Abstract
The growing evidence of literature, correlating the importance of balancing noninfectious microbes inhabiting our bodies in disease and health, has given birth to a new field of medicine—microbiome therapeutics. Composition of microbiome evolves with us right from birth, impacted by several factors like an individual’s genetic makeup, quantity and quality of the different foods that we consume, and the environment that we interact with. A change in the composition of our microbiome (gut, skin, lung, gastric, vaginal, oral) may trigger or predispose us to a disease condition before clinical manifestation of symptoms. The current trend to better understand these correlations in health and disease is by leveraging metagenomics, metabolomics, data mining, artificial intelligence, and machine learning tools for human microbiome diagnostics. Encouraging results have been obtained with therapeutic strategies using prebiotics, probiotics, signaling molecules, antimicrobial peptides, and microbiome transplant in alleviating disease symptoms and promoting well-being. This is generating increased interest in the medical and scientific community and awareness in the public. The emerging concepts of ‘smart sampling’ using 3D printed devices, engineering diagnostic and therapeutic bacteria using synthetic biology, and using microbiome engineering to restore niche-specific balance are some additional paths that scientists are pursuing to arrive at a viable solution. In this article, we will address the challenges and potential solutions of microbiome diagnostics and therapeutics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Hundred trillion invisible living beings (grouped together as the microbiome) live inside humans. A delicate balance between the quality and quantity of the microbes that live inside our body is vital for our well-being. Growing scientific evidence suggests that the microbiome is intricately connected to human health, wellness, and treatment of certain disorders. As the cells of our bodywork tirelessly to perform the necessary functions for us to live, so do the microbial communities within our body right from our birth. They keep performing jobs that benefit us like breaking down foods, aiding nutrient absorption, protecting against pathogens, and aiding in immunity. According to scientific reports, around 1000 different species of bacteria reside in our gut; therefore, any imbalance (‘dysbiosis’) can result in a variety of problems to human health. An estimate suggests that 85% ‘good bacteria’ and 15% ‘bad bacteria’ are tolerated by the human gut; deviations from this ratio cause perturbations in the digestive system and various illnesses, also affecting our own immunity. Many of us would have experienced that when doctors prescribe antibiotics to kill harmful bacteria that invade us occasionally, they also recommend consuming probiotics as supplements. This is for the simple reason that while antibiotics do an excellent job killing ‘bad bacteria,’ they also eliminate some of the ‘good bacteria’ from our system, thus creating an imbalance. This results in diarrhea and many other gastrointestinal problems. Supplementing with probotics helps in restoring the loss of the ‘good bacteria’ and gradual reversal of symptoms associated with gut dysbiosis.
2 Need for Human Microbiome-Based Diagnostics
According to the World Health Organization, a state of good health is not actually the absence of a disease; rather, it is a state of complete mental, physical, and social well-being (WHO 1946). Based on patient examination, clinical history, associated symptoms, and diagnostic tests, health professionals diagnose the nature of the illness. They prescribe appropriate therapeutics, life style changes, or a combination of both, to treat the illness and improve the health of the patient. Diagnostic is a symptom or characteristic of value in diagnosis. Microbiome diagnostics, pertaining to the identification of the imbalance (if any) in the human microbiome, has been more of a research endeavor in the past. With the growing evidence in literature, of changing microbiome profiles (both abundance and diversity) correlations in different disease conditions, its cause and consequences on effectiveness of pharmaceuticals (Vieira-Silva et al. 2020); the importance of microbiome diagnostics as a companion in the clinical medical diagnostics tool box is emerging rapidly (Raes 2016). Diagnostic applications of the microbiome in the past were focused on the pathogenic microbes, but we now know the relevance of monitoring nonpathogenic microbial components of commensals associated with many noncommunicable chronic diseases. For example, Hollister et al. (2019), were capable to clearly differentiate children with irritable bowel syndrome, also called as I.B.S., a chronic condition, from the children who were healthy, by profiling the intestinal microbes, their genes/pathways, and metabolites. With the whole world reeling under the COVID-19 pandemic, a contagious disease whose pathophysiology is not yet completely understood, microbiome diagnostics has the power to query the changes happening at the molecular level in niche-specific microbiomes in both cross-sectional and longitudinal studies in patients (He et al. 2020). The knowledge gathered will empower research scientists and health professionals to build an appropriate repertoire to counter the pathology of SARS-CoV2.
Human orifices, and organs like the gut, lung, and skin, are abundant with niche-specific microbial species including bacteria, archaea, fungi, virus, and protozoa (mainly gut). Each one of us acquires a largely distinctive microbiome early in life. The same may persist with us for years or may undergo changes in compositional diversity/abundance. Such changes may correlate with a change in the environment, health status, or lifestyle. The niche-specific microbiome components are known to differ between environments and populations (Integrative HMP (iHMP) Research Network Consortium 2019), but certain indicator species are conserved across human populations studied around the world. This suggests that a symbiotic relationship between these indigenous organisms and human physiology cannot be ignored. However, the ‘cause-consequence’ problem of perturbations in the microbiome that are associated with a health condition remains to be understood in detail in several diseases. Some questions still remain: Are the molecular components of an individual’s microbiome responsible for health outcomes? How do they combine with and maintain critical physiological processes like the immune system and metabolism?
Recently, gathered evidence clearly suggests that the composition of the gut microbiome has a correlative effect via modulating at least the brain, lung, and liver. Many reviews have described these microbiomes with respect to their modulation and interplay with host factors at a greater depth.
Classically, culturing microorganisms and their identification from various clinical samples were the basis of ‘germ theory’ for any etiological agent and the earliest tool for microbiome diagnosis. But we now know the unculturable plethora of microbes that inhabit our body, and efforts are ongoing in ‘culturomics’ to narrow this gap by combining extensive laboratory culturing conditions followed by identification using mass spectrometry (Lagier et al. 2012; Lagier et al. 2018). Attempts at using real-time PCR as a diagnostic for microbiome composition (Ott 2004) at a time when Next-Gen sequencing (NGS) was just launched could not give the true picture of the microbiome since it estimated the abundance of only the dominant 20 pathogenic and commensal species in the intestinal bacterial flora. The advent of NGS has revolutionized the approach that researchers have at their disposal today and together with MALDI-TOF mass spectrometry, it is poised to take microbiome diagnosis to the next level with precise study designing, controls, unbiased analysis of data and reporting. A desirable outcome that would immensely help clinicians to serve their patient better would be the availability of NGS backed biomarker diagnostic assays for a disease condition or its prediction. How to identify these biomarkers? Novel noninvasive diagnostic biomarkers for colorectal cancer diagnosis have been successfully identified (Liang et al. 2017) based on metagenome sequencing analysis of fecal bacterial marker candidates and adapted for a qPCR assay.
3 Challenges in Design, Analysis, and Interpretation
For niche-specific microbiome diagnostics, it would be ideal to have reference ranges of microbial species or their metabolites (in a healthy or faulty microbiome) that a doctor could use for differential diagnosis. Among human microbiomes studied worldwide, the gut microbiome has probably been researched the most due to the ease of sample collection, abundance of microbes, and apriori knowledge. Unfortunately, due to many unresolved factors, the availability of a dependable diagnostic test, based on microbiome analysis, is still in the developmental stage. Though there is an abundance of microbiome data, the taxonomic changes identified in a disease are not consistent across different studies. The underlying reasons for the incoherence may have arisen due to variations in sample population (including diet, lifestyle) and the different technological approaches used in the diverse studies. In addition, a key problem in the field is to define the ‘healthy’ microbiome, owing to the large degree of variation in the microbiome composition among healthy individuals. Hence to stay relevant, all efforts toward identifying microbial markers for disease diagnostics must be based on comparisons with parallel control groups of healthy individuals (Versalovic et al. 2017).
An ambitious project launched in 2007 in the USA (Turnbaugh et al. 2007), termed the HMP or the National Institutes of Health’s Human Microbiome Project, was a one of its kind, large-scale initiatives to resolve the burning issues mentioned above (Gevers et al. 2012a, b). The first phase of the program involved generating massive amount of data and putting together the different analysis platforms to determine the composition of the ‘healthy’ microbiome (absence of evident disease). A baseline adult population (Huttenhower et al. 2012; Lloyd-Price et al. 2017) and ‘demonstration’ populations with specific disease states were studied to determine characteristic ranges (for some populations) of various microbiome-host parameters. The parameters that were included are as follows: (1) combinations of metabolic functions that are either ubiquitous or specific to the strain; (2) enzymatic repertoires; (3) some host factor, such as race or ethnicity. Information generated comprised of nucleotide sequences of microorganisms and human population (http://hmpdacc.org), protocols for body-wide microbiome sampling and data generation (Aagaard et al. 2013), and computational methods for microbiome analysis and epidemiology (Gevers et al. 2012a, b; Markowitz et al. 2012; Faust et al. 2012). This is a rich community resource for the scientific community. A striking revelation from the HMP1 was that the taxonomic classification of the microbiome was unable to explain host health or disease phenotype; molecular functional analysis of the microbial population or understanding personalized strain-specific makeup was a better correlate (Human Microbiome Project Consortium 2012).
Studies of the gut microbiome from healthy cohorts of other countries such as Denmark (Qin et al. 2010), China (Zhang et al. 2019), and India (Dhakan et al. 2019) have corroborated the same based on observations of some degree of functional redundancy in microbiomes in spite of compositional differences. Diet was a significant player in modulating the composition in these studies.
4 An Informed Approach to Next-Gen Sequencing-Based Microbiome Diagnostic Design and Evaluation
Metabolite-related profiling studies are beyond the chapter scope; hence, we are limiting ourselves to nucleic acid-based diagnostics here. Given the diversity of the human microbiome, challenge of limited clinical specimen size, and the large number of samples in cohort studies, Next-Gen sequencing-based approaches (Levy and Myers 2016) are the current method of choice for nucleic acid-based microbiome diagnostics (Fig. 10.1). In order to establish baseline ranges of taxonomic diversity in the HMP1 study, encompassing within and between body sites analyses, to decipher functional commonalities and signature strains across various subjects, NGS-based approaches were adopted. Sequencing profiles based on 16S rRNA gene sequences of 5577 samples and 681 shotgun metagenomes spanning up to 18 body sites and three time points each from 242 healthy adults were analyzed (Human Microbiome Project Consortium 2012). The study was extended (HMP1-II) to 2355 total shotgun metagenomes from 265 healthy adults to identify niche-specific and host-associated microbial community functions and to quantify strain personalization and retention dynamics over time (Lloyd-Price et al. 2017). Subsequently, three iHMP clinical studies served as models of microbiome-associated conditions, wherein the biological properties of both the microbiome and host were studied longitudinally. Microbial community compositions, transcriptomes and proteomes of the microbiomes, global metabolome, and immune and clinical markers from the host were analyzed to generate datasets. Among the conditions, vaginal microbiome of the mother associated with preterm birth, gut microbiome of subjects with inflammatory bowel disease, and gut/nasal microbiomes of type 2 diabetics were chosen. (NIH Human Microbiome Portfolio Analysis Team 2019; Integrative HMP (iHMP) Research Network Consortium 2019).
A schematic illustration of few popular methods employed in the microbiome analysis. Sequence-based profiling (top and middle-left panels) has more widespread reach due to the speed, higher throughput, sensitivity, specificity, and the ability to detect smallest signals and even dead bacteria. Culture-based profiling (bottom-left panel) is complementary to the sequence-based profiling; a prototype graph of MALDI-Biotyper is shown, different bacteria have different spectrum and scores. Real-time PCR (top-left panel) is very useful for diagnosis and quantification if the sequence of the organism or a biomarker for a particular disease is known. Next-generation sequencing (middle-left panel) has led to data explosion, due to continuous advancement in the field. It is broadly categorized into two types—(a) Targeted amplicon sequencing (top-right panel), where 16S/18S ribosomal RNA gene is used as phylogenetic markers for identification of microbes. General flow of the process is shown; (b) Holistic approach (bottom-right panel), where the entire genome/metabolites are used for the identification of microbes. It can be further divided into three categories: (1) shotgun based metagenomics, where DNA or genome is the target. General flow of the process is shown, where taxonomic identification is done either by mapping the sequences to the reference genome or assembling the sequences to get gene profile and hence the probable functional pathways; (2) metatranscriptomics, where the transcribed RNA is the target (expression profile) which gives an insight into active pathways/gene; (3) metabolomics, where the metabolites produced by the microbes are the target (not shown here) (Allaband et al. 2019; Schlaberg 2020)
Country-specific microbiome databases are required to construct meaningful correlations in disease and for the comparison of healthy and diseased individuals. The following critical parameters need attention for any microbiome diagnostic:
-
1.
Design: The role of the clinician, statistician, epidemiologist, and research scientist is critical in identifying:
-
(a)
The sample population size, age group, and location consisting of healthy and affected subjects (clinical diagnosis based, patient consent, diet, clinical history of self and family, medications, supplements, lifestyle, ability to comprehend instructions).
-
(b)
Which niche to study? (Skin/oral/nasal/gastric/gut/vaginal) Will simultaneous blood tests be needed to record any other parameters?
-
(c)
Timeline of study: Longitudinal study or cross-sectional study, sampling frequency.
-
(d)
Appropriate sampling, storage, and transportation: home/clinic collection, preferred time of collection, clear instruction on what and how to collect (stool/urine/sputum); addition of appropriate stabilizer/inhibitor for stabilizing nucleic acids; proper storage at desired temperature (4 °C or − 20 °C).
-
(e)
Controls: Inclusion of a mock community of several microbes (bacteria/fungi/virus) at varying abundances as a positive control for the process and LoD (limit of detection) determination; a reagent control with sterile water or saline in place of clinical sample to serve as a negative control.
-
(a)
-
2.
Data generation and analysis:
-
(a)
Isolation of nucleic acid (DNA/RNA/both): Commercially available kits or laboratory-developed protocol best suited for the clinical sample type can be evaluated for nucleic acid extraction yield, quality, and removal of inhibitors from mock community to give a statistically sound representation of the richness and abundance of microbial species. Once the isolation protocol meets the quality requirements, the same can be applied for the clinical samples in the study. Efficient conversion of the labile RNA to a more stable cDNA is critical for RNA genomes (RNA viruses) or for transcriptomic analysis of the sample. Methods that are easily adaptable for upscaling and automation are highly desirable to make the process efficiency user agnostic and predictive with turn-around times.
-
(b)
Sequencing methodology and platform (targeted amplicon-based/shotgun metagenomics/metatranscriptomics): Generally, sequencing library preparation for targeted amplicon-based sequencing includes a polymerase chain reaction step using DNA/cDNA, to generate amplicons of the targeted genetic marker with adapters. The shotgun metagenomics/metatranscriptomics library preparation approach on the other hand is not targeted, but includes a size selection of the double-stranded DNA/cDNA prior to adapter ligation using a series of enzymatic and mechanical manipulations as directed by the manufacturer. Details on the choice of sequencing methodology and platform are out of scope of this chapter. Briefly, NGS platforms can be categorized into two major categories: short-read (e.g., Illumina, Ion Torrent) or long-read (e.g., Pacific Biosciences (PacBio), Oxford Nanopore’s MinION) sequencing (Fig. 10.1). For taxonomic profiling, DNA-based targeted amplification of 16S/18S rRNA gene variable region, panel of gene targets, and shotgun metagenomics are commonly used. For molecular function-based querying of the microbiome and to distinguish active from dormant metabolic state, targeted transcript-based or metatranscriptomic methodology is followed. The read depth coverage and sequencing of single reads or paired reads are some other criteria that are taken into consideration.
-
(c)
Data processing tools and analysis of data: Upon data acquisition from the sequencers, performing several quality control checks is critical to prepare the data for downstream analytics. Examples are data trimming and the removal of poor-quality reads. Two primary approaches to taxonomic profiling of analysis can be employed. These include de novo assembly-based and ‘read alignment to reference-based’ methods. Many assembly software such as metaSPADES (Nurk et al. 2017) and MEGAHIT (Li et al. 2015, 2016) can be used to reconstruct genomes from metagenomics sequence data. Once draft genomes are assembled, software such as CONCOCT (Alneberg et al. 2014), or MetaBat (Kang et al. 2015) can perform contig binning and taxonomic profiling. Examples of read-based taxonomic profiling software include Kraken (Wood and Salzberg 2014) and MetaPhlAn2 (Truong et al. 2015). Computational software such as QIIME (Caporaso et al. 2010) and MOTHUR (Schloss et al. 2009) are most commonly employed for targeted amplicon sequence data analysis using operational taxonomic unit (OTU)-based analyses. Aligned read pairs form contigs, followed by clustering of contigs into OTUs based on similarity to reference sequence in a database such as Greengenes (DeSantis et al. 2006) or SILVA (Pruesse et al. 2007) for taxonomic classification. Following classification, community structure as measured via alpha and beta diversity can be examined.
-
(a)
-
3.
Interpretation of data for diagnostics:
-
(a)
Correlation and association based: Comparison of the microbial diversity and relative abundance in the healthy group vs. the disease group to derive statistically significant correlations and associations using bioinformatic and statistical tools that take metadata into consideration is critical for unbiased interpretation. This topic will not be covered in detail here.
-
(b)
Database richness and accounting for microbiota interactive network: Ensuring database richness and updating for taxonomy and disease associations for microbiome profiles from various studies are needed to translate the benefit in diagnostic reporting.
-
(c)
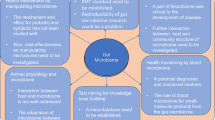
Predictive medicine: Since precise microbiome diagnostic needs to be population-specific, every nation may need to determine the microbiome biomarkers for disease diagnosis/prognosis/treatment that is relevant to their population for best patient outcomes. Using Big Data analytics and machine intelligence to discover those correlations and validate them is paving the way for predictive medicine (Fig. 10.2). The following possibilities are very encouraging for the future of medicine:
-
Identify unforeseen mechanistic insights of treatment.
-
Identify associations not yet detected by humans.
-
Identify biomarkers defining a patient’s response to treatment.
-
Predict synergism/antagonisms of combination therapies and dosage effects.
-
Potentially minimize side effects and maximize efficacy of treatment.
-
Predictive modeling for the diagnosis and treatment of diseases.
-
Developing noninvasive microbiome-based diagnostics with the help of AI.
