Abstract
Retinoblastoma (Rb) is the most common intraocular cancer of childhood with an incidence of 1 in 15,000 live births or about 12 per million children ages 0–4 years [1, 2]. Retinoblastoma accounts for 2% of all childhood cancers and approximately 8% percent of cancer in the first 4 years of life [3]. Worldwide there are 9000 new cases annually with the greatest number of cases seen in Asia and Africa where the birth rate is higher [4]. However the rate per live birth remains the same and no significant gender or racial predilection for the development of Rb has been described [5]. Maternal nutrition [6], HPV infection [7, 8], and advanced paternal age [9] have been suggested as predisposing etiologies but have not been definitively confirmed. Worldwide, the survival of children with retinoblastoma has improved [10] however disparities in the treatment and survival of children with this ocular cancer remain [11–16]. The treatment of Retinoblastoma continues to evolve with a focus on more localized therapies to spare systemic toxicity. Nonetheless, there remains a critical need for personalized therapies for retinoblastoma. Retinoblastoma was one of the first cancers with a known genetic underpinning due to a mutation in the RB1 retinoblastoma tumor suppressor gene (RB1) [17, 18], and has provided enormous insights into cancer biology; however, because this tumor cannot be safely biopsied, we still know very little about the genetic, genomic and epigenetic changes in this tumor that may affect treatment and prognosis for eye salvage [19]. This chapter will discuss the diagnosis, staging, and current treatment paradigms for retinoblastoma as well as discuss the future of this disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Retinoblastoma
- Optical coherence tomography
- Intravitreal melphalan
- Intravitreal chemotherapy
- Toxicity
- Personalized medicine
- Cell-free DNA
- Invisible tumors
- Cone precursors
- Tumor-suppressor gene
-
Retinoblastoma is the most common intraocular tumor in childhood
-
The management of this tumor has changed significantly over the past several years including advances in local delivery of chemotherapy with intravitreal injection now done via safety enhanced techniques
-
Seeding regression is nearly 100% with intravitreal chemotherapy however toxicity has been reported and the mechanisms and risk-factors have not yet been elucidated
-
Advances have also been made in the use of hand-held Optical Coherence Tomography for diagnosis and monitoring
-
The high-resolution imaging provided by OCT enables enhanced detection of tumors, including those that are “invisible’ on fundoscopy.
-
Optical coherence tomography has demonstrated the potential to detect recurrences masked by retinal scars
-
Imaging of small tumors may help us better understand the cell of origin for retinoblastoma
-
Retinoblastoma is known to have a genetic underpinning secondary to a mutation in the RB1 tumor-suppressor gene; nonetheless the genetic, genomic and epigenetic changes at the level of the tumor have not been readily assessed or correlated with clinical features or prognosis due to the inability to biopsy this tumor. This is a broad area for future research.
3.1 Retinoblastoma
Retinoblastoma (Rb) is the most common intraocular cancer of childhood with an incidence of 1 in 15,000 live births or about 12 per million children ages 0–4 years [1, 2]. Retinoblastoma accounts for 2% of all childhood cancers and approximately 8% percent of cancer in the first 4 years of life [3]. Worldwide there are 9000 new cases annually with the greatest number of cases seen in Asia and Africa where the birth rate is higher [4]. However the rate per live birth remains the same and no significant gender or racial predilection for the development of Rb has been described [5]. Maternal nutrition [6], HPV infection [7, 8], and advanced paternal age [9] have been suggested as predisposing etiologies but have not been definitively confirmed. Worldwide, the survival of children with retinoblastoma has improved [10] however disparities in the treatment and survival of children with this ocular cancer remain [11,12,13,14,15,16]. The treatment of retinoblastoma continues to evolve with a focus on more localized therapies to spare systemic toxicity. Nonetheless, there remains a critical need for personalized therapies. Retinoblastoma was one of the first cancers with a known genetic underpinning due to a mutation in the RB1 retinoblastoma tumor suppressor gene (RB1) [17, 18], and has provided enormous insights into cancer biology; however, because this tumor cannot be safely biopsied, we still know very little about the genetic, genomic and epigenetic changes in this tumor that may affect treatment and prognosis for eye salvage [19]. This chapter will discuss the diagnosis, staging, and current treatment paradigms for retinoblastoma as well as discuss the future of this disease.
3.2 Diagnosis and Staging
The most common presenting sign of retinoblastoma is leukocoria, or loss of the red reflex, followed by strabismus [20]. Because retinoblastoma is rare, and screening requires a dilated, or low-light examination for loss of the normal red reflex, retinoblastoma remains undiagnosed until the cancer is quite advanced. Sometimes the tumor progresses to that point that it undergoes massive intratumoral necrosis and the child presents with significant ocular and periocular inflammation mimicking endophthalmitis or preseptal/orbital cellulitis [21].
Any child with leukocoria, strabismus or periocular inflammation should undergo a dilated fundus examination by an ophthalmologist followed by an examination under anesthesia (EUA) for any concern for retinoblastoma. The differential diagnosis of retinoblastoma includes other causes of leukocoria, such as Coats, persistent fetal vasculature (PFV), retinal astrocytic hamartoma, retinopathy of prematurity (ROP), familial exudative vitreoretinopathy (FEVR), retinal detachment, endophthalmitis, toxocariasis, toxoplasmosis, (old) vitreous hemorrhage and cataract [22].
On clinical examination, classically, retinoblastoma demonstrates single or multiple creamy white nodular retinal-based masses with prominent intralesional blood vessels. There are three primary clinical patterns of retinoblastoma growth: endophytic, exophytic and rarely diffuse infiltrating wherein a distinct mass is not seen. Endophytic growth occurs when the tumor grows from the retina into the vitreous cavity and is frequently associated with vitreous seeding, wherein small pieces of the tumor break off and proliferate in the vitreous compartment [23]. Exophytic growth occurs when the tumor expands in the subretinal space causing exudative retinal detachments and subretinal seeding. This growth pattern is more likely to demonstrate invasion to the choroid, a known risk factor for orbital relapse and metastatic disease [23]. Advanced tumors generally demonstrate a combination of these growth patterns. Pathognomonic features of retinoblastoma include intralesional calcium and tumor seeding, in the vitreous and subretinal spaces. This can occur either at diagnosis or in association with a tumor recurrence. More information on diagnosis can be found in Chap. 1.
3.3 Imaging Modalities for Retinoblastoma
Imaging modalities can be critical in the diagnosis of retinoblastoma. The most commonly used modality is b-scan ultrasonography which frequently demonstrates a dome-shaped retinal mass with diffuse intralesional calcium [24]. Calcium can often be most clearly seen on Computed Tomography (CT) scans, however it is not recommended that this modality be used if there is a suspicion for retinoblastoma given exposure to radiation in a child with a possible cancer predisposition syndrome [25, 26].
Magnetic resonance imaging (MRI) is the preferred imaging modality for most clinicians and recommended at initial staging. On T1 imaging, retinoblastoma is slightly hyperintense (bright) to the vitreous. The tumor demonstrates moderate to marked enhancement. On gadolinium-enhanced T1 weighted images, finely dispersed areas of low signal intensity correspond to areas of calcification. On T2 weighted imaging, the tumor is classically dark compared with the vitreous. The partially calcified areas may appear as hypointense foci [27]. Aside from its diagnostic value, MRI is performed with three main goals: (1) determine whether there is optic nerve extension, which is often better seen on fat-suppressed imaging [28] (2) extraocular/orbital extension, and (3) trilateral or tetralateral retinoblastoma. Trilateral retinoblastoma refers to a concomitant primitive midline neuroectodermal tumor (PNET) in the pineal region or the suprasellar cistern. Trilateral disease is found in 1.2–6.7% of patients with retinoblastoma and can be found at diagnosis or during/after treatment of the ocular disease [29]. Tetralateral (or quadrilateral) retinoblastoma is defined as the present of intraocular retinoblastoma and tumor in both suprasellar and pineal regions. MRI is often used as continued screening for these CNS tumors up to the age of 3 years (5 years in some centers). Post-enucleation enhancement in the orbit and the cut end of the optic nerve has been described on MRI and can be seen in these screening evaluations. Without a mass or clinical signs of orbital recurrence, this should be considered a benign finding [30]. Fluorescein angiography (FA) can also be critical in the diagnosis of retinoblastoma, particularly when Coat’s disease is also being considered on the differential. Classically, FA demonstrates changes in the caliber of both small and large caliber vessels, with intratumoral retinal leakage and neo-vascularization of the iris [31]. General information on the imaging modalities available for choroidal tumors can be found in Chap. 4.
