Abstract
Functional foods and nutraceuticals frequently contain viable probiotic strains that, at certain titers, are considered to be responsible of beneficial effects on health. Recently, it was observed that secreted metabolites might play a key role in this respect, especially in immunomodulation. Exopolysaccharides produced by probiotics, for example, are used in the food, pharmaceutical, and biomedical fields, due to their unique properties. Lactobacillus brevis CD2 demonstrated the ability to inhibit oral pathogens causing mucositis and periodontal inflammation and to reduce Helycobacter pylori infections. Due to the lack of literature, for this strain, on the development of fermentation processes that can increase the titer of viable cells and associated metabolites to industrially attractive levels, different batch and fed-batch strategies were investigated in the present study. In particular, aeration was shown to improve the growth rate and the yields of lactic acid and biomass in batch cultures. The use of an exponential feeding profile in fed-batch experiments allowed to produce 9.3 ± 0.45 × 109 CFU/mL in 42 h of growth, corresponding to a 20-fold increase of viable cells compared with that obtained in aerated batch processes; moreover, also increased titers of exopolysaccharides and lactic acid (260 and 150%, respectively) were observed. A purification process based on ultrafiltration, charcoal treatment, and solvent precipitation was applied to partially purify secreted metabolites and separate them into two molecular weight fractions (above and below 10 kDa). Both fractions inhibited growth of the known gut pathogen, Salmonella typhimurium, demonstrating that lactic acid plays a major role in pathogen growth inhibition, which is however further enhanced by the presence of Lact. brevis CD2 exopolysaccharides. Finally, the EPS produced from Lact. brevis CD2 was characterized by NMR for the first time up to date.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organic acids (lactic or acetic acid), bacteriocins, and other compounds originated from lactic acid bacteria (LAB), such as exopolysaccharides (EPSs) contribute to their antimicrobial properties [1]. It is known that EPSs are high molecular weight and biodegradable polymers, synthesized by LAB, that are not used by the producer microorganisms themselves as energy sources [2]. Beyond their function as adhesive molecules, EPSs have been used in the production of several fermented foods, thickeners, stabilizers, emulsifiers, and gelling or water-binding agents [3]. In fact, their rheological properties make the EPSs particularly useful in food science/engineering (e.g., thickeners, preservatives) and even in the field of mechanical engineering (e.g., bio-lubricants, drug reducers). In addition, EPSs from food-grade organisms, such as LAB, have a potential as food supplements and as functional food ingredients with some claimed beneficial human health effects, and several papers indicated that this is due to the ability of the producer microorganisms to function as probiotic [4,5,6,7,8]. Probiotics, in fact, were demonstrated to possess activity against gastrointestinal disorders and inflammatory bowel diseases and to prevent allergies [9,10,11]. Furthermore, probiotic therapies were gradually applied to prevent and alleviate infectious diseases, therefore limiting antibiotics overuse and misuse also due to the emergence of antibiotic-resistant strains [12]. Many studies have also reported the immunostimulatory [8, 13,14,15] and antitumor activity [16,17,18,19] and the capacity to lower blood cholesterol [20, 21]. Extensive research has been focused on the EPSs that have the potential to be produced at industrial scale since the main difficulties to full commercialization are their production costs, mostly related to substrates and downstream processing [22]. In particular, several studies focused on the optimization of cultivation conditions in bioreactors by first identifying the best process parameters in batch mode, and then further increasing biomass and related exopolysaccharide concentrations in fed-batch experiments to respond to the strains’ specific metabolic requirements [23,24,25].
Lact. brevis is an obligate heterofermentative LAB isolated from plants, food and human microbiota, and several strains showed probiotic properties [26,27,28]; moreover, recently, some of them also emerged as efficient producers of γ-aminobutyric acid (GABA) that has a central function in the human nervous system and reduces the threat of type 1 diabetes [26, 29, 30]. In Lact. brevis KB290 that is one of the most characterized strains, a three-gene set that contributes to its probiotic properties, namely EPS production, cell aggregation, and bile resistance, was recently described [31]. This strain showed tolerance to gastrointestinal juices [27, 32], improvement of gut health [33, 34], and of immune function [35].
Lact. brevis CD2 is a strain that is normally present in the human mouth and intestinal flora and is also commonly found in dairy products [36]. This strain was observed to reduce the intragastric load of Helicobacter pylori [37] and to have anti-inflammatory activity on periodontal diseases and bone loss [38]; in addition, in a phase II pilot study, the efficacy of Lact. brevis CD2 lozenges in preventing oral mucositis in leukemia patients undergoing high-dose chemotherapy was highlighted by Sharma et al. [36].
Due to the lack of literature on the optimization of Lact. brevis CD2 growth and correlated production of secreted metabolites with biotechnological applications, in this work, we initially evaluated different growth conditions and carbon sources consumption in bottle experiments, and next focused on the development of batch and fed-batch fermentation processes on 2 L of controlled bioreactors. In particular, exponential feeding profiles were applied, based on the substrate consumption rate observed during the log phase, to avoid growth inhibition and overflow metabolism. Since EPS determines strain-specific probiotic properties, it was also interesting to study a preliminary purification process for further characterization and evaluation of antimicrobial activity. Usually, the recovery of extracellular microbial polysaccharides from the culture broth needs cell removal by centrifugation or microfiltration on membranes with a cut-off of 0.22 μm followed by precipitation with organic solvents such as ethanol, isopropanol, or acetone; the precipitated polymer is finally dried [39]. In this work, after centrifugation, membrane processes have been used for the concentration and recovery of products released in the fermentation broth, in order to decrease the amount of organic solvent to be used for the precipitation of exopolysaccharides, reducing process costs and above all the environmental impact. Lact. brevis CD2 was previously shown to inhibit periodontal and gastric pathogens [37, 40]; to study, its behavior also toward gut pathogens, we tested the antimicrobial activity of the fermentation products on Salmonella typhimurium and a conspicuous growth inhibiting effect of the released low molecular weight metabolites, was found.
Overall, in this research project, we performed fermentations and downstream processes, using Lact. brevis CD2, in order to improve biomass production and purify antimicrobial compounds such as lactic acid and exopolysaccharides to evaluate their potential to inhibit growth of other microbial strains. In particular, the antimicrobial activity of the fermentation products was tested on the pathogen Salmonella typhimurium revealing a conspicuous growth inhibiting effect of the released low molecular weight metabolites. Moreover, the structure of the released EPS was established by glycosyl analysis and 1H-NMR spectrum.
