Abstract
Morphologically different, three bacterial strains, capable of decolorizing Reactive Blue 59 were isolated from dye effluent contaminated soil sample, collected from Ichalkaranji, India. The individual bacterial strains viz. Bacillus odysseyi SUK3, Morganella morganii SUK5 and Proteus sp. SUK7 decolorized Reactive Blue 59 (50 mg l−1) completely within 60, 30, 24 h, respectively, while the bacterial consortium PMB11 of these strains required 3 h for the complete decolorization. The decolorization was confirmed by UV–Vis spectroscopy. Further, the biodegradation of Reactive Blue 59 in to different metabolites was confirmed by High performance liquid chromatography and Fourier transform infrared spectroscopy analysis. Significant increase in the activity of aminopyrine N-demethylase (AND) in the individual as well consortium cells, obtained after decolorization showed involvement of AND in the decolorization process. Phytotoxicity studies, revealed the nontoxic nature of the degraded metabolites of Reactive Blue 59 indicating effectiveness of bacterial consortium PMB11 for the treatment of textile effluent containing Reactive Blue 59.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes and pigments are extensively used in the textile industries. The discharge of highly colored synthetic dye effluents from the industries can result in serious environmental pollution problems. Poor analytical methods and the complexity of manufacturing and retailing are also contributing to the environmental damage caused by these coloring compounds. In aquatic systems, the dyes undergo various reactions and the alterations to their chemical structures can result in the formation of new xenobiotic compounds, which may be more or less toxic than the parental compounds [43]. The treatment of textile wastewater is essential before discharging the wastewater into a receiving water body. Some of the azo, xanthene and anthroquinone dyes are known to be very toxic and mutagenic to the living organisms [26, 29].
The general approach of bioremediation is to improve the natural degradation capacity of the native organisms. Biodegradation is an environmental friendly and cost competitive alternative to chemical decomposition processes [14, 8]. During the past two decades, several physico-chemical decolorization techniques have been reported, few, however, have been accepted by the textile industries [9, 32]. Moreover, the physical and chemical methods have disadvantages of being highly expensive, coupled with the formation of sludge and the emission of toxic substances [19, 37]. The ability of microorganisms to carry out dye decolorization has recently received much attention. Microbial decolorization of dyes is a cost-effective method for removing them from the environment [27, 42]. Recent research has exposed the survival of a wide variety of microorganisms including white rot fungi, bacteria and mixed cultures capable of decolorizing a wide range of dyes [2, 3]. Microbial consortia are usually used without analyzing the constituent microbial populations for environmental remediation and complexity of the microbial consortium enables them to act on a variety of pollutants [44]. The present day bioremediation relies up on the pollutant degrading capacities of naturally occurring microbial consortia in which bacteria play central role [24, 31]. Several bacteria capable of dye decolorization, either individually or in consortia, have been reported [13, 17, 28, 45].
The present study deals with the isolation of textile dye decolorizing bacteria and development of the bacterial consortium PMB11 to degrade Reactive Blue 59. Reactive Blue 59 is one of the monochlorosulfonated azo dye and has a very large consumption rate in the textile dyeing processes. Reactive Blue 59 has both aromatic sulfonic and azo groups contributing in the xenobiotic nature. The dye is soluble in water and has a λ max of 590 nm. This article, reports studies on the Reactive Blue 59 decolorization by individual bacterial strains as well as consortium PMB11. We have also reported the enzymes involved in the decolorization process. Assessment of the toxicity of Reactive Blue 59 and its degradation metabolites was carried out by phytotoxicity studies.
Materials and methods
Isolation, screening and identification of microorganisms
Isolation of bacterial species was carried out from soil contaminated with textile processing and dye manufacturing unit in Ichalkaranji (India), by an enrichment culture technique. The morphologically distinct bacterial strains were selected for the dye decolorization study.
The identification of dye degrading bacterial strains were carried out on the basis of its morphological, biochemical and 16S rDNA analysis. All the biochemical tests were performed with specific requirements for each test.
Phylogenetic analysis and sequence alignment
The 16S rDNA sequence was initially analyzed at NCBI server (http://www.ncbi.nlm.nih.gov) using BLAST (blastn) tool and corresponding sequences were downloaded and evolutionary history was inferred using the Neighbor-joining method [35]. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) was shown next to the branches [10]. The phylogenetic tree was linearized assuming equal evolutionary rates in all lineages [39]. The clock calibration to convert distance to time was 0.01 (time/node height). The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method [40] and are in the units of the number of base substitutions per site. Phylogenetic analyses were conducted in MEGA4 [41].
