take two anomeric configurations, meaning that the number of combinations rises to eight. Furthermore, a galactose moiety can occur in both furanose and pyranose forms; thus, there are 16 possible isomeric structures of this Gal-Man disaccharide. As the number of linkages expands, so does the seemingly endless possible combinations. In contrast to proteins and nucleic acids, glycans are not limited in their molecular structure, as they can branch three-dimensionally. Neither are glycans constrained by genetic templates as is the case with nucleic acids and proteins. This structural diversity (and flexibility) has led to the emerging science of glycobiology and its application in medicine in the development of targetted drugs.
The nomenclature of carbohydrates has been described fully in a series of publications authorized by the International Union of Pure and Applied Chemistry (IUPAC). Detailed information on currently accepted nomenclature and its historical development can be accessed on-line (Queen Mary University of London, 2008). This web site details the 1996 Recommendations of the IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN) of the IUPAC and the International Union of Biochemistry and Molecular Biology (IUBMB). The full 1996 recommendations have been published (IUPAC, 1996) and are available as a pdf file at www.iupac.org/publications/pac/1996/pdf/6810x1919.pdf.
5.4 Synthesis and Manufacturing Methods
The synthesis of oligosaccharides from simple sugars has been studied extensively using classical methods of carbohydrate chemistry. The use of enzymes in the synthesis of oligosaccharides has now overtaken direct chemical pathways because of the ability of enzymes to be specific in the formation of particular linkages between monomers. The scope of the earlier work on oligosaccharide synthesis has been ably summarized by Bailey (1965) and Pazur (1970). Chemical synthetic methods based on lactose have been discussed by Thelwall (1997).
The interest of the food industry in these ingredients since 1980 has resulted in the development of manufacturing methods, most of which are based on enzymatic conversions, and only limited purification steps to reduce cost. Highly purified product is not warranted for most food applications. However, products of greater purity and specific structure are necessary in some applications, particularly those for pharmaceutical use.
Prebiotic oligosaccharides are prepared by several methods. Some are extracted from plant materials and used directly, e.g., inulin, some resistant starches, soybean and many dietary fibres. Others are modified enzymatically after extraction of the crude parent feedstock from plants (e.g., xylo-oligosaccharides and some fructo-oligosaccharides).
β-Galacto-oligosaccharides are generally synthesized from lactose by transgalactosylation of lactose by the enzyme β-galactosidase. In contrast, lactosucrose is prepared by a different enzymatic route. Here, lactose, in the presence of sucrose, acts as the acceptor of fructose in a transfructosylation reaction catalysed by the enzyme β-fructofuranosidase. Fructo-oligosaccharides are also synthesized from sucrose by similar enzymatic routes.
Lactulose and lactitol are prepared by two different chemical syntheses from lactose. Lactulose is prepared by alkaline isomerization of lactose, while lactitol is synthesized by catalytic hydrogenation of lactose. Lactulose can also be prepared by an enzymatic route, though this is not used commercially. Lactobionic acid is prepared by dehydrogenation of lactose at high pH using a metal catalyst. However, it can also be prepared in high yield by microbial bioconversion and by enzymic oxidation of lactose. Tagatose was prepared initially from galactose by alkaline isomerization in a manner parallel to the preparation of the disaccharide, lactulose from lactose, but it is now manufactured by an enzymatic route from lactose.
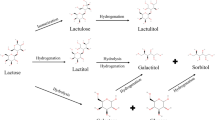
The basis of the preparation of galacto-oligosaccharides, lactulose, lactitol and lactosucrose from lactose is shown in Figure 5.1.
5.4.1 β-Galacto-oligosaccharides
A typical flow chart for the manufacture of galacto-oligosaccharides is shown in Figure 5.2. Similar methods are used by the main galacto-oligosaccharide manufacturers (Yakult, Friesland Foods Domo and Nissin Sugar). The process depends on reversing the normal degradative hydrolytic action of β-galactosidase. Instead of producing glucose and galactose from the feedstock lactose, the enzyme is “pushed” into synthesis of a mixture of tri, tetra and penta galacto-oligosaccharides. This is achieved by having a high concentration of lactose in solution (e.g., 400 g/L or higher). This is achievable only if the temperature is elevated to between 50 and 80°C and the enzyme chosen to catalyse the reaction must be active at these temperatures. The enzyme chosen must also have minimal hydrolytic activity to avoid the simultaneous formation of excessive amounts of glucose and galactose. The β-galactosidases used may produce either β1→4 or β1→6 linkages in the oligosaccharide chain. The ratio of products containing either linkage can be controlled by using dual enzyme systems. Generally, the production of trisaccharides predominates, and thus trisaccharides are dominant in most commercial GOS products. Enzymes derived from Bacillus circulans or Cryptococcus laurentii will produce 4′-galactosyllactose, while enzymes from Aspergillus oryzae or Streptococcus thermophilus will produce 6′-galactosyllactose. The former enzymes are used by the Nissin Company to produce their Cup-Oligo product which is a β1→4 galactosyl lactose, whereas Yakult Pharmaceuticals have used the latter enzymes for their Oligomate products which have predominantly β1→6 linkages, although Yakult now also uses B. circulans to produce β1→4 linked product. Thus, the choice of enzyme is crucial to the outcome of the commercial process. Similar considerations apply to the production of fructo-oligosaccharides from sucrose. Figure 5.2 shows the steps required for the simple production of a mixture of oligosaccharides (tri- to hepta-saccharides), lactose, glucose and galactose, when no purification steps are included. Additional separation of oligosaccharides, from the substrate lactose, and the hydrolytic products, glucose and galactose, can be achieved, but is an expensive process. Separation is usually by a chromatographic process. Following the batch reaction with enzymes, the product mixture is decolourized and demineralized, then filtered and concentrated to produce either a syrup or a powder. Attempts to reduce enzyme costs have been made by immobilization of the enzymes, but it is believed that this is not used commercially. A wide range of microorganisms continue to be evaluated as sources of suitable β-galactosidases. For example, the enzyme from Sterigmatomyces elviae has given high oligosaccharide yields from lactose at 200 g/L at 85°C. Active patenting activity occurs in this area in particular. Mahoney (1997) listed the wide range of microbial species which have β-galactosidases able to utilize lactose. Since that publication, considerable research has been performed on new sources of thermostable enzymes, the development of recombinant enzymes and enzymes from Bifidobacterium species. However, the choice of a suitable commercial enzyme depends on its ability to perform the reaction at a high temperature, and this will continue to determine the selection of the enzyme.
5.4.1.1 Type of Enzyme and Mechanisms of Reaction
Two types of enzyme have been used in the preparation of oligosaccharides, the glycosyltransferases (EC 2.4 series) and the glycosidases (EC 3.2 series). The commercial processes used in the food industry which are described in the previous section all use glycosidases.
Glycosyltransferases: Glycosyltransferases catalyse the stereo- and regio-specific transfer of a monosaccharide from a donor substrate (such as a glycosyl nucleotide) to an acceptor substrate. The specificity of such reactions is high and good yields can be obtained. The presence of multiple functional groups and stereo isomers in complex oligosaccharides makes them difficult and time-consuming to synthesize by organic chemistry. Chemical methods for the preparation of peptides and oligonucleotides have been developed which are robust and automated. This has not been possible for the chemical preparation of complex oligosaccharides and glyco-conjugates. Thus, researchers have turned to enzymatic methods.
Enzymatic synthesis of oligosaccharides using glycosyltransferases of the Leloir pathway can overcome the problems encountered in the chemical synthesis of specific oligosaccharides. However, these enzymatic syntheses require sugar nucleotides and glycosyltransferases, both of which have not been readily available or, at least, not cost-effectively. Such synthetic methods will not be covered in detail in this chapter, but are well reviewed elsewhere (Prenosil et al., 1987a; Nilsson, 1988; Rastall and Bucke, 1992; Koizumi et al., 1998). Koizumi et al. (1998) discuss the large-scale production of uridine 5′-diphosphate (UDP)-galactose and globotriose. Globotriose is the trisaccharide portion of a verotoxin receptor and has the structure α-d-Gal (1→4)-β-d-Gal (1→4)-d-Glc.
The instability of glycosyltransferases as synthetic reagents has led some workers to develop immobilized glycosyltransferases for oligosaccharide synthesis (Rastall and Bucke, 1992). For specific synthesis of oligosaccharides of medical interest, a large number of different glycosyltransferases are required, and their availability is a limiting factor. As more glycosyltransferases are isolated from nature or formed by recombinant techniques, it has become increasingly possible to form the specific physico-chemical structures required.
The usual substrates for galactosyltransferases are UDP-galactose and either a free N-acetylglucosamine or an N-acetylglucosamine bound to a protein molecule. The complexity and cost of methods involving glycosyltransferases have precluded their use in food applications to date. However, several companies are interested in the production of longer chain, specifically tailored oligosaccharides for use in the functional food sector. The main application of synthesis systems using glycosyltransferases remains in the biomedical and pharmaceutical areas.
In 2007, the Sigma-Aldrich Company (USA) listed four glycosyltransferases (Table 5.3) in their catalogue, which were also available in different formulations and as kits with accompanying substrates and cofactors (Sigma-Aldrich, 2007). Other major biochemical supply houses carry some glycosyltransferase products. There are also a number of specialist glycoscience commercial groups worldwide, some of which are developing specific oligosaccharide pharmaceuticals, others providing specialist analytical services in the area and others offering custom manufacture and the supply of specific oligosaccharides (see Playne, 2002b for details).
Glycosidases: Glycosidases can transfer the glycosyl moiety of a substrate to hydroxyl acceptors. Hydrolysis represents merely a special case where water serves as the hydroxyl acceptor. Most glycosidases are able to catalyse either hydrolysis or transglycosidation, with the reaction outcome dependent on the relative abundance of the hydroxyl acceptors. Glycosidases are broadly classified as exo- and endo-glycosidases. The exo-glycosidases act on the linkage at the non-reducing end of the saccharide chain, whereas the endo-glycosidases act on the glycosidic linkages within the saccharide molecule. Glycosidases are less specific in their ability to catalyse certain cleavages than the glycosyltransferases and are available from a wide range of sources, e.g., viruses, microorganisms, plant and animal cells. They do not require expensive cofactors for synthesis reactions and generally are regarded as suitable for the synthesis of short-chain oligosaccharides. The use of glycosidases as synthetic enzymes dates back many years, but only since 1980 has their use become of major commercial importance.
Glycosidase reversal can be achieved by a kinetic approach or by an equilibrium approach. The kinetic approach recognizes that the hydrolysis of glycosidic bonds is a two-stage process involving a covalently linked sugar–enzyme intermediate. For oligosaccharide synthesis, the covalent bond is cleaved by a nucleophilic displacement reaction by an acceptor molecule. In this case, the normal acceptor molecule, water, is replaced by an alcohol or by a carbohydrate.
The equilibrium approach is based on the fact that, in principle, all enzymatically catalysed reactions are reversible. To synthesize oligosaccharides by this method requires, therefore, the use of very high concentrations of the sugar substrate to reduce the water activity. High temperatures are normally used (a) to speed up the otherwise rather slow reaction and (b) to increase the solubility of the sugar substrate so that a high concentration in solution can be achieved. The enzyme is protected from denaturation by the high temperatures, the stabilizing effects of the high sugar and low water concentrations (Johansson et al., 1989). The temperature tolerance of the enzyme also depends on its source. The manufacturing processes for food-grade oligosaccharide mixtures described in Section 5.4.1 use the equilibrium principle and the glycosidase, β-galactosidase.
The microbial β-galactosidases (β-d-galactoside galactohydrolase, EC 3.2.1.23) have been well studied, but it is often not appreciated that their catalytic action is best described as a transgalactosylation rather than a hydrolysis. The enzyme transfers the galactose moiety of a β-galactoside to an acceptor containing a hydroxyl group. If the acceptor is water, then galactose is formed, but if lactose is used as the acceptor molecule, then new oligosaccharides will be synthesized. The different microbial origins of β-galactosidases not only determine their temperature tolerance but also the characteristic mixture of di- to hexa-saccharides formed and whether a β1→4 or a β1→6 linkage is formed. The formation of oligosaccharides reaches a time-course maximum during a batch reaction. Continuation of the reaction will lead to the hydrolysis of the formed oligosaccharides to monosaccharides (Smart, 1993).
Allolactose (β-d-Gal-(1→6)-d-Glu) is always formed initially in the reaction to produce oligosaccharides from lactose (Prenosil et al., 1987a). Galactobiose (β-d-Gal-(1→6)-d-Gal) is also formed, but allolactose is the dominant initial product. A range of higher oligosaccharides is then formed, including galactotriose, [4-β-galactobiosylglucose, 6-β-galactobiosylglucose and 6-β-galactotriosylglucose (Asp et al., 1980; Rastall and Bucke (1993)]. The products of synthesis from lactose have been thoroughly reviewed by Prenosil et al. (1987a). Table 5.4 shows the numerous possible reaction products from lactose resulting from hydrolysis, internal rearrangement and transgalactosylation.
Methods to enhance the effectiveness and reduce the cost of processing lactose using β-galactosidase have been examined intensively in recent years. They include immobilization of the enzyme by encapsulation, by entrapment in fibre matrices, in gels or on semi-permeable membranes. A variety of adsorption and covalent attachment techniques have also been described.
5.4.1.2 Microorganisms Used to Produce β-Galactosidases (Lactase)
Historically, β-galactosidase has been an important industrial enzyme due to its many applications in the dairy industry. These include the hydrolysis of lactose to prevent symptoms of lactose intolerance, lactose crystallization problems in processed foods, in cheese manufacture, cheese whey waste reduction, improvement of whey protein concentrates and in ethanol production. Since about 1985, the use of lactases for the production of oligosaccharides has added a new dimension, which has required a reassessment of the properties of the range of lactases available in different microorganisms. A summary review of lactose hydrolysis processes using lactases has been compiled by Sienkiewicz and Riedel (1990), including the use of permeabilization agents, immobilization of enzymes and membrane systems.
β-Galactosidases are generally found only in microorganisms and in the mammalian intestinal tract. However, there are some exceptions, such as the lactase derived from the marine mollusc, Charonia lampas. The choice of enzyme to use for the production of lactose hydrolysis products and of galacto-oligosaccharides is critical to a commercial process for the reasons outlined earlier. The use of these enzymes commercially for many years in lactose hydrolysis has resulted in the ready commercial availability of a number of well-described sources. Dominant among these is the yeast lactase from Kluyveromyces spp. and the fungal lactase from Aspergillus spp. For the large-scale commercial production of galacto-oligosaccharides, other microbial sources are used also (e.g., B. circulans, S. thermophilus, C. laurentii and S. elviae). A list of microorganisms used to produce lactase is shown in Table 5.5. The enzymes differ in their temperature optimum and their optimum pH, as well as their preference in cleavage of β(1→3) or β(1→4) linkages. This information, where it is known, is included in Table 5.5.
5.4.1.3 Some Suppliers of β -Galactosidases (EC 3.2.1.23)
Examples of some suppliers of the enzyme are listed in Table 5.6. Generally, the fungal lactases function optimally at low pH and a relatively high temperature, whereas the yeast lactases perform best at neutral pH and a lower temperature. The yeast lactases respond to nutrient additions of magnesium and potassium ions.
5.4.1.4 Investigations of the Major Transgalactosylation Enzyme Sources
Kluyveromyces marxianus syn. K. fragilis and syn. Candida pseudotropicalis is an anamorph of Candida kefyr.
and Kluyveromyces lactis syn. K. marxiansus var. lactis. is an anamorph of Candida sphaerica (NCBI, 2007)
These two yeasts were described in earlier years as belonging to the genus Saccharomyces and also have a number of synonymous names. Saccharomyces is still used sometimes as the generic name for these species. The β-galactosidase in these species is intracellular, and production is inducible. Thus, enzyme activity increases rapidly in the presence of carbohydrate sources, such as lactose, galactose or lactobionic acid. The activity is growth-associated and displays optimal activity at neutral pH and a temperature of about 40°C. The regulation of enzyme activity in these yeasts is complex and responds to ionic concentrations of a range of elements, namely, K, Mn and Mg. The enzymes are inhibited by Na+ and Ca2+. Finkelman (1989) has described the process of strain selection of yeasts for maximal enzyme activity. Although these yeast lactases suffer from low acid and temperature tolerance, and operate at neutral pH, this industrial disadvantage is outweighed by the high yields, the ease of cultivation and the established safety record of the yeasts. Yeast lactases from Kluyveromyces remain the most popular source of the enzyme. There is considerable strain-to-strain variation in the activity of the β-galactosidase present in the strain, and much research has been conducted to select the best strains.
One of the first descriptions of the use of these enzymes for the production of galacto-oligosaccharides is that of Roberts and Pettinati (1957).
Recently, Rodriguez et al. (2006) expressed the intracellular β-galactosidase of k. lactis as an extracellular enzyme in Aspergillus niger.
5.4.1.4.1 Aspergillus oryzae and Aspergillus niger
β-Galactosidases from both of these fungal species have been used for the production of galacto-oligosaccharides, but the enzyme from A. oryzae has been the one mostly used in industry to produce galacto-oligosaccharides. Consequently, it has been studied extensively (Toba and Adachi, 1978; Toba et al., 1985; Prenosil et al., 1987b; Sienkiewicz and Riedel, 1990).
Enzymes from A. oryzae include glycosidases which may act as α-galactosidases or as β-galactosidases. They can also express α-mannosidase activity and can perform fructosidation as well as galactosidation reactions. Their β-galactosidase is extracellular, in contrast to the intracellular enzyme in yeasts.
The mould lactases of Aspergillus are more thermostable and acid stable than the Kluyveromyces lactases and are less exacting in their requirements for activators and stabilizers. They are produced by solid-substrate fermentation and, hence, the enzyme yields and activities are lower than those obtained with Kluyveromyces fermentations. In immobilized enzyme systems, these Aspergillus lactases show good stability. From an industrial viewpoint, their maximum operating temperature under long-term exposure was still considered too low for optimal reaction kinetics.
5.4.1.4.1.1 Bacillus circulans
Bacillus circulans is an aerobic, Gram-positive, spore-forming organism that has been investigated as a source of active enzymes for a number of biotechnological products. Nakanishi et al. (1983) and Mozaffar et al. (1984) first reported that β-galactosidase from B. circulans synthesizes oligosaccharides from lactose, but did not describe their structures. Subsequently, these have been described further by Sakai et al. (1992) and Usui et al. (1993) who reported that this β-galactosidase preferentially formed a β(1→4) linked galactosyl-disaccharide. Yanahira et al. (1995) described 11 oligosaccharides formed in the reaction, including 5 newly described oligosaccharides ranging in size up to an octasaccharide. They noted that although 95% of the trisaccharide initially formed was a 4′-galactosyl lactose, after time, trisaccharides with 3, 2 and 6 linkages occurred. This finding emphasizes the importance of the duration of the reaction on the final structural composition of the oligosaccharides formed. Griffiths and Muir (1978) examined the thermostability of the β-galactosidase of a thermophilic Bacillus and compared the properties of the enzyme in whole cells and in cells entrapped in polyacrylamide gel. They noted optimal activity of the enzyme at 65–75°C and at pH 6.2–6.6.
5.4.1.4.1.2 Streptococcus thermophilus
S. thermophilus is used widely in fermented dairy foods and is a GRAS organism. The use of non-GRAS status bacteria limits their commercial use in many countries. For example, the use of Escherichia coli strains is not permitted in the food industry in most countries. The β-galactosidase of S. thermophilus is a useful enzyme for commercial hydrolysis of lactose in milk and cheese whey (Greenberg and Mahoney, 1982). These authors pointed out that the thermal stability of the enzyme is greater in milk and whey than in buffer. The enzyme from this bacterium is more heat stable than that of K. marxianus (Linko et al., 1992) and it is produced intracellularly. The inhibitors and stimulants of the enzyme have been studied (Greenberg and Mahoney, 1982). The (1→3) glycosyl linkage is cleaved first by this enzyme, but later it also cleaves the (1→4) and (1→6) linkages.
The ability of the β-galactosidase of S. thermophilus to undergo transgalactosylation reactions was recognized by Toba et al. (1981) and by Greenberg and Mahoney (1983) and further studied by Smart and co-workers in more detail (Smart, 1989, 1991, 1993; Garman et al., 1996). Playne et al. (1993) have also described successful laboratory production of oligosaccharide mixtures from lactose using a crude enzyme extract from a strain of S. thermophilus. Oligosaccharide production was higher than that by commercial enzymes from Aspergillus and Kluyveromyces. The company, Yakult Honsha Co., Ltd., uses a β-galactosidase from S. thermophilus as well as an enzyme from A. oryzae in a two-stage process to produce their oligosaccharide product (Matsumoto et al., 1993).
Because of its long-standing importance in the manufacture of yoghurt and in other fermented dairy products, a considerable body of knowledge has been established for S. thermophilus. The complete genomic sequences for two strains have been determined (Bolotin et al., 2004). This genomic analysis allows a new understanding of its biochemistry and physiology.
5.4.1.4.1.3 Lactobacillus Species
Most species of Lactobacillus possess high β-galactosidase activity. Thus, there have been a number of investigations of enzymes from this source and their possible application for the production of β-galacto-oligosaccharides (Toba et al., 1981). Garman et al. (1996), in a study of a number of species of Lactobacillus and S. thermophilus, found that a strain of Lb. delbrueckii subsp. bulgaricus possessed a β-galactosidase with transgalactosylation activity similar to the enzyme from S. thermophilus. As an example of the use of lactobacilli, Kobayashi et al. (1990) patented a method for producing a processed milk containing galacto-oligosaccharide. In their patent, milk was treated with a β-galactosidase derived from S. thermophilus or Lb. delbrueckii subsp. bulgaricus so as to change at least 15% of the lactose in the milk into galacto-oligosaccharide. The Lactobacillus enzyme was found to be useful as it performed transgalactosylation reactions even when the lactose concentration was quite low. Other enzyme sources, such as that from the well-established A. oryzae, act largely hydrolytically when the lactose is in low concentrations as found in milk.
