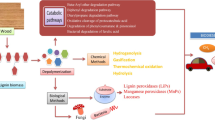
Abstract
Lignin, an aromatic polymer present in lignocellulosic biomasses, is conventionally viewed as a waste by-product of the pulp, paper, and other industries that use plant biomass as feedstocks. More recently, lignin has been reported as a renewable feedstock whose valorization generates several renewable aromatic intermediates, which can be used as carbon sources to synthesize other value-added chemicals, polymers, fuels, and other oxidized products. In this endeavor, the lignin’s recalcitrance, complex structure, and heterogeneity are major impediments that not only make microbial depolymerization exceptionally challenging but also generate phenolic compounds which inhibit microbial fermentation. Furthermore, during lignin’s breakdown, a heterogeneous complex mixture of low-molecular-weight aromatic monomers is released, representing additional hurdles when striving to recover the desired compound selectively. Nevertheless, in nature, some microorganisms are competent to funnel the heterogeneous stream of central aromatic intermediates into a single, pure compound. Centered on lignin, this chapter starts with a generalized description of the structure and types of commercially available lignin (e.g., lignosulphonates or sulfonated lignin, kraft lignin, soda lignin, organosolv lignin, and biorefinery lignin). Next, the slate of aromatic intermediatory compounds formed down the lignin-degrading β-ketoadipate pathway is briefly presented. Finally, this chapter summarizes few case studies related to the production of a high value-added chemical, vanillin, and a biopolymer, polyhydroxyalkanoates (PHAs), using lignin or its derivatives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Lignocellulosic biomass (LCB) from agricultural and forest wastes is an abundant renewable feedstock, well-suited for biofuel, biopolymers, and other biomaterials production. LCB is largely composed of cellulose, hemicellulose, and lignin. Normally, cellulose and hemicellulose cover approximately two-thirds of LCB. The remaining material is lignin and its derivatives. Chemical or physiochemical pre-treatment makes the cellulose and hemicellulose fraction readily available for enzymatic saccharification and subsequent production of liquid and gaseous biofuels (e.g., alcohols, hydrogen). Lignin and its derivatives are released as by-products (referred to as “crude lignin”). Annually, the total amount of lignin generated commercially as a bioproduct in biorefineries and other industries such as paper, pulp, and the ethanol industry is estimated to be 108 tons (Bajwa et al. 2019; Xu et al. 2019). Much of this lignin is consumed on-site, being formed into pellets for steam and electricity generation. However, when burned as a fuel, crude lignin does not reach its full commercial potential and releases greenhouse gases into the environment. Hence, alternative usage of lignin and its derivatives to synthesize biobased products is desirable and is associated with the lowest ecological impacts. Lignin’s valorization for new product development such as aromatic macromolecules, biopolymers, biofuels, and bio-oils is essential to the aging pulp and paper industries, which must expand their commodities portfolio to retain their vitality.
Today, the isolation of lignin from the lignocellulosic biomasses is no longer a barrier, as specific industrial pulping processes—mainly the sulfite, soda, kraft, and organosolv processes—are well established as a technology (see Sect. 6.2 for further details). However, further depolymerization and fragmentation of lignin into its constituent aromatic building blocks or its derivatives are still a challenge. Lignin is highly heterogeneous and non-uniform in its composition, and its thermochemical valorization leads to the synthesis of multiple product species, necessitating rigorous separation and purification measures to obtain a single target product (Xu et al. 2019). For controlled degradation of lignin, microbial-based methods are becoming increasingly attractive because microbes possess (a) the ability to make and break bonds selectively and (b) diverse metabolic pathways, which can be used to channel lignin’s assorted aromatic building blocks, derived intermediates, and other fermentation residuals into a particular target compound. Nevertheless, lignin’s depolymerization and subsequent fragmentation release certain phenolics into the media that often inhibit the fermentation and product formation. Furthermore, many of the targeted chemicals are toxic to microbial metabolism. Hence, the use of cell-free systems, such as enzyme extracts or microbial strains having tolerance to the phenolics or aromatic compounds, is being investigated by scientists for attaining the desired conversion rate and the associated molar yields. Efforts to discover alternative microbial pathways that can lead to easy bioconversions while avoiding the generation of side products are also an area of active research.
Today, the production and separation of high added-value compounds from lignin due to their chemistry and properties is an evolving domain of valuable research to both scientific and industrial communities. For sustainable industrial biorefineries, it is important that all the chief components of the lignocellulose, including lignin, be transformed to higher value compounds. This paradigm shift is essential for biorefineries and forestry-based industries to stay competitive. In this chapter, a comprehensive summary of the biological solutions to unlock the potential of lignin for the production of a wide range of bio compounds is presented.
6.2 Structure and Types of Lignin
Lignin is a high-molecular-weight macromolecular found in the cell wall of woods and plants. It is the third largest naturally occurring polymer on the earth after cellulose and chitin (Abdelaziz et al. 2019). Lignin is extremely branched in its structure, composed of three primary phenylpropanoid alcohols (monolignol phenolic units): coniferyl alcohol (G), sinapyl alcohol (S), and p-coumaryl alcohol (H), that are modified with a variety of functional groups such as methoxy (O–CH3), carboxyl (R-COOH), hydroxyl (R-OH) and carbonyl (C=O) (Chatterjee and Saito 2015). There are some complex linkages between the three phenylpropanoid units that provide lignin a dense, hydrophobic structure resistant to depolymerization by enzymes (Abdelaziz et al. 2016; Chatterjee and Saito 2015; Horwath 2015). These include the carbon-carbon bond (C-C), ether bond (C-O-C), C-O bonds of α- and β-arylalkyl ethers, and bonds that OH groups can make with other polysaccharides (cellulose and hemicellulose) and proteins (extensins) in the plant’s cell wall. The β-O-4 linkage dominates, represent approximately 45–50% of the linkages, followed by 5–5 (18–25%), β-5 (9–12%), and the β-1 (7–10%) linkage (Strassberger et al. 2014). While these tight linkages necessitate harsh pretreatments to depolymerize lignin, they make lignin one the most sturdy macromolecule on the planet and are estimated to hold about 95 billion tons of carbon (Chatterjee and Saito 2015). Due to its high carbon content, lignin is considered a significant replacement for fossil fuels. It has also been explored as a renewable bioresource for the synthesis of carbon-based chemicals, and has promising potential as a constituent in polymer blends and composites. However, the ultimate end usage of lignin depends on its properties, which vary according to source and type. Commercially available lignin includes lignosulphonates, kraft lignin, soda lignin, organosolv lignin, and biorefinery lignin.
6.2.1 Lignosulphates
In the industries which separate lignin from cellulose, different oxidative pulping methods are employed. The first and the most widely adopted separation methodology is sulfite pulping, where sulfur dioxide or an acidic bisulfite/sulfite solution is used to soften the plant material and remove lignin as lignosulphonates (Hintz 2001). Lignosulphates are sulfur-containing lignin that behaves as a polar ionic molecule, soluble in water but insoluble in organic solvents. Their single most extensive usage is in the concrete industry, where lignosulphonates are used as plasticizers and allow concrete to be made with 15% less water, which also creates more rigid concrete while retaining its capacity to flow (Gargulak et al. 2015; Vazquez and Pique 2016). Lignosulphonates have the power to decrease the viscosity of the solutions and provide hydration. They also find usage in the production of linoleum flooring and plaster (Vazquez and Pique 2016). Lignosulphonates possess good binding, dispersing, and emulsifying abilities that make them useful additives in tanning, leather, pesticides, fertilizers, wax emulsions, dyes, pigments, oil drilling mud, coal briquettes, and food industry (WebArchive 2003).
Chemical oxidation of sulfonated lignin at elevated temperature (up to 160 °C) and pressure (10 bar) has a value for the production of low-molecular-weight artificial flavoring agents such as vanillin and vanillic acid (4-hydroxy-3-methoxy benzoic acid) (Pacek et al. 2013; Richter et al. 1945). The global market potential of vanillic acid (in 2020) was USD 1189.9 million (Fox40 2020). Dimethyl sulphoxide (DMSO) is another high-utilization chemical derived from lignosulphonates (Macfarlane et al. 2014). Hence, lignosulphonates have immense market potential. However, the sulfite coking process by which they are produced is environmentally costly, and lignosulphonates contain sulfur and hemicellulose (Abdelaziz et al. 2016), making them less pure. It is their property of being soluble in water that distinguishes them from other lignin streams. Due to their anionic sulfate groups, lignosulphonates can scavenge metals and keep them dissolved in solutions. This property has an advantage in agriculture where lignosulphonates can keep metals available to plants (preventing them from precipitating out as insoluble compounds), and also in drinking water systems, where scaly metals deposition on the walls of the water systems can be avoided (WebArchive 2003). Moreover, with their ability to immobilize metals, the utilization of lignosulphonates for bioremediation of wastewater cannot be ruled out.
