Abstract
Diabetic cardiomyopathy (DCM) or diabetes-induced cardiac dysfunction is a direct consequence of uncontrolled metabolic syndrome and occurs worldwide. However, the underlying cellular and molecular mechanisms remain poorly understood. Recently, exosomes have attracted considerable interest for their use as efficient, targeted, and non-immunogenic delivery systems for biological molecules or pharmacotherapies. This review will summarize the fast-developing field of the regulation and function of exosomes in DCM, affording valuable insights and therapeutic opportunities in combatting diabetes-related cardiac disorder for modern human health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM), presenting as a metabolic dysfunction, is a disorder in which either the insulin secretion of pancreatic β islet cells is impaired (type 1 diabetes mellitus, T1DM), insulin resistance is present (type 2 diabetes mellitus, T2DM), or there is a combination of the two. Currently, the uncontrolled rapid growth of DM represents a global burden. It is well established that patients with DM, in particular T2DM, are more than twice as likely to develop cardiovascular complications, including atherosclerosis, stroke, and coronary artery disease [1]. Except for large vessel injuries, heart failure occurring in diabetic patients with microangiopathy, referred to as diabetic cardiomyopathy (DCM), is also the leading cause of death in diabetic patients [2]. Currently, numerous molecular mechanisms have been proposed to contribute to the development of DCM following various animal models of T1DM and T2DM, including altered insulin signaling, oxidative stress, inflammation, apoptosis/necrosis, autophagy, mitochondrial dysfunction, lipotoxicity, impaired Ca2+ handling, fibrosis, increased fatty acid utilization, and miRNAs [3,4,5,6,7,8]. These potential mechanisms have been widely proposed and studied, and some interventions have suggested beneficial effects on associated pathological features of DCM in clinical patients [3, 5, 7]. However, some mechanisms, such as miRNAs and autophagy, remain unclear and need further investigation. Thus, a crucial need remains to delineate the basic mechanisms of DCM and to translate promising strategies to clinical interventions.
Extracellular vesicles (EVs) are small vesicles (50 nm to 2 μm) released from the surface of diverse cell types into different body fluids including plasma, saliva, milk, tears, semen, and urine [9]. EVs include nanovesicles, microvesicles, and apoptotic bodies, which are produced by different cells and mechanisms. The term “exosome” was first used to describe submicron-sized lipid vesicles released from cells in 1981 [10]. Exosomes (50–100 nm), initially thought to be small nanovesicles by which maturating sheep reticulocytes discard obsolete cellular components [11], now viewed as a homogenous population of EVs that are released by numerous cell types including cardiomyocytes, cardiac fibroblasts, endothelial cells, inflammatory cells, and resident stem cells [12] upon fusion of multivesicular bodies with cell membranes [13]. Growing evidence has shown that exosomes play essential roles not only in human physiology and homeostasis but also in the pathogenesis of human diseases including DCM by carrying different molecules including lipids, mRNA, miRNA, membrane-bound proteins, and other regulatory RNAs [14, 15]. Exosomes are therefore regarded as a promising therapeutic approach for DCM.
In this review, we provide an introduction to DCM and review existing research on the role of exosomes in DCM, thus identifying potential future research themes and highlight the potential therapeutic strategies for DCM.
Diabetic Cardiomyopathy
DCM in humans is characterized by abnormal diastolic function along with subtle changes in systolic function such as reduced longitudinal fiber contractility. Pathologically, DCM is associated with endothelial dysfunction, myocyte hypertrophy, necrosis, apoptosis, and increased deposition of fibrosis in the interstitial regions [16]. Actually, in the early stage of diabetes, high-glucose levels in the bloodstream can result in endothelial dysfunction and microvascular rarefaction, and impaired endothelial cells (ECs) can lead to cardiomyocyte apoptosis and myocardial contractile disorder. Moreover, damaged cardiomyocytes in turn further deteriorate insufficient myocardial angiogenesis, finally giving rise to heart failure. Therefore, intermediators mediating the crosstalk between cardiomyocytes and ECs in hyperglycemia are of significant importance [17].