-
Create virtual preemptive and predictive in silico testing of safer, more effective therapeutics.
-
-
(a)
A schematic illustration showing the future of artificial intelligence (AI)/machine learning (ML) in microbiome diagnostics and therapeutics. Artificial intelligence has the potential of removing the ‘one-size-fit-all’ stigma in the medical field. The progress made in sequencing technology has led to an increased interest in microbiome data and can lead to DNA-based specific diagnostics. The integration of high-resolution sequencing data and the patient metadata or clinical data can serve as model for machine learning, which can help in detecting/identifying patterns or biomarkers or insights missed by the human eye. AI/ML is still in nascent phase, and it has huge potential to revolutionize the medical field. However, caution is needed, in terms of training dataset as well as ethics and privacy (Espinoza 2018; Leber et al. 2017; Topçuoğlu et al. 2020)
Innovations in the field from designing sampling devices, sequencing platforms with better and cheaper technologies, and algorithms for data analysis are forward-facing. Most studies of the gut microbiome study fecal sample, which may not be the best representation of the whole gut microbiota. It is interesting to note that an ingestible, biocompatible, 3D-printed microengineered battery-less pill has shown promise in vitro and in animal models including primates (Rezaei Nejad et al. 2019) to aid in this sampling process.
5 The Healthy Gut Microbiome
Among the inhabitant microbes of our gut, bacteria are the most predominant. Though there are ∼1000 different bacterial species colonizing our gut, only about 330 of them have been characterized and classified so far. The top inhabiting phyla consists of strict anaerobes (with their relative abundance in parentheses): Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%) (Gill et al. 2006; Bäckhed et al. 2012). The constantly changing microbiome responds to our lifestyle modifications, such as diet and exercise, and displays perturbations accordingly (Qin et al. 2010; David et al. 2014; Wu et al. 2011).
Among the benefits we derive from our gut bacteria, commensal species such as Lactobacillus plantarum helps in regulating the integrity of intestinal epithelium, which acts as the first physical barrier for enteric pathogens. Short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate play vital roles in the gut microbiome homeostasis and host immunity. The SCFAs are produced from the breakdown of polysaccharides (mainly dietary fiber) by specific bacteria (El Kaoutari et al. 2013; Canfora et al. 2015; Koh et al. 2016; Miyamoto et al. 2016). It is the tuning of the biochemical pathways in the specific bacteria that result in different by-products though starting with the same dietary fiber source. The major acetate producers belong to the genus Streptococcus, Prevotella, Bifidobacterium, and Clostridium to name a few (Rey et al. 2010). Propionate is produced by Bacteroides spp., Salmonella spp., Dialister spp., Veillonella spp., Roseburia inulinivorans, Coprococcus catus, Blautia obeum, etc., (Louis and Flint 2017). Bacteria belonging to Lachnospiraceae, Ruminococcaceae, and Acidaminococcaceae families are the major butyrate producers in the gut (Duncan et al. 2002). It is reported that the SCFAs along with G-protein coupled receptor 41 (GPR41) and GPR43 present in intestinal epithelial cells can modulate satiety and food cravings (Kim et al. 2013a, b; Ang and Ding 2016). Higher uptake of nutrients is facilitated by suppressing intestinal mobility transit by the secretion of peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) stimulated by SCFAs (Chambers et al. 2018; Ang and Ding 2016).
The major representatives of archaea in the gut microbiome are different species of methanogens and halophiles. Some examples and their relative abundance among gut methanogenic archaea include Methanobrevibacter smithii (94%), M. stadtmanae (23%), Candidatus Methanomethylophilus alvus, and Candidatus Methanomassiliicoccus intestinalis (Dridi et al. 2009). Gaseous by-products such as methane and hydrogen are generated by the anaerobes inhabiting the bowel.
The signaling among the gut microbiota, the gut, and the brain by metabolites occurs via neuronal pathways which involves both the central and enteric nervous systems, along with the circulatory system (Cryan and Dinan 2012; Mohajeri et al. 2018). Thus, the significance of the gut microbiome in health and disease is being appreciated like never before by scientists, clinicians, nutritionists, and the informed public.
The healthy adult gut microbiota is highly tolerant in accommodating minor perturbations with respect to its diversity and abundance, due to a temporary change, such as in eating habits, life style, or environment. In a study conducted over a course of 5 years, the individual gut microbiome displayed 60% strain level conservation, where the major contributors were the members of the phyla Bacteroidetes and Actinobacteria (Faith et al. 2013). This conservation or ‘longitudinal stability’ along with the diversity of the microbiome at individual levels or ‘interpersonal diversity’ is capable of assigning an unique ‘microbial fingerprint’ to every individual, based on the identification of >80% of the individuals microbiome composition (Franzosa et al. 2015). Despite its resilience potential, the recent studies indicate ‘dysbiosis’ of the microbiome to be associated with a major change(s), such as onset of disease, surgery, or antibiotic treatment (Morgan et al. 2012). Some examples of noncommunicable diseases, where correlations between the gut microbiome profile and disease status have been elucidated, will be discussed in subsequent sections of this chapter.
Developing robust microbiome-based therapeutics to restore microbiome balance, maintain the same over a period of time, and prevent relapses of dysbiosis poses a few challenges that need interdisciplinary approaches to offer viable and effective solutions.
6 Microbiome Therapeutics
We all consume curd/yogurt in our regular food habits, but it was the curiosity and observation of Elie Metchnikoff, who wondered how a rural Bulgarian community with limited resources for living were able to live longer. He later found out that by manipulating the microbiome, one can increase the life and health spans of humans. He is the father of probiotics.
Traditionally, people in Europe and Japan have relied on fermented food products and the active ingredients that give the health benefits in fermented food products, are the microbes that constitute the food products.
In today’s world with high stress, reduced sleep, unbalanced diet, and lack of exercise, it is increasingly important to balance the gut microbiome through supplementation of probiotics. Probiotics not only help in balancing the ‘good bacteria’ but also keep the ‘bad bacteria’ away. This in turn helps the digestive system and overall health of an individual.
We also know that imbalance in the microbiome is the underlying cause of many disease conditions. The progressing field of microbiome diagnostics will be best matched, when ‘Microbiome Therapeutics’ can be customized based on the need of every patient with the right combination of prebiotic, probiotic, and supplements (Fig. 10.3).
Functional foods have health benefits above and beyond their nutritional value, e.g., providing prebiotics and probiotics which help in balancing the microbiome. Probiotics (right panel) are the live microbes which are beneficial for health and can be obtained from various foods which act as live culture for these beneficial microbes. Prebiotics (left panel) are the food for these live microorganism (probiotics) present in different food and are associated with the growth of beneficial bacteria. (Green et al. 2020; Markowiak and Ślizewska 2017; McBurney et al. 2019; Pandey et al. 2015; Rezac et al. 2018; Terpou et al. 2019)
6.1 Prebiotics Support Probotics
According to the International Scientific Association of Probiotics and Prebiotics, prebiotics are defined as ‘a substrate that is selectively utilized by host microorganisms conferring a health benefit’ (Gibson et al. 2017). In our microbiome, they promote the absorption of ion and trace element such as that of calcium, iron, and magnesium and modulate cytokine and secretory immunoglobulin A production, via mechanisms involving microbial metabolic products (Holscher 2017). Inulin, fructooligosaccharides (FOS), galactooligosaccharides (GOS), and human milk oligosaccharides (HMOS) are a few prebiotic ingredients in our diet that have a strong correlation in keeping body weight under check (Kim et al. 2019). They are found to stimulate the growth of Bifidobacteria and Lactobacillus species, thus enhancing the availability of SCFAs within the microbiome. Higher SCFA level positively influences satiety and food consumption via improved GLP-1, PYY, and ghrelin production (Cerdó et al. 2019).
6.2 Probiotics in Food and as Supplements
Natural probiotics can be obtained from food sources such as curd/yoghurt and fermented foods. Food products that contain probiotics are yoghurt, kefir, cheese, tempeh, kimchi, miso, sauerkraut, and some soy beverages. Freeze-dried bacteria in the form of tablets, capsules, powders and sachets, and ampoules containing bacterial spores, are available commercially from cultured organisms at a defined composition and abundance (Table 10.1, Alfano et al. 2020). In addition, probiotic-fortified foods are also available like juices, chocolates, flour, and cereal. Food and Drug Administration, USA (FDA), regulations allow probiotics to be sold as supplements and not like drugs, for healthy people. For people with illnesses such as irritable bowel syndrome, inflammatory bowel disease, diarrhea (both infectious and antibiotic-induced), urinary tract infections, and eczema, doctor-prescribed probiotics may be given. Pregnant women, infants, young children, and immunocompromised patients should be given probiotics with caution.
6.3 Postbiotics
Postbiotics is a therapeutically attractive emerging field that deals with the use of nonviable by-products of microbial growth (metabolites, cell lysis components, enzymes) that have an added health benefit when consumed. Unlike probiotics, which are based on the administration of live organisms, postbiotics are administered in alignment with pharmacokinetic and pharmacodynamic properties. They are being explored since they are abundant at most body sites, are suitable for different routes of administration, have low toxicity potential, are stable in the systemic circulation, and scale up friendly. Bacterial exopolysaccharides (EPS) from Bifidobacterium and Lactobacilli and extracellular vesicles (EVs) from Akkermansia muciniphila and commensal Escherichia coli are some such postbiotic examples (Wegh et al. 2019). There are several downsides such as pleiotropic effects, shorter half lives, and high cell-type specificity, which need further exploration and well-designed studies to evaluate them for therapeutics.
6.4 Fecal Microbiota Transplantation (FMT)
Fecal microbiota transplantation (FMT) is a procedure of administering donor fecal suspension into the colon of a diseased recipient, thus aiming to restore the disturbed gut microbiota and the associated disease. The first application of FMT in modern medicine was reported in 1965 for Clostridioides difficile colitis. Though FMT has benefited many to cure chronic conditions, it is not advisable for all conditions of gut dysbiosis and for all categories of patients. Selection of patients for whom FMT is effective is an important concern since long-term safety of the procedure and outcome for the patient needs careful consideration. Nevertheless, more data from well-designed studies will help in determining the efficacy and safety of the procedure.
6.5 Research-Driven Probiotics: The Future
The rationale that the naturally occurring human-associated microorganisms offer myriad of health benefits, is the basis of probiotic therapies. A systematic and well-designed research on specific probiotics designed for an individual is needed (Mimee et al. 2016). This would be possible through the studies on microbiome and microbiome diagnostics. A precise quantization of the microbiome components, the dosage, and regimens all require advanced science. The need of the hour is to develop microbiome-based recommendations for probiotics and probiotics prescribed for specific diseases classified as ‘prescription probiotics. Preparing them will involve large-scale culturing and identification of various bacterial species, their long-term storage, and potency testing of the probiotic cocktails tailored to an individual’s need. A futuristic probiotic application wherein the probiotic strain can be used for directly delivering anti-inflammatory and intestinal epithelial repair factors to the intestinal tract will allow correction of multiple aberrations in a unified manner.
Genetically engineered probiotics: The use of genetically modified organisms (GMO) in human microbiome therapeutics is still farfetched due to the associated regulatory clearances needed for their safety. Nevertheless, researchers have tested the concept in animal models and have met with success. The probiotic E. coli Nissle 1917 was altered to be used as a prophylactic, in order to inhibit virulence of Vibrio cholerae. (Hamady et al. 2010). A genetically modified derivative of a vaginal commensal Lactobacillus jensenii able to prevent transmission of chimeric simian/human immunodeficiency virus (SHIV) in a rhesus macaque model, when administered is another encouraging study (Motta et al. 2012) demonstrating the utility of engineered probiotic strains. A common side effect of chemotherapy ‘oral mucositis,’ a condition involving ulcerative lesions, is shown to be benefited from the topical application of an altered L. lactis engineered to secrete trefoil factor-1. Data from an early clinical trial for the treatment of the condition displayed good tolerance among patients and could be effective at reducing prevalence (Limaye et al. 2013).
Engineered ‘designer’ consortia: This concept is based on building a collection of well-characterized probiotic strains that can be custom combined in the laboratory based on the attributes desired for the therapeutic consortia. One such example is elaborated here. Bacteria in the gut generate urease which convert the urea produced by the liver to ammonia and carbon dioxide. Patients with liver deficiency, neurotoxicity and encephalopathy are found to be associated with accumulation of systematic ammonia. In a study, mouse models were treated with antibiotic and polyethylene glycol, resulting in reduction of endogenous microbiota. This was followed by transplantation with a defined microbial community with low urease activity. The microbiota reconstitution was successful in altering community-wide metabolic activity of urea that remained stable for months (Shen et al. 2015). Such designer consortia for human use may be seen in trials soon in the near future.
7 The Role of Microbiome Diagnostics and Therapeutics in a Few Disease Scenarios
In this section, select disease conditions primarily influenced by the gut microbiome dysbiosis are presented. A brief pathophysiology of the condition, it’s associated microbiome dysbiosis profile and suggested therapeutics for correcting the dysbiosis is summarized in the following sections (Fig. 10.4). Oral, vaginal, skin, and respiratory microbiome modulations are out of scope of this chapter.
7.1 Atherosclerosis
Atherosclerosis, a chronic inflammatory disease, and major contributor in CVDs (cardiovascular diseases), is associated with plaque formation consisting of accumulated modified lipids, calcified regions, neurotic cores, inflamed smooth muscle cells, endothelial cells, leukocytes, foam cells and impaired lipid metabolism and endothelial functions (Frostegård 2013). CVD is one of the leading causes of disease and death globally (Benjamin et al. 2018).
Atherosclerosis has been linked to intestinal microbes due to a substance called trimethylamine oxide in recent studies (Chen et al. 2016). In 2015, Cleveland Clinic researchers observed that lecithin and L-carnitine which is present in red meat, egg yolk, etc., can be converted to TMAO wherein intestinal microbes play an important part thus promoting atherosclerosis and speeding up the pathological process of cerebrovascular diseases (Wang et al. 2015a, b).
What’s the connect? Intestinal microbes can absorb foods rich in lecithin, choline, and carnitine to produce trimethylamine (TMA, a colorless gas of a foul odor), which is oxidized by flavin monooxygenase (FMO, FMO3 with highest activity) to TMAO in the liver. Atherosclerosis is linked with increased TMAO in the blood (Koeth et al. 2013), which in turn is linked to the diet, intestinal microbes, FMO3 activity, gender, and heredity of host (Seldin et al. 2016). Some mechanisms which TMAO uses to develop atherosclerosis are hampering cholesterol reverse transportation (Koeth et al. 2013), up-regulating the expression of macrophages CD36 and scavenger receptor A1 (SR-A1), encouraging foam cell formation, down-regulating the expression of cholesterol absorption targets ABCG5/8 and NPC1L1 affecting cholesterol metabolism, reducing the expression of cytochrome P450 (CYP) 7A1 and 27A1 in the liver, which in turn reduces the transport of bile acid and clearance of cholesterol and activates monocytes via mitogen-activated kinase and nucleic acid factor-κB signaling pathway by developing vascular inflammation (Seldin et al. 2016). Thus, if the density, richness, and diversity of gut microbiota are improved, it can help in prevention and treatment of atherosclerosis. People having lower species richness and diversity in the gut are prone to develop atherosclerosis (Menni et al. 2018).
Atherosclerotic patients are found to have a gut microbiota that is less fermentative and more inflammatory (Jie et al. 2017). Firmicutes and Bacteroides are the major taxa present which seem to be remarkably constant (Huttenhower et al. 2012; Faith et al. 2013). However, in patients with atherosclerosis, Escherichia coli, Klebsiella spp., Enterobacter aerogenes, Streptococcus spp., Lactobacillus salivarius, Solobacterium moorei, and Atopobium parvulum were found to be increased, whereas Bacteroides spp., Prevotella copri, and Alistipes shahii were found to be depleted (Jie et al. 2017).
Therapeutics that help: The three ‘p’ known for the well-being of gut are probiotics, prebiotics, and polyphenols, as these help regulate the gut microbiota composition (Marchesi et al. 2016). Since small intestine cannot absorb polyphenols directly, the bioavailability of polyphenols depends on the gut microbiota and their ability to convert it into components which can be absorbed by the small intestine (Duda-Chodak et al. 2015). Few of the polyphenols which have shown some potential mechanism in atherosclerosis are protocatechuic acid (PCA), quercetin-3-glucuronide, 2,4,5-trimethoxycinnamic acid, gallic acid, and equol (Pieczynska et al. 2020). Resveratrol, a natural phenolic phytochemical, works by reducing TMAO levels by promoting the growth of commensal bacteria such as Bacteroides, Lactobacillus, and Bifidobacterium (Jung et al. 2009; Qiao et al. 2014). The decrease in TMAO levels by resveratrol associated with inhibited development of atherosclerosis has also been proven in vivo (Chen et al. 2016).
As with prebiotics, probiotics like Lactobacillus plantarum and E. aerogenes could lower the production of TMAO and attenuate the formation of atherosclerotic abrasion in ApoE/mice (Qiu et al. 2017, 2018). Another prebiotic, mannan oligosaccharide (MOS) supplement, was found to regulate gut microbiota by lowering plasma cholesterol levels and improving atherosclerotic plaques in high cholesterol diet-fed mice (Hoving et al. 2018). Stimulation of Akkermansia in ApoE −/− mice was linked with berberine, which is found to be effective against atherosclerosis (Zhu et al. 2018). The endothelial function in ApoE −/− mice seems to be improved by administering ITFs, a prebiotic (Inulin-type fructans), as supplement. These inulin-type fructans enhance the formation of butyrate and protect against atherosclerosis formation (Watzl et al. 2005; Catry et al. 2018) as per the recent studies.
Both fish oil and flaxseed oil are found to reduce TMAO by enhancing SCFAs production and lowering LPS generation by microbes, and fish oil seems to be more productive (He et al. 2019). Research suggests that calorie-controlled diet integrated with supervised exercise lowers TMAO levels considerably (Erickson et al. 2019).
FMT is another therapy; however, it comes with risk as well. For example, while beneficial flora is getting transferred, so could the endotoxins or infectious agents present in the donor, and this could start new gastrointestinal complications. This is the reason it has found limited use as treatment for CVD patients (De Leon et al. 2013; Brandt 2013). Further research is needed to take a look at whether or not FMT probably prolongs different aspects of cardiometabolic disorders. Instead of fecal contents, the transplantation of particular group of microbes can be a rational opportunity to FMT. To better define the optimal fecal microbial preparation, dosing, and method of delivery, further research needs to be conducted (Sanchez-Rodriguez et al. 2020).