6.2.2 Kraft Lignin
Compared to sulfite pulping, the more modern pulping process is the Kraft process, where the plant material is treated with a mixture of water, sodium hydroxide (NaOH), and sodium sulfide (Na2S) at 140–180 °C to break the linkages binding lignin with cellulose and hemicellulose. The lignin extracted during this process is known as Kraft lignin which accounts for about 85% of the total lignin production in the world (Chen 2015). Approximately 630,000 tons of kraft lignin is produced annually, and most of its utilization is for combustion. Its high value utilization as an additive and binder for improvement of the properties of resins, foams, printing inks, fertilizers, and adhesives, only stands at 2% of the total kraft lignin produced (Macfarlane et al. 2014). Like lignosulphonate, kraft lignin has reactive sulfate groups attached to its phenyl rings. Also, it is associated with hemicellulose, making it impure. Nevertheless, there is an ongoing push to use kraft lignin for producing activated carbon and as a low-cost raw material for carbon fiber synthesis. It has an expected compound annual growth rate (CAGR) of 7% (John 2020).
6.2.3 Soda or Alkaline Lignin
Produced by one of the simplest pulping processes, soda or alkaline lignin is produced as a by-product in the pulp industry using sodium hydroxide (NaOH) or a mixture of NaOH and anthraquinone at 150–170 °C (Macfarlane et al. 2014). On average, soda pulping yields approximately 80% of lignin from the wood samples (USDA 2020). Performing a steam explosion before soda pulping can enhance the lignin extraction percentage to 90% (USDA 2020). While the lignin from this process is sulfur-free, it still contains hemicellulose. It has comparatively high aliphatic and phenolic content than lignosulphonates and kraft lignin (Abdelaziz et al. 2016). Hence, aromatic resins are the key chemicals expected to be derived from the use of soda lignin as the substrate. Also, because they are free from sulfur, soda lignin can be utilized as binders in animal feed (Macfarlane et al. 2014).
6.2.4 Organosolv Lignin
Organosolv is the most eco-friendly pulping process that uses organic solvents (e.g., ethanol, acetone, methanol, acetylene glycol, etc.) to efficiently depolymerize wood into lignin, hemicellulose, and cellulose fractions. Organosolv lignin is of high quality and purity, with <1 wt.% residual carbohydrate content (Strassberger et al. 2014). Organosolv lignin is free from sulfur and other impurities. Hence, it can directly be used to produce specialty value-added products via a more environmentally friendly method. Organosolv lignin has a highly homogenous nature, with its composition being much closer to native lignin (Tribot et al. 2019). It is very rich in phenolic content and is highly hydrophobic, which allows it to be spun into fibers directly, without blending with other polymers (Macfarlane et al. 2014). Also, because of its purity, organosolv lignin can be exploited for its antioxidant, antibiotic, and antitumor properties in the cosmetics, medicinal, and pharmaceutical sectors (Macfarlane et al. 2014). To date, organosolv lignin is the most attractive lignin in terms of its quality. However, the commercial realization of the organosolv processes is presently marginal, probably as a result of high process costs, and is not readily available at volume (Abdelaziz et al. 2016; Thoresen et al. 2020).
6.2.5 Biorefinery Lignin
The lignin produced as a by-product in biorefineries using second-generation lignocellulosic feedstocks is another source of lignin, with an annual production of approximately 100 kilotons (Bajwa et al. 2019). The biorefinery lignin, produced by a hydrolytic pretreatment (acidic, thermal, or enzymatic) of underutilized lignocellulose is essentially sulfur-free. However, some hemicellulose is still linked with it through ester, glycosidic, ether, or carbon-carbon bonds (Hansen et al. 2013). These bonds are responsible for the hydrophilic surface properties of lignin, which limits its applications in some industries, including the range of polymer resins it can be effectively combined within biocomposite applications. Nevertheless, biorefinery lignin holds the potential to be precipitated from the rest of the biomass in the form of an insoluble, amorphous, solid residue that has a significant amount of protein attached to it and that can be used as animal feed (Hansen et al. 2013). In the future, removing carbohydrate impurities and polishing lignin by enzymes could yield low-molecular-weight lignin with higher purity and hydrophobicity suitable for various industrial applications.
Over the years, lignin separated from lignocellulosic biomass by different biomass conversion technologies has been given distinct names, as in cellulolytic enzyme lignin, produced by cellulolytic enzyme treatment of pretreated agricultural residue (Tian et al. 2017; Zhang et al. 2010); Bjorkman lignin, produced by treatment of lignocellulose with neutral organic solvents (Bjorkman 1954; Obst and Kirk 1988); Klason lignin, produced by treatment of lignocellulose with sulphuric acid followed by removal of ash (Chen 2015; Obst and Kirk 1988), etc. (Retsina et al. 2013).
From this section, it is evident that varieties of industrial lignins exist in the market whose properties and structures depend on the pulping or the coking process (Table 6.1). These lignins are directly suitable for a range of applications, from low-density combustion fuel to binder and blender additives to make high-strength and durable concrete, cardboard, and papers. Lately, the use of lignin in its purest form as an antioxidant has been recognized. Furthermore, in 2007, scientists from the Pacific Northwest National Laboratory (PNNL) released a report evaluating the opportunity for using lignin for the derivation of certain macromolecules, higher aromatic monomers, and oxidized compounds by breaking the lignin’s polymeric structure (Holladay et al. 2007). The report presented a case that lignin produced in biorefineries has a high economic opportunity to generate large revenues (USD 12–35 billion) by producing a variety of co-products (Holladay et al. 2007). This strategy would entail the choice of technology for selective fragmentation of lignin into low molecular weight monomers and oligomers, which can be further biocatalyzed by microorganisms, via cellular assimilation, into value-added compounds.
Frequently, the microorganisms that depolymerize lignin into its constituent monolignols proceed to polymerize these intermediates into other renewable chemicals using their versatile metabolic pathways. Section 6.3 touches on some of the high-value lignin monomers and oligomers synthesized by microbial/enzymatic-based lignin depolymerization without going into the technical details of these processes. Section 6.4 discusses some final value-added chemicals produced by microorganisms via cellular assimilation of such lignin intermediates via the β-ketoadipate pathway.
6.3 Mono-and Oligomers as Intermediates from Lignin Depolymerization
In nature, microorganisms existing in symbiotic association with plants have evolved mechanisms to degrade and utilize lignocellulosic biomass. These plant degrading microbes release a repertoire of extracellular enzymes collectively termed ligninolytic enzymes (comprising laccase, superoxide dismutase, oxidoreductases, and peroxidases) that oxidize phenolic units in lignin, but not the non-phenolic compounds (Datta et al. 2017; Govil et al. 2020a; Janusz et al. 2017). Some mediators such as p-coumaric acid, 2,2′-azino-di(3-ethylbenzthiazoline-6-sulfonic acid, vanillin, and syringaldehyde, can enhance the oxidative capacity of laccase itself to oxidize the non-phenolic units in lignin (Abdelaziz et al. 2016). In addition, some low-molecular-weight secondary metabolites produced by microbes during lignin degradation, such as benzoic acid, veratryl alcohol (MnO2), oxalate, and 2-chloro-1,4-dimethoxybenzene, can aid the further breakdown of the phenolic and non-phenolic groups in lignin (Datta et al. 2017; Janusz et al. 2017; Shimada et al. 1981). These metabolites are slowly metabolized by the microorganisms and accumulate in the reaction solutions. Other metabolites such as flavonoids, tannins, and lignans are parts of plants themselves. They are also known to initiate bond scissions in lignin via an oxidative and reductive cascade of reactions (Janusz et al. 2017).
Over the years, many studies have reported the biodegradation of lignin by microorganisms and their enzymes, and significant progress has been made in understanding these processes (Abdelaziz et al. 2016; Lee et al. 2019). Literature is also available that details the pathways for lignin valorization. The pathways suggest that during the breakdown of lignin, various low-molecular-weight aromatic compounds can be formed depending on the composition of the lignin and the depolymerization method used. The most common of these monolignols are p-coumaric acid, caffeic acid, ferulic acid, guaiacol, syringic acid, syringaldehyde, phenol, benzoic acid, and vanillic acid (Abdelaziz et al. 2016). Tang et al. (2015) reported production of 8.04 mg, 0.88 mg, 0.63 mg, 0.34 mg, and 0.29 mg of hydroxybenzoic acid, syringaldehyde, vanillic acid, p-coumaric acid, and ferulic acid, respectively, from each gram of oil palm empty fruit bunch lignin. Chen et al. (1982) reported the generation of vanillic acid, veratric acid, and various benzoic acid derivatives during degradation of spruce wood lignin by white-rot fungus Phanerochaete chrysosporium (Chen et al. 1982). The production of phenolic oligomers containing about seven phenylpropane units from kraft lignin when subjected to degradation by the fungus Trametes versicolor has also been reported (Reid 1998). Guaiacol, benzoic acid, and vanillic acid were identified as significant intermediates when a member of the genus Acetoanaerobium degraded kraft lignin. In the same study, ferulic acid, syringic acid, and benzenepropanoic acid were also detected, which were considered the final products after intermediate stage degradation (Duan et al. 2016). Consistent with these observations, similar degradation products have also been found in other studies, some of which are summarized in Table 6.2.