5.4.1.4.1.4 Bifidobacterium Species
Tzortzis et al. (2005a) have used whole cells of B. bifidum NCIMB 41171 to produce galacto-oligosaccharides from lactose. Optimum enzyme activity occurred at pH 6.8–7.0 and 40°C. A 50% (w/w) solution of lactose gave a 20% mixture of oligosaccharides. The mixture comprised 25% disaccharides (other than lactose), 35% trisaccharides, 25% tetrasaccharides and 15% pentasaccharides. These proportions seemed to be produced consistently by this organism. Current interest in bifidobacteria as a source of β-galactosidase may be generated by the ability of at least some strains of Bifidobacterium to produce higher proportions of longer chain oligosaccharides than most other lactases studied to date.
Van Laere et al. (2000) studied the ability of several strains of Bifidobacterium to metabolize a GOS mixture, which had been purified so that it contained 6% tri-, 17% tetra-, 37% penta-, 27% hexa- and 8.5% hepta-oligosaccharide. These proportions were different from most commercial GOS products which contain predominantly trisaccharide and only low concentrations of the higher oligosaccharides. This study found that Bifidobacterium adolescentis DSM 20083 could utilize the higher oligosaccharides better than other species. Thus, it may be that longer chain oligosaccharides are of value for the manufacture of particular symbiotic mixtures of prebiotic and probiotic, aimed to stimulate the growth of particular strains of bifidobacteria.
Rabiu et al. (2001) have shown, in a study of five strains of Bifidobacterium, that their β-galactosidases predominantly produced (1→6) GalP linkages as opposed to the (1→4) linkages generated by the β-galactosidases of many microbial species (see Table 5.4). These authors also noted the production of unusual higher chain oligosaccharides by these bifidobacterial enzymes. Tzortzis et al. (2005b) also recorded mostly Gal β(1→6) Gal linkages in their oligosaccharide mixture produced by B. bifidum NCIMB 41171.
5.4.1.4.2 Other Yeasts
5.4.1.4.2.1 Cryptococcus laurentii
The production of galactose transfer products by C. laurentii IFO 609 has been examined by Onishi and Yokozeki (1992). The enzyme of this species produces a 4′-galactosyl lactose from lactose. A yield of 47 g/L of 4′-galactosyl lactose was produced from 100 g/L of lactose in studies using C. laurentii OKN-4 conducted by the Nissin Sugar Manuf. Co. Ltd., Japan (Ozawa et al., 1991; Ohtsuka et al., 1992).
This yeast can be an opportunistic pathogen in immuno-compromised patients. It can cause superficial infections and is described as causing fungemia. It has been implicated in meningitis and is regarded as a human pathogen. Thus, its direct use as a food-processing aid is restricted.
5.4.1.4.2.2 Sporobolomyces singularis (syn. Bullera singularis)
It has long been recognized that this basidiomycetous yeast possesses a β-hexosidase able to behave with galactosidase-like activity (Gorin et al., 1964). The nature of this action has been investigated in detail by Ishikawa et al. (2005). It seems that the basidiomycetous yeasts commonly possess a β-glucosidase which performs like a β-galactosidase when presented with lactose as a substrate and is a strong producer of galacto-oligosaccharides. Dombou et al. (1994) described a method for the production of galacto-oligosaccharides from lactose using basidiomycetous yeasts. Preferred yeasts belonged to the genera of Rhodotorula, Pichia, Sporobolomyces, Kluyveromyces, Debaryomyces, Candida, Torulopsis, Cryptococcus, Trichosporon, Lipomyces and Brettanomyces. The inventors preferred an isolate of Lipomyces starkeyi for the description of the invention.
The purification and biochemical properties of a galacto-oligosaccharide-producing β-galactosidase from B. singularis has been described by Cho et al. (2003). Shin et al. (1995, 1998) examined optimal culture conditions for B. singularis and continuous production of galacto-oligosaccharides in a chitosan-immobilized system for the enzyme.
5.4.1.4.2.3 Sterigmatomyces elviae
The basidiomycete species Sterigmatomyces has been investigated for its potential as a source of a β-galactosidase for the production of galacto-oligosaccharides (Onishi and Tanaka, 1995; Onishi et al., 1995). The main transgalactosylation product was a 4′-galactosyl lactose. In comparison with a number of other bacteria and yeasts, the authors considered the enzyme from S. elviae CBS 8119 to be the best galacto-oligosaccharide producer. The other high-yielding enzymes were from the yeast species Rhodotorula minuta and Sirobasidium magnum. With the enzyme from the Sterigmatomyces sp. strain, the optimum reaction temperature was 80°C, but the yield was only 37%, which occurred when cells were permeabilized with toluene and resting cells used to produce the oligosaccharide. An improved fermentation system where cell growth consumed excess glucose resulted in an increased yield of 64%. Onishi and Tanaka (1995) purified the β-galactosidase from the Sterigmatomyces strain and examined its properties in more detail. They noted that the yeast would not grow above 40°C despite having an optimum temperature for the enzyme in the toluene-permeabilized resting cells of 80°C. They found that optimal pH for activity was between 4.5 and 5.0. The thermostable nature of the enzyme from a mesophile was found to be unusual as most thermostable enzymes were derived from thermophiles. The enzyme was similar to that found in C. laurentii except that it was far more thermo-tolerant, and for this reason was regarded as a superior enzyme.
5.4.1.4.2.4 Rhodotorula minuta
Onishi and Tanaka (1996) reported the properties of a glycosidase that can produce galacto-oligosaccharides from a strain of this yeast species. This basidomycete is found in the environment and in dairy products. It may colonize plants, humans and other mammals. While being considered as a common contaminant, Rhodotorula may infect individuals with predisposing risk factors. For this reason, it may not be approved by regulatory authorities for the production of a food-processing aid.
5.4.1.4.2.5 Geotrichum amycelium (syn. Trichosporon ovoides)
This is another basidiomycetous yeast claimed to be able to produce substantial yields of galacto-oligosaccharides from lactose. However, there is only limited published information on this enzyme activity of the species (Onishi et al., 1995).
5.4.1.4.2.6 Sirobasidium magnum
Research conducted at the laboratories of the Ajinomoto Co. Inc. in Japan has demonstrated the potential of this basidiomycetous yeast as a source of a β-galactosidase to produce galacto-oligosaccharide (Onishi et al., 1996) A 4′-galactosyl lactose was produced with a yield of over 200 g/L at up to 50°C using toluene-treated cells to improve cell wall permeability and using glucose oxidase to remove glucose formed as a hydrolysis product. In the following year, the group purified the β-galactosidase and found that the optimum conditions for enzyme activity were 65°C and pH 4.5–5.5 (Onishi and Tanaka, 1997).
A patent for the production of galacto-oligosaccharides was taken out by Onishi and Yokozeki (1992); it included Sirobasidium with a number of other species.
5.4.1.4.3 The Extremophiles
Microorganisms isolated from environmentally extreme conditions are of potential use for the industrial production of galacto-oligosaccharides. A number of these have been studied with this objective. It would be a major advantage if enzymatic conversions to oligosaccharides by the equilibrium route could be performed at a very high temperature under acidic conditions. A number of thermophiles have been examined for suitable β-galactosidases, including strains of Thermus, Thermoaerobacter, Sulfolobus and Thermotoga spp. Examples are given below.
5.4.1.4.3.1 Sulfolobus solfataricus
Sulfolobales are hyperthermophilic archaea from terrestrial volcanic sites that grow in sulfur-rich hot acid springs, with optimum growth at 75–80°C and pH 2–3. S. solfataricus grows optimally at temperatures ranging from 70 to 90°C and at pH 2–4. It can grow either lithoautotrophically by oxidizing sulphur or chemoheterotrophically on reduced carbon compounds. Pisani et al. (1990) studied the properties of a β-galactosidase in the species and found it to be thermostable. Grogan (1991) examined in more detail the properties of the β-glycosidase present and found that the same enzyme exhibited both β-galactosidase and β-glucosidase activity. He also noted the optimal reaction temperature to be 77–87°C and the optimal pH for β-galactosidase activity to be pH 4.9. Other thermophilic microorganisms such as Thermotoga maritima can also produce galacto-oligosaccharides by a thermostable recombinant β-galactosidase (She et al., 2001).
5.4.1.4.3.2 Thermotoga maritima
Thermotoga maritima, a rod-shaped bacterium belonging to the order Thermotogales, was originally isolated from a geothermal marine sediment. The organism has an optimum growth temperature of 80°C. The species metabolizes many simple and complex carbohydrates.
Ji et al. (2005) prepared a recombinant β-galactosidase from a strain of T. maritima in E. coli. They determined the stability and productivity of this enzyme at a range of pH and up to 95°C. Optimal conditions were pH 6 at 80°C in the presence of manganese ions. Lactose at 500 g/L yielded 91 g/L galacto-oligosaccharides in 300 min with 1.5 units enzyme/ml.
5.4.1.4.3.3 Thermus Species
The production of galacto-oligosaccharides has also been explored in strains of Thermus species. Recombinant production of a thermostable β-glucosidase, expressed in E. coli K 12, has been used to investigate the production of galacto-oligosaccharides from lactose. The yield of galacto-oligosaccharides from 300 g/L lactose at 70°C was 40% and trisaccharides comprised two-thirds of the products formed (Akiyama et al., 2001). Choi et al. (2004a) have also developed a similar method with the production of a recombinant enzyme from a β-glycosidase in Thermus caldophilus.
5.4.1.4.4 Thermoactinomycetes
5.4.1.4.4.1 Saccharopolyspora rectivirgula
Some thermoactinomycetes have been shown to produce β-galactosidases which have high β-d-galactosyltransferase activity, high heat stability and which can act in the neutral pH range. Some strains from the genera Saccharopolyspora, Thermomonospora and Thermoactinomyces have been found to possess effective enzymes. In particular, a strain of S. rectivirgula has been studied (Nakayama et al., 1992, 1993; Nakao et al., 1994). Nakayama et al. (1993) pointed out that other β-galactosidases derived from species such as Paecilomyces variori also possess high heat stability and are capable of repeated use at high temperatures, but that the Paecilomyces enzyme is not suitable for many applications because it is effective only at low pH (3.5). The most advantageous property of the Saccharopolyspora enzyme is its heat stability over long periods. Nakao et al. (1994) reported a 41% yield of galacto-oligosaccharides from 1.75 M lactose.
Saccharopolyspora rectivirgula was previously named Faenia rectivirgula. Species of Saccharopolyspora are implicated as causal agents of infection for a condition known as farmer’s lung disease. In nature, the species is found in mouldy hay. It can also cause allergic reactions in humans.
5.4.1.5 Development of Modified Galacto-oligosaccharide Structures
There is considerable scope for the development of novel galacto-oligosaccharides with specific functionalities for human health. Oligosaccharides have been developed to act as alternative receptors to absorb lectin-like toxins from toxigenic bacteria (VTEC, ETEC, Clostridium difficile) in the gut. Zopf and Roth (1996) have discussed the use of oligosaccharides as anti-infective agents, using a decoy oligosaccharide in the mucous layer to bind the pathogen’s carbohydrate-binding proteins. They claim that such oligosaccharides (as found in human milk) seem to prevent pathogens attaching to intestinal mucosa. Kunz and Radloff (1996) state that “Human oligosaccharides are considered to be soluble receptor analogues of epithelial cell surfaces, participating in the non-immunological defence system of human milk-fed infants”. Their view is that these specialized oligosaccharides are potent inhibitors of bacterial adhesion to epithelial cells. Thus, there is scope for specifically designed galacto-oligosaccharides to be able to act as anti-infective reagents, particularly in the small intestine. However, care needs to be taken to ensure that the oligosaccharide did not also interfere with colonization in the large intestine by the normal intestinal microbiota, including any added probiotics.
Thus, there are opportunities for the synthesis of galacto-oligosaccharides which more closely mimic the structures found in human milk. Human milk contains 5–12 g/L oligosaccharides, and colostrum has even higher concentrations, but cow’s milk contains very little (0.03–0.06 g/L) and most of that is as sialyl lactose. The monomers of human milk oligosaccharides are d-glucose, d-galactose, N-acetylglucosamine, l-fucose and sialic acid (N-acetyl neuraminic acid). The core oligosaccharide molecule normally carries lactose at its reducing end and generally has the following structure:
Both fucose and sialic acid can attach in a number of different ways to this core. Predominantly, fucose attaches with an α1→2 linkage to galactose, but with α1→3/4 linkages to N-acetylglucosamine. Readers are referred to Kunz and Rudloff (2002) and Boehm and Stahl (2003) for more details on possible oligosaccharide structures. The major oligosaccharides in human milk are lacto-N-tetraose and lacto-N-fucopentaose (Kunz and Radloff, 1996) (see also Chapter 8).
There is scope for manufacturers to develop cost-effective procedures to produce a range of fuco-galacto-oligosaccharides. At present, procedures are multi-step processes and are expensive (Crout and Vic, 1998). Of relevance to this chapter is the possibility of economic manufacture of fucose-containing and N-acetylglucosamine-containing galacto-oligosaccharides.
The use of recombinant DNA technology to modify the metabolic pathways of microorganisms for oligosaccharide synthesis is complex, and hence expensive. It also involves the use of sugar nucleotides, such as UDP, as cofactors in the enzyme reactions. Use of whole microbial cells reduces costs, and concentrations of the synthesized oligosaccharide obtained are increasing (Ruffing and Chen, 2006). For example, Koizumi et al. (1998) achieved a yield of 188 g/L of the galacto-oligosaccharide, globotriose (Galα1→4Galβ1→4Glc) and no oligosaccharide by-products were observed. The synthesis simultaneously produced high yields of UDP-galactose from galactose and orotic acid.
Crout and Vic (1998) favoured the use of glycosidases over glycosyltransferases because they are better suited to cheap synthesis methods. They also point out that exo-glycosidases currently used commercially only allow glycosyl transfer at the non-reducing terminal monomer of the substrate, and this restricts the types of oligosaccharide structures that can be produced. They advocate the use of endo-glycosidases which may allow branched structures to be formed. To improve the reverse hydrolysis required for this type of synthesis, organic solvents have been used in a number of studies (Crout and Vic, 1998). The use of glycosidases with novel approaches may lead to synthesis of useful new oligosaccharides.
For the synthesis of a fucose-containing galacto-oligosaccharide, it will first be necessary to produce the l-fucose required. l-fucose occurs in nature only at low concentrations and is present in plant species of the Convolvulaceae family. On the other hand, d-fucose is found in some seaweeds or can be produced from d-galactose. Fucose is a hexose deoxy sugar (Figure 5.3) and can be formed using an α-fucosidase. While one can envisage synthesis of d-fucose from d-galactose, it is difficult to see how l-fucose could be formed easily.
Economic sources of N-acetylglucosamine may be chitin in the shells of crustaceans. β-1,4-Linked N-acetyl-d-glucosamine can be derived readily from chitosan (Izume et al., 1992) and it may be feasible to link it enzymatically to galacto-oligosaccharides during their synthesis. There is much scope to investigate such methods in order to produce a new generation of modified galacto-oligosaccharide products. Chemical structures of l- and d-fucose, d-glucose, d-galactose, N-acetylglucosamine, N-acetyl neuraminic acid are shown in Figure 5.3.
5.4.2 Lactulose
The disaccharide, lactulose (Figure 5.4), is made from lactose by a semi-synthetic isomerization reaction under alkaline conditions. Lactulose can also be formed in small quantities in milk which has been heat-treated, e.g., UHT milk. Lactulose is used primarily as a pharmaceutical, but also as a functional food ingredient. The Solvay Company started manufacturing lactulose in Europe in the 1960s and produces about 50% of world production (see Table 5.8). A considerable body of research has evolved over the last 40 years to improve yields and the purity and safety of the product, and reduce costs. The essential principle of the synthesis has remained the same – alkaline isomerization using heated solutions. These isomerization reactions to convert lactose to lactulose all produce a number of side products derived from lactose, principally galactose, epi-lactose, tagatose and fructose. Epi-lactose is another isomer of lactose (4-O-β-d-galactopyranosyl-d-mannose).
Lactulose was first prepared by a Lobry deBruyn–Alberda van Ekenstein alkaline isomerization of lactose in dilute Ca(OH)2 solution (Montgomery and Hudson, 1930). Strong alkalis, such as NaOH, and strong organic bases have also been used in the isomerization reaction. All these methods resulted in low yields of lactulose because of side reactions which lead to the formation of a number of unwanted products. Separation and purification methods were required to remove the unwanted products and the brownish colour occurring due to these side products.
Subsequent methods for producing lactulose included complexing reagents, such as aluminate (Guth and Tumerman, 1970) and borate ions (Mendicino, 1960; Carubelli, 1966; Kozempel and Kurantz, 1994; Carobbi et al., 2001), to shift the transient equilibrium established during base-catalysed isomerization in favour of the ketose. The yield of lactulose was much improved, but both aluminates and borates were difficult to remove from the reaction mixture. A very high ratio of borate to sugar (e.g., 50:1) was required for optimum yields of lactulose. Under such conditions, removal of the borate was very difficult, and the cost of borate proved prohibitive. This meant that methods to recycle the borate had to be devised.
Considerable research has been conducted to improve the alkaline isomerization process. Efforts have concentrated on reducing the costs of the complexing agent, borate (Hicks et al., 1986). For example, Dorscheid and Krumbholz (1991) used a combination of electrodialysis and ion exchange to purify the lactulose and recycle the borate. Other methods have been devised to remove borate by precipitation as insoluble salts (Fu and Song, 1993). The yield of lactulose has often been quite low (<20%).
Amines have been used as catalysts for the isomerization process. However, only the tertiary amine, triethylamine, avoided the problems of the primary and secondary amines, which formed adducts with reducing sugars (Parrish, 1970)
Later, the combination of the isomerization of lactose in the presence of borate (at a molar ratio of 1:1) with a tertiary amine (Hicks and Parrish, 1980; Hicks, 1981) gave 90% yield of lactulose with a minimal use of borate and reduced the formation of side products.
Other reagents such as basic magnesium salts and sodium hydrosulphite have also been used (Carobbi et al., 1985). Whilst offering some advantages, these compounds also created new problems of purification and of disposal of spent materials in an environmentally friendly way.
Attempts have been made to develop continuous reaction systems, as opposed to batch reactors. One example is the dual reactor tank system coupled to a tubular reactor described by Kozempel and Kurantz (1994). The yield of lactulose from lactose was 70% in this system.
Developing commercially viable processes has proved difficult, but the use of borate appears to be the preferred approach, mainly because high yields are obtained. Effective separation of lactulose from the product mix has proved very difficult to achieve economically. Most pharmaceutical-grade lactulose syrups contain substantial concentrations of lactose, epi-lactose and galactose.
Research has been conducted to develop better methods to obtain lactulose in powder or crystalline forms. As lactulose is highly soluble in water, it is difficult to obtain in the form of a stable powder and it is generally produced as a syrup. Nevertheless, complicated processes have been developed to prepare powdered or crystallized products. Crystallization methods using alcohols, such as ethanol and methanol, have been developed to obtain lactulose crystals. Highly pure lactulose crystals can be obtained, but contamination with alcohol remains a problem. Another problem is the presence of lactose, galactose and other side products which have to be removed also, and this adds to the cost. Alternative methods that avoid the use of alcohols have been developed. These are based on seeding crystals into the concentrated syrup and then freezing the mixture. The Morinaga Company has developed a process to form crystalline lactulose trihydrate. This product, it is claimed, is purer and more stable under atmospheric storage conditions than anhydrous lactulose or the monohydrate. The process is based on a crystallization precipitation procedure (Tomito et al., 1994).
Lactulose can also be prepared by an enzymatic route (Lee et al., 2004), but this route is not used currently for commercial production.
5.4.3 Lactosucrose (β-d-Gal-(1→4)-α-d-Glu-(1→2)-β-d-Fru)
Lactosucrose is a trisaccharide produced enzymatically by transfer of the fructosyl moiety of sucrose to lactose as an acceptor molecule. The structure is shown in Figure 5.4. The enzyme used is a β-fructofuranosidase (EC 3.2.1.26). It is known to conduct the transfructosylation reaction, as well as hydrolysis, in a manner analogous to that described for β-galactosidase. Similarly, the microbial origin of the fructofuranosidase is important. Enzymes from different sources exhibit different degrees of acceptor types and specificity. Some may prefer lactose as an acceptor, others sucrose. Thus, research has been conducted into the characteristics of the enzyme from a number of microorganisms (Takaichi et al., 1995; Choi et al., 2004b; Park et al., 2005). The reaction conditions, such as substrate concentration and reaction time, are also important (Fujita et al., 1992a). The β-fructofuranosidase is sometimes called “levansucrase”. Microorganisms belonging to the genera Arthrobacter and Bacillus are mostly used both in research and industrially to produce β-fructofuranosidase (Fujita et al., 1992b; Hara et al., 1992; Kawase et al., 2001; Pilgrim et al., 2001). A variety of methods have been used in production – some in which whole microbial cells are used, others have used crude cell extracts and still others have used purified enzyme. Fujita et al. (1992a) describe an industrial method for producing lactosucrose using β-fructofuranosidase from Arthrobacter sp. strain K1, which seems to be the one used by Ensuiko Sugar Refining Co., Ltd. and by the Hayashibara Company. These two Japanese-based companies are the main producers of commercial lactosucrose. The yield of lactosucrose from the two substrates (sucrose and lactose) is low (5–30% of total sugars in the reactants). By careful selection of strains and the use of optimal conditions, batch reactions have been able to produce 181 g lactosucrose from 225 g sucrose and 225 g lactose (40% conversion), but the concentration of lactosucrose in the reactor has only been about 18% (see Park et al., 2005). Those authors used a strain of B. subtilis at pH 6 and 55°C for a 10 h reaction. This result is typical of the published data for batch reactions. Kawase et al. (2001), who described the production of lactosucrose using a simulated moving bed reactor, claim that this continuous process increases the yield to 56% compared to 48% typical of a batch fermentation.
