Abstract
Dye is one of the integral parts of human civilization. It has been used in day-to-day life from prehistoric periods. There are more than ten thousand different types of dyes present in the market which are used in industries related to food, textile, paint, cosmetics, paper and pharmaceuticals. Most of the recent dyes are synthetic in nature and have xenobiotic, toxic, mutagenic and cancer-causing properties. There are various classes of dyes based on their chemical structure or based on their mode of action, some common classes of dyes which are used in industries are azo dyes, vat dyes, acidic dyes, basic dyes, reactive dyes, disperse dyes and others Theses dyes after being used in the various process are discharged to various water resources by various industries without proper treatment or by partial treatment, which leads to water pollution and affects the aquatic ecosystem and human health. Several conventional physicochemical methods based on principles of coagulation, membrane filtration, oxidation, reverse osmosis and others have been used but these methods are associated with several drawbacks related to cost, complexity, end product and efficiency. These limitations can be overcome by using biological methods in which various microflora having suitable properties are used. Methods using microorganisms are commercially viable, have a low initial investment, simple and ecologically suitable. Degradation of dyes by microflora can be achieved by either biosorption or enzymatic action. There are several oxidizing and reducing enzymes produced by microflora are used in dye decolourization with effective result. This chapter focuses on various biological methods for dye decolourization, advantages of using the biological method over conventional methods and the future in the field of dye removing by microflora.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Dyes are aromatic compounds which are used in our day to day life and have a deep impact on human civilization, it has been used in a various way and has a serious impact in our daily life. More than a million tons of dyes are synthesized and consumed in various industries every year. Dyes can be utilized to produce various goods from industries associated with paper, textile, leather food, cosmetics, pharmaceutical and paints [1]. Harmful effects of these dyes on the environment and human health have been found in several reports and studies, which showed toxic, allergic, mutagenic and carcinogenic nature of dyes to animal, plants and humans beings [2]. Due to partial degradation of azo dye (most commonly used dye in industries), aromatic amines are generated which are harmful to both animals and plants [3]. The hazardous nature of these dye in the environment is enhanced due to their association with other pollutants released by industries in water resources without proper treatment. In case of some dyes, they can combine with metals like copper, nickel, iron, boron, cobalt and others to form a complex compound which helps to provide them stability and increases the fixation process. These metal-dye complex are not only harmful in nature but also have the ability to accumulate and increase the organic load. The exotoxic nature and bioaccumulating properties of dye in aquatic life ultimately reach to humans through the food chain and lead to several diseases like skin allergy, dermatitis, skin cancer, asthma, respiratory tract irritation, kidney damage, liver damage, genetic mutation, photodynamic damage and several different types of cancers [4].
There are several techniques and approaches to degrade or decolourize dyes. some of the old, traditional, conventional and well understood and some are new, complex and need a good establishment. Most traditional methods are physiochemical methods which use the principle of flocculation, precipitation, oxidation, reduction, electrolysis, membrane filtration, reverse osmosis and others. These methods are almost understood, but have various drawbacks related to cost-effectiveness, source utilization, the large amount of sludge production, toxic compound production and harmful effect on environmental. These drawbacks of physicochemical approaches forced to develop new methods of dye degradation with the help of microbes. It is because these microbes can survive in any condition, have highly adaptive nature, consume fewer resources and energy and do not harm ecology. These microbes which are the microflora are isolated, identified and studied exhibit highly successful dye removal. It has been also reported that this microorganism produces several enzymes which have the potentials for dye degradation. It is considered that microbes are an alternative and highly effective solution for biodegradation of dyes globally. When these microflorae are coupled with other methods like membrane technology, their ability to degrade dyes will enhance more. The exploration in the field of genetics, metabolomics and proteomic not only enhances the existing approaches but also widens the field for biodegradation of dyes by microflora [5].
This chapter aims at reviewing the current trends in dye removal by microflora. Firstly, the major dyes used in industries and their harmful effects are discussed. The involvement of microflora in dye removal has opened a new area in biodegradation process of dye, with ecofriendly and highly effective results.
2 Major Dyes Used in Industries
Dyes are organic colourants, which are used in our day-to-day life from prehistoric periods. It has been used for various purposes from food additive to textile industries. These can be natural or synthetic, the first evidence of using dye was dated 4000 years ago in Egyptian mummy which was indigo [6]. More than ten thousand of different dyes are being used in industries related to textile, paper, food, leather, paint, pharmaceuticals and cosmetics. Due to advancements in techniques and competition in market industries are mostly using synthetic dyes like azo dyes, direct dyes, reactive dyes to develop a product which is more attractive to customers. In the last decade, various environmental issues have been reported due to dye which has affected several textile dye industries.
Dyes isolated from natural sources require mordants during their application as they have low binding affinities. This reason for natural dye makes them not suitable for industrial applications [7]. Whereas synthetic dyes are derived from natural dyes and are modified version, which are most commonly used in industries. The first synthetic dye was produced in 1871 as picric acid from natural dye indigo. Classification of dyes can be done based upon the chemical structure, chromophore present and their actions. Based upon the chemical structure it can be classified as dyes with Azo group, dyes with Nitro group, dyes with Nitroso group, dyes with Indigo group, dyes with Phthalein group, dyes with Triphenylmethane group and dyes with Anthraquinone group as given in Table 1, and based upon the mode of action as Azoic dyes, Vat dyes, Direct dyes, Dispersed dyes, Sulphur dyes, Reactive dyes, Acidic dyes and Basic dyes.
2.1 Azoic Dyes
Dyes which contain azo linkage in their chemical structure are called as azo dyes. The number of azo linkages can be different in numbers so, the classification of azo dye is also done based on the number of azo bonds present in the molecule as monoazo dyes, diazo dyes, triazo dyes and polyazo dyes. These dyes are considered as the largest class among dyes which consist of seventy percent among all dyes used in industries related to food, textile, leather, paper, cosmetics, paint and pharmaceuticals [8]. Azo dyes can be obtained naturally and synthetically in diverse forms. According to a report published by [9], there are more than two thousand different types of azo dyes used in industries and more than a million-ton azo dyes are produced yearly. The simple coupling reaction of azo dyes, diverse structural variations, suitable adaptability property based on different application makes them one of the suitable dyes for industrial application. More than fifty thousand tons of these dyes are discarded from various industries in environment yearly. Azo dyes on partial degradation are transformed to aromatic compound like azoamine. These azoamines are harmful and toxic in nature. Some common examples of azo dyes are Allura red AC, Alcian yellow, acid orange, acid red, etc (Table 2).
