Abstract
Rationale
There is a significant interest in the NMDA-receptor antagonist ketamine due to its efficacy in treating depressive disorders and its induction of psychotic-like symptoms that make it a useful tool for modeling psychosis. Pharmacological MRI in awake nonhuman primates provides a highly translational model for studying the brain network dynamics involved in producing these drug effects.
Objective
The present study evaluated ketamine-induced changes in functional connectivity (FC) in awake rhesus monkeys. The effects of ketamine after pretreatment with the antipsychotic drug risperidone were also examined.
Methods
Functional MRI scans were conducted in four awake adult female rhesus monkeys during sub-anesthetic i.v. infusions of ketamine (0.345 mg/kg bolus followed by 0.256 mg kg−1 h−1 constant infusion) with and without risperidone pretreatment (0.06 mg/kg). A 10-min window of stable BOLD signal was used to compare FC between baseline and drug conditions. FC was assessed in specific regions of interest using seed-based cross-correlation analysis.
Results
Ketamine infusion induced extensive changes in FC. In particular, FC to the dorsolateral prefrontal cortex (dlPFC) was increased in several cortical and subcortical regions. Pretreatment with risperidone largely attenuated ketamine-induced changes in FC.
Conclusions
The results are highly consistent with similar human imaging studies showing ketamine-induced changes in FC, as well as a significant attenuation of these changes when ketamine infusion is preceded by pretreatment with risperidone. The extensive increases shown in FC to the dlPFC are consistent with the idea that disinhibition of the dlPFC may be a key driver of the antidepressant and psychotomimetic effects of ketamine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine has an extraordinary profile of pharmacological effects which make it a key research target for advancing the understanding and treatment of several psychiatric disorders, including schizophrenia and mood disorders. Sub-anesthetic infusions of ketamine have been shown to produce schizophrenia-like symptoms in healthy human volunteers (Frohlich and Van Horn 2014; Krystal et al. 1994) while also generating a rapid antidepressant response in patients with treatment-resistant depression (Berman et al. 2000; Krystal et al. 2013) at identical doses. Thus, ketamine challenge is currently being used experimentally both as a way to model schizophrenia and as a treatment for depression and other mood disorders. However, the mechanisms by which ketamine produces these effects remain incompletely understood. As a non-competitive N-methyl-d-aspartate glutamate receptor antagonist, ketamine produces widespread effects throughout the brain (De Simoni et al. 2013; Deakin et al. 2008). The global scope of the ketamine response poses a challenge to the identification of the specific brain circuitry responsible for producing the psychotomimetic and antidepressant effects of ketamine.
One way to probe specific brain circuitry underlying the psychotomimetic and antidepressant effects of ketamine is to employ pharmacological MRI (phMRI) to measure changes in brain functional connectivity (FC) networks induced by ketamine administration. While ketamine has been shown to increase FC globally (Driesen et al. 2013a; Joules et al. 2015), changes in FC between specific regions have been found to correlate with the subjective effects of ketamine (Dandash et al. 2015; Driesen et al. 2013a). Indeed, this regional specificity may be particularly important because both schizophrenia (Meyer-Lindenberg 2010; Woodward et al. 2011) and mood disorders (Anand et al. 2009; Greicius et al. 2007; Wessa et al. 2014) have been associated with alterations in resting-state FC networks albeit in distinct regional patterns (Chai et al. 2011; Goya-Maldonado et al. 2016). Thus, measuring changes to FC with phMRI provides an excellent tool for examining the effects of ketamine on specific brain circuitry and how it relates to psychiatric disorders. Recently, this technique has become even more powerful following the successful development of an apparatus and methodology (Murnane and Howell 2010) to conduct phMRI studies in conscious rhesus monkeys (Maltbie et al. 2016; Murnane et al. 2015; Murnane and Howell 2010).
Nonhuman primate (NHP) models offer distinct advantages for studying cognitive dysfunction and psychopathology (Phillips et al. 2014). Both schizophrenia (Lewis and Lieberman 2000) and mood disorders (Mayberg 2003) are characterized in part by altered processing in prefrontal cortex (PFC) and limbic circuits, and NHPs represent an excellent animal model because their behavioral repertoires are sophisticated and their PFC is closely aligned with humans (Preuss 1995). We recently reported that the blood oxygenation level-dependent (BOLD) activation to ketamine infusion produced a response pattern in conscious NHPs (Maltbie et al. 2016) that was highly concordant with effects observed in human studies (De Simoni et al. 2013; Deakin et al. 2008). Further, we also reported (Maltbie et al. 2016) that pretreatment with the antipsychotic drug risperidone attenuated the ketamine response to a similar magnitude and extent as observed in humans (Doyle et al. 2013). This indicates that NHPs may provide a highly translational animal model for studying the effects of ketamine as well as the pharmacological interaction between ketamine and other compounds.