Organisms and culture conditions
The pure cultures of isolates were maintained at 4 °C and each strain was sub cultured monthly on nutrient agar slants having composition (g l−1); peptone 10, NaCl 5, beef extract 3 and agar 25. These cultures were used for decolorization studies after inoculating in 250 ml Erlenmeyer flask, containing 100 ml nutrient broth having similar composition as above except agar. All the decolorization studies were carried out under static condition at 30 °C temperature unless otherwise stated.
Development of bacterial consortium
Bacterial isolates were mixed in different combinations and their ability to decolorize Reactive Blue 59 was studied. To develop a consortium, bacterial isolates (5% v/v) was inoculated in 100 ml nutrient broth and the flasks were incubated at 30 °C for 24 h, which was used in further studies because of its higher decolorization potential, as compared with the individual isolates. The bacterial combination, that showed dye decolorization in short time, was selected as the potent consortium for further decolorization studies and was named as PMB11.
Dyes and chemicals
Reactive Blue 59 and other reactive dyes used in decolorization studies were obtained from local textile industry Ichalkaranji, India. Bacterial identification kit purchased from Himedia, India. 2,2′-Azino-bis (3-ethyl benzothiazoline-6-sulfonic acid) (ABTS) was obtained from Sigma Aldrich, USA. Tartaric acid was obtained from BDH Chemicals India. Nicotinamide Adenine Dinucleotide reduced disodium salt (NADH), n-Propanol and catechol from SRL Chemicals, India.
Decolorization experiments
All the decolorization experiments were performed in the three sets. Decolorization of Reactive Blue 59 was studied under static condition at 30 °C in 250 ml Erlenmeyer flasks containing 100 ml nutrient medium. Decolorization experiments were performed by addition of Reactive Blue 59 (50 mg l−1) into 24 h grown individual cultures as well as bacterial mixed cultures. Aliquot (3 ml) withdrawn after decolorization was centrifuged (6,000 rpm, 10 min) and residual dye content (%) in the supernatant was measured at 590 nm. Decolorization was expressed in terms of percentage and was calculated as:
Preparation of cell-free extract
Cells were harvested (10,000 rpm at 4 °C for 20 min) and suspended in 50 mM potassium phosphate buffer (pH 7.4). Cell suspension (100 mg ml−1) was chilled properly, gently homogenized and sonicated, keeping the sonifier output at 40 amp, giving five strokes each of 30 s, at 2 min intervals (Sonics vibra Cell, Germany), at 4 °C. This cell homogenate was used for enzyme assays.
Enzyme assays
Biotransformation enzymes viz. lignin peroxidase (LiP), laccase, tyrosinase, NADH-dichlorophenolindophenol reductase (NADH-DCIP reductase) and aminopyrine N-demethylase (AND) were studied. Activities of LiP, laccase and tyrosinase were assayed spectrophotometrically in the cell-free extract at room temperature where reference blank contained all the components except enzyme (0.2 ml), in triplicates and average rates were calculated. One unit of enzyme activity was defined as a change in absorbance unit min−1mg protein−1.
Lignin peroxidase activity was assayed by the procedure of Shanmugam et al. [38]. It was determined by monitoring the formation of propanaldehyde at 300 nm. Laccase activity was assayed by the procedure of Hatvani and Mecs [12], by monitoring the formation of oxidized ABTS at 420 nm. Tyrosinase activity was determined by modified procedure of Zhang and Flurkey [46], by monitoring the formation of catechol quinone at 495 nm in a reaction mixture (2 ml) containing 0.01% catechol in 0.1 M phosphate buffer (pH 7.4).
NADH-dichlorophenolindophenol reductase activity was determined using a procedure reported earlier by Salokhe and Govindwar [36]. The reduction of DCIP was calculated using the extinction coefficient of 19 mM cm−1. AND activity was determined procedure reported by Jadhav and Govindwar [18].