Materials and Methods
Bacterial Strain and Media
The strain Lact. brevis CD2 was supplied by Bioteknet s.p.a (Naples, Italy) in the framework of a collaboration with VSL laboratories (VSL Pharmaceuticals Inc. Towson, MD, USA). It was stored and maintained in 20% v/v glycerol stock solutions at − 80 °C. All medium components and salts were supplied by Sigma-Aldrich (St. Louis, MO, USA). Yeast extract was furnished by Organotechnie (La Corneuve, France), while ammonium hydroxide and sulfuric acid were purchased by Carlo Erba (Milan, Italy). The standard medium used in fermentation consisted of a basal salt medium (sodium acetate, ammonium sulfate, monobasic potassium phosphate, dibasic potassium phosphate, citric acid, magnesium and manganese sulfate, and tween 80) supplemented with fructose (25 g/L) as carbon source and yeast extract (15 g/L) as nitrogen source. The pathogenic strain used was the gram-negative Salmonella enterica subs. enterica serovar typhimurium (ATCC® 14028GFP™), available in the Clinical Microbiology Section of the Department of Experimental Medicine (University of Campania L. Vanvitelli).
Bottle and Bioreactor Fermentation Processes
Growth optimization experiments were run in 100 mL pyrex bottles on MRS medium initially in static and agitation (100 rpm) with an initial pH equal to 6 and a temperature of 32 °C. Carbon source evaluation was conducted by supplementing the medium with 25 g/L of fructose, glucose, or sucrose in agitation at pH 6 and 32 °C.
Before each bioreactor experiment, one stock (about 20 OD600nm) of Lact. brevis CD2 was added to 0.2 L of MRS medium supplemented with fructose in a 0.2 L bottle, and incubated in a rotary air shaker (model Minitron, Infors, Basel, Switzerland) at 32 °C and 100 rpm for 8 h. The inoculum size was 10% (v/v). The culture was transferred to a Biostat CT plus (Sartorius Stedim, Gottingen, Germany) bioreactor containing 1.8 L of the same medium, grown at 32 °C, pH 6, 100 rpm. Batch experiments were run with (0.75 vvm) and without air supply. Batch with pulse and fed-batch experiments were both run at 32 °C, pH 6, 100 rpm with constant air sparging at 0.75 vvm. In both types of experiments, the feed was added when the concentration of fructose initially present in the reactor (25 g/L) was below 5 g/L. In the first case, the fructose concentration was restored by adding a single pulse of concentrated solution. Fed-batch experiments instead used an exponential profile ranging from 2 to 3.2 g/L·h.
Sample Preparation and Quantification of Organic Acids
Samples during batch and fed-batch fermentation processes were withdrawn throughout the experiments. The broth was centrifuged at 5400 ×g (Avanti J-20 XP, Beckman Coulter, Brea, CA, USA) in order to separate the biomass and recover the supernatant. A supernatant of 1 mL was then UF/DF on 3 kDa centrifugal filter devices (Centricon, Amicon, Sigma-Aldrich) at 10000 ×g and concentrated about 5-fold. Permeates were analyzed by an HPAE-PAD ionic chromatographic system (model ICS-3000, Dionex, Thermo Fisher Scientific, Waltham, MA, USA), to quantify the residual carbon source, and by HPLC (model STH 575, Dionex), to measure the organic acids produced as previously reported [41].
Downstream Process
At the end of the fermentation process, the broth was centrifuged at 5000 ×g and 4 °C for 30 min and the supernatant was collected for the following downstream procedures. The supernatant was ultrafiltered, on 10 kDa cut-off membranes with a filtering area of 0.1 m2 (Sartorius Stedim, Gottingen, Germany). The system used for the tangential flow filtration process was a Sartoflow alpha (Sartorius Stedim, Gottingen, Germany), equipped with a 10-L steel tank, and pressure gauges on the inlet, and retentate lines. Additionally, a thermostatic bath kept a constant temperature of 20–25 °C. The retentate was treated on 2% p/v activated charcoal and then precipitated with 3 volumes of 96% v/v ethanol.
Quantification of Exopolysaccharides
Exopolysaccahrides were quantified by using the phenol-sulfuric acid method [42] a simple and rapid colorimetric assay to determine total carbohydrates in a sample. The method detects virtually all classes of carbohydrates, including mono-, di-, oligo-, and polysaccharides; however, the absorptivity of the different carbohydrates varies. Thus, the results must be expressed arbitrarily in terms of one carbohydrate. Pentoses during hydrolysis are then dehydrated to furfural, and hexoses to hydroxymethylfurfural. These compounds then react with phenol to produce a yellow-gold color. In this research, the calibration curve was obtained with standard solutions of D (+) glucose at concentrations ranging from 0.01–0.1 mg/mL. Briefly, 200 μL of standards are placed in a reaction tube with 200 μL of aqueous solution of phenol 5% w/v. Then, 1 mL of concentrated sulfuric acid (98% w/w) is added and the reaction tube is quickly closed. After vigorous stirring, the reaction is carried out for 30 min at 30 °C and then sample absorbance is read at 490 nm using distilled H2O as blank.
Characterization of Exopolysaccharides: Molecular Weight, Polydispersity, and Intrinsic Viscosity Determination by SEC-TDA
The chromatographic analyses of samples were performed using the SEC–TDA 305 equipment by Viscotek (Malvern, Milan, Italy). It was equipped with a triple detector array module including a refractive 11 index detector (RI), a four-bridge viscosimeter (VIS), and a laser detector (LS) made of a right-angle light scattering 12 (RALS) detector and a low-angle light scattering (LALS) one, as previously reported [43]. The OmniSEC software program was used for the acquisition and analysis of the Viscotek data. Two TSK–GEL GMPWXL columns (Tosoh Bioscience, Tokyo, Japan Cat. No. 8–08025, hydroxylated polymethacrylate base material, 100–1000 Å pore size, 13 μm mean particle size, 7.8 × 30.0 cm) in series that were preceded by a TSK–GEL guard column GMPWXL (Tosoh Bioscience, Cat. No. 08033, 12 μm mean particle size, 6.0 × 4.0 cm) were used. An isocratic elution with 0.1 M NaNO3 aqueous solution (pH 7.0) at a flow rate of 0.6 mL/min was carried out. Analyses were performed at 40 °C with a running time of 50′. The data were analyzed with a hypothesis of a dn/dc equal to 0.146 mL/g according to Petry et al. [44]. Universal calibration for the determination of K1, K2, and K3 was performed by using a polyethylene oxide (PEO) standard (22 kDa PolyCAL, Viscotek). However, given that the constants were dependent on hardware parameters, and due to the sophisticated system (e.g., mirrors, windows of analysis, four-capillary bridge VIS) and the related difficulty in accurately determining these parameters, the most precise evaluation was obtained by analyzing external standards of known characteristics (e.g., molecular weight, polydispersity, intrinsic viscosity, dn/dc).
