Abstract
The performance of a single-chamber microbial fuel cell (MFC) using wastewater containing phenol as the anodic fuel was evaluated. The evaluation was performed considering the effects of the presence of different phenol concentrations in the anodic fuel and the external resistance at which the cells were adapted. Maximum power and current densities of 49.8 mW m−2 and 292.8 mA m−2 were obtained, respectively. Microbial diversity on the anode surface remained relatively stable when the phenol concentration was increased. Pseudomonas sp. was the most abundant microorganism in the MFC, followed by the genus Geobacter and Shewanella. Phenol degradation was mainly conducted by bacteria present in the wastewater, and its presence did not affect the electricity generation. The operation of the MFC with a resistance different to the adaptation resistance produced lower current and power densities; however, the variation in external resistances did not adversely affect the phenol degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A microbial fuel cell (MFC) is a device that can convert the chemical energy from organic matter to electricity in a single step [1–3]. It has been proposed that operating an MFC, in a single-chamber, without an ion exchange membrane may lead to a significant internal ohmic resistance reduction [4]. Also, in a single-chamber air-cathode MFC, the mass transport loss is reduced due to the direct supply of oxygen from ambient air to the electrode, minimizing the energy required to drive the hydrodynamic flow. This design also makes the MFC structure relatively simple and compact, eliminating the inherent problems associated with the MFC resistances [5]. In the simplest configuration of a single-chamber, air-cathode MFC, the anode and cathode are placed on either side of a tube, with the anode sealed against a flat plate and the cathode exposed to air on one side and water on the other [3]. Hydrostatic pressure on the cathode could make it leak water, but that can be minimized by applying a floating cathode on top of the cell and the anode at the bottom.
MFCs are influenced by the operational parameters of the system, such as configuration [3], hydraulic retention time, pH, and external load [6, 7], but not much information is available regarding how the external resistance, used during colonization and start-up, influences their performance. It is important to understand the parameters that affect the MFC operation, especially when this technology will be applied for industrial wastewaters degradation. In this sense, the use of MFCs for the removal of recalcitrant wastes present in industrial wastewaters, such as dyes, pesticides, explosives, and polyalcohols, has been previously reported [8]. Furan derivatives and phenolic compounds, which can be directly generated from hydrolysis of lignocellulosic biomass, were evaluated as substrates in a single-chamber MFC. It was found that the electricity generation was inhibited when these compounds were added at concentrations less than 0.2 mM [9]. It was observed that when phenol was used as a fuel in a two-chamber MFC, electricity was successfully produced using ferricyanide as the terminal electron acceptor [10]. The maximal power densities were 9.1 and 28.3 W m−3 for MFCs using phenol and a glucose–phenol mixture, respectively. The influence of different initial carbon sources (glucose, sodium acetate, and sodium propionate) on the acclimation of an MFC degrading phenol have been evaluated [11]. It was found that the MFC inoculated using glucose exhibited the highest power density.
The aim of this paper is to evaluate the performance of a single-chamber MFC with a floating cathode degrading phenol and, in particular, considering the effects of the presence of different phenol concentrations in the anodic fuel and the external resistance at which the cells were adapted.
Experimental
MFC Configuration
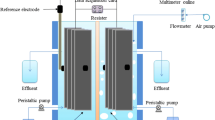
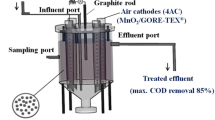
The configuration of the cells used in the experiments can be described as follows. The single-chamber, air-cathode, membrane-less MFCs were constructed using an acrylic tube (7 cm in length) that was closed at the bottom. The cylinder served as the MFC chamber. The cathode was supported on the top through a polyethylene floating ring, while the anode was placed at the bottom of the MFC. The volume of the MFC was 120 mL. The anode (5.8 cm in diameter) was made of carbon paper (30 % wet proofing; E-Tek, USA). The cathode (4.1 cm in diameter) was made of type S carbon paper (E-Tek, USA), which contained 0.5 mg cm−2 Pt on the water-facing side. The current collectors were made using platinum wires.