Commercially, many of these degradation products of lignin are valued chemicals, and they can find industrial applications. For instance, syringaldehyde is an aromatic aldehyde with valued antioxidant, bioactive (antimicrobial), and antioncogenic activity and is used in cosmetics, pharmaceuticals, food, paper, and pulp industries. Moreover, syringaldehyde is also a promising laccase and peroxidase mediator that can enhance these enzymes’ activity by almost six-fold (Mohamad Ibrahim et al. 2012). p-coumaric acid possesses excellent anti-infection, anti-inflammatory, and antioxidant activities that can help it protect against conditions of oxidative stress (Shen et al. 2019). Ferulic acid also possesses antithrombic, antimicrobial, anticancer, antidiabetic, and immunostimulant properties, and finds applications in cosmetics, pharmaceuticals, food, and health industries (Kumar and Pruthi 2014). Guaiacol and its derivatives are valuable as additives in mucoactive, antiseptic, and anesthetic agents. Indeed, lignin and the phenolic compounds derived from it have a total market value of approximately USD 732 million, projected to reach USD 913 million by 2025 (Bajwa et al. 2019). However, despite the potential to synthesize such promising aromatics, vanillin stays at present the only marketable aromatic product of lignin produced using microbial sources.
6.4 Final Value-Added Chemicals Production from Lignin Intermediates
Ideally, the production of these lignin intermediates is linked to each other via the β-ketoadipate pathway. One intermediate gets converted to the other rapidly via a cascade of reactions. Hence, most of the time, lignin breakdown is a complex mixture of low-molecular-weight aromatic monomers. It is, in fact, difficult to synthesize a particular aromatic compound from lignin at sufficient concentration. This has a negative prospect for the valorization of lignin to generate a definable aromatic compound. However, Ohta et al. (2017) showed that highly specific aromatic monomers can be synthesized from lignin by controlling the reactions using selective enzymes. In their study, the authors achieved the exclusive synthesis of monomers with a phenylpropane moiety (e.g., guaiacylhydroxylpropanone (GHP), syringylhydroxylpropanone, SHP)) using four β-O-4-cleaving enzymes (two short-chain dehydrogenase/reductase, two glutathione S-transferases with β-etherase activity) isolated from a Novosphingobium strain in one pot (Ohta et al. 2017). Prim et al. (2003) showed that the production of 4-ethyl phenol, a phenolic compound responsible for aroma in wine, is possible from hydroxycinnamic acids (e.g., ferulic, p-coumaric, sinapyl acid, and caffeic acids) using phenolic acid decarboxylase from Bacillus sp. BP-7, with no accumulation of any side products (Prim et al. 2003). A similar conversion was achieved using a decarboxylase gene from Bacillus licheniformis (Hu et al. 2015). Furthermore, phenolic acid decarboxylase activity from Bacillus amyloliquefaciens has been demonstrated for the selective synthesis of p-hydroxystyrene from p-coumaric acid (Jung et al. 2013).
6.4.1 Specific Case of Vanillin Production
As mentioned earlier, because of lignin’s heterogeneity, many side products are formed along with the desired compound during lignin processing and intermediate production. To avoid this issue, studies have been conducted to examine microbial enzymes which can bioconvert a particular metabolite into other monomeric aromatic compounds. In this regard, processes that lead to the production of vanillic acid are one of the most intensively studied enzymes and enzymatic processes. Vanillic acid is a food preservative and a natural precursor for vanillin production - an aromatic compound responsible for the characteristic vanilla flavor, which has enormous consumer demand. Organic vanillin obtained from plants is high-priced (approximately USD 3000/kg) and has a net worth of more than USD1 billion annually (Li and Rosazza 2000). Vanillin synthesized chemically is comparatively cheaper (USD 11/kg) though it is not considered natural under US legislation (Ashengroph et al. 2011). Hence, cheaper production of natural vanillin using microbial transformations has increasingly been attempted and has immense economic potential, owing to the spur in demand of bio-vanillin as a flavoring base in foods, pharmaceuticals, and cosmetic industries (Luziatelli et al. 2019) (Fig. 6.1).
Production of vanillin from Ferulic acid and Isoeugenol. ehy Eugenol hydroxylase, calA Coniferyl alcohol dehydrogenase, calB Coniferyl aldehyde dehydrogenase, fcs Feruloyl-CoA synthetase, ech Enoyl-SCoA hydratase/lyase, vdh Vanillin dehydrogenase. (Source of data depicting compound annual growth rate of Vanillin: Persistence Market Research Report, released Match 2020 (PMR 2020))
For years, the most exploited feedstock for vanillic acid’s biological production is ferulic acid (a hydroxycinnamic acid found attached to hemicellulose via several ester linkages). Some of the microbial enzymes that have been studied for this purpose include enzymes belonging to the superfamily feruloyl enoyl-SCoA hydratase/lyase (EC 4.2.1.101) and feruloyl-CoA synthetase (EC 6.2.1.34)) expressed by Pseudomonas fluorescens (Gasson et al. 1998; Leonard et al. 2006), Pseudomonas sp. Strain HR199 (Overhage et al. 1999), Pseudomonas putida KT2440 (Plaggenborg et al. 2003), plant species Glechoma hederacea and Vanilla planifolia (Gallage et al. 2014; Negishi et al. 2009), Amycolatopsis sp. strain HR167 (Achterholt et al. 2000), Streptomyces sp. NL15-2K (Nishimura et al. 2018; Yang et al. 2013) and strain V1 (Hua et al. 2007b), Streptomyces setonii (Muheim and Lerch 1999), Delftia acidovorans (Plaggenborg et al. 2001), Rhodotorula rubra (Huang et al. 1993), Halomonas elongate (Abdelkafi et al. 2006), Bacillus subtilis (Gurujeyalakshmi and Mahadevan 1987), and Aspergillus niger CGMCC0774 and Pycnoporus cinnabarinus CGMCC1115 (Zheng et al. 2007). In the two-step conversion process, feruloyl-CoA synthetase (fcs) converts ferulic acid into feruloyl CoA and subsequently enoyl-SCoA hydratase/lyase (ech) mediates the conversion of feruloyl CoA to vanillin and acetyl CoA (Fig. 6.1). Table 6.3 summarizes the studies where the natural host strain or the recombinant strains expressed the enzymes that could transform ferulic acid to vanillin and yields up to 28.3 g/L were achieved. In the future, cloning an efficient ferulic acid esterase gene that can release ferulic acid from hemicellulose (Bugg et al. 2011) in such clones can create a path for producing vanillin directly from hemicellulose.
As such, ferulic acid is an excellent nontoxic precursor for vanillin synthesis. A high concentration of ferulic acid can be fed to the microorganisms without inhibiting microbial growth (Muheim and Lerch 1999). However, ferulic acid is costly, and this has prompted research where eugenol (a plant-derived phenylpropanoid) is being considered as a cheaper (USD 5/kg) and a more abundant vanillin precursor. Several microbial strains and their enzymes have been shown to aid in the biotransformation of eugenol into vanillin, including Rhodococcus rhodochrous (Chatterjee et al. 1999), Serratia marcescens DSM30126 (Rabenhorst and Hopp 1991), strains of the genus Bacillus (Hua et al. 2007a; Shimoni et al. 2000; Zhang et al. 2006), strains of the genus Pseudomonas (Kasana et al. 2007; Unno et al. 2007; Yamada et al. 2007), and Candida galli strain PGO6 (Ashengroph et al. 2011). Lately, the use of cell-free extracts rich in characteristic enzymes for biocatalysis of eugenol into vanillin has also been attempted. For example, vanillyl alcohol oxidase enzyme (vaoA) from Penicillium simplicissimum CBS 170.90 has successfully been used for the biocatalytic conversion of 1 g/L of eugenol into vanillin (0.24 g/L, the molar yield of 10%) and vanillic acid (1.1 g/L, the molar yield of 44%) (Ashengroph et al. 2011). vaoA (Vanillyl-alcohol oxidase) gene when cloned into a recombinant Escherichia coli, that had already been transformed with the genes encoding coniferyl alcohol dehydrogenase and coniferyl aldehyde dehydrogenase from Pseudomonas sp. strain HR199, produced 0.3 g of vanillin per liter of the fermentation medium from eugenol (Overhage et al. 2003).
