Abstract
Bacillus sp. has proven to be a goldmine of diverse bioactive lipopeptides, finding wide-range of industrial applications. This review highlights the importance of three major families of lipopeptides (iturin, fengycin, and surfactin) produced by Bacillus sp. and their diverse activities against plant pathogens. This review also emphasizes the role of non-ribosomal peptide synthetases (NRPS) as significant enzymes responsible for synthesizing these lipopeptides, contributing to their peptide diversity. Literature showed that these lipopeptides exhibit potent antifungal activity against various plant pathogens and highlight their specific mechanisms, such as siderophore activity, pore-forming properties, biofilm inhibition, and dislodging activity. The novelty of this review comes from its comprehensive coverage of Bacillus sp. lipopeptides, their production, classification, mechanisms of action, and potential applications in plant protection. It also emphasizes the importance of ongoing research for developing new and enhanced antimicrobial agents. Furthermore, this review article highlights the need for future research to improve the production efficiency of these lipopeptides for commercial applications. It recognizes the potential for these lipopeptides to expand the field of biological pest management for both existing and emerging plant diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical pest management has been a prominent agricultural practice for over a century. The utilization of pesticides has led to a notable 39% reduction in global crop losses, as demonstrated in various studies [1,2,3]. In nations with subtropical climates like India, storage pests pose a significant challenge, resulting in postharvest losses and a decline in product quality. In the natural environment, plants consistently encounter an array of biotic and abiotic challenges that hinder their growth and overall productivity [4, 5]. The extensive application of chemical fertilizers and agrochemicals raises concerns about their long-term implications. Addressing this issue will be pivotal in enhancing agricultural yield and output, particularly as the world's population grows at an unprecedented rate [6, 7].
Plants are exposed to billions of bacteria within their natural environment, including Bacillus sp., which infiltrate and occupy various compartments and chambers within plant tissues [8, 9]. These compartments comprise the rhizosphere, endosphere, phyllosphere, and rhizoplane [4, 8,9,10]. Consequently, biopesticides have emerged as the preferred choice for pest management, supplanting synthetic pesticides [11]. This shift is attributed to their superior pest control capabilities and diverse array of modes of action [12].
While chemical fungicides have provided a solution for years, their widespread use has led to environmental degradation and raised human health concerns [2, 3, 13]. The rising dependence of farmers on chemical fertilizers further jeopardizes ecosystem stability and soil fertility [1]. At the forefront of sustainable agricultural practices are Plant Growth-Promoting Rhizobacteria (PGPR), offering a range of benefits, including improved crop yields and enhanced soil quality [8, 14]. Within this landscape, Bacillus spp. stands out as a prominent PGPR in soil. It employs mechanisms such as biofilm formation, induced systemic resistance (ISR), and lipopeptide production to empower plants in dealing with both biotic and abiotic stress [15].
The spotlight is on lipopeptides, owing to their compelling potential in diverse pharmaceuticals, agriculture, chemicals, and food sectors. Their amphipathic structures underscore their significance, boasting a spectrum of bioactivities encompassing antibacterial, antiviral, antifungal, and anticancer properties. Among these, Bacillus subtilis takes centre stage as a favoured microorganism for producing active lipopeptides. Its rapid growth, modest nutritional requirements, human and animal safety, and potent antibacterial attributes render it a cornerstone of various industries, including agriculture, food production, medicine, and feed manufacturing [16, 17].
In the domain of Bacillus species, a wide array of lipopeptides can be identified, encompassing surfactins, iturins, and fengycins [18]. These naturally occurring lipopeptides manifest as an array of isoforms and analogs, each with its distinctive structural fingerprint. Over more than two decades, this class of compounds has undergone rigorous scrutiny encompassing isolation, synthesis, purification, structural characterization, bioactivity assessment, and potential applications. Particularly, the groups of Bacillus lipopeptides—surfactants, fengycins, and iturins—have gained attention for their antagonistic properties against an array of potential phytopathogens, including fungi, oomycetes and bacteria, positioning them as potent biocontrol agents [19]. Inhibiting many fungal infections, Bacillus spp. metabolites have further emphasised their efficacy in safeguarding plant health [19].
The uniqueness of this manuscript lies in its thorough exploration of Bacillus lipopeptides, including surfactins, fengycins, and iturins, showcasing their antagonistic attributes against potential phytopathogens and their role as potent biocontrol agents. The manuscript places a strong emphasis on highlighting the efficacy of Bacillus spp. metabolites in restraining fungal infections and bolstering the protection of plant health.
Classification of lipopeptide produced by Bacillus
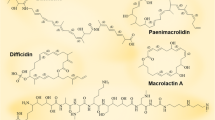
A subclass of microbial surfactants known as lipopeptides comprises compounds such as iturin, surfactin, fengycin, kurstakin, and lichenysin [16] (Fig. 1). Lipopeptides are classified based on amino acid sequences and types of and different strains of Bacillus spp. that produces lipopeptides, like B. cereus, B. subtilis, B. pumilus, B. amyloliquefaciens, B. globigii, B. thuringiensis, B. licheniformis, and B. megaterium and are used to classify lipopeptides surfactants.
Classification of lipopetides (cyclic nonribosomally synthesized peptide) from Bacillus sp. All the structures were procured from https://pubchem.ncbi.nlm.nih.gov/
Iturin
Iturins, cyclic lipopeptides, feature hydrophilic C-terminal heptapeptides and hydrophobic N-terminal β-amino fatty acids synthesized by certain strains of Bacillus bacteria [16, 20]. Their aliphatic chain contains 14 to 17 carbons, along with a chiral LDDLLDL sequence (L-Asn-D-Tyr-D-Asn-L-Gln-L-Pro-D-Asn-L-Ser) within the peptide structure [21]. Amino acid variations account for the significant polymorphism in iturins. Notable members of this group include iturin A, iturin D, iturin C, iturin E, bacillomycin D, bacillomycin L, bacillomycin F, mycosubtilin, and bacillomycin Lc (Fig. 1). Iturin’s structure varies by producing Bacillus strains, with common amino acids including isoleucine and aspartic acid. Nonribosomal peptide synthetases (NRPSs) encoded in the Bacillus genome mediate its biosynthesis, including the incorporation of the fatty acid chain. Besson et al. [22] investigated L's structure using infrared spectroscopy. Iturin A D-lipopeptide synthesized by B. subtilis demonstrated the presence of turns in Iturin A. Its structure was discovered as a result of the findings: Iturin A forms a closed-loop structure with fatty acid, β-AA and D-heptapeptide is an alternative L-lipopeptide [23]. Functionally, iturin showcases antifungal, antibacterial, and plant growth-promoting activities [24]. It disrupts fungi and bacteria cell membranes by interacting with their lipid components, leading to cell lysis. Additionally, it stimulates plant growth by improving nutrient uptake, protecting plants from pathogens, and fostering root development.
