Abstract
Two kinds of novel phosphorus-containing polyether toughening agents were synthesized and characterized by 1H nuclear magnetic resonance (1H NMR) and Fourier transform infrared spectra (FTIR). Afterwards, a series of phenolic foams with different loadings of phosphorus-containing toughening agents were prepared. The apparent density and scanning electron microscopy (SEM) results showed that the addition of 5 wt% toughening agents increased the expansion ratio and promoted the formation of uniform cells. The limiting oxygen index (LOI) values of modified phenolic foams decreased with the increase of modifier content, but it still remained at 40% even if the amount of modifier loadings was 10 wt%. UL-94 results showed all samples can pass V0 rating, indicating the modified foams still have great flame retardance. Microscale combustion calorimetry (MCC) results indicated that the peak heat release rate (PHRR) and total heat release (THR) of the modified foams were reduced by 42% and 35%, respectively, compared to the pure phenolic foams. Moreover, the thermal stability of samples was investigated by thermogravimetric analysis (TGA). The mechanical properties were evaluated and correlated with composition and structural features.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the disaster of high building fire, which caused by the flammability of polymer insulation materials, has attracted increasing attention. As is well known, polymeric materials, such as polyurethane foams [1] and polystyrene foams [2], are widely used in the building thermal insulation materials due to their low density and low thermal conductivity. However, the fatal flaws of these polymer materials are their high flammability [3]. Moreover, these foams generate melting droplets that can promote the flame propagation and toxic gases which can lower the survival of human beings during fire [4]. Therefore, phenolic foams (PF) have been spotlighted due to its low thermal conductivity, excellent fire resistant performance, low water absorption, and low generation of toxic gas during combustion [5–8]. However, the biggest weakness of phenolic foams is its brittleness and powdering, which severely restricts its applications [9]. It is therefore very important to study the toughening technology of phenolic foams.
To overcome the brittleness of phenolic foams, many methods have been developed to toughen phenolic foams in the past few decades. According to the earlier literatures [10], mainly include three categories: chemical modification, inert fillers, and fiber reinforcement. Among them, chemical modification has attracted extensive attention in recent years for their notable toughening effect. The main toughening mechanism of chemical modification is introducing flexible chains into the rigid backbone chains of phenolic resin to enhance its toughness. Normally, the incorporation of the toughening agents, including long chain dicarboxylic acid [11], polyurethane prepolymer [12] and polyethyleneglycol [13], always shows some improvements of toughness. However, the excellent fire retardant performance of phenolic foam itself will deteriorate after the addition of these toughening agents. Therefore, it’s very necessary to make the toughening agents have good flame retardant properties.
Phosphorus-containing compounds, as high effective halogen-free flame retardants, have received considerable attention [14–16]. These compounds mainly act on condensed phase flame retardant mechanism. Phosphorus-containing flame retardants are generally classified into two types: additive type and reactive type. The former displays many drawbacks such as leaching and poor compatibility with polymer materials, while the latter can overcome these shortcomings due to the stable chemical bonds that formed between reactive phosphate monomers and polymer matrix [17].
In this study, two kinds of novel phosphorus-containing toughening agents for phenolic foams were synthesized. Phosphorus-containing polyethyleneglycol 400 (PPEG400) was synthesized from phenyl dichlorophosphate (PDCP) and Polyethyleneglycol 400 (PEG400, \( \overline M \) n =400). Phosphorus-containing polyethyleneglycol 600 (PPEG600) was synthesized from phenyl dichlorophosphate (PDCP) and Polyethyleneglycol 600 (PEG600, \( \overline M \) n =600). Then a series of phenolic foams with different loadings of phosphorus-containing toughening agents were prepared. The structure of the phosphorus-containing toughening agents was characterized by 1H nuclear magnetic resonance (1H NMR) and Fourier transform infrared spectra (FTIR). The flame retardant and thermal properties of the samples were evaluated by limiting oxygen index (LOI), UL-94 vertical burning test, microscale combustion calorimetry (MCC), and thermogravimetric analysis (TGA). Moreover, the mechanical performance is evaluated and correlated with composition and structural features.
