Abstract
Autosomal dominant conditions are known to be associated with advanced paternal age, and it has been suggested that retinoblastoma (Rb) also exhibits a paternal age effect due to the paternal origin of most new germline RB1 mutations. To further our understanding of the association of parental age and risk of de novo germline RB1 mutations, we evaluated the effect of parental age in a cohort of Rb survivors in the United States. A cohort of 262 Rb patients was retrospectively identified at one institution, and telephone interviews were conducted with parents of 160 survivors (65.3%). We classified Rb survivors into three groups: those with unilateral Rb were classified as sporadic if they had no or unknown family history of Rb, those with bilateral Rb were classified as having a de novo germline mutation if they had no or unknown family history of Rb, and those with unilateral or bilateral Rb, who had a family history of Rb, were classified as familial. We built two sets of nested logistic regression models to detect an increased odds of the de novo germline mutation classification related to older parental age compared to sporadic and familial Rb classifications. The modeling strategy evaluated effects of continuous increasing maternal and paternal age and 5-year age increases adjusted for the age of the other parent. Mean maternal ages for survivors classified as having de novo germline mutations and sporadic Rb were similar (28.3 and 28.5, respectively) as were mean paternal ages (31.9 and 31.2, respectively), and all were significantly higher than the weighted general US population means. In contrast, maternal and paternal ages for familial Rb did not differ significantly from the weighted US general population means. Although we noted no significant differences between mean maternal and paternal ages between each of the three Rb classification groups, we found increased odds of a survivor being in the de novo germline mutation group for each 5-year increase in paternal age, but these findings were not statistically significant (de novo vs. sporadic ORs 30–34 = 1.7 [0.7–4], ≥35 = 1.3 [0.5–3.3]; de novo vs. familial ORs 30–34 = 2.8 [1.0–8.4], ≥35 = 1.6 [0.6–4.6]). Our study suggests a weak paternal age effect for Rb resulting from de novo germline mutations consistent with the paternal origin of most of these mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retinoblastoma (Rb) is a malignant tumor of the retina that occurs in children typically under the age of five. It is estimated that Rb affects 1:15,000 births in the United States (US) (Abramson and Schefler 2004; Lin and O’Brien 2009). RB1 is a tumor suppressor gene involved in regulating the cell cycle, and malignant tumors occur in retinal cells with mutations in both copies of the RB1 gene. Approximately 25–35% of Rb cases are bilateral, affecting both eyes. Individuals who inherit an RB1 mutation in an autosomal dominant manner from a parent or who have a de novo germline mutation, typically develop bilateral Rb. Approximately 65–75% of Rb cases are unilateral, occurring in only one eye, and occur sporadically when “two hits” occur in the same retinal cell (Knudson 1971). Approximately 10% of all Rb cases can be attributed to family history with autosomal dominant inheritance. In addition, family history information and genetic testing has revealed that some unilateral cases (10–15%) involve a germline mutation in the RB1 gene (Lohmann et al.1997; Lohmann and Gallie 2004; Newsham et al. 2009; Richter et al. 2003).
Several autosomal dominant genetic conditions are now known to be associated with advanced paternal age (generally considered to be 40–45 years of age or older) (Thacker 2004; Sartorius and Nieschlag 2010). These include Marfan syndrome, achondroplasia, and Apert syndrome, among others. It has also been suggested that there is a paternal age effect with Rb, albeit a weaker effect than the conditions mentioned above (Kühnert and Nieschlag 2004; Risch et al. 1987; Sivakumaran et al., 2000), Further, it has been estimated that 85% of new RB1 germline mutations are paternal in origin, therefore, it would be expected that older paternal age might be related to the appearance of de novo Rb (Dryja et al. 1989; Kato et al. 1994; Zhu et al. 1989).
There have been a few studies in the literature regarding paternal/maternal age effects but no definitive determination of whether these effects are related to the occurrence of Rb (Bunin et al. 1989, DerKinderen et al. 1990; Johnson et al. 2009; Matsunaga et al. 1990; Moll et al. 1996; Pellié et al. 1973, Yip et al. 2006). This study aims to further our understanding of the association of parental age and risk of de novo germline RB1 mutations by evaluating parental age in a cohort of Rb survivors in the US. Our hypothesis is that Rb survivors with a de novo germline mutation are more likely to have a father of advanced paternal age when compared with survivors of sporadic or familial Rb, and the general population.
