Abstract
With the increasing need to conserve the perishable energy sources many techno-innovative ideas have been looked upon to retain as well as enhance the availability of energy sources. Bioenergy is one such source of renewable energy that concentrates on biological wastes as a source of energy. A plant microbial fuel cell (PMFC) is an advanced form of microbial fuel cell, which uses living plants to generate bioelectricity. Apart from being a renewable source, it is also an in-situ sustainable source of bioenergy. Over the past decade a lot of novel designs have been tried for the PMFCs. The basic design of the PMFC model is that of the sediment PMFC using rice paddy. It was tested in Japan in 2007 which attained a power density of 5.75 mW/m2. Another design is that of a dual chamber plant MFC with common cord grass which was tested by M. Helder et al. in 2009 that achieved 222 mW/m2 power density. A novel tubular design was tested to make it cost effective with reed mannagrass by R. Timmers et al. in 2012 that gained a power density of 60 mW/m2. As such, there are various possible models for different plant species. This article describes the different designs of PMFC with various possible anode and cathode combinations.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Depletion of natural resources with a rapidly changing climate has forced mankind to develop and exploit new possibilities in obtaining sustainable and reliable energy sources. Renewable technologies like solar, hydro, wind and bioenergy have already been implemented in daily life. Market share of biofuels, such as bioethanol, biodiesel and bioelectricity is increasing (Eisentraut 2010). However bio energy is not always sustainable. Deforestation and competition with food production on arable land are the two frequent disadvantages (Haveliek and Schneider 2011). Thus, many countries focus on increased usage of renewable energy, as it helps curb difficulties faced due to greenhouse gas emissions and reducing global warming by replacing conventional energy sources (Panswar et al. 2011; Arent et al. 2011; Swift-Hook 2013).When renewable energy sources are considered for electricity generation, they provide benefits for managerial techniques for the economic growth, as well as for environment (Varun et al. 2009). The various bioenergy sources discovered till date are: biodiesel, biohydrogen, bioethanol, biogas, microbial fuel cell and plant microbial fuel cells.
Depending on the source of bioenergy, the biofuels are classified as 1st generation biofuels that are obtained from edible crop seeds, 2nd generation biofuels which are obtained from non-edible seeds or energy crops, the 3rd generation biofuels which are algae based biofuels and the 4th generation biofuels that are advanced biofuels (Daroch et al. 2013). In 1911, a new source for bioenergy generation was discovered which exploits microorganisms for electricity production. They are the Microbial fuel cells (MFCs).
1.1 Microbial Fuel Cells
Microbial fuel cell is a biological fuel cell that utilises the microorganisms to generate electricity. These microbes breakdown the organic compounds contained in the electrolyte system into electrons and protons (Liu et al. 2004). A general setup of an MFC consists of two chambers separated by a membrane. The anode is placed in the anode chamber where the necessary fuel is supplied. Oxidation reaction takes place where the organic compounds are broken into electrons and H+ protons (Park and Gregory 2000). An oxidizer is supplied to the cathode chamber where a reduction reaction takes place near the cathode. Figure 17.1 gives a brief setup of the microbial fuel cell.
Unlike other conventional fuel cells, MFCs utilise biological matter as its nutrient and energy source (Chaudhuri and Lovley 2003). In general MFCs can be classified as mediated or mediator-less cells based on the addition of electron carriers in the anode chamber. Mediator-less fuel cell has better control on the fuel cell and provides a higher efficiency potential (Liu et al. 2004). MFC has many possible advantages over conventional fuel cells, which is the reason why increasing amount of research is being conducted on it. Recent advances in MFCs show that plants are introduced in the electrolyte system to boost the current generation.