Finally, in the last several years, the advent of hand-held Optical Coherence Tomography (OCT), which allows for acquisition of images from an anesthetized, supine patient, has brought OCT into the realm of pediatric ocular oncology. The application of OCT has become critical for the management of retinoblastoma including detecting normal retinal anatomy underneath tumors, monitoring treatment response, identification of very small ‘invisible’ tumors, as well as early retinoblastoma recurrences [32,33,34,35,36,37,38,39,40,41]. As shown in Fig. 3.1, small retinoblastoma tumors appear as a smooth round, grey homogenous lesion involving the outer retina; there is ‘tenting’ of the overlying inner retinal layers. Larger tumors demonstrate posterior shadowing artifacts as well as more extensive involvement of the inner retinal layers [37]. It is difficult to distinguish retinoblastoma from benign retinocytomas on OCT, however, longitudinal perspective showing stability of the lesion can aid the clinician in making appropriate management decisions [42]. Use of OCT helps define other associated features such as subretinal fluid, intraretinal fluid, or intra-tumoral calcifications which may be difficult to assess when significant structural distortions from the tumor obscure the normal adjacent retina. For example, the ability to identify a fovea that has not been damaged by direct tumor involvement and/or associated fluid suggests that the future vision in the eye may be intact and thus the eye may merit salvaging therapy. Imaging of the vitreous with OCT imaging can also be valuable in clinical decision making. Even in the era of intravitreal injections of chemotherapy, vitreous seeding is the main cause of tumor relapse and requires more aggressive management. Clinically, vitreous seeds may be difficult to detect if they present as very fine dust type-seeds scattered just anterior to the retina. While difficult to detect on fundoscopy, these are well visualized with OCT [43]. As shown in Fig. 3.2, OCT can be used to image vitreous seeding of various morphologies including large spherical, hollow seeds, with posterior shadowing on the retina [44].
OCT features of small retinoblastoma. (1A) Color fundus photograph of the left eye demonstrates 3 retinoblastoma tumors. (1B) Color fundus photograph of the left eye demonstrates 3 retinoblastoma tumors, marked by the arrows. The smallest tumor, barely visible on fundoscopy, lies just superior to the optic nerve. Rb1-3: Spectral-domain OCT of the three tumors shows homogenous dome shaped masses with overlying inner retinal draping. Tumor #3 is located in the outer retina involving the outer nuclear and possibly the outer plexiform layer. The inner nuclear (INL) and inner plexiform layer (IPL) drape over the tumor. There is also an outer retinal abnormality in all tumors affecting the external limiting membrane (ELM), inner segment-outer segment junction (ISOS), ellipsoid zone (EZ), and interdigitation zone (IZ). There is shadowing on OCT from the retinal vessels overlying the tumor which are also seen clinically. (2A–F) Optical Coherence Tomography (OCT) montage of images through the smallest lesion moving from the superior aspect towards the optic nerve. (2A) The lateral aspect of the larger tumor is seen peripherally. (2B) Most superior aspect of lesion. The inner nuclear layer (INL), outer nuclear layer (ONL) and outer plexiform layer (OPL) are shown in (2B, 2E) with the OPL and INL seen draping over the edges of the small tumor (Adapted from Berry et al. [40]. Reproduced with permission)
Imaging of a spherical Seed in retinoblastoma. The clinical applications of handheld Optical Coherence Tomography (OCT) imaging (Bioptigen, USA) for retinoblastoma continue to evolve and include characterization of small tumors, tumor recurrences, evaluation of seeding and retinal anatomy. OCT done at staging examination of the left eye in a 13-month old child diagnosed with bilateral retinoblastoma (Group C right eye, Group D left eye) demonstrated normal foveal architecture, preretinal dusting of small hyper-reflective seeds and a hollow reflective cystic structure floating above the retina, with shadowing posteriorly, which correlated clinically with a large translucent spherical seed in the vitreous cavity (asterisk) (Adapted Berry et al. [44]. Reproduced with permission)
As smaller and smaller tumors are imaged, OCT may also help us understand the cell of origin for retinoblastoma, which remains unclear. An early description of small tumors on OCT described lesions “centered in the inner nuclear layer (INL)” that appeared to “consume” the middle layer while “sparing of the outer retinal layer” [36]. This led the authors of that paper to conclude that the cell responsible for retinoblastoma originated from the INL. However, the smallest lesions imaged to date appear to involve mostly the outer nuclear layer (ONL) and outer plexiform layer (OPL) with some extension into the INL. This supports the hypothesis that retinoblastoma shares a cellular lineage with the photoreceptors [40]. This is further supported by the characteristic finding of the normal outer plexiform and inner retinal layers “draping” over the outer retinal tumor [45]. Involvement of inner retinal structures may be from migration of malignant cells towards the inner retina and blood supply or general tumor expansion from the ONL. Imaging of small retinoblastoma tumors that primarily involve the outer nuclear layer is consistent with in vivo studies that suggest that the retinoblastoma cells of origin are the cone precursor cells. These cells are exquisitely sensitive to loss of a functional retinoblastoma protein [46, 47].
3.4 Grouping & Staging
Since the advent of the chemotherapy era in the late 1990s intraocular retinoblastoma has been classified according to the International Intraocular Retinoblastoma Classification (IIRC) described by Murphree [48]. The classification scheme separates intraocular retinoblastoma into five groups (A–E) based on size, location and presence of fluid, seeding and other clinical features. IIRC Group A eyes can generally be treated with local therapy only (laser therapy), with each classification progressively advancing until Group E, which is an eye functionally destroyed by tumor and according to the classification should be considered for salvage therapy only in very rare situations such as bilateral Group E eyes. Group A is associated with the greatest likelihood and Group E the least likelihood of ocular salvage based on chemoreduction protocols [49,50,51,52]. Recently however this has been supplanted with the AJCC 8th edition classification for retinoblastoma which includes a 4th factor to the TNM staging, H, for heredity and is the first cancer in the AJCC to have such a staging. This new staging classification is discussed in more detail in Chap. 1.
At most centers, staging consists of clinical examination and MRI (see imaging) however bone marrow aspiration or lumbar puncture may also be performed in patients if there is concern regarding the extent of disease such as extraocular extension, cerebrospinal fluid (CSF) or bone marrow metastases. This is not done routinely if the disease is confined to the globe.
3.5 Treatment
In the early 1900s the only successful treatment for retinoblastoma was enucleation. This treatment is still used today for advanced disease or recurrent tumors poorly responsive to other therapies, particularly in the setting of poor visual prognosis. However, given that 40% of new retinoblastoma cases involve both eyes this treatment modality was devastating for many children and thus attempts at globe salvage were undertaken.
3.6 EBRT
Historically external beam radiotherapy (EBRT) was a mainstay of treatment for many years however it is now rarely used except in certain salvage situations. Increased risk of orbital bony hypoplasia, which was very difficult to remedy cosmetically, and worse, second primary tumors, particularly in patients younger than 12 months of age, led clinicians to seek alternative therapies [53]. Even without EBRT, patients with heritable retinoblastoma are at an increased risk of many other types of second primary cancers throughout life including bony and soft tissue sarcomas and melanoma [10, 53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72].
Retinoblastoma survivors with a familial germline mutation are at slightly higher risk of a second primary tumor compared with those with a de novo germline mutation, in particular melanoma [69]. For germline patients, the risk of developing a second primary malignancy outside of the eye is approximately 30% at 40 years after the initial diagnosis [65, 67]. Unfortunately, patients at risk for a second non-ocular tumor are at risk of third and fourth tumors with increasing mortality regardless of EBRT exposure [73, 74].
EBRT has remained in the treatment arsenal for vitreous seeding, a common cause of relapse after systemic or intra-arterial chemotherapy [75]. However, a new treatment modality that involves intravitreal injection of chemotherapy has largely made this obsolete (see below). Recalcitrant retinal recurrences (particularly in an only remaining eye) may also still be treated with EBRT.
3.7 Systemic Chemoreduction
As an alternative to EBRT, Gallie, Murphree and several other ocular oncologists pioneered the use of chemotherapy in the 1990s [76, 77]. It has since become the backbone of treatment for retinoblastoma.
Systemic chemotherapy given for retinoblastoma is generally described as chemoreduction as the goal of chemotherapy administration is to shrink the tumor so that focal consolidative therapy (e.g. laser and/or cryotherapy) may be effective [78]. Focal consolidative therapy is directly destructive to tumor cells; it may also be used to augment penetration of chemotherapy into the eye [79]. Since the early 1990s, systemic 3-drug chemotherapy, along with local consolidation therapy, is a common and well documented therapy for globe salvage for patients with retinoblastoma [80].
Various regimens are used for systemic therapy, most typically carboplatin, vincristine, and etoposide with 3–6 cycles being given based on the extent of disease. Success rates for advanced Group D eyes are reported at approximately 50% with chemoreduction and local consolidation [51, 52]. At Children’s Hospital Los Angeles, the chemoreduction protocol consists of intravenous carboplatin 390 mg/m2 (13 mg/kg for children <36 months) × 2 days, etoposide 150 mg/m2 (5 mg/kg for <36 months) × 2 days, and vincristine 1.5 mg/m2 (0.05 mg/kg for <36 months) × 1 day, for 6 cycles every 28 days (i.e. CEV). Infants less than 6 months of age at diagnosis receive a modified dosing regimen with a 50% decrease in all agents for the first cycle [81]. Children with Group B eyes (less advanced) are treated with three initial cycles [82]. The therapy is augmented with local consolidation therapy, which includes diode or argon laser therapy (532 nm or 810 nm laser), and/or cryotherapy often used for larger lesions anterior to the equator.
