Abstract
Lactose is a common natural disaccharide composed of galactose and glucose molecules. It is mainly found in the whey, the by-product of cheese and casein industries. As the supply of lactose far exceeds demand, a lot of lactose was discarded as the waste every year, which not only leads to resource waste, but also causes environmental pollution. Therefore, the deep processing of lactose as the feedstock has become a hot research topic. The lactose-derived sugar alcohols, including lactitol, sorbitol, and galactitol, have shown great potential applications not only in food manufacture, but also in pharmaceutical, cosmetic, and material fields. In this paper, we focus on the property, physiological effect, production, and application of the lactose-derived sugar alcohols.
Key points
• The deep processing of lactose as the feedstock has become a hot research topic.
• The lactose-derived sugar alcohols show great application values.
• Recent advances in the lactose-derived sugar alcohols are reviewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
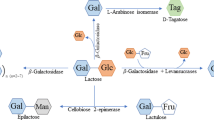
Lactose, β-D-galactopyranosyl-(1 → 4)-D-glucose, composed of glucose and galactose, is an abundant disaccharide in nature. It is widely present in the whey, which is the by-product of cheese and casein manufacture. In fact, the annual output of global lactose is more than 1 million tons, and this value is still increasing. However, due to lactose intolerance and other reasons, the commercial value of lactose is not high. Every year, huge amounts of lactose are thrown away as the waste together with whey. But the discarded lactose without recovery will cause serious environmental pollution (Marwaha and Kennedy 1988). Thus, the further utilization of lactose has become one of the current hot topics (Xiao et al. 2019b).
Because of its chemical structure and large-scale production, lactose has been widely used as the feedstock to produce high-value lactose-derived products. Usually, the utilizations of lactose are performed through hydrolysis (glucose and galactose), hydrogenation (mainly lactitol), oxidation (lactobionic acid), isomerization (lactulose and epilactose), and transglycosylation reactions (galacto-oligosaccharides and lactosucrose) (Cheng and Martinez-Monteagudo 2019). The lactose-derived sugars, including lactulose, epilactose, galacto-oligosaccharides, lactosucrose, and D-tagatose, were already described in our previous review (Xiao et al. 2019a).
In recent years, with the improvement of human lives, healthy diets become the focus of the public. Therefore, the low-calorie sugar alcohols with special physiological functions have been widely used as the sweeteners, flavor enhancers, humectants, and cooling agents in food industries, such as xylitol, sorbitol, mannitol, erythritol, and maltitol (Chen et al. 2020). Besides, some sugar alcohols also display the great potential as building blocks in high-value-added derivatives.
The lactose-derived sugar alcohols, including lactitol, sorbitol, galactitol, and lactulitol, are traditionally synthesized from lactose through hydrolysis and hydrogenation (Fig. 1). Lactitol is the primary product hydrogenation of lactose, while very small amount of lactulitol is also found at the same time. In addition, lactitol might be further hydrolyzed and hydrogenated to sorbitol and galactitol (Martinez-Monteagudo et al. 2019). Lactitol and sorbitol have already been applied in food industries for many years, as well as in cosmetic, toiletries, and pharmaceutical fields. Galactitol could also be used for the production of rare sugar or pharmaceutical intermediates. In this manuscript, the recent advances in the property, physiological effect, production, and application of the lactose-derived sugar alcohols were reviewed. It should be noted that, since there are almost no research reports on lactulitol, the related contents are not discussed in this review.
Lactitol
Properties of lactitol
Lactitol is a 12-carbon monoclinic polyol with no reducing activity. It is not present in nature, and usually obtained from lactose through chemical-catalyzed hydrogenation. In fact, solid-state lactitol exists in different crystalline forms, including one amorphous form, two anhydrate, and three hydrate forms. In most cases, it exists as lactitol monohydrate form, produced from the lactitol slurry through the slow crystallization method (Martinez-Monteagudo et al. 2019). The melting points of lactitol vary depending on the crystalline forms. For lactitol monohydrate, the melting point ranges from 93 to 100 °C, which is influenced by the grinding and drying extent (Halttunen et al. 2001). Lactitol displays about 40% relative sweetness as sucrose and a mild clean sweet taste without aftertaste. It only has 2.4 kcal/g calorie value, which is about 60% as calorie as sucrose (Table 1). Lactitol is well soluble in water and dimethyl sulfoxide (DMSO), slightly soluble in ethanol, but almost insoluble in chloroform, ether, and ethyl acetate. At room temperature, its solubility is similar to sucrose in water. Because of the absence of a carbonyl group in its chemical structure, lactitol displays a relatively high stability in high temperature and wide pH ranges (pH 3–9). Due to its unique physical and chemical properties, lactitol is widely used in low-sweetness foods (Kummel and Brokx 2001).