2.2 Vat Dyes
These are the dyes named based on the method of application in textile industries. This dye is very effective in colouring fibres made from cellulose, mostly cotton. They show the property of unparalleled fastness in context to various agents like detergent, bleach and light, which is due to the insoluble nature of the vat dye in water. When these dyes are applied for other fibres which may be synthetic, shows no effective result. The binding affinity of vat dye with fibres is due to their selective diffusion behavior. This selective nature of this dye arises nonuniform shades when used in synthetic fibres.
The best example of vat dyes is indigo, which is a natural dye, isolated from the indigo plant Indigofera. There are various derivatives of indigo dye based on the modification by halogenation process which is further classified in various classes as indigoid, thiondigoid, anthraquinones and others. The preferable characteristics of vat dyes are light and wet fastness which is suitable for textile industries.
2.3 Sulphur Dyes
Sulphur dyes are usually utilized in paper and textile industries. These dyes were first reported in 1966 and were found very effective for cotton and cellulose fibres. It is reported that the annual production of sulphur dye is 120,000 tons which is the highest percentage of production among groups of dyes. Black sulphur is the most commonly used dye for cellulosic fibres [10]. Classification of sulphur dye is based on the method used for sulfurization and the initial material used [11]. The initial compound during the synthesis of sulphur dyes includes mostly aromatic compounds like benzene, azobenzenes with a functional group like hydroxy, nitro, nitroso and others. Whereas the method of sulfurization includes reactions like oxidation, reduction, substitution and ring formation. Sulphur dyes are commonly used for fibres made with cellulose, synthetic fibres blend with cotton, some paper industries and leather industries [12]. These dyes are cost-effective, excellent in light and wet fastness ensures the wide application and heavy use of these dyes.
2.4 Acidic Dyes
These dyes are predominantly used and have higher efficiency in acidic environmental condition at the pH range of 3–7, these are generally used along with formic or acetic acids. The acidic strength of environment applied depends upon the individual property of dye as these dyes are diverse in nature. Some of the acidic dyes have metal complexes and are mostly used to colour nylon, wool and silk [13]. The binding property of acidic dyes depends on the sulfonated group, which makes them polar in nature and water-soluble. The interaction between dyes and wool is due to ionic force and Vander wall force, these forces of interaction provide the fastness property to these dyes. Indian ink, Nigrosoin and Congo red are the most common examples of acid dyes.
2.5 Basic Dyes
Basic dyes are mostly used to impart colour to paper, nylon, acrylic polymers and some modified polyesters. As these dyes have poor mobility they are often used with retarders. These are polar dyes and can easily dissolve in water. It produces cations which interact with the fibres and polymers by electrostatic force. These dyes can be positively charged or can have delocalized charge as present in triarylmethane, xanthenes and acridine dyes. Methylene blue, Thionine and Crystal violet are the most common examples of this class.
2.6 Disperse Dyes
These dyes are synthetic in nature which targets hydrophobic compounds [11]. These are mostly used as a commercial mixture in large amount along with the large quantity of water, as they are insoluble or partially soluble in nature, most of the dyes remain in the water bath and generate a large volume of wastewater. Due to non-ionic nature of these dyes, they form an aqueous dispersion and are commonly used for the substrates like polyester, nylon, acrylic fibre, cellulose acetate [14].
2.7 Reactive Dyes
Dyes with the functional group and polar nature are known as Reactive dyes. They mostly contain functional group like dichloro-s-triazine which can make the covalent bond with their substrates when applied in textile industries [15]. These dyes show excellent light and wash fastness on cotton which makes them desirable for industrial uses [16]. These dyes are commonly used to stain wool, cotton and nylon. One of the major problems associated with these dyes is poor dye fixation.
3 Harmful Impact of Dyes in the Environment
Dyes are used in various manufacturing industries and the waste generated from it are discarded in water resources. These dyes are causative agents for environmental degradation and human diseases. It not only affects ecological niches but also harms water resources and animal health [4]. The dyes are recalcitrant in nature and they bioaccumulate, sediment and then gets mixed up in the public water supply chain [17]. In case of partial degradation of azo dyes, aromatic azoamines are generated. These amines are toxic and cancer-causing in nature [17]. Xenobiotic nature of some dyes impacts the ecosystem and hinders the structure and functionality of both aquatic flora, fauna and human health when it is exposed for a longer duration. In most cases, dyes are used with metal complex for their higher binding affinity. Metal associated which have a higher half-life and contains metals like copper, nickel, cobalt, chromium and lead [18]. when these metal associated dyes are relished in an aquatic habitat, it is bioaccumulated in fish tissues and ultimately reach to the human organ through food chain causing various pathophysiological condition [4]. It is found that chromium present in the dye causes oxidative stress to plants and harm the growth by affecting photosynthesis and carbon dioxide assimilation (Fig. 1; Table 3).
Synthetic dyes such as 2-Naphtylamine and benzidine, used in textile industries are responsible for higher incidence and prevalence rate of disease like bladder cancer [19]. The harmful effect of these textile dye range from dermatitis to problem related to the CNS [4]. Some dyes can affect at the cellular level where it can hinder the function of enzymes. In the case of textile workers, dermatitis, rhinitis, asthma, conjunctivitis and other allergic reactions are seen due to exposure with dye [11]. The genotoxicity of these dyes can lead to chromosomal aberration and have the potential for mutagenesis [11]. Dyes like Azure-B, commonly used in industries can intercalate DNA structure, interact with the cell membrane and other cellular organelles. This dye also affects the function of cellular enzymes like monoamine oxidase A and glutathione reductase which plays important role in human behaviour and cellular homeostasis respectively. Dispersed Red 1 dye shows the mutagenic property in hepatoma cells and in human lymphocytes during several studies. Orange 1 dye can induce DNA damage by supporting basepair substitution and a frameshift mutation.