While generally safe and well tolerated, systemic toxicity is known to occur. This leads to dangerous cytopenias, peripheral neuropathies [83], hearing loss [84] and rarely secondary leukemias [85,86,87,88]. Because of this, localized methods of chemotherapeutic delivery were touted to increase chemotherapeutic efficacy and minimize systemic toxicity. The main focus of this has been intra-arterial delivery of chemotherapy directly to the eye via the ophthalmic artery.
3.8 Intra-arterial Chemotherapy
Local methods of intra-arterial delivery of chemotherapeutic agents were pioneered by Suzuki and Kaneko in Japan [89] and Abramson and Gobin in the United States [79, 90,91,92,93,94]. Abramson and colleagues modified the Japanese protocol wherein, under general anesthesia, a cannula is introduced through the femoral artery and advanced to but not through the os of the ophthalmic artery. Fluoroscopy is used to confirm the position of the catheter before the chemotherapy is infused into the artery, approximately over 30 min [79]. Typically the initial agent of choice is melphalan although carboplatin, and/or topotecan can also be used. Initial doses are melphalan 0.4 mg/kg (with a maximum starting dose of 5 mg), carboplatin 50 mg and topotecan 0.2–4 mg [95].
This technique has been termed super selective ophthalmic artery chemotherapy or ophthalmic artery chemosurgery. Using Melphalan, Abramson and colleagues reported eye salvage rates superior to systemic chemoreduction, especially when used as primary versus second-line therapy for recurrent disease [95]. Group D eyes treated with intra-arterial chemotherapy have been reported with salvage rates ranging from 36% to 100% [96], with several large series of showing a rate between 78% and 100% [97,98,99,100,101,102,103,104]. Prospectively evaluated success rates for advanced Group D eyes have been reported as 100% when this is used as primary therapy [104]; however this has not been repeatable at all centers. A randomized clinical trial has been recommended to determine the efficacy and safety of this approach, as well as to determine the optimum chemotherapeutic agent. The Children’s Oncology Group recently closed a trial of intra-arterial chemotherapy for patients with unilateral Group D disease, however results have not yet been reported.
Side effects, mostly vascular in nature, have also been described including ciliary flush, sectoral occlusive choroidopathy, and concerns exist about potential for stroke with this method of delivery [105,106,107]. There is also a learning curve with this technique with higher rates of vascular complications reported early on [108]. The total dose of whole body radiation given with multiple fluoroscopies also remains undefined. A more concerning trend, however, is that with greater success in salvage more advanced eyes are being treated with local chemotherapy only, this there may be an increased risk of both metastatic disease and orbital recurrences. A meta-analysis by Yousef et al. of intra-arterial chemotherapy for retinoblastoma found multiple reports of metastatic disease, while others have found equal, but relatively higher rates of metastatic disease and orbital relapse regardless of whether salvage therapy is attempted [109,110,111,112,113,114]. In general, as long as enucleation is reserved for the most advanced eyes, attempts at salvage therapy with enucleation for persistent or recurrent disease appear to be safe [115]. No therapy, including primary enucleation with adjuvant systemic chemotherapy, has been shown to completely eliminate the risk for metastatic disease. Thankfully, this remains a relatively rare event following a diagnosis of retinoblastoma confined to the intraocular space, regardless of treatment modality.
3.9 Intravitreal Chemotherapy
Seeding is one of the main indications for secondary enucleation in eyes that undergo attempted salvage therapy. Prior to 2012 recurrent seeding after chemotherapy was treated with EBRT, or if visual potential was poor, it may prompt enucleation in order to spare the child from radiation. This paradigm shifted dramatically in 2012 when Francis Munier introduced his safety-enhanced technique for intravitreal injection of chemotherapy which included a paracentesis with extraction of aqueous humor to lower the intraocular pressure prior to injection [116, 117]. This was not the first time that attempts had been made at injection of medication, chemotherapy or vectors had been made into the vitreous in retinoblastoma eyes [118, 119]. In fact, it was first described in 1962 by Ericson and Rosengren but reports of extraocular spread limited its use [120]. With the addition of the safety measures proposed by Munier, which were intended to lower the intraocular pressure and thus prevent reflux of intraocular fluid, intravitreal injection of melphalan has since been found to be safe when considering the risk of extraocular spread, and highly effective in eradicating vitreous seeding [89, 116, 117, 121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136]. As with any new therapy, the ideal dose of melphalan is not yet known and ranges widely from 8 to 50 μg with an equal range of number and interval of injection, although the average range is 20–30 μg given weekly until there is clearance of seeds [123, 124, 132]. Success rates are near 100% in eradicating vitreous seeds, and globe salvage even in advanced eyes is on par with previous studies using EBRT to control seeding [135, 137], however both anterior and posterior toxicity, loss of ERG function in doses over 30 μg and acute hemorrhagic retinopathy with devastating consequences have been reported (Fig. 3.3) [127, 134, 138, 139]. The mechanism of toxicity for the hemorrhagic retinopathy has been hypothesized to involve induction of a posterior vitreous detachment followed by retrohyaloidal injection of the medication causing toxicity secondary to concentration against the retina [134]. This however will require further research to better elucidate the mechanism for this acute toxicity. Nonetheless, the overall efficacy and relative safety of intraocular injection of chemotherapy have led to recent reports of use intracamerally for anterior segment seeding but the long term safety and outcomes of this are not yet known [140].
Intravitreal melphalan toxicity. (1A, 2A) Fundus photographs of the left eye from patient 1 and right eye from patient 2 revealing partially treated retinoblastoma with associated vitreous seeding located in the macula and nasal to the optic disc respectively. (1B, 2B) Fundus photographs from patient 1 and patient 2, respectively, revealing diffuse retinal edema with associated intra and preretinal hemorrhages. (1C, 2C) Mid-phase fluorescein angiography of the left eye from patient 1 and the right eye from patient 2, respectively, revealing areas of blockage corresponding to the areas of retinal hemorrhage and intraocular tumor, with vascular sheathing. (1D, 2D) Fundus photographs at follow-up of the patient 1 and patient 2, respectively revealing diffuse chorioretinal atrophy in the posterior pole with a demarcation between the normal and atrophic retina. (a) Schematic rendition of an eye with retinoblastoma and associated vitreous seeding. The blue line represents the hyaloid face; the red line represents the retina and the yellow mass represents the tumor. (b–d) shows injection of melphalan (green) into the vitreous cavity via the pars plana. (e) represents a localized posterior hyaloid detachment over the tumor. (f, g) Injection of melphalan into the subhyaloid space due to the presence of a partial detachment. (h) Treated retinoblastoma (orange) with retinal atrophy (brown line) and resolution of vitreous seeding. (Adapted from Aziz et al. [134]. Reproduced with permission)
Given the new treatment paradigm for seeding, a new classification has been proposed, describing three patterns of vitreous seeding, based on their clinical morphology, which have prognostic significance [141]. These patterns include “dust,” (type 1) which are small fine granules or vitreous haze, “spheres,” (type 2) which are spherical vitreous opacities, which may have a translucent or opaque center, and “clouds,” (type 3) which are dense, sheet-like collections of vitreous opacities. Seeds can occur in any of four vitreous compartments: retrohyaloidal, vitreous, subretinal, or anterior chamber. The seed classification has been shown to predict the number and overall dose of intravitreal melphalan injections required for local control [137, 142]. A retrospective study with a cohort of 28 patients found that at a mean dose of 25–30 μg, eyes with dust required three injections, eyes with spheres required four injections and eyes with clouds required the most number of injections (median 6) although some eyes responded to only one injection in all classification. Cloud type seeds also required the highest cumulative dose of melphalan [137]. These findings were similar to a larger study by Francis et al. that demonstrated dust type seeding required fewer injections and a lower overall dose than spheres and both lower than clouds for complete regression [142]. (Fig. 3.4) These studies found that the spherical seed class was the most likely to demonstrate recurrence and thus took the longest for complete clinical clearance (Fig. 3.5). This finding is supported by recent histopathologic correlation which found that spherical seeds can be composed of either non-necrotic viable retinoblastoma cells or an outer rim of active cells with a necrotic center however both types actively disperse viable cells which make them most likely to recur. This research also found that the cloud-type seed may take the longest to clear clinically but is mostly composed of macrophages and non-viable necrotic seeds and thus may not actually require more injections than the other seed classes [143].