Physiological effects of lactitol
In recent years, there have been many studies on the physiological function of lactitol. Only 2% lactitol was absorbed and metabolized in the small intestine by passive diffusion, while the vast majority of lactitol reached the colon without digestion (Natah et al. 1997). The lactitol was slowly fermented in the large intestine acting as the energy source for colonic microflora. Then, it was transformed into short-chain fatty acids (SCFAs), lactic acid, butyric acid, CO2, and a little H2. As this result, lactitol was proven as an important prebiotic to stimulate Lactobacilli and Bifidobacteria growth (Finney et al. 2007). What is more, the combination of the prebiotics lactitol and probiotics Lactobacillus acidophilus NCFM displayed symbiotic beneficial effects on the microbiotal composition and mucosal functions (Ramos-Ramos et al. 2020). Unlike other sugar alcohol, lactitol is well tolerated. No undesirable laxative symptoms were observed when the intake was less than 20 g per day (Miller et al. 2014). As fermented in the mouth, the addition of lactitol could avoid the damage to tooth enamel and reduce the incidence of caries (Loveren and C. 2004). Besides, since lactitol was hardly absorbed in the small intestine, lactitol ingestion exhibited nearly no impact on blood glucose or insulin levels, suggesting that lactitol was a desirable sugar-free sweetener for diabetics (Drakoularakou et al. 2007). In addition, lactitol was one of the most common laxative agents for chronic constipation, because it could enhance the intestinal osmolality, lead to higher fecal volume and moisture content, and stimulate the peristalsis in large intestine (Li et al. 2020). According to many clinical evidence, lactitol was regarded as an efficient drug for the treatment of hepatic encephalopathy, because it could reduce oxidative injury and decrease gut-derived endotoxemia in patients with chronic viral hepatitis (Chen et al. 2013).
Production of lactitol
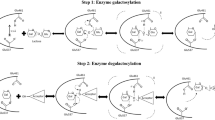
Lactitol is the primary lactose-derived sugar alcohol; however, it could not be found in the nature. The main method for lactitol production is catalytic hydrogenation from lactose, in which hydrogen reduction occurred at the carbonyl group of the glucose part. About 100 years ago, Senderens obtained lactitol from lactose hydrogenation catalyzed by active nickel, which was the first report about lactitol (Senderens 1920). After that, with the innovation of catalyst materials and optimization of catalytic conditions, chemical synthesis of lactitol developed rapidly (Coughlin and Nickerson 1975).
In chemically catalyzed reactions, the transition metals, reaction temperature, and pressure play the key role. These experimental factors could significantly influence the yield, selectivity, and by-product formation in lactose hydrogenation (Cheng and Martinez-Monteagudo 2019). Generally, the elevated temperatures and high hydrogen pressure could favor the lactitol formation. The hydrogenation process was usually carried out by changing the hydrogen pressure between 20 and 70 bar, at the temperature range from 110 to 150 °C. Because of the good selectivity for lactitol (> 90%), sponge nickel catalyst was previously used in industrial process of lactose hydrogenation. However, nickel-based catalysts were limited by the deactivation problem caused by fast nickel leaching and weak catalyst sintering. To solve this problem, the modified ruthenium-based, instead of nickel-based catalysts, are widely used in lactitol production. The ruthenium-based catalysts were usually supported by magnesium oxide (MgO), titanium dioxide (Ru/TiO2), alumina (Ru/Al2O3), silica gel (Ru/gel), cross-linked polystyrene (Ru/CP), hypercross-linked polystyrene (Ru/HPS), and activated carbon (Ru/C) (Kuusisto et al. 2008). Among these ruthenium-based catalysts, Ru/C (> 98%), Ru/HPS (> 97%), and Ru/CP (> 95%) displayed obviously higher selectivity for lactitol than the others. Overall, ruthenium-based catalysts were more active and stable than nickel-based catalysts. Recently, the ruthenium-nickel bimetallic nanohybrids on TiO2 (Ru–NiO/TiO2) were prepared, which could efficiently catalyze the lactose hydrogenation to lactitol. It displayed the highest selectivity (99.4%) for lactitol at almost complete conversion of lactose. What is more, the bimetallic Ru–NiO/TiO2 catalyst could be revered and reused for four times with no activity loss (Mishra et al. 2018).