1.2 Plant Microbial Fuel Cell (PMFC)
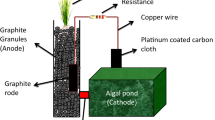
Plants release a considerable amount of organic compounds (C6H12O6) during the process of photosynthesis. About 50–60% of the fixed carbon is transferred from the leaves to the roots, depending on the type of plant, the growth structure and the surrounding environmental conditions (Lynch and Whipps 1990). Bacteria which are electrochemically active , present around the roots breakdown this organic matter, releasing electrons. Electricity is generated when an electron acceptor or an electrode is placed in the vicinity of these bacteria. The electrode material with a higher potential is used as an anode. The plant is allowed to grow in the anode region where the anodic environment is made favourable for the plant growth. Figure 17.2 provides the basic design of a plant microbial fuel cell.
Electricity generation takes place in two steps. (i) In the anode chamber, electrons are released wherein they are taken up by the anode and transferred to the cathode by an external circuit through a load (Timberlake 2009). (ii) The electrons combine with the protons that permeate through the membrane forming water in the cathode region.
Equations 17.1, 17.2 and 17.3 gives the anode and cathode reactions as follows:
Anode:
Cathode:
Net Reaction:
The power generation in a PMFC is less (approx. 10 W/m2) as compared to wind (5–7.7 MW/m2) and solar (4.5–7.5 MW/m2); however it is cheaper as compared to the both of them.But, the impact on the environment of both wind turbines and solar panels is high and at the same time is a source of social debate (Mc Gowan and Connors 2000; Kazmerski 2006). PMFCs aim at electricity generation while sustaining the natural environment by providing in-situ bioenergy. It provides continuous electricity for a week without using any substrates and with energy conservation.
The diversity of microbes is considerably high in PMFC. The conversion of rhizodeposits to current is mainly due to the interactions between fermentative microorganisms and the electrochemically active bacteria . Optimization of plant species, cell design and nutrient media are essential to enhance the current production in terms of power density (Lu et al. 2015). Demarcating exudates and rhizodeposits in plants is difficult, hence, root exudates are defined as all the organic substances released by healthy and intact roots of the plant into the lower soil. The root exudates comprise of carbohydrates, amino acids, amides, aliphatic acids, aromatic acids, fatty acids, and vitamins (Grayston et al. 1997). The nutrient acquisition mainly depends on the release of exudates by the roots in the lower soil (Marschener 1998) (Fig. 17.3).
2 Material Selection
2.1 Selection of Plants for PMFC
The efficiencies of energy conversion are highly depending on the plant species. Hence Selection of plants has to be done precisely. Flowering plants consume more organic matter for pollen formation, hence are substituted by marshland grasses. Table 17.1 show various plants suitable for PMFC.
Plants like Spathyphilum (peace lily) have a flower like formation, but are known to withstand soaked soil for a good duration. Examples of such plants under study are those of Spartina anglica, Glyceria maxima, Oryza sativa, Arundinella anomala and Musa acuminate.
2.2 Selection of Electrodes
The electrodes can provide support for the growth of biocatalyst and connection. They act as electron acceptors completing the circuit. Hence, selection of the electrodes surface is very crucial. Just like Plant free MFC, electrode material with higher potential behaves as an anode. Table 17.2 gives a list of the possible materials of electrodes and other components.
Proper placing of the anode and cathode is crucial as the distance between the anode and cathode affects the proton diffusion between the two (Cheng et al. 2006). Also, cathode modification with catalyst accelerates oxygen reduction (Tender et al. 2002). In PMFCs the potential of the cell is dependent on the maximum theoretical voltage of the cell, current density and the internal resistance of the cell, all with respect to the geometric area of the anode or the membrane. Acetate is generally added to supply substrate to the microbe culture in the cell to enhance electricity generation (Kaku et al. 2008).
3 Classification of PMFC
PMFCs are generally of two types, single chambered and dual chambered. The most basic form of a plant MFC is the ‘sediment type PMFC’ which consists of a single chamber incorporating both the electrodes in the same chamber. The dual chamber PMFC consists of an anode and a cathode chamber separated by a proton exchange membrane. Figure 17.4 explains the various classifications of PMFC models.