Seeding classification and treatment response. Illustration summarizing vitreous seed classification and response to intravitreal melphalan: number of injections received, time to response, and median dose of melphalan per injection. (Adapted from Francis et al. [142]. Reproduced with permission)
Kaplan-Meier curves for seed regression based on classification. Median time to seed regression was 3 weeks for dust (range 1–24 weeks), 8 weeks for spheres (range 5–75) and 11 weeks for clouds (range 1–32 weeks) which was significant (p = 0.07). There was 100% seeding regression was seen in all classes of seed. Spheres were the most likely to recur and thus have the widest range from time to regression. (Adapted from Berry et al. [137]. Reproduced with permission)
3.10 Adjuvant Systemic Chemotherapy for High-Risk Histopathologic Features
There is no clear consensus on whether or not patients with high-risk pathologic features (such as massive choroidal invasive, post-laminar optic nerve invasion, and scleral invasion) should receive post enucleation adjuvant chemotherapy. A meta-analysis by Kim suggests that the risk with isolated post laminar involvement is 16% and with concomitant massive choroidal invasion the risk increases to nearly 33% and thus adjuvant chemotherapy is recommended and given at most centers [144]. Despite the lack of consensus on these features, there is general acceptance that optic-nerve invasion with involvement of the resection margin or bulky extra-scleral spread are highly predictive for extraocular relapse; in these scenarios, post-enucleation adjuvant systemic chemotherapy is indicated [145,146,147].
3.11 Orbital Relapse and Metastatic Disease
Orbital relapse occurs in about 4% of patients after primary enucleation, most within 12 months [148]. These patients are at increased risk for metastatic disease. Treatment for orbital recurrence (confined to the orbit) is systemic high-dose multi-agent chemotherapy as well as orbital radiotherapy. Patients with disseminated metastatic disease such as bone-marrow involvement receive high-dose 3–4 agent chemotherapy, bone marrow transplantation, and may also receive intrathecal radiotherapy [148].
Metastatic disease, while rare in the developed world, had remained largely fatal until recent trials of intensive multimodal therapy (including high-dose multi-agent chemotherapy and radiotherapy to bulky sites) with autologous hematopoietic stem cell rescue have shown success with reported 5-year event-free survival >60% [149,150,151,152,153,154,155].
3.12 Genetic Disease and Personalized Medicine for Retinoblastoma
Retinoblastoma is a known genetic disease. Tumorigenesis is initiated by a mutation in the RB1 gene on chromosome 13q, which was the first tumor suppressor gene to be discovered [18, 156,157,158,159,160,161]. The inheritance pattern of retinoblastoma was instrumental in the discovery of the RB tumor suppressor gene [162]. Based on clinical genetics, there are three forms of Retinoblastoma: familial (10%), sporadic heritable (30%) in which a new mutation in the suppressor gene is present in all/many cells of the body, and non-heritable (60%) wherein both mutations in the RB1 gene occur as somatic events in the tumor. Due to familial cases of retinoblastoma, it was known that retinoblastoma harbored a genetic underpinning. However, the genetic locus for this tumor was only elucidated in the past few decades. In 1971, Knudson first described the “two-hit hypothesis” suggesting that the RB gene requires mutations in both active gene copies in order for a child to manifest retinoblastoma [162]. The hypothesis suggested that in heritable cases a germline mutation was present in all (or most) cells of the body, and that a second somatic mutation was needed for tumor formation. Germline cases represent approximately 40% of retinoblastoma cases [163]. These patients tend to have bilateral and multifocal tumors and have a significantly increased risk for secondary tumors including pinealoblastoma (PNET). Their future offspring have a 45% chance of developing retinoblastoma (due to penetrance, it Is not 50%) [164].
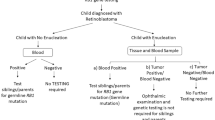
In the non-heritable cases, both mutations in the RB1 gene occur as somatic events, thereby explaining why somatic disease was always unilateral, unifocal and often seen in older children. Additionally, it did not predispose to second primary cancers. Approximately 15% of unilateral cases are found to have germline mutations [163, 165]. Some children may present with unilateral disease but subsequently progress asynchronously to bilateral involvement. Thus, serum genetic testing to determine whether the initial mutation is germline is critical to the management of these patients and families. It is recommended that children have genetic testing, particularly in the setting of unilateral disease regardless of age at presentation [166]. Further information on the genetics of retinoblastoma and testing for these mutations can be found in Chap. 2
Investigating the tumor suppressor pathway regulated by RB1 has provided unprecedented insights into the genetic mechanisms of tumorigenesis, not only for retinoblastoma but also for virtually all human cancers. Despite this, we have not been able to leverage the growing field of cancer genomics for retinoblastoma patients which has dramatically impacted the care of breast, lung and prostate cancer patients [167,168,169,170,171,172,173,174,175]. This is primarily because we cannot safely biopsy this tumor and thus cannot correlate the genetic and epigenetic changes at the level of the tumor with clinical outcomes. Thus, since the 1990s the only real change in the management of retinoblastoma has been a focus on more localized delivery of chemotherapy to the eye with no role for tumor-derived genetic factors in the initial management of this disease. Preliminary research has, however, suggested that there are factors that predispose to increased tumor anaplasia, which correlates with a poor prognosis [176, 177]. Further studies have shown tumor-derived cell-free-DNA (cfDNA) is present in the aqueous humor of advanced retinoblastoma eyes. This cfDNA can be reliably collected, amplified and even evaluated for chromosomal alterations (e.g. small regions of gains and losses) [19]. It is also known from studies on tumor tissue, that chromosomal copy number alteration (gains and losses of partial sections of chromosomes) is a common secondary genomic change that allows for tumor progression [178, 179]. Interestingly, the tumor-derived cfDNA in the aqueous mimics the changes at the level of the tumor suggesting that the aqueous can be used as a liquid biopsy, or ‘surrogate biopsy’ for retinoblastoma (Fig. 3.6). This allows for the unique opportunity to evaluate these chromosomal changes in the tumor DNA found in the aqueous (e.g. before an eye has been enucleated) so that they can be associated with clinical tumor features, response to therapy and prognosis. Given that a paracentesis is now routinely performed before each injection of intravitreal melphalan (see intravitreal chemotherapy) and no cases of extraocular spread have been reported [180], it appears that a paracentesis is safe to perform in eyes with retinoblastoma even during active therapy. This method of isolating cfDNA from the aqueous has been termed the ‘surrogate liquid tumor biopsy’ and is the first time that retinoblastoma tumor-derived DNA has been isolated without enucleation from eyes undergoing salvage therapy [19]. While this research is early, it may finally allow for identification of the RB1 mutation in (nearly) all patients without enucleation and finally allow for genotypic-phenotypic correlation and important prognostic information. Evaluation of the aqueous humor during treatment for eyes with retinoblastoma has allowed for identification of a possible biomarker that portends a poor prognosis for globe salvage with therapy. In this study the authors found that identification of an increased copy number on a segment of chromosome 6p (termed gain of 6p) was associated with a 10× increased risk of the eye requiring enucleation after failed attempts at eye salvage [181]. Genes on 6p (DEK, E2F) are known to play a role in retinoblastoma tumorigenesis but the exact mechanism of 6p gain, or even the single gene player, is not yet known [182]. It will be important to obtain aqueous humor at diagnosis from eyes undergoing salvage and to evaluate the outcomes of these children prospectively before fully elucidating the prognostic impact of this potential biomarker. While there have been great strides in the diagnosis, imaging, staging and the local delivery of chemotherapy to augment globe salvage for these patients, there has been a complete lack of targeted therapies. The ‘surrogate liquid tumor biopsy’ may finally allow for the development of personalized medical therapy for retinoblastoma patients. Thus, we are entering into an exciting landscape for the management of this disease.
Chromosomal copy number variation profiles from the aqueous humor and tumor composites. Chromosomal copy number variations (CNV) in single samples from the aqueous humor compared to Summative tumor CNV by Kooi et al., CNV from aqueous cfDNAs from 3 patients (a) compared to a summation of CNV profiles from 71 retinoblastoma tumor tissue samples from Kooi et al. [178]. (b) Show a very similar pattern of chromosomal gains and losses typical for retinoblastoma. (Adapted from Kooi et al. [178] and Berry et al. [19]. Reproduced with permission)
References
Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93(1):21–3.
Seregard S, Lundell G, Svedberg H, Kivela T. Incidence of retinoblastoma from 1958 to 1998 in Northern Europe: advantages of birth cohort analysis. Ophthalmology. 2004;111(6):1228–32.
Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017;18(6):719–31.
Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93(9):1129–31.
Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet. 2012;379(9824):1436–46.
Lombardi C, Ganguly A, Bunin GR, Azary S, Alfonso V, Ritz B, et al. Maternal diet during pregnancy and unilateral retinoblastoma. Cancer Causes Control. 2015;26(3):387–97.