Subjects and methods
The retrospectively defined cohort used for this study consists of 262 Rb patients diagnosed from January 1, 1985 through December 31, 1996 at a medical center in New York, NY. This cohort is currently part of a larger study of secondary cancer incidence and cause-specific mortality in long-term Rb survivors (Kleinerman et al. 2005; Yu et al. 2009). Hospital records were used to identify study subjects and to collect medical history and treatment data. Demographic data, including biological parental age at the birth of the Rb patient, were collected via telephone interviews conducted in 1998. Of the original 262 Rb patients, 4 did not survive after 1 year and 13 had died by the time of the interview in 1998. Therefore, parents of 245 survivors were eligible to be interviewed for the study and parents of 160 (65.3%) survivors agreed to participate. We did not identify statistically significant differences between the respondents and non-respondents for hereditary status, year of birth or sex of their child; however, a higher proportion of respondents were Caucasian (p < 0.001) and reported a family history of Rb (p = 0.02). Family history was defined as a first or second degree relative with Rb.
We excluded one individual from the analysis who was born in 1950 because all other members of the cohort were born between 1975 and 1996. Maternal age data were not available for two survivors and paternal age for five survivors, but all other available data for these survivors were included. Therefore, we analyzed 159 of the 160 survivors for this study.
The cohort survivors were grouped into one of three Rb classifications: survivors with unilateral Rb were classified as sporadic if they had no or unknown family history of Rb, survivors with bilateral Rb were classified as having a de novo germline mutation if they had no or unknown family history of Rb, and survivors with unilateral or bilateral Rb, who had a family history of Rb, were classified as familial. The sporadic and de novo germline mutation classifications based on laterality of Rb tumors are an approximation, because mutation testing data were not available for the cohort.
In this study, we have included Rb survivors with a family history of Rb because this group likely has children at the same age as parents in the general population. Published mean maternal age data for the US are available for the years 1970–2000 (Mathews and Hamilton 2002), however, mean paternal age data are not, so we calculated mean paternal age from public use files available from the US Centers for Disease Control and Prevention, National Center for Health Statistics Reproductive Statistics Branch, Division of Vital Statistics (US CDC data) for births for the years 1975–1996. We also calculated mean maternal age for each year from 1975 to 1996 and then compared these data to those published by Mathews and Hamilton (2002) and found our calculations to be accurate to within 0.1 years. Paternal age data for the US were missing for 11.1% of fathers in 1975 up to a high of 16.9% of fathers in 1991. For comparison to the mean parental ages in our cohort, we calculated a weighted mean for mean maternal and paternal age in the US general population based on the years of birth of our cohort of survivors.
Statistical analysis
Associations between categorical predictors and the three Rb classification groups were assessed with either Chi-square tests or the Freeman–Halton extension of the Fisher’s exact test when small expected cell frequencies were noted. Two sets of nested logistic regression models were built to look for increased odds of a de novo germline mutation classification versus sporadic and familial Rb associated with increased maternal and paternal age. The first modeling strategy looked for effects of continuous increasing maternal and paternal age while controlling for differences in race (White, African-American, Hispanic, other/unknown) and age of the other parent. To assess nonlinear age effects, a second set of models using parental age groupings of <30, 30–34, and ≥35 years were subsequently analyzed while controlling for differences in race and age of the other parent. We used parental age <30 years as our reference group because we had a limited number of fathers and mothers less than age 25. All differences between means were compared using either one sample t tests or one-way ANOVAs. For all tests, statistical significance was declared for two tailed p values p < 0.05. All calculations were performed using SAS Enterprise Guide version 4.22, SAS version 9.2 (Cary, NC, USA).
Results
We classified 75 (47.2%) survivors as sporadic, 46 (28.9%) as de novo germline mutation and 38 (23.9%) as familial (Table 1). We found no noteworthy differences between sex, race, year of birth, age of mother at birth, and age of father at birth for each of the three groups. Laterality and family history differed for each of the Rb classification groups by definition. As expected, de novo germline mutation and familial cases of Rb were more likely to be diagnosed at less than 1 year of age as compared to sporadic cases (p < 0.001).
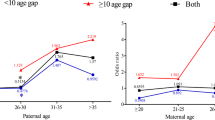
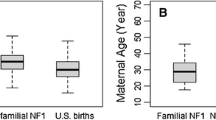
Mean maternal and paternal ages for the de novo germline mutation classification were significantly higher than the weighted mean maternal (28.3 vs. 26.2 years, p = 0.003) and paternal (31.9 vs. 29.3 years p = 0.007) ages of the general US population (Table 2). Similarly, maternal and paternal ages for the sporadic Rb classification group were significantly higher than the mean maternal and paternal ages of the general US population (p < 0.001 and p = 0.01, respectively). Conversely, the parental ages for the familial classification group did not differ significantly from the general US population. Although the three Rb classification groups did not differ significantly from each other for maternal (p = 0.2) or paternal (p = 0.3) mean ages, the mean maternal and paternal ages for both de novo and sporadic Rb groups were similar (28.3 and 28.5, and 31.9 and 31.2, respectively) whereas the mean ages for the familial parents were approximately 1.7 years younger than mothers of presumed sporadic and de novo survivors and approximately 1.8 years younger than the average age for fathers of presumed sporadic and de novo survivors.
