Abstract
Global food security is become central focus, specifically because of threatening plant diseases caused by fungal phyto-pathogens and massive economic losses thereof. In the context of bio-control of fungal phyto-pathogens, lipopeptides produced by Bacillus sp. have been studied well. The three families of Bacillus lipopeptides are surfactin, iturins and fengycins, confirmed for their antagonistic activities against various fungal phyto-pathogens. In recent past lipopeptides produced by Pseudomonas sp. has also proven effective bio-control agents, specifically against fungal phyto-pathogens. On other hand echinocandins are novel class of antifungal lipopeptides produced by various Aspergillus sp., used successfully in treatment of serious fungal infections and currently in clinical trials. Here we summarized all available information and data of lipopeptides in focus of their use as bio-control agent for plant protection as well as in treatment of fungal diseases in human caused by different pathogenic fungi.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Host defense peptides or antimicrobial peptides (AMPs) play an essential protective role in the innate immune system of all organisms. These antimicrobial peptides are multifunctional compounds with multiple utilities (Franco 2011). The increasing resistance of bacteria and fungi to multiple antibiotics is a major public health concern worldwide that leads to enormous efforts for developing new antibiotics with new modes of action. Two promising families of drugs that meet these criteria are antimicrobial peptides (AMPs) and lipopeptides (Boman 1998; Zasloff 2002). Lipopeptides differ from AMPs as the earlier are produced only in bacteria and fungi but later is produced by all forms of life. Lipopeptides are small molecules produced during cultivation on various carbon sources. They are formed as short linear peptides linked with a lipid tail or other lipophilic molecules to form cyclic structures (Arnusch et al. 2012; Raaijmakers et al. 2010; Mandal et al. 2013).
Polymyxin A was the first antimicrobial lipopeptide described in detail. It was isolated in the year 1949 from the bacterium Bacillus polymyxa (Mandal et al. 2013) which is reclassified as Paenibacillus polymyxa. However, the effective lipopeptide production as a bio-surfactant was first reported from Bacillus subtilis IAM1213 (Jones 1949). Since then, various types of antimicrobial lipopeptides have been isolated from other Bacillus strains and their biosynthesis and antimicrobial activity studied in detail (Arima et al. 1968; Peypoux et al. 1984; Grangemard et al. 1999; Rivardo et al. 2009; Tareq et al. 2014). Other genera like Pseudomonas, Streptomyces, and Aspergillus representing Gram-negative bacteria, actinobacteria, and fungi are also reported for lipopeptide production (Cochrane and Vederas 2014; Morikawa et al. 2000; Higginbotham and Murphy 2010; Rao et al. 2013; Nielsen and Sørensen 2003). Recently, lipopeptides have received great attention for their cytotoxic, antitumor, immunosuppressant, and surfactant properties apart from antimicrobial property (Sørensen et al. 2001; Cameotra and Makkar 2004; Donadio et al. 2007; Song et al. 2011). Some of them like daptomycin (Song et al. 2014), caspofungin (Robbel and Marahiel 2010), micafungin (Ngai et al. 2011), and anidulafungin (Emiroglu 2011) have even reached the market with antibiotic status. Lipopeptides showed remarkable membrane-active and surface-interface properties resulting in a number of excellent biological activities, which are of great relevance in health care and biotechnology-based processes. These properties make lipopeptides as potent candidate drugs for the resolution of a number of global issues in medicine industry and environmental protection (George and Reboli 2012).
4.1.1 Origin and Structural Diversity of Lipopeptides
4.1.1.1 Lipopeptides Produced by Gram-Positive Bacteria (Bacillus sp.)
Broadly there are three types of lipopeptides, namely, surfactin, iturin, and fengycin, that are produced by various Bacillus species.
4.1.1.1.1 Iturin
Iturins usually have a molecular mass of ∼1.1 kDa. They consist of two major parts: a peptide part composed of short amino acid sequence and carbon hydrophobic tail. This composition clearly indicates an amphiphilic character of these compounds and suggests cell membrane-based mechanism of action (Banat et al. 2010). The iturins are cyclic peptides containing seven amino acids (heptapeptide) linked to a fatty acid (\( \beta \)-amino) chain that varies between C14 and C17 carbon molecules. Bacillomycin D and bacillomycin L are important members of the iturin family and peptide-lipid antibiotics. Structural determination indicated that bacillomycins D and L consist of a heptapeptide chain linked to a liposoluble β-amino acid. Further aspartyl, glutamyl, asparaginyl, and glutarninyl amino acids were found as peptidic moieties (Arima et al. 1968; Stachelhaus et al. 1995; Moyne et al. 2001). Bacillomycin Lc, a new antifungal antibiotic of the iturin class, was isolated from a strain of Bacillus subtilis. The structure determined by chemical and spectrometric analyses showed differences from bacillomycin L. It also exhibited sequence changes from aspartate-1 to asparagine-1 and from glutamine-5 to glutamate-5 (Tenoux et al. 1991). Bacillomycin F, another variant, also displayed differences with other members of the iturin family. In fact, it is a mixture of homologous peptide-lipids, essentially containing C51H80N12014 and C52N82N12014. The lipid moiety consists of minor isoCI5, anteisoC15 β-amino acids, and major isoC16, isoC17 and anteisoC17 p-amino acids (Eshita et al. 1995). Mycosubtilin, a peptide-lipid antibiotic, was isolated from Bacillus subtilis. It was identified as a cyclopeptide composed of seven α-amino acids in an LDDLLDL sequence closed by a β-amino acid linkage similar to that found in other antibiotics of the iturin group (Peypoux et al. 1985). The unique biological and physicochemical properties make these molecules interesting for biocontrol, food, and pharmaceutical applications (Fig. 4.1).
4.1.1.1.2 Surfactin
Surfactin (∼1.36 kDa) is an amphipathic cyclic heptapeptide composed of Glu-Leu-Leu-Val-Asp-Leu-Leu (ELLVDLL) with the chiral sequence LLDLLDL interlinked with \( \beta \)-hydroxy fatty acid with the chain length of 12–16 carbon atoms to form a cyclic lactone ring structure (Jacques 2011). The order of amino acids and the size of lipid moiety differ in a variety of surfactins (Tsan et al. 2007). In surfactin molecules while the hydrophobic amino acids are located at positions 2, 3, 4, 6, and 7, the Glu and Asp residues are located at positions 1 and 5, respectively. Usually, surfactin isoforms coexist in the cell as a mixture of several peptidic variants with different aliphatic chain lengths and differ in their biological properties accordingly (Roongsawang et al. 2002). However, the culture conditions decide the pattern of amino acids and \( \beta \)-hydroxy fatty acids in the surfactin molecules (Raaijmakers et al. 2010). The \( \beta \)-turn may be formed by an intramolecular hydrogen bond; thus, the \( \beta \)-sheet may depend on an intermolecular hydrogen bond (Fzb et al. 2004). The lipopeptide lichenysin produced by B. licheniformis structurally resembles surfactin of B. subtilis, but the glutamic acid at position 1 in surfactins is replaced by glutaminyl residue in lichenysins. This local variation caused significant changes in various properties of the molecule compared to surfactin. Lichenysin exhibits superior surfactant power, low critical micellar concentration, and higher hemolytic activity. Lichenysin is also a better chelating agent because of increased association constants with Ca2+ and Mg2+ by a factor of 4 and 16, respectively (Peypoux et al. 1984; Romero et al. 2007a). Pumilacidins A, B, C, D, E, F, and G are cyclic acyl heptapeptide composed of a β-hydroxy fatty acid, 2 L-leucine, 2 D-leucine, L-glutamic acid, L-aspartic acid, and L-isoleucine (or L-valine) (Grangemard et al. 2001). They were first isolated from the culture broth of a strain of B. pumilus.
A surfactant named BL-86 is produced by B. licheniformis strain 86. It is a mixture of lipopeptides with the major component size ranging between 979 and 1,091 Da with variation in increments of 14 Da. The variation in molecular weight represents changes in the number of methylene groups of the lipid and/or peptide portion of the surfactant. It showed an ester carbonyl structure, which could be a part of a lactone ring connecting the β-position of the lipid to one of the carbonyl groups in the peptide (Naruse et al. 1990).
4.1.1.1.3 Fengycin
Fengycin represents the third family of lipopeptides after surfactin and iturin and is also called plipastatin (Akpa et al. 2001). These are bioactive lipopeptides produced by several strains of B. subtilis F-29-3. Fengycins effectively inhibit the growth of filamentous fungi but are ineffective against yeast and bacteria. The inhibition is antagonized by sterols, phospholipids, and oleic acid; however, addition of two other unsaturated fatty acids is found to increase the antifungal effect. Fengycins are broadly classified into two groups that differed by one amino acid. Fengycin A is composed of 1 D-Ala, 1 L-Ile, 1 L-Pro, 1 D-allo-Thr, 3 L-Glu, 1 D-Tyr, 1 L-Tyr, and 1 D-Orn, whereas fengycin B D-Val was replaced by D-Ala (Horowitz and Griffin 1991; Akpa et al. 2001). These bioactive molecules are lipodecapeptides containing a lactone ring with \( \beta \)-hydroxy fatty acid chain (either saturated or unsaturated). As mentioned the structure of fengycin contains a peptide chain of ten amino acids, cyclic decapeptides formed by lactonization (Il et al. 2010). The fatty acid moieties vary from C14 to C17 carbon atoms. The presence of different fatty acids yields different homologous compounds and isomers. Members of fengycin family also exhibit heterogeneity at the sixth position in peptide moiety. Based on variations in single amino acid (at the sixth position), fengycins are classified into two classes, namely, fengycin A and fengycin B. While fengycin A contains Ala at sixth position, it is replaced by valine in the case of fengycin B (Vanittanakom and Loeffler 1986; Bonmatin et al. 2003).
4.1.1.2 Lipopeptides Produced by Gram-Negative Bacteria (Pseudomonas sp.)
4.1.1.2.1 Viscosinamide
Viscosinamide produced by Pseudomonas fluorescens DR54 showed antagonistic properties against plant pathogenic fungi like Pythium ultimum and Rhizoctonia solani. However, bio-surfactant properties of viscosinamide differed from the known bio-surfactant viscosin that contained glutamine rather than glutamate at the second amino acid position. Isolation and determination of structure of this new compound with antibiotic and antifungal properties reveals promising perspectives for the application of P. fluorescens DR54 as a biological control agent. Tests performed in plants also showed that purified viscosinamide reduced the aerial mycelium development of both P. ultimum and R. solani (Lee et al. 2010; De Faria et al. 2011; Nielsen et al. 1999).