Purification of Exopolysaccharides
A sample (30 mg) of the retentate from ultrafiltration was dissolved in deionized water and loaded on a Sephacryl S-400 (Sephacryl S400 HR, Ge-Healthcare, Chicago, IL, USA) column (1 cm × 115 cm) and eluted with 50 mM ammonium hydrogen carbonate pH 8 (flow 12 mL/h). Fractions containing carbohydrates were detected by phenol-sulfuric acid, pooled, and lyophilized (5 mg).
Monosaccharide Composition Analysis
The purified EPS sample was subjected to glycosyl composition analysis by means of GC-MS of acetylated methyl glycosides [45]. Briefly, 0.5 mg of EPS were treated with 1.25 M HCl in dry methanol for 16 h at 80 °C. The methanol layer was dried and acetylated with Ac2O and pyridine (100 °C, 30 min). The derivatives were analyzed using Agilent Technologies gas chromatograph 7820A equipped with a mass selective detector 5977B and an HP-5 capillary column (Agilent, Santa Clara, CA, USA, 30 m × 0.25 mm i.d., flow rate 1 mL/min, He as carrier gas). Acetylated methyl glycosides were analyzed using the following temperature program: 140 °C for 3 min, 140 °C → 240 °C at 3 °C/min. The monosaccharides were identified by comparison of their GC column retention times with those of authentic standards.
NMR Spectroscopy Analysis
Samples were analyzed according to Casillo et al. [46]. In particular, they were dissolved in 0.5 mL of D2O and 1H NMR were recorded at 298 K by using a Bruker 600 MHz spectrometer.
Antimicrobial Activity
To test antimicrobial activity of UF retentate and permeate obtained from batch and FB processes, Salmonella typhimurium was grown in tryptic soy agar (TSA Oxoid, Cambrige, UK) overnight at 37 °C in aerobic conditions. After 24 h, a single colony from overnight cultures was resuspended in tryptic soy broth (TSB, Oxoid, Cambrige, UK) and cultivated until the exponential phase of growth corresponding to a final concentration of approximately 108 CFU/mL. Each assay was performed using an inoculum of strain at 108 CFU/mL and the inoculum was confirmed by vital plate counts on TSA [47]. The culture of S. typhimurium at exponential phase was centrifuged at 2455 ×g for 10 min, washed twice with phosphate buffered saline (PBS, Sigma-Aldrich, St. Louis, MO, USA) and resuspended in buffered peptone water (BPW, Oxoid). Successively, 100 μL of log phase bacterial suspension (108 CFU/mL) were transferred into 5 mL sample (retentates and permeates) and incubated overnight at 37 °C. After incubation, the treated samples were serially diluted in BPW and 10 μL aliquots of the undiluted and 10-fold serially diluted samples were plated in triplicate on TSA plates to count the viable bacteria. The plates were incubated at 37 °C for 24 h to assess the colony-forming units, as the inhibitory effect of retentate and permeate samples on bacterial growth was evaluated by counting the CFU/mL obtained after the treatment [48].
Results
Medium Optimization and Bioreactor Bioprocess Development
Growth of Lact. brevis CD2 was initially studied in 100 mL bottles by keeping constant the pH and the temperature (6, 32 °C) and evaluating the effect of agitation versus static conditions and carbon source type. Results reported in Table 1 show a clear improvement of the total biomass, viability, and productivity of about 3.3-, 7-, and 8-fold, respectively, when the culture was incubated in shaking conditions. The replacement of fructose with either glucose or sucrose did not improve strain performance (Table 1); therefore, fructose was selected as the main carbon source for successive bioreactor experiments.
Table 2 and Fig. 1 summarize results obtained in a 2-L batch, batch with pulse and fed-batch fermentations. All fermentations were conducted under controlled pH and temperature conditions. Worst results were obtained in batch experiments without air supply; in fact, in these conditions the amount of viable cells by the end of the process was similar to that obtained in agitated bottle experiments (1.3 ± 0.35 × 108 CFU/mL). Moreover, as shown in the figure also the rates of growth, sugar consumption and lactic acid production were slower and the carbon source was not completely consumed by the end of the process (Table 2, Fig. 1). However, the yield of lactic acid on consumed fructose was similar to that obtained in the other fermentation conditions.
Fermentation processes of Lact. brevis CD2 grown on a semidefined medium containing fructose as main C source. Experiments were performed on a 2-L bioreactor at 32 °C, pH 6, and 0.75 vvm of air sparging. a and b Full circle, batch with air, OD600nm and fructose, respectively; full squares, batch without air, OD600nm and fructose, respectively; empty circle, batch with air, viability and lactic acid, respectively; empty squares, batch without air, viability and lactic acid, respectively. c and d Full triangle, batch with pulse, OD600nm and fructose, respectively; full diamonds, fed-batch, OD600nm and fructose, respectively; empty triangle, batch with pulse, viability and lactic acid, respectively; empty diamonds, fed-batch, viability and lactic acid, respectively. Data are representative of three biological replicates
Aerated batch processes resulted in a total average CFU/mL of about 4.5 × 108 ± 1.1 × 107 CFU/mL, with a final OD600nm of about 13.1 ± 0.3; lactic acid produced reached a maximum titer of 22.01 ± 0.03 g/L after 21 h of growth. Fructose was completely consumed after about 16–18 h of growth with an average consumption rate of 1.19 ± 0.02 g/L·h, and the yields of biomass on substrate, Yx/s and of product on substrate, YLA/fru are reported in Table 2. At the end of the process, the amount of total protein was 0.31 ± 0.03 g/L.