UV–Vis spectral analysis, HPLC and FTIR
Metabolites produced by biodegradation of the Reactive Blue 59 were extracted with equal volumes of ethyl acetate. The extract was dried over anhydrous Na2SO4 and evaporated solvent in rotary evaporator. The crystals obtained were dissolved in small volume of high performance liquid chromatography (HPLC) grade methanol and the sample was used for Fourier transform infrared spectroscopy (FTIR) and HPLC analysis. UV–Vis spectral analysis was carried out using Hitachi UV–Vis spectrophotometer (UV 2800) and changes in its absorption spectrum (400–800 nm) were recorded. The supernatant samples of the individual bacterial strains and consortium obtained at 0 h and after decolorization were subjected to spectral analysis between 400 and 800 nm. HPLC analysis was performed in an isocratic Waters 2690 system equipped with dual absorbance detector, using C18 column (4.6 mm × 250 mm) and HPLC grade methanol as a mobile phase.
Toxicity study
The degradation metabolites of Reactive Blue 59 extracted in ethyl acetate were dried and dissolved in water to form the final concentration of 1,000 ppm for phytotoxicity studies. The phytotoxicity study was carried out at room temp. (33 ± 2°C) in relation to Phaseolus mungo and Triticum aestivum seeds (ten seeds) by watering separately 5 ml samples of Reactive Blue 59 and its degradation product (1,000 ppm) per day. Control set was carried out using plain water at the same time. Length of plumule (shoot), radical (root) and germination (%) was recorded after 7 days.
Statistical analysis
Data were analyzed by One-way analysis of variance with Tukey kramer multiple comparisons test. Values are mean of three experiments. Readings were considered significant when P was ≤0.05.
Result and discussion
Isolation and identification
Morphologically different bacterial strains, having remarkable Reactive Blue 59 degradation capacity, were isolated from the soil sample from Ichalkaranji, India. Bacterial cultures SUK5, SUK7 were found to be Gram-negative short rods and bacterial culture SUK3 consisted of Gram-positive long rod. The identification of the strains was done on the basis of morphological, biochemical characteristics (Table 1) and 16 S rDNA gene sequence. Bacterial strains SUK3, SUK5 and SUK7 were identified as, Bacillus odysseyi SUK3 (EU760698), Morganella morganii SUK5 (EU760699) and Proteus sp. SUK7 (EF541142), respectively. Then the various combinations were made using these isolates to improve the decolorization of the Reactive Blue 59. Out of these, consortium PMB11 was selected for further studies.
Phylogenetic position of isolates
To analyze the phylogenetic position, the 16 S rDNA sequence of the strain SUK3 (650 bp, EU760698), SUK5 (888 bp, EU760699) and SUK7 (755 bp, EF541142) were determined. Figure 1a, b, c showed the phylogenetic relationship between the isolated bacterial strains and other related bacteria found in the GenBank database. The homology assay result indicated that the strain SUK3, SUK5 and SUK7 were in the phylogenetic branch of the genus Bacillus, Morganella and Proteus, respectively.
Phylogenetic tree constructed by the neighbor-joining method, showing the phylogenetic relationship of Bacillus odysseyi SUK3, Morganella morganii SUK5 and Proteus sp. SUK7 and other species of the genus Bacillus, Morganella and Proteus, respectively. Number at nodes shows the level of bootstrap support based on data for 1,000 replications. Bar, 0.01 substitutions per nucleotide position and numbers in bracket represent GenBank accession numbers
Decolorization of Reactive Blue 59
Decolorization occurred only when a carbon and nitrogen sources were available in the growth medium, similar results were obtained by [7, 27]. When pure cultures of these isolates were tested individually for their decolorization ability in liquid medium, these cultures showed complete decolorization of Reactive Blue 59 (50 mg l−1). The individual strain showed the ability to decolorize Reactive Blue 59 (50 mg l−1) 89, 90 and 82% within 60, 30 and 24 h respectively, while the bacterial consortium PMB11 decolorized 92% within 3 h. The use of microbial consortia was found to be advantageous than the pure cultures in the decolorization of synthetic dyes. When all bacterial cultures were mixed and inoculated together in liquid medium, complete decolorization of Reactive Blue 59 was observed in short time as compared to individual bacterial strains. The similar results were previously reported with a consortium named NBNJ6, in decolorization of Direct Red-81 dye where as not any of these cultures individually showed complete decolorization of Direct Red-81, even on extended incubation [20]; that suggesting a synergistic role of the bacterial species in decolorization [23, 32]. The individual strains may attack the dye molecule at different positions or may use degradation products produced by another strain for further degradation [7, 11].
