Abstract
Phenolic foams are well-established polymeric materials in the industry, mostly because of their inherent properties such as high thermal stability, thermal insulation, high compressive strength, and lower density, which makes these materials extremely suitable for thermal insulations in houses and constructions. This book chapter discusses the materials and chemistry of phenolic foams and the designs of procedures to target specific properties and applications. The chemical versatility of the phenolic ring is the key factor that allows many chemical modifications to improve brittleness, friability, flame-retardancy, and even self-healing properties. The sources of phenols, which can be both petrochemicals as well as renewable resources, are another strong aspect that pushed the scientific community and industries into the research and applications of these materials. Hence, throughout this chapter, a deep analysis of the materials and chemistry of phenolic foams is presented to aid the understanding of their properties, providing a wide scope of structure–property relation to accurately design future experiments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The term polymer is derived from the Greek word “poly” meaning “many”, and “mer” means “units or parts”. A polymer is a large molecule made of repeating structural units called monomers. Generally, these repeating units contain carbon, hydrogen, and other elements such as O, N, S, F, Cl, P, Si, etc. Polymeric materials have become essential and playing a ubiquitous role in human life. They have a very important role in the modernization of our life and are found almost everywhere in daily use such as in clothes, shoes, bikes, cars, toys, electronic devices, bedding, couches, cars, constructions to name a few. There are two major types of polymeric materials namely thermoplastic and thermoset (Fig. 1). Thermoplastics are the polymers that become soft when exposed to heat and return to their original conditions after cooling. Monomers in thermoplastic are held together by relatively weak intermolecular forces. A broad class of thermoplastics like polyethylene, polypropylene, polystyrene, polycarbonate, polyvinyl chloride, and polylactic acid has been used in foam-making processes. The word “foam” originates from the medieval German word “veim” meaning “froth”. A thermosetting plastic remains solid after heating and cannot be reshaped by cooling and because of this property, they are hard to reuse or recycle using conventional methods. Thermoset polymers are mechanically stronger than thermoplastic and can be used for high-temperature applications due to their three-dimensional network and a high degree of cross-linking. Due to high strength, durability, and resistance to heat, they find their wide applications in automobiles, construction, varnishes, glues, etc.
The polymeric foams are additionally classified as rigid, semi-rigid, semi-flexible, or flexible depending upon their compositions, cellular morphology, physical, and thermal distinctiveness (Fig. 2). The above mention classification of polymeric materials is due to the rigidity of polymeric backbone, chemical composition, degree of cross-linking, degree of crystallinity, etc. Foamed polyvinyl chloride was perhaps the foremost thermoplastic material that was made available in the market during World War II. After the importance of polyvinyl chloride in the market, polyurethane and polystyrene foams were synthesized and made available in the market. In 1862 Alexander Parkes synthesized cellulose nitrate (celluloid). The primary cellular polymer to be positioned in the market was sponged rubber, which was developed in 1914. It was synthesized using natural rubber latex with the addition of gas-producing substances like sodium and ammonium carbonate or sodium polysulfide [1].
In 1872, German Scientist Adolph Von Baeyer first discovered the phenol–formaldehyde resin. Later in 1907, the father of phenolic resin L. H. Baekeland synthesized phenolic resins (Bakelite) [2]. After the development of phenolic resins, it was commercialized in the early 1940s by the German industry as a balsa material substitute [3]. After the commercialization of Bakelite and its uses, much later bisphenol-A (polycarbonate and epoxide resin) mainly became an important part of the development of phenolic resin materials. Over the past couple of decades, phenolic polymers have been attracted to manufacturers because of their uses in insulating products and flame-resistant materials. Before 1968, resols and novolacs were the major products obtained from the reaction between phenols and formaldehyde. D’Alessandro synthesized improved phenolic resin foam using polyhalogenated saturated fluorocarbons. The polymeric foams are available in different shapes such as boards, sheets, slabs, blocks, molded shapes, 3D shapes, etc. Due to their several advanced and tunable properties, polymeric materials have emerged as potential materials for commercial applications.
Due to social and economic impact, the global market of the polymer industries has increased tremendously. The leading producers and consumer countries of polymeric foam are North America, Europe, and Japan. In recent years, Latin American and other Asian countries (such as Argentina, Mexico, Brazil, India, South Korea, etc.) have also been started producing polymeric foams. The applications of polymers phenolic foams are now not only limited to household goods but also has been used in a variety of industries such as aviation, aerospace, medical devices, etc. [4]. Phenolic foams have unique properties such as low inflammability, high-quality thermal insulation, outstanding flame resistance, less smoke density, and non-toxicity [3]. Phenolic foams are versatile polymers used in a variety of applications such as structural strength, thermal-fire-moisture resistance, etc. Applications of polymeric foams are dependent upon their classification. Generally, flexible foams are used in packaging, footwear, cushioning, automotive interiors, carpet, textiles, and fibers, etc. while rigid foams are used in insulating windows, doors, appliances (e.g. refrigerator), sealants, plumbing pipes, etc.
The general composition and method for preparing phenolic foams involve mixing a liquid phenolic resole, a blowing agent, a catalyst, a surfactant, and optical additives as well as an acid curing agent into a considerably uniform composition [3, 5, 6]. The physical and mechanical properties of foams considerably vary from those of solid matter. Due to the different range of properties, foams have a larger scope of applications as compared with solid materials. In all commercial foams, phenolic foams are well known as rigid thermoset foams. Phenolic foams own high thermal stability with good thermal insulation, admirable fire resistance, and low toxic gases during combustion [5]. The properties of foams are also dependent upon the cell geometry (i.e. open/closed cells). Open cells are useful for sound insulation and closed cells are more appropriate for thermal insulation. But, phenolic foam production by several industries has faced many issues ranging from waste discard, recyclability, and the depletion of the ozone layer by the chlorofluorocarbons which are used as blowing agents [1]. Different attempts are also made to convert phenolic foams into useful monolithic nitrogen-containing activated carbon foams [7]. Lightweight carbon with red mud hybrid foam was synthesized using phenolic resin as a carbon source and red mud as filler from industrial wastes [4]. The pioneering approach in the synthesis has been attempted such as bark-derived phenol–formaldehyde resins [8]. There are emerging areas in the synthesis of lignin-derived bio-based polymers for flame retardants and mechanical strengths [9]. The use of renewable sources for the production of phenolic foams has great importance in the present situation. The efforts in the synthesis of phenolic foams using a renewable source such as cardanol (a byproduct of the cashew industry) [10], glycerides of coconut oil [6] were reported in the literature. Though the polymeric foams have an environmental impact, it is necessary to modify the process and overcome the issues with renewable sources. In the forthcoming years, novel approaches such as halogen-free, phosphorus, nitrogen-based, and bio-based polymeric foams may get placed on the conventional type of production of the phenolic foams.
2 Materials and Chemistry of Phenolic Foams
The phenolic foams were officially introduced in the market around 1940 as a replacement for wooden-based materials such as balsa [3]. Phenolic foams present several advantages such as high thermal and environmental stability, low thermal conductivity, and improved flame retardancy. In phenolic foams, carbonization takes place around 500 °C leading to a formation of a protective char layer that prevents the spread of fire. This phenomenon explains why phenolic foams present flame resistance properties [11]. Also, they present high thermal stability allowing them to remain functional under low temperatures as −200 or as high as 200 °C [12, 13]. Their low thermal conductivity also plays a role in their properties by slowing down the heat transfer, which ends up improving thermal stability [14]. Despite that, phenolic foams lost some space for wide applications compared with other polymers such as polystyrene, polyvinyl chloride, polyurethanes because of the lack of mechanical properties, usually presenting a lower compressive strength and friability in comparison to other polymers [3]. However, after the Montreal and Kyoto protocols, both industry and scientific community focused their efforts on finding eco-friendly materials [15]. Fortunately, phenolic foams have a low heat and smoke release when burned along with the low toxicity of the smoke, which is mostly composed of carbon dioxide. Because of that many new materials are being employed along with different synthetic approaches, which are discussed through this session.