Consequently, iturin finds applications in agriculture, biotechnology, and medicine. According to the published reports, iturin has shown strong antifungal activity against fungal pathogens like Alternaria alternata, Botrytis cinerea and Penicillium expansum [25,26,27]. Its potential as a biocontrol agent against fungal and bacterial pathogens, its role in enhancing plant health and crop yields, and its environmentally friendly profile make iturin an attractive alternative to synthetic pesticides and antimicrobial agents [18].
Fengycin
Fengycin, a cyclic lipopeptide synthesized primarily by Bacillus subtilis and related strains, belongs to the lipopeptide family alongside antimicrobial agents like iturin and surfactin [27]. Its structure consists of a cyclic heptapeptide with seven amino acids and a hydrophobic fatty acid tail that grants amphiphilic properties [28]. The specific composition of fengycin varies among Bacillus strains, incorporating alternating L- and D-amino acids like leucine, valine, and hydroxyvaline [28]. This lipopeptide's biosynthesis is governed by Non Ribosomal Peptide Synthetases (NRPSs), encoded in the Bacillus genome, which assemble amino acids into the cyclic peptide structure while integrating the fatty acid chain [29, 30].
Fengycin's remarkable attributes include potent antifungal activity against plant pathogens and filamentous fungi [31]. This action stems from its disruption of fungal membranes, leading to cell demise. Leveraging this quality, fengycin holds potential as a biocontrol agent against plant fungal diseases in agriculture [32]. Its environmentally friendly nature is owed to its natural bacterial origin and limited toxicity to non-target organisms [18, 24]. This property aligns with its exploration as an eco-friendly alternative to synthetic fungicides, contributing to sustainable disease management in crops [33].
Surfactin
Surfactin, synthesized by specific Bacillus strains like Bacillus subtilis, is a cyclic lipopeptide with versatile properties encompassing surfactant, antimicrobial, and biosurfactant functions [18]. Its structure includes a cyclic heptapeptide ring linked to a hydrophobic fatty acid tail, typically containing seven L- and D-amino acids. The hydrophobic tail commonly features a β-hydroxy fatty acid chain [34].
The amino acid sequence in surfactin's peptide ring can vary among Bacillus strains, often including amino acids such as leucine, valine, aspartic acid, and glutamic acid. This hydrophobic tail contributes to its interaction with lipid membranes, augmenting its surfactant qualities. Produced through non-ribosomal peptide synthetases (NRPSs), surfactin’s biosynthesis involves assembling amino acids into the cyclic peptide structure and attaching the fatty acid tail.
Surfactin is notable for its surface-active properties, lowering surface tension and forming micelles, which find applications in industries like detergents and emulsifiers. Additionally, surfactin displays potent antimicrobial actions against various microorganisms, disrupting cellular membranes by integrating its hydrophobic tail into the lipid bilayer, resulting in membrane permeabilization and cell death.
Surfactin's effectiveness extends to inhibiting biofilm formation, offering potential solutions to biofilm-related issues in different industries [35]. Its eco-friendly nature is attributed to its natural origin and biodegradability. Thus, surfactin is applied across sectors, including agriculture (as a biopesticide), bioremediation (for oil spill cleanup), cosmetics (as an emulsifier), and pharmaceuticals (for drug delivery)[34, 36]. Bacillus spp., including Bacillus subtilis, B. amyloliquefaciens, and B. velezensis, all make surfactin [36, 37].
Other Lipopeptides procured from Bacillus sp.
All these metabolites comprise a gFA or FA connected to a partially cyclic or cyclic peptide chain by an amide or ester connection.
Licheniformin
Licheniformin, from Bacillus licheniformis, is a surfactin-like lipopeptide with antimicrobial potency. It has a cyclic structure blending a cyclic peptide with a lipid tail, enabling it to disrupt microbial membranes effectively. Due to its membrane-disrupting ability, it combats a range of microorganisms including bacteria, fungi, and viruses. Notably, licheniformin dislodges biofilms, though microbial communities resistant to standard antimicrobials, making it a candidate for biofilm-related infections. While antiviral potential has been noted, further research is necessary. Licheniformin’s biofilm disruption and antimicrobial traits spark interest for potential use in biotechnology, encompassing fields like food preservation and medical devices to address microbial contamination and biofilm concerns [20, 31].
Kurstakins
Kurstakins, cyclic lipopeptides from Bacillus species like Bacillus subtilis, possess a unique structure, potent antimicrobial abilities, and broad application potential. With a peptide ring and lipid tail arrangement, they exhibit amphiphilic properties to interact in diverse environments [31]. These compounds effectively combat bacteria and fungi by disrupting cell membranes, causing cell death. They also disrupt biofilms, making them valuable against biofilm-related issues. Due to their antimicrobial and biofilm-disrupting properties, Kurstakins hold promise for biotechnological use in agriculture, food safety, medical devices, and pharmaceuticals. Diverse Bacillus strains yield varied kurstakin analogs, each with distinct traits for exploring various functions. Kurstakins contribute to research on natural antimicrobials, supplementing the study of potential industrial and medical applications. Ongoing research is crucial for comprehending mechanisms, optimizing production, and uncovering full applications [38].
Locillomycins
Locillomycins, produced by specific Bacillus subtilis strains, are lipopeptide antibiotics known for their intricate structure, strong antibacterial efficacy, and potential uses. Their complex design links a lipid tail to a cyclic peptide core, conferring amphiphilic qualities for interaction in varied environments. These lipopeptides are potent against Gram-positive bacteria, hindering cell wall biosynthesis and causing cell demise. Their unique mode of action suggests promise in countering antibiotic resistance. Locillomycins hold potential for medical and agricultural applications, including novel antibiotics for combating resistant strains and use in plant protection [31]. Various Bacillus subtilis strains yield diverse locillomycin analogs, each with distinctive attributes. Ongoing research is essential to grasp their mechanisms, optimize production, and explore comprehensive applications [39].
Pumilacidin
Pumilacidin is another lipopeptide produced by Bacillus species, particularly Bacillus pumilus. It has been found to possess antimicrobial activity against various pathogens, including bacteria and fungi. Pumilacidin is not a surfactin either; it is a cyclic lipopeptide with a complex structure, and its antimicrobial properties are of interest for potential therapeutic applications.