Batch with pulse and fed-batch experiments, in the presence of air, were performed in order to increase the number of CFU/mL, and the concentration of lactic acid and exopolysaccharide accumulated by the end of the process. Overall, the fructose consumption and lactic acid production rates in both conditions were similar to that observed during the batch process, whereas a higher concentration of biomass and of all metabolic products was obtained (Table 2). In batch with pulse experiments, a concentrated solution of fructose was added in a single shot, restoring the initial titer of fructose in the reactor; during fed-batch experiments, the feed started when the concentration of fructose was below 5 g/L on average. This led to a slightly higher final titer of lactic acid and to a 2.6-fold increase of viable cells leading also to higher productivities (Table 2). At the end of the process, the amount of total proteins in the supernatants was of about 0.34 ± 0.03 and 0.56 ± 0.07 g/L, respectively.
Downstream Process
Figure 2 shows the flowchart of the downstream process applied in the work. After centrifugation, the supernatant was treated on 10 kDa cut-off membranes. The flux and the other parameters observed during the ultrafiltration process are reported in Table 3.
A slight increase of the TMP and a flux decrease over time was observed when treating the supernatants recovered from FB processes, coupled to a longer process duration, as expected (Table 3). Before the following characterizations, samples were further purified and concentrated by charcoal treatment and ethanol precipitation.
Quantification and Molecular Weight Determination of the Secreted Exopolysaccharide
The phenol-sulfuric acid method was used to determine total carbohydrates in each sample, and data obtained from batch and fed-batch processes are reported in Table 4.
After the purification process, the molecular weight of the polysaccharides produced by Lact. brevis CD2 was evaluated by SEC-TDA (Table 5). The chromatogram reported in Fig. 3 indicates the presence of three species of about 95, 33, and 14 kDa in the sample derived from the fed-batch processes. The second peak (33 kDa) is the most abundant of the three.
Purification of EPS and Partial Characterization
The UF retentate was purified by gel filtration chromatography, and the monosaccharide composition was obtained by GC-MS of acetylated methyl glycosides. The GC chromatogram revealed that the EPS was mainly constituted by neutral sugars (Table 6). The variety of monosaccharides suggests an heteropolysaccharide structure, in which the mannose is the predominant component. The presence of a heteropolysaccharide was confirmed by the 1H NMR spectrum (Fig. 4). In fact, in the anomeric region ranging from 4.6 to 5.5 ppm, there are several signals indicating different monosaccharide arrangements.
Antimicrobial Activity
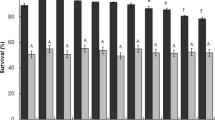
The concentrated supernatant and the permeate obtained by ultrafiltration were tested for the antimicrobial activity against S. typhimurium. Figure 5a shows the effect of UF retentate and permeate samples on the inhibition of S. typhimurium growth also in relation to the concentration of lactic acid present in the sample (Fig. 5b). Pure lactic acid at 5 and 10 g/L was also tested during the experiment. In particular, among all samples, the UF permeates mostly reduced pathogen growth; the samples obtained from ultrafiltering the FB supernatant that contained the highest concentration of lactic acid (46.1 g/L) decrease by about 73-fold the number of S. typhymurium viable cells, demonstrating best inhibitory performances among all samples tested.
Discussion
The important role of lactic acid bacteria and their metabolites in the bio-preservation of different types of food has attracted considerable interest already since the 1990s, as attested by the wide number of scientific reports concerning the antimicrobial activity of organic acids (primarily lactic acid), exopolysaccharides, and bacteriocins [49]. To boost the production of these metabolites, different fermentation strategies, using simple carbon sources or renewable materials, and diverse substrate feeding modes were investigated [23, 50,51,52,53]. However, this aspect was not thoroughly examined for Lact. brevis, so far. Different Lact. brevis strains were used as cell factories for the production of metabolites such as 1,3-propanediol, GABA, and vitamin B12 by using mainly batch fermentation processes under anaerobic conditions [54,55,56]. These experiments demonstrated the strain’s ability to use different substrates (e.g., glucose, glycerol, glutamate) and address parts of them not only to the production of lactic acid but also to that of metabolites with interesting applications in several fields [54,55,56]. The use of cheap plant biomasses was also investigated, for example, Lact. brevis ATCC367 showed the ability to co-ferment glucose and xylose, and produced lactic acid with a yield of 0.52 g/g and a final concentration of about 19 g/L in anaerobic batch experiments [57].
The main purpose of the present project was the optimization of growth of Lact. brevis CD2 and the development of improved batch and fed-batch fermentation processes, for the production of lactic acid and exopolysaccharides. We therefore initially investigated strain performance in small-scale bottle experiments maintaining the pH and temperature set at 6 and 32 °C, respectively, based on the previously reported fermenter experiments [58]. Li and coworkers [58], in fact, studied the effect of pH, temperature, and glutamate concentration on growth and GABA biosynthesis in Lact. brevis NCL912, and in terms of biomass production, the best results were obtained between 30 and 35 °C and at pH 6. Lactobacillus are facultative anaerobes or microaerophilic strains, so we initially compared static toward agitated growth conditions. Culture agitation resulted in a 7-fold higher amount of viable cells and faster growth rates, indicating that Lact. brevis CD2 could benefit from an improved oxygen availability. We also evaluated biomass production on three different carbon sources and identified fructose as the most efficient one. In fact, glucose may not be the preferred carbohydrate source for heterofermentative LAB, for example, Lact. brevis 123–20 was previously described as facultative fructophilic, to indicate that it grew more efficiently on fructose compared with glucose [59]. Glucose dissimilation was, however, enhanced in the presence of external electron acceptors [59].
We also studied the influence of dissolved oxygen in the medium in controlled batch experiments on the 2-L scale. Heterofermentative species including Lact. brevis metabolize both hexoses and pentoses via phosphoketolase (PK) pathway, leading to different end products (i.e., CO2, lactate and acetate, or ethanol) depending on the NADH/NAD+ balance. Aerobic growth has been long known to improve the growth yield and change end products of metabolism in heterofermentative LAB such as Lact. casei, Lact. bulgaricus, and Lact. plantarum [60,61,62], and also Lact. brevis, since oxygen acts as an alternative electron acceptor for the regeneration of NAD+ via H2O- or H2O2-generating NADH oxidase, thus increasing the ATP yield by making acetyl-phosphate available for substrate level phosphorylation [63]. The experiments reported here demonstrated that a higher pO2 improved by about 3-fold the CFU/mL compared with those obtained in batch experiments without air supply. Air sparging almost doubled the growth rate and therefore also the fructose consumption rate, while the YLA/fru was similar to that obtained in the absence of air. A similar behavior was observed for Lact. brevis ATCC 367 that showed complete carbon source consumption, shorter log phase, and a 3-fold higher cell density during aerobic growth on glucose, as compared with anaerobic conditions in batch. Moreover, upon glucose exhaustion, the strain showed the conversion of lactate to acetate [64]; also in this work, a partial lactate consumption was observed either in batch with pulse experiments, when fructose concentration was equal to zero (Fig. 1, 22 h and 40 h of growth), or in fed-batch experiments when no residual fructose was present in the tank (data not shown).