In our analysis of continuous parental age, there was no statistically significant effect of maternal or paternal age when the odds of having a presumed de novo germline mutation were compared to the odds of having sporadic Rb or familial Rb. The odds ratios for almost all scenarios were close to 1.0, even when the analysis was adjusted for race and the age of the other parent.
When we examined the odds of having a presumed de novo germline mutation versus the odds of having familial Rb by 5-year age groups for paternal age, we found increased odds of having a de novo germline mutation versus the odds of having either sporadic Rb or familial Rb (Table 3). The effect was highest for the 30–34 paternal age group but no effects were significant, even when adjusted for race and age of the other parent (Table 3). We found a non-significant increase in the odds of having a presumed de novo germline mutation versus the odds of having familial Rb, for maternal ages 30–34 (OR = 2.8 [0.9–8.8]). However, the odds for maternal age greater than 35, was reduced (OR = 0.8 [0.2–3.3]).
Discussion
In this study, we have assessed the influence of older parental age on Rb caused by de novo autosomal dominant mutations using a cohort of Rb survivors diagnosed and treated at one institution. To our knowledge, this is the first study to evaluate parental age effects for three different categories of Rb (hereditary Rb resulting from a de novo germline mutation, sporadic Rb, and familial Rb inherited from an affected parent), and investigate parental age differences between these three groups.
Although we found no significant differences between maternal and paternal ages when comparing the three Rb groups, there was some evidence of a signal for older paternal age and the odds of a de novo germline mutation based on the modeling. We also noted that mean parental ages related to de novo germline mutations and sporadic Rb, but not familial Rb, were higher than the general population. This finding supports the notion that, in general, those with familial Rb reproduce at ages similar to the general population, but have a 50/50 chance of passing on the mutated RB1 allele with each pregnancy. It was not surprising to find younger parental ages for familial Rb cases when compared to sporadic Rb because this was reported in a previous study (Yip et al. 2006). However, the similarity of mean maternal and paternal ages for de novo germline mutations and sporadic Rb was unexpected, because it has not been previously reported. Although some of the unilateral patients may have been misclassified as sporadic, there is no obvious explanation why these ages are similar. In addition, paternal age data for the US were missing for 11.1% of fathers in 1975 up to a high of 16.9% of fathers in 1991. We believe that our estimate of paternal age may be an underestimate due to the number of mothers under the age of 25 who report no age of biological father on birth certificates. For example, in 2006 there was no information regarding the biological father’s age for 25% of births in women less than 25 years of age (Martin, et al. 2009). If the mean age of fathers in the general population is even younger than that presented in our data, the differences presented between our classification groups and the general population would likely be even more significant.
When comparing our study with previous studies regarding parental age data for Rb (see Table 4), it is important to note that we compared our hospital-based cohort to the general population in our analysis of mean maternal and paternal age. We did not use matched general population controls for our logistic regression analysis nor did we use incidence data for Rb in the United States. Unlike previous studies, we compared the odds of being in the de novo germline mutation classification group versus the sporadic and familial Rb groups given increasing parental age in our cohort of individuals. Our comparisons of parental age to general population means were similar to findings reported by DerKinderen et al. (1990) and Moll et al. (1996) for de novo mutations, but in contrast to these studies, we also saw significant differences for sporadic non-hereditary Rb.
Although our comparisons of parental age to general population means were similar to previously reported findings, there are several limitations to our study. The classifications performed for our study were accomplished by proxy with laterality and family history information. Thus, some unilateral survivors in our cohort may have a de novo mutation that predisposed them to Rb and would be misclassified in our study. In our cohort, we had relatively few parents over the age of 35 years making it difficult to estimate the odds of having a child with a de novo mutation for fathers of advanced paternal age (greater than 40–45 years of age). The theory behind de novo mutations and advanced paternal age is that errors occur in mitotic divisions during male spermatogenesis (Thacker 2004; Sartorius and Nieschlag 2010). Although our results suggested a pattern of increased odds of a de novo mutation with paternal age, we believe this is a preliminary finding that should be followed up with additional analysis. In addition, our study compared survivors from one referral hospital in New York City to all births in the US general population rather than to matched controls. Our study also did not include patients who died from Rb and thus may have missed more seriously affected patients. Of the survivor’s families contacted, only 65% of the cohort agreed to participate; having more subjects would allow for statistical power to determine if the patterns we observed in this analysis are valid. In addition, more complete information on subject characteristics, such as race and socioeconomic status, would allow for a more detailed analysis of potential confounders. Additional research could also consider the influence of other covariates such as environmental exposures of one or both parents that could increase the risk of de novo RB1 mutations (Bunin et al. 2011).