Cyclic depsipeptides
Bacillus species produce a variety of cyclic depsipeptides like plipastatins, difficidins, and bacillomycins. These compounds often have antimicrobial properties and can disrupt microbial membranes. They are being investigated for their potential in food preservation, agriculture, and medicine.
Bacylisin
Bacylisin is a lipopeptide produced by Bacillus amyloliquefaciens that exhibits antibacterial activity against Gram-positive bacteria. It has shown potential as an alternative to antibiotics in animal production and agriculture.
Mycosubtilin
Mycosubtilin is another lipopeptide produced by Bacillus subtilis with antifungal properties. It has been explored for its use in agricultural biocontrol against fungal pathogens.
Bacitracin
Bacitracin is a well-known peptide antibiotic produced by Bacillus licheniformis and Bacillus subtilis. It is used in medicine to prevent bacterial infections and in animal feed to promote growth.
Biosynthesis of lipopeptides
Lipopeptides are a class of molecules that combine the characteristics of both lipids (fats) and peptides (amino acid chains). They often have interesting biological and pharmaceutical properties due to their amphiphilic nature, allowing them to interact with hydrophilic (water-loving) and hydrophobic (water-repelling) environments [20]. The biosynthesis of lipopeptides can involve a combination of enzymatic and non-enzymatic processes [40, 41]. Lipopeptide biosynthesis involves intricate processes merging amino acids and lipids, yielding versatile biological and pharmaceutical potential molecules. Initiated by ATP-dependent aminoacyl-tRNA synthetases, amino acids bind to tRNAs, forming aminoacyl-tRNAs. Lipopeptides often arise from multi-enzyme Non Ribosomal Peptide Synthetases (NRPS), with modules incorporating amino acids into growing chains and domains guiding activation, selection, and bond formation (Fig. 2).
Simultaneously, fatty acids elongate through enzymes like fatty acid synthase, paralleling peptide synthesis. An enzymatic NRPS domain, as part of the complex or separate, attaches a fatty acid or lipid to the peptide as it reaches a threshold length [31, 37]. Subsequent modifications, e.g., cyclization, oxidation, and glycosylation, reshape the lipopeptide's structure and bioactivity [31, 37]. Following assembly, lipopeptides exit cells via transport systems or secretion pathways. Biosynthesis varies across organisms and types, using ribosomal, non-ribosomal, or mixed processes guided by intended function [42]. Research continually unveils insights into lipopeptide biosynthesis, propelled by their unique attributes for drug development, antimicrobials, and more, offering an exciting frontier for scientific advancement [19]. Genetic engineering has been employed to enhance strain qualities; however, it has proven challenging to clone and produce the genes that encode the proteins necessary for lipopeptide biosynthesis [31, 37].
The analysis, extraction, and purification stages are crucial for obtaining high-quality lipopeptides from microbial sources [43, 44]. Lipopeptide analysis characterises their structure, weight, and properties through mass spectrometry, NMR, and chromatography, shedding light on structural variations and potential activities [20, 45]. Lipopeptide separation from microbial cultures employs solvent extraction, solid-phase extraction, and liquid–liquid extraction methods, with efficient techniques like organic solvent extraction ensuring optimal recovery and minimal damage [21, 41, 46]. Subsequent purification removes impurities from crude extracts, often utilizing chromatography methods like RP-HPLC that isolate lipopeptides based on hydrophobicity [40]. This results in high-purity compounds suitable for detailed characterization and diverse applications [18].
In the latest research findings, employing liquid culture and promoter-exchanged B. subtilis THY-7 to upregulate the Pg3 gene has demonstrated the highest yield of Bacillus lipopeptides, reaching 9.74 g/L [47]. In another investigation conducted by Dang et al. [40], the production of the antifungal lipopeptide iturin A from Bacillus amyloliquefaciens LL3 was enhanced through the optimization of growth conditions and metabolic engineering. The iturin A yield was elevated to 99.73 mg/L through a response surface approach for fermentation condition optimisation. Kim et al. [41] employed both wild-type and mutant strains of Bacillus velezensis to inhibit the growth and spore germination of Botrytis cinerea. The secondary metabolites from culture broths of these Bacillus strains were analyzed using HPLC (Agilent1100) with a C18 column. In the study by Malfanova et al. [48], bacterial cells were grown at 28 °C for 5 days, followed by centrifugation at 13,000 rpm for 10 min to extract lipopeptides. The resulting supernatant was acidified with strong HCl, and the acid precipitate was then extracted with methanol (Fig. 3).
Naik et al. (37) employed a distinctive method for isolating cyclic lipopeptides using Bacillus velezensis. Specifically, they cultivated 200 ml of Sphaerulina musiva spores in conjunction with bacterial culture EB14 on a sterile paper disc, and subsequently incubated the setup at room temperature. Following a 72-h incubation period, agar plugs weighing 300 mg were extracted from the growth inhibition zone’s bacterial and fungal sections. To process these agar plugs, an acetonitrile/water-based approach was adopted. The compounds extracted from these agar plug samples were then analysed using a Fourier Transform Ion Cyclotron Resonance Mass Spectrometer [44].
In a separate research effort, Liu's laboratory purified and extracted C16-Fengycin from Bacillus amyloliquefaciens. This procedure involved utilizing fmb60 fermentation broth media, which was acidified to a pH of 2.0 using 6 M HCl. Subsequent steps included an overnight precipitation process and centrifugation to isolate the acid precipitate, followed by reconstitution in methanol. The isolation of C16-Fengycin A was achieved by utilizing a high-performance liquid chromatography (HPLC) system subsequent to its detection [29, 49].
Therefore, the mass production of lipopeptides requires a multidisciplinary approach involving microbiology, fermentation technology, chemistry, engineering, and quality assurance [46].
Although additional research is necessary to enhance the biosynthesis efficiency of Bacillus lipopeptides for industrial applications, these advancements provide essential insights for the formulation of strategies aimed at the large-scale production of these lipopeptides.
Bacillus lipopeptide interactions concerning biological management of plant diseases
Effect of lipopeptides in plant tissue colonization
In the rhizosphere, microorganisms thrive in the presence of a wide variety of low molecular weight chemicals and biomolecules frequently released from plants’ roots [9]. Various rhizosphere bacteria colonize the majority of plants planted in the field. Some bacteria connected with plants are categorised as beneficial microorganisms based on how they affect plant performance. These free-living bacteria that inhabit the rhizosphere soil include plant-growth-promoting rhizobacteria (PGPR), which produce a range of antifungal metabolites and plant-growth-promoting traits [50]. However, given the paucity of studies in this area, there is an urgent need to comprehend the role of endophytes in plants’ simultaneous abiotic and biotic stress tolerance [51].