Since P. ultimum is a root pathogen, its growth inhibition using the viscosinamide has been studied on sugar beet seeds. Seeds infected with P. ultimum and P. fluorescens DR54 have improved plant emergence after 7 days compared to a control without the biocontrol strain. The impact of P. fluorescens DR54 on the growth and activity of P. ultimum was also studied by direct microscopy after staining with the vital fluorescent dye like Calcofluor White and fluorescein diacetate. P. fluorescens DR54 caused reduction in P. ultimum mycelial density, oospore formation, and intracellular activity. Further, P. ultimum oospore formation was completely absent in the presence of P. fluorescens DR54. In vitro studies confirmed that purified viscosinamide induced encystment of Pythium zoospores (De Faria et al. 2011; Nielsen et al. 1999; Thrane et al. 1999) (Fig. 4.2).
4.1.1.2.2 Tolaasin
Tolaasin (tolaasin I, tolaasin II, and tolaasins A, B, C, D, and E are the available analogs) and few other metabolites are produced by P. tolaasii. All these analogs showed differences in their amino acid composition. All observed lipodepsipeptides of bacterial origin are maintained by the β-hydroxy octanoyl phi chain at the N-terminus, except tolaasin A, in which the acyl moiety is a gamma-carboxy butanoyl phi moiety (Mazzola et al. 2009; Andolfi et al. 2008). P. tolaasii is a causal organism of brown blotch disease to Agaricus bisporus and causes yellowing of Pleurotus ostreatus (Molinaro et al. 2003; Nutkins et al. 1991; Moquet et al. 1996; Cho et al. 2007).
Tolaasin is a lipodepsipeptide, found to be produced by a pathogen of mushroom species. During development of lysis bioassay for tolaasin, it was also found to cause hemolysis. Hemolysis by tolaasin involves a dose-dependent manner that is maximal between pH 6.0 and 7.0 and increased with increase in temperature. Tolaasin can also be produced in a growth-dependent manner, and its production always initiates during exponential growth of P. tolaasii and continued till the stationary phase. Ultrastructural studies performed to identify the mechanism of action showed disruption of the A. bisporus plasma membrane and vacuole membranes by tolaasin. Multilamellar liposomes inoculated with tolaasin demonstrated loss of lytic activity and prevented tolaasin-induced hemolysis, suggesting tolaasin induces partitions and forms pores in erythrocyte membranes that cause lysis by a colloid osmotic mechanism. Tolaasin is phytotoxic when infiltrated into leaves of Nicotiana tabacum and was shown to be active against a range of basidiomycetes and Gram-positive bacteria (Soler-Rivas and Arpin 1999; Bassarello et al. 2004; Coraiola et al. 2006).
4.1.1.2.3 Tensin
Tensin is an antifungal cyclic lipopeptide produced by P. fluorescens strain 96.578. The molecular formula is C67H116N12O20, representing an exact molecular weight of 1408.84 Da. The NMR data obtained for tensin predicted it as a cyclic lipopeptide composed of a 3-hydroxydecanoyl residue in combination with 11 amino acid residues that include 5 leucine (Leu), 1 isoleucine (Ile), 1 aspartic acid (Asp), 1 glutamine (Gln), 1 glutamic acid (Glu), 1 threonine (Thr), and 1 serine (Ser) amino acids (Rainey et al. 1991). P. fluorescens is already used as a biocontrol agent against fungi such as R. solani. Various studies suggested that tensin is effective against radial growth of R. solani (Cho et al. 2010).
4.1.1.2.4 Syringotoxin and Syringopeptin
Syringotoxin is produced by P. syringae pv. syringae which is a known pathogen of various species of citrus trees. Peptide moiety of bioactive molecule contains amino acids in order of Ser-Dab-Gly-Hse-Orn-aThr-Dhb-(3-OH)Asp-(4-Cl)Thr with the terminal carboxy group closing a macrocyclic ring on the OH group of the N-terminal Ser (Nielsen et al. 2000). Syringopeptin, another major phytotoxic antibiotic produced by P. syringae pv. syringae, is also a lipodepsipeptide. The amino acid sequence is Ser-Ser-Dab-Dab-Arg-Phe-Dhb-4(Cl)Thr-3(OH)Asp with the beta-carboxy group of the C-terminal residue closing a macrocyclic ring on the OH group of the N-terminal Ser (Couillerot et al. 2009; Ballio et al. 1990) (Fig. 4.3).
4.1.1.2.5 Pseudophomins
The pseudophomins are also cyclic lipodepsipeptides grouped into pseudophomins A and B. They are isolated from P. fluorescens strain BRG100, a bacterium with potential application as a biocontrol agent for plant pathogens and agricultural weeds. Pseudophomin B showed higher antifungal activity against the phytopathogens such as Phoma lingam/Leptosphaeria maculans and Sclerotinia sclerotiorum. In contrast, pseudophomin A showed stronger inhibition toward green foxtail (Setaria viridis) and induced root germination than pseudophomin B (Segre et al. 1989; Galonić et al. 2007).
4.1.1.2.6 Pseudomycins
Pseudomycins are peptide antimycotics that are isolated from P. syringae, another plant-associated bacterium. Pseudomycins contain three analogs classified as A–C that contain amino acids hydroxyl aspartic acid, aspartic acid, serine, arginine, lysine, and diaminobutyric acid. The molecular mass of pseudomycins A–C is 1,224, 1,208, and 1,252 Da, respectively, as determined by plasma desorption mass spectrometry. Pseudomycin D, on the other hand, has a molecular mass of 2,401 Da and is more complex than pseudomycins A–C (Wilson Quail et al. 2002; Pedras et al. 2003). Pseudomycin A is the predominant peptide of the pseudomycin family that possesses selective phytotoxicity and is effective against human pathogen Candida albicans (Harrison et al. 1991).
4.1.1.2.7 Massetolide A
Massetolide A is isolated from P. fluorescens SS101, identified as a biocontrol agent. P. fluorescens SS101 was effective in preventing infection of tomato (Lycopersicon esculentum) leaves by P. infestans and significantly reduced the expansion of existing late blight lesions. Purified massetolide A displayed significant control of P. infestans both locally and systemically via induced resistance. This study suggests that the cyclic lipopeptide massetolide A is a metabolite with versatile functions in the ecology of P. fluorescens SS101 and in interactions with tomato plants and the late blight pathogen P. infestans (Ballio et al. 1994; Sun et al. 2001). Thus, the number of Pseudomonas strains has shown promising results in biological control of late blight caused by P. infestans.
4.1.1.3 Fungal Lipopeptides
4.1.1.3.1 Echinocandins
The echinocandins produced by fungi comprise a new class of antifungal lipopeptide agents. Echinocandins isolated for the first time from Aspergillus rugulosus and A. nidulans exhibited antifungal and anti-yeast activities (Tran et al. 2007; De Bruijn et al. 2008). Compounds under this group are amphiphilic, cyclic hexapeptides with an N-linked acyl lipid side chain. The molecular weight of these compounds is around 1,200 Da (Tóth et al. 2012). So far, three echinocandin compounds have been determined for structural elucidation. While the echinocandin B (ECB) structure was determined by chemical degradation studies in combination with X-ray crystallographic analysis, structures of echinocandins C and D were elucidated by their conversion into a common intermediate isoform derived from the echinocandin B (Benz et al. 1974; Denning 2003). They are all acylated by a linoleic acid molecule at the N-terminus. The synthesis of echinocandins C and D was carried out using the stereocontrolled synthesis of all constituents such as (2S,3S,4S)-3-hydroxy-4-methylproline, (2S,3R)-3-hydroxyhomotyrosine, and γ,δ-dihydroxyornithine (Benz et al. 1974; Messik and Oberthür 2013). The common feature to these lipopeptides is a cyclic peptide and an N-terminal fatty acid group; however, the fatty acid group is usually either branched or unbranched with a chain length of 14–18 carbon atoms. Both the cyclic structure (Shamala et al. 1976; Kurokawa and Ohfune 1993) and an acyl side chain are essentially required for the biological activity of the compound. This emphasizes the importance of the acyl group for the mechanism of antimicrobial action (Journet et al. 1999; Rodriguez et al. 1999; Debono et al. 1989).
Deacylation of ECB has been accomplished using an Actinoplanes utahensis culture to design new derivatives with enhanced efficiency (Debono et al. 1989). The biological activity was restored with the reintroduction of the N-acyl group that might play certain structural requirements. Indeed, small groups such as acetyl, benzoyl, or cyclohexanoyl were effective in restoring activity, and also increase in the chain length from C12 up to C17–C18 was proportional to the increase in activity (Debono et al. 1995).
The echinocandin drugs available in the market consist of three agents, caspofungin (Cancidas; Merck and Co., Whitehouse Station, New Jersey, USA), micafungin (Mycamine; Astellas Pharmaceuticals, Grand Island, New York, USA), and anidulafungin (Eraxis; Pfizer Pharmaceuticals, New York, USA). Echinocandins exhibit antimicrobial activity by blocking the synthesis of 1-3-β-D-glucan, a critical component of the fungal cell wall, through noncompetitive inhibition of the enzyme 1-3-β-D-glucan synthase. Osmotic disruption after loss of cell wall integrity is mainly responsible for fungicidal activity (Boeck et al. 1989; Hobbs et al. 1988). The echinocandin antifungal spectrum is restricted with few exceptions to Candida spp. and Aspergillus spp. Echinocandin antifungal agents have been studied in various clinical settings ranging from invasive, oral, and esophageal candidiasis and for treatment of invasive aspergillosis. At present echinocandin drugs are available only as intravenous injections in the market (Fig. 4.4).
-
(a)
Caspofungin
Caspofungin (caspofungin acetate) is a semisynthetic water-soluble lipopeptide produced as a fermentation product of the fungus Glarea lozoyensis. This also belongs to the echinocandin family and is a derivative of pneumocandin Bo. Studies demonstrated that caspofungin has antifungal activity against yeasts of the genus Candida (including isolates resistant to azoles and amphotericin B). This also includes species of the genus Candida that are resistant (Candida krusei) or less susceptible to azoles (Candida dubliniensis, Candida glabrata) or resistant to amphotericin B (Diekema et al. 2005; Douglas 2006; Barchiesi et al. 1999). Several species of Aspergillus and certain dimorphic fungi, such as Histoplasma, Blastomyces, and Coccidioides, were also inhibited by caspofungin (Vazquez et al. 1997; Bachmann et al. 2002; Kurtz et al. 1994). In vitro and in vivo experiments confirmed the additive or synergic activity of caspofungin with amphotericin B and triazoles against different fungi. It also possesses activity against Pneumocystis carinii (Marco et al. 1998).
