Abstract
Wood was rapidly liquefied at the supercritical temperature of phenol. Under these conditions, wood was liquefied by over 90% for 0.5 min, and the combined phenol content of the obtained liquefied wood reached about 75%. The effects of various reaction conditions on liquefaction were investigated. With increases in reaction temperature, phenol/wood weight ratio, and the charged mass-to-reactor capacity (w/v) ratio, the amount of methanol-insoluble residue decreased and combined phenol content increased. The range of molecular weights and polydispersity of the products obtained after the time at which sufficient liquefaction was achieved were from 400 to 600 and from 1.5 to 2.5, respectively. Wood showed a marked decomposition to low molecular weight components early in the reaction, and then the molecular weight increased slightly with increasing reaction time. The properties of liquefied wood were investigated and compared with those obtained with conventional liquefaction methods. Combined phenol content was similar to that obtained by other liquefaction methods, except the sulfuric acid–catalyzed method, which resulted in flow properties comparable to those of other liquefaction methods. The flexural strength of moldings prepared using liquefied wood was also comparable to those prepared by other liquefaction methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A number of studies have been performed on supercritical fluid technology in processes involving the decomposition of polymeric materials and hazardous waste materials (Lee and Peart 1992; Martino and Savage 1997; Takahashi et al. 1999; Yalpani 1993; Yuan and Olesik 1997). This technology has also been applied to biomass conversion to useful chemical components. A prominent example is cellulose (a major component of biomass) conversion to glucose, from which most petroleum products can be produced by hydrolytic treatment with supercritical water (Adschiri et al. 1993; Arai 1995; Kabyemela et al. 1997; Sasaki et al. 1997). In the supercritical state, the physical and chemical properties of water can be controlled and manipulated with slight changes in temperature or pressure. The reactions occur extremely rapidly in supercritical water, and high yields of hydrolysis products can be obtained.
Wood liquefaction has been conducted by two representative methods: acid-catalyzed liquefaction at moderate temperature (120–180°C) and noncatalyzed liquefaction at elevated temperature (250°C) (Alma et al. 1995a, 1998; Lee et al. 2000b; Lin et al. 1994; Pu and Shiraishi 1993a, 1993b, 1993c). The application of liquefied wood to Novolak and resol-type phenolic resin (Alma et al. 1995b, 1996; Lee et al. 2000a, 2002a, 2002b; Ono et al. 1996) and the liquefaction mechanism of the representative components of wood (such as cellulose and lignin) by using their model compounds (Lin et al. 1997a, 1997b, 1997c; Yamada et al. 1996) has also been studied. However, in these methods, a considerably long reaction time of 30–180 min is required to accomplish sufficient liquefaction of wood.
Therefore, in this study we attempted to liquefy wood at the subcritical and supercritical temperature range of phenol to reduce the time required. To our knowledge, there have been no previous reports of wood liquefaction at these extreme temperatures of phenol. Phenol is in a supercritical state if its temperature and pressure exceed 421°C and 60.5 atm, respectively. This temperature is higher and the pressure is lower than the critical point of supercritical water. Various properties of the liquefied wood thus obtained were compared with those of liquefied wood obtained in conventional liquefaction methods.
Experimental materials
Wood meal (birch, Betula maximomawiczii Regel, 20 to 80 mesh) was dried in an oven at 105°C for 24 h and then kept in a desiccator at room temperature before use. Wood flour of 200-mesh pass size (Hitachi Chemical, Japan) was used as filler for molding. Methanol, used for the measurement of the amount of combined phenol, was of HPLC grade, and tetrahydrofuran (THF), used as an eluting solvent for gel permeation chromatography (GPC), was of extra-pure grade containing 0.03% stabilizer (2,6-Di-t-butyl-4-methyl-phenol). All other chemicals used were reagent grade and were obtained from commercial sources.