Screening of various textile dyes for decolorization
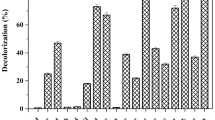
Dyes of different structures were often used in the textile processing industry, and therefore, the effluents from the industry are markedly variable in composition. A nonspecific biological process may be very important for treatment of textile effluents. As shown in Table 2, the bacterial isolates and consortium PMB11 decolorized all the ten different reactive textile dyes tested. There was rapid decolorization observed for all dyes used in the study by bacterial consortium PMB11 as compared to individual bacterial strains. The complete decolorization of dyes by bacterial consortium PMB11 occurred within 24 h. The slower decolorization with individual strains was due to structural differences [27, 34], higher molecular weight and presence of inhibitory groups likes –NO2 and –SO3Na in the dyes [15, 25]. The present study confirms the ability of consortium PMB11 to decolorize ten structurally different reactive textile dyes with decolorization efficiency of more than 80% in short time. Thus, bacterial consortium PMB11 could be successfully employed for the treatment of Reactive Blue 59 bearing industrial wastewater as it has prominent capacity to degrade other different dyes.
UV–Vis spectral analysis
UV–Vis spectral analysis has been used to confirm that decolorization process of Reactive Blue 59 was due to biodegradation, and is not merely the visual decolorization [1]. Spectrophotometrically analysis of the Reactive Blue 59, showed maximum absorbance at 590 nm and decrease in the absorbance of samples withdrawn after decolorization using individual bacterial strains and consortium PMB11 (Fig. 2). If dye removal is attributed to biodegradation, either the major visible light absorbance peak would completely disappear or a new peak will appear [5]. These results indicate that the color removal by an isolated bacterial strains and its consortium PMB11 may be largely attributed to the biodegradation.
High performance liquid chromatography analysis
High performance liquid chromatography analysis of Reactive Blue 59 and the metabolites extracted after its decolorization by consortium PMB11 showed peaks at different retention times. Reactive Blue 59 showed major peak at retention time 1.676 min (Fig. 3a), where as metabolites extracted after decolorization showed additional five peaks at retention times 1.038, 1.710, 1.340, 1.483 and 1.885 min (Fig. 3b). HPLC analysis is confirming the biodegradation of Reactive Blue 59 in to different metabolites.
Fourier transform infrared spectroscopy analysis
Results of FTIR analysis of control and samples obtained after decolorization showed various peaks. The FTIR spectrum of control Reactive Blue 59 displays peak at 3,479 cm−1 for the intramolecular hydrogen bonding aromatic -OH and O–H stretching. Peak at 2,924 cm−1 for C–H stretching, of alkyl acetals and peak at 2,359 cm−1 for N–H stretching of amines. A peak at 1,479 cm−1 for aromatic homocyclic compound and C=O in plain vibrations, a peak at 1,049 cm−1 for S=O stretching of sulfonic acid. Peak at 883 cm−1, 802 cm−1, 632 cm−1 are for 1,2,4-trisubstituted benzene, benzene ring with three adjacent H and C–Cl stretching, respectively. This conform the structure of Reactive Blue 59 as a monochloro sulfonated azo dye. The degradation metabolites of Reactive Blue 59 using consortium PMB11 showed peak at 3,223 cm−1 for secondary amides, peak at 2,956 cm−1 for C–H stretching of alkanes. Peak at 2,926 cm−1 for alkanes, 1,670 cm−1 for C=O stretching of tertiary amides, 1,649 cm−1 for C=C and C=N stretching and presence of amide bond, peak at 1,458 cm−1 for N=O stretching of nitrosamines. Peak at 1,313 cm−1 for S = O stretching and peak at 752 cm−1 for C–Cl stretching indicating presence of alkyl chloride. It indicates formation of nitrosamines, alkyl chloride, secondary and tertiary amides after decolorization (Fig. 4a).