The β(1,3)-D-Glucan is found to be an essential component of the cell walls of numerous fungal species as the solid three-dimensional matrix formed by chains of β(1,3)-D-glucan gives shape and mechanical strength to the cell wall. Caspofungin blocks the synthesis of β(1,3)-D-glucan by noncompetitive inhibition of the enzyme β(1,3)-D-glucan synthase and showed potential antifungal effects (Pfaller et al. 1998; Zhang et al. 2006; Georgopapadakou 1997). Clinical trials proved caspofungin to be well tolerated and effective for invasive aspergillosis patients, oropharyngeal candidiasis, esophageal candidiasis, and invasive candidiasis. It showed an efficacy that is equivalent to amphotericin B (Robbel and Marahiel 2010; Douglas 2001; Georgopapadakou 2001; Groetzner et al. 2008; Groll and Walsh 2001; Herbrecht et al. 2010; Mora-Duarte et al. 2002). Experimental studies carried out in several models showed correlation with the in vitro susceptibility data of caspofungin.
Several studies have also demonstrated that caspofungin is effective against disseminated candidiasis in immune-competent or immunosuppressed mice (Kartsonis et al. 2005a; Keating and Figgitt 2003; Abruzzo et al. 1997). Experimental data confirmed its efficacy in immunosuppressed mice infected with in vitro fluconazole-resistant or dose-dependent susceptible isolates of C. krusei, C. glabrata, and C. albicans (Graybill et al. 1997a, b). Caspofungin administered (0.05–5 mg/kg/day) by the intravenous or intraperitoneal route into the infected animals significantly prolonged the survival and reduced the renal fungal load.
Several experimental studies of invasive aspergillosis in immunocompromised rodents have demonstrated the efficacy of caspofungin (Abruzzo et al. 2000; Sionov et al. 2006; Chabrol et al. 2010; Kartsonis et al. 2005b). However, caspofungin did not protect mice against lethal infection with C. neoformans even at high doses of 40 mg/kg/day. These experimental data confirm the resistance of C. neoformans observed in vitro (Kartsonis et al. 2005a).
Caspofungin was compared with the reference treatment trimethoprim plus sulfamethoxazole in mice pretreated with steroids and infected with P. carinii (Maertens et al. 2004) by administering through subcutaneous or oral route. Results suggested 90 % reduction in number of pulmonary cysts in subcutaneous administration. Though similar results were observed for the reference treatment, the effect was more rapid with caspofungin (4 days) when compared to reference treatment (14 days). However, the oral route of administration was always found to be less effective for caspofungin. Moreover, a prophylactic effect was observed in the same models upon daily subcutaneous administration of caspofungin (Madureira et al. 2007; Powles et al. 1998).
-
(b)
Micafungin
Micafungin is used worldwide in chemotherapy of life-threatening fungal infections. It is the second approved antifungal agent in the echinocandin series. FR901379, which is known as a seed compound of micafungin, was first discovered at Fujisawa Pharmaceutical Co., Ltd, Japan, in the year 1989 (Fortún et al. 2009). Micafungin is a water-soluble antifungal agent with molecular weight of 1292.26 Da that was derived from Coleophoma empetri (Spreghini et al. 2012; Hashimoto 2009; Mikamo et al. 2000). The water solubility of micafungin is attributed to a sulfate moiety in the molecule. Micafungin is a potent inhibitor of 1,3-β-D-glucan synthase, an enzyme involved in cell wall biosynthesis in several pathogenic fungal species. Micafungin acts in a concentration-dependent manner as a noncompetitive inhibitor in the formation of the enzyme 1,3-β-D-glucan synthase. This enzyme is necessary for synthesis of 1,3-β-D-glucan, a glucose polymer crucial to the structure and integrity of the cell wall of several fungal pathogens (Tóth et al. 2012; Barrett 2002; Vicente et al. 2003; Onishi et al. 2000; Tawara et al. 2000). Fungal cells that are unable to synthesize this polysaccharide cannot maintain their shape and lack adequate rigidity to resist osmotic pressure, which results in fungal cell lysis. This mechanism is found to be unique to the echinocandin class of antifungal agents. It contains the potential in additive or synergistic activity with other antifungals like polyenes and azoles. Apart from cell wall structure, glucan is also involved in cell growth and division (Nishiyama et al. 2002). Micafungin demonstrates a prolonged concentration-dependent post-antifungal effect. Fungal cell walls are usually made up of chitin as a major component along with mannoproteins. The selective antifungal effect of echinocandin activity is because of varied quantities of fungal cell wall components among different fungal species (Wiederhold et al. 2008; Groll et al. 2001). Of significance, the cell walls of zygomycetes and cryptococci lack 1,3-β-D-glucan, which explains the poor activity of echinocandins, including micafungin, against selective fungal pathogens (Vicente et al. 2003; Andes et al. 2008).
Micafungin is the second antimicrobial agent in the echinocandins class that is approved for use in clinical practice. Though it is a potent antifungal, it showed slow fungicidal activity against clinical isolates like Candida albicans, C. dubliniensis, C. tropicalis, C. glabrata, and C. krusei. The activity was somewhat higher (MIC90s) for C. parapsilosis, C. lusitaniae, and C. guilliermondii (Vicente et al. 2003; Mariné et al. 2007; Nakai et al. 2002; Laverdiere et al. 2002). Micafungin did not show activity against basidiomycetous yeasts, Cryptococcus neoformans or Trichosporon species in vitro, but it displayed inhibitory activity against Aspergillus species at lower concentrations than amphotericin B and itraconazole (Vicente et al. 2003; Andes et al. 2008; Laverdiere et al. 2002; Ostrosky-Zeichner et al. 2003).
Although clinical data are still lacking, the role of micafungin appears to be similar to that of caspofungin. The initial approval has been granted for treatment of esophageal candidiasis and prophylaxis in subjects with neutropenia. Pharmacokinetic and pharmacodynamic studies revealed no adverse effects, and safety is reported similar to those of other agents in the echinocandin class.
4.1.1.4 Clinical Efficacy
Micafungin is found to be clinically effective in treatment of Candida and Aspergillus infections as most trials that were conducted in Japan, South Africa, the United States, Latin America, and Germany produced positive results. The US FDA has approved micafungin as a therapeutic agent for the treatment of esophageal candidiasis. However, the FDA did not approve micafungin to use for children as safety and efficacy studies in this population have not been established.
Candida Infections
The safety and efficacy of micafungin in the treatment of esophageal candidiasis in AIDS patients was evaluated (Uchida et al. 2000; Fujie et al. 2001), and doses of micafungin were determined as 12.5, 50, 75, and 100 mg/day through intravenous injection. Clinical improvement was noted for patients who received 75 or 100 mg/day showing favorable clinical and endoscopic outcomes with rapid response within a duration of 3–5 days (Fujie et al. 2001; Pettengell et al. 2004). In a multinational, double-blind, non-inferiority study, a single high dose of micafungin (150 mg/day) with fluconazole (200 mg/day) (Fujie et al. 2001) also showed similar results. In another open-label, non-comparative study of micafungin for the treatment of candidemia patients, the overall success was noted in 83.2 % of patients (De Wet et al. 2004). Similarly, results of several smaller trials also supported the efficacy of micafungin in the management of candidemia (Kohno et al. 2013; Denning et al. 2006). An interesting report noted that a successful outcome with topical application of micafungin is in the treatment of refractory yeast-related corneal ulcers (Hachem et al. 2008).
Mold Infections
Although data is available on using micafungin for the treatment of refractory aspergillosis, it is from open trials and not examined in a randomized, controlled manner. In a phase 2 multinational study, efficacy of micafungin as primary or salvage therapy for invasive aspergillosis was assessed (Kohno et al. 2004). Another study involving stem cell transplant (SCT) recipients evaluated the safety and efficacy of micafungin in combination with other antifungal drugs for the treatment of refractory aspergillosis (De Wet et al. 2004; Matsumoto et al. 2005). Overall, significant Aspergillus-infected patients had a satisfactory response. In addition, several case reports have described success with micafungin in severely compromised hosts with refractory aspergillosis (Moretti et al. 2014; Kontoyiannis et al. 2009; Ota et al. 2004). Considering the poor prognosis in patients with refractory aspergillosis, the aforementioned data from these unpublished trials and case reports are encouraging. Most available data were in the setting of refractory aspergillosis with micafungin used as salvage therapy in combination with other drugs. Data for its use as monotherapy, particularly for the initial treatment of invasive aspergillosis, are lacking.
A phase 3, large, randomized, double-blind, multi-institutional comparative study was conducted in the United States and Canada that evaluated the efficacy of micafungin as prophylaxis during the pre-engraftment period of neutropenia (Yokote et al. 2004; Chandrasekar et al. 2004). However, whether micafungin should be preferred to fluconazole as prophylaxis during the pre-engraftment period in SCT recipients and whether the improved long-term survival seen with fluconazole will also be seen with micafungin are important questions that need to be addressed (Van Burik et al. 2004; Hiramatsu et al. 2009).
Urinary Sepsis
Candida glabrata is frequently resistant to fluconazole, and in advanced renal failure, the safe use of this and other recommended drugs is limited. Patients suffering from renal and severe urinary sepsis by C. glabrata are successfully treated with micafungin. In fact, amphotericin B, fluconazole, and flucytosine are recommended for effective treatment of symptomatic candiduria (Marr et al. 2000; De Pauw and Donnelly 2007). However, in renal failure these agents are contradicted, and due to significant toxicity risk, their utilities are substantially limited. Fluconazole is extensively excreted by the kidneys reaching high urinary concentrations. Non-C. albicans species, notably C. glabrata, is nowadays frequently resistant to fluconazole therapy. Echinocandins are potent antifungal agents used to treat these strains as they exert activity by inhibiting β-D-glucan synthase(Franco 2011; Zasloff 2002), a major component of the fungal cell wall (Ullmann et al. 2012). Micafungin, like the entire class of echinocandin drugs, has a broad spectrum of activity on Candida species, and it is now considered a first-line therapy in candidiasis. In general, micafungin is well tolerated and has a low potential of drug interaction, its clearance is independent from glomerular filtration, and there are no contradictions for dose adjustment even in severe renal failures. All echinocandins are highly protein bound (99 %) and share the major pharmacokinetic disadvantage of poor glomerular filtration and tubular secretion resulting in very low urinary concentrations (Jones 1949; Arima et al. 1968). For that reason, even if they have a very favorable profile in terms of efficacy and safety, their use seems precluded in fungal urinary tract infections. However, despite micafungin being minimally excreted in urine, its wide distribution in many organs and tissues, including the kidneys, liver, and spleen, has been shown in animal models (Nishiyama et al. 2002; Mermel et al. 2009; Sucher et al. 2009). The observed tissue concentrations were severalfold in excess of the MIC against clinical isolates of Candida spp. and Aspergillus spp. Few reports in the literature are available on the safe and effective use of echinocandins (i.e., caspofungin and micafungin) in candiduria (Petraitis et al. 2002; Niwa et al. 2004; Lagrotteria et al. 2007; Kauffman 2005). Of these, six were of candiduria successfully treated with caspofungin (collected by Merck Research Laboratories retrospectively from a phase 2–3 clinical study). To our knowledge, only three cases of candiduria treated with micafungin are reported in the literature. There are some features in treatment where firstly the patient was treated with a dose of 200 mg daily of micafungin (a dose higher than the standard recommended dose) without adverse effects. The choice of using a dosage greater than usual was empirical and was motivated by the possibility of achieving higher renal tissue concentration with such dosage. In conclusion the present case shows that micafungin can achieve clinically relevant fungal sterilization even in urine and there is room to consider micafungin in the armamentarium against C. glabrata urinary infections, notably in the context of renal failure where other antifungal effective drugs are unsafe or contradicted.