MFC Operation
The MFCs were inoculated with municipal wastewater (containing 258 ± 20 mg chemical oxygen demand (COD L−1) that had been settled for 30 min, to remove suspended solids, from the university campus (Queretaro, Mexico). The substrate was complemented with sodium acetate (100 mg L−1) and different phenol concentrations. The MFCs were operated in batch mode at room temperature (23 ± 2 °C). The contents of the MFCs were exchanged after 48 h, when the voltage decreased up to 80 % of the observed maximum value.
The study was divided in two parts. In the first experiment, the influence of phenol concentration on the performance of the MFC was evaluated. For that, an MFC (MFC-ph) was operated with increasing concentrations of phenol in the influent (25, 50, 100, and 200 mg L−1) to determine whether inhibition affects the MFC performance. The concentration was increased when, in three consecutive cycles, up to 80 % of the initial phenol was degraded. The external resistance was held constant at 1,000 Ω. Kinetics experiments were performed with 200-mg phenol/L to evaluate the contribution of phenol degradation to the voltage generation using two MFC adapted under identical conditions, but tested at opened circuit (OC) and closed circuit (CC, 1,000 Ω resistance). Results are the average of four degradation cycles.
In the second experiment, the performance of the MFC was evaluated considering the effect of the external resistance on the anode colonization. Two MFCs were adapted to a mixture of wastewater, acetate, and 200-mg phenol L−1. One MFC (MFC-220) was adapted with an external resistance of 220 Ω, while the other MFC (MFC-1000) was adapted with an external resistance of 1,000 Ω. Then, the external resistance was increased in the MFC-220 to 460 and 1,000 Ω, while in the MFC-1000 was decreased to 460 and 220 Ω. Each resistance was maintained for three cycles, and the average value was reported. Each cycle lasted 48 h.
Measurements and Calculations
COD and pH were measured according to standard methods [12]. The phenol concentration was measured taking samples and processing them offline using a modification of the colorimetric technique of 4-aminoantipyrine. A dilution of the filtered sample was made to obtain the adequate concentration of phenol, and the standard procedure was followed as described in previous studies [13]. The potential was measured using an NI-USB-6009 data acquisition system (National Instruments, USA) connected to a computer. The power density of the MFC at different external resistances was determined using the polarization curve measurements. The external resistance was changed from 10 to 10,000 Ω, and the corresponding voltage generated was measured using a multimeter. The data were taken after the readings stabilized. Based on the external resistance and the voltage, the current density generated was calculated according to Eq. (1) and the power density, (W/m2) was calculated according to Eq. (2). Coulombic efficiency was calculated according to [3] using Eq. (3):
Where R is the resistance, V is the voltage, and A is the area of the anode.
Where CE is the coulombic efficiency, C p is the total electrons gained, C t is the theoretical electricity production, M is the molecular weight of oxygen, I is the current, F is the Faraday’s constant, b = 4 is the number of electrons exchanged per mole of oxygen, v An is the volume of liquid in the cell compartment, and ΔCOD is the change in total soluble COD (including acetate, wastewater, and phenol) over time t.
Microbial Community Analysis
Four biomass samples were taken from the MFC-ph just before the initial concentration was increased (25, 50, 100, and 200 mg L−1). For each condition, two samples were collected, one from the surface of the anode and the other one from the liquid phase. The samples were analyzed by PCR-DGGE. Total DNA was extracted and purified from the samples using MoBio UltraClean Soil DNA Kit according to the manufacturer’s instruction. The DNA extraction was verified by means of an agarose gel at 1 %. The PCR was performed using 12.5 μL of TopTaq PCR Master Mix (Qiagen), 5 μL of template DNA, 10 mM of each primer, 4 μL of MgCl2 (50 mM), and 6.5 μL of RNases-free water. Two universal bacteria primer sets were used: 46F-GC (CGCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGGCCTAACACATGCAAGTC) and 534R (ATTACCGCGGCTGCTGG). The following conditions were used for the PCR amplification: 2 min at 94 °C for initial denaturalization and 30 cycles (1 min at 94 °C, 1 min at 54 °C for alignment and 1 min at 72 °C for elongation) and 7 min at 72 °C for the final extension step. PCR reactions were performed in a Gradient Palm-Cycler (Corbett research). The PCR amplification products were confirmed by electrophoresis with a 1 % (w v −1) agarose gel.