Experimental
Materials
Phenyl dichlorophosphate (PDCP) was provided by Huiyuan Chemical Industry Corporation (Zhengzhou, China) and freshly distilled before use. Triethylamine (TEA) was obtained from the Sinopharm Chemical Reagent Co. (Shanghai, China), and purified by distillation. Tetrahydrofuran (THF) using as solvent was refluxed with sodium, and then distilled before use. Polyethyleneglycol 400 (PEG400), polyethyleneglycol 600 (PEG600), phosphoric acid and p-toluenesulfonic acid (PTSA) were all of reagent grade and purchased from Sinopharm Chemical Reagent Co. N-pentane, used as blowing agent, was obtained by Tianjing Guangfu Chemical Research Institute (Tianjing, China). Resol-type phenolic resin (viscosity, 25 °C: 2500 ~ 3000 mPa·S, solid content: 75%) was supplied by Tengzhou Huahai Building Materials Group Co., Ltd (Shandong, China). Modified silicon oil (colorless transparent liquid) was obtained from commercial sources and used as received.
Synthesis
Synthesis of phosphorus-containing polyethyleneglycol (PPEG)
PDCP (21.1 g, 0.1 mol), was dissolved in 40 ml dry THF in a 100 ml conical flask fitted with a magnetic stirrer. Then, triethylamine (21.25 g, 0.21 mol) was added into the above blends and the system was cooled to 0 °C and stirred for 20 min. In the meantime, PEG600 (126 g, 0.21 mol) was dissolved in 100 ml dry THF in a 500 ml three-neck flask equipped with a nitrogen inlet, a dropping funnel, a calcium chloride dry tube and a mechanical stirrer. The mixture of PDCP, TEA and THF was added dropwise to the above reaction flask at 0 °C using an ice bath, and then kept at 0 °C for 4 h with constant agitation under nitrogen. Finally, the mixture was warmed to room temperature, stirred for another 10 h. The triethylamine hydrochloride salt by-product was removed by filtration. Then the solution was rotary evaporated to remove the solvent and the unreacted reactants under reduced pressure. A light yellow viscous liquid was obtained and was called PPEG600. The synthesis process of PPEG400 was similar with that of PPEG600. An instrumental analysis of PPEG600 and PPEG400 was carried out by Fourier transform infrared (FTIR) spectroscopy and 1H nuclear magnetic resonance (1H NMR).
-
PPEG600: FTIR (KBr, cm-1): 3415 (−OH), 3067 (aromatic = C-H), 2869 (−CH2-), 1591, 1489 and 1456 (aromatic C = C), 1293 (P = O), 1108 (C-O-C), 1035 (P-O-C), 949 (P-O-Ph); 1H NMR (400 MHz, CDCl3, ppm): 7.1-7.4 (5H, Ph-H), 4.27 (4H, -CH2-OH), 3.65 (76H, -O-CH2-O-), 3.04 (2H, -OH), 1.22 (4H, P-O-CH2).
-
PPEG400: FTIR (KBr, cm-1): 3415 (−OH), 3067 (aromatic = C-H), 2871 (−CH2-), 1591, 1490 and 1458 (aromatic C = C), 1293 (P = O), 1116 (C-O-C), 1035 (P-O-C), 949 (P-O-Ph); 1H NMR (400 MHz, CDCl3, ppm): 7.1-7.4 (5H, Ph-H), 4.29 (4H, -CH2-OH), 3.65 (76H, -O-CH2-O-), 3.3 (2H, -OH), 1.37 (4H, P-O-CH2). All of synthesis route was illustrated in Scheme 1.
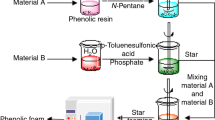
Preparation of the samples of phenolic foam
A certain percentage of resol-type phenolic resin, silicon oil (defoamer), and PPEG (toughening agent) were added into a 500 ml plastic beaker at room temperature, and then stirred with high-speed mechanical mixer for about 25 s. The mixture was then mixed with n-pentane (foaming agent) and curing agent (phenol sulfonic acid: phosphoric acid: water = 2:1:2), and then stirred at high speed for 30 s. The obtained viscous mixture was poured into a foaming mould quickly, and cured at 80 °C [18, 19] for 2 h. The sample was cut precisely and used for the fire and mechanical testing. The composition of toughness phenolic foams was listed in Table 1. Other samples were prepared in the same procedures.
