Abstract
In this chapter, we highlight the role of brain imaging techniques (e.g., magnetic resonance imaging [MRI]) in studying ketamine abuse. In the past two to three decades, brain imaging studies demonstrated deficits in brain circuits related to drug addiction and drug abuse. This chapter begins with a brief introduction of structural and functional brain imaging techniques such as computed tomography (CT) and electroencephalogram (EEG). Then, we give a brief introduction of ketamine abuse in mainland China before introducing structural MRI and functional MRI and reviewing the application of structural MRI study for ketamine abusers (including reduction of gray matter volume and disruption of white matter integrity) and functional MRI study for ketamine abusers (including alternation of regional homogeneity (ReHo) of resting-state brain activity, functional connectivity by resting-state fMRI, task-based fMRI). Finally, we discuss the implication for medical use of ketamine by brain imaging study, especially its rapid-acting glutamatergic antidepressant effects and the “ketamine model” of psychosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain imaging
- Magnetic resonance imaging
- Structural MRI
- Functional MRI
- Chronic ketamine use
- Ketamine abusers
1 Brain Imaging Techniques
Brain imaging or neuroimaging techniques, by either directly or indirectly imaging the structure or function of the brain, have offered us a window to view noninvasively the human brain activity underlying complex affect, behavior, and cognition (Casey et al. 2005). Within the past two or three decades, by uncovering the inner workings of the human brain or revealing the neurological changes that result from brain diseases, these technological advances have accelerated the translation of basic neuroscience discoveries into precision medicine and targeted therapy in clinical management of brain diseases, which have made huge advances in cognitive and behavioral neuroscience.
Conceptually, brain imaging techniques can be classified into two broad categories: structural imaging and functional imaging (Hirsch et al. 2015). Structural imaging is used to reveal the anatomical properties of the brain, detect brain damage and abnormalities, and diagnose the gross intracranial disease and injury. Functional imaging is used to diagnose metabolic diseases and lesions on a finer scale or for neurological and cognitive psychology researches to identify brain areas and underlying brain processes that are linked to a particular cognitive or a behavioral task.
Here, we introduce some commonly used structural and functional brain imaging tools.
Structural brain imaging techniques:
-
1.
Computed tomography (CT) or computed axial tomography (CAT) is a widely used approach that is typically used for viewing gross brain abnormalities, cerebral vascular accidents, and bone structure. However, it requires exposure to differential absorption of X-rays and has relatively low spatial resolution.
-
2.
Magnetic resonance imaging (MRI) is a relatively accessible approach to visualize the soft tissues of brain such as gray matter and white matter with good spatial resolution. But it cannot be used in patients with metallic implants such as certain cardiac pacemakers or implantable cardioverter defibrillators.
-
3.
Diffusion-based MRI (e.g., diffusion tensor imaging (DTI)) is an approach that is used for detecting detailed information of white matter integrity or microstructure and delineating white matter pathways that connect different regions of the brain. However, it requires complex image analyses and is sensitive to patient movement.
Functional brain imaging techniques:
-
1.
Functional MRI (fMRI) and arterial spin labeling (ASL) can localize the brain activity associated with performing a cognitive task and/or behavior. It relies on the paramagnetic properties of oxygenated and deoxygenated hemoglobin to view images of blood flow changes associated with the regional activation of neurons during cognitive or behavioral tasks. The main drawback is its limited temporal resolution ability.
-
2.
Positron emission tomography (PET) is an approach that is used to monitor the brain activity as well as the metabolism associated with performing a cognitive task and/or behavior by measuring emissions from radioactively labeled, metabolically active chemicals (e.g., fludeoxyglucose F 18) that have been injected into the bloodstream. However, it requires the injection of radioactive tracers, which is a main drawback.
-
3.
Electroencephalogram (EEG) and evoked related potentials (ERP) can directly record the underlying electrical brain activity that is associated with a cognitive task and/or behavior with good temporal resolution. However, these techniques have poor spatial resolution compared with fMRI and require a complicated analysis of the acquired data.
Many other brain imaging techniques are also available, such as the functional near-infrared spectroscopy (fNIRS) and magnetoencephalogram (MEG). In this chapter, we focus on MRI (structural and functional) techniques because these are most widely used techniques in drug abuse and addiction (Parvaz et al. 2011). This chapter mainly focuses on structural and functional brain imaging of ketamine abusers. We briefly introduce ketamine abuse in mainland China before reviewing neuroimaging studies of ketamine addiction and abuse.