Endophytes can infiltrate and colonise host plant tissues by horizontal transmission from the soil to the plants or vertical sowing techniques. The colonisation of endophytes has long been thought to be a passive process. When the endophyte penetrates the host plant, the host plant recognizes it, and signal molecules crosstalk begins. Root exudates play a crucial role in various plant–microbe interactions and significantly impact soil microbial communities, nutrient cycling, and plant health [9]. These positive relationships increase plant health and agricultural productivity by increasing nutrient availability, producing hormones stimulating plant growth, lowering diseases from pathogens or pests, or strengthening resilience to environmental stress [50]. Additionally, due to their amphiphilic surfactant-like properties, the synergistic impact of several lipopeptides (mostly iturins, surfactins, and fengycins) exhibited antiadhesive action, reducing colonization and promoting biofilm dispersion of harmful bacteria [52]. Putisovin I and II, two Pseudomonas fluorescens lipopeptides, are known to inhibit the development of new biofilms and the degradation of existing biofilm [52]. According to Abdallah et al., [53] a combination of lipopeptides synthesized by Bacillus amyloliquefaciens successfully suppressed an Agrobacterium tumefaciens biofilm. The combination might prevent tumour growth once a pathogen adheres to a tomato plant. The experiment also resulted in dislodging existing biofilms and inhibiting new biofilm development. In agriculture, the antiadhesive qualities of certain lipopeptide may also be employed to lessen phytopathogen adhesion and their colonisation effectiveness, which is particularly important for the emergence of many plant diseases [53].
Biocontrol and stimulation of immunity
Host vulnerability, pathogen virulence, and environmental variables make up the classic disease triangle of plant pathogens. The disease triangle is influenced by Bacillus spp. both directly and indirectly. For example, indirect approaches include biofilm development, plant growth stimulation, space and nutrient competition, and ISR. Bacillus strains that generate lipopeptides form biofilms around plant roots, enhancing rhizosphere antibacterial activity. Bacillus subtilis forms biofilms and produces a variety of toxins to battle pathogenic species, creating its own environment [15]. Insects and microorganisms are among the many species that come into contact of plants from their natural surroundings ). Depending on the pathogen's biology and lifestyle, the plant activates a layer of defence systems in response to infection [54]. Microbe Associated Molecular Pattern (MAMPS) and Pattern-Recognition Receptors (PRRs) work together to detect microbial chemicals and produce an explicit defence response. The timing and kind of an immune response determine its type, with secondary responses being more potent than primary ones. Plants’ second defence responses, systemic acquired resistance (SAR) and ISR, are thought to be mediated by diverse phytopathogens. When any harmful pathogen attacks a plant, it slows down the plant’s metabolic activities and stimulates its defence system, resulting in the development of a long-term SAR. Plant roots treated with a specific plant growth-promoting bacteria (PGPB) have been shown to have a protective effect on plants, protecting an aerial plant part from a range of detrimental bacteria. Priming is a physiological stage in which a plant’s defence capacity is increased [15].
Salicylic acid (SA), a crucial component of SAR, is not the only signal favouring systemic immunity over local resistance. Therefore, it was discovered that lipopeptides may also stimulate plant innate immunity (Fig. 4) [55]. These signals interact to alter systemic immunity in signalling networks relevant to SAR and perhaps ISR. Pipecolic acid or its presumably bioactive derivative N-hydroxy-pipecolic acid drives the non-SA SAR pathway. This mechanism also regulates the growth of inter-plant defences by allowing SAR-induced plants to release volatile organic compounds recognised as defence cues by neighboring plants [56].
Schematic representation of effects of lipopeptides in plant defense stimulation and antimicrobial activities. Lipopeptide recognition triggers fundamental signaling activities, such as the MAPK phosphorylation cascade. These early signals activate transcription factors, leading to the production of defense genes (TF). Consequently, the resistance to microorganisms is triggered through defensive mechanisms such as PR protein accumulation and cell wall reinforcement. Lipopeptide-induced plant immunity relies on the contact between lipopeptides and the plant membrane, rather than a protein receptor. Through direct incorporation into the microbial plasma membrane, lipopeptides also exert direct antibacterial effects. These insertions cause cells to lose their shape, resulting in the development of pores. Cellular components are released as pores form, leading to microbial cell death
ISR stands apart from systemic acquired resistance (SAR), vital to bolstering plant health and diminishing reliance on chemical pesticides [5, 51, 57]. Inducing prompt and potent immune responses against pathogen invasions, plants employ systemic long-distance signalling to defend proximal tissue upon ISR activation. Therefore, biocontrol agents (BCAs) help control pests and diseases by boosting the plant immune system. Several BCAs have already shown that they can produce ISR. By strengthening defence systems, beneficial bacteria, such as Bacillus spp., can help plants acquire broad-spectrum disease resistance [50]. There have been some encouraging developments in biological control thanks to applying certain antagonistic (BCAs), notably Bacillus spp. Bacillus velezensis, B. subtillis, B. altitudinis, and other bacteria are effective against fungal (Aspergillus fumigatus, Achromobacter xylosoxidans, Pyricularia oryzae, Magnaporthe oryzae), viral, and ISR invasion [21, 58,59,60]. For instance, numerous tomato defence-related genes were induced to transcript levels by the Bacillus subtilis strain HA1-CF (PAL, PR-1, HQT, and CHS), suggesting that these genes may be involved in induced systemic resistance in tomato against Tobacco Mosaic Virus (TMV) resistance [61]. One of the most significant ailments affecting the Brassicaceae family is clubroot, which is brought on by the parasite Plasmodiophora brassicae. As biocontrol agents to control soil-borne illnesses, Bacillus spp. that generate lipopeptide antibiotics are frequently utilised [62]. Bacillus halotolerans QTH8 has long been used as a biocontrol agent for controlling wheat crown rot disease caused by Fusarium pseudograminearum [63]. The Bacillus spp. ability to combat blast disease through direct antagonistic interactions and inducing Induced Systemic Resistance (ISR) in roots. Rice strains from the Bacillus altitudinis and B. velezensis groups may prevent the Pyricularia oryzae caused blast disease [58] (Table 1).