There is a major problem with the presence of other carbohydrate products and residual amounts of unused lactose and sucrose in the process. Thus, it is typical for crude product to be decolourized, demineralized and purified by column chromatography using a strongly acidic cation-exchange resin. A fermentation method to remove monosaccharides, like glucose, has also been used. The lactosucrose is spray dried (Hara et al., 1992, 1994a). The aim is to obtain a purified solution of lactosucrose of 45%, w/v, prior to spray drying. The two major producers sell lactosucrose as a powder with different degrees of purity (40, 55 or about 70% lactosucrose in the powder). Thus, a considerable concentration of other carbohydrates remains in the product.
In a series of publications, Petzelbauer et al. (1999, 2000, 2002a,b) used thermostable purified enzymes from S. solfataricus and Pyrococcus furiosus to produce lactosucrose in a continuous stirred-tank reactor at 70°C, coupled to a cross-flow ultrafiltration module. They also used an immobilized enzyme system. Their data do not show that they were able to achieve the yields obtained in commercial systems. However, they demonstrated the stability of these thermostable enzymes at high temperatures for a prolonged period under realistic bioprocessing conditions.
The properties of the β-fructofuranosidase extracted from Arthrobacter sp. K1 have been described by Fujita et al. (1990a). The enzyme has an isoelectric point of 4.3, an optimum pH of 6.5–6.8, but the enzyme remains stable between pH 5.5 and 10.00. It has an optimum temperature of 55°C, but is stable between 45 and 60°C. It is inhibited by several heavy metals. Fujita et al. (1990b) also describe the acceptor specificity for a wide range of mono- and oligo-saccharides and glycosides.
5.4.4 Lactitol
Lactitol (Figure 5.5) is well established as a replacement sweetener for low-calorie foods. In recent years, interest in it as a prebiotic carbohydrate has developed. This sugar alcohol was discovered in 1912 and was first used in foods in the 1980s. It is formed when lactose is hydrogenated in the presence of Raney-nickel catalyst. The conversion of a sugar to a sugar alcohol always involves the reduction of a carbonyl group. The preferred reducing agent is hydrogen gas under high pressure (e.g., 40 bar) at 100°C in the presence of a nickel catalyst. This synthesis has been used widely at both laboratory and industrial scale for many years. Ipatiew (1912) first produced a lactitol syrup by such a process, but lactitol was first crystallized by Senderens (1920). Lactitol is produced either as a syrup, as dihydrate or monohydrate crystals or in anhydrous form. Van Velthuijsen (1979) has described a typical modern industrial process and specifications of the product obtained in such processes. Less than 2.5% other polyols and 0.1% reducing sugars are present in the food-grade lactitol products which are 97.5% lactitol. Thus, this compound is sold as a much purer product for food-grade use than galacto-oligosaccharides, lactosucrose and lactulose. The food grades of these other products all contain substantial amounts of other reactant and product carbohydrates. Lactitol can also be prepared by reducing lactose using NaBH4 (Scholnick et al., 1975; Saijonmaa et al., 1978), but industrially, lactitol is prepared by the catalytic hydrogenation process (Van Velthuijsen, 1979). The reaction is carried out in an autoclave at over 40 bar and over 100°C. A lactose solution of 30–40% is used. The ratio of Raney-nickel catalyst to lactose is critical for efficient conversion. After hydrogenation is completed, the catalyst is sedimented and filtered. The lactitol solution is treated with an ion-exchange resin and activated carbon, and the purified solution is concentrated. Crystals of lactitol are removed from the mother liquor by centrifugation and the process repeated. After repeated crystallization, the mother liquor can be used as a 64% syrup of lactitol (Van Velthuijsen, 1979; Booy, 1987).
Preparation of derivatives of lactitol has also been studied. Polyalcohol esters can be used as non-ionic emulsifying agents. For example, sorbitan esters and sucrose esters of fatty acids are well known. Van Velthuijsen (1979) described the preparation of esters like lactitol palmitate and their properties and possible applications as laundry detergents.
Research has also demonstrated the formation of a range of oligosaccharides from lactitol using β-galactosidase (Yanahira et al., 1992); they were able to form six different oligosaccharides – all as a trisaccharide containing a lactitol unit. The general chemistry and properties of the sugar alcohols are described by Benson (1978). Comprehensive reviews on lactitol are by Van Velthuijsen (1979), Booy (1987) and Mesters and Brokx (2000).
5.4.5 Lactobionic Acid
Lactobionic acid is formed by oxidation of lactose and is 4-O-d-galactopyranosyl-d-gluconic acid (Figure 5.5). Heterogeneous catalytic oxidation and microbiological/enzymatic oxidation of lactose have been researched. The facile dehydrogenation of lactose at high pH over a noble metal catalyst is used commercially (Figure 5.1).
The aldehyde group of the glucose in the lactose molecule is oxidized to the carboxyl group by either (a) chemical oxidation or (b) biochemical oxidation. Electrolytic methods of oxidation are also possible. For chemical oxidation, a mild treatment with hypobromite or hypoiodite produces an equilibrium mixture of lactobionic acid and its δ-lactone. Biochemical oxidation can be achieved with enzymes isolated from microorganisms or by using the microorganisms themselves for the bioconversion. For the latter process, a number of species of Pseudomonas have been studied extensively (Sienkiewicz and Riedel, 1990). Some examples of recent research are given below.
An ultrafiltration membrane bioreactor was used in batch, fed-batch or continuous modes to oxidize aldose sugars to their corresponding aldonic acids. The enzyme used for the reaction was glucose-fructose oxidase obtained from a Zymomonas mobilis strain. The enzyme was selective and a high yield of lactobionic acid was obtained from lactose (Satory et al., 1997).
A novel enzymatic process to convert lactose to lactobionic acid has been developed using cellobiose dehydrogenase. The electron acceptor in the reaction is regenerated in a continuous process with laccase, a copper-containing oxidase. Specific productivity to lactobionic acid was high at 25 g/L/h/kU (Ludwig et al., 2004).
Miyamoto et al. (2000) reported the laboratory-scale production of lactobionic acid from cheese whey and from lactose in fed-batch cultures of Pseudomonas sp. LS13-1. The yield of lactobionic acid was reported to exceed 80%. The reaction time was 155 h, and concentrations achieved reached 290 g/L lactobionic acid.
5.4.6 Tagatose
Galactose, produced by hydrolysis of lactose and removal of the glucose co-product, is used as the substrate to produce tagatose (Figures 5.1 and 5.6). The galactose is isomerized under alkaline conditions, using, for example, Ca(OH)2, to form tagatose. The mixture is purified and solid tagatose is produced by crystallization. Considerations for the efficient production of tagatose parallel those described in Section 5.4.2 for the production of lactulose from lactose. This was the original commercial method used by Spherix Inc. and there are patents that describe it in more detail (Beadle et al., 1991, 1992).
More recently, enzymatic methods have been developed (Bertelsen et al., 2006), including the use of thermostable isomerases (Pyun et al., 2005; Hansen et al., 2006). An enzymatic method is currently used by the present producer of food-grade tagatose, NUTRILAB, in Belgium and the process has been described by Bertelsen et al. (2006). A 40% solution of lactose is passed through an enzyme bioreactor at an elevated temperature. A thermostable β-galactosidase (lactase) is used for hydrolysis. The stream is then treated chromatographically to remove glucose and lactose, with the lactose being recycled. The eluate containing mainly galactose is passed through a second enzyme bioreactor. In this case, a thermostable l-arabinose isomerase is used to convert the galactose to d-tagatose. The eluate typically contains about 15% tagatose, 35% galactose and 50% glucose. It is again treated chromatographically to remove glucose and galactose, and the galactose stream is recycled. The eluate contains the tagatose product. Various configurations of this process have been tested, including the incorporation of both enzyme systems in one bioreactor. The thermostable enzymes are obtained from a number of thermophilic microorganisms, such as Bacillus, Sulfolobus, Thermoanaerobacter, Thermus and Pyrococcus. Kim and his colleagues in South Korea have published a suite of papers on improvements to the enzymatic process for the production of tagatose, and this research has been summarized by Kim (2004).
5.5 Commercial Producers and Products
5.5.1 Estimated Production of the Lactose Derivatives
There have been a number of reviews in which the manufacturing processes for oligosaccharides, major commercial oligosaccharide manufacturers and the tonnage of the different oligosaccharide products produced have been summarized (Matsumoto et al., 1993; Playne and Crittenden, 1996; Crittenden and Playne, 1996). Estimates made in 1994/1995 of the annual global production of different oligosaccharides and allied products were galacto-oligosaccharides 12,000–14,000 t; lactulose 20,000 t; lactosucrose 1,600 t (Playne and Crittenden, 1996). Estimates from a survey conducted in 2004 were that annual global tonnages were galacto-oligosaccharides12,000–14,000 t; lactulose 40,000 t; lactosucrose 3,000 t; lactitol 10,000 t; lactobionic acid no data; and tagatose 500 t (3A Business Consulting, 2005). The same authors predicted that world production in 2009 would be galacto-oligosaccharides 21,000 t; lactulose 45,000 t; lactitol 11,000 t; and tagatose 800 t. Thus, annual growth rates around 10% or more were predicted for galacto-oligosaccharides and for tagatose, with slower growth rates for lactulose and lactitol. However, a higher estimate for lactulose production of over 50,000 t annually has also been published (LFRA, 2000).
In-house production of galacto-oligosaccharides in a number of countries makes it difficult to obtain precise data on world production. Such production is used directly for other products such as animal feed and infant milk foods. We estimate that total world production of all the above lactose-derived products in 2009 will be 83,000–90,000 t. In addition, there will be substantial production of lactose hydrolysate and galactose.
5.5.2 β -Galacto-oligosaccharides
Galacto-oligosaccharides (GOSs) have been manufactured commercially since the mid-1980s, principally by three Japanese companies – Yakult Honsha Co. Ltd., Snow Brand Milk Products and Nissin Sugar Manufacturing Co. Ltd. Commercial production in Europe commenced in 1995 in the Netherlands. In-house production has also occurred elsewhere, including in Australia and New Zealand.
The early establishment of a gut microflora in babies dominated by bifidobacteria, particularly in breast-fed babies, has been attributed to the presence of oligosaccharides in human milk. Although highly diverse, these human milk oligosaccharides have a backbone that is structurally similar to GOS. Hence, the inclusion of GOS as bifidogenic factors in infant products has been an important driver of the commercial production of GOS. The companies principally involved in the manufacture and marketing of GOS are Yakult Honsha Co Ltd., Nissin Sugar Manufacturing Co. Ltd. and Friesland Foods Domo (formerly Borculo Domo Ingredients) (Table 5.7). Yakult produces three galacto-oligosaccharide products: Oligomate 55 (syrup), Oligomate 55P (powder) and TOS-100 (a purified powder containing 99% oligosaccharides). Nissin also produces a syrup (Cup-Oligo H70) and a powder (Cup-Oligo P), while Friesland Foods Domo produces a syrup, Vivinal GOS. On the other hand, Snow Brand has now sub-contracted production and includes galacto-oligosaccharides in infant milk formula powders (e.g., P7 powder containing 1.2% oligosaccharide). With the exception of the purified powder produced by Yakult, the other commercial food-grade galacto-oligosaccharides all contain 40–70% of tri- and tetra-saccharides.
5.5.3 Lactulose
The two major manufacturers of lactulose are Solvay Pharmaceuticals and Morinaga Milk Industry. The Solvay production seems to be geared towards pharmaceutical applications, while Morinaga’s production emphasizes uses in the food and feed markets. The other manufacturers of note are shown in Table 5.8. Commercially, lactulose is available in either dried form (powder, crystals or granulated) or as a syrup of 50–72%, w/v, lactulose.
5.5.3.1 Purity of Lactulose
Just as galacto-oligosaccharides are normally sold as impure mixtures, this is also the case with lactulose, in which substantial amounts of galactose and lactose are usually present in the commercial products. Examples are given for some Solvay products. The Solvay syrup product which contains 667 g lactulose/L contains <59 g lactose, <112 g galactose, <45 g epi-lactose, <14 g tagatose and <7 g fructose. The crystalline pharmaceutical product contains >95% lactulose, <2% lactose, <2.5% galactose, <3% tagatose and <1.5% epi-lactose [Solvay, personal communication June 1995]. In a more recent analysis, their Canadian factory reported that relative to lactulose, the Solvay 667 g/L syrup contains <3% tagatose, <1% fructose, <15% galactose, <7% epi-lactose, <9% lactose [Canlac certif. analysis, Sept. 2003].
5.5.4 Lactosucrose
The manufacture of lactosucrose appears to be restricted to the two companies listed in Table 5.9. However, a number of other Japanese companies distribute the product (e.g., Maruha Corporation) and develop new applications (e.g., Otsuka Pharmaceutical Co. Ltd., Tokyo). Lactosucrose production was developed in Japan as a cooperative venture between Ensuiko, Hayashibara and Bifermin Pharmaceutical Co. Ltd.
5.5.5 Lactitol
The two major world manufacturers are PURAC Biochem and Danisco. Some 11,000 t are produced annually, and the growth rate is around 5% per annum. The price per kg is approximately US$2.00–2.50. Thus, it is a much cheaper product than food-grade GOS and lactosucrose, but similar to prices for lactulose syrup.
The Hayashibara Company of Okayama, Japan, took out early patents on lactitol production. Manufacturers are listed in Table 5.10.
5.5.6 Lactobionic Acid
Both Solvay, Germany, and Friesland Foods Domo, Netherlands, are major producers of the relatively small tonnage of lactobionic acid produced annually (see Tables 5.7 and 5.8 for the addresses of these companies).
5.5.7 Tagatose
The development of tagatose as a commercial product has followed a tortuous path, since its discovery. Licensing disagreements have dominated its development. The original inventor was Dr Gilbert Levin who formed BioSpherix Inc. in 1967. Later, the name was changed to Spherix Inc. In 1988, a patent was taken out on the use of d-tagatose as a sweetener and the product developed in-house until 1996. In 1996, a license was granted to the Danish firm, MD Foods, to develop the food uses for tagatose, while Spherix retained the rights to non-food uses. MD Foods later took over the Swedish company, Arla Foods, and used its name. A period of slow development occurred as Arla gathered evidence and sought listing of d-tagatose as a GRAS ingredient. This was granted by the US Food and Drug Administration in 2001. As no product was on the market by 2002, the agreement between Spherix and Arla Foods was re-negotiated with new royalty agreements. Meanwhile in 2002, Arla Foods had commenced a joint venture with the German sugar producer, Nordzucker, to manufacture tagatose at a plant near Hannover, Germany. The joint venture company was SweetGredients GmbH & Co. KG. Commercial production of tagatose began in 2003 and the product was marketed as Gaio®tagatose. After an active period of global marketing of the product, SweetGredients decided to put tagatose production on hold in March 2006 as they had not been able to create a large enough market for the sweetener and manufacturing was closed down in Germany. Later in 2006, the Belgian Company NUTRILAB NV took over the stocks and project from Arla Foods and began to set up a new manufacturing site with a new enzymatic process for manufacture. The new product brand name is NUTRILATOSE. The process is being scaled-up to produce up to 10,000t annually. The plant is planned for completion by late 2009. The original tagatose patents assigned to Spherix Inc. expired in 2006 in the USA and 2007 in Europe.
Global annual production capacity is currently estimated to be about 500 t, but this is expected to increase markedly after 2009. The market may grow quite quickly for this product in the health foods area for low GI foods. Manufacturers are listed in Table 5.11.
5.6 Analytical Methods
The choice of analytical methods for complex carbohydrates will depend on the required outcomes. For example, it may suffice to use a simple HPLC procedure with water as solvent and a standard ion-exchange column, such as the BioRad HPX87C (calcium form). This will lead to adequate separations of monomer sugars and of disaccharides and the 3–6 unit oligosaccharides (Crittenden and Playne, 2002). However, usually it will not differentiate isomers. In many cases, this may not be necessary. More sophisticated highly alkaline systems with pulsed amperometric detection (PAD) will give improved separations. More complex approaches are necessary if full structural analysis is necessary. Some useful references to the analysis of monomer sugars and oligosaccharides are Townsend et al. (1988), Hardy and Townsend (1988), Lee (1990), Reddy and Bush (1991) and Van Riel and Olieman (1991). A standard method (method 2001.02) for analysis of galacto-oligosaccharides in foods has been developed (AOAC, 2006). This method is based on high-performance anion-exchange chromatography (HPAEC) using a sodium acetate gradient under alkaline conditions. The eluate is monitored using pulsed amperometric detection (PAD) with a gold electrode. A commonly used apparatus is a Bio-LC system (Dionex Corporation, Sunnyvale, California) (for example, see Hardy and Townsend, 1988; Quemener et al., 1994; van Laere et al., 2000; De Slegte et al., 2002).
Prior to the widespread adoption of HPLC methods, oligosaccharides were commonly analysed by gas chromatography of derivatized monomer sugars. Samples were methanolized (methanolic 0.5 M HCl, 80°C, 24 h), N-acetylated and trimethylsilyated. The glycosides so formed were separated by gas chromatography (e.g., Lemoine et al., 1997). Other methods for preparing methylated derivatives have been described (Rabiu et al., 2001). Thin layer chromatography has also been used widely for qualitative and semi-quantitative analyses of mono- and oligo-saccharides (e.g., Rabiu et al., 2001). NMR spectroscopy has been used for analysis of carbohydrates where structural elucidation of complex products is needed (see Thelwall, 1997). Yanahira et al. (1995) examined the composition and structure of galactosaccharides using methylation analysis, mass spectrometry and NMR spectroscopy. Lemoine et al. (1997) carried out extensive studies using NMR spectroscopy to examine the structures of extracellular polysaccharides of S. thermophilus. Boehm and Stahl (2003) have provided an excellent overview of analytical methods suitable for glyco-conjugates present in milk. They review methods for chromatography, mass spectrometry, spectroscopy, electrophoresis and separation techniques, such as crystallization and filtration.
Traditionally, dietary fibre was considered to comprise largely of plant cell walls, namely cellulose and lignin (Van Soest, 1978). Southgate et al. (1978) defined dietary fibre as “the sum of lignin and the polysaccharides that are not digested by the endogenous secretions of the human digestive tract”. This physiologically based definition includes both water-soluble and -insoluble constituents. Thus, fructo- and galacto-oligosaccharides and possibly other prebiotic carbohydrates would be considered as “dietary fibre”. Appropriate methods of analysis of the water-soluble components have long been a challenge for analysts. Thebaudin et al. (1997) discussed the heterogeneous composition of this fraction and methods of analysis in more detail. The 1998 Codex guidelines on food labelling defined dietary fibre as “edible plant or animal material not hydrolysed by the endogenous enzymes of the human digestive tract” (Codex Alimentarius, 1998). The historical development of the dietary fibre concept, its definition and the ensuing development of suitable analytical methods has been discussed extensively (AACC, 2001; IFST, 2007). The present accepted definition, which has been adopted widely by food regulators and manufacturers, includes generalized health benefits based mainly on epidemiological data generated primarily from studies using fruits, vegetables and whole grain cereal foods. This definition is
Dietary fiber is the edible parts of plants or analogous carbohydrates that are resistant to digestion and absorption in the human small intestine with complete or partial fermentation in the large intestine. Dietary fiber includes polysaccharides, oligosaccharides, lignin and associated plant substances. Dietary fibers promote beneficial physiological effects including laxation and/or blood cholesterol attenuation and/or blood glucose attenuation (AACC, 2001).
In 2006, FAO/WHO commissioned experts to further consider the definition of dietary fibre. They recommended that the definition be restricted to “dietary fibre consists of intrinsic plant cell wall polysaccharides”, thus restricting the previous all-encompassing physiological definition. This recommendation was considered in late 2007 (IFST, 2007). What does this all mean for the analysis of galacto-oligosaccharides?
For food labelling reasons, manufacturers of fructo- and galacto-oligosaccharides have been keen to be able to label those ingredients as dietary fibre. The standard official method of analysis of dietary fibre adopted in 1985 was AOAC method 985.29. Because the method was inadequate for fructans, complementary methods were developed, such as AOAC 997.08 (based on ion-exchange chromatography) and AOAC 999.03 (an enzymatic/spectrophotometric method). Quemener et al. (1994) developed a method for measurement of trans-galacto-oligosaccharides by enzymatic treatment with β-galactosidase, followed by high-performance anion-exchange chromatography, using PAD (HPAEC-PAD). This method was tested further in collaborative studies (De Slegte et al., 2002) and adopted as the official AOAC method (AOAC 2001.02). It has been published as Method 32-33 by the AACC (2002). The chromatography supply company, Dionex Corporation, has recently released an Application Note No. 155 which examines the use of different potential waveforms in the PAD and improves the current method.
