Abstract
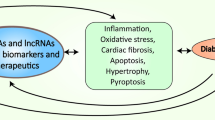
Diabetic cardiomyopathy (DCM) is the leading cause of morbidity and mortality in diabetic population worldwide, characteristic by cardiomyocyte hypertrophy, apoptosis and myocardial interstitial fibrosis and eventually developing into heart failure. Non-coding RNAs, such as microRNAs (miRNAs), circular RNAs (circRNAs), long non-coding RNAs (lncRNAs) and other RNAs without the protein encoding function were emerging as a popular regulator in various types of processes during human diseases. The evidences have shown that miRNAs are regulators in diabetic cardiomyopathy, such as insulin resistance, cardiomyocytes apoptosis, and inflammatory, especially their protective effect on heart function. Besides that, the functions of lncRNAs and circRNAs have been gradually confirmed in recent years, and their functions in DCM have become increasingly prominent. We highlighted the nonnegligible roles of non-coding RNAs in the pathological process of DCM and showed the future possibilities of these non-coding RNAs in DCM treatment. In this chapter, we summarized the present advance of the researches in this filed and raised the concern and the prospect in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Diabetic cardiomyopathy (DCM) was the specific abnormality of myocardial structure and function in diabetic patients, and does not coexist with cardiovascular diseases such as coronary artery disease and hypertension [1]. DCM was characterized by myocardial dilation, hypertrophy, decreased left ventricular diastolic, systolic function, and eventually develops into heart failure. About 75% of the human genomic DNA sequence can be transcribed, and nearly 74% of the transcripts are non-coding RNAs, which play an important role in maintaining the normal physiological function and the occurrence and progress of diseases in organisms. Various non-coding RNAs have been shown to be involved in regulating the occurrence of DCM, including miRNA and other types of non-coding RNA. In this review, we summarized current studies of non-coding RNA and DCM.
1 Introduction of Diabetic Cardiomyopathy
There were two types of diabetes which named type 1 and type 2 diabetes. Type 1 diabetes was caused by insufficient insulin secretion and type 2 diabetes was caused by insulin resistance and gestational-related diabetes mellitus. The complications of diabetes, including diabetic microangiopathy and diabetic macroangiopathy, were the main causes of disability and death of diabetes mellitus. The incidence of diabetes was increased year by year due to the increased obesity population and rapid ageing population. According to the data from International Diabetes Alliance research project, near 451 million people worldwide suffer from diabetes in 2017. And it was estimated that 693 million people around the world will be diagnosed as diabetes by 2045. As the complication of diabetes mellitus, diabetic cardiomyopathy (DCM) received more attention by clinicians [2]. In 1972, Rubler observed 4 diabetic patients with congestive heart failure, and found that these patients had no other potential causes of heart failure, such as coronary artery disease, dilated cardiomyopathy or hypertension, except diabetes mellitus [3]. Both type 1 and type 2 diabetes could be complicated with DCM, associated with the main pathological changes, including cardiomyocyte hypertrophy, extracellular matrix deposition, myocardial microvascular basement membrane thickening, and interstitial fibrosis [4, 5]. Until now, there is no corresponding clinical guidelines or consensus for diagnosis and management of patients with DCM [6]. The diagnosis of DCM still belongs to exclusive diagnosis, mainly diagnosed by clinical history of diabetes mellitus, manifestations and symptoms of cardiac dysfunction, combined with laboratory examinations such as echocardiography [7]. With the excluded of coronary heart disease, hypertensive heart disease, dilated cardiomyopathy and other heart diseases. According to the changes of cardiac structure and cardiac function, DCM can be divided into three stages. Stage 1, there are changes in cardiac structure and no changes in diastolic function. Left ventricular ejection fraction (EF) is normal and subclinical. Changes of cardiac structure are aggravated in stage 2, with ventricular hypertrophy, myocardial fibrosis, decreased ventricular diastolic function in cardiac function, and gradually abnormal systolic function, EF value < 50%. Cardiac structural changes further aggravated in stage 3, cardiac microvascular changes, ventricular hypertrophy and myocardial fibrosis further aggravated, with global diastolic and systolic disorders occurred [8, 9].
Comprehensive treatment, including lifestyle interventions, were currently used in the treatment of DCM [10]. Quitting smoking, limiting alcohol, controlling salt intake, optimizing diet and moderate exercise were first proposed [11,12,13,14,15]. The injection of metformin, thiazolidinediones and glucagon-like polypeptide (GLP-1) analogues not only made effects on diabetes mellitus, especially improved insulin resistance and promoted glucose uptake and utilization, but also resisted myocardial cell damage and prevented cardiac remodeling [16]. Other medications that were used in cardiovascular system were also considered to have therapeutic effects on DCM, including renin-angiotensin-aldosterone system (RASS) inhibitors, beta-blockers, calcium channel antagonists, statins, and trimetazidine. According to the latest ADA/AHA guidelines, RASS inhibitors should be used as first-line drugs in patients with diabetes mellitus and hypertension [17]. Although drug therapy could improve the progression of DCM, the negative effects of drug could not be avoided. A comprehensive treatment was still in an urgent need.
2 Current Research on the Pathogenesis of DCM
The pathogenesis of DCM has not been fully elucidated till now. Abnormal insulin signal transduction, metabolic disorders, microangiopathy, myocardial interstitial fibrosis, imbalance of calcium regulation, and cardiac autonomic neuropathy might be involved in the occurrence and development of DCM. Thus, enhanced understanding of DCM will provide clues to prevent the occurrence and diagnosis of DCM.
2.1 Insulin Resistance and Abnormal Insulin Metabolic Signaling
Insulin resistance in myocardial was a metabolic and functional disorder accompanied by the development of DCM. In normal heart, insulin affected the mammalian target of rapamycin (mTOR)–S6 kinase 1(S6K1) pathway to regulate myocardial metabolism through the targeting of phosphatidylinositol 3 kinase/protein kinase B signaling pathway [18, 19]. Insulin could also regulate glucose transport, glycolysis, glycogen synthesis, protein synthesis, lipid metabolism in cardiac myocytes, and affect myocardial contractile function [20]. Different from the regulation of cardiomyocyte in physiological states, insulin resistance leaded to the imbalance between myocardial metabolism and growth activity, which was mainly regulated by mitogen-activated protein kinase signaling pathway [21]. It has been found that impaired insulin-mediated glucose uptake occurs before impaired insulin-activated protein kinase B signaling pathway in insulin-resistant animal models, and this defect was caused by the decrease of glucose transporter 4 and abnormal translocation of glucose transporter 4 membrane (GLUT4) [22, 23]. Insulin resistance in cardiomyocytes could lead to functional disorders and metabolic changes. The inhibition of insulin signal transduction in cardiomyocytes was one of the markers of DCM. Insulin resistance during the development of DCM was associated with the increased risk of left ventricular hypertrophy and heart failure.
2.2 Direct Myocardium Injury by Metabolic Disorder
Metabolic disorder, mainly referred to hyperglycemia and glucotoxicity, was an important factor to trigger DCM [24]. Metabolic disorder, could first cause the biological changes on cardiac myocytes, which lead to subclinical myocardial dysfunction. Then the dysfunction developed into myocardial small vessel disease, microcirculation disorder and cardiac autonomic neuropathy, and eventually heart failure occurred. Glycolipid metabolism disorder that owing to interactions between lipid metabolic disorder and hyperglycemia in diabetic patients could directly affect the function of mitochondria, and the dysfunction of mitochondria future affected the metabolism of cardiac myocytes and caused the dysfunction of cardiomyocytes, which was an important reason for the occurrence and development of DCM [25,26,27].