Besides externally providing electron acceptors, other ways of increasing biomass production in LAB require growing cells under sugar limitation or restoring a functional electron transfer chain. Lact. brevis ATCC 367 for example was demonstrated to have 3 out of 4 of the cytochrome cyd genes, and Lact. brevis B306 was reported to require both heme and menaquinone supplementation for aerobic growth stimulation in agitated shake flasks [65]. Aerated fed-batch cultivations have been widely exploited to increase biomass and metabolite production in different LAB species [66,67,68]. These strategies allow to keep substrate levels low and avoid substrate and product inhibition. Therefore, in order to further boost biomass and metabolite titers, in this study the process was prolonged either by the addition of a concentrated sugar feed in one shot or by following an exponential feeding profile, with a low residual concentration of fructose in the reactor. This approach allowed to increase the growth rate (and therefore the substrate consumption rate), and it allowed to obtain a 21- and 2.6-fold higher concentration of viable cells at the end of the process compared with batch and batch with pulse experiments.
As compared with batch processes, the concentration of proteins and secreted polysaccharides were about 1.8 and 3.5 times higher, respectively, in fed-batch processes, whereas only a slight (20%) but significant increase was found in respect to the one-shot feed addition. Overall, the titers of biomass, LA and EPS, and process productivities reached in the present study, were not reported for Lact. brevis CD2 up to date to our knowledge. Besides, improving the titer of viable biomass that is a critical aspect of industrial probiotic production, we also focused on the purification and characterization of low molecular weight metabolites, such as EPS. Generally, recovery of the microbial extracellular polysaccharides from the culture broth is achieved by centrifugation or filtration, followed by precipitation with organic solvents such as methanol, ethanol, isopropanol, or acetone [69, 70]. Cell removal is facilitated by dilution of the culture broth through addition of deionized water before centrifugation/filtration. However, this approach increases operating costs, because considerably higher volumes of cell-free supernatant are generated, and consequently, higher volumes of precipitating agent are required [71]. The preliminary purification strategy used in this study for the treatment of batch and FB supernatants was based on membrane processes. The latter are considered preferential initial steps of downstream treatments, since they allow reaching elevated concentrations of high molecular weight molecules of interest, their partial purification being among the least expensive procedures that can be applied at this stage of downstream treatments. This aspect is important since this phase represents very often the most expensive part of the whole manufacturing process. The choice of the membrane processes also aimed to separate, recover, and test products with different molecular weights for their antimicrobial activity. The supernatant recovered after batch and FB processes was ultrafiltered on 10 kDa membranes, thus obtaining a concentrated solution rich in exopolysaccharides and a permeate containing organic acids such as lactic acid or small proteins. In fact, there has been lately much interest in the potential health benefits of these compounds as possible active ingredients in nutraceuticals and functional foods. During the UF treatments, we found a flux decrease and a TMP increase mainly during processing of the fed-batch supernatant, clearly due to the higher viscosity of the broth for the presence of higher exopolysaccharide levels; the latter in fact may be responsible of the gel layer formation and of polarization phenomena involved in the clogging of the membrane. Overall, however, only a slight (5%) reduction of the flow in the two processes was observed due to the very large membrane surface area in respect to the amount of treated volume, that almost prevented membrane clogging (Table 3).
Suzuki et al. [27] described the composition of the EPS produced by Lact. brevis KB290, in which glucose and N-acetyl glucosamine were reported as the main components. After further purification, the EPS produced by Lact. brevis CD2 was characterized here, for the first time, revealing the co-existence of three species of different Mw. GC-MS and 1H-NMR indicated the presence of a heteropolysaccharide composed of several monosaccharides, with mannose as a predominant component. Such features designate a different structure with respect to that revealed from monosaccharide composition previously detected for Lact. brevis KB290.
Abdelzez et al. [26] previously demonstrated the antimicrobial activity of two Lact. brevis strains, namely KLDS 1.0727 and KLDS 1.0373, against foodborne pathogens by analyzing the diameter of the formed clear zones on agar plates. Lact. brevis CD2 was shown to inhibit growth of oral and stomach pathogens [36, 37, 72]. In this work, the high and low Mw samples recovered from the ultrafiltration processes were separately tested for their antimicrobial potential against S. typhimurium, to test the ability of Lact. brevis CD2, to also counteract growth of a gut pathogen, and to speculate on the molecules that could be responsible of this activity. Results indicated that all permeates more strongly inhibited pathogenic growth compared with retentates; in particular, samples derived from the processing of fed-batch supernatants more efficiently reduced the growth of S. typhimurium, probably due to the high concentration of lactic acid and to the presence of low molecular weight proteins. In order to understand if the presence of exopolysaccharides in the samples also had an impact on the growth of S. typhimurium, the effect of pure lactic acid at concentrations similar to those found in the batch and FB retentates, namely 5 and 10 g/L, was also analyzed. As indicated in Fig. 5a and b, lactic acid plays a major role in pathogen growth inhibition, which is however further enhanced by the presence of Lact. brevis CD2 exopolysaccharides.
Overall, a biotechnological process for the production and recovery of metabolites of biotechnological interest, based on modern fermentation and purification technologies, was developed using Lact. brevis CD2. EPS and lactic acid produced by Lact. brevis fermentations were tested for their antimicrobial activity toward a gut pathogenic microorganism, proving their potentiality as producers of interesting probiotic molecules for food and nutraceutical products and also extending the array of Lact. brevis CD2 possible targets. In addition, a preliminary structural characterization of the EPS produced by the strain was also provided.