Exosomes and Diabetic Cardiomyopathy
Exosomes Released from Diabetic Cardiomyocytes Induce Detrimental Effects
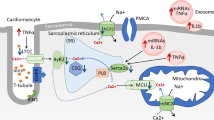
Studies have recently shown that exosomes can induce either detrimental or beneficial effects on myocardium, depending on the contents enclosed [18, 19]. A study by Wang et al. demonstrated that exosomes released from diabetic cardiomyocytes (induced by a streptozotocin (STZ)-mediated type 1 diabetes model in vivo and a high glucose-induced cardiomyocyte injury model in vitro) carry detrimental components that are able to initiate a cascade of cardiovascular complications, including ventricular dysfunction, cardiac fibrosis, and cardiomyocyte apoptosis [20]. Neighboring cells, such as ECs, receive these detrimental effect signals, leading to impaired angiogenesis. HSP20, a chaperone protein from the heat-shock protein (HSP) family that plays an essential role in cellular intrinsic defense, may induce the production of exosomes in cardiomyocytes by directly targeting Tsg101 [20]. Moreover, GW4869, an inhibitor that blocks the release of exosomes from cardiac cells in vivo, preserved cardiac function and endothelial angiogenesis in a STZ-induced type 1 DCM model [20]. In addition to T1DM, exosomes released from cardiomyocytes in adult Goto-Kakizaki (GK) rats, a commonly used animal model of T2DM, also exhibited impaired angiogenesis by inhibiting EC proliferation, migration, and tube-like formation. Exosomes derived from diabetic cardiomyocytes were transferred to ECs, leading to upregulation of miR-320 and downregulation of IGF-1, HSP20, and Ets2 [21].
In addition to impaired cardiac function and vascular formation, exosomes released from diabetic cardiomyocytes also have injurious effects on embryonic development. Research by Shi et al. documented that exosomes released from heart blood in diabetic pregnant mice (induced by intraperitoneal injection of STZ) induced a systemic and significant developmental deficiency, especially increased risk of congenital heart defects (CHDs). By using a combinatory application of labeled exosomes and nanoscale gold particles, exosomes were confirmed to cross the maternal- fetal barrier as shown by their presence in the fetus and placenta. More importantly, exosomes derived from a diabetic pregnant mice could cause a high incidence of embryonic deformity by tail vein injection into a normal pregnant mice. RNA-sequencing analysis of exosomal miRNA in a diabetic pregnant mice demonstrated that 218 miRNAs were significantly differentially expressed; 126 miRNAs were upregulated, and 92 miRNAs were downregulated from their levels in wild-type mice. Among these miRNAs, miR-122-5p, miR-192-5p, miR-99a-5p, miR-328-3p, miR-423-3p, and miR-133a-5p were significantly increased, and miR-423-5p, miR-23a-3p, miR-320-3p, miR-146a-5p, miR-221-3p, miR-30c-5p, and miR-350-3p were decreased in diabetic exosomes [22]. Interestingly, miR-320 expression was found to be downregulated in serum-derived exosomes in a T1DM rat model, while a study by Wang et al. demonstrated that cardiomyocytes exert anti-angiogenic effects in a T2DM rat model through exosomal transfer of miR-320 into ECs, which suggested that exosomes from different sources or in different environments have diverse functions.
