Abstract
Rationale
Subanaesthetic ketamine infusion in healthy volunteers induces experiences redolent of early psychosis, including changes in the experience of one’s own body. It is not clear, however, whether repeated self-administration of ketamine has a sustained effect on body representation that is comparable to that found during acute administration.
Objectives
We sought to establish whether chronic ketamine use resulted in disturbances to sense of body ownership.
Methods
Following on from our work on the effects of acute ketamine infusion, we used the rubber hand illusion (RHI) to experimentally manipulate the sense of body ownership in chronic ketamine users, compared to healthy controls.
Results
Chronic ketamine users experienced the RHI more strongly and reported more body-image aberrations, even though they had not recently taken the drug.
Conclusions
These findings suggest that the chronic ketamine model for psychosis models more long-lasting changes in sense of ownership, perhaps more akin to schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The N-methyl-d-aspartate (NMDA) receptor antagonist ketamine has, in recent years, gained credence as a useful for model for psychosis (Corlett et al. 2007, 2011; Krystal et al. 1994). Controlled subanaesthetic infusion of the drug in healthy, drug-naïve, volunteers results in a transient psychopathology reminiscent of both the positive and negative symptoms of schizophrenia (Javitt and Zukin 1991). In particular, ketamine infusion has a profound impact on cognitive processing, causing elements of thought disorder and disorganisation (Adler et al. 1998; Krystal et al. 1994; Malhotra et al. 1996) and aberrant executive functioning (Krystal et al. 2000). Furthermore, acute ketamine impacts upon memory processing (see Newcomer and Krystal 2001 for a thorough review), specifically, verbal working memory (Adler et al. 1998; Honey et al. 2003, 2004), spatial working memory (Rowland et al. 2005) and the encoding of source memory (Honey et al. 2005, 2006).
Acute ketamine also induces profound disturbances in perceptual experience (Duncan et al. 2001; Oye et al. 1992; Stone et al. 2006) having an impact, for example, on the experience of visual illusions (Stone and Pilowsky 2006), the experience of objects (for example, sharpness and definition) and colours, such as increased brightness or blurring (Pomarol-Clotet et al. 2006) and the perceptual salience of objects in the environment (Krystal et al. 1994; Oye et al. 1992; Vollenweider et al. 1997a, b). It affects, moreover, the perception of time (Coull et al. 2011; Pomarol-Clotet et al. 2006; Stone and Pilowsky 2006) and of one’s body and its movement (Pomarol-Clotet et al. 2006) with a corresponding disturbance in the sense of agency over one’s actions (Moore et al. 2011, 2013) and ownership (Morgan et al. 2011). Moreover, ketamine elicits feelings of depersonalisation and unreality (Duncan et al. 2001), general dissociation (Stone and Pilowsky 2006) from one’s body (Oye et al. 1992) and a breakdown in the sense of self (Vollenweider et al. 1997b).
While these observations were made in controlled, clinical, environments, they are consistent with reports of dissociative feelings during recreational use. At their most profound condition, these entail feelings of detachment from reality (the ‘k-hole’) and hallucinations. It is harder to establish the impact of repeated ketamine use on cognitive processing and bodily experience with experimenter-administered ketamine in healthy volunteers, given ethical and safety concerns. The research that does exist was completed outside of a controlled clinical setting and examines chronic (frequent users over a sustained period of time) and recreational (infrequent users, usually in a social context) ketamine users (Morgan and Curran 2012).
Repeated ketamine abuse appears to have long-lasting cognitive effects, for example, on episodic memory, even with cessation or reduction of drug use (Curran and Morgan 2000; Morgan et al. 2004a). Similarly, schizophrenia-like symptoms are present even without recent administration of the drug, although effects may vary (Uhlhaas et al. 2007) that showed that delusional ideation was higher in ketamine users compared to controls at a 3-day follow-up, but dissociative experiences were not. Furthermore, both schizophrenia-like and dissociative symptoms were elevated in ketamine users 3 days after taking the drug, in comparison to polydrug users (Curran and Morgan 2000). Three years later, these participants had reduced their ketamine use, and their symptoms had overall reduced, yet they still scored higher than polydrug controls on measures of psychosis-like experiences, perceptual alterations and bodily symptoms but not on a dissociative experiences scale (Morgan et al. 2004a). In contrast, another study by Morgan and colleagues reported no differences between ketamine users and polydrug controls for either schizophrenia-like or dissociative experiences, at a 3-day follow-up, despite an initial difference (Morgan et al. 2004b). Thus, the existing literature does not provide an obvious or precise picture of the long-term impact of ketamine on the presence of symptoms and behaviour. Rather, it appears that ketamine has a differential impact upon dissociative symptoms and delusional ideation, both immediately after taking the drug and at follow-up. Moreover, this impact could vary dependent upon the frequency of ketamine use and the specific symptom that is assessed.
Considering the data reviewed above, it could be inferred that the delusional ideation associated with ketamine use is more persistent than the dissociative experiences, which are more transitory. However, one study reported above focuses specifically on subjective and bodily experience (measured using the Subjective Effects Scale) as separate to general dissociative experiences scores (assessed by the Clinician-Administered Dissociative States Scale) and reports that body symptoms persist but dissociative experiences scores do not (Morgan et al. 2004a). This is an important distinction to make particularly in light of more recent findings that the frequency of lifetime ketamine use correlates with changes to bodily experience and, in particular, the sensation that one feels disconnected from one’s body (Wilkins et al. 2011, 2012). It is likely then that persistent bodily alterations linked to ketamine, distinct from general symptoms of dissociation, arise following a long period of (frequent) drug abuse perhaps more akin to chronic schizophrenia than the prodromal period that acute ketamine is thought to model. By this explanation, dissociative experiences would not persist in recreational or infrequent users when not on the drug, reflected in the research findings summarised above. Moreover, this differential impact of acute ketamine from chronic use is consistent with the argument that acute ketamine impacts upon inferential processing (i.e. transient dissociative effects), whereas chronic ketamine use results in disrupted learning mechanisms (longer term disturbances to one’s behavioural model about their body) similar to acute episodes of schizophrenia and chronic schizophrenia, respectively.