The present study evaluated further the validity of the NHP model by investigating the effects of ketamine on resting-state FC in conscious rhesus monkeys. The effects of ketamine on FC in brain networks known to be affected by schizophrenia and mood disorders were examined and compared with findings from the literature in human subjects. The effect of pretreatment with risperidone on FC changes induced by ketamine was also examined. The findings present important precursors for future research investigating the mechanisms by which ketamine produces psychotomimetic and antidepressant effects as well as a potential model for evaluating novel antipsychotics.
Materials and methods
Animals and surgery
A complete description of the animals, surgery, and habituation protocol employed in this study has been described in detail in the companion paper (Maltbie et al. 2016). Briefly, four adult female rhesus monkeys (Macaca mulatta) were included in the study. They were all initially naïve to any experimental drugs underwent in the same experiments. Animal use procedures were in strict accordance with the National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of Emory University. In order to habituate the animals to the MRI environment, all subjects were extensively and gradually habituated to all procedures necessary for these experiments over a period of several months.
MRI data acquisition
A complete description of the MRI imaging methods employed is discussed in the companion paper (Maltbie et al. 2016). Briefly, the monkeys lay prone in a custom-built restraint cradle (Murnane and Howell 2010) attached to a NHP head coil. In each scanning session, BOLD MRI images were collected utilizing a whole-brain gradient echo single-shot echo planar imaging (EPI) sequence (TR/TE/FA = 3000 ms/32 ms/90°; 1.5 × 1.5 × 1.5 mm resolution; 1100 measurements). A low-resolution T1-weighted (T1w) anatomic scan was acquired with a 3D MPRAGE sequence (TR/TE/TI/FA = 2300 ms/2.7 ms/800 ms/8°; 1.5 × 1.5 × 1.5 mm resolution) to assist in spatial normalization. Further for each monkey, a set of seven high-resolution (0.5 × 0.5 × 0.5 mm) T1w 3D MPRAGE anatomic scans were acquired in a separate scanning session and averaged together to yield a final high-quality anatomic image for anatomic reference and spatial normalization.
Drug-infusion protocols
The drug-infusion protocol is described in detail in the companion paper (Maltbie et al. 2016). Briefly, each subject underwent two 55-min pharmacological MRI scans in separate scanning sessions. In both sessions (one with and one without pretreatment with risperidone (0.06 mg/kg, i.v.) administered 1 h prior to the MRI session), there was a 1-min baseline followed by a 1-min bolus i.v. infusion of 0.345 mg/kg of ketamine followed by 53 min continuous infusion of 0.256 mg kg−1 h−1 ketamine. This ketamine dosing regimen was chosen to target a plasma concentration of 100 ng/mL, which has been used in a previous FC study (Dandash et al. 2015). The literature (Muly et al. 2012) suggested that a dose of 0.1 mg/kg of risperidone would correspond closely to a clinical maintenance dose; however, this dose induced sleep in two of the animals. Administration of a dose one half-log unit lower (0.03 mg/kg) was found to be well tolerated in all four animals, as was an intermediate dose of 0.06 mg/kg. The intermediate dose of 0.06 mg/kg of risperidone produced the expected behavioral response, as detailed in Maltbie et al. (2016), and was selected for use in the imaging experiments.
The results from the phMRI study (Maltbie et al. 2016) showed that the ketamine-induced phMRI signal increase across the brain peaked around 4–5 min after the onset of drug infusion (t on) and remained relatively steady until around 18–20 min after the onset of drug infusion. Also, all the monkeys exhibited the least motion during this time window. Hence, a steady-state block of phMRI time-series from 7 to 16 s after t on was selected to assess functional connectivity networks in the brain during ketamine infusion with/without pretreatment with risperidone.