For lactose hydrogenation, the primary catalytic products were lactitol at a conversion yield of about 90%, with small amount of lactulitol as the byproduct. Lactitol might be further hydrolyzed and hydrogenated to sorbitol and galactitol at a yield of 4.6–4.8%. After the whole hydrogenation catalysis, the filtration separation and ion-exchange resin purification were carried out, followed by lactitol concentration and crystallization. Now, the industrial production of lactitol in the world is mainly located in the USA, China, and Europe (Germany, United Kingdom, Switzerland, and Latvia) (Martinez-Monteagudo et al. 2019).
Application of lactitol
Lactitol has been widely used in food industries, due to its good properties and good food safety. For the regulation status, in 1983, lactitol was designated approved as a safe product by the Joint FAO/WHO Expert Committee of Food Additives (JECFA). In 1993, lactitol was classified as generally regarded as safe by the US Food and Drug Administration (FDA). In 2008, it was also allowed as a food additive (sweetening agent E966) in the EU countries. Besides, its use was also approved in many other countries, such as China, Japan, Canada, Australia, Brazil, and Argentina.
Owing to its low calories and moderate sweetness, lactitol is usually used as a bulk sweetener and ideal sucrose substitute in beverage, bakery, candy, and dessert manufacture. While in high-sweetness foods, lactitol is normally used via the combination with high-intensity sweeteners, such aspartame, sodium cyclamate, acesulfame-K, or saccharin sodium, nowadays, sugar-free chocolate becomes very popular, and anhydrous lactitol has been successfully applied in chocolate manufacture. Anhydrous lactitol shows good stability and low cooling effect, providing a top quality and flavor. Lactitol has mild clean taste and caries prevention effect; hence, it is supplied in sugar-free chewing gum together with other sugar alcohols. Besides, the high glass transition temperature and low hygroscopicity make it suitable for the hard candy and sugar-free tablets. In bakery products, as the result of low hygroscopicity, the addition of lactitol could maintain the hardness and brittleness and extend the shelf life (Psimouli and Oreopoulou 2012). In addition, the supplement of lactitol dihydrate displayed excellent cryoprotective effects on fish proteins during frozen storage of surimi (Sych et al. 1991).
Not only in food industries, lactitol also shows broad applications in many other fields, such as biotechnology pharmaceutical, animal feed, material science, and cultural relics preservation (Fig. 2). Because of its excellent physiological functions, lactitol has been already used for the treatment of diabetes (Olli et al. 2016), viral hepatitis (Chen et al. 2013), and chronic constipation (Vanderdonckt and Ravelli 1993). In addition, lactitol could be used as the raw material for D-gulose production through microbial and chemical methods. When fermentation using lactitol as sole carbon source, lactitol was firstly oxidized to the intermediate 3-ketolactitol by Agrobacterium tumefaciens M31, while 3-ketolactitol could be further converted to D-gulose and D-sorbitol by chemical catalysis (Morimoto et al. 2013). It was also reported that the synergistic supplement of lactitol and lactic acid bacteria could enhance the short-chain fatty acid and gas production from intestinal fermentation, which might improve the feed utilization of animal feeding (Piva et al. 2005). In material science fields, a series of lactitol-based polyether polyol (LPEP) were prepared and used in drug delivery system to control the release rates of bioactive compounds (Han et al. 2000). Additionally, lactitol is regarded as the potential surfactants and emulsifier, although there is no commercial application yet (Drummond and Wells 1998). Recently, it was also reported that lactitol showed great potential in preservation of cultural relics. Using pre-impregnation of lactitol and trehalose before freeze-drying, it could effectively reduce the shrinkage and deformation of the waterlogged archeological wood and improve the overall stability of cultural relics (Babinski 2015; Majka et al. 2017).
Sorbitol
Properties of sorbitol
Sorbitol, also known as glucitol, is a six-carbon sugar alcohol and existed naturally in any appreciable quantity, such as vegetables, fruits, tobacco, and seaweed (Fang et al. 2020). Sorbitol is the epimer of mannitol at C-2 position. While similar, these two sugar alcohols have different properties and applications. Sorbitol is a white, crystalline compound, and the solid-state sorbitol has four crystalline forms (α, β, γ, and δ) and one glass transition form (Ɛ). These different forms of sorbitol display very obviously different properties, for instance hygroscopicity, hardness, compressibility, and stability (Nezzal et al. 2009). Due to its high melting temperature, low hygroscopicity, and good stability, the γ-form sorbitol is preferred by the food and medicine industries. Crystalline sorbitol, except δ-form, exhibits well compressibility and is widely used in making tablets. It is well soluble in water, slightly soluble in methanol, ethanol, and acetic acid, but almost insoluble in chloroform and ether. With changes in the relative humidity of the surrounding environment, sorbitol gains or loses moisture slowly, which makes it excellent humectants in baked goods. In common with other sugar alcohols, sorbitol is relative stable and does not participate in the in Maillard browning reactions. Sorbitol is about 60% as sweet as sucrose and shows a smooth mouthfeel with a cool, sweet, and pleasant flavor. Sorbitol is usually regarded as a nutritive sweetener and provides dietary energy of 2.6 kcal/g, which is one-third fewer calories compared with sucrose (Table 1).