Measurements and characterization
1H nuclear magnetic resonance (NMR) spectra
1H nuclear magnetic resonance (NMR) spectra was recorded on an AVANCE 400 Bruker spectrometer at room temperature with chloroform-d as the solvent.
Fourier transform infrared (FTIR) spectroscopy
Fourier transform infrared (FTIR) spectroscopy was obtained at 4 cm−1 resolution and averages of spectra were obtained from at least 16 scans in the standard wavenumber range of 400–4000 cm−1 by Nicolet 6700 spectrometer (Nicolet Instrument Company, USA) using KBr pellets.
Limiting oxygen index (LOI)
The limiting oxygen index (LOI) test was carried out with an HC-2 oxygen index meter (LOI analysis instrument company, Jiangning county, China). The test was measured according to ASTM D2863. The samples used for the test were 100 × 10 × 10 mm 3.
UL-94 vertical burning test
The vertical burning test was conducted by a CZF-II horizontal and vertical burning tester (Jiang Ning Analysis Instrument Company, China). The specimens which used were 130 × 12.7 × 3 mm3 according to UL94 test ASTM D3801 standard.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA) was carried out using a Q5000IR (TA Instruments) thermo-analyzer instrument at a linear heating rate of 20 °C/min under an air flow. The weight of all samples was kept within 3–10 mg in an open Pt pan and heated from room temperature to 700 °C.
Microscale combustion calorimeter (MCC)
The combustion properties such as the heat release rate (HRR) and the total heat release (THR) were evaluated using a microscale combustion calorimeter (GOVMARK MCC-2). Samples weighing 4 ± 1 mg were heated to 650 °C at a heating rate of 1 °C/s in a stream of nitrogen flowing at 80 ml/min. The combustor temperature was set at 900 °C and oxygen/nitrogen flow rate was set at 20/80 ml/ml. The reported data are averages of three tests and the experimental error was ±5%.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM; KYKY1010B, Shanghai Electron Optical Technology Institute, China) was used to study the cellular structure of phenolic foams, which was obtained by thin sectioning. The slices were adhibitted on the copper plate and then coated with gold/palladium alloy.
Compressive and bending tests
The compressive and bending properties were tested with a WSM-20 KB universal testing machine (Changchun, China) according to GB/T 8813–2008 and GB/T8812.1-2007, respectively. At least three samples were tested to obtain average values. The density was determined using the dimensions and the weight of the foam.
Results and discussion
Synthesis and characterization of phosphorus-containing toughening agents (PPEG400 and PPEG600)
PPEG400 and PPEG600 were synthesized by the esterification of PDCP with TEA, followed by the reaction with PEG400 and PEG600 respectively, as shown in Scheme 1. These two compounds were characterized by 1H NMR and FTIR. From the 1H NMR spectra of PPEG600, the chemical shift at 1.22 ppm is attributed to P-O-CH3, and the hydroxy proton appears at 3.04 ppm. The methylene protons in the macromolecular chains of PEG appear at 3.65 ppm, and the methylol protons appear at 4.27 ppm. Aromatic signals appear at 7.3-7.1 ppm. The 1H NMR spectra of PPEG400 was similar as that of PPEG600.
From the FTIR spectra of PPEG600, the most significant absorption can be observed at 3415 cm-1 and 2869 cm-1 corresponding to O-H stretching vibration of PPEG600 and C-H stretching vibration of -CH2- groups, respectively. The absorption band at around 3067 cm-1 is attributed to stretching vibration of = C-H groups on aromatic ring. The absorptions at 1591, 1489 and 1456 cm-1 are attributed to the skeletal vibration of aromatic ring. The stretching vibration of P = O occurs at 1293 cm-1. Furthermore, the absorptions at 1108 cm-1, 1035 cm-1 and 949 cm-1 are assigned to the stretching vibration of C-O-C, P-O-C and P-O-Ph, respectively [20–22]. Likewise, the FTIR spectra of PPEG400 show similar absorption as that of PPEG600. The above results confirmed the PPEG structure as shown in Scheme 1.