Baseline resting-state fMRI
Apart from the phMRI scans mentioned above, two 10-min resting-state fMRI (rsFMRI) scans were acquired in a separate session. The second rsFMRI scan was employed to assess FC during resting baseline for all the monkeys, to ensure that monkeys were relaxed and maximally acclimatized to the scanning environment.
fMRI data quality control
The fMRI image time-series data were examined for large motions defined as more than 0.5 mm frame-to-frame displacement. If a given monkey exhibited motion above this threshold in more than 10 % of the fMRI volumes within the 10 min of a drug treatment dataset employed to assess functional connectivity, the scan was repeated in another session, and the motion-corrupted dataset was discarded. None of the scans acquired for these experiments exceeded this threshold and thus no scanning sessions were discarded or repeated.
fMRI data analysis
Preprocessing and spatial normalization
MRI data analysis was conducted with AFNI (Cox 1996) and FSL (Smith et al. 2004) software packages as well as in-house Matlab™ (Natick, MA) scripts. The fMRI time-series images were first corrected for distortions introduced by magnetic field inhomogeneities, temporally shifted to account for differences in slice acquisition times and registered to a base volume to account for motion. Each subject’s averaged high-resolution high SNR T1w anatomic was registered to the INIA19 NHP template atlas (Rohlfing et al. 2012) using an affine image registration algorithm. For each subject, the motion-corrected EPI drug-infusion fMRI time-series was aligned to the low-resolution T1w anatomic (lores-anat) acquired in the same session with a rigid registration algorithm and then aligned to INIA19 template brain through the warp calculated in the alignment of the lores-anat to the high-resolution T1w anatomic in INIA19 co-ordinate space. The resultant EPI time-series were further denoised by replacing spikes in signal intensity resulting from motion and other spurious noise sources which exceeded 3.5 times median absolute deviation from time-series baseline with the 5 timepoint median of the EPI time-series centered at each spike. Finally, the denoised EPI time-series were spatially smoothed with a full-width at half-maximum (FWHM) = 3 mm isotropic Gaussian filter. As a quality control step, time-series volumes that exhibited more than 0.5 mm frame-to-frame displacement were censored from functional connectivity analysis.
Functional connectivity analysis
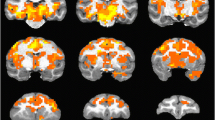
Seed based cross-correlation analysis (CCA) was employed to assess the strength of functional connectivity networks during each of the drug treatment conditions and baseline. A priori seed regions of interest (ROIs) of areas implicated in schizhophrenia (Frangou 2014; Meyer-Lindenberg 2010; Minzenberg et al. 2009) and mood disorders (Anand et al. 2009; Mayberg 2003; Phillips and Swartz 2014) were demarcated on the INIA19 NHP atlas based on associated NeuroMaps labels (Rohlfing et al. 2012). Left (as well as right) hemisphere ROIs of the entire dorsolateral prefrontal cortex (dlPFC), orbital frontal cortex (OFC), subgenual cingulate (SgC), nucleus accumbens (NAcc), and amygdala (Amyg) and posterior cingulate cortex (PCC) were demarcated by appropriately aggregating and segregating NeuroMaps-labeled areas pertaining to each of the ROIs (see Fig. 1). Since averaging all voxels within large ROIs can lead to enhanced sensitivity to motion artifacts (Power et al. 2012) and global signal (Saad et al. 2012), each ROI was subdivided into 3 × 3 × 3 mm non-overlapping sub-ROIs and all EPI voxel time-series within each sub-ROI were averaged to construct sub-ROI reference vectors. The z-transformed cross-correlation coefficient (CC) maps of all constituent sub-ROIs of a given ROI (e.g., dlPFC) were averaged to construct subject-level functional connectivity (FC) maps for that ROI. Due to the small sample size (N = 4) nonparametric statistical analysis was employed to assess brain FC. Group-level FC maps for each ROI, for each session were constructed with appropriate Wilcoxon one-sample signed rank test (nonparametric equivalent of one-sample t test) on the individual subject FC maps for the corresponding ROI. Group-level differences in FC between ketamine, baseline and ketamine after pretreatment with risperidone (RispKet) for each ROI were assessed with the Wilcoxon signed rank test (nonparametric equivalent of paired t test) on the individual subject FC maps for the corresponding ROI. The Wilcoxon signed rank one-sample and between condition z-maps were clustered, and the significance of activations accounting for multiple comparisons are derived by means of Monte Carlo simulation of the process of image generation, spatial correlation of voxels, intensity thresholding, masking and cluster identification (Forman et al. 1995) through the 3dClustSim program implemented in AFNI software. All the significant reported in the “Results” are corrected for multiple comparisons at p < 0.05 unless otherwise indicated.
A priori seed ROI masks drawn based on INIA19 NHP atlas: Red dorsolateral prefrontal cortex; violet orbitofrontal cortex; blue subgenual cingulate cortex; yellow nucleus accumbens; green posterior cingulate cortex. Slice locations indicate INIA19 NHP atlas coordinates. Only left hemishphere seed ROIs are highlighted for clarity
Results
Blood plasma drug levels
Blood samples taken immediately after scanning were analyzed for plasma levels of ketamine and risperidone. Blood plasma results have been published in the companion paper (Maltbie et al. 2016). Plasma ketamine ranged from 72 to 129 ng/mL in all scans and averaged 104 ng/mL in scans with ketamine alone and 96 ng/mL in scans where ketamine followed risperidone pretreatment. This ketamine dosage was sufficient to produce a significant drug effect in every scan.
