Abstract
The chapter deals with the brief introduction to water and energy crisis. Further, it presents a detailed introduction of microbial fuel cell (MFC) technology discussing its various components, types and different substrates treatable in MFC with various terminal electron acceptors utilised in it along with critical literature survey of last 5 years. As the fossil fuels stand on the edge of extinction, studies for new and potential renewable resources have become a focus of scientific research. The need to look for alternatives brought the scientific community to the domain of waste harvesting to generate potential renewable resources that can take the reign of energy generation from fossil fuels to lead the human civilisation to new highs in near future. MFC technology has recently garnered considerable attention due to its unique nature to create a symbiotic relationship between the wastewater and electric output. It works on the principle of redox reaction where the wastewater present in the anodic compartment generates electrons to flow through the circuit and produce current. An MFC system can treat a wide variety of waste streams ranging from simple substrate like glucose/acetate to more complex substrates like domestic/industrial wastewater. Several novel designs of MFC have been suggested over the years with the same basic idea of anaerobic anode and aerobic cathode. MFC presents a promising approach for waste treatment contrary to the conventional technologies with zero energy input, low sludge production, compact designing with no movable parts for easy handling and efficient performance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 The Major Concerns
Water and energy are the two critical and extensively expanding demands of the twenty-first century’s sustainable society. Scarcity or lack of freshwater, according to World Health Organisation (WHO), affects more than 40% of the world’s population [192]. Water finds application in every aspect of life ranging from agriculture to domestic and industrial processes. These water utilising sources release effluents with undesirable pH, colour, odour, temperature and chemicals, termed as wastewater. This wastewater when mixed with fresh water and utilised for different purposes causes various health hazards. Clean and freshwater is a basic need of all living organisms. However, the toxicants such as dyes, pesticides, drugs and metal ions when present in the potable water induce severe environmental and health issues. They have high bioaccumulation potential, which is another serious and important concern. Figure 1 presents some facts about water—its distribution and pollution (WHO and UN-Water sites) [148, 159]. Recently, wastewater released from various sources like domestic and factory effluent has gained attention as a potential resource of renewable energy to harvest electric energy and offset the wastewater treatment cost, which is otherwise high for conventional treatment technologies.
Further, the energy demand is continuously increasing around the world and most of this demand is fulfilled through fossil fuels. The development of all the economies around the world has been supported by these fuels for centuries by expanded industrialisation. However, with the limited availability, threatened depletion and the drastic consequence of fossil fuels on the environment (greenhouse gases (GHGs) emission), focus has shifted to sustainable and renewable sources of energy, a much-needed alternative to fulfil the energy demand efficiently and cleanly. Nonetheless, data released by International Energy Agency (IEA) in 2017 [62] reflected that the renewable resources only account for around 23% of the total energy produced (Fig. 2). Biofuels, in the recent years, have drawn worldwide attention in this context. In near future, bioelectricity can serve as a potential source of fuel to serve the purpose of energy requirement on a larger scale. It is a renewable source of energy with potential to become a replacement of fossil fuels for power production in future [96]. Thus, it can serve as GHG neutral alternative to the conventional energy sources (e.g., fossil fuels), which release heavy amounts of GHGs into the atmosphere thus intensifying global warming [100, 157]. Industrial and agricultural wastewater has high organic content that can be utilised to derive energy. Data released by IEA in 2017 reflected, however, that these resources (biomass and waste) only account for around 2% of the total energy produced globally (Fig. 2). Also, country-wise energy generation data suggest that Asian countries like China and India have high energy demand, which is expected to grow in the near future as per IEA energy outlook 2019 [63] (Fig. 3).
India is among the top 10 countries in the world facing the worst water crisis as nearly 54% population of India lives under water stress. With heavy consumption, 21 cities (including Delhi, Chennai) are estimated to run out of groundwater in very near future. By 2030, early 40% of Indian population may be affected with nearly no access to fresh drinking water as water demand will rise from 650 in 2008 to 1498 billion cubic meters by 2030. On the other hand, with increasing development in both urban and rural sectors of the country, the energy demand is continuously increasing with the overall energy consumption of 1561 TWh in 2018 as compared to 1317 TWh in 2015. By 2040, the Indian share of global energy demand is estimated to increase by 2 times and with coal being the major source, the CO2 emission will simultaneously be roughly doubled worsening its effect of environment. India is in urgent need to counter these growing concerns in an environmentally friendly way to pave way for a better future.
2 Renewable Sources of Energy
The world has seen different eras of energy from charcoal to coal to oil era of twentieth century (Fig. 4). Owing to their threatened depletion and environmental effects, these non-renewable resources are being replaced with more renewable ones.
Resources that can be repeatedly replenished in nature and do not run out with time are known as renewable resources, and energy produced from these resources is known as renewable energy or clean energy. Solar, wind, tidal, hydro and geothermal energies have been successfully employed to harvest renewable energy around the world. Figure 5 presents the flowchart of the various techniques available for generating renewable energy. Recently, bioenergy has garnered significant attention as a potential renewable energy source marking twenty-first century as the beginning of era of bioenergy. Bioenergy is the energy derived from biomass, which ranges from food waste and plants to wastewater [125].
In the recent years, as the direct derivation of renewable energy from the waste has become a potential option, bioelectrochemical systems have played a significant role in extraction of usable energy from waste.
Different bioelectrochemical systems have been developed over the years with microbial fuel cell (MFC) being the most explored technology. Figure 6 presents the number of papers published on different bioenergy techniques (MFC, MDC, MEC, and BES) while Fig. 7 shows the papers published on MFC between 2015 and January 2020 according to Web of Science data search.
3 Microbial Fuel Cell Technology
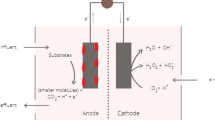
The need and interest in finding the sustainable alternatives to the non-renewable sources of energy that are cost-effective and performance efficient have brought the focus of the scientists across the world to MFC technology (Fig. 8). MFC is a technology capable of harvesting energy from organic and inorganic chemical wastes in the form of electricity. The technology has garnered significant attention because of its capability of harvesting energy from waste with almost zero energy input [79]. MFC can be defined as the technology that utilises active microbial population to catalyse the oxidation of organic and inorganic waste to harvest energy in the form of bioelectricity. It acts as a connecting link between microbial metabolism and electrochemistry of the system [79]. The first idea of harvesting electric energy with the aid of microorganisms’ dates back to the early twentieth century when Potter and co-workers in 1911 suggested that living cultures like E. coli and Sacchromyces can assist in the production of electricity. However, the work did not gain much attention until the 1931 work by Cohen, who connected MFCs in series producing voltage larger than 35 V [157]. Further, NASA in 1960s researched the applications of MFCs in space missions. Later, in 1980s, Allen and Bennetto discovered that the performance of MFC can be greatly improved by using mediators to support electron conduction from microbes to the electrodes. However, the instability and toxicity of the mediators offered obstructions in their practical application. A major breakthrough came when Kim et al. in 1999 [63] showed that the electricity conduction does not require mediators and some microbial communities can transfer electrons to the electrodes directly using microbial metabolites. Bruce Logan and team are considered to be the first to develop a laboratory-scale MFC [48, 124]. The basic principle of MFC is based on the redox reaction taking place within the system where the oxidation taking place at the anode generates electrons and protons lowering the redox potential at the anode. The electrons produced travel through the external circuit while the protons migrate across the membrane to the cathode where they are accepted by the terminal electron acceptor (TEA) at higher redox potential. The flow of electron across the circuit as a result of developed potential gradient generates electrical energy [56]. The oxidation product formed in the anodic chamber during the process is carbon dioxide and as most of the carbon dioxide in the renewable biomass is originally coming from the fixation of atmospheric carbon dioxide through photosynthesis, no net carbon emission is made into the environment by MFC [39]. However, the electricity generation can be dramatically inhibited if oxygen contaminates the anodic chamber affecting the performance of anaerobic microbes adversely, which can be avoided by maintaining completely anaerobic conditions in the anodic chamber [39, 120].
MFC is a systematic arrangement of various components that collectively work to run the system. The five main building blocks of MFC are presented in Fig. 9.
Anode
Anodic chamber is one of the core elements of MFC as all the pre-requisites for degrading the biomass is provided in this chamber like the substrate to be degraded, microorganisms, mediators, and electron accepting anode. Bio-anode is sometimes described as the defining part of the MFC and can often act as the limiting factor for the MFC performance [124]. The choice of anode material also affects the performance as the shape, structure and material of the anode can affect the factors like bacterial adhesion, electron transfer and substrate metabolism. Some crucial properties to be considered while choosing a potential anode are its conductivity, biocompatibility, chemical stability in the electrolyte and specific surface area. Carbonaceous materials like graphite rods and plates, carbon paper, carbon cloth, carbon mesh, carbon felt, carbon fibre brush, reticulated vitreous carbon are the most widely used electrode material because of their high stability, conductive and biocompatible nature with simultaneous high surface area [96].
Considering that anode has significant impact on the overall performance of MFC, several strategies have been employed over the years to modify the anode in a bid to improve the MFC power output. Strategies have been adopted to increase the biocompatibility and surface area of the anode through acid, ammonia and heat treatment. In 2007, Cheng and Logan improved the output power density (PD) of the carbon cloth utilising MFC by treatment with ammonia and enhancing the surface charge on the electrode [25]. Cai et al. [14] compared the performance of MFC utilising a plain, nitric acid-treated and heat-treated carbon cloth electrodes and observed that the heat-treated electrode outperformed the other two and the improvement in the power output was mainly attributed to the improved biocompatibility of the electrode [14]. Furthermore, other researchers employed the techniques of nanoengineering to modify anodes where, conducting polymers like polyaniline (PANI) and polypyrrole (PPy) and carbon-based nanomaterials are the prime area of focus. Lai et al. [81] performed the electropolymerisation of PANI on carbon cloth and observed enhanced electrochemical activity, increased roughness for better biofilm formation with a relatively sustainable and reproducible MFC power output [81]. Qiao et al. [117] successfully reported carbon nanotube (CNT)/PANI as a feasible electrode material [117]. Zou et al. [196] harvested electric energy from E. coli catalysed MFC utilising a CNT/PPy coated carbon paper electrode [196]. Zhang et al. [190] studied the performance of graphene-modified stainless steel mesh anode and observed 18 folds improvement in the performance as compared to plain stainless-steel mesh as a result of enhanced electron transfer efficiency owing to improved surface area and better bacterial adhesion [190]. More recently, Tang et al. [142] used anthraquinone-2 sulfonate immobilised conductive polypyrrole hydrogel to significantly enhance the power output and reduce the charge transfer resistance of electrodes [142].
Another relevant factor that must be kept in mind while selecting anode is its potential. It defines the MFC power generation by monitoring the bacterial community and substrate interaction. Electrochemical analysis suggests that to achieve highest electric output the anode should have a low while cathode should have high electrode potential. However, the bioelectrochemical studies suggest a better bacterial colonisation at more positive anodic potential. Thus, to maximise the MFC current and power output performance, a careful tuning of anode potential is crucial [124]. Pinto et al. [115] investigated the effect of anodic potential on biofilm formation and electroactivity of Shewanella oneidensis and observed that the negative potential of −0.3 V favoured the mediated electron transfer while the positive anodic potential of +0.3 V favours faster colonisation of the microbial population on the electrode [115].
Cathode
Cathode is responsible for transferring the electrons travelling from anodic to the cathodic chamber and subsequently to TEA. Oxygen is generally the most frequently used electron acceptor owing to its ease of accessibility, free cost, high oxidation potential with no possibility of production of poisonous chemical waste as the end product of oxygen reduction is water [147]. However, the sluggish reduction kinetics of oxygen makes it a restricting agent in MFCs owing to the large potential loss and thus acts as a major bottleneck in the electrical applications of MFC. Potassium ferricyanide is another popular electron acceptor that shows significant improvement in the power output, which could be due to the improved mass transfer rate and reduced activation energy required for the cathodic reaction [114]. However, ferricyanide shows a major drawback of regeneration and needs refilling frequently [120]. Several other TEAs have been explored over the years. Dai et al. [31] explored the potential of sodium bromate as an electron acceptor and observed the catholyte pH to decrease with the increasing concentration of sodium bromate thereby improving the performance of MFC [31]. Kumar et al. [80] compared the performance of four different electron acceptors namely buffered ferric chloride, potassium hexacyanoferrate, potassium dichromate and phosphate buffer solution (PBS) and observed the highest bioelectric performance in case of ferric chloride with the output PD of 308.7 mW/m2. It was also reported that the internal resistance of MFC with different catholytes decreased in the order of potassium dichromate > hexacyanoferrate > ferric chloride > PBS [80]. Oon et al. [111] compared the performance of different monoazo (New Coccine and Acid Orange 7) and diazo dyes (Reactive Red 120 and Reactive green 19) as potential electron acceptors and reported that the decolorisation rate was nearly 50% higher for monoazo dyes as compared to the diazo dyes and the decolorisation and power output followed the trend; New Coccine > Acid Orange 7 > Reactive Red 120 > Reactive green 19 suggesting that the structure of the dye affected the decolorisation rate and power output [111]. Among the variety of TEAs explored so far, the free and easy access to oxygen still makes it the most opted electron acceptor. In a bid to enhance the oxygen reduction reaction rate (ORR) and lower the activation energy, the use of appropriate catalyst becomes important. Platinum (Pt) is the most widely known and explored catalyst showing a higher ORR calatalytic activity [12, 43, 116]. However, the use of Pt catalyst tremendously increases the operating cost of MFC and presents a major drawback of substrate poisoning in certain solutions [59]. Recently a variety of materials have been explored as ORR catalysts. For MFC, an ideal ORR catalyst should be cost-effective (keeping MFCs economically feasible), durable, synthesisable on large scale, and showing elevated catalytic activities. The catalysts used in cathode can be broadly divided into two categories, namely, (i) abiotic catalysts and (ii) biotic or biocatalysts [180]. The abiotic catalysts can be further subdivided into carbon-based catalysts like carbon black (CB), activated carbon (AC), carbon nanofibres (mainly CNTs) and graphene; metal-based catalysts; metal-carbon hybrids; and metal-nitrogen-carbon hybrid.