Phenol is a white translucent solid with a melting temperature of 43 °C and a boiling point of 182 °C. Phenol chemistry is based on the resonance of the aromatic ring with the hydroxyl group at the ε (epsilon) position, which can be activated under an alkaline environment to donate electrons to the ring at o (ortho) and p (para) positions turning these regions in reactive nucleophilic centers. The resonance structure of phenol is described in Fig. 3 [16, 17]. These active sites can react with formaldehyde and yield mono, di, or tri-substituted compounds with higher functionality of hydroxyl groups (Fig. 4). The attachment of methylol functions increases the reactivity of the ring due to the electron donor effect. These activating groups increase the reactivity of the aromatic ring allowing it to react promptly as nucleophilic species [18]. The increase of functionality allows these types of structures to be used as starting materials to synthesize either branched or cross-linked polymeric foams. The hydroxy-methylphenol derivatives can react with phenols to form phenolic polymers (Fig. 4) [19].
The different types of functionalization that can be performed on a phenol molecule can provide phenolic foams with a range of properties [19]. Commercially, the components of the phenolic foams are sold as an aqueous solution with stabilizers such as methanol in different ratios of formaldehyde and phenol (F/P) [20]. The F/P ratio is an important parameter that determines the type of phenolic resin. For example, novolacs resins are formed when a higher concentration of phenol and low pH is used, while resol type of phenolic resins are prepared using a higher concentration of formaldehyde catalyzed under higher pH (Fig. 5) [21]. Resol types of phenolic materials are not as stable as novolacs types due to the formation of a cross-linked structure at a higher temperature. Cross-linking is promoted by the presence of methylol groups in the structure [16, 22]. The phenol compound itself presents vast chemistry that can be employed in many ways as discussed. However, many other phenolic compounds are also used in both industry and research. The chemical structure of some common phenols is given in Fig. 6.
Synthesis of resol and novolac phenolic resins and respective conditions. “Adapted with permission [21]. Copyright (2015) American Chemical Society.”
In general, phenolic foams are prepared by reacting phenols with isocyanates. The reactivity of phenols toward isocyanates mostly depends on the solvents and substituents groups on the phenolic compound. A study concluded that the use of aprotic and polar organic solvents such as cyclohexanone demonstrated the highest reaction rate followed by 1,4-dioxane, while the lowest reaction rate was observed for xylene. For the substituent groups, it was observed that electron-withdrawing groups in the phenolic compounds prompted the reaction between the hydroxyl and isocyanate. It was believed that due to the withdrawing effect of electrons on the O–H bond, the oxygen could pull the electron from the hydrogen turning it into a partial positively charged. In that condition, the electron-rich nitrogen from the isocyanate group (-NCO) is prompt to attack the hydrogen and yield the urethane linkage [23]. Besides the understanding of the proper chemical structure that yields the highest reaction rate, there are some other external factors such as reaction temperature [24], time [25], concentration and type of catalyst [25, 26], pH [26], and formaldehyde/phenol ratio [27,28,29], which can affect the output of the reaction. Other factors such as low reactivity and steric hindrance of phenolic compounds could also affect the properties of phenolic foams. For example, lignin, a low-cost bio-derived material with many phenolic groups, can be used to obtain phenolic foams, however, its low reactivity due to steric hindrance and poor dispersibility in most solvents prohibit its industrial applications. It has been demonstrated that low molecular weight lignin presents more reactive sites to allow the methylol reaction, which is the main requirement to obtain phenolic foams [30, 31]. A previous report proposed the depolymerization process to obtain a lower molecular weight lignin macromolecule for phenolic foams [32]. The procedure consisted of depolymerization of the lignin catalyzed by sulfuric and solid acid (HZSM-5), followed by phenolation and hydroxymethylation. After the lower molecular weight, lignin functionalized with phenolic and methylol was obtained, it was cross-linked by using a resol resin. The schematics are described in Fig. 7 [32].
Schematics for the depolymerization of lignin through acid catalysis followed by phenolation and hydroxymethylation. Concluded with cross-link reaction with Resol resin to obtain the phenolic foam. “Adapted with permission [32]. Copyright (2018) Elsevier.”
One of the main sources for the extraction of phenol is coal tar, which started at the end of the nineteenth century and is still considered as a primary source for obtaining phenol with a combination of other approaches [33,34,35]. Coal tar is a mixture of condensed organic compounds as leftovers from coal carbonization. Phenol can be separated with a sodium hydroxide treatment to precipitate it as sodium phenolate. After extraction, it can return to the phenol form through bubbling of carbon dioxide [22]. Phenols are also produced through liquid effluents from the gasification processes of gas-making plants [36, 37]. A report even demonstrated a 93% efficiency of phenol removal from wastewater by using methyl isobutyl ketone [38]. In earlier days, phenol got huge attention due to its first important applications in the synthesis of Aspirin (acetylsalicylic acid) back in 1898 and Bakelite in 1910 [2]. Following that trend, Bisphenol A was also scaling up in the productions, which demanded new approaches to synthesize these phenolic compounds for various applications [22]. The predominant synthetic route to obtain phenol nowadays is named Hock process or cumene process, which yields phenol and acetone as a byproduct [39]. This process is used to produce most of the phenol worldwide (~97%). The starting material for this process is synthesized by reacting benzene and propylene, which is then oxidized in air yielding cumene hydroperoxide (CHP). A strong inorganic acid is then used as a catalyst to decompose cumene hydroperoxide into phenol and acetone. The mechanism of this process is shown in Fig. 8 [39].
Synthetic route for phenol and acetone through cumene named Hock process or acetone process [39]
Several different approaches were developed along the time. During the 1960s, Dow and California Research Corp simultaneously developed a commercial process to synthesize phenol from toluene [22]. In another approach, benzene was used as a starting material for the synthesis of phenol [22, 40]. In this process, benzene was oxidized and catalyzed by N2O and zeolite (ZSM-5), respectively, at high temperatures (300–450 °C) to produce a large quantity of phenol [22, 40]. This process was developed by the Boreskov Institute of Catalysis and later commercialized by Solutia Industries [22, 40]. Another method uses chlorobenzene as the starting material that goes through hydrolysis to synthesis phenol. In another approach, ammonia was used to convert benzene into phenol, however, due to relatively low conversion efficiency, these methods have not been applied to the industry [40].
Although the production of phenol using petrochemical sources is a well-established process, the fluctuating price of petrochemicals and thus phenolic compounds and growing concerns for the environment encourage scientists and researchers to find alternative sources for commercial production of phenol. There are some materials from bio-sources such as lignin, oil from cashew nutshell, etc., which can be used as alternative sources for phenol compounds. Lignin, a natural phenolic compound widely found in most plants in large amounts, is mostly used for the production of phenols. The main feedstocks for lignin are woods [41, 42], forest [43], agricultural [44, 45], and pulp industry residues [46,47,48,49]. The most relevant points that reinforce the use of lignin for industrial applications are the abundance and competitive price. However, the quality of some bio-renewable phenolic foams and resins still has some disparity in properties compared with the foams derived using petrochemicals along with the difficult separation process to obtain pure phenol [41]. Hence, they can be used to complement the formulation of final products to reduce cost and keep satisfactory properties [50]. As mentioned, lignin is a polymeric structure mostly considered as a macromolecule, thus to obtain phenol and oligomeric derivatives from lignin, a depolymerization process is required. The fast pyrolysis process to obtain phenol-based compounds from lignin is the mostly used method that takes place through pyrolysis of lignin between 400 and 600 °C at a fast heating rate along with quick condensation of the liquids that are formed. This process yields around 75 wt.% of phenolic compounds that can be used for the synthesis of phenolic foams and resins [51, 52]. The extraction of phenol through lignin also generates other substances such as syringol, guaiacol, sinapyl, and coniferyl alcohol as shown in Fig. 9 [50]. Their chemistry is highly related to their substituent groups in a way that oxygen atoms adjacent to the hydroxyl groups may increase the polarization by increasing the reactivity [53].