The exact nature of the relationship between ketamine use and its psychotomimetic symptoms, as reported to date, thus remains unclear. It is possible that the psychotomimetic properties of ketamine when taken recreationally are not as transient as those observed in a clinical setting, but the dissociative effects are, and this is modulated by the frequency of drug use. Frequent users are also largely unconcerned by the potential implications of ketamine for schizotypal behaviours and thereby could be unaware of such behaviours. This reveals an inherent problem to the existing data, namely, it is largely subjective. In this study, therefore, we aim to address this issue by making use of a paradigm that investigates bodily experience both objectively and subjectively (the rubber hand illusion) as well as self-report measures of symptoms.
The rubber hand illusion (RHI) is a simple paradigm in which a fake rubber hand is felt to be one’s own hand when the real hand is hidden from view. The illusion is induced using synchronous visual and tactile stimulation to the index finger of both the rubber hand and the real hand, applied using brushstrokes. Hence, what the participant sees matches what they feel, and they come to report that the fake hand could be their own. Consequently, temporal asynchrony between the two percepts attenuates the illusion (Tsakiris and Haggard 2005) indicating that sense of ownership (the knowledge that my body is my own), captured by the RHI, is reliant upon coherence between multisensory inputs and consistency with existing body representations: Using a wooden block, for example, abolishes the illusion (Costantini and Haggard 2007; Tsakiris 2010). The illusion is commonly measured using self-report measures of subjective experiences as well as a more objective measure of accuracy in hand localisation.
Of particular importance is that the RHI is elicited more strongly and quickly in patients with schizophrenia (Peled et al. 2000, 2003; Thakkar et al. 2011) and, more recently, in healthy participants with schizotypal personality traits (Germine et al. 2013). In the case of Thakkar et al. (2011), one patient even reported an out-of-body experience during the RHI session. Furthermore, following subanaesthetic infusion of ketamine in healthy participants, the RHI was felt more strongly than placebo infusion (Morgan et al. 2011). These findings give us strong justification for using the RHI as a tool to investigate bodily disturbances in psychosis. An initial question is whether chronic ketamine users also experience the RHI more strongly, even when they are not under the acute influence of the drug. We therefore explored this question in the current study using a case-control design.
Methods and materials
Participants
Ketamine group
26 participants (two female) with a mean age of 25.1 years (SD = 2.2) were recruited from three drug rehabilitation centres: the Kangda Voluntary Drug Rehabilitation Centre (Hunan Province), the Department of Addiction Medicine (Hunan Brain Hospital) and the Guangzhou Baiyun Psychological Hospital. All participants met criteria for lifetime ketamine dependence as determined through the Structured Clinical Interview and were diagnosed using the Diagnostic and Statistical Manual of Mental Disorders—IV—TR (DSM-IV-TR) (American Psychiatric Association 2000). Exclusion criteria were comorbid substance dependence (any polydrug use approximated fewer than 10 occasions and thus did not meet criteria for dependence), including solvent abuse or intravenous drug infusion for medical purposes, but excluding nicotine. In total, 88.5 % of the ketamine group reported smoking an average of 15.6 cigarettes per day for an average of 9 years (SD 5.05). It has been shown that nicotine does not impact upon the effect of ketamine in humans (D’Souza et al. 2012; Mathalon et al. 2014; Knott et al. 2012; Deroza et al. 2012); hence, this factor is not entered into analysis. Prior to the testing session, participants were required to abstain from ketamine use for 24 h although this was not objectively verified. Self-reported ketamine use, for all participants, ranged from four times per week up to 8 times per day for the past month. Those 21 participants who reported using ketamine on a daily basis had a mean craving level of 5.03 (using a scale of 0–10 and with a range of 0–10 reported). The experimenter and participant also made an estimate in grams of the total ketamine use over the participant’s lifetime (mean 4,688.38 g, SD 4,542.08).
Healthy controls
20 participants (two female) with a mean age of 24.4 years (SD = 2.9) were recruited using targeted site sampling, poster advertisement and snowball sampling referrals. Exclusion criteria included as follows: being of a minority ethnic group (rather than Han Chinese), diagnosis of a learning disability, current or previous diagnosis of disorder of the central nervous system, any medical condition or disease which might have impacted upon the central nervous system, previous head injury with skull fracture or loss of consciousness for longer than 10 min, any physical disorder that might impact upon the study, family history of psychiatric disorder and current or previous recreational use of psychoactive drugs excluding alcohol (and prescription drugs but including substance abuse or dependence). Healthy controls (45 %) reported smoking an average of 10 cigarettes per day, for an average of 6.4 years (SD 2.55).
Personality traits
All participants completed the Chapman scales of Magical Ideation (Eckblad and Chapman 1983) and Perceptual Aberration (Chapman et al. 1978) and the Peters Delusions Inventory—21 items (Peters et al. 2004). These are typically used to measure the presence of schizotypal thoughts or behaviours in healthy people.
This study was approved and conducted in accordance with guidelines issued by the Second Xiangya Hospital of the Central South University Review Board.
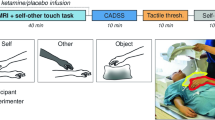
The rubber hand illusion set-up
The participant sat at a table with their right hand hidden from view in an open-side box that was placed on the table, with the life-sized rubber hand placed 15 cm to the left side of the participant’s real hand. Participants wore a blue latex glove on their right hand, to match the visual appearance of the rubber hand, which also wore a blue latex glove. A black cloak was draped over their shoulder, occluding their right arm from view thereby preventing the participant from gaining any information as to the position of their real hand. An electric motor was used to power two revolving paintbrushes, positioned to apply brushstrokes to the right index fingers of both hands at a consistent rhythm of approximately 1 Hz. These two brushes moved either synchronously in time, i.e. the observed touch on the rubber hand matched the felt touch on the real hand (synchronous condition), or asynchronously, such that the participant observed touch every other second to feeling touch thereby creating a notable temporal delay. The rubber hand was hidden until the experimental condition was started.