CB has been widely used in MFC as a metal catalyst supporting material. CNTs have garnered significant attention because of its high surface area and electric conductivity. The CNT-based catalysts can be easily tuned by doping with other components to achieve desired properties [173]. Also, CNT-based catalysts have been reported to have better durability than Pt-based catalysts [180]. Graphene has also recently received a lot of attention because of its higher stability and conductivity [136]. Graphene is comparable to CNTs in terms of cost-effectiveness but still lags behind the AC [180]. Macheri et al. [102] studied the performance of zirconium oxide (ZrO2)/CB cathode with different concentrations of ZrO2 (0, 25, 50, 75 and 100%) and observed that the ORR catalytic activity of ZrO2/CB cathode with 25 wt% of ZrO2 was better compared to the other cathodes with the maximum PD of 600 mW/m2 in a single chambered MFC. Also, the study reported that the assembling cost of ZrO2/CB catalyst was 15 times less than Pt/CB cathode thereby decreasing the operating cost of the MFC [102]. Das et al. [34] synthesised a metal-based ORR catalyst using surface modified ferrite with Co and Zn in 1:1 ratio and observed the results to be comparable to 10% Pt/C-based catalyst with the maximum PD of 176.42 mW/m2, a high Coulombic efficiency (CE) of 43.3% and maximum COD removal efficiency of 87% [34]. A novel Fe/N doped graphene/CNT composite was synthesised by Wang et al. [150]. The study revealed the synthesised nanocomposite to possess improved electrogenic and high electrocatalytic activity in the neutral PBS medium for ORR. They also reported that the synthesised composite displays higher MFC power output of 1210 mW/m2 as compared to the Pt/C catalyst (1080 mW/m2). The results of the work performed by Wang et al. concluded that the Fe-N/G with CNT possesses enhanced ORR capacity compared to Pt/C, which could be due to the high pyridine doped CNT or increased ORR active sites [150]. In another recently published study, a low-cost AC supported F-N-C catalyst was synthesised and used as cathode, which improved the PD of MFC by around 33% as compared to the plain AC. The synthesised composite also displayed good stability with no surface morphology change during the experiment [171]. Further, in the research carried out by Majidi et al. [98], an α-MnO2 nanowire supported carbon vulcan was employed as ORR cathode catalyst. The study reported that the α-MnO2/carbon vulcan can serve as an effective and economically feasible Pt free MFC catalyst on large scale because of its increased redox activity owing to its surface structure and increased surface area [98].
Microorganisms can also play the role of active ORR catalyst by acting as electron shuttle between cathode and TEA. Studies have revealed that the microbial community can transfer the electrons through one or multiple pathways [180]. Several studies have demonstrated that a variety of substrates can be treated using pure and mixed culture bio-cathodic MFCs [60, 65, 163, 185, 186]. The performance of the bio-cathode can be influenced by the initial catholyte concentration of dissolved oxygen (DO). In one of the recent studies, the PD for nitrogen wastewater treating MFC was highest for anoxic bio-cathode [52]. The uniqueness of the bio-cathodes lies in the variety of microbial communities interacting with the system to achieve the desired goals. Cao et al. [15] developed a photobio-cathode by illuminating the developed bio-cathode to directly fix dissolved CO2 or bicarbonate as electron acceptor. The MFC utilising the bio-cathode produced 15 folds higher PD than MFC working with the plain carbon cathode with the maximum achieved PD of 750 mW/m2 [15]. In another study Wang et al. [156], used an air diffusion bio-cathode to accelerate the ORR in MFCs. The study confirmed the improved ORR in MFC with bio-cathode than abiotic MFC with enhanced current and power densities. The study, however, also revealed that the use of bio-cathode decreased the biodiversity within the system [156]. In a very recent study performed by Izadi et al. [64], a gas diffusion bio-cathode was developed for MFC enriched with iron oxidising bacteria. The study reported an enhanced PD of 1.02 W/m2 compared to that of 0.59 W/m2 PD produced using Pt catalyst in continuously operated MFC. The study further revealed that the gas diffusion electrode (GDE) improved the mass transfer and the MFC performance and provided a reproducible and fast startup for bio-cathodic MFC [64]. Like bio-anodes, the activity of the bio-cathodes can be enhanced by modifying the bio-cathode to improve its surface area, biocompatibility and conductivity using appropriate material. Chen et al. [23] developed a microbially in situ synthesised reduced graphene oxide bio-cathode that significantly improved the ORR [23]. Table 1 presents recent advances and modification in electrodes used in MFC and also shown in Fig. 10.
Membrane
Membranes or separators are significantly intrinsic part of MFC physically barring the oxidation and reduction reactions taking place in the anodic and cathodic chambers. In MFC, these separators allow the selective permeation of protons from anodic chamber (where they are produced) to cathodic chamber (where they are consumed). The separators also help in controlling the oxygen diffusion to the anaerobic anodic chamber which could be detrimentally affected by the oxygen penetration [120]. However, the incorporation of the membranes in the MFC system also presents some hurdles like pH splitting caused by the increasing cathodic pH and decreasing anodic pH due to the slow movement of protons from one to the other chamber [87]. Also, the membranes add to the cost of the MFC setup making upto 38% of the capital cost and also increase the internal resistance of the system [49, 58]. A wide variety of materials have been used as separators in MFC like glass wool, ceramics, nanoporous filter, salt bridge and ion exchange membranes (IEMs) [26, 74, 87, 132, 166, 179, 187] beside others. However, the IEMs remain the most widely used separators because of their high conductivity and selective permeability. In MFCs, the most commonly used membranes are the cation exchange membrane (CEMs) or more precisely the proton exchange membranes (PEMs). The positive ions are attracted and permitted to allow through these membranes as they are composed of the backbone of negatively charged groups [120]. Nafion, a fluoropolymer based on sulfonated tetrafluoroethylene group has been widely used in various studies [131]. It has a SO3− (sulfonate) group attached to it imparting high proton conductivity. Nafion, however, has the drawbacks of high cost, easily susceptible to biological and chemical biofouling as well as pH splitting [21, 87]. Ghasemi et al. [48] compared the performance of treated, untreated and biofouled Nafion membranes and observed the performance to be following the trend of treated > untreated > biofouled membrane with the CE of the treated membrane being 2.32 and 4.15 times better than untreated and biofouled membranes, respectively [49]. Ultrex is another commonly used PEM. Ultrex CMI-7000 is a strong acid membrane composed of gel polystyrene and divinylbenzene cross-linked polymer with sulphonic acid (SO3H−) as the active functional group. Although the membrane is compatible with Nafion in terms of mechanical strength, conductivity and affordability, the higher resistance posed by the membrane lowers its performance [139]. Zirfon, composed of 85:15 wt% of hydrophilic zirconium oxide (ZrO2) and polysulfone, is an anion exchange membrane (AEM), which outperforms the specific resistance of Nafion membrane and is cheaper. However, it presents a major drawback of high oxygen penetration to the anodic chamber thereby affecting the performance of the MFC system [120, 139]. In a very recent study performed by Wang et al. [152], four different IEMs, bipolar exchange membrane (BEM), CEM, PEM and AEM were investigated for chromium removal and electricity generation in MFC. It was observed to follow the trend BPM > AEM > CEM > PEM with PEM displaying pH splitting [152]. San-Martin et al. [128] compared the performances of different commercially available IEMs, Nafion-117, Ultrex CMI-7000, Zirfon Perl UTP and Fumasep FKE and FKB. The study reported Nafion and Ultrex to possess high thermal stability while the other tested membranes displayed better resistance to biofouling. The study further concluded that the electrochemical performance in BES was maintained by all the membranes tested [128]. Verily, an ideal MFC membrane should showcase following characteristics of absolute substrate crossover and oxygen diffusion control, cost-effective with low internal resistance and resistant to biofouling [5]. Recently, novel membrane materials have been synthesised and tested that can possibly be cheaper and perform compatibly with the commercially available membranes. A novel sulphonated polyether ether ketone membrane (SPEEK) was synthesised and compared for its performance with the Nafion-117 by Ayyaru and Dharmalingam [8]. The study reported the synthesised membrane to display high PD and CE with retarded substrate losses emphasising the potential of electrode in enhancing the performance of MFC system greatly [8]. A polybenzimidazole-based novel PEM has been recently synthesised and tested as a potential separator in MFC and its performance was compared with Nafion-117. The membrane reportedly performed better in terms of power output, durability and treatment efficiency as compared to Nafion. The membrane also showed inhibition to surface bacterial adhesion thus preserving the MFC from biofouling, which is a major drawback with Nafion [5].
4 Biofilm Formation and Microbial Communities Involved in MFC
In order to generate electricity in MFC, the electrons originated by the reduction of organic substrates need to be transferred to the anode, which acts as an electron acceptor. To fulfil this, the electrons are required to be transferred extracellularly. The shuttling of electron between microbes and electrodes is known as extracellular electron transfer (EET) [77]. Potter and Cohen were the first to observe the microbial property of EET in early 1900s. A wide variety of microbial communities have been studied over the years to possess the ability to transfer electrons extracellularly. The microbes performing this process of electron transfer are commonly known as Exoelectrogens. Geobacter and Shewanella have been the most extensively studied EET performing microbial species. These are gram negative metal-reducing microbes, performing EET via multihaem c-type cytochrome to transfer the electrons to the metals via direct contact [27]. In MFC, these microbes perform the EET to transfer the electrons to anode instead of metal as electron acceptor in the similar fashion. Some widely known examples of exoelectrogens are Rhodoferax ferrireducen, Shewanella putrefacien, Geobacter sulfurreducen, Geobacter metallireducen [67]. Wrighton et al. [160] first gave the exoelectrogenic evidence of gram-positive bacteria using Thermincola potens strain. Recently, a novel pure culture of gram-positive P. freudenreichii was confirmed to behave as exoelectrogen in an H-shaped mediatorless MFC [122]. In a study conducted by Wang et al. [149], E. coli was successfully employed to harvest current in MFC with excellent power output of 547 mW/m2 [149] while [167] isolated Citrobacter sp. from MFC as potential exoelectrogen [167]. Studies have suggested mixed culture to be favourable in utilising complex substrates [112]. The electron transport between microbes and electrodes can take place via different routes and has been reported to be broadly categorised in three sections: short-range direct transfer, long-range direct transfer and indirect electron transfer employing special redox-active molecules commonly called mediators to shuttle the electrons between microbes and electron acceptor or electrode [175]. In MFC start-up, electron transport process to the anode is considered as the rate-limiting step as the biofilm developed on anode plays a crucial role in EET [189]. Four main types of proteins have been identified so far to be involved in EET: Porin-cytochrome complex, a complex of porin and redox protein; surface-bound cytochromes; nanowires; and miscellaneous redox proteins [30, 126]. In case of short-range direct transfer, the physical contact of the developed biofilm with the electrode is a pre-requisite to conduct electron. The microbial community in the biofilm adheres to the electrode and transfer the electrons via the involvement of outer membrane redox multiheme molecules called cytochrome c proteins [69]. In such electron transfer processes, as the physical contact is an important criterion for electron conduction, the microbial community only in the closed proximity/physically adhering to the electron (monolayer) is able to perform electron transport thereby limiting the MFC performance. A study evinced that the outer membrane c-cytochrome, OmcA and MtrC are determining factor for Shewanella species to generate electricity [160]. In case of G. Sulfurreducens, outer membrane cytochromes OmcE, OmcB, OmcZ and OmcS have been reported to have role in electron transport [27, 126]. In the high current producing bioflms, OmcZ has been observed to play a major role. The homogenously carried electron transport through biofilm involves OmcZ while OmcB moderates the electron transport heterogeneously between biofilm and electrode [78]. OmcF has also been reported to play indirect role in current production without directly influencing electron transfer by regulating the synthesis of appropriate proteins like OmcB, OmcE and OmcS by monitoring the relevant gene transcription [33]. Moreover, it has been reported that the microbial communities in developed biofilm have higher dominance of cytochrome as compared to the lag phase [79].