Phenolic derivatives from lignin sources. “Adapted with permission [50]. Copyright (2008) Elsevier.”
Since the use of phenol as a starting monomer for phenolic-formaldehyde resins and plastics, it has made a huge impact in the industries. In addition to its applications in phenolic-formaldehyde resins and plastics industries, it got quick attention to polyurethane industries. Phenol-based polyols for polyurethane industries provide many possible variations yielding end products with superior mechanical and flame retardant properties. The popularity of phenol-based compounds as polyols for polyurethanes is due to the high reactivity of phenolic -OH groups toward isocyanate. Other materials such as catalysts, surfactants, and blowing agents are also very important for the foaming industries [54]. The phase separation between the less dense and polar polyols and the denser and non-polar isocyanate is believed to slow down the reaction rate, therefore, it becomes necessary to implement catalysts and surfactants [54, 55]. The most used catalysts are usually tertiary amines such as 1,4-diazabicyclo[2.2.2] octane (DABCO) and triethylamine (TEA) [56]. Another type of catalyst often used is organometallic compounds such as dibutyltindilaurate (DBTDL) and stannous octoate. Organic acids like p-toluene sulfonic acid and phenol sulfonic acid are used along with inorganic acids in the phenolic foams. The chemical structure of some common catalysts is shown in Fig. 10. The role of these catalysts is to polarize the bonds from both isocyanate and phenol/polyols creating an intermediate that rearranges to form the urethane linkage [57,58,59]. The catalyst enhances their reactivity allowing the reaction to occur faster and at room temperature. Their mechanism is described in Fig. 11 [57, 60,61,62].
Mechanism of bond polarization through the use of a and b organo-metallic and c and d tertiary amine catalysts. “Adapted with permission [60]. Copyright (2007) Elsevier”
The polarization effect of tin-based catalysts is more pronounced in aromatic isocyanates such as diphenylmethanediisocyanate (MDI) or toluene diisocyanate (TDI) compared with aliphatic ones such as hexamethylenediisocyanate (HDI) or isophoronediisocyanate (IPDI). However, for the zirconium-based catalyst, the observed reactivity was the same regardless of the type of isocyanate used [55]. A novel type of catalyst that has been employed in the formulation of polyurethanes is the CuCo2O4/graphitic carbon nitride, which diminishes the production of CO and imparts flame-retardant properties, which can add a synergy effect for phenolic foams that inherently present this property [63].
Other important components for foam formulation are the surfactants that are responsible for improving the superficial area between the reactants allowing them to mix properly, working in pairs with the catalyst. Along with that they also grant stable formation of the foam by controlling the pore size to obtain a regular cellular morphology. This factor highly influences the mechanical properties of the foams and their interactions with fillers [55]. Surfactants are mostly silicon-based compounds such as silicone oils, nonylphenolethoxylates, polydimethylsiloxane-polyoxyalkylene along with some organic compounds. The non-ionic surfactants are known to improve the superficial area and are easier to include in the formulation due to their fixed value of critical micelle concentration [64]. In general, a low concentration (0.5–5 wt%) of surfactants is required in phenolic foams. An appropriate amount of surfactants is required to maintain the cellular structure of the foams. Low concentration may destabilize the structure while a higher concentration of the surfactants may increase the chance of collapsing the cellular structure along with an increase in the cost of production. A study developed an approach that does not require the use of surfactant because in some cases, they present low molecular weight, an increase in delamination, and corrosion can be observed [65, 66].
Blowing agents like surfactants also play an important role in controlling the morphology and cell structure of the foam. They can react with the isocyanate forming a urea function and liberating carbon dioxide that gets entrapped within the structure of the cell while increasing the overall volume of the foam. Carbon dioxide acts as in-situ blowing agents, especially for resol-based phenolic foams. Before the international regulations, many types of chlorofluorocarbons (CFCs) were used as blowing agents; however, the depletion of the ozone layer due to their exaggerated use forced the international regulation to restrict their distribution. After that, H2O appeared as a suitable and environmentally friendly chemical blowing agent for the foaming process. Hydrocarbon-based blowing agents such as cyclopentane, n-pentane, iso-pentane, iso-butane, hexane, etc. can also be employed in phenolic foams due to their low boiling points [67]. Figure 12 shows the structure of some common blowing agents used in phenolic foams. The structure of a phenolic foam largely depends on the type of blowing agent used during the foaming process. For example, highly volatile blowing agents will produce open-celled foams. Curing agents are also used during the curing process of the foam to provide cross-linking in the structure. Organic acids such as toluene sulfonic acid, benzene sulfonic acid, xylene sulfonic acid, phenol sulfonic acid, and inorganic acids such as hydrochloric acid, sulfuric acid, phosphoric acid are commonly used as a curing agent in phenolic foams.
Other components such as plasticizers can be also added into a polyurethane formulation that reduces the hardness of the materials. The decrease of brittleness in foam can be acquired by implementing a bulky and flexible group in the chain in one of the reagents, usually the polyol or through the physical blend. The large and flexible group may allow the polymeric chains to decrease the brittleness of the polymer [68, 69], along with pigments for implementing color and aesthetics [70], fillers to reduce cost and improve the desired properties [71], flame retardants, and smoke suppressants [72, 73]. As discussed earlier, phenols are aromatic rings with a hydroxyl group that presents versatile chemistry. Mannich’s reaction is one of the most studied procedures in phenol chemistry. In basic terms, it consists of a phenol dissolved in a solution of formaldehyde and water along with the addition of an amine-based compound such as diethanolamine that chemically attaches to the aromatic ring through the formation of a methylene bridge. The scientific community still exploits the parameters that influence this reaction such as the amount of formaldehyde/amine ratio, pH, temperature, and kinetics [74,75,76]. Cardanol oil has been reported in many studies as a renewable and viable option for epoxy and phenol–formaldehyde resins as well as phenolic polyurethanes [77]. Cardanol is obtained from cashew nut shell liquid and the main constituents of cashew nut shell liquid are cardanol, cardol, and 2-methylcardol as shown in Fig. 13 [78]. Cardanol is composed of several aromatic oils that present a phenol group with variations of aliphatic chains in the m position with degrees of unsaturation [79].
The main component of cashew nutshell liquid [78]
Mannich’s reaction was performed to convert cardanol oil into a polyol for making polymeric foams. The procedure consisted of three synthetic steps. First, the reaction between para-formaldehyde and diethanolamine was carried out to synthesize N-(2-hydroxyethyl)-1,3-oxazolidine, also mentioned as intermediate of Mannich’s reaction. The second step was the reaction of this intermediate compound with the aromatic ring in the cardanol oil, performing an electrophilic substitution at ortho and para positions. In the third step, the cardanol-based polyol was further functionalized with propylene oxide to increase the functionality of the polyol from 3 to 5. The synthetic route is described in Fig. 14 [79]. This approach presents some advantages such as increase the functionality of the polyol without an excessive increase of viscosity. This is due to the formation of the intermediate, N-(2-hydroxyethyl)-1,3-oxazolidine, that prevents the condensation reaction between formaldehyde and phenol, while still acting as a Mannich base due to the presence of an iminium cation and alternating state between cyclic and open structure, hence, facilitating the processing to obtain the foams (Fig. 15) [75, 77, 79]. The foams obtained through this method provide biodegradability, flame retardancy, and satisfactory mechanical properties compared with petrochemical-based foams.