Denaturing gradient gel electrophoresis (DGGE) was performed using a DCode Universal Mutation Detection System (BioRad Laboratories) as described by Muyzer et al. [14], with denaturant gradients ranging from 40 to 60 %. Electrophoresis was conducted in a ×0.5 TAE (40 mM Tris, 40 mM acetic acid, and 10 mM EDTA 2Na•2H2O) buffer solution at 80 V and 60 °C for 17 h. The gel was stained with SYBR Green (Invitrogen) and visualized in a Gel Logic 200 Imaging System (Eastman Kodak Company). DGGE bands were manually excised from the gel and placed in 80 μl of RNase-free water (Sigma-Aldrich). DNA was eluted through a freezing-and-thawing procedure: samples were stored at −20 °C for 16 h, then at room temperature for 3 h, and finally thawing overnight at 4 °C. The eluted DNA fragments were re-amplified with primer sets 46F (GCCTAACACATGCAAGTC)/534R. The products were confirmed by electrophoresis on a 1 % (w v −1) agarose gel. Successfully re-amplified PCR products were sequenced. The identification of the obtained sequences was performed by the use of the BLAST software (NCBI). Relative microbial abundances were estimated on bacterial DGGE gel using band intensities by a gel electrophoresis image analysis software GelAnalyzer 2010a (GelAnalyzer.com). DGGE was analyzed, taking into account the relative microbial abundances, in order to obtain the Ochiai-Barkman’s similarity index, Simpson 1-D, Shannon-Wiener’s diversity index (H′), dominance (D), and evenness (E), using the PAST software [15].
Results and Discussion
Influence of Phenol on the MFC Performance
Phenol Removal
The MFC-ph was colonized using wastewater and sodium acetate as substrate. After 8 cycles, stable operation was observed. A maximum voltage of 349 ± 48 mV and a coulombic efficiency of 12 ± 4 % were observed. Then, phenol was added. Regardless of the initial phenol concentration, high phenol removal efficiencies were obtained in the MFC-ph (Fig. 1a). Figure 1a shows that the increase of phenol concentration did not affect the removal efficiency up to 100 mg L−1 of phenol. There was a slight increase in phenol removal, from 80 to 97 %, as the initial phenol concentration was increased from 25 to 100 mg L−1. When the MFC-ph was operated with 200-mg phenol L−1, some instability was observed regarding phenol removal attaining 61 ± 5 %. Under this condition, the voltage observed was 349 ± 40 mV and the coulombic efficiency was 13 ± 5 %. Voltages generated in our results are in the same range of those reported by other authors. Song et al. [11], using a single-chamber MFC, found a phenol removal from 77 to 88 % when an initial concentration of 600-mg phenol L−1 was applied; under this condition, the voltage generated varied from 193 to 242 mV and the coulombic efficiencies varied from 1 to 3 %. These lower performances were explained because, at higher concentrations, phenol can be oxidized by other anaerobic microorganisms that consume electrons.
The polarization curve (Fig. 1b) shows that the maximum power density was 54 mW m−2. This value is much higher than the power densities obtained with other MFC configurations. Luo et al. [10] obtained 6 mW m−2 using a two-chamber MFC with an aqueous air cathode, a 1,000 Ω resistance, and 400-mg phenol L−1 as the sole source of carbon. These differences can be attributed to the different cell configuration and the presence of readily biodegradable organic matter (acetate and wastewater) used in the present study.
Microbial Community Analysis
Bacterial DGGE patterns are presented in the supplementary material (Fig S1). Eleven bands were observed and six bands were successfully re-amplified and sequenced. The majority of the species found in the community are related to microorganisms previously reported in different MFCs [16–19]. Similarity of bacterial communities existing in the samples of the anode was very high presenting an Ochiai-Barkman’s index higher than 0.89 between the samples (Fig S1).