The long-range direct transfer, on the other hand, is able to conduct electrons from microbes to the electrodes more efficiently and rapidly by the development of an electroactive layer. Unlike short-range transfer, in this case, the electron transport is performed via special pili-like electron carrying structures produced by exoelectrogens like Geobacter and Shewanella species. These produced filaments allow the electrons to be conducted through a more complex multilayered biofilm without the restriction of physical monolayer contact [78]. The nanowires produced by Geobacter sp. are comprised of pili protein that have been reported to have metal-like conductivity because of their structural backbone of aromatic amino acids maintaining pi-pi orbital overlapping for electron delocalisation [30]. On the other, nanowires produced by Shewanella sp., unlike Geobacter are comprised not of pili but are extension of outer membrane and follow an alternative mechanism of conduction called electron hopping. In this model, the electrons hop along the chain of cytochromes that accounts for the current conduction [77]. It has been reported that the nanowire production by Geobacter sp. increases when electrode is used as electron acceptor in MFC. Also the effect of temperature and pH change has been observed to be consistent with metal-like materials [99]. Species like Shewanella and Geobacter can perform electron conduction via three different modes of mediation through outer membrane cytochrome, nanowires or via self-synthesised electron shuttles or mediators [77]. The electron transfer achieved via mediators is known as indirect electron transfer or mediated electron transfer [69]. Mediators play a crucial role of electron shuttling between the microbes and the electrodes. Mediator can be divided into two main categories of endogenous and exogenous. An endogenous mediator is a microbial synthesised substance while an exogenous mediator is an externally added redox substance that plays the role of electron shuttle. Endogenous soluble electron mediators are excreted by microbes like G. fermentas, P. aeruginosa, S. oneidensis and L. lactis. G. fermentas produces riboflavin, P. aeruginosa secretes pyocyanin and phanazine-1-carboxamide while S. oneidensis synthesise riboflavin and flavin mononucleotide as extracellar mediators [78]. Exogenous electron mediators are utilised by microbial communities like D. desulfuricans, E. coli, P. fluoroscens, P. vulgaris, P. micorbilis beside others. Several studies have reported the use of 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), redox dyes and ferricyanide as exogenous electron shuttles [69, 191].
Electron flow in MFC can take place bidirectionally: from microbes to electrode (anode) and electrode (cathode) to microbes. Microbial species like Geobacter and Shewanella can potentially transfer electrons bidirectionally. Moreover, biocommunities like C. pasteurianum, A. colcoacenticus, A. ferrooxidans apart from Geobacter and Shewanella can perform electron transfer from cathode to microbes [27, 77, 78]. The electron transfer mechanism of Geobacter in case of cathode to microbe has been reported to be completely different from microbe to anode electron transfer. Periplasm located cytochrome PccH is a potential candidate in cathode to microbe electron transport by Geobacter. On the other hand, Shewanella has been reported to follow the same electron transfer mechanism involving flavin and riboflavin with c-cytochrome in reverse direction [32, 140]. Methods like chemical, high temperature or surface binding pre-treatment of electrodes and anode modification affect the development of biofilm and thus performance of MFC [77]. CNT/PPy-modified electrode has been reported to enhance the power output from MFC metabolising glucose substrate as a result of better electrode-bacteria interaction on modified electrode [196]. Lui et al. [95] reported PEDOT (poly (3,4-ethylenedioxythiophene) to increase the active sites for bacterial catalytic reactions and improve MFC performance [95]. Further, the addition of biosurfactants like rhamnolipid to anolyte has been recently reported to increase the development of biofilm and electron transfer resulting in an improved MFC performance [189]. Heavy metal ions like Cu2+ and Cd2+ added to the anodic chamber can potentially enhance the riboflavin electron shuttle synthesis and effectively improved the EET and MFC performance [168]. Figure 11 presents the pictorial representation of various modes of electron transfer.
5 Types of MFC
Various different MFC configurations have been developed over the years in a bid to achieve high and sustainable performance in longer run and at larger scale. The MFC configurations are mainly categorised into two groups: air-cathode MFCs and liquid-feed MFCs. The most common MFC configurations are single and dual chambered MFCs. The following section discusses some common MFC configurations developed over the years (Fig. 12).
Single Chambered MFCs (SCMFCs)
This is the most basic configuration of MFC and falls under the category of air-cathode MFC. SCMFC is comprised of a single anodic anaerobic chamber with anode separated through a membrane to an air cathode (placed at outer surface of the reactor). The electrodes are connected using external circuits. The anodic microbial community feeds on the added substrate and release electrons, which flow through the circuit generating electric current. The transported electrons are accepted by oxygen as TEA [39, 69]. SCMFCs have the benefit over dual chambered MFCs of reduced cost of catholyte and aeration as air-cathode MFCs take atmospheric oxygen as TEA [109]. Membrane-less SCMFCs have also been constructed to further lower the operational cost in various studies; however, membrane-less MFCs pose the problem of lower performance because of anodic oxygen diffusion [134, 195].
Dual Chambered MFCs (DCMFCs)
DCMFCs, like SCMFCs are also basic and widely used configuration that mainly falls under the category of liquid feed cathode. DCMFC consists of two separate anaerobic anodic and aerobic cathodic chambers separated by the membrane (mainly PEM) to avoid liquid and oxygen diffusion and allow protons to pass through it to the cathodic chamber. DCMFCs can simultaneously treat two different waste streams in anodic and cathodic chambers respectively (e.g. degradation of organic-rich substrate in anode and metal recovery via reduction in cathode) [69]. Different designs of DCMFC have been developed like H-shaped and rectangular DCMFC [20, 82, 184].
Tubular Chambered MFCs
The reactor is mainly a cylindrical single chambered system comprised of a folded membrane surrounded by the cathode/anode. The anodic chamber is inoculated with anaerobic sludge and organic substrate is added. Rabaey et al. [118] constructed a continuous tubular MFC with graphite granules and graphite mat as anode and cathode, respectively. The cathode was dripped over with ferricyanide solution as electron acceptor [118]. Cheng et al. [22] constructed mini air-cathode tubular MFCs to treat benzene contaminated groundwater. The three mini-tubular MFCs were comprised of folded anode surrounded membrane with internal air-cathodes. The systems were dipped in the benzene contaminated water. The results revealed the considerable performance in terms of treatment and power generation [22]. Liao et al. [88] reported a novel method to improve the performance of tubular MFC by employing a rotating carbon brush anode resulting in a dramatic power output of 210 W/m3 [88]. Tubular MFC has the potential advantage over others of enhanced scalability in continuously operated systems with increased sludge retention and diminished hydraulic retention time thereby reducing the overall operating cost [69, 124].
Up-flow MFCs (UMFC)
He et al. [57] combined the benefits of upflow anaerobic sludge bed reactor (UASB) of long biomass retention and high treatment efficiency to MFC. A UMFC consists of two cylindrical compartments separated by membrane with cathodic compartment located on top of anodic compartment. The anodic feed is allowed to recirculate in the system at defined rate. He et al. treated a common carbohydrate, sucrose to harvest electrical output [57]. Xylose wastewater was treated in an UMFC with AEM and flat graphite electrodes and revealed that decreasing HRT improved the power output at optimum recirculation rate [83]. Recently, single chambered UMFCs have gained attention across globe as a potential approach to reduce the operating cost and high maintenance and difficulty in upscaling and [143] successfully treated Acid orange 7 azo dye in one such single chambered UMFC [143]. Another study demonstrated that the KCl concentration plays an important role in enhancing the anode to cathode electron transfer while chemical oxygen demand (COD) increase has derogatory effect on the voltage recovery as excessive biofilm formation increases the internal resistance [144].
Staked MFCs
The systematic arrangement of individual MFCs in series and parallel pattern is collectively known as stacked MFCs. In this type of configuration, individual fuel cells arrange to form a battery fuel cell. The arrangement does not affect the individual CE of MFC but improves the overall performance of the newly arranged system [69]. Both stacked connections show potential applications in MFC performance with series connection directly affecting the output voltage of the system compared to the individual units. On the other hand, in parallel arrangement voltage stabilisation with enhanced current output is observed [7]. Winfield et al. [158] studied the effect of electronic configuration on the hydraulically connected MFCs and observed both configurations to improve the output performance compared to the individual cells however, in parallel connection the power and current output were 2 and 10 folds higher than series stack, which was proposed to be result of shunt losses in series stack because of fluidic and electrical connections. One of the biggest drawbacks of stacking is voltage reversal in the connected MFCs as it can severely damage the bio-anode and limit the performance. Voltage reversal has been suggested to be caused by kinetics imbalance at electrodes. To overcome another issue of proton accumulation in the anodic chamber of MFC, hybrid stack of hydraulically connected SC and DC MFCs were constructed. Compared to the individual stacks of SC and DC, the hybrid SC/DC stack was reported to generate higher electric output. The proton accumulation was proposed to be monitored due to the oxygen diffusion through SCMFC cathode to acetate metabolising biofilm [170].
Sediment MFCs (SMFC)
SMFC harvest energy by connecting marine sediments with seawater. They generate energy from anaerobic sediments and aerobic water electropotential difference. To construct a SMFC, anaerobic anode is buried into the sediments and connected to the overlying aerobic cathode. The organic matter in sediments is metabolised by anaerobes and the electrons produced are collected at anode and transported to cathode thereby generating current [69, 194]. The first SMFC was constructed by Reimer and co-workers [123]. Rate of organic loading influence the performance of SMFC. Zhao et al. [193] studied the effect of different organic loading rates on MFC performance and observed that the excess loading can lead to biogas accumulation causing system to break down while the low loading limits the power output [193]. Another study suggested that high polarity organic contaminants are preferably treated in SMFCs [165]. The removal of toxic waste (mercury, silver and zinc) present in the sediments was studied using SMFC and reported to achieve more than 80% removal of all contaminants with successful energy generation [1].
Constructed wetland MFCs (CW-MFCs)
Constructed wetland (CW) is a widely employed wastewater treatment technology allowing the treatment by taking advantages of natural processes like filtration. CW-MFC is an emerging technology capable of harvesting energy while performing waste treatment. The wetlands (CW-MFCs) are constructed by burying anode in the depth and allowing the cathode to be exposed freely to the available oxygen. Anaerobic and aerobic conditions in wetland prevail throughout the depth with anaerobic conditions maintained in the deeper depths and aerobic conditions present at the surface of wetlands from atmospheric oxygen penetration [37]. Plants in wetlands perform filtration and adsorption of wastewater while degradation is performed by anaerobic and aerobic microbes [194]. Araneda et al. [6] performed the treatment of greywater (wastewater generated in households and office buildings) in CW-MFCs with Phragmites australis as wetland plant and matrix of gravel achieving the maximum PD of 719.57 mW/m2 and COD removal efficiency of 91.7% [6].
6 Substrates in MFC
Several kinds of wastewaters have been investigated in MFC to study their treatment and effect on different factors like PD, COD removal and CE. A wide range of wastewaters treated in the MFC can be broadly divided into Defined or Simple Substrate and Undefined or Complex Substrate. MFCs can treat waste both anaerobically in anodic chamber and aerobically in cathodic chamber. In anodic chamber, the substrate added is fed upon by anaerobes and oxidised to yield electrons and protons. Alternatively in the cathodic chamber, the aerobic microbial community transfers the electrons to the externally added TEAs, which are thereby reduced to simpler less harmful products.
Defined or Simple Substrate
Various pure simple organic contaminants have been utilised over the years to harvest electrical output. Carbohydrates, amino acids and volatile fatty acids are the simplest metabolic fuels that make up the more complex waste among which carbohydrates are the most commonly utilised MFC metabolite. These simple metabolites or pure substrates commonly referred to as synthetic wastewater have been tested in MFCs as electron donor to generate electric output. Acetate is the simplest and most commonly used carbon source reported in various MFC studies. Acetate and butyrate have been compared for electric output in a SCMFC and acetate was observed to generate 66% higher power output than butyrate fed MFC [92]. In another study, mechanism of glucose metabolisation in MFC was studied and reported to involve the syntrophic relation of fermenters and electrogens. The fermenters act on glucose to produce hydrogen and acetate which are acted upon by electrogens to produce carbon dioxide, protons and electrons [46]. Catal et al. [17] studied energy generation from 12 monosaccharides including 1 aldonic acid (gluconic acid), 2 uronic acids (glucouronic acid and galacturonic acid), 3 pentoses (arabinose, xylose and ribose) and 6 hexoses (fructose, glucose, galactose, rhamnose, fucose and mannose) using mixed microbial community. The results presented more than 80% COD removal of all monosaccharides. Mannose and glucouronic acid were observed to produce lowest and highest power output, respectively [17]. The results demonstrated a wide range of monosaccharides as potential fuel in MFC for electricity generation. Glucose (fermentable) and acetate (non-fermentable) remain the most evaluated MFC substrate however the lower performance from fermentable substrate compared to non-fermentable substrate has been suggested to be the consequence of denser biofilm metabolising fermentable substrate [112].