Anand B, Ramesh C, Appaji L, Kumari BS, Shenoy AM, Nanjundappa, et al. Prevalence of high-risk human papillomavirus genotypes in retinoblastoma. Br J Ophthalmol. 2011;95(7):1014–8.
Shetty OA, Naresh KN, Banavali SD, Shet T, Joshi R, Qureshi S, et al. Evidence for the presence of high risk human papillomavirus in retinoblastoma tissue from nonfamilial retinoblastoma in developing countries. Pediatr Blood Cancer. 2012;58(2):185–90.
Mills MB, Hudgins L, Balise RR, Abramson DH, Kleinerman RA. Mutation risk associated with paternal and maternal age in a cohort of retinoblastoma survivors. Hum Genet. 2012;131(7):1115–22.
Tamboli D, Topham A, Singh N, Singh AD. Retinoblastoma: a SEER dataset evaluation for treatment patterns, survival, and second malignant neoplasms. Am J Ophthalmol. 2015;160(5):953–8.
Gichigo EN, Kariuki-Wanyoike MM, Kimani K, Nentwich MM. Retinoblastoma in Kenya: survival and prognostic factors. Ophthalmologe. 2015;112(3):255–60.
Waddell KM, Kagame K, Ndamira A, Twinamasiko A, Picton SV, Simmons IG, et al. Clinical features and survival among children with retinoblastoma in Uganda. Br J Ophthalmol. 2015;99(3):387–90.
Waddell KM, Kagame K, Ndamira A, Twinamasiko A, Picton SV, Simmons IG, et al. Improving survival of retinoblastoma in Uganda. Br J Ophthalmol. 2015;99(7):937–42.
Chawla B, Hasan F, Azad R, Seth R, Upadhyay AD, Pathy S, et al. Clinical presentation and survival of retinoblastoma in Indian children. Br J Ophthalmol. 2016;100(2):172–8.
Li SY, Chen SC, Tsai CF, Sheu SM, Yeh JJ, Tsai CB. Incidence and survival of retinoblastoma in Taiwan: a nationwide population-based study 1998-2011. Br J Ophthalmol. 2016;100(6):839–42.
Temming P, Arendt M, Viehmann A, Eisele L, Le Guin CH, Schundeln MM, et al. How eye-preserving therapy affects long-term overall survival in heritable retinoblastoma survivors. J Clin Oncol. 2016;34(26):3183–8.
Benedict WF, Murphree AL, Banerjee A, Spina CA, Sparkes MC, Sparkes RS. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983;219(4587):973–5.
Dryja TP, Friend S, Weinberg RA. Genetic sequences that predispose to retinoblastoma and osteosarcoma. Symp Fundam Cancer Res. 1986;39:115–9.
Berry JL, Xu L, Murphree AL, Krishnan S, Stachelek K, Zolfaghari E, et al. Potential of aqueous humor as a surrogate tumor biopsy for retinoblastoma. JAMA Ophthalmol. 2017;135(11):1221–30.
Abramson DH, Beaverson K, Sangani P, Vora RA, Lee TC, Hochberg HM, et al. Screening for retinoblastoma: presenting signs as prognosticators of patient and ocular survival. Pediatrics. 2003;112(6 Pt 1):1248–55.
Foster BS, Mukai S. Intraocular retinoblastoma presenting as ocular and orbital inflammation. Int Ophthalmol Clin. 1996;36(1):153–60.
Maki JL, Marr BP, Abramson DH. Diagnosis of retinoblastoma: how good are referring physicians? Ophthalmic Genet. 2009;30(4):199–205.
Nawaiseh I, Al-Hussaini M, Alhamwi A, Meyar M, Sultan I, Alrawashdeh K, et al. The impact of growth patterns of retinoblastoma (endophytic, exophytic, and mixed patterns). Turk Patoloji Derg. 2015;31(1):45–50.
Novotny A, Krasny J. Diagnosis of retinoblastoma using ultrasound. Cas Lek Cesk. 1990;129(12):364–5.
Kaufman LM, Mafee MF, Song CD. Retinoblastoma and simulating lesions. Role of CT, MR imaging and use of Gd-DTPA contrast enhancement. Radiol Clin N Am. 1998;36(6):1101–17.
Danziger A, Price HI. CT findings in retinoblastoma. AJR Am J Roentgenol. 1979;133(4):695–7.
Razek AA, Elkhamary S. MRI of retinoblastoma. Br J Radiol. 2011;84(1005):775–84.
Ainbinder DJ, Haik BG, Frei DF, Gupta KL, Mafee MF. Gadolinium enhancement: improved MRI detection of retinoblastoma extension into the optic nerve. Neuroradiology. 1996;38(8):778–81.
de Jong MC, Kors WA, de Graaf P, Castelijns JA, Moll AC, Kivela T. The incidence of trilateral retinoblastoma: a systematic review and meta-analysis. Am J Ophthalmol. 2015;160(6):1116–26 e5.
Kim JW, Madi I, Lee R, Zolfaghari E, Jubran R, Lee TC, et al. Clinical significance of optic nerve enhancement on magnetic resonance imaging in enucleated retinoblastoma patients. Ophthalmol Retina. 2017;1(5):369–74.
Kim JW, Ngai LK, Sadda S, Murakami Y, Lee DK, Murphree AL. Retcam fluorescein angiography findings in eyes with advanced retinoblastoma. Br J Ophthalmol. 2014;98(12):1666–71.
Shields CL, Mashayekhi A, Luo CK, Materin MA, Shields JA. Optical coherence tomography in children: analysis of 44 eyes with intraocular tumors and simulating conditions. J Pediatr Ophthalmol Strabismus. 2004;41(6):338–44.
Shields CL, Materin MA, Shields JA. Review of optical coherence tomography for intraocular tumors. Curr Opin Ophthalmol. 2005;16(3):141–54.
Sony P, Garg SP. Optical coherence tomography in children with retinoblastoma. J Pediatr Ophthalmol Strabismus. 2005;42(3):134.
Yousef YA, Shroff M, Halliday W, Gallie BL, Heon E. Detection of optic nerve disease in retinoblastoma by use of spectral domain optical coherence tomography. J AAPOS. 2012;16(5):481–3.
Rootman DB, Gonzalez E, Mallipatna A, Vandenhoven C, Hampton L, Dimaras H, et al. Hand-held high-resolution spectral domain optical coherence tomography in retinoblastoma: clinical and morphologic considerations. Br J Ophthalmol. 2013;97(1):59–65.
Cao C, Markovitz M, Ferenczy S, Shields CL. Hand-held spectral-domain optical coherence tomography of small macular retinoblastoma in infants before and after chemotherapy. J Pediatr Ophthalmol Strabismus. 2014;51(4):230–4.
Pierro L, De Francesco S, Hadjistilianou D, Casalino G, Fusco F, Sergenti J, et al. Spectral-domain optical coherence tomography appearance of a posterior pole retinoma. J Pediatr Ophthalmol Strabismus. 2014;51(5):320.
Hasanreisoglu M, Dolz-Marco R, Ferenczy SR, Shields JA, Shields CL. Spectral domain optical coherence tomography reveals hidden fovea beneath extensive vitreous seeding from retinoblastoma. Retina. 2015;35(7):1486–7.
Berry JL, Cobrinik D, Kim JW. Detection and intraretinal localization of an ‘invisible’ retinoblastoma using optical coherence tomography. Ocul Oncol Pathol. 2016;2(3):148–52.
Park K, Sioufi K, Shields CL. Clinically invisible retinoblastoma recurrence in an infant. Retin Cases Brief Rep. 2017;13(2):108–10.
Malhotra PP, Bhushan B, Mitra A, Sen A. Spectral-domain optical coherence tomography and fundus autofluorescence features in a case of typical retinocytoma. Eur J Ophthalmol. 2015;25(6):e123–6.
Seider MI, Grewal DS, Mruthyunjaya P. Portable optical coherence tomography detection or confirmation of ophthalmoscopically invisible or indeterminate active retinoblastoma. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):965–8.
Berry JL, Anulao K, Kim JW. Optical coherence tomography imaging of a large spherical seed in retinoblastoma. Ophthalmology. 2017;124(8):1208.
Saktanasate J, Vongkulsiri S, Khoo CT. Invisible retinoblastoma. JAMA Ophthalmol. 2015;133(7):e151123.
Xu XL, Singh HP, Wang L, Qi DL, Poulos BK, Abramson DH, et al. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature. 2014;514(7522):385–8.
Xu XL, Fang Y, Lee TC, Forrest D, Gregory-Evans C, Almeida D, et al. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell. 2009;137(6):1018–31.
Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin N Am. 2005;18(1):41–53.
Kaliki S, Shields CL, Rojanaporn D, Al-Dahmash S, McLaughlin JP, Shields JA, et al. High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120(5):997–1003.
Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol. 2006;17(3):228–34.
Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The international classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–80.
Berry JL, Jubran R, Kim JW, Wong K, Bababeygy SR, Almarzouki H, et al. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer. 2013;60(4):688–93.
Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998;105(4):573–9.
Abramson DH, Ronner HJ, Ellsworth RM. Second tumors in nonirradiated bilateral retinoblastoma. Am J Ophthalmol. 1979;87(5):624–7.
Abramson DH, Ellsworth RM, Kitchin FD, Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984;91(11):1351–5.
Eng C, Li FP, Abramson DH, Ellsworth RM, Wong FL, Goldman MB, et al. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85(14):1121–8.
Moll AC, Imhof SM, Bouter LM, Kuik DJ, Den Otter W, Bezemer PD, et al. Second primary tumors in patients with hereditary retinoblastoma: a register-based follow-up study, 1945-1994. Int J Cancer. 1996;67(4):515–9.
Li FP, Abramson DH, Tarone RE, Kleinerman RA, Fraumeni JF Jr, Boice JD Jr. Hereditary retinoblastoma, lipoma, and second primary cancers. J Natl Cancer Inst. 1997;89(1):83–4.
Moll AC, Imhof SM, Bouter LM, Tan KE. Second primary tumors in patients with retinoblastoma. A review of the literature. Ophthalmic Genet. 1997;18(1):27–34.
Rubin CZ, Rosenfield NS, Abramson SJ, Abramson DH, Dunkel IJ. The location and appearance of second malignancies in patients with bilateral retinoblastoma. Sarcoma. 1997;1(2):89–93.
Dunkel IJ, Gerald WL, Rosenfield NS, Strong EW, Abramson DH, Ghavimi F. Outcome of patients with a history of bilateral retinoblastoma treated for a second malignancy: the memorial sloan-kettering experience. Med Pediatr Oncol. 1998;30(1):59–62.
Hasegawa T, Matsuno Y, Niki T, Hirohashi S, Shimoda T, Takayama J, et al. Second primary rhabdomyosarcomas in patients with bilateral retinoblastoma: a clinicopathologic and immunohistochemical study. Am J Surg Pathol. 1998;22(11):1351–60.
Abramson DH. Second nonocular cancers in retinoblastoma: a unified hypothesis. The Franceschetti Lecture. Ophthalmic Genet. 1999;20(3):193–204.
Schlienger P, Campana F, Vilcoq JR, Asselain B, Dendale R, Desjardins L, et al. Nonocular second primary tumors after retinoblastoma: retrospective study of 111 patients treated by electron beam radiotherapy with or without TEM. Am J Clin Oncol. 2004;27(4):411–9.
Marees T, Moll AC, Imhof SM, de Boer MR, Ringens PJ, van Leeuwen FE. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer I. 2008;100(24):1771–9.
Sheen V, Tucker MA, Abramson DH, Seddon JM, Kleinerman RA. Cancer screening practices of adult survivors of retinoblastoma at risk of second cancers. Cancer. 2008;113(2):434–41.
Balaguer J, Harto M, Serra I, Oltra S, HernAndez M, Castel V. Mortality from second tumors among long-term survivors of retinoblastoma: an 87 case series report. Pediatr Blood Cancer. 2009;53(5):811.
Woo KI, Harbour JW. Review of 676 second primary tumors in patients with retinoblastoma: association between age at onset and tumor type. Arch Ophthalmol. 2010;128(7):865–70.
Kleinerman RA, Yu CL, Little MP, Li Y, Abramson D, Seddon J, et al. Variation of second cancer risk by family history of retinoblastoma among long-term survivors. J Clin Oncol. 2012;30(9):950–7.
MacCarthy A, Bayne AM, Brownbill PA, Bunch KJ, Diggens NL, Draper GJ, et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951-2004. Br J Cancer. 2013;108(12):2455–63.
Rodjan F, Graaf P, Brisse HJ, Verbeke JI, Sanchez E, Galluzzi P, et al. Second cranio-facial malignancies in hereditary retinoblastoma survivors previously treated with radiation therapy: clinic and radiologic characteristics and survival outcomes. Eur J Cancer. 2013;49(8):1939–47.
Fujiwara T, Fujiwara M, Numoto K, Ogura K, Yoshida A, Yonemoto T, et al. Second primary osteosarcomas in patients with retinoblastoma. Jpn J Clin Oncol. 2015;45(12):1139–45.
Abramson DH, Melson MR, Dunkel IJ, Frank CM. Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology. 2001;108(10):1868–76.
Melson MR, Dunkel IJ, Frank CM, Abramson DH. Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Invest Ophthalmol Vis Sci. 2000;41(4):S469.
Kaneko A, Suzuki S. Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol. 2003;33(12):601–7.
Gallie BL, Budning A, DeBoer G, Thiessen JJ, Koren G, Verjee Z, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch Ophthalmol. 1996;114(11):1321–8.
Murphree AL, Villablanca JG, Deegan WF, Sato JK, Malogolowkin M, Fisher A, et al. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114(11):1348–56.
Shields JA, Shields CL, De Potter P. Photocoagulation of retinoblastoma. Int Ophthalmol Clin. 1993;33(3):95–9.
Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115(8):1398–404.
Shields CL, De Potter P, Himelstein BP, Shields JA, Meadows AT, Maris JM. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114(11):1330–8.
Berry JL, Jubran R, Lee TC, Murphree AL, Lee D, Kim JW. Low-dose chemoreduction for infants diagnosed with retinoblastoma before 6 months of age. Ocul Oncol Pathol. 2015;1:103–10.
Zhu DBJL, Ediriwickrema L, Wong K, Lee TC, Murphree AL, Kim JW, Jubran R. Long-term outcomes of group b eyes in patients with retinoblastoma treated with short-course chemoreduction: experience from Children’s Hospital Los Angeles/University of Southern California. Ocul Oncol Pathol. 2016;2:105–11.
Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416–30.
Jehanne M, Lumbroso-Le Rouic L, Savignoni A, Aerts I, Mercier G, Bours D, et al. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer. 2009;52(5):637–43.
Weintraub M, Revel-Vilk S, Charit M, Aker M, Pe’er J. Secondary acute myeloid leukemia after etoposide therapy for retinoblastoma. J Pediatr Hematol Oncol. 2007;29(9):646–8.
Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. 2007;114(7):1378–83.
Nishimura S, Sato T, Ueda H, Ueda K. Acute myeloblastic leukemia as a second malignancy in a patient with hereditary retinoblastoma. J Clin Oncol. 2001;19(21):4182–3.
Rizzuti AE, Dunkel IJ, Abramson DH. The adverse events of chemotherapy for retinoblastoma: what are they? Do we know? Arch Ophthalmol. 2008;126(6):862–5.
Suzuki S, Aihara Y, Fujiwara M, Sano S, Kaneko A. Intravitreal injection of melphalan for intraocular retinoblastoma. Jpn J Ophthalmol. 2015;59(3):164–72.
Klufas MA, Gobin YP, Marr B, Brodie SE, Dunkel IJ, Abramson DH. Intra-arterial chemotherapy as a treatment for intraocular retinoblastoma: alternatives to direct ophthalmic artery catheterization. AJNR Am J Neuroradiol. 2012;33(8):1608–14.
Abramson DH, Marr BP, Brodie SE, Dunkel I, Palioura S, Gobin YP. Ophthalmic artery chemosurgery for less advanced intraocular retinoblastoma: five year review. PLoS One. 2012;7(4):e34120.
Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery). Ophthalmology. 2010;117(8):1623–9.
Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Bilateral superselective ophthalmic artery chemotherapy for bilateral retinoblastoma: tandem therapy. Arch Ophthalmol. 2010;128(3):370–2.
Brodie SE, Pierre Gobin Y, Dunkel IJ, Kim JW, Abramson DH. Persistence of retinal function after selective ophthalmic artery chemotherapy infusion for retinoblastoma. Doc Ophthalmol. 2009;119(1):13–22.
Abramson DH, Daniels AB, Marr BP, Francis JH, Brodie SE, Dunkel IJ, et al. Intra-arterial chemotherapy (ophthalmic artery chemosurgery) for group D retinoblastoma. PLoS One. 2016;11(1):e0146582.
Chung CY, Medina CA, Aziz HA, Singh AD. Retinoblastoma: evidence for stage-based chemotherapy. Int Ophthalmol Clin. 2015;55(1):63–75.
MacCarthy A, Birch JM, Draper GJ, Hungerford JL, Kingston JE, Kroll ME, et al. Retinoblastoma: treatment and survival in Great Britain 1963 to 2002. Br J Ophthalmol. 2009;93(1):38–9.
Naseripour M, Nazari H, Bakhtiari P, Modarres-zadeh M, Vosough P, Ausari M. Retinoblastoma in Iran: outcomes in terms of patients’ survival and globe survival. Br J Ophthalmol. 2009;93(1):28–32.
Dimaras H, Dimba EA, Gallie BL. Challenging the global retinoblastoma survival disparity through a collaborative research effort. Br J Ophthalmol. 2010;94(11):1415–6.