3.1 Sediment PMFC
A sediment PMFC consists only of one chamber that maintains both the anode and the cathode. Figure 17.5 depicts the basic model of the sediment PMFCs. The cathode is however exposed to the atmosphere near the top soil (Letebvre et al. 2008). The absence of the proton exchange membrane makes the system very cost effective. But the rate of current generation is very low due to high diffusion rates of the electrons and proton (Chiao et al. 2006).
3.2 Rooftop PMFC
An advanced form of a PMFC is a rooftop system, which combines the advantages of green roofs with that of electricity generation (Helder et al. 2013). Rooftop PMFCs are a type of single chamber PMFCs wherein they incorporate the electrode-plant assembly onto the household roofs. As the area of application is large and to make it cost affective, the use of a membrane is eliminated. As seen in Fig. 17.6, it generally employs grass species which grow in extensive covers.
Other advantages of a rooftop PMFC is that it prevents rainwater runoff, provides higher aesthetic value, preserves biodiversity, improves the oxygen content in the atmosphere and decreases the temperature within cities (Strik et al. 2011).
3.3 Cylindrical PMFC
A cylindrical PMFC consists of two concentric cylinders wherein the anode chamber is held in the cathode chamber (Logan et al. 2006).The two chambers are separated with a Nafion 117A membrane. Cocopeat is used in the anodic chamber and a graphite rod acts as the anode. The cathode chamber is filled with water and a graphite sheet is placed at the base as cathode (Fig. 17.7).
3.4 Tubular PMFC
Reduction of the anode material is very essential for the cost effective models. On reducing the anode material comparable power outputs per meter square of the membrane surface areas can be achieved. Cylindrical PVC T-piece can be used to create an air tight container fixed with PVC discs at both ends. A tubular ultrafiltration membrane is held through the centre of both discs. Anode is placed outside the membrane in the tube while the cathode is placed inside the ultrafiltration membrane (Timmers et al. 2013). Figure 17.8 depicts the tubular PMFC design. The system can be tried for anode materials of graphite felt and graphite granules.
3.5 Flat Plate PMFC
A flat porous plate PMFC can be constructed using acrylic sheets. The anode is made from three graphite felt layers, stacked with plastic rings in between them. The three layers are connected using golden wire as current collector. Cathode consists of one layer of graphite felt and catholyte is constantly circulated through the cathode. System is controlled by external resistances ranging from 500 to 1000 𝛺 (Park and Gregory 2000; Helder et al. 2012) (Fig. 17.9).
3.6 Dual Chambered Box Type PMFC
In a box type dual chambered PMFC, the anode and cathode chambers are separated with a Nafion 117A membrane. Both the anode and cathode chambers are of box shape having equal dimensions. The desired plant is potted along with the anode in the anode chamber, while still water is maintained in the cathode chamber. Graphite sheets and cocopeat-water mixture can be used as electrode material and anolyte respectively (Fig. 17.10).
4 Measurement Techniques
For considering a PMFC in an application , it is necessary to determine the performance parameters. The following are the measured parameters of the plant in beaker sediment MFC system
-
Voc: Open Circuit Voltage (Volts)
-
Isc: Short circuit Current (mA/m2GA)
-
V: Generated Voltage (Volts)
-
Rext: External Resistance (Ω)
The three main performance parameters are the current density (I), the internal resistance (Rint) and the power density (P) of the PMFC. All these parameters are evaluated with respect to the anode geometric area (GA). Anode which comprises of mostly carbon cloth or graphite sheets is expensive. Transforming the PMFC into a cost efficient and easy accessible system is a prime motive. Hence, the performance parameters are evaluated with respect to the GA of the anode (Strik et al. 2008). Equations 17.4, 17.5 and 17.6 provide the performance parameters necessary for the evaluation of the PMFC.