Ketamine-induced changes in FC to dlPFC
Ketamine-induced (Supplementary Fig. 1) widespread changes in dlPFC FC with a number of different brain regions. When compared with the baseline session, ketamine induced (Fig. 2, Table 1) significantly (p < 0.05) increased dlPFC FC with areas involved in affective processing and mood regulation: e.g., ventral anterior cingulate (vACC), SgC, amygdala, NAcc, anterior insula, anterior superior temporal gyrus (STG), caudate, putamen, and dlPFC. In addition, dlPFC FC to sensorimotor and attention areas: e.g., premotor cortex (PMC), primary motor (M1) and somatosensory (S1) cortices, supplementary motor area (SMA), cingulate gyrus, and posterior STG also increased significantly from baseline during ketamine infusion.
Pretreatment with risperidone (0.06 mg/kg) an hour prior to infusion of ketamine (RispKet), significantly attenuated (Fig. 2; Table 1) the effects of ketamine. During the RispKet session, dlPFC FC was significantly decreased, when compared with ketamine, to limbic and affective processing areas: SgC, NAcc, caudate, putamen, amygdala, anterior insula, anterior STG, and vlPFC. At the same time, dlPFC FC to superior parietal lobule (SPL) was increased when compared with ketamine. However, RispKet still resulted in increased dlPFC FC compared with baseline (Table 2) with some of the same affective processing and sensorimotor areas as ketamine, albeit with reduced extent.
Ketamine-induced changes in FC to SgC
During ketamine infusion, the SgC exhibited (Supplementary Fig. 2) significant (p < 0.05) FC with limbic and affective processing areas, as well as dlPFC, SMA, premotor cortex, STG, and supramarginal gyrus (SMG). Ketamine induced higher SgC FC compared with baseline in a number of these areas; however, ketamine > baseline effects were significant (p < 0.05) (Table 1) only in dlPFC, and some sensorimotor areas: premotor cortex, STG, insula, and cerebellum.
The RispKet session exhibited (Fig. 3; Table 1) significantly decreased SgC FC when compared with ketamine to limbic and affective processing areas: vACC, OFC, SgC, amygdala, NAcc, caudate, putamen, and anterior insula, in addition to dlPFC and sensorimotor areas. Interestingly, the RispKet session exhibited (Fig. 3; Table 1) decreased SgC FC with limbic and affective processing areas also compared with baseline.
Ketamine-induced changes in FC to amygdala
During ketamine infusion, the amygdala exhibited (Supplementary Fig. 3) significant (p < 0.05) FC to limbic and affective processing areas. Ketamine induced significantly enhanced amygdala FC (Table 1) with SgC, ventral striatum, anterior ventral putamen, caudate, and contralateral amygdala when compared with baseline. Amygdala FC with posterior default mode network (DMN) areas (PCC and lateral parietal cortex) was attenuated compared with baseline during ketamine infusion (Supplementary Fig. 3), but the effect was not significant. During the RispKet session, the amygdala displayed (Fig. 4; Table 1) significantly decreased FC compared with ketamine to areas involved in limbic and affective processing: NAcc, caudate, putamen, thalamus, amygdala, anterior insula, and STG, apart from dlPFC and sensorimotor and associative/DMN areas: premotor cortex, insula, STG, secondary somatosensory cortex, SFC, parietal SMG, and SPL. Further, during the RispKet session, the amygdala showed (Fig. 4; Table 1) significantly reduced FC with these same regions when compared with baseline.
Ketamine-induced changes in FC to nucleus accumbens (ventral striatum)
During ketamine infusion, the NAcc exhibited (Supplementary Fig. 4) significant (p < 0.05) FC to striatal and temporal limbic and affective processing areas as well as dlPFC, attention, sensorimotor, and DMN areas. Ketamine-induced NAcc FC was (Fig. 5; Table 1) significantly increased compared with baseline to limbic and affective processing areas: caudate, putamen, amygdala, anterior insula, and anterior STG, in addition to PCC, cingulate, and SFC. During the RispKet session, the NAcc displayed significantly decreased (Fig. 4; Table 1) FC compared with ketamine with all of these above areas and as well as dlPFC, SMA, and ACC.