References
Vesterlund S, Paltta J, Lauková A, Karp M, Ouwehand AC (2004) Rapid screening method for the detection of antimicrobial substances. J Microbiol Methods 57:23–31. https://doi.org/10.1016/j.mimet.2003.11.014
Mahdhi A, Leban N, Chakroun I, Chaouch MA, Hafsa J, Fdhila K, Mahdouani K, Majdoub H (2017) Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb Pathog 109:214–220. https://doi.org/10.1016/j.micpath.2017.05.046
Tapan Kumar S (2012) Microbial extracellular polymeric substances: production, isolation and applications. Iosr-phr 2:276–281. https://doi.org/10.9790/3013-0220276281
Schiraldi C, Valli V, Molinaro A, Cartenì M, De Rosa M (2006) Exopolysaccharides production in Lactobacillus bulgaricus and Lactobacillus casei exploiting microfiltration. J Ind Microbiol Biotechnol 33:384–390. https://doi.org/10.1007/s10295-005-0068-x
Caggianiello G, Kleerebezem M, Spano G (2016) Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl Microbiol Biotechnol 100:3877–3886. https://doi.org/10.1007/s00253-016-7471-2
Khalil ES, Abd Manap MY, Mustafa S, Alhelli AM, Shokryazdan P (2018) Probiotic properties of exopolysaccharide-producing Lactobacillus strains isolated from Tempoyak. Molecules. 23:1–20. https://doi.org/10.3390/molecules23020398
Abid Y, Casillo A, Gharsallah H, Joulak I, Lanzetta R, Corsaro MM, Attia H, Azabou S (2019) Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int J Biol Macromol 108:719–728. https://doi.org/10.1016/j.ijbiomac.2017.10.155
Rahbar Saadat Y, Yari Khosroushahi A, Pourghassem Gargari B (2019) A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr Polym 217:79–89. https://doi.org/10.1016/j.carbpol.2019.04.025
Toh ZQ, Anzela A, Tang ML, Licciardi PV (2012) Probiotic therapy as a novel approach for allergic disease. Front Pharmacol 3:171–184. https://doi.org/10.3389/fphar.2012.00171
Sarowska J, Choroszy-Król I, Regulska-Ilow B, Frej-Mądrzak M, Jama-Kmiecik A (2013) The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv Clin Exp Med 22:759–766
Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I, Bruno G, Petito V, Laterza L, Cammarota G, Gaetani E, Sgambato A, Gasbarrini A (2013) Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int 435268:1–9. https://doi.org/10.1155/2013/435268
Fang F, Xu J, Li Q, Xia X, Du G (2018) Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol 18:221–229. https://doi.org/10.1186/s12866-018-1369-3
Hosono A, Lee JW, Ametani A, Natsume M, Hirayama M, Adachi T, Kaminogawa S (1997) Characterization of a water-soluble polysaccharide fraction with immunopotentiating activity from Bifidobacterium adolescentis M101-4. Biosci Biotechnol Biochem 61:312–316. https://doi.org/10.1271/bbb.61.312
Li H, Qiu T, Huang G, Cao Y (2010) Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb Cell Factories 9:85–91. https://doi.org/10.1186/1475-2859-9-85
Karamese M, Aydin H, Sengul E, Gelen V, Sevim C, Ustek D, Karakus E (2016) The Immunostimulatory effect of lactic acid bacteria in a rat model. Iran J Immunol 13:220-228. IJIv13i3A7
Kitazawa H, Toba T, Itoh T, Kumano N, Adachi S, Yamaguchi T (1991) Antitumoral activity of slime-forming, encapsulated Lactococcus lactis subsp. cremoris isolated from scandinavian ropy sour milk, Viili. Anim Sci Technol 63:277–283. https://doi.org/10.3168/jds.S0022-0302(93)77483-4
Riaz Rajoka MS, Zhao H, Mehwish HM, Li N, Lu Y, Lian Z, Shao D, Jin M, Li Q, Zhao L, Shi J (2019) Anti-tumor potential of cell free culture supernatant of Lactobacillus rhamnosus strains isolated from human breast milk. Food Res Int 123:286–297. https://doi.org/10.1016/j.foodres.2019.05.002
Chuah LO, Foo HL, Loh TC, Mohammed Alitheen NB, Yeap SK, Abdul Mutalib NE, Abdul Rahim R, Yusoff K (2019) Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement Altern Med 19:114–125. https://doi.org/10.1186/s12906-019-2528-2
Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V, Namdar A (2019) Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol 234:17127–17143. https://doi.org/10.1002/jcp.28473
Nakajima H, Suzuki Y, Kaizu H (1992) Cholesterol lowering activity of ropy fermented milk. J Food Sci:1365–2621. https://doi.org/10.1111/j.1365-2621.1992.tb06848.x
Kitazawa H, Harata T, Uemura J, Saito T, Kaneko T, Itoh T (1998) Phosphate group requirement for mitogenic activation of lymphocytes by an extracellular phosphopolysaccharide from Lactobacillus delbrueckii ssp. bulgaricus. Int J Food Microbiol 40:169–175. 10.1016/s0168-1605(98)00030-0
Freitas F, Alves VD, Paism J, Costa N, Oliveira C, Mafrac L, Hilliou L, Oliveira R, Reis MAM (2009) Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour Technol 100:859–865. https://doi.org/10.1016/j.biortech.2008.07.002
Shaofeng D, Tianwei T (2006) L-lactic acid production by Lactobacillus casei fermentation using different fed-batch feeding strategies. Process Biochem 41:1451–1454. https://doi.org/10.1016/j.procbio.2006.01.014
Oleksy-Sobczak M, Klewicka E (2019) Optimization of media composition to maximize the yield of exopolysaccharides production by Lactobacillus rhamnosus strains. Probiotics Antimicrob Proteins:1–10. https://doi.org/10.1007/s12602-019-09581-2
Liu Q, Huang X, Yang D, Si T, Pan S, Yang F (2016) Yield improvement of exopolysaccharides by screening of the Lactobacillus acidophilus ATCC and optimization of the fermentation and extraction conditions. EXCLI J 15:119–133. https://doi.org/10.17179/excli2015-356
Abdelazez A, Abdelmotaal H, Evivie SE, Melak S, Jia FF, Khoso MH, Zhu ZT, Zhang LJ, Sami R, Meng XC (2018) Screening potential probiotic characteristics of Lactobacillus brevis strains in vitro and intervention effect on type I diabetes in vivo. Biomed Res Int 2018:1–20. https://doi.org/10.1155/2018/7356173