Most azo and the nitro group-containing dyes have shown neoplastic as well as carcinogenic properties some of the examples are Sudan I cause neoplastic liver nodule, Red 9 causes sarcoma in the liver, mammary gland, bladder and hematopoietic system, crystal violet can cause mitotic poisoning which leads to hepatocarcinoma, uterus, ovary and bladder carcinoma [20]. Various dyes have significant effects on the digestive tract, renal system, respiratory system and multiple organs [19]. More than 4000 dyes have been studied for their toxicity out of which 100 dyes have the ability to develop cancer in human [21].
4 Conventional Methods for Dye Decolourization
Dyes released from various industries as an affluent have various harmful effects on both ecosystem and human health. In most of the cases, industries discharged effluent without proper treatment or partial treatment which contaminate several water resources. There are several conventional physiochemical processes used by several industries to treat their discharged some of them are adsorption, membrane filtration, ozonization, electrochemical oxidation, photo-electrocatalysis, coagulation, flocculation, advanced oxidation and photocatalysis [22]. These mentioned methods have been proved effective in dye removing process from discharge wastewater in industries but they have several drawbacks associated with these techniques which can be related to cost, complex infrastructure, inefficient results, toxic byproducts, amount of sludge produced and production of a secondary pollutant. Several industries use two or more physiochemical methods commonly to reduce these drawbacks and get effective results. In spite of combining various physicochemical methods, the results obtained at the end are not promising due to recalcitrant and resistive nature of dye to the degradation process [23]. In the present scenario, the need and based on futuristic approach wastewater treatment process are not only focusing towards the quality of ecosystem, human health but also they are concerned towards utilizing and reusing the treated wastewater in day to day life to fulfil the growing demand of water. These physiochemical processes of dye degradation resulted in the production of aromatic amino compounds from azo dyes. These azo amines have the potential to induce mutation can alter human DNA and can lead to various disease like cancer. Some approaches to conventional physicochemical methods used in dye decolourization are explained briefly (Fig. 2).
4.1 Adsorption Methods
It is the process which is based on the principle of adsorption where activated charcoal or carbon are used along with cobs, sawdust or other absorbing materials to remove dyes. In this process, the efficiency of the result depends on the quality of absorbents used and based on the quality of activated carbon. After the process of dye removal, the absorbent is a pollutant that needed to be disposed of properly [24].
4.2 Coagulation Methods
It is the process in which various chemical compounds such as coagulants are used which generally form precipitate or flocs. During this process, the flocs with the proper size, weight and strength are settled at the bottom which is removed. In this process, a large amount of sludge with various chemical compounds are generated which are pollutant and toxic in nature so further treatment is needed before discarding those sludge [23].
4.3 Membrane Filtration Methods
It is the process in which membrane with specialized pore size is used which works on the principle of filtration where dyes are filtered by membrane by reverse osmosis techniques. In this process nano or microfiltration technology are used for efficient separation of dye. As these methods include advanced technology, it has a high initial cost [25].
4.4 Oxidation Methods
It is the process in which dyes are oxidized to degrade or transformed into various state, with the help of various methods and compounds like ozone, hydrogen peroxide, Fenton’s reagent, photolysis with ultraviolet light and sonolysis. The major drawback of these methods is limited lifetime, ineffective for insoluble dyes and produce a large amount of sludge [23].
4.5 Electrochemical Methods
There are several electrochemical approaches for dyes degradation but most of the common approach is electrochemical oxidation of dyes, where electrochemical cell is used along with electrodes to oxidize the dye at the anode. As this process depends on the various parameters like pH, minerals content, temperature, the voltage applied, the concentration of dyes, anode material and operating condition, it is difficult and complex to standardize these processes for an effective result [22].
5 Involvement of Microflora in Dye Removing
5.1 Bacteria
Bacteria are tiny microorganisms with higher capabilities in fields of bioremediation. Various studies have been done to identify bacteria with the capabilities which can decolourise dyes present in the environment [26]. These types of bacteria are present and can be isolated from various niches like soil, water, animal excreta and contaminated food. These microorganisms having high efficiency in the process of decolourization and degradation of dyes are easy to culture and maintain [27]. Bacteria can decompose the dyes either by aerobic or anaerobic mechanism or both to give an effective result [28]. As these microbes are useful for a wide range of dyes, eco-friendly in nature, cost-effective and produce less sludge are considering as one of the effective methods for dye bioremediation.
5.1.1 Pure Culture of Bacteria
Bacteria used in decolourization process of dyes mostly used their enzyme which breaks several bonds in dye and helps in the degradation process. Harmful aromatic azoamine which are generated during the degradation of dyes in case of the physicochemical process is completely removed in aerobic or anaerobic condition by bacteria [29]. Most of the common type of dye used in industries are azo dyes and these are found resistive in nature during bacterial degradation process in aerobic condition as presence of oxygen prevents breakage of the azo bond [30] but there are several bacteria like Bacillus subtillis, Bacillus cereus, Aeromonas hydrophillia, Acinetobacter sp., S. hominis, S. aureus which can degrade azo dyes effectively [31,32,33]. In some cases, bacteria have developed oxygen insensitive enzymes like azorductase which can degrade azo dyes even in an oxygen-rich environment [34]. Extremophiles can grow and degrade dyes in extreme environmental’ conditions with high pH, temperature, salinity and presence of xenobiotic compound. Some bacteria like Staphylococcus Exigubacterium, Aeromonas hydrophila can degrade dyes in high salt concentration, Geobacillus stearothermophilus UCP 986 and Bacillus badius can degrade dyes in high temperature [28]. There are several bacterial species identified and studied which have dye degradation ability which is briefly given in the supplementary Table 4.