2 Nonmedical Use of Ketamine in Mainland China
During the last three or four decades, drug abuse spread quickly following its reemergence as a national problem in China (Liu et al. 2016). The number of registered drug abusers increased from 70,000 in 1990 to more than one million by the end of 2005 (Michels et al. 2007). According to China drug situation report 2018, the number of registered drug abusers doubled in the next decade to more than two million by the end of 2018, and the number of abusers of “new” types of drugs including methamphetamine (accounting for 56.1%) and ketamine (accounting for 2.6%) exceeded the number of abusers of opioids (www.nncc626.com). Illicit drug trafficking and production have swept in mainland China, particularly in southern China, which have caused many problems (such as the spread of HIV) for both abusers and the community (Fang et al. 2006).
In mainland China, ketamine was rescheduled from Schedule II in 2001 to Schedule I in 2007 (Liao et al. 2017). It is commonly abused through snorting. Figure 1 shows a typical way of making and snorting ketamine powder. Long-term ketamine abuse often results in bladder dysfunction (Tsai et al. 2009), sleeping disturbances (Tang et al. 2015a), cognitive impairment (Chan et al. 2013; Morgan et al. 2004a, b; Tang et al. 2013), depression (Li et al. 2017a; Tang et al. 2013), and even schizophrenia-like symptoms (Liao et al. 2016a; Tang et al. 2015b). Furthermore, a line of study reported brain structural and functional alterations in ketamine abusers (Liao et al. 2010, 2011, 2012, 2016b, 2018; Wang et al. 2013). This chapter reviews the available structural and functional neuroimaging (mainly MRI) studies of ketamine abusers in detail.
3 Brain Imaging of Ketamine Abusers
Magnetic resonance imaging (MRI) is one of the most commonly used techniques to examine the brain activity in healthy brain and brain with disease, such as addiction or other mental illness. It is often divided into structural MRI and functional MRI (fMRI) (Symms et al. 2004). The field strength of the magnet is measured in tesla (T), and the magnetic strength for most clinical or research MRI scanners is 1.5 or 3 T. Most studies indicate that 3.0-T MRI scanner performs as good as or better than 1.5-T MRI scanner (Wood et al. 2012).
3.1 Magnetic Resonance Imaging
In the magnetic field, the nuclear spins of certain atoms within an object are oriented either parallel or antiparallel to the main magnetic field and produce a secondary spin or wobble (precession) of nuclei around the main or static magnetic field with a certain frequency called the Larmor frequency (named after the Irish physicist and mathematician Joseph Larmor, 1857–1942). The Larmor or precessional frequency in MRI is related to the strength of the magnetic field (the B0 field). Magnetic resonance occurs when a radio frequency (RF) pulse produced at the (tissue specific) Larmor frequency, exciting and then raising the nuclear spin orientation of hydrogen atoms from lower to higher energy states. By rotating the magnetization, the RF field is switched off and the magnetization once again freely precesses around the direction of the original magnetization. This exchange of energy between spin states is called resonance, and a computer displays the different resonance characteristics of various tissue types as an image. This time-dependent precession produces a current in the receiver RF coil, and the exponentially decaying resultant current (the free induction decay) constitutes the MR signal. The amount of the signal that is used to compose an image is proportional to the magnetic field strength of the scanner. (Signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), spatial resolution, and temporal resolution are commonly used technical terms to describe MRI image quality.) During the energy transition period, magnetization returns to its original equilibrium state (relaxation), characterized by T1 and T2 time constants, which depend on physical and chemical characteristics unique to the tissue type. The T1 and T2 differences between different tissue types (e.g., gray matter and white matter) produce a high-contrast MR image (Lauterbur 1973; Mansfield and Maudsley 1977).
3.1.1 Structural MRI
Structural MRI is a noninvasive technique that is used for examining the anatomy and pathology of the brain (as opposed to fMRI). It produces images that can be used for clinical radiological reporting as well as for diagnosis or research with detailed analysis. Structural MRI includes high-resolution imaging, T2 relaxation measurement, T2-weighted imaging, T1 relaxation measurement, magnetization transfer imaging, and diffusion imaging (Symms et al. 2004). Some MRI scan sequences are volumetric, and the volumes of regional gray matter and white matter often change considerably in individuals with brain diseases.
3.1.2 Functional MRI
Since its inception in 1990, functional MRI (fMRI) has been used to map human brain function noninvasively and rapidly with full brain coverage, and with relatively high spatial and temporal resolution. John Belliveau, Bruce Rosen et al. introduced it by using gadolinium as a contrast agent (Belliveau et al. 1990; Rosen et al. 1990). This was then immediately followed by a series of fMRI studies using the “blood oxygen level dependent” (BOLD) signal (Hoge et al. 1999; Kwong et al. 1992; Ogawa et al. 1990), which is the best known oxygen sensitive contrast agent. BOLD contrast results from a change in the magnetic field surrounding the red blood cells depending on the oxygen state of the protein hemoglobin, which is used for the indirect measurement of the brain activity.