Physical properties of PPEG-modified phenolic foams
Apparent density
The apparent density curves of phenolic foams modified by PPEG400 and PPEG600 at different PPEG content are shown in Fig. 1. It can be seen that adding 3 wt% and 5 wt% PPEG into phenolic foams can reduce the apparent density. Among them, the apparent density of phenolic foam with 5 wt% PPEG had reached its lowest point, indicating that adding 5 wt% PPEG into phenolic foam can increase the expansion ratio. This may be due to the PPEG itself, because it is also one kind of surfactants that can decrease the surface tension of phenolic resins. In the high-speed mixing process, the existence of PPEG improves the dispersion of foaming agent in phenolic resin, and is helpful to the formation of bubbles. Moreover, it can reduce the diffusion of gas, and make the bubble cell more stable. Consequently, the bubbles grow uniformity at foaming temperature, resulting in the decrease of apparent density of phenolic foams. Figure 1 also shows that the apparent density of phenolic foam begins to increase when the addition of PPEG content is higher than 5%. This may be explained by that the amount of PPEG acting as surfactant has reached its saturation point. Moreover, there are two hydroxy groups on both ends of PPEG molecular chains, the hydroxy group can dehydrate with hydroxymethyl of resol during the curing process. The PPEG molecular chains may act as halters and restrict the movement of resol resin during the foaming process. Therefore, excess addition of PPEG has no good to the increase of expansion ratio.
Microstructure of phenolic foams
The microstructure of the phenolic foams is observed by SEM. The cell geometry, cell size and the effect of the addition of PPEG600 on the microstructure of phenolic foams are examined. As shown in Fig. 2a and c, the foams consist of a majority of closed cells. The cells of the phenolic foams modified by PPEG600 are relatively spherical, and the distribution of cells is more uniform. However, the shape of pure foam cells is ellipsoid-like and not very uniform, meaning that the addition of PPEG600 is beneficial to the formation of uniform cells. From Fig. 2b and d, the average cell size is determined to be about 300 μm and the cell walls are very thin. Moreover, there are some fragments attached to cell walls, which are caused by the slicing procedure for SEM test.
Fire-resistant behavior
The fire-resistant behavior of PF and PF/PPEG systems is evaluated by UL-94 vertical burning test and limiting oxygen index (LOI), as show in Fig. 3. It is clear that all samples can pass the V0 rating, indicating the modified foams still remain great flame retardance. The LOI values of both PPEG400 and PPEG600 modified phenolic foams decrease gradually with increasing the PPEG loading. As is well known, phenolic foam has a very high LOI value due to a large number of benzene rings on the backbone chains of phenolic resin. It is difficult to burn because of its easily charring when exposed to the flame. However, after modification, the incorporation of PPEG reduces the LOI value of phenolic foam owing to the flammability of the polyether molecular chains. The more the addition amount of PPEG, the lower the LOI value of PF/PPEG system. In addition, the LOI values of PF/PPEG600 are always higher than those of PF/PPEG400 at the same loading.
Flammability of the foams evaluated by MCC
To study the combustion behaviors of modified phenolic foams, PF systems modified with PPEG600 were chosen for MCC test. The heat release rate (HRR) curves of the samples are shown in Fig. 4. Associated data for the PF/PPEG600 systems are listed in Table 2. All HRR curves of samples show very low heat release rate values (< 55 W/g), indicating that phenolic foam release very small quantity of heat during combustion. The HRR curve of neat PF shows a dull peak around 535 °C. Associated data for PF are peak heat release rate (PHRR) = 53 W/g, total heat release (THR) = 13.3 kJ/g. In the case of PF/PPEG600 systems, the HRR curves show two dull peaks at around 300 °C and 530 °C owing to the heat releasing of polyether chain and phenolic chain, respectively. With the increase of PPEG600 content, the PHRR around 300 °C increases gradually, while the PHRR around 530 °C decreases obviously. This can be explained by the increase of phosphorus content and its heat release restraining capability. Moreover, from the MCC data, the PHRR and THR of the PF modified with 10 wt% PPEG600 reach the lowest values. Compare with the neat PF, the PHRR and THR are reduced by 42% and 35%, respectively.