5.1.2 Consortium of Bacteria
Utilization of single bacteria species might not provide suitable result but the use of bacteria consortium gives effective result in biodegradation and mineralization process. In this process, multiple bacteria play a synergistic role in dye degradation [36]. The consortium is made of prepared either by a combination of two or more species of bacteria, fungi or both [35]. The harmful compounds generated as the intermediate product during the degradation process are transformed into nontoxic compounds as complementary bacteria used in this process. Some bacterial consortium like Enterobacter cloacae and Enterococcus casseliflavus combinedly degrade Orange II in 15 min [38], Bacillus odysseyi, Morganella morgani and Proteus sp., combinedly can degrade reactive blue in less than 3 h [39], Bacillus flexus, Bacillus cereus, Bacillus cytotoxicus can degrade Direct Blue 151 and Red within 5 days. In the same way, other microorganisms which can be used to make consortium are Stenotrophomonas acidaminiphila, Pseudomonas fluorescence, Bacillus cereus, Pseudomonas putida, Micrococcus luteus, Paenibacillus polymyxa, Providencia sp., Pseudomonas aeuroginosa, Arthrobacter, B. cerreus, Pseodomonas sp., B. megaterium, Rizobium, M. glutamicus [40,41,42]. These bacteria in the consortium can degrade and decolourize various dyes like Acid Red 88, Reactive Violet 5R and others.
5.2 Fungi
The process of bioremediation with the help of fungus is known as mycoremediation. There are several fungi which have the capability to degrade various dyes either by biosorption or by utilization of several enzymes. Broadly fungi used for degradation or decolourization of dyes are classified as filamentous fungus and yeast. Utilization of fungi in the field of bioremediation has been initiated and various study has been done to identify different species and strains of fungi which are important in this approach.
5.2.1 Filamentous Fungi
The process of biodegradation of dyes with the help of fungi has been studied and found that various soluble, insoluble, phenolic and nonphenolic dye can be decolourized effectively by them [43]. It has also been demonstrated that degradation of aromatic dyes by fungus is the secondary metabolic response when there is a lack of nutrient source, fungi used these compounds as an alternative resource of energy. These fungi can convert various organic compounds with the help of multiple cellular enzymes by the process of conversion reaction which includes hydroxylation of dyes. There are various filamentous fungi having the capability of degradation of various dyes, some of them are like P. chryososporium, Curvularia lunata can degrade Indigo dyes up to 95%, Hypocrea koingii can degrade five dyes including reactive violet, Red–black, Dark Navy and others. There are other fungi which produce several oxidizing and reducing enzymes which degrade dyes in a non-specific manner. Most common examples of enzyme-producing fungi are Trametes sp., Armillaria sp., P. chyryosporium, white-rot fungus and ligninolytic fungus of basidiomycetes class [44,45,46].
It is well understood and accepted that fungi are very efficient in the bioremediation process of various dyes, where they use various approaches like biosorption or enzymatic degradation of dyes [47]. The efficiency of the result is affected by various factors some can be abiotic like pH, temperature, carbon source, time, nutrient accessibility, salt concentration, oxygen concentration and nitrogen sources [48]. it has been also found that utilization of filamentous fungi for textile effluent containing synthetic dye in large amount cause problem sometimes.
5.2.2 Yeast
Yeast is also a type of fungi which have a higher impact on the process of biodegradation and fermentation. Yeasts have adopted various methods for decolourization of various dyes some of the approaches are adsorption based and some are associated with enzymatic degradation [49]. It has been found that utilization of yeast in the biodegradation process has advantageous effect over utilization of bacteria during the process as the yeast can grow rapidly as bacteria and can also grow in adverse environmental conditions [50]. Some dye-degrading fungi are Candida zeylanoides can degrade several azo dyes, Ascomycetes yeast species like Candida tropicalis (Violet 3), Debaryomyces polymorphous (Reactive Black 5) and Issatchenkia occidentalis. Candida oleophila can degrade reactive black [8]. Most suitable condition for yeast for degradation of dyes is an acidic environment, as it has been observed that various yeast like Candida albicans degrades Direct violet dye mostly at 2.5 pH, Candida tropicalis degrade violet 3 at 4 pH with best results.
5.3 Algae
Algae are one of the most influencing living micro-flora which have a higher impact in process of bioremediation which is highly used to treat various textile effluent containing various pollutants among which dyes are one of the major parts. It has been proved from various study that algae can offer a solution for the global environmental problem. In most of the cases algae used three different approaches for decolourization of dyes where they utilize chromophore to develop biomass, it has been seen that algal biomass and growth is not inhibited due to presence of pollutant-like dyes in an industrial effluent [52].
Algae decolourizes dyes by the process of adsorption or enzymatic reaction. Dye degrading algae mostly belongs to Blue-green algae, diatoms and green algae groups. Mostly Oscillatoria and Chlorella have higher potential for colour decolourization and production of CO2 and H2O [53]. In some case of algae they produce enzymes as azoreductase which breaks the azo bond present in azo dyes, some species of algae like Oscillatoria curviceps produces enzymes like azoreductase, polyphenol oxidase and laccase which can degrade acid Black dye, Chara and Scenedesmus obliquus from green algae can also enzymatically degrade Congo Red and Crystal violet, Chara vulgaris and S. quadricauda can degrade a large range of textile dye when they are immobilized on alginate [54]. In the case of bacterial and fungal bioremediation process of dyes, various supplements are needed to be supplied like carbon source, oxygen but in the case of algal bioremediation, no extra supplement is needed.
6 Methods of Dye Removal by Microflora
The biological process of dye degradation and decolourization is considered as one of the best approaches in removing dyes from textile effluent, industrial waste and contaminated water resources. This approach utilizes various micro-flora like bacteria, fungi, algae or combination of all for an effective outcome by degrading almost every type of dyes. In most of the cases of a biological approach, microorganisms remove dyes by two approaches which can be either by biosorption or by biodegradation. In both scenarios, it has been found that utilization of microbes for wastewater treatment are ecologically suitable and requires a minimum initial investment. The biological approach has been also found to have the potential to overcome various disadvantages and drawbacks possessed by the physicochemical approach.