Experimental design
All participants experienced one block of synchronous, and one of asynchronous, visuotactile stimulation presented in counterbalanced order. Each block of stimulation comprised a 2-min induction period followed by 3 min in which participants made judgments about hand location. Two measures of the illusion were used: proprioceptive drift and subjective experience.
Proprioceptive drift
Before seeing the rubber hand or receiving stimulation, participants made a baseline judgment about the location of their hand, followed by four further judgments at 1-min intervals. Judgments of location were verbal reports made in relation to a ruler, placed on top of the box. Drift was calculated as the change in perceived location from the baseline measurement.
Subjective experience
Following each condition, participants responded to nine statements that describe perceptual experiences (including ‘I felt as if the rubber hand were my hand’, ‘It seemed as if I were feeling the touch of the paintbrush in the location where I saw the rubber hand touched’ and ‘It seemed as though the touch I felt was caused by the paintbrush touching the rubber hand’) thought to capture the rubber hand illusion (Botvinick and Cohen 1998). Responses were made using a scale of 1 (disagree completely) to 5 (agree completely).
Measures of symptoms
All participants completed a number of questionnaires that assessed their current symptoms, in particular, dissociative experiences. In keeping with Morgan et al. (2011), we used the Clinician-Administered Dissociative States Scale (CADSS) (Bremner et al. 1998) and Brief Psychiatric Rating Scale (BPRS) (Overall and Klett 1972). For the CADSS scale, the ketamine group was asked to make two responses, first, based on their current state (‘current’) and, second, based upon retrospective evaluation of their ketamine-induced state (‘ketamine’).
Results
All statistical tests were two tailed unless accompanied by a strong a priori hypothesis (where one-tailed tests were used and this is indicated accordingly). Bonferroni corrections were not used as it was felt that the interdependence of the measures used would have made this an unnecessarily stringent correction; therefore, an alpha level of p < .001 is applied.
Participants
Although global cognition was not independently assessed, the years of education for each participant were recorded. On average, the ketamine group received 11.6 years (SD 2.5, range 8–16, 95 % confidence interval (CI) 10.52,12.58), whilst the healthy control group received 12.5 years (SD 2.3, range 9–16, 95 % CI 11.37,13.53). The means did not significantly differ, paired t test t (19) = 1.056, p = .304 (95 % CI −2.683,0.883), so no further analysis was conducted. The demographic characteristics and the questionnaires are summarized in Table 1.
Preliminary analysis: baseline accuracy of hand position estimation
The purpose of this analysis was to determine whether ketamine users were less accurate in locating their hand prior to the onset of the illusion-inducing stimulation and thus whether there might be an impact of non-specific localisation difficulties on the accuracy of the drift measure. This was assessed in two ways. First, the difference between the experimenter’s estimate of baseline location and that of the participant was assessed across both participant groups. The root-mean-square difference for the control group (standard error of the mean (SEM); synchronous 0.196 and asynchronous 0.209), p = .088, and the ketamine group (SEM; synchronous 0.195 and asynchronous 0.192), p = .834, did not significantly differ from each other prior to stimulation, t test. Furthermore, the difference in the ketamine group was not greater than controls in either the synchronous condition, p = .142, or the asynchronous condition, p = .630. Second, we investigated whether this difference in baseline estimate (i.e. a measure of initial inaccuracy) was correlated with the total drift recorded. No significant correlation was found in either the control group or the ketamine group, for either stimulation condition. Any change in perceived hand location can thus be attributed to experimental manipulation and not to a non-specific difficulty in hand localisation in the ketamine users.
The rubber hand illusion
The data violated assumptions for normality; hence, non-parametric tests (Mann-Whitney U to compare the groups and Wilcoxon signed-ranks to compare stimulation conditions) are reported; however, to investigate whether an interaction was present, parametric analyses (mixed analysis of variance (ANOVA)) are also reported.
Proprioceptive drift
The change in perceived hand location over time is presented in Fig. 1. This shows the magnitude of drift to increase over time. Consequently, the final drift measure was entered into analysis on the assumption that this represented the total magnitude of drift.
First, a 2 × 2 × 2 (within-subject factor of stimulation, between-subject factors of group and stimulation order) mixed ANOVA was conducted. A main effect of stimulation, F (1, 42) = 32.098, p = .000 (partial eta-squared .433), and of group, F (1, 42) = 52.488, p = .000 (partial eta-squared .556), is reported with a significant interaction between the two factors. There was neither a main effect of stimulation order, F (1, 42) = .936, p = .339 (partial eta-squared .022), nor stimulation × stimulation order, F (1, 42) = 4.428, p = .041 (partial eta-squared .095), nor a group by stimulation order interaction, F (1, 42) = .252, p = .252 (partial eta-squared).
The total drift was bigger in the ketamine group compared to the control group for the synchronous, Mann-Whitney U = 69.500, Z = 4.252, p = .000, and asynchronous, Mann-Whitney U = 21.00, Z = 5.330, p = .000, conditions. The Wilcoxon signed-ranks test showed that the total drift was greater following synchronous rather than asynchronous stimulation in the control group, Z = 3.23, p = .001, and the ketamine group, Z = 4.13, p = .000.
Subjective experience
The subjective experience was assessed using the traditional questionnaire comprising nine statements describing perceptual experiences and sensations. Agreement with each statement was recorded on a scale of one (disagree entirely) to five (agree entirely). The mean across all nine questions (overall index) was calculated along with a more focused subscale of statements 1 to 3 (illusion index). These are represented in Fig. 2. Both parametric and non-parametric analyses were again conducted.
A repeated-measures ANOVA (stimulation × group × stimulation order) showed a main effect of stimulation, F (1, 42) = 80.03, p = .000 (partial eta-squared .656). There was no main effect of stimulation order, F (1, 42) = .000, p = .987 (partial eta-squared .000) but there was of group, F (1, 42) = 42.239, p = .000 (partial eta-squared .501). There was neither group × stimulation order interaction, F (1, 42) = .016, p = .900 (partial eta-squared .000), nor group × stimulation interaction, F (1, 42) = .053, p = .820, nor a stimulation × stimulation order interaction, F (1, 42) = 5.302, p = .026 (partial eta-squared .112).