How the gut dysbiosis and TMAO derived from the microbiota participate in atherosclerosis is yet to be cleared (Zhu et al. 2020a, b). New strategies to prevent or treat the disease can be developed by better understanding of gut microbiota composition, to the development of atherosclerosis (Pieczynska et al. 2020).
7.2 Hypertension
Hypertension is among the chief causes of cardiovascular disease and is responsible for global deaths (Go et al. 2014). Although the procedure of how gut microbiota is involved with hypertension is not quite clear, SCFAs and oxidized low-density lipoprotein (ox-LDL) are believed to take some part in it (Ma et al. 2018).
What’s the connect? Obese pregnant women with lower blood pressure have shown increase in butyrate-producing bacteria (Gomez-Arango et al. 2016). Gut dysbiosis was improved by fiber and acetate supplementation in a study on hypertensive mice. It led to a surge in Bacteroides acidifaciens, which seems to have a defensive role in hypertension/heart failure (Marques et al. 2017). GPR41, GPR43, and GPR109AA are the three G-protein-coupled receptors (GPCRs), regulated by SCFAs. Another type expressed in the kidney is olfactory receptor 78 (Olfr78) regulated by acetate and propionate (Tan et al. 2017). The GPCRs regulated pathways of host can be stimulated by SCFAs which effects the secretion of renin and in turn effects the blood pressure (Furusawa et al. 2013; Pluznick et al. 2013).
Oxidation of LDL causes vasoconstriction leading to hypertension through gut dysbiosis (Packer et al. 2014), by boosting the expression of pro-inflammatory cytokines which induces oxidative stress triggering the Ox-LDL stimulation (Chawla et al. 2011; Peluso et al. 2012). Oxidation of L-arginine by nitric oxide synthase produces nitric oxide (NO) (Ma et al. 2006). Production of NO and endothelin-1 which maintains basic vascular tension and cardiovascular system homeostasis (Boulanger and Lüscher 1990) is hampered due to higher levels of ox-LDL which causes hypertension (Subah Packer 2007). Another cause of hypertension is chronic low-grade inflammation (Schiffrin 2014), which occurs due to depletion in microbial gene richness (Cotillard et al. 2013). Chronic probiotic intake decreases preeclampsia associated with hypertension (Brantsaeter et al. 2011).
Therapeutics that help: The composition of gut microbiota is altered by consuming β-glucan in such a way that it reduces the risk markers associated with CVD as per the single-blind randomized trial (Hoving et al. 2018). When Lactobacilli fermented milk was consumed by hypertensive humans, it lowered their blood pressure (Seppo et al. 2003). It is observed in human trials that consumption of at least 1011 colony-forming units along with multiple species of probiotics for 8 weeks decreases both systolic and diastolic blood pressures (Khalesi et al. 2014). Long-term administration of probiotics of various Lactobacillus bacteria such as Lactobacillus fermentum CECT5716 (LC40), Lactobacillus coryniformis CECT5711 (K8), and Lactobacillus gasseri CECT5714 (LC9) could decrease systolic blood pressure in hypertensive rats (Gómez-Guzmán et al. 2015). Phenylacetyl glutamine, a gut microbiota-derived metabolite, is negatively linked with pulse wave velocity and systolic blood pressure (Menni et al. 2015).
7.3 Obesity
Obesity is the manifestation of accumulated fat and is correlated with the progression of many diseases of metabolic origin like cardiovascular disease, type 2 diabetes mellitus, cancer, and nonalcoholic fatty liver disease (Kim et al. 2019). The fact that obese people live 7 years shorter than nonobese people is quite alarming (Van Hul et al. 2018).
What’s the connect? Studies from humans and animals have clearly shown a correlation between obesity and gut microbiome. Some examples are as follows: (1) decreased gut diversity (Baothman et al. 2016); (2) increased Firmicutes and decreased Bacteroidetes (Koliada et al. 2017; Mariat et al. 2009; Greenhill 2015). This increased Firmicutes/Bacteroidetes (F/B) ratio facilitates the energy extraction, and this in turn effects the energy storage in the adipose tissue (Mariat et al. 2009; Bell 2015). A significant increase in Enterobacteriaceae was observed in obesity (Balamurugan et al. 2010).
There exist a few other mechanisms for the functioning of gut microbiome in influencing obesity. Examples include bile acids that actively help in resolving fat uptake from diet in the small intestine, but they also hamper the growth of different commensal bacteria such as Lactobacilli and Bifidobacteria by disorganizing their membrane permeability (Ridlon et al. 2006; Kurdi et al. 2006). Acetate, propionate, and butyrate (SCFAs) which are eventually consumed by various organisms are estimated to have a production rate of 80–200 kcal/day (Riley et al. 2013). Decrease in butyrate-producing bacteria along with reduced intake of dietary carbohydrates such as polysaccharides, vegetable oligosaccharides, and resistant starch was observed in obese patients (Canfora et al. 2019). Another study gave similar results where the levels of fecal butyrate, SCFAs, and Bifidobacterium were found to be reduced considerably in obese patients who consumed less fiber (Brinkworth et al. 2009). Metabolic endotoxemia is defined as a chronically high plasma LPS disorder at 10–50 times less than the septic conditions of LPS and is a high-fat dietary elevation of plasma lipopolysaccharide (LPS) as termed by Cani et al. (2007). Dietary increases of endotoxin were linked to enhanced fat deposit, systemic and tissue-specific inflammation, and resistance to insulin (Cani et al. 2007; Amar et al. 2008).
Therapeutics that help: Prebiotics regulate the gut microbiome composition by improving lipid metabolism which is also seen in short-chain FOS treatment in diet-induced obese mice (Cluny et al. 2015) and are known to have antiobesity effects (Barengolts 2016; Nicolucci and Reimer 2017; Delzenne et al. 2011). Animals treated with oligofructose displayed reduction in both triglyceride level and adipose tissue mass (Cluny et al. 2015). It is also reported that α-cyclodextrins supplementation in obese mice (diet-induced) resulted in inflection of gut microbiota and SCFA production (Nihei et al. 2018). Human breast milk is enriched with milk oligosaccharides and serves as wonderful prebiotics candidate. As a prebiotic, it promotes the growth of beneficial bacteria such as Bacteroides and Bifidobacterium and hinders the pathogens such as Campylobacter jejuni, Helicobacter pylori, and E. coli (Newburg 2000).
Recent studies have shown that when compared with placebo-treated control animals, supplementation of Bifidobacterium species such as B. breve B3, B. infantis, and B. longum and Lactobacillus species such as L. rhamnosus, L. casei strain Shirota [LAB13], L. gasseri, and L. plantarum has shown obliteration of weight gain, fat deposits, and white adipose tissue (Barengolts 2016; Kim et al. 2019).
In another study, treatment of obese adults with L. gasseri (SBT2055 and BNR17) exhibited reduction in visceral adipose tissue as well as waist size (Kadooka et al. 2010; Kim et al. 2018). Similar study was reported by Pedret et al, where intervention with Bifidobacterium animalis subspecies. Lactis CECT 8145 reduced waist size, waist circumference/height ratio, and BMI considerably (Pedret et al. 2019). L. rhamnosus CGMCC1.3724 therapy displayed weight loss in obese women but nothing significant in obese men (Sanchez-Rodriguez et al. 2020).
Diet is a major player in obesity and has associations with gut microbiota (Brahe et al. 2016). It has been shown that the various diet styles such as western, vegetarian, gluten-free, and the Mediterranean diet disturb gut diversity (Lazar et al. 2019). The Western diet which comprises of high amount of sugar, salt, saturated fats, refined grains, and high fructose corn syrup with lesser amount of fiber is responsible for decrease in total gut microbiota amount along with reduction in beneficial bacteria such as Lactobacillus sp. and Bifidobacterium sp., thereby promoting inflammation and changing gut microbiota to obese pattern (Bell 2015; Statovci et al. 2017). One of the mechanisms can be improving energy harvesting by increase in Firmicutes for promoting better caloric absorption leading to weight gain (King et al. 2012).
Plant-based diet such as vegetarian and vegan diets is rich in dietary fiber and entails plant-derived products which is known to trigger an increase in the abundance of protective microbiota. This diet promotes an increase in (1) Bifidobacteria and Lactobacillus; known intestinal barrier protectors, (2) Faecalibacterium prausnitzii and Roseburia; butyrate producers, and a decrease in Escherichia coli and Enterobacter cloacae; inflammation-inducing lipopolysaccharide-producing bacteria, thus ultimately preventing obesity (Tomova et al. 2019; Glick-Bauer and Yeh 2014). Mediterranean diet is majorly comprised of vegetables, olive oil, fruits, a modest amount of poultry, with limited consumption of red meat and dairy products. This dietary habit correlates with higher abundance of Lactobacillus, Bifidobacterium, and Prevotella in the gut, which helps in preventing obesity by improving lipid and cholesterol profiles (Garcia-Mantrana et al. 2018; Coelho and Cândido 2019). Korean traditional diet consists of high amounts of vegetables, fermented foods with modest consumption of legumes and fish. Such a diet helps to prevent obesity by increasing abundance of Bacteroides (Bacteroidaceae) and Bifidobacterium (Bifidobacteriaceae-Actinobacteria) while decreasing Prevotella (Prevotellaceae) (Baik 2018; Lim et al. 2015). With the help of high-fiber diet, obesity can be managed through intestinal SCFA dependent modulation of downstream pathways (Barathikannan et al. 2019). In high-fat diet (HFD) fed mice, when HFD is replaced by treatment with L. rhamnosus GG, it reduces adiposity via the heightened production of adiponectin, thereby protecting the animal from insulin resistance as well as helping in diminishing liver adiposity (Kim et al. 2013a, b). Pasteurized nonviable Akkermansia muciniphila as a prebiotic treatment showed an increased ability to reduce the development of fat mass along with insulin resistance and dyslipidemia in mice (Plovier et al. 2017; Depommier et al. 2019).
7.4 Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is marked by hepatic steatosis and may advance to an inflammatory condition called nonalcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma. Gut microbiome and certain host factors have been linked to this condition (Grabherr et al. 2019).
What’s the connect: A large variation in terms of phylum, family, and genus was observed between healthy controls and NASH patients in several studies. NAFLD patients display upregulation and downregulation of a large array of organisms; the organisms which are enriched are Bacteroides, Ruminococcus, Lactobacillus (Genus), E. coli (Species), Lactobacillaceae (Family), and Proteobacteria (Phylum), while the downregulated organisms are Oscillibacter, Prevotella, Ruminococcus, Coprococcus (Genus), Faecalibacterium prausnitzii (Species), Actinobacteria, Bacteroidetes, and Firmicutes (Phylum) (Loomba et al. 2017; Del Chierico et al. 2017; Boursier et al. 2016; Da Silva et al. 2018).
NAFLD may be associated with low abundance of Faecalibacterium prausnitzii, a butyrate-producing bacterium from Firmicutes phylum, which was associated with >5% fat hepatic content and increased adipose tissue inflammation (Munukka et al. 2014, 2017). A gram-negative Proteobacterium Bilophilia wadsworthia, has been shown to aggravate high fat diet induced metabolic dysfunctions in mice. The mechanism followed is that it lowers the butyrate metabolism, which leads to disruption of the gut barrier (interrupted tight junctions), thus allowing the LPS circulation from the gut lumen into the incoming portal vein of the liver. Once there, it releases a pro-inflammatory cytokine by acting on the hepatic macrophages and promotes a reduction of bile acids production. All of these leads to a disrupted microbiota and hence the heightened release of LPSs (Feng et al. 2017; Natividad et al. 2018).
Helicobacter pylori is a gram-negative Proteobacterium which is responsible for immune resistance contributing to NAFLD. H. pylori infection can increase the chances of NAFLD development. However, to comprehend the association between H. pylori and NAFLD progression, more clinical studies are needed (Wijarnpreecha et al. 2018; Ning et al. 2019).
In a healthy state, ethyl alcohol is continuously being produced in the gut. In the healthy individual, it gets metabolized in the liver by alcohol dehydrogenase (ADH)/other hepatic enzymes. However, when alcohol-producing bacteria like Klebsiella pneumoniae increase in the gut, they produce reactive oxygen species (ROS) constantly due to exceeding of liver detoxification capacity which promotes hepatic inflammation, often ending in steatohepatitis (Yuan et al. 2019).
Obese and NAFLD animals have shown lesser abundance of Akkermansia muciniphila, a gram-negative bacterium from the phylum Verrucomicrobia with mucin degrading capacity than in their healthy counterparts (Everard et al. 2013; Zhao et al. 2017).
Therapeutics that help: The consumption of processed foods and beverages containing fructose was seen higher in NAFLD patients (Chen et al. 2017). NAFLD progression is also associated with lower fiber, polyphenols, vitamins (Vitamin D), and mineral nutrients (calcium) intake (Van Herck et al. 2017; Wehmeyer et al. 2016). Polyphenols like quercetin, epigallocatechin gallate, anthocyanins, and resveratrol have also been found to be protective (Wrzosek et al. 2013).
It has been reported that administration of a cocktail of Lactobacillus acidophilus ATCC B3208, Bifidobacterium lactis DSMZ 32,269, Bifidobacterium bifidum ATCC SD6576 and Lactobacillus rhamnosus DSMZ 21,690 to adolescents for 12 weeks in the form of probiotic capsules, resulted in substantial decrease in ALT (Alanine aminotransferase), lipid profile and intrahepatic fat content compared to placebo group. In another study, efficacy of ‘Symbiter,’ containing 14 alive probiotic strains of Lactobacillus + Lactococcus, Bifidobacterium, Propionibacterium, and Acetobacter, is assessed in NAFLD patients and has shown to improve hepatic steatosis, aminotransferase activity, TNF-α, and IL6 levels (Kobyliak et al. 2018). Another multistrain probiotic VSL#3 has been found to protect the integrity of intestinal barrier and diminish endotoxemia and oxidative/nitrosative stress, thus improving liver pathology in patients suffering from various chronic liver diseases (Loguercio et al. 2005). VSL#3 contains Bifidobacterium longum, and it adjusts gut microbiota in such a way that increases the production of conjugated linoleic acid (CLA); this further impacts fatty acid composition in the liver and in a way plays a significant role in therapeutic interventions (Meroni et al. 2019). When Bifidobacterium longum is administered in combination with prebiotic fructo-oligosaccharides (FOS), it considerably improves the metabolic and inflammatory markers and fibrosis scores in NASH patients (Malaguarnera et al. 2012).
7.5 Type 2 Diabetes
Diabetes mellitus (DM) is marked by chronic hyperglycaemia, as a result of deficits either in insulin secretion, or in insulin action, or both. 90–95% of diabetes cases are of type 2 (T2D) (Woldeamlak et al. 2019).
What’s the connect? Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, and Roseburia were found to be negatively related with T2D, while Ruminococcus, Fusobacterium, and Blautia were connected positively with T2D (Gurung et al. 2020). Patients treated with metformin or after undergoing gastric bypass surgery have found to be negatively associated with B. adolescentis, B. bifidum, B. pseudocatenulatum, B. longum, and B. dentium (Wu et al. 2017; Murphy et al. 2017). Bacteroides intestinalis, Bacteroides 203, and Bacteroides vulgatus were decreased in T2D patients and Bacteroides stercoris were enriched after sleeve gastrectomy (SG) surgery in T2D patients with diabetes remission (Wu et al. 2011; Murphy et al. 2017; Zhang et al. 2013; Karlsson et al. 2013).
Investigations have reported a negative association of Roseburia inulinivorans, Roseburia_272, and one unclassified OTU from this genus, with disease (Murphy et al. 2017; Zhang et al. 2013; Karlsson et al. 2013). Lower frequencies of Faecalibacterium were reported in patients in two case–control studies (Gao et al. 2018; Salamon et al. 2018). Also, after different types of antidiabetic treatments ranging from metformin and herbal medicine to bariatric surgery, decreased their abundance (Tong et al. 2018; Murphy et al. 2017). Half of the T2D studies showed that out of these five genera, Bacteroides, Bifidobacterium, Roseburia, Faecalibacterium, and Akkermansia, at least one is reduced, suggesting that they have a role which goes beyond serving as a biomarker (Gurung et al. 2020). There is an increase in organisms like L. acidophilus, L. gasseri, and L. salivarius, and reduction in L. amylovorus in T2D patients which signify species specificity (Karlsson et al. 2013; Graessler et al. 2013; Forslund et al. 2015). L. acidophilus, L. plantarum, and L. reuteri were found to have lower frequencies when compared with controls in this disease (Suceveanu et al. 2018).
Patients with T2D show raised levels of pro-inflammatory cytokines, chemokines, and inflammatory proteins. Also, increased gut permeability allows for passage of gut microbe-derived products into the blood. This causes metabolic endotoxemia, effects glucose homeostasis and insulin resistance in liver, muscle, and fat, and in addition affects the digestion of sugars and production of gut hormones that regulate glucose metabolism (Gurung et al. 2020). The microbes, like Fusobacterium nucleatum and Ruminococcus gnavus which are potentially harmful in T2D, induces various inflammatory cytokines (Yang et al. 2017a, b; Hall et al. 2017), in other inflammatory diseases.
The Firmicutes to Bacteroidetes ratio along with proportions of phylum Firmicutes and class Clostridia was reduced, while class Betaproteobacteria was increased in T2DM patients as per one of the case–control study (Larsen et al. 2010). Also, Faecalibacterium prausnitzii and genus Blautia were diminished in T2DM patients (Navab-Moghadam et al. 2017; Inoue et al. 2017). Increase in serum fructosamine is associated with the decrease in Prevotellaceae and increase in Enterobacteriaceae (Li et al. 2019a, b).