Future work regarding Rb and parental age should include mutation status gathered from genetic testing rather than by proxy with laterality and family history, since some unilateral survivors, classified as sporadic in our cohort, may in fact have a de novo mutation that predisposed them to Rb. Additional studies of parental age and Rb could be accomplished by randomly selecting individuals diagnosed with Rb from the general population regardless of vital status, classifying Rb patients with respect to mode of inheritance and genetic testing, and comparing the patient sample to a sample of general population controls matched to cases on birth year. A dataset that includes mutation status, rather than the by proxy classifications presented herein, would allow for a more precise analysis of the differences in parental ages of the three Rb classification groups, given an adequate number of patients to achieve statistical power. Although prior studies have benefited from larger sample sizes, we believe this is the first study to include familial inherited Rb in an analysis of parental age. Familial, inherited Rb may be a more appropriate control in this study, as opposed to sporadic Rb, in that individuals with familial germline Rb mutations have children at similar ages to the general population, and Rb occurs due to the 50% risk to offspring of an RB1 mutation carrier. This similarity in ages for the familial Rb classification group and the general US population was also seen in the mean parental age data shown in Table 2.
Overall, our findings show that, as previously reported for other countries, the mean parental age of Rb survivors with a presumed de novo mutation is statistically significantly higher than the mean age of the general US population. The similarity of mean maternal and paternal ages for presumed de novo germline mutations as well as for presumed sporadic Rb was unexpected and deserves further attention, because there is no obvious explanation for this similarity. Our study suggests a greater paternal rather than maternal contribution to RB1 mutations, perhaps during gametogenesis, for the de novo germline mutations; however, we have insufficient data for investigating paternal age over 40 years to test this hypothesis further. Our findings do not indicate statistically significant effects for advanced paternal age and thus would not be appropriate for use in genetic counseling at this time.
References
Abramson D, Schefler A (2004) Update on retinoblastoma. Retina 24(6):828–848
Bunin GR, Meadows AT, Emanuel BS, Buckley JD, Woods WG, Hammond GD (1989) Pre- and postconception factors associated with sporadic heritable and nonheritable retinoblastoma. Cancer Res 49(20):5730–5735
Bunin GR, Felice MA, Davidson W, Friedman DL, Shields CL, Maidment A, O’Shea M, Nichols KE, Leahey A, Dunkel IJ, Jubran R, Rodriguez-Galindo C, Schmidt ML, Weinstein JL, Goldman S, Abramson DH, Wilson MW, Gallie BL, Chan HS, Shapiro M, Cnaan A, Ganguly A, Meadows AT (2011) Medical radiation exposure and risk of retinoblastoma resulting from new germline RB1 mutation. Int J Cancer 128(10):2393–2404. doi:10.1002/ijc.25565
DerKinderen DJ, Koten JW, Tan KE, Beemer FA, Van Romunde LK, Den Otter W (1990) Parental age in sporadic hereditary retinoblastoma. Am J Ophthalmol 110(6):605–609
Dryja TP, Mukai S, Petersen R, Rapaport JM, Walton D, Yandell DW (1989) Parental origin of mutations of the retinoblastoma gene. Nature 339(6225):556–558. doi:10.1038/339556a0
Johnson KJ, Carozza SE, Chow EJ, Fox EE, Horel S, McLaughlin CC, Mueller BA, Puumala SE, Reynolds P, Von Behren J, Spector LG (2009) Parental age and risk of childhood cancer: A pooled analysis. Epidemiology 20(4):475–483. doi:10.1097/EDE.0b013e3181a5a332
Kato MV, Ishizaki K, Shimizu T, Ejima Y, Tanooka H, Takayama J, Kaneko A, Toguchida J, Sasaki MS (1994) Parental origin of germ-line and somatic mutations in the retinoblastoma gene. Hum Genet 94(1):31–38