Physiological effects of sorbitol
Sorbitol could be slowly absorbed in the small intestine passively, and the absorption speed was much lower than that of glucose and fructose. After absorption, sorbitol was firstly transformed into fructose by sorbitol dehydrogenase (SDH), and then converted into fructose 6-phosphate, finally metabolized by the glycolytic pathway (Adcock and Gray 1957). The sorbitol metabolism was not regulated by insulin and did not cause an increase in blood glucose level. According to glycemic response test, the glycemic index (GI) of sorbitol is ultra-low at about 10, which belongs to low GI component (less than 50). As this result, it was used as a sugar alternative for diabetics. In addition, the unabsorbed sorbitol could stimulate bowel peristalsis through osmotic pressure, which displays diuretic and laxative effects (Godswill 2017). Usually, the ingested sugar could be fermented on the teeth surface by the oral bacteria, which produced acid and eventually led to the dental caries or tooth decay. Sorbitol was not fermented by the oral bacteria, so it was regarded as non-cariogenic (Rafeek et al. 2019). It is allowed to consume on 40 g of sorbitol daily, but less than 10 g per single dose (Yao et al. 2014).
Production of sorbitol
Sorbitol is one of the most common sugar alcohols and is widespread in plants and fruits, such as in pears, apples, peaches, and grapes. Although found widely in nature, the commercial production of sorbitol is currently the catalytic hydrogenation of glucose, starch, or cellulose (Zhang et al. 2013). In essence, starch or cellulose is first converted into glucose, and then subsequently hydrogenated to sorbitol. The transition metals, such as Ru, Ni, Rh, Pt, or Pd, were used as the catalysts in the hydrogenation process, while the Ru catalysts showed higher hydrogenation activity than others. The hydrogen pressure ranged from 40 and 120 bar, while reaction temperature ranged from 130 to 150 °C. There are so many researches, so the chemical hydrogenation of glucose is not discussed in detail here. Due to its chemical structure, sorbitol could be produced from lactose via glucose or lactitol as the intermediate. It was reported that, using Cu/SiO2 as the catalyst, lactose could be effectively hydrolyzed and hydrogenated to a mixture of sorbitol and galactitol with a yield of 75–86% (Zaccheria et al. 2017).
Recently, the bioproduction of sorbitol has attracted many attention (Rice et al. 2020). In previous studies, sorbitol could be converted from fructose by the facultative anaerobic Zymomonas mobilis fermentation via glucose-fructose oxidoreductase (GFOR). By treatment with 10% (v/v) toluene, the free Z. mobilis cells produced 290 g/L sorbitol and 283 g/L gluconic acid from a 60% sugar solution (300 g/L fructose and 300 g/L glucose), with yields of 96.7% and 94.3%, respectively (Chun and Rogers 1988). Furthermore, using the recombinant Z. mobilis strain harboring the pHW20a-gfo for GFOR over-expression, nearly 2-fold increase of the fermentation activity was obtained in a batch fermentation, when compared with the wild-type strains (Liu et al. 2010). What is more, the bioengineering approaches on lactate dehydrogenase (LDH)-deficient mutants of lactic acid bacteria, such as Lactobacillus plantarum and Lactobacillus casei, were developed for sorbitol production from glucose, which enhanced sorbitol production (overexpress sorbitol-6-phosphate dehydrogenase, S6PDH), blocked the sorbitol consumption (deactivate the sorbitol phosphotransferase system genes, PTS sorbitol), and reduced other by-products (disrupt mannitol-1-phosphate dehydrogenase, M1PDH) (Ladero et al. 2007). In addition, using the engineered L. casei mutants, it could produce sorbitol from lactose directly, with a conversion rate of 9.4% (De Boeck et al. 2010).
Application of sorbitol
As one of the most commercial and common sugar alcohols available, sorbitol was used in a wide range of food, pharmaceutical, cosmetic manufacture. Sorbitol was approved as safe by the JECFA, with an acceptable daily consumption of ‘not specified’, which was the safest category. In addition, it was affirmed as GRAS by FDA, and also allowed to be used as a food additive by EU and many countries, for example China, Japan, Canada, and Australia.