Several attempts have been made to produce vanillin with a minimum of co-products from lignin as the starting substrate. For example, Reiter et al. (2013) recombinantly expressed three β-O-4-cleaving enzymes, a Cα-dehydrogenase, a β-etherase, and a glutathione lyase from the proteobacterium Sphingobium sp. SYK6 in E. coli BL21, and obtained the production of 58.2 mg/L of vanillin from softwood lignin with a small amount of ferulic acid as the co-product (Reiter et al. 2013). Exclusive production of vanillin from wheat straw lignocellulose was also reported in a study by Sainsbury et al. (2013), where a mutant strain of Rhodococcus jostii RHA1 deficient in gene vanillin dehydrogenase was found to accumulate up to 96 mg/L of vanillin, together with minor quantities of ferulic acid and 4-hydroxybenzaldehyde (Sainsbury et al. 2013). These studies show that the exclusive production of specific phenolic compounds from lignin is possible, provided the application of targeted pathway engineering can control the biocatalytic routes for lignin breakdown. However, the reported yield and concentration of products produced via this route are below 1 g/L and do not qualify as economically efficient biotransformation. In these studies, the reported yield of vanillin production of less than 1 g/L and associated efficiency of less than 10%, was due to the toxic effects of vanillin on the cellular systems at concentrations above 1 g/L, leading to its quick metabolization by the microorganism into vanillic acid and vanillyl alcohol using vanillin dehydrogenase (EC 1.2.1.67) (Ashengroph and Amini 2017). Hence, in microbial hosts, the respective alcohol or the respective acid typically gets accumulated rather than vanillin.
To enhance the bioconversion yield of vanillin from eugenol or ferulic acid, the use of static growth conditions is the first strategy adopted where resting cells have been shown to delay degradation of vanillin to vanillic acid. Here, with the resting cells of Psychrobacter sp. strain CSW4, a vanillin concentration of 1.28 g/L (molar yield of 13.8%) was achieved by Ashengroph et al. (2012). Secondly, researchers have also, from time to time, reported the isolation of a few strains from nature that are resistant to the toxic effects of vanillin, and hence can accumulate more vanillin in a reduced reaction time. Pseudomonas chlororaphis CDAE5 is one such strain that has demonstrated the potential to be a suitable candidate for biotechnological production of vanillin from isoeugenol. Another study has shown Pseudomonas chlororaphis CDAE5 to produce 1.2 g/L of vanillin, with a molar yield of 13% (Kasana et al. 2007). Much higher productivity of vanillin has been reported by Ashengroph and Amini (2017), where the yeast Trichosporon asahii transformed 5 g/L of isoeugenol into 2.4 g/L of vanillin with a 52.5% molar yield (Ashengroph and Amini 2017). Even cell-free extracts rich in a designated enzyme have been investigated for the process. Enzyme lipoxygenase is useful for this biocatalysis approach, where Liu et al. (2020) tested a method for the synthesis of vanillin from isoeugenol and eugenol using soybean lipoxygenase (lipoxidase). The reported production of vanillin in this study was 2.68 g/L. A European patent dating back to 1991 claimed a process for the preparation of vanillin (10–15 g/L) from eugenol or isoeugenol using lipoxidase (Markus et al. 1992), an enzyme from Glycine max (soybean) that is now commercially available from Sigma Aldrich (L6632 and L7395).
To augment these processes and improve productivity, the metabolic engineering of microbial strains already known to have the tolerance to vanillin toxicity has been documented in the literature. Specifically, Amycolatopsis sp. ATCC 39116, a gram-positive Actinobacteria, was engineered with the deletion of its vanillin dehydrogenase-encoding (vdh) gene that codes for vanillin catabolism enzyme, vanillin dehydrogenase. This mutation decreased the catabolism of vanillin to vanillic acid by 90%, and resulted in an increase of the total vanillin production to 2.2 g/L from ferulic acid, with a molar yield of 80.9% (Fleige et al. 2016). The same group achieved a vanillin concentration of 19.3 g/L (molar yield of 94.9%) by constitutively expressing two of the vanillin anabolism genes fcs (coding for feruloyl-coenzyme A (CoA) synthetase) and ech (enoyl-CoA hydratase/aldolase) in the same stain of Amycolatopsis sp. 39116 (Fleige et al. 2016). The transcription of ech and fcs eliminated the adaptation phase in the host. Moreover, by using an improved fed-batch feeding strategy, the group could attain an even higher concentration of vanillin, 22.3 g/L, which is the highest vanillin concentration reported to date from any of the native wild host strains (Fleige et al. 2016). This study shows that improvements in vanillin yield using whole cells are possible through the right combination of strategies involving optimization of the fermentation parameters with resting cells and metabolic engineering. The identical strategy of inactivating vanillin dehydrogenase and overexpressing feruloyl-coenzyme A (CoA) synthetase and enoyl-CoA hydratase/aldolase in Pseudomonas sp. have been documented by Graf and Altenbuchner (2014). However, here the authors observed vanillin metabolism to vanillic acid, despite knockout of the vanillin dehydrogenase gene (vdh). Hence, additional inactivation of a molybdate transporter gene was done in their study, which led to the complete prevention of vanillin degradation. However, the concentration of vanillin achieved in their study was only 1.2 g/L (Graf and Altenbuchner 2014). This indicates that each microbial strain has a characteristic tolerance to the amount of vanillin it can produce in the system. The highest concentration of vanillin production of vanillin has been achieved at 1.2 g/L with any Pseudomonas strain to date.
Genetic engineering strategies have also been tried with recombinant E. coli (transformed with vanillin synthesizing genes) as the preferred candidate for cost-effective vanillin synthesis because it has a well-studied and understood fermentation process and has no vanillin degradation pathway (Lee et al. 2009). Lee et al. induced an E. coli host transformed with feruloyl-CoA synthetase (fcs) and enoyl-CoA hydratase/aldolase (ech) genes to produce more vanillin by amplifying a glt gene encoding citrate synthase in it. During the vanillin synthase from ferulic acid, acetyl-CoA is a concomitant by-product whose accumulation impedes feruloyl-CoA’s forward reaction to vanillin. The enzyme citrate synthase bio transforms acetyl-CoA into CoA and helps the vanillin synthesis reaction to be pulled forward by eliminating product inhibition. Therefore, in their study, by overexpressing the gltA gene, 1.98 g/L of vanillin was produced, which was almost twofold more than the vanillin production of 0.91 g/L obtained by the E. coli without gltA amplification (Lee et al. 2009). In another study, Yoon et al. (2007) followed a two-step strategy to enhance vanillin production in an E. coli harboring fcs and ech genes transformed from Amycolatopsis sp. strain HR104. First, they generated mutants of E. coli that were vanillin resistant, and second, they used XAD-2 resin for the adsorption and removal of released toxic vanillin from the medium. This combined engineering strategy increased the vanillin production from the recombinant host to 2.9 g/L, which was three-fold higher than that for its wild-type strain without the use of the resin (Yoon et al. 2007). Indeed, the utility of adsorbent resins with microporous structures can be observed from a study by Hua et al. (2007a, b) where vanillin produced by ferulic acid biotransformation by Streptomyces sp. strain V-1 was adsorbed on the resins, leading to high production of 19.2 g/L along with ease of its downstream processing (Hua et al. 2007b). With a similar concept of obtaining the product adsorbed onto a resin (HD8), Zhao et al. (2006) also obtained decent production of vanillin (8.1 g/L) from isoeugenol. Recently, Luziatelli et al. (2019) reported production of approximately 4.9 g/L concentration of vanillin from ferulic acid in recombinant E.coli transformed with fcs and ech genes from a Pseudomonas strain, using the concept of resting cells, optimization of the bioprocess variables after using response surface methodology (RSM), and a unique solid-liquid separation system which had ferulic acid entrapped into 1.75% w/v agarose gel cylinders (Luziatelli et al. 2019). Here, in contrast to the product’s adsorption, the substrate was immobilized for its steady release in the media. Overall, these studies largely demonstrate that product inhibition could be well sidestepped by the addition of adsorbent resins in the fermentation systems.
Finally, in terms of using genetically engineered strains as the hosts, the study by Yamada et al. (2007) is worth mentioning in which the authors cloned a rare isoeugenol monooxygenase gene from a Pseudomonas putida strain IE27 into E. coli BL21. With the expression of just a single gene in E. coli, the concentration of 28.3 g/L of vanillin was realized from 230 mM isoeugenol in 6 h (Yamada et al. 2008). The achieved concentration of 28.3 g/L was the highest concentrations of vanillin ever reported in the literature from the use of either recombinant or native cells or cell-free extracts. Although it was nearly close to the production attained using wild Amycolatopsis sp. 39116 (22.3 g/L) by Fleige et al. (2016). The use of E. coli as the host eliminates the complications associated with the use of Amycolatopsis like microorganisms that are spore formers and do not have the requisite Generally recognized As Safe (GRAS) status. Infact, Kaur et al. (2014) have reported heterologous expression of fcs and ech genes in a lactic acid bacterium, Pediococcus acidilactici BD16, with GRAS status. In their study, the authors could recover 3.14 mM of vanillin within 20 min from 1.08 mM ferulic acid (Kaur et al. 2014).