In the past three decades, with extraordinary features (e.g., widespread availability, noninvasive nature, relatively low cost, and good spatial resolution), fMRI was applied in an exceptionally large number of studies in the cognitive neurosciences, clinical psychiatry/psychology, and presurgical planning to find biomarkers for disease (Bush et al. 1999; Fleisher et al. 2009), to monitor therapy (Stoeckel et al. 2014), or to study pharmacological efficacy (Honey and Bullmore 2004). The use of fMRI at rest has also enabled researchers to investigate resting functional connectivity of the human brain (Biswal et al. 1995). Measures of resting functional connectivity have been shown to be sensitive to brain diseases including drug and nondrug addiction (Fedota and Stein 2015; Hong et al. 2013; Sutherland et al. 2012). The limitations of fMRI include high susceptibility of the BOLD response to several nonneural and imaging artifacts, especially due to its low SNR and low temporal resolution.
By comparing brain structure, function, and metabolism between drug-abusing and non-abusing individuals, brain imaging techniques enable researchers to observe the disruption of ketamine or other drug abusers’ brain activity and allows us to better understand the mechanisms of addiction (Volkow et al. 2014).
3.2 Structural MRI Study of Ketamine Abusers
In the past two decades, there was considerable evidence that many psychiatric disorders (e.g., schizophrenia (Canu et al. 2015; Van Erp et al. 2018), bipolar disorder (Hibar et al. 2016; Strakowski et al. 1999), major depressive disorder (Schmaal et al. 2017), autism spectrum disorder (Sparks et al. 2002), obsessive–compulsive disorder (Fouche et al. 2017; Jenike et al. 1996), and attention-deficit/hyperactivity disorder (Seidman et al. 2005)) are associated with either increased or decreased regional brain volumes compared with gender- and age-matched healthy subjects. These brain structural abnormalities were also reported among ketamine abusers. Until now, only a few clinical structural MRI findings, however, have reported that the long-term effects of ketamine abuse is associated with the reduction of gray matter volume and the disruption of white mater integrity.
to assess regional gray matter volume reduction in ketamine abusers, voxel-based morphometry in conjunction with statistical parametric mapping on the structural magnetic resonance images of 41 ketamine abusers and 44 drug-naive control individuals in mainland China was used. This study found reduction of dorsal prefrontal gray matter after long-term repeated ketamine abuse. In particular, this study revealed significant reduction in gray matter volume in left superior frontal gyrus and right middle frontal gyrus of ketamine abusers in comparison with age-matched healthy volunteers (see Fig. 2). Furthermore, the duration of ketamine abuse was negatively correlated with the reduction of gray matter volume in bilateral frontal cortex, whereas the estimated total lifetime ketamine consumption was negatively correlated with the reduction of gray matter volume in left superior frontal gyrus. The association between frontal gray matter loss and the duration of ketamine use or cumulative doses of ketamine may suggest a dose-dependent effect of long-term abuse of the drug (Liao et al. 2011). Neuroimaging studies in other drug addictive users have also identified dysfunction of the prefrontal cortex, which suggests the involvement of this brain region in drug addiction (Goldstein and Volkow 2011).
Several studies, mainly in China and the UK, uncovered the disruption of white matter in chronic ketamine abusers. A study with the same Chinese sample (41 ketamine-dependent subjects and 44 drug-free healthy volunteers), using in vivo diffusion tensor magnetic resonance imaging data, measured white matter volumes. This study also found bilateral frontal and left temporoparietal white matter alterations associated with long-term ketamine abuse (see Fig. 3). Furthermore, frontal white matter fractional anisotropy (FA is a scalar value between 0 and 1 that describes the degree of anisotropy of a diffusion process.) correlated with the severity of drug use (as measured by estimating totally abused ketamine). This study provided first evidence for dose-dependent abnormalities of white matter in bilateral frontal and left temporoparietal regions following long-term ketamine abuse. These findings further suggest a microstructural basis for the changes in cognition and experience observed with chronic ketamine abuse (Liao et al. 2010).
Another study in the UK measured indices of white matter microstructural integrity and connectivity in 16 ketamine abusers and 16 poly-drug-using controls. The study used probabilistic tractography to quantify alterations in corticosubcortical connectivity associated with repeated ketamine abuse. This study found a decrease in the axial diffusivity profile of white matter in the right hemisphere network of white matter regions in ketamine abusers compared with poly-drug abusers. For ketamine abusers, this study found that the frequency of dissociative experiences reported by chronic ketamine users associated positively with the changes of connectivity in the caudate nucleus and the lateral prefrontal cortex. These findings further confirmed that ketamine abuse may be associated with widespread alternation of white matter integrity. It also suggests that white matter pathways between subcortical and prefrontal cortical regions may in part predict individual differences in the frequency of dissociative experiences due to ketamine abuse (Roberts et al. 2014).
