Abstract
Background
Chronic intestinal inflammation alters host physiology and could lead to colorectal cancer (CRC). We have previously reported beneficial effects of the probiotic strain of Lactobacillus casei BL23 in different murine models of intestinal inflammation. In addition, there is an emerging interest on the potential beneficial effects of probiotics to treat CRC. We thus explored whether L. casei BL23 displays protective effects on CRC.
Methods
Mice were subcutaneously injected with 1,2-dimethylhydrazine (DMH) weekly during 10 weeks and orally administered with L. casei BL23 in the drinking water until the 10th week. Multiple plaque lesions in the large intestine were observed macroscopically and counted and intestinal tissues were also histologically analyzed. Finally, T-cell populations and cytokine production were evaluated after co-incubation of L. casei BL23 with spleen cells from non-treated mice to determine the immuno-modulatory effects of this bacterium.
Results
Our results show that oral treatment with this probiotic bacterium modulates host immune responses and significantly protect mice against DMH-induced CRC. This protection may be associated with the modulation of regulatory T-cells towards a Th17-biased immune response accompanied by the expression of regulatory cytokines (IL-6, IL-17, IL-10 and TGF-β), as demonstrated in L. casei BL23-treated splenocytes, but also with the colonic expression of IL-22 observed in vivo on L. casei BL23-treated mice; suggesting the induction of a fine-tune Th17-biased response.
Conclusions
Altogether our results reveal the high potential of L. casei BL23 to treat CRC and opens new frontiers for the study of immunomodulatory functions of probiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer death worldwide [1]. Currently, some studies have demonstrated that both probiotic bacteria and gut microbiota [2] could be an alternative approach to prevent and treat a number of human diseases including cancer and CRC [3, 4]. Probiotics have been defined by the Food and Agriculture Organization (FAO) of the United Nations World Health Organization (WHO) as “live microorganisms which, when administered in adequate amounts, confer health benefits on the host” [5]. The majority of probiotics belong to the group of lactic acid bacteria (LAB), being Lactobacillus the most common genus. In humans, some strains of Lactobacillus are present in the vagina and the gastrointestinal tract (GIT), where they make up a small portion of the gut microbiota and are thus considered as commensal microorganisms. Also, some strains of lactobacilli are used for the production of yogurt, cheese, beer, wine, cider and other fermented foods, as well as animal feeds, such as silage [6]. Most importantly, several studies have reported potential therapeutic properties of Lactobacillus spp., including anti-inflammatory [7, 8] and anti-cancer [8–12] activities. Furthermore, it has been shown that IBD patients have a completely different intestinal microbiota compared to healthy individuals [13]. The predominance of potentially harmful bacteria as well as a decrease of beneficial bacterial species such as lactobacilli and bifidobacteria has been identified in the intestinal microbiota of IBD patients. Thus, manipulating the abnormal intestinal microbiota to decrease the more pathogenic species and enhance the concentration and metabolic activity of the beneficial species could be an alternative therapy for IBD [13, 14]. For example, it has been reported that administration of Lactobacillus reuteri prevented colitis in IL-10 KO mice by increasing the number of LAB in the GIT [7]. In another trial, orally administered L. salivarius UCC118 reduced prevalence of mucosal inflammatory activity and CRC in IL-10 KO mice by modifying the intestinal microbiota in these animals [15]. Yogurt has also received much attention as a potential cancer-preventing agent in the diet. The results of in vitro assays, animal studies, and human clinical trials have demonstrated that some strains of lactobacilli and their fermented products (such as yogurt) could reduce the risks of certain types of cancer and inhibit the growth of certain tumors [16–20]. In addition, some LAB and their metabolites have been shown to inhibit the proliferation of tumor cells by direct or indirect interactions [21–23].
Although intervention studies with probiotics have yielded promising results, it remains difficult to draw general conclusions. This is probably due to the highly variable nature of the probiotics used. Indeed, bacteria from the same species might have opposite effects and consequently the health benefit from one probiotic strain cannot be extrapolated to others. Thus, deciphering the precise molecular mechanisms supporting probiotic action, and potential side effects, are required to delineate the optimal use of probiotics as supplementary therapy.
Previously, we have demonstrated anti-inflammatory effects of the probiotic strain of L. casei BL23, in two different models of chemically-induced colitis in mice [24, 25]. In addition, we have also observed that L. casei ATCC 393 strain (a L. casei BL23-closely related strain) displays anti-tumoral properties in a mouse allograft model of human papilloma virus (HPV)-induced cancer [26]. Here, we have investigated the beneficial effects of orally administered L. casei BL23 after oral administration in a mouse model of CRC.