-
(c)
Anidulafungin
Anidulafungin is a semisynthetic product of echinocandin B which is a fermentation product of the mold A. nidulans. It was developed by Eli Lily, underwent preclinical and clinical studies at Vicuron Pharmaceuticals, and was sold to Pfizer and marketed under the name Eraxis™. Anidulafungin (Eraxis; Pfizer) is the newest echinocandin antifungal approved by the US Food and Drug Administration recently and currently used for the treatment of esophageal candidiasis, candidemia, and deep-tissue candidiasis.
Anidulafungin is a novel echinocandin and has several advantages over existing antifungals. The unique features of anidulafungin are its slow degradation in humans where it undergoes biotransformation rather than being metabolized. It has potent in vitro activity against Aspergillus and Candida species, including the strains that resist fluconazole or amphotericin B. Results of various clinical trials indicate anidulafungin as effective in treatment of esophageal candidiasis, including azole-refractory disease. The results of a recent study comparing fluconazole versus anidulafungin demonstrated the superiority of anidulafungin in the treatment of candidemia and invasive candidiasis (IC). Studies evaluating the concomitant use of anidulafungin, amphotericin B, voriconazole, or cyclosporine did not show significant drug-drug interactions or any adverse effects. To date, anidulafungin appears to have an excellent safety profile. Based on the early clinical experience, it appears that anidulafungin will be a valuable asset in the management of serious and difficult-to-treat fungal infections.
Anidulafungin has potent in vitro fungicidal activity against a broad range of Candida species, including C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. famata, C. rugosa, and C. stellatoidea (Table 4.1) (Cochrane and Vederas 2014; Morikawa et al. 2000; Higginbotham and Murphy 2010). Anidulafungin is also effective against species of Candida that are intrinsically resistant to azoles (C. krusei), amphotericin B (C. lusitaniae), or other echinocandins (C. parapsilosis) (Rao et al. 2013; Nielsen and Sørensen 2003). Anidulafungin has also demonstrated excellent in vitro activity against several species of Aspergillus (Table 4.2) (Nielsen and Sørensen 2003; Sørensen et al. 2001; Cameotra and Makkar 2004). Anidulafungin also demonstrates additive effects in vitro in combination with amphotericin B against species of the genera Aspergillus and Fusarium by synergistic activity when combined with itraconazole or voriconazole against Aspergillus species (Donadio et al. 2007; Song et al. 2011).
Mechanism of Action
Anidulafungin is a semisynthetic lipopeptide synthesized from fermentation products of A. nidulans. The compound is a noncompetitive inhibitor of 1,3-β-D-glucan synthase, which results in the selective inhibition of the synthesis of glucan, a major structural component of the cell wall of many pathogenic fungi that is absent in mammalian cells. A difference in glucan content determines the excellent activity of anidulafungin in fungi and the paucity of adverse effects in humans (Rivardo et al. 2009).
4.1.2 Lipopeptides Against Fungal Phytopathogens
Some bacterial species live in association with plant roots and other parts. The natural antagonistic property showed by these microorganisms (notably belonging to the Bacillus and closely related Paenibacillus genera and Pseudomonas sp.) has emerged as promising alternatives to reduce the use of chemical pesticides and other toxic substances in agriculture (Sobel et al. 2000; McSpadden Gardener 2004; Cawoy et al. 2011; Jacobsen et al. 2004). The disease protection properties of these microorganisms rely on three main traits.
The first and foremost among these is a high ecological fitness to efficiently colonize in the roots, which is a prerequisite to proficiently compete for space and nutrients in the microenvironment of the rhizosphere. Second is their capacity to secrete highly active antimicrobial substances with strong antagonistic activity toward various plant pathogens. The third is their ability to trigger plant immune response in tissues which imparts resistance state that makes the host less susceptible to subsequent infection (Pérez-García et al. 2011; Lugtenberg and Kamilova 2009; Chen et al. 2000). The efficient antimicrobial substance production which leads to direct antagonism of phytopathogens is a key biocontrol mechanism (Berendsen et al. 2012; Chen et al. 2009). Antimicrobial production efficiency and a high rhizosphere fitness possibly explain the strong biocontrol potential of Bacillus both in vitro and under field conditions for their successful marketing commercially (Rückert et al. 2011; Pal and Mc Spadden Gardener 2006; Janisiewicz and Korsten 2002; Larkin and Tavantzis 2013; Spadaro and Gullino 2005; Király et al. 2008). Among the Bacillus antimicrobial products, cyclic lipopeptides (LPs) such as surfactin, iturin, and fengycin families are of high interest not only due to their high production rate in bioreactors by B. subtilis or B. amyloliquefaciens but also because of the secretion of these compounds in relevant amounts under natural conditions in the rhizosphere environment (Yang et al. 2013; Kinsella et al. 2009; Nihorimbere et al. 2009, 2012; Dietel et al. 2013; Yaryura et al. 2008) (Fig. 4.5).
Panel 1: Fungicidal activities of the lipopeptides (C14-KLLK and C16-KLLK) upon artificial infection of gray mold (Botrytis cinerea) on cucumber leaves for 4 days after infection and 3 days after treatment. Panel 2: Cucumber fruits infected with B. cinerea conidia 3 days after the last treatment with double-distilled water (A) or 30 mg/l C14-KLLK (B). Panel 3: Corn leaves infected with Cochliobolus heterostrophus, untreated (A) or treated with 30 mg/l C14-KLLK (Debois et al. 2014)
One of the B. subtilis strain designated as JA was found to antagonize the growth of Gibberella zeae. The electrospray ionization mass spectrometry (ESI/MS) analysis of the product revealed the antifungal lipopeptide production by this strain. A mutant of this strain exhibited favorable properties including the high yield of antifungal lipopeptide production with relatively faster growth over the parent strain, which suggested that this strain would be a promising biocontrol candidate in agriculture (Makovitzki et al. 2007).
The potential of B. subtilis strain M4 at protecting plants against fungal diseases was demonstrated in different pathosystems, and fengycin-like lipopeptide production was reported by this strain. The protective effect against damping-off of bean seedlings which is caused by Pythium ultimum (gray mold of apple and in other postharvest diseases) was demonstrated. The protection ability was also established by the strong biocontrol activity of lipopeptide-enriched extracts applied onto infected tissues of plants. Apart from this, root pre-inoculation with strain M4 enabled the host plant to respond more efficiently to subsequent pathogen infection on leaves and other parts of the plant. The mechanisms by which Bacillus spp. suppress disease in infected plants are yet to be discovered. These antifungal properties of stain M4 emphasize the interest of B. subtilis as a pathogen antagonist and plant defense-inducing agent. The production of cyclic fengycin-type lipopeptides may be closely related to the expression of these two biocontrol qualities (Liu et al. 2005).
Apart from members of Bacillus genus, a Chromobacterium sp. strain C61 that displayed antifungal activities was also used successfully as a biocontrol agent for various plant diseases under field conditions. The analysis of C61 culture filtrates exposed an antifungal cyclic lipopeptide, chromobactomycin, that contained a unique nonameric peptide ring. The chromobactomycin inhibited the growth of several phytopathogenic fungi in vitro as well as plant applications. It significantly reduced disease severity caused by several pathogens and inhibited the mycelial growth of R. solani, Pyricularia grisea, B. cinerea, Alternaria longipes, and C. gloeosporioides (Ongena et al. 2005) (Fig. 4.6).
Chemical structure of chromobactomycin. Dhb dihydroxybutyric acid, Gln glutamine, Gly glycine, His histidine, HMS β-hydroxymyristate, Thr threonine,Tyr tyrosine (Ongena et al. 2005)
Isolation and characterization of bacterial antagonist for use as biological control of phytopathogenic fungi like rice blast fungus has been studied. The antimicrobial substance was further purified and characterized the antifungal molecule produced by the antagonist. The strain was isolated from soil and identified as B. licheniformis BC98 that showed high antagonist antifungal activity against the rice blast fungus Magnaporthe grisea. This bacterial strain also inhibited the growth of other phytopathogens such as Curvularia lunata and Rhizoctonia bataticola. Biochemical and mass studies of biologically active fractions revealed it as a lipopeptide with molecular mass of 1,035 Da. However, it was identified as surfactin after NMR analysis. Microscopic analysis of the effect of the antagonist on M. grisea revealed bulbous hyphae showing patchy and vacuolated cytoplasm under the electron microscope. The antagonist inhibited germination of M. grisea, and therefore it is considered as a potential candidate for control of rice blast disease (Romero et al. 2007b).
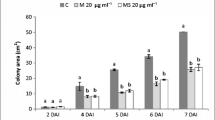
Podosphaera fusca is the main causal agent of cucurbit powdery mildew, and four Bacillus subtilis strains, UMAF6614, UMAF6619, UMAF6639, and UMAF8561, were found to inhibit the growth of P. fusca. Therefore, they were studied for their ability to suppress the disease on melon. Experiments were performed using detached leafs and seedling assays. They were also further subjected to elucidate the mode of action involved in their biocontrol performance. Three lipopeptide antibiotics, i.e., surfactin, fengycin, and iturin A or bacillomycin, were identified in butanolic extracts of B. subtilis culture filtrate. The purified lipopeptide fractions (bacillomycin, fengycin, and iturin A) have shown strong inhibitory effects on P. fusca conidia germination and provided interesting evidence of their presumed involvement in the antagonistic activity. This also suggested that the iturin and fengycin families of lipopeptides have a major role in the antagonism of B. subtilis toward P. fusca (Alvarez et al. 2012a) (Fig. 4.7).