By employing MRI, a study from the south of China illustrated the possible disrupted brain regions due to ketamine abuse by comparing 21 ketamine addicts with three age-matched healthy controls. This study revealed the lesions in many regions (e.g., prefrontal, parietal, occipital, limbic, brainstem, and corpus striatum) of the brain of ketamine abusers (Wang et al. 2013).
Several animal studies using monkeys or rats (Li et al. 2017b; Sun et al. 2014; Yeung et al. 2010; Zou et al. 2009) have also shown that brain structural, especially frontal cortex, disruption is associated with long-term exposure to ketamine.
3.3 Functional MRI Study of Ketamine Abusers
Only a few studies measured the long-term effects of ketamine abuse on brain functional alternations. One resting-state fMRI demonstrated the alterations in regional homogeneity (ReHo) of resting-state brain activity in long-term ketamine abusers. This study examined such effects on spontaneous brain dynamics between 41 patients with ketamine dependence and 44 healthy control subjects by resting-state fMRI. The results showed that compared with healthy controls, ketamine abusers displayed decreased ReHo in the right anterior cingulate cortex and increased ReHo in left precentral frontal gyrus. This study also showed negative correlations between increased ReHo in precentral frontal gyrus and estimated totally abused ketamine in their lifetime and levels of ketamine craving. The findings from this study indicate that ketamine abuse might be associated with alterations in the functional connectivity of medial and lateral prefrontal cortices (Liao et al. 2012).
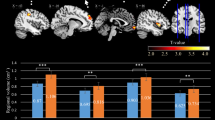
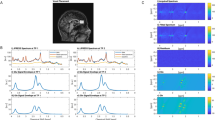
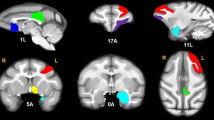
Decreased thalamocortical connectivity also has been observed in chronic ketamine abusers, which may suggest its role in the mechanistic “switch” from recreational abuse to dysregulated ketamine addiction. In this study, 41 ketamine abusers and 89 healthy control subjects were enrolled to measure the functional connectivity within the cerebral cortex by resting-state fMRI. This study found that ketamine abusers showed significantly decreased (but not increased) connectivity between the thalamic nuclear groups and the cortical regions of interest (ROI), including the prefrontal cortex, the motor cortex/supplementary motor area, and the posterior parietal cortex (see Fig. 4). This finding provided the first evidence of abnormal thalamocortical connectivity of resting state brain activity in long-term ketamine abusers (Liao et al. 2016b).
The diffusively decreased thalamocortical connectivity in ketamine-dependent subjects. (a) The six cortical regions of interest. (b) Thalamocortical connectivity of the drug-free control subjects. (c) Thalamocortical connectivity of the ketamine-dependent subjects. (d) Group differences in thalamocortical connectivity for the drug-free control subjects and the ketamine-dependent subjects
Ketamine abuse-related cues may induce craving in ketamine-dependent patients. A study measured regional brain activation to ketamine, cigarette smoking, and sexual activity. This functional MRI study recruited 40 smokers with ketamine abuse, 45 smokers without ketamine abuse, and 44 non-ketamine abuse noncigarette smoking healthy controls to view ketamine use-related cigarette smoking and sexual films. This task-based fMRI found that smokers with ketamine abuse showed significant increased activation in regions of anterior cingulate cortex and precuneus in response to ketamine cues. They also showed reduced activation in cerebellum and middle temporal cortex in response to sexual cues. Smokers (both with and without ketamine abuse) showed increased activation in the right precentral frontal cortex in response to smoking cues. Non-ketamine users (both smokers without ketamine abuse and controls) showed increased activation of cerebellum and middle temporal cortex when they were viewing sexual cues (see Fig. 4). These findings indicated the engagement of distinct neural circuitry for ketamine-related stimuli (compared with cigarette smoking stimuli or sexual stimuli) in ketamine abusers. While smokers (for both with and without ketamine abuse) showed overlapping differences in activation for smoking cues (Liao et al. 2018), which may be partly due to the interaction effects of ketamine and nicotine in multiple brain regions. Nicotine substantially ameliorated the effects of ketamine on anterior cingulate regional cerebral blood flow (rCBF), and those effects may link to psychosis, reward, and addictive behaviors (Rowland et al. 2010).
Besides measuring functional MRI, the consequences of ketamine abuse in the human brain function have also been studied using positron emission tomography (PET) in a group of 14 recreational chronic ketamine users and matched healthy subjects. The study found that chronic ketamine abuse exhibited a regionally selective upregulation of D1 receptor availability in the dorsolateral prefrontal cortex (Narendran et al. 2005). Moreover, functional MRI of the brains in adolescent monkeys with chronic exposure to ketamine showed regional brain functional changes, particularly in the prefrontal dopaminergic system – a system critically involved in working memory and executive function (Yu et al. 2012). Thus, long-term ketamine administration may involve neurodegenerative process similar to that of aging and/or Alzheimer’s disease, which was demonstrated in both animal (Yeung et al. 2010) and human (Chan et al. 2013) studies.