Materials and methods
Bacterial strains
Lactobacillus casei BL23 [27] was grown in MRS medium (Difco, USA) at 37 °C without shaking. Lactococcus lactis MG1363 [28] was grown in M17 medium (Difco, USA) supplemented with 1 % glucose (GM17) at 30 °C without shaking.
TC-1 tumor model
C57BL/6 mice (females, 6–8 weeks of age; Janvier, Le Genest Saint Isle, France) were maintained at the animal care facilities of the National Institute of Agricultural Research (IERP, INRA, Jouy-en-Josas, France) under specific pathogen-free conditions. Mice were housed under standard conditions for a minimum of 1 week before experimentation. All experiments were performed in accordance with European Community rules and approved by the Animal Care Committee COMETHEA (Comité d’Ethique en Expérimentation Animale du Centre INRA de Jouy-en-Josas and AgroParisTech, Jouy en Josas, France).
Establishment of tumors was performed using an HPV-16 tumor model in which injection of a mouse (C57BL/6) lung tumor line TC-1 provokes lethal tumors as previously described [29]. The TC-1 cell line was generated by transduction with a retroviral vector expressing HPV16 E6/E7 plus a retrovirus expressing activated c-Ha-ras [30]. TC-1 cells were grown in RPMI medium 1640 (Lonza, Switzerland) supplemented with 10 % fetal calf serum (FCS), 50 units/ml penicillin, 50 g/ml streptomycin, and 0.4 mg/ml G418.
Preparation of live bacterial inocula and TC-1 mice challenge
Lactobacillus casei BL23 was grown as described above until OD600 = 0.5 and cells harvested by centrifugation at 3,000×g at 4 °C. After two washing steps with PBS, the pellet was resuspended in PBS to a final concentration of 1 × 109 CFU. Groups of mice (n = 8) were administered intranasally (i.n.) with 10 μl (5 μl was administered with a micropipette into each nostril) of L. casei BL23 at 1 × 108 CFU/μl on days 0, 14, and 28. Control mice received identical quantities of PBS. L. casei BL23-treated mice were challenged 7 days after the last immunization (day 35) by subcutaneous (s.c.) injection in the right rear flank with 1 × 105 TC-1 cells in 100 μl of sterile PBS. The dimensions of the tumor at the site of injection were measured every week in two perpendicular directions with a caliper, and the tumor volume was estimated as: (length × width2)/2 [29].
DMH-CRC model and feeding protocol
BALB/c mice (females, 6 weeks old, weighing 22–25 g) were obtained from the inbred closed colony maintained at the Centro de Referencia para Lactobacilos (CERELA-CONICET, San Miguel de Tucuman, Argentina) under specific pathogen-free conditions. Animal were maintained in a room with a 12-h light/dark cycle at 18 ± 2 °C. Animal protocol was approved by the Animal Protection Committee of CERELA (CRL-BIOT-LI-20141A), and all experiments comply with the current laws of Argentina.
To induce colon tumors, mice were injected with the carcinogen 1,2-dimethylhydrazine (DMH, Sigma, St. Louis, MO, USA). Each mouse received s.c. 20 mg DMH/kg/week (in 100 μl of sterile PBS) weekly during 10 weeks. Approximately 60 % of mice from DMH group (without treatment) developed tumors 5–6 months after the first injection.
For the feeding protocol, either L. lactis MG1363 or L. casei BL23 cultures were washed twice with saline solution (0.15 M NaCl) resuspended in sterile non-fat milk and administered 1 % (v/v) in the drinking water of the mice. For enumeration, dilutions of samples were plated on appropriate media and incubated at 30 °C or 37 °C (for L. lactis and L. casei, respectively) for 48 h. Under these conditions, mice drink on average 3–5 ml per day (1 ± 0.4 × 109 CFU/mouse) and no difference in water or food consumption was observed between the different groups. Moreover, since this is a long-duration model (6 months), the overall water consumption does not vary, even if some mice drink more some days than others, this is compensated as a function of time as observed by average daily water intakes that do not vary. The control group (DMH group) consisted of mice that received non-fat milk diluted in the drinking water under the same conditions as the test groups. Bacterial suspensions were given ad libitum in drinking water (suspensions were prepared freshly every day), starting the day of the first DMH injection, during 6 months (until the end of the experiment).
The mice were separated into three groups: (1) DMH group, mice injected with DMH to induce tumor growth and without bacterial administration; (2) DMH-L. lactis MG1363 group, mice injected with DMH and receiving L. lactis MG1363, and (3) DMH-L. casei BL23 group, mice injected with DMH and receiving L. casei BL23. All groups were fed ad libitum with a balanced rodent diet (Cooperation, containing 32 % protein, 5 % fat, 2 % fiber and 60 % nitrogen-free extract). Each experimental group consisted of 30-35 mice.