Therapeutics that help: There have been many animal studies conducted for diabetes. An improved glucose tolerance is seen in several Bifidobacterium spps, i.e., B. bifidum, B. longum, B. infantis, B. animalis, B. pseudocatenulatum, B. Breve (Le et al. 2015; Moya-Pérez et al. 2015; Kikuchi et al. 2018; Aoki et al. 2017; Wang et al. 2015a, b). In another study, an improvement in glucose tolerance and insulin resistance was seen in diabetic mice by providing Bacteroides acidifaciens and Bacteroides uniformis (Yang et al. 2017a, b; GauffinCano 2012). In human studies, progress in type 2 diabetes-related symptoms is seen by administration of L. sporogenes, L. casei Shirota, and L. reuteri used as monoprobiotics (Hulston et al. 2015; Simon et al. 2015; Asemi et al. 2014, 2016). Lactobacilli works more effectively as a part of probiotic cocktail than given individually in majority of cases (Ejtahed et al. 2012; Asemi et al. 2013; Tajabadi-Ebrahimi et al. 2016; Mohamadshahi et al. 2014). Among the several Lactobacillus species that have been tested as probiotics, L. plantarum, L. reuteri, L. casei, L. curvatus, L. gasseri, L. paracasei, L. rhamnosus, and L. Sakei have shown favorable effects on T2D in mice models (Gurung et al. 2020).
There have been different mechanisms found to reduce gut permeability like (1) Administering Bacteroides vulgatus and B. Dorei species (Yoshida et al. 2018); (2) Butyrate, produced by Faecalibacterium, via serotonin transporters and PPAR-g pathways (Kinoshita et al. 2002); (3) Akkermansia muciniphila, probiotic bacterium using extracellular vesicles which improve intestinal tight junctions via AMPK activation in epithelium (Chelakkot et al. 2018).
There has been an emerging focus on interactions between microbiota and antidiabetic drugs in microbiome research (Gurung et al. 2020). Different combinations have been used and been effective than when administered alone in reducing/improving T2D parameters, like reduced hyperglycemia, adiposity, improved fasting blood glucose, glucose tolerance, and insulin resistance in different studies. For example, (1) probiotic Bifidobacterium animalis ssp. lactis 420, prebiotic polydextrose in amalgamation with sitagliptin in diabetic mice (Salamon et al. 2018); (2) prebiotic polysaccharide in combination with metformin and sitagliptin in Zucker diabetic rats’ study (Reimer et al. 2014); (3) combination of a prebiotic and metformin in streptozotocin-induced diabetic mice (Zheng et al. 2018).
There has been inconsistency in identification of T2D-associated microbiota in humans because of several elements such as geographic location, race, culture, health status, and drug use. Stool samples are the preferred choice for microbiota analysis in most of the studies due to difficulties in sampling from human intestine, but, however, the stool sample does not represent entire gut microbiome profile (Gurung et al. 2020).
It has been observed that vegetables, fruits, dietary fibers, and medicinal plants reduce or prevent T2DM by raising the level of SCFAs and fine-tuning gut microbiota. Capsaicin can increase the Firmicutes to Bacteroidetes ratio, Roseburia number, and at the genus level reduce the quantities of Bacteroides and Parabacteroides which could reduce pro-inflammatory cytokines, such as TNF-α and IL-6 in obese diabetic mice (Song et al. 2017). Pumpkin polysaccharide can exert its antidiabetic effect by increase of selective bacteria, like Bacteroidetes, Prevotella, and Deltaproteobacteria in mice (Liu et al. 2018a, b). The Lactobacillus rhamnosus GG fermented carrot juice can improve T2DM in rats by increasing Oscillibacter and Akkermansia (Hu et al. 2019). The antidiabetic activity of Momordica charantia (bitter melon) is improved by Lactobacillus fermentation, which in turn can increase Bacteroides caecigallinarwn, Bacteroides thetaiotaomicron, Prevotella loescheii, Prevotella oralis, and Prevotella melaninogenica (Gao et al. 2018). The phlorizin in many fruits could regulate the blood glucose level by reducing serum LPS and insulin resistance, increasing butyric acid as well as increasing Akkermansia muciniphila and Prevotella (Mei et al. 2016). It has been reported that the extracts from cinnamon bark can improve glucose tolerance and insulin resistance by reducing Peptococcus, and the extracts from grape pomace in diabetic mice could decrease Desulfovibrio and Lactococcus, and increase Allobaculum and Roseburia (Van Hul et al. 2018). The inulin reduces the effects of T2DM in diabetic mice by increasing Cyanobacteria and Bacteroides and decreasing Deferribacteres and Tenericutes (Li et al. 2019a, b).
7.6 Inflammatory Bowel Disease (IBD)
IBD is a long-lasting heterogeneous, GI tract associated disorder, characterized by an inflammatory process and materializing in the form of Crohn’s disease (CD) or ulcerative colitis (UC) in different patients and differentially diagnosed by clinicians. Factors that could influence host–microbiome homeostasis such as host genetics, host immune system, shifts in diet, exposure to antimicrobials, urbanization, westernization (Statovci et al. 2017) of life style, and many more could predispose and trigger IBD.
What’s the connect? IBD was one of the three conditions that was explored in a longitudinal study of a cohort of 132 individuals over a period of 1 year in the Human Microbiome Program (iHMP) and is the most comprehensive study till date (Lloyd-Price et al. 2017). Interestingly, the dysbiosis consisted of changes in transcription profile of several microbial species and associated changes in host biochemical parameters such as considerable transition in acylcarnitine pools and bile acids and heightened levels of serum antibody. IBD patients go through a cycle of dysbiosis and non-dysbiosis phases. Taxonomic perturbations during dysbiosis included reduction in obligate anaerobes such as Faecalibacterium prausnitzii and Roseburia hominis and the increase in facultative anaerobes such as E. coli in Crohn’s disease.
Therapeutics that help: Probiotics, especially Bifidobacteria, are linked to suppression of mucosal inflammation in animal models of IBD. In humans, however, the evidence for probiotic efficacy is less positive for maintenance of remission of CD, based on meta-analysis of eight clinical trials, mainly due to variance in study design. Inclusion of probiotics with conventional treatment for UC did not improve the overall remission rates in mild-to-moderate UC, but it was possible to generate a slight decrease in disease activity. The mixture VSL#3 (composed of four lactobacillus strains; three from bifidobacteria and S. thermophilus) has shown positive effects in UC treatment, whereas indications for probiotic efficacy in CD are low. At the current stage, a conclusion has been reached that use of probiotics in IBD cannot be recommended. Studies have been conducted using a diversity of bacterial strains in different clinical situations, but only a few patients have been enrolled in these studies. Hence, more randomized trials with statistically sound study design may give us better clarity (Jadhav et al. 2020).
Among microbially derived products that have been shown to be protective in colitis, polysaccharide A from Bacteroides fragilis and butyrate from Clostridial spp. induce peripheral regulatory T cells. Favorable effects of FMT on UC (compared to control treatment) have been published. However, there are many parameters such as donor selection, administration routes, frequency of FMT and the development of easy-to-administer formulation, that need to be optimized and validated before FMT is ready to be offered as a standard therapy for UC.
7.7 Irritable Bowel Syndrome (IBS)
IBS is a disorder associated with the gastrointestinal tract and is marked by a long-lasting, persistent discomfort and pain in abdomen and, with altered bowel behaviors. Mainly based on the stool pattern, IBS patients can be categorized into (1) IBS with constipation (IBS-C), (2) IBS with diarrhea (IBS-D), (3) IBS with mixed bowel habits (IBS-M), and (4) unclassified IBS. The other associated comorbidities linked with IBS are profoundly psychiatric in nature such as depression, anxiety, and somatoform disorders (Chong et al. 2019).
What’s the connect? In a healthy gut, the mucus epithelium and homeostatic immune responses limit microbes both symbiotic and commensal from breaching the epithelial barrier surface. Nevertheless, when the influx of any agent breaches the barrier, provoking intense immune reactions, it leads to severe inflammation. This in turn impacts the intestinal environment including the alteration in the composition of gut microbiota (Pédron et al. 2016). Such underlying conditions with wider consequences on the gut neuromuscular junction and gut–brain axis could contribute to IBS pathogenesis. Gut dysbiosis accompanying the changes from beneficial bacteria to pathogens in the human gut has been reported (Carroll et al. 2011, 2012). It is not surprising that IBS patients are highly deficient in the beneficial activities of Lactobacilli and Bifidobacteria (Bellini et al. 2014). Interestingly, there seems to be a direct correlation between the severity of IBS with low levels of exhaled methane, decreased microbial richness, absence of Methanobacteriales, and enrichment with Bacteroides enterotypes compared to that in controls (Tap et al. 2017).
Disease conditions with altered gut mycobiome profile are attracting the attention of researchers, especially in immunocompromised patients. IBS is another condition where the connection of microbiome alterations and the occurrence of visceral hypersensitivity indicate the role of gut microbiome in the disease pathogenesis (Botschuijver et al. 2017).
Therapeutics that help: Probiotics specially Lactobacillus plantarum is found to be most beneficial in IBS by improving visceral sensitivity, intestinal permeability, and inflammation (Ohman and Simrén 2013). Maintaining a food and symptom diary can help patients determine which foods trigger symptoms. Specialized diets such as where fermentable oligo-, di- and monosaccharide, and polyol (FODMAPs) are less may improve symptoms in individual IBS patients, though the safety of this diet such as the possibility of malnutrition needs to be monitored in the long term (Ferreira et al. 2020).
7.8 Fecal Microbial Transplantation in Clostridium difficile Infections
The commensal microbes of the gut have the capacity to defend against pathogenic invasion by either competing for resources or waging microbial warfare. Consumption of prescribed broad-spectrum antibiotics while controlling the ‘bad infection’ eliminates these beneficial commensals from the gut. Some individuals are then predisposed to opportunistic pathogens such as Clostridium difficile resulting in diarrhea and associated symptoms. Van Nood in 2014 reviewed the efficacy of FMT in treating a variety of dysbiotic states (van Nood et al. 2014). It is encouraging that FMT treatment showed around 90% success rate in treating C. difficile-induced severe GI dysbiosis. In a patient with persistent C. difficile, associated with diarrhea, the fecal microbiota both 2 weeks and 1-month post-FMT consisted mostly of the bacteria from the healthy donor and normalized the patient’s bowel function, thus demonstrating the success of the treatment. Until systematic trials evaluate both safety and effectiveness of FMT, its efficacy is currently on a case-by-case basis. In order to enhance treatment reliability and mitigate safety concerns, it is important to identify the minimal subset of microbes needed to achieve therapeutic efficacy and formulate the treatment using appropriate guidelines (Petrof et al. 2013).
7.9 Chronic Kidney Disease
Chronic kidney disease (CKD) is emerging health problem, and its occurrence is due to important risk factors like diabetes, hypertension, and obesity affecting about 10%of the population worldwide (Hall et al. 2014). The microbiome has recently gained lot of importance and known to impart an important contribution in health and disease (Hobby et al. 2019).
What’s the connect: Renal failure may occur due to high levels of urea in blood, and in the presence of intestinal bacteria, it is converted to ammonia by urease produced by intestinal bacteria leading to overgrowth of bacterial families. Patients with end-stage renal disease (ESRD) have shown to have an expansion of uricase and indole-forming and p-cresyl-forming enzyme-producing bacteria compared to healthy controls (Wong et al. 2014). The most extensively studied gut-derived uremic toxins, i.e., p-cresyl sulfate and indoxyl sulfate, arise from the colon with increased concentration and decline in renal function (Aronov et al. 2011; Ramezani and Raj 2014). In one study, the patients with ESRD have shown largest increase in Actinobacteria, Firmicutes, and Proteobacteria compared to healthy controls (Vaziri et al. 2013). Bifidobacterium catenulatum, Bifidobacterium longum, Bifidobacterium bifidum, Lactobacillus plantarum, Lactobacillus paracasei, and Klebsiella pneumoniae (Wang et al. 2012) are hardly seen in patients on peritoneal dialysis.
Research from different groups have shown that patients undergoing hemodialysis have higher number of Enterobacteria and Enterococci, facultative anaerobic bacteria, and lower abundance particularly in the genera Bifidobacteria with a related higher abundance of Clostridium perfringens than healthy controls (Kieffer et al. 2016).
In CKD and ESRD, patients who have reduced intake of fiber and decreased colonic time have encountered comorbidities such as diabetes (Yasuda et al. 2002). The disintegration of the epithelial barrier of the gut is mainly due to ammonia and ammonium hydroxide derived from urea which allows absorption of the toxins produced by microbes, thus leading to systemic inflammation which lead to anemia, protein wasting, and cardiovascular disease (Wong et al. 2014).
Therapeutics that help: In rats with reduced renal function, AST-120, an oral adsorbent, helps in restoring the epithelial tight junction proteins by absorbing uremic toxins produced in gut and thus reducing the levels of endotoxin and markers of oxidative stress and inflammation (Redman et al. 2014). There may be increase in SCFA-producing bacteria due to increased intake of resistant starches reducing loss of renal function, interstitial fibrosis, renal tubular damage, and activation of pro-inflammatory molecules (Vaziri et al. 2014).
The serum levels of indoxyl sulfate have been reduced due to administration of B. Longum orally (Takayama et al. 2003). In hemodialysis patients, plasma levels of indoxyl sulfate have been reduced due to increase in dietary fiber (Sirich et al. 2014). In vitro studies and in clinical studies, indoxyl sulfate has been reduced due to Streptococcus thermophilus (Vitetta et al. 2019).
The metabolism of gut microbiota of CKD patients can be modulated by dietary fiber which helps in improving CKD by reducing uremic toxins and also improves uremic symptoms and comorbidities in dialysis patients. However, more clinical studies are needed to be carried out in order to evaluate the multidimensional benefits of a fiber-rich diet in CKD and to determine the effect of different kinds of fiber in terms of quality as well as quantity (Camerotto et al. 2019).
7.10 Cancer
What’s the connect? Gut microbiome has played a significant role in tumor development and effective anticancer therapies (Heshiki et al. 2020). Case in point is the gut flora metabolizing bile acids which then redistribute and regulate the employment of natural killer T cells to tumorous liver cells (Ma et al. 2018). Another example is those gut microbes which metabolize estrogen and hence potentially altering the risk of postmenopausal estrogen receptor-positive breast cancer (Kwa et al. 2016). Another case would be the microbes which take glucocorticoids, present in the gut and urinary tract of men (leading to prostate cancer), and metabolize it to produce 11-oxyandrogens (Devendran et al. 2017, 2018; Zimmermann et al. 2019).
Gut microbiota can act as a tumor promoter (Vivarelli et al. 2019). Helicobacter pylori accounts for 90% of gastric cancers and is also considered to be a component of stomach microbiome (Xavier et al. 2020). CagA protein (cytotoxin associated gene A) from Helicobacter pylori was the first bacteria-derived protein to have a role in human cancer (Hatakeyama 2017). It assists in increase of gastric cancer by inducing the breakdown of p53 in gastric epithelial cells, by interfering with the host’s AKT pathway (Buti et al. 2011). Beside this CagA protein, there are others as well such as Fusobacterium nucleatum-derived effector adhesin A (FadA) and Bacteroides fragilis-derived metalloproteinase toxin (MP toxin) which promote cell proliferation and cancerogenic transformation of affected host’s cells by interacting with epithelial E-cadherin of host, directly or indirectly hampering the intercellular junctions and triggering β-catenin signaling (Murata-Kamiya et al. 2007; Rubinstein et al. 2013; Wu et al. 2007).
Similarly, Salmonella enterica effector avirulence protein A (AvrA) with its intrinsic deubiquitinase activity activates β-catenin thereby enhancing cell proliferation and promoting colonic tumorigenesis (Lu et al. 2014). Escherichia coli-derived colibactin and cytolethal distending toxin (CDT), when released near gastrointestinal epithelium, release toxins. This creates break in double-stranded DNA within the host’s epithelial cells, thus inducing a temporary cell cycle arrest, contributing to genomic instability which ultimately leads to tumor initiation and progression in those predisposed cells (Lara-Tejero 2000). This is generally encountered during the dysbiosis of gut microbiome led by pathogenic infections (Halazonetis 2004; Frisan 2016).
H. pylori colonization may harm human health by causing gastroesophageal reflux disease and its sequelae, Barrett’s esophagus, and adenocarcinoma of the esophagus (Blaser and Atherton 2004) and benefit human health (by protecting against asthma, multiple sclerosis, and IBD (Chen et al. 2017; Kira and Isobe 2019; Piovani et al. 2019; Gravina et al. 2018; Hosseininasab Nodoushan and Nabavi 2019).
There are bacteria which block tumorigenesis inhibiting immune effectors and thus induce cancer formation. Fusobacterium nucleatum indirectly helps in onset of cancer by inhibiting its own hosts natural killer (NK) cells (Gur et al. 2015). Few microbiota may obstruct the host hormones metabolism. Clostridium leptum and Clostridium coccoides secrete β-glucuronidase and increased level of this enzyme when coupled with gut dysbiosis results in deconjugating liver-catabolized and plant-based estrogens. This in turn allows to bind and trigger the estrogen receptors leading to cell proliferation in estrogen related tissues, i.e., as breast and endometrium. There is a heightened risk of breast cancer due to intake of estrogen hormones (Fernández et al. 2018).
Some gut microbiota act as a tumor suppressor. Microbial-derived SCFAs like butyrate and propionate are able to exhibit an anticancer effect by inhibiting host’s tumor cells histone deacetylases (Vivarelli et al. 2019). Case in point is the extensively studied LPS (bacterial lipopolysaccharide), which triggers the surface toll-like receptor 4 (TLR4) on the host’s cell. This starts a immune response (T-cell mediated) against cancer cells (Paulos et al. 2007). Similarly, the Salmonella enterica-derived monophosphoryl lipid A (MPL) is presently used in the vaccine as adjuvant, against anticervical carcinoma (Paavonen et al. 2009). Furthermore, pyridoxine, a group B vitamin from bacteria, is capable of stimulating antitumoral immunosurveillance in host (Aranda et al. 2015). The chapter dedicated to the role of the microbiome in immunotherapy will shed more light on that aspect.
Western diet can cause increase in intestinal tumors due to high animal fat and protein which increases bile secretion into the GI tract (Goncalves et al. 2019). Bilophila wadsworthia metabolizes taurine into acetate and ammonia, along with release of hydrogen sulfide, a carcinogenic gas (Ridlon et al. 2006). In colorectal cancer, Fusobacterium nucleatum is one of the most predominant bacteria (Bullman et al. 2017).