The relative microbial abundance index (Fig. 2) showed that Pseudomonas sp. (20–26 % in anode and 14–23 % in liquid) was the more abundant genus present in the MFC, followed by Geobacter and Shewanella (17–24 % and 17–19 %, respectively). Several species of genus Pseudomonas have been related to a high capacity to degrade efficiently phenol and its derivatives [17]. It has also been demonstrated that Pseudomonas spp. produce phenazine-based metabolites in the anodes of MFCs which improves the electricity generation [17]. Microorganisms of the genus Geobacter and Shewanella have been shown to play a key role in the MFC performance. These microorganisms transfer electrons from inside the cell to extracellular acceptors via c-type cytochromes, biofilms, and highly conductive pili (nanowires) [20]. It has been shown that the Geobacter sp. has the ability to oxidize acetate completely (94–100 % coulombic efficiency), whereas Shewanella oneidensis has only partial oxidation ability (56 %–69 %) [21].
Other bands were related to Gordonia polyisoprenivorans and to the genus Burkholderia, reported to be present in municipal wastewaters [18, 22]. G. polyisoprenivorans was found in all the samples, with a low relative abundance, and its presence can be explained because of the wastewater employed during the anode colonization. The band related to the genus Burkholderia only appear in the liquid samples of the MFCs, but at the lower phenol concentrations (25 and 50 mg L−1). Burkholderia has been described as an exoelectrogenic microorganism [18]. Bands 2 (related to Desulfovibrio vulgaris), 8, 9, and 10 (not sequenced) were present in some samples, but they only represent between 3.1 and 8.5 % of the total microbial abundance.
In general, a diverse community with high stability was observed in all the cases. The communities in the anode showed a H′ diversity index of 1.774 ± 0.071, while in the liquid was 1.954 ± 0.169 (Table 1). The microbial diversity in anode remained relatively stable; however, the diversity in liquid was reduced gradually from 2.118 to 1.749 due to the increase in the phenol concentration. High evenness in the communities was observed (E, 0.799–0.984), matching with a low dominance (D, 0.127–0.199), suggesting that there was not a single dominant species. The Simpson 1-D index was higher than 0.8 in all the cases, indicating that there is a high probability that two individuals, randomly selected from a sample, belong to different species.
Phenol Degradation Under Opened and Closed Circuit
Experiments performed at OC and CC were conducted in order to determine whether phenol removal is related to the MFC performance or due to the ability of bacteria to degrade the toxin. The experiments for this phase were conducted in two cells adapted and operated under the same conditions, except during the degradation test where one cell was maintained in OC and the other in CC. No significant differences were observed during phenol degradation kinetic at OC and CC (Fig. 3). Phenol degradation percentages were 62.3 ± 1.3 % and 66.3 ± 2.7 % for OC and CC, respectively. A slightly higher phenol degradation rate (11 %) was obtained with the CC than the OC condition (2.94 ± 0.08 mg phenol L−1 h−1 vs. 2.65 ± 0.12 mg phenol L−1 h−1). However, a significant higher organic matter removal (measured as COD) was found for the CC operation (61.9 ± 2.9 %) than with the OC experiment (48.3 ± 1.6 %). These results suggest that the phenol is degraded by bacteria, such as Pseudomonas, present in the wastewater rather than electrogenic bacteria forming the anode biofilm. The better removal of organic matter in the CC cell indicates that the acetate, as well as the organic matter present in the wastewater, is utilized by the electrogenic microorganisms to generate electricity. It is important to consider that although phenol presents a reduced participation in power generation, it is concomitantly and efficiently removed with the other organic matter in the MFC.