Because of its low calories and cool sweet taste, sorbitol was widely used in sugar-free candy, chocolate, and beverage. Besides, sorbitol displayed the anti-caries properties, which brought the main application in gum, toothpaste, and mouthwash. It was also an excellent humectants and anti-crystallizing agent available in bakery products. Additionally, sorbitol could lower the freezing point, which made it suitable in ice cream production (Sheet et al. 2014). In surimi and meat processing, sorbitol was usually as the water retention agent, anti-freezing agent, and cryoprotect agent (Dey and Dora 2011). In particular, sorbitol exhibited low glycemic response; as this result, it was an important sucrose alternative in the diets of diabetics.
What is more, sorbitol also showed many non-food applications (Fig. 2). For example, it was an important intermediate for the manufacture of L-ascorbic acid (vitamin C), which occupied nearly 15% of world sorbitol production every year (Pappenberger and Hohmann 2013). It was reported that sorbitol was an efficient laxative, because it could drew water in the large intestine and stimulate bowel movements (Izzy et al. 2016). In modern cosmetics, it was usually used as the humectant and thickener. Most importantly, sorbitol could be degraded into isosorbide and lower alcohols (ethylene glycol, propylene glycol, and glycerol). These lower alcohols were the important chemical raw materials, while isosorbide was a key bio-based chemical compound in biomedicine, cosmetic, and polymer material industries (Zhang et al. 2013).
Galactitol
Physiological effects of galactitol
Galactitol, also known as dulcitol (basic physicochemical properties shown in Table 1), is a metabolic breakdown product of galactose and is involved in metabolism of galactose. In the principal pathway for galactose metabolism, also called Leloir pathway, galactose was firstly phosphorylated to galactose-1-phosphate (Gal1P) by galactokinase; subsequently, Gal1P was converted to UDP-galactose and glucose-1-phosphate through replacement reaction with UDP-glucose catalyzed by Gal1P uridyl transferase; after that, the UDP-galactose was transformed to UDP-glucose by UDP-galactose 4-epimerase; finally, UDP-glucose was recycled and converted to glucose-1-phosphate and entered the glucose metabolism pathway. However, a small amount of galactose was converted to galactitol by aldose reductase, and then transformed to galactonic acid by galactose dehydrogenase (Liu et al. 2000). Deficiencies of these galactose metabolism-related enzymes, especially Gal1P uridyl transferase, would lead to the galactosemia disease. This disease causes the accumulation of Gal1P and galactose in the body. The accumulated galactose might be reduced to galactitol, while the galactitol in the lens would cause cataract disease (Leslie 2003). In a worse case, it might lead to liver damage, renal failure, and growth retardation for infants (Berry et al. 2001).
Production of galactitol
Galactitol is another important lactose-derived alcohol. Although it can be produced from galactose by chemical hydrogenation, the bioproduction of galactitol also attracted many researchers’ attention. It was firstly reported that several yeast could produce galactitol when growing on galactose. Muniruzzaman et al. constructed a new method for galactitol production from lactose. In this method, lactose was first transformed into galactose by commercial β-D-galactosidase, subsequently converted to galactitol by Mycobacterium smegmatis SMDU strains (Muniruzzaman et al. 1994). Using the engineered Saccharomyces cerevisiae, 3.5 g/L galactitol was obtained from 40 g/L of lactose directly by microbial fermentation (Liu et al. 2019). Recently, it was reported that Rhodosporidium toruloides could produce 8.4 g/L galactitol from 40 g/L galactose when growing in nitrogen-rich medium. Interestingly, when growing in nitrogen-poor medium, R. toruloides produced not only galactitol but also lipids (Jagtap et al. 2019).
Application of galactitol
Generally, galactitol was widely applied in the production of rare sugar or pharmaceutical intermediates (Fig. 2). Galactitol is mainly used to produce D-tagatose by galactitol dehydrogenase (GDH) via C-2 oxidation (Lu et al. 2019). After optimization of the induction condition, 3.16 g/L D-tagatose was obtained from 20 g/L galactitol through Gluconobacter oxydans fermentation (Manzoni et al. 2001). It was also found that the addition of glycerol could enhance the GDH activity and increase D-tagatose production, and 4.4 g/L D-tagatose was produced from 22.5 g/L galactitol (Rollini and Manzoni 2005). Among many recombinant GDH, Rhizobium leguminosarum GDH displayed the highest activity towards galactitol, with a specific activity of 13.5 U/mg and conversion rate of 72% (Jagtap et al. 2014). In addition, galactitol could be converted to D-sorbose by Pseudomonas sp. fermentation with 70% conversion ratio. In this process, galactitol was firstly dehydrogenated to D-tagatose, and then epimerized to D-sorbose (Khan et al. 1992). Besides, L-tagatose could be produced from galactitol by GDH oxidation with conversion rate of 78% (Huwig et al. 1997). What is more, 6.96 g/L hexanoic acid was obtained from 15 g/L galactitol through Clostridium sp. fermentation (Jeon et al. 2013). Recently, the galactitol-based polymers exhibited great potential applications in bone regeneration and drug delivery (Natarajan et al. 2017).