Application of lipopeptides in agriculture
While iturins have not been utilized to treat physical ailments, they are well known as biological control agents for plant diseases due to their antifungal effect and ability to create plant resistance [39]. The antifungal mechanism of iturin involves several key processes: disruption of cell membranes, ion imbalance, production of reactive oxygen species (ROS), inhibition of fungal spore germination, and alteration of fungal membrane composition. The antifungal action of the iturin family is primarily broad-spectrum, with limited antibacterial activity. Antifungal activity of iturin-producing Bacillus velezensis strains was extensive, including antagonistic abilities against Aspergillus fumigatus, Pyricularia oryzae, and Fusarium oxysporium [21, 41, 58]. Iturin A is a type of biosurfactant produced mostly by Bacillus subtilis with broad antifungal activity [21, 27, 29]. Iturin A, generated by B. subtilis played a key role in inhibiting Aspergillus carbonarius by altering the fungal cell structure and disrupting the fungi's energy, transport, and osmotic pressure metabolisms [67]. The literature claims that various B. subtilis strains produce Cyclic Lipopeptides (CLPs) [25, 28, 41]. Bacillus velezensis, for example, produces iturin, but Bacillus subtilis and Bacillus amyloliquefaciens HZ-12 produce iturin A, a sub-compound of iturin [68]. As a result, even against the same disease, various strains have distinct effects. Rice is protected against Rhizoctonia solani by B. subtilis RFB104 or B. subtilis BBG111 [70]. Furthermore, depending on the plant/pathogen relationship under consideration, the activity of the same strain can vary. B. subtilis BBG111, for example, can protect rice from the necrotrophic R. solani but not from the hemibiotrophic Magnaporthe oryzae [70]. As a result, it is critical to decipher the ways that each pathosystem works, as well as the substances that each strain's active ingredients are, emphasising those that have promise for use in industry [71]. Fengycin is another type of cyclic lipopeptide produced by certain strains of Bacillus subtilis. Like iturin, fengycin also exhibits antimicrobial activity, particularly against various fungal and bacterial pathogens. The antimicrobial activity of fengycin is primarily attributed to its interactions with cell membranes and other cellular components. Fengycin-type cyclopeptides are excellent suppressors of crop diseases produced by pathogenic fungus and oomycetes such as Fusarium graminearum, Fusarium solani, Plasmodiophora brassicae, Candida albicans, and Magnoporthe grisea, among others [32, 49, 59, 62, 66]. The antibiotic fengycin has potent antifungal properties and inhibits the progression of pathogens, particularly filamentous fungi [28]. The antifungal properties of the fengycin family are the most common. Fengycin activates specific plant species or host–pathogen relationships [28, 29]. For example, the Rice (Oryza sativa L.) is stimulated to go into a defence mode in response to Magnaporthe oryzae is dependent on fengycin produced by Bacillus subtilis BJ-1 [60].
Bacillus sp. as a commercial biocontrol agent
Bacillus sp. is commercially used as a biocontrol agent for plant protection and is employed globally to boost agricultural production yield. The selection of bacterial species capable of producing secondary metabolites that, in ideal environments, primarily inhibit fungal development formed the basis of the conventional method for producing such commercial products. These species must be produced in large quantities to support long-term self-storage [72]. Products made from Bacillus are crucial for creating environmentally friendly agriculture and low-toxicity biocontrol insecticides. One of the most important parts of biological control is the formulation of stable, effective biocontrol agents. Aqueous suspensions, wettable powders, oil flowable, and other formulations of biocontrol agents have been employed. The materials that passed the screening process were made Bacillus subtilis XZ18-3 wettable powder with the following ingredients: 30.0% kaolin, 4.0% polyvinyl alcohol, 8.0% Tween-80, 2.0% polyethylene glycol, and 100% fermentation broth. Since mycelia are the primary pathogen growth form and wettable powders are more stable, they have been shown in some investigations to outweigh the drawbacks of liquid agents. The wettable powder of Bacillus subtilis XZ18-3 produced an 88.28% efficient pathogen control through screening and orthogonal optimization [73, 74]. Korangi Alleluya et al. [75], investigated the Bacillus lipopeptide-mediated biocontrol of peanut stem rot caused by Athelia rolfsii. The genera Agrobacterium, Bacillus, and Pseudomonas contain most bacterial strains used as fungicides, bactericides, and bio pesticides [19, 76]. Due to their numerous therapeutic effects and stable spores, which ensure lengthy shelf lives, Bacillus spp. account for 50% of commercial items now on the market (Table 2).
Future perspectives and conclusions
The increasing societal emphasis on environmentally friendly chemicals is driving the possibility of a new market for biopesticides. These biopesticides, made up of biodegradable biosurfactants, could be safer alternatives to highly toxic synthetic chemical pesticides. To gain a deeper understanding of the molecular and biochemical processes, the development of advanced mathematical modelling supported by bioinformatics is crucial. Recent advances in genetic engineering and synthetic biology have enhanced the efficiency and precision of lipopeptide production, opening avenues to target significant pathogenic systems. Another important area for exploration is the reduction of production costs in industrial settings. Lipopeptides of bacterial origin hold great promise in offering a versatile and extensive platform to combat present and future plant pathogens.
Lipopeptides exhibit a remarkable array of applications, making them highly valuable compounds. Bacillus-derived lipopeptides have found practical use in biotechnology, pharmaceuticals, food, and cosmetics due to their properties, such as emulsification, antibacterial action, and surfactant capabilities. Despite the encouraging prospects of lipopeptide production and utilization, their adoption at a larger scale is hindered by their expensive production processes. Furthermore, although research indicates potential benefits like thrombolytic, anticancer, and anti-inflammatory properties, there is a need for greater emphasis on clinical trials and anti-inflammatory effectiveness. The fate of lipopeptides in agriculture encompasses their interactions with plants, pathogens, soil microorganisms, and the broader environment. Understanding how lipopeptides behave within agricultural systems is crucial for maximizing their benefits while minimizing potential risks or unintended consequences. Future research should corroborate existing findings from published studies, enhancing the understanding and application of these remarkable substances. In conclusion, due to their broad applicability, significant efforts are required to harness the potential of Bacillus-derived lipopeptides fully.
Data availability
The data associated with this article will be available with corresponding authors and available on request.