Detrimental Exosomes Can Be Reversed by Mediators in DCM
As demonstrated in a study by Wang et al., HSP20 was responsive to both acute and chronic hyperglycemia in mouse hearts, suggesting that decreased HSP20 contributes to different developmental stages of DCM. Of interest, the deleterious exosomes released from cardiomyocytes in diabetic hearts can be converted to protective exosomes in a transgenic mice with cardiac-specific HSP20 overexpression, thus restoring cardiac function under hyperglycemic conditions. Myocardial overexpression of HSP20 induced both qualitative and quantitative alterations in the composition and number of the exosomes secreted by cardiomyocytes. The altered exosomes were detected to contain cellular protective proteins, including phosphorylated Akt, SOD1, and survivin, which could be delivered to ECs, therefore promoting myocardial angiogenesis and alleviating oxidative stress, fibrosis, and apoptosis in the diabetic hearts [20]. Therefore, it would be interesting to characterize the molecular changes induced in HSP20 transgenic cardiomyocytes under diabetic conditions in depth and define what mediates the switch from releasing detrimental exosomes to beneficial ones. In addition, HSP20 is reported to be directly inhibited by miR-320 at the posttranscriptional level [21]. As miR-320 was found to be increased in a T2DM rat model, the exosomal miR-320-HSP20 signaling pathway can be regarded as a novel target for the treatment of cardiac injury induced by hyperglycemia (Fig. 1).
Exercise has been described as a “polypill,” mitigating obese or diabetic complications [23, 24]. Exercise can also diminish cardiac dysfunction in diabetic patients, but the underlying molecular mechanisms still remain unknown [25, 26]. Recently, a study by Chaturvedi et al. demonstrated that compared to the db/db mouse group (T2DM model mice), there was a robust release of exosomes through budding into the lumen of the vessels in the db/db exercise group [27]. Furthermore, Safdar A et al. pointed out that the content of circulatory EVs, including exosomes, increases in an intensity-dependent manner in response to endurance exercise [28]. An underlying analysis confirmed that exosomes derived from exercised cardiomyocytes downregulated the levels of MMP9 by upregulating miR-29b and miR-455 and prevented the downstream detrimental effects of MMP9 that lead to cardiac fibrosis and myocyte uncoupling [27]. However, the role of exosomes in exercise has not been shown and needs to be investigated further in future research.
Furthermore, in addition to the reversal of the detrimental effects of exosomes in pathological myocardium by exogenous mediators, beneficial paracrine effects of exosomes from unstimulated rats or humans can also be reversed. A recent study by Davidson et al. showed that plasma exosomes from non-diabetic mice could protect rat hearts from ischemia /reperfusion (IR) injury both in vitro and in vivo. Moreover, exosomes directly protected primary cardiomyocytes against IR injury in vitro through the interaction of HSP70 with sarcolemmal TLR4 by activating the ERK1/2 and P38/MAPK cardioprotective pathways and phosphorylation of HSP27, a member of the highly cytoprotective family of HSPs. However, plasma exosomes from GK rats were found to have lost the ability to activate cardioprotective signaling pathways without affecting exosome production and morphology. Of interest, cardioprotective signaling can be activated in cardiomyocytes from diabetic rats using exosomes from non-diabetic animals [29]. We speculate that diabetes and hyperglycemia could impair cardioprotective signaling roles that have been ascribed to exosomes, and exosomes in unstimulated rats or humans exert a continual “tonic” beneficial effect on the heart that may be modified in response to stress such as high glucose.
Exosomes: a Novel Biomarker and Therapy for DCM?
Currently, exosomes have attracted considerable attention for their potential use as efficient, targeted, and non-immunogenic delivery systems, either as biomarkers or therapeutics for disease [30, 31]. Increased numbers of exosomes can be observed in patients with atherosclerosis, insulin resistance, T2DM, myocardial infarction, and stroke. Moreover, the protein or RNA cargo, especially miRNAs, of exosomes offer additional potential not only as biomarkers in disease but also as vehicles for delivering activated substances [32,33,34,35]. The heart is a terminally differentiated organ, meaning that there is little division of cardiomyocytes in response to injury. Stem cell-derived exosome therapy has been intensively investigated in cardiac regeneration [36]. A study by Lai et al. reported that exosomes released from human embryonic stem cell (ESC)-derived mesenchymal stem cells (ESC-MSCs) could protect against myocardial IR injury both in vitro and in vivo [37]. The protective effects were sustainable, as shown by improved cardiac function after 28 days [38]. Furthermore, an increase in the activity of kinases Akt and GSK3α/β was detected 1 h after exosome administration until the following day, and these kinases are reported to be cardioprotective [38]. In another study by Yu et al., exosomes released from MSCs overexpressing GATA4 preserved cardiac contractile function and reduced infarct size after direct injection into rats at the time of infarction. The mechanisms were attributed to increased levels of miR-19a by inhibition of PTEN, which directly increased the activation of Akt and ERK [39]. However, little research has focused on exosomes as therapy for DCM; therefore, EC-derived exosomes may appear advantageous for preventing the evolution of DCM or ischemic complications of diabetes.