Sample collection in DMH-CRC model
Five animals from each group were sacrificed monthly by cervical dislocation. Large intestines were removed and their contents collected with 500 μl of PBS containing Complete Mini EDTA-free Protease Inhibitor Cocktail (Roche Molecular Biochemicals), centrifuged (8,000×g, 10 min, 4 °C), and supernatants were stored at −80 °C until further analysis.
Evaluation of intestinal damages in the DMH-CRC model
Multiple plaque lesions (MPL) in the large intestine were observed macroscopically and counted. Intestinal tissues were then prepared for histological evaluation as previously described [25]. Serial paraffin sections of 4 µm were made and stained with haematoxylin–eosin (H&E) for light microscopy examination. MPL were observed in microscopy, measured and their area were calculated and separated in two categories, <0.1 and >0.1 µm2.
Tissues were analyzed and scored microscopically for inflammatory damage as previously described [31] with some modifications considering the tumor presence. The criteria were: (1) loss of mucosal architecture (0, absent; 1, mild; 2, severe); (2) cellular infiltration (0, none; 1, in muscularis mucosae; 2, in lamina propria (LP); 3, in serosa); (3) muscle thickening (0, muscle < 1/2 of mucosal thickness; 1, muscle = 1/2–3/4 of mucosal thickness; 2, muscle = mucosal thickness; 3 = all muscle); (4) goblet cell depletion (0, absent; 1, present); (5) crypt abscess formation (0, absent; 1, present); and (6) tumor (0, absent; 1, present). The score of each variable was added.
Cytokine analysis in the DMH-CRC model
Samples obtained from the intestinal contents were assayed with the BD Cytometric Bead Array (CBA) Mouse Inflammation Kit (BD Bioscience, San Diego, CA, USA) to measure interleukin-6 (IL-6), IL-10, Monocyte Chemoattractant Protein-1 (MCP-1), interferon-γ (IFN-γ), tumor necrosis factor (TNF), and IL-12p70 protein levels, following the manufacturer’s instructions. The concentration of each cytokine from the intestinal fluid of each mouse was obtained, and the results were expressed in relation to the protein concentration measured in the sample. Total protein content of the samples was determined using the Bio-Rad Protein Assay based on the method of Bradford [32]. Cytokine ratios for each mouse were also determined.
In vitro and in vivo analysis of primary immune response induced by L. casei BL23
Lactobacillus casei BL23 was grown as described above and incubated at 37 °C until cultures reached an OD600 = ~1.0. Bacteria were pelleted by centrifugation, washed three times with PBS, and adjusted to a concentration of 1 × 107 CFU/ml in RPMI supplemented with 10 % FCS and 2 mM l-glutamine. In vitro experiments were performed using fresh naïve mouse spleen cells, as previously described [33] with some modifications. Briefly, splenocytes were stimulated with L. casei BL23 at a multiplicity of infection (MOI) = 10 (i.e., 10 bacteria:1 cell) for 1 h at 37 °C 10 % CO2. After 1 h, cells were recovered and washed with supplemented RPMI containing 50 µg/ml of gentamicin, 100 units/ml of penicillin, and 100 µg/ml of streptomycin. Cell incubation was pursued for 4 days. After that, cells were pelleted and supernatant recovered for cytokine analyses and stored at −80 °C until use. Cells were recovered, washed again, and used for immune cell phenotyping.
For the in vivo experiments, we followed a protocol previously described for this bacterium [34]. Briefly, groups of mice (n = 6) were orally administered with five consecutive doses (day 1–5) of either L. casei BL23 or PBS (as a control). Animals were sacrificed 3 days after the last administration (day 8). Intestine was recovered, fat cleared and Peyer’s Patches (PP) removed. Colonic segments of ~1 cm (~10–15 mg) were collected, snap frozen in liquid nitrogen, and stored at −80 °C. Splenic cells were recovered for cellular analysis.
Immune cell phenotyping was performed by flow cytometry using a Guava EasyCyte 5HT Flow Cytometer (Merck-Millipore) instrument and Guava Express Pro Software (CytoSoft v. 5.3). Samples were first examined in all light scatter patterns and fluorescence channels to confirm quality and abnormal populations were excluded. Unlabeled processed samples were used for reporting percentage of positive cells. Cell viability was measured through Guava Viacount Reagent (Millipore, Temecula, CA). Lymphocytes were typed as follow: Treg were defined as FoxP3+ (gated CD4+); Th17 as CD4+ RORg+; Th1 as CD4+Tbet+; Th2 as CD4+GATA3+. The antibodies used were: anti-CD4-FITC (clone GK1.5 eBioscience), 24G2 (CD16/CD32) (rat IgG2a lambda, clone 93, eBioscience), Foxp3 MAC’S Miltenyi Biotch (ref 130-093-014), GATA3-PE clone TWAJ eBioscience, T-bet-PerCP clone 4B10, Santa Cruz, RORgamma-FITC, clone 46419, Imgenex, anti-CD4-PECy7 (clone GK1.5, eBioscience).