The Mann-Whitney U test showed that the ketamine group reported a stronger illusion than the control group for the overall score, synchronous U = 19.500, Z = 5.341, p = .000 and asynchronous U = 49.500, Z = 4.682, p = .000, and the illusion score, synchronous U = 40.000, Z = 4.951, p = .000 and asynchronous U = 79.000, Z = 4.037, p = .000.
The Wilcoxon signed-ranks test showed that the overall index was higher in the synchronous condition for the ketamine group, Z = 4.189, p = .000, and the control group, Z = 3.251, p = .000. The same was true for the illusion index in the ketamine group, Z = 4.207, p = .000, and the control group, Z = 3.425, p = .000.
Ketamine use and the illusion
During their clinical interview, the participant made an estimate of the total ketamine used (in g), together with the experimenter. This value was entered into correlation analysis (using non-parametric tests because data were not normally distributed) with both measures of the illusion. Adhering to a 1 % level of significance, total ketamine use did not significantly correlate with any measure of the illusion although total drift approached significance for the synchronous, p = .538, p = .005, and the asynchronous, ρ = .509, p = .008, conditions.
The ketamine users also reported the frequency of use for the 30 days prior to the testing session. Frequency per week was calculated, with a range of 4.5 times per week to 28 times per week and a mean of 16.8 times per week. Again, using a 1 % level of significance, none of these relationships was statistically significant.
Psychosis proneness and the illusion
Peters Delusions Inventory
Participants made yes/no responses to 21 items that were then converted to a total score out of 21. The Mann-Whitney U test was used to compare scores between the two groups. The ketamine group (mean score 9.3, SD 4.6) scored significantly higher than controls (mean score 3.8, SD 2.4), U (46) = 73.5, Z = −4.152, p < .001. For all ‘yes’ responses, participants rated their feelings of distress, preoccupation and conviction with that belief on a scale of 1–5, giving a total possible score of 1–5 for each subscale. Ketamine users (mean score 25.0, SD 14.7) scored higher on the distress (mean score 7.8, SD 7.0), U (46) = 57.0, Z = −4.504, p < .001, preoccupation, U (46) = 60.0, Z = −4.435, p < .001, and conviction subscales U (46) = 68.5, Z = −4.247, p < .001.
Chapman scales
For both scales, participants made true/false responses that were converted to a score of ‘1’ for true and ‘0’ for false, for loaded statements. The Mann-Whitney U test was used to compare total scores.
Chapman Magical Ideation
The total score was not significantly different between the groups, U (46) = 181.5, Z = −1.745, p = .081.
Perceptual Aberration
Ketamine users scored higher than controls, U (46) = 86.5, Z = −3.855, p < .001.
Symptoms
CADSS scores were analysed using comparison of (1) the control group responses with those made by the ketamine group based on their current experience (current), (2) controls with ketamine users’ retrospective evaluation of their ketamine experience (ketamine) and (3) current with ketamine scores.
-
1.
The sum of responses to 19 statements, made on a scale of 0–4, was calculated as the total CADSS score for each group (maximum possible score of 76). A Mann-Whitney U test showed that the total score did not significantly differ between groups, U (46) = 242.0, Z = −502, p = .615. A total score was calculated for subscales of derealisation, depersonalisation and amnesia. No significant differences were found between groups; hence, this score is not entered into further analysis.
-
2.
Ketamine users gave a retrospective evaluation of their ketamine state. Items 12 (does this interview seem to be taking much longer than you would have expected?) and 14 (have there been things which have happened during this interview that you cannot account for?) were excluded as these related to the current experience. A total score for controls and the ketamine group (ketamine) using the remaining 17 items was calculated (maximum possible score of 68). The Mann-Whitney U test showed that the ketamine score was significantly higher than the control,
-
3.
Wilcoxon signed-ranks showed that the ketamine scores were significantly higher than the current (17 items). No further analysis of the subscales was carried out due to the reduced items in the scale.
Correlations between RHI experience and CADSS
We explored the relationship, using Spearman’s rho (two tailed), between the total CADSS score and the measures of the illusion. There were a number of statistically significant findings; however, closer inspection of scatterplots means that we cannot be confident that these are not driven by outliers. For example, in many cases, 75 % or more of the participants had a score less than 0. As such, we report those correlations showing a clear spread of data.
The overall index reported by the ketamine group in the asynchronous condition showed a weak correlation for the ketamine total CADSS score, ρ (26) = .494, p = .010 (Fig. 3) as well as for the illusion index, ρ = .453, p = .020 (Fig. 3).
The magnitude of drift and the total CADSS ketamine score approached significance for the synchronous, ρ = .499, p = .009, and asynchronous, ρ = .548, p = .004, conditions (see Fig. 4).
Ketamine use and psychosis proneness
The scores for measures of psychosis proneness were entered in correlation analysis with the total ketamine use over the participant’s lifetime as well as frequency of use in the 30 days prior to testing. The ketamine CADSS score and ketamine frequency, ρ = .540, p = .004, and ketamine used, ρ = .564, p = .003, r (26) = .495, p = .010, approached significance.
Discussion
It was hypothesised that chronic ketamine users would experience a stronger RHI, compared with healthy controls, even when not having recently taken the drug. As expected, chronic ketamine users scored higher on measures of schizophrenia-like behaviours and of dissociative symptoms, compared with healthy controls. The RHI was experienced more strongly by the ketamine group for both synchronous and asynchronous stimulation conditions, for both measures of proprioceptive drift and subjective experience. Finally, it appears that the greater the presence of dissociative symptoms when on ketamine, the stronger the RHI when off ketamine.
The rubber hand illusion
Previously, we have found that ketamine administered intravenously to healthy controls resulted in larger changes in perceived hand location compared to when participants received placebo infusion, for both synchronous and asynchronous stimulation (Morgan et al. 2011). A similar pattern is again revealed with chronic ketamine use; proprioceptive drift was bigger, irrespective of synchrony, than in healthy controls. Nonetheless, although ketamine users demonstrated reduced sensitivity to mismatches between multisensory inputs, changes were still greater when inputs were synchronous than when they were asynchronous. Hence, it appears that the discrepancy in visuotactile experience, caused by temporal asynchrony, is not so subtle that it is not distinguished by ketamine users at all but perhaps attenuated such that they show some degree of the illusion even when such a discrepancy occurs. Likewise, ketamine users reported more illusory changes captured by the subjective experience measure than controls, for both synchronous and asynchronous stimulation. Within groups, the illusion was stronger when stimulation was synchronous. Again, this pattern is reminiscent of our findings for acute ketamine.