Challenges in Exosome Research and Future Prospects
With T2DM reaching epidemic proportions and cardiovascular disease (CVD) being the major cause of death worldwide, novel therapeutic strategies are urgently needed to provide cell and tissue repair systems to the myocardium. Exosomes are proposed to play an important albeit variably described role not only in cardiac physiology and homeostasis but also in the pathogenesis of major CVD. However, currently, research examining the role of exosomes in DCM is still in its infancy. Emerging work, especially that involving the three underlying challenges facing scientists, is ongoing.
First, growing attention is now being focused on exosome-mediated cell-cell communication, which has been largely overlooked previously. Exosomes can mediate local and systemic cell communication through interacting with surrounding cells by horizontal transfer of information, such as proteins, mRNAs, and miRNAs, and are well known to induce physiological changes in recipient cells [9, 40]. Exosomes from a specific cell of origin can selectively bind and be internalized by certain target cell types [41]. However, mechanisms by which individual exosomes interact with recipient cells are unknown. Furthermore, in the therapeutic potential of exosomes investigations, reducing the side effects of therapies has been widely concerned. This unanswered question needs to be urgently solved in the future.
Second, as the research by Wang et al. demonstrated, injection of therapeutic exosomes retained long-lasting functional effects for 6 weeks, suggesting that exosomes can transmit durable information between cardiac cells in DCM [20]. However, the precise distribution and half-life of individual exosomes delivered from an external source is still an unexplored question yet to be elucidated.
Third, as mentioned above, circulating exosomes can be used as functional biomarkers or mediators transmitting active RNAs or proteins. However, there are several blood and vascular cell types including inflammatory cells, platelets, ECs, and erythrocytes in the circulation, and the contribution of different cell types to the protection observed with plasma exosomes remains debated within the cardiovascular research community.
Fourth, no clear characterizations and markers are available for exosomes from different cell types. Currently, exosomes-associated proteins such as CD9, CD63, CD81, Tsg101 (tumor susceptibility gene 101), Alix (apoptosis-linked gene 2 interacting protein X), and Hsp70 (heat shock 70kda protein) are commonly used as markers to further verify exosomes [42], although recent studies suggest that some of these markers are not specific for exosomes as previously thought [43]. It should be addressed that, due to their endosomal origin, many exosomes do express similar membrane markers [44] which makes it difficult to identify cell types of origin. Profiling of the contents of exosomes may potentially be informative in the identification of specific biomarkers.
Fifth, detection of the role of exosomes in vivo is complicated by difficulties in labeling endogenous exosomes, tracing their dynamic movement, and defining target cells without interfering with their function. Recently, significant progress has been made in imaging single exosomes by a Cre-loxP system. This system can fluorescently trace Cre-reporter cells that have taken up exosomes released by Cre recombinase-expressing cells in vivo and in vitro [45]. However, the development of techniques to detect the function and location of specific exosome subpopulations in vivo is ongoing. Therefore, in future studies, exosome regulation and function in vivo need to be further explored, which may one day provide novel-therapeutic opportunities in combatting the heavy burden of DCM.
References
Harcourt, B. E., Penfold, S. A., & Forbes, J. M. (2013). Coming full circle in diabetes mellitus: from complications to initiation. Nature Reviews Endocrinology, 9, 113–123.