Therapeutics that help: The use of probiotics synergistically with pain killers such as opioids is being studied (Rousseaux et al. 2007). Many probiotics have shown a potential antineoplastic activity. For example, ferrichrome, a Lactobacillus casei-derived metabolite, is able to trigger apoptosis in tumor cells via direct activation of JNK pathway (Konishi et al. 2016). It has been appeared in several studies that Lactobacilli may eliminate cancerous or precancerous cells by stimulation of immune cells like NK cells or dendritic cells (DC) or TH1 response in the host (Lenoir et al. 2016; Lee et al. 2004; Baldwin et al. 2010; Takagi et al. 2008). Lactobacillus casei when orally administered reduces the reappearance of superficial bladder cancer (Aso and Akazan 1992). Lactobacillus rhamnosus GG (LGG) is a gut-resident bacterium which modulates several proliferation pathways of host, for example, mTOR or WNT (Taherian-Esfahani et al. 2016), and thus wield either antiproliferative effects or antimetastatic effects (Orlando et al. 2016; Nouri et al. 2016; Zhao et al. 2017; Behzadi et al. 2017; Chen et al. 2017). This can influence immune system of host, consequently helping in eliminating newly developing cancer cells (Gamallat et al. 2016). LGG can exert an anti-inflammatory profile by triggering an immune response within the normal untransformed gut epithelium (Suzuki et al. 2017) as well as changing gene expression in intestinal porcine epithelial cells and intestine myofibroblasts (Taranu et al. 2018; Uribe et al. 2018). LGG can serve as an apt candidate as a possible adjuvant in integrated anticancer therapies (Vivarelli et al. 2019).
The main purpose of giving probiotics especially Lactobacilli, to cancer patients, is to reestablish the levels and function of the beneficial bacteria which gets exhausted after the treatments, thus restoring the microbiota balance in the patients’ gut (Zitvogel et al. 2018). At the same time, probiotics may possess a likely risk of development of opportunistic bacteria/infection and exhibit antibiotic resistance when administered to immunocompromised cancer patients (Vanderhoof and Young 2008; Redman et al. 2014).
In 2014, it was observed in a double-blind control trial that Bifilact (Lactobacillus acidophilus LAC361 and Bifidobacterium longum BB536) administration could considerably reduce both moderate and severe diarrhea induced by the pelvic radiation treatment (Demers et al. 2014). On similar front, in 2015, a probiotic formulation Colon Dophilus (a cocktail of ten different strains) was evaluated in a clinical trial in metastatic CRC patients treated with irinotecan-based chemotherapy. Prevention of diarrhea in such patients suggested that the administration of such probiotics facilitates in the reduction of the occurrence as well as severity of diarrhea and gastrointestinal toxicity induced by the chemotherapy and is considered to be safe (Mego et al. 2015). In 2016, it was observed that probiotic Saccharomyces boulardii has the ability to downregulate pro-inflammatory cytokines in treated patients, with no beneficial effects on the postoperative infection rates (Consoli et al. 2016). In 2017, it was observed that the epigenetic patterns of tumor tissue from its baseline can be altered, with potential therapeutic benefits by the administration of Bifidobacterium lactis and Lactobacillus acidophilus to CRC patients (Hibberd et al. 2017).
7.11 Mental Disorders
Mood disorders, such as depression and bipolar disorder (BD), have known to cause significant individual and socioeconomic burdens affecting around 10% of the world’s population (Wittchen 2012). When compared to the normal population, people with mood disorder incline toward higher mortality rates and reduced life span (Angst et al. 1999; Kessing et al. 2015).
What’s the connect? The human gut has trillions of bacterial which are known to play a critical role in communication between gut and brain via influencing neural, immune, and endocrine pathways (Dupont et al. 2020). Patients with a variety of psychiatric disordered such as BD, schizophrenia, depression, and autism spectrum disorder display a significant difference in the composition of the gut microbiome. Thus, there is renewed interest in treating the gut microbiome as a potential therapeutic target in psychiatry. Psychobiotics, a recently coined term, refers to favorable microbes that may provide value in diagnosed mental disorders cases. The term refers to ‘a live microorganism that, when taken in adequate amounts, produces a beneficial health benefit in patients suffering from psychiatric illness’ (Dinan et al. 2013). The research on gut microbiome related to mood disorders is still in its nascent stage. Although growing evidence suggest that alteration in the gut microbiome plays a significant role in patients with mood disorders, the cause–effect relationship is yet to be determined. In major depressive disorder (MDD) and bioplar disorder, a consistent surge in the abundance of Actinobacteria and Enterobacteriaceae and a decline in Faecalibacterium were observed in different studies (Sowa-Kućma et al. 2018). Raised levels of Clostridium sp. have constantly been observed in patients with autism spectrum disorder. These discoveries imply that in patients with mood disorders, a decrease in bacterial genera which produces short-chain fatty acids and an increase in pro-inflammatory genera can be linked to chronic, low-grade systemic inflammation. On the other hand, till now, a clear relation between gut microbiota composition and anxiety disorders has been not been reported (Groen et al. 2018).
Exploring microbiome therapeutics: Changing the diet and incorporating probiotics/prebiotics in order to enhance the beneficial bacteria in the gut in both healthy and patient groups are likely to improve mood and decrease anxiety. Diet and food habit/pattern lead to the formation of gut microbiota which in turn regulate the host inflammation and thrombosis, causing brain disorders (Zhu et al. 2020a, b). For depression and other mental disorders, omega-3 fatty acids, ions such as zinc and magnesium, and plant phytochemicals are found to be more relevant nutrients (Kaplan et al. 2015; Trebatická and Ďuračková 2015; Haider et al. 2015; Bouayed et al. 2007; Solanki et al. 2015). Epidemiological studies have stated that traditional diet styles lead toward better mental health and hence reduce the risk of depression (Jacka et al. 2011; Jacka et al. 2010; Sánchez-Villegas et al. 2009; Skarupski et al. 2013; Rienks et al. 2013).
Food rich in polyphenol such as green tea, coffee, cocoa, curcumin, and other polyphenol-rich foods is connected with better mood, less fatigue, and hence reduced risk of depression in humans (Pham et al. 2014; Sathyapalan et al. 2010; Pase et al. 2013; Yu et al. 2015). These foods in preclinical settings prevent dysbiosis by inducing the growth of beneficial bacteria (Seo et al. 2015; Mills et al. 2015; Massot-Cladera et al. 2012; McFadden et al. 2015). It emerges that microbially transformed phytochemicals (e.g., quercetin after it has been subjected to fermentation) can alter the gut microbiota in healthy ways (i.e., growth of bifidobacteria and decrease in the ratio of Firmicutes to Bacteroidetes) (Parkar et al. 2013). Honey is an important diet constituent, high in phytochemicals such as phenolic acid and that of flavonoid families (Alvarez-Suarez et al. 2013), and has antidepressant and anxiolytic properties too (Azman et al. 2015; Mijanur Rahman et al. 2014). Honey flavonoids when stimulated by LPS hinder the release of pro-inflammatory cytokines, e.g., tumor necrosis factor alpha and interleukin-1 beta from microglia in a significant manner (Candiracci et al. 2012).
Free radicals or reactive oxygen species (ROS) lead to oxidative stress and trigger the pro-inflammatory avalanche, which involved IL-6 and CRP, both linked with depression. Several dietary antioxidants such as curcumin, ascorbic acid, carotenoids, and flavonoids are found to diminish depressive symptoms, either directly or linked to a lower occurrence of depression (Christy Harrison 2020).
Fermentation leads to transformation of bioavailable phytochemical structures and peptides which could lead to novel therapeutics (Selhub et al. 2014). It has been reported that consumption of fermented food results in reduced social anxiety, particularly in people with high neuroticism scores (Hilimire et al. 2015). Fermented food has heat-inactivated microorganisms, and their structural parts may also have significant effects on intestinal ecosystems (Yang et al. 2014).
7.12 Rheumatoid Arthritis
Rheumatoid arthritis (RA), an autoimmune disorder, is dependent on/controlled by/related to several factors, both genetic and environmental. Since it targets the self-antigens present in the synovium, cartilage, and bone, it leads to destruction of the joints and hence functional disability in most patient (Halpern et al. 2009; Tran et al. 2005). Majority of the drugs available for the treatment of RA today (including DMRDs or disease-modifying antirheumatic drugs) target cytokines, nonspecific immune suppression, or T-cell and B-cell activation (Isaacs 2008; Kumar and Banik 2013; Benjamin et al. 2018). Besides the genetic factors, diet, smoking, and stress are some of the environmental factors which influence the microbiota diversity/composition and hence the onset/outcome of RA.
What’s the connect? The onset of autoimmunity may be associated with gastrointestinal tract. It is found that microbial composition in subjects with early rheumatoid arthritis differed from controls, with a reduction of bacteria from the family Bifidobacterium and Bacteroides (Vaahtovuo et al. 2008), and a noticeable increase of species from the genus Prevotella (Bernard 2014). Most of the studies indicated that treatment with MTX and HCQ leads to partial restoration of dysbiotic microbiomes to normal or alteration to increase abundance of beneficial microbial members (Zhang et al. 2015). Methotrexate reduced the abundance of Enterobacteriaceae and partially restored the gut microbiota in patients. Hydroxychloroquine increased the gut microbial species’ richness and diversity. Hydroxychloroquine plus doxycycline treatment led to the reduction in abundance of phylum Bacteroidetes and Firmicutes (Bodkhe et al. 2019).
Recent studies in humans and mouse models of arthritis indicate that periodontal disease is linked with a heightened risk of RA (Scher et al. 2012; Arvikar et al. 2013; de Aquino et al. 2014). The presence of periodontitis is associated with anti-CCP antibody levels in RA patients (Dissick et al. 2010). Treatment of periodontitis enhances the disease activity of RA (Ortiz et al. 2009; Al-Katma et al. 2007). It is found that periodontal bacteria such as P. Gingivalis and A. actinomycetemcomitans induce the production of anti-CCP antibodies, which in turn leads to the arthritis thus suggesting the link of periodontal bacteria with RA pathogenesis. Another evidence comes from the finding that DNA of oral cavity bacteria such as Fusobacterium nucleatum and Porphyromonas gingivalis has been detected in the synovial fluid of RA patients (Reichert et al. 2013; Stephanie et al. 2012). In order to establish the mechanistic link of the oral cavity bacteria with that of development of RA in humans, more studies are needed. Oral microbiome and periodontal disease are described in another chapter in further detail.
Exploring microbiome therapeutics: Randomized controlled trials with probiotic bacteria like Lactobacillus rhamnosus, (Pineda et al. 2011; Hatakka et al. 2003), Lactobacillus casei (Vaghef-Mehrabany et al. 2014), Bacillus coagulans (Mandel et al. 2010), Lactobacillus reuteri, Lactobacillus acidophilus, and Bifidobacterium bifidum have been carried out to determine their ability to treat RA. Randomized controlled trials with probiotic bacteria have been studied for their ability to treat RA, like Lactobacillus rhamnosus, (Pineda et al. 2011; Hatakka et al. 2003) Lactobacillus casei (Vaghef-Mehrabany et al. 2014), Bacillus coagulans (Mandel et al. 2010) Lactobacillus reuteri, Lactobacillus acidophilus, and Bifidobacterium bifidum. These probiotics have appeared to be effective for RA patients and are considered safe (Zamani et al. 2016). However, since there is limited data on the interaction between drug and microbiomes and probiotics for RA, further studies are needed for a probiotic to be used for immune homeostasis. This could be helpful in personalized medicine where a tailor-made probiotic (targeted organism/metabolite which is in low abundance) can be supplemented to create immune homeostasis in patients.
8 Conclusion
Acknowledging the impact that the microbiome has on human health and well-being by scientific and nonscientific communities has allowed us to get curious and explore this area in our own way. The existing approaches of microbiome diagnostics and therapeutics need to evolve keeping in pace with current technologies. New microbiome diagnostic marker panels developed based on Next-Gen sequencing can be used to diagnose (and potentially prognos) disease by clinicians. Predictive medicine using AI/ML algorithm-based analysis of patient’s microbiome could predict the outcome of treatment options. To revolutionize microbiome therapeutics, the treatment options should move from general to personalized ones. Such personalized treatment strategy can be created based on the gut microbiome of an individual (patient-specific). The treatment options can range from ‘precision probiotics’ where a specific microbial cocktail can be administered to alter the species richness or ‘precision prebiotics’ where targeted microbial nutrition is given to promote growth of certain microbes or ‘personalized dietary interventions’ or ‘targeted subtractive theory’ (Fig. 10.5). Microbiome time series analysis to follow-up treatment success and establishment of normobiosis will allow early intervention and tweaking of treatment plans for better patient outcomes. Nevertheless, though the challenges are many, it is achievable. It will be thrilling to witness this microbiome-based therapeutic model being adopted in routine clinical practice and becoming a true translational discipline in the near future!
A schematic illustration showing various aspects associated with microbiome. Microbiome balance is affected by many factors (left panel) and leads to disease condition (top-right panel). The use of broad range antimicrobial may lead to further disbalance the microbiome by killing the good bacteria along with the targeted bad ones. Microbiome diagnostic (bottom-left panel) can reveal the upregulated/downregulated microbes in disease condition. The advent in sequencing leads to better diagnostic, and the better the diagnostic, the better will be the therapeutics (bottom-left panel). Therapeutics involves the microbes either directly (live microbes) or indirectly (metabolites from bacteria or products to support the growth of good bacteria). (Allaband et al. 2019; Espinoza 2018; FitzGerald and Spek 2020; Raes 2016)
References
Aagaard K, Petrosino J, Keitel W, Watson M, Katancik J, Garcia N, Patel S, Cutting M, Madden T, Hamilton H, Harris E, Gevers D, Simone G, McInnes P, Versalovic J (2013) The human microbiome project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J 27:1012–1022
Alfano A, Perillo F, Fusco A, Savio V, Corsaro MM, Donnarumma G, Schiraldi C, Cimini D (2020) Lactobacillus brevis CD2: fermentation strategies and extracellular metabolites characterization. Probiotics Antimicrob Proteins 12(4):1542–1554
Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD (2007) Control of periodontal infection reduces the severity of active rheumatoid arthritis. JCR J Clin Rheumatol 13:134–137
Allaband C, McDonald D, Vázquez-Baeza Y, Minich JJ, Tripathi A, Brenner DA, Loomba R, Smarr L, Sandborn WJ, Schnabl B, Dorrestein P, Zarrinpar A, Knight R (2019) Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin Gastroenterol Hepatol 17(2):218–230
Alneberg J, Bjarnason BS, de Bruijn I, Schirmer M, Quick J, Ijaz UZ, Lahti L, Loman NJ, Andersson AF, Quince C (2014) Binning metagenomic contigs by coverage and composition. Nat Methods 11:1144–1146
Álvarez-Mercado AI, Navarro-Oliveros M, Robles-Sánchez C, Plaza-Díaz J, Sáez-Lara MJ, Muñoz-Quezada S, Fontana L, Abadía-Molina F (2019) Microbial population changes and their relationship with human health and disease. Microorganisms 7:1–27
Alvarez-Suarez J, Giampieri F, Battino M (2013) Honey as a source of dietary antioxidants: structures, bioavailability and evidence of protective effects against human chronic diseases. Curr Med Chem 20:621–638
Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferriéres J (2008) Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 87:1219–1223
Ang Z, Ding JL (2016) GPR41 and GPR43 in obesity and inflammation–protective or causative? Front Immunol 7
Angst J, Angst F, Stassen HH (1999) Suicide risk in patients with major depressive disorder. J Clin Psychiatry 60(2):57–62. discussion 75-76, 113–116
Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, Hattori M, Koga Y (2017) A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep 7:43522
Aranda F, Bloy N, Pesquet J, Petit B, Chaba K, Sauvat A, Kepp O, Khadra N, Enot D, Pfirschke C, Pittet M, Zitvogel L, Kroemer G, Senovilla L (2015) Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene 34:3053–3062
Aronov PA, Luo FJ-G, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW (2011) Colonic contribution to uremic solutes. J Am Soc Nephrol 22:1769–1776
Arvikar SL, Collier DS, Fisher MC, Unizony S, Cohen GL, McHugh G, Kawai T, Strle K, Steere AC (2013) Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther 15:R109
Asemi Z, Alizadeh S-A, Ahmad K, Goli M, Esmaillzadeh A (2016) Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 35:819–825
Asemi Z, Khorrami-Rad A, Alizadeh S-A, Shakeri H, Esmaillzadeh A (2014) Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 33:198–203
Asemi Z, Zare Z, Shakeri H, Sabihi S, Esmaillzadeh A (2013) Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab 63:1–9
Aso Y, Akazan H (1992) Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. Urol Int 49:125–129
Azman K, Othman Z, Zakaria R, Abd Aziz C, Al-Rahbi B (2015) Tualang honey improves memory performance and decreases depressive-like behavior in rats exposed to loud noise stress. Noise Health 17:83
Bäckhed F, Fraser CM, Ringel Y et al (2012) Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe 12(5):611–622
Baik I (2018) Forecasting obesity prevalence in Korean adults for the years 2020 and 2030 by the analysis of contributing factors. Nutr Res Pract 12:251
Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AMS, Ramakrishna BS (2010) Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr 103:335–338
Baldwin C, Millette M, Oth D, Ruiz MT, Luquet F-M, Lacroix M (2010) Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer 62:371–378
Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M (2016) The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis 15:108