Table 1 presents a summary of regional brain structural and functional disruption in ketamine abusers.
4 Implication for Medical Use of Ketamine by Brain Imaging Study
In addition to its abuse potential, ketamine is commonly used for medical purposes (Morgan et al. 2012). Ketamine is a noncompetitive antagonist at the glutamatergic N-methyl-d-aspartate (NMDA) receptor that is most widely used injectable anesthetic agent in veterinary medicine (Clarke and Hall 1990; Young et al. 1993). It is also used in specialist anesthesia, particularly pediatrics and field medicine for human (Cartwright and Pingel 1984; Green and Johnson 1990). Furthermore, ketamine has a role in pain management in both human and veterinary medicine (Elia and Tramèr 2005). Ketamine, as an N-methyl-d-aspartate (NMDA) receptor antagonist, has been found to induce schizophrenia-type symptoms in humans. Thus, there are also a line of experimental studies using brain imaging to explore the “ketamine model” of psychosis (Deakin et al. 2008; Honey et al. 2008). As ketamine can block bursting in the lateral habenula to rapidly relieve depressive symptoms (Yang et al. 2018), it is used today for the treatment of depression, particularly for treatment-resistant depression (Aan Het Rot et al. 2012; Katalinic et al. 2013; Rosenblat et al. 2019). Recently, it was also found to be promising for addiction relapse prevention (e.g., heroin and alcohol addiction) as it reduced symptoms of depression (Ezquerra-Romano et al. 2018).
A series of pharmacological MRI study examined ketamine’s pharmacological effects on brain function, mainly to explore its potent and fast-acting antidepressant effects and uncover the neural mechanisms of psychosis and schizophrenia (as it produces similar symptoms).
4.1 For Depression
The abuse potential and psychotomimetic effects of ketamine may be linked with the dopaminergic system that also associated with antidepressant effects. Traditional antidepressants require several weeks to show their therapeutic effects. A single subanesthetic dose of the N-methyl-d-aspartate glutamate receptor antagonist ketamine, however, can produce significant clinical improvement within hours, getting the approval of Food and Drug Administration (FDA) of esketamine nasal spray for patients with treatment-resistant depression. Ketamine as a rapid-acting glutamatergic antidepressant, its novel treatment mechanisms are emerging a line of research (Krystal et al. 2013). The antidepressant properties of (R,S)-ketamine have also relevance for the development of next-generation, rapid-acting antidepressants (Zanos et al. 2016).
Subanesthetic doses of ketamine increase dopamine levels in the frontal cortex, in the striatum, and in the nucleus accumbens in rodents (Kokkinou et al. 2017). Applying 1H-MRS to 10 major depressive disorder (MDD) patients, however, did not observe the association between ketamine infusion with changes in occipital amino acid neurotransmitter (AANt) content, which indicates that these changes are not a correlate of ketamine’s antidepressant action (Valentine et al. 2011). A study using resting-state fMRI to investigate the intrinsic brain networks in MDD patients with ketamine treatment showed that ketamine significantly increased global signal regression (GBCr) in the PFC and reduced GBCr in the cerebellum, which may serve as a putative marker for successful treatment and a target for antidepressants’ development (Abdallah et al. 2017). However, it is important to be aware of ketamine abuse and addiction potential, even in MDD patients who received ketamine for antidepressant purposes (Bonnet 2015). In the future, research should explore more details about ketamine’s acute and chronic antidepressant effects on neuropharmacological and cognitive levels.
4.2 For Psychosis and Schizophrenia
On the contrary, several studies indicate that subanesthetic doses of ketamine impair prefrontal cortex (PFC) function in the rat and produce schizophrenia-like symptoms and dissociative states, including impaired performance of frontal lobe-sensitive tests, which is similar to cognitive impairment (Morgan and Curran 2006), persistent dissociative, depressive, and delusional thinking (Sassano-Higgins et al. 2016) by long-term ketamine use. It is suggested that ketamine may disrupt PFC function partly by interacting with dopaminergic neurotransmission in this region. By increasing the release of glutamate, thereby stimulating postsynaptic non-NMDA glutamate receptors, it probably impairs cognitive functions associated with PFC as a result (Moghaddam et al. 1997). Intravenous ketamine (1-min bolus of 0.26 mg/kg) to healthy individuals induced a rapid, focal, and unexpected decrease of the brain activity in ventromedial frontal cortex, including orbitofrontal cortex and subgenual cingulate, which strongly predicted its dissociative effects and increased activity in regions of midposterior cingulate, thalamus, and temporal cortex (Deakin et al. 2008).