Nevertheless, the recombinant strains are associated with certain disadvantages such as their genetic instability, inappropriate genetic tools, and high cost associated with the cloning, transformation, or recombination. Hence, immobilization of vanillin-producing microbial cells as biofilms has gained attention as yet another strategy due to their exceptional operational stabilities when persistent bioconversion times are required, high cell concentrations, and tolerance against harsh environments. Yan et al. (2016) attempted biocatalysis of ferulic acid to vanillin in a packed bed bioreactor, which had Bacillus subtilis cells immobilized as biofilms on the carbon fiber textiles (CFT); their vanillin’s reported production was 1.84 g/L, with the hydraulic retention time of just 20 h (Yan et al. 2016). Therefore, their process represents a faster production of vanillin in stable biotransformation where recycling or recovery of the immobilized biomass presents a potential economic advantage.
6.4.2 Specific Case of Polyhydroxyalkanoates (PHA) Production
Within the biopolymer group, polyhydroxyalkanoates or PHAs that are synthesized directly by microorganisms, are the plastic materials of the 21st century. PHAs are deposited intracellularly within the bacteria during the stress conditions, as energy storage or carbon reserves, (Getachew and Woldesenbet 2016). Their monomer building blocks, formed mainly from saturated or unsaturated hydroxy alkanoic acids, can vary in length from C3 to C14 carbon atoms with a variety of straight or branched chain aliphatic or aromatic side groups. Typically, the structure of PHAs depends on the feedstock monomers available together with the substrate specificity of the PHA synthase (PhaC).
There are reports available where microorganisms have been isolated in nature that grow on lignin as the sole carbon sources and transform derivatives of lignin to (R)-3-hydroxyacyl-CoA (3HA-CoA) via fatty acid de novo biosynthesis pathways for the biosynthesis of PHA. Pandoraea sp. ISTKB is one such strain tested for its ability to degrade lignin and use the released derivatives for PHA (Kumar et al. 2017). For this testing, the authors grew ISTKB on kraft lignin and its lignin derivatives, particularly syringol, vanillic acid, 4-hydroxybenzoic acid (4-HBA), p-coumaric acid, and 2,6-dimethoxyphenol (DMP), as the only carbon sources in the media, aerobically under nutrient-limited conditions for 6 days at 30 °C, pH 8, and 185 rpm. The concentration of PHA that Pandoraea sp. ISTKB accumulated was 246 mg/L with 4-HBA, followed by 170 mg/L with p-coumaric acid, 72 mg/L with p-coumaric acid, 69 mg/L with DMP, and 18 mg/L with kraft lignin (Kumar et al. 2017). Their results indicate that the bacterium’s PHA accumulation decreased with an increase in the substrate’s structural complexity. 4-HBA is an intermediate produced down the p-coumaric acid degradation pathway and seemingly had the simplest structure. Hence, ISTKB accumulated maximum PHA of the type poly (3-hydroxybutyric-co-hydroxyvaleric) acid P(HB-co-HV) with 4-HBA, and least with lignin (Kumar et al. 2017). Previously, with Ralstonia eutropha H16 as the model strain, Satoshi et al. (2014) reported similar results. The authors tested the capacity of H16 to synthesize P (HB-co-HV) from a variety of lignin derivatives. However, it was with 4-HBA and 3-HBA as the substrates that maximum PHA at 63 wt.% and 65 wt.% was accumulated by R. eutropha H16 (Tomizawa et al. 2014). With other intermediates, such as vanillic acid, ferulic acid, and p-coumaric acid, cell growth inhibition and PHA accumulation were observed (Tomizawa et al. 2014). Inhibition of cell growth was also observed in a γ-proteobacterium marine isolate Oceanimonas doudoroffii in the presence of p-coumaric acid, vanillic acid, ferulic acid, caffeic acid, and gallic acid. With O. doudoroffii, the authors reported reasonable PHA production (short-chain length polyhydroxyvalerate (PHV), 1.9 wt.% with sinapinic acid, and 2.7 wt.% with syringic acid (Numata and Morisaki 2015), which are again the simpler derivatives produced during lignin degradation.
In contrast to the aforementioned observations, where microorganisms tend to be inhibited by lignin, strains of the genus Pseudomonas have been reported for PHA production from lignin itself. For instance, P. putida KT2440 has been demonstrated to produce 150 mg/L medium chain-length (C6-C14) polyhydroxyalkanoates (mcl-PHAs) from alkaline pretreated corn liquor rich in lignin (32% wt./wt.) and extractives of lignin (23% wt./wt. of p-coumaric acid, vanillic acid, and ferulic acid) by Linger et al. (2014). The mcl-PHA biopolymer produced by P. putida KT2440 has a molecular weight of 124 kDa and has side chains comprising 3-hydroxydecanoic acid (55%), 3-hydroxyoctanoic acid (22%), 3-hydroxydodecanoic acid (16%), 3-hydroxytetradecancoic acid (4%), and 3-hydroxyhexanoic acid (3%) (Linger et al. 2014). Alkaline pretreated lignin (APL) from corn stover was also used to support P. putida KT2440, P. putida mt-2, and Cupriavidus necato growth and PHA accumulation (Salvachúa et al. 2015). Therein, the P. putida KT2440 and P. putida mt-2 synthesized 52 mg/L and 60 mg/L of mcl-PHA, respectively. C. necator was found to accumulate 162 mg/L of short-chain length polyhydroxy butyrate (PHB) (Salvachúa et al. 2015). More recently, Pandoraea sp. B-6 has also been shown to carry the potential to bio convert kraft lignin into PHA. Strain B-6 was shown to degrade 40% of kraft lignin in barely 4 days, with a resultant 24.7% accumulation of scl-PHB (Liu et al. 2019a). Similar findings were reported by Shi et al. (2017) with Cupriavidus basilensis B-8, which could accumulate 128 mg/L of PHB from kraft lignin (without any pretreatment) as the sole carbon source in 7 days (Shi et al. 2017). The authors in the study also highlighted the utility of fed-batch in enhancing PHB production during the bioconversion, as 319.4 mg/L of PHB was reported with 5 g/L of lignin using the fed-batch mode of fermentation (Shi et al. 2017).
Overall, these studies lay a solid foundation for pursuing bioconversion of lignin-rich streams to value-added biopolymers (Fig. 6.2). Generally, lignin and its aromatic intermediates are recalcitrant and toxic, and have been found to impede the fermentation and bioconversions in the host. In the future, optimization of culture conditions, the use of innovative fermentation modes, isolating new microbial strains, and metabolically engineering existing strains may prove useful for enhancing the yield and percent accumulation of PHA inside the host when lignin or its intermediates are used as the carbon source. Recently, CRISPR/Cas9n-based tool was used to engineer Pseudomonas putida KT2440 to produce a higher amount of mcl-PHA (270 mg/L) using ferulic acid as the feedstock. In yet another bioengineering study, Lin et al. (2016) improved the tolerance and productivity of Pseudomonas putida strain A514 toward lignin and its derivative vanillin by overexpressing a gene that codes for the VanAB enzyme of the β-ketoadipate pathway that is explicitly induced in the presence of vanillic acid (Lin et al. 2016). The group further channelized the vanillin bioconversion towards mcl-PHA synthesis by overexpressing phaB and phaC genes in A514. The results indicated that the modified A514 could accumulate 65 mg/L PHA, with a yield of 73.5% per CDW compared with 54% in the wild strain of A514. After this two-step metabolic engineering, A514 was also able to accumulate 75 mg/L mcl PHA (C8-C14) with kraft lignin, which is significantly more recalcitrant than the processed APL lignin (Lin et al. 2016). In addition, the authors reported that enhanced PHA production occurred through the complete growth cycle for both nitrogen-limiting and nitrogen-excess conditions. Moreover, the composition of the produced mcl-PHA had side chains ranging from C8-C14, which in itself is valued highly for its ability to be a precursor for the synthesis of jet fuels (C8-C16) (Lin et al. 2016). Wang et al. (2018) enhanced the PHA production of Pseudomonas putida strain A514 to 246 mg/L from vanillin by further overexpression of long-chain fatty acid-CoA ligase (alkK), and 3-hydroxyacyl-acyl carrier protein (ACP) thioesterase (phaG). In the same study, these genes were identified after an extensive study of genomics, transcriptomics, and proteomics data of A514 grown on vanillin under nitrogen-limited conditions (Wang et al. 2018). Thus, molecular insights based on omics study can help identify schemes to enhance PHA production from lignin or its derivatives.