Barathikannan K, Chelliah R, Rubab M, Daliri EB-M, Elahi F, Kim D-H, Agastian P, Oh S-Y, Oh DH (2019) Gut microbiome modulation based on probiotic application for anti-obesity: a review on efficacy and validation. Microorganisms 7:456
Barengolts E (2016) Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endocr Pract 22:1224–1234
Behzadi E, Mahmoodzadeh Hosseini H, Imani Fooladi AA (2017) The inhibitory impacts of lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb Pathog 110:1–6
Bell DSH (2015) Changes seen in gut bacteria content and distribution with obesity: causation or association? Postgrad Med 127:863–868
Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P (2014) Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. World J Gastroenterol 20(27):8807–8820
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P (2018) Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 137
Bernard NJ (2014) Prevotellacopri associated with new-onset untreated RA. Nat Rev Rheumatol 10:2–2
Blaser MJ, Atherton JC (2004) Helicobacter pylori persistence: biology and disease. J Clin Invest 113:321–333
Bodkhe R, Balakrishnan B, Taneja V (2019) The role of microbiome in rheumatoid arthritis treatment. Ther Adv Musculoskelet Dis 11:1759720X1984463
Botschuijver et al (2017) Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gasteroenterology 153(4):1026–1039
Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R (2007) Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J Neurol Sci 262:77–84
Boulanger C, Lüscher TF (1990) Release of endothelin from the porcine aorta inhibition by endothelium-derived nitric oxide. J Clin Invest 85:587–590
Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM (2016) The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63:764–775
Brahe LK, Astrup A, Larsen LH (2016) Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Adv Nutr 7:90–101
Brandt LJ (2013) FMT: first step in a long journey. Am J Gastroenterol 108:1367–1368
Brantsaeter AL, Myhre R, Haugen M, Myking S, Sengpiel V, Magnus P, Jacobsson B, Meltzer HM (2011) Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian mother and child cohort study. Am J Epidemiol 174:807–815
Brinkworth GD, Noakes M, Clifton PM, Bird AR (2009) Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr 101:1493
Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T, Chipashvili O, Hagan T, Walker M, Ramachandran A, Diosdado B, Serna G, Mulet N, Landolfi S, Ramon Y, Cajal S, Fasani R, Aguirre AJ, Ng K, Elez E, Ogino S, Tabernero J, Fuchs CS, Hahn WC, Nuciforo P, Meyerson M (2017) Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358:1443–1448
Buti L, Spooner E, Van der Veen AG, Rappuoli R, Covacci A, Ploegh HL (2011) Helicobacter pylori cytotoxin-associated gene a (cag a) subverts the apoptosis-stimulating protein of p 53 (ASPP2) tumor suppressor pathway of the host. Proc Natl Acad Sci 108:9238–9243
Camerotto C, Cupisti A, D’Alessandro C, Muzio F, Gallieni M (2019) Dietary fiber and gut microbiota in renal diets. Nutrients 11:2149
Candiracci M, Piatti E, Dominguez-Barragán M, García-Antrás D, Morgado B, Ruano D, Gutiérrez JF, Parrado J, Castaño A (2012) Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J Agric Food Chem 60:12304–12311
Canfora EE, Jocken JW, Blaak EE (2015) Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11:577–591
Canfora EE, Meex RCR, Venema K, Blaak EE (2019) Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol 15:261–273
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Carroll IM, Ringel-Kulka T, Keku TO, Chang Y-H, Packey CD, Sartor RB, Ringel Y (2011) Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol-Gastrointest Liver Physiol 301(5):G799–G807
Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y (2012) Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 24(6):521–e248
Catry E, Bindels LB, Tailleux A, Lestavel S, Neyrinck AM, Goossens J-F, Lobysheva I, Plovier H, Essaghir A, Demoulin J-B, Bouzin C, Pachikian BD, Cani PD, Staels B, Dessy C, Delzenne NM (2018) Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 67:271–283
Cerdó T, García-Santos J, Bermúdez M, Campoy C (2019) The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 11:635
Chambers ES, Preston T, Frost G, Morrison DJ (2018) Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep 7:198–206
Chawla A, Nguyen KD, Goh YPS (2011) Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 11:738–749
Chelakkot C, Choi Y, Kim D-K, Park HT, Ghim J, Kwon Y, Jeon J, Kim M-S, Jee Y-K, Gho YS, Park H-S, Kim Y-K, Ryu SH (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 50:e450–e450
Chen C, Xun P, Tsinovoi C, He K (2017) Accumulated evidence on helicobacter pylori infection and the risk of asthma. Ann Allergy Asthma Immunol 119:137–145
Chen M, Yi L, Zhang Y, Zhou X, Ran L, Yang J, Zhu J, Zhang Q, Mi M (2016) Resveratrol attenuates trimethylamine- N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 2016:7
Chen Q, Wang T, Li J, Wang S, Qiu F, Yu H, Zhang Y, Wang T (2017b) Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD). Nutrients 9:96
Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC (2019) The microbiome and irritable bowel syndrome - a review on the pathophysiology, current research and future therapy. Front Microbiol 10:1–23
Cluny NL, Eller LK, Keenan CM, Reimer RA, Sharkey KA (2015) Interactive effects of oligofructose and obesity predisposition on gut hormones and microbiota in diet-induced obese rats. Obesity 23:769–778
Coelho OGL, Cândido FG, de CG AR (2019) Dietary fat and gut microbiota: mechanisms involved in obesity control. Crit Rev Food Sci Nutr 59:3045–3053
Consoli MLD, da Silva RS, Nicoli JR, Bruña-Romero O, da Silva RG, de Vasconcelos GS, Correia MITD (2016) Randomized clinical trial. J Parenter Enteral Nutr 40:1114–1121
Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto J-M, Renault P, Doré J, Zucker J-D, Clément K, Ehrlich SD (2013) Dietary intervention impact on gut microbial gene richness. Nature 500:585–588
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712
Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE, Lou W, Allard JP (2018) Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep 8:1466
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563
de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FAJ, Pruijn GJM, Marijnissen RJ, Walgreen B, Helsen MM, van den Bersselaar LA, de Molon RS, Campos MJA, Cunha FQ, Cirelli JA, van den Berg WB (2014) Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1–driven Th17 response. J Immunol 192:4103–4111
De Leon LM, Watson JB, Kelly CR (2013) Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol 11:1036–1038
Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G, Dallapiccola B, Miccheli A, Alisi A, Putignani L (2017) Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 65:451–464
Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD (2011) Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 7:639–646
Demers M, Dagnault A, Desjardins J (2014) A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr 33:761–767
Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen J-P, de Vos WM, Cani PD (2019) Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25:1096–1103
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072
Devendran S, Méndez-García C, Ridlon JM (2017) Identification and characterization of a 20β-HSDH from the anaerobic gut bacterium Butyricicoccus desmolans ATCC 43058. J Lipid Res 58:916–925
Devendran S, Mythen SM, Ridlon JM (2018) The desA and desB genes from Clostridium scindens ATCC 35704 encode steroid-17, 20-desmolase. J Lipid Res 59:1005–1014
Dhakan DB, Maji A, Sharma AK et al (2019) The unique composition of Indian gut microbiome, gene catalogue, and associated fecal metabolome deciphered using multi-omics approaches. Gigascience 8(3):giz004
Dinan TG, Stanton C, Cryan JF (2013) Psychobiotics: a novel class of psychotropic. Biol Psychiatry 74:720–726
Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, Mikuls TR, Amdur RL, Richards JS, Kerr GS (2010) Association of Periodontitis with rheumatoid arthritis: a pilot study. J Periodontol 81:223–230
Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M (2009) High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One 4(9):e7063
Duda-Chodak A, Tarko T, Satora P, Sroka P (2015) Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr 54:325–341
Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ (2002) Acetate utilization and Butyryl coenzyme A (CoA): acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol 68:5186–5190
Dupont HL, Jiang Z-D, Dupont AW, Utay NS (2020) The intestinal microbiome in human health and disease. Trans Am Clin Climatol Assoc 131:178–197
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V (2012) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28:539–543
El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B (2013) The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 11(7):497–504
Erickson M, Malin S, Wang Z, Brown J, Hazen S, Kirwan J (2019) Effects of lifestyle intervention on plasma trimethylamine N-oxide in obese adults. Nutrients 11:179
Espinoza JL (2018) Machine learning for tackling microbiota data and infection complications in immunocompromised patients with cancer. J Intern Med 284:189–192
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci 110:9066–9071
Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI (2013) The Long-term stability of the human gut microbiota. Science 341:1237439
Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, Huttenhower C (2012) Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol 8:e1002606
Feng Z, Long W, Hao B, Ding D, Ma X, Zhao L, Pang X (2017) A human stool-derived Bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog 9:59
Fernández M, Reina-Pérez I, Astorga J, Rodríguez-Carrillo A, Plaza-Díaz J, Fontana L (2018) Breast cancer and its relationship with the microbiota. Int J Environ Res Public Health 15:1747
Ferreira AI, Garrido M, Castro-Poças F (2020) Irritable bowel syndrome: news from an old disorder. GE Port J Gastroenterol 27(4):255–268
FitzGerald MJ, Spek EJ (2020) Microbiome therapeutics and patent protection. Nat Biotechnol 38:806–810
Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Krogh Pedersen H, Arumugam M, Kristiansen K, Yvonne Voigt A, Vestergaard H, Hercog R, Igor Costea P, Roat Kultima J, Li J, Jørgensen T, Levenez F, Dore J, Bjørn Nielsen H, Brunak S, Raes J, Hansen T, Wang J, Dusko Ehrlich S, Bork P, Pedersen O (2015) Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528:262–266
Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, Bohannan BJM et al (2015) Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci 112:E2930–E2938
Frisan T (2016) Bacterial genotoxins: the long journey to the nucleus of mammalian cells. Biochim Biophys Acta Biomembr 1858:567–575
Frostegård J (2013) Immunity, atherosclerosis and cardiovascular disease. BMC Med 11:117
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450
Gamallat Y, Meyiah A, Kuugbee ED, Hago AM, Chiwala G, Awadasseid A, Bamba D, Zhang X, Shang X, Luo F, Xin Y (2016) Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed Pharmacother 83:536–541
Gao R, Zhu C, Li H, Yin M, Pan C, Huang L, Kong C, Wang X, Zhang Y, Qu S, Qin H (2018) Dysbiosis signatures of gut microbiota along the sequence from healthy, young patients to those with overweight and obesity. Obesity 26:351–361
Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC (2018) Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol 2018:9
Gauffin Cano P, Santacruz A, Moya Á, Sanz Y (2012) Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS One 7:e41079
Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW, Nelson KE, White O, Methé BA, Huttenhower C (2012a) The human microbiome project: a community resource for the healthy human microbiome. PLoS Biol 10:e1001377
Gevers D, Pop M, Schloss PD, Huttenhower C (2012b) Bioinformatics for the human microbiome project. PLoS Comput Biol 8:e1002779
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD et al (2017) Expert consensus document: the international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Amp Hepatol 14:491
Gill SR, Pop M, Deboy RT et al (2006) Metagenomic analysis of the human distal gut microbiome. Science 312(5778):1355–1359
Glick-Bauer M, Yeh M-C (2014) The health advantage of a vegan diet: exploring the gut microbiota connection. Nutrients 6:4822–4838
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2014) Heart disease and stroke statistics—2014 update. Circulation 129:5
Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M (2016) Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 68:974–981
Gómez-Guzmán M, Toral M, Romero M, Jiménez R, Galindo P, Sánchez M, Zarzuelo MJ, Olivares M, Gálvez J, Duarte J (2015) Antihypertensive effects of probiotics lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res 59:2326–2336
Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang S-K, Murphy CJ, Pauli C, Morris R, Taylor S, Bosch K, Yang S, Wang Y, Van Riper J, Lekaye HC, Roper J, Kim Y, Chen Q, Gross SS, Rhee KY, Cantley LC, Yun J (2019) High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363:1345–1349
Grabherr F, Grander C, Effenberger M, Adolph TE, Tilg H (2019) Gut dysfunction and non-alcoholic fatty liver disease. Front Endocrinol (Lausanne) 10:5
Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong M-L, Xu A, Chavakis T, Bornstein AB, Ehrhart-Bornstein M, Lamounier-Zepter V, Lohmann T, Wolf T, Bornstein SR (2013) Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics 13:514–522
Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M (2018) Helicobacter pylori and extragastric diseases: a review. World J Gastroenterol 24:3204–3221
Green M, Arora K, Prakash S (2020) Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci 21:5
Greenhill C (2015) Firmicutes and Bacteroidetes involved in insulin resistance by mediating levels of glucagon-like peptide 1. Nat Rev Endocrinol 11:254–254
Groen RN, de Clercq NC, Nieuwdorp M, Hoenders HJR, Groen AK (2018) Gut microbiota, metabolism and psychopathology: a critical review and novel perspectives. Crit Rev Clin Lab Sci 55:283–293
Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklić K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O (2015) Binding of the Fap 2 protein of fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42:344–355
Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N (2020) Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51:102590
Haider S, Naqvi F, Batool Z, Tabassum S, Sadir S, Liaquat L, Naqvi F, Zuberi NA, Shakeel H, Perveen T (2015) Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res Bull 115:1–8
Halazonetis TD (2004) Constitutively active DNA damage checkpoint pathways as the driving force for the high frequency of p53 mutations in human cancer. DNA Repair (Amst) 3:1057–1062
Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur T, Lagoudas GK, Vatanen T, Fornelos N, Wilson R, Bertha M, Cohen M, Garber J, Khalili H, Gevers D, Ananthakrishnan AN, Kugathasan S, Lander ES, Blainey P, Vlamakis H, Xavier RJ, Huttenhower C (2017) A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med 9:103
Hall J, Juncos L, Wang Z, Hall M, do Carmo J, da Silva A (2014) Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014:75
Halpern MT, Cifaldi MA, Kvien TK (2009) Impact of adalimumab on work participation in rheumatoid arthritis: comparison of an open-label extension study and a registry-based control group. Ann Rheum Dis 68:930–937
Hamady ZZR, Scott N, Farrar MD, Lodge JPA, Holland KT, Whitehead T, Carding SR (2010) Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut 59:461–469
Harrison C (2020) Of microbes and mental health: eating for mental wellness. https://asm.org/Articles/2020/February/Of-Microbes-and-Mental-Health-Eating-for-Mental-We#:~:text=Evidence%20suggests%20that%20probiotics%20mayto%20many%20mental%20health%20disorders
Hatakeyama M (2017) Structure and function of helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc Japan Acad Ser 93:196–219
Hatakka K, Martio J, Korpela M, Herranen M, Poussa T, Laasanen T, Saxelin M, Vapaatalo H, Moilanen E, Korpela R (2003) Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis – a pilot study. Scand J Rheumatol 32:211–215
He Y, Wang J, Li F, Shi Y (2020) Main clinical features of COVID-19 and potential prognostic and therapeutic value of the microbiota in SARS-CoV-2 infections. Front Microbiol 11:1302
He Z, Hao W, Kwek E, Lei L, Liu J, Zhu H, Ma KY, Zhao Y, Ho HM, He W-S, Chen Z-Y (2019) Fish oil is more potent than flaxseed oil in modulating gut microbiota and reducing trimethylamine- N -oxide-exacerbated atherogenesis. J Agric Food Chem 67:13635–13647
Heshiki Y, Vazquez-Uribe R, Li J, Ni Y, Quainoo S, Imamovic L, Li J, Sørensen M, Chow BKC, Weiss GJ, Xu A, Sommer MOA, Panagiotou G (2020) Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome 8:28
Hibberd AA, Lyra A, Ouwehand AC et al (2017) Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol 4(1):e000145
Hilimire MR, DeVylder JE, Forestell CA (2015) Fermented foods, neuroticism, and social anxiety: an interaction model. Psychiatry Res 228:203–208
Hobby GP, Karaduta O, Dusio GF, Singh M, Zybailov BL, Arthur JM (2019) Chronic kidney disease and the gut microbiome. Am J Physiol Physiol 316:F1211–F1217
Hollister EB, Oezguen N, Chumpitazi BP, Luna RA, Weidler EM, Rubio-Gonzales M, Dahdouli M, Cope JL, Mistretta T-A, Raza S, Metcalf GA, Muzny DM, Gibbs RA, Petrosino JF, Heitkemper M, Savidge TC, Shulman RJ, Versalovic J (2019) Leveraging human microbiome features to diagnose and stratify children with irritable bowel syndrome. J Mol Diagn 21:449–461
Holscher HD (2017) Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8:172–184
Hosseininasab Nodoushan S, Nabavi A (2019) The interaction of Helicobacter pylori infection and type 2 diabetes mellitus. Adv Biomed Res 8:15
Hoving LR, Katiraei S, Heijink M, Pronk A, van der Wee-Pals L, Streefland T, Giera M, Willems van Dijk K, van Harmelen V (2018) Dietary mannan oligosaccharides modulate gut microbiota, increase fecal bile acid excretion, and decrease plasma cholesterol and atherosclerosis development. Mol Nutr Food Res 62:1700942
Hu R, Zeng F, Wu L, Wan X, Chen Y, Zhang J, Liu B (2019) Fermented carrot juice attenuates type 2 diabetes by mediating gut microbiota in rats. Food Funct 10:2935–2946
Hulston CJ, Churnside AA, Venables MC (2015) Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. Br J Nutr 113:596–602
Human Microbiome Project Consortium (2012) Structure, function and diversity of the healthy human microbiome. Nature 486(7402):207–214. https://doi.org/10.1038/nature11234. PMID: 22699609; PMCID: PMC3564958
Huttenhower C, Gevers D, Knight R et al (2012) Structure, function and diversity of the healthy human microbiome. Nature 486:207–214
Inoue R, Ohue-Kitano R, Tsukahara T, Tanaka M, Masuda S, Inoue T, Yamakage H, Kusakabe T, Hasegawa K, Shimatsu A, Satoh-Asahara N (2017) Prediction of functional profiles of gut microbiota from 16S rRNA metagenomic data provides a more robust evaluation of gut dysbiosis occurring in Japanese type 2 diabetic patients. J Clin Biochem Nutr 61:217–221