Influence of the External Resistance on the Anode Colonization
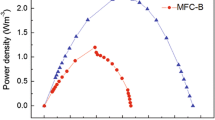
Table 2 shows the current, power, and opened circuit voltage reached by both MFCs. In general, there was a reduction of pH, from 6.8 at the initial to 6.5, at the end of the cycle, associated with the metabolic activity during the degradation of the organic matter. The opened circuit voltage for both MFCs was comprised between 412 and 478 mV (Table 2). Higher opened circuit voltages were obtained for the MFC-1000 (471.6 ± 9.3 mV) than for the MFC-220 (423.0 ± 9.8 mV), indicating only 11 % difference. These results agree with Zhang et al. [23], who reported a decrease in the opened circuit voltage when the external resistance decreased from that at which the MFC was adapted. However, it was found a significant difference in the cell’s performance depending on the resistance to which they were adapted (Table 2 and Fig. 4). Results indicated that the operation of an MFC under a given condition, but functioning with a resistance different to the adaptation resistance, generates lower current and power densities. For example, using an external resistance of 1,000 Ω (with the MFC-1000), the generated current and power densities were 292.8 mA m−2 and 49.8 mW m−2, respectively. However, 40.1 and 35.7 % less current and power densities were observed when the same 1,000 Ω resistance was used in the MFC-220 (Table 2). For the case of the intermediate resistance (460 Ω), much lower differences were obtained. It has already been discussed that the biofilm present in the anode can be sensitive to the external resistance change. Variations in the microbial community structure were found as a consequence of the variation of external resistance [24].
Effect of increasing and decreasing external resistance on the polarization curves. a The MFC was adapted using an external resistance of 220 Ω and then the resistance was increased to 460 and 1,000 Ω; b The MFC was adapted using an external resistance of 1,000 Ω and then the resistance was decreased to 460 and 220 Ω
The power densities obtained in this study varied from 34.5 to 49.8 mW m−2 and were in the same range reported in other studies concerning phenol degradation. Song et al. [11] reported power densities of 16.5 mW m−2, when phenol at 600 mg L−1 was used as the only substrate, and 37.3 mW m−2 when the cells were supplemented with glucose. Luo et al. [10] obtained 6 mW m−2 when an air cathode and 400-mg phenol L−1 were used. Power generation from phenol was relatively lower than values obtained with other substrates such as glucose in which 494 mW m−2 was obtained using an air-cathode MFC [4]. The recalcitrance of phenol degradation might have resulted in low power densities.
Phenol degradation and organic matter removal (as COD) were not significantly affected by the adaptation to the external resistance nor by the increase or decrease of the external resistance (Fig. 5). Maximum organic matter removal and phenol degradation were 47.4 ± 6.3 % and 49.2 ± 4.2 %, respectively. The CE as a function of the external resistance is presented in Table 2. The CE was not significant affected by the operational condition of the cell. Maximum values were obtained at the lower resistances, regardless of whether the resistance was increased or decreased. At the best condition, a CE of 21.2 and 39.3 % was observed for the MFC-220 and the MFC-1000, respectively. These results can be explained considering the nature of the organic matter fed to the cells. The MFCs were operated with a mixture of wastewater (258-mg COD L−1), acetate (106-mg COD L−1), and phenol (476-mg COD L−1). Under this condition, the easily biodegradable organic matter (wastewater and acetate) contributed with 43.3 %, the rest of the initial COD was phenol. As discussed, the phenol degradation contribution was mainly performed by the bacteria present in the wastewater rather used for electricity generation. Thus, despite that cells were fed with 840-mg COD L−1, only 43 % of this initial organic matter contributed effectively to the electricity production. The low CE observed is attributed to the low electrons gained by the system since, in the cells, not all the COD was available.
Conclusions
High phenol removal efficiencies were obtained in a single-chamber microbial fuel. The increase in phenol (up to 200 mg L−1) concentration did not affect the electricity generation in the MFC. Pseudomonas sp. was the most abundant microorganism in the MFC, followed by Geobacter and Shewanella. The microbial diversity in anode remained relatively stable when the phenol concentration was increased. Phenol presented a reduced participation in power generation; however, it was concomitantly and efficiently removed with the other organic matter in the MFC. The electricity production was strongly influenced by the external resistance at which the cell was adapted. The operation of the MFC with a resistance different to the adaptation resistance produced lower current and power densities; however, the variation in external resistances did not adversely affect the phenol degradation.