References
Raj A, Kumar A (2022) Recent advances in assessment methods and mechanism of microbe-mediated chlorpyrifos remediation. Environ Res 214(Pt 4):114011. https://doi.org/10.1016/j.envres.2022.114011
Malla MA, Dubey A, Kumar A, Yadav S (2023) Unlocking the biotechnological and environmental perspectives of microplastic degradation in soil-ecosystems using metagenomics. Process Saf Environ Prot 170:372–379. https://doi.org/10.1016/j.psep.2022.11.084
Malla MA, Dubey A, Kumar A, Yadav S (2022) Metagenomic analysis displays the potential predictive biodegradation pathways of the persistent pesticides in agricultural soil with a long record of pesticide usage. Microbiol Res 261:127081. https://doi.org/10.1016/j.micres.2022.127081
Dubey A, Malla MA, Khan F et al (2019) Soil microbiome: a key player for conservation of soil health under changing climate. Biodivers Conserv 28(8–9):2405–2429. https://doi.org/10.1007/s10531-019-01760-5
Dubey A, Kumar A, Abd_Allah EF, Hashem A, Khan ML (2019) Growing more with less: Breeding and developing drought resilient soybean to improve food security. Ecol Indic 105(March):425-437. https://doi.org/10.1016/j.ecolind.2018.03.003
Patel S, Sangeeta S (2019) Pesticides as the drivers of neuropsychotic diseases, cancers, and teratogenicity among agro-workers as well as general public. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-3642-2
Reichenberger S, Bach M, Skitschak A, Frede HG (2007) Mitigation strategies to reduce pesticide inputs into ground- and surface water and their effectiveness. A review Sci Total Environ 384(1–3):1–35. https://doi.org/10.1016/j.scitotenv.2007.04.046
Kumar A, Dubey A (2020) Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J Adv Res 24:337–352. https://doi.org/10.1016/j.jare.2020.04.014
Dubey A, Malla MA, Kumar A, Dayanandan S, Khan ML (2020) Plants endophytes: unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit Rev Biotechnol 40(8):1210–1231. https://doi.org/10.1080/07388551.2020.1808584
Anand G, Bhattacharjee A, Shrivas VL, Dubey S, Sharma S (2021) ACC deaminase positive Enterobacter-mediated mitigation of salinity stress, and plant growth promotion of Cajanus cajan: a lab to field study. Physiol Mol Biol Plants. https://doi.org/10.1007/s12298-021-01031-0
Tripathi YN, Divyanshu K, Kumar S et al (2020) Biopesticides: current status and future prospects in India. In: Keswani C, ed. Bioeconomy for Sustainable Development. Springer Singapore 2020:79–109. https://doi.org/10.1007/978-981-13-9431-7_6
Salazar B, Ortiz A, Keswani C, et al (2022) Bacillus spp. as Bio-factories for antifungal secondary metabolites: innovation beyond whole organism formulations. Microb Ecol 86:1–24 https://doi.org/10.1007/s00248-022-02044-2
Malla MA, Dubey A, Yadav S, Kumar A, Hashem A, Abd Allah EF (2018) Understanding and designing the strategies for the microbe-mediated remediation of environmental contaminants using omics approaches. Front Microbiol 9:1132. https://doi.org/10.3389/fmicb.2018.01132
Dubey A, Kumar A, Khan ML (2020) Role of Biostimulants for enhancing abiotic stress tolerance in Fabaceae Plants. In: Mirza Hasanuzzaman, Susana Araújo SSG, ed. The Plant Family Fabaceae. Springer Singapore 223–236. https://doi.org/10.1007/978-981-15-4752-2_8
Mahapatra S, Yadav R, Ramakrishna W (2022) Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J Appl Microbiol 132(5):3543–3562. https://doi.org/10.1111/jam.15480
Wang Z, Liu C, Shi Y, et al (2023) Classification, application, multifarious activities and production improvement of lipopeptides produced by Bacillus. Crit Rev Food Sci Nutr 1–14. https://doi.org/10.1080/10408398.2023.2185588
Li Z, Fernandez KX, Vederas JC, Gänzle MG (2023) Composition and activity of antifungal lipopeptides produced by Bacillus spp. in daqu fermentation. Food Microbiol 111:104211. https://doi.org/10.1016/j.fm.2022.104211
Kaspar F, Neubauer P, Gimpel M (2019) Bioactive secondary metabolites from Bacillus subtilis: a comprehensive review. J Nat Prod 82(7):2038–2053. https://doi.org/10.1021/acs.jnatprod.9b00110
Sreedharan SM, Rishi N, Singh R (2023) Microbial lipopeptides: properties, mechanics and engineering for novel lipopeptides. Microbiol Res 271:127363. https://doi.org/10.1016/j.micres.2023.127363
Mnif I, Rajhi H, Bouallegue A, Trabelsi N, Ghribi D (2022) Characterization of Lipopeptides biosurfactants produced by a newly isolated strain Bacillus subtilis ZNI5: potential environmental application. J Polym Environ 30(6):2378–2391. https://doi.org/10.1007/s10924-021-02361-6
Xiong ZR, Cobo M, Whittal RM, Snyder AB, Worobo RW (2022) Purification and characterization of antifungal lipopeptide produced by Bacillus velezensis isolated from raw honey. PLoS One 17(4):e0266470. https://doi.org/10.1371/journal.pone.0266470
Besson F, Michel G, Claude U, Lyon B (1986) They differ from iturin A by the presence of a free carboxyl group in iturin D and a car- boxymethyl group in iturin E. October XL(4):437–442
Wan C, Fan X, Lou Z, Wang H, Olatunde A, Rengasamy KRR (2021) Iturin: cyclic lipopeptide with multifunction biological potential. Crit Rev Food Sci Nutr 1–13. https://doi.org/10.1080/10408398.2021.1922355
de Souza Freitas F, de Assis Coelho, Lage T, Ayupe BAL, de Paula Siqueira T, de Barros M, Tótola MR (2020) Bacillus subtilis TR47II as a source of bioactive lipopeptides against Gram-negative pathogens causing nosocomial infections. 3 Biotech 10(11):474. https://doi.org/10.1007/s13205-020-02459-z
Etchegaray A, De Castro BC, De Melo IS et al (2008) Effect of a highly concentrated lipopeptide extract of Bacillus subtilis on fungal and bacterial cells. Arch Microbiol 190(6):611–622. https://doi.org/10.1007/s00203-008-0409-z
Abbo AS, Osman Idris M, Hammad AM (2014) The antifungal effects of four tomato rhizosphere Bacillus spp. against Alternaria alternata. Int J Sci Res 3 (7):1324–1328 https://www.ijsr.net/archive/v3i7/MDIwMTQxMTQ1.pdf