Hyperglycemia can induce myocardial injury through direct and indirect pathways, which was the key factor in the progression of DCM. Mitochondrial damage, which was induced by hyperglycemia, was mainly related to abnormal polyol pathway activation. This damage future increased the expression of advanced glycation end products (AGEs), the activation of hexamine pathway and protein kinase C pathway. AGEs accumulation leaded to development of cardiac fibrosis and stiffness, increased connective tissue cross-linking, and impaired diastolic relaxation. AGE receptors (AGERs) on the cell surface were activated by AGEs, and then the expression of various inflammatory mediators was increased. Ultimately, these lead to the deposition of matrix components through the mitogen-activated protein kinase (MAPK) and Janus kinase (JAK) signaling pathways [28, 29].
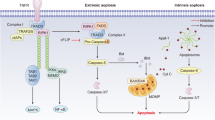
In addition, dysfunction of cardiac myocytes and accumulation of abnormal substances caused by energy utilization disorders could also lead to DCM. In physiological state, fatty acid oxidation (FAO) provided nearly 70% of the energy required for cardiomyocytes, and glycolysis was another 30–40% source of energy, while almost all of the energy of diabetic patients came from the oxidation of non-esterified fatty acids due to the impairment of glucose utilization [30, 31]. These leaded to accumulation of lipid metabolites, such as diacylglycerols, ceramides, uncoupling protein 3, and the production of reactive oxygen species in mitochondria and peroxidase bodies in cardiac myocytes affecting myocardial energy supply, inducing inflammation, and leading to myocardial fibrosis, myocardial cell necrosis and myocardial dysfunction. In addition, ceramide was the intracellular apoptotic messenger, which induced cardiomyocyte apoptosis by activating NF-κB translocation to the nucleus, up-regulating inducible nitric oxide synthase and activating cysteine protease [32, 33]. Besides, ceramide directly activated atypical PKCs to phosphorylate and inhibit the insulin metabolic through Akt signaling, attenuating GLUT4 translocation and insulin-induced glucose uptake [34].
2.3 Calcium Ion Regulation Imbalance
The mechanism of calcium regulation was related to early concealed ventricular systolic dysfunction in DCM. Calcium ion levels in cardiac myocytes mainly depend on the calcium channels in cell membranes and sarcoplasmic reticulum. In diabetic individuals, oxidative stress caused by accumulation of toxic metabolites from disordered lipid metabolism might be the main cause of the imbalance of calcium regulation [35]. Lipid toxicity weakened calcium uptake in sarcoplasmic reticulum and other calcium exchange activities, and decreased calcium processing capacity in cardiomyocytes through inhibited ATPase activity on the cardiomyocyte membranes [36]. Some studies shown that the activities of sarcoplasmic reticulum calcium ATPase isomer 2, carnitine receptor and sodium-calcium exchanger in DCM patients are significantly reduced, which further reduced the release of sarcoplasmic reticulum stored calcium ions and the recovery of calcium ions from diastolic sarcoplasmic reticulum, resulting in the accumulation of calcium ions in the cytoplasm of end-diastolic myocardial cells, the decrease of myocardial compliance, and the impairment of myocardial diastolic and systolic functions [37]. In addition, the increase of AGEs induced by hyperglycemia could also lead to imbalance of calcium regulation in cardiac myocytes and affect myocardial contractile function ultimately [38, 39].
2.4 Mitochondrial Dysfunction and Oxidative Stress
The swelling and fragmentation of mitochondria in diabetic patients might impaired mitochondrial function, suggesting the involvement of impaired mitochondrial morphology and dysfunction in the pathogenesis of DCM. The function of mitochondrial has been altered due to metabolic disorder in DCM patients. As mentioned above, increased fatty acid uptake and beta-oxidation during diabetic cardiomyopathy might exceed mitochondrial respiratory capacity. Hence, mitochondria played an important role in abnormal energy metabolism and accumulation of toxic lipid products in cardiomyocytes [40].
Cardiomyocytes not only decomposed fatty acids, but also accumulated intermediate products and phospholipids of glycolysis pathway. The increased fatty acid concentration in cardiomyocyte of DCM patients could induce the activation of peroxisome proliferator-activated receptor alpha (PPAR-alpha) [41]. These promoted the expression of fatty acid oxidation and its uptake genes, which inhibited the pyruvate dehydrogenase kinase activation and impaired the oxidative capacity of glucose, in order to increase the uptake of fatty acids by mitochondria, increase myocardial oxygen consumption and reduce heart rate [42]. Therefore, mitochondria metabolized fatty acids accompanied with the increased cardiac oxygen consumption, resulting in changes in cardiac structure and function, leading to DCM.
ROS came from NADPH oxidases, xanthine oxidase, uncoupling of nitric oxide synthase, the process of arachidonic acid metabolism and microsomal P-450 enzymes [43]. Diabetes mellitus resulted in a large number of ROS aggregation, due to the rapid increase of ROS production and the relative inadequacy of antioxidant capacity [44]. ROS induced by diabetes could lead to structural damage of myocardial mitochondria, and further damage mitochondrial function by inducing the opening of mitochondrial membrane permeability channels [45]. ROS produced by mitochondria could also induced DCM mainly through PKC signaling pathways and hexosamine pathway [46, 47].
2.5 Other Pathophysiological Mechanisms of DCM
Microangiopathy and vascular injury DCM were independent of coronary artery disease, which were mainly manifested as microangiopathy. Microangiopathy was an important pathological change in the development of diabetes mellitus [48], which mainly included morphological changes of vascular endothelial cells, reduction of mitochondria and capillary basement membrane thickening, small artery thickening, capillary microaneurysm and decrease of capillary density [49]. The typical characteristics of microangiopathy were microcirculation disturbance. Cardiac microangiopathy might occur prior to clinical symptoms in DCM patients, leading to chronic myocardial ischemia, extensive focal myocardial necrosis, and even heart failure, cardiogenic shock and sudden death. The damage of vascular endothelial cells and vascular smooth muscle cells that were caused by oxidative stress were the main cause of pathological changes of blood vessels in diabetic patients, and might be the most important initiating events of cascade reaction of vascular pathological changes [50].
Autophagy was inhibited at high glucose concentration (e.g. diabetes), which might be related to the development of DCM [51]. Autophagy in response to cardiac energy stress was mediated by a network of AMPK and insulin signaling pathways, which were the main regulator of cellular and systemic energy balance [52]. As a response to exercise, hypoxia, oxidative stress and glucose deficiency, the AMPK and insulin signaling pathways were activated to accommodate the increase in intracellular insulin and AMP/ATP ratios. New evidence suggested that AMPK regulated not only cell energy, but also other cellular processes, including protein synthesis, cell growth and autophagy [53,54,55]. The activity of AMPK decreased at high glucose concentration, leading to autophagy disorder [56]. In streptozotocin-induced type 1 diabetic mice, overexpression of alpha-MHC-Beclin 1could activate autophagy of cardiomyocytes, thus further accelerate the diabetes mellitus induced cardiomyocytes damage [57]. However, there existed debate in the autophagic changes that were found in diabetes and its complications, different results were observed in different tissues. Therefore, further research was needed before potential drugs could be used clinically.
3 Non-coding RNAs and DCM
Non-coding RNAs were mainly defined as a class of RNA that did not encode proteins, including RNAs with definite functions, such as rRNAs, tRNAs, snRNAs, snoRNAs, and microRNAs (miRNAs), as well as RNAs with unknown functions. These RNAs shared some common features, such as they could perform biological functions at the RNA level without being translated into proteins after their transcribed from the genome. Long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) were the novel members of non-coding RNA family, whose functions and regulatory approaches have not been completely revealed. This suggested that non-coding RNA was still of great values in the diagnosis, evaluation and treatment of DCM. The current research progress in this field will be introduced here.