The FTIR spectra of degradation metabolites by B. odysseyi SUK3 has displays peak at 2,927 cm−1 for C–H asymmetric stretching, peak at 1,682 cm−1 for C–N stretching of oximes. Peak at 1,443 cm−1 for N=O stretching of nitrosamine, 1,281 cm−1 peak for C–OH stretching of secondary alcohol indicating formation of oximes, nitrosamines and secondary alcohol. The FTIR spectra of the degradation metabolites using M. morganii SUK5 displays peak at 3,412 cm−1 for free N–H stretching of amides. Peak at 2,925 cm−1 showing asymmetrical C–H stretching, peak at 2,853 cm−1 for C–H asymmetrical stretching of aldehyde. Peak at 1,676 cm−1 for C=N stretching of acyclic α-β unsaturated amines. Peak at 1,453 cm−1 for N=O stretching of nitrosoamines. This indicates formation of amides, aldehydes, unsaturated amines and nitrosamines. The FTIR spectra of degradation metabolites using Proteus sp. SUK7 showed the peak at 3,407 cm−1 for N–H stretching and peak at 2,925 cm−1 for C–H stretch of asymmetric CH2. Peak at 1,662 cm−1 for C=N stretching and peak at 1,457 cm−1 for CH2 asymmetric bend. This indicates dye is degraded and alkyl benzene and secondary amines are produced (Fig. 4b).
Thus, the metabolites formed by consortium PMB11, as compared to individual bacterial strains, are found to be more ecofriendly as they are participating in the normal metabolism of bacteria.
Enzymatic analysis
Decolorization of different textile dyes by an isolated bacterial strains resulted in the specific influence on the status of biotransformation enzymes. Several bacterial enzymes that can be used in bioremediation include mainly oxidative enzymes such as mono and di-oxygenases. In addition, various reductases such as cytochrome c reductase, NADH-DCIP reductase, MG reductase [33] and N-demethylase to mineralize synthetic dyes [4]. Oxidation of sulfonated dyes by using LiP [6], similarly, fungal laccases were identified for their ability to decolorize synthetic dyes (anthraquinone, azo, indigo and triarylmethane) [30].
Studies on the biodegradation of textile dyes focused primarily on the decolorization of Reactive Blue 59 due to enzymatic actions. The data shown in Table 3 represents the enzyme activities present in the control cells and the cells obtained after the decolorization. LiP, laccase, tyrosinase, NADH-DCIP reductase and AND were found to be present in the control cells. After decolorization significant increase the activity of AND in the B. odysseyi SUK3, Proteus sp. SUK7 and bacterial consortium PMB11 as well as significantly induced laccase in all the bacterial cells except in consortium PMB11. LiP and tyrosinase has found to be induced in M. morganii SUK5, additionally, the consortium PMB11 showed induction in LiP. The activity of the NADH-DCIP reductase induced in B. odysseyi SUK3 and consortium PMB11 after decolorization. These indicate the involvement of AND and LiP enzymes in the decolorization process using the consortium PMB11, at this set of conditions. Complete decolorization of dye might be due to induction of AND that suggests the prominent role of AND in the decolorization process, supporting earlier observations [16, 21].
Toxicity study
Seed germination and plant growth bioassays are the most common techniques used to evaluate the phytotoxicity [22]. Thus, it was of prime interest to assess the phytotoxicity of dye and its metabolites after degradation by bacterial consortium PMB11. Germination (%) of the both Triticum aestivum and Phaseolus mungo seeds was less with Reactive Blue 59 treatment as compared to its degradation metabolites and plain water. The length of plumule and radicle were significantly affected (Table 4) by Reactive Blue 59 than its degradation metabolites, indicating less toxic nature of degradation metabolites as compared to dye. Hence, phytotoxicity studies revealed biodegradation of Reactive Blue 59 by bacterial consortium PMB11 resulted in the detoxification of dye.