An enzymatic method to determine the lactulose content of milk in the presence of lactose has been adopted by the IDF (1995). The method is suitable for determining lactulose in the presence of much higher concentrations of lactose. Nagendra and Rao (1992) published a colorimetric method to measure lactulose in the presence of lactose. However, the HPAEC-PAD method described above is suitable for the determination of lactulose, lactosucrose, lactitol and tagatose, as well as complex mixtures of oligosaccharides.
Lactulose is often determined by enzymatic methods using kits supplied by companies like Merck and Boehringer–Mannheim. The principle of the determination is to first hydrolyse the lactulose with a galactosidase to fructose and galactose, then to convert the fructose in the presence of ATP with a hexokinase to fructose-6-phosphate. The fructose-6-phosphate is then converted to glucose-6-phosphate using phosphoglucose isomerase. Finally, in a reaction of the glucose-6-phosphate with NADP using glucose-6-phosphate dehydrogenase, the amount of NADPH formed is measured spectrophotometrically.
5.7 Properties
5.7.1 Properties of Oligosaccharides
Food-grade oligosaccharides are not pure products, but are mixtures containing oligosaccharides of different degrees of polymerization (dp), the parent polysaccharide or disaccharide and monomer sugars. A typical commercial product composition for an unpurified GOS may comprise glucose and galactose 10%; disaccharides (lactose) 70%; trisaccharides 18.7%; tetrasaccharides 1.2%; and pentasaccharides 0.06%. More advanced manufacturing processes result in much higher concentrations of galacto-oligosaccharides in the product. For example, Oligomate 50 produced by the Yakult Company contains 50–52% galacto-oligosaccharides, 10–13% lactose and 36–39% monosaccharides (Matsumoto et al., 1993). Purified products are available from many manufacturers, but costs increase considerably because of the extra processing steps required. Purified products usually contain between 85 and 99% galacto-oligosaccharides. It is obvious that there are important differences in the properties and actions of food-grade oligosaccharide mixtures depending on the mixture purchased. The applications of oligosaccharides as components in foods will therefore vary considerably.
General properties of oligosaccharides are listed in Table 5.12. The data provided by producers, not all of which have been possible to reproduce in this review, usually include the following factors: sweetness (relative to sucrose), viscosity, water activity, moisture retention, osmotic pressure, freezing point depression, heat stability, storage stability at different pH values and colouring by heat.
Most producers also provide safety and toxicological data for acute, sub-acute and chronic toxicity. Data are also commonly provided to demonstrate changes in colonic microbial flora following ingestion of oligosaccharides. Usually, substantial increases in bifidobacteria are demonstrated relative to the other bacterial classes measured.
The physico-chemical properties of oligosaccharides are largely dependent on the molecular weight of the particular oligosaccharide product. These effects are shown in Table 5.13.
The susceptibility to hydrolysis of oligosaccharides during passage through the gastrointestinal tract is an important characteristic. This has been determined in a range of in vitro model systems of the stomach and small intestine of humans. Different types of oligosaccharides have been subjected to regimes such as (a) hydrolysis by human saliva (50%, v/v, saliva, 30 min exposure, pH 7.0, 37°C); (b) hydrolysis by gastric acid (4 h exposure, pH 2.0, 37°C in acid/pepsin); (c) hydrolysis by pancreatic and brush border carbohydrases (17%, v/v, porcine pancreatic and duodenal homegenates for 1 h exposure, pH 7.0, 37°C). Carbohydrate analysis before and after exposure by HPLC then determines the degree of hydrolysis. Tests such as these have shown that xylo-oligosaccharide mixtures, palatinose condensates, commercial galacto-oligosaccharide mixtures and lactulose are very resistant to hydrolysis, whereas lactosucrose, along with gentio-oligosaccharides, soybean oligosaccharides, fructo-oligosaccharide mixtures and inulin oligosaccharides are hydrolysed slightly by such conditions (Crittenden and Playne, 1997, unpublished data).
5.7.2 Properties of Lactulose
Lactulose solution is a yellowish, odourless clear syrup with a sweet taste. Dry lactulose is a white, odourless crystalline powder. Lactulose is soluble in water, but poorly soluble in methanol and insoluble in ether. Its solubility in water is 76.4% (w/w) at 30°C, rising to 86% at 90°C. The melting point is 168.5–170.0°C. Its sweetness is classified as 0.48–0.62 that of sucrose. It is 1.5 times sweeter than lactose. Acid hydrolysis of lactulose yields galactose and fructose. Unlike lactose, lactulose cannot be hydrolysed by human intestinal enzymes. Lactulose can be fermented by some human colonic bacteria and acts as a prebiotic ingredient (Mizota et al., 1987; Tamura et al., 1993). Heat sterilization is a common step in food manufacture. Lactulose is stable and little decomposed when heated to 130°C for 10 min at low pH. This relatively high stability makes it a suitable ingredient for normal food processing.
5.7.3 Properties of Lactosucrose
Lactosucrose is very soluble in water (up to 3670 g/L at 25°C). It has a sweetness relative to sucrose of 0.3. It is stable for 1 h at pH 4.5 and 120°C. It is most stable at neutral pH and remains fairly stable at pH 3 at 80°C. As a powder, it has a high moisture-retaining capacity and is highly hygroscopic.
Lactosucrose acts as a prebiotic carbohydrate and is not readily digested by intestinal enzymes in the stomach and small intestine. It is selectively fermented by Bifidobacterium in the human colon.
5.7.4 Properties of Lactitol
Lactitol is an odourless, colourless, sweet, non-hygroscopic and stable sugar alcohol. As it lacks a reactive carbonyl group, which is characteristic of sugar molecules, it does not participate in Maillard browning reactions with amino groups. It occurs in both monhydrate and dihydrate crystalline forms as pure products with good flowability. Lactitol is stable under both alkaline and acid conditions and at high temperatures likely to be encountered during food processing. Its melting point is 146°C and it is very soluble in water. The sweetening power of lactitol is about 40% of sucrose and the calorific contribution of lactitol after it is metabolized by colonic bacteria is less than 2.4 cal g–1 (10 kJ) compared to 4.0 cal g–1 (17 kJ) for sucrose.
Lactitol is not hydrolysed or absorbed in the small intestine and passes through to the colon. Lactitol also acts as a prebiotic carbohydrate and enhances the growth of certain groups of bacteria (e.g., bifidobacteria) in the colon.
5.7.5 Properties of Lactobionic Acid
Lactobionic acid is best described as a natural polyol acid with a formula weight of 358.30 Da and a pK a of 3.8. It is soluble in water as a free acid or as a salt. The molecule possesses multifunctional groups and thus acts as a metal ion chelator and can sequester calcium (Abbadi et al., 1999). Lactobionic acid inhibits the production of hydroxyl radicals as a result of its iron-chelating capacity and it functions as an antioxidant in tissues. It has a sweet taste despite being a weak acid. Lactobionic acid can be dehydrated to a lactone.
Lactobionic acid is hygroscopic and forms a gel containing about 14% water with atmospheric moisture. Concentrated solutions will form gels and films. Its superior water-retention ability is valuable in cosmetic applications. Its main uses derive from its important chelating and emulsifying properties.
5.7.6 Properties of d-tagatose
d-Tagatose is a monosaccharide with a formula weight of 180.16 Da; it is a white solid with a melting point of 133–135°C. It has a similar texture to sucrose and is 92% as sweet, but its calorific value (1.5 kcal/g) is only 38% that of sucrose since it is metabolized differently from sucrose. Its indigestibility in the stomach and small intestine results in a minimal effect on blood glucose levels in humans. Values for the absorption of tagatose in the small intestine vary widely from 20 to about 80% (Normen et al., 2001). The unabsorbed fraction is then completely fermented in the colon by some intestinal microorganisms. Those genera able to ferment tagatose included Enterococcus and Lactobacillus, but not Bifidobacterium. Many dairy lactic acid bacteria, such as lactococci and streptococci, fermented tagatose readily (Bertelsen et al., 2001). It is claimed that its fermentation in the colon results in an increased production of butyrate relative to that of acetate and propionate (Laerke and Jensen, 1999, Laerke et al., 2000; Bertlesen et al., 1999). It has a glycaemic index of only 3 compared to 100 for glucose. Its glycaemic index is markedly lower than those for other natural sugars and most sugar alcohols. It is claimed to have prebiotic properties (Bertlesen et al., 1999, 2001), but its inability to be fermented by Bifidobacterium species limits its application as a prebiotic. However, it is non-cariogenic and non-plaque forming.
5.8 Uses and Applications
5.8.1 Galacto-oligosaccharides
The major uses of galacto-oligosaccharides are in beverages and in infant milk formula, follow-on formula and infant foods. These are usually provided at about 6 g/L of infant formula and are normally supplied as a mixture of nine parts galacto-oligosaccharide and one part fructo-oligosaccharide. A recent major Dutch study with 120 women, half of whom were breast-feeding and half of whom were bottle-feeding, was conducted by Bakker-Zierikzee et al. (2005). The data supported the benefit of adding these oligosaccharides, as they were able to mimic the metabolic and microbial effects normally found in breast-fed babies.
Oligosaccharides are also used widely in confectionery and in bread-making, for their physico-chemical properties, as well as claimed health-enhancing properties. Obviously, oligosaccharides also provide textural and sensory properties to foods (see Table 5.14). They are being used increasingly as ingredients of synbiotic foods, for example, their use in yoghurt and yoghurt drinks.
The use of oligosaccharides in the livestock and pet food industries is also increasing. In the latter case, GOSs are used to improve bowel consistency and to provide firmer and less malodorous faeces. Prebiotic ingredients are finding increased use in the chicken, pig and calf industries for improving health and minimizing the use of antibiotics.
5.8.2 Lactulose
Most lactulose is used in the pharmaceutical industry, but increasingly it is finding application as a prebiotic ingredient in functional foods. Lactulose is generally classified as a drug, the prescription or non-prescription status of which depends on the country. However, it has now been approved for use as a prebiotic in several countries (Italy, Netherlands and Japan) and may be sold as either a food or drink additive.
Lactulose is used to treat constipation and hepatic encephalopathy. It is the principal anti-constipation drug worldwide. Lactulose has exhibited some ability to ameliorate symptoms of idiopathic, as well as infectious, inflammatory bowel disease and this may be due to its prebiotic effects on colonic bacteria. There are some early indications in animal models that it may aid in reducing the incidence of colon tumours and hence colon cancer.
Applications listed by Morinaga are (a) as a prebiotic ingredient in infant foods, (b) as a pharmaceutical for remission of hyperammonemia and chronic constipation, (c) as a sweetener for low-calorie dietary foods and (d) as an additive for cattle and pet feed. A variety of yoghurts, drinks, ice cream and infant formula have been developed by Morinaga Milk Industry.
5.8.3 Lactosucrose
Lactosucrose has been used as a prebiotic ingredient in a range of food products which have attained FOSHU status in Japan with 15 products approved by 2005. It is used as a sweetener as well as a prebiotic in a range of beverages, including coffee and tea. It is used in confectionaries, desserts, sweets, bakery products and yoghurts. Its use is largely confined to Japan, but marketing into the USA has commenced. It also has a use for improving bowel consistency and faecal odour. Research has also been performed on its use in pet food, particularly for reducing faecal odour (Fujimori, 1992).
5.8.4 Lactitol
The principal use of lactitol is as a low-calorie sweetener in foodstuffs. It is used in a range of low-energy and low-fat foods. Its high stability makes it popular for bakery applications, although it is not able to participate in the Maillard reaction. It is used in sweets, chocolates, biscuits and ice cream where it competes with other sugar alcohols, such as sorbitol, mannitol and xylitol. It is used in glycaemic foods for diabetics and is also recognized as not causing dental caries. Lactitol has also found application in pharmaceuticals as an alternative to lactulose and as a cryoprotectant in surimi. Its low hygroscopicity and high viscosity give it advantages in chocolate and confectionary manufacture. It has an emerging use in pre- and pro-biotic functional foods.
5.8.5 Lactobionic Acid
In the pharmaceutical industry, lactobionic acid is used to deliver erythromycin intravenously and also in calcium supplementation. A major commercial use is its role in organ preservation fluids during transplantation procedures in hospitals. Lactobionic acid is used in the “Wisconsin transplantation solution” because its metal-chelating properties reduce oxidative damage to tissue during storage and preservation of organs caused by some metal ions. Lactobionic acid can also be an ingredient in chlorohexidine-based disinfectants. It is able to suppress tissue damage caused by oxygen radicals and is used to assist wound healing. In skincare, it has a use as a dermal care cosmetic and possesses a number of useful properties for this purpose.
Emerging applications are as an acidulant with a sweet taste; a filler in cheese production; use as a calcium carrier in functional drinks; as a co-builder in detergents; and in corrosion protection. Lactobionic acid amides have been investigated for use in the surfactant industry. Lactobionic acid N-alkylamides have been proposed for use in corrosion inhibition.
5.8.6 Tagatose
The main use of tagatose is as a sweetener with a low calorific content. Its main market appears to be in diabetic foods, as it does not increase blood glucose or insulin levels, and it has a very low GI value. It may also be useful in weight loss diets to help overcome widespread obesity in Western human populations. It has the advantage of being of similar sweetness to sucrose, but with a low contribution to energy supply.
Tagatose has some potential as a prebiotic carbohydrate, but evidence for its effectiveness relative to competing products is not yet available. Its ability to be fermented in the colon to produce enhanced concentrations of butyrate is of interest in relation to colonic cancers, and this requires further investigation (Bertlesen et al., 1999; Topping and Clifton, 2001).
5.9 Physiological and Health Effects
A major driver for the development of lactose derivatives has been the identification of numerous physiological properties beneficial to health. Some of these physiological effects have therapeutic value for specific disorders while other properties are potentially beneficial to the population at large. Hence, lactose derivatives have found applications both as pharmaceuticals and as functional food ingredients. Despite a diversity of chemical structures, the lactose derivates developed to date share a number of common physiological traits important to their health benefits. These molecules are universally
-
non-digestible
-
non-metabolizable
-
poorly fermented by bacteria in the mouth
-
fermented by bacteria in the intestinal tract.
These physiological characteristics contribute to health benefits both at specific sites in the body and systemically. The benefits demonstrated, or proposed, include prevention of dental caries, roles in weight management, improved defecation frequency and consistency and a range of other effects stemming from modification of the composition and/or activity of the intestinal microbiota (prebiotic effects). Of course, differences in the chemical structures of the various lactose derivatives also mean that they have differing specific actions and potencies. The following sections outline the known and purported health benefits of lactose derivates and what we know about their mechanisms of action.
5.9.1 Dental Health
Lactitol is one of a number of sugar alcohols that have found applications in products where sweetness is desired without stimulating cariogenic bacterial activity in the mouth. Other examples include xylitol, sorbitol, mannitol and maltitol, with the first two examples the most widely used. Sugar alcohols are not fermented by bacteria in the mouth and so are non-acidogenic and do not lead to bacterial exo-polysaccharide production that contributes to dental plaque (van Loveren, 2004). Since they are small molecules that are not digested, they cannot be consumed in large amounts due to intestinal side effects (discussed later) and so are not used as bulk sweeteners, but rather in products such as chewing gums and toothpaste. The other lactose derivatives discussed in this chapter are also non-cariogenic, but are not widely used specifically for oral health.
5.9.2 Prevention and Treatment of Constipation
Lactulose (10–20 g/day) is used widely as a pharmaceutical to treat constipation and has proven efficacy in a number of placebo-controlled trials (Fernández-Bañares 2006; Quah et al., 2006) even in patients with chronic constipation. Since it is a relatively small molecule that is not digested or absorbed, lactulose has an osmotic effect, trapping fluid, accelerating transit in the small bowel and increasing ileocaecal flow. A recent human clinical study has also shown that therapeutic doses of lactulose produce a prolonged tonic contraction in the gut that may also be involved in the laxative effect (Jouët et al., 2006). Since any carbohydrate that reaches the large bowel should have a laxative effect (Macfarlane et al., 2006), the other lactose derivatives also have benefits in the prevention and treatment of constipation. GOSs too have been demonstrated to improve stool frequency and consistency in infants and adults, though it is used more in functional foods as opposed to pharmaceutical applications. Possible mechanisms of action of lactose derivatives in alleviating constipation are shown in Figure 5.7.
5.9.3 Treatment of Hepatic Encephalopathy
Hepatic encephalopathy (HE) is a neuropsychiatric condition driven by liver dysfunction that includes a spectrum of symptoms ranging from subtle changes in cognition and personality to lethargy, stupor and coma (Dbouk and McGuire, 2006).
Although the precise underlying mechanisms remain debated, elevated ammonia concentrations in plasma and the central nervous system, caused by the inability of the liver to clear ammonia generated by the gut microbiota, are considered key to its pathogenesis (Prasad et al., 2007). Lactulose and lactitol are front-line therapeutic agents in HE because they limit both ammonia production in, and absorption from, the colon. The fermentation of these carbohydrates when dosed at relatively high levels results in a diversion of ammonia to microbial protein production. Additionally, acidification of the colonic lumen inhibits urease-positive and deaminating bacteria (implicated in intestinal ammonia production), increases intestinal transit and reduces absorption of ammonia from the intestinal lumen (Bongaerts et al., 2005; Dbouk and McGuire, 2006). Lactulose and lactitol have similar efficacy, although lactitol is more palatable and produces more rapid results with fewer side effects (Dbouk and McGuire, 2006).
5.9.4 Prebiotic Effects
Some of the main physiological effects of lactose derivatives stem from their impact on the composition and activities of the intestinal microbiota. Our intestinal tract is colonized by a complex ecosystem of microorganisms that increase in numbers from 102–104 per gram of contents in the stomach to 106–108 per gram in the small intestine and 1010–1012 per gram in the colon (McCartney and Gibson, 2006). Far from being inconsequential to our lives, these microorganisms are in a symbiotic relationship with their host and are highly important to our health and well-being. They provide us with a barrier to infection by intestinal pathogens (Bourlioux et al., 2003), much of the metabolic fuel for our colonic epithelial cells (Topping and Clifton, 2001), and contribute to normal immune development and function (Blum and Schiffrin, 2003; Tlaskalova-Hogenova et al., 2004). Intestinal bacteria have also been implicated in the aetiology of some chronic diseases of the gut such as inflammatory bowel disease (IBD) (Cummings et al., 2003; Marteau et al., 2003). As we age, changes occur in the composition of the intestinal microbiota that may contribute to an increased level of undesirable microbial metabolic activity and subsequent degenerative diseases of the intestinal tract (Saunier and Doré, 2002; Guarner and Malagelada, 2003).
Modifying the composition of the intestinal microbiota to restore or maintain a beneficial population of microorganisms would appear to be a reasonable approach in cases where a deleterious or sub-optimal population of microorganisms has colonized the gut. The two genera most often proposed as beneficial bacteria with which to augment the intestinal microbiota are the lactobacilli and bifidobacteria, both of which are numerically common members of the human intestinal microbiota. Two approaches are used to increase the number or proportion of these bacteria in the gut. The first is by directly supplementing the intestinal microbiota by consuming live bacteria, “probiotics”, in foods or pharmaceuticals. The second is by consuming dietary components, “prebiotics”, which selectively stimulate the proliferation and/or activity of these purportedly beneficial organisms that are already resident within the intestinal microbiota (Gibson et al., 2005). Most prebiotics identified to date are non-digestible, fermentable carbohydrates, particularly oligosaccharides and include the lactose derivatives, GOSs and lactulose.
Despite a diversity of structures, most prebiotics stimulate the proliferation of bifidobacteria in particular and are sometimes referred to as “bifidus factors” or “bifidogenic factors” (Table 5.1). A number of largely prophylactic health targets have been proposed for prebiotics stemming from alterations to the composition or fermentative activity of the microbiota in response to the availability of a selectively utilized carbohydrate source. These include protection against enteric infections, increased mineral absorption, immunomodulation for the prevention of allergies and gut inflammatory conditions (Figure 5.8), and trophic effects of short-chain fatty acids (SCFA) on the colonic epithelium, faecal bulking and reduced toxigenic microbial metabolism that may reduce risk factors for colon cancer (Figure 5.9).
5.9.4.1 Applications of GOSs in Infant Nutrition
The composition of the human intestinal microbiota changes naturally with age, and in early infancy the microbiota is believed to be particularly important in correct functioning of the gut and maturation of the immune system. Indeed, differences have been observed between the composition of the microbiota in allergic and healthy infants including reduced numbers of bifidobacteria and a more adult-like profile of Bifidobacterium species (Kirjavainen and Reid, 2006). Bifidobacteria colonize the human intestinal tract during or soon after birth and in breast-fed infants eventually dominate the microbiota (Harmsen et al., 2000). The numerical dominance of bifidobacteria is induced by bifidogenic components in breast milk, including oligosaccharides (Harmsen et al., 2000; Mountzouris et al., 2002). Indeed, human milk oligosaccharides (HMOs) are the original prebiotics. The concentration of HMOs in breast milk (5–12 g/L) is about 100 times that found in cow’s milk (0.03–0.06 g/L) (Kunz et al., 2000; Boehm and Stahl, 2007), representing the third largest solid component behind lactose and fat (Bruzzese et al., 2006). That mothers direct so much energy towards components of breast milk that are not directly metabolized by the infant, but rather selectively feed and direct the composition of the microbiota, shows evolutionarily just how important the infant gut microbiota is to well-being.