6.1 Biosorption by Microflora
Biosorption is the natural process in which living organisms uptake certain compounds and accumulate as biomass. This approach for removal of toxic dyes from water resources is successfully done by microflora [55]. The phenomenon of biosorption is possible due to presence of various functional groups such as –COOH, –OH, –PO4, –NH2 and others in the lipids and heteropolysaccharides, in the cell wall of microflora which makes them polar in nature and helps in interaction with charged group of dyes [2]. There are various microbial species like Corynebacterium glutamicum can absorb Reactive Red 4 dye, Bacillus weihenstephannsis can remove congo red by this approach`. Similarly, fungi are also used as dye decolourizer by this approach. In addition to these algae are considered as one of the best biosorbents of dyes with highly effective result. The choice of algae for dye degradation is due to their availability in diverse habitat, cell wall with higher surface area and with higher interactive affinity with dyes, which can attract dyes electrostatically and forms different types of complex [56]. In case of dead algae, there is no nutrient demand and can be used, stored for longer duration and can be regenerated with the help of organic solvents when needed so, they are considered more effective tools in comparison to living algae [57]. In case of living algae large amount of nutrient sources are required to sustain the development and physiological process, to do so heavy investment are required to maintain them in bioreactors of wastewater treatment plants but once they are dehydrated or dried their physiological process is stopped at that environmental condition where they either form spores or adapt different mechanism to sustain that environment. In this condition, these algae can be regenerated when they are required in bioreactors by providing suitable environmental condition. These inactive forms of algae are easy to manage as they do not require the extra resources of nutrient and have higher storage life due to their adaptability to environment, so these forms are considered as highly effective tools in process of dye degradation where they can be used whenever they are needed.
The phenomenon of biosorption not only depends on the charged groups present in the cell wall of microbes but also on the pH of the media or water containing dyes, temperature of the environment, ionic strength of the solution, the time of contact in between dye and the microorganism, the material used as absorbent, type and structure of dyes present in contaminated water, the concentration of dyes, inhibitors present in the water and the type of microorganism used for degradation process [51]. This process of dye removal is selective, cost-effective, efficient and work in low concentration with very appreciable results. These all benefits of biosorption method for dyes degradation proved it to be a better approach over presently existing physiochemical methods. Only drawbacks associated with this method is an early saturation and no control over the process. In the process of biosorption, the microflora used for dye degradation have low volume capacity, i.e. they can only adsorb very less amount of dye due to higher surface by volume ratio leading to less saturation time of the bed, which also result in frequent replacement of bed causing increase cost and effort in dye removing technology [58]. Different microflora uses different methods for Biosorpion, as these all methods depend upon the type of microorganism used, their physiological condition and other abiotic factors. These all biotic and abiotic factors cannot be fully controlled in an in-vitro condition like bioreactors and thus can not be fully controlled [59].
6.2 Enzymatic Decolourization by Microflora
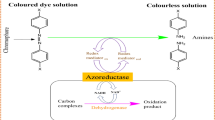
One of the most effective approaches in dyes decolourization and degradation is the utilization of various enzymes for wastewater treatment. There are already coexisting several physiochemical approaches which work on principles of coagulation, adsorption, chemical treatment and ionic extraction for removing dyes but due to their various drawbacks and problems liked with them related to cost, toxic compound as a byproduct. In the case of the physicochemical process, a large amount of sludge is generated which also pollutes the environment. Enzymatic methods became an alternative approach for removing several dyes. There are several enzymes produced by various microbes which break bonds of dyes and showed various chemical reaction for dye degradation [37]. The enzymes used for dyes decolourization are broadly divided as reducing and oxidizing based on their action (Fig. 5).
6.2.1 Reducing Enzyme
Enzymes are proteins made from different amino acids and have catalytic activity for various physiological processes. These enzymes which are effective in dye decolourization are mostly produced by microbes like bacteria, algae, fungi and yeast [28]. These reductase enzymes are highly effective in reduced conditions. one of the best examples of these enzymes is azoreductase which breaks azo bond present in azo dye and breaks the compound into aromatic amines which further degrade into nontoxic compounds. The function of these reductase enzymes depends on various reducing equivalents like Nicotinamide adenine dinucleotide phosphate (NADPH), Nicotinamide adenine dinucleotide hydride (NADH) and Flavin adenine dinucleotide (FADH). It has been also found that in various situation dyes are bound with several other functional groups leading in increase molecular weight which inhibits the transportation of those compound through cell membrane and in that case it has been found that those complex forms of dye are degraded by reductase enzyme which suggests that decolourization of dyes with enzymes do not depend upon the effectiveness of cellular intake of dyes [60]. There are various enzymes identified based upon reducing equivalence and are called as NADH-DCIP reductases, FMN-dependent reductase, NADH-dependent reductases, NADPH-dependent reductase and FMN-independent reductase. Whereas it has been also found that NADHDCIP reductase is a marker enzyme of bacteria and fungi having the potential to degrade various xenobiotic compound [61].
6.2.2 Oxidizing Enzymes
In the same way as reducing enzymes degrade dyes by the process of reduction, oxidizing enzymes decolourize the dye by an oxidation process. There are several oxidizing enzymes like cellobiose dehydrogenase, dye decolourizing peroxidase, laccase, tyrosinase, lignin peroxidase, N-demethylase, manganese peroxidase and polyphenol oxidase secreted by microbes which are effective in dyes degradation. These oxidizing enzymes are found in different species of bacteria, filamentous fungi, yeast and algae, where they breakdown various dyes during their metabolic process [32]. These microbes convert the dye into less toxic product and then remove from wastewater in form of radical and insoluble product [62]. one of the most common examples of oxidizing enzymes is peroxidase, which is iron-containing enzyme and generally found in the various microorganism. In the same way lignin peroxidase and horseradish peroxidase found in Penicillium chrysosporium can oxidize Methylene blue and Azure B dye. In recent studies, versatile peroxidase has been isolated and placed with lignin peroxidase and manganese peroxidase in ligninolytic peroxidase family [63]. Versatile peroxidase can oxidise Mn2+ to Mn3+, whereas laccases are copper oxidizing enzyme and can degrade copper-containing dyes, these are nonspecific in nature and can oxidize various dyes even in absence of electron acceptors like oxygen. Enzymes like polyphenol oxidase have a tetrameric structure with four copper atoms and two binding sites in each molecule of tetrameric structure, which helps in removing various dyes like from textile effluents. Multiple bacteria secreting these type of enzymes are G. geotrichum, B. laterosporus, consortium GG-BL which uses different types of the enzyme for the degradation of multiple dyes.