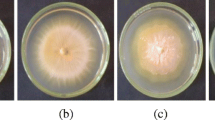
Powdery mildew symptoms on melon leaves following treatments with butanolic extracts of cell-free filtrates of the antagonistic strain Bacillus subtilis UMAF6639. Treatments are 1 untreated control, 2 nutrient broth control, 3 washed cells from stationary phase, 4 cell-free filtrate nondiluted, and 5 and 6 cell-free filtrates 1:4- and 1:16-fold diluted, respectively. Panels are compositions of photographs of leaves inoculated with conidia of Podosphaera fusca and treated as described, taken 7 (a) and 16 (b) days after treatments (Fzb et al. 2004)
The antifungal compounds identified as iturin, surfactin, and fengycin isoforms produced by two previously isolated Bacillus sp. strains, ARP23 and MEP218, were found to be effective against Sclerotinia sclerotiorum. These strains were further assessed for their ability to control sclerotinia stem rot in soybean. The field trials showed effective results. While the surfactin C15 and fengycin A (C16–C17) and B (C16) isoforms were produced by strain ARP23, the major lipopeptide produced by strain MEP218 was iturin A C15. Mycelial growth, morphology, and sclerotial germination were altered in the presence of lipopeptides. Foliar application of Bacillus amyloliquefaciens strains on soybean plants prior to S. sclerotiorum infection also revealed significant protection against sclerotinia stem rot. Strains ARP23 and MEP218 were renamed or identified as strains belonging to the species B. amyloliquefaciens and were concluded to produce antifungal compounds belonging to the cyclic lipopeptide family. As sclerotinia stem rot was considered as one of the most severe soybean diseases worldwide, these results proposed the potential of B. amyloliquefaciens strains ARP23 and MEP218 to control plant diseases caused by S. sclerotiorum as biocontrol agent (Kim et al. 2014) (Fig. 4.8).
Systemic protection against Botrytis cinerea B05 infection by synthetic ultrashort lipopeptides in cucumber and Arabidopsis seedlings. (a) The first leaf of cucumber plants (white arrows) was infiltrated with 100 μl of water (MOCK) or 100 μl of a 12.5 M solution of P1 24 h before inoculation of the second and third leaves with B. cinerea mycelium. Symptoms were assessed 3–4 days after infection. (b) Arabidopsis rosette leaves 3–4 days after infection with B. cinerea spores. Four leaves from each plant rosette were treated with 100 μl of water (MOCK) or 100 μl of 12.5 M peptides. Pathogen inoculation was done on non-treated leaves. Three to four days later, infected leaves were detached and analyzed (Tendulkar et al. 2007)
4.2 Conclusion
Lipopeptides are amphiphilic molecules with diverse physiochemical properties. Because of their broad-spectrum antimicrobial activities, they are used as various biocontrol agents. Moreover, presence-specific amino acid residues import certain functions to the lipopeptides that are important for biological activity. Though the molecular pattern is not conserved in all lipopeptides, structural homologues are interestingly more active in comparison to others. In fact, this is the reason why some Bacillus strains are more efficient than others against pathogenic strains. Therefore, the identification and understanding of putative receptors and their molecular mechanism is essential. However, efficiency of biological control properties of lipopeptides can be improved by using different combinations of production mechanism or by improving the production strains or in combination. Large-scale production of these lipopeptides is essential for biotechnological studies including therapeutic applications.
References
Abruzzo GK, Flattery AM, Gill CJ, Kong L, Smith JG, Pikounis VB, Balkovec JM, Bouffard AF, Dropinski JF, Rosen H, Kropp H, Bartizal K (1997) Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother 41:2333–2338
Abruzzo GK, Gill CJ, Flattery AM, Kong L, Leighton C, Smith JG, Pikounis VB, Bartizal K, Rosen H (2000) Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob Agents Chemother 44:2310–2318
Akpa E, Jacques P, Wathelet B, Paquot M, Fuchs R, Budzikiewicz H, Thonart P (2001) Influence of culture conditions on lipopeptide production by Bacillus subtilis. Appl Biochem Biotechnol 91–93:551–561
Alvarez F, Castro M, Príncipe A, Borioli G, Fischer S, Mori G, Jofré E (2012) The plant-associated Bacillus amyloliquefaciens strains MEP 218 and ARP 23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J Appl Microbiol 112:159–174
Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J (2008) In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 52:3497–3503
Andolfi A, Cimmino A, Lo Cantore P, Iacobellis NS, Evidente A (2008) Bioactive and structural metabolites of Pseudomonas and Burkholderia species causal agents of cultivated mushrooms diseases. Perspect Med Chem 2008:81–112
Arima K, Kakinuma A, Tamura G (1968) Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem Biophys Res Commun 31:488–494
Arnusch CJ, Ulm H, Josten M, Shadkchan Y, Osherov N, Sahl HG, Shai Y (2012) Ultrashort peptide bioconjugates are exclusively antifungal agents and synergize with cyclodextrin and amphotericin B. Antimicrob Agents Chemother 56:1–9
Bachmann SP, Patterson TF, López-Ribot JL (2002) In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J Clin Microbiol 40:2228–2230
Ballio A, Bossa F, Collina A, Gallo M, Iacobellis NS, Paci M, Pucci P, Scaloni A, Segre A, Simmaco M (1990) Structure of syringotoxin, a bioactive metabolite of Pseudomonas syringae pv. syringae. FEBS Lett 269:377–380
Ballio A, Bossa F, Di Giorgio D, Ferranti P, Paci M, Pucci P, Scaloni A, Segre A, Strobel GA (1994) Novel bioactive lipodepsipeptides from Pseudomonas syringae: the pseudomycins. FEBS Lett 355:96–100
Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444
Barchiesi F, Schimizzi AM, Fothergill AW, Scalise G, Rinaldi MG (1999) In vitro activity of the new echinocandin antifungal, MK-0991, against common and uncommon clinical isolates of Candida species. Eur J Clin Microbiol Infect Dis 18:302–304
Barrett D (2002) From natural products to clinically useful antifungals. Biochim Biophys Acta – Mol Basis Dis 1587:224–233
Bassarello C, Lazzaroni S, Bifulco G, Lo Cantore P, Iacobellis NS, Riccio R, Gomez-Paloma L, Evidente A, Tolaasins A-E (2004) Five new lipodepsipeptides produced by Pseudomonas tolaasii. J Nat Prod 67:811–816
Benz VF, Knüsel F, Nüesch J, Treichler H, Voser W, Nyfeler R, Keller-Schierlein W (1974) Echinocandin B, ein neuartiges polypeptid-antibioticum aus Aspergillus nidulans var. echinulatus: Isolierung und bausteune. Helv Chim Acta 57:2459–2477
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Boeck LD, Fukuda DS, Abbott BJ, Debono M (1989) Deacylation of echinocandin B by Actinoplanes utahensis. J Antibiot (Tokyo) 42:382–388
Boman HG (1998) Gene-encoded peptide antibiotics and the concept of innate immunity: an update review. Scand J Immunol 48:15–25
Bonmatin J-M, Laprévote O, Peypoux F (2003) Diversity among microbial cyclic lipopeptides: iturins and surfactins. Activity-structure relationships to design new bioactive agents. Comb Chem High Throughput Screen 6:541–556
Cameotra SS, Makkar RS (2004) Recent applications of biosurfactants as biological and immunological molecules. Curr Opin Microbiol 7:262–266
Cawoy H, Bettiol W, Fickers P, Ongena M (2011) Bacillus-based biological control of plant diseases. In: Pesticides in the modern world – pesticides use and management. InTech, Croatia, pp 273–303
Chabrol A, Cuzin L, Huguet F, Alvarez M, Verdeil X, Linas MD, Cassaing S, Giron J, Tetu L, Attal M, Récher C (2010) Prophylaxis of invasive aspergillosis with voriconazole or caspofungin during building work in patients with acute leukemia. Haematologica 95:996–1003
Chandrasekar PH, Cutright JL, Manavathu EK (2004) Efficacy of voriconazole plus amphotericin B or micafungin in a guinea-pig model of invasive pulmonary aspergillosis. Clin Microbiol Infect 10:925–928
Chen C, Belanger RR, Benhamou N, Paulitz TC (2000) Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol Mol Plant Pathol 56:13–23
Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Süssmuth R, Piel J, Borriss R (2009) Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol 140:27–37
Cho K-H, Kim S-T, Kim Y-K (2007) Purification of a pore-forming peptide toxin, tolaasin, produced by Pseudomonas tolaasii 6264. J Biochem Mol Biol 40:113–118
Cho KH, Wang HS, Kim YK (2010) Temperature-dependent hemolytic activity of membrane pore-forming peptide toxin, tolaasin. J Pept Sci 16:85–90
Cochrane SA, Vederas JC (2014) Lipopeptides from Bacillus and Paenibacillus spp.: a gold mine of antibiotic candidates. Med Res Rev 36:4–31
Coraiola M, Lo Cantore P, Lazzaroni S, Evidente A, Iacobellis NS, Dalla Serra M (2006) WLIP and tolaasin I, lipodepsipeptides from Pseudomonas reactans and Pseudomonas tolaasii, permeabilise model membranes. Biochim Biophys Acta – Biomembr 1758:1713–1722
Couillerot O, Prigent-Combaret C, Caballero-Mellado J, Moënne-Loccoz Y (2009) Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett Appl Microbiol 48:505–512
De Bruijn I, De Kock MJD, De Waard P, Van Beek TA, Raaijmakers JM (2008) Massetolide A biosynthesis in Pseudomonas fluorescens. J Bacteriol 190:2777–2789
De Faria AF, Stéfani D, Vaz BG, Silva ÍS, Garcia JS, Eberlin MN, Grossman MJ, Alves OL, Durrant LR (2011) Purification and structural characterization of fengycin homologues produced by Bacillus subtilis LSFM-05 grown on raw glycerol. J Ind Microbiol Biotechnol 38:863–871
De Pauw BE, Donnelly JP (2007) Prophylaxis and aspergillosis – has the principle been proven? N Engl J Med 356:409–411
De Wet N, Llanos-Cuentas A, Suleiman J, Baraldi E, Krantz EF, Della Negra M, Diekmann-Berndt H (2004) A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin Infect Dis 39:842–849