In contrast to breast-fed infants, infants fed traditional cow’s milk-based formulae develop a more mixed intestinal microbiota, with lower counts of bifidobacteria and higher counts of clostridia and enterococci (Adlerberth, 1999). Formula-fed infants also have been observed to have higher faecal ammonia and other potentially harmful bacterial products (Heavey et al., 2003; Edwards and Parrett, 2002). The bifidogenic effect of HMOs can be emulated using oligosaccharides such as GOSs or fructo-oligosaccharides (FOSs). In fact, most Japanese infant formula manufacturers have been supplementing their products with prebiotic oligosaccharides for many years (Boehm et al., 2005).
GOSs share some structural similarities with the backbone of HMOs, although HMOs are considerably more complex, with over 200 different structures identified based on a variable combination of glucose, galactose, sialic acid, fucose and/or N-acetylglucosamine, with varied sizes and linkages accounting for the considerable variety (Kunz et al., 2000). In recent years, a number of studies have investigated the effect of infant formula containing 8 g/L of a mixture of 9:1 GOSs:FOSs on the intestinal microbiota of infants. The GOSs:FOSs mixture was designed to mimic the molecular size distribution of HMOs (Knol et al., 2005). Summarized in Figure 5.10, they show that feeding this formula induced a microbiota similar to that of breast-fed infants, both in terms of composition of the overall microbiota and the proportion and species composition of lactobacilli and bifidobacteria (which tends to be more adult-like in traditional formula-fed infants) (Rinne et al., 2005; Haarman and Knol, 2005, 2006; Knol et al., 2005). The oligosaccharide-containing formula also resulted in a reduced faecal pH and a SCFA profile that mimicked breast milk (dominated by acetate) and was different from that produced by traditional infant formulae (higher in butyrate and propionate) (Boehm et al., 2005; Fanaro et al., 2005; Knol et al., 2005; Bakker-Zierikzee et al., 2005). Feeding the oligosaccharide formula also resulted in an improved stool consistency and frequency, similar to breast-fed infants, while having no adverse impacts on measures of infant growth or other adverse effects (Boehm et al., 2005; Bruzzese et al., 2006; Ziegler et al., 2007). Finally, the oligosaccharide-supplemented formula showed a positive effect on the level of protective secretory IgA in the gut, increasing concentrations in comparison to standard formula (Bakker-Zierikzee et al., 2005). These studies demonstrate that formulae supplemented with the GOSs:FOSs can mimic many of the physiological impacts of HMOs. Now the challenge remains to establish if these translate into benefits for clinical end-points such as incidence of infections or the prevalence of allergy.
The inclusion of a prebiotic mixture of 9:1 galacto-oligosaccharides (GOSs) to fructo-oligosaccharides (FOSs) mimics many of the effects of human milk oligosaccharides (HMOs) on the composition and activity of the human infant intestinal microbiota. In contrast, traditional infant formulae containing no oligosaccharides drive a more adult-like microbiota and stool composition.
5.9.4.2 Impacts on the Microbiota Composition in Adults
Following weaning, the composition of the colonic microbiota becomes increasingly complex. In adulthood, the microbiota consists of more than 500 different species, although it is dominated by 30–40, and its composition becomes quite stable (McCartney and Gibson, 2006). Bifidobacteria remain numerically important, although their proportion of total microbes declines from 60 to 80% in breast-fed infants to 1–5% in adults. Feeding prebiotics to adults typically induces 10- to 100-fold increases in the size of the intestinal Bifidobacterium population (Crittenden, 1999). However, a range of factors may influence the magnitude of any increase in Bifidobacterium numbers, the most important being the initial size of the population in the intestinal tract. In individuals colonized by an already large population of bifidobacteria (in the order of 108 cfu/g faeces), prebiotic consumption appears not to increase Bifidobacterium numbers further (Rao, 1999).
Of the lactose derivatives, lactulose has the best accumulated evidence of prebiotic effects on the adult microbiota. A number of well-controlled feeding trials with lactulose have consistently shown bifidogenic effects, which have been detected using both traditional microbiological culture methods and molecular ecology techniques (Ballongue et al.,1997; Tuohy et al., 2002; Bouhnik et al., 2004a; De Preter et al., 2006; Vanhoutte et al., 2006). GOSs have also demonstrated prebiotic effects in adults, but the bifidogenic impact of GOSs in infants has not been as consistently replicated in adult feeding trials (summarized by Rastall, 2006). DNA-based measurements of the composition of the bacterial community in the gut do not necessarily reflect the metabolic activity of specific strains. Using RNA-DGGE, this activity can be monitored (Tannock et al., 2004). Those authors found that feeding adults a dose of GOSs as low as 2.5 g/day did change the metabolic activity of bifidobacteria. Unlike FOSs and inulin, these lactose derivatives also appear to increase the numbers of lactobacilli in faeces. Additionally, these increases have often been accompanied by concomitant reductions in putrefactive and pathogenic bacterial populations. Effective doses have usually been 10–20 g/day, although lactulose has been reported to have bifidogenic effects also at lower doses (Terada et al., 1992; Bouhnik et al., 2004b).
Lactitol also has been shown to have prebiotic effects in adults similar to lactulose (Ballongue et al., 1997; Kummel and Brokx, 2001; Chen et al., 2007) although it is possibly slightly less potent (Ballongue et al., 1997). Additionally, there is some evidence of prebiotic action by lactosucrose from a number of small human feeding trials (Fujita et al., 1991; Yoneyama et al., 1992; Hara et al., 1994b; Ohkusa et al., 1995).
Overall, there is evidence that the consumption of some lactose derivatives can result in changes in the composition of the intestinal microbiota in adults. The observed increases in the numbers of saccharolytic, acidogenic bacteria and reductions in the numbers of putrefactive and potentially pathogenic bacteria could be reasonably hypothesized to benefit health. However, a clear link between these changes in the microbiota composition and improved health clinical end-points in adults is yet to be established.
5.9.4.3 Fermentation of Lactose Derivatives by the Intestinal Microbiota
In addition to modifying population dynamics, prebiotic lactose derivatives also modify the activity of the microbiota by providing a source of readily fermentable carbohydrate. It may be this dietary fibre-like characteristic of modifying the fermentative activity of the existing microbiota that is the important factor in providing a number of health benefits to consumers. The proposed health effects of prebiotics believed to be largely contingent on modifications to the metabolic activity of the microbiota include reductions in risk factors for colon cancer, increased mineral absorption, improved lipid metabolism and increased resistance to intestinal pathogens.
5.9.4.4 Colon Cancer
The intestinal microbiota has a number of biochemical activities relevant to colon cancer risk that relate to the composition and activity of different bacterial populations. Hence, lactose-derivative prebiotics may have a role in reducing risk factors for colon cancer. Proposed mechanisms include supplying the colonic epithelium with SCFA (particularly butyrate); suppression of microbial protein metabolism, bile acid conversion and other mutagenic and toxigenic bacterial reactions; and immunomodulation (Figure 5.8).
A number of studies on humans and animals have shown that the consumption of lactulose (Terada et al., 1992; Ballongue et al., 1997; De Preter et al., 2004) and GOSs (Rowland and Tanaka, 1993; Kawakami et al., 2005) improves the colonic environment in terms of reducing the levels of mutagenic enzyme activities (e.g., β-glucuronidase and azoreductase) and bacterial metabolites (e.g., secondary bile acids, phenols and indoles) that are purportedly associated with risk of colon cancer. However, the quantitative importance of these markers to eventual cancer development remains to be established.
Protection by prebiotics against the development of pre-neoplastic lesions and tumours in rodent models of colon carcinogenesis has also been reported for lactulose (Rowland et al., 1996; Challa et al., 1997), while in a human feeding study, administration of lactulose reduced the recurrence of colonic adenomas in patients who had had surgery to remove adenomas (Ponz de Leon and Roncucci, 1997). However, these results are very preliminary and a great deal more research is required to discern if the consumption of lactulose has a protective function against colorectal cancer.
5.9.4.5 Mineral Absorption
Encouraging results have been reported for the impact of fermentable carbohydrates, including GOSs and lactulose, on increasing mineral absorption from the gut. The precise mechanisms of prebiotic-mediated improvements in mineral uptake remain unclear, but fermentative activities of the microbiota, including the production of SCFA and reductions in luminal pH, are believed to be involved in improving mineral solubility and extending the site of mineral absorption further into the colon (Morohashi, 2002) (Figure 5.11). In rat studies, increased calcium uptake has lead to improved bone mineralization for animals fed GOSs (Chonan et al., 1995) or lactulose (Mizota, 1996), and improved calcium uptake has also been reported in human feeding studies using these two lactose derivatives (Van den Heuvel et al., 1999; 2000). Though preliminary, the results to date suggest that the consumption of lactulose or GOSs may improve calcium uptake and further research is warranted to investigate links between long-term consumption and improved bone density in humans at risk of developing osteoporosis.
5.9.4.6 Colonization Resistance
The ability of lactose derivatives to improve colonization resistance and prevent bacterial infections in the gut has not been explored thoroughly, but results so far indicate a potential application for lactulose and NDOs in this function. Özaslan et al. (1997) observed lower caecal overgrowth and translocation of E. coli in rats with obstructive jaundice when they were fed lactulose, while Bovee-Oudenhoven et al. (1997) reported that the consumption of lactulose increased colonization resistance against the invasive pathogen, Salmonella enteritidis, in rats. Indeed, the consumption of high doses (up to 60 g/day) of lactulose is effective in eliminating salmonella from the intestinal tract of chronic human carriers and it is used as a pharmaceutical for this purpose in some countries (Schumann, 2002). The mode of action is speculated to be acidification of the gut that prevents the growth of this acid-sensitive pathogen. In a placebo-controlled study, Chen et al. (2007) reported that feeding lactitol significantly reduced plasma levels of endotoxin in a group of patients with chronic viral hepatitis. Endotoxaemia is closely correlated with the disturbance of the gut flora and the decline of colonization resistance in these patients. Consuming lactitol significantly increased populations of bifidobacteria and lactobacilli, perhaps contributing to an improved intestinal barrier.
Another mechanism by which oligosaccharides may provide protection against enteric infections is through competitive inhibition of pathogen adherence to the mucosa. Oligosaccharides can act as structural mimics of the pathogen-binding sites, which are often carbohydrate epitopes. HMOs act in this way to block the initial binding of a range of pathogens to inhibit colonization (Gibson et al., 2005; Shoaf et al., 2006). In vitro experiments using epithelial cell culture models (Tzortzis et al., 2005b; Shoaf et al., 2006) and in vivo monkey challenge experiments (Gibson et al., 2005) have shown that GOSs and lactulose have the ability to interfere with the adhesion of enteropathogenic E. coli. The ability rapidly to install or restore colonization resistance where the intestinal microbiota has been perturbed may prove to be an effective use of lactose derivatives in the future. Possible mechanisms by which prebiotics may increase colonization resistance are illustrated in Figure 5.12.
5.9.4.7 Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) describes a group of chronic, severe, relapsing inflammatory conditions of the gut that includes Crohn’s disease and ulcerative colitis. A genetic predisposition to develop an over-zealous inflammatory immune response to components of the intestinal microbiota has been implicated in its aetiology (Schultz et al., 2004). Elimination of specific bacterial antigens, immunomodulation and trophic effects of SCFA on the intestinal epithelium have all been proposed as mechanisms by which prebiotics could alleviate symptoms (Figure 5.9). The size of the intestinal Bifidobacterium population has been shown to be relatively small (Favier et al., 1997; Linskens et al., 2001) in subjects afflicted with IBD, although cause and effect links between disease and a diminished intestinal Bifidobacterium population remain to be established. Using different rodent models of IBD, Rumi et al. (2004) and Camuesco et al. (2005) ameliorated inflammation by feeding lactulose. Camuesco et al. (2005) noted that the improvement was associated with an increase in the numbers of bifidobacteria and lactobacilli. However, using the same model as Camuesco et al. (2005), Holma et al. (2002) observed no reduction in inflammation by intervention with galacto-oligosaccharides despite an increase in Bifidobacterium numbers. While the results with lactulose are encouraging, the scientific literature is unfortunately littered with examples of treatments for IBD that have worked in animal models, only to fail dismally in human studies. Despite this, further research into the efficacy of lactulose in the treatment of IBD is warranted.
5.9.4.8 Glycaemic Index, Weight Management and Serum Lipids
Since lactose derivatives are sweet and not digested, they have a low calorific value and are used as low-energy, low glycaemic index sweeteners that are also suitable for diabetic individuals. Additionally, several other physiological effects resulting from the consumption of non-digestible, fermentable carbohydrates have been reported that impact on weight management and serum lipid profiles. A number of prebiotic carbohydrates, including lactose derivatives (Ferchaud-Roucher et al., 2005; Shimomura et al., 2005; Vogt et al., 2006), have been shown to reduce serum triglyceride level in animal and human studies. The mechanism by which lowering of serum lipids and cholesterol may occur has been speculated to be regulation of host de novo lipogenesis through SCFA (particularly acetate) absorbed from the gut (Williams and Jackson, 2002) or by reduced intestinal fat absorption (Shimomura et al., 2005) (Figure 5.10). Although promising, not all feeding studies in humans using prebiotic carbohydrates have resulted in clinically significant changes in serum lipid profiles, and further research is needed to identify responsive populations, interactions with diet and to show benefits of dietary intervention in clinical end-points such as impact of cardiovascular disease.
Preliminary data have also suggested that lactulose (Ferchaud-Roucher et al., 2005; Brighenti et al., 2006) and lactitol (Juśkiewicz et al., 2006) could improve insulin sensitivity. In a small human feeding trial, Brighenti et al. (2006) demonstrated that feeding a meal containing lactulose diminished post-prandial blood glucose response in a subsequent meal (“second meal effect”) by reducing competition by non-esterified fatty acids for glucose disposal, and to a minor extent, by affecting intestinal motility. The potential of fermentable carbohydrates in the management of metabolic disorders linked to insulin resistance may warrant further study.
5.9.5 Farm and Companion Animals
Prebiotic oligosaccharides, including GOSs, have been evaluated for use in both farm animal feeds and for companion animals, especially dogs. The advantage of use with dogs is the positive impact of feeding oligosaccharides on reduced odour and improved volume and consistency of faeces (Swanson and Fahey, 2006). With respect to farm animals, prebiotics have been studied for their potential to replace antibiotics in maintaining high-feed conversion efficiencies and also to suppress methane production by ruminants (Mwenya et al., 2004a,b; Sar et al., 2004; Santoso et al., 2004).
5.10 Product Safety, Dose Rates and Regulatory Issues
5.10.1 Galacto-oligosaccharides
The regulatory regimes for non-digestible carbohydrates have been under active review in many countries in recent years. A marked development since 2000 has been the inclusion of most non-digestible oligosaccharides in the category “dietary fibre” (see definition in Section 5.6). This has been a landmark development, because it allows recognition of these products as having some health benefits. Prior recognition in a regulatory sense had been restricted to Japan, where for many years the functional food sector was regulated by the FOSHU system.
In Japan, the occurrence of an ageing population and the likely national health costs were recognized much earlier than in Western countries. So by 1991, a special category of FOSHU regulations was introduced. By December 2007, there were 755 FOSHU-approved products. Those with gastrointestinal claims, which include products containing prebiotics such as FOS, lactosucrose and lactulose, accounted for 51% of sales in 2007 (Bailey, 2008). FOSHU is controlled by the Japan Health Food and Nutrition Food Association. It has produced English-language versions of its guide, brochure and list of approved products.
The use of GOSs as an optional ingredient in infant milk formulae and infant foods has been the subject of intensive regulatory inquiry and its acceptance varies among countries. Revised Codex standards were released in November 2006 (Codex ALINORM 07/30/26). Readers are referred to the Codex website (www.codexalimentarius.net) for current standards (CODEX STAN 72-1981; and 156-1987).
5.10.1.1 Safety Issues and Dose Rates
Safety of use must always be dominant in the development of new food products. Fortunately, it is well established that GOSs and lactulose are safe, even at high doses. Galacto-oligosaccharides are considered safe ingredients as they are constituents of human milk and can also be produced in the gut by intestinal bacteria from ingested lactose. Acute toxicity tests in rats have shown that ingestion of more than 15 g/kg body weight of β1→4 galacto-oligosaccharide was needed for LD50. No adverse symptoms were recorded in a chronic toxicity test when 1.5 g/kg bodyweight were fed for 6 months. Non-mutagenicity was confirmed by the AMES-Salmonella and Rec assay (Matsumoto et al., 1993; Sako et al., 1999). However, excessive intakes (> 30 g/day in adults) can lead to flatulence, cramping and osmotic diarrhoea, particularly with the shorter chain oligosaccharides. There is some evidence of adaptation to oligosaccharides by individuals, who can then eat double the normal recommended maximum doses without symptoms (Schoterman, 2001). Furthermore, other non-digestible carbohydrates are present as natural components in many vegetable food sources. Estimates have been published of expected intakes of fructo-oligosaccharides and inulin from such sources. It is likely that an intake around 8 g/day per adult human may be normal.
Recommended effective doses of added oligosaccharides in adult humans usually range from 10 to 20 g/day. With the slower-fermenting inulin or resistant starch, an intake of up to 40 g/day is acceptable. With the short-chain oligosaccharides, few ill-effects have been recorded in adults with intakes of 25–30 g/day, but adaptation was sometimes necessary. These intakes are all additional to what might be derived from other food components in a normal diet. Bifidogenic responses are not always found when oligosaccharides are added to the diet of humans. This occurs when the populations of bifidobacteria in the colon are already relatively high (Alander et al., 2001).
5.10.2 Lactulose
Most preparations of lactulose contain substantial concentrations of lactose, epi-lactose and galactose. Thus, the use of lactulose is contraindicated in those who require low lactose or low galactose diets. In some countries, such as the USA, lactulose is a prescription drug and its use is limited to prescribed medical use. Those who develop gastrointestinal disturbances, such as bloating, discomfort, flatulence and diarrhoea, with dietary fibre should exercise caution in using lactulose. Acute and sub-acute toxicity data for lactulose have been summarized by Mizota et al. (1987) and are similar to that for sucrose. In 1992, the Ministry of Health and Welfare in Japan approved lactulose as a useful ingredient in specified foods currently being sold for health use (FOSHU). Thus, in 1995, a soft drink, “Mai-asa Sohkai”, containing 4 g lactulose per carton, was approved as “a beverage made from lactulose which properly supports the proliferation of Bifidobacterium in the intestines to maintain good condition of the bowels” (Anon., 1997).
As an anti-constipation drug, the normal daily dose for adults is 20 g lactulose. It is also established that ingestion of lactulose at higher doses can result in diarrhoea.
5.10.2.1 Toxicological Data
Canlac, a subsidiary of Solvay Pharmaceuticals, has measured toxic effects of Lactulose USP concentrate (693 g/L). The results of these studies can be summarized as:
-
Acute toxicity – by oral route, LD50 in rats, 30–45 mg/kg
Chronic toxicity
-
oral route (diet), after prolonged exposure, in rats, 15 ml/kg, no observed effect.
-
oral route (diet), after prolonged exposure, in dogs, target organ: gastrointestine, 4 ml/kg, no observed effect
-
no effect on fertility
-
no teratogenic effect.
The lactulose concentrate was not hazardous under normal conditions of handling and use. Ecotoxicity tests were also measured. In summary, no adverse environmental effects were found, and it was not regarded as hazardous for the aquatic environment (Anon., 2003).
5.10.3 Lactosucrose
The minimum effective dose of lactosucrose is 5 g/day for an adult. When it is taken in large amounts, lactosucrose may cause a rise in gastrointestinal osmotic pressure and induce diarrhoea. Its maximum no-effect dose is 0.6 g/kg bodyweight. An optimum dose is considered to be between 5 and 36 g/day for an adult human (Oku and Tsuji, unpublished).
5.10.4 Lactitol
The European Community listed lactitol as a permitted sweetener (E 966) (EC Directive 96/83/EC) and the USA FDA has accepted lactitol for GRAS listing. Lactitol is approved as a sweetener in over 30 countries.
Lactitol was last evaluated in 1983 by the Joint FAO/WHO Expert Committee on Food Additives. A range of short- and long-term studies on its absorption, distribution and excretion in biochemical studies and in animal models were examined. Toxicological studies were examined including studies on carcinogenicity, dermal irritation, eye irritation, mutagenicity, reproduction and teratogenocity (for details, see JECFA, 2008; Inchem, 2008). In humans, lactitol at 24 g/day orally is tolerated well by healthy and diabetic persons. It does not alter blood glucose level. Higher doses (50 g/day) cause diarrhoea. It was concluded that lactitol has only a very low general toxicity following large doses. The Expert Committee did not believe that lactitol at the normal levels of intake likely to be experienced presented a hazard to health. No acceptable daily intake for man (ADI) was specified by the committee, and the establishment of a numerical figure was not deemed necessary.
A biodegradability test based on dissolved organic carbon showed complete degradation within 5 days.
5.10.5 Lactobionic Acid
The regulatory status in the food industry is limited. It is approved as a food additive in the form of its calcium salt. Its use is as a firming agent in dry pudding mixes [Code of Federal Regulations, Title 21, Vol. 3 (21CFR 172.5), US Food and Drug Administration]. However, it has many non-food uses, such as in detergents, chelating agents and in cosmetics. It also is accepted by the US Food and Drug Administration as an inactive ingredient for medical use in organ transplantation solutions.
5.10.6 Tagatose
Tagatose was listed in 2001 as a GRAS ingredient by the US Food and Drug Administration. The Food Standards Agency in the UK recognized tagatose as a novel food ingredient in the EU, and this is expected to be ratified soon. Also, it has been approved for food use in Brazil, Korea, Australia and New Zealand, and approval in Mexico, Japan and Canada is expected shortly.