Analysis of gene expression profile on colonic cells
Total RNA from colon tissue was extracted from individual mice using RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions using an Ultra-Turrax T8 homogenizator (IKA Labortechnik, Staufen, Germany) for tissue homogenization. Total RNA was quantified using a NanoDrop ND-1000 Spectrophotometer apparatus (Thermo Fisher Scientific, Inc, USA), and 2 µg were used to synthesize the cDNA using SuperScript II reverse transcriptase (Thermo Fisher Scientific, Inc, USA) following the manufacturer’s protocol. The qPCR was performed in a real-time thermocycler (StepOnePlus system, Applied Biosystems) using Takyon Rox SYBR dTTP Blue master mix (Eurogentec, Liège, Belgium) and the set of primers described in Table 1. The thermal cycling conditions included: a 2 min incubation at 50 °C followed by a 5 min pre-incubation at 95 °C, and 40 cycles consisting of denaturation for 15 s at 95 °C, and 45 s at 58 °C for primer annealing and polymerase extension. Melting curve analysis was used to confirm specific replicon formation. The data were normalized relative to the expression of β-actin by applying the introduced algorithm (the 2−ΔΔCT method) using the naïve group as calibrator [38]. All the reactions were performed in triplicates.
Statistical analysis
All data are expressed as mean values and standard deviations and were analyzed either by two-way repeated-measures analysis of variance with Bonferroni’s multiple comparison test to compare the difference between groups or by Mann–Whitney analysis of variance to compare groups with control using Prism software. p < 0.05 was considered significant.
Results
Antitumor effects of L. casei BL23 in a mouse allograft model of HPV-induced cancer
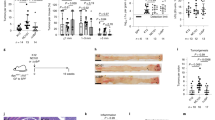
In order to determine whether L. casei BL23 (a probiotic strain with well-known anti-inflammatory properties) also displays anti-tumoral effects, we first tested this strain in the TC-1 mouse allograft model of HPV-induced cancer, a tumor model widely used in our laboratory [26]. As shown in Fig. 1, 100 % of mice receiving PBS developed aggressive tumors, which killed them within 35 days (after challenge), with a median tumor size of 3.0 ± 1 cm3. Strikingly, 40 % of mice (3/8) treated with L. casei BL23 strain remained tumor free over the 35-day test period, and in the 60 % tumor-bearing mice (5/8), the median size (0.4 ± 0.2 cm3) was significantly lower than the one measured in PBS-treated mice (Fig. 1). Altogether these results confirm that besides the anti-inflammatory effects, L. casei BL23 also possesses anti-tumor properties.
Tumor protection experiments with L. casei BL23 strain in the TC-1 mouse allograft model of HPV-induced cancer. Mice (n = 8) were i.n. treated with 1 × 109 CFU of L. casei BL23 resuspended in 10 µl of PBS (5 µl were administered with a micropipette into each nostril) on days 0, 14, and 28. Control mice received PBS (10 µl). Seven days after the last administration (day 35), a challenge with the tumoral cell line TC-1 was performed, and the presence and size of the tumor was monitored once a week. Data are represented as individual tumors volumes from mice at the end of week 10 (median of the size is shown by bars), Student’s t test, *p < 0.001
Lactobacillus casei BL23 reduces DMH-associated CRC
Since IBD is highly associated with an increased risk of colorectal cancer (CRC), especially when lesions are present in the colon, and considering the anti-inflammatory and anti-tumor potential of L. casei BL23 strain, we evaluated the protective effects of this probiotic bacterium in a DMH-induced CRC model. The number of MPL counted macroscopically was only significantly different between the groups that received L. casei and the other two groups (DMH control and DMH-L. lactis) in samples obtained at the 4th month (Fig. 2a). Moreover, microscopic observation of MPL showed significant differences in their sizes between the groups (Figs. 2b, 3). Indeed, analysis of histologic damage in the samples obtained from control mice (DMH group) showed an inflammation of the large intestine, characteristic of this model. In addition, mice from this group showed an increase in intestinal damage through the time of the experiment. In the two last months (samples obtained at 5th and 6th months) animals showed severe loss of mucosal architecture, important cellular inflammation and thickness of muscle, depletion of goblet cells, and the presence of crypt abscess formation (Table 2). The microscopic observation of MPL showed the predominance of mononuclear cells, and their measurement indicated that more than 50 % of these infiltrating cells occupied areas bigger than 0.1 µm2. Some of the MPL occupied areas of 0.4–0.5 µm2 (Fig. 3). It was also observed microscopically that there were more MPL than for the counts performed macroscopically; however, some of them, were smaller than 0.1 µm2. Similar results were obtained in mice treated with L. lactis MG1363. The number of mice with tumors was similar in these two groups (2–3 mice); however, the histological lesions observed in several mice at month 5 or 6 suggest that these animals could have developed tumors if they had not been sacrificed.