References
Adcock L, Gray C (1957) The metabolism of sorbitol in the human subject. Biochem J 65(3):554–560
Babinski L (2015) Dimensional changes of waterlogged archaeological hardwoods pre-treated with aqueous mixtures of lactitol/trehalose and mannitol/trehalose before freeze-drying. J Cult Herit 16(6):876–882
Berry GT, Hunter JV, Wang ZY, Dreha S, Mazur A, Brooks DG, Ning C, Zimmerman RA, Segal S (2001) In vivo evidence of brain galactitol accumulation in an infant with galactosemia and encephalopathy. J Pediatr 138(2):260–262
Chen C, Yu X, Lu H, Xiao D, Mao W, Li L (2013) Antioxidant protective effects of lactitol against endotoxemia in patients with chronic viral hepatitis. Mol Med Rep 7(2):401–405
Chen M, Zhang W, Wu H, Guang C, Mu W (2020) Mannitol: physiological functionalities, determination methods, biotechnological production, and applications. Appl Microbiol Biotechnol 104:6941–6951
Cheng SY, Martinez-Monteagudo SI (2019) Hydrogenation of lactose for the production of lactitol. Asia Pac J Chem Eng 14(1):e2275
Chun U, Rogers P (1988) The simultaneous production of sorbitol from fructose and gluconic acid from glucose using an oxidoreductase of Zymomonas mobilis. Appl Microbiol Biotechnol 29(1):19–24
Coughlin JR, Nickerson T (1975) Acid-catalyzed hydrolysis of lactose in whey and aqueous solutions. J Dairy Sci 58(2):169–174
De Boeck R, Sarmiento-Rubiano LA, Nadal I, Monedero V, Pérez-Martínez G, Yebra MJ (2010) Sorbitol production from lactose by engineered Lactobacillus casei deficient in sorbitol transport system and mannitol-1-phosphate dehydrogenase. Appl Microbiol Biotechnol 85(6):1915–1922
Dey SS, Dora KC (2011) Suitability of chitosan as cryoprotectant on croaker fish (Johnius gangeticus) surimi during frozen storage. J Sci Food Agric 48(6):699–705
Drakoularakou A, Hasselwander O, Ouwehand AC (2007) Lactitol, an emerging prebiotic: functional properties with a focus on digestive health. Food Sci Technol Bull Funct Foods 3(7):71–80
Drummond CJ, Wells D (1998) Nonionic lactose and lactitol based surfactants: comparison of some physico-chemical properties. Colloid Surface A 141(1):131–142
Fang T, Cai Y, Yang Q, Ogutu CO, Liao L, Han Y (2020) Analysis of sorbitol content variation in wild and cultivated apples. J Sci Food Agric 100(1):139–144
Finney M, Smullen J, Foster HA, Brokx S, Storey DM (2007) Effects of low doses of lactitol on faecal microflora, pH, short chain fatty acids and gastrointestinal symptomology. Eur J Nutr 46(6):307–314
Godswill AC (2017) Sugar alcohols: chemistry, production, health concerns and nutritional importance of mannitol, sorbitol, xylitol, and erythritol. Int J Adv Acad Res 3:31–66
Halttunen H, Rajakyla E, Nurmi J, Perkkalainen P, Pitkanen I (2001) Comparison of two melting range analysis methods with lactitol monohydrate. Thermochim Acta 380(1):55–65
Han JH, Krochta JM, Kurth MJ, Hsieh YL (2000) Lactitol-based poly(ether polyol) hydrogels for controlled release chemical and drug delivery systems. J Agric Food Chem 48(11):5278–5282
Huwig A, Emmel S, Jakel G, Giffhorn F (1997) Enzymatic synthesis of L-tagatose from galactitol with galactitol dehydrogenase from Rhodobacter sphaeroides D. Carbohydr Res 305(3–4):337–339
Izzy M, Malieckal A, Little E, Anand S (2016) Review of efficacy and safety of laxatives use in geriatrics. World J Gastrointest pharmacol Therap 7(2):334–342
Jagtap SS, Singh R, Kang YC, Zhao HM, Lee JK (2014) Cloning and characterization of a galactitol 2-dehydrogenase from Rhizobium legumenosarum and its application in D-tagatose production. Enzym Microb Technol 58:44–51
Jagtap SS, Bedekar AA, Liu JJ, Jin YS, Rao CV (2019) Production of galactitol from galactose by the oleaginous yeast Rhodosporidium toruloides IFO0880. Biotechnol Biofuels 12(1):250