Methods
Liquefaction reaction procedure
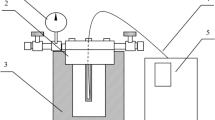
The reaction equipment was a special small pressure-proof autoclave (200 kgf/cm2 guarantee) and stirrer heated using a molten salt bath. The autoclave used in this study was not equipped to measure the internal temperature of the reactor. Thus, although the reactor heat-up time is a very important parameter in dealing with short reaction times, the reaction time in this study indicates the time including reactor heat-up time. After charging 5–7 g of wood and phenol into the 10-ml autoclave, the autoclave was dipped into a molten salt bath (250–450°C) and allowed to stand for 0.3–10 min. After the reaction time, the autoclave was immediately withdrawn from the molten salt bath and cooled in a large volume of iced water. All operations were performed by automatic control. The resulting reaction mixture was poured into methanol. The diluted solution was filtered through a glass fiber filter under reduced pressure. The methanol-insoluble residue was dried to a constant weight at 105°C for 24 h and weighed for the determination of the percent residue. The filtered methanol-soluble part was analyzed by high-performance liquid chromatography (Shimadzu LC-10A) equipped with an UV absorbance detector (SPD-10A) to quantify the unreacted phenol. An ODS-II reverse phase column (Shimadzu) was used, and the mobile phase was methanol/water (1/2 v/v) by using a flow rate of 1.0 ml/min. The methanol-insoluble residue and the combined phenol was calculated using the following two equations: R(%)=(W r/W 0)×100, and CP(%)=(W 1−W 2)/(W 0−W r)×100. Here R is the residue percentage; W 0, the weight of the starting wood; W r, the weight of the wood residue; CP, the amount of the combined phenol; W 1, the starting weight of the phenol; and W 2, the weight of unreacted phenol. The quantity of phenol reacted with unliquefied residue was disregarded without measuring it in this study.
Molecular weight distribution
The molecular weight of the liquefied wood was determined using a gel permeation chromatograph (Nippon Bunko GPC-900) equipped with a differential refractometer R401 detector. THF was used as the mobile phase at a flow rate of 1.0 mL/min with 7.0 MPa pressure.
Thermal flow properties
A flow tester (Shimadzu CFT-500A) was used to measure the thermal flow properties of the phenolated wood. The starting temperature was 50°C, and the heating rate was 10°C/min. Melt viscosity was determined at 120–160°C under a pressure of 5.0 MPa.
Preparation and flexural properties of moldings prepared using liquefied wood
Phenolated wood was mixed mechanically with wood flour filler, hexamethylenetetramine (HMTA, curing agent), calcium hydroxide (accelerating agent), and zinc stearate (lubricating agent). The obtained mixture was molded at 180°C under 50 MPa pressure for 5 min. The flexural strength of the molded specimens was measured using an Autograph AGS-5kNG (Shimadzu) and calculated by the Shikibu program.
Results and discussion
Effects of reaction conditions
The effects of reaction temperature on liquefaction were examined over the range 250–450°C for 2 min of reaction time. The results are shown in Fig. 1. With increasing temperature up to 421°C, the amount of methanol-insoluble residue decreased almost linearly to 5%. However, there were no marked changes in the liquefaction result in the supercritical region, i.e., from 421°C to 450°C. For 2 min of reaction time, it is considered that the critical liquefaction reaction was conducted in the range 350°–421°C.
Previously, it was reported that sufficient liquefaction (below 20% of residue) takes at least 30 min in noncatalyzed conditions at an elevated temperature (250°C) and acid-catalyzed liquefaction at moderate temperature (Alma et al. 1998; Lee et al. 2000a; Lin et al. 1994; Pu and Shiraishi 1993a). In comparison with these earlier results, liquefaction was possible at the supercritical temperature in a very short time. This led to the current study of wood liquefaction at the supercritical temperature of phenol. Combined phenol contents at temperatures below 350°C were higher than those close to the critical point. Above 350°C, the combined phenol content was between 60 and 80%.