Suzuki S, Yakabe T, Suganuma H, Fukao M, Saito T, Yajima N (2013) Cell-bound exopolysaccharides of Lactobacillus brevis KB290: protective role and monosaccharide composition. Can J Microbiol 59:549–555. https://doi.org/10.1139/cjm-2013-0115
Kishi A, Uno K, Matsubara Y, Okuda C, Kishida T (1996) Effect of the oral administration of Lactobacillus brevis subsp. coagulans on interferon-alpha producing capacity in humans. J Am Coll Nutr 15:408–412. https://doi.org/10.1080/07315724.1996.10718617
Wu Q, Shah NP (2017) High γ-aminobutyric acid production from lactic acid bacteria: emphasis on Lactobacillus brevis as a functional dairy starter. Crit Rev Food Sci Nutr 57:3661–3672. https://doi.org/10.1080/10408398.2016.1147418
Wu Q, Shap NP (2018) Restoration of GABA production machinery in Lactobacillus brevis by accessible carbohydrates, anaerobiosis and early acidification. Food Microbiol 69:151–158. https://doi.org/10.1016/j.fm.2017.08.006
Fukao M, Oshima K, Morita H, Toh H, Suda W, Kim SW, Suzuki S, Yakabe T, Hattori M, Yajima N (2013) Genomic analysis by deep sequencing of the probiotic Lactobacillus brevis KB290 harboring nine plasmids reveals genomic stability. PLoS One 8(3):1–10. https://doi.org/10.1371/journal.pone.0060521
Fukao M, Zendo T, Inoue T, Nakayama J, Suzuki S, Fukaya T, Yajima N, Sonomoto K (2019) Plasmid-encoded glycosyltransferase operon is responsible for exopolysaccharide production, cell aggregation, and bile resistance in a probiotic strain, Lactobacillus brevis KB290. J Biosci Bioeng S1389-1723(19):30063–30065. https://doi.org/10.1016/j.jbiosc.2019.04.008
Murakami K, Habukawa C, Nobuta Y, Moriguchi N, Takemura T (2012) The effect of Lactobacillus brevis KB290 against irritable bowel syndrome: a placebo controlled double-blind crossover trial. Biopsychosoc Med 6:16–23. https://doi.org/10.1186/1751-0759-6-16
Fuke N, Aizawa K, Suganuma H, Takagi T, Naito Y (2017) Effect of combined consumption of Lactobacillus brevis KB290 and b-carotene on minor diarrhea-predominant irritable bowel syndrome-like symptoms in healthysubjects: a randomised, double-blind, placebo-controlled, parallel-group trial. Int J Food Sci Nutr 68:973–986. https://doi.org/10.1080/09637486.2017.1311843
Waki N, Matsumono M, Fukui Y, Suganuma H (2014) Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among school children. Lett Appl Microbiol 59:565–571. https://doi.org/10.1111/lam.12340
Sharma A, Tilak T, Bakhshi S, Raina V, Kumar L, Chaudhary SP, Sahoo RK, Gupta R, Thulkar S (2017) Lactobacillus brevis CD2 lozenges prevent oral mucositis in patients undergoing high dose chemotherapy followed by haematopoietic stem cell transplantation. SMO Open 1(6):e000138. https://doi.org/10.1136/esmoopen-2016-000138
Linsalata M, Russo F, Berloco P, Caruso ML, Matteo GD, Cifone MG, Simone CD, Ierardi E, Di Leo A (2004) The influence of Lactobacillus brevis on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter 9:165–172. https://doi.org/10.1111/j.1083-4389.2004.00214.x
Riccia DN, Bizzini F, Perilli MG, Polimeni A, Trinchieri V, Amicosante G, Cifone MG (2007) Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis 13:376–378. https://doi.org/10.1111/j.1601-0825.2006.01291.x
Shrikant AS, Parag SS, Ishwar BB, Rekha S (2007) Fermentative production, downstream processing and applications. Food Technol Biotechnol 45:107–118
Vuotto C, Barbanti F, Mastrantonio P, Donelli G (2014) Lactobacillus brevis CD2 inhibits Prevotella melaninogenica biofilm. Oral Dis 20:668–674. https://doi.org/10.1111/odi.12186
Cimini D, Restaino OF, Catapano A, De Rosa M, Schiraldi C (2010) Production of capsular polysaccharide from Escherichia coli K4 for biotechnological applications. Appl Microbiol Biotechnol 85:1779–1787. https://doi.org/10.1007/s00253-009-2261-8
Scott TA, Melvin EH (1953) Determination of dextran with anthrone. Anal Chem 25:1656–1661. https://doi.org/10.1021/ac60083a023
Restaino OF, Cimini D, Cassese E, Ventriglia R, Alfano A, Marrazzo A, D’ambrosio S, Barbuto Ferraiuolo S, De Rosa M, Schiraldi C (2019) Molecular weight determination of heparosan- and chondroitin-like capsular polysaccharides: figuring out differences between wild type and engineered Escherichia coli strains. Appl Microbiol Biotechnol 103:6771–6782. https://doi.org/10.1007/s00253-019-09969-8
Petry S, Furlan S, Waghorne E, Saulnier L, Cerning L, Maguin E (2003) Comparison of the thickening properties of four Lactobacillus delbrueckii subsp. bulgaricus strains and physicochemical characterization of their exopolysaccharides. FEMS Microbiol Lett 221:285–291. https://doi.org/10.1016/S0378-1097(03)00214-3
Casillo A, Ståhle J, Parrilli E, Sannino F, Mitchell DE, Pieretti G, Lanetta R, Parrilli M, Widmalm G, Tutino ML, Corsaro MM (2017) Structural characterization of an all-amino sugar-containing capsular polysaccharide from Colwellia psychrerythraea 34H. Antonie Leeuwenhoek 110:1377–1387. https://doi.org/10.1007/s10482-017-0834-6
Casillo A, Parrilli E, Sannino F, Mitchell DE, Gibson MI, Marino G, Lanzetta R, Parrilli M, Cosconati S, Novellino E, Randazzo A, Tutino ML, Corsaro MM (2017) Structure-activity relationship of the exopolysaccharide from a psychrophilic bacterium: a strategy for cryoprotection. Carbohydr Polym 156:364–371. https://doi.org/10.1016/j.carbpol.2016.09.037
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Valgas C, de Souza SM, Smânia EFA, Smânia A Jr (2007) Screening methods to determine antibacterial activity of natural products. Braz J Microbiol 38:369–380. https://doi.org/10.1590/S1517-83822007000200034
Lindgren SW, Dobrogosz WJ (1990) Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol Rev 87:149–164. https://doi.org/10.1111/j.1574-6968.1990.tb04885.x