3.1 miRNA and Pathogenesis of DCM
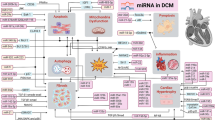
Among all non-coding RNAs, miRNAs were one of most concerned regulatory RNAs. The classical way of miRNAs function was through binding to complementary sequences on the target gene mRNA to take effect as a post-transcriptional inhibitor of target gene expression [58]. Although the latest research reported the non-canonical molecular mechanisms of miRNA, the follow-up functional researches were still underway [59]. Their functions covered a wide range of aspects, including organism growth, development, maintenance of homeostasis and disease occurrence. In the past decade, there have been continuous studies on the role of miRNAs in the development and progression of DCM. The expression pattern of miRNAs during diabetic cardiomyopathy were revealed in 2011. 19 miRNAs and 16 miRNAs expression were detected by miRNA array and real-time RT-PCR in diabetic heart. GO pathway analyze and target gene analyze also showed the close relationship above miRNA and the Typical pathological process of DCM, such as cardiac hypertrophy and myocardial fibrosis [60]. In 2013, researchers identified 43 different expression miRNAs in mice heart from streptozotocin induced DCM, which 37 miRNAs were downregulated and 6 miRNAs were upregulated. Ultimately, the decrease of miR-1, miR-499, miR-133a, and miR-133b and increase of miR-21 were identified by RT-qPCR. Interestingly, miR-1, miR-499, miR-133a, and miR-133b were also involved in the antioxidant effect that were produced by N-acetylcysteine (NAC)-treatment in DCM [61].
3.1.1 miRNAs were Involved in Cardiomyocytes Injury or Cell Survival
Among the members of the miR-30 family, miR-30d and miR-30c have been reported to play different roles in DCM. It was reported that decreased expression of miR-30c could lead to the activation of p53/p21 pathway and cardiomyocyte apoptosis. Downregulation of miR-30c was a mediator of myocardial hypertrophy in response to high glucose condition by upregulation of Cdc42 and Pak1 genes [62]. Through its target gene PGC-1β miR-30c could also affect cardiomyocyte apoptosis, which was produced by affecting the utilization of glucose and the accumulation of lipids in cardiomyocytes [63]. Interestingly, miR-30d was identified as a regulator of cardiomyocyte pyroptosis, the pro-inflammatory programmed cardiomyocyte death in DCM rat model. Beside miR-30 family, miR-9 was another regulating miRNA of ventricular cardiomyocytes pyroptosis, whose rised a potential therapeutic target for DCM [64]. In addition, knockdown MiR-195 could extenuated the cardiac dysfunction and cardiomyocytes apoptosis in diabetic mice [65].
3.1.2 miRNA Related to DCM Induced Cardiac Injury
As the second found miRNA family in C. elegans, Let-7 functioned during tissue development, metabolic process, aging and immunology function [66]. lin28/let-7 was a typical regulator of insulin-PI3K-mTOR signaling in skeletal muscles, which was the largest metabolic organ in the human body [67]. Let-7 also restrained the amino acid-sensing pathway to inhibit mTOR-induced anabolism and autophagic catabolism [68]. The overexpression of let-7 revealed glucose intolerance and insulin insensitivity in mice [67, 69]. Inhibition of let-7 family was found as a therapeutic method against ischemia-reperfusion injury in diabetic rats via improving glucose uptake and insulin resistance.
miR-21 was a novel miRNA related to virous pathological changes in the heart, such as miR-21-3p regulated sepsis-associated or aging induced cardiac dysfunction. Overexpression of miR-21 that was induced by high glucose increased macrophage apoptosis, which participated in atherosclerosis. The inhibition of miR-21 leaded to weight loss in db/db mice by targeting TGFRB2, PTEN, and Sprouty1 and 2, which provided a safety and effective method to get a weight control in animal model. Long-term miR-21 knockout also abolished the effect on heart induced by obesity, reduced cardiac function and cardiac fibrosis [70]. In addition to disorders of lipid metabolism induced cardiomyopathy, cardiomyocyte apoptosis associated with glucose metabolism was also regulated by miR-21. Under the high glucose-stimulated, miR-21 targeted DSFP8 to activate the p38 pathway and c-Jun N-terminal kinase (JNK)/stress-activated kinase (SAPK) pathway, resulting in cardiac fibroblast proliferation and collagen synthesis [71].
Hyperglycemia could affect the action potential of cardiomyocytes and lead to abnormal systolic and diastolic function of myocardium through induce the expression of miR-1/133 in cardiomyocytes and inhibit the genes encoding slowly activating delayed rectifier potassium channel- KCNE1 and KCNQ1. miR-133a overexpression in heart reversed the diabetes collagen synthesis induced cardiac fibrosis and repaired the heart function. The expression of miR-133a in cardiomyocytes was negatively correlated with the expression of fibronectin 1, collagen type IV α1, connective tissue growth factor, fibroblast growth factor and TGF-β1. In diabetic hearts, the expression of miR-133a was down-regulated, resulting in an increased risk of myocardial fibrosis [72]. Cardiac-specific miR-133a overexpression mice alleviated diabetes mellitus induced cardiac fibrosis by inhibiting ERK1/2 and SMAD-2 phosphorylation [73]. Application of miR-133a treatment in vivo. could significantly extenuated cardiac hypertrophy, fibrosis and Type 1 diabetes mellitus induced systolic dysfunction [74].
miR-143/145 cluster was an effector of activin A, whose released from epicardial adipose tissue was closely related to T2DM. Precursor-miR-143 overexpression decreased insulin-stimulated glucose uptake in cardiomyocytes due to Akt phosphorylation. On the contrary, miR-145 had no effect on the glucose uptake and utilization in cardiomyocytes. An activin A-p38-miR-143/145-ORP8 axis was built in regulating glucose uptake by insulin, which was directly related to insulin resistance during the development of T2DM [75]. However, the function of this pathway in heart failure and remodeling during DCM was still needed to be discussed.
miR-451 expression level was upregulated in T2DM heart, and this upregulation of miR-451 expression was time and dose dependent. Knockdown miR-451 alleviated the lipotoxicity in cardiomyocytes by suppression of the LKB1/AMPK pathway. Cardiac function recovery accompanied by decreasingthe concentration of lipid metabolism intermediate and reactive oxygen species production [76]. miR-503 participated in the protective effect of phase II enzyme inducer in DCM rat, and related to the antioxidant effect on cardiomyocytes [77].
It was reported that miR-155 is involved in metabolic diseases and inflammation disease [78,79,80,81]. miR-155 was a direct blocker of IL-13-induced anti-inflammatory type 2 macrophage by inhibiting the expression of IL-13Rα1. As an important contributor of excessive inflammatory response, miR-155 overexpression was also detected in the virus infected heart [82]. A covalent complex of gold nanoparticle (AuNP) and thiol-modified antago-miR-155 was made in a resent study, which transported miR-155 directly to macrophages. As a high specificity messenger of macrophage, AuNP-based miR-155 antagonist was injected into the ovariectomized female mice diabetic mouse model, which promoted M2 macrophages polarization in vivo. Then, recovered DCM induced cardiac function, mitigated coordinating inflammation, apoptosis, and fibrosis [83].
Endothelial to mesenchymal transition (EMT) was a phenotypic change during endothelial injury, which was closely related to cardiac fibrosis and existed in the pathogenesis of DCM. Specific overexpression of miR-200b in endothelial cells were detected to be induced in diabetic mice heart. Meanwhile, endothelial miR-200b overexpression blocked the EMT, further protected cardiac systolic function in diabetic mice.