To examine the effects of reaction time, the liquefaction temperature was set at 421°C, and the phenol/wood weight ratio was either 2 or 3. Liquefaction was conducted for 0.3–10 min, and the results obtained are shown in Fig. 2. The amount of methanol-insoluble residue was markedly decreased to approximately 10% for 0.5 min of reaction time. Minimum methanol-insoluble residues were 9% at 1 min and 5% at 2 min at phenol/wood weight ratios of 2 and 3, respectively. After this point, the methanol-insoluble residue content increased slightly with increasing reaction time.
The combined phenol content increased slightly with increasing reaction time. This increase was probably due to the phenolation of decomposed wood components. At 10 min, the combined phenol content reached 72% and 51% at phenol/wood weight ratios of 3 and 2, respectively. These values were comparable to those obtained by conventional liquefaction methods referenced above.
Figure 3 shows the results of experiments at 450°C, above the supercritical temperature of phenol. The minimum methanol-insoluble residue content was observed at an earlier reaction time, within 30 seconds or less, by increasing reaction temperature by 29°C. The same tendency was observed with the results obtained at 421°C, and the combined phenol content increasing slightly with increasing reaction time.
To examine the effects of the phenol/wood weight ratio, liquefaction was conducted with different weight ratios from 1 to 5 at 421°C for 2 min. The results obtained are shown in Fig. 4. As the weight ratio increased to 3, the methanol-insoluble residue content abruptly decreased to about 5%, and further decreased to 3% with an increase in the weight ratio to 5. Combined phenol content increased to 75% at a weight ratio of 3, and then leveled off, indicating that further increases in the combined phenol content were difficult at supercritical temperature. On the other hand, it has been found that the combined phenol content was increased to above 200% by increasing the weight ratio to 5 in the sulfuric acid–catalyzed method (Lin et al. 1994).
The changes in pressure in the supercritical state are very important, as are changes in the temperature. The pressure of the experiments was not recorded, so the charged mass (wood and phenol)-to-reactor capacity (w/v) ratio was used to examine the effects of the change of pressure on liquefaction indirectly. Figure 5 shows the effects of the charged mass-to-reactor capacity ratio on the amount of methanol-insoluble residue and combined phenol content. By increasing the charged mass-to-reactor capacity ratio from 5 to 7, the amount of methanol-insoluble residue was decreased to 5%, and the combined phenol content was increased to 66%. However, at a charged mass-to-reactor capacity ratio above 7, there were no significant changes in either amount of methanol-insoluble residue or combined phenol content.
Molecular weight and polydispersity
The molecular weight and polydispersity of liquefied wood are the fundamental parameters that can markedly influence the chemical and physical properties of the liquefied wood–based resin. Figure 6 shows the effects of reaction time on the molecular weight (Mw) and polydispersity (Mw/Mn) of liquefied wood obtained at 421°C at phenol/wood weight ratios of 2 and 3. Molecular weight and polydispersity were markedly decreased in the initial stage (within 1 min) of the reaction, after which small increases occurred with increasing reaction time at both weight ratios. These results indicated that wood components rapidly undergo extensive phenolysis decomposition and are phenolated by the reaction of phenol and decomposed wood components. Although the recondensation of the decomposed wood components themselves is also possible, this was considered to be only a minor reaction. The range of molecular weights and polydispersity obtained after the time at which sufficient liquefaction was achieved were from 400 to 600 and from 1.5 to 2.5, respectively. These values were lower than those obtained using conventional liquefaction methods. In the case of sulfuric acid–catalyzed liquefaction, the tendency toward increasing molecular weight is intensified with longer reaction time (Lin et al. 1997d). This can be attributed to recondensation of the decomposed wood components, resulting in the high molecular weight of liquefied wood. In the noncatalyzed liquefaction method at supercritical temperature, however, phenolysis decomposition of wood seems to be the preferred reaction rather than recondensation because of the extreme temperature and short duration.