Protection effects of L. casei BL23 strain in the DMH-associated CRC model. a In the CRC murine model, mice injected with DMH were fed with a suspension of L. lactis MG1363 or L. casei BL23 (control mice did not received DMH). For MPL counts, mice (n = 5 per group) were sacrificed every month and MPL in the large intestine were observed and counted macroscopically. Data are represented as grouped MPL number and STD from mice at months 3–6. b Microscopic damage score in a murine model of CRC. Mice (n = 5 per group) were sacrificed every month and intestinal tissues were prepared for histological evaluation. Data are represented as grouped microscopic score and STD from tissues at month 3–6. Anova and Bonferroni post hoc test **p < 0.01
Mice that received L. casei BL23 showed a lower damage score, especially in the samples obtained at the end of the experiment (month 6) compared to the other groups (DMH and DMH-L. lactis) (Fig. 2b). At months 4 and 6, mice fed with L. casei BL 23 decreased significantly in the histological damage compared to the DMH group. The lack of significant differences in the samples from month 5 was due to one animal with tumors that increased the standard deviation. Removing this mouse, the rest of mice showed predominantly small MPL (more than 80 %) that occupied a tissue area lower than 0.1 µm2. The presence of larger MPL was only observed in some mice with areas between 0.10 and 0.15 µm2.
As shown in Fig. 3 (representative histological pictures from each group at month 5 and 6 are presented), samples from the DMH group present multiple plaque lesions (MPL) with a predominance of mononuclear cells in the LP. Small infiltrates are shown with dotted arrows and the bigger ones (whole arrows) were observed macroscopically and counted as MPL, as it is observed in the picture representative for month 5. In the two large MPL there are infiltrative cells that occupied the LP and destroyed the Lieberkühn’s glands. A great vascular congestion was observed, basal membrane was fragmented and metaplasia and dysplasia appeared. A microphotography of tumor tissue obtained from a mouse at month 6 is also shown. Similarly, the representative pictures for the DMH-L. lactis group show the infiltrative cells (arrow for month 5), loss of the goblet cells, big MPL (arrow for month 6), and the destruction of the Lieberkühn’s glands.
The samples from the DMH-L. casei BL23 group show multifocal cell infiltrates (arrows) observed in the LP, some of them are larger than others, accompanied by areas without inflammation and glandular structure conserved. There is not observed the same depletion of goblet cells as those in DMH and DMH-L. lactis groups (especially at month 6).
Cytokine analysis in the intestinal contents showed that tumor development was accompanied by an inflammatory status, with high levels of MCP-1 (Fig. 4a) and TNF-α (Fig. 4b) in the samples from both DMH and DMH-L. lactis groups. In contrast, mice receiving L. casei BL23 significantly decreased these pro-inflammatory cytokines in the intestinal samples and increased the levels of the anti-inflammatory cytokine IL-10, compared to other groups (Fig. 4c). Thus, L. casei BL23 administration is associated with a significant increase in IL-10/TNF-α ratio when compared with DMH and DMH-L. lactis groups (Fig. 4d).
Cytokine analysis from the intestinal contents of mice used in a model of CRC from months 5 and 6. a MCP-1, b TNF-α and c IL-10 are expressed as cytokine concentration in relation to total protein concentration. d A ratio between IL-10 concentration and TNF-α concentration. Non-parametric Mann–Whitney test *p < 0.05
L. casei BL23 elicits a fine-tune Th17-biased immune response
To further decipher the mechanism by which L. casei BL23 may protect against DMH-induced CRC, we analyzed the impact of this bacterium on the T-cell population (Fig. 5) and cytokine production (Fig. 6) after co-incubation with spleen cells from non-treated mice (ex vivo analysis). Cells were first analyzed for the expression of CD4, and then the percentage of Foxp3+ (Treg) and RORg+ (Th17) cells within this CD4+ population was evaluated by flow cytometry. L. casei BL23-treated splenocytes show a Th17-biased response as revealed by a significantly elevated percentage of Th17 cells (Fig. 5a) and decreased levels of Treg (Fig. 5b). These observations were correlated with a higher production of IL-17 (Fig. 6a), IL-6 (Fig. 6b), and TGF-β (Fig. 6c) by L. casei BL23-treated splenocytes compared to PBS-treated cells, thus confirming a microenvironment favorable to Th17 differentiation. However, we also found a significantly higher production of anti-inflammatory IL-10 in L. casei BL23-treated splenocytes compared to the PBS-treated cells (Fig. 6D) suggesting that a fine balanced immune response might be generated by this bacterium.