These findings are particularly intriguing because they directly contradict the hypothesis that the RHI, and thus ownership, depends largely upon temporal congruity between multiple sensory inputs (Botvinick and Cohen 1998; Tsakiris and Haggard 2005). Given these consistent findings across the objective and subjective indices of the illusion, it is likely that multisensory integration is still key to the RHI, but, in schizophrenia and with ketamine, the way in which multiple sensory inputs are processed is altered. As a result, the RHI is induced when not expected for the healthy population, and ownership is more easily manipulated in schizophrenia, even in the asynchronous condition, for example, Thakkar et al. (2011). The question remains, however, as to how this occurs.
Irrespective of the RHI, ketamine induces dissociative experiences that alter the experience of the self and of the body (Duncan et al. 2001; Oye et al. 1992; Pomarol-Clotet et al. 2006; Stone and Pilowsky 2006; Vollenweider et al. 1997a). It is possible therefore that the findings reported here arise due to a non-specific effect of ketamine that renders the user incapable of accurately locating their body and thus more likely to incorporate a false hand into their body schema. We used two different analyses to address this potential confounder and to ascertain if our findings are specific to the illusion. In both cases, ketamine users were capable of locating their body prior to the onset of the illusion. We can therefore be confident that any changes in perceived location of their hand arise due to manipulations of the illusion and not a non-specific effect of ketamine. Hence, there must be another, more precise explanation for how ketamine alters bodily related processing and thus what we observe on the RHI.
It is possible that the visual representation of tactile experience is made more salient by ketamine, and consequently, a visual capture of touch occurs (Pavani et al. 2000). In the case of the asynchronous condition, this means that the error signal between the visual representation of the brushstroke and the actual tactile experience of that touch, created by mismatches in timing, is reduced. Consequently, the own-hand experience is recalibrated to the position of the rubber hand and bigger drift is recorded. By this explanation, there are clear changes to the parameters that usually govern bottom-up sensory processes, i.e. visuotactile integration or a visual enhancement of touch (Longo et al. 2008). Multisensory integration by itself though is not sufficient to induce the illusion in healthy people (rotating the hand reduces the illusion, for example, Costantini and Haggard (2007); hence, other, more top-down processes are also important.
Ketamine is thought to alter the balance between top-down and bottom-up bodily processing. Specifically, top-down control (depending on stored representations of one’s body) is weakened by the drug making the body schema more malleable which, coupled with increased salience of bottom-up sensory percepts (like the visual representation of touch), mean that the rubber hand is more readily incorporated into bodily experience. Thus, a stronger RHI is reported irrespective of synchrony, for both measures. Whilst this explanation is theoretically feasible, it does not account for the specific mechanisms underpinning these processes and what is it exactly about multisensory integration that drives these changes.
It has been reported that the amplitude of gamma-band oscillations is augmented with acute ketamine administration (Hong et al. 2010) and in the rubber hand illusion, particularly for synchronous stimulation (Kanayama et al. 2007, 2009). We suggested previously (Morgan et al. 2011) that this specific brain activity provides a tentative mechanism by which the illusion is elicited to a greater degree on ketamine, a behavioural finding that we replicate here. In contradiction, there is evidence to suggest that chronic schizophrenia is characterised by reduced electrical activity in the gamma band (Kocsis et al. 2013). Nonetheless, the critical consensus is that the cognitive deficits observed in schizophrenia could be underpinned by altered gamma-band oscillations (Díez et al. 2014; Tan et al. 2013; Perez et al. 2013) and that gamma-band activity is functionally relevant for cognitive processing including perceptual integration (Bosman et al. 2014; Moratti et al. 2014; Başar 2013). One particular finding, for example, reports that gamma-band oscillations predicted the strength of audiovisual integration (Hipp et al. 2011).
Furthermore, in schizophrenia, somatosensory evoked potentials over multisensory processing areas were altered in comparison to healthy controls, during the RHI (Peled et al. 2003). It is possible then that gamma-band oscillations that are implicated in multisensory integration are fundamentally altered in acute and in chronic ketamine such that distinct sensory inputs are processed together even with temporal mismatches between these inputs such as in the asynchronous condition. We recognise that this hypothesis is highly speculative, but we argue that it provides an intriguing hypothesis for understanding changes to bodily experience with ketamine administration.
There are two potential confounding factors worth noting. The first potential confounding factor is polysubstance use. In our sample, we have carefully excluded comorbid substance dependents (any polydrug use approximated fewer than 10 occasions and thus did not meet criteria for dependence). The second one is nicotine use. In the present study, nicotine use does differ between groups in terms of the proportion of smokers (about twice as many in the ketamine group) and of use within the subgroup of smokers in each group (ketamine users who smoke do so more than controls who smoke). The effects of nicotine are complex and are not concluded. There are some studies that implicate a role for nicotinic receptors in regulating thalamo-cortical gamma-band oscillations and potential relations to hallucinatory phenomena (see review Behrendt 2003; Behrendt and Young 2004). However, there are also some studies that show a lack of an effect of nicotinic manipulations on the effects of acute ketamine (D’Souza et al. 2012) and this on the independence of ketamine and nicotine effects on electrophysiology (Mathalon et al. 2014).
It may be then that patients with schizophrenia or ketamine users aberrantly integrate multiple, distinct, sensory inputs due to altered time perception (underpinned by gamma-band oscillations). Recently, for example, Foucher et al. (2007) found that patients with schizophrenia can only perceive two sensory stimuli (visual and auditory) as separate if they are further apart in time than for healthy controls. In the RHI, patients with schizophrenia may require a visual and a tactile percept to be further apart in time than healthy controls. As a result, they experience the illusion even in asynchronous conditions due to hyper-binding of distinct sensory inputs, a mechanism underpinned by gamma-band oscillations. Moreover, the tactile experience to their own hand is bound to the visual representation of the rubber hand due to greater reliability of visual input in multisensory processing more generally (hence a visual capture of touch). Ketamine administration may similarly impact upon the multisensory processing in the RHI thereby accounting for the findings that we report here.