Thermal stability
Figure 5 shows the (a) TGA and (b) DTG curves of pure PF and phenolic foams with various contents of PPEG600 at the linear heating rate of 20 °C/min under air atmosphere, and the corresponding values are listed in Table 3. It is clearly seen that the pure PF begins to decompose around 356 °C and its thermal degradation is only composed of only one main step with the maximum weight loss temperatures (Tmax) of 532 °C. Compared with pure PF, the modified phenolic foams are composed of two main steps. The first stage is in the temperature range of 250–350 °C, and the maximum weight loss temperatures (Tmax) of this step is around 320 °C. This could be caused by the degradation of PPEG. The second stage is similar as the main decomposition step of pure PF. From Table 3, it can be also seen that as the PPEG600 content increases, the residue left at 700 °C increases significantly. Moreover, the char yield at 700 °C for modified PF with 10 wt% PPEG600 content is 13.8% which is much higher than that of pure PF. This is mainly owning to the existence of phosphorus-containing polyether, which acts as char-forming catalyst during the decomposition process. Therefore, the incorporation of PPEG600 can improve the degradation stability of the PF/PPEG600 systems at higher temperature and promote the formation of char residue.
To further study the difference of thermal stability between PF/PPEG400 and PF/PPEG600, their TG and DTG curves at 5 wt% loading are compared, as shown in Fig. 6 and Table 4. As can be seen, the char yield at 700 °C for PF/PPEG400 is 17.15%, which is higher than 11.27% of PF/PPEG600 and 8.9% of PF. Because of the chain length of PPEG400 is shorter than that of PPEG600, so the phosphorus content of the former is higher than that of the latter at the same weight. So the char residue left at 700 °C for PF/PPEG400 is relatively higher.
Mechanical properties
Compression performance
Figure 7 plots compressive strength of phenolic foams as a function of the content of PPEG400 and PPEG600. For the PF/PPEG600 systems, the compressive strength increases at first and then ceases to increase as the content of PPEG600 further increases. The compressive strength of PF/PPEG400 increases gradually as the content of PPEG400 increases. Both of the two kinds of foams show higher compressive strength compare to the pure phenolic foam, indicating that the incorporation of suitable PPEG600 and PPEG400 can improve the toughness of the PPEG/PF systems.
It is well known that the strength of the phenolic foams depends on the foam structure, such as the size of cells, the homogeneity of cells, the toughness of cell wall and the close-cell ratio. It can be seen that the sample containing 5 wt% PPEG600 has the highest compressive strength value which increases significantly by 30.4% compared to that of the pure PF. However, the strength no longer increases when the PPEG600 content increases to 10 wt%, indicating that there is an optimum addition quantity of modifier to improve the compressive strength of phenolic foam.
Bending performance
Table 5 lists the bending results of the pure PF and PPEG modified PF, including flexural strength, bending elastic modulus and bending defection. Their relationship can be described as the following Eqs. 1 and 2.
Where R is flexural strength (kPa), F R is maximum load (kN), L is span between the two support (mm), b is width of the sample (mm), d is thickness of the sample.
Where E is bending elastic modulus (kPa), L is span between the two support (mm), b is width of the sample (mm), d is thickness of the sample, F t is load corresponding to the deformation.
It is seen that the flexural strength of most of PF systems modified with PPEG400 and PPEG600 decreases compare to the pure PF, and the bending elastic modulus also decreases slightly after modification. However, the bending defection increases significantly after modification, especially the sample with 5 wt% PPEG600. PF modified by 5 wt% PPEG600 has the maximal bending defection value 5.078 mm, increased by 47.7% compare to the pure PF, indicating that the incorporation of PPEG600 and PPEG400 improves the flexibility of phenolic foam.