Miki, T., Yuda, S., Kouzu, H., & Miura, T. (2013). Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Failure Reviews, 18, 149–166.
Costantino, S., Paneni, F., Luscher, T. F., & Cosentino, F. (2016). MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. European Heart Journal, 37, 572–576.
Ouwens, D. M., Boer, C., Fodor, M., et al. (2005). Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia, 48, 1229–1237.
Williams, S. A., Tappia, P. S., Yu, C. H., Bibeau, M., & Panagia, V. (1998). Impairment of the sarcolemmal phospholipase D-phosphatidate phosphohydrolase pathway in diabetic cardiomyopathy. Journal of Molecular and Cellular Cardiology, 30, 109–118.
Khanra, R., Dewanjee, S., T, K.D., et al. (2015). Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. Journal of Translational Medicine, 13, 6.
Pan, Y., Wang, Y., Zhao, Y., et al. (2014). Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabete, 63, 3497–3511.
Bugger, H., & Abel, E. D. (2014). Molecular mechanisms of diabetic cardiomyopathy. Diabetologia, 57, 660–671.
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., & Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9, 654–659.
Trams, E. G., Lauter, C. J., Salem Jr., N., & Heine, U. (1981). Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et Biophysica Acta, 645, 63–70.
Johnstone, R. M. (2005). Revisiting the road to the discovery of exosomes. Blood Cells, Molecules & Diseases, 34, 214–219.
Chistiakov, D. A., Orekhov, A. N., & Bobryshev, Y. V. (2016). Cardiac extracellular vesicles in normal and infarcted heart. International Journal of Molecular Sciences, 5 17(1).
McKelvey, K. J., Powell, K. L., Ashton, A. W., Morris, J. M., & McCracken, S. A. (2015). Exosomes: mechanisms of uptake. Journal of Circulating Biomarkers, 4, 7.
Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of Cell Biology, 200, 373–383.
Montecalvo, A., Larregina, A. T., Shufesky, W. J., et al. (2012). Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood, 119, 756–766.
Joshi, M., Kotha, S. R., Malireddy, S., et al. (2014). Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Molecular and Cellular Biochemistry, 386, 233–249.
Wan, A., & Rodrigues, B. (2016). Endothelial cell-cardiomyocyte crosstalk in diabetic cardiomyopathy. Cardiovascular Research, 111, 172–183.
Lawson, C., Vicencio, J. M., Yellon, D. M., & Davidson, S. M. (2016). Microvesicles and exosomes: new players in metabolic and cardiovascular disease. The Journal of Endocrinology, 228, R57–R71.
Huang-Doran, I., Zhang, C. Y., & Vidal-Puig, A. (2016). Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends in Endocrinology and Metabolism: TEM, 28, 3–18.
Wang, X., Gu, H., Huang, W., et al. (2016). Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabete, 65, 3111–3128.
Wang, X., Huang, W., Liu, G., et al. (2014). Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. Journal of Molecular and Cellular Cardiology, 74, 139–150.
Shi, R., Zhao, L., Cai, W., et al. (2017). Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochemical and Biophysical Research Communications, 483, 602–608.
Siamilis, S., Jakus, J., Nyakas, C., et al. (2009). The effect of exercise and oxidant-antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord, 47, 453–457.
Staiano, A. E., Beyl, R. A., Hsia, D. S., Katzmarzyk, P. T., & Newton, R. L. (2017). Twelve weeks of dance exergaming in overweight and obese adolescent girls: transfer effects on physical activity, screen time, and self-efficacy. Journal of Sport and Health Science, 6, 4–10.
Wang, H., Bei, Y., Lu, Y., et al. (2015). Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1alpha and Akt activation. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 35, 2159–2168.
Sacre, J. W., Jellis, C. L., Jenkins, C., et al. (2014). A six-month exercise intervention in subclinical diabetic heart disease: effects on exercise capacity, autonomic and myocardial function. Metabolism, 63, 1104–1114.