Naseripour M. Retinoblastoma survival disparity: the expanding horizon in developing countries. Saudi J Ophthalmol. 2012;26(2):157–61.
Al-Nawaiseh I, Jammal HM, Khader YS, Jaradat I, Barham R. Retinoblastoma in Jordan, 2003-2013: ocular survival and associated factors. Ophthalmic Epidemiol. 2014;21(6):406–11.
Moreno F, Sinaki B, Fandino A, Dussel V, Orellana L, Chantada G. A population-based study of retinoblastoma incidence and survival in Argentine children. Pediatr Blood Cancer. 2014;61(9):1610–5.
Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepalli SA, Caywood EH, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121(7):1453–60.
Munier FL, Mosimann P, Puccinelli F, Gaillard MC, Stathopoulos C, Houghton S, et al. First-line intra-arterial versus intravenous chemotherapy in unilateral sporadic group D retinoblastoma: evidence of better visual outcomes, ocular survival and shorter time to success with intra-arterial delivery from retrospective review of 20 years of treatment. Br J Ophthalmol. 2017;101(8):1086–93.
Abruzzo T, Patino M, Leach J, Rahme R, Geller J. Cerebral vasoconstriction triggered by sympathomimetic drugs during intra-atrerial chemotherapy. Pediatr Neurol. 2013;48(2):139–42.
Shields CL, Bianciotto CG, Jabbour P, Griffin GC, Ramasubramanian A, Rosenwasser R, et al. Intra-arterial chemotherapy for retinoblastoma: report No. 2, treatment complications. Arch Ophthalmol. 2011;129(11):1407–15.
Marr B, Gobin PY, Dunkel IJ, Brodie SE, Abramson DH. Spontaneously resolving periocular erythema and ciliary madarosis following intra-arterial chemotherapy for retinoblastoma. Middle East Afr J Ophthalmol. 2010;17(3):207–9.
Dalvin LA, Ancona-Lezama D, Lucio-Alvarez JA, Masoomian B, Jabbour P, Shields CL. Primary intra-arterial chemotherapy for retinoblastoma in the intravitreal chemotherapy era: five years of experience. Ocul Oncol Pathol. 2019;5:139–46.
Mathew AA, Sachdev N, Staffieri SE, McKenzie JD, Elder JE. Superselective intra-arterial chemotherapy for advanced retinoblastoma complicated by metastatic disease. J AAPOS. 2015;19(1):72–4.
Yousef YA, Soliman SE, Astudillo PP, Durairaj P, Dimaras H, Chan HS, et al. Intra-arterial chemotherapy for retinoblastoma: a systematic review. JAMA Ophthalmol. 2016;134(5):584–91.
Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129(6):732–7.
Shields CL, Lally SE, Leahey AM, Jabbour PM, Caywood EH, Schwendeman R, et al. Targeted retinoblastoma management: when to use intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Curr Opin Ophthalmol. 2014;25(5):374–85.
Yannuzzi NA, Francis JH, Abramson DH. Incidence of orbital recurrence after enucleation or ophthalmic artery chemosurgery for advanced intraocular retinoblastoma--reply. JAMA Ophthalmol. 2016;134(1):114–5.
Yannuzzi NA, Francis JH, Marr BP, Belinsky I, Dunkel IJ, Gobin YP, et al. Enucleation vs ophthalmic artery chemosurgery for advanced intraocular retinoblastoma: a retrospective analysis. JAMA Ophthalmol. 2015;133(9):1062–6.
Berry JL, Kogachi K, Aziz HA, McGovern K, Zolfaghari E, Murphree AL, et al. Risk of metastasis and orbital recurrence in advanced retinoblastoma eyes treated with systemic chemoreduction versus primary enucleation. Pediatr Blood Cancer. 2017;64(4):26270.
Munier FL, Gaillard MC, Balmer A, Soliman S, Podilsky G, Moulin AP, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96(8):1078–83.
Munier FL, Gaillard MC, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: recent advances and perspectives. Saudi J Ophthalmol. 2013;27(3):147–50.
Ildefonso CJ, Kong L, Leen A, Chai SJ, Petrochelli V, Chintagumpala M, et al. Absence of systemic immune response to adenovectors after intraocular administration to children with retinoblastoma. Mol Ther. 2010;18(10):1885–90.
Chevez-Barrios P, Chintagumpala M, Mieler W, Paysse E, Boniuk M, Kozinetz C, et al. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol. 2005;23(31):7927–35.
Ericson LA, Rosengren BH. Tests of intravitreous chemotherapy in retinoblastoma. Acta Ophthalmol. 1962;40:121–2.
Seregard S, Kock E, af Trampe E. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. Br J Ophthalmol. 1995;79(2):194–5.
Kivela T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118(8):1689.
Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268–71.
Shields CL, Fulco EM, Arias JD, Alarcon C, Pellegrini M, Rishi P, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye (Lond). 2013;27(2):253–64.
Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2013;97(10):1231–6.
Sun YB, Hui P, Punyara K, Bi MC, Li SH, Teng SY, et al. Intravitreal injection of melphalan in the treatment of retinoblastoma with vitreous cavity seeding. Chin Med J. 2013;126(8):1587.
Francis JH, Schaiquevich P, Buitrago E, Del Sole MJ, Zapata G, Croxatto JO, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: a preclinical and clinical study. Ophthalmology. 2014;121(9):1810–7.
Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132(8):936–41.
Lawson BM, Saktanasate J, Say EA, Shields CL. Intravitreal chemotherapy provides control for massive vitreous seeding from retinoblastoma. J Pediatr Ophthalmol Strabismus. 2014;51:e92–4.
Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2014;98(3):292–7.
Manjandavida FP, Shields CL. The role of intravitreal chemotherapy for retinoblastoma. Indian J Ophthalmol. 2015;63(2):141–5.
Tuncer S, Balci O, Tanyildiz B, Kebudi R, Shields CL. Intravitreal lower-dose (20 microg) melphalan for persistent or recurrent retinoblastoma vitreous seeds. Ophthalmic Surg Lasers Imaging Retina. 2015;46(9):942–8.
Francis JH, Marr BP, Brodie SE, Gobin P, Dunkel IJ, Abramson DH. Intravitreal melphalan as salvage therapy for refractory retinal and subretinal retinoblastoma. Retin Cases Brief Rep. 2016;10(4):357–60.
Aziz HA, Kim JW, Munier FL, Berry JL. Acute hemorrhagic retinopathy following intravitreal melphalan injection for retinoblastoma: a report of two cases and technical modifications to enhance the prevention of retinal toxicity. Ocul Oncol Pathol. 2017;3(1):34–40.
Berry JL, Shah S, Bechtold M, Zolfaghari E, Jubran R, Kim JW. Long-term outcomes of Group D retinoblastoma eyes during the intravitreal melphalan era. Pediatr Blood Cancer. 2017;64:26696.
Kiratli H, Koc I, Varan A, Akyuz C. Intravitreal chemotherapy in the management of vitreous disease in retinoblastoma. Eur J Ophthalmol. 2017;27(4):423–7.
Berry JL, Bechtold M, Shah S, Zolfaghari E, Reid M, Jubran R, et al. Not all seeds are created equal: seed classification is predictive of outcomes in retinoblastoma. Ophthalmology. 2017;124(12):1817–25.
Francis JH, Marr BP, Brodie SE, Abramson DH. Anterior ocular toxicity of intravitreous melphalan for retinoblastoma. JAMA Ophthalmol. 2015;133(12):1459–63.
Francis JH, Brodie SE, Marr B, Zabor EC, Mondesire-Crump I, Abramson DH. Efficacy and toxicity of intravitreous chemotherapy for retinoblastoma: four-year experience. Ophthalmology. 2017;124(4):488–95.
Munier FL. Intracameral chemotherapy (melphalan) for aqueous seeding in retinoblastoma: bicameral injection technique and related toxicity in a pilot case study. Ocul Oncol Pathol. 2017;3:149–55.
Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35(4):193–207.
Francis JH, Abramson DH, Gaillard MC, Marr BP, Beck-Popovic M, Munier FL. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology. 2015;122(6):1173–9.
Amram AL, Rico G, Kim JW, Chintagumpala M, Herzog CE, Gombos DS, et al. Vitreous seeds in retinoblastoma: clinicopathologic classification and correlation. Ophthalmology. 2017;124(10):1540–7.
Kim JW. Retinoblastoma: evidence for postenucleation adjuvant chemotherapy. Int Ophthalmol Clin. 2015;55(1):77–96.
Magramm I, Abramson DH, Ellsworth RM. Optic nerve involvement in retinoblastoma. Ophthalmology. 1989;96(2):217–22.
Shields CL, Shields JA, Baez KA, Cater J, De Potter PV. Choroidal invasion of retinoblastoma: metastatic potential and clinical risk factors. Br J Ophthalmol. 1993;77(9):544–8.