3.2 Other Non-coding RNAs and DCM
3.2.1 lncRNAs in the Diagnose and Pathological Process of DCM
Long non-coding RNAs (lncRNAs) was a class longer than 200 nucleotides RNA, which without protein-coding function. lncRNAs had many epigenetic forms of regulation, including DNA methylation, histone modification and regulation of miRNA [84, 85]. lncRNAs played important roles in chromosome modification, X-chromosome silencing, genomic imprinting, transcriptional interference, transcriptional activation and intranuclear transport [86]. According to the mechanism of action of lncRNA, lncRNAs had the following functions according to the mechanisms of action: (1) Transcription occured in the upstream promoter region of the protein-coding gene, which interfered with the expression of downstream genes (e.g. SER3 gene in yeast). (2) Regulated the expression of downstream gene expression by means of RNA polymerase II inhibition or mediating chromatin remodeling and histone modification (e.g. p15AS in mice) [87]. (3) By forming complementary double-stranded with the mRNA of coding genes, lncRNAs interfered with the cleavage of RNA, thus produced different forms of cleavage. (4) By forming complementary double-stranded with transcripts of protein-coding genes, the expression level of genes was regulated by attracting endogenous siRNA through DICER. (5) By binding to specific proteins, lncRNAs transcripts could regulate the activity of corresponding proteins. (6) As a structural component, lncRNAs forms nucleic acid-protein complexes with proteins [88]. (7) By binding to a specific protein, changed the localization of this protein. (8) As a precursor molecule of small RNA, such as miRNA and piwi-interacting RNA (piRNA) [89,90,91].
As a research hotspot in the field of non-coding RNAs, the role of lncRNAs in DCM has been gradually revealed [92]. Downregulated expression of lncRNA homeobox transcript antisense RNA (HOTAIR) was detected in the heart and serum of DCM patients. Further studies have found that HOTAIR enhances the viability of cardiomyocytes by activating PI3K/Akt pathway, which may provide a possible treatment of DCM. The latest research focused on the involvement of HOTAIR in animal DCM model and the molecular mechanism. In STZ-induced mouse DCM model, specific overexpression of HOTAIR in cardiac myocytes could improve cardiac function, reduce myocardial death, inflammation and oxidative stress [93].
As one of the first reported lncRNAs, lncRNA-H19 was well studied on its biological functions and the mechanism of its interaction with other molecules [94]. The encoding gene of lncRNA-H19 and the insulin-like growth factor 2 were from the same gene cluster, indicating the potential relationship of lncRNA-H19 with metabolism and blood glucose regulation [95]. Decreased IGF2 and lncRNA-H19 in pancreatic might play a key role in the repair of islet ultrastructure and function in offspring of gestational diabetes mellitus [96]. Interestingly, there was a double negative feedback between lncRNA-H19 and its target miRNA let-7 in participating the glucose metabolism in muscle cells. More concretely, as a sponge of let-7, lncRNA-H19 was decreased in the muscle of DM patients, while let-7 was increased. lncRNA H19-miR-675-VDAC1 was recognized as the mediate axis in regulating the function of lncRNA H19 in cardiomyocytes [97]. In DCM, overexpression of lncRNA-H19 reduced inflammation and oxidative stress, protected myocardial cells from apoptosis, inhibited autophagy. The inhibitory effect of lncRNA H19 on autophagy of cardiomyocytes was mediated by its direct binding with EZH2 and restraining DIRAS3 transcription [98].
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was related to tumor cell growth invasion and metastasis. It was also recognized as a significantly upregulated lncRNA in diabetic rats. Inhibition of MALAT1 in rat heart could protect heart from DCM induced dysfunction by significant reducing the cardiomyocytes apoptosis [99]. Besides that, MALAT1 knockdown also alleviated the DCM early characteristic inflammation response [100]. The up-regulation of MALAT1 in cardiomyocytes and hearts of hyperglycemic mice could be counteracted by nitric oxide [101].
Other lncRNAs have also been found to be involved in the development of DCM. Most of the studies are concerned about the effects of lncRNA on cardiomyocyte survival, including apoptosis, pyroptosis or autophagy. Overexpression of myocardial infarction associated transcript (MIAT) was detected in the DCM mice and showed protective effect on cardiac function. Similar to other LncRNA, MIAT functioned as a competing endogenous of miR-22-3p, resulting in the activation of target gene DAPK2 [102]. Inhibition of Kcnq1ot1 reduced cardiomyocytes pyroptosis hence affecting the function of cardiac function in mice DCM model, which was also identified as a sponge lncRNA of miR-214-3p, leading to the increase of caspase-1 and IL-1β [103].
Although lncRNAs have been found to be associated with ventricular remodeling, especially cardiac fibrosis, the relationship between lncRNAs and DCM-induced myocardial fibrosis has been less discussed [104, 105]. As an cardiac fibroblasts (CFs) enriched lncRNA in heart, lncRNA Crnde (CRNDE) was significantly negatively related to cardiac fibrosis in patients with cardiac fibrosis or in DCM mice. Overexpression CRNDE could reduce the marker gene of myofibroblast expression in TGF-β induced CFs and alleviate DCM-related cardiac fibrosis. At the same time, left ventricular function was partial recovery by CRNDE overexpression through formatting a Smad3-Crnde negative feedback [106].
lncRNAs were specific markers to predict the occurrence of DCM in well-controlled type 2 diabetes patients. Circulating long intergenic non-coding RNA predicting cardiac remodeling (LIPCAR) was negatively correlated with diastolic function(E/A peak flow), while serum smooth muscle and endothelial cell-enriched migration/differentiation-associated long noncoding RNA (SENCR) were significantly related to cardiac remodeling in patient with uncomplicated type 2 diabetes. All the above motioned results demonstrated that lncRNAs was valuable tools for recognizing the cardiomyopathy at a preclinical stage, especially for the screening for the first diagnosis patients or the well-controlled patients [107].
3.2.2 CircRNAs as Potential Tools for DCM
Circular RNAs (circRNAs) were the important members of non-coding RNAs. Although circRNAs werediscovered in organisms as early as 1979, the functional research of circRNAs has been zigzag [108]. Because most of the circRNAs were derived from gene exons, researchers used probes to capture known exon sequences and then conducted in-depth sequencing. Through this way, more than 3000 circRNAs were identified in 2000 clinical samples. However, because this method retained linear RNA, the researchers also found that the expression of circRNAs has no obvious relationship with the number of RNA produced by parent gene. Thus, the change of expression of cyclic RNA could not be simply attributed to the change of the expression of the mother gene. It might contain more complex generation and regulation mechanisms. In the past 10 years, the function of circRNAs has been gradually revealed. circRNAs were implicated in the development of metabolic disease and cardiovascular disease such as coronary heart disease, pathological cardiac hypertrophy and cardiac remodeling [109, 110]. Studies described the circRNAs expression pattern in human endothelial cells after high glucose stimulation. 95 circRNAs different expressed were observed in different group, which may be involved in the process of the endothelial dysfunction in diabetes mellitus. But unfortunately, there was no research to explain the role of a specific circRNA in the pathogenesis of DCM.
4 Conclusion
Different models will have certain influence during the process of studying the pathogenesis of DCM. TIDM and T2DM have the greatest impact on cardiomyopathy. Although both of them involve oxidative stress, inflammation, cardiomyocyte apoptosis and high glucose stimulation induced hypertrophy, the role of insulin resistance could not be ignored [111]. In addition, high fat and lipid metabolism disorders also played a role in the pathogenesis of DCM, which needed further clarification. Considering the current research situation of the relationship between non-codingRNAs and DCM, there were still many valuable problems need to be further discussed.