Proteins also form an important constituent of wastewater like domestic wastewater. MFC has been studied to harvest electricity from nitrogen-containing wastewater. Cystein, a proteinogenic amino acid has been employed as electron donor in DCMFC [97]. Yang et al. [169] tested eight amino acids namely L-Asparagine, DL-Alanine, L-Aspartic acid, L-Arginine, L-Glutamic acid, L-Histidine, L-lysine and L-Serine as substrate in SCMFC and observed L-Serine and DL-Alanine to generate highest and lowest PD, respectively [169]. In a recent study [82], treated P-nitroaniline in DCMFC in the presence and absence of co-substrate. The result revealed that both PD and COD removal efficiency decreased as the concentration of P-nitroaniline in anodic chamber increased; however, the addition of co-substrate improved the power output by two folds [82]. In another study, urea as a form of total ammonia nitrogen was successfully treated in SCMFC upto the concentration of 3490 mg/L with more than 80% achievable nitrogen removal. The PD was reported to increased upto 69% on increasing the total ammonia nitrogen concentration from 80 to 3490 mg/L. However, further increase has detrimental effect on MFC performance [153].
Undefined or Complex Substrate
Real wastewater discarded from domestic, municipal, agricultural and industrial sources are not pure but rather complex consisting of variety of treatable components. MFC owing to its ability of treating wastewater with simultaneously harvesting energy has been employed to test a wide range of waste streams. A few of the complex substrates have been discussed in this section. Agro-food waste like brewery, manure, swine and dairy are generally very rich in organic content and show high biodegradability. Dairy wastewater is rich in biodegradable content with sugar making upto 97% of the total COD [112]. It was treated in long run in DCMFC for 2.5 months with successful remediation of high COD substrate achieving a maximum PD of 27 W/m3 [19]. Swine wastewater has been treated in CW-MFCs while maintaining upflow-downflow scheme. The observed results showed 70 and 75% increase in PD and ammonia removal efficiency compared to the continuous upflow system [38]. Winery wastewater, an important agro-industrial waste is rich in biodegradable organics. Using this wastewater, Pentaedo et al. [114] harvested power output of upto 465 mW/m2 in a DCMFC [113].
Food waste is referred to the food losses occurring in the food chain. Food waste is mainly rich in carbohydrates. Nearly 1/3rd of the food globally produced per year is lost as waste [84]. Thus, food waste garners a lot of attention for high biodegradability, potential for energy and inexhaustibility. Food waste is disposed from both domestic and commercial sectors and pose serious environmental concerns like odour, toxic gas emission and contamination of groundwater. Goud et al. [51] treated canteen-based food waste in SCMFC and successfully harnessed electrical energy via anaerobic bio-treatment. The study revealed that optimum organic loading rate affects the MFC output performance [51]. A similar study performed by Li et al. [84] revealed that the aromatic and hydrophilic fractions of canteen-based food waste were more readily and preferentially degraded than non-aromatic compounds and neutral fractions [84]. In another study, MFC treated orange peel waste to generate PD of 358 mW/m2 while simultaneously achieving a COD removal efficiency above 80% [105]. Domestic and municipal wastewater have been of major interest among research working in the field of energy recovery. Domestic wastewater can be treated in MFC successfully with upto 80% COD removal and power recovery within a hydraulic retention range of 3–33 h at an influent strength of 50–220 mg/L [93]. Septic wastewater is rich in COD content, which is potentially convertible to useful energy. Yazdi et al. [174] studied the treatment of septic wastewater in stack MFC and obtained the PD of 142 mW/m2 from three parallel connected units [174]. Urine in another study was used as MFC substrate to generate electric output and simultaneously recover struvite, a phosphate fertiliser present in urine [176].
Further, the decomposition of organic waste and rainwater percolation at landfills sites generates organic constituent rich waste called Landfill Leachate. Landfill Leachate is heavily polluted wastewater that contains organic/inorganic and heavy metal waste capable of heavily contaminating groundwater through percolation. MFC can potentially treat this waste to generate power output. In one of such study, leachate was treated in a SCMFC to obtain a maximum open circuit potential and specific PD of 1.29 V and 1513 mW/m2. The results also revealed that the volumetric PD increase with increase in surface area while area-specific PD decrease with increase in electrode surface area [135]. Moharir and Tembhurkar [108] studied the effect of recirculating food waste leachate anolyte to generate the highest PD of 29.23 mW/m2 and achieving COD removal efficiency of 65.76% suggesting an increase in PD and COD removal on anolyte recirculation [108].
Textile industry is one of the largest and most complex industries generating tonns of recalcitrant toxic waste every year that is rich in organic content and a potential resource of energy. Azo dyes are the most commonly used dye (making upto 60%) in textile industry that is extremely toxic, carcinogenic and mutagenic in nature. These factors make textile wastewater a priority candidate among wastewater treatment researchers. MFC has been widely employed in dye treatment. Khan et al. [70] successfully treated two azo dyes viz. Reactive Orange 16 and Acid Navy Blue R in MFC with simultaneous energy recovery [70]. Congo Red has been treated in the presence of 3 different co-substrate (glucose, acetate and ethanol) and observed the highest PD of 103 mW/m2 with glucose followed by acetate and ethanol respectively [16]. In a recently conducted study, textile effluent and Scarlett RR dye were treated in phytobed MFC with Chrysopogon zizanioides and Typha angustifolia plants to enhance the COD, TDS and colour reduction [66].
Industrial wastewater is a huge source of contamination to water bodies affecting the biotic communities present in the ecosystem. A collective wastewater sample consisting of waste from chemical, metal, vegetable oil, glass and marble and other industries was treated to produce the maximum voltage output of 890 mV [2]. Other industrial wastewaters treated are rice mill water [10], soak liquor [121], neomycin sulphate antibiotic [18] and surgical cotton industry [141]. A comparative analysis of some common MFC substrates has been presented in Table 2.
7 Terminal Electron Acceptors
In MFCs, electron flow occurs from lower redox potential (anode) to higher redox potential (cathode). The availability of appropriate TEA that overcomes potential losses makes the cell thermodynamically favourable for electron flow. A good TEA reflects the properties of low cost, ease of availability, sustainability in biotic/abiotic environments for prolonged duration, fast kinetics and high redox potential [56]. The most commonly used TEA in MFC by far is oxygen owing to its easy availability, high redox potential and sustainable nature. However, a wide range of alternate TEAs have been studied in MFCs like dyes, metal ions and others. The TEA employed in MFCs can be broadly divided into inorganic and organic compounds. Oxygen is the most widely used inorganic electron acceptor and has played the role of TEA in various studies [11, 41, 45, 94, 119]. It can be supplied to the electrode by either directly exposing electrode to the air or by aerating the cathodic compartment [147]. Poor oxygen-electrode interaction/contact and slow reduction rate of oxygen on plain carbon-based electrode limit the performance of MFC. The reduction reaction can be improved by modifying carbon electrodes with the help of ORR enhancing catalysts. The redox potential of nitrate is comparable to oxygen making it a potential TEA. Biologically catalysed denitrification was first performed by Clauwaert et al. [29] yielding the power output of 8 W/m3 and removing upto 0.146 kgNO3—Nm−3d−1 [29]. Wang et al. [151] studied nitrate treatment and suggested that the feed-drain frequency enhances the nitrification-denitrification efficiency [151]. The nitrite accumulation at cathode has been observed as a major issue in denitrification due to its health concern and should be achieved to minimise denitrification. The environmental conditions like pH, DO and insufficient electron donors do not significantly affect the nitrite accumulation. [138] modelled denitrification yielding parameter to design bio-cathode performing higher denitrification [138]. Wu et al. [164] design a novel MFC with aerated electroconductive membrane bio-cathode to improve nitrification-denitrification [164]. Ferricyanide is another popular TEA used in various studies owing to its high redox potential [35, 103]. However, ferricyanide has a disadvantage of frequent chemical regeneration limiting its use as electron acceptor [104]. Several other metal ions have been reduced in MFC like Cr(VI) [54, 76], bromate (BrO3−) [31], Fe(III) [80], heavy metal ions like Cu2+, Hg2+, Zn2+, Cd2+, Pb2+ and Cr2+, SO42−, percarbonate (HCO3−) and Mn7+ [40, 44, 54, 133, 155, 161]. Simultaneous nitrate and perchlorate removal has also been achieved in MFC [65]. Chlorella-based bio-cathode has been employed in MFC for Cd2+ removal [188]. Buffered catholytes especially PBS has been used in MFCs to maintain pH balance. Saline water has also been studied as potential catholyte [3]. Various organic contaminants have also been employed as TEA in cathode compartment of MFC. A variety of textile dyes have been used as potential electron acceptors and reduced at MFC cathode. Oon et al. [111] successfully employed various mono and diazo dyes as TEA and observed 50% higher decolorisation for mono than diazo dyes [111]. Nitrobenzene, nitrophenol, ethanoamine have also been tested as cathodic electron acceptor generating significant voltage output [191, 192]. Phenol and chlorophenols (CPs) are colourless organic compounds that are potential carcinogens. Khan et al. have explored the bioremediation of these contaminants at cathode in MFCs [71, 72, 74, 75].
8 Challenges
MFC on many fronts has emerged as a technology, which is suitable and sustainable in harvesting energy from waste in an eco-friendly way. Development of commercial prototypes like Cambarian innovation and initiatives of companies like Pilus Energy and Emefcy has brought the technology a step closer to commercialisation. However, issues concerning low power output, electron transfer efficiency or CE, understanding of suitable wastefeed and microbial processes taking place within the system still present challenges in scaling up of the technology. Factors like wastewater concentration, composition, unwanted biomass, side processes like methanogenesis, optimum pH, diffusion across separators, incomplete biodegradation limits the waste treatment in MFC [5].
The efficiency of electrical recovery (CE) is another major concern regarding MFC scaleup. Six major reasons for low CE as suggested by Pandey et al. [88, 112] are:
-
(a)
Electron diversion to non-exoelectrogens.
-
(b)
Substrate consumption by competitive pathways like methanogenesis.
-
(c)
Metabolic inhibition of biomass because of toxicant and proton buildup.
-
(d)
Substrate lockup of electrons.
-
(e)
Low transfer efficiency to/from electrodes.
-
(f)
Inappropriate separators causing electrolyte/oxygen diffusion to the other chamber.
As the interaction of biomass and electrode plays a crucial role in MFC, designing appropriate electrode with high biocompatibility is a major challenge. This chapter has made attempts to discuss the various electrode modification advances made recently to design suitable electrodes that can effectively tackle fouling, corrosion and enhance activity. Further the cost of MFC components is still too high for practical implication and suitable, economically feasible alternatives are much needed to make the technology commercially feasible [9].
9 Conclusion
Water and energy are undoubtedly the most essential contributing factors of the society. As per a Slovakian proverb “Water is the first and foremost medicine” and clean water is the basic right of all living souls inhibiting the earth. Energy on the other hand is a mean to derive the society forward. The continuously growing society with rapid industrialisation has led to one major issue of water contamination making it unfit for daily activities. MFC technology has earned the focus of the world research as a potential technique linking the basic needs of the mankind. It treats wastewater and harvest energy in the form of electric power from the organic/inorganic contaminants. MFC technology promises to be a carbon-neutral clean source of renewable energy. The article reviews the role of different configurational aspect of MFC on its performance. From the article, it can be concluded that MFC can achieve high performance by optimising the factors such as electrode used, membrane and microbes involved, and selection and concentration of substrates being treated. However, for the practical implementation of this technology, further research in the field of longevity, system fouling, role of internal resistance and microbial kinetics needs to be deeply explored.