Debois D, Jourdan E, Smargiasso N, Thonart P, De Pauw E, Ongena M (2014) Spatiotemporal monitoring of the antibiome secreted by Bacillus biofilms on plant roots using MALDI mass spectrometry imaging. Anal Chem 86:4431–4438
Debono M, Abbott BJ, Fukuda DS, Barnhart M, Willard KE, Molloy RM, Michel KH, Turner JR, Butler TF, Hunt AH (1989) Synthesis of new analogs of echinocandin B by enzymatic deacylation and chemical reacylation of the echinocandin B peptide: synthesis of the antifungal agent cilofungin (LY121019). J Antibiot (Tokyo) 42:389–397
Debono M, Turner WW, LaGrandeur L, Burkhardt FJ, Nissen JS, Nichols KK, Rodriguez MJ, Zweifel MJ, Zeckner DJ, Gordee RS, Tang J, Parr TR (1995) Semisynthetic chemical modification of the antifungal lipopeptide echinocandin B (ECB): structure-activity studies of the lipophilic and geometric parameters of polyarylated acyl analogs of ECB. J Med Chem 38:3271–3281
Denning DW (2003) Echinocandin antifungal drugs. Lancet 362:1142–1151
Denning DW, Marr KA, Lau WM, Facklam DP, Ratanatharathorn V, Becker C, Ullmann AJ, Seibel NL, Flynn PM, van Burik JAH, Buell DN, Patterson TF (2006) Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect 53:337–349
Diekema DJ, Petroelje B, Messer SA, Hollis RJ, Pfaller MA (2005) Activities of available and investigational antifungal agents against Rhodotorula species. J Clin Microbiol 43:476–478
Dietel K, Beator B, Budiharjo A, Fan B, Borriss R (2013) Bacterial traits involved in colonization of Arabidopsis thaliana roots by Bacillus amyloliquefaciens FZB42. Plant Pathol J 29:59–66
Donadio S, Monciardini P, Sosio M (2007) Polyketide synthases and nonribosomal peptide synthetases: the emerging view from bacterial genomics. Nat Prod Rep 24:1073–1109
Douglas CM (2001) Fungal beta(1,3)-D-glucan synthesis. Med Mycol 39(Suppl 1):55–66
Douglas CM (2006) Understanding the microbiology of the Aspergillus cell wall and the efficacy of caspofungin. Med Mycol 44:95–99
Emiroglu M (2011) Micafungin use in children. Expert Rev Anti Infect Ther 9:821–834
Eshita SM, Roberto NH, Beale JM, Mamiya BM, Workman RF (1995) Bacillomycin Lc, a new antibiotic of the iturin group: isolations, structures, and antifungal activities of the congeners. J Antibiot (Tokyo) 48:1240–1247
Fortún J, Martín-Dávila P, Montejo M, Muñoz P, Cisneros JM, Ramos A, Aragón C, Blanes M, San Juan R, Gavaldá J, Llinares P (2009) Prophylaxis with caspofungin for invasive fungal infections in high-risk liver transplant recipients. Transplantation 87:424–435
Franco OL (2011) Peptide promiscuity: an evolutionary concept for plant defense. FEBS Lett 585:995–1000
Fujie A, Iwamoto T, Sato B, Muramatsu H, Kasahara C, Furuta T, Hori Y, Hino M, Hashimoto S (2001) FR131535, a novel water-soluble echinocandin-like lipopeptide: synthesis and biological properties. Bioorg Med Chem Lett 11:399–402
Fzb S, Koumoutsi A, Chen X, Henne A, Liesegang H, Hitzeroth G, Franke P, Vater J, Borriss R (2004) Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus. J Bacteriol 186:1084–1096
Galonić DP, Barr EW, Walsh CT, Bollinger JM, Krebs C (2007) Two interconverting Fe(IV) intermediates in aliphatic chlorination by the halogenase CytC3. Nat Chem Biol 3:113–116
George J, Reboli AC (2012) Anidulafungin: when and how? The clinician’s view. Mycoses 55:36–44
Georgopapadakou NH (1997) Antifungals targeted to the cell wall. Expert Opin Investig Drugs 6:147–150
Georgopapadakou NH (2001) Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin Investig Drugs 10:269–280
Grangemard I, Bonmatin JM, Bernillon J, Das BC, Peypoux F (1999) Lichenysins G, a novel family of lipopeptide biosurfactants from Bacillus licheniformis IM 1307: production, isolation and structural evaluation by NMR and mass spectrometry. J Antibiot (Tokyo) 52:363–373
Grangemard I, Wallach J, Maget-Dana R, Peypoux F (2001) Lichenysin: a more efficient cation chelator than surfactin. Appl Biochem Biotechnol 90:199–210
Graybill JR, Najvar LK, Luther MF, Fothergill AW (1997a) Treatment of murine disseminated candidiasis with L-743,872. Antimicrob Agents Chemother 41:1775–1777
Graybill JR, Bocanegra R, Luther M, Fothergill A, Rinaldi MJ (1997b) Treatment of murine Candida krusei or Candida glabrata infection with L- 743,872. Antimicrob Agents Chemother 41:1937–1939
Groetzner J, Kaczmarek I, Wittwer T, Strauch J, Meiser B, Wahlers T, Daebritz S, Reichart B (2008) Caspofungin as first-line therapy for the treatment of invasive aspergillosis after thoracic organ transplantation. J Heart Lung Transplant 27:1–6
Groll AH, Walsh TJ (2001) Caspofungin: pharmacology, safety and therapeutic potential in superficial and invasive fungal infections. Expert Opin Investig Drugs 10:1545–1558
Groll AH, Mickiene D, Petraitis V, Petraitiene R, Ibrahim KH, Piscitelli SC, Bekersky I, Walsh TJ (2001) Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin lipopeptide micafungin (FK463) in rabbits. Antimicrob Agents Chemother 45:3322–3327
Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I (2008) The changing epidemiology of invasive candidiasis: Candida glabrata and candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112:2493–2499
Hansen M, Thrane C, Olsson S, Sørensen J, Srensen J (2000) Confocal imaging of living fungal hyphae challenged with the fungal antagonist viscosinamide. Mycologia 92:216–221
Harrison L, Teplow DB, Rinaldi M, Strobel G (1991) Pseudomycins, a family of novel peptides from Pseudomonas syringae possessing broad-spectrum antifungal activity. J Gen Microbiol 137:2857–2865
Hashimoto S (2009) Micafungin: a sulfated echinocandin. J Antibiot (Tokyo) 62:27–35
Herbrecht R, Maertens J, Baila L, Aoun M, Heinz W, Martino R, Schwartz S, Ullmann AJ, Meert L, Paesmans M, Marchetti O, Akan H, Ameye L, Shivaprakash M, Viscoli C (2010) Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant 45:1227–1233
Higginbotham SJ, Murphy CD (2010) Identification and characterisation of a Streptomyces sp. isolate exhibiting activity against methicillin-resistant Staphylococcus aureus. Microbiol Res 165:82–86
Hiramatsu Y, Maeda Y, Fujii N, Saito T, Nawa Y, Hara M, Yano T, Asakura S, Sunami K, Tabayashi T, Miyata A, Matsuoka KI, Shinagawa K, Ikeda K, Matsuo K, Tanimoto M (2009) Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol 88:588–595
Hobbs M, Perfect J, Durack D (1988) Evaluation of in vitro antifungal activity of LY121019. Eur J Clin Microbiol Infect Dis 7:77–80
Horowitz S, Griffin WM (1991) Structural analysis of Bacillus licheniformis 86 surfactant. J Ind Microbiol 7:45–52
Jacobsen BJ, Zidack NK, Larson BJ (2004) The role of bacillus-based biological control agents in integrated pest management systems: plant diseases. Phytopathology 94:1272–1275
Jacques P (2011) Surfactin and other lipopeptides from Bacillus spp. In: Biosurfactants. Springer, Berlin, pp 57–92
Janisiewicz WJ, Korsten L (2002) Biological control of postharvest diseases of fruits. Annu Rev Phytopathol 40:411–441
Jones TSG (1949) Chemical evidence for the multiplicity of the antibiotics produced by Bacillus polymyxa. Ann N Y Acad Sci 51:909–916
Journet M, Cai D, Dimichele LM, Hughes DL, Larsen RD, Verhoeven TR, Reider PJ (1999) Semisynthesis of an antifungal lipopeptide echinocandin. J Org Chem 64:2411–2417
Kartsonis N, Killar J, Mixson L, Hoe CM, Sable C, Bartizal K, Motyl M (2005a) Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob Agents Chemother 49:3616–3623
Kartsonis NA, Saah AJ, Lipka CJ, Taylor AF, Sable CA (2005b) Salvage therapy with caspofungin for invasive aspergillosis: results from the caspofungin compassionate use study. J Infect 50:196–205
Kauffman CA (2005) Candiduria. Clin Infect Dis 41(Suppl 6):S371–S376
Keating GM, Figgitt DP (2003) Caspofungin: a review of its use in oesophageal candidiasis, invasive candidiasis and invasive aspergillosis. Drugs 63:2235–2263
Kim PI, Ryu J, Kim YH, Chi YT (2010) Production of biosurfactant lipopeptides iturin A, fengycin, and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol 20:138–145
Kim HJ, Choi HS, Yang SY, Kim IS, Yamaguchi T, Sohng JK, Park SK, Kim JC, Lee CH, Gardener BM, Kim YC (2014) Both extracellular chitinase and a new cyclic lipopeptide, chromobactomycin, contribute to the biocontrol activity of Chromobacterium sp. C61. Mol. Plant Pathol 15:122–132
Kinsella K, Schulthess CP, Morris TF, Stuart JD (2009) Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil Biol Biochem 41:374–379
Király L, Hafez YM, Fodor J, Király Z (2008) Suppression of tobacco mosaic virus-induced hypersensitive-type necrotization in tobacco at high temperature is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. J Gen Virol 89:799–808
Kohno S, Masaoka T, Yamaguchi H, Mori T, Urabe A, Ito A, Niki Y, Ikemoto H (2004) A multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in Japan. Scand J Infect Dis 36:372–379
Kohno S, Izumikawa K, Yoshida M, Takesue Y, Oka S, Kamei K, Miyazaki Y, Yoshinari T, Kartsonis NA, Niki Y (2013) A double-blind comparative study of the safety and efficacy of caspofungin versus micafungin in the treatment of candidiasis and aspergillosis. Eur J Clin Microbiol Infect Dis 32:387–397
Kontoyiannis DP, Ratanatharathorn V, Young JA, Raymond J, Laverdière M, Denning DW, Patterson TF, Facklam D, Kovanda L, Arnold L, Lau W, Buell D, Marr KA (2009) Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis: short communication. Transpl Infect Dis 11:89–93
Kurokawa N, Ohfune Y (1993) Synthetic studies on antifungal cyclic-peptides, echinocandins – stereoselective total synthesis of echinocandin-d via a novel peptide coupling. Tetrahedron 49:6195–6222
Kurtz MB, Heath IB, Marrinan J, Dreikorn S, Onishi J, Douglas C (1994) Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-??-D-glucan synthase. Antimicrob Agents Chemother 38:1480–1489