Human and animal studies indicate that NMDA receptor hypofunction has been implicated in the pathophysiology of psychosis and schizophrenia and diminishes the inhibitory control of PFC output neurons. The findings of the present study suggest that both chronic ketamine users (Narendran et al. 2005) and schizophrenia patients (Laruelle et al. 2003) display the same endophenotypic trait – upregulated D1 receptor expression in the dorsolateral PFC, which supports the hypothesis that this alteration might be secondary to NMDA dysfunction in schizophrenia. For example, a 4-T 1H proton magnetic resonance spectroscopy (1H-MRS) study with ketamine administration in 10 healthy subjects found increased glutamatergic activity in the anterior cingulate, which may associate with acute hypofunctional NMDA receptor state (Rowland et al. 2005). Animal study also found its NMDA receptor hypofunction. Thus, reducing this effect may be critical for the treatment of psychosis and schizophrenia (Homayoun and Moghaddam 2007).
5 Conclusion
Brain imaging technology has had a tremendous impact on evaluating the neural mechanism associated with acute or chronic administration of ketamine. It provided a basic knowledge of ketamine’s medical use- or abuse-related brain circuits and the related cognitive and behavior outcomes. However, a major caveat remains in the uncertainty is its long-term safety, such as how to use ketamine for MDD treatment while limiting its psychotomimetic and dissociative side effects as well as addiction and abuse potential. Novel analytical approaches for brain imaging data should also be developed to facilitate the translation of findings from the research to the clinical setting, promoting the medical benefits of ketamine’s medical use while preventing from its addiction and abuse potential. In the future, the insights provided by neuroimaging studies of ketamine could contribute to biomarker development for its effective treatment of MDD and even other disorders, and to new approaches to discover better treatment strategies.
References
Aan Het Rot M, Zarate CA Jr, Charney DS, Mathew SJ (2012) Ketamine for depression: where do we go from here? Biol Psychiatry 72:537–547
Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY (2017) Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42:1210
Belliveau JW, Rosen BR, Kantor HL, Rzedzian RR, Kennedy DN, McKinstry RC, Vevea JM, Cohen MS, Pykett IL, Brady TJ (1990) Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med 14:538–546
Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541
Bonnet U (2015) Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs 47:276–285
Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J (1999) Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry 45:1542–1552
Canu E, Agosta F, Filippi M (2015) A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res 161:19–28
Cartwright P, Pingel S (1984) Midazolam and diazepam in ketamine anaesthesia. Anaesthesia 39:439–442
Casey BJ, Tottenham N, Liston C, Durston S (2005) Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 9:104–110
Chan KW, Lee TM, Siu AM, Wong DP, Kam C-M, Tsang SK, Chan CC (2013) Effects of chronic ketamine use on frontal and medial temporal cognition. Addict Behav 38:2128–2132
Clarke K, Hall L (1990) A survey of anaesthesia in small animal practice: AVA/BSAVA report. J Assoc Vet Anaesth Great Britain Ireland 17:4–10
Deakin JW, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco–magnetic resonance imaging study. Arch Gen Psychiatry 65:154–164
Elia N, Tramèr MR (2005) Ketamine and postoperative pain—a quantitative systematic review of randomised trials. Pain 113:61–70
Ezquerra-Romano II, Lawn W, Krupitsky E, Morgan C (2018) Ketamine for the treatment of addiction: evidence and potential mechanisms. Neuropharmacology 142:72–82
Fang YX, Wang YB, Shi J, Liu ZM, Lu L (2006) Recent trends in drug abuse in China. Acta Pharmacol Sin 27:140–144
Fedota JR, Stein EA (2015) Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci 1349:64
Fleisher AS, Sherzai A, Taylor C, Langbaum JB, Chen K, Buxton RB (2009) Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage 47:1678–1690
Fouche J-P, Du Plessis S, Hattingh C, Roos A, Lochner C, Soriano-Mas C, Sato JR, Nakamae T, Nishida S, Kwon JS (2017) Cortical thickness in obsessive–compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br J Psychiatry 210:67–74
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652
Green SM, Johnson NE (1990) Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med 19:1033–1046
Hibar D, Westlye LT, van Erp TG, Rasmussen J, Leonardo CD, Faskowitz J, Haukvik UK, Hartberg CB, Doan NT, Agartz I (2016) Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry 21:1710
Hirsch GV, Bauer CM, Merabet LB (2015) Using structural and functional brain imaging to uncover how the brain adapts to blindness. Ann Neurosci Psychol 2:5
Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB (1999) Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med 42:849–863
Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500
Honey G, Bullmore E (2004) Human pharmacological MRI. Trends Pharmacol Sci 25:366–374
Honey GD, Corlett PR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, McKenna PJ, Bullmore ET, Menon DK, Fletcher PC (2008) Individual differences in psychotic effects of ketamine are predicted by brain function measured under placebo. J Neurosci 28:6295–6303
Hong S-B, Zalesky A, Cocchi L, Fornito A, Choi E-J, Kim H-H, Suh J-E, Kim C-D, Kim J-W, Yi S-H (2013) Decreased functional brain connectivity in adolescents with internet addiction. PLoS One 8:e57831
Jenike MA, Breiter HC, Baer L, Kennedy DN, Savage CR, Olivares MJ, O’Sullivan RL, Shera DM, Rauch SL, Keuthen N (1996) Cerebral structural abnormalities in obsessive-compulsive disorder: a quantitative morphometric magnetic resonance imaging study. Arch Gen Psychiatry 53:625–632
Katalinic N, Lai R, Somogyi A, Mitchell PB, Glue P, Loo CK (2013) Ketamine as a new treatment for depression: a review of its efficacy and adverse effects. Aust N Z J Psychiatry 47:710–727
Kokkinou M, Ashok AH, Howes OD (2017) The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry 23:59
Krystal JH, Sanacora G, Duman RS (2013) Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73:1133–1141
Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R (1992) Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci 89:5675–5679
Laruelle M, Kegeles LS, Abi-Dargham A (2003) Glutamate, dopamine, and schizophrenia. Ann N Y Acad Sci 1003:138–158
Lauterbur PC (1973) Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature 242:190–191
Li C-SR, Zhang S, Hung C-C, Chen C-M, Duann J-R, Lin C-P, Lee TS-H (2017a) Depression in chronic ketamine users: sex differences and neural bases. Psychiatry Res Neuroimaging 269:1–8
Li Q, Shi L, Lu G, Yu H-L, Yeung F-K, Wong N-K, Sun L, Liu K, Yew D, Pan F (2017b) Chronic ketamine exposure causes white matter microstructural abnormalities in adolescent cynomolgus monkeys. Front Neurosci 11:285
Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, Liu T, Chen X, Fletcher PC, Hao W (2010) Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain 133:2115–2122
Liao Y, Tang J, Corlett PR, Wang X, Yang M, Chen H, Liu T, Chen X, Hao W, Fletcher PC (2011) Reduced dorsal prefrontal gray matter after chronic ketamine use. Biol Psychiatry 69:42–48
Liao Y, Tang J, Fornito A, Liu T, Chen X, Chen H, Xiang X, Wang X, Hao W (2012) Alterations in regional homogeneity of resting-state brain activity in ketamine addicts. Neurosci Lett 522:36–40
Liao Y, Qi C, Wu Q, Tang J (2016a) Psychiatric symptoms in individuals who use ketamine versus methamphetamine—implications for glutamatergic and dopaminergic model for schizophrenia: a cohort study. Lancet 388:S67
Liao Y, Tang J, Liu J, Xie A, Yang M, Johnson M, Wang X, Deng Q, Chen H, Xiang X (2016b) Decreased thalamocortical connectivity in chronic ketamine users. PLoS One 11:e0167381
Liao Y, Tang Y-l, Hao W (2017) Ketamine and international regulations. Am J Drug Alcohol Abuse 43:495–504
Liao Y, Johnson M, Qi C, Wu Q, Xie A, Liu J, Yang M, Huang M, Zhang Y, Liu T (2018) Cue-induced brain activation in chronic ketamine-dependent subjects, cigarette smokers, and healthy controls: a task functional magnetic resonance imaging study. Front Psych 9:88
Liu Y, Lin D, Wu B, Zhou W (2016) Ketamine abuse potential and use disorder. Brain Res Bull 126:68–73
Mansfield P, Maudsley AA (1977) Medical imaging by NMR. Br J Radiol 50:188–194
Michels II, Fang Y-x, Zhao D, Zhao L-y, Lu L (2007) Comparison of drug abuse in Germany and China. Acta Pharmacol Sin 28:1505
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Morgan CJ, Curran HV (2006) Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl) 188:408–424
Morgan CJ, Monaghan L, Curran HV (2004a) Beyond the K-hole: a 3-year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction 99:1450–1461
Morgan CJ, Riccelli M, Maitland CH, Curran HV (2004b) Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug Alcohol Depend 75:301–308
Morgan CJ, Curran HV, Drugs ISC o (2012) Ketamine use: a review. Addiction 107:27–38
Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M, Kegeles LS, Talbot PS, Huang Y, Hwang D-R (2005) Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry 162:2352–2359
Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 87:9868–9872
Parvaz MA, Alia-Klein N, Woicik PA, Volkow ND, Goldstein RZ (2011) Neuroimaging for drug addiction and related behaviors. Rev Neurosci 22:609–624
Roberts RE, Curran HV, Friston KJ, Morgan CJ (2014) Abnormalities in white matter microstructure associated with chronic ketamine use. Neuropsychopharmacology 39:329