Ketamine-induced changes in FC to other ROIs
Table 1 lists the between-session differences in FC to the two other ROIs described in the methods. RispKet session FC of OFC (like those of dlPFC, NAcc, SgC, amygdala) was significantly attenuated compared with ketamine in limbic and affective processing areas. Also, RispKet session FC of OFC (like the FCs of SgC and amygdala) with limbic and affective processing areas was significantly reduced compared with baseline.
Finally, during ketamine infusion, the PCC (which is a hub of the DMN) exhibited increased FC with striatal limbic and affective processing areas: NAcc, anterior caudate and putamen; as well as with SMA and cingulate compared with baseline. Pretreatment with risperidone decreased ketamine-induced PCC FC to limbic areas. Further, the RispKet session showed significantly higher PCC FC with anterior DMN regions and retrosplenial cortex compared with baseline while inducing decreased FC to affective processing regions.
Ketamine-induced interhemispheric asymmetry in FC networks
Table 2 lists the regions in the brain, for each seed ROI, which exhibited significant differences in FC between the seed’s left and right hemisphere homologs, under each scan condition. At baseline, very few regions exhibited preferential FC to any given hemisphere. Ketamine induced a breakdown in hemispheric symmetry in FC in all the seed ROIs examined except NAcc (Table 2; Fig. 6). Ketamine induced increased FC of left dlPFC (compared with its right hemisphere homolog) with areas in sensorimotor, salience, and limbic networks. RispKet increased left dlPFC FC with bilateral nucleus reuniens and increased right dlPFC FC to right amygdala, compared with their contralateral homologs.
Group Wilcoxon signed rank test results showing regions with significant (cluster-level p < 0.05) differences between FC to left and right hemisphere dlPFC (left) and amygdala (right) seed ROIs during ketamine and RispKet session. Left dlPFC/amygdala FC > right dlPFC/amygdala FC (red); right dlPFC/amygdala FC > Left dlPFC/amygdala FC (blue). Slice locations indicate INIA19 NHP atlas coordinates
Ketamine induced increased FC of right amygdala (compared with left amygdala) to limbic and salience monitoring network regions (Table 2; Fig. 6). RispKet exhibited increased right amygdala FC with posterior default mode network as well as cingulate and hippocampus. Both ketamine and RispKet sessions exhibited increased left OFC FC, left PCC FC, and right SgC FC with a number of brain areas compared with the respective seeds’ contralateral homologs.
Discussion
The results described above reveal a number of interesting changes in brain FC network characteristics induced by ketamine, and the modulation of ketamine-induced changes in FC network by pretreatment with risperidone. Sub-anesthetic ketamine infusion at similar doses is employed in human subjects both as a model for schizophrenia (Frohlich and Van Horn 2014; Krystal et al. 1994) and as a treatment for depression (Berman et al. 2000; Krystal et al. 2013). While previous studies have shown ketamine-induced changes to FC, each has employed either a global analysis (Anticevic et al. 2015; Driesen et al. 2013a; Joules et al. 2015) or examined FC changes to seeds in a single region (Dandash et al. 2015; Driesen et al. 2013b; Grimm et al. 2015). The present study provides a more extensive analysis of the effects of ketamine on cortico-limbic connectivity than has been previously published.
The strongest brain FC network changes induced by ketamine were in connection with the dlPFC, a region strongly implicated in schizophrenia (Arnsten et al. 2012; Meyer-Lindenberg 2010; Meyer-Lindenberg et al. 2005; Minzenberg et al. 2009), bipolar disorder (Anticevic et al. 2013; Frangou et al. 2008), and major depression (Concerto et al. 2015; Dutta et al. 2014). Ketamine induced significant increases in dlPFC FC with frontal, striatal, and temporal areas of limbic and affective processing networks in addition to sensorimotor and attention networks. Increases in FC between dlPFC and hippocampus have been reported in both humans and rats (Grimm et al. 2015), and the widespread increase in FC from baseline observed in this study could be related to the disinhibition of dlPFC after ketamine administration that has been reported by many systems neuroscience studies (Arnsten et al. 2012; Wang and Arnsten 2015). Pretreatments with the antipsychotic drug risperidone attenuated ketamine-induced increases in FC to dlPFC from a number of regions, especially limbic and affective processing areas. This indicates that one mechanism of risperidone action is to counteract the effects of dlPFC disinhibition.