Current density (mA/m2GA)
Internal Resistance (Ω/m2 GA)
Power density (mW/m2GA)
5 Polarization Curves
Based on the current densities and the power densities , polarization curves were obtained for different values of resistances like 50𝛺, 100𝛺, 500𝛺, 1000𝛺, 1500𝛺 and 2000𝛺. The polarization curves are necessary, for they determine the maximum power density of the system. From the maximum power density, one can obtain the internal resistance of the system. The internal resistance of the PMFC is the external resistance corresponding to the maximum power density in the polarization curve. In order to enhance the performance of the system, the internal resistance needs to be reduced by maintaining a constant resistance (value maintained to that of internal resistance) across the circuit.
6 Model Reviews
MFC performance of PMFCs varies with its difference in its design (Table 17.3). A preliminary MFC performance test for the voltage it can generate and the current produced for different resistances is essential for the selection of suitable plant for a PMFC setup. This data provides us with the plants individual power density with respect to the anode GA. Current and power densities are measured in terms of the anode GA, so as to determine the systems dependence on the anode material (Strik et al. 2008).
Determining the internal resistance is quite crucial. Polarization curves are drawn with the current and voltages at different resistance values for the systems. Internal resistance can be obtained from polarisation curves, which is equivalent to the external resistance at max power density . N. Kaku, et al. obtained the polarisation curve for rice paddy electricity generation system, with max power density of 5.75 mW/m2 (Kaku et al. 2008).
An initial incubation period of 50–100 days or an open cell voltage of 0.4 V, whichever is attained earlier, is necessary for producing an electrical current. This is mainly due to various reasons such as omission of nutrients, release of oxygen, conducted through the ‘aerenchyma’, scavenging the electrons collected at the anode or lack of an adapted anodic microbial consortium (Schamphelaire et al. 2008). Experimental evidences show that bright sunny days give higher output compared to dull rainy days (Kaku et al. 2008). The amount of biomass produced above ground determines the functioning ability of the plant in the PMFC system. Experimental data by M. Helder et al. determines how the above ground biomass values in form of total leave and stem length increases during the time of study (Helder et al. 2010).
A novel design tubular PMFC was tested by R. Timmers et al. (2013). The design mostly concentrated on reducing the anode material by incorporating it in the form of a tubular roll. The design achieved a maximum power density of 60 mW/m2 during initial polarization. K. Wetser et al. (2015) used a dual cathode chamber flat plate PMFC system for salt water species of S.anglica (Wetser et al. 2015).
7 Conclusion
Recent advances and extensive research has been carried out in developing the technique of PMFC. Innovative techniques were involved to improve the power density and match the current crop based electricity systems. Four designs were discussed depicting their functional parameters. Power density as high as 222 mW/m2 anode GA was obtained for a dual chamber cylindrical PMFC. Also, reducing the anode material in a tubular design, power density of 60 mW/m2 is achieved. Yet, further fundamental research and technological integration is needed to display the full plant power electricity potential. After which complete environmental and economic analysis can be done.