7 Advantages Due to the Microflora Over Conventional Physicochemical Methods for Dye Degradation
There are several conventional physicochemical methods used by several industries for decolourization and degradation of various dyes which are released as an effluent which is latter discharged in water resources after proper treatment. Traditional methods for treatment of waste dyes can be based on membrane filtration, adsorption, coagulation, flocculation, oxidation and electrochemical. As these methods have various drawbacks related to them, which is overcome by biological methods where various microorganism are used for dyes degradation. It has been seen that the utilization of these microflorae is highly cost-effective as the operating cost, infrastructure cost and maintenance of resources are less [64]. In the case of physicochemical methods used for removing dye are economically not viable, as they need well-established infrastructure, advanced equipment and have high maintenance cost [36]. In the case of physicochemical methods, the large volume of sludge is generated as an end product which is difficult to manage and dispose but in case of the biological method this challenge is not faced.
When these methods are compared based on their impact on the environment it is found that during the processing of dyes with physicochemical methods, various toxic intermediate products and final products are generated due to partial degradation of lower efficiency. They also produce a large volume of sludge which act as secondary pollutant contaminating various natural resources and aquatic life [65]. Whereas in case of microbial approach they are considered eco-friendly as living microorganism or enzyme released by microorganism are used in these methods for decolourization of dye without production of any toxic substance [64]. biological methods utilize very less amount of water and energy while decomposition and degradation of dyes in comparison of physicochemical methods. It has been also revealed from several studies that physicochemical methods are less efficient in dye removing in comparison to biological methods as it has been seen that these physicochemical cannot completely degrade dye, metal-dye complex and recalcitrant dyes and produce intermediate products which are further needed to be processed [65], Whereas complete degradation and mineralization of dyes are done in case of biological methods under certain environmental condition [66]. These studies suggest that microbial methods of dye degradation utilize fewer resources and energy with an effective result without harming the environment or without producing pollutant in comparison to traditional physicochemical methods.
8 Future of Dye Degradation Method by the Microflora
Microflora is considered as one of the effective solutions for bioremediation. These organisms are eco-friendly, highly adaptive and can be found in almost every habitat throughout the globe. The efficiency and capability of these organisms are highly appreciable, due to their positive impact in dyes degradation process it is needed to develop and standardize various methods and technologies for effective outcomes. As these biological methods involve various microorganism, which cannot be controlled during the process of degradation but their growth and metabolic activity can be enhanced by providing exact nutritional requirement and environmental condition by the process of biostimulation. In some cases, the hybrid technology can be also developed in which both microbial and physicochemical technology are hybridized for complete degradation of dyes or complex of dyes [37]. it has been studied that the combination of techniques can overcome each other’s limitation and can provide an effective result. These microbes can be also combined with membrane technology which will increase the surface area and exposure time (contact time). As the higher surface area and higher contact time, higher the rate of degradation.
In recent time the molecular approach towards the exploration of the microbial world by the techniques associated with metagenomics, transcriptomics, proteomics and metabolomics. These approaches to study and explore microorganism not only provides sufficient data to look and understand microorganism deeply at the molecular level but it will also help to develop various strategy to manipulate other microbes with the help of genetic engineering and metabolic engineering [67]. Multiple crucial genes, proteins and enzymes can be identified and used to build strategy or method through which complete degradation of an industrial effluent containing dye can be achieved [5]. Nanotechnology coupled with the microbial method can be another strategy for complete degradation of pollutants like dyes. These all approaches for the improvement of microbial associated biodegradation of dyes is to achieve cost-effective, highly efficient clean technology in the upcoming future.
References
Hussain RA, Badshah A, Raza B, Saba S (2016) Functional metal sulfides and selenides for the removal of hazardous dyes from Water. J Photochem Photobiol B 159:33–41
Das A, Mishra S, Verma VK (2015) Enhanced biodecolorization of textile dye remazol navy blue using an isolated bacterial strain Bacillus pumilus HKG212 under improved culture conditions. J Biochem Technol 6(3):962–969
Jadhav UU, Dawkar VV, Ghodake GS, Govindwar SP (2008) Biodegradation of Direct Red 5B, a textile dyes by newly isolated Comamonas sp. UVS. J Hazard Mater 158:507–516. https://doi.org/10.1016/j.jhazmat.2008.01.099
Khan S, Malik A (2018) Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. 25(5):4446–4458
Zhang L, Pan J, Liu L, Song K, Wang Q (2019) Combined physical and chemical activation of sludge-based adsorbent enhances Cr (VI) removal from wastewater. J Clean Prod 238:117904
Berton G, Gordon S (1983) Immunology 49:705
Agarwal P (2009) Application of natural dyes on textiles
Lucas MS, Dias AA, Sampaio A, Amaral C, Peres JA (2007) Water Res 41:1103–1109
Fatima M, Farooq R, Lindström RW, Saeed M (2017) A review on biocatalytic decomposition of azo dyes and electrons recovery. J Mol Liq 246:275–281. ISSN: 0167-7322. https://doi.org/10.1016/j.molliq.2017.09.063
Wang M, Yang J, Wang H (2001) Dyes Pigments 50:243–246
Benkhay S, M’rabet S, EI Harfi A (2020) A review on classifications, recent synthesis and applications of textile dyes. Inorg Chem Commun 115:107891. ISSN:1387-7003
Shore J (1995) Cellulosics dyeing, society of dyers and colourists
Nunn DM (1979) The dyeing of synthetic-polymer and acetate fibres. Dyers Co. Publications Trust
Clark M (2011) Handbook of textile and industrial dyeing: principles, processes and types of dyes.