Kleinerman RA, Tucker MA, Tarone RE, Abramson DH, Seddon JM, Stovall M, Li FP, Fraumeni JF Jr (2005) Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: An extended follow-up. J Clin Oncol 23(10):2272–2279. doi:10.1200/JCO.2005.05.054
Knudson AG Jr (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 68(4):820–823
Kühnert B, Nieschlag E (2004) Reproductive functions of the ageing male. Hum Reprod Update 10(4):327–339. doi:10.1093/humupd/dmh030
Lin P, O’Brien JM (2009) Frontiers in the management of retinoblastoma. Am J Ophthalmol 148(2):192–198. doi:10.1016/j.ajo.2009.04.004
Lohmann DR, Gallie BL (2004) Retinoblastoma: revisiting the model prototype of inherited cancer. Am J Med Genet C Semin Med Genet 129C(1):23–28. doi:10.1002/ajmg.c.30024
Lohmann DR, Gerick M, Brandt B, Oelschlager U, Lorenz B, Passarge E, Horsthemke B (1997) Constitutional rb1-gene mutations in patients with isolated unilateral retinoblastoma. Am J Hum Genet 61(2):282–294. doi:10.1086/514845
Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Mathews TJ (2009) Births: final data for 2006. Natl Vital Stat Rep 57(7):1–102
Mathews TJ, Hamilton BE (2002) Mean age of mother, 1970–2000. Natl Vital Stat Rep 51(1):1–13
Matsunaga E, Minoda K, Sasaki MS (1990) Parental age and seasonal variation in the births of children with sporadic retinoblastoma: a mutation-epidemiologic study. Hum Genet 84(2):155–158
Moll AC, Imhof SM, Kuik DJ, Bouter LM, Den Otter W, Bezemer PD, Koten JW, Tan KE (1996) High parental age is associated with sporadic hereditary retinoblastoma: The Dutch retinoblastoma register 1862–1994. Hum Genet 98(1):109–112
Newsham IF, Hadjistilianou T, Cavenee WK (2009) Retinoblastoma. In: Amberger J, Bocchini CA, Scott AF, Hamosh A (eds) Mckusick’s online mendelian inheritance in man (omim). Nucleic Acids Res 37 (database issue):D793–D796. doi:10.1093/nar/gkn665
Pellié C, Briard ML, Feingold J, Frézal J (1973) Parental age in retinoblastoma. Humangenetik 20:59–62
Richter S, Vandezande K, Chen N, Zhang K, Sutherland J, Anderson J, Han L, Panton R, Branco P, Gallie B (2003) Sensitive and efficient detection of rb1 gene mutations enhances care for families with retinoblastoma. Am J Hum Genet 72(2):253–269. doi:10.1086/345651
Risch N, Reich EW, Wishnick MM, McCarthy JG (1987) Spontaneous mutation and parental age in humans. Am J Hum Genet 41(2):218–248
Sartorius GA, Nieschlag E (2010) Paternal age and reproduction. Hum Reprod Update 16(1):65–79. doi:10.1093/humupd/dmp027
Sivakumaran TA, Ghose S, Kumar H, Sethi A, Kucheria K (2000) Parental age in Indian patients with sporadic hereditary retinoblastoma. Ophthalmic Epidemiol 7 (4):285-291
Thacker PD (2004) Biological clock ticks for men, too: genetic defects linked to sperm of older fathers. JAMA 291(14):1683–1685. doi:10.1001/jama.291.14.1683
Yip BH, Pawitan Y, Czene K (2006) Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol 35(6):1495–1503. doi:10.1093/ije/dyl177
Yu CL, Tucker MA, Abramson DH, Furukawa K, Seddon JM, Stovall M, Fraumeni JF Jr, Kleinerman RA (2009) Cause-specific mortality in long-term survivors of retinoblastoma. J Natl Cancer Inst 101(8):581–591. doi:10.1093/jnci/djp046
Zhu XP, Dunn JM, Phillips RA, Goddard AD, Paton KE, Becker A, Gallie BL (1989) Preferential germline mutation of the paternal allele in retinoblastoma. Nature 340(6231):312–313. doi:10.1038/340312a0
Acknowledgments
We are grateful for the support of the Stanford University School of Medicine Master’s Program in Human Genetics and Genetic Counseling. This research was supported in part by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Conflict of interest
The authors declare that they have no conflict of interest and that this study was conducted in compliance with the laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mills, M.B., Hudgins, L., Balise, R.R. et al. Mutation risk associated with paternal and maternal age in a cohort of retinoblastoma survivors. Hum Genet 131, 1115–1122 (2012). https://doi.org/10.1007/s00439-011-1126-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-011-1126-2