Conclusion
In this work, we successfully synthesized two kinds of phosphorus-containing toughening agents, PPEG400 and PPEG600, to modify the phenolic foams. The flammability and mechanical properties of phenolic foams modified by PPEG400 and PPEG600 were studied.
Though the incorporation of PPEG reduced the LOI value of phenolic foam, it still remained at 40% when the loading of toughening agents was 10 wt%. UL-94 results showed all samples can pass V0 rating, indicating the modified foams still have great flame retardance. From the MCC results, the peak heat release rate (PHRR) and total heat release (THR) of sample modified with 10 wt% PPEG600 content were decreased by 42% and 35%, respectively, compared to the pure PF. TGA results showed that the PF composites possessed lower initial decomposition temperature but higher char residues than pure PF, mainly owing to the degradation of PPEG in advance and the catalysis of the carbonization of phenolic foam. Moreover, the addition of 5 wt% PPEG600 increased the expansion ratio and promoted the formation of uniform cells. Besides, the compression and bending results showed the incorporation of 5 wt% PPEG600 and PPEG400 can improve the toughness of the PPEG/PF systems.
References
Sarier N, Onder E (2008) Thermochim Acta 475:15–21
Ozkan DB, Onan C (2011) Appl Energ 88:1331–1342
Ji GQ, Wang JP (2010) Proceedings of the 7th International Conference on Tall Buildings 331–340.
Stec AA, Hull TR (2011) Energ Buildings 43:498–506
Guo QG, Lei SW, Zhang DQ, Shi JL, Liu L, Wei XH (2010) J Appl Polym Sci 117:3545–3550
Shen HB, Lavoie AJ, Nutt SR (2003) Compos Part a-Appl S 34:941–948
Shen HB, Nutt S (2003) Compos Part a-Appl S 34:899–906
Lei SW, Guo QG, Zhang DQ, Shi JL, Liu L, Wei XH (2010) J Appl Polym Sci 117:3545–3550
Rangari VK, Hassan TA, Zhou YX, Mahfuz H, Jeelani S, Prorok BC (2007) J Appl Polym Sci 103:308–314
Auad ML, Zhao LH, Shen HB, Nutt SR, Sorathia U (2007) J Appl Polym Sci 104:1399–1407
Choi MH, Byun HY, Chung IJ (2002) Polymer 43:4437–4444
Li T, Zhang L, Jiang HM, Chen JZ, Li XF (2010) Thirteenth national conference on packaging engineering. Tncpe 13:137–141
Wang FY, Ma CCM, Wu WJ (2001) J Appl Polym Sci 80:188–196
Liu YY, Wang SJ, Xiao M, Meng YZ (2007) Res J Chem Environ 11:84–103
Wang LC, Jiang JQ, Jiang PK, Yu JH (2010) J Polym Res 17:891–902
Wu K, Song L, Wang ZZ, Hu Y (2009) J Polym Res 16:283–294
Wang X, Hu Y, Song L, Xing WY, Lu HD, Lv P, Jie GX (2010) Polymer 51:2435–2445
Nutt SR, Shen HB (2005) U.S.Patent 6,841,584.
Orpin M (2010) U.S. Patent 7,718,751.
Braun U, Balabanovich AI, Schartel B (2006) Polymer 47:8495–508
Wu K, Hu Y, Song L, Lu HD, Wang ZZ (2009) Ind Eng Chem Res 48:3150–7
Wu K, Song L, Hu Y, Lu HD, Kandola BK, Kandare E (2009) Prog Org Coat 65:490–7
Acknowledgment
The work was financially supported by National Basic Research Program of China (973 Program) (2012CB719701), the joint fund of National Natrual Science Foundation of China (NSFC) and Civil Aviation Administration of China (CAAC) (No. 61079015) and the Program for Specialized Research Fund for the Doctoral Program of Higher Education (201003402110006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Wang, X., Yuan, H. et al. Fire performance and mechanical properties of phenolic foams modified by phosphorus-containing polyethers. J Polym Res 19, 9831 (2012). https://doi.org/10.1007/s10965-012-9831-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9831-7