Gaffer HE (2013) Carbohyd Polym 97:138–142
Gao Y, Cranston R (2008) Text Res J 78:60–72
Giri BS, Raza N, Roy K, Kim KH, Rai BN et al (2018) Recent advancements in bioremediation of dye: current status and challenges. Bioresour Technol 253:355–367
Christie RM (2001) Colour chemistry. Royal Society of Chemistry, United Kingdom
Christie RM (2007) Environmental aspects of textile dyeing. Elsevier
Pohanish RP (2017) Sittig’s handbook of toxic hazardous chemicals and carcinogens. Elsevier, Amsterdam; William Andrew, Cambridge
Lacasse K, Baumann W (2012) Textile chemicals: environmental data and facts. Springer, Dortmund
Gupta VK, Khamparia S, Tyagi I, Jaspal D, Malviya A (2015) Decolorization of mixture of dyes: a critical review. Glob J Environ Sci Manag 1(1):71–94
Ayanda OS, Nelana SM, Naidoo EB (2018) Ultrasonic degradation of aqueous phenolsulfonphthalein (PSP) in the presence of nano-Fe/H2O2. Ultrason Sonochem 47:29–35
Zhao B, Shang Y, Xiao W, Dou C, Han R (2014) Adsorption of Congo red from solution using cationic surfactant modified wheat straw in column model. J Environ Chem Eng 2:40–45
Ahmad AL, Harris WA, Ooi BS (2012) Removal of dye from wastewater of textile industry using membrane technology. J Teknologi 36:31–44
Celik L, Ozturk A, Abdullah MI (2012) Biodegradation of Reactive Red 195 azo dye by the bacterium Rhodopseudomonas palustris 51ATA. Afr J Microbiol Res 6:120–126. https://doi.org/10.5897/AJMR11.1059
Ning X, Yang C, Wang Y, Yang Z, Wang J, Li R (2014) Decolorization and biodegradation of the azo dye Congo Red by an isolated Acinetobacter baumannii YNWH 226. Biotechnol Bioprocess Eng 19:687–695. https://doi.org/10.1007/s12257-013-0729-y
Misal SA, Lingojwar DP, Shinde RM, Gawai KR (2011) Purification and characterization of azoreductase from alkaliphilic strain Bacillus badius. Process Biochem 46:1264–1269. https://doi.org/10.1016/j.procbio.2011.02.013
Joshi T, Iyengar L, Singh K, Garg S (2008) Isolation, identification and application of novel bacterial consortium TJ-1 for the decolorization of structurally different azo dyes. Bioresour Technol 99:7115–7121. https://doi.org/10.1016/j.biortech.2007.12.074
Ola IO, Akintokun AK, Akpan I, Omomowo IO, Areo VO (2010) Aerobic decolorization of two reactive azo dyes under varying carbon and nitrogen source by Bacillus cereus. Afr J Biotechnol 9:672–677. https://doi.org/10.5897/AJB09.1374
Zhiqiang C, Wenjie Z, Jiangtao M, Jinyan C (2015) Biodegradation of azo dye Disperse Orange S-RL by a newly isolated strain Acinetobacter sp. SRL8. Water Environ Res 87:516–523. https://doi.org/10.2175/106143014X13975035526068
Pan H, Feng J, Cerniglia CE, Chen H (2011) Effects of Orange II and Sudan III azo dyes and their metabolites on Staphylococcus aureus. J Ind Microbiol Biotechnol 38:1729–1738. https://doi.org/10.1007/s10295-011-0962-3
Singh RP, Singh PK, Singh RL (2014) Bacterial decolorization of textile azo dye Acid Orange by Staphylococcus hominis RMLRT03. Toxicol Int 21:160–166. https://doi.org/10.4103/0971-6580.139797
Lim SL, Chu WL, Phang SM (2010) Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour Technol 101(19):7314–7322. https://doi.org/10.1016/j.biortech.2010.04.092
Kolekar YM, Nemade HN, Markad VL (2012) Decolorization and biodegradation of azo dye Reactive Blue 59 by aerobic granules. Bioresour Technol 104:818–822. https://doi.org/10.1016/j.biortech.2011.11.046
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2010) Decolorization and biodegradation of reactive dyes and dye wastewater by a developed bacterial consortium. Biodegradation 21:999–1015. https://doi.org/10.1007/s10532-010-9360-1
Singh RL, Singh PK, Singh RP (2015) Enzymatic decolorization and degradation of azo dyes—a review. Int Biodeterior Biodegrad 104:21–31. https://doi.org/10.1016/j.ibiod.2015.04.027
Chan GF, Rashid NAA, Koay LL, Chang SY, Tan WL (2011) Identification and optimization of novel NAR-1 bacterial consortium for the biodegradation of Orange II. Insight Biotechnol 1:7–16. https://doi.org/10.5567/IBIOT-IK.2011.7.16
Patil PS, Shedbalkar UU, Kalyani DC, Jadhav JP (2008) Biodegradation of Reactive Blue 59 by isolated bacterial consortium PMB11. J Ind Microbiol Biotechnol 35:1181–1190. https://doi.org/10.1007/s10295-008-0398-6
Tony BD, Goyal D, Khanna S (2009) Decolorization of textile azo dyes by aerobic bacterial consortium. Int Biodeterior Biodegrad 63:462–469. https://doi.org/10.1016/j.ibiod.2009.01.003
Ruiz-Arias A, Juarez-Ramirez C, De los Cobos-Vasconcelos D, Ruiz-Ordaz N, Salmeron-Alcocer A, Ahuatzi-Chacon D, Galindez-Mayer J (2010) Aerobic biodegradation of a sulfonated phenylazonaphthol dye by a bacterial community immobilized in a multistage packed-bed BAC reactor. Appl Biochem Biotechnol 162:1689–1707. https://doi.org/10.1007/s12010-010-8950-z
Qu Y, Shi S, Ma F, Yan B (2010) Decolorization of Reactive Dark Blue K-R by the synergism of fungus and bacterium using response surface methodology. Bioresour Technol 101:8016–8023. https://doi.org/10.1016/j.biortech.2010.05.025
Porri A, Baroncelli R, Guglielminetti L, Sarrocco S (2011) Fusarium oxysporum degradation and detoxification of a new textile glyco-conjugate azo dye (GAD). Fungal Biol 115:30–37. https://doi.org/10.1016/j.funbio.2010.10.001
Hadibarata T, Yusoff ARM, Aris A, Salmiati HT, Kristanti RA (2012) Decolorization of azo, triphenylmethane and anthraquinone dyes by laccase of a newly isolated Armillaria sp. F022. Water Air Soil Pollut 223:1045–1054. https://doi.org/10.1007/s11270-011-0922-6