Jeon BS, Moon C, Kim BC, Kim H, Um Y, Sang BI (2013) In situ extractive fermentation for the production of hexanoic acid from galactitol by Clostridium sp BS-1. Enzym Microb Technol 53(3):143–151
Khan AR, Takahata S, Okaya H, Tsumura T, Izumori K (1992) D-Sorbose fermentation from galactitol by Pseudomonas sp. ST 24. J Ferment Bioeng 74(3):149–152
Kummel KF, Brokx S (2001) Lactitol as a functional prebiotic. Cereal Foods World 46(9):424–429
Kuusisto J, Mikkola J-P, Sparv M, Wärnå J, Karhu H, Salmi T (2008) Kinetics of the catalytic hydrogenation of D-lactose on a carbon supported ruthenium catalyst. Chem Eng J 139(1):69–77
Ladero V, Ramos A, Wiersma A, Goffin P, Schanck A, Kleerebezem M, Hugenholtz J, Smid EJ, Hols P (2007) High-level production of the low-calorie sugar sorbitol by Lactobacillus plantarum through metabolic engineering. Appl Environ Microbiol 73(6):1864–1872
Leslie ND (2003) Insights into the pathogenesis of galactosemia. Annu Rev Nutr 23(1):59–80
Li X, Zhang X, Wu X, Lan Y, Xu L, Meng X, Li J (2020) Beneficial effects of lactitol on the composition of gut microbiota in constipated patients. J Digest Dis (accepted 21:445–453. https://doi.org/10.1111/1751-2980.1291
Liu G, Hale GE, Hughes CL (2000) Galactose metabolism and ovarian toxicity. Reprod Toxicol 14(5):377–384
Liu C, Dong H, Zhong J, Ryu DD, Bao J (2010) Sorbitol production using recombinant Zymomonas mobilis strain. J Biotechnol 148(2–3):105–112
Liu J, Zhang G, Kwak S, Oh EJ, Yun EJ, Chomvong K, Cate JHD, Jin YS (2019) Overcoming the thermodynamic equilibrium of an isomerization reaction through oxidoreductive reactions for biotransformation. Nat Commun 10(1):1–8
Loveren V, C. (2004) Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res 38(3):286–293
Lu F, Xu W, Zhang W, Guang C, Mu W (2019) Polyol dehydrogenases: intermediate role in the bioconversion of rare sugars and alcohols. Appl Microbiol Biotechnol 103(16):6473–6481
Majka J, Babinski L, Olek W (2017) Sorption isotherms of waterlogged subfossil scots pine wood impregnated with a lactitol and trehalose mixture. Holzforschung 71(10):813–819
Manzoni M, Rollini M, Bergomi S (2001) Biotransformation of D-galactitol to tagatose by acetic acid bacteria. Process Biochem 36(10):971–977
Martinez-Monteagudo SI, Enteshari M, Metzger L (2019) Lactitol: production, properties, and applications. Trends Food Sci Technol 83:181–191
Marwaha S, Kennedy J (1988) Whey—pollution problem and potential utilization. I J Food Sci Technol 23(4):323–336
Miller LE, Tennilä J, Ouwehand AC (2014) Efficacy and tolerance of lactitol supplementation for adult constipation: a systematic review and meta-analysis. Clin Exp Gastroenterol 7:241
Mishra DK, Dabbawala AA, Truong CC, Alhassan SM, Jegal J, Hwang JS (2018) Ru-NiOx nanohybrids on TiO2 support prepared by impregnation-reduction method for efficient hydrogenation of lactose to lactitol. J Ind Eng Chem 68:325–334
Morimoto K, Shimonishi T, Miyake S, Takata G, Izumori K (2013) Preparation of D-gulose from disaccharide lactitol using microbial and chemical methods. Biosci Biotechnol Biochem 77(2):253–258
Muniruzzaman S, Itoh H, Yoshino A, Katayama T, Izumori K (1994) Biotransformation of lactose to galactitol. J Ferment Bioeng 77(1):32–35
Natah SS, Hussien KR, Tuominen JA, Koivisto VA (1997) Metabolic response to lactitol and xylitol in healthy men. Am J Clin Nutr 65(4):947–950
Natarajan J, Movva S, Madras G, Chatterjee K (2017) Biodegradable galactitol based crosslinked polyesters for controlled release and bone tissue engineering. Mater Sci Eng C Mater Biol Appl 77:534–547
Nezzal A, Aerts L, Verspaille M, Henderickx G, Redl A (2009) Polymorphism of sorbitol. J Cryst Growth 311(15):3863–3870
Olli K, Saarinen MT, Forssten SD, Madetoja M, Herzig KH, Tiihonen K (2016) Independent and combined effects of lactitol, polydextrose, and bacteroides thetaiotaomicron on postprandial metabolism and body weight in rats fed a high-fat diet. Front Nutr 3:15