Rosen BR, Belliveau JW, Vevea JM, Brady TJ (1990) Perfusion imaging with NMR contrast agents. Magn Reson Med 14:249–265
Rosenblat JD, Carvalho AF, Li M, Lee Y, Subramanieapillai M, McIntyre RS (2019) Oral ketamine for depression: a systematic review. J Clin Psychiatry 80:18r12475
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM (2005) Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162:394–396
Rowland LM, Beason-Held L, Tamminga CA, Holcomb HH (2010) The interactive effects of ketamine and nicotine on human cerebral blood flow. Psychopharmacology (Berl) 208:575–584
Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M (2016) A review of ketamine abuse and diversion. Depress Anxiety 33:718–727
Schmaal L, Hibar D, Sämann P, Hall G, Baune B, Jahanshad N, Cheung J, Van Erp T, Bos D, Ikram M (2017) Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol Psychiatry 22:900
Seidman LJ, Valera EM, Makris N (2005) Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1263–1272
Sparks B, Friedman S, Shaw D, Aylward EH, Echelard D, Artru A, Maravilla K, Giedd J, Munson J, Dawson G (2002) Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59:184–192
Stoeckel LE, Garrison KA, Ghosh SS, Wighton P, Hanlon CA, Gilman JM, Greer S, Turk-Browne NB, deBettencourt MT, Scheinost D (2014) Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage Clin 5:245–255
Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER (1999) Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 56:254–260
Sun L, Li Q, Li Q, Zhang Y, Liu D, Jiang H, Pan F, Yew DT (2014) Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys. Addict Biol 19:185–194
Sutherland MT, McHugh MJ, Pariyadath V, Stein EA (2012) Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage 62:2281–2295
Symms M, Jäger H, Schmierer K, Yousry T (2004) A review of structural magnetic resonance neuroimaging. J Neurol Neurosurg Psychiatry 75:1235–1244
Tang W, Liang H, Lau C, Tang A, Ungvari GS (2013) Relationship between cognitive impairment and depressive symptoms in current ketamine users. J Stud Alcohol Drugs 74:460–468
Tang J, Liao Y, He H, Deng Q, Zhang G, Qi C, Cui H, Jiao B, Yang M, Feng Z (2015a) Sleeping problems in Chinese illicit drug dependent subjects. BMC Psychiatry 15:28
Tang J, Morgan HL, Liao Y, Corlett PR, Wang D, Li H, Tang Y, Chen J, Liu T, Hao W (2015b) Chronic administration of ketamine mimics the perturbed sense of body ownership associated with schizophrenia. Psychopharmacology (Berl) 232:1515–1526
Tsai TH, Cha TL, Lin CM, Tsao CW, Tang SH, Chuang FP, Wu ST, Sun GH, Yu DS, Chang SY (2009) Ketamine-associated bladder dysfunction. Int J Urol 16:826–829
Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G (2011) The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS. Psychiatry Res Neuroimaging 191:122–127
Van Erp TG, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, Pearlson GD, Yao N, Fukunaga M, Hashimoto R (2018) Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatry 84:644–654
Volkow ND, Wang G-J, Fowler JS, Tomasi D, Baler R (2014) Neuroimaging of addiction. In: Imaging of the human brain in health and disease. Elsevier, Amsterdam, pp 1–26
Wang C, Zheng D, Xu J, Lam W, Yew D (2013) Brain damages in ketamine addicts as revealed by magnetic resonance imaging. Front Neuroanat 7:23
Wood R, Bassett K, Foerster T, Spry C, Tong L (2012) 1.5 tesla magnetic resonance imaging scanners compared with 3.0 tesla magnetic resonance imaging scanners: systematic review of clinical effectiveness. CADTH Technol Overviews 2:e2201
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H (2018) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554:317
Yeung L, Wai MS, Fan M, Mak Y, Lam W, Li Z, Lu G, Yew DT (2010) Hyperphosphorylated tau in the brains of mice and monkeys with long-term administration of ketamine. Toxicol Lett 193:189–193
Young LE, Bartram D, Diamond MJ, Gregg AS, Jones R (1993) Clinical evaluation of an infusion of xylazine, guaifenesin and ketamine for maintenance of anaesthesia in horses. Equine Vet J 25:115–119
Yu H, Li Q, Wang D, Shi L, Lu G, Sun L, Wang L, Zhu W, Mak YT, Wong N (2012) Mapping the central effects of chronic ketamine administration in an adolescent primate model by functional magnetic resonance imaging (fMRI). Neurotoxicology 33:70–77
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481
Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W (2009) Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci 108:149–158
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Liao, Y., Hao, W. (2020). Brain Imaging of Ketamine Abusers. In: Hashimoto, K., Ide, S., Ikeda, K. (eds) Ketamine. Springer, Singapore. https://doi.org/10.1007/978-981-15-2902-3_2
Download citation
DOI: https://doi.org/10.1007/978-981-15-2902-3_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2901-6
Online ISBN: 978-981-15-2902-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)