References
Aksu Z (2003) Reactive dye bioaccumulation by Saccharomyces cerevisiae. Process Biochem 38:1437–1444. doi:10.1016/S0032-9592(03)00034-7
Asgher M, Shah SAH, Ali M, Legge RL (2006) Decolorization of some reactive textile dyes by white rot fungi isolated in Pakistan. World J Microbiol Biotechnol 22:89–93. doi:10.1007/s11274-005-5743-6
Banat IM, Nigam P, Singh D, Marchant R (1996) Microbial decolorization of textile dye containing effluents, a review. Bioresour Technol 58:217–227. doi:10.1016/S0960-8524(96)00113-7
Cha C, Doerge DR, Cerniglia CE (2001) Biotransformation of malachite green by the fungus Cunninghamella elegans. Appl Environ Microbiol 67:4358–4360. doi:10.1128/AEM.67.9.4358-4360.2001
Chen KC, Jane YW, Liou DJ, Hwang SCJ (2003) Decolorization of the textile dyes by newly isolated bacterial strains. J Biotechnol 101:57–68. doi:10.1016/S0168-1656(02)00303-6
Chivukula M, Spadaro JT, Renganathan V (1995) Lignin peroxidase catalyzed oxidation of sulfonated azo dyes generates novel sulfophenyl hydroperoxides. Biochemistry 34:7765–7772. doi:10.1021/bi00023a024
Coughlin MF, Kinkle BK, Tepper A, Bishop PL (1997) Characterization of aerobic azo dye degrading bacteria and their activity in biofilms. Water Sci Technol 36:215–220. doi:10.1016/S0273-1223(97)00327-2
Cuoto SR, Rosales E, Sanroman MA (2006) Decolorization of synthetic dyes by Trametes hirsuta in expanded-bed reactors. Chemosphere 62:1558–1563
Da Silva CG, Faria JL (2003) Photochemical and photocatalytic degradation of an azo dye in aqueous solution by UV irradiation. J Photochem Photobiol Chem 155:133–143. doi:10.1016/S1010-6030(02)00374-X
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evol Int J Org Evol 39:783–791. doi:10.2307/2408678
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971. doi:10.1016/j.envint.2004.02.001
Hatvani N, Mecs I (2001) Production of laccase and manganese peroxidase by Lentinus edodes on malt containing byproduct of the brewing process. Process Biochem 37:491–496. doi:10.1016/S0032-9592(01)00236-9
Haug W, Schmidt A, Nortemann B, Hempel DC, Stolz A, Knackmuss HJ (1991) Mineralization of the sulfonated azo dye Mordant Yellow 3 by a 6-aminonaphthalene-2-sulfonate-degrading bacterial consortium. Appl Environ Microbiol 57:3144–3149
He F, Hu W, Li Y (2004) Biodegradation mechanisms and kinetics of azo dye 4BS by a microbial consortium. Chemosphere 57:293–301. doi:10.1016/j.chemosphere.2004.06.036
Hu T, Wu SC (2001) Assessment of the effect of azo dye RP2B on the growth of nitrogen fixing cyanobacterium Anabena sp. Bioresour Technol 77:93–95. doi:10.1016/S0960-8524(00)00124-3
Itoh K, Kitade Y, Kobayashi S, Nakanishi M, Yatome C (1998) Demethylation of acridine orange by Arthrobacter globiformis. Bull Environ Contam Toxicol 60:781–785. doi:10.1007/s001289900694
Itoh K, Kitade Y, Nakanishi M, Yatome C (2002) Decolorization of Methyl red by a mixed culture of Bacillus sp. and Pseudomonas stutzeri. J Environ Sci Health 37:415–421. doi:10.1081/ESE-120002838
Jadhav JP, Govindwar SP (2006) Biotransformation of malachite green by Saccharomyces cerevisiae MTCC 463. Yeast 23:315–323. doi:10.1002/yea.1356
Johnson RF, Zenhausen A, Zollinger H (1978) In: Mark HF, Mcketta JJ, Othmer DF Jr, Standen A (eds) Krik-Othmer, 2nd edn. Encyclopedia of chemical technology, vol 2. Wiley, Hoboken, pp 868–910
Junnarkar N, Murty DS, Bhatt NS, Madamwar D (2006) Decolorization of diazo dye Direct Red 81 by a novel bacterial consortium. World J Microbiol Biotechnol 22:163–168. doi:10.1007/s11274-005-9014-3
Kalme SD, Parshetti GK, Jadhav SU, Govindwar SP (2007) Biodegradation of benzidine based dye Direct Blue-6 by Pseudomonas desmolyticum NCIM 2112. Bioresour Technol 98:1405–1410. doi:10.1016/j.biortech.2006.05.023
Kapanen A, Itavaara M (2001) Ecotoxicity tests for compost applications. Ecotoxicol Environ Saf 49:1–16. doi:10.1006/eesa.2000.1927
Knapp JS, Newby PS (1995) The microbiological decolorization of an industrial effluent containing a diazo linked chromophore. Water Res 29:1807–1809. doi:10.1016/0043-1354(94)00341-4