Based on the results of the current study, there was no doubt that miRNAs could regulate DCM. This regulation ran through the entire process of DCM from initial to advanced heart failure. DCM shared a common pathological process with many cardiac injury diseases. High glucose and oxidative stress damaged cardiomyocytes in early stage of disease, which leading to apoptosis or even necrosis of cardiomyocytes. At the late stage of DCM induced heart failure, the main pathological manifestations were cardiac hypertrophy and ventricular remodeling. In addition, metabolic changes in the heart were closely related to DCM, including abnormal glycometabolism and lipid metabolism, which was also related to the discovery of miRNAs function in the above pathological processes. For example, the miR-155 described above was a classical metabolic syndrome-related miRNA [112, 113]. MiR-133a was a muscle-specific miRNA, and its association with cardiac fibrosis has been reported already. These conclusions were confirmed in the cardiac fibrosis that was induced by DCM. We made it clear that these miRNAs were closely related to DCM. However, almost no miRNA was obtained that was specific related to DCM. Indicated that no breakthrough has been made either in solving the specific target of miRNAs treatment or in finding specific markers again. According to the classical definition, non-coding RNA was a kind of RNA without coding function in transcriptome. However, recent studies found that some non-coding RNAs or its precursors can also be translated [114, 115]. This brought more possibilities in exploring the function and mechanism of non-coding RNA in human diseases. The functions of circRNAs and its relationship with diseases were the hotspots of current research, especially after the redefinition of circRNA as a functional RNA. The potential of circRNAs was not only embodied in its subunits, but also in its coding and homeopathic regulation of nearby genes [116].
However, the research on non-coding RNAs as a biomarker of DCM was relatively rare, which may be due to the following reasons: (1) In clinical, compared with other diseases such as myocardial infarction, the diagnosis of diabetic cardiomyopathy often required comprehensive clinical history, cardiac ultrasonography and other results to diagnose. Also, fewer cases of definite diagnosis were obtained. (2) Diagnosed patients have often entered the stage of heart failure, that too many confounding factors existed. (3) DCM was mostly developed on the basis of type 2 diabetes mellitus in clinical patients. However, more attention has been paid to type 1 diabetes mellitus induced DCM in experimental research, which was different between clinical and basic research.
In addition, conservativeness of non-coding RNAs among species was also one of the bottlenecks to its application in clinical treatment in the future. This made it more difficult for the transformation from animal model to clinical practice. Therefore, a small amount of non-coding RNAs, which was found to be highly conservative among species, could be the target of future therapeutic strategies developing.
In conclusion, the pathogenesis of DCM is not completely clear at present. The research on the relationship between non-coding RNAs and DCM has revealed the strong role of non-coding RNAs in the pathogenesis of DCM. Research on this direction is a meaningful perspective to explore the pathogenesis of DCM in the future.
References
Tao L, Shi J, Yang X, Yang L, Hua F. The exosome: a new player in diabetic cardiomyopathy. J Cardiovasc Transl Res. 2019;12(1):62–7.
Ernande L, Audureau E, Jellis CL, Bergerot C, Henegar C, Sawaki D, Czibik G, Volpi C, Canoui-Poitrine F, Thibault H, Ternacle J, Moulin P, Marwick TH, Derumeaux G. Clinical implications of echocardiographic phenotypes of patients with diabetes mellitus. J Am Coll Cardiol. 2017;70(14):1704–16.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602.
Rawshani A, Rawshani A, Sattar N, Franzen S, McGuire DK, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, Rosengren A, Gudbjornsdottir S. Relative prognostic importance and optimal levels of risk factors for mortality and cardiovascular outcomes in type 1 diabetes mellitus. Circulation. 2019;139(16):1900–12.
Armstrong AC, Ambale-Venkatesh B, Turkbey E, Donekal S, Chamera E, Backlund JY, Cleary P, Lachin J, Bluemke DA, Lima JA, Group DER. Association of Cardiovascular Risk Factors and Myocardial Fibrosis with Early Cardiac Dysfunction in type 1 diabetes: the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2017;40(3):405–11.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC, Jr., Virani SS, Williams KA, Sr., Yeboah J, Ziaeian B (2019) 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation:CIR0000000000000678.
Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P, Mao X, Huang K, Xie Z, Zou MH. Hyperglycemia-driven inhibition of AMP-activated protein kinase alpha2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation. 2019;139(16):1913–36.
Dillmann WH. Diabetic cardiomyopathy. Circ Res. 2019;124(8):1160–2.
Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124(1):121–41.
Palomer X, Pizarro-Delgado J, Vazquez-Carrera M. Emerging actors in diabetic cardiomyopathy: heartbreaker biomarkers or therapeutic targets? Trends Pharmacol Sci. 2018;39(5):452–67.
Aaron CP, Tandri H, Barr RG, Johnson WC, Bagiella E, Chahal H, Jain A, Kizer JR, Bertoni AG, Lima JA, Bluemke DA, Kawut SM. Physical activity and right ventricular structure and function. The MESA-right ventricle study. Am J Respir Crit Care Med. 2011;183(3):396–404.
Redberg RF, Greenland P, Fuster V, Pyorala K, Blair SN, Folsom AR, Newman AB, O’Leary DH, Orchard TJ, Psaty B, Schwartz JS, Starke R, Wilson PW. Prevention conference VI: diabetes and cardiovascular disease: writing group III: risk assessment in persons with diabetes. Circulation. 2002;105(18):e144–52.
Song G, Chen C, Zhang J, Chang L, Zhu D, Wang X. Association of traditional Chinese exercises with glycemic responses in people with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. J Sport Health Sci. 2018;7(4):442–52.
Le S, Mao L, Lu D, Yang Y, Tan X, Wiklund P, Cheng S. Effect of aerobic exercise on insulin resistance and central adiposity disappeared after the discontinuation of intervention in overweight women. J Sport Health Sci. 2016;5(2):166–70.
Li L, Zhang S, Dobson J. The contribution of small and large sensory afferents to postural control in patients with peripheral neuropathy. J Sport Health Sci. 2019;8(3):218–27.
Barakat GM, Nuwayri-Salti N, Kadi LN, Bitar KM, Al-Jaroudi WA, Bikhazi AB. Role of glucagon-like peptide-1 and its agonists on early prevention of cardiac remodeling in type 1 diabetic rat hearts. Gen Physiol Biophys. 2011;30(1):34–44.
Cryer MJ, Horani T, DiPette DJ. Diabetes and hypertension: a comparative review of current guidelines. J Clin Hypertens (Greenwich). 2016;18(2):95–100.
Suhara T, Baba Y, Shimada BK, Higa JK, Matsui T. The mTOR Signaling pathway in myocardial dysfunction in type 2 diabetes mellitus. Curr Diab Rep. 2017;17(6):38.
Bar L, Feger M, Fajol A, Klotz LO, Zeng S, Lang F, Hocher B, Foller M. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc Natl Acad Sci U S A. 2018;115(22):5804–9.
Wang Q, Ren J. mTOR-independent autophagy inducer trehalose rescues against insulin resistance-induced myocardial contractile anomalies: role of p38 MAPK and Foxo1. Pharmacol Res. 2016;111:357–73.
Kumphune S, Chattipakorn S, Chattipakorn N. Roles of p38-MAPK in insulin resistant heart: evidence from bench to future bedside application. Curr Pharm Biotechnol. 2013;19(32):5742–54.
Ginion A, Auquier J, Benton CR, Mouton C, Vanoverschelde JL, Hue L, Horman S, Beauloye C, Bertrand L. Inhibition of the mTOR/p70S6K pathway is not involved in the insulin-sensitizing effect of AMPK on cardiac glucose uptake. Am J Phys Heart Circ Phys. 2011;301(2):H469–77.
Schwenk RW, Angin Y, Steinbusch LK, Dirkx E, Hoebers N, Coumans WA, Bonen A, Broers JL, van Eys GJ, Glatz JF, Luiken JJ. Overexpression of vesicle-associated membrane protein (VAMP) 3, but not VAMP2, protects glucose transporter (GLUT) 4 protein translocation in an in vitro model of cardiac insulin resistance. J Biol Chem. 2012;287(44):37530–9.
Shao Y, Chernaya V, Johnson C, Yang WY, Cueto R, Sha X, Zhang Y, Qin X, Sun J, Choi ET, Wang H, Yang XF. Metabolic diseases downregulate the majority of histone modification enzymes, making a few Upregulated enzymes novel therapeutic targets—“sand out and gold stays”. J Cardiovasc Transl Res. 2016;9(1):49–66.