References
Abbas SZ, Rafatullah M, Khan MA, Siddiqui MR (2019) Bioremediation and electricity generation by using open and closed sediment microbial fuel cells. Front Microbiol 10:1–12. https://doi.org/10.3389/fmicb.2018.03348
Abbasi U, Jin W, Pervez A, Bhatti ZA, Tariq M, Shaheen S, Iqbal A, Mahmood Q (2016) Anaerobic microbial fuel cell treating combined industrial wastewater: correlation of electricity generation with pollutants. Bioresour Technol 200:1–7. https://doi.org/10.1016/j.biortech.2015.09.088
Ahn Y, Logan BE (2013) Saline catholytes as alternatives to phosphate buffers in microbial fuel cells. Bioresour Technol 132:436–439. https://doi.org/10.1016/j.biortech.2013.01.113
An BM, Song YH, Shin JW, Park JY (2016) Two-chamber microbial fuel cell to simultaneously remove ethanolamine and nitrate. Desalin Water Treat 57:7866–7873. https://doi.org/10.1080/19443994.2015.1083482
Angioni S, Millia L, Bruni G, Ravelli D, Mustarelli P, Quartarone E (2017) Novel composite polybenzimidazole-based proton exchange membranes as efficient and sustainable separators for microbial fuel cells. J Power Sour 348:57–65. https://doi.org/10.1016/j.jpowsour.2017.02.084
Araneda I, Tapia NF, Allende KL, Vargas IT (2018) Constructed wetland-microbial fuel cells for sustainable greywater treatment. Water (Switzerland) 10:1–9. https://doi.org/10.3390/w10070940
Asensio Y, Mansilla E, Fernandez-Marchante CM, Lobato J, Cañizares P, Rodrigo MA (2017) Towards the scale-up of bioelectrogenic technology: stacking microbial fuel cells to produce larger amounts of electricity. J Appl Electrochem 47:1115–2112. https://doi.org/10.1007/s10800-017-1101-2
Ayyaru S, Dharmalingam S (2011) Development of MFC using sulphonated polyether ether ketone (SPEEK) membrane for electricity generation from waste water. Bioresour Technol 102:11167–11171. https://doi.org/10.1016/j.biortech.2011.09.021
Babu S, Srikanth S, Mohan SV, Pant D (2015) Continuous mode operation of microbial fuel cell (MFC) stack with dual gas diffusion cathode design for the treatment of dark fermentation effluent. Int J Hydrog Energy 40:12424–12435. https://doi.org/10.1016/j.ijhydene.2015.07.049
Behera M, Jana PS, More TT, Ghangrekar MM (2010) Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry 79:228–233. https://doi.org/10.1016/j.bioelechem.2010.06.002
Biffinger JC, Pietron J, Ray R, Little B, Ringeisen BR (2007) A biofilm enhanced miniature microbial fuel cell using Shewanella oneidensis DSP10 and oxygen reduction cathodes. Biosens Bioelectron 22:1672–1679. https://doi.org/10.1016/j.bios.2006.07.027
Buitrón G, Moreno-Andrade I (2014) Performance of a single-chamber microbial fuel cell degrading phenol: effect of phenol concentration and external resistance. Appl Biochem Biotechnol 174:2471–2481. https://doi.org/10.1007/s12010-014-1195-5
Burkitt R, Whiffen TR, Yu EH (2016) Iron phthalocyanine and MnOx composite catalysts for microbial fuel cell applications. Appl Catal B Environ 181:279–288. https://doi.org/10.1016/j.apcatb.2015.07.010
Cai H, Wang J, Bu Y, Zhong Q (2013) Treatment of carbon cloth anodes for improving power generation in a dual-chamber microbial fuel cell. J Chem Technol Biotechnol 88:623–628. https://doi.org/10.1002/jctb.3875
Cao X, Huang X, Liang P, Boon N, Fan M, Zhang L, Zhang X (2009) A completely anoxic microbial fuel cell using a photo-biocathode for cathodic carbon dioxide reduction. Energy Environ Sci 2:498–501. https://doi.org/10.1039/b901069f
Cao Y, Hu Y, Sun J, Hou B (2010) Explore various co-substrates for simultaneous electricity generation and Congo red degradation in air-cathode single-chamber microbial fuel cell. Bioelectrochemistry 79:71–76. https://doi.org/10.1016/j.bioelechem.2009.12.001
Catal T, Li K, Bermek H, Liu H (2008) Electricity production from twelve monosaccharides using microbial fuel cells. J Power Sour 175:196–200. https://doi.org/10.1016/j.jpowsour.2007.09.083
Catal T, Yavaser S, Enisoglu-Atalay V, Bermek H, Ozilhan S (2018) Monitoring of neomycin sulfate antibiotic in microbial fuel cells. Bioresour Technol 268:116–120. https://doi.org/10.1016/j.biortech.2018.07.122
Cecconet D, Molognoni D, Callegari A, Capodaglio AG (2018) Agro-food industry wastewater treatment with microbial fuel cells: energetic recovery issues. Int J Hydrog Energy 43:500–511. https://doi.org/10.1016/j.ijhydene.2017.07.231
Cetinkaya AY, Ozkaya B, Taskan E, Karadag D, Cakmakci M (2016) The production of electricity from dual-chambered microbial fuel cell fueled by old age leachate. Energy Sour Part A Recov Util Environ Eff 38:1544–1552. https://doi.org/10.1080/15567036.2013.843041
Chae KJ, Choi M, Ajayi FF, Park W, Chang IS, Kim IS (2008) Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 22:169–176. https://doi.org/10.1021/ef700308u
Chang SH, Wu CH, Wang RC, Lin CW (2017) Electricity production and benzene removal from groundwater using low-cost mini tubular microbial fuel cells in a monitoring well. J Environ Manag 193:551–557. https://doi.org/10.1016/j.jenvman.2017.02.053
Chen J, Hu Y, Huang W, Zhang L (2017) Enhanced electricity generation for biocathode microbial fuel cell by in situ microbial-induced reduction of graphene oxide and polarity reversion. Int J Hydrog Energy 42:12574–12582. https://doi.org/10.1016/j.ijhydene.2017.03.012
Chen X, Cui D, Wang X, Wang X, Li W (2015) Porous carbon with de fi ned pore size as anode of microbial fuel cell. Biosens Bioelectron 69:135–141. https://doi.org/10.1016/j.bios.2015.02.014
Cheng S, Logan BE (2007) Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem Commun 9:492–496. https://doi.org/10.1016/j.elecom.2006.10.023
Cheraghipoor M, Mohebbi-Kalhori D, Noroozifar M, Maghsoodlou MT (2021) Production of greener energy in microbial fuel cell with ceramic separator fabricated using native soils: effect of lattice and porous SiO2. Fuel 284:118938
Choi O, Sang BI (2016) Extracellular electron transfer from cathode to microbes: application for biofuel production. Biotechnol Biofuels 9:1–14. https://doi.org/10.1186/s13068-016-0426-0
Choudhury P, Ray RN, Bandyopadhyay TK, Basak B, Muthuraj M, Bhunia B (2021) Process engineering for stable power recovery from dairy wastewater using microbial fuel cell. Int J Hydrog Energy 46(4):3171–3182
Clauwaert P, Rabaey K, Aelterman P, De SL, Pham TH, Boeckx P, Boon N, Vrestraete W (2007) Biological denitrification in microbial fuel cells. Environ Sci Technol 41:3354–3360
Costa NL, Clarke TA, Philipp LA, Gescher J, Louro RO, Paquete CM (2018) Electron transfer process in microbial electrochemical technologies: the role of cell-surface exposed conductive proteins. Bioresour Technol 255:308–317. https://doi.org/10.1016/j.biortech.2018.01.133
Dai H, Yang H, Liu X, Zhao Y, Liang Z (2016) Performance of sodium bromate as cathodic electron acceptor in microbial fuel cell. Bioresour Technol 202:220–225. https://doi.org/10.1016/j.biortech.2015.12.008
Dantas JM, Campelo LM, Duke NEC, Salgueiro CA, Pokkuluri PR (2015) The structure of PccH from Geobacter sulfurreducens—A novel low reduction potential monoheme cytochrome essential for accepting electrons from an electrode. FEBS J 282:2215–2231. https://doi.org/10.1111/febs.13269
Dantas JM, Silva MA, Pantoja-Uceda D, Turner DL, Bruix M, Salgueiro CA (2017) Solution structure and dynamics of the outer membrane cytochrome OmcF from Geobacter sulfurreducens. Biochim Biophys Acta Bioenerg 1858:733–741. https://doi.org/10.1016/j.bbabio.2017.03.007
Das I, Noori MT, Bhowmick GD, Ghangrekar MM (2018) Synthesis of bimetallic iron ferrite Co0.5Zn0.5Fe2O4 as a superior catalyst for oxygen reduction reaction to replace noble metal catalysts in microbial fuel cell. Int J Hydrog Energy 43:19196–19205. https://doi.org/10.1016/j.ijhydene.2018.08.113
Deeke A, Sleutels THJA, Hamelers HVM, Buisman CJN (2012) Capacitive bioanodes enable renewable energy storage in microbial fuel cells. Environ Sci Technol 46:3554–3560
Di Palma L, Bavasso I, Sarasini F, Tirillò J, Puglia D, Dominici F, Torre L (2018) Synthesis, characterization and performance evaluation of Fe3O4/PES nano composite membranes for microbial fuel cell. Eur Polym J 99:222–229. https://doi.org/10.1016/j.eurpolymj.2017.12.037
Doherty L, Zhao Y, Zhao X, Hu Y, Hao X, Xu L, Liu R (2015) A review of a recently emerged technology: constructed wetland—Microbial fuel cells. Water Res 85:38–45. https://doi.org/10.1016/j.watres.2015.08.016
Doherty L, Zhao Y, Zhao X, Wang W (2015) Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem Eng J 266:74–81. https://doi.org/10.1016/j.cej.2014.12.063
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25:464–482. https://doi.org/10.1016/j.biotechadv.2007.05.004
Eaktasang N, Kang CS, Lim H, Kwean OS, Cho S, Kim Y, Kim HS (2016) Production of electrically-conductive nanoscale filaments by sulfate-reducing bacteria in the microbial fuel cell. Bioresour Technol 210:61–67. https://doi.org/10.1016/j.biortech.2015.12.090
Elmekawy A, Srikanth S, Vanbroekhoven K, De WH, Pant D (2014) Bioelectro-catalytic valorization of dark fermentation effluents by acetate oxidizing bacteria in bioelectrochemical system (BES). J Power Sour 262:183–191. https://doi.org/10.1016/j.jpowsour.2014.03.111
Erable B, Oliot M, Lacroix R, Bergel A, Serov A, Kodali M, Santoro C, Atanassov P (2018) Iron-Nicarbazin derived platinum group metal-free electrocatalyst in scalable-size air-breathing cathodes for microbial fuel cells. Electrochim Acta 277:127–135. https://doi.org/10.1016/j.electacta.2018.04.190
Feng Y, Yang Q, Wang X, Logan BE (2010) Treatment of carbon fiber brush anodes for improving power generation in air—cathode microbial fuel cells. J Power Sour 195:1841–1844. https://doi.org/10.1016/j.jpowsour.2009.10.030
Forrestal C, Huang Z, Ren ZJ (2014) Percarbonate as a naturally buffering catholyte for microbial fuel cells. Bioresour Technol 172:429–432. https://doi.org/10.1016/j.biortech.2014.09.014
Freguia S, Rabaey K, Yuan Z, Keller J (2007) Non-catalyzed cathodic oxygen reduction at graphite granules in microbial fuel cells. Electrochim Acta 53:598–603. https://doi.org/10.1016/j.electacta.2007.07.037
Freguia S, Rabaey K, Yuan Z, Keller J (2008) Syntrophic processes drive the conversion of glucose in microbial fuel cell anodes. Environ Sci Technol 42:7937–7943
Geetanjali, Rani R, Sharma D, Kumar S (2019) Optimization of operating conditions of miniaturize single chambered microbial fuel cell using NiWO4/graphene oxide modified anode for performance improvement and microbial communities dynamics. Bioresour Technol 285:121337. https://doi.org/10.1016/j.biortech.2019.121337
Ghasemi M, Daud WRW, Hassan SHA, Oh SE, Ismail M, Rahimnejad M, Jahim JM (2013) Nano-structured carbon as electrode material in microbial fuel cells: a comprehensive review. J Alloys Compd 580:245–255. https://doi.org/10.1016/j.jallcom.2013.05.094
Ghasemi M, Wan Daud WR, Ismail M, Rahimnejad M, Ismail AF, Leong JX, Miskan M, Ben Liew K (2013) Effect of pre-treatment and biofouling of proton exchange membrane on microbial fuel cell performance. Int J Hydrog Energy 38:5480–5484. https://doi.org/10.1016/j.ijhydene.2012.09.148