Synthetic route for cardanol-based polyol. “Adapted with permission [79]. Copyright (2012) Springer”
Alternating structures of N-(2-hydroxyethyl)-1,3-oxazolidine for open and cyclic form. “Adapted with permission [79]. Copyright (2012) Springer.”
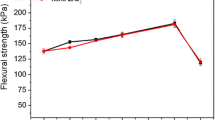
Another interesting approach utilized cardanol oil modified with a phosphorus-based compound, named 10-(2,5-dihydroxyphenyl)-9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO), to improve the inherent flame-retardant properties of the phenolic foams [80]. Also, a silicon-based segment originated from polydimethylsiloxane was attached to improve the mechanical properties by reducing the brittleness that usually accompanies the phenolic foams. A relevant factor that cardanol-based foams impart is the plasticizer effect on mechanical properties that reduces brittleness. This phenomenon occurs due to the presence of the 15 carbon aliphatic unsaturated chain attached to the aromatic ring. It allows the polymeric chains to slip pass each other while increasing the free volume of the foams [81]. The procedure to obtain the cardanol polyol functionalized with DOPO consisted of an epoxidation of the unsaturation of the aliphatic chain of cardanol oil followed by ring-opening with DOPO. Along with that, the phenolic hydroxyl reacted with epichlorohydrin followed by hydrolysis, hence, making the polyol inherent flame-retardant [82]. As previously discussed, the viscosity is an important parameter that has to be controlled to allow a proper mixing between the components of the foams. In this case, the isocyanate, hexamethylenediisocyanate (HDI) was added in excess to react with polyethylene glycol. This approach increased the linearity and flexibility of the chains, which is reflected as ease processing and more flexibility for the phenolic foam, by reducing the natural brittleness and pulverization.
The general trend for research nowadays is leaning toward a greener and environmentally friendly approach. In that line, many researchers are finding a middle ground by partially replacing petrochemical compounds with environmentally friendly fillers into industrial phenolic foams to improve their property. As an example, a facile procedure to improve the mechanical properties of phenolic resol resin by blending it with around 2 wt.% of cellulose fibers was reported [83]. It demonstrated an effective increase in the modulus and compressive strength of the foam even surpassing other well-known mechanical reinforce composites of aramids and glass fibers [13, 84]. The previous procedure is often described as physical toughening, which is the blending of the filler with the foaming components without chemical reaction as a means to enhance the mechanical properties. Compounds such as fibers and rubbers can be employed for this end [84,85,86]. It has some advantages such as ease processing, facile procedures, and usually lower cost. However, the distribution of the filler is usually not regular throughout the foam, which may cause inner defects. Besides, the effects of the toughening may decrease over time since the filler tends to migrate, which creates irregularities that may deteriorate other properties. A different approach that can be used to counter this situation is the chemical toughening. In this case, the toughening agent is chemically bonded to the structure of the foam. It brings the positive points of longer term efficiency and proper dispersion through the foam, avoiding irregularities [87]. A novel phosphorus-based phenolic polyol was synthesized by using phenyl dichlorophosphate (PDCP) as the toughening agent. The PDCP reacted with trimethylamine at 0 °C to form a good leaving group for the further reaction with polyethylene glycol to obtain the polyol that was mixed with industrial phenol resin. The synthesis can be described in Fig. 16 [88].
Synthesis of phosphorus-based polyol for the enhancement of mechanical and flame retardant properties. “Adapted with permission [88]. Copyright (2012) Springer”
A concern that usually comes with both physical and chemical fillers is the deterioration of other properties that were not targeted. For example, some toughening agents may cause an increase in the flammability of the foam. As a way to avoid this scenario, this approach yielded a polyol that increased both mechanical as well as the flame retardant properties of the foam [88]. A follow-up procedure implemented silicon at the polymeric chain of the polyol, as a means to improve compressive strength, friability, and thermal stability [89]. Following the same trend, another seldom approach includes the use of boron as a toughening and flame retardant agent. A report described a procedure that used boric acid (BA) to react with polyethylene glycol (PEG) at 110–150 °C dissolved in xylene to form a branched polyol (PEG-BA). This material was then cured with a phenolic resin to obtain an inherent flame-retardant and toughened foam after the reaction with isocyanate. The reaction is described in Fig. 17 [87]. Finally, it is notable that the synthesis of phenolic foams allows many possibilities, including the source of starting materials that can be petrochemicals, mostly resol and novolacs resins, or renewable, mostly cardanol oil and lignin. The factors like low cost, abundance, inherent flame-retardant properties, and ease modification of synthetic procedures prompt its use as a viable polymeric material for large-scale applications.
Synthesis of a boron-based branched polyol with further curing process with phenolic resin. “Adapted with permission [87]. Copyright (2015) American Chemical Society”
1,3-dihydroxybenzene know as resorcinol is a derivative of phenol that has a huge commercial application in phenolic foams. Its condensate form with formaldehyde is used to improve the adhesion on rubber and tires and allows curing at room temperature [90]. The main synthetic route to obtain resorcinol consists of the sulfonation process of benzene with sulfur trioxide at 150 °C in the presence of sulfuric acid as a catalyst [90]. The obtained product is epsilon and meta substituted aromatic ring with sulfonate groups more precisely, benzene-1,3-disulfonate. This compound is then placed to react with sodium hydroxide at 350 °C. The product obtained at the end of this reaction is extracted for the organic phase with diisopropylether. Finally, it is distilled to obtain the purified resorcinol. The points that limit the use of resorcinol are related to the cost of production and safety concerns. Despite that, previous studies have obtained a copolymer based on lignin–resorcinol–glyoxal. The synthesis was performed under mild alkali media with stirring of the three monomers (Fig. 18) [91].
Facile synthesis for lignin–resorcinol–glyoxal copolymer resin for further implementation in the polymeric matrix. “Adapted with permission [91]. Copyright (2020) Elsevier”
As previously described, the Mannich’s reaction is an extremely valuable tool when it comes to the synthesis of phenolic resins. The chemical attachment of amino alcohol through the methylene bridge, usually in the ortho position of the phenol, increases the functionality of the polyol making it suitable for foaming reactions [77]. A general procedure of this reaction can be performed with the mixture of the phenol and the amine-functionalized reagent such as alcohol. Then a formaldehyde or paraformaldehyde solution can be added with a concentration of around 25–37%. The temperature should be maintained around 50–70 °C and digesting for around 2 h. After the completion of the reaction, both water as a byproduct of the reaction as well as formaldehyde is removed through vacuum distillation around 90–120 °C. It is important to mention that the lower temperature range reduces the tendency of condensation to form oligomers. This condensate is nothing but an oligomer of two or three phenolic groups; however, it causes an increase in the viscosity, which may harden the processing. Previous studies on this reaction established that it occurs in a two-step process, in which an ammonium cation is formed between the formaldehyde and the amine reaction (primary or secondary). The ammonium cation then reacts with the phenolate anions at ortho and/or para positions following a mechanism based on SN1 [92, 93]. Another type of mechanism that follows the SN2 is also possible. In this scenario, there is a coupling of formaldehyde with two molecules of the alkylamine compound, leading to a neutral molecule. Then, the phenolate anion attacks the methylene group, which is under an electron-withdrawing effect of the two amine groups. SN1 is prompt to happen when the reaction mixture is under acidic media, hence forming amine salts. Oppositely, SN2 can occur under basic or neutral media.