Gu J, Yan X, Dai X, Wang Y, Lin Q, Xiao J, Zhou S, Zhang J, Wang K, Zeng J, Xin Y, Barati MT, Zhang C, Bai Y, Li Y, Epstein PN, Wintergerst KA, Li X, Tan Y, Cai L. Metallothionein preserves Akt2 activity and cardiac function via inhibiting TRB3 in diabetic hearts. Diabetes. 2018;67(3):507–17.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Yin Y, Zheng Z, Jiang Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed Pharmacother. 2019;109:2070–7.
Pei Z, Deng Q, Babcock SA, He EY, Ren J, Zhang Y. Inhibition of advanced glycation endproduct (AGE) rescues against streptozotocin-induced diabetic cardiomyopathy: role of autophagy and ER stress. Toxicol Lett. 2018;284:10–20.
Saleh A, Smith DR, Tessler L, Mateo AR, Martens C, Schartner E, Van der Ploeg R, Toth C, Zochodne DW, Fernyhough P. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Exp Neurol. 2013;249:149–59.
Fang YH, Piao L, Hong Z, Toth PT, Marsboom G, Bache-Wiig P, Rehman J, Archer SL. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle’s cycle. J Mol Med. 2012;90(1):31–43.
Bergman HM, Lindfors L, Palm F, Kihlberg J, Lanekoff I. Metabolite aberrations in early diabetes detected in rat kidney using mass spectrometry imaging. Anal Bioanal Chem. 2019;411(13):2809–16.
He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA, Yang Q. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012;126(14):1705–16.
Feuerstein GZ. Apoptosis in cardiac diseases--new opportunities for novel therapeutics for heart diseases. Cardiovasc Drugs Ther. 1999;13(4):289–94.
Simon JN, Chowdhury SA, Warren CM, Sadayappan S, Wieczorek DF, Solaro RJ, Wolska BM. Ceramide-mediated depression in cardiomyocyte contractility through PKC activation and modulation of myofilament protein phosphorylation. Basic Res Cardiol. 2014;109(6):445.
Ilatovskaya DV, Blass G, Palygin O, Levchenko V, Pavlov TS, Grzybowski MN, Winsor K, Shuyskiy LS, Geurts AM, Cowley AW Jr, Birnbaumer L, Staruschenko A. A NOX4/TRPC6 pathway in Podocyte calcium regulation and renal damage in diabetic kidney disease. J Am Soc Nephrol. 2018;29(7):1917–27.
Liu Y, Steinbusch LKM, Nabben M, Kapsokalyvas D, van Zandvoort M, Schonleitner P, Antoons G, Simons PJ, Coumans WA, Geomini A, Chanda D, Glatz JFC, Neumann D, Luiken J. Palmitate-induced Vacuolar-type H(+)-ATPase inhibition feeds forward into insulin resistance and contractile dysfunction. Diabetes. 2017;66(6):1521–34.
Bugger H, Riehle C, Jaishy B, Wende AR, Tuinei J, Chen D, Soto J, Pires KM, Boudina S, Theobald HA, Luptak I, Wayment B, Wang X, Litwin SE, Weimer BC, Abel ED. Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. J Mol Cell Cardiol. 2012;52(5):1019–26.
Hegab Z, Mohamed TMA, Stafford N, Mamas M, Cartwright EJ, Oceandy D. Advanced glycation end products reduce the calcium transient in cardiomyocytes by increasing production of reactive oxygen species and nitric oxide. FEBS Open Bio. 2017;7(11):1672–85.
Yan D, Luo X, Li Y, Liu W, Deng J, Zheng N, Gao K, Huang Q, Liu J. Effects of advanced glycation end products on calcium handling in cardiomyocytes. Cardiology. 2014;129(2):75–83.
Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, Ikeda S, Shirakabe A, Sadoshima J. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res. 2019;124(9):1360–71.
Liu F, Song R, Feng Y, Guo J, Chen Y, Zhang Y, Chen T, Wang Y, Huang Y, Li CY, Cao C, Zhang Y, Hu X, Xiao RP. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor alpha. Circulation. 2015;131(9):795–804.
Pedersen MT, Vorup J, Bangsbo J. Effect of a 26-month floorball training on male elderly’s cardiovascular fitness, glucose control, body composition, and functional capacity. J Sport Health Sci. 2018;7(2):149–58.
Rezende F, Prior KK, Lowe O, Wittig I, Strecker V, Moll F, Helfinger V, Schnutgen F, Kurrle N, Wempe F, Walter M, Zukunft S, Luck B, Fleming I, Weissmann N, Brandes RP, Schroder K. Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radic Biol Med. 2017;102:57–66.
Elbatreek MH, Pachado MP, Cuadrado A, Jandeleit-Dahm K, Schmidt H. Reactive oxygen comes of age: mechanism-based therapy of diabetic end-organ damage. Trends Endocrinol Metab. 2019;30(5):312–27.
Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31(22):2741–8.
Yang YC, Tsai CY, Chen CL, Kuo CH, Hou CW, Cheng SY, Aneja R, Huang CY, Kuo WW. Pkcdelta activation is involved in ROS-mediated mitochondrial dysfunction and apoptosis in Cardiomyocytes exposed to advanced Glycation end products (Ages). Aging Dis. 2018;9(4):647–63.
Rajamani U, Essop MF. Hyperglycemia-mediated activation of the hexosamine biosynthetic pathway results in myocardial apoptosis. Am J Phys Cell Phys. 2010;299(1):C139–47.
Adameova A, Dhalla NS. Role of microangiopathy in diabetic cardiomyopathy. Heart Fail Rev. 2014;19(1):25–33.
McGrath GM, McNeill JH. Cardiac ultrastructural changes in streptozotocin-induced diabetic rats: effects of insulin treatment. Can J Cardiol. 1986;2(3):164–9.
Yuan J, Tan JTM, Rajamani K, Solly EL, King EJ, Lecce L, Simpson PJL, Lam YT, Jenkins AJ, Bursill CA, Keech AC, Ng MKC. Fenofibrate rescues diabetes-related impairment of ischemia-mediated angiogenesis by PPARalpha-independent modulation of Thioredoxin-interacting protein. Diabetes. 2019;68(5):1040–53.
Niu C, Chen Z, Kim KT, Sun J, Xue M, Chen G, Li S, Shen Y, Zhu Z, Wang X, Liang J, Jiang C, Cong W, Jin L, Li X. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy. 2019;15(5):843–70.
Kjobsted R, Roll JLW, Jorgensen NO, Birk JB, Foretz M, Viollet B, Chadt A, Al-Hasani H, Wojtaszewski JFP. AMPK and TBC1D1 regulate muscle glucose uptake after – but not during – exercise and contraction. Diabetes. 2019;292(5):1308–17.
Yoon KJ, Zhang D, Kim SJ, Lee MC, Moon HY. Exercise-induced AMPK activation is involved in delay of skeletal muscle senescence. Biochem Biophys Res Commun. 2019;512(3):604–10.
Sanchez-Lopez E, Zhong Z, Stubelius A, Sweeney SR, Booshehri LM, Antonucci L, Liu-Bryan R, Lodi A, Terkeltaub R, Lacal JC, Murphy AN, Hoffman HM, Tiziani S, Guma M, Karin M. Choline uptake and metabolism modulate macrophage IL-1beta and IL-18 production. Cell Metab. 2019;29(6):1350–1362.e7.
Zhao H, Li T, Wang K, Zhao F, Chen J, Xu G, Zhao J, Li T, Chen L, Li L, Xia Q, Zhou T, Li HY, Li AL, Finkel T, Zhang XM, Pan X. AMPK-mediated activation of MCU stimulates mitochondrial Ca(2+) entry to promote mitotic progression. Nat Cell Biol. 2019;21(4):476–86.