Goel M, Chovelon JM, Ferronato C, Bayard R, Sreekrishnan TR (2010) The remediation of wastewater containing 4-chlorophenol using integrated photocatalytic and biological treatment. J Photochem Photobiol B Biol 98:1–6. https://doi.org/10.1016/j.jphotobiol.2009.09.006
Goud RK, Babu PS, Mohan SV (2011) Canteen based composite food waste as potential anodic fuel for bioelectricity generation in single chambered microbial fuel cell (MFC): bio-electrochemical evaluation under increasing substrate loading condition. Int J Hydrog Energy 36:6210–6218. https://doi.org/10.1016/j.ijhydene.2011.02.056
Guo J, Cheng J, Li B, Wang J, Chu P (2019) Performance and microbial community in the biocathode of microbial fuel cells under different dissolved oxygen concentrations. J Electroanal Chem 833:433–440. https://doi.org/10.1016/j.jelechem.2018.12.015
Guo X, Zhan Y, Chen C, Cai B, Wang Y, Guo S (2016) Influence of packing material characteristics on the performance of microbial fuel cells using petroleum refinery wastewater as fuel. Renew Energy 87:437–444. https://doi.org/10.1016/j.renene.2015.10.041
Habibul N, Hu Y, Wang Y, Chen W, Yu H, Sheng G (2016) Bioelectrochemical Chromium (VI) removal in plant-microbial fuel cells. Environ Sci Technol 50:3882–3889. https://doi.org/10.1021/acs.est.5b06376
Harewood AJT, Popuri SR, Cadogan EI, Lee C-H, Wang C-C (2017) Bioelectricity generation from brewery wastewater in a microbial fuel cell using chitosan/biodegradable copolymer membrane. Int J Environ Sci Technol 14:1535–1550. https://doi.org/10.1007/s13762-017-1258-6
He CS, Mu ZX, Yang HY, Wang YZ, Mu Y, Yu HQ (2015) Electron acceptors for energy generation in microbial fuel cells fed with wastewaters: a mini-review. Chemosphere 140:99–105. https://doi.org/10.1016/j.chemosphere.2015.03.059
He Z, Minteer SD, Angenent LT (2005) Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ Sci Technol 39:5262–5267. https://doi.org/10.1021/es0502876
Hernández-Flores G, Poggi-Varaldo HM, Solorza-Feria O (2016) Comparison of alternative membranes to replace high cost Nafion ones in microbial fuel cells. Int J Hydrog Energy 41:23354–23362. https://doi.org/10.1016/j.ijhydene.2016.08.206
Hirooka K, Ichihashi O, Takeguchi T (2018) Sodium cobalt oxide as a non-platinum cathode catalyst for microbial fuel cells. Sustain Environ Res 28:322–325. https://doi.org/10.1016/j.serj.2018.07.002
Hsu L, Masuda SA, Nealson KH, Pirbazari M (2012) Evaluation of microbial fuel cell Shewanella biocathodes for treatment of chromate contamination. RSC Adv 2:5844–5855. https://doi.org/10.1039/c2ra20478a
Iannaci A, Sciarria TP, Mecheri B, Adani F, Licoccia S, Epifanio AD (2017) Power generation using a low–cost sulfated zirconium oxide based cathode in single chamber microbial fuel cells. J Alloys Compd 693:170–176. https://doi.org/10.1016/j.jallcom.2016.09.159
International Energy Agency F (2017) World gross electricity production, by source
International Energy Agency F (2019) World Energy Outlook 2019
Izadi P, Fontmorin JM, Fernández LFL, Cheng S, Head I, Yu EH (2019) High performing gas diffusion biocathode for microbial fuel cells using acidophilic iron oxidizing bacteria. Front Energy Res 7:1–10. https://doi.org/10.3389/fenrg.2019.00093
Jiang C, Yang Q, Wang D, Zhong Y, Chen F, Li X, Zeng G, Li X, Shang M (2017) Simultaneous perchlorate and nitrate removal coupled with electricity generation in autotrophic denitrifying biocathode microbial fuel cell. Chem Eng J 308:783–790. https://doi.org/10.1016/j.cej.2016.09.121
Kadam SK, Watharkar AD, Chandanshive VV, Khandare RV, Jeon BH, Jadhav JP, Govindwar SP (2018) Co-planted floating phyto-bed along with microbial fuel cell for enhanced textile effluent treatment. J Clean Prod 203:788–798. https://doi.org/10.1016/j.jclepro.2018.08.336
Kashima H, Regan JM (2015) Facultative nitrate reduction by electrode-respiring Geobacter metallireducens biofilms as a competitive reaction to electrode reduction in a bioelectrochemical system. Environ Sci Technol 49:3195–3202. https://doi.org/10.1021/es504882f
Khalid S, Alvi F, Fatima M, Aslam M, Riaz S, Farooq R, Zhang Y (2018) Dye degradation and electricity generation using microbial fuel cell with graphene oxide modified anode. Mater Lett 220:272–276. https://doi.org/10.1016/j.matlet.2018.03.054
Khan MD, Khan N, Sultana S, Joshi R, Ahmed S, Yu E, Scott K, Ahmad A, Khan MZ (2017) Bioelectrochemical conversion of waste to energy using microbial fuel cell technology. Process Biochem 57:141–158. https://doi.org/10.1016/j.procbio.2017.04.001
Khan MZ, Singh S, Sultana S, Sreekrishnan TR, Ahammad SZ (2015) Studies on the biodegradation of two different azo dyes in bioelectrochemical systems. New J Chem 39:5597–5604. https://doi.org/10.1039/C5NJ00541H
Khan N, Anwer AH, Ahmad A, Sabir S, Khan MZ (2020) Investigating microbial fuel cell aided bio-remediation of mixed phenolic contaminants under oxic and anoxic environments. Biochem Eng J 155:107485
Khan N, Anwer AH, Ahmad A, Sabir S, Sevda S, Khan MZ (2019) Investigation of CNT/PPy-modified carbon paper electrodes under anaerobic and aerobic conditions for phenol bioremediation in microbial fuel cells. ACS Omega 5(1):471–480
Khan N, Anwer AH, Khan MD, Azam A, Ibhadon A, Khan MZ (2021) Magnesium ferrite spinels as anode modifier for the treatment of Congo red and energy recovery in a single chambered microbial fuel cell. J Hazard Mater 410:124561
Khan N, Khan MD, Ansari MY, Ahmad A, Khan MZ (2019) Bio-electrodegradation of 2,4,6-trichlorophenol by mixed microbial culture in dual chambered microbial fuel cells. J Biosci Bioeng 127:353–359. https://doi.org/10.1016/j.jbiosc.2018.08.012
Khan N, Khan MD, Nizami AS, Rehan M, Shaida MA, Ahmad A, Khan MZ (2018) Energy generation through bioelectrochemical degradation of pentachlorophenol in microbial fuel cell. RSC Adv 8(37):20726–20736
Kim C, Lee CR, Song YE, Heo J, Choi SM, Lim DH, Cho J, Park C, Jang M, Kim JR (2017) Hexavalent chromium as a cathodic electron acceptor in a bipolar membrane microbial fuel cell with the simultaneous treatment of electroplating wastewater. Chem Eng J 328:703–707. https://doi.org/10.1016/j.cej.2017.07.077
Kumar A, Hsu LHH, Kavanagh P, Barrière F, Lens PNL, Lapinsonnière L, Lienhard JH, Schröder U, Jiang X, Leech D (2017) The ins and outs of microorganism-electrode electron transfer reactions. Nat Rev Chem 1:1–13. https://doi.org/10.1038/s41570-017-0024
Kumar R, Singh L, Wahid ZA, Din MFM (2015) Exoelectrogens in microbial fuel cells toward bioelectricity generation: a review. Int J Energy Res 39:1048–1067. 10.1002/er
Kumar R, Singh L, Zularisam AW, Hai FI (2018) Microbial fuel cell is emerging as a versatile technology: a review on its possible applications, challenges and strategies to improve the performances. Int J Energy Res 42:369–394. https://doi.org/10.1002/er.3780
Kumar SS, Malyan SK, Bishnoi NR (2017) Performance of buffered ferric chloride as terminal electron acceptor in dual chamber microbial fuel cell. J Environ Chem Eng 5:1238–1243. https://doi.org/10.1016/j.jece.2017.02.010
Lai B, Tang X, Li H, Du Z, Liu X, Zhang Q (2011) Power production enhancement with a polyaniline modified anode in microbial fuel cells. Biosens Bioelectron 28:373–377. https://doi.org/10.1016/j.bios.2011.07.050
Lai C, Li B, Chen M, Zeng G, Huang D, Qin L, Liu X, Cheng M, Wan J, Du C, Huang F, Liu S, Yi H (2018) Simultaneous degradation of P-nitroaniline and electricity generation by using a microfiltration membrane dual-chamber microbial fuel cell. Int J Hydrog Energy 43:1749–1757. https://doi.org/10.1016/j.ijhydene.2017.11.025
Lay CH, Kokko ME, Puhakka JA (2015) Power generation in fed-batch and continuous up-flow microbial fuel cell from synthetic wastewater. Energy 91:235–241. https://doi.org/10.1016/j.energy.2015.08.029
Li H, Tian Y, Zuo W, Zhang J, Pan X, Li L, Su X (2016) Electricity generation from food wastes and characteristics of organic matters in microbial fuel cell. Bioresour Technol 205:104–110. https://doi.org/10.1016/j.biortech.2016.01.042
Li J, Liu G, Zhang R, Luo Y, Zhang C, Li M (2010) Electricity generation by two types of microbial fuel cells using nitrobenzene as the anodic or cathodic reactants. Bioresour Technol 101:4013–4020. https://doi.org/10.1016/j.biortech.2009.12.135
Li L, Liu S, Wang H, Yang P (2018) Application of graphene-cobalt/nickel composite-carbon cloth modified electrode in microbial fuel cell. Environ Eng Sci 35:1173–1184. https://doi.org/10.1089/ees.2017.0543
Li WW, Sheng GP, Liu XW, Yu HQ (2011) Recent advances in the separators for microbial fuel cells. Bioresour Technol 102:244–252. https://doi.org/10.1016/j.biortech.2010.03.090
Liao Q, Zhang J, Li J, Ye D, Zhu X, Zhang B (2015) Increased performance of a tubular microbial fuel cell with a rotating carbon-brush anode. Biosens Bioelectron 63:558–561. https://doi.org/10.1016/j.bios.2014.08.014
Lin C, Wu C, Huang W, Tsai S (2015) Evaluation of different cell-immobilization strategies for simultaneous distillery wastewater treatment and electricity generation in microbial fuel cells. Fuel 144:1–8. https://doi.org/10.1016/j.fuel.2014.12.009
Lin T, Bai X, Hu Y, Li B, Yuan Y, Song H (2017) Synthetic Saccharomyces cerevisiae-Shewanella oneidensis consortium enables glucose-fed high-performance microbial fuel cell. Am Inst Chem Eng 63:1830–1838. https://doi.org/10.1002/aic
Liu D, Chang Q, Gao Y, Huang W, Sun Z, Yan M, Guo C (2020) High performance of microbial fuel cell afforded by metallic tungsten carbide decorated carbon cloth anode. Electrochim Acta 330:135243. https://doi.org/10.1016/j.electacta.2019.135243
Liu H, Cheng S, Logan BE (2005) Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ Sci Technol 39:658–662. https://doi.org/10.1021/es048927c
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285. https://doi.org/10.1021/es034923g
Liu S, Feng X, Gu F, Li X, Wang Y (2017) Sequential reduction/oxidation of azo dyes in a three-dimensional biofilm electrode reactor. Chemosphere 186:287–294
Liu X, Wu W, Gu Z (2015) Poly (3,4-ethylenedioxythiophene) promotes direct electron transfer at the interface between Shewanella loihica and the anode in a microbial fuel cell. J Power Sour 277:110–115. https://doi.org/10.1016/j.jpowsour.2014.11.129
Logan BE, Hamelers B, Rozendal R, Schroder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192. https://doi.org/10.1021/es0605016
Logan BE, Murano C, Scott K, Gray ND, Head IM (2005) Electricity generation from cysteine in a microbial fuel cell. Water Res 39:942–952. https://doi.org/10.1016/j.watres.2004.11.019
Majidi MR, Shahbazi Farahani F, Hosseini M, Ahadzadeh I (2019) Low-cost nanowired α-MnO2/C as an ORR catalyst in air-cathode microbial fuel cell. Bioelectrochemistry 125:38–45. https://doi.org/10.1016/j.bioelechem.2018.09.004
Malvankar NS, Lovley DR (2014) Microbial nanowires for bioenergy applications. Curr Opin Biotechnol 27:88–95. https://doi.org/10.1016/j.copbio.2013.12.003
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sustain Energy Rev 45:540–555. https://doi.org/10.1016/j.rser.2015.02.032
Mashkour M, Rahimnejad M, Pourali SM, Ezoji H, ElMekawy A, Pant D (2017) Catalytic performance of nano-hybrid graphene and titanium dioxide modified cathodes fabricated with facile and green technique in microbial fuel cell. Prog Nat Sci Mater Int 27:647–651. https://doi.org/10.1016/j.pnsc.2017.11.003