5.11 Conclusions
5.11.1 Future Directions and Challenges
The manufacture of GOSs and the other lactose-derived compounds is in the process of rapid expansion globally. A major reason for this expansion in production in recent years has been the general regulatory acceptance of the addition of GOSs to infant milk formulae and infant foods. More broadly, the ability to include these ingredients under the heading “dietary fibre” on food labels has enabled some health benefits to be stated. However, as discussed earlier, there is now some doubt whether oligosaccharides will remain classified as dietary fibres.
As further clinical and animal studies are conducted, a range of non-digestible carbohydrates are gaining medical acceptance as prebiotics, which are able to confer some health benefits. This includes GOSs and lactulose. The evidence is less well established for lactosucrose and lactitol. Lactobionic acid and tagatose are not considered to be prebiotics, although unsubstantiated claims have been made for tagatose.
Evidence exists that β-galactosidases obtained from certain microorganisms (e.g., B. bifidum) tend to produce higher proportions of the longer chain galacto-oligosaccharides. These mixtures tend to be used more specifically by certain species of Bifidobacterium (Rabiu et al., 2001; Tzortzis et al., 2005a). This search for prebiotic carbohydrates with greater selectivity by probiotic bacteria is of high interest to manufacturers, and offers scope for the development of genuine synbiotic products. The ultimate in synbiotic combinations will include oligosaccharides that can not only benefit the proliferation and activity of the specific probiotic strains in the colon, but also protect those bacteria during manufacture, formulation and storage, and during gastrointestinal transit.
The efficiency of oligosaccharide synthesis is also being improved by modifying the β-galactosidase molecule by deletion of amino acid residues in the protein. This has converted the enzyme to being predominantly a transgalactosylation enzyme rather than a hydrolytic enzyme (Jorgensen et al., 2001). This truncated enzyme performed equally well at lactose concentrations ranging from 10 to 40%. Thus, there is much scope for modifying the chain length of oligosaccharides synthesized and to do this with high yields.
A number of the health benefits of lactose derivatives are well established, including the pharmaceutical applications of lactulose and lactitol as laxatives and treatments for hepatic encephalopathy. It is clear too that ingestion of GOSs and lactulose can modify the composition and activity of the intestinal microbiota, and there is some evidence also for prebiotic effects of lactitol and lactosucrose.
Increasing the numbers of bifidobacteria or lactobacilli in the intestinal microbiota of individuals with an unfavourable intestinal microbial balance appears with our current understanding of the human intestinal microbiota to be a reasonable approach to promoting intestinal health. However, basic research into the composition and role of different microbial populations within the intestinal microbiota in health and disease is an essential prerequisite for the development of appropriate prebiotic strategies. Little is currently known of the sub-genus changes in bifidobacterial populations that can be induced by non-digestible carbohydrates, or if such changes are important in a health context. A better understanding of what constitutes a "healthy" intestinal microbiota composition, and which microbial groups and activities are definitively involved in health and disease, will allow the development of prebiotics with specifically targeted health effects in the future. The recent research showing how infant formulae supplemented with GOSs can emulate many of the effects of human milk oligosaccharides on the infant microbiota and faecal consistency provides encouraging evidence for a useful role in infant nutrition. The challenge remains to link the observed changes in the intestinal microbiota with clinical end-points that clearly demonstrate a health benefit.
The galactose moiety in oligosaccharides is important in mammalian cell biology (Kobata, 1996). There is considerable interest in the development of novel galactosyl structures. This is evidenced by the emergence of a large number of glycoscience companies developing carbohydrate-based drugs. For example, there are opportunities to develop cost-effective manufacturing processes for fucose-containing and N-acetylglucosamine-containing galacto-oligosaccharides.
The fact that lactose derivatives are non-digestible and are fermented by the intestinal microbiota to SCFA possibly underpins many of the potential health benefits of these compounds. There is preliminary evidence for a range of health benefits, including improved mineral absorption, protection against colorectal cancer and positive impacts on insulin resistance and serum lipid concentrations. Further studies to elucidate mechanisms of action and to demonstrate clinical benefits in controlled feeding studies are certainly warranted.
References
3A Business Consulting. 2005. Global market analysis of whey and lactose products 2004–2009 (report published November 2005) J.M. Morks Gade 1, DK-8100 Aarhus C, Denmark.
AACC. 2001. The definition of dietary fiber. Report of the Dietary Fiber Definition Committee to the Board of Directors of the American Association of Cereal Chemists. Cereal Foods World 46, 112–126.
AACC. 2002. Method 32-33. Determination of trans-galacto-oligosaccharides in selected food products by ion- exchange chromatographic method. In: Approved Methods of the American Association of Cereal Chemists, 10th edition, p. 8.
Abbadi, A., Gotlieb, K.F., Meiberg, J.B.M., Peters, J.A., Van Bekkum, H. 1999. New Ca-sequestering materials based on the oxidation of the hydrolysis products of lactose. Green Chem. 1, 231–235.
Adlerberth, I. 1999. Establishment of a normal intestinal microflora in the newborn infant. In: Probiotics, Other Nutritional Factors and Intestinal Microflora (L.A. Hansen, R.H. Yolken, eds.), pp. 63–78, Nestle Nutrition Workshop Series, Vol. 42, Nestec Ltd, Vevey. Lippincott-Raven, Philadelphia.
Akiyama, K., Takase, M., Horikoshi, K., Okonogi, S. 2001. Production of galactooligosaccharides from lactose using a β-glucosidase from Thermus sp. Z-1 – recombinant production of thermostable β-glucosidase, purification, and use in galacto-oligosaccharide preparation, Biosci. Biotechnol. Biochem. 65, 438–441.
Alander, M., Matto, J., Kneifel, W., Johansson, M., Kogler, B., Crittenden, R., Mattila-Sandholm, T., Saarela, M. 2001. Effect of galacto-oligosaccharide supplementation on human faecal microflora and on survival and persistence of Bifidobacterium lactis Bb-12 in the gastrointestinal tract. Int. Dairy J. 11, 817–825.
Anon. 1997. Lactulose. Brochure of Morinaga Milk Industry Co., Ltd., Tokyo, Japan.
Anon. 2003. Material Safety Data Sheet for Lactulose Concentrate, rev. 2, dated 18 Sept. 2003, Canlac Division of Solvay Pharma, Quebec, Canada.
Asp, N.G., Burval, A., Dahlquist, A., Hallgren, P., Lundblad, A. 1980. Oligosaccharide formation during hydrolysis of lactose with Saccharomyces lactis lactase (Maxilact ®). II. Oligosaccharide structures. Food Chem. 5, 147–153.
AOAC International. 2006. Official Methods of Analysis, 18th edition revised. Method 2001.02.
Bailey, R. (2008). Japan: An established market for nutraceuticals. Nutraceuticals World Nov. 2008.
Bailey, R.W. 1965. Oligosaccharides, Pergamon Press, Oxford.
Bakker-Zierikzee, A.M., Alles, M.S., Knol, J., Kok, F.J., Tolboom, J.J.M., Bindels, J.G. 2005. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br. J. Nutr. 94, 783–790.
Ballongue, J. 1998. Bifidobacteria and probiotic action. In: Lactic Acid Bacteria- Microbiology and Functional Aspects (S. Salminen, A. Von Wright, eds.), 2nd edition, Marcel Dekker, New York, pp. 519–587.
Ballongue, J., Schumann, C., Quignon, P. 1997. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand. J. Gastroenterol. 32 Suppl. 222, 41–44.
Beadle, J.R., Saunders, J. P., Wadja Jr., T.J. 1991. Process for manufacturing tagatose, US Patent 5,002,612. March 26, 1991. Assigned to Biospherics Inc.
Beadle, J.R., Saunders, J.P., Wadja Jr., T.J. 1992. Process for manufacturing tagatose, US Patent 5,078,796. 7 January 1992. Assigned to Biospherics Inc.
Benson, F.R. 1978. Sugar alcohols. In: Kirk-Othmer Encyclopaedia of Chemical Technology (E. Ecteroth, ed.), Vol. 1, 3rd Edition, pp. 754–778, Wiley, New York.
Bertelsen, H., Andersen, H., Tvede, M. (2001). Fermentation of D-tagatose by human intestinal bacteria and dairy lactic acid bacteria. Microbial Ecol. Health Dis. 13, 87–95.
Bertelsen, H., Eriknauer, K., Bottcher, K., Christensen, J.S., Stougaard, P., Hansen, O.C., Jorgensen, F. 2006. Process for manufacturing of tagatose. US Patent 6,991,923 B2, issued Jan 31, 2006, assigned to Arla Foods Amba.
Bertlesen, H., Jensen, B.B., Buemann, B. 1999. D-tagatose – a novel low-calorie bulk sweetener with prebiotic properties. World Rev. Nutr. Dietetics 85, 98–109.
Blum, S., Schiffrin, E. J. 2003. Intestinal microflora and homeostasis of the mucosal immune response: Implications for probiotic bacteria? Curr. Iss. Intest. Microbiol. 4, 53–60.
Boehm, G., Stahl, B. 2003. Oligosaccharides. In: Functional Dairy Products (T. Mattila-Sandholm, M. Saarela, eds.), pp. 203– 243, Woodhead Publishing/CRC Press.
Boehm, G., Stahl, B. 2007 Oligosaccharides from milk . J. Nutr., 137, Suppl 847S–849S.
Boehm, G., Stahl, B., Jelinek, J., Knol, J., Miniello, V., Moro, G.E. 2005. Prebiotic carbohydrates in human milk and formulas. Acta Paed. 94 Suppl., 18–21.
Bolotin, A., et al. 2004. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nature Biotechnol. 22, 1554–1558.
Bongaerts, G., Severijnen, R., Timmerman, H. 2005. Effect of antibiotics, prebiotics and probiotics in treatment for hepatic encephalopathy. Med. Hypotheses 64, 64–68.
Booy, C.J. 1987. Lactitol – a New Food Ingredient. Bulletin 212, International Dairy Federation, Brussels, pp. 62–68.
Bouhnik, Y., Raskine, L., Simoneau, G., Vicaut, E., Neut, C., Flourié, B., Brouns, F., Bornet, F. R. 2004a. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am. J. Clin. Nutr. 80, 1658–1664.
Bouhnik, Y., Attar, A., Joly, F.A., Riottot, M., Dyard, F., Flourie B. 2004b. Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. Eur. J. Clin. Nutr. 58, 462–466.
Bourlioux, P., Koletzko, B., Guarner, F., Braesco, V. 2003. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. Am. J. Clin. Nutr. 78, 675–683.
Bovee-Oudenhoven, I.M.J., Termont, D.M.S.L., Heidt, P.J., Van der Meer, R. 1997. Increasing the intestinal resistance of rats to the invasive pathogen Salmonella enteritidis: additive effects of dietary lactulose and calcium. Gut 40, 497–504.
Brighenti, F., Benini, L., Del Rio, D., Casiraghi, C., Pellegrini, N., Scazzina, F., Jenkins, D.J.A., Vantini, I. 2006. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am. J. Clin. Nutr. 83, 817–822.
Bruzzese, E., Volpicelli, M., Squaglia, M., Tartaglione, A., Guarino, A. 2006. Impact of prebiotics on human health. Digest Liver Dis. 38 Suppl 2, S283–S287.
Burvall, A., Asp, N.G., Dahlquist, A. 1979. Oligosaccharide formation during hydrolysis of lactose with Saccharomyces lactis lactase (Maxilact ®) Part 1. Food Chem. 4, 243–250.
Camuesco, D., Peran, L., Comalada, M., Nieto, A., Di Stasi., L.C., Rodriguez-Cabezas, M.E., Concha, A., Zarzuelo, A., Galvez, J. 2005. Preventative effects of lactulose in the trinitrobenzenesulphonic acid model of rat colitis. Inflammatory Bowel Dis. 11, 265–271.
Carobbi, R., Bimbi, G., Cipolletti, G. 2001. Process for the preparation of a lactulose syrup by lactose isomerization. US Patent 6,214, 124, issued Aug 10, 2001, assigned to Inalco Spa.
Carobbi, R., Miletti, S., Franci, V. 1985. Process for preparing lactulose from lactose, in the form of a syrup or a crystalline product. U.S. Pat. No. 4,536, 221, issued Aug. 20, 1985, assigned to SIRAC Spa.
Carubelli, R. 1966. Transformation of disaccharides during borate ion-exchange chromatography. Isomerization of lactose into lactulose. Carbohydr. Res. 2, 480–485.
Challa, A., Rao, D.R., Chawan, C.B., Shackelford, L.1997. Bifidobacterium longum and lactulose suppress azoxymethane-induced colonic aberrant crypt foci in rats. Carcinogenesis 18, 517–521.
Chen, C., Li, L., Wu, Z., Chen, H., Fu, S. 2007. Effects of lactitol on intestinal microflora and plasma endotoxin in patients with chronic viral hepatitis. J. Infect. 54, 98–102.
Cho, Y.J., Shin, H.J., Bucke, C. 2003. Purification and biochemical properties of a galactooligosaccharide producing β-galactosidase from Bullera singularis. Biotechnol. Lett. , 25, 2107–2111.
Choi, J.J., Kwon, S.T., Oh, E.J. 2004a. Method for synthesis of galactooligosaccharide using Thermus caldophilus gk24 β-glycosidase and high-level expression system of recombinant Thermus caldophilus gk-24 β-glycosidase used in the same method – plasmid mediated gene transfer and expression in Escherichia coli for recombinant saccharide production. Korean Patent KR 2004033748, issued 28 Apr 2004.
Choi, H-J., Kim, C.S., Kim, P., Jung, H-C., Oh, D-K. 2004b. Lactosucrose bioconversion from lactose and sucrose by whole cells of Paenibacillus polymyxa harbouring levansucrase activity. Biotechnol. Prog. 20, 1876–1879.
Chonan, O., Matsumoto, K., Watanuki, M. 1995. Effect of galacto-oligosaccharides on calcium absorption and preventing bone loss in ovariectomized rats. Biosci. Biotechnol. Biochem. 59, 236–239.
Codex Alimentarius. 1998. Food Labelling Complete Texts. FAO/WHO, Rome.
Crittenden, R.G. 1999. Prebiotics. In: Probiotics: A Critical Review (G.W. Tannock, ed.), pp. 141– 156, Horizon Scientific Press, Wymondham, UK.
Crittenden, R.G., Playne, M.J. 1996. Production, properties and applications of food-grade oligosaccharides. Trends Food Sci. Technol. 7, 353–361.
Crittenden, R.G., Playne, M.J. 2002. Purification of food-grade oligosaccharides using immobilised cells of Zymomonas mobilis. Appl. Microbiol. Biotechnol. 58, 297–302.
Croft-Hill, A. 1898. Reversible zymohydrolysis. J. Chem. Soc. LXIII, 634–658.
Crout, D.H.G., Vic, G. 1998. Glycosidases and glycosyl transferases in glycoside and oligosaccharide synthesis. Curr. Opinion Chem. Biol. 2, 98–111.
Cummings, J.H., Macfarlane, G.T., Macfarlane, S. 2003. Intestinal bacteria and ulcerative colitis. Curr. Iss. Intest. Microbiol. 4, 9–20.
Dbouk, N., McGuire, B.M. 2006. Hepatic encephalopathy: a review of its pathophysiology and treatment. Curr. Treatment Options Gastroenterol. 9, 464–474.
De Preter, V., Geboes, K., Verbrugghe, K., De Vuyst, L., Vanhoutte, T., Huys, G., Swings, J., Pot, B., Verbeke, K. 2004. The in vivo use of the stable isotope-labelled biomarkers lactose-[N-15]ureide and [H-2(4)]tyrosine to assess the effects of pro- and prebiotics on the intestinal flora of healthy human volunteers. Br. J. Nutr. 92, 439–446.
De Preter, V., Vanhoutte, T., Huys, G., Swings, J., Rutgeerts, P., Verbeke, K. 2006. Effect of lactulose and Saccharomyces boulardii administration on the colonic urea-nitrogen metabolism and the bifidobacteria concentration in healthy human subjects. Alimentary Pharmacol. Therapeutics 23, 963–974.
De Slegte, J., et al. 2002. Determination of trans-galactooligosaccharides in selected food products by ion-exchange chromatography: collaborative study. J. Ass. Off. Anal. Chem. Internat. 85, 417–423.
Dombou, M., Tomioka, I., Tsurutani, R., Kitabatake, S., Nakajima, H. 1994. Method for production of a growth factor for Bifidobacterium sp. US Patent 5,294, 546, issued 15 March 1994, assigned to Unitika Ltd, Japan.
Dorscheid, R.E., Krumbholz, M.J. 1991. Method of manufacturing lactulose. US Patent 5,071, 530, issued 10 Dec 1991, assigned to Duphar International Research B.V.
Edwards, C.A., Parrett, A.M. 2002. Intestinal flora during the first months of life: new perspectives. Br. J. Nutr. 88(Suppl 1), S11–S18.
Fanaro, S., Boehm, G., Garssen, J., Knol, J., Mosca, F., Stahl, B., Vigi, V. 2005. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatrica 94, 22–26.
Favier, C., Neut, C., Mizon, C., Cortot, A., Colombel, J.F., Mizon, J. 1997. Fecal beta-D-galactosidase production and bifidobacteria are decreased in Crohn's disease. Digestive Dis. Sci. 42, 817–822.
Ferchaud-Roucher, V., Pouteau, E., Piloquet, H., Zaïr, Y., Krempf, M. 2005. Colonic fermentation from lactulose inhibits lipolysis in overweight subjects. Am. J. Physiol. 289, E716–E720.
Fernández-Bañares, F. 2006. Nutritional care of the patient with constipation. Clin. Gastroenterol. 20, 575–587.
Finkelman, M.A.J. 1989. Yeast strain development for extracellular enzyme production. In: Yeast Strain Selection (C.J. Panchal, ed.), pp. 185– 223, Marcel Dekker Inc., New York.
Fu, W., Song, K. 1993. Removal of boric acid catalyst in lactose isomerization. Chinese Patent CN 1,073,668.
Fujimori, I. 1992. Pet food, US Patent 5,294, 458, issued Mar 15, 1994, assigned to Maruha Corporation and Ensuiko Sugar Refining Co., Ltd., Japan.
Fujita K., Hara K., Hashimoto H., Kitahata S. 1990a. Purification and some properties of β-fructofuranosidase I from Arthrobacter sp. K-1. Agric. Biol. Chem. 54, 913–919.
Fujita, K., Hara, K., Hashimoto, H., Kitahata, S. 1990b. Transfructosylation catalyzed by β-fructofuranosidase I from Arthrobacter sp. K-1 Agric. Biol. Chem. 54, 2655–2661.
Fujita, K., Hara, K., Hashimoto, H., Kitahata, S. 1992a. Method for the preparation of fructose-containing oligosaccharide US Patent 5,089, 401, issued Feb 18, 1992, assigned to Ensuiko Sugar Refining Co Ltd., Japan.
Fujita K, Kitahata S, Hara, K., Hashimoto, H. 1992b. Production of lactosucrose and its properties. In: Carbohydrates in Industrial Synthesis (M.A. Clarke, ed.), pp. 68– 76, Proc. Symp. Div. Carbohyd. Chem. Amer. Chem. Soc., Bartens, Berlin.
Fujita, K., Hara, K., Sakai, S., Miyake, T., Yamashita, M., Tsunstomi, Y., Mitsuoka, Y. 1991. Effects of 4-β-D-galactosylsucrose (lactosucrose) on intestinal flora and its digestibility in humans. J. Jap. Soc. Starch Sci. 38, 249–255.
Garman, J., Coolbear, T., Smart, J.B. 1996. The effect of cations on the hydrolysis of lactose and the transferase reactions catalysed by β-galactosidase from six strains of lactic acid bacteria. Appl. Microbiol. Biotechnol. 46, 22–27.
German Culture Collection. 2007. www.dsmz.de/, accessed October 15, 2007.
Gibson, G.R., McCartney, A.L., Rastall, R.A. 2005. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 93, 31–34.
Gibson, G. R., Probert, H. M., Van Loo, J.A.E., Roberfroid, M.B. 2004. Dietary modulation of the human colonic microflora: updating the concept of prebiotics. Nutr. Res. Rev. 17, 257–259.
Gibson, G.R., Roberfroid, M.B. 1995. Dietary modulation of the human colonic microbiota – introducing the concept of prebiotics. J.Nutr. 125, 1401–1412.
Gorin, P.A., Spencer, J.F.T., Phaff, H.J. 1964. The structures of galactosyl-lactose and galactobiosyl-lactose produced from lactose by Sporobolomyces singularis. Can. J. Chem. 42, 1341–1344.
Greenberg, N.A., Mahoney, R.R. 1982. Production and characterization of β-galactosidase from Streptococcus thermophilus. J. Food Sci. 47, 1824–1828, 1835.
Greenberg, N.A., Mahoney, R.R. 1983. Formation of oligosaccharides by β-galactosidase from Streptococcus thermophilus. Food Chem. 10, 195–204.
Griffiths, M.W., Muir, D.D. 1978. Properties of a thermostable β-galactosidase from a thermophilic Bacillus: Comparison of the enzyme activity of whole cells, purified enzyme and immobilized whole cells. J. Sci. Food Agric. 29, 753–761.
Grogan, D.W. (1991). Evidence that β-galactosidase of Sulfobus solfataricus is only one of several activities of a thermostable β-D-glucosidase. Appl. Environ. Microbiol. 57, 1644–1649.
Guarner, F., Malagelada, J.R. 2003. Gut flora in health and disease. Lancet 361, 512–519.