Lagrotteria D, Rotstein C, Lee CH (2007) Treatment of candiduria with micafungin: a case series. Can J Infect Dis Med Microbiol 18:149–150
Larkin RP, Tavantzis S (2013) Use of biocontrol organisms and compost amendments for improved control of soilborne diseases and increased potato production. Am J Potato Res 90:261–270
Laverdiere M, Hoban D, Restieri C, Habel F (2002) In vitro activity of three new triazoles and one echinocandin against Candida bloodstream isolates from cancer patients. J Antimicrob Chemother 50:119–123
Lee SC, Kim SH, Park IH, Chung SY, Subhosh Chandra M, Choi YL (2010) Isolation, purification, and characterization of novel fengycin S from bacillus amyloliquefaciens LSC04 degrading-crude oil. Biotechnol Bioproc Eng 15:246–253
Liu J, Liu M, Wang J, Yao JM, Pan RR, Yu ZL (2005) Enhancement of the Gibberella zeae growth inhibitory lipopeptides from a Bacillus subtilis mutant by ion beam implantation. Appl Microbiol Biotechnol 69:223–228
Liu XY, Yang SZ, Mu BZ (2008) Isolation and characterization of a C12-lipopeptide produced by Bacillus subtilis HSO 121. J Pept Sci 14:864–875
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Madureira A, Bergeron A, Lacroix C, Robin M, Rocha V, de Latour RP, Ferry C, Devergie A, Lapalu J, Gluckman E, Socié G, Ghannoum M, Ribaud P (2007) Breakthrough invasive aspergillosis in allogeneic haematopoietic stem cell transplant recipients treated with caspofungin. Int J Antimicrob Agents 30:551–554
Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, Sable CA, Kartsonis NA, Ngai A, Taylor A, Patterson TF, Denning DW, Walsh TJ (2004) Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis: Off Publ Infect Dis Soc Am 39:1563–1571
Makovitzki A, Viterbo A, Brotman Y, Chet I, Shai Y (2007) Inhibition of fungal and bacterial plant pathogens in vitro and in planta with ultrashort cationic lipopeptides. Appl Environ Microbiol 73:6629–6636
Mandal SM, Barbosa AEAD, Franco OL (2013) Lipopeptides in microbial infection control: scope and reality for industry. Biotechnol Adv 31:338–345
Marco F, Pfaller MA, Messer SA, Jones RN (1998) Activity of MK-0991 (l-743,872), a new echinocandin, compared with those of LY303366 and four other antifungal agents tested against blood stream isolates of Candida spp. Diagn Microbiol Infect Dis 32:33–37
Mariné M, Serena C, Pastor J, Quindós G, Carrillo AJ, Guarro J (2007) In vitro activity of micafungin combined with itraconazole against Candida spp. Int J Antimicrob Agents 30:463–465
Marr KA, Seidel K, Slavin MA, Bowden RA, Schoch HG, Flowers ME, Corey L, Boeckh M (2000) Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood 96:2055–2061
Matsumoto Y, Dogru M, Goto E, Fujishima H, Tsubota K (2005) Successful topical application of a new antifungal agent, micafungin, in the treatment of refractory fungal corneal ulcers: report of three cases and literature review. Cornea 24:748–753
Mátyus E, Blaskó K, Fidy J, Tieleman DP (2008) Structure and dynamics of the antifungal molecules Syringotoxin-B and Syringopeptin-25A from molecular dynamics simulation. Eur Biophys J 37:495–502
Mazzola M, De Bruijn I, Cohen MF, Raaijmakers JM (2009) Protozoan-induced regulation of cyclic lipopeptide biosynthesis is an effective predation defense mechanism for Pseudomonas fluorescens. Appl Environ Microbiol 75:6804–6811
McSpadden Gardener BB (2004) Ecology of Bacillus and Paenibacillus spp. in agricultural systems. Phytopathology 94:1252–1258
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJA, Sherertz RJ, Warren DK (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45
Messik F, Oberthür M (2013) Total synthesis of the antifungal agent echinocandin c. Angew Chem Int Ed Engl 52:5871–5875
Mikamo H, Sato Y, Tamaya T (2000) In vitro antifungal activity of FK463, a new water-soluble echinocandin-like lipopeptide. J Antimicrob Chemother 46:485–487
Molinaro A, Bedini E, Ferrara R, Lanzetta R, Parrilli M, Evidente A, Lo Cantore P, Iacobellis NS (2003) Structural determination of the O-specific chain of the lipopolysaccharide from the mushrooms pathogenic bacterium Pseudomonas tolaasii. Carbohydr Res 338:1251–1257
Moquet F, Mamoun M, Olivier JM (1996) Pseudomonas tolaasii and tolaasin: comparison of symptom induction on a wide range of Agaricus bisporus strains. FEMS Microbiol Lett 142:99–103
Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, Lupinacci R, Sable C, Kartsonis N, Perfect J (2002) Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 347:2020–2029
Moretti S, Bozza S, Massi-Benedetti C, Prezioso L, Rossetti E, Romani L, Aversa F, Pitzurra L (2014) An immunomodulatory activity of micafungin in preclinical aspergillosis. J Antimicrob Chemother 69:1065–1074
Morikawa M, Hirata Y, Imanaka T (2000) A study on the structure-function relationship of lipopeptide biosurfactants. Biochim Biophys Acta – Mol Cell Biol Lipids 1488:211–218
Moyne AL, Shelby R, Cleveland TE, Tuzun S (2001) Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J Appl Microbiol 90:622–629
Nakai T, Uno J, Otomo K, Ikeda F, Tawara S, Goto T, Nishimura K, Miyaji M (2002) In vitro activity of FK463, a novel lipopeptide antifungal agent, against a variety of clinically important molds. Chemotherapy 48:78–81
Naruse N, Tenmyo O, Kobaru S, Kamei H, Miyaki T, Konishi M, Oki T (1990) Pumilacidin, a complex of new antiviral antibiotics. Production, isolation, chemical properties, structure and biological activity. J Antibiot (Tokyo) 43:267–280
Ngai AL, Bourque MR, Lupinacci RJ, Strohmaier KM, Kartsonis NA (2011) Overview of safety experience with caspofungin in clinical trials conducted over the first 15 years: a brief report. Int J Antimicrob Agents 38:540–544
Nielsen TH, Sørensen J (2003) Production of cyclic lipopeptides by Pseudomonas fluorescens strains in bulk soil and in the sugar beet rhizosphere. Appl Environ Microbiol 69:861–868
Nielsen TH, Christophersen C, Anthoni U, Sørensen J (1999) Viscosinamide, a new cyclic depsipeptide with surfactant and antifungal properties produced by Pseudomonas fluorescens DR54. J Appl Microbiol 87:80–90
Nielsen TH, Thrane C, Christophersen C, Anthoni U, Sørensen J (2000) Structure, production characteristics and fun gel antagonism of tensin – a new antifungal cyclic lipopeptide from Pseudomonas fluorescens strain 96.578. J Appl Microbiol 89:992–1001
Nihorimbere V, Fickers P, Thonart P, Ongena M (2009) Ecological fitness of Bacillus subtilis BGS3 regarding production of the surfactin lipopeptide in the rhizosphere. Environ Microbiol Rep 1:124–130
Nihorimbere V, Cawoy H, Seyer A, Brunelle A, Thonart P, Ongena M (2012) Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol Ecol 79:176–191
Nishiyama Y, Uchida K, Yamaguchi H (2002) Morphological changes of Candida albicans induced by micafungin (FK463), a water-soluble echinocandin-like lipopeptide. J Electron Microsc (Tokyo) 51:247–255
Niwa T, Yokota Y, Tokunaga A, Yamato Y, Kagayama A, Fujiwara T, Hatakeyama J, Anezaki M, Ohtsuka Y, Takagi A (2004) Tissue distribution after intravenous dosing of micafungin, an antifungal drug, to rats. Biol Pharm Bull 27:1154–1156
Nutkins JC, Mortishire-Smith RJ, Packman LC, Brodey CL, Rainey PB, Johnstone K, Williams DH (1991) Structure determination of tolaasin, an extracellular lipodepsipeptide produced by the mushroom pathogen, Pseudomonas tolaasii Paine. J Am Chem Soc 113:2621–2627
Ongena M, Jacques P, Touré Y, Destain J, Jabrane A, Thonart P (2005) Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl Microbiol Biotechnol 69:29–38
Onishi J, Meinz M, Thompson J, Curotto J, Dreikorn S, Rosenbach M, Douglas C, Abruzzo G, Flattery A, Kong L, Cabello A, Vicente F, Pelaez F, Diez MT, Martin I, Bills G, Giacobbe R, Dombrowski A, Schwartz R, Morris S, Harris G, Tsipouras A, Wilson K, Kurtz MB (2000) Discovery of novel antifungal (1,3)-beta-D-glucan synthase inhibitors. Antimicrob Agents Chemother 44:368–377
Ostrosky-Zeichner L, Rex JH, Pappas PG, Hamill RJ, Larsen RA, Horowitz HW, Powderly WG, Hyslop N, Kauffman CA, Cleary J, Mangino JE, Lee J (2003) Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother 47:3149–3154
Ota S, Tanaka J, Kahata K, Toubai T, Kondo K, Mori A, Toyoshima N, Musashi M, Asaka M, Imamura M (2004) Successful micafungin (FK463) treatment of invasive pulmonary aspergillosis in a patient with acute lymphoblastic leukemia in a phase II study. Int J Hematol 79:390–393
Pal KK, McSpadden Gardener B (2006) Biological control of plant pathogens. Plant Health Instr 2:1117–1142
Pedras MSC, Ismaila N, Quail JW, Boyetchko SM (2003) Structure, chemistry, and biological activity of pseudophomins A and B, new cyclic lipodepsipeptides isolated from the biocontrol bacterium Pseudomonas fluorescens. Phytochemistry 62:1105–1114
Pérez-García A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol 22:187–193
Petraitis V, Petraitiene R, Groll AH, Roussillon K, Hemmings M, Lyman CA, Sein T, Bacher J, Bekersky I, Walsh TJ (2002) Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother 46:1857–1869
Pettengell K, Mynhardt J, Kluyts T, Lau W, Facklam D, Buell D, Baraldi E, Botes ME, Kluyts T, Malan DM, Elizabeth P, Mynhardt J, Pettengell K, Ross D, Smego RA, Soni P, Van Der Westhuizen IP, Marais C, Webber C, Lau W, Facklam D, Buell D (2004) Successful treatment of oesophageal candidiasis by micafungin: a novel systemic antifungal agent. Aliment Pharmacol Ther 20:475–481
Peypoux F, Besson F, Michel G, Delcambe L (1981) Structure of bacillomycin D, a new antibiotic of the iturin group. Eur J Biochem 118:323–327
Peypoux F, Pommier MT, Das BC, Besson F, Delcambe L, Michel G (1984) Structures of bacillomycin D and bacillomycin L peptidolipid antibiotics from Bacillus subtilis. J Antibiot (Tokyo) 37:1600–1604
Peypoux F, Marion D, Maget-Dana R, Ptak M, Das BC, Michel G (1985) Structure of bacillomycin F, a new peptidolipid antibiotic of the iturin group. Eur J Biochem 153:335–340