Mecheri B, Iannaci A, D’Epifanio A, Mauri A, Licoccia S (2016) Carbon-supported zirconium oxide as a cathode for microbial fuel cell applications. ChemPlusChem 81:80–85. https://doi.org/10.1002/cplu.201500347
Mehdinia A, Ziaei E, Jabbari A (2014) Multi-walled carbon nanotube/SnO2 nanocomposite: a novel anode material for microbial fuel cells. Electrochim Acta 130:512–518. https://doi.org/10.1016/j.electacta.2014.03.011
Mink JE, Hussain MM (2013) Sustainable design of high- performance microsized microbial fuel cell with carbon nanotube anode and air cathode. ACS Nano 7:6921–6927
Miran W, Nawaz M, Jang J, Lee DS (2016) Conversion of orange peel waste biomass to bioelectricity using a mediator-less microbial fuel cell. Sci Total Environ 547:197–205. https://doi.org/10.1016/j.scitotenv.2016.01.004
Miran W, Rasool K, Nawaz M, Kadam A, Shin S, Heo J, Jang J, Lee S (2016) Simultaneous electricity production and direct red 80 degradation using a dual chamber microbial fuel cell. Desalin Water Treat 57:9051–9059. https://doi.org/10.1080/19443994.2015.1049410
Mohamed SN, Thomas N, Tamilmani J, Boobalan T, Matheswaran M, Kalaichelvi P, Alagarsamy A, Pugazhendhi A (2020) Bioelectricity generation using iron (II) molybdate nanocatalyst coated anode during treatment of sugar wastewater in microbial fuel cell. Fuel 277:118119
Moharir PV, Tembhurkar AR (2018) Effect of recirculation on bioelectricity generation using microbial fuel cell with food waste leachate as substrate. Int J Hydrog Energy 43:10061–10069. https://doi.org/10.1016/j.ijhydene.2018.04.072
Munoz-Cupa C, Hu Y, Xu C, Bassi A (2021) An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci Total Environ 754:142429
Noori MT, Mukherjee CK, Ghangrekar MM (2017) Enhancing performance of microbial fuel cell by using graphene supported V2O5-nanorod catalytic cathode. Electrochim Acta 228:513–521. https://doi.org/10.1016/j.electacta.2017.01.016
Oon YS, Ong SA, Ho LN, Wong YS, Oon YL, Lehl HK, Thung WE, Nordin N (2017) Microbial fuel cell operation using monoazo and diazo dyes as terminal electron acceptor for simultaneous decolourisation and bioelectricity generation. J Hazard Mater 325:170–177. https://doi.org/10.1016/j.jhazmat.2016.11.074
Pandey P, Shinde VN, Deopurkar RL, Kale SP, Patil SA, Pant D (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723. https://doi.org/10.1016/j.apenergy.2016.01.056
Penteado ED, Fernandez-Marchante CM, Zaiat M, Cañizares P, Gonzalez ER, Rodrigo MAR (2016) Energy recovery from winery wastewater using a dual chamber microbial fuel cell. J Chem Technol Biotechnol 91:1802–1808. https://doi.org/10.1002/jctb.4771
Penteado ED, Fernandez-Marchante CM, Zaiat M, Gonzalez ER, Rodrigo MA (2017) On the effects of ferricyanide as cathodic mediator on the performance of microbial fuel cells. Electrocatalysis 8:59–66. https://doi.org/10.1007/s12678-016-0334-x
Pinto D, Coradin T, Laberty-Robert C (2018) Effect of anode polarization on biofilm formation and electron transfer in Shewanella oneidensis/graphite felt microbial fuel cells. Bioelectrochemistry 120:1–9. https://doi.org/10.1016/j.bioelechem.2017.10.008
Pu K-B, Ma Q, Cai WF, Chen Q-Y, Wang YH, Li F-J (2018) Polypyrrole modified stainless steel as high performance anode of microbial fuel cell. Biochem Eng J 132:255–261. https://doi.org/10.1016/j.bej.2018.01.018
Qiao Y, Li CM, Bao SJ, Bao QL (2007) Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J Power Sour 170:79–84. https://doi.org/10.1016/j.jpowsour.2007.03.048
Rabaey K, Clauwaert P, Aelterman P, Verstraete W (2005) Tubular microbial fuel cells for efficient electricity generation. Environ Sci Technol 39:8077–8082
Rabaey K, Read ST, Clauwaert P, Freguia S, Bond PL, Blackall LL, Keller J (2008) Cathodic oxygen reduction catalyzed by bacteria in microbial fuel cells. ISME J 2:519–527. https://doi.org/10.1038/ismej.2008.1
Rahimnejad M, Adhami A, Darvari S, Zirepour A, Oh S-E (2015) Microbial fuel cell as new technology for bioelectricity generation: a review. Alex Eng J. https://doi.org/10.1016/j.aej.2015.03.031
Rajeswari S, Vidhya S, Krishnaraj RN, Saravanan P, Sundarapandiyan S, Maruthamuthu S, Ponmariappan S, Vijayan M (2016) Utilization of soak liquor in microbial fuel cell. Fuel 181:148–156. https://doi.org/10.1016/j.fuel.2016.04.121
Reiche A, Sivell J lynn, Kirkwood KM (2016) Electricity generation by Propionibacterium freudenreichii in a mediatorless microbial fuel cell. Biotechnol Lett 38:51–55. https://doi.org/10.1007/s10529-015-1944-8
Reimers CE, Girguis P, Stecher HA, Tender LM, Ryckelynck N, Whaling P (2006) Microbial fuel cell energy from an ocean cold seep. Geobiology 4:123–136. https://doi.org/10.1111/j.1472-4669.2006.00071.x
Rinaldi A, Mecheri B, Garavaglia V, Licoccia S, Di Nardo P, Traversa E (2008) Engineering materials and biology to boost performance of microbial fuel cells: a critical review. Energy Environ Sci 1:417–429. https://doi.org/10.1039/b806498a
Rittmann BE (2008) Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioeng 100:203–212. https://doi.org/10.1002/bit.21875
Rotaru AE, Shrestha PM, Liu F, Markovaite B, Chen S, Nevin KP, Lovley DR (2014) Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl Environ Microbiol 80:4599–4605. https://doi.org/10.1128/AEM.00895-14
Samsudeen N, Radhakrishnan TK, Matheswaran M (2015) Bioelectricity production from microbial fuel cell using mixed bacterial culture isolated from distillery wastewater. Bioresour Technol 195:242–247. https://doi.org/10.1016/j.biortech.2015.07.023
San-Martín MI, Carmona FJ, Alonso RM, Prádanos P, Morán A, Escapa A (2019) Assessing the ageing process of cation exchange membranes in bioelectrochemical systems. Int J Hydrog Energy 44:25287–25296. https://doi.org/10.1016/j.ijhydene.2019.08.004
Santoro C, Kodali M, Kabir S, Soavi F, Serov A, Atanassov P (2017) Three-dimensional graphene nanosheets as cathode catalysts in standard and supercapacitive microbial fuel cell. J Power Sour 356:371–380. https://doi.org/10.1016/j.jpowsour.2017.03.135
Sekar AD, Jayabalan T, Muthukumar H, Chandrasekaran NI, Mohamed SN, Matheswaran M (2019) Enhancing power generation and treatment of dairy waste water in microbial fuel cell using Cu-doped iron oxide nanoparticles decorated anode. Energy 172:173–180. https://doi.org/10.1016/j.energy.2019.01.102
Selvaraj D, Somanathan A, Jeyakumar R, Kumar G (2020) Generation of electricity by the degradation of electro-Fenton pretreated latex wastewater using double chamber microbial fuel cell. Int J Energy Res 44(15):12496–12505
Sevda S, Dominguez-Benetton X, Graichen FHM, Vanbroekhoven K, De WH, Sreekrishnan TR, Pant D (2016) Shift to continuous operation of an air-cathode microbial fuel cell long-running in fed-batch mode boosts power generation. Int J Green Energy 13:71–79. https://doi.org/10.1080/15435075.2014.909363
Shen J, Huang L, Zhou P, Quan X, Puma GL (2017) Correlation between circuital current, Cu (II) reduction and cellular electron transfer in EAB isolated from Cu (II)-reduced biocathodes of microbial fuel cells. Bioelectrochemistry 114:1–7. https://doi.org/10.1016/j.bioelechem.2016.11.002
Sirinutsomboon B (2014) Modeling of a membraneless single-chamber microbial fuel cell with molasses as an energy source. Int J Energy Environ Eng 5:1–9. https://doi.org/10.1007/s40095-014-0093-5
Sonawane JM, Adeloju SB, Ghosh PC (2017) Landfill leachate: a promising substrate for microbial fuel cells. Int J Hydrog Energy 42:23794–23798. https://doi.org/10.1016/j.ijhydene.2017.03.137
Song R-B, Zhao C-E, Jiang L-P, Abdel-halim ES, Zhang J-R, Zhu J-J (2016) Bacteria-affinity 3D macroporous graphene/MWCNTs/Fe3O4 foams for high-performance microbial fuel cells. ACS Appl Mater Interfaces 8:16170–16177. https://doi.org/10.1021/acsami.6b03425
Srikanth S, Kumar M, Singh D, Singh MP, Das BP (2016) Electro-biocatalytic treatment of petroleum refinery wastewater using microbial fuel cell (MFC) in continuous mode operation. Bioresour Technol 221:70–77. https://doi.org/10.1016/j.biortech.2016.09.034
Srinivasan V, Weinrich J, Butler C (2016) Nitrite accumulation in a denitrifying biocathode microbial fuel cell. Environ Sci Water Res Technol 2:344–352. https://doi.org/10.1039/c5ew00260e
Srinophakun P, Thanapimmetha A, Plangsri S, Vetchayakunchai S, Saisriyoot M (2017) Application of modified chitosan membrane for microbial fuel cell: roles of proton carrier site and positive charge. J Clean Prod 142:1274–1282. https://doi.org/10.1016/j.jclepro.2016.06.153
Sydow A, Krieg T, Mayer F, Schrader J, Holtmann D (2014) Electroactive bacteria—Molecular mechanisms and genetic tools. Appl Microbiol Biotechnol 98:8481–8495. https://doi.org/10.1007/s00253-014-6005-z
Tamilarasan K, Banu JR, Jayashree C, Yogalakshmi KN, Gokulakrishnan K (2017) Effect of organic loading rate on electricity generating potential of upflow anaerobic microbial fuel cell treating surgical cotton industry wastewater. J Environ Chem Eng 5:1021–1026. https://doi.org/10.1016/j.jece.2017.01.025
Tang X, Ng HY (2017) Anthraquinone-2-sulfonate immobilized to conductive polypyrrole hydrogel as a bioanode to enhance power production in microbial fuel cell. Bioresour Technol 244:452–455. https://doi.org/10.1016/j.biortech.2017.07.189
Thung WE, Ong SA, Ho LN, Wong YS, Ridwan F, Oon YL, Oon YS, Lehl HK (2015) A highly efficient single chambered up-flow membrane-less microbial fuel cell for treatment of azo dye Acid Orange 7-containing wastewater. Bioresour Technol 197:284–288. https://doi.org/10.1016/j.biortech.2015.08.078
Thung WE, Ong SA, Ho LN, Wong YS, Ridwan F, Oon YL, Oon YS, Lehl HK (2018) Sustainable green technology on wastewater treatment: the evaluation of enhanced single chambered up-flow membrane-less microbial fuel cell. J Environ Sci (China) 66:295–300. https://doi.org/10.1016/j.jes.2017.05.010
Tiwari BR, Ghangrekar MM (2018) Electricity production during distillery wastewater treatment in a microbial fuel cell equipped with low cost PVA-nafion-borosilicate membrane. J Clean Energy Technol, 6:155–158. https://doi.org/10.18178/jocet.2018.6.2.452
Tofighi A, Rahimnejad M, Ghorbani M (2019) Ternary nanotube α-MnO2/GO/AC as an excellent alternative composite modifier for cathode electrode of microbial fuel cell. J Therm Anal Calorim 135:1667–1675. https://doi.org/10.1007/s10973-018-7198-7
Ucar D, Zhang Y, Angelidaki I (2017) An overview of electron acceptors in microbial fuel cells. Front Microbiol 8:1–14. https://doi.org/10.3389/fmicb.2017.00643
United Nations (2019) Water facts-UN water, pp 1–14
Wang CT, Chen WJ, Huang RY (2010) Influence of growth curve phase on electricity performance of microbial fuel cell by Escherichia coli. Int J Hydrog Energy 35:7217–7223. https://doi.org/10.1016/j.ijhydene.2010.01.038
Wang D, Ma Z, Xie Y, Zhang M, Zhao N, Song H (2018) Fe/N-doped graphene with rod-like CNTs as an air-cathode catalyst in microbial fuel cells. RSC Adv 8:1203–1209. https://doi.org/10.1039/c7ra11613f
Wang H, Liu J, He W, Qu Y, Li D, Feng Y (2016) Energy-positive nitrogen removal from reject water using a tide-type biocathode microbial electrochemical system. Bioresour Technol 222:317–325. https://doi.org/10.1016/j.biortech.2016.09.090