Gond SK, Bergen MS, Torres MS, White JF (2015) Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol Res 172:79–87. https://doi.org/10.1016/j.micres.2014.11.004
Vanittanakom N, Loeffler W, Koch U, Jung G (1986) Fengycin–a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot (Tokyo) 39(7):888–901. https://doi.org/10.7164/antibiotics.39.888
Liu Y, Lu J, Sun J et al (2019) C16-Fengycin A affect the growth of Candida albicans by destroying its cell wall and accumulating reactive oxygen species. Appl Microbiol Biotechnol 103(21–22):8963–8975. https://doi.org/10.1007/s00253-019-10117-5
Chitarra GS, Breeuwer P, Nout MJR, Van Aelst AC, Rombouts FM, Abee T (2003) An antifungal compound produced by Bacillus subtilis YM 10–20 inhibits germination of Penicillium roqueforti conidiospores. J Appl Microbiol 94(2):159–166. https://doi.org/10.1046/j.1365-2672.2003.01819.x
Théatre A, Hoste ACR, Rigolet A et al (2022) Bacillus sp: a remarkable source of bioactive lipopeptides. Adv Biochem Eng Biotechnol 181:123–179. https://doi.org/10.1007/10_2021_182
Hanif A, Zhang F, Li P et al (2019) Fengycin Produced by Bacillus amyloliquefaciens FZB42 Inhibits Fusarium graminearum Growth and Mycotoxins Biosynthesis. Toxins (Basel) 11(5):295. https://doi.org/10.3390/toxins11050295
Ait Kaki A, Smargiasso N, Ongena M et al (2020) Characterization of New Fengycin Cyclic Lipopeptide Variants Produced by Bacillus amyloliquefaciens (ET) Originating from a Salt Lake of Eastern Algeria. Curr Microbiol 77(3):443–451. https://doi.org/10.1007/s00284-019-01855-w
Kisil OV, Trefilov VS, Sadykova VS, Zvereva ME, Kubareva EA (2023) Surfactin: its biological activity and possibility of application in agriculture. Appl Biochem Microbiol 59(1):1–13. https://doi.org/10.1134/S0003683823010027
Fenibo EO, Ijoma GN, Selvarajan R, Chikere CB (2019) Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms 7(11):581. https://doi.org/10.3390/microorganisms7110581
Perez KJ, Viana JDS, Lopes FC et al (2017) Bacillus spp. Isolated from Puba as a Source of Biosurfactants and Antimicrobial Lipopeptides. Front Microbiol 8:61. https://doi.org/10.3389/fmicb.2017.00061
Wang C, Cao Y, Wang Y, Sun L, Song H (2019) Enhancing surfactin production by using systematic CRISPRi repression to screen amino acid biosynthesis genes in Bacillus subtilis. Microb Cell Fact 18(1):90. https://doi.org/10.1186/s12934-019-1139-4
Diallo MM, Vural C, Şahar U, Ozdemir G (2021) Kurstakin molecules facilitate diesel oil assimilation by Acinetobacter haemolyticus strain 2SA through overexpression of alkane hydroxylase genes. Environ Technol (United Kingdom) 42(13):2031–2045. https://doi.org/10.1080/09593330.2019.1689301
Luo C, Chen Y, Liu X et al (2019) Engineered biosynthesis of cyclic lipopeptide locillomycins in surrogate host Bacillus velezensis FZB42 and derivative strains enhance antibacterial activity. Appl Microbiol Biotechnol 103(11):4467–4481. https://doi.org/10.1007/s00253-019-09784-1
Dang Y, Zhao F, Liu X et al (2019) Enhanced production of antifungal lipopeptide iturin A by Bacillus amyloliquefaciens LL3 through metabolic engineering and culture conditions optimization. Microb Cell Fact 18(1):68. https://doi.org/10.1186/s12934-019-1121-1
Kim YT, Kim SE, Lee WJ et al (2020) Isolation and characterization of a high iturin yielding Bacillus velezensis UV mutant with improved antifungal activity. Gupta V, ed. PLoS One 15(12):e0234177. https://doi.org/10.1371/journal.pone.0234177
Baltz RH (2008) Biosynthesis and genetic engineering of lipopeptide antibiotics related to daptomycin. Curr Top Med Chem 8(8):618–638. https://doi.org/10.2174/156802608784221497
Ma Z, Zhang S, Zhang S et al (2020) Isolation and characterization of a new cyclic lipopeptide surfactin from a marine-derived Bacillus velezensis SH-B74. J Antibiot (Tokyo). https://doi.org/10.1038/s41429-020-0347-9
Naik S, Palys S, Di Falco M et al (2021) Isolation and Characterization of Bacillus velezensis EB14, an Endophytic Bacterial Strain Antagonistic to Poplar Stem Canker Pathogen Sphaerulina musiva and Its Interactions with the Endophytic Fungal Microbiome in Poplar. PhytoFrontiers™ 1(3):229–238. https://doi.org/10.1094/PHYTOFR-10-20-0023-R
Chen H, Wang L, Su CX, Gong GH, Wang P, Yu ZL (2008) Isolation and characterization of lipopeptide antibiotics produced by Bacillus subtilis. Lett Appl Microbiol. https://doi.org/10.1111/j.1472-765X.2008.02412.x
Inès M, Dhouha G (2015) Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides 71:100–112. https://doi.org/10.1016/j.peptides.2015.07.006
Jiao S, Li X, Yu H, Yang H, Li X, Shen Z (2017) In situ enhancement of surfactin biosynthesis in Bacillus subtilis using novel artificial inducible promoters. Biotechnol Bioeng 114(4):832–842. https://doi.org/10.1002/bit.26197
Malfanova N, Kamilova F, Validov S et al (2011) Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed. Microb Biotechnol 4(4):523–532. https://doi.org/10.1111/j.1751-7915.2011.00253.x
Li Y, Héloir M-C, Zhang X et al (2019) Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol Plant Pathol 20(8):1037–1050. https://doi.org/10.1111/mpp.12809
Lahlali R, Ezrari S, Radouane N et al (2022) Biological control of plant pathogens: a global perspective. Microorganisms 10(3):596. https://doi.org/10.3390/microorganisms10030596
Dubey A, Saiyam D, Kumar A, Hashem A, Abduallah EF, Khan ML (2021) Bacterial root endophytes: Characterization of their competence and plant growth promotion in soybean (glycine max (L.) merr.) under drought stress. Int J Environ Res Public Health 18(3):1–20. https://doi.org/10.3390/ijerph18030931
Malviya D, Sahu PK, Singh UB et al (2020) Lesson from ecotoxicity: revisiting the microbial lipopeptides for the management of emerging diseases for crop protection. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph17041434
Ben Abdallah D, Tounsi S, Gharsallah H, Hammami A, Frikha-Gargouri O (2018) Lipopeptides from Bacillus amyloliquefaciens strain 32a as promising biocontrol compounds against the plant pathogen Agrobacterium tumefaciens. Environ Sci Pollut Res Int 25(36):36518–36529. https://doi.org/10.1007/s11356-018-3570-1