At a sensory level then, the long-term impact of ketamine could be to augment gamma-band oscillations, in conjunction with a wider window for multisensory integration. The result is that altered sensory processing manipulates bodily experience more easily, accounting for the dissociative symptoms associated with ketamine and disturbances in the self in schizophrenia. The ownership-specific effects captured by the RHI follow from both altered sensory processing and less robust pre-existing body representations. Here, even without recent ketamine administration, we observe behaviours redolent of schizophrenia and acute ketamine experience. To be precise, top-down processes such as belief formation appear altered resulting in higher delusional ideation more generally (akin to subjectively experiencing a fake hand as one’s own) and bodily experience aberrations (such as the sensory representation of a fake hand as one’s own). Let us now consider the implications for a broader model for psychosis and, in particular, our understanding of disrupted ownership in schizophrenia.
The RHI experience for chronic ketamine users is reminiscent of the acute ketamine experience; thus, we have employed a similar explanation using top-down/bottom-up alterations (Morgan et al. 2011). The difference is that in this case, the RHI experience is elicited even without recent administration of the drug. One explanation for this is that acute ketamine disrupts inferential reasoning, whilst repeated ketamine use alters learning (Corlett et al. 2011), such that learned priors are aberrantly updated (hence are weaker) thereby resulting in a greater reliance on sensory information. More precisely, reduced top-down modulation by learned prior information means that internal body representations are much more malleable even without recent administration of the drug. Together with alterations in sensory processing, bodily experience is disrupted and a rubber hand is adopted into the body schema, reminiscent of delusional beliefs in schizophrenia. Furthermore, the presence of temporary dissociative effects (that are not body specific and so might not drive the same updating of prior information) could reveal a propensity to body-specific changes captured in the RHI through repeated ketamine use.
To address this hypothesis, we measured the presence of dissociative symptoms and the interaction between symptoms and RHI experience. Using the CADSS, Ketamine users were asked to make two responses to each CADSS item providing a lifetime score and a ketamine state score. Drift in both conditions was found to be greater in those ketamine users who reported more dissociative symptoms when on ketamine. The presence of dissociative symptoms in the ketamine state also made it more likely that ketamine users would experience a strong subjective RHI illusion, both for the overall score and illusion-specific effect in the asynchronous condition. Moreover, the overall RHI experience was strongest for those who report dissociative symptoms even in their lifetime score. This provides further support that sensory processing in chronic ketamine users is altered, and this has body-specific effects such as the likelihood of incorporating a fake hand (albeit temporarily) into the body schema.
It is worth noting that we cannot establish whether the behaviours reported above were driving factors in ketamine use initially, i.e. a tendency to report odd experiences might indicate pleasure-seeking behaviour (and thus drug use) more generally. As such, ketamine users may demonstrate traits that are reminiscent of schizophrenia in much the way that non-drug users also do. However, there is evidence to suggest that the frequency and amount of ketamine used have a direct impact on the delusional behaviours reported. Consequently, the significant correlation between the RHI and dissociative experiences means that the relationship between trait behaviours, state symptoms and RHI remains of value and interest to us.
Psychosis proneness
Existing findings indicate that delusional ideation is higher in ketamine users even when ketamine has not recently been administered, at a 3-day follow-up (Curran and Morgan 2000; Morgan et al. 2004a; Uhlhaas et al. 2007). Consistent with this, chronic ketamine users in this study scored higher on a measure of delusional ideation even after abstaining from ketamine for the 24 h prior to the testing session. The ketamine group members were also notably more affected by their beliefs than healthy controls, as would be expected in schizophrenia (Peters et al. 2004). In contrast, another measure of unusual beliefs or belief-like experiences, the Magical Ideation scale (Eckblad and Chapman 1983), elicited higher scores for the ketamine group, but these were not statistically significant. This could simply be a result of different items in these two scales. Overall, these findings support the premise that ketamine use elevates tendencies to develop odd beliefs and experience unusual perceptions.
Chronic ketamine users also reported more odd perceptual experiences, captured using a measure that was developed specifically for body-image aberration in schizophrenia but has also been applied to non-clinical populations (Chapman et al. 1978). This included items such as ‘I have felt as though my head or limbs were somehow not my own’. It is a measure that has not been used in chronic ketamine users to date (that we are aware of) and is a more precise measure of trait bodily alterations than general state dissociative experiences that have been captured using the Dissociative Experiences Scale (Morgan et al. 2010) or the CADSS (Curran and Monaghan 2001). The existing findings with these measures suggest that dissociative experiences are transient but might predict body-specific changes as suggested above, whilst delusional ideation is more persistent. Here, however, even without recent administration of the drug, ketamine users report more body aberrations than controls consistent with our RHI findings. This is consistent with reports that the frequency of ketamine use correlates with out-of-body experiences on ketamine (Wilkins et al. 2011), indicating that repeated ketamine use can result in more long-lasting changes to bodily experience.
As well as body-specific changes (and in an effort to demonstrate consistency with the existing literature), dissociative symptoms were measured using the CADSS. Our findings support the notion that general dissociative symptoms are transient as CADSS scores were only higher when the ketamine group members imagined having taken the drug and not when in their ketamine-free state. There is thus a distinction between body-specific dissociative alterations that are evident in the ketamine-free state and non-specific dissociation that is exclusive to the ketamine state. Importantly, however, these scores were correlated with the RHI experience suggesting that the presence of general dissociative experiences could be a precursor to more persistent, bodily specific alterations.
Summary
In conclusion, we sought to establish whether repeated ketamine use resulted in bodily disturbances, measured using self-report and the rubber hand illusion, akin to those following acute ketamine administration. We found that chronic ketamine users experienced the RHI more strongly and reported more body-image aberrations even when they have not recently taken the drug. These findings suggest that the chronic ketamine model for psychosis models more long-lasting changes, perhaps more akin to schizophrenia.