Chaturvedi, P., Kalani, A., Medina, I., Familtseva, A., & Tyagi, S. C. (2015). Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. Journal of Cellular and Molecular Medicine, 19, 2153–2161.
Safdar, A., Saleem, A., & Tarnopolsky, M. A. (2016). The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nature Reviews Endocrinology, 12, 504–517.
Davidson, S. M., Riquelme, J. A., Takov, K., et al. (2018). Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. Journal of Cellular and Molecular Medicine, 22, 141–151.
Lu, M., Yuan, S., Li, S., Li, L., Liu, M., & Wan, S. (2018). The exosome-derived biomarker in atherosclerosis and its clinical application. Journal of Cardiovascular Translational Research.
Deddens, J. C., Vrijsen, K. R., Colijn, J. M., et al. (2016). Circulating extracellular vesicles contain miRNAs and are released as early biomarkers for cardiac injury. Journal of Cardiovascular Translational Research, 9, 291–301.
Aurelian, S. M., Cheta, D. M., & Onicescu, D. (2014). Microvesicles - potential biomarkers for the interrelations atherosclerosis/type 2 diabetes mellitus. Romanian Journal of Morphology and Embryology = Revue roumaine de morphologie et embryologie, 55,(3) 1035–1039.
Muller, G. (2012). Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes, metabolic syndrome and obesity, 5, 247–282.
Hulsmans, M., & Holvoet, P. (2013). MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovascular Research, 100, 7–18.
Bei, Y., Chen, T., Banciu, D. D., Cretoiu, D., & Xiao, J. (2017). Circulating exosomes in cardiovascular diseases. Advances in Experimental Medicine and Biology, 998, 255–269.
Ni, J., Sun, Y., & Liu, Z. (2018). The potential of stem cells and stem cell-derived exosomes in treating cardiovascular diseases. Journal of Cardiovascular Translational Research.
Lai, R. C., Arslan, F., Lee, M. M., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 4, 214–222.
Arslan, F., Lai, R. C., Smeets, M. B., et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research, 10, 301–312.
Yu, B., Kim, H. W., Gong, M., et al. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. International Journal of Cardiology, 182, 349–360.
Thery, C. (2011). Exosomes: secreted vesicles and intercellular communications. F1000 Biology Reports, 3, 15.
Denzer, K., van Eijk, M., Kleijmeer, M. J., Jakobson, E., de Groot, C., & Geuze, H. J. (2000). Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. Journal of Immunology (Baltimore, Md. : 1950), 165, 1259–1265.
Xu, R., Greening, D. W., Zhu, H. J., Takahashi, N., & Simpson, R. J. (2016). Extracellular vesicle isolation and characterization: toward clinical application. The Journal of Clinical Investigation, 126, 1152–1162.
Kowal, J., Arras, G., Colombo, M., et al. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America, 113, E968–E977.
Brisson, A. R., Tan, S., Linares, R., Gounou, C., & Arraud, N. (2017). Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets, 28, 263–271.
Zomer, A., Steenbeek, S. C., Maynard, C., & van, Rheenen. J. (2016). Studying extracellular vesicle transfer by a Cre-loxP method. Nature Protocols, 11, 87–101.
Funding
This work was supported by the grants from National Natural Science Foundation of China (81700343 to Lichan Tao), Natural Science Foundation of Jiangsu Province of China (BK20170296 to Lichan Tao), and Changzhou High-Level Medical Talents Training Project (2016ZCL J020 to Fei Hua).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This review does not contain any studies with animals performed by any of the authors.
Additional information
Associate Editor Enrique Lara-Pezzi oversaw the review of this article
Rights and permissions
About this article
Cite this article
Tao, L., Shi, J., Yang, X. et al. The Exosome: a New Player in Diabetic Cardiomyopathy. J. of Cardiovasc. Trans. Res. 12, 62–67 (2019). https://doi.org/10.1007/s12265-018-9825-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-018-9825-x