References
Li, Z. J., Zhang, X. W., Zeng, Y. X., & Lei, L. C. (2009). Bioresource Technology, 100, 2551–2555.
Ieropoulos, L., Greenman, J., Melhuish, C., & Hart, J. (2005). Journal of Power Sources, 145, 253–256.
Logan, B. E., Hamelers, B., Rozendal, R. A., Schrorder, U., Keller, J., Freguia, S., Aelterman, P., Verstraete, W., & Rabaey, K. (2006). Environmental Science and Technology, 40, 5181–5192.
Liu, H., & Logan, B. E. (2004). Environmental Science and Technology, 38, 4040–4046.
Rismani-Yazdi, H., Carver, S. M., Christy, A. D., & Tuovinen, I. H. (2008). Journal of Power Sources, 180, 683–694.
Jadhav, G. S., & Ghangrekar, M. M. (2009). Bioresource Technology, 100, 717–723.
Aelterman, P., Versichele, M., Marzorati, M., Boon, N., & Verstraete, W. (2008). Bioresource Technology, 99, 8895–8902.
Huang, L. P., Cheng, S. A., & Chen, G. H. (2011). Journal of Chemical Technology and Biotechnology, 86, 481–491.
Catal, T., Fan, Y. Z., Li, K. C., Bermek, H., & Liu, H. (2008). Journal of Power Sources, 180, 162–166.
Luo, H. P., Liu, G. L., Zhang, R. D., & Jin, S. (2009). Chemical Engineering Journal, 147, 259–264.
Song, T. S., Wu, X. Y., & Zhou, C. C. (2014). Bioprocess and Biosystems Engineering, 37, 133–138.
American Public Health Association, American Water Works Association, Water Environment Federation. (2005). Standard methods for the examination of water and wastewater, 21st ed. Washington D.C.
Buitrón, G., Schoeb, M. E., Moreno-Andrade, I., & Moreno, J. A. (2005). Water Research, 39, 1015–1024.
Muyzer, G., Teske, A., Wirsen, C. O., & Jannasch, H. W. (1995). Archives of Microbiology, 164, 165–172.
Hammer, Ø., Harper, D.A.T., & Ryan, P.D. (2001). Palaeontologia Electronica, 4, art.4. 1–9.
Du, Z., Li, H., & Gu, T. (2007). Biotechnology Advances, 25, 464–482.
Pham, T. H., Boon, N., De Maeyer, K., Höfte, M., Rabaey, K., & Verstraete, W. (2008). Applied Microbiology and Biotechnology, 80, 985–993.
Quan, X. C., Quan, Y. P., & Tao, K. (2012). Chemical Engineering Journal, 210, 150–156.
Lovley, D. R. (2008). Current Opinion in Biotechnology, 19, 564–571.
Franks, A. E., & Nevin, K. (2010). Energies, 3, 899–919.
Krastanov, E., Alexieva, Z., & Yemendzhiev, H. (2013). Engineering in Life Science, 13, 76–87.
Shen, F. T., Huang, H. R., Arun, A. B., Lu, H. L., Lin, T. C., Rekha, P. D., & Young, C. C. (2007). Canadian Journal of Microbiology, 53, 768–774.
Zhang, L., Zhu, X., Li, J., Liao, Q., & Ye, D. D. (2011). Journal of Power Sources, 196, 6029–6035.
Lyon, D. Y., Buret, F., Vogel, T. M., & Monier, J. M. (2010). Bioelectrochemistry, 78, 2–7.
Acknowledgments
This research was supported by DGAPA-UNAM through project PAPIIT (IN104710). The technical assistance of Carlos Cervantes Astorga and Jaime Perez Trevilla is acknowledged.
Conflict of Interest
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 583 kb)
Rights and permissions
About this article
Cite this article
Buitrón, G., Moreno-Andrade, I. Performance of a Single-Chamber Microbial Fuel Cell Degrading Phenol: Effect of Phenol Concentration and External Resistance. Appl Biochem Biotechnol 174, 2471–2481 (2014). https://doi.org/10.1007/s12010-014-1195-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1195-5