References
Arent DJ, Wise A, Gelman R (2011) The status and prospects of renewable energy for combating global warming. Energy Econ 33:584–593
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediator-less microbial fuel cells. Nat Biotechnol 21:1229–1234
Cheng S, Liu H, Logan BE (2006) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40:2426–2432
Chiao M, Blam K, Lewei L (2006) Micro machined microbial and photosynthetic fuel cells. J Micromechanical Micro Eng 16:2547–2553
Daroch M, Geng S, Wang G (2013) Recent advances in liquid biofuel production from algal feed stocks. Appl Energy 102:1371–1381
Eisentraut A (2010) Sustainable production of second generation biofuels. IEA Energy papers, France, vol 1, pp 1–221
Grayston SJ, Vaughan D, Jones D (1997) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56
Havliek P, Schneider UA (2011) Global land-use implications of first and second generation biofuel targets. Energy Policy 39:5690–5702
Helder M, Strik D, Hamelers H (2010) Concurrent bioelectricity and biomass production in three plant microbial fuel cells. J Bioresource Technol 101:3541–3547
Helder M, Strik DP, Hamelers HV, Kuijken RCP, Buisman CJ (2012) Year round performance of the flat-plate plant-microbial fuel cell. Bioresour Technol 104:417–423
Helder M, Strik D, Timmers R (2013) Resilience of roof-top plant-microbial fuel cells during Dutch winter. J Biomass and Bioenergy 51:1–7
Kaku N, Yoneawa N, Kodama Y (2008) Plant/microbe cooperation for electricity generation in a rice paddy field. J Appl Microbiol Biotechnol 79:43–49
Kazmerski LL (2006) Solar photovoltaic R&D at the tipping point: a 2005 technology overview. J Electron Spectrosc Relat Phenom 150:105–135
Lefebvre O, Al-Mamun A, Ooi WK, Tang Z, Chua DH, Ng HY (2008) An insight into cathode options for microbial fuel cells. Water Sci Technol 57:2031–2037
Liu H, Ramnarayanan R, Logan R (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285
Logan BE, Hamelers HWM, Rozendal R, Schröder U (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Lu L, Xing D, Ren Z (2015) Microbial community structure accompanied with electricity production in a constructed wetland plant MFC. J Bioresource Biotechnol 195:115–121
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant and Soil 129:1–10
Marschener H (1998) Role of root growth, arbuscular mycorrhiza, and root exudates for the efficiency in nutrient acquisition. Field Crop Res 56:203–207
McGowan JG, Connors SR (2000) Windpower: a turn of the century review. Annu Rev Energy Environ 25:147–197
Panswar NL, Kaushik SC, Kothari S (2011) Role of renewable energy sources in environmental protection: a review. Renew Sustain Energy Rev 15:1513–1524
Park DH, Gregory Z (2000) Electricity generation in microbial fuel cells using neutral red as an Electronophore. Appl Environ Microbiol 66:1292–1297
Schamphelaire LVD, Bossche H, Dand S (2008) Microbial fuel cell generating electricity from Rhizodeposits of Rice plants. J Environ Sci Technol 42:3053–3058
Strik DPBTB, Hamelers HVM, Snel JFH, Buisman CJN (2008) Green electricity production with living plants and bacteria in a fuel cell. Int J Energy Resources 32:870–876
Strik DPBTB, Timmers RA, Helder M, Steinbusch KJJ, Hamelers HVM, Buisman CJN (2011) Microbial solar cells: applying photosynthetic and electrochemically active organisms. Trends Biotechnol 29:41–49
Swift-Hook DT (2013) The case for renewables apart from global warming. Renew Energy 49:147–150
Tender LM, Reimers CE, Stecher HA, Holmes DE, Bond DR (2002) Harnessing microbially generated power on the seafloor. Nat Biotechnol 20:821–825
Timberlake KC (2009) Basic chemistry, 3rd edn. Prentice Hall, Upper Saddle River
Timmers R, Strik D, Hamelers H (2013) Electricity generation by a novel design tubular plant microbial fuel cell. J Biomass Bioenergy 51:60–67
Varun, Parakash R, Bhat IK (2009) Energy, economics and environmental impacts of renewable energy systems. Renew Sustain Energy Rev 13:2716–2721
Wetser K, Sudirjo E, Buisman C (2015) Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. J Appl Energy 137:151–157
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd
About this chapter
Cite this chapter
Borker, M., Suchithra, T.V., Srinivas, M., Jayaraj, S. (2017). Sustainable Bioelectricity Generation from Living Plants. In: Patra, J., Vishnuprasad, C., Das, G. (eds) Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-10-6847-8_17
Download citation
DOI: https://doi.org/10.1007/978-981-10-6847-8_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6846-1
Online ISBN: 978-981-10-6847-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)