Sen K, Pakshirajan K, Santra SB (2012) Modelling the biomass growth and enzyme secretion by the white rot fungus Phanerochaete chrysosporium: a stochastic based approach. Appl Biochem Biotechnol 167:705–713. https://doi.org/10.1007/s12010-012-9720-x
Asgher M, Yasmeen Q, Iqbal HMN (2013) Enhanced decolorization of solar Brilliant Red 80 textile dye by an indigenous white rot fungus Schizophyllum commune IBL-06. Saudi J Biol Sci 20:347–352. https://doi.org/10.1016/j.sjbs.2013.03.004
Karthikeyan K, Nanthakumar K, Shanthi K, Lakshmanaperumalsamy P (2010) Response surface methodology for optimization of culture conditions for dye decolorization by a fungus Aspergillus niger HM11 isolated from dye affected soil. Iran J Microbiol 2:213–222
Grinhut T, Salame TM, Chen Y, Hadar Y (2011) Involvement of ligninolytic enzymes and Fenton-like reaction in humic acid degradation by Trametes sp. Appl Microbiol Biotechnol 91:1131–1140. https://doi.org/10.1007/s00253-011-3300-9
Yu Z, Wen X (2005) Screening and identification of yeasts for decoloring synthetic dyes in industrial wastewater. Int Biodeterior Biodegrad 56:109–114. https://doi.org/10.1016/j.ibiod.2005.05.006
Hai FI, Yamamoto K, Nakajima F, Fukushi K (2012) Application of a GAC-coated hollow fiber module to couple enzymatic degradation of dye on membrane to whole cell biodegradation within a membrane bioreactor. J Membr Sci 389:67–75. https://doi.org/10.1016/j.memsci.2011.10.016
Ambrosio ST, Vilar JC, da Silva CAA, Okada K, Nascimento AE, Longo RL (2012) A biosorption isotherm model for the removal of reactive azo dyes by inactivated mycelia of Cunninghamella elegans UCP542. Molecules 17:452–462. https://doi.org/10.3390/molecules17010452
Dubey SK, Dubey J, Mehra S, Tiwari P, Bishwas AJ (2011) Potential use of cyanobacterial species in bioremediation of industrial effluents. Afr J Biotechnol 10:1125–1132
Acuner E, Dilek FB (2004) Treatment of Tectilon Yellow 2G by Chlorella vulgaris. Process Biochem 39:623–631. https://doi.org/10.1016/S0032-9592(03)00138-9
Chu WL, Yike-Chu S, Siew-Moi P (2009) Use of immobilised Chlorella vulgaris for the removal of color from textile dyes. J Appl Phycol 21:641–648. https://doi.org/10.1007/s10811-008-9396-3
Bhatnagar A, Sillanpaa M (2010) Utilization of agroindustrial and municipal waste materials as potential adsorbents for water treatment: a review. Chem Eng J 157:277–296. https://doi.org/10.1016/j.cej.2010.01.00
Donmez G, Asku Z (2002) Removal of chromium(VI) from saline wastewater by Dunaliella species. Process Biochem 38:751–762. https://doi.org/10.1016/S0032-9592(02)00204-2
Fu Y, Viraraghavan T (2001) Fungal decolorization of dye wastewaters: a review. Bioresour Technol 79:251–262. https://doi.org/10.1016/S0960-8524(01)00028-1
Hammaini A, Ballester A, Blazquez MI, Gonzalez F, Munoz J (2002) Effect of the presence of lead on the biosorption of copper, cadmium and zinc by activated sludge. Hydrometallurgy 67:109–116. https://doi.org/10.1016/S0304-386X(02)00157-3
Aksu Z, Donmez G (2003) A comparative study on the biosorption characteristics of some yeast for Remazol Blue reactive dye. Chemosphere 50:1075–1083. https://doi.org/10.1016/S0045-6535(02)00623-9
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of color from textile wastewater using whole bacterial cells: a review. Dyes Pigments 58:179–196. https://doi.org/10.1016/S0143-7208(03)00064-0
Bhosale S, Saratale G, Govindwar S (2006) Mixed function oxidase in Cunninghamella blakesleeana (NCIM-687). J Basic Microb 46:444–448. https://doi.org/10.1002/jobm.200510117
Torres E, Bustos-Jaimes I, Le Borgne S (2003) Potential use of oxidative enzymes for the detoxificat -ion of organicpollutants. Appl Catal B Environ 46:1–15. https://doi.org/10.1016/S0926-3373(03)00228-5
Martinez AT (2002) Molecular biology and structure function of lignin-degrading heme peroxidases. Enzyme Microb Technol 30:425–444. https://doi.org/10.1016/S0141-0229(01)00521-X
Dong H, Guo T, Zhang W, Ying H, Wang P, Wang Y, Chen Y (2019) Biochemical characterization of a novel azoreductase from Streptomyces sp.: application in eco-friendly decolorization of azo dye wastewater. Int J Biol Macromol 140:1037–1046
Guo G, Li X, Tian F, Liu T, Yang F, Ding K, Wang C (2020) Azo dye decolorization by a halotolerant consortium under microaerophilic conditions. Chemosphere 244:125510
Rathod J, Dhebar S, Archana G (2017) Efficient approach to enhance whole cell azo dye decolorization by heterologous overexpression of Enterococcus sp. L2 azoreductase (azoA) and Mycobacterium vaccae formate dehydrogenase (FDH) in different bacterial systems. Int Biodeterior Biodegrad 124:91–100
An Q, Cheng J, Wang Y, Zhu M (2020) Performance and energy recovery of single and two stage biogas production from paper sludge: Clostridium thermocellumaugmentation and microbial community analysis. Renew Energy 148:214–222
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, B., Christina, E. (2022). Mechanism and Techniques of Dye Removal by Microflora. In: Muthu, S.S., Khadir, A. (eds) Dye Biodegradation, Mechanisms and Techniques. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-16-5932-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-5932-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5931-7
Online ISBN: 978-981-16-5932-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)