Guth, J.H., Tumerman, L. 1970. Method of making lactulose. US Patent 3,546,206. Dec. 8, 1970, assigned to Kraftco Corp.
Gyorgy, P., Norris, R.F., Rose, C.S. 1954a. Bifidus factor I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch. Biochem. Biophys. 48, 193–201.
Gyorgy, P., Kuhn, R., Rose, C.S., Zilliken, F. 1954b. Bifidus factor II. Its occurrence in milk from different species and in other natural products. Arch. Biochem. Biophys. 48, 202–208.
Haarman, M., Knol, J. 2005. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 71, 2318–2324.
Haarman, M., Knol, J. 2006. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 72, 2359–2365.
Hansen, O.C., Jorgensen, F., Stougaard, P., Bertelsen, H., Bottcher, K., Christensen, H.J.S., Eriknauer, K. 2006. Thermostable isomerase and use hereof, in particular for producing tagatose. US Patent 7,052, 898, issued 30 May 2006, assigned to Bioneer A/S.
Hara, K., Fujita, K., Yamashita, M., Tsunetomi, Y., Sakai, S., Miyake, T. 1992. Process for preparing lactosucrose high-content powder. US Patent 5,130, 239, issued July 14, 1992, assigned to KK Hayashibara SKK, and Ensuiko SKK.
Hara, K., Fujita, K., Yamashita, M., Tsunetomi, Y., Sakai, S., Miyake, T. 1994a. Process for preparing a powder having a high concentration of lactosucrose and use of said powder. US Patent 5,296, 473, March 22, 1994, assigned to KK Hayashibara SKK.
Hara, H., Li, S.-T., Sasaki, M., Maruyama, T., Terada, A., Ogata, Y., Fujita, K., Ishigami, H., Hara, K., Fujimori, I., Mitsuoka, T. 1994b. Effective dose of lactosucrose on fecal flora and fecal metabolites of humans. Bifidobacteria Microfl. 13, 51–63.
Hardy, M.R., Townsend, R.R. 1988. Separation of positional isomers of oligosaccharides and glycopeptides by high-performance anion-exchange chromatography with pulsed amperometric detection. Proc. Natl. Acad. Sci., USA 85, 3289–3293.
Harmsen, H.J.M., Wildeboer-Veloo, A.C.M., Raangs, C.G., Wagendorp, A.A., Klijn, N., Bindels, J.G., Welling, G.W. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 30, 61–67.
Heavey, P.M., Savage, S.A., Parrett, A., Cecchini, C., Edwards, C.A., Rowland, I.R. 2003. Protein-degradation products and bacterial enzyme activities in faeces of breast-fed and formula-fed infants. Br. J. Nutr. 89, 509–515.
Hicks, K.B. 1981. Ketose sugars from aldose sugars. U.S. Pat. No. 4,273, 922, issued Jun. 16, 1981, assigned to USA.
Hicks, K.B., Parrish, F.W. 1980. A new method for the preparation of lactulose from lactose. Carbohydr. Res. 82, 393–397.
Hicks, K.B., Simpson, G.L., Bradbury, A.G.W. 1986. Removal of boric acid and related compounds from solutions of carbohydrates with boron-selective resin (IRA-743). Carbohydr. Res. 147, 39–48.
Holma, R., Juvonen, P., Asmawi, M.Z., Vapaatalo, H., Korpela, R. 2002. Galacto-oligosaccharides stimulate the growth of bifidobacteria but fail to attenuate inflammation in experimental colitis in rats. Scand. J. Gastroenterol. 37, 1042–1047.
Inchem. 2008. International Programme on Chemical Safety (IPCS) and the Canadian Centre for Occupational Health and Safety (CCOHS) website IPCS INCHEM. www.inchem.org/pages/jecfa.html accessed 17 April 2008.
IFST. 2007. Information Statement on Dietary Fibre. (Institute of Food Science & Technology Trust Fund, London, U.K.) 10 pages. www.ifst.org/, accessed 5 June 2007.
IDF. 1995. Provisional Standard. Milk. Determination of lactulose content. Enzymatic method. International Dairy Federation, Brussels, Standard 175.
Ipatiew, W. 1912. Katalytische Reaktionen bei hohen Temperaturen und Drucken. XXV Berichte der Deutschen Chemischen Gesselschaft. 45, 3218–3226 [in German].
Ishikawa, E., Sakai, T., Ikemura, H., Matsumoto, K., Abe, H. 2005. Identification, cloning, and characterization of a Sporobolomyces singularis β-galactosidase-like enzyme involved in galacto-oligosaccharide production. J. Biosci.Bioeng. 99, 331–339.
IUPAC. 1996. Nomenclature of carbohydrates. Pure Appl. Chem. 68, 1919–2008.
Izume, M., Nagae, S., Kawagishi, H., Ohtakara, A. 1992. Preparation of N-acetylchitooligosaccharides from enzymatic hydrolyzates of chitosan. Biosci. Biotech. Biochem. 56, 1327–1328.
JECFA. 2008. Joint FAO/WHO Expert Committee on Food Additives. www.who.int/ipcs/food/jecfa/en/, accessed 17 April 2008.
Ji, E.S., Park, N.H., Oh, D.K. 2005. Galacto-oligosaccharide production by a thermostable recombinant β-galactosidase from Thermotoga maritima – Thermotaga maritima recombinant thermostable β-galactosidase production for galacto-oligosaccharide preparation. World J. Microbiol. Biotechnol. 21, 759–764.
Johansson, E., Hedbys, L., Mosbach, K., Larsson, P.-O. 1989. Studies of the reversed-mannosidase reaction in high concentrations of mannose. Enz. Microbial. Technol. 11, 347–352.
Jorgensen, F., Hansen, O.C., Stougaard, P. 2001. High efficiency synthesis of oligosaccharides with a truncated β-galactosidase from Bifidobacterium bifidum. Appl. Microbiol. Biotechnol. 57, 647–652.
Jouët, P., Sabate, J.-M., Cuillerier, E., Coffin, B., Lemann, M., Jian, R., Flourie, B. 2006. Low-dose lactulose produces a tonic contraction in the human colon. Neurogastroenterol. Motility 18, 45–52.
Juśkiewicz, J., Klewicki, R., Zdunczyk, Z. 2006. Consumption of galactosyl derivatives of polyols beneficially affects cecal fermentation and serum parameters in rats. Nutr. Res. 26, 531–536.
Kawakami, K., Makino, I., Asahara, T., Kato, I., Onoue, M. 2005. Dietary galacto-oligosaccharides mixture can suppress serum phenol and p-cresol levels in rats fed tyrosine diet. J. Nutr. Sci. Vitaminol. 51, 182–186.
Kawase, M., Pilgrim, A., Araki, T., Hashimoto, K. 2001. Lactosucrose production using a simulated moving bed reactor. Chem. Eng. Sci. 56, 453–458.
Kim, P. 2004. Current studies on biological tagatose production using L-arabinose isomerase: A review and future prospects. Appl. Microbiol. Biotechnol. 65, 243–249.
Kirjavainen, P.V., Reid, G. 2006. The infant intestinal microbiota in allergy. In: Gastrointestinal Microbiology (A.C. Ouwehand, E. E. Vaughan, eds.), pp. 189– 205, Taylor and Francis, New York.
Knol, J., Boehm, G., Lidestri, M., Negretti, F., Jelinek, J., Agosti, M., Stahl, B., Marini, A., Mosca, F. 2005. Increase of faecal bifidobacteria due to dietary oligosaccharides induces a reduction of clinically relevant pathogen germs in the faeces of formula-fed preterm infants. Acta Paed. 94, 31–33.
Kobata, A. 1996. Glycobiology. In: Encyclopaedia of Molecular Biology and Molecular Medicine (R.A. Meyers, ed.), Volume 2, pp. 446– 457, VCH, Weinheim, Germany.
Kobayashi, Y., Kan, T., Terashima, T. 1990. Method for producing processed milk containing galactooligosaccharide. U.S. Patent 4944952. issued 31 July 1990; assigned to K.K. Yakult Honsha.
Koizumi, S., Endo, T., Tabata, K., Ozaki, A. 1998. Large-scale production of UDP-galactose and globotiose by coupling metabolically engineered bacteria. Nature Biotechnol. 16, 847–850.
Kozempel, M.F., Kurantz, M.J. 1994. The isomerization kinetics of lactose to lactulose in the presence of borate. J. Chem. Tech. Biotechnol. 59, 25–29.
Kummel, K.F., Brokx, S. 2001. Lactitol as a functional prebiotic. Cereal Foods World 46, 424–429.
Kunz, C., Rudloff, S. 1996. Strukturelle und funktionelle aspekte von oligosacchariden in frauenmilch. Z. Ernahrungswiss. 35, 22–31.
Kunz, C., Rudloff, S. 2002. Health Benefits of Milk-Derived Carbohydrates. Bulletin 375, International Dairy Federation, Brussels, pp. 72–79.
Kunz, C., Rudloff, S., Baier, W., Klein, N., Strobel, S. 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Ann. Rev. Nutr. 20, 699–722.
Laerke, H.N., Jensen, B.B. 1999. D-Tagatose has low small intestinal digestibility but high large intestinal fermentability in pigs. J. Nutr. 129, 1002–1009.
Laerke, H.N., Jensen, B.B., Hojsgaard, S. 2000. In vitro fermentation pattern of D-tagatose is affected by adaptation of the microbiota from the gastrointestinal tract of pigs. J. Nutr. 130, 1772–1779.
LFRA. 2000. Ingredients Handbook: Prebiotics and probiotics (G. Gibson, F. Angus, eds.), Leatherhead Food Research Association Ltd., Surrey, UK.
Lee, Y.C. (1990). High performance anion-exchange chromatography for carbohydrate analysis. Anal. Biochem. 189, 151–162.
Lee, Y-J., Kim, C.S., Oh, D-K. 2004. Lactulose production by β-galactosidase in permeabilized cells of Kluyveromyces lactis. Appl. Microbiol. Biotechnol. 64, 787–793.
Lemoine, J., Chirat, F., Wieruszeski, J.-M, Strecker, G., Favre, N., Neeser, J.-R. 1997. Structural characterization of the exocellular polysaccharides produced by Streptococcus thermophilus SFi39 and SFi12. Appl. Environ. Microbiol., 63, 3512–3518.
Linko, S., Enwald, S., Vahvaselka, M., Mayra-Makinen, A. 1992. Optimization of the production of β-galactosidase by an autolytic strain of Streptococcus salivarius subsp. thermophilus. Ann. New York Acad. Sci. 672, 588–594.
Linskens, R.K., Huijsdens, X.W., Savelkoul, P.H.M., Vandenbroucke-Grauls, C.M.J.E., Meuwissen, S.M.G. 2001. The bacterial flora in inflammatory bowel disease: Current insights in pathogenesis and the influence of antibiotics and probiotics. Scand. J. Gastroenterol. 36, S29–S40.
Ludwig, R., Ozga, M., Zamocky, M., Peterbauer, C., Kulbe, K.D., Haltrich, D. 2004. Continuous enzymatic regeneration of electron acceptors used by flavoenzymes: Cellobiose dehydrogenase-catalyzed production of lactobionic acid as an example. Biocatal. Biotransform. 22, 97–104.
Macfarlane, S., Macfarlane, G.T., Cummings, J.H. 2006. Review article: prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Therapeut. 24, 701–714.
Mahoney, R.R. 1997. Lactose: enzymatic modification. In: Advanced Dairy Chemistry (P.F. Fox, ed.), 2nd Edition, Vol. 3, pp. 77–125, Chapman & Hall, London.
Mahoney, R.R., Whitaker, J.R. 1978. Purification and physico-chemical properties of β-galactosidase from Kluyveromyces fragilis. J. Food Sci. 43, 584–591.
Mahoney, R.R., Nickerson, T.A., Whitaker, J.R. 1975. Selection of strain, growth conditions, and extraction procedures for optimum production of lactase from Kluyveromyces fragilis. J. Dairy Sci. 58, 1620–1629.
Marteau, P., Seksik, P., Shanahan, F. 2003. Manipulation of the bacterial flora in inflammatory bowel disease. Best Practice Res: Clin. Gastroenterol. 17, 47–61.
Matsumoto, K., Kobayashi, Y., Ueyama, S., Watanabe, T., Tanaka, R., Kan, T., Kuroda, A., Sumihara, Y. 1993. Galactooligosaccharides. In: Oligosaccharides. Production, Properties, and Applications (T. Nakakuki, ed.), Japanese Technology Reviews Vol 3, No. 2, pp. 90–106, Gordon and Breach, Science Publishers, Basel.
McCartney A.L., Gibson, G.R. 2006. The normal microbiota of the human gastrointestinal tract: history of analysis, succession, and dietary influences. In: Gastrointestinal Microbiology (A.C. Ouwehand, E.E. Vaughan, eds.), pp. 51–73, Taylor and Francis, New York.
Mendicino, J. F. (1960). Effect of borate on the alkali-catalyzed isomerisation of sugars. J. Am. Chem. Soc., 82, 4975–4979.
Mesters, P.H.J., Brokx, S. 2000. Lactitol: a functional prebiotic. Functional Foods 2000 Conference Proceedings, The Hague, The Netherlands (F. Angus, C. Miller, eds.), pp. 173–189, Leatherhead Publishing, UK.
Meyer, D., Tungland, B. 2001. Non-digestible oligosaccharides: their physiological effects and health implications. In: Advanced Dietary Fibre Technology (B.V. McCleary, L. Prosky, eds.), pp. 455– 470, Blackwell Publishing, Oxford, UK.
Minami,Y., Yazawa, K., Tamura, Z., Tanaka, T., Yamamoto, T. 1983. Selectivity of utilization of galactosyl-oligosaccharides by bifidobacteria. Chem. Pharm. Bull. 31, 1688–1691.
Miyamoto, Y., Ooi, T., Kinoshita, S. 2000. Production of lactobionic acid from whey by Pseudomonas sp. LS13-1. Biotechnol. Lett. 22, 427–430.
Mizota, T., Tamura, Y., Tomita, M., Okonogi, S. 1987. Lactulose as a Sugar with Physiological Significance. Bulletin 212, International Dairy Federation, Brussels, pp. 69–76.
Mizota, T. 1996. Lactulose as a growth promoting factor for Bifidobacterium and its physiological aspects. Bulletin 313, International Dairy Federation, Brussels, pp. 43–48.
Modler, H.W., McKellar, R.C., Yaguchi, M. 1990. Bifidobacteria and bifidogenic factors. Can. Inst. Food Sci. Technol. 23, 29–41.
Montgomery, E.M., Hudson, C.S. 1930. Relations between rotatory power and structure in the sugar group. XXVII. Synthesis of a new disaccharide ketose (lactulose) from lactose. J. Am. Chem. Soc. 52, 2101–2106.
Morohashi, T. 2002. The effect on bone of stimulated intestinal mineral absorption following fructooligosaccharide consumption in rats. Biosci. Microfl. 21, 21–25.
Mountzouris, K.C., McCartney, A.L., Gibson, G.R. 2002. Intestinal microflora of human infants and current trends for its nutritional modulation. Br. J. Nutr. 87, 405–420.
Mozaffar, Z., Nakanishi, K., Matsuno, R., Kamikubo, T. 1984. Purification and properties of β-galactosidase from Bacillus circulans. Agric. Biol. Chem. 48, 3053–3061.
Mwenya, B., Santoso, B., Sar, C., Gamo, Y., Kobayashi, T., Arai, I., Takahashi, J. 2004a. Effects of including β1-4 galacto-oligosaccharides, lactic acid bacteria or yeast culture on methanogenesis as well as energy and nitrogen metabolism in sheep. Anim. Feed Sci. Technol. 115, 313–326.
Mwenya, B., Zhou, X., Santoso, B., Sar, C., Gamo, Y., Kobayashi, T., Takahashi, J. 2004b. Effects of probiotic-vitacogen and β1-4 galacto-oligosaccharides supplementation on methanogenesis and energy and nitrogen utilization in dairy cows. Asian-Austral. J. Anim. Sci. 17, 349–354.
Nagendra, R., Rao, S.V. 1992. An improved colorimetric method for the estimation of lactulose in lactose-lactulose mixtures. Food. Chem. 43, 399–402.
Nakakuki, T. 2002. Present status and future of functional oligosaccharide development in Japan. Pure Appl. Chem. 74, 1245–1251.
Nakanishi, K., Matsuno, R., Torii, K., Yamamoto, K., Kamikubo, T. 1983. Properties of immobilized β-galactosidase from Bacillus circulans. Enz. Microb. Technol. 5, 115–120.
Nakao, M., Harada, M., Kodama, Y., Nakayama, T., Shibano, Y., Amachi, T. 1994. Purification and characterization of a thermostable β-galactosidase with high transgalactosylation activity from Saccharopolyspora rectivirgula. Appl. Microb. Biotechnol. 40, 657–663.
Nakayama, T., Kodama, Y., Amano, N., Nakao, M., Shibano, Y., Amachi, T. 1992. Heat-resistant β-galactosyltransferase, its production process and its use. United States Patent 5153128. issued 10 June 1992; assigned to Suntory ltd., Osaka, Japan.
Nakayama, T., Kodama, Y., Amano, N., Nakao, M., Shibano, Y., Amachi, T. 1993. Process for producing novel heat-resistant β-galactosyltransferase. U.S.Patent 5,234,828, issued 10 Aug 1993; assigned to Suntory Ltd, Osaka, Japan.
NCBI. 2007. National Center for Biological Information, US National Library of Medicine, USA, Taxonomy browser (www.ncbi.nlm.nih.gov), accessed 13 August 2007.
Nilsson, K.G.I. 1988. Enzymatic synthesis of oligosaccharides. Trends Biotechnol. 6, 256–264.
Normen, L., Laerke, H. N., Jensen, B.B., Langkilde, A.M., Andersson, H. 2001. Small-bowel absorption of D-tagatose and related effects on carbohydrate digestibility: an ileostomy study. Am. J. Clin. Nutr. 73, 105–110.
Ohkusa, T., Ozaki,Y., Sato, C., Mikuni, K., Ikeda, H. 1995. Long-term ingestion of lactosucrose increases Bifidobacterium sp. in human fecal flora. Digestion 56, 415–420.
Ohtsuka, K., Oki, S., Owaza, O., Uchida, T. 1992. [Isolation and cultural conditions of galactooligosaccharide producing yeast Cryptococcus laurentii]. Hakkokogaku Kaishi 66, 225–233.
Ohtsuka, K., Tanoh, A., Ozawa, O., Kanematsu, T., Uchida, T., Shinke, R. 1990. Purification and properties of a β-galactosidase with high galactosyl transfer activity from Cryptococcus laurentii. J. Ferment. Bioeng. 70, 301–307.
Onishi, N., Kira, I., Yokozeki, K. 1996. Galacto-oligosaccharide production from lactose by Sirobasidium magnum CBS6803. Lett. Appl. Microbiol. 23, 253–256.
Onishi, N., Tanaka, T. 1995. Purification and properties of a novel thermostable galacto-oligosaccharide-producing β-galactosidase from Sterigmatomyces elviae CBS 8119. Appl. Environ. Microbiol. 61, 4026–4030.
Onishi, N., Tanaka, T. 1996. Purification and properties of a galacto- and gluco-oligosaccharide-producing β-glycosidase from Rhodotorula minuta IFO879. J. Ferment. Bioeng. 82, 439–443.
Onishi, N., Tanaka, T. 1997. Purification and characterization of galacto-oligosaccharide producing β-galactosidase from Sirobasidium magnum. Lett. Appl. Microbiol. 24, 82–86.
Onishi, N., Yamashiro, A., Yokozeki, K. 1995. Production of galacto-oligosaccharide from lactose by Sterigmatomyces elviae CBS 8119. Appl. Environ. Microbiol. 61, 4022–4025.
Onishi, N., Yokozeki, K. 1992. Method for producing galactose transfer products. US Patent 5149640; issued Sept 22, 1992, assigned to Ajinomoto Co., Inc.
Özaslan, C., Türkçapar, A.G., Kesenci, M., Karayalçin, K., Yerdel, M.A., Bengisun, S., Törüner, A. 1997. Effect of lactulose on bacterial translocation. Eur. J. Surg. 163, 463–467.
Ozawa,O., Ohtsuka, K., Uchida, T., Usami, S. 1991. 4′-Galactosyl lactose production in a jar fermentor by Cryptococcus laurentii OKN-4. J. Ferm. Bioeng. 72, 309–310.
Park, N.-H., Choi, H.-J., Oh, D.-K. 2005. Lactosucrose production by various microorganisms harbouring levansucrase activity. Biotechnol. Lett. 27, 495–497.
Parrish, F.W. 1970. Isomerization of glucose, maltose and lactose with amino compounds. US Patent 3,514,327. May 26, 1970, assigned to US Army.
Pazur, J.H. 1970. Oligosaccharides. In: The Carbohydrates: Chemistry and Biochemistry (W. Pigman, D. Horton, A. Herp, eds.), pp. 69–137, Vol. IIA, 2nd edition, Academic Press, New York.
Pazur, J.H. 1953. The enzymatic conversion of lactose into galactosyl oligosaccharides. Science 117, 355–356.
Pazur, J.H. 1954. The mechanism of enzymatic synthesis of galactosyl oligosaccharides. J. Biol. Chem. 208, 439–444.
Pazur, J.H., Tipton, C.L., Budovich, T., Marsh, J.M. 1958. Structural characterization of products of enzymatic disproportionation of lactose. J. Am. Chem. Soc. 80, 119–121.