Ketamine infusion also increased the FC of SgC, amygdala, and OFC to limbic and affective processing networks. Disruptions in FC within these cortico-limbic networks has been shown repeatedly in mood disorders (Anand et al. 2009; Mayberg 2003; Phillips and Swartz 2014) and the ketamine-induced increases in connectivity in these networks may be related to its efficacy as an antidepressant. Risperidone pretreatment substantially attenuated ketamine-induced FC within affective processing network. In fact, ketamine-RispKet effects of FC in limbic and affective processing networks were much stronger in extent than ketamine-baseline effects because the RispKet session exhibited reduced FC within affective processing networks compared with baseline. This indicates that risperidone is acting to decrease the functional connections in limbic networks, which may be related to some of the side effects associated with risperidone treatment (Miyamoto et al. 2005).
Ventral striatum (NAcc) plays an important role in motivation and mood regulation. The results of this study showed that ketamine induced increased FC of ventral striatum with amygdala and temporal affective processing regions as well as the default mode network. These effects were counteracted by pretreatment with risperidone, and in fact, reductions in FC between striatum and parietal regions of the DMN have been associated with alleviation in symptoms following antipsychotic treatment (Sarpal et al. 2015).
Finally, PCC (which is a major hub in the DMN) exhibited increased FC with NAcc and striatal affective processing regions after ketamine infusion. The DMN shows abnormal FC in both mood disorders (Greicius et al. 2007; Ongur et al. 2010) and schizophrenia (Rotarska-Jagiela et al. 2010; Woodward et al. 2011). Pretreatment with risperidone attenuated these effects while increasing PCC FC to anterior DMN regions. This result was reinforced by the RispKet-baseline comparison which revealed increased PCC FC to DMN regions and reduced PCC FC with affective processing regions during RispKet.
Rhesus macaque resting-state FC networks are known to show little difference between contralateral and ipsilateral FC (Adachi et al. 2012; Hutchison et al. 2012). The FC networks examined in the baseline session in this study were consistent with prior studies exhibiting little hemispheric asymmetry. However, ketamine infusion resulted in significant departures from hemispheric symmetry in most of the FC networks examined in this study. These results are consistent with breakdown in interhemispheric symmetry of FC networks in human models of schizophrenia and other mood disorders (Guo et al. 2013; Zhang et al. 2015). RispKet reduced the hemispheric asymmetry in FC networks induced by ketamine for the dlPFC and amygdala seeds.
Overall, these results indicate that ketamine induces hyperconnectivity in NHP functional brain networks associated with emotional regulation, cognitive control, and motivation. These findings are highly consistent with similar studies of the effects of ketamine on FC in concordant human brain networks (Anticevic et al. 2015; Dandash et al. 2015; Driesen et al. 2013a). Further, pretreatment with risperidone significantly attenuated the effects of ketamine, which is also consistent with findings in human subjects (Joules et al. 2015).
Insights into the results of the ketamine drug-infusion phMRI study
In the recently published companion paper (Maltbie et al. 2016), we reported sustained robust BOLD phMRI response to ketamine infusion in a number of cortical and subcortical regions including anterior and mid-cingulate cortex, anterior STG, SMA, and thalamus, which were consistent with results of human studies on BOLD response to ketamine infusion induced (De Simoni et al. 2013; Doyle et al. 2013). Ketamine-induced BOLD phMRI response to dlPFC was significant but not as strong as the above-mentioned areas. Thus, the fact that the strongest changes in FC with respect to baseline during sustained ketamine infusion occurs in brain networks connected to dlPFC indicates that FC is sensitive to brain mechanisms distinct from mere blood flow response to ketamine infusion. This result is consistent with studies in human subjects (Khalili-Mahani et al. 2015) which report spatial heterogeneity between CBF and FC network changes after ketamine infusion. Indeed, since FC network alterations induced by ketamine administration are consistent with those caused by schizophrenia (Anticevic et al. 2015; Driesen et al. 2013a) measures of functional connectivity in brain networks may in fact elucidate more pertinent information regarding disease mechanisms than pure phMRI BOLD activation maps regarding the effects of ketamine on brain function.
Limitations
Ketamine challenge is not an exact model of schizophrenia (Cohen et al. 2015). One recent paper suggests that this may be due to changes over the time course of disease pathology, and the ketamine model may be consistent with early-but-not-chronic-schizophrenia (Anticevic et al. 2015). No experiments were done to test the effects of risperidone alone on FC of brain networks. While this may be an interesting future direction, to our knowledge, no studies have examined the effects of risperidone challenge (or other acute second generation antipsychotic) alone on FC, though several studies have investigated the effects of chronic antipsychotic treatment (Hadley et al. 2014; Kraguljac et al. 2016). Finally, the small sample size (N = 4) is another limitation of this study, rendering it not amenable to more sophisticated statistical analyses which may lend more accurate and quantitative estimates of the effects of ketamine on brain function networks.