Liu S, Suffita JM (1993) Ecology and evolution of microbial populations for bioremediation. Trends Biotechnol 11:344–352. doi:10.1016/0167-7799(93)90157-5
Mohandass R, Bhaskar A, Kalavathy S, Devilaksmi S (2007) Biodecolorization and biodegradation of Reactive Blue by Aspergillus sp. Afr J Biotechnol 6:1441–1445
Moller P, Wallin H (2000) Genotoxic hazards of azo pigments and other colorants related to 1-phenylazo-2-hydroxynaphthalene. Mutat Res 462:13–30. doi:10.1016/S1383-5742(99)00090-3
Moosvi S, Keharia H, Madamwar D (2005) Decolorization of textile dye Reactive Violet by a newly isolated bacterial consortium RVM 11.1. World J Microbiol Biotechnol 21:667–672. doi:10.1007/s11274-004-3612-3
Moosvi S, Kher X, Madamwar D (2007) Isolation characterization and decolorization of textile dyes by a mixed bacterial consortium JW-2. Dyes Pigments 74:723–729. doi:10.1016/j.dyepig.2006.05.005
Nigam P, Banat IM, Singh D, Marchant R (1996) Microbial process for the decolorization of textile effluent containing azo, diazo and reactive dyes. Process Biochem 31:435–442. doi:10.1016/0032-9592(95)00085-2
Nyanhongo GS, Gomes J, Guebitz GM, Zvauya R, Read J, Steiner W (2002) Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res 36:1449–1456. doi:10.1016/S0043-1354(01)00365-7
O’Neill C, Lopez A, Esteves S, Hawkes FR, Hawkes DL, Wilcox S (2000) Azo-dye degradation in an anaerobic–aerobic treatment system operating on simulated textile effluent. Appl Microbiol Biotechnol 53:249–254. doi:10.1007/s002530050016
Okazaki S, Nagasawa S, Goto M, Furusaki S, Wariishi H, Tanaka H (2002) Decolorization of azo and anthraquinone dyes in hydrophobic organic media using microperoxidase-11 entrapped in reversed micelles. Biochem Eng J 12:237–241. doi:10.1016/S1369-703X(02)00074-8
Parshetti GK, Kalme SD, Saratale GD, Govindwar SP (2006) Biodegradation of Malachite green by Kocuria rosea MTCC 1532. Acta Chim Slov 53:492–498
Paszcezynski A, Pasti-Grigsby M, Goszceynski S, Crawford R, Crawford DL (1992) Mineralization of sulfonated azo dyes and sulfanilic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl Environ Microbiol 58:3598–3604
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Salokhe MD, Govindwar SP (1999) Effect of carbon source on the biotransformation enzymes in Serratia marcescens. World J Microbiol Biotechnol 15:259–263. doi:10.1023/A:1008875404889
Senan RC, Abraham TE (2004) Bioremediation of textile azo dyes by aerobic bacterial consortium. Biodegradation 15:275–280. doi:10.1023/B:BIOD.0000043000.18427.0a
Shanmugam V, Kumari M, Yadav KD (1999) n-Propanol as a substrate for assaying the lignin peroxidase activity of Phaenerochaete chrysosporium. Indian J Biochem Biophys 36:39–43
Takezaki N, Rzhetsky A, Nei M (2004) Phylogenetic test of the molecular clock and linearized trees. Mol Biol Evol 12:823–833
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035. doi:10.1073/pnas.0404206101
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
Verma P, Madamwar D (2003) Decolorization of synthetic dyes by a newly isolated strain of Serratia marcescens. World J Microbiol Biotechnol 19:615–618. doi:10.1023/A:1025115801331
Vijaya PP, Sandhya S (2003) Decolorization and complete degradation of methyl red by a mixed culture. Environmentalist 23:145–149. doi:10.1023/A:1024839805387
Watanabe K, Baker PW (2000) Environmentally relevant microorganisms. J Biosci Bioeng 89:1–11. doi:10.1016/S1389-1723(00)88043-3
Yatome C, Yamada S, Ogawa T, Matsui M (1993) Degradation of crystal violet by Nocardia coralline. Appl Microbiol Biotechnol 38:565–569
Zhang X, Flurkey W (1997) Phenoloxidases in Portabella mushrooms. J Food Sci 62:97–100. doi:10.1111/j.1365-2621.1997.tb04376.x
Acknowledgments
Ms P.S. Patil one of the authors is thankful to Department of Microbiology, Shivaji University, Kolhapur for awarding the Departmental Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, P.S., Shedbalkar, U.U., Kalyani, D.C. et al. Biodegradation of Reactive Blue 59 by isolated bacterial consortium PMB11. J Ind Microbiol Biotechnol 35, 1181–1190 (2008). https://doi.org/10.1007/s10295-008-0398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0398-6