Analysis of T-cell population after co-incubation of spleen cells from non-treated-mice and L. casei BL23. Splenocytes were stimulated with L. casei BL23 at MOI = 10 for 1 h. After co-incubation, cells were washed and incubated for 4 more days before immune cell phenotyping. a Percentage of CD4+Tbet+ lymphocyte, b percentage of CD4+GATA3+ lymphocytes, c percentage of CD4+RORg+ lymphocytes and d percentage of CD4+FoxP3+ lymphocytes. Non parametric Mann–Whitney test *p < 0.05
Analysis of cytokine production after co-incubation of spleen cells from non-treated-mice with L. casei BL23. Splenocytes were stimulated with L. casei BL23 at MOI = 10 for 1 h. After co-incubation, cells were washed and incubated for 4 more days before supernatant recovery and cytokines analysis. a IL-17, b IL-6, c TGF-β and d IL-10 are expressed as cytokine concentration (pg/ml). Non-parametric Mann–Whitney test *p < 0.05
To confirm these observations, we performed in vivo experiments. After oral administration of L. casei BL23 (see “Materials and methods” section), splenic cells were recovered and analyzed by flow cytometry. According to the ex vivo results, significantly lower levels of Treg were observed in splenocytes from L. casei BL23-treated mice (Fig. 7d). Consistently, mice treated with L. casei BL23 showed a slight increase of Th17 cells, suggesting that L. casei BL23 trigger a Th17/Treg mixed-type immune response (Fig. 7c, d).
Analysis of the T-cell population of spleen cells from nontreated-mice and mice administered by L. casei BL23. a Percentage of CD4+Tbet+ lymphocyte, b percentage of CD4+GATA3+ lymphocytes, c percentage of CD4+RORg+ lymphocytes and d percentage of CD4+FoxP3+ lymphocytes. Non-parametric Mann–Whitney test *p < 0.05
The Th17-biased immune response induced by L. casei BL23 is associated with local expression of IL-22
To better understand the type of Th17-biased response elicited by L. casei BL23, we decided to analyze the cytokine expression profile on colonic cells. Surprisingly, lower levels of IL-17 were measured in colon samples from L. casei BL23 treated-mice compared to controls (Fig. 8a). Consistently, oral treatment with L. casei BL23 reduces the expression of Th17-related cytokines, TGF-β (Fig. 8b) and IL-6 (Fig. 8c), whereas IL-23 expression (a cytokine involved in Th17 differentiation) remains unchanged (Fig. 8e). Interestingly, L. casei BL23 significantly increases the IL-22 expression (a cytokine usually associated with Th17-cells) (Fig. 8d).
Analysis of cytokine gene expression in mice treated with L. casei BL23. RNA was isolated from colonic tissue and relative mRNA expression of a IL-17, b TGF-β, c IL-6, d IL-22 and e IL-23 were measured by RT-qPCR. Results are reported as fold change relative to naïve animals (fold change value 1) and data represent mean ± SEM of n = 6 mice/group. Non-parametric Mann–Whitney test *p < 0.05
Discussion
The main aim of this study was to evaluate the anti-cancer (and more particularly the anti-CRC) properties of L. casei BL23. Indeed, we have recently observed that a wild-type L. casei ATCC 393 strain (a L. casei BL23-closely related strain) had anti-tumoral effects in the TC-1 mouse allograft model of HPV-induced cancer [26]. Here, we demonstrated that L. casei BL23 displays similar anti-cancer properties than does L. casei ATCC 393 strain in the TC-1 mouse allograft model of HPV-induced cancer. Then, we demonstrated that this probiotic bacterium significantly protected mice against DMH-induced CRC. The mechanism by which this strain appears to protect against CRC involves the induction of a fine-regulated immune response that seems different at the local and systemic levels. Indeed, in response to L. casei BL23 stimulation, splenic cells result in an increase of Th17 population with a shared reduction of Treg cells. In contrast, the results concerning IL-17 expression at the local level suggest a reduction in Th17 population (or at least those which produce IL-17), since we found a significant increase of IL-22 (a cytokine typically associated with a Th17-biased response). These results are unexpected since both a diminution in Th17 and an increase in Treg populations are most related to a beneficial effect in cancer (including CRC) [39–42]. However, although the inhibition of Treg in L. casei BL23-treated splenocytes (ex vivo analysis, Fig. 5d) and in splenocytes from L. casei BL23-treated mice (in vivo analysis, Fig. 7d) seem contradictory to the reported anti-cancer effects of this T-cell population [41], a recent study has described how impairment in Foxp3+ Treg promotes anti-tumor immunity [43]. In this context, recent observations put the light on the Treg/Th17 plasticity paradigm, suggesting that a response to specific-stimuli might be adjustable to environmental conditions [44]. More recently, an interesting study reports the impact of microbiota in the induction of a subset of Treg cells that also express RORγt (a Th17 lineage-specific transcription factor) and known as type 3 immunity [45]. Thus, we cannot rule out the probability that L. casei BL23 could be involved in the induction of this type of cells. In addition, the ability of L. casei BL23 to induce innate lymphoid cells, which also produce IL-22 at intestinal level, deserves further investigation [46].