Generally, for synthesizing high-molecular-weight PHA copolymers, expensive odd fatty acids (propionic acid, valeric acid) are supplemented in the medium as co-carbon substrates (Govil et al. 2020b). This co-addition of additional feedstocks not only increases the cost of production of PHA copolymer but also has a low yield and accumulation of copolymers compared to the addition of homopolymers. However, the studies discussed in this section suggest that the lignin or its aromatic derivatives can serve as low-cost platform precursors for synthesizing not only scl-copolymers like P(HV) and P(HB-co-HV) but also mcl-PHAs with side chains ranging from 3-hydroxyhexanoic acid to hydroxy-tetra decanoate.
Currently, the valorization process of lignin to PHA has an important drawback. The concentration and the yield of the produced PHA are low, being in the tens or low hundreds of milligrams per liter. The principal reason for this low yield is related to the low reactivity and assimilation of lignin by the microbial hosts. However, a study published in 2019 showed that co-utilization of lignin with a limited amount of glucose could facilitate lignin biocatalysis to PHA. The authors used this concept to produce the record titer of 1.5 g/L of PHA by the synergistic bioconversion of lignin and residual sugar released during corn stover pretreatment and hydrolysis by the Pseudomonas putida strain KT2440 (Liu et al. 2019b). This shows that lignin-based biorefinery sustainability is conceivable by applying innovative yet straightforward concepts.
6.5 Conclusion and Future Directions
Lignin, a polyaromatic macromolecule, is one of the most underutilized fractions of the lignocellulosic biomasses, whose valorization to fine chemicals has a much higher economic and environmental benefit than burning it for heat and electricity. The abundance of aromatic monomers in its skeletal structure makes lignin a promising substrate for biocatalysis into an array of value-added products such as aromatic biomolecules, biopolymers, bio-oil, and biofuels. Today, lignin is also considered a commendable, environmental-friendly component or additive in the preparation of epoxy resins, fire-retardants, antioxidants, adhesives, and concrete admixtures. Hence, the production of lignin-derived co-products can support, and enhance the profitability of second-generation biorefineries, and related industries. With the advancement of multi-omics knowledge, the sophisticated metabolic pathways essential for lignin degradation are being elucidated in detail. In the future, engineering of these pathways in microbes for the overproduction of useful intermediates such as ferulic acid, vanillin, and guaiacol is foreseen. In addition, the central target for the near future should be the improvement of the technology for separating lignin efficiently and cost-effectively from lignocellulosic biomass.
References
Abdelaziz OY, Brink DP, Prothmann J, Ravi K, Sun M, García-Hidalgo J, Sandahl M, Hulteberg CP, Turner C, Lidén G, Gorwa-Grauslund MF (2016) Biological valorization of low molecular weight lignin. Biotechnol Adv 34(8):1318–1346
Abdelaziz OY, Meier S, Prothmann J, Turner C, Riisager A, Hulteberg CP (2019) Oxidative depolymerisation of lignosulphonate lignin into low-molecular-weight products with Cu–Mn/δ-Al2O3. Top Catal 62(7):639–648
Abdelkafi S, Sayadi S, Ben Ali Gam Z, Casalot L, Labat M (2006) Bioconversion of ferulic acid to vanillic acid by Halomonas elongata isolated from table-olive fermentation. FEMS Microbiol Lett 262(1):115–120
Achterholt S, Priefert H, Steinbüchel A (2000) Identification of Amycolatopsis sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 54(6):799–807
Ahmad M, Taylor CR, Pink D, Burton K, Eastwood D, Bending GD, Bugg TD (2010) Development of novel assays for lignin degradation: comparative analysis of bacterial and fungal lignin degraders. Mol Biosyst 6(5):815–821
Ashengroph M, Amini J (2017) Bioconversion of isoeugenol to vanillin and vanillic acid using the resting cells of Trichosporon asahii. 3 Biotech 7(6):358–358
Ashengroph M, Nahvi I, Zarkesh-Esfahani H, Momenbeik F (2011) Pseudomonas resinovorans SPR1, a newly isolated strain with potential of transforming eugenol to vanillin and vanillic acid. New Biotechnol 28(6), 656–64
Ashengroph M, Nahvi I, Zarkesh-Esfahani H, Momenbeik F (2012) Conversion of isoeugenol to vanillin by Psychrobacter sp. strain CSW4. Appl Biochem Biotechnol 166(1):1–12
Bajwa DS, Pourhashem G, Ullah AH, Bajwa SG (2019) A concise review of current lignin production, applications, products and their environmental impact. Ind Crop Prod 139:111526
Barghini P, Di Gioia D, Fava F, Ruzzi M (2007) Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb Cell Factories 6(1):13
Bjorkman A (1954) Isolation of lignin from finely divided wood with neutral solvents. Nature 174(4440):1057–1058
Bugg TD, Ahmad M, Hardiman EM, Rahmanpour R (2011) Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep 28(12):1883–1896
Chatterjee S, Saito T (2015) Lignin-derived advanced carbon materials. ChemSusChem 8(23):3941–3958
Chatterjee, T., De, B.K., and , Bhattacharyya, D.K. 1999. Microbial conversion of isoeugenol to vanillin by Rhodococcus rhodochrous. Indian J Chem Sect B, 38B(5), 538–541
Chen H (2015) Chapter 3—Lignocellulose biorefinery feedstock engineering. In: Chen H (ed) Lignocellulose biorefinery engineering. Woodhead Publishing, Sawston, pp 37–86
Chen CL, Chang HM, Kirk TK (1982) Aromatic acids produced during degradation of lignin in spruce wood by Phanerochaete chrysosporium. Department of Wood and Paper Science, North Carolina State University, Raleigh, NC; Forest Products Lab., USDA Forest Service, Madison, WI
Chen YH, Chai LY, Zhu YH, Yang ZH, Zheng Y, Zhang H (2012) Biodegradation of Kraft lignin by a bacterial strain Comamonas sp. B-9 isolated from eroded bamboo slips. J Appl Microbiol 112(5):900–906
Datta R, Kelkar A, Baraniya D, Molaei A, Moulick A, Meena RS, Formanek P (2017) Enzymatic degradation of lignin in soil: a review. Sustainability 9(7):1163
Di Gioia D, Luziatelli F, Negroni A, Ficca AG, Fava F, Ruzzi M (2011) Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J Biotechnol 156(4):309–316
Duan J, Huo X, Du WJ, Liang JD, Wang DQ, Yang SC (2016) Biodegradation of Kraft lignin by a newly isolated anaerobic bacterial strain, Acetoanaerobium sp. WJDL-Y2. Lett Appl Microbiol 62(1):55–62
Fleige C, Meyer F, Steinbüchel A (2016) Metabolic engineering of the Actinomycete Amycolatopsis sp. strain ATCC 39116 towards enhanced production of natural vanillin. Appl Environ Microbiol 82(11):3410–3419
Fox40 (2020) Vanillic Acid (CAS 121-34-6) Market Size Latest Report 2020 Segment by manufacturers, Type, Applications and Dynamics
Gallage NJ, Hansen EH, Kannangara R, Olsen CE, Motawia MS, Jørgensen K, Holme I, Hebelstrup K, Grisoni M, Møller BL (2014) Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat Commun 5:4037
Gargulak JD, Lebo SE, McNally TJ (2015) Lignin. in: Kirk-Othmer encyclopedia of chemical technology, pp 1–26
Gasson MJ, Kitamura Y, McLauchlan WR, Narbad A, Parr AJ, Parsons EL, Payne J, Rhodes MJ, Walton NJ (1998) Metabolism of ferulic acid to vanillin. A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J Biol Chem 273(7):4163–4170
Getachew A, Woldesenbet F (2016) Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes 9:509
Govil T, Saxena P, Samanta D, Singh SS, Kumar S, Salem DR, Sani RK (2020a) Adaptive enrichment of a thermophilic bacterial isolate for enhanced enzymatic activity. Microorganisms 8(6):871
Govil T, Wang J, Samanta D, David A, Tripathi A, Rauniyar S, Salem DR, Sani RK (2020b) Lignocellulosic feedstock: a review of a sustainable platform for cleaner production of nature’s plastics. J Clean Prod 270:122521