Wang Y, Xiong H, Liu D, Hill C, Ertay A, Li J, Zou Y, Miller P, White E, Downward J, Goldin RD, Yuan X, Lu X. Autophagy inhibition specifically promotes epithelial-mesenchymal transition and invasion in RAS-mutated cancer cells. Autophagy. 2019;15(5):886–99.
Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60(6):1770–8.
Wang L, Lv Y, Li G, Xiao J. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci. 2018;7(4):433–41.
Dragomir MP, Knutsen E, Calin GA. SnapShot: unconventional miRNA functions. Cell. 2018;174(4):1038–1038.e1031.
Diao X, Shen E, Wang X, Hu B. Differentially expressed microRNAs and their target genes in the hearts of streptozotocin-induced diabetic mice. Mol Med Rep. 2011;4(4):633–40.
Yildirim SS, Akman D, Catalucci D, Turan B. Relationship between downregulation of miRNAs and increase of oxidative stress in the development of diabetic cardiac dysfunction: junctin as a target protein of miR-1. Cell Biochem Biophys. 2013;67(3):1397–408.
Raut SK, Kumar A, Singh GB, Nahar U, Sharma V, Mittal A, Sharma R, Khullar M. miR-30c mediates upregulation of Cdc42 and Pak1 in diabetic cardiomyopathy. Cardiovasc Drugs Ther. 2015;33(3):89–97.
Yin Z, Zhao Y, He M, Li H, Fan J, Nie X, Yan M, Chen C, Wang DW. MiR-30c/PGC-1beta protects against diabetic cardiomyopathy via PPARalpha. Cardiovasc Diabetol. 2019;18(1):7.
Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, Youker KA, Kishore R, Krishnamurthy P. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun. 2016;471(4):423–9.
Zheng D, Ma J, Yu Y, Li M, Ni R, Wang G, Chen R, Li J, Fan GC, Lacefield JC, Peng T. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia. 2015;58(8):1949–58.
Bussing I, Slack FJ, Grosshans H. Let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14(9):400–9.
Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Consortium D, Investigators M, Altshuler D, Daley GQ. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94.
Dubinsky AN, Dastidar SG, Hsu CL, Zahra R, Djakovic SN, Duarte S, Esau CC, Spencer B, Ashe TD, Fischer KM, MacKenna DA, Sopher BL, Masliah E, Gaasterland T, Chau BN, Pereira de Almeida L, Morrison BE, La Spada AR. Let-7 coordinately suppresses components of the amino acid sensing pathway to repress mTORC1 and induce autophagy. Cell Metab. 2014;20(4):626–38.
Frost RJ, Olson EN. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc Natl Acad Sci U S A. 2011;108(52):21075–80.
Seeger T, Fischer A, Muhly-Reinholz M, Zeiher AM, Dimmeler S. Long-term inhibition of miR-21 leads to reduction of obesity in db/db mice. Obesity (Silver Spring). 2014;22(11):2352–60.
Liu S, Li W, Xu M, Huang H, Wang J, Chen X. Micro-RNA 21Targets dual specific phosphatase 8 to promote collagen synthesis in high glucose-treated primary cardiac fibroblasts. Am J Cardiol. 2014;30(12):1689–99.
Nandi SS, Duryee MJ, Shahshahan HR, Thiele GM, Anderson DR, Mishra PK. Induction of autophagy markers is associated with attenuation of miR-133a in diabetic heart failure patients undergoing mechanical unloading. Am J Transl Res. 2015;7(4):683–96.
Chen S, Puthanveetil P, Feng B, Matkovich SJ, Dorn GW 2nd, Chakrabarti S. Cardiac miR-133a overexpression prevents early cardiac fibrosis in diabetes. J Cell Mol Med. 2014;18(3):415–21.
Nandi SS, Shahshahan HR, Shang Q, Kutty S, Boska M, Mishra PK. MiR-133a mimic alleviates T1DM-induced systolic dysfunction in Akita: an MRI-based study. Front Physiol. 2018;9:1275.
Blumensatt M, Greulich S, Herzfeld de Wiza D, Mueller H, Maxhera B, Rabelink MJ, Hoeben RC, Akhyari P, Al-Hasani H, Ruige JB, Ouwens DM. Activin a impairs insulin action in cardiomyocytes via up-regulation of miR-143. Cardiovasc Res. 2013;100(2):201–10.
Kuwabara Y, Horie T, Baba O, Watanabe S, Nishiga M, Usami S, Izuhara M, Nakao T, Nishino T, Otsu K, Kita T, Kimura T, Ono K. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ Res. 2015;116(2):279–88.
Miao Y, Wan Q, Liu X, Wang Y, Luo Y, Liu D, Lin N, Zhou H, Zhong J. miR-503 is involved in the protective effect of phase II enzyme inducer (CPDT) in diabetic cardiomyopathy via Nrf2/ARE Signaling pathway. Biomed Res Int. 2017;2017:9167450.
Virtue A, Johnson C, Lopez-Pastrana J, Shao Y, Fu H, Li X, Li YF, Yin Y, Mai J, Rizzo V, Tordoff M, Bagi Z, Shan H, Jiang X, Wang H, Yang XF. MicroRNA-155 deficiency leads to decreased atherosclerosis, increased White adipose tissue obesity, and non-alcoholic fatty liver disease: a NOVEL MOUSE MODEL OF OBESITY PARADOX. J Biol Chem. 2017;292(4):1267–87.
Johnson C, Ct D, Virtue A, Gao T, Wu S, Hernandez M, Singh L, Wang H, Yang XF. Increased expression of Resistin in MicroRNA-155-deficient White adipose tissues may be a possible driver of metabolically healthy obesity transition to classical obesity. Front Physiol. 2018;9:1297.
Wang L, Zhang N, Wang Z, Ai DM, Cao ZY, Pan HP. Decreased MiR-155 level in the peripheral blood of non-alcoholic fatty liver disease patients may serve as a biomarker and may influence LXR activity. Vitro Cell Dev Biol Plant. 2016;39(6):2239–48.
Miller AM, Gilchrist DS, Nijjar J, Araldi E, Ramirez CM, Lavery CA, Fernandez-Hernando C, McInnes IB, Kurowska-Stolarska M. MiR-155 has a protective role in the development of non-alcoholic hepatosteatosis in mice. PLoS One. 2013;8(8):e72324.
Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SL, Hazebroek M, van Leeuwen R, Gijbels MJ, Wijnands E, Biessen EA, De Winther MP, Stassen FR, Carmeliet P, Kauppinen S, Schroen B, Heymans S. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res. 2012;111(4):415–25.
Jia C, Chen H, Wei M, Chen X, Zhang Y, Cao L, Yuan P, Wang F, Yang G, Ma J. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int J Nanomedicine. 2017;12:4963–79.
Li D, Kular L, Vij M, Herter EK, Li X, Wang A, Chu T, Toma MA, Zhang L, Liapi E, Mota A, Blomqvist L, Serezal IG, Rollman O, Wikstrom JD, Bienko M, Berglund D, Stahle M, Sommar P, Jagodic M, Landen NX. Human skin long noncoding RNA WAKMAR1 regulates wound healing by enhancing keratinocyte migration. Proc Natl Acad Sci U S A. 2019;116(19):9443–52.
Cheedipudi SM, Matkovich SJ, Coarfa C, Hu X, Robertson MJ, Sweet M, Taylor M, Mestroni L, Cleveland J, Willerson JT, Gurha P, Marian AJ. Genomic reorganization of Lamin-associated domains in cardiac Myocytes is associated with differential gene expression and DNA methylation in human dilated cardiomyopathy. Circ Res. 2019;124(8):1198–213.