Petuely, F. 1957. Bifidusflora bei flaschenkindern durch bifidogene substanzen (Bifidusfaktor). Z. Kinderheilkunde 79, 174–179.
Petzelbauer, I., Nidetzky, B., Haltrich, D., Kulbe, K.D. 1999. Development of an ultra high temperature process for the enzymatic hydrolysis of lactose. I. The properties of two thermostable β-glycosidases. Biotechnol. Bioeng. 64, 322–332.
Petzelbauer I., Zeleny, R., Reiter, A., Kulbe, K.D., Nidetzky, B. 2000. Development of an ultra high temperature process for the enzymatic hydrolysis of lactose. II. Oligosaccharide formation by two thermostable β-glycosidases. Biotechnol. Bioeng. 69, 140–149.
Petzelbauer, I., Splechtna, B., Nidetzky, B. 2002a. Development of an ultra high temperature process for the enzymatic hydrolysis of lactose. III. Utilization of two thermostable β-glycosidases in a continuous ultrafiltration membrane reactor and galacto-oligosaccharide formation under steady-state conditions. Biotechnol. Bioeng. 77, 394–404.
Petzelbauer, I., Kuhn, B., Splechtna, B., Kulbe, K.D., Nidetzky, B. 2002b. Development of an ultra high temperature process for the enzymatic hydrolysis of lactose. IV. Immobilization of two thermostable β-glycosidases and optimization of a packed-bed reactor for lactose conversion. Biotechnol. Bioeng. 77, 619–631.
Pilgrim, A., Kawase, M., Ohashi, M., Fujita, K., Murakami, K., Hashimoto, K. 2001. Reaction kinetics and modeling of the enzyme-catalyzed production of lactosucrose using β-fructofuranosidase from Arthrobacter sp. K-1. Biosci. Biotechnol. Biochem. 65, 758–765.
Pisani, F. M., Rella, R., Raia, C., Rozzo, C., Nucci, R., Gambacotra, A., DeRosa, M., Rossi, M. 1990. Thermostable β-galactosidase from the archaebacterium Sulfolobus solfataricus: purification and properties. Eur. J. Biochem. 187, 321–328.
Playne, M.J., Morel, B., Dimopoulos, A. 1993. Production of carbohydrate-based functional foods using enzyme and fermentation technologies. Proc. 11th Australian Biotechnol. Conf., Perth, pp. 207–209.
Playne, M.J., Crittenden, R. 1996. Commercially-available Oligosaccharides. Bulletin 313, International Dairy Federation, Brussels. pp.10–22.
Playne, M.J. 2002a. Galacto-oligosaccharides. In: Encyclopaedia of Dairy Sciences (H. Roginski, J.W. Fuquay, P.F. Fox eds.), pp. 1151–1158, Academic Press, San Diego, CA, USA.
Playne, M.J. 2002b. Glycoscience: oligosaccharides as drugs, functional foods, and receptors in the gut. Australas. Biotechnol. 12, 35–37.
Ponz de Leon, M., Roncucci, L. 1997. Chemoprevention of colorectal tumours: role of lactulose and of other agents. Scand. J. Gastroenterol. 32 Suppl. 222, 72–75.
Prasad, S., Dhiman, R.K., Duseja, A., Chawla, Y.K., Sharma, A., Agarwal, R. 2007. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 45, 549–559.
Prenosil, J.E., Stucker, E., Bourne, J.R. 1987a. Formation of oligosaccharides during enzymatic lactose: Part 1: State of art. Biotechnol. Bioeng. 30,1019–1025.
Prenosil, J.E., Stucker, E., Bourne, J.R. 1987b. Formation of oligosaccharides during enzymatic lactose hydrolysis and their importance in a whey hydrolysis process: Part II: Experimental. Biotechnol. Bioeng. 30, 1026–1031.
Pyun, Y.R., Kim, B.C., Lee, H.S., Lee, D.W., Lee, Y.H. 2005. Thermostable L-arabinose isomerase and process for preparing D-tagatose. US Patent 6,933,138 issued 23 Aug. 2005; assigned to CJ Corp.
Quah, H.M., Ooi, B.S., Seow-Choen, F., Sng, K.K., Ho, K.S. 2006. Prospective randomized crossover trial comparing fibre with lactulose in the treatment of idiopathic chronic constipation. Tech. Coloproctol. 10, 111–114.
Queen Mary University of London 2008. www.chem.qmul.ac.uk/iupac/2carb/, accessed 15 April 2008.
Quemener, B., Thibault, J.C., Coussement, P. 1994. Determination of inulin and oligofructose in food-products, and integration in the AOAC method for measurement of total dietary fiber. Lebensm. Wiss. Technol. 27, 125–132.
Rabiu, B.A., Jay, A.J., Gibson, G.R., Rastall, R.A. 2001. Synthesis and fermentation properties of novel galacto-oligosaccharides by β-galactosidases from Bifidobacterium species. Appl. Environ. Microbiol. 67, 2526–2530.
Rao, A.V. 1999. Dose-response effects of inulin and oligofructose on intestinal bifidogenesis effects. J. Nutr. 129 Suppl 7, 1442–1445.
Rastall, R.A. 2006. Galacto-oligosaccharides as prebiotics. In: Prebiotics: Development and Application (G.R. Gibson, R.A. Rastall, eds.), pp. 101– 110, John Wiley & Sons, Ltd, Chichester, England.
Rastall, R.A., Bucke, C. 1992. Enzymatic synthesis of oligosaccharides. Biotechnol. Gen. Eng. Rev. 10, 253–281.
Reddy, G.P., Bush, C.A. 1991. High-performance anion exchange-chromatography of neutral milk oligosaccharides and oligosaccharide alditols derived from mucin glycoproteins. Anal. Biochem. 198, 278–284.
Rinne, M.M., Gueimonde, M., Kalliomäki, M., Hoppu, U., Salminen, S.J., Isolauri, E. 2005. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol. Med. Microbiol. 43, 59–65.
Roberfroid, M. 2007. Prebiotics: the concept revisited. J. Nutr. 137 Suppl. 2, 830S–837S.
Roberts, H.R., Pettinati, J.D. 1957. Oligosaccharide production, concentration effects in the enzymatic conversion of lactose to oligosaccharides. J. Agric. Food Chem. 5, 130–134.
Rodriguez, A.P., Leiro, R.F., Trillo, C., Cerdan, E., Siso, I.G., Becerra, M. 2006. Secretion and properties of a hybrid Kluyveromyces lactis – Aspergillus niger β-galactosidase. Microbial Cell Factories 5, 41 (accessed at www.microbialcellfactories.com/content/5/1/41.).
Rowland, I.R., Bearne, C.A., Fischer, R., Pool-Zobel, B.L. 1996. The effect of lactulose on DNA damage induced by DMH in the colon of human flora-associated rats. Nutr. Cancer 26, 37–47.
Rowland, I.R., Tanaka. R. 1993. The effects of transgalactosylated oligosaccharides on gut flora metabolism in rats associated with a human faecal microflora. J. Appl. Bacteriol. 74, 667–674.
Ruffing, A., Chen, R.R. 2006. Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis. Microbial Cell Factories 5, 25 (accessed at www.microbialcellfactories.com/content/5/1/25).
Rumi, G., Tsubouchi, R., Okayama, M., Kato, S., Mózsik, G., Takeuchi, K. 2004. Protective effect of lactulose on dextran sulfate sodium-induced colonic inflammation in rats. Digest. Dis. Sci. 49, 1466–1472.
Saijonmaa, T., Heikonen, M., Kreula, M., Linko, P. 1978. Preparation and characterization of milk sugar alcohol, lactitol. Milchwissenschaft 33, 733–735.
Saito, T., Kato, S., Maeda, T., Suzuki, S., Iijima, S., Kobayashi, T. 1992. Overproduction of thermostable β-galactosidase in Escherichia coli, its purification and molecular structure. J. Ferment. Bioeng. 74, 12–16.
Sakai, K., Katsumi, R., Ohi, H., Usui, T., Ishido, Y. 1992. Enzymatic syntheses of N-acetyllactosamine and N-acetylallolactosamine by the use of β-D-galactosidases. J. Carbohydr. Chem., 11, 553–565.
Sako, T., Matsumoto, K., Tanaka, R. 1999. Recent progress on research and applications of non-digestible galacto-oligosaccharides. Int. Dairy J. 9, 69–80.
Santoso, B., Mwenya, B., Sar, C., Gamo, Y., Kobayashi, T., Morikawa, R., Kimura, K., Mizukoshi, H., Takahashi, J. 2004. Effects of supplementing galacto-oligosaccharides, Yucca schidigera or nisin on rumen methanogenesis, nitrogen and energy metabolism in sheep. Livestock Production Sci. 91, 209–217.
Sar, C., Santoso, B., Gamo, Y., Kobayashi, T., Shiozaki, S., Kimura, K., Mizukoshi, H., Arai, I., Takahashi, J. 2004. Effects of combination of nitrate with β1-4 galacto-oligosaccharides and yeast (Candida kefyr) on methane emission from sheep. Asian-Australasian J. Animal Sci. 17, 73–79.
Satory, M., Furlinger, M., Haltrich, D., Kulbe, K.D., Pittner, F., Nidetzky, B. 1997. Continuous enzymatic production of lactobionic acid using glucose-fructose oxidoreductase in an ultrafiltration membrane reactor. Biotechnol. Lett. 19, 1205–1208.
Saunier, K., Doré, J. 2002. Gastrointestinal tract and the elderly: functional foods, gut microflora and healthy ageing. Dig. Liver Dis. 34, suppl S 19–S24.
Scholnick, F., Ben-Et, G., Sucharski, M.K. Maurer, E.W., Linfield, W.M. 1975. Lactose-derived surfactants: II. Fatty esters of lactitol. J. Am. Oil Chem. Soc. 52, 256–258.
Schoterman, H.C. 2001. Galactooligosaccharides: properties and health aspects. In: Advanced Dietary Fibre Technology (D.B.V. McCleary, L. Prosky, eds.), pp. 494– 502, Blackwell Publishing, UK.
Schultz, M., Timmer, A., Herfarth, H.H., Sartor, R.B., Vanderhoof, J.A., Rath, H.C. 2004. Lactobacillus GG in inducing and maintaining remission of Crohn's disease. BMC Gastroenterol. 4, 5 (15 March 2004).
Schumann, C. 2002. Medical, nutritional and technological properties of lactulose. An update. Eur. J. Nutr. 41 Suppl 1, 17–25.
Senderens, J.B. 1920. Hydrogenation catalytique du lactose Compt. Rend. 170, 47–50 [in French].
She, Q., et al. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 98, 7835–7840.
Shimomura, Y., Maeda, K., Nagasaki, M., Matsuo, Y., Murakami, T., Bajotto, G., Sato, J., Seino, T., Kamiwaki, T., Suzuki, M. 2005. Attenuated response of the serum triglyceride concentration to ingestion of a chocolate containing polydextrose and lactitol in place of sugar. Biosci. Biotechnol. Biochem. 69, 1819–1823.
Shin, H.J., Park, J.M., Yang, J.W. 1995. Optimum culture condition of Bullera singularis for galactooligosaccharide production. Kor. J. Appl. Microbiol. Biotechnol. 23, 593–598.
Shin, H.J., Park, J.M., Yang, J.W. 1998. Continuous production of galacto-oligosaccharides from lactose by Bullera singularis β-galactosidase immobilized in chitosan beads. Process Biochem. 33, 787–792.
Shoaf, K., Mulvey, G.L., Armstrong, G.D., Hutkins, R.W. 2006. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 74, 6920–6928.
Sienkiewicz, T., Riedel, C.-L. 1990. Whey and Whey Utilization, 2nd edn. Verlag Th. Mann, Gelsenkirchen-Buer, Germany.
Sigma-Aldrich. 2007. Biofiles Vol. 2, No. 1, pp. 4–5.
Smart, J.B. 1989. Physiologically interesting side reactions of β-galactosidase. Proc. 8th Australian Biotechnol. Conf., Sydney, pp. 337–340.
Smart, J.B. 1991. Transferase reactions of the β-galactosidase from Streptococcus thermophilus. Appl. Microbiol. Technol. 34, 495–501.
Smart, J.B. 1993. Transferase Reactions of β-galactosidases – New Product Opportunities. Bulletin 289, International Dairy Federation, Brussels, pp. 16–22.
Southgate, D.A.T., Hudson, G.J., Englyst, H. 1978. The analysis of dietary fibre- the choices for the analyst. J. Sci. Food Agric. 29, 979–988.
Swanson, K.S., Fahey Jr, G.C. 2006. Prebiotic impacts on companion animals. In: Prebiotics: Development and Application (G.R. Gibson, R.A. Rastall, eds.), pp. 213– 236, John Wiley & Sons, Ltd, Chichester, England.
Takaichi, A., Okamoto, T., Azuma, Y., Watanabe, Y., Matsumoto, T., Miyata, K.,Sakamoto, S., Okamatsu, H. et al. 1995. Food composition for inhibiting the formation of an intestinal putrefactive product. US Patent 5,455,235., Oct 3, 1995, assigned to Otsuka Pharmaceutical Co., Ltd.
Tamura, Y., Mizota, T., Shimamura, S., Tomita, M. 1993. Lactulose and its Application to the Food and Pharmaceutical Industries. Bulletin 289, International Dairy Federation, Brussels. pp. 43–53.
Tamura, Z. 1983. Nutriology of bifidobacteria. Bifidobacteria Microflora 2, 3–16.
Tanaka, R., Matsumoto, K. 1998. Recent Progress on Prebiotics in Japan, Including Galacto-oligosaccharides. Bulletin 336, International Dairy Federation, Brussels, pp. 21–27.
Tannock, G.W., Munro, K., Bibiloni, R., Simon, M.A., Hargreaves, P., Gopal, P., Harmsen, H., Welling, G. 2004. Impact of consumption of oligosaccharide-containing biscuits on the fecal microbiota of humans. Appl. Environ. Microbiol. 70, 2129–2136.
Terada, A., Hara, H., Katoaka, M., Mitsuoka, T. 1992. Effect of lactulose on the composition and metabolic activity of human faecal flora. Microbial Ecol. Health Dis. 5, 43–50.
Thebaudin, J.Y., Lefebvre, A.C., Harrington, M., Bourgeois, C.M. 1997. Dietary fibres: nutritional and technological interest. Trends Food Sci. Technol. 8, 41–48.
Thelwall, L.A.W. 1997. Lactose: chemical derivatives. In: Advanced Dairy Chemistry (P.F. Fox, ed.), pp. 39– 76, Vol. 3, 2nd Edition, P.F. Fox, ed., Chapman & Hall, London.
Tlaskalova-Hogenova, H., Stepankova, R., Hudcovic, T., Tuckova, L.,Cukrowska, B., Lodinova-Zadnkova, R., Kozakova, H., Rossmann, P., Bartova, J., Sokol, D., Funda, D.P., Borovska, D., Rehakova, Z., Sinkora, J., Hofman, J., Drastich, P., Kokesova, A. 2004. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 93, 97–108.
Toba, T., Adachi, S. 1978. Hydrolysis of lactose by microbial β-galactosidases. Formation of oligosaccharide with special reference to 2-O-β-D-galactopyranosyl-D-glucose. J. Dairy Sci. 61, 33–38.
Toba, T., Tomita, Y., Itoh, T., Adachi, S. 1981. β-Galactosidases of lactic acid bacteria: characterization by oligosaccharides formed during hydrolysis of lactose. J. Dairy Sci. 64, 185–192.
Toba, T., Yokota, A., Adachi, S. 1985. Oligosaccharide structures formed during the hydrolysis of lactose by Aspergillus oryzae β-galactosidase. Food Chem. 16, 147–162.
Tomito, M., Shimamura, S., Tamura, Y., Mizota, T., Nakano, S., Suzawa, I. 1994. Crystalline lactulose trihydrate and a method for its manufacture. US Patent 5304251, publication date Apr. 19, 1994, assigned to Morinaga Milk Industry Co., Ltd.
Topping, D.L., Clifton, P.M. 2001. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064.
Townsend, R.R., Hardy, M.R., Hindsgaul, O., Lee, Y.C. 1988. High-performance anion-exchange chromatography of oligosaccharides using pellicular resins and pulsed amperometric detection. Anal. Biochem. 174, 459–470.
Tuohy, K.M., Ziemer, C.J., Klinder, A., Knobel, Y., Pool-Zobel, B.L., Gibson, G.R. 2002. A human volunteer study to determine the prebiotic effects of lactulose powder on human colonic microbiota. Microbial Ecol. Health Dis. 14, 165–173.
Tzortzis, G., Goulas, A.K., Gibson, G.R. 2005a. Synthesis of prebiotic galactooligosaccharides using whole cells of a novel strain, Bifidobacterium bifidum NCIMB 41171. Appl. Microbiol. Biotechnol. 68, 412–416.
Tzortzis, G., Goulas, A.K., Gee, J.M., Gibson, G.R. 2005b. A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. J. Nutr. 135, 1726–1731.
Usui T., Kubota, S., Ohi, H. 1993. A convenient synthesis of β-galactosyl disaccharide derivatives using the β-D-galactosidase from Bacillus circulans. Carbohydr. Res. 244, 315–323.
Van den Heuvel, E.G., Muijs, T., van Dokkum, W., Schaafsma, G. 1999. Lactulose stimulates calcium absorption in post-menopausal women. J. Bone Mineral Res. 14, 1211–1216.
Van den Heuvel, E.G., Schoterman, M.H.C., Muijs, T. 2000. Transgalactooligosaccharides stimulate calcium absorption in post-menopausal women. J. Nutr. 130, 2938–2942.
Vanhoutte, T., De Preter, V., De Brandt, E., Verbeke, K., Swings, J., Huys, G. 2006. Molecular monitoring of the fecal microbiota of healthy human subjects during administration of lactulose and Saccharomyces boulardii. Appl. Environ. Microbiol. 72, 5990–5997.
Van Laere, K.M.J., Abee, T., Schols, H.A., Beldman, G., Voragen, A.G.J. 2000. Characterization of a novel β-galactosidase from Bifidobacterium adolescentis DSM 20083 active towards transgalactooligosaccharides. Appl. Environ. Microbiol. 66, 1379–1384.
Van Loveren, C. 2004. Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res. 38, 286–293.
Van Riel, J., Olieman, C. 1991. Selectivity control in the anion-exchange chromatographic determination of saccharides in dairy products using pulsed amperometric detection. Carbohyd. Res. 215, 39–46.
Van Soest, P.J. 1978. Dietary fibers: their definition and nutritional properties. Am. J. Clin. Nutr. 31 Suppl., S12–S20.
Van Velthuijsen, J.A. 1979. Food additives derived from lactose: lactitol and lactitol palmitate. J. Agric. Food Chem. 27, 680–686.
Vogt, J.A., Ishii-Schrade, K.B., Pencharz, P.B., Jones, P.J.H., Wolever, T.M.S. 2006. L-Rhamnose and lactulose decrease serum triacylglycerols and their rates of synthesis, but do not affect serum cholesterol concentrations in men. J. Nutr. 136, 2160–2166.
Wallenfels, K. 1951. Enzymatische synthese von oligosacchariden aus disacchariden. Naturwissenschaften 38, 306–307.
Williams, C.M., Jackson, K.G. 2002. Inulin and oligofructose: effects on lipid metabolism from human studies. Br. J. Nutr. 87, Suppl 2, S261–S264.
Yanahira, S., Suguri, T., Yakabe, T., Ikeuchi, Y., Hanagata, G., Deya, E. 1992. Formation of oligosaccharides from lactitol by Aspergillus oryzae β-D-galactosidase. Carbohyd. Res. 232, 151–159.
Yanahira, S., Kobayashi, T., Suguri, T., Nakakoshi, M., Miura, S., Ishikawa, H., Nakajima, I. 1995. Formation of oligosaccharides from lactose by Bacillus circulans β-galactosidase. Biosci. Biotechnol. Biochem. 59, 1021–1026.
Yazawa, K., Tamura, Z. 1982. Search for sugar sources for selective increase of bifidobacteria. Bifidobacteria Microflora, 1, 34–44.
Yazawa, K., Imai, K., Tamura, Z. 1978. Oligosaccharides and polysaccharides specifically utilizable by bifidobacteria. Chem. Pharm. Bull. (Tokyo) 26, 3306–3311.
Yoneyama, M., Mandai, T., Aga, H., Fujii, K., Sakai, S., Katayama, Y. 1992. Effects of 4-β-d-galactosylsucrose (lactosucrose) intake on intestinal flora in healthy humans. J. Jap. Soc. Nutr. Food Sci. 45, 101–107.
Ziegler, E., Vanderhoof, J.A., Petschow, B., Mitmesser, S.H., Stolz, S.I., Harris, C.L., Berseth, C.L. 2007. Term infants fed formula supplemented with selected blends of prebiotics grow normally and have soft stools similar to those reported for breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 44, 359–364.
Zopf, D., Roth, S. 1996. Oligosaccharide anti-infective agents. Lancet 347, 1017–1021.
Acknowledgments
Ms Janet Werkmeister and staff of the Information Resource Centre, Dairy Australia, Melbourne and Ms Bee Thia of the CSIRO Ian Wark Laboratory are thanked for their bibliographic assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Playne, M., Crittenden, R. (2009). Galacto-oligosaccharides and Other Products Derived from Lactose. In: McSweeney, P., Fox, P. (eds) Advanced Dairy Chemistry. Springer, New York, NY. https://doi.org/10.1007/978-0-387-84865-5_5
Download citation
DOI: https://doi.org/10.1007/978-0-387-84865-5_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-84864-8
Online ISBN: 978-0-387-84865-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)