Wang Y, Tashiro Y, Sonomoto K (2015) Fermentative production of lactic acid from renewable materials: recent achievements, prospects, and limits. J Biosci Bioeng 119:10–18. https://doi.org/10.1016/j.jbiosc.2014.06.003
Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, Kamran M, Ehsan N, Mehmood S (2014) Recent trends in lactic acid biotechnology: a brief review on production to purification. J Radiat Res Appl Sc 7:222–229. https://doi.org/10.1016/j.jrras.2014.03.002
Aguirre-Ezkauriatza EJ, Aguilar-Yáñez JM, Ramírez-Medrano A, Alvarez MM (2010) Production of probiotic biomass (Lactobacillus casei) in goat milk whey: comparison of batch, continuous and fed-batch cultures. Bioresour Technol 101:2837–2844. https://doi.org/10.1016/j.biortech.2009.10.047
Juturu V, Wu JC (2018) Microbial production of bacteriocins: latest research development and applications. Biotechnol Adv 36:2187–2200. https://doi.org/10.1016/j.biotechadv.2018.10.007
Vivek N, Aswathi TV, Sven PR, Pandey A, Binod P (2017) Self-cycling fermentation for 1,3-propanediol production: comparative evaluation of metabolite flux in cell recycling, simple batch and continuous processes using Lactobacillus brevis N1E9.3.3 strain. J Biotechnol 259:110–119. https://doi.org/10.1016/j.jbiotec.2017.07.033
Hasegawa M, Yamane D, Funato K, Yoshida A, Sambongi Y (2018) Gamma-aminobutyric acid fermentation with date residue by a lactic acid bacterium, Lactobacillus brevis. J Biosci Bioeng 125:316–319. https://doi.org/10.1016/j.jbiosc.2017.10.003
Xie C, Coda R, Chamlagain B, Varmanen P, Piironen V, Katina K (2019) Co-fermentation of Propionibacterium freudenreichii and Lactobacillus brevis in wheat bran for in situ production of vitamin B12. Front Microbiol 10:1541–1551. https://doi.org/10.3389/fmicb.2019.01541
Zhang Y, Vadlani PV (2015) Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J Biosci Bioeng 119:694–699. https://doi.org/10.1016/j.jbiosc.2014.10.027
Li CY, Lin HC, Lai CH, Lu JJ, Wu SF, Fang SH (2011) Immunomodulatory effects of Lactobacillus and Bifidobacterium on both murine and human mitogen-activated T cells. Int Arch Allergy Immunol 156:128–136. https://doi.org/10.1159/000322350
Neveling DP, Endo A, Dicks LMT (2012) Fructophilic Lactobacillus kunkeei and Lactobacillus brevis isolated from fresh flowers, bees and bee-hives. Curr Microbiol 65:507–515. https://doi.org/10.1007/s00284-012-0186-4
Alfano A, Donnarumma G, Cimini D, Fusco A, Marzaioli I, De Rosa M, Schiraldi C (2015) Lactobacillus plantarum: microfiltration experiments for the production of probiotic biomass to be used in food and nutraceutical preparations. Biotechnol Prog 31:325–333. https://doi.org/10.1002/btpr.2037
Schiraldi C, Adduci V, Valli V, Maresca C, Giuliano M, Lamberti M, Carteni M, De Rosa M (2003) High cell density cultivation of probiotics and lactic acid production. Biotechnol Bioeng 2003:213–222. https://doi.org/10.1002/bit.10557
Schlothauer RC, Morgan AJ, Rademacher I, Christensen T, Martel I (2004) Use of lactobacillus to produce exopolysaccharides in food and pharmaceutical compositions WO2004013343 (A2)
Sun Kang T, Korber DR, Tanaka T (2013) Regulation of dual glycolytic pathways for fructose metabolism in heterofermentative Lactobacillus panis PM1. Appl Environ Microbiol 79:7818–7826. https://doi.org/10.1128/AEM.02377-13
Guo T, Zhang L, Xin Y, Xu Z, He H, Kong J (2017) Oxygen-inducible conversion of lactate to acetate in heterofermentative Lactobacillus brevis ATCC 367. Appl Environ Microbiol 17:83–104. https://doi.org/10.1128/AEM.01659-17
Brooijmans R, Smit B, Santos F, Riel JV, deVos WM, Hugenholtz J (2009) Heme and menaquinone induced electron transport in lactic acid bacteria. Microb Cell Factories 8:28–38. https://doi.org/10.1186/1475-2859-8-28
Callewaert R, De Vuyst L (2000) Bacteriocin production with Lactobacillus amylovorus DCE471 is improved and stabilized by fed-batch fermentation. Appl Environ Microbiol 66:606–613
Castro LP, Bernardez PF, Guerra NP, Guseva EV, Fick M (2007) Fed-batch pediocin production on whey using different feeding media. Enzym Microb Technol 41:397–406
Racine FM, Saha BC (2007) Production of mannitol by Lactobacillus intermedius NRRL B-3693 in fed-batch and continuous cell-recycle fermentations. Process Biochem 42:1609–1613
Polak-Bereckaa M, Waśko A, Skrzypekb H, Kreftc A (2013) Production of exopolysaccharides by a probiotic strain of Lactobacillus rhamnosus: biosynthesis and purification methods. Acta Aliment 42:220–228. https://doi.org/10.1556/AAlim.42.2013.2.9
Bajaj BI, Survase SA, Saudagar PS, Singhal RS (2007) Gellan gum: fermentative production, downstream processing and applications. Food Technol Biotechnol 45:341–354
Freitas F, Alves VD, Reis AM (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29:388–398. https://doi.org/10.1016/j.tibtech.2011.03.008
Maekawa T, Hajishengallis G (2014) Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. Periodontal Res 49:785–791. https://doi.org/10.1111/jre.12164
Acknowledgments
The authors gratefully acknowledge Enrico Maria Cacciapuoti for fruitful discussions on fermentation and downstream processing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Bioteknet s.p.a. is a public body of which the L. Vanvitelli University is the majority shareholder.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alfano, A., Perillo, F., Fusco, A. et al. Lactobacillus brevis CD2: Fermentation Strategies and Extracellular Metabolites Characterization. Probiotics & Antimicro. Prot. 12, 1542–1554 (2020). https://doi.org/10.1007/s12602-020-09651-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09651-w