The functionalization of phenol through Mannich’s reaction is a valid strategy because it allows the introduction of primary hydroxyl groups as the R groups, which are known to be more reactive toward isocyanate. This not only improves the reaction rate for the formation of the foam but also increases the functionality of the starting material. Hence, a variety of different structures can be obtained. On top of that, the addition of a tertiary amine to the structure acts as a self-catalyst by increasing the foaming process [77, 94]. As an example of this approach, a previous study reported the synthesis of a novel Mannich polyol in three steps. In the first step, a Friedel–Crafts alkylation was performed between phenol and limonene (Fig. 19). Oxazolidines were synthesized by the reaction of formaldehyde and alkanolamines in the second step of this process (Fig. 20). The third and final step consisted of the synthesis of Mannich polyol through the reaction with propylene oxide to increase the functionality of the Mannich polyol [94]. The obtained polyols were suitable to make rigid foams with satisfying mechanical, thermal, and physical properties.
Friedel–Crafts reaction between phenol and limonene. “Adapted with permission [94]. Copyright (2015) Springer”
Variations of Mannich bases. “Adapted with permission [94]. Copyright (2015) Springer”
Finally, it is notable that the synthesis of phenolic foams allows many possibilities, including the source of starting materials that can be petrochemicals, mostly resol and novolacs resins, or renewable, mostly cardanol oil and lignin. The factors like low cost, abundance, inherent flame-retardant properties, and ease modification of synthetic procedures prompt their use as viable polymeric materials for large-scale applications.
3 Chemistry-Based Applications of Phenolic Foams
The properties of phenolic foams such as inherent flame retardancy, rigidity, low thermal conductivity, high thermal stability, and low emission of toxic smoke allow their use as thermal insulators and fire protection materials for buildings and constructions. On top of that, it can also be applied as adhesives, sealants, coatings, elastomers, binders, and foams [23, 95]. The property–structure relation is crucial to understand how these materials fit for certain applications along with the versatility of phenolic foam’s chemistry that broadens the range of experiments that can be designed. Thus, the following session discusses the main parameters to obtain the desired properties based on the chemical structure of the starting materials, more specifically into how the functionalization of phenolic groups influences their properties.
The functionalization of the phenolic polyol highly influences the properties of the foams. Accompanied to that, most of the phenolic starting materials are functionalized resins such as resoles and novolacs as previously discussed. Thus, it is important to understand what are the parameters that influence their properties, when it comes to design a procedure. One of the starting points is the formaldehyde/phenol ratio (F/P). A study found that under excess of formaldehyde catalyzed by alkaline media, less base was required to carry out the reaction [26]. It was also observed that for an F/P ratio of around 2.5, the polymerization happened to a lower degree [26]. For this scenario, the extra amount of formaldehyde was volatilized, which increased the porosity and shrinkage of the phenolic foam. Hence, it may hurt the mechanical properties and increase friability. Therefore, the proper control of F/P plays an important role in the properties of the phenolic foams [96]. Accompanied to that study, it was found that catalysts also might prompt a type of chemical structure. The influence of triethanolamine as a catalyst in the reaction between formaldehyde and phenol to obtain phenolic resins was studied [26]. It was observed that besides the obvious influence at the rate of reaction and condensation time, the catalyst had a slight influence in the structure. The higher population of transition state intermediates seemed to be prompted the aromatic ring substitution to occur more at ortho position rather than para. This difference in structure may impart changes on the mechanical properties, since ortho-ortho substitution may increase the size of dandling groups if compared with ortho-para substitution due to lesser bulky groups hanging on the polymeric structure [77].
The chemistry of phenolic materials is highly related to its reactive sites. In an ideal condition, a phenol compound may present functionality of three, those being the phenolic hydroxyl, ortho and para positions for nucleophilic substitution. This condition allows cross-linking to take place. This is often desired to enhance the mechanical and thermal properties of materials, which can turn them into thermosets if the crosslinking degree is high enough. The rigidity and thermal stability of phenolic foams are in part due to their inherent cross-linking capability. Previous studies have demonstrated that in a real scenario, the functionality of a phenol would be around 2.38–2.7 [27, 97, 98]. Thus, from an experimental perspective, an increase of the F/P ratio of at least 1.3 is required to prompt the cross-linking reaction. A previous study reported that cross-linking reactions occur mostly at para-para positions [27].
Even though the cross-link of phenolic foams is one of their signature features for a rigid structure with high compressive strength, it is also what makes it brittle and friable. To solve that issue, the synthesis of composites of phenolic foams is a viable option to obtain materials that can absorb mechanical stress without breaking. For that, multi-walled carbon nanotubes have been employed. Their large structure and many reactive sites lead to covalent bonds that diminish the stress during compression [99]. Also, the high friability is an undesirable property that comes with phenolic foams. One approach that can prevent this phenomenon is the addition of aramid compounds [99]. Another recent approach involves self-healing foams, smart devices, and recyclable composites, which are based on the concept of covalent adaptable networks or also named dynamic cross-linked chains. Polymer can be synthesized to present the reversibility of bonds. This feature shifts the properties of the material making it able to be reprocessed and acquire self-healing properties, which are not possible for highly cross-linked materials with permanent covalent bonds [100]. The reversible break and formation of these bonds can happen through Diels–Alder [101,102,103,104], transesterification [105,106,107], phenol-urethanes [108, 109], and in many otherways [108, 110,111,112,113]. To design an experiment with these properties, some well-known monomers are employed, such as bisphenols. They consist of two phenolic rings linked by a chemical group between them such as isopropylene, hexafluoroisopropylene, and sulfone. From the first to latter, higher is the electron-withdrawing effect. The higher this effect, the easier to disrupt and change the chemical bonds of the structure, also lower the temperature for that phenomenon to take place [114].
Another possible option that has been highlighted around the scientific community is the chemical functionalization with the attachment of a flexible segment into the structure of the phenolic resin. The reactivity of the phenolic ring allows many chemical modifications, which are greatly responsible for imparting the desired properties into the phenolic foams. The presence of an aliphatic chain could decrease the rigidity of the molecular structure. Hence, allowing the final polymer to become tougher instead of brittle. Fortunately, cardanol oil, as previously demonstrated, is a starting material that already presents an unsaturated alkyl chain at the meta position [115]. This feature promotes two aspects of the material. First, it decreases the brittleness improving the mechanical properties. Second, it provides an extra reactive site, which is the double bonds at the aliphatic chain, allowing further chemical modifications [115]. The low rigidity is an important factor for applications that require mechanical energy absorption. Another important feature of chemical functionalization is the increase of reactivity that fastens both the foaming and curing process. It highly affects the application of the phenolic polyurethanes since it adds a profitable aspect for large-scale applications such as thermal insulation for buildings and constructions. As previously discussed, the Mannich’s reaction in phenolic compounds implements a methylene bridge along with a secondary or tertiary alkyl amine segment. The presence of these amine groups creates a self-catalyzed process for the resins, hence, notably increases the foaming reaction between hydroxyl and isocyanates groups [94]. This quick reaction allows it to be used in a “spray” foaming process. However, along with a fast reaction rate, proper control of viscosity is also important. Because, in one hand, low viscosity allows easy flow and a mixture of components, hence, a more homogenous structure of foaming. On the other hand, higher viscosity provides better control of cellular structure and may avoid coalescence during the foaming process [22, 116, 117].
External components that do not directly participate in the foaming reaction also have a strong impact on the properties of foams. The formulation of the components to manufacture foam is one of the key factors that allow the same material to acquire different properties. For the case of phenolic foams, the implementation of blowing agents and/or volatile solvents can influence the size of the pores, which bears great importance for applications such as decontamination of radioisotopes and water contaminated with heavy metals [118, 119]. The blowing agents also have a direct effect on the density of the foam, which enables them to obtain materials that are light as well as rigid [3]. The final cell structure is also relevant for the range of applications of the foam. For example, open cells are used for the soundproof environment while closed ones tend to have higher thermal insulation and better flame retardancy [120]. Finally, this session presented the main factors that influence the properties of phenolic foams regarding their chemical structure and the possible approaches to tune the features of the final material according to the experimental goal.