References
Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A (1998) Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry 43:811–816
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR), 4th edn. American Psychiatric Association, Washington, DC
Başar E (2013) A review of gamma oscillations in healthy subjects and in cognitive impairment. Int J Psychophysiol 90(2):99–117
Behrendt RP (2003) Hallucinations: synchronisation of thalamocortical gamma oscillations underconstrained by sensory input. Conscious Cogn 12(3):413–451
Behrendt RP, Young C (2004) Hallucinations in schizophrenia, sensory impairment, and brain disease: a unifying model. Behav Brain Sci 27(6):771–787
Bosman CA, Lansink CS, Pennartz CM (2014) Functions of gamma-band synchronization in cognition: from single circuits to functional diversity across cortical and subcortical systems. Eur J Neurosci 39(11):1982–1999
Botvinick M, Cohen J (1998) Rubber hands ‘feel’ touch that eyes see. Nature 391:756
Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM (1998) Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J Trauma Stress 11:125–136
Chapman LJ, Chapman JP, Raulin ML (1978) Body-image aberration in Schizophrenia. J Abnorm Psychol 87:399–407
Corlett PR, Honey GD, Fletcher PC (2007) From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol 21:238–252
Corlett PR, Honey GD, Krystal JH, Fletcher PC (2011) Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology 36:294–315
Costantini M, Haggard P (2007) The rubber hand illusion: sensitivity and reference frame for body ownership. Conscious Cogn 16:229–240
Coull JT, Morgan H, Cambridge VC, Moore JW, Giorlando F, Adapa R, Corlett PR, Fletcher PC (2011) Ketamine perturbs perception of the flow of time in healthy volunteers. Psychopharmacology (Berlin) 218:543–556
Curran HV, Monaghan L (2001) In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction 96:749–760
Curran HV, Morgan C (2000) Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 95:575–590
D’Souza DC, Ahn K, Bhakta S, Elander J, Singh N, Nadim H, Jatlow P, Suckow RF, Pittman B, Ranganathan M (2012) Nicotine fails to attenuate ketamine-induced cognitive deficits and negative and positive symptoms in humans: implications for schizophrenia. Biol Psychiatry 72(9):785–794
Deroza PF, Ghedim FV, Heylmann AS, de Luca RD, Budni J, Souza RP, Quevedo J, Zugno AI (2012) Effect of cigarette smoke exposure in the behavioral changes induced by ketamine. Schizophr Res 141(1):104–105
Díez A, Suazo V, Casado P, Martín-Loeches M, Perea MV, Molina V (2014) Frontal gamma noise power and cognitive domains in schizophrenia. Psychiatry Res 221(1):104–113
Duncan EJ, Madonick SH, Parwani A, Angrist B, Rajan R, Chakravorty S, Efferen TR, Szilagyi S, Stephanides M, Chappell PB, Gonzenbach S, Ko GN, Rotrosen JP (2001) Clinical and sensorimotor gating effects of ketamine in normals. Neuropsychopharmacology 25:72–83
Eckblad M, Chapman LJ (1983) Magical ideation as an indicator of schizotypy. J Consult Clin Psychol 51:215–225
Foucher JR, Lacambre M, Pham BT, Giersch A, Elliott MA (2007) Low time resolution in schizophrenia lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophr Res 97:118–127
Germine L, Benson TL, Cohen F, Hooker CI (2013) Psychosis-proneness and the rubber hand illusion of body ownership. Psychiatry Res 207:45–52
Hipp JF, Engel AK, Siegel M (2011) Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron 69(2):387–396
Honey RA, Turner DC, Honey GD, Sharar SR, Kumaran D, Pomarol-Clotet E, McKenna P, Sahakian BJ, Robbins TW, Fletcher PC (2003) Subdissociative dose ketamine produces a deficit in manipulation but not maintenance of the contents of working memory. Neuropsychopharmacology 28:2037–2044
Honey RA, Honey GD, O’Loughlin C, Sharar SR, Kumaran D, Bullmore ET, Menon DK, Donovan T, Lupson VC, Bisbrown-Chippendale R, Fletcher PC (2004) Acute ketamine administration alters the brain responses to executive demands in a verbal working memory task: an FMRI study. Neuropsychopharmacology 29:1203–1214
Honey GD, Honey RA, Sharar SR, Turner DC, Pomarol-Clotet E, Kumaran D, Simons JS, Hu X, Rugg MD, Bullmore ET, Fletcher PC (2005) Impairment of specific episodic memory processes by sub-psychotic doses of ketamine: the effects of levels of processing at encoding and of the subsequent retrieval task. Psychopharmacology (Berlin) 181:445–457
Honey GD, O’Loughlin C, Turner DC, Pomarol-Clotet E, Corlett PR, Fletcher PC (2006) The effects of a subpsychotic dose of ketamine on recognition and source memory for agency: implications for pharmacological modelling of core symptoms of schizophrenia. Neuropsychopharmacology 31:413–423
Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, Lahti AC (2010) Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 35:632–640
Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308
Kanayama N, Sato A, Ohira H (2007) Crossmodal effect with rubber hand illusion and gamma-band activity. Psychophysiology 44:392–402
Kanayama N, Sato A, Ohira H (2009) The role of gamma band oscillations and synchrony on rubber hand illusion and crossmodal integration. Brain Cogn 69:19–29
Knott V, Shah D, Millar A, McIntosh J, Fisher D, Blais C, Ilivitsky V (2012) Nicotine, auditory sensory memory, and sustained attention in a human ketamine model of schizophrenia: moderating influence of a hallucinatory trait. Front Pharmacol 3:172
Kocsis B, Brown RE, McCarley RW, Hajos M (2013) Impact of ketamine on neuronal network dynamics: translational modeling of schizophrenia-relevant deficits. CNS Neurosci Ther 19(6):437–447
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Krystal JH, Bennett A, Abi-Saab D, Belger A, Karper LP, D’Souza DC, Lipschitz D, Abi-Dargham A, Charney DS (2000) Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry 47:137–143
Longo MR, Schuur F, Kammers MP, Tsakiris M, Haggard P (2008) What is embodiment? A psychometric approach. Cognition 107:978–998
Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A (1996) NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 14:301–307