Peypoux F, Pommier MT, Marion D, Ptak M, Das BC, Michel G (1986) Revised structure of mycosubtilin, a peptidolipid antibiotic from Bacillus subtilis. J Antibiot (Tokyo) 39:636–641
Pfaller MA, Marco F, Messer SA, Jones RN (1998) In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis 30:251–255
Powles MA, Liberator P, Anderson J, Karkhanis Y, Dropinski JF, Bouffard FA, Balkovec JM, Fujioka H, Aikawa M, Mcfadden D, Schmatz D (1998) Efficacy of MK-991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob Agents Chemother 42:1985–1989
Raaijmakers JM, de Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062
Rainey P, Brodey C, Johnstone K (1991) Biological properties and spectrum of activity of tolaasin, a lipodepsipeptide toxin produced by the mushroom pathogen Pseudomonas tolaasii. Physiol Mol Plant 39:57–70
Rao M, Wei W, Ge M, Chen D, Sheng X (2013) A new antibacterial lipopeptide found by UPLC-MS from an actinomycete Streptomyces sp. HCCB10043. Nat Prod Res 27:2190–2195
Rivardo F, Turner RJ, Allegrone G, Ceri H, Martinotti MG (2009) Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl Microbiol Biotechnol 83:541–553
Robbel L, Marahiel MA (2010) Daptomycin, a bacterial lipopeptide synthesized by a nonribosomal machinery. J Biol Chem 285:27501–27508
Rodriguez MJ, Vasudevan V, Jamison JA, Borromeo PS, Turner WW (1999) The synthesis of water soluble prodrugs analogs of echinocandin B. Bioorg Med Chem Lett 9:1863–1868
Romero D, De Vicente A, Olmos JL, Dávila JC, Pérez-García A (2007a) Effect of lipopeptides of antagonistic strains of Bacillus subtilis on the morphology and ultrastructure of the cucurbit fungal pathogen Podosphaera fusca. J Appl Microbiol 103:969–976
Romero D, de Vicente A, Rakotoaly RH, Dufour SE, Veening J-W, Arrebola E, Cazorla FM, Kuipers OP, Paquot M, Pérez-García A (2007b) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant Microbe Interact 20:430–440
Roongsawang N, Thaniyavarn J, Thaniyavarn S, Kameyama T, Haruki M, Imanaka T, Morikawa M, Kanaya S (2002) Isolation and characterization of a halotolerant Bacillus subtilis BBK-1 which produces three kinds of lipopeptides: Bacillomycin L, plipastatin, and surfactin. Extremophiles 6:499–506
Rückert C, Blom J, Chen X, Reva O, Borriss R (2011) Genome sequence of B. amyloliquefaciens type strain DSM7T reveals differences to plant-associated B. amyloliquefaciens FZB42. J Biotechnol 155:78–85
Segre A, Bachmann RC, Ballio A, Bossa F, Grgurina I, Iacobellis NS, Marino G, Pucci P, Simmaco M, Takemoto JY (1989) The structure of syringomycins A1, E and G. FEBS Lett 255:27–31
Shamala N, Row TNG, Venkatesan K (1976) Crystal and molecular structure of allo-4-hydroxy-L-proline dihydrate. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem B32:3267–3270
Sionov E, Mendlovic S, Segal E (2006) Efficacy of amphotericin B or amphotericin B-intralipid in combination with caspofungin against experimental aspergillosis. J Infect 53:131–139
Sobel JD, Kauffman CA, McKinsey D, Zervos M, Vazquez JA, Karchmer AW, Lee J, Thomas C, Panzer H, Dismukes WE (2000) Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. The National Institute of Allergy and Infectious Diseases (NIAID) Mycoses Study Group. Clin Infect Dis 30:19–24
Soler-Rivas C, Arpin N (1999) The effects of tolaasin, the toxin produced by Pseudomonas tolaasii on tyrosinase activities and the induction of browning in Agaricus bisporus fruiting. Mol Plant 55:21–28
Song Y-C, Chou A-H, Homhuan A, Huang M-H, Chiang S-K, Shen K-Y, Chuang P-W, Leng C-H, Tao M-H, Chong P, Liu S-J (2011) Presentation of lipopeptide by dendritic cells induces anti-tumor responses via an endocytosis-independent pathway in vivo. J Leukoc Biol 90:323–332
Song YC, Liu HH, Chen IH, Chen HW, Chong P, Leng CH, Liu SJ (2014) A purified recombinant lipopeptide as adjuvant for cancer immunotherapy. Biomed Res Int 2014:349783
Sørensen D, Nielsen TH, Christophersen C, Sørensen J, Gajhede M (2001) Cyclic lipoundecapeptide amphisin from Pseudomonas sp. strain DSS73. Acta Crystallogr C Cryst Struct Commun 57:1123–1124
Spadaro D, Gullino ML (2005) Improving the efficacy of biocontrol agents against soilborne pathogens. Crop Prot 24:601–613
Spreghini E, Orlando F, Sanguinetti M, Posteraro B, Giannini D, Manso E, Barchiesia F (2012) Comparative effects of micafungin, caspofungin, and anidulafungin against a difficult-to-treat fungal opportunistic pathogen, Candida glabrata. Antimicrob Agents Chemother 56:1215–1222
Stachelhaus T, Schneider A, Marahiel MA (1995) Rational design of peptide antibiotics by targeted replacement of bacterial and fungal domains. Science 269:69–72
Sucher AJ, Chahine EB, Balcer HE (2009) Echinocandins: the newest class of antifungals. Ann Pharmacother 43:1647–1657
Sun X, Zeckner DJ, Current WL, Boyer R, McMillian C, Yumibe N, Chen SH (2001) N-acyloxymethyl carbamate linked prodrugs of pseudomycins are novel antifungal agents. Bioorg Med Chem Lett 11:1875–1879
Tareq FS, Lee MA, Lee HS, Lee YJ, Lee JS, Hasan CM, Islam MT, Shin HJ (2014) Gageotetrins A-C, noncytotoxic antimicrobial linear lipopeptides from a marine bacterium bacillus subtilis. Org Lett 16:928–931
Tawara S, Ikeda F, Maki K, Morishita Y, Otomo K, Teratani N, Goto T, Tomishima M, Ohki H, Yamada A, Kawabata K, Takasugi H, Sakane K, Tanaka H, Matsumoto F, Kuwahara S (2000) In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother 44:57–62
Tendulkar SR, Saikumari YK, Patel V, Raghotama S, Munshi TK, Balaram P, Chattoo BB (2007) Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. J Appl Microbiol 103:2331–2339
Tenoux I, Besson F, Michel G (1991) Studies on the antifungal antibiotics: bacillomycin D and bacillomycin D methylester. Microbios 67:187–193
Thrane C, Olsson S, Harder Nielsen T, Sørensen J (1999) Vital fluorescent stains for detection of stress in Pythium ultimum and Rhizoctonia solani challenged with viscosinamide from Pseudomonas fluorescens DR54. FEMS Microbiol Ecol 30:11–23
Thrane C, Harder Nielsen T, Neiendam Nielsen M, Sørensen J, Olsson S (2000) Viscosinamide-producing Pseudomonas fluorescens DR54 exerts a biocontrol effect on Pythium ultimum in sugar beet rhizosphere. FEMS Microbiol Ecol 33:139–146
Tóth V, Nagy CT, Pócsi I, Emri T (2012) The echinocandin B producer fungus Aspergillus nidulans var. roseus ATCC 58397 does not possess innate resistance against its lipopeptide antimycotic. Appl Microbiol Biotechnol 95:113–122
Tran H, Ficke A, Asiimwe T, Höfte M, Raaijmakers JM (2007) Role of the cyclic lipopeptide massetolide a in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol 175:731–742
Tsan P, Volpon L, Besson F, Lancelin JM (2007) Structure and dynamics of surfactin studied by NMR in micellar media. J Am Chem Soc 129:1968–1977
Uchida K, Nishiyama Y, Yokota N, Yamaguchi H (2000) In vitro antifungal activity of a novel lipopeptide antifungal agent, FK463, against various fungal pathogens. J Antibiot (Tokyo) 53:1175–1181
Ullmann AJ, Cornely OA, Donnelly JP, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Garbino J, Groll AH, Herbrecht R, Hope WW, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Cuenca-Estrella M (2012) ESCMID guideline for the diagnosis and management of Candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. Clin Microbiol Infect 18:1–8
Van Burik J-AH, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, Bunin N, Wall DA, Hiemenz JW, Satoi Y, Lee JM, Walsh TJ (2004) Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 39:1407–1416
Vanittanakom N, Loeffler W (1986) Fengycin – a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot (Tokyo) XXXIX:888–901
Vazquez JA, Lynch M, Boikov D, Sobel JD (1997) In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob Agents Chemother 41:1612–1614
Vicente MF, Basilio A, Cabello A, Peláez F (2003) Microbial natural products as a source of antifungals. Clin Microbiol Infect 9:15–32
Wiederhold NP, Cota JM, Frei CR (2008) Micafungin in the treatment of invasive candidiasis and invasive aspergillosis. Infect Drug Resist 1:63–77
Wilson Quail J, Ismail N, Soledade M, Pedras C, Boyetchko SM (2002) Pseudophomins A and B, a class of cyclic lipodepsipeptides isolated from a Pseudomonas species. Acta Crystallogr C Cryst Struct Commun 58:o268–o271
Yang P, Sun Z x, Liu S y, Lu H x, Zhou Y, Sun M (2013) Combining antagonistic endophytic bacteria in different growth stages of cotton for control of Verticillium wilt. Crop Prot 47:17–23
Yaryura PM, León M, Correa OS, Kerber NL, Pucheu NL, García AF (2008) Assessment of the role of chemotaxis and biofilm formation as requirements for colonization of roots and seeds of soybean plants by Bacillus amyloliquefaciens BNM339. Curr Microbiol 56:625–632
Yokote T, Akioka T, Oka S, Fujisaka T, Yamano T, Hara S, Tsuji M, Hanafusa T (2004) Successful treatment with micafungin of invasive pulmonary aspergillosis in acute myeloid leukemia, with renal failure due to amphotericin B therapy. Ann Hematol 83:64–66
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zhang JC, Dai JY, Fan J, Wu XP (2006) The treatment of pneumocystis Carinii pneumonia with caspofungin in elderly patients: a case report and literature review. Zhonghua Jie He He Hu Xi Za Zhi 29:463–465
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer India
About this chapter
Cite this chapter
Baindara, P., Korpole, S. (2016). Lipopeptides: Status and Strategies to Control Fungal Infection. In: Basak, A., Chakraborty, R., Mandal, S. (eds) Recent Trends in Antifungal Agents and Antifungal Therapy. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2782-3_4
Download citation
DOI: https://doi.org/10.1007/978-81-322-2782-3_4
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2780-9
Online ISBN: 978-81-322-2782-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)