Isaacs JD (2008) Therapeutic T-cell manipulation in rheumatoid arthritis: past, present and future. Rheumatology 47:1461–1468
Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS (2011) The association between habitual diet quality and the common mental disorders in community-dwelling adults. Psychosom Med 73:483–490
Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O’Reilly SL, Nicholson GC, Kotowicz MA, Berk M (2010) Association of western and traditional diets with depression and anxiety in women. Am J Psychiatry 167:305–311
Jadhav P, Jiang Y, Jarr K, Layton C, Ashouri JF, Sinha SR (2020) Efficacy of dietary supplements in inflammatory bowel disease and related autoimmune diseases. Nutrients 12(7):2156
Jie Z, Xia H, Zhong S-L, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, Zhang D, Su Z, Fang Z, Lan Z, Li J, Xiao L, Li J, Li R, Li X, Li F, Ren H, Huang Y, Peng Y, Li G, Wen B, Dong B, Chen J-Y, Geng Q-S, Zhang Z-W, Yang H, Wang J, Wang J, Zhang X, Madsen L, Brix S, Ning G, Xu X, Liu X, Hou Y, Jia H, He K, Kristiansen K (2017) The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8:845
Jung CM, Heinze TM, Schnackenberg LK, Mullis LB, Elkins SA, Elkins CA, Steele RS, Sutherland JB (2009) Interaction of dietary resveratrol with animal-associated bacteria. FEMS Microbiol Lett 297:266–273
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T (2010) Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64:636–643
Kang DD, Froula J, Egan R, Wang Z (2015) MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. Peer J 3:e1165
Kaplan BJ, Rucklidge JJ, Romijn A, McLeod K (2015) The emerging field of nutritional mental health. Clin Psychol Sci 3:964–980
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F (2013) Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498:99–103
Kessing LV, Vradi E, McIntyre RS, Andersen PK (2015) Causes of decreased life expectancy over the life span in bipolar disorder. J Affect Disord 180:142–147
Khalesi S, Sun J, Buys N, Jayasinghe R (2014) Effect of probiotics on blood pressure. Hypertension 64:897–903
Kho ZY, Lal SK (2018) The human gut microbiome—a potential controller of wellness and disease. Front Microbiol 9:1–23
Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, Nazertehrani S, Moore ME, Marco ML, Martin RJ, Adams SH (2016) Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Physiol 310:F857–F871
Kikuchi K, Ben Othman M, Sakamoto K (2018) Sterilized bifidobacteria suppressed fat accumulation and blood glucose level. Biochem Biophys Res Commun 501:1041–1047
Kim B, Choi H-N, Yim J-E (2019) Effect of diet on the gut microbiota associated with obesity. J Obes Metab Syndr 28:216–224
Kim J, Yun JM, Kim MK, Kwon O, Cho B (2018) Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: a randomized, double-blind, placebo-controlled trial. J Med Food 21:454–461
Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH (2013a) Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145:396–406
Kim S-W, Park K-Y, Kim B, Kim E, Hyun C-K (2013b) Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem Biophys Res Commun 431:258–263
King DE, Mainous AG, Lambourne CA (2012) Trends in dietary fiber intake in the United States, 1999-2008. J Acad Nutr Diet 112:642–648
Kinoshita M, Suzuki Y, Saito Y (2002) Butyrate reduces colonic paracellular permeability by enhancing PPARγ activation. Biochem Biophys Res Commun 293:827–831
Kira J, Isobe N (2019) Helicobacter pylori infection and demyelinating disease of the central nervous system. J Neuroimmunol 329:14–19
Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, Dynnyk O (2018) A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J Gastrointestin Liver Dis 27:41–49
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL (2013) Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19:576–585
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-Chain fatty acids as key bacterial metabolites. Cell 165:1332–1345
Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, Gavalko Y, Dorofeyev A, Romanenko M, Tkach S, Sineok L, Lushchak O, Vaiserman A (2017) Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol 17:120
Konishi H, Fujiya M, Tanaka H, Ueno N, Moriichi K, Sasajima J, Ikuta K, Akutsu H, Tanabe H, Kohgo Y (2016) Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat Commun 7:12365
Kosti I, Lyalina S, Pollard KS, Butte AJ, Sirota M (2020) Meta-analysis of vaginal microbiome data provides new insights into preterm birth. Front Microbiol 11:1–13
Kumar P, Banik S (2013) Pharmacotherapy options in rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord 6:5558
Kurdi P, Kawanishi K, Mizutani K, Yokota A (2006) Mechanism of growth inhibition by free bile acids in Lactobacilli and Bifidobacteria. J Bacteriol 188:1979–1986
Kwa M, Plottel CS, Blaser MJ, Adams S (2016) The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst 108(8):djw029
Lagier J-C, Armougom F, Million M, Hugon P, Pagnier I, Robert C, Bittar F, Fournous G, Gimenez G, Maraninchi M, Trape J-F, Koonin EV, La Scola B, Raoult D (2012) Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect 18:1185–1193
Lagier J-C, Dubourg G, Million M, Cadoret F, Bilen M, Fenollar F, Levasseur A, Rolain J-M, Fournier P-E, Raoult D (2018) Culturing the human microbiota and culturomics. Nat Rev Microbiol 16:540–550
Lara-Tejero M (2000) A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354–357
Larsen N, Vogensen FK, van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5(e):9085
Lazar V, Ditu L-M, Pircalabioru GG, Picu A, Petcu L, Cucu N, Chifiriuc MC (2019) Gut microbiota, host organism, and diet trialogue in diabetes and obesity. Front Nutr 6:21
Le TKC, Hosaka T, Nguyen TT, Kassu A, Dang TO, Tran HB, Pham TP, Tran QB, Le THH, Da Pham X (2015) Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed Res 36:63–70
Leber A, Hontecillas R, Abedi V, Tubau-Juni N, Zoccoli-Rodriguez V, Stewart C, Bassaganya-Riera J (2017) Modeling new immunoregulatory therapeutics as antimicrobial alternatives for treating Clostridium difficile infection. Artif Intell Med 78:1–13
Lee J-W, Shin J-G, Kim EH, Kang HE, Yim IB, Kim JY, Joo H-G, Woo HJ (2004) Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of lactobacillus casei and Bifidobacterium longum. J Vet Sci 5:41–48
Lenoir M, del Carmen S, Cortes-Perez NG, Lozano-Ojalvo D, Muñoz-Provencio D, Chain F, Langella P, de Moreno de LeBlanc A, LeBlanc JG, Bermúdez-Humarán LG (2016) Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J Gastroenterol 51:862–873
Levy SE, Myers RM (2016) Advancements in next-generation sequencing. Annu Rev Genomics Hum Genet 17:95–115
Li D, Liu C-M, Luo R, Sadakane K, Lam T-W (2015) MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31:1674–1676
Li D, Luo R, Liu C-M, Leung C-M, Ting H-F, Sadakane K, Yamashita H, Lam T-W (2016) MEGAHIT v1.0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 102:3–11
Li K, Zhang L, Xue J, Yang X, Dong X, Sha L, Lei H, Zhang X, Zhu L, Wang Z, Li X, Wang H, Liu P, Dong Y, He L (2019a) Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct 10:1915–1927
Li X, Gan, Sun, Meng, Shang, Mao & Li (2019b) Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods 8, 440
Liang Q, Chiu J, Chen Y, Huang Y, Higashimori A, Fang J, Brim H, Ashktorab H, Ng SC, Ng SSM, Zheng S, Chan FKL, Sung JJY, Yu J (2017) Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin Cancer Res 23:2061–2070
Lim MY, Rho M, Song Y-M, Lee K, Sung J, Ko G (2015) Stability of gut enterotypes in Korean monozygotic twins and their association with biomarkers and diet. Sci Rep 4:7348
Limaye SA, Haddad RI, Cilli F, Sonis ST, Colevas AD, Brennan MT, Hu KS, Murphy BA (2013) Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer 119:4268–4276
Liu G, Liang L, Yu G, Li Q (2018a) Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int J Biol Macromol 115:711–717
Liu Q, Tang G-Y, Zhao C-N, Feng X-L, Xu X-Y, Cao S-Y, Meng X, Li S, Gan R-Y, Li H-B (2018b) Comparison of antioxidant activities of different grape varieties. Molecules 23:2432
Liu RT, Walsh RFL, Sheehan AE (2019) Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev 102:13–23
Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, Brady A, Creasy HH, McCracken C, Giglio MG, McDonald D, Franzosa EA, Knight R, White O, Huttenhower C (2017) Strains, functions and dynamics in the expanded human microbiome project. Nature 550:61–66
Loguercio C, Federico A, Tuccillo C, Terracciano F, Auria MV, De Simone C, Blanco CDV (2005) Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 39:540–543
Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen C-H, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE (2017) Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 25:1054–1062
Louis P, Flint HJ (2017) Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 19:29–41
Lu R, Wu S, Zhang Y, Xia Y, Liu X, Zheng Y, Chen H, Schaefer KL, Zhou Z, Bissonnette M, Li L, Sun J (2014) Enteric bacterial protein Avr a promotes colonic tumorigenesis and activates colonic beta-catenin signaling pathway. Oncogene 3:e105–e105
Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF (2018) Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360:5931
Ma FX, Zhou B, Chen Z, Ren Q, Lu SH, Sawamura T, Han ZC (2006) Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J Lipid Res 47:1227–1237
Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, Galvano F (2012) Bifidobacterium longum with Fructo-oligosaccharides in patients with non-alcoholic steatohepatitis. Dig Dis Sci 57:545–553
Mandel DR, Eichas K, Holmes J (2010) Bacillus coagulans: a viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement Altern Med 10:1
Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A (2016) The gut microbiota and host health: a new clinical frontier. Gut 65:330–339
Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet J-P (2009) The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 9:123
Markowiak P, Ślizewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021
Markowitz VM, Chen I-MA, Chu K, Szeto E, Palaniappan K, Jacob B, Ratner A, Liolios K, Pagani I, Huntemann M, Mavromatis K, Ivanova NN, Kyrpides NC (2012) IMG/M-HMP: a metagenome comparative analysis system for the human microbiome project. PLoS One 7:e40151
Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM (2017) High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135:964–977
Massot-Cladera M, Pérez-Berezo T, Franch A, Castell M, Pérez-Cano FJ (2012) Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch Biochem Biophys 527:105–112
McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, Verbeke K, Walter J, Latulippe ME (2019) Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J Nutr 149:1882–1895
McFadden R-MT, Larmonier CB, Shehab KW, Midura-Kiela M, Ramalingam R, Harrison CA, Besselsen DG, Chase JH, Caporaso JG, Jobin C, Ghishan FK, Kiela PR (2015) The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm Bowel Dis 21:2483–2494
Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B, Beniak J, Medvecova L, Lagin A, Svetlovska D, Spanik S, Zajac V, Mardiak J, Drgona L (2015) Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med 23:356–362
Mei X, Zhang X, Wang Z, Gao Z, Liu G, Hu H, Zou L, Li X (2016) Insulin sensitivity-enhancing activity of Phlorizin is associated with lipopolysaccharide decrease and gut microbiota changes in obese and type 2 diabetes (db/db) mice. J Agric Food Chem 64:7502–7511
Menni C, Lin C, Cecelja M, Mangino M, Matey-Hernandez ML, Keehn L, Mohney RP, Steves CJ, Spector TD, Kuo C-F, Chowienczyk P, Valdes AM (2018) Gut microbial diversity is associated with lower arterial stiffness in women. Eur Heart J 39:2390–2397
Menni C, Mangino M, Cecelja M, Psatha M, Brosnan MJ, Trimmer J, Mohney RP, Chowienczyk P, Padmanabhan S, Spector TD, Valdes AM (2015) Metabolomic study of carotid–femoral pulse-wave velocity in women. J Hypertens 33:791–796
Meroni M, Longo M, Dongiovanni P (2019) The role of probiotics in nonalcoholic fatty liver disease: a new insight into therapeutic strategies. Nutrients 11:2642
Mijanur Rahman M, Gan SH, Khalil MI (2014) Neurological effects of honey: current and future prospects. Evid-Based Complement Altern Med 2014:1–13
Mills CE, Tzounis X, Oruna-Concha M-J, Mottram DS, Gibson GR, Spencer JPE (2015) In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br J Nutr 113:1220–1227
Mimee M, Citorik RJ, Lu TK (2016) Microbiome therapeutics — advances and challenges. Adv Drug Deliv Rev 105:44–54
Miyamoto J, Kasubuchi M, Nakajima A, Irie J, Itoh H, Kimura I (2016) The role of short-chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens 25:379–383
Mohajeri MH, La Fata G, Steinert RE, Weber P (2018) Relationship between the gut microbiome and brain function. Nutr Rev 76:481–496
Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani G-A, Mohammadi F (2014) Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4:83–88
Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV et al (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13:R79
Motta J-P, Bermudez-Humaran LG, Deraison C, Martin L, Rolland C, Rousset P, Boue J, Dietrich G, Chapman K, Kharrat P, Vinel J-P, Alric L, Mas E, Sallenave J-M, Langella P, Vergnolle N (2012) Food-grade bacteria expressing Elafin protect against inflammation and restore colon homeostasis. Sci Transl Med 4:158ra144
Moya-Pérez A, Neef A, Sanz Y (2015) Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS One 10:e0126976
Munukka E, Pekkala S, Wiklund P, Rasool O, Borra R, Kong L, Ojanen X, Cheng SM, Roos C, Tuomela S, Alen M, Lahesmaa R, Cheng S (2014) Gut-adipose tissue axis in hepatic fat accumulation in humans. J Hepatol 61:132–138
Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, Hänninen A, Vuopio J, Huovinen P, Jalkanen S, Pekkala S (2017) Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME 11:1667–1679
Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Azuma T, Hatakeyama M (2007) Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 26:4617–4626
Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M (2017) Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg 27:917–925
Natividad JM, Lamas B, Pham HP, Michel M-L, Rainteau D, Bridonneau C, da Costa G, van Hylckama VJ, Sovran B, Chamignon C, Planchais J, Richard ML, Langella P, Veiga P, Sokol H (2018) Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun 9:2802
Navab-Moghadam F, Sedighi M, Khamseh ME, Alaei-Shahmiri F, Talebi M, Razavi S, Amirmozafari N (2017) The association of type II diabetes with gut microbiota composition. Microb Pathog 110:630–636
Newburg DS (2000) Oligosaccharides in human milk and bacterial colonization. J Pediatr Gastroenterol Nutr 30:8–17
Nicolucci AC, Reimer RA (2017) Prebiotics as a modulator of gut microbiota in paediatric obesity. Pediatr Obes 12:265–273
NIH Human Microbiome Portfolio Analysis Team (2019) A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007-2016. Microbiome 7(1):31
Nihei N, Okamoto H, Furune T, Ikuta N, Sasaki K, Rimbach G, Yoshikawa Y, Terao K (2018) Dietary α-cyclodextrin modifies gut microbiota and reduces fat accumulation in high-fat-diet-fed obese mice. Bio Factors 44:336–347
Ning L, Liu R, Lou X, Du H, Chen W, Zhang F, Li S, Chen X, Xu G (2019) Association between helicobacter pylori infection and nonalcoholic fatty liver disease: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol 31:735–742
Nouri Z, Karami F, Neyazi N, Modarressi MH, Karimi R, Khorramizadeh MR, Taheri B, Motevaseli E (2016) Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J 18:127–134
Nurk S, Meleshko D, Korobeynikov A, Pevzner PA (2017) Meta SPAdes: a new versatile metagenomic assembler. Genome Res 27:824–834
Ohman, Simrén (2013) Intestinal microbiota and its role in irritable bowel syndrome (IBS). Curr Gastroenterol Rep 15(5):323
Orlando A, Linsalata M, Russo F (2016) Antiproliferative effects on colon adenocarcinoma cells induced by co-administration of vitamin K1 and Lactobacillus rhamnosus GG. Int J Oncol 48:2629–2638
Ortiz P, Bissada NF, Palomo L, Han YW, Al-Zahrani MS, Panneerselvam A, Askari A (2009) Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol 80:535–540
Ott SJ (2004) Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53(5):685–693
Paavonen J, Naud P, Salmerón J, Wheeler C, Chow S-N, Apter D, Kitchener H, Castellsague X, Teixeira J, Skinner S, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki F, Schwarz T, Poppe W, Bosch F, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G (2009) Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314
Packer CS, Rice AE, Johnson TC, Pelaez NJ, Temm CJ, Potter GV, White WA, Roth AH, Dominguez JH, Peterson RG (2014) Oxidized low density lipoprotein (OX-LDL) induced arterial muscle contraction signaling mechanisms. Open Hypertens 6:20–26
Pandey KR, Naik SR, Vakil BV (2015) Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol 52:7577–7587
Parkar SG, Trower TM, Stevenson DE (2013) Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 23:12–19
Pase MP, Scholey AB, Pipingas A, Kras M, Nolidin K, Gibbs A, Wesnes K, Stough C (2013) Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. J Psychopharmacol 27:451–458
Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP (2007) Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest 117:2197–2204
Pedret A, Valls RM, Calderón-Pérez L, Llauradó E, Companys J, Pla-Pagà L, Moragas A, Martín-Luján F, Ortega Y, Giralt M, Caimari A, Chenoll E, Genovés S, Martorell P, Codoñer FM, Ramón D, Arola L, Solà R (2019) Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes (Lond) 43:1863–1868
Pédron T, Nigro G, Sansonetti PJ (2016) From homeostasis to pathology: decrypting microbe-host symbiotic signals in the intestinal crypt. Philos Trans R Soc Lond B Biol Sci 371(1707):20150500
Peluso I, Morabito G, Urban L, Ioannone F, Serafi M (2012) Oxidative stress in atherosclerosis development: the central role of LDL and oxidative burst. Endocrine, Metab Immune Disord Targets 12:351–360
Petrof EO, Claud EC, Gloor GB, Allen-Vercoe E (2013) Microbial ecosystems therapeutics: a new paradigm in medicine? Benefic Microbes 4(1):53–65
Pham NM, Nanri A, Kurotani K, Kuwahara K, Kume A, Sato M, Hayabuchi H, Mizoue T (2014) Green tea and coffee consumption is inversely associated with depressive symptoms in a Japanese working population. Public Health Nutr 17:625–633
Pieczynska MD, Yang Y, Petrykowski S, Horbanczuk OK, Atanasov AG, Horbanczuk JO (2020) Gut microbiota and its metabolites in atherosclerosis development. Molecules 25:594
Pineda ML, Thompson SF, Summers K et al (2011) A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit 17:CR347–CR3354
Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S (2019) Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 157:647–659
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KCH, Aalvink S, Martinez LO, Dumas M-E, Maiter D, Loumaye A, Hermans MP, Thissen J-P, Belzer C, de Vos WM, Cani PD (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23:107–113
Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ (2013) Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci 110:4410–4415
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glockner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196
Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G (2014) Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct 5:1241
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J-M, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65
Qiu L, Tao X, Xiong H, Yu J, Wei H (2018) Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct 9:4299–4309
Qiu L, Yang D, Tao X, Yu J, Xiong H, Wei H (2017) Enterobacter aerogenes ZDY01 attenuates choline-induced trimethylamine N-oxide levels by remodeling gut microbiota in mice. J Microbiol Biotechnol 27:1491–1499
Raes J (2016) Microbiome-based companion diagnostics: no longer science fiction? Gut 65:896–897
Ramezani A, Raj DS (2014) The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25:657–670
Redman MG, Ward EJ, Phillips RS (2014) The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol 25:1919–1929
Reichert S, Haffner M, Keyßer G, Schäfer C, Stein JM, Schaller H-G, Wienke A, Strauss H, Heide S, Schulz S (2013) Detection of oral bacterial DNA in synovial fluid. J Clin Periodontol 40:591–598
Reimer RA, Grover GJ, Koetzner L, Gahler RJ, Lyon MR, Wood S (2014) Combining sitagliptin/metformin with a functional fiber delays diabetes progression in Zucker rats. J Endocrinol 220:361–373
Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, Gordon JI (2010) Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem 285:22082–22090
Rezac S, Kok CR, Heermann M, Hutkins R (2018) Fermented foods as a dietary source of live organisms. Front Microbiol 9:1785
Rezaei Nejad H, Oliveira BCM, Sadeqi A, Dehkharghani A, Kondova I, Langermans JAM, Guasto JS, Tzipori S, Widmer G, Sonkusale SR (2019) Ingestible osmotic pill for in vivo sampling of gut microbiomes. Adv Intell Syst 1(5):1900053
Ridlon JM, Kang D-J, Hylemon PB (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259
Rienks J, Dobson AJ, Mishra GD (2013) Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr 67:75–82
Riley LW, Raphael E, Faerstein E (2013) Obesity in the United States–dysbiosis from exposure to low-dose antibiotics? Front Public Health 1:69
Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel J-F, Ardid D, Desreumaux P (2007) Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13:35–37
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW (2013) Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its Fad A adhesin. Cell Host Microbe 14:195–206
Salamon D, Sroka-Oleksiak A, Kapusta P, Szopa M, Mrozińska S, Ludwig-Słomczyńska AH, Wołkow PP, Bulanda M, Klupa T, Małecki MT, Gosiewski T (2018) Characteristics of the gut microbiota in adult patients with type 1 and 2 diabetes based on the analysis of a fragment of 16S rRNA gene using next-generation sequencing. Polish Arch Intern Med 128:336
Sanchez-Rodriguez E, Egea-Zorrilla A, Plaza-Díaz J, Aragón-Vela J, Muñoz-Quezada S, Tercedor-Sánchez L, Abadia-Molina F (2020) The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients 12:605
Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, Schlatter J, Lahortiga F, Majem LS, Martínez-González MA (2009) Association of the Mediterranean Dietary Pattern with the incidence of depression. Arch Gen Psychiatry 66:1090
Sathyapalan T, Beckett S, Rigby AS, Mellor DD, Atkin SL (2010) High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J 9:55
Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, Lipuma L, Attur M, Pillinger MH, Weissmann G, Littman DR, Pamer EG, Bretz WA, Abramson SB (2012) Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum 64:3083–3094
Schiffrin EL (2014) Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 126:267–274
Schlaberg R (2020) Microbiome diagnostics. Clin Chem 66(1):68–76
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM (2016) Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc 5(2):e002767
Selhub EM, Logan AC, Bested AC (2014) Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol 33:2
Seo D-B, Jeong HW, Cho D, Lee BJ, Lee JH, Choi JY, Bae I-H, Lee S-J (2015) Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J Med Food 18:549–556
Seppo L, Jauhiainen T, Poussa T, Korpela R (2003) A fermented milk high in bioactive peptides has a blood pressure–lowering effect in hypertensive subjects. Am J Clin Nutr 77:326–330
Shen T-CD, Albenberg L, Bittinger K, Chehoud C, Chen Y-Y, Judge CA, Chau L, Ni J, Sheng M, Lin A, Wilkins BJ, Buza EL, Lewis JD, Daikhin Y, Nissim I, Yudkoff M, Bushman FD, Wu GD (2015) Engineering the gut microbiota to treat hyperammonemia. J Clin Invest 125:2841–2850
Simon M-C, Strassburger K, Nowotny B, Kolb H, Nowotny P, Burkart V, Zivehe F, Hwang J-H, Stehle P, Pacini G, Hartmann B, Holst JJ, Mac Kenzie C, Bindels LB, Martinez I, Walter J, Henrich B, Schloot NC, Roden M (2015) Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: a proof of concept. Diabetes Care 38:1827–1834
Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW (2014) Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 9:1603–1610
Skarupski KA, Tangney CC, Li H, Evans DA, Morris MC (2013) Mediterranean diet and depressive symptoms among older adults over time. J Nutr Health Aging 17:441–445
Solanki N, Alkadhi I, Atrooz F, Patki G, Salim S (2015) Grape powder prevents cognitive, behavioral, and biochemical impairments in a rat model of posttraumatic stress disorder. Nutr Res 35:65–75
Song J-X, Ren H, Gao Y-F, Lee C-Y, Li S-F, Zhang F, Li L, Chen H (2017) Dietary capsaicin improves glucose homeostasis and alters the gut microbiota in obese diabetic Ob/Ob mice. Front Physiol 8:602
Sowa-Kućma M, Styczeń K, Siwek M, Misztak P, Nowak RJ, Dudek D, Rybakowski JK, Nowak G, Maes M (2018) Are there differences in lipid peroxidation and immune biomarkers between major depression and bipolar disorder: effects of melancholia, atypical depression, severity of illness, episode number, suicidal ideation and prior suicide attempts. Prog Neuro-Psychopharmacol Biol Psychiatry 81:372–383
Statovci D, Aguilera M, Mac Sharry J, Melgar S (2017) The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol 8:838
Stephanie T, Alia C, Ali A, Ahmed EH, Steven F (2012) Identification of oral bacterial DNA in synovial fluid of arthritis patients with native and failed prosthetic joints. J Clin Rheumatol 18(3):117–121
Subah Packer C (2007) Estrogen protection, oxidized LDL, endothelial dysfunction and vasorelaxation in cardiovascular disease: new insights into a complex issue. Cardiovasc Res 73:6–7. https://doi.org/10.1016/j.cardiores.2006.11.013
Suceveanu AI, Stoian AP, Parepa I, Voinea C, Hainarosie R, Manuc D, Nitipir C, Mazilu L, Suceveanu AP (2018) Gut microbiota patterns in obese and type 2 diabetes (T2D) patients from Romanian Black Sea coast region. Rev Chim 69:2260–2267
Suzuki C, Aoki-Yoshida A, Aoki R, Sasaki K, Takayama Y, Mizumachi K (2017) The distinct effects of orally administered lactobacillus rhamnosus GG and Lactococcus lactis subsp. lactis C59 on gene expression in the murine small intestine. PLoS One 12:e0188985
Taherian-Esfahani Z, Abedin-Do A, Nouri Z, Mirfakhraie R, Ghafouri-Fard S, Motevaseli E (2016) Lactobacilli differentially modulate mTOR and Wnt/β-catenin pathways in different cancer cell lines. Iran J Cancer Prev 9(3):e5369
Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, Raygan F, Karamali F, Razzaghi R, Taheri S, Asemi Z (2016) A randomized controlled clinical trial investigating the effect of Synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes 125:21–27
Takagi A, Ikemura H, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T (2008) Relationship between the in vitro response of dendritic cells to lactobacillus and prevention of tumorigenesis in the mouse. J Gastroenterol 43:661–669
Takayama F, Taki K, Niwa T (2003) Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis 41:S142–S145
Tan JK, McKenzie C, Mariño E, Macia L, Mackay CR (2017) Metabolite-sensing G protein–coupled receptors—facilitators of diet-related immune regulation. Annu Rev Immunol 35:371–402
Tap et al (2017) Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 152(1):111–123
Taranu I, Marin D, Braicu C, Pistol G, Sorescu I, Pruteanu L, Neagoe IB, Vodnar D (2018) In vitro transcriptome response to a mixture of lactobacilli strains in intestinal porcine epithelial cell line. Int J Mol Sci 19(7):1923
Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N (2019) Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 11:01591
The Integrative HMP (iHMP) Research Network Consortium (2019) The integrative human microbiome project. Nature 569:641–648
Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H (2019) The effects of vegetarian and vegan diets on gut microbiota. Front Nutr 6:47
Tong X, Xu J, Lian F, Yu X, Zhao Y, Xu L, Zhang M, Zhao X, Shen J, Wu S, Pang X, Tian J, Zhang C, Zhou Q, Wang L, Pang B, Chen F, Peng Z, Wang J, Zhen Z, Fang C, Li M, Chen L, Zhao L (2018) Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional chinese herbal formula: a multicenter, randomized, open label clinical trial. MBio 9:e02392
Topçuoğlu BD, Lesniak NA, Ruffin MT, Wiens J, Schloss PD (2020) A framework for effective application of machine learning to microbiome-based classification problems. MBio 1:1–13
Tran CN, Lundy SK, Fox DA (2005) Synovial biology and T cells in rheumatoid arthritis. Pathophysiology 12:183–189
Trebatická J, Ďuračková Z (2015) Psychiatric disorders and polyphenols: can they be helpful in therapy? Oxid Med Cell Longev 2015:1–16
Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, Tett A, Huttenhower C, Segata N (2015) MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods 12(10):902–903
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI (2007) The human microbiome project. Nature 449(7164):804–810
Uribe G, Villéger R, Bressollier P, Dillard RN, Worthley DL, Wang TC, Powell DW, Urdaci MC, Pinchuk IV (2018) Cell Microbiol 20(11):e12871
Vaahtovuo J, Munukka E, Korkeamäki M, Luukkainen R, Toivanen P (2008) Fecal microbiota in early rheumatoid arthritis. J Rheumatol 35:1500–1505
Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, Sharif S-K, Asghari-Jafarabadi M, Zavvari S (2014) Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 30:430–435
Van Herck M, Vonghia L, Francque S (2017) Animal models of nonalcoholic fatty liver disease—a Starter’s guide. Nutrients 9:1072
Van Hul M, Geurts L, Plovier H, Druart C, Everard A, Ståhlman M, Rhimi M, Chira K, Teissedre P-L, Delzenne NM, Maguin E, Guilbot A, Brochot A, Gérard P, Bäckhed F, Cani PD (2018) Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Metab 314:E334–E352
van Nood E, Speelman P, Nieuwdorp M, Keller J (2014) Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol 30(1):34–39
Vanderhoof JA, Young R (2008) Probiotics in the United States. Clin Infect Dis 46:S67–S72
Vaziri ND, Liu S-M, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, Kieffer DA, Adams SH, Martin RJ (2014) High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 9:e114881
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen T-H, Andersen GL (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83:308–315
Versalovic J, Dore J, Guarner F, Luna RA, Ringel Y (2017) Microbiome-based diagnostics: ready for applications in laboratory medicine? Clin Chem 63:1674–1679
Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, Forslund SK, Assmann K, Valles-Colomer M, Nguyen TTD, Proost S, Prifti E, Tremaroli V, Pons N, Le Chatelier E, Andreelli F, Bastard J-P, Coelho LP, Galleron N, Hansen TH, Hulot J-S, Lewinter C, Pedersen HK, Quinquis B, Rouault C, Roume H, Salem J-E, Søndertoft NB, Touch S, Dumas M-E, Ehrlich SD, Galan P, Gøtze JP, Hansen T, Holst JJ, Køber L, Letunic I, Nielsen J, Oppert J-M, Stumvoll M, Vestergaard H, Zucker J-D, Bork P, Pedersen O, Bäckhed F, Clément K, Raes J (2020) Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581:310–315
Vitetta L, Llewellyn H, Oldfield D (2019) Gut dysbiosis and the intestinal microbiome: Streptococcus thermophilus a key probiotic for reducing uremia. Microorganisms 7(8):228. https://doi.org/10.3390/microorganisms7080228
Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G, Libra M (2019) Gut microbiota and cancer: from pathogenesis to therapy. Cancers (Basel) 11:38
Wang I-K, Lai H-C, Yu C-J, Liang C-C, Chang C-T, Kuo H-L, Yang Y-F, Lin C-C, Lin H-H, Liu Y-L, Chang Y-C, Wu Y-Y, Chen C-H, Li C-Y, Chuang F-R, Huang C-C, Lin C-H, Lin H-C (2012) Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl Environ Microbiol 78:1107–1112
Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van Hylckama Vlieg JE, Strissel K, Zhao L, Obin M, Shen J (2015a) Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME 9:1–15
Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK et al (2015b) Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163:1585–1595
Watzl B, Girrbach S, Roller M (2005) Inulin, oligofructose and immunomodulation. Br J Nutr 93:S49–S55
Wegh C, Geerlings SY, Knol J, Roeselers G, Belzer C (2019) Postbiotics and their potential applications in early life nutrition and beyond. Int J Mol Sci 20(19):4673
Wehmeyer MH, Zyriax B-C, Jagemann B, Roth E, Windler E, Schulze zur Wiesch J, Lohse AW, Kluwe J (2016) Nonalcoholic fatty liver disease is associated with excessive calorie intake rather than a distinctive dietary pattern. Medicine (Baltimore) 95:e3887
WHO (1946) Preamble to the constitution of WHO as adopted by the international health conference, New York, 19 June−22 July 1946; signed on 22 July 1946 by the representatives of 61 States (Official Records of WHO, no. 2, p 100) and entered into force on 7 April 1948
Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Manatsathit W, Jaruvongvanich V, Ungprasert P (2018) Helicobacter pylori and risk of nonalcoholic fatty liver disease. J Clin Gastroenterol 52:386–391
Wittchen H-U (2012) The burden of mood disorders. Science 338:15
Woldeamlak B, Yirdaw K, Biadgo B (2019) Role of gut microbiota in type 2 diabetes mellitus and its complications: novel insights and potential intervention strategies. Korean J Gastroenterol 74:314
Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND (2014) Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-Chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39:230–237
Wood DE, Salzberg SL (2014) Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15:R46
Wrzosek L, Miquel S, Noordine M-L, Bouet S, Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, Langella P, Thomas M (2013) Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11:61
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD (2011) Linking Long-term dietary patterns with gut microbial enterotypes. Science 334:105–108
Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23:850–858
Wu S, Rhee K-J, Zhang M, Franco A, Sears CL (2007) Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and -secretase-dependent E-cadherin cleavage. J Cell Sci 120:1944–1952
Xavier JB, Young VB, Skufca J, Ginty F, Testerman T, Pearson AT, Macklin P, Mitchell A, Shmulevich I, Xie L, Caporaso JG, Crandall KA, Simone NL, Godoy-Vitorino F, Griffin TJ, Whiteson KL, Gustafson HH, Slade DJ, Schmidt TM, Walther-Antonio MRS, Korem T, Webb-Robertson B-JM, Styczynski MP, Johnson WE, Jobin C, Ridlon JM, Koh AY, Yu M, Kelly L, Wargo JA (2020) The cancer microbiome: distinguishing direct and indirect effects requires a systemic view. Trend Cancer 6:192–204
Yang H, Xia H, Ye Y, Zou W, Sun Y (2014) Probiotic Bacillus pumilus SE5 shapes the intestinal microbiota and mucosal immunity in grouper Epinephelus coioides. Dis Aquat Organ 111:119–127
Yang J-Y, Lee Y-S, Kim Y, Lee S-H, Ryu S, Fukuda S, Hase K, Yang C-S, Lim HS, Kim M-S, Kim H-M, Ahn S-H, Kwon B-E, Ko H-J, Kweon M-N (2017a) Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol 10:104–116
Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer M-P, Wang P, Cai S, Goel A, Qin H, Ma Y (2017b) Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor−κB, and up-regulating expression of micro RNA-21. Gastroenterology 152:851–866
Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, Tochikubo O (2002) Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis 39:1292–1299
Yoshida N, Emoto T, Yamashita T, Watanabe H, Hayashi T, Tabata T, Hoshi N, Hatano N, Ozawa G, Sasaki N, Mizoguchi T, Amin HZ, Hirota Y, Ogawa W, Yamada T, Hirata K (2018) Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 138:2486–2498
Yu J-J, Pei L-B, Zhang Y, Wen Z-Y, Yang J-L (2015) Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder. J Clin Psychopharmacol 35(4):406–410
Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, Cheng W, Li B, Li H, Lin W, Tian C, Zhao J, Han J, An D, Zhang Q, Wei H, Zheng M, Ma X, Li W, Chen X, Zhang Z, Zeng H, Ying S, Wu J, Yang R, Liu D (2019) Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab 30:675–688
Zamani B, Golkar HR, Farshbaf S, Emadi-Baygi M, Tajabadi-Ebrahimi M, Jafari P, Akhavan R, Taghizadeh M, Memarzadeh MR, Asemi Z (2016) Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Int J Rheum Dis 19:869–879
Zhang W, Li J, Lu S et al (2019) Gut microbiota community characteristics and disease-related microorganism pattern in a population of healthy Chinese people. Sci Rep 9:1594
Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L (2013) Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One 8:e71108
Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, Lan Z, Chen B, Li Y, Zhong H, Xie H, Jie Z, Chen W, Tang S, Xu X, Wang X, Cai X, Liu S, Xia Y, Li J, Qiao X, Al-Aama JY, Chen H, Wang L, Wu Q, Zhang F, Zheng W, Li Y, Zhang M, Luo G, Xue W, Xiao L, Li J, Chen W, Xu X, Yin Y, Yang H, Wang J, Kristiansen K, Liu L, Li T, Huang Q, Li Y, Wang J (2015) The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 21:895–905
Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J (2017) Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol 58:1–14
Zheng J, Li H, Zhang X, Jiang M, Luo C, Lu Z, Xu Z, Shi J (2018) Prebiotic Mannan-oligosaccharides augment the hypoglycemic effects of metformin in correlation with modulating gut microbiota. J Agric Food Chem 66:5821–5831
Zhu L, Zhang D, Zhu H, Zhu J, Weng S, Dong L, Liu T, Hu Y, Shen X (2018) Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe −/− mice. Atherosclerosis 268:117–126
Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G, Jin L, Chen X (2020a) The progress of gut microbiome research related to brain disorders. J Neuroinflammation 17:25. https://doi.org/10.1186/s12974-020-1705-z
Zhu Y, Li Q, Jiang H (2020b) Gut microbiota in atherosclerosis: focus on trimethylamine N-oxide. APMIS 128:353–366
Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL (2019) Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 570:462–467
Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF (2018) The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 359:1366–1370
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dasgupta, N., Srivastava, A., Rao, A., Murugkar, V., Shroff, R., Das, G. (2021). Microbiome Diagnostics and Interventions in Health and Disease. In: Bramhachari, P.V. (eds) Microbiome in Human Health and Disease. Springer, Singapore. https://doi.org/10.1007/978-981-16-3156-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-16-3156-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3155-9
Online ISBN: 978-981-16-3156-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)