4 Conclusion and Future Lookout
Throughout this chapter, the importance of phenolic foams and derivate materials in terms of their chemistry and properties were discussed. Phenolic foams are known for their high compressive strength, thermal stability, and flame retardancy properties accompanied by low emission of toxic smoke. The starting materials for phenolic resins can be obtained from petrochemical sources such as phenol, bisphenols, m-cresols, and other variations. However, renewable sources such as cardanol, castor oil, and lignin that are promising materials with satisfactory products and tunable properties. Their greatest concern goes around their brittleness and friability, which demanded efforts from the scientific community to develop new synthetic approaches and alternative materials to nullify these drawbacks. Some of these techniques include chemical modification with flexible segments, blending with other materials to create composites, and changes in formulation for proper control of properties. This is all possible due to the chemical versatility of phenolic compounds. Still, there are many undiscovered possibilities for phenolic materials, since the chemistry of phenolic ring allows many possibilities. However, phenolic foams are already responsible for a good part of the market related to thermal insulation for household applications due to their easy source and high reactivity. As research progresses new applications for phenolic foams were found, for example, the capture of radioisotopes and removal of heavy metals from water, which will bear great importance for future scenarios.
References
Khemani KC (1997) Polymeric foams: an overview. ACS Symp Ser 669:1–7
Baekeland LH (1909) Original papers: the synthesis, constitution, and uses of bakelite. Ind Eng Chem 1:149–161
Mougel C, Garnier T, Cassagnau P, Sintes-Zydowicz N (2019) Phenolic foams: a review of mechanical properties, fire resistance and new trends in phenol substitution. Polymer (Guildf) 164:86–117
Kumar R, Sharma A, Pandey A, Chaudhary A, Dwivedi N, Shafeeq MM, Mondal DP, Srivastava AK (2020) Lightweight carbon-red mud hybrid foam toward fire-resistant and efficient shield against electromagnetic interference. Sci Rep 10:1–12
Li X, Wang Z, Wu L, Tsai T (2016) One-step: In situ synthesis of a novel α-zirconium phosphate/graphene oxide hybrid and its application in phenolic foam with enhanced mechanical strength, flame retardancy and thermal stability. RSC Adv 6:74903–74912
Paruzel A, Michałowski S, Hodan J, Horák P, Prociak A, Beneš H (2017) Rigid polyurethane foam fabrication using medium chain glycerides of coconut oil and plastics from end-of-life vehicles. ACS Sustain Chem Eng 5:6237–6246
Zhao W, Gao H, Fan M (2015) Synthesis of novel monolithic activated carbons from phenol-urea-formaldehyde resin. RSC Adv 5:104936–104942
Li B, Feng SH, Niasar HS, Zhang YS, Yuan ZS, Schmidt J, Xu C (2016) Preparation and characterization of bark-derived phenol formaldehyde foams. RSC Adv 6:40975–40981
Yang H, Yu B, Xu X, Bourbigot S, Wang H, Song P (2020) Lignin-derived bio-based flame retardants toward high-performance sustainable polymeric materials. Green Chem 22:2129–2161
Suresh KI (2013) Rigid polyurethane foams from cardanol: synthesis, structural characterization, and evaluation of polyol and foam properties. ACS Sustain Chem Eng 1:232–242
Mouritz AP, Mathys Z (1999) Post-fire mechanical properties of marine polymer composites. Compos Struct 47:643–653
Kim D-K, Lee S-B (2006) Properties and thermal characteristics of phenol foam for heat insulating materials. Appl Chem Eng 17:357–360
Desai A, Nutt SR, Alonso MV (2008) Modeling of fiber-reinforced phenolic foam. J Cell Plast 44:391–413
Kim M, Choe J, Lee DG (2016) Development of the fire-retardant sandwich structure using an aramid/glass hybrid composite and a phenolic foam-filled honeycomb. Compos Struct 158:227–234
Madhava Sarma K, Bankobeza GM (2000) Montreal protocol on substances that deplete the ozone layer. Nairobi Kenya
Hu X-M, Zhao Y-Y, Cheng W-M (2015) Effect of formaldehyde/phenol ratio (F/P) on the properties of phenolic resins and foams synthesized at room temperature. Polym Compos 36:1531–1540
Pizzi A, Mittal KL (2003) Handbook of adhesive technology, revised and expanded. CRC Press
Haupt RA, Sellers TJ (1994) Characterizations of phenol-formaldehyde resol resins. Ind Eng Chem Res 33:693–697
Fontanille M, Gnanou Y (2014) Chimie et physico-chimie des polymères. DUNOD
Chevalier M (2006) Phénoplastes ou phénols-formols PF. Tech l’ingénieur, Fasc A3435 A 3436
Bessire BK, Lahankar SA, Minton TK (2015) Pyrolysis of phenolic impregnated carbon ablator (PICA). ACS Appl Mater Interfaces 7:1383–1395
Pilato L (2010) Phenolic resins: a century of progress. Springer
Yang P, Han Y, Li T (2010) Effect of substituents and solvents on phenol-isocyanate urethane reaction. Adv Mater Res 150–151:23–26
Astarloa Aierbe G, Echeverrı́a JM, Riccardi CC, Mondragon I (2002) Influence of the temperature on the formation of a phenolic resol resin catalyzed with amine. Polymer (Guildf) 43:2239–2243
Grenier-Loustalot M-F, Larroque S, Grande D, Grenier P, Bedel D (1996) Phenolic resins: 2. Influence of catalyst type on reaction mechanisms and kinetics. Polymer (Guildf) 37:1363–1369
Astarloa-Aierbe G, Echeverrı́a JM, Vázquez A, Mondragon I (2000) Influence of the amount of catalyst and initial pH on the phenolic resol resin formation. Polymer (Guildf) 41:3311–3315
Manfredi LB, de la Osa O, Galego Fernández N, Vázquez A (1999) Structure–properties relationship for resols with different formaldehyde/phenol molar ratio. Polymer (Guildf) 40:3867–3875
Astarloa Aierbe G, Echeverrı́a JM, Martin MD, Etxeberria AM, Mondragon I (2000) Influence of the initial formaldehyde to phenol molar ratio (F/P) on the formation of a phenolic resol resin catalyzed with amine. Polymer (Guildf) 41:6797–6802
Grenier-Loustalot M-F, Larroque S, Grenier P (1996) Phenolic resins: 5. Solid-state physicochemical study of resoles with variable FP ratios. Polymer (Guildf) 37:639–650
Tejado A, Peña C, Labidi J, Echeverria JM, Mondragon I (2007) Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour Technol 98:1655–1663
Wang G, Chen H (2014) Carbohydrate elimination of alkaline-extracted lignin liquor by steam explosion and its methylolation for substitution of phenolic adhesive. Ind Crops Prod 53:93–101
Wang G, Liu X, Zhang J, Sui W, Jang J, Si C (2018) One-pot lignin depolymerization and activation by solid acid catalytic phenolation for lightweight phenolic foam preparation. Ind Crops Prod 124:216–225
Zhao Y, Mao X, Li W, Gu X, Wang G (2017) Study on extraction phenol from coal tar with high flux centrifugal extractor. Int J Coal Sci Technol 4:333–341
Jiao T, Zhuang X, He H, Li C, Chen H, Zhang S (2015) Separation of phenolic compounds from coal tar via liquid-liquid extraction using amide compounds. Ind Eng Chem Res 54:2573–2579