Properties
Generally, the properties of liquefied wood are dependent on the liquefaction conditions such as reaction temperature, time, and catalyst species used (Alma et al. 1995a, 1996; Lin et al. 1994). The thermal flow properties of liquefied wood and flexural strengths of liquefied wood-based moldings were investigated and compared with those obtained by conventional liquefaction methods. The results are summarized in Table 1. The combined phenol content was found to affect the flow properties of liquefied wood. The flow temperature and melt viscosity increased with increases in the amount of combined phenol. The introduction of phenol groups by hydroxyl phenylation of the wood decomposition products would cause interactions among the molecular motions of liquefied wood. The combined phenol contents obtained by the procedure described here were similar to those obtained with other liquefaction methods, except the sulfuric acid–catalyzed method, resulting in flow properties and molding flexural strength comparable to those obtained with other liquefaction methods.
Conclusions
A new method has been developed to liquefy wood rapidly in the supercritical temperature of phenol. Under these conditions, wood was liquefied to over 90% in very short reaction times, and the combined phenol contents of the liquefied wood thus obtained were similar to those obtained by conventional liquefaction methods (except the sulfuric acid–catalyzed method). Reaction conditions such as phenol/wood weight ratio and the charged mass-to-reactor capacity (w/v) ratio affected liquefaction rates and products. Early in the reaction, wood was markedly decomposed to low molecular weight components, and then the molecular weight increased slightly with increasing reaction time. The flow properties of liquefied wood and flexural strength of moldings were comparable to those obtained by conventional liquefaction methods.
Further consideration of energy efficiency is required in that although this method uses high temperature and pressure, liquefaction is possible in a very short time. The development of continuous liquefaction equipment that can be used in supercritical phenol will be beneficial in the production of phenolated wood.
References
Adschiri T, Hirose S, Malaluan R, Arai K (1993) Noncatalytic conversion of cellulose in supercritical and subcritical water. J Chem Eng Jpn 26:676–680
Alma MH, Yoshioka M, Yao Y, Shiraishi N (1995a) Some characterizations of hydrochloric acid catalyzed phenolated wood-based materials. Mokuzai Gakkaishi 41:741–748
Alma MH, Yoshioka M, Yao Y, Shiraishi N (1995b) Preparation of oxalic acid-catalyzed resinified phenolated wood and its characterization. Mokuzai Gakkaishi 41:1122–1131
Alma MH, Yoshioka M, Yao Y, Shiraishi N (1996) The preparation and flow properties of HCL-catalyzed phenolated wood and its blend with commercial Novolak resin. Holzforschung 50:85–90
Alma MH, Yoshioka M, Yao Y, Shiraishi N (1998) Preparation of sulfuric acid-catalyzed phenolated wood resin. Wood Sci Technol 32:297–308
Arai K (1995) Biomass conversion in supercritical water for chemical recycle. Energy Resources 16:175–180 (in Japanese)
Kabyemela BM, Takigawa M, Adschiri T, Malaluan RM, Arai K (1997) Mechanism and kinetics of cellulose decomposition in sub- and supercritical water. In: Proc 4th international symposium on supercritical fluids, Sendai, Japan, 11–14 May 1997
Lee HH, Peart TE (1992) Supercritical carbon dioxide extraction of resin and fatty acids from sediments at pulp mill sites. J Chromatogr A 594:309–315
Lee SH, Yoshioka M, Shiraishi N (2000a) Preparation and properties of phenolated corn bran (CB)/phenol/formaldehyde co-condensed resin. J Appl Polym Sci 77:2901–2907