Pappenberger G, Hohmann HP (2013) Industrial production of L-ascorbic acid (vitamin C) and D-isoascorbic acid, in biotechnology of food and feed additives. Springer, pp 143–188
Piva A, Casadei G, Gatta PP, Luchansky JB, Biagi G (2005) Effect of lactitol, lactic acid bacteria, or their combinations (synbiotic) on intestinal proteolysis in vitro, and on feed efficiency in weaned pigs. Can J Anim Sci 85(3):345–353
Psimouli V, Oreopoulou V (2012) The effect of alternative sweeteners on batter rheology and cake properties. J Sci Food Agric 92(1):99–105
Rafeek R, Carrington CVF, Gomez A, Harkins D, Torralba M, Kuelbs C, Addae J, Moustafa A, Nelson KE (2019) Xylitol and sorbitol effects on the microbiome of saliva and plaque. J Oral Microbiol 11(1):1536181
Ramos-Ramos JC, Lazaro-Perona F, Arribas JR, Garcia-Rodriguez J, Mingorance J, Ruiz-Carrascoso G, Borobia AM, Pano-Pardo JR, Herruzo R, Arnalich F (2020) Proof-of-concept trial of the combination of lactitol with Bifidobacterium bifidum and Lactobacillus acidophilus for the eradication of intestinal OXA-48-producing Enterobacteriaceae. Gut Pathog 12(1):1–18
Rice T, Zannini E, Arendt EK, Coffey A (2020) A review of polyols–biotechnological production, food applications, regulation, labeling and health effects. Crit Rev Food Sci Nutr 60(12):2034–2051
Rollini M, Manzoni M (2005) Bioconversion of D-galactitol to tagatose and dehydrogenase activity induction in Gluconobacter oxydans. Process Biochem 40(1):437–444
Senderens J (1920) Catalytic hydrogenation of lactose. Comptes Rendus 170:47–50
Sheet BS, Artık N, Ayed MA, Abdulaziz OF (2014) Some alternative sweeteners (xylitol, sorbitol, sucralose and stevia). Karaelmas Sci Eng J 4(1):63–70
Sych J, Lacroix C, Carrier M (1991) Determination of optimal level of lactitol for sSurimi. J Food Sci 56(2):285–290
Vanderdonckt J, Ravelli GP (1993) Lactitol as an alternative to harsh (irritant) laxatives. An exploratory, open pilot-study in chronic functional constipation. Acta Therap 19(3):295–308
Xiao Y, Chen Q, Guang C, Zhang W, Mu W (2019a) An overview on biological production of functional lactose derivatives. Appl Microbiol Biotechnol 103(9):3683–3691
Xiao Y, Chen Q, Shakhnovich EI, Zhang W, Mu W (2019b) Simulation-guided enzyme discovery: a new microbial source of cellobiose 2-epimerase. Int J Biol Macromol 139:1002–1008
Yao CK, Tan HL, van Langenberg DR, Barrett JS, Rose R, Liels K, Gibson PR, Muir JG (2014) Dietary sorbitol and mannitol: food content and distinct absorption patterns between healthy individuals and patients with irritable bowel syndrome. J Hum Nutr Diet 27:263–275
Zaccheria F, Mariani M, Scotti N, Psaro R, Ravasio N (2017) Catalytic upgrading of lactose: a rest raw material from the dairy industry. Green Chem 19(8):1904–1910
Zhang J, Li J, Wu S, Liu Y (2013) Advances in the catalytic production and utilization of sorbitol. Ind Eng Chem Res 52(34):11799–11815
Funding
This study was funded by the National Natural Science Foundation of China (No. 31801583 and 31922073), the Natural Science Foundation of Jiangsu Province (No. BK20180607), and the National First-Class Discipline Program of Food Science and Technology of China (JUFSTR20180203).
Author information
Authors and Affiliations
Contributions
WZ wrote the manuscript. JC collected references and drew the figures. HW and QC contributed to manuscript editing. WM designed, supervised, and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Chen, J., Chen, Q. et al. Sugar alcohols derived from lactose: lactitol, galactitol, and sorbitol. Appl Microbiol Biotechnol 104, 9487–9495 (2020). https://doi.org/10.1007/s00253-020-10929-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10929-w