While antipsychotics, such as risperidone, are administered chronically in the treatment of schizophrenia, only acute administration was considered in this study. The therapeutic response occurs gradually, with symptoms decreasing over a period of days and weeks (Agid et al. 2003). However, the effects of risperidone are mediated primarily via D2 and 5-HT2 receptor antagonism with high occupancy levels being achieved within hours of acute administration (Nyberg et al. 1993) and there is evidence suggesting that D2 receptor occupancy on the second day of treatment is predictive of the eventual clinical response (Catafau et al. 2006). Thus, the acute effects of risperidone should provide important insights into the validity of our translational model.
The endocrine status of the female subjects featured in this study was not monitored. To the knowledge of the authors, no sex differences have been previously reported for the psychotomimetic effects of ketamine in humans. While it is possible that the endocrine status of the subjects affected the results, the evidence in the literature suggests that any effects should be minimal. A review (Cyr et al. 2001) found no effects of ovariectomy on NMDARs in brain regions other than the hippocampus. Further, a recent study in rats showed no effect of ovariectomy on the disruption of prepulse inhibition by ketamine (van den Buuse et al. 2015).
Conclusion
In conclusion, this study adds to previous evidence that phMRI in NHPs can provide a highly translational model for studying the effects of ketamine, which may provide new insights into multiple brain disorders and could also be used for evaluating novel antipsychotics. Indeed, measures of FC may provide more information regarding the effects of ketamine on brain circuitry than general ketamine-induced BOLD response changes. The finding that the dlPFC exhibits such broad and robust increases in functional connectivity implies that altered processing in this region may be a critical driver of the behavioral effects of ketamine.
References
Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, Miyamoto K, Miyashita Y (2012) Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex 22:1586–1592
Agid O, Kapur S, Arenovich T, Zipursky RB (2003) Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 60:1228–1235
Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M (2009) Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res 171:189–198
Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, Glahn DC (2013) Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 73:565–573
Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, Repovs G, Murray JD, Driesen NR, Morgan PT, Xu K, Wang F, Krystal JH (2015) N-methyl-d-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 77:569–580
Arnsten AF, Wang MJ, Paspalas CD (2012) Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76:223–239
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Catafau AM, Corripio I, Perez V, Martin JC, Schotte A, Carrio I, Alvarez E (2006) Dopamine D2 receptor occupancy by risperidone: implications for the timing and magnitude of clinical response. Psychiatry Res 148:175–183
Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, Cohen BM, Ongur D (2011) Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 36:2009–2017
Cohen SM, Tsien RW, Goff DC, Halassa MM (2015) The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res 167:98–107
Concerto C, Lanza G, Cantone M, Ferri R, Pennisi G, Bella R, Aguglia E (2015) Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: a six-month clinical follow-up study. Int J Psychiatry Clin Pract 19:252–258
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T (2001) Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Brain Res Rev 37:153–161
Dandash O, Harrison BJ, Adapa R, Gaillard R, Giorlando F, Wood SJ, Fletcher PC, Fornito A (2015) Selective augmentation of striatal functional connectivity following NMDA receptor antagonism: implications for psychosis. Neuropsychopharmacology 40:622–631
De Simoni S, Schwarz AJ, O’Daly OG, Marquand AF, Brittain C, Gonzales C, Stephenson S, Williams SC, Mehta MA (2013) Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. NeuroImage 64:75–90
Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 65:154–164
Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O’Daly OG, Williams SC, Mehta MA (2013) Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. J Pharmacol Exp Ther 345:151–160
Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH (2013b) Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry 18:1199–1204
Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D’Souza DC, Gueorguieva R, He G, Leung HC, Ramani R, Anticevic A, Suckow RF, Morgan PT, Krystal JH (2013a) The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology 38:2613–2622
Dutta A, McKie S, Deakin JF (2014) Resting state networks in major depressive disorder. Psychiatry Res 224:139–151
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–647
Frangou S (2014) A systems neuroscience perspective of schizophrenia and bipolar disorder. Schizophr Bull 40:523–531
Frangou S, Kington J, Raymont V, Shergill SS (2008) Examining ventral and dorsal prefrontal function in bipolar disorder: a functional magnetic resonance imaging study. Eur Psychiatry 23:300–308
Frohlich J, Van Horn JD (2014) Reviewing the ketamine model for schizophrenia. J Psychopharmacol 28:287–302
Goya-Maldonado R, Brodmann K, Keil M, Trost S, Dechent P, Gruber O (2016) Differentiating unipolar and bipolar depression by alterations in large-scale brain networks. Hum Brain Mapp 37:808–818