Meng H, Ge C-T, Ren N-N, Ma W-Y, Lu Y-Z, Li C-X (2014) Complex extraction of phenol and cresol from model coal tar with polyols, ethanol amines, and ionic liquids thereof. Ind Eng Chem Res 53:355–362
Li Z, Wu M, Jiao Z, Bao B, Lu S (2004) Extraction of phenol from wastewater by N-octanoylpyrrolidine. J Hazard Mater 114:111–114
Medir M, Arriola A, Mackay D, Giralt F (1985) Phenol recovery from water effluents with mixed solvents. J Chem Eng Data 30:157–159
Yang C, Qian Y, Zhang L, Feng J (2006) Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem Eng J 117:179–185
Rao TSS, Awasthi S (2007) Oxidation of alkylaromatics. E J Chem 4:185364
Weber M, Weber M (2010) Phenols. In: Phenolic resins: a century of progress. Springer, pp 9–23
Amen-Chen C, Pakdel H, Roy C (2001) Production of monomeric phenols by thermochemical conversion of biomass: a review. Bioresour Technol 79:277–299
Chum H, Diebold J, Scahill J, Johnson D, Black S, Schroeder H, Kreibich RE (1989) Biomass pyrolysis oil feedstocks for phenolic adhesives. In: Adhesives from renewable resources. American Chemical Society, pp 11–135
Russell JA, Riemath WF, Pasco both of W (1984) Method for making adhesive from biomass. United States Patents
Lee SH, Ohkita T (2003) Rapid wood liquefaction by supercritical phenol. Wood Sci Technol 37:29–38
Khan MA, Ashraf SM, Malhotra VP (2004) Development and characterization of a wood adhesive using bagasse lignin. Int J Adhes Adhes 24:485–493
Çetin NS, Özmen N (2002) Use of organosolv lignin in phenol–formaldehyde resins for particleboard production: I. Organosolv lignin modified resins. Int J Adhes Adhes 22:477–480
Çetin NS, Özmen N (2002) Use of organosolv lignin in phenol-formaldehyde resins for particleboard production: II. Particleboard production and properties. Int J Adhes Adhes 22:481–486
Alonso MV, Oliet M, Rodrı́guez F, Garcı́a J, Gilarranz MA, Rodrı́guez JJ (2005) Modification of ammonium lignosulfonate by phenolation for use in phenolic resins. Bioresour Technol 96:1013–1018
Ono H-K, Sudo K (1989) Wood adhesives from phenolysis lignin. In: Lignin. American Chemical Society, pp 25–334
Effendi A, Gerhauser H, Bridgwater AV (2008) Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew Sustain Energy Rev 12:2092–2116
Chum HL, Black SK, Diebold JP, Kreibich RE (1993) Phenolic compounds containing/neutral fractions extract and products derived therefrom from fractionated fast-pyrolysis oils
Chum H, Kreibich R (1992) Process for preparing phenolic formaldehyde resole resin products derived from fractionated fast-pyrolysis oils
Kresta JE, Garcia A, Frisch KC, Linden G (1981) Model studies of phenolic-urethane polymers. In: Urethane chemistry and applications. American Chemical Society, pp 27–403
Sonnenschein MF (2014) Polyurethanes: science, technology, markets, and trends. John Wiley & Sons
Akindoyo JO, Beg MDH, Ghazali S, Islam MR, Jeyaratnam N, Yuvaraj AR (2016) Polyurethane types, synthesis and applications-a review. RSC Adv 6:114453–114482
Burdeniuc J, Kamzelski A (2009) Blowing catalyst compositions containing hydroxyl and surface active groups for the production of polyurethane foams. US Patent 7,495,131 1:1–40
Britain JW, Gemeinhardt PG (1960) Catalysis of the isocyanate-hydroxyl reaction. J Appl Polym Sci 4:207–211
Lai Y-C, Dvornić PR, Lenz RW (1982) Exactly alternating silarylene-siloxane polymers. 4. Step-growth polymerization reactions with dichlorosilane monomers. J Polym Sci Polym Chem Ed 20:2277–2288
Sasaki N, Yokoyama T, Tanaka T (1973) Properties of isocyanurate-type crosslinked polyurethanes. J Polym Sci Polym Chem Ed 11:1765–1779
Chattopadhyay DK, Raju K (2007) Structural engineering of polyurethane coatings for high performance applications. Progr Polym Sci 32:352–418
Chattopadhyay DK, Sreedhar B, Raju KVSN (2005) Effect of chain extender on phase mixing and coating properties of polyurethane ureas. Ind Eng Chem Res 44:1772–1779
Hepburn C (2012) Polyurethane elastomers. Springer, Netherlands
Shi Y, Yu B, Zhou K, Yuen RKK, Gui Z, Hu Y, Jiang S (2015) Novel CuCo2O4/graphitic carbon nitride nanohybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic polyurethane nanocomposites. J Hazard Mater 293:87–96
Zheng J, Luo J, Zhou D, Shen T, Li H, Liang L, Lu M (2010) Preparation and properties of non-ionic polyurethane surfactants. Colloids Surf A Physicochem Eng Asp 363:16–21
Jin L, Liu Z, Xu Q, Li Y (2006) Preparation of soap-free cationic emulsion using polymerizable surfactant. J Appl Polym Sci 99:1111–1116
Lu Y, Xia Y, Larock RC (2011) Surfactant-free core–shell hybrid latexes from soybean oil-based waterborne polyurethanes and poly(styrene-butyl acrylate). Prog Org Coatings 71:336–342
Singh SN (2001) Blowing agents for polyurethane foams. Rapra Technology
Petroviƈ ZS, Cvetković I, Hong D, Wan X, Zhang W (2008) Polyester polyols and polyurethanes from ricinoleic acid. J Appl Polym Sci 108:1184–1190
Nieschlag HJ, Wolff IA (1971) Industrial uses of high erucic oils. J Am Oil Chem Soc 48:723–727
Liu Z, Bai G, Huang Y, Ma Y, Du F, Li F, Guo T, Chen Y (2007) Reflection and absorption contributions to the electromagnetic interference shielding of single-walled carbon nanotube/polyurethane composites. Carbon N Y 45:821–827
Taheri N, Sayyahi S (2016) Effect of clay loading on the structural and mechanical properties of organoclay/HDI-based thermoplastic polyurethane nanocomposites. e-Polymers 16:65–73
Patel RH, Shah MD, Patel HB (2011) Synthesis and characterization of structurally modified polyurethanes based on castor oil and phosphorus-containing polyol for flame-retardant coatings. Int J Polym Anal Charact 16:107–117
Zhang M, Luo Z, Zhang J, Chen S, Zhou Y (2015) Effects of a novel phosphorus–nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym Degrad Stab 120:427–434
Nobles WL, Potti ND (1968) Studies on the mechanism of the mannich reaction. J Pharm Sci 57:1097–1103
Tramontini M, Angiolini L (1994) Mannich bases-chemistry and uses. CRC Press
Short EL, Tychopoulos V, Tyman JHP (1992) Long chain phenols—part 30: a rate study of the mannich reaction of phenols (with particular reference to 3-pentadecylphenol). J Chem Technol Biotechnol 53:389–396
Ionescu M (2006) Mihail Ionescu: chemistry and technology of polyols for polyurethanes. Polimeri 26:218–218
Vasapollo G, Mele G, Del Sole R (2011) Cardanol-based materials as natural precursors for olefin metathesis. Molecules 16:6871–6882
Ionescu M, Wan X, Bilić N, Petrovic ZS (2012) Polyols and rigid polyurethane foams from cashew nut shell liquid. J Polym Environ 20:647
Bo C, Wei S, Hu L, Jia P, Liang B, Zhou J, Zhou Y (2016) Synthesis of a cardanol-based phosphorus-containing polyurethane prepolymer and its application in phenolic foams. RSC Adv 6:62999–63005
Rekha N, Asha SK (2008) Synthesis and FTIR spectroscopic investigation of the UV curing kinetics of telechelic urethane methacrylate crosslinkers based on the renewable resource—cardanol. J Appl Polym Sci 109:2781–2790