Yasui N, Kawamoto H, Fujiwara M, Aihara Y, Ogawa C, Hosono A, et al. High-dose chemotherapy for high-risk retinoblastoma: clinical course and outcome of 14 cases in the National Cancer Center, Japan. Bone Marrow Transplant. 2015;50(2):221–4.
Kim JW, Kathpalia V, Dunkel IJ, Wong RK, Riedel E, Abramson DH. Orbital recurrence of retinoblastoma following enucleation. Br J Ophthalmol. 2009;93(4):463–7.
Kremens B, Wieland R, Reinhard H, Neubert D, Beck JD, Klingebiel T, et al. High-dose chemotherapy with autologous stem cell rescue in children with retinoblastoma. Bone Marrow Transplant. 2003;31(4):281–4.
Dunkel IJ, Chan HS, Jubran R, Chantada GL, Goldman S, Chintagumpala M, et al. High-dose chemotherapy with autologous hematopoietic stem cell rescue for stage 4B retinoblastoma. Pediatr Blood Cancer. 2010;55(1):149–52.
Dunkel IJ, Jubran RF, Gururangan S, Chantada GL, Finlay JL, Goldman S, et al. Trilateral retinoblastoma: potentially curable with intensive chemotherapy. Pediatr Blood Cancer. 2010;54(3):384–7.
Dunkel IJ, Khakoo Y, Kernan NA, Gershon T, Gilheeney S, Lyden DC, et al. Intensive multimodality therapy for patients with stage 4a metastatic retinoblastoma. Pediatr Blood Cancer. 2010;55(1):55–9.
Matsubara H, Makimoto A, Higa T, Kawamoto H, Sakiyama S, Hosono A, et al. A multidisciplinary treatment strategy that includes high-dose chemotherapy for metastatic retinoblastoma without CNS involvement. Bone Marrow Transplant. 2005;35(8):763–6.
Lee SH, Yoo KH, Sung KW, Kim JY, Cho EJ, Koo HH, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in children with bilateral advanced retinoblastoma. Bone Marrow Transplant. 2008;42(6):385–91.
Girnius S, Seldin DC, Skinner M, Finn KT, Quillen K, Doros G, et al. Hepatic response after high-dose melphalan and stem cell transplantation in patients with AL amyloidosis associated liver disease. Haematologica. 2009;94(7):1029–32.
Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, et al. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323(6089):643–6.
Tien HF, Chuang SM, Chen MS, Lee FY, Hou PK. Cytogenetic evidence of multifocal origin of a unilateral retinoblastoma. A help in genetic counseling. Cancer Genet Cytogenet. 1989;42(2):203–8.
Lee SY, Jeon DG, Lee JS, Hwang CS, Huh K, Lee TW, et al. Deletion of Rb1 gene in late osteosarcoma from survivor of unilateral retinoblastoma--a case report. J Korean Med Sci. 1996;11(1):94–8.
Kim JW, Lee CG, Han SM, Kim KS, Kim JO, Lee JM, et al. Loss of heterozygosity of the retinoblastoma and p53 genes in primary cervical carcinomas with human papillomavirus infection. Gynecol Oncol. 1997;67(2):215–21.
Fung YK, Murphree AL, T'Ang A, Qian J, Hinrichs SH, Benedict WF. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987;236(4809):1657–61.
Benedict WF, Fung YK, Murphree AL. The gene responsible for the development of retinoblastoma and osteosarcoma. Cancer. 1988;62(8 Suppl):1691–4.
Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–3.
American Academy of P, Section on O, American Association for Pediatric O, Strabismus, American Academy of O, American Association of Certified O. Red reflex examination in neonates, infants, and children. Pediatrics. 2008;122(6):1401–4.
Abramson DH, Schefler AC. Update on retinoblastoma. Retina. 2004;24(6):828–48.
Ayari-Jeridi H, Moran K, Chebbi A, Bouguila H, Abbes I, Charradi K, et al. Mutation spectrum of RB1 gene in unilateral retinoblastoma cases from Tunisia and correlations with clinical features. PLoS One. 2015;10(1):e0116615.
Berry JL, Lewis L, Zolfaghari E, Green S, Le BHA, Lee TC, et al. Suprasellar arachnoid cyst presenting with bobble-head doll movements: a report of 3 cases. Neurol India. 2018;51(3):407–9.
Tang G, Paik S, Baehner FL, Liu Q, Jeong JH, Kim SR, et al. Prognostic impact of the 21-gene recurrence score (RS) on disease-free and overall survival of node-positive, ER-positive breast cancer patients (pts) treated with adjuvant chemotherapy: results from NSABP B-28. J Clin Oncol. 2012;30(27_suppl):1.
Torres-Torres B, Soberino J, Rios S, Gonzalez Rivas CS, Ruiz Vozmediano J, Castillo L, et al. The prognostic value of detection methylation level panel of gene in DNA circulating after and before treatment in patients with breast cancer. J Clin Oncol. 2012;30(30_suppl):67.
Tian S, Wang C, Chang HH, Sun J. Identification of prognostic genes and gene sets for early-stage non-small cell lung cancer using bi-level selection methods. Sci Rep. 2017;7:46164.
Peretti U, Ferrara R, Pilotto S, Kinspergher S, Caccese M, Santo A, et al. ALK gene copy number gains in non-small-cell lung cancer: prognostic impact and clinico-pathological correlations. Respir Res. 2016;17(1):105.
Lopes GL, Vattimo EF, Castro Junior G. Identifying activating mutations in the EGFR gene: prognostic and therapeutic implications in non-small cell lung cancer. J Bras Pneumol. 2015;41(4):365–75.
Tao KY, Li XX, Xu WZ, Wang Y, Zhu SM, Xie HX, et al. Prognostic role of apoptosis-related gene functional variants in advanced non-small-cell lung cancer patients treated with first-line platinum-based chemotherapy. Onco Targets Ther. 2015;8:147–55.
Hauke RJ Jr, Sissung TM, Figg WD. Discussing the predictive, prognostic, and therapeutic value of germline DNA-repair gene mutations in metastatic prostate cancer patients. Cancer Biol Ther. 2017;18(8):545–6.
Berg KD. The prognostic and predictive value of TMPRSS2-ERG gene fusion and ERG protein expression in prostate cancer biopsies. Dan Med J. 2016;63:12.
Kulda V, Topolcan O, Kucera R, Kripnerova M, Srbecka K, Hora M, et al. Prognostic significance of TMPRSS2-ERG fusion gene in prostate cancer. Anticancer Res. 2016;36(9):4787–93.
Mendoza PR, Specht CS, Hubbard GB, Wells JR, Lynn MJ, Zhang Q, et al. Histopathologic grading of anaplasia in retinoblastoma. Am J Ophthalmol. 2015;159(4):764–76.
Mendoza PR, Geisert EE, Ziesel AC, Specht CS, Hubbard GB, Wells JR, et al. Gene expression profiling of retinoblastoma and retinocytoma. Invest Ophthalmol Vis Sci. 2015;56:3444.
Kooi IE, Mol BM, Massink MP, Ameziane N, Meijers-Heijboer H, Dommering CJ, et al. Somatic genomic alterations in retinoblastoma beyond RB1 are rare and limited to copy number changes. Sci Rep. 2016;6:25264.
Kooi IE, Mol BM, Massink MP, de Jong MC, de Graaf P, van der Valk P, et al. A meta-analysis of retinoblastoma copy numbers refines the list of possible driver genes involved in tumor progression. PLoS One. 2016;11(4):e0153323.
Francis JH, Abramson DH, Ji X, Shields CL, Teixeira LF, Schefler AC, et al. Risk of extraocular extension in eyes with retinoblastoma receiving intravitreous chemotherapy. JAMA Ophthalmol. 2017;135(12):1426–9.
Berry JL, Xu L, Kooi I, Murphree AL, Prabakar RK, Reid M, et al. Genomic cfDNA analysis of aqueous humor in retinoblastoma predicts eye salvage: the surrogate tumor biopsy for retinoblastoma. Mol Cancer Res. 2018;16(11):1701–12.
Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer. 2007;46(7):617–34.
Acknowledgments
Dr. Berry has grant support from the National Cancer Institute of the National Institute of Health Award Number K08CA232344, The Robert E. and May R. Wright Foundation, The Knights Templar Eye Foundation, the American Cancer Society #IRG-16-181-57, Hyundai Hope on Wheels and the Childhood Eye Cancer Trust.
Disclosure Statement The authors have no financial disclosures or conflicts of interests to disclose regarding the materials presented herein.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Berry, J.L. (2020). Changing Trends in Retinoblastoma Management and What Is in Store for the Future. In: Khetan, V. (eds) Intraocular Tumors. Springer, Singapore. https://doi.org/10.1007/978-981-15-0395-5_3
Download citation
DOI: https://doi.org/10.1007/978-981-15-0395-5_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0394-8
Online ISBN: 978-981-15-0395-5
eBook Packages: MedicineMedicine (R0)