Wang CY, Jegu T, Chu HP, Oh HJ, Lee JT. SMCHD1 merges chromosome compartments and assists formation of super-structures on the inactive X. Cell. 2018;174(2):406–21.. e425
Lin N, Chang KY, Li Z, Gates K, Rana ZA, Dang J, Zhang D, Han T, Yang CS, Cunningham TJ, Head SR, Duester G, Dong PD, Rana TM. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol Cell. 2014;53(6):1005–19.
Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S, Jiang Z, Xu J, Liu Q, Cao X. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344(6181):310–3.
Xu M, Chen X, Lin K, Zeng K, Liu X, Pan B, Xu X, Xu T, Hu X, Sun L, He B, Pan Y, Sun H, Wang S. The long noncoding RNA SNHG1 regulates colorectal cancer cell growth through interactions with EZH2 and miR-154-5p. Mol Cancer. 2018;17(1):141.
Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494–504.
Mohammad F, Pandey RR, Nagano T, Chakalova L, Mondal T, Fraser P, Kanduri C. Kcnq1ot1/Lit1 noncoding RNA mediates transcriptional silencing by targeting to the perinucleolar region. Mol Cell Biol. 2008;28(11):3713–28.
Ma C, Luo H, Liu B, Li F, Tschope C, Fa X. Long noncoding RNAs: a new player in the prevention and treatment of diabetic cardiomyopathy? Diabetes Metab Res Rev. 2018;34(8):e3056.
Gao L, Wang X, Guo S, Xiao L, Liang C, Wang Z, Li Y, Liu Y, Yao R, Liu Y, Zhang Y. LncRNA HOTAIR functions as a competing endogenous RNA to upregulate SIRT1 by sponging miR-34a in diabetic cardiomyopathy. J Cell Physiol. 2019;234(4):4944–58.
Liu R, Li X, Zhu W, Wang Y, Zhao D, Wang X, Gurley EC, Liang G, Chen W, Lai G, Pandak WM, Lippman HR, Bajaj JS, Hylemon PB, Zhou H. Cholangiocyte-derived exosomal LncRNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis. Hepatology. 2019;70(4):1317–35.
Tarnowski M, Tkacz M, Czerewaty M, Poniewierska-Baran A, Grymula K, Ratajczak MZ. 5Azacytidine inhibits human rhabdomyosarcoma cell growth by downregulating insulinlike growth factor 2 expression and reactivating the H19 gene product miR675, which negatively affects insulinlike growth factors and insulin signaling. Int J Oncol. 2015;46(5):2241–50.
Ding GL, Wang FF, Shu J, Tian S, Jiang Y, Zhang D, Wang N, Luo Q, Zhang Y, Jin F, Leung PC, Sheng JZ, Huang HF. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61(5):1133–42.
Li X, Wang H, Yao B, Xu W, Chen J, Zhou X. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Sci Rep. 2016;6:36340.
Zhuo C, Jiang R, Lin X, Shao M. LncRNA H19 inhibits autophagy by epigenetically silencing of DIRAS3 in diabetic cardiomyopathy. Oncotarget. 2017;8(1):1429–37.
Zhang M, Gu H, Xu W, Zhou X. Down-regulation of lncRNA MALAT1 reduces cardiomyocyte apoptosis and improves left ventricular function in diabetic rats. Int J Cardiol. 2016;203:214–6.
Zhang M, Gu H, Chen J, Zhou X. Involvement of long noncoding RNA MALAT1 in the pathogenesis of diabetic cardiomyopathy. Int J Cardiol. 2016;202:753–5.
Bacci L, Barbati SA, Colussi C, Aiello A, Isidori AM, Grassi C, Pontecorvi A, Farsetti A, Gaetano C, Nanni S. Sildenafil normalizes MALAT1 level in diabetic cardiomyopathy. Endocr Rev. 2018;62(1):259–62.
Zhou X, Zhang W, Jin M, Chen J, Xu W, Kong X. lncRNA MIAT functions as a competing endogenous RNA to upregulate DAPK2 by sponging miR-22-3p in diabetic cardiomyopathy. Cell Death Dis. 2017;8(7):e2929.
Yang F, Qin Y, Wang Y, Li A, Lv J, Sun X, Che H, Han T, Meng S, Bai Y, Wang L. LncRNA KCNQ1OT1 mediates Pyroptosis in diabetic cardiomyopathy. Cell Physiol Biochem. 2018;50(4):1230–44.
Qu X, Du Y, Shu Y, Gao M, Sun F, Luo S, Yang T, Zhan L, Yuan Y, Chu W, Pan Z, Wang Z, Yang B, Lu Y. MIAT is a pro-fibrotic Long non-coding RNA governing cardiac fibrosis in post-infarct myocardium. Sci Rep. 2017;7:42657.
Chen Z, Li C, Lin K, Cai H, Ruan W, Han J, Rao L. Non-coding RNAs in cardiac fibrosis: emerging biomarkers and therapeutic targets. Am J Cardiol. 2018;25(6):732–41.
Zheng D, Zhang Y, Hu Y, Guan J, Xu L, Xiao W, Zhong Q, Ren C, Lu J, Liang J, Hou J. Long noncoding RNA Crnde attenuates cardiac fibrosis via Smad3-Crnde negative feedback in diabetic cardiomyopathy. FEBS J. 2019;286(9):1645–55.
de Gonzalo-Calvo D, Kenneweg F, Bang C, Toro R, van der Meer RW, Rijzewijk LJ, Smit JW, Lamb HJ, Llorente-Cortes V, Thum T. Circulating long-non coding RNAs as biomarkers of left ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci Rep. 2016;6:37354.
Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280(5720):339–40.
Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, Zhou LY, Sun T, Wang M, Yu T, Gong Y, Liu J, Dong YH, Li N, Li PF. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37(33):2602–11.
Gomes CPC, Salgado-Somoza A, Creemers EE, Dieterich C, Lustrek M, Devaux Y, Cardiolinc network. Circular RNAs in the cardiovascular system. Noncoding RNA Res. 2018;3(1):1–11.
Leonska-Duniec A, Jastrzebski Z, Zarebska A, Maciejewska A, Ficek K, Cieszczyk P. Assessing effect of interaction between the FTO A/T polymorphism (rs9939609) and physical activity on obesity-related traits. J Sport Health Sci. 2018;7(4):459–64.
Mahdavi R, Ghorbani S, Alipoor B, Panahi G, Khodabandehloo H, Esfahani EN, Razi F, Meshkani R. Decreased serum level of miR-155 is associated with obesity and its related metabolic traits. Clin Lab Med. 2018;64(1):77–84.
Bacci M, Giannoni E, Fearns A, Ribas R, Gao Q, Taddei ML, Pintus G, Dowsett M, Isacke CM, Martin LA, Chiarugi P, Morandi A. miR-155 drives metabolic reprogramming of ER+ breast Cancer cells following Long-term Estrogen deprivation and predicts clinical response to aromatase inhibitors. Cancer Res. 2016;76(6):1615–26.
Lauressergues D, Couzigou JM, Clemente HS, Martinez Y, Dunand C, Becard G, Combier JP. Primary transcripts of microRNAs encode regulatory peptides. Nature. 2015;520(7545):90–3.
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CC, Xiao X, Wang Z. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–41.
Liu CX, Li X, Nan F, Jiang S, Gao X, Guo SK, Xue W, Cui Y, Dong K, Ding H, Qu B, Zhou Z, Shen N, Yang L, Chen LL. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell. 2019;177(4):865–80.
Competing Financial Interests
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Xia, L., Song, M. (2020). Role of Non-coding RNA in Diabetic Cardiomyopathy. In: Xiao, J. (eds) Non-coding RNAs in Cardiovascular Diseases. Advances in Experimental Medicine and Biology, vol 1229. Springer, Singapore. https://doi.org/10.1007/978-981-15-1671-9_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-1671-9_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1670-2
Online ISBN: 978-981-15-1671-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)