Previous studies have reported that LAB display protective effects against CRC by reinforcing and modulating the host immune response. However in this study we show for the first time that a fine-tuned Th17-biased immune response could be related to the L. casei BL23 beneficial effect in the DMH-induced CRC. Nevertheless, further studies are necessary to better understand this phenomenon. Yet, our findings open new frontiers for the study of the immunomodulatory functions of probiotics since most of their beneficial effects are attributed to the paradigm: probiotics promote Treg cells and suppress Th17 populations. In addition, we cannot discard that LAB may also modify luminal secretions, reinforce the mucosal barrier, disturb epithelial cell proliferation, and reduce the exposure to toxic and carcinogenic compounds in the colon (for review see [39]).
In conclusion, our findings suggested that L. casei BL23, a probiotic anti-inflammatory strain, could be an attractive potential agent for CRC prevention and/or therapy. Indeed, L. casei BL23 is a good model for commercially used strains of probiotics (such as those present in several dairy products) and deserves attention for potential therapeutic applications.
Change history
08 September 2020
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s00535-015-1158-9.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6.
Uccello M, Malaguarnera G, Basile F, D’Agata V, Malaguarnera M, Bertino G, et al. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012;12(Suppl 1):S35.
Kahouli I, Tomaro-Duchesneau C, Prakash S. Probiotics in colorectal cancer (CRC) with emphasis on mechanisms of action and current perspectives. J Med Microbiol. 2013;62(Pt 8):1107–23.
Joint FAO/WHO Working Group report on drafting guidelines for the evaluation of probiotics in food. London: Food and Agriculture Organization. 2002. http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed 04 Jan 2016.
Bernardeau M, Vernoux JP, Henri-Dubernet S, Gueguen M. Safety assessment of dairy microorganisms: the Lactobacillus genus. Int J Food Microbiol. 2008;126(3):278–85.
Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116(5):1107–14.
Khazaie K, Zadeh M, Khan MW, Bere P, Gounari F, Dennis K, et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA. 2012;109(26):10462–7.
Kato I, Endo K, Yokokura T. Effects of oral-administration of Lactobacillus casei on antitumor responses induced by tumor resection in mice. Int J Immunopharmacol. 1994;16(1):29–36.
Aragon F, Carino S, Perdigon G, de Moreno de LeBlanc A. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with Lactobacillus casei CRL 431. J Immunother. 2015;38(5):185–96.
Hu J, Wang C, Ye L, Yang W, Huang H, Meng F, et al. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. J Biosci. 2015;40(2):269–79.
Aragon F, Carino S, Perdigon G, de Moreno de LeBlanc A. The administration of milk fermented by the probiotic Lactobacillus casei CRL 431 exerts an immunomodulatory effect against a breast tumour in a mouse model. Immunobiology. 2014;219(6):457–64.
Martin R, Miquel S, Ulmer J, Kechaou N, Langella P, Bermudez-Humaran LG. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Fact. 2013;12:71.
de Moreno de LeBlanc A, LeBlanc JG. Effect of probiotic administration on the intestinal microbiota, current knowledge and potential applications. World J Gastroenterol. 2014;20(44):16518–28.
O’Mahony L, Feeney M, O’Halloran S, Murphy L, Kiely B, Fitzgibbon J, et al. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15(8):1219–25.
Lankaputhra WE, Shah NP. Antimutagenic properties of probiotic bacteria and of organic acids. Mutat Res. 1998;397(2):169–82.
Reddy BS. Prevention of colon cancer by pre- and probiotics: evidence from laboratory studies. Br J Nutr. 1998;80(4):S219–23.
Brady LJ, Gallaher DD, Busta FF. The role of probiotic cultures in the prevention of colon cancer. J Nutr. 2000;130(2S Suppl):410S–4S.