Wang H, Song X, Zhang H, Tan P, Kong F (2020) Removal of hexavalent chromium in dual-chamber microbial fuel cells separated by different ion exchange membranes. J Hazard Mater 384:121459. https://doi.org/10.1016/j.jhazmat.2019.121459
Wang L, Xie B, Gao N, Min B, Liu H (2017) Urea removal coupled with enhanced electricity generation in single-chambered microbial fuel cells. Environ Sci Pollut Res 24:20401–20408. https://doi.org/10.1007/s11356-017-9689-7
Wang R, Yan M, Li H, Zhang L, Peng B, Sun J, Liu D, Liu S (2018) FeS2 nanoparticles decorated graphene as microbial-fuel-cell anode achieving high power density. Adv Mater 30:1–7. https://doi.org/10.1002/adma.201800618
Wang X, Li J, Wang Z, Tursun H, Liu R, Gao Y, Li Y (2016) Increasing the recovery of heavy metal ions using two microbial fuel cells operating in parallel with no power output. Environ Sci Pollut Res 23:20368–20377. https://doi.org/10.1007/s11356-016-7045-y
Wang Z, Zheng Y, Xiao Y, Wu S, Wu Y, Yang Z, Zhao F (2013) Analysis of oxygen reduction and microbial community of air-diffusion biocathode in microbial fuel cells. Bioresour Technol 144:74–79. https://doi.org/10.1016/j.biortech.2013.06.093
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85:849–860. https://doi.org/10.1007/s00253-009-2246-7
Winfield J, Ieropoulos I, Greenman J (2012) Investigating a cascade of seven hydraulically connected microbial fuel cells. Bioresour Technol 110:245–250. https://doi.org/10.1016/j.biortech.2012.01.095
World Health Organization (2019) Fact sheet on drinking water. World Health Organization, Geneva. https://www.who.int/en/news-room/fact-sheets/detail/drinking-water
Wrighton KC, Thrash JC, Melnyk RA, Bigi JP, Byrne-Bailey KG, Remis JP, Schichnes D, Auer M, Chang CJ, Coates JD (2011) Evidence for direct electron transfer by a gram-positive bacterium isolated from a microbial fuel cell. Appl Environ Microbiol 77:7633–7639. https://doi.org/10.1128/AEM.05365-11
Wu MS, Xu X, Zhao Q, Wang ZY (2017) Simultaneous removal of heavy metals and biodegradation of organic matter with sediment microbial fuel cells. RSC Adv 7:53433–53438. https://doi.org/10.1039/c7ra11103g
Wu S, He W, Yang W, Ye Y, Huang X, Logan BE (2017) Combined carbon mesh and small graphite fiber brush anodes to enhance and stabilize power generation in microbial fuel cells treating domestic wastewater. J Power Sour 356:348–355. https://doi.org/10.1016/j.jpowsour.2017.01.041
Wu X, Ren X, Owens G, Brunetti G, Zhou J, Yong X, Wei P, Jia H (2018) A facultative electroactive chromium (VI)-reducing bacterium aerobically isolated from a biocathode microbial fuel cell. Front Microbiol 9:1–10. https://doi.org/10.3389/fmicb.2018.02883
Wu Y, Yang Q, Zeng Q, Ngo HH, Guo W, Zhang H (2017) Enhanced low C/N nitrogen removal in an innovative microbial fuel cell (MFC) with electroconductivity aerated membrane (EAM) as biocathode. Chem Eng J 316:315–322. https://doi.org/10.1016/j.cej.2016.11.141
Xia C, Xu M, Liu J, Guo J, Yang Y (2015) Sediment microbial fuel cell prefers to degrade organic chemicals with higher polarity. Bioresour Technol 190:420–423. https://doi.org/10.1016/j.biortech.2015.04.072
Xu L, Zhao Y, Tang C, Doherty L (2018) Influence of glass wool as separator on bioelectricity generation in a constructed wetland-microbial fuel cell. J Environ Manag 207:116–123. https://doi.org/10.1016/j.jenvman.2017.11.035
Xu S, Liu H (2011) New exoelectrogen Citrobacter sp. SX-1 isolated from a microbial fuel cell. J Appl Microbiol 111:1108–1115. https://doi.org/10.1111/j.1365-2672.2011.05129.x
Xu YS, Zheng T, Yong XY, Zhai DD, Si RW, Li B, Yu YY, Yong YC (2016) Trace heavy metal ions promoted extracellular electron transfer and power generation by Shewanella in microbial fuel cells. Bioresour Technol 211:542–547. https://doi.org/10.1016/j.biortech.2016.03.144
Yang Q, Wang X, Feng Y, Lee H, Liu J, Shi X, Qu Y, Ren N (2012) Electricity generation using eight amino acids by air-cathode microbial fuel cells. Fuel 102:478–482. https://doi.org/10.1016/j.fuel.2012.04.020
Yang W, Li J, Ye D, Zhang L, Zhu X, Liao Q (2016) A hybrid microbial fuel cell stack based on single and double chamber microbial fuel cells for self-sustaining pH control. J Power Sour 306:685–691. https://doi.org/10.1016/j.jpowsour.2015.12.073
Yang W, Wang X, Rossi R, Logan BE (2020) Low-cost Fe–N–C catalyst derived from Fe (III)-chitosan hydrogel to enhance power production in microbial fuel cells. Chem Eng J 380:122522. https://doi.org/10.1016/j.cej.2019.122522
Yang Z, Pei H, Hou Q, Jiang L, Zhang L, Nie C (2018) Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: nutrient, organics removal and bioenergy production. Chem Eng J 332:277–285. https://doi.org/10.1016/j.cej.2017.09.096
Yazdi AA, D’Angelo L, Omer N, Windiasti G, Lu X, Xu J (2016) Carbon nanotube modification of microbial fuel cell electrodes. Biosens Bioelectron 85:536–552. https://doi.org/10.1016/j.bios.2016.05.033
Yazdi H, Liliana A-G, Ren ZJ (2015) Pluggable microbial fuel cell stacks for septic wastewater treatment and electricity production. Bioresour Technol 180:258–263. https://doi.org/10.1016/j.biortech.2014.12.100
Yong XY, Yan ZY, Shen HB, Zhou J, Wu XY, Zhang LJ, Zheng T, Jiang M, Wei P, Jia HH, Yong YC (2017) An integrated aerobic-anaerobic strategy for performance enhancement of Pseudomonas aeruginosa-inoculated microbial fuel cell. Bioresour Technol 241:1191–1196. https://doi.org/10.1016/j.biortech.2017.06.050
You J, Greenman J, Melhuish C, Ieropoulos I (2016) Electricity generation and struvite recovery from human urine using microbial fuel cells. J Chem Technol Biotechnol 91:647–654. https://doi.org/10.1002/jctb.4617
You S, Gong X, Wang W, Qi D, Wang X, Chen X, Ren N (2016) Enhanced cathodic oxygen reduction and power production of microbial fuel cell based on noble-metal-free electrocatalyst derived from metal-organic frameworks. Adv Energy Mater 6:1–9. https://doi.org/10.1002/aenm.201501497
Yu J, Park Y, Kim B, Lee T (2015) Power densities and microbial communities of brewery wastewater-fed microbial fuel cells according to the initial substrates. Bioprocess Biosyst Eng 38:85–92. https://doi.org/10.1007/s00449-014-1246-x
Yu Y, Ndayisenga F, Yu Z, Zhao M, Lay CH, Zhou D (2019) Co-substrate strategy for improved power production and chlorophenol degradation in a microbial fuel cell. Int J Hydrog Energy 44:20312–20322. https://doi.org/10.1016/j.ijhydene.2019.05.221
Yuan H, Hou Y, Abu-Reesh IM, Chen J, He Z (2016) Oxygen reduction reaction catalysts used in microbial fuel cells for energy-efficient wastewater treatment: a review. Mater Horizons 3:382–401. https://doi.org/10.1039/c6mh00093b
Zhai DD, Fang Z, Jin H, Hui M, Kirubaharan CJ, Yu YY, Yong YC (2019) Vertical alignment of polyaniline nanofibers on electrode surface for high-performance microbial fuel cells. Bioresour Technol 288:121499. https://doi.org/10.1016/j.biortech.2019.121499
Zhang B, Jiang Y, Han J (2017) A flexible nanocomposite membrane based on traditional cotton fabric to enhance performance of microbial fuel cell. Fibers Polym 18:1296–1303. https://doi.org/10.1007/s12221-017-7191-y
Zhang C, Liang P, Yang X, Jiang Y, Bian Y, Chen C, Zhang X, Huang X (2016) Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosens Bioelectron 81:32–38. https://doi.org/10.1016/j.bios.2016.02.051
Zhang F, He Z (2012) Simultaneous nitrification and denitrification with electricity generation in dual-cathode microbial fuel cells. J Chem Technol Biotechnol 87:153–159. https://doi.org/10.1002/jctb.2700
Zhang G, Zhao Q, Jiao Y, Wang K, Lee DJ, Ren N (2012) Biocathode microbial fuel cell for efficient electricity recovery from dairy manure. Biosens Bioelectron 31:537–543. https://doi.org/10.1016/j.bios.2011.11.036
Zhang L, Fu G, Zhang Z (2019) Simultaneous nutrient and carbon removal and electricity generation in self-buffered biocathode microbial fuel cell for high-salinity mustard tuber wastewater treatment. Bioresour Technol 272:105–113. https://doi.org/10.1016/j.biortech.2018.10.012
Zhang W, Xie B, Yang L, Liang D, Zhu Y, Liu H (2017) Brush-like polyaniline nanoarray modified anode for improvement of power output in microbial fuel cell. Bioresour Technol 233:291–295. https://doi.org/10.1016/j.biortech.2017.02.124
Zhang Y, He Q, Xia L, Li Y, Song S (2018) Algae cathode microbial fuel cells for cadmium removal with simultaneous electricity production using nickel foam/graphene electrode. Biochem Eng J 138:179–187. https://doi.org/10.1016/j.bej.2018.07.021
Zhang Y, Jiang J, Zhao Q, Gao YZ, Wang K, Ding J, Yu H, Yao Y (2017) Accelerating anodic biofilms formation and electron transfer in microbial fuel cells: Role of anionic biosurfactants and mechanism. Bioelectrochemistry 117:48–56. https://doi.org/10.1016/j.bioelechem.2017.06.002
Zhang Y, Mo G, Li X, Zhang W, Zhang J, Ye J, Huang X, Yu C (2011) A graphene modified anode to improve the performance of microbial fuel cells. J Power Sour 196:5402–5407. https://doi.org/10.1016/j.jpowsour.2011.02.067
Zhang Y, Sun J, Hou B, Hu Y (2011) Performance improvement of air-cathode single-chamber microbial fuel cell using a mesoporous carbon modified anode. J Power Sour 196:7458–7464. https://doi.org/10.1016/j.jpowsour.2011.05.004
Zhao F, Rahunen N, Varcoe JR, Chandra A, Avignone-rossa C, Thumser AE (2008) Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environ Sci Technol 42:4971–4976
Zhao Q, Li R, Ji M, Ren ZJ (2016) Organic content influences sediment microbial fuel cell performance and community structure. Bioresour Technol 220:549–556. https://doi.org/10.1016/j.biortech.2016.09.005
Zhao Q, Yu H, Zhang W, Kabutey FT, Jiang J, Zhang Y, Wang K, Ding J (2017) Microbial fuel cell with high content solid wastes as substrates: a review. Front Environ Sci Eng 11:13. https://doi.org/10.1007/s11783-017-0918-6
Zhu G, Onodera T, Tandukar M, Pavlostathis SG (2013) Simultaneous carbon removal, denitrification and power generation in a membrane-less microbial fuel cell. Bioresour Technol 146:1–6. https://doi.org/10.1016/j.biortech.2013.07.032
Zou Y, Xiang C, Yang L, Sun L, Xu F, Cao Z (2008) A mediatorless microbial fuel cell using polypyrrole coated carbon nanotubes composite as anode material. Int J Hydrog Energy 33:4856–4862. https://doi.org/10.1016/j.ijhydene.2008.06.061
Acknowledgements
The authors wish to acknowledge Department of Chemistry, Aligarh Muslim University, Aligarh for providing necessary research facilities. Authors are also thankful to Science and Engineering Research Board (SERB), University Grants Commission for departmental research support in the form of DRS II Grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Khan, N., Anwer, A.H., Khan, M.Z. (2022). Microbial Fuel Cells—A Sustainable Approach to Clean Energy and Wastewater Remediation. In: Ahmad, A., Mohamad Ibrahim, M.N., Yaqoob, A.A., Mohd Setapar, S.H. (eds) Microbial Fuel Cells for Environmental Remediation. Sustainable Materials and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-19-2681-5_18
Download citation
DOI: https://doi.org/10.1007/978-981-19-2681-5_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2680-8
Online ISBN: 978-981-19-2681-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)