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007) Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437
Grimm O, Gass N, Weber-Fahr W, Sartorius A, Schenker E, Spedding M, Risterucci C, Schweiger JI, Bohringer A, Zang Z, Tost H, Schwarz AJ, Meyer-Lindenberg A (2015) Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology 232:4231–4241
Guo S, Kendrick KM, Zhang J, Broome M, Yu R, Liu Z, Feng J (2013) Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression. NeuroImage Clin 2:818–826
Hadley JA, Nenert R, Kraguljac NV, Bolding MS, White DM, Skidmore FM, Visscher KM, Lahti AC (2014) Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 39:1020–1030
Hutchison RM, Gallivan JP, Culham JC, Gati JS, Menon RS, Everling S (2012) Functional connectivity of the frontal eye fields in humans and macaque monkeys investigated with resting-state fMRI. J Neurophysiol 107:2463–2474
Joules R, Doyle OM, Schwarz AJ, O’Daly OG, Brammer M, Williams SC, Mehta, MA (2015) Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacol (Berl)
Khalili-Mahani N, Niesters M, van Osch MJ, Oitzl M, Veer I, de Rooij M, van Gerven J, van Buchem MA, Beckmann CF, Rombouts SA, Dahan A (2015) Ketamine interactions with biomarkers of stress: a randomized placebo-controlled repeated measures resting-state fMRI and PCASL pilot study in healthy men. NeuroImage 108:396–409
Kraguljac NV, White DM, Hadley JA, Visscher K, Knight D, Ver Hoef L, Falola B, Lahti AC (2016) Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. NeuroImage Clin 10:146–158
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Krystal JH, Sanacora G, Duman RS (2013) Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73:1133–1141
Lewis DA, Lieberman JA (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28:325–334
Maltbie E, Gopinath K, Urushino N, Kempf D, Howell L (2016) Ketamine-induced brain activation in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233:961–972
Mayberg HS (2003) Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207
Meyer-Lindenberg A (2010) From maps to mechanisms through neuroimaging of schizophrenia. Nature 468:194–202
Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF (2005) Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62:379–386
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009) Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66:811–822
Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10:79–104
Muly EC, Votaw JR, Ritchie J, Howell LL (2012) Relationship between dose, drug levels, and D2 receptor occupancy for the atypical antipsychotics risperidone and paliperidone. J Pharmacol Exp Ther 341:81–89
Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL (2015) Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology 232:745–754
Murnane KS, Howell LL (2010) Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods 191:11–20
Nyberg S, Farde L, Eriksson L, Halldin C, Eriksson B (1993) 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology 110:265–272
Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF (2010) Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res 183:59–68
Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML (2014) Why primate models matter. Am J Primatol 76:801–827
Phillips ML, Swartz HA (2014) A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 171:829–843
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154
Preuss TM (1995) Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci 7:1–24
Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, Pfefferbaum A (2012) The INIA19 template and NeuroMaps Atlas for primate brain image parcellation and spatial normalization. Front Neuroinflammation 6:27
Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE (2010) Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res 117:21–30
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32
Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, Gallego JA, Kane JM, Szeszko PR, Malhotra AK (2015) Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 72:5–13
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219
van den Buuse M, Mingon RL, Gogos A (2015) Chronic estrogen and progesterone treatment inhibits ketamine-induced disruption of prepulse inhibition in rats. Neurosci Lett 607: 72–6.
Wang M, Arnsten AF (2015) Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neurosci Bull 31:191–197
Wessa M, Kanske P, Linke J (2014) Bipolar disorder: a neural network perspective on a disorder of emotion and motivation. Restor Neurol Neurosci 32:51–62
Woodward ND, Rogers B, Heckers S (2011) Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res 130:86–93
Zhang J, Kendrick KM, Lu G, Feng J (2015) The fault lies on the other side: altered brain functional connectivity in psychiatric disorders is mainly caused by counterpart regions in the opposite hemisphere. Cereb Cortex 25:3475–3486
Acknowledgments
The authors declare no competing financial interests. This research was supported by P51OD11132 (Yerkes National Primate Research Center), Sunovion Pharmaceutical, Ltd. (LLH) and DA031246 (LLH). Special thanks to the Yerkes Imaging Center, technicians Marisa Olsen and Juliet Brown, and Christopher Muly, MD, PhD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kaundinya Gopinath and Eric Maltbie contributed equally to this work.
Electronic supplementary material
ESM 1
(DOCX 6898 kb)
Rights and permissions
About this article
Cite this article
Gopinath, K., Maltbie, E., Urushino, N. et al. Ketamine-induced changes in connectivity of functional brain networks in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233, 3673–3684 (2016). https://doi.org/10.1007/s00213-016-4401-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4401-z