Nguyen NH, Trotel-Aziz P, Villaume S et al (2020) Bacillus subtilis and Pseudomonas fluorescens Trigger Common and Distinct Systemic Immune Responses in Arabidopsis thaliana Depending on the Pathogen Lifestyle. Vaccines 8(3). https://doi.org/10.3390/vaccines8030503
Lin R, Zhang Q, Yin L et al (2022) Isolation and characterization of a mycosubtilin homologue antagonizing Verticillium dahliae produced by Bacillus subtilis strain Z15. PLoS One 17(6):e0269861. https://doi.org/10.1371/journal.pone.0269861
Vlot AC, Sales JH, Lenk M et al (2021) Systemic propagation of immunity in plants. New Phytol 229(3):1234–1250. https://doi.org/10.1111/nph.16953
Kumar A, Dubey A, Malla MA, Dames J (2021) Pyrosequencing and phenotypic microarray to decipher bacterial community variation in Sorghum bicolor (L.) Moench rhizosphere. Curr Res Microb Sci 2(2020):100025. https://doi.org/10.1016/j.crmicr.2021.100025
Lam VB, Meyer T, Arias AA, Ongena M, Oni FE, Höfte M (2021) Bacillus Cyclic Lipopeptides Iturin and Fengycin Control Rice Blast Caused by Pyricularia oryzae in Potting and Acid Sulfate Soils by Direct Antagonism and Induced Systemic Resistance. Microorganisms 9(7):1441. https://doi.org/10.3390/microorganisms9071441
Zhang L, Sun C. Fengycins (2018) Cyclic Lipopeptides from Marine Bacillus subtilis Strains, Kill the Plant-Pathogenic Fungus Magnaporthe grisea by Inducing Reactive Oxygen Species Production and Chromatin Condensation. Master ER, ed. Appl Environ Microbiol 84(18). https://doi.org/10.1128/AEM.00445-18
He Y, Zhu M, Huang J, Hsiang T, Zheng L (2019) Biocontrol potential of a Bacillus subtilis strain BJ-1 against the rice blast fungus Magnaporthe oryzae. Can J Plant Pathol 41(1):47–59. https://doi.org/10.1080/07060661.2018.1564792
El-Gendi H, Al-Askar AA, Király L, Samy MA, Moawad H, Abdelkhalek A (2022) Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 8(4). https://doi.org/10.3390/horticulturae8040301
Guo S, Zhang J, Dong L et al (2019) Fengycin produced by Bacillus subtilis NCD-2 is involved in suppression of clubroot on Chinese cabbage. Biol Control 136:104001. https://doi.org/10.1016/j.biocontrol.2019.104001
Li S, Xu J, Fu L et al (2022) Biocontrol of wheat crown rot using Bacillus halotolerans QTH8. Pathog (Basel, Switzerland) 11(5). https://doi.org/10.3390/pathogens11050595
Chishti Z, Ahmad Z, Zhang X, Jha SK (2021) Optimization of biotic and abiotic factors liable for biodegradation of chlorpyrifos and their modeling using neural network approaches. Appl Soil Ecol 166:103990
Jiang M, Pang X, Liu H et al (2021) Iturin A induces resistance and improves the quality and safety of harvested cherry tomato. Molecules 26(22). https://doi.org/10.3390/molecules26226905
Liu W, Sun C (2021) C17-fengycin B, produced by deep-sea-derived Bacillus subtilis, possessing a strong antifungal activity against Fusarium solani. J Oceanol Limnol 39(5):1938–1947. https://doi.org/10.1007/s00343-020-0215-2
Jiang C, Li Z, Shi Y et al (2020) Bacillus subtilis inhibits Aspergillus carbonarius by producing iturin A, which disturbs the transport, energy metabolism, and osmotic pressure of fungal cells as revealed by transcriptomics analysis. Int J Food Microbiol 330:108783. https://doi.org/10.1016/j.ijfoodmicro.2020.108783
Xu Y, Cai D, Zhang H et al (2020) Enhanced production of iturin A in Bacillus amyloliquefaciens by genetic engineering and medium optimization. Process Biochem 90:50–57. https://doi.org/10.1016/j.procbio.2019.11.017
Desmyttere H, Deweer C, Muchembled J et al (2019) Antifungal activities of Bacillus subtilis Lipopeptides to Two Venturia inaequalis strains possessing different Tebuconazole sensitivity. Front Microbiol 10(OCT):1–10
Chandler S, Van Hese N, Coutte F, Jacques P, Höfte M, De Vleesschauwer D (2015) Role of cyclic lipopeptides produced by Bacillus subtilis in mounting induced immunity in rice (Oryza sativa L.). Physiol Mol Plant Pathol 91:20–30. https://doi.org/10.1016/j.pmpp.2015.05.010
Vu HNT, Nguyen DT, Nguyen HQ et al (2018) Antimicrobial and cytotoxic properties of bioactive metabolites produced by Streptomyces cavourensis YBQ59 Isolated from Cinnamomum cassia Prels in Yen Bai Province of Vietnam. Curr Microbiol. https://doi.org/10.1007/s00284-018-1517-x
Dimopoulou A, Theologidis I, Varympopi A et al (2021) Shifting Perspectives of Translational Research in Bio-Bactericides: Reviewing the Bacillus amyloliquefaciens Paradigm. Biology (Basel) 10(11):1202. https://doi.org/10.3390/biology10111202
Yi Y, Luan P, Liu S et al (2022) Efficacy of Bacillus subtilis XZ18–3 as a biocontrol agent against Rhizoctonia cerealis on Wheat. Agriculture 12(2). https://doi.org/10.3390/agriculture12020258
Chen S, Deng Y, Chang C et al (2015) Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Sci Rep 5(1):8784. https://doi.org/10.1038/srep08784
Korangi Alleluya V, Argüelles Arias A, Ribeiro B et al (2023) Bacillus lipopeptide-mediated biocontrol of peanut stem rot caused by Athelia rolfsii. Front Plant Sci 14. https://doi.org/10.3389/fpls.2023.1069971
Etesami H, Jeong BR, Glick BR (2023) Biocontrol of plant diseases by Bacillus spp. Physiol Mol Plant Pathol 126:102048. https://doi.org/10.1016/j.pmpp.2023.102048
Acknowledgements
AK gratefully acknowledges DST-SERB for financial support obtained through the project grant of (CRG/2021/003696), New Delhi, Govt of India.
Author information
Authors and Affiliations
Contributions
DS and AD prepared the draft of the manuscript. The manuscript was overseen and revised by MAM and AK.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Luis Augusto Nero
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saiyam, D., Dubey, A., Malla, M.A. et al. Lipopeptides from Bacillus: unveiling biotechnological prospects—sources, properties, and diverse applications. Braz J Microbiol 55, 281–295 (2024). https://doi.org/10.1007/s42770-023-01228-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01228-3