Graf N, Altenbuchner J (2014) Genetic engineering of Pseudomonas putida KT2440 for rapid and high-yield production of vanillin from ferulic acid. Appl Microbiol Biotechnol 98(1):137–149
Gupta VK, Minocha AK, Jain N (2001) Batch and continuous studies on treatment of pulp mill wastewater by Aeromonas formicans. J Chem Technol Biotechnol 76(6):547–552
Gurujeyalakshmi G, Mahadevan A (1987) Dissimilation of ferulic acid byBacillus subtilis. Curr Microbiol 16(2):69–73
Hansen MAT, Jørgensen H, Laursen KH, Schjoerring JK, Felby C (2013) Structural and chemical analysis of process residue from biochemical conversion of wheat straw (Triticum aestivum L.) to ethanol. Biomass Bioenergy 56:572–581
Hintz HL (2001) Paper: pulping and bleaching. In: Buschow KHJ, Cahn RW, Flemings MC, Ilschner B, Kramer EJ, Mahajan S, Veyssière P (eds) Encyclopedia of materials: science and technology. Elsevier, Oxford, pp 6707–6711
Holladay JE, Bozell JJ, White JF, Johnson D (2007) Top value-added chemicals from biomass. Volume II—results of screening for potential candidates from biorefinery lignin. Pacific Northwest National Laboratory
Horwath W (2015) Chapter 12—Carbon cycling: the dynamics and formation of organic matter. In: Paul EA (ed) Soil microbiology, ecology and biochemistry, 4th edn. Academic, Boston, pp 339–382
Hu H, Li L, Ding S (2015) An organic solvent-tolerant phenolic acid decarboxylase from Bacillus licheniformis for the efficient bioconversion of hydroxycinnamic acids to vinyl phenol derivatives. Appl Microbiol Biotechnol 99(12):5071–5081
Hua D, Ma C, Lin S, Song L, Deng Z, Maomy Z, Zhang Z, Yu B, Xu P (2007a) Biotransformation of isoeugenol to vanillin by a newly isolated Bacillus pumilus strain: identification of major metabolites. J Biotechnol 130(4):463–470
Hua D, Ma C, Song L, Lin S, Zhang Z, Deng Z, Xu P (2007b) Enhanced vanillin production from ferulic acid using adsorbent resin. Appl Microbiol Biotechnol 74(4):783–790
Huang Z, Dostal L, Rosazza JP (1993) Mechanisms of ferulic acid conversions to vanillic acid and guaiacol by Rhodotorula rubra. J Biol Chem 268(32):23954–23958
Janusz G, Pawlik A, Sulej J, Swiderska-Burek U, Jarosz-Wilkolazka A, Paszczynski A (2017) Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev 41(6):941–962
Jing D, Jidong L, Yiping W, Wenjing D (2016) Kraft lignin biodegradation by Dysgonomonas sp. WJDL-Y1, a new anaerobic bacterial strain isolated from sludge of a pulp and paper mill. J Microbiol Biotechnol 26(10):1765–1773
John B (2020) Kraft lignin market analysis share size and growth demand by 2020–2025
Jung DH, Choi W, Choi KY, Jung E, Yun H, Kazlauskas RJ, Kim BG (2013) Bioconversion of p-coumaric acid to p-hydroxystyrene using phenolic acid decarboxylase from B. amyloliquefaciens in biphasic reaction system. Appl Microbiol Biotechnol 97(4):1501–1511
Kasana RC, Sharma UK, Sharma N, Sinha AK (2007) Isolation and identification of a novel strain of Pseudomonas chlororaphis capable of transforming isoeugenol to vanillin. Curr Microbiol 54(6):457–461
Kaur B, Chakraborty D, Kumar B (2014) Metabolic engineering of Pediococcus acidilactici BD16 for production of vanillin through ferulic acid catabolic pathway and process optimization using response surface methodology. Appl Microbiol Biotechnol 98(20):8539–8551
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep (Amst) 4:86–93
Kumar M, Singhal A, Verma PK, Thakur IS (2017) Production and characterization of polyhydroxyalkanoate from lignin derivatives by Pandoraea sp. ISTKB. ACS Omega 2(12):9156–9163
Lee EG, Yoon SH, Das A, Lee SH, Li C, Kim JY, Choi MS, Oh DK, Kim SW (2009) Directing vanillin production from ferulic acid by increased acetyl-CoA consumption in recombinant Escherichia coli. Biotechnol Bioeng 102(1):200–208
Lee S, Kang M, Bae J-H, Sohn J-H, Sung BH (2019) Bacterial valorization of lignin: strains, enzymes, conversion pathways, biosensors, and perspectives. Front Bioeng Biotechnol 7:209–209
Leonard PM, Brzozowski AM, Lebedev A, Marshall CM, Smith DJ, Verma CS, Walton NJ, Grogan G (2006) The 1.8 A resolution structure of hydroxycinnamoyl-coenzyme A hydratase-lyase (HCHL) from Pseudomonas fluorescens, an enzyme that catalyses the transformation of feruloyl-coenzyme A to vanillin. Acta Crystallogr D Biol Crystallogr 62(Pt 12):1494–1501
Li T, Rosazza JP (2000) Biocatalytic synthesis of vanillin. Appl Environ Microbiol 66(2):684–687
Lin L, Cheng Y, Pu Y, Sun S, Li X, Jin M, Pierson EA, Gross DC, Dale BE, Dai SY, Ragauskas AJ, Yuan JS (2016) Systems biology-guided biodesign of consolidated lignin conversion. Green Chem 18(20):5536–5547
Linger JG, Vardon DR, Guarnieri MT, Karp EM, Hunsinger GB, Franden MA, Johnson CW, Chupka G, Strathmann TJ, Pienkos PT, Beckham GT (2014) Lignin valorization through integrated biological funneling and chemical catalysis. Proc Natl Acad Sci U S A 111(33):12013–12018
Liu D, Yan X, Si M, Deng X, Min X, Shi Y, Chai L (2019a) Bioconversion of lignin into bioplastics by Pandoraea sp. B-6: molecular mechanism. Environ Sci Pollut Res Int 26(3):2761–2770
Liu Z-H, Shinde S, Xie S, Hao N, Lin F, Li M, Yoo CG, Ragauskas AJ, Yuan JS (2019b) Cooperative valorization of lignin and residual sugar to polyhydroxyalkanoate (PHA) for enhanced yield and carbon utilization in biorefineries. Sustain Energy Fuels 3(8):2024–2037
Liu H-M, Zou Y, Yao C-Y, Yang Z (2020) Enzymatic synthesis of vanillin and related catalytic mechanism. Flavour Fragr J 35(1):51–58
Luziatelli F, Brunetti L, Ficca AG, Ruzzi M (2019) Maximizing the efficiency of vanillin production by biocatalyst enhancement and process optimization. Front Bioeng Biotechnol 7(279)
Macfarlane AL, Mai M, Kadla JF (2014) Chapter 20—Bio-based chemicals from biorefining: lignin conversion and utilisation. In: Waldron K (ed) Advances in biorefineries. Woodhead Publishing, Sawston, pp 659–692
Markus HP, Peters MLJ, Roos R (1992) Process for the preparation of phenylaldehydes, (Ed.) Q.I. BV, Vol. EP0542348A2. Europe
Miller J (2016) Lignin: technology, applications, and markets. RISI Latin American Pulp & Paper Outlook Conference
Mohamad Ibrahim, M.N., Sriprasanthi, R.B., Shamsudeen, S., Adam, F., and , Bhawani, S.A. 2012. A concise review of the natural existance, synthesis, properties, and applications of syringaldehyde. Bioresour Technol, 7(3), 4377–4399
Muheim A, Lerch K (1999) Towards a high-yield bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 51(4):456–461
Negishi O, Sugiura K, Negishi Y (2009) Biosynthesis of vanillin via ferulic acid in Vanilla planifolia. J Agric Food Chem 57(21):9956–9961
Nishimura M, Kawakami S, Otsuka H (2018) Molecular cloning and characterization of vanillin dehydrogenase from Streptomyces sp. NL15-2K. BMC Microbiol 18(1):154–154
Numata K, Morisaki K (2015) Screening of marine Bacteria to synthesize Polyhydroxyalkanoate from lignin: contribution of lignin derivatives to biosynthesis by Oceanimonas doudoroffii. ACS Sustain Chem Eng 3(4):569–573
Obst JR, Kirk TK (1988) Isolation of lignin. In: Methods in enzymology, vol. 161. Academic, pp 3-12
Ohta Y, Hasegawa R, Kurosawa K, Maeda AH, Koizumi T, Nishimura H, Okada H, Qu C, Saito K, Watanabe T, Hatada Y (2017) Enzymatic specific production and chemical functionalization of Phenylpropanone platform monomers from lignin. ChemSusChem 10(2):425–433
Overhage J, Priefert H, Steinbüchel A (1999) Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl Environ Microbiol 65(11):4837–4847
Overhage J, Steinbüchel A, Priefert H (2003) Highly efficient biotransformation of eugenol to ferulic acid and further conversion to vanillin in recombinant strains of Escherichia coli. Appl Environ Microbiol 69(11):6569–6576
Pacek AW, Ding P, Garrett M, Sheldrake G, Nienow AW (2013) Catalytic conversion of sodium lignosulfonate to vanillin: engineering aspects. Part 1. Effects of processing conditions on vanillin yield and selectivity. Ind Eng Chem Res 52(25):8361–8372