Bo C, Hu L, Jia P, Liang B, Zhou J, Zhou Y (2015) Structure and thermal properties of phosphorus-containing polyol synthesized from cardanol. RSC Adv 5:106651–106660
Del Saz-Orozco B, Alonso MV, Oliet M, Domínguez JC, Rodriguez F (2015) Mechanical, thermal and morphological characterization of cellulose fiber-reinforced phenolic foams. Compos Part B Eng 75:367–372
Shen H, Nutt S (2003) Mechanical characterization of short fiber reinforced phenolic foam. Compos Part A Appl Sci Manuf 34:899–906
Huang Y-J, Wang C-H, Huang Y-L, Guo G, Nutt SR (2010) Enhancing specific strength and stiffness of phenolic microsphere syntactic foams through carbon fiber reinforcement. Polym Compos 31:256–262
Kaynak C, Cagatay O (2006) Rubber toughening of phenolic resin by using nitrile rubber and amino silane. Polym Test 25:296–305
Liu L, Fu M, Wang Z (2015) Synthesis of boron-containing toughening agents and their application in phenolic foams. Ind Eng Chem Res 54:1962–1970
Yang H, Wang X, Yuan H, Song L, Hu Y, Yuen RKK (2012) Fire performance and mechanical properties of phenolic foams modified by phosphorus-containing polyethers. J Polym Res 19:9831
Yang H, Wang X, Yu B, Yuan H, Song L, Hu Y, Yuen RKK, Yeoh GH (2013) A novel polyurethane prepolymer as toughening agent: preparation, characterization, and its influence on mechanical and flame retardant properties of phenolic foam. J Appl Polym Sci 128:2720–2728
Gardziella A, Pilato LA, Knop A (2013) Phenolic resins: chemistry, applications, standardization, safety and ecology. Springer Berlin Heidelberg
Rao G-S, Nabipour H, Zhang P, Wang X, Xing W, Song L, Hu Y (2020) Lightweight, hydrophobic and recyclable carbon foam derived from lignin–resorcinol–glyoxal resin for oil and solvent spill capture. J Mater Res Technol 9:4655–4664
Kleinman EF, Volkmann RA (2015) comprehensive organic synthesis II. Choice Rev Online 52:52-4219-52–4219
Leonard NJ, Paukstelis JV (1963) Direct synthesis of ternary iminium salts by combination of aldehydes or ketones with secondary amine salts 1,2. J Org Chem 28:3021–3024
Gupta RK, Ionescu M, Wan X, Radojcic D, Petroviƈ ZS (2015) Synthesis of a novel limonene based Mannich polyol for rigid polyurethane foams. J Polym Environ 23:261–268
Hu X, Cheng W, Wang D (2014) Properties and applications of novel composite foam for blocking air leakage in coal mine. Russ J Appl Chem 87:1099–1108
Knop A, Böhmer V, Pilato LA (2013) Phenolic resins: chemistry, applications and performance. Springer Berlin Heidelberg
Lenghaus K, Qiao GG, Solomon DH (2001) The effect of formaldehyde to phenol ratio on the curing and carbonisation behaviour of resole resins. Polymer (Guildf) 42:3355–3362
Vazquez A, Adabbo HE, Williams RJJ (1984) Statistics of resols. Ind Eng Chem Prod Res Dev 23:375–379
Yuan J, Zhang Y, Wang Z (2015) Phenolic foams toughened with crosslinked poly (n-butyl acrylate)/silica core-shell nanocomposite particles. J Appl Polym Sci 132
Shi J, Zheng T, Zhang Y, Guo B, Xu J (2020) Reprocessable cross-linked polyurethane with dynamic and tunable phenol-carbamate network. ACS Sustain Chem Eng 8:1207–1218
Zhang G, Zhao Q, Yang L, Zou W, Xi X, Xie T (2016) Exploring dynamic equilibrium of diels-alder reaction for solid state plasticity in remoldable shape memory polymer network. ACS Macro Lett 5:805–808
Zhang Y, Dai Z, Han J, Li T, Xu J, Guo B (2017) Interplay between crystallization and the Diels-Alder reaction in biobased multiblock copolyesters possessing dynamic covalent bonds. Polym Chem 8:4280–4289
Zeng C, Seino H, Ren J, Hatanaka K, Yoshie N (2013) Bio-based furan polymers with self-healing ability. Macromolecules 46:1794–1802
Liu Y-L, Chuo T-W (2013) Self-healing polymers based on thermally reversible Diels-Alder chemistry. Polym Chem 4:2194–2205
Montarnal D, Capelot M, Tournilhac F, Leibler L (2011) Silica-like malleable materials from permanent organic networks. Science (80- ) 334:965–968
Capelot M, Unterlass MM, Tournilhac F, Leibler L (2012) Catalytic control of the vitrimer glass transition. ACS Macro Lett 1:789–792
Han J, Liu T, Hao C, Zhang S, Guo B, Zhang J (2018) A Catalyst-free epoxy vitrimer system based on multifunctional hyperbranched polymer. Macromolecules 51:6789–6799
Shi J, Zheng T, Guo B, Xu J (2019) Solvent-free thermo-reversible and self-healable crosslinked polyurethane with dynamic covalent networks based on phenol-carbamate bonds. Polymer (Guildf) 181:121788
Cao S, Li S, Li M, Xu L, Ding H, Xia J, Zhang M, Huang K (2017) A thermal self-healing polyurethane thermoset based on phenolic urethane. Polym J 49:775–781
Fang Z, Zheng N, Zhao Q, Xie T (2017) Healable, reconfigurable, reprocessable thermoset shape memory polymer with highly tunable topological rearrangement kinetics. ACS Appl Mater Interfaces 9:22077–22082
Zhang L, Rowan SJ (2017) Effect of sterics and degree of cross-linking on the mechanical properties of dynamic poly(alkylurea–urethane) networks. Macromolecules 50:5051–5060
Chen J-H, Hu D-D, Li Y-D, Meng F, Zhu J, Zeng J-B (2018) Castor oil derived poly(urethane urea) networks with reprocessibility and enhanced mechanical properties. Polymer (Guildf) 143:79–86
Chen J-H, Hu D-D, Li Y-D, Zhu J, Du A-K, Zeng J-B (2018) Castor oil-based high performance and reprocessable poly(urethane urea) network. Polym Test 70:174–179
Wicks DA, Wicks ZW (1999) Blocked isocyanates III: Part A. Mechanisms and chemistry. Prog Org Coatings 36:148–172
Voirin C, Caillol S, Sadavarte NV, Tawade BV, Boutevin B, Wadgaonkar PP (2014) Functionalization of cardanol: towards biobased polymers and additives. Polym Chem 5:3142–3162
Yang C, Zhuang ZH, Yang ZG (2014) Pulverized polyurethane foam particles reinforced rigid polyurethane foam and phenolic foam. J Appl Polym Sci 131:39734
Jing S, Li T, Li X, Xu Q, Hu J, Li R (2014) Phenolic foams modified by cardanol through bisphenol modification. J Appl Polym Sci 131:39942
Gao L, Li C, Wang C, Cui J, Zhou L, Fang S (2019) Structure and luminescent property of polyurethane bonded with Eu3+-complex. J Lumin 212:328–333
Lei S, Guo Q, Zhang D, Shi J, Liu L, Wei X (2010) Preparation and properties of the phenolic foams with controllable nanometer pore structure. J Appl Polym Sci 117:3545–3550
Eaves D (2004) Handbook of polymer foams. Choice Rev Online 42:42-0962-42–0962
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
de Souza, F.M., Palve, A.M., Gupta, R.K. (2022). Materials and Chemistry of Phenolic Foams. In: P.K, S., M.S., S., Thomas, S. (eds) Phenolic Based Foams. Gels Horizons: From Science to Smart Materials. Springer, Singapore. https://doi.org/10.1007/978-981-16-5237-0_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-5237-0_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5236-3
Online ISBN: 978-981-16-5237-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)