Burns AJ, Rowland IR. Anti-carcinogenicity of probiotics and prebiotics. Curr Issues intest Microbiol. 2000;1(1):13–24.
de Moreno de LeBlanc A, Perdigon G. The application of probiotic fermented milks in cancer and intestinal inflammation. Proc Nutr Soc. 2010;69(3):421–8.
Reddy BS, Rivenson A. Inhibitory effect of Bifidobacterium longum on colon, mammary, and liver carcinogenesis induced by 2-amino-3-methylimidazo[4,5-f]quinoline, a food mutagen. Cancer Res. 1993;53(17):3914–8.
Hirayama K, Rafter J. The role of probiotic bacteria in cancer prevention. Microbes Infect. 2000;2(6):681–6.
Commane D, Hughes R, Shortt C, Rowland I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat Res. 2005;591(1–2):276–89.
Rochat T, Bermudez-Humaran L, Gratadoux JJ, Fourage C, Hoebler C, Corthier G, et al. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on DSS-induced colitis in mice. Microb Cell Fact. 2007;6:22.
de Moreno de LeBlanc A, LeBlanc JG, Perdigon G, Miyoshi A, Langella P, Azevedo V, et al. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J Med Microbiol. 2008;57(1):100–5.
Ribelles P, Benbouziane B, Langella P, Suarez JE, Bermudez-Humaran LG. Protection against human papillomavirus type 16-induced tumors in mice using non-genetically modified lactic acid bacteria displaying E7 antigen at its surface. Appl Microbiol Biotechnol. 2013;97(3):1231–9.
Maze A, Boel G, Zuniga M, Bourand A, Loux V, Yebra MJ, et al. Complete genome sequence of the probiotic Lactobacillus casei strain BL23. J Bacteriol. 2010;192(10):2647–8.
Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154(1):1–9.
Bermudez-Humaran LG, Cortes-Perez NG, Lefevre F, Guimaraes V, Rabot S, Alcocer-Gonzalez JM, et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol. 2005;175(11):7297–302.
Lin KY, Guarnieri FG, StaveleyOCarroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56(1):21–6.
Santiago C, Pagan B, Isidro AA, Appleyard CB. Prolonged chronic inflammation progresses to dysplasia in a novel rat model of colitis-associated colon cancer. Cancer Res. 2007;67(22):10766–73.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Mason KM, Bigley NJ, Fink PS. Development of a novel in vitro co-culture system for studying host response to native bacterial antigens. J Immunol Methods. 1998;211(1–2):147–58.
Hazebrouck S, Przybylski-Nicaise L, Ah-Leung S, Adel-Patient K, Corthier G, Langella P, et al. Influence of the route of administration on immunomodulatory properties of bovine beta-lactoglobulin-producing Lactobacillus casei. Vaccine. 2009;27(42):5800–5.
Yang M, Yang C, Mine Y. Multiple T cell epitope peptides suppress allergic responses in an egg allergy mouse model by the elicitation of forkhead box transcription factor 3- and transforming growth factor-beta-associated mechanisms. Clin Exp Allergy. 2010;40(4):668–78.
Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, et al. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol. 2010;184(12):6799–806.
Cardoso CR, Provinciatto PR, Godoi DF, Ferreira BR, Teixeira G, Rossi MA, et al. IL-4 regulates susceptibility to intestinal inflammation in murine food allergy. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G593–600.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8.
De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncog. 2015;34(27):3493–503.
Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183(7):4169–75.
Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69(13):5490–7.
Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–71.
Liu Y, Wang L, Predina J, Han R, Beier UH, Wang LC, et al. Inhibition of p300 impairs Foxp3(+) T regulatory cell function and promotes antitumor immunity. Nat Med. 2013;19(9):1173–7.
Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21(3):274–80.
Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, et al. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. 2015;349(6251):989–93.
Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, et al. RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12(4):320–6.
Acknowledgments
Marion Lenoir was a recipient of an ABIES Grant. Diego Muñoz-Provencio was the recipient of a fellowship from the “Fundación Alfonso Martín Escudero, Spain”. Daniel Lozano-Ojalvo acknowledges his FPU Grant (MECD) and financial support through AGL2014-59771R project. This work was partially funded by ECOS-Sud Program (Action No. A11S02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Marion Lenoir, Silvina del Carmen and Alejandra de Moreno de LeBlanc have contributed equally to this work.
About this article
Cite this article
Lenoir, M., del Carmen, S., Cortes-Perez, N.G. et al. RETRACTED ARTICLE: Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J Gastroenterol 51, 862–873 (2016). https://doi.org/10.1007/s00535-015-1158-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1158-9