Plaggenborg R, Steinbüchel A, Priefert H (2001) The coenzyme A-dependent, non-beta-oxidation pathway and not direct deacetylation is the major route for ferulic acid degradation in Delftia acidovorans. FEMS Microbiol Lett 205(1):9–16
Plaggenborg R, Overhage J, Steinbüchel A, Priefert H (2003) Functional analyses of genes involved in the metabolism of ferulic acid in Pseudomonas putida KT2440. Appl Microbiol Biotechnol 61(5):528–535
PMR (2020) Bio vanillin market, vol. 2021. Persistence Market Research
Prim N, Pastor FI, Diaz P (2003) Biochemical studies on cloned Bacillus sp. BP-7 phenolic acid decarboxylase PadA. Appl Microbiol Biotechnol 63(1):51–56
Rabenhorst J, Hopp R (1991) Process for the preparation of vanillin Patent application, EP0405197
Raj A, Krishna Reddy MM, Chandra R (2007) Identification of low molecular weight aromatic compounds by gas chromatography–mass spectrometry (GC–MS) from Kraft lignin degradation by three Bacillus sp. Int Biodeterior Biodegradation 59(4):292–296
Reid ID (1998) Fate of residual lignin during delignification of Kraft pulp by trametes versicolor. Appl Environ Microbiol 64(6):2117–2125
Reiter J, Strittmatter H, Wiemann LO, Schieder D, Sieber V (2013) Enzymatic cleavage of lignin β-O-4 aryl ether bonds via net internal hydrogen transfer. Green Chem 15(5):1373–1381
Retsina T, Pylkkanen V, Nelson K, Szczepanik M (2013) Processes and apparatus for lignin separation in biorefineries, vol. US20140034047A1. Granbio Intellectual Property Holdings LLC. USA
Richter JS, Brink DL, Diddams DG, Peter O (1945) Process for making vanillin, vol. US2434626A. Salvo Chemical Corp. USA
Sainsbury PD, Hardiman EM, Ahmad M, Otani H, Seghezzi N, Eltis LD, Bugg TD (2013) Breaking down lignin to high-value chemicals: the conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem Biol 8(10):2151–2156
Salvachúa D, Karp EM, Nimlos CT, Vardon DR, Beckham GT (2015) Towards lignin consolidated bioprocessing: simultaneous lignin depolymerization and product generation by bacteria. Green Chem 17(11):4951–4967
Shen Y, Song X, Li L, Sun J, Jaiswal Y, Huang J, Liu C, Yang W, Williams L, Zhang H, Guan Y (2019) Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed Pharmacother 111:579–587
Shi Y, Yan X, Li Q, Wang X, Liu M, Xie S, Chai L, Yuan J (2017) Directed bioconversion of Kraft lignin to polyhydroxyalkanoate by Cupriavidus basilensis B-8 without any pretreatment. Process Biochem 52:238–242
Shimada M, Nakatsubo F, Kirk TK, Higuchi T (1981) Biosynthesis of the secondary metabolite veratryl alcohol in relation to lignin degradation in Phanerochaete chrysosporium. Arch Microbiol 129(4):321–324
Shimoni E, Ravid U, Shoham Y (2000) Isolation of a Bacillus sp. capable of transforming isoeugenol to vanillin. J Biotechnol 78(1):1–9
Strassberger Z, Tanase S, Rothenberg G (2014) The pros and cons of lignin valorisation in an integrated biorefinery. RSC Adv 4(48):25310–25318
Tang P-L, Hassan O, Maskat MY, Badri K (2015) Production of monomeric aromatic compounds from oil palm empty fruit bunch fiber lignin by chemical and enzymatic methods. Biomed Res Int 2015:–891539
Thoresen PP, Matsakas L, Rova U, Christakopoulos P (2020) Recent advances in organosolv fractionation: towards biomass fractionation technology of the future. Bioresour Technol 306:123189
Tian D, Hu J, Chandra RP, Saddler JN, Lu C (2017) Valorizing recalcitrant cellulolytic enzyme lignin via lignin nanoparticles fabrication in an integrated biorefinery. ACS Sustain Chem Eng 5(3):2702–2710
Tomizawa S, Chuah J-A, Matsumoto K, Doi Y, Numata K (2014) Understanding the limitations in the biosynthesis of polyhydroxyalkanoate (PHA) from lignin derivatives. ACS Sustain Chem Eng 2(5):1106–1113
Tribot A, Amer G, Abdou Alio M, de Baynast H, Delattre C, Pons A, Mathias J-D, Callois J-M, Vial C, Michaud P, Dussap C-G (2019) Wood-lignin: supply, extraction processes and use as bio-based material. Eur Polym J 112:228–240
Unno T, Kim SJ, Kanaly RA, Ahn JH, Kang SI, Hur HG (2007) Metabolic characterization of newly isolated Pseudomonas nitroreducens Jin1 growing on eugenol and isoeugenol. J Agric Food Chem 55(21):8556–8561
USDA (2020) United States Department of Agriculture Agricultural Marketing Service|National Organic Program Document Cover Sheet
Vazquez A, Pique TM (2016) Chapter 5—Biotech admixtures for enhancing Portland cement hydration. In: Pacheco-Torgal F, Ivanov V, Karak N, Jonkers H (eds) Biopolymers and biotech admixtures for eco-efficient construction materials. Woodhead Publishing, Sawston, pp 81–98
Vyrides I, Agathangelou M, Dimitriou R, Souroullas K, Salamex A, Ioannou A, Koutinas M (2015) Novel Halomonas sp. B15 isolated from Larnaca Salt Lake in cyprus that generates vanillin and vanillic acid from ferulic acid. World J Microbiol Biotechnol 31(8):1291–1296
Wang X, Lin L, Dong J, Ling J, Wang W, Wang H, Zhang Z, Yu X (2018) Simultaneous improvements of Pseudomonas cell growth and polyhydroxyalkanoate production from a lignin derivative for lignin-consolidated bioprocessing. Appl Environ Microbiol 84(18):e01469–e01418
WebArchive (2003) Lignins—products with many uses, vol. 2020. Way Back Machine. Lignin Institute
Xu Z, Lei P, Zhai R, Wen Z, Jin M (2019) Recent advances in lignin valorization with bacterial cultures: microorganisms, metabolic pathways, and bio-products. Biotechnol Biofuels 12(1):32
Yamada M, Okada Y, Yoshida T, Nagasawa T (2007) Biotransformation of isoeugenol to vanillin by Pseudomonas putida IE27 cells. Appl Microbiol Biotechnol 73(5):1025–1030
Yamada M, Okada Y, Yoshida T, Nagasawa T (2008) Vanillin production using Escherichia coli cells over-expressing isoeugenol monooxygenase of Pseudomonas putida. Biotechnol Lett 30(4):665–670
Yan L, Chen P, Zhang S, Li S, Yan X, Wang N, Liang N, Li H (2016) Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci Rep 6(1):34644
Yang W, Tang H, Ni J, Wu Q, Hua D, Tao F, Xu P (2013) Characterization of two Streptomyces enzymes that convert Ferulic acid to vanillin. PLoS One 8(6):e67339
Yoon SH, Lee EG, Das A, Lee SH, Li C, Ryu HK, Choi MS, Seo WT, Kim SW (2007) Enhanced vanillin production from recombinant E. coli using NTG mutagenesis and adsorbent resin. Biotechnol Prog 23(5):1143–1148
Zhang Y, Xu P, Han S, Yan H, Ma C (2006) Metabolism of isoeugenol via isoeugenol-diol by a newly isolated strain of Bacillus subtilis HS8. Appl Microbiol Biotechnol 73(4):771–779
Zhang A, Lu F, Sun R-C, Ralph J (2010) Isolation of cellulolytic enzyme lignin from wood Preswollen/dissolved in dimethyl sulfoxide/N-Methylimidazole. J Agric Food Chem 58(6):3446–3450
Zhao L-Q, Sun Z-H, Zheng P, He J-Y (2006) Biotransformation of isoeugenol to vanillin by Bacillus fusiformis CGMCC1347 with the addition of resin HD-8. Process Biochem 41(7):1673–1676
Zheng L, Zheng P, Sun Z, Bai Y, Wang J, Guo X (2007) Production of vanillin from waste residue of rice bran oil by Aspergillus niger and Pycnoporus cinnabarinus. Bioresour Technol 98(5):1115–1119
Acknowledgments
We acknowledge support from the CNAM-Bio-Center and the South Dakota Governor’s Office of Economic Development. The authors also gratefully acknowledge support from the National Science Foundation (Award #1736255, #1849206, and #1920954) and the Department of Chemical and Biological Engineering at the South Dakota Mines.
Conflicts of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Govil, T., Vaughn, M., Salem, D.R., Sani, R.K. (2022). Biorefining of Lignin Wastes: Modularized Production of Value-Added Compounds. In: Saini, J.K., Sani, R.K. (eds) Microbial Biotechnology for Renewable and Sustainable Energy. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-16-3852-7_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-3852-7_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3851-0
Online ISBN: 978-981-16-3852-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)