Mathalon DH, Ahn KH, Perry EB Jr, Cho HS, Roach BJ, Blais RK, Bhakta S, Ranganathan M, Ford JM, D’Souza DC (2014) Effects of nicotine on the neurophysiological and behavioral effects of ketamine in humans. Front Psychiatry 5:3
Moore JW, Turner DC, Corlett PR, Arana FS, Morgan HL, Absalom AR, Adapa R, de WS, Everitt JC, Gardner JM, Pigott JS, Haggard P, Fletcher PC (2011) Ketamine administration in healthy volunteers reproduces aberrant agency experiences associated with schizophrenia. Cogn Neuropsychiatry 16:364–81
Moore JW, Cambridge VC, Morgan H, Giorlando F, Adapa R, Fletcher PC (2013) Time, action and psychosis: using subjective time to investigate the effects of ketamine on sense of agency. Neuropsychologia 51:377–384
Moratti S, Méndez-Bértolo C, Del-Pozo F, Strange BA (2014) Dynamic gamma frequency feedback coupling between higher and lower order visual cortices underlies perceptual completion in humans. J Neuroimaging 86:470–479
Morgan CJ, Curran HV (2012) Ketamine use: a review. Addiction 107:27–38
Morgan CJ, Monaghan L, Curran HV (2004a) Beyond the K-hole: a 3-year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction 99:1450–1461
Morgan CJ, Riccelli M, Maitland CH, Curran HV (2004b) Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug Alcohol Depend 75:301–308
Morgan CJ, Muetzelfeldt L, Curran HV (2010) Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction 105:121–133
Morgan HL, Turner DC, Corlett PR, Absalom AR, Adapa R, Arana FS, Pigott J, Gardner J, Everitt J, Haggard P, Fletcher PC (2011) Exploring the impact of ketamine on the experience of illusory body ownership. Biol Psychiatry 69:35–41
Newcomer JW, Krystal JH (2001) NMDA receptor regulation of memory and behavior in humans. Hippocampus 11:529–542
Overall JE, Klett CJ (1972) Applied multivariate analysis. McGraw Hill, New York
Oye I, Paulsen O, Maurset A (1992) Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 260:1209–1213
Pavani F, Spence C, Driver J (2000) Visual capture of touch: out-of-the-body experiences with rubber gloves. Psychol Sci 11:353–359
Peled A, Ritsner M, Hirschmann S, Geva AB, Modai I (2000) Touch feel illusion in schizophrenic patients. Biol Psychiatry 48:1105–1108
Peled A, Pressman A, Geva AB, Modai I (2003) Somatosensory evoked potentials during a rubber-hand illusion in schizophrenia. Schizophr Res 64:157–163
Perez VB, Roach BJ, Woods SW, Srihari VH, McGlashan TH, Ford JM, Mathalon DH (2013) Early auditory gamma-band responses in patients at clinical high risk for schizophrenia. Suppl Clin Neurophysiol 62:147–162
Peters E, Joseph S, Day S, Garety P (2004) Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI). Schizophr Bull 30:1005–1022
Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, McKenna PJ, Bullmore ET, Fletcher PC (2006) Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry 189:173–179
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM (2005) Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162:394–396
Stone JM, Pilowsky LS (2006) Psychopathological consequences of ketamine. Br J Psychiatry 189:565–566
Stone JM, Erlandsson K, Arstad E, Bressan RA, Squassante L, Teneggi V, Ell PJ, Pilowsky LS (2006) Ketamine displaces the novel NMDA receptor SPET probe [(123) I] CNS-1261 in humans in vivo. Nucl Med Biol 33:239–243
Tan HR, Lana L, Uhlhaas PJ (2013) High-frequency neural oscillations and visual processing deficits in schizophrenia. Front Psychol 4:621
Thakkar KN, Nichols HS, McIntosh LG, Park S (2011) Disturbances in body ownership in schizophrenia: evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLoS One 6:e27089
Tsakiris M (2010) My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia 48:703–712
Tsakiris M, Haggard P (2005) The rubber hand illusion revisited: visuotactile integration and self-attribution. J Exp Psychol Hum Percept Perform 31:80–91
Uhlhaas PJ, Millard I, Muetzelfeldt L, Curran HV, Morgan CJ (2007) Perceptual organization in ketamine users: preliminary evidence of deficits on night of drug use but not 3 days later. J Psychopharmacol 21:347–352
Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J (1997a) Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 7:25–38
Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J (1997b) Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18 F] fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol 7:9–24
Wilkins LK, Girard TA, Cheyne JA (2011) Ketamine as a primary predictor of out-of-body experiences associated with multiple substance use. Conscious Cogn 20:943–950
Wilkins LK, Girard TA, Cheyne JA (2012) Anomalous bodily-self experiences among recreational ketamine users. Cogn Neuropsychiatry 17:415–430
Financial disclosures
This study was supported by the Natural Science Foundation of China (Grant No. 30900486 and 81371480 to J.T., 81100996 to Y.L., 81471361 and 81271484 to X.C., 81271499 to Y.T., 81130020 to W.H.), the National Key Basic Research and Development Program (973) (Grant No. 2012CB517904 to X.C., 2009CB522007 to W.H.), Doctoral Fund of the Ministry of Education of China (20100162110046 to W.H.). Philip Corlett reports receiving research support from Astra Zeneca Pharmaceuticals, LP, and consulting for Pfizer Pharmaceuticals and Janssen Pharmaceuticals. His contribution was made possible by the Connecticut Mental Health Center as well as the Department of Mental Health and Addiction Services. He is supported by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Paul Fletcher was funded by the Wellcome Trust and the Bernard Wolfe Health Neuroscience Fund. Paul Fletcher reports receiving consultancy fees from GlaxoSmithKline and an honorarium for a lecture from Astra Zeneca. However, the funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jinsong Tang and Hannah L. Morgan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tang, J., Morgan, H.L., Liao, Y. et al. Chronic administration of ketamine mimics the perturbed sense of body ownership associated with schizophrenia. Psychopharmacology 232, 1515–1526 (2015). https://doi.org/10.1007/s00213-014-3782-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3782-0