Lee SH, Yoshioka M, Shiraishi N (2000b) Liquefaction and product identification of corn bran (CB) in phenol. J Appl Polym Sci 78:311–318
Lee SH, Teramoto Y, Shiraishi N (2002a) Acid-catalyzed liquefaction of waste paper in the presence of phenol and its application to Novolak-type phenolic resin. J Appl Polym Sci 83:1473–1481
Lee SH, Teramoto Y, Shiraishi N (2002b) Resol-type phenolic resin from liquefied phenolated wood and its application to phenolic foam. J Appl Polym Sci 84:468–472
Lin L, Yoshioka M, Yao Y, Shiraishi N (1994) Liquefaction of wood in the presence of phenol using phosphoric acid as a catalyst and the flow properties of the liquefied wood. J Appl Polym Sci 52:1629–1636
Lin L, Yao Y, Yoshioka M, Shiraishi N (1997a) Liquefaction mechanism of lignin in the presence of phenol at elevated temperature without catalysts: studies on β–O-4 lignin model compound. 1. Structural characterization of the reaction products. Holzforschung 51:316–324
Lin L, Yao Y, Yoshioka M, Shiraishi N (1997b) Liquefaction mechanism of lignin in the presence of phenol at elevated temperature without catalysts: studies on β–O-4 lignin model compound. 2. Reaction pathway. Holzforschung 51:325–332
Lin L, Yao Y, Yoshioka M, Shiraishi N (1997c) Liquefaction mechanism of lignin in the presence of phenol at elevated temperature without catalysts: studies on β–O-4 lignin model compound. 3. Multi-condensation. Holzforschung 51:333–337
Lin L, Yao Y, Yoshioka M, Shiraishi N (1997d) Molecular weights and molecular weight distributions of liquefied wood obtained by acid-catalyzed phenolysis. J Appl Polym Sci 64:351–357
Martino CJ, Savage PE (1997) Supercritical water oxidation kinetics, products, and pathways for CH3- and CHO-substituted phenols. Ind Eng Chem Res 36:1391–1400
Ono H, Yamada T, Hatano Y, Motohashi KJ (1996) Adhesives from water paper by means of phenolation. J Adhes 59:135–145
Pu S, Shiraishi N (1993a) Liquefaction of wood without a catalyst. 1. Time course of wood liquefaction with phenols and effects of wood/phenol ratios. Mokuzai Gakkaishi 39:446–452
Pu S, Shiraishi N (1993b) Liquefaction of wood without a catalyst. 2. Weight loss by gasification during wood liquefaction, and effects of temperature and water. Mokuzai Gakkaishi 39:453–458
Pu S, Shiraishi N (1993c) Liquefaction of wood without a catalyst. 4. Effect of additives, such as acid, salt, and neutral organic solvent. Mokuzai Gakkaishi 40:824–829
Sasaki M, Kabyemela B, Adschiri T, Malaluan R (1997) Cellulose hydrolysis in supercritical water. In: Proc 4th international symposium on supercritical fluids, Sendai, Japan, 11–14 May 1997
Takahashi K, Sato Y, Kato K, Nishi S (1999) Decomposition of aromatic polyamide using supercritical water. Polym Prepr (Jpn) 48:3288–3289
Yalpani M (1993) Supercritical fluids: puissant media for the modification of polymers and biopolymers. Polymer 34:1102–1105
Yamada T, Ono H, Ohara S, Yamaguchi A (1996) Characterization of the products resulting from direct liquefaction of cellulose. 1. Identification of intermediates and the relevant mechanism in direct phenol liquefaction of cellulose in the presence of water. Mokuzai Gakkaishi 42:1098–1104
Yuan H, Olesik SV (1997) Supercritical fluid and enhanced-fluidity liquid extraction of phenolics from river sediment. J Chromatogr A 764:265–277
Acknowledgement
The authors wish to express their appreciation to Nobuo Shiraishi and Akira Kimura, emeritus professors of Kyoto University, Japan, for their critical reading of and comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.H., Ohkita, T. Rapid wood liquefaction by supercritical phenol. Wood Sci Technol 37, 29–38 (2003). https://doi.org/10.1007/s00226-003-0167-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-003-0167-7