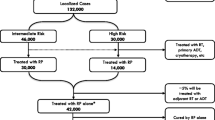
Abstract
Biochemical recurrence after radical prostatectomy (RP) is associated with risk factors including Gleason score, preoperative PSA level, tumor stage, seminal vesicle invasion, and positive surgical margins. The 5-year biochemical progression rate for patients with these characteristics has been estimated to be as high as 50–70%. Two treatment approaches for the postoperative management of these patients are adjuvant radiation therapy in men with an undetectable PSA or observation followed by early salvage radiation therapy in men with persisting or rising PSA after initially postoperative undetectable values.
Three randomized phase III trials demonstrated a nearly 20% absolute benefit for biochemical progression-free survival after adjuvant radiation therapy (ART) (60–64 Gy) compared to a “wait and see” policy. The greatest benefit has been revealed in patients with positive margins and pT3 tumors.
SRT can be offered to patients with rising PSA after RP. Of these patients, 30–70% will experience a decrease in their PSA to an undetectable range, and in about 40–50% of these patients, the PSA will remain stable after 5 years.
At the present time, there are no published randomized trials to compare ART versus SRT.
The purpose of this chapter is to review the rationale, results, and possible side effects for the two treatment approaches ART and SRT.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Radical Prostatectomy
- Gleason Score
- Radiation Therapy Oncology Group
- Positive Surgical Margin
- Salvage Radiation Therapy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
14.1 Introduction
Radical prostatectomy (RP) and radiation therapy are the two major first-line therapeutic options for patients with prostate cancer, with best results achieved in patients with organ-confined disease. Recurrence of prostate cancer after RP has been associated with multiple factors including Gleason score, prostate-specific antigen (PSA) level before surgery, tumor stage, infiltration of the seminal vesicles, or positive surgical margins (Chun et al. 2006; Swindle et al. 2005; Salomon et al. 2003; Pinto et al. 2006). However, biochemical recurrence is a common event even in patients with favorable prognostic factors.
Following RP, PSA should become undetectable within 4–6 weeks, as serum half-life of PSA is approximately 2–3 days (Stamey et al. 1987). Persistent serum PSA levels after RP indicate residual prostatic tissue, either malignant or benign (BPH). In the former case, such levels predate clinically evident disease and do correlate well with disease progression.
A PSA increase of ≥0.2 ng/ml is a common definition of progression of disease following RP (Heidenreich et al. 2011; Wenz et al. 2010). It occurs in up to 50% of patients with pT3/4 tumors, and this value ranges up to 70% in case of pT3 tumors with positive surgical margins and/or positive pelvic lymph nodes (Roehl et al. 2004; Stephenson et al. 2009). The rate of biochemical progression after 7 years for patients with organ-confined tumors (pT2) and positive surgical margins is about 25% (Stephenson et al. 2009).
Vital tumor tissue was histopathologically proven by biopsies from the urethrovesical anastomosis in 35–55% of all patients, with rising PSA after RP without clinical correlates suggestive of recurrent tumor (Shekarriz et al. 1999).
The optimal management of patients with clinical and pathologic features of increased risk for developing a biochemical recurrence continues to be a source of controversy. Two treatment approaches for the postoperative management of these patients are adjuvant radiation therapy in men with an undetectable PSA or observation followed by early salvage radiation therapy in men with persisting or rising PSA after initially postoperative undetectable values.
The purpose of this chapter is to review the rationale, results, and possible side effects for the different treatment approaches ART and SRT.
14.2 Adjuvant Radiation Therapy
14.2.1 Randomized Clinical Trials
Three randomized phase III trials demonstrated a nearly 20% absolute benefit for biochemical progression-free survival (bNED) after adjuvant radiation therapy (60–64 Gy) compared with a “wait and see” policy, mostly for pT3 cN0 or pN0 tumors (Table 14.1). The greatest benefit (30% bNED after 5 years) has been demonstrated in patients with positive margins and pT3 tumors (Bolla et al. 2005; Thompson et al. 2006; Van der Kwast et al. 2007; Wiegel et al. 2009a). In the meantime, 10-year follow-up data of the EORTC trial were reported and confirmed these results (Bolla et al. 2010).
In the prospective study of the South Western Oncology Group (SWOG), overall survival was improved from 13.5 years without to 15.2 years with adjuvant radiation therapy (Thompson et al. 2009). Notably, central pathologic review on the outcome at 5 years in the EORTC trial showed that only surgical margin status caused a statistically significant interaction with the treatment effect, to such an extent that the treatment benefit in patients with negative margins did not remain significant. The hazard ratio for the treatment benefit in the group with negative surgical margins was 0.87 (P = 0.601) compared to 0.38 (P < 0.0001) in the group with positive surgical margins according to the review pathology. Excluding the patients with a PSA of >0.2 ng/ml after prostatectomy, the hazard ratio for postoperative irradiation was 1.11 (P = 0.740) and 0.29 (P < 0.0001) for the patients with negative and positive margins, respectively (Van der Kwast et al. 2007). This benefit was also seen in the real adjuvant situation, when the PSA was undetectable before the start of radiation therapy (Wiegel et al. 2009a and 2009b). In the trial of the German Cancer Society, 159 patients were randomized into the observation and 148 into the adjuvant irradiation arm (60 Gy in 30 fractions over 6 weeks). After a median follow-up of nearly 5 years, there was a significant benefit from adjuvant radiation therapy for bNED: 72% versus 54% (P < 0.03). In the subgroup of pT3 R1 tumors, this benefit increased from 18% to 28% (Wiegel et al. 2009a).
It is notable that the three randomized studies have used different definitions of biochemical progression: SWOG: PSA > 0.4 ng/ml, EORTC: PSA > 0.2 ng/ml, ARO: PSA > 0.05 ng/ml.
Consequently, biochemical recurrences (as an increase of the PSA out of the undetectable range) were detected earlier in the EORTC and the ARO study. In the light of that, the apparently worse results of the ARO study could be explained (Table 14.1).
It is well known that the location, the extent, and the number of positive surgical margins after radical prostatectomy are significant predictors of biochemical progression after radical prostatectomy. The investigators of the Cleveland Clinic/Ohio found in their retrospective series of 7,160 patients treated with radical prostatectomy 1,540 patients with positive margins. The 7-year progression-free probability was 60% in those patients, resulting in an hazard ratio for biochemical recurrence of 2.3 in the case of positive surgical margins compared with negative margins. There was also an increased risk of biochemical recurrence in patients with multiple versus solitary positive surgical margins (HR 1.4) and extensive versus focal positive surgical margins (adjusted HR 1.3) (Stephenson et al. 2009). From the data of the randomized trials mentioned above, these patients with positive margins and pT3-tumors do stand to profit mostly from postoperative radiation therapy.
In the EORTC trial, when the data of patients with pT2 tumors and positive surgical margins were analyzed, there was a significant benefit of 5-year biochemical progression-free survival rate in the irradiated group (76.4% vs. 52.2% in the wait-and-see group) (Bolla et al. 2005). However, these data come from a subgroup analysis, and biochemical progression-free survival was not the primary end point of this study. Therefore, the results must be interpreted with caution. The possible benefit of radiotherapy must be weighed out carefully in consideration of potential late effects as erectile dysfunction.
14.2.2 Definition of Clinical Target Volume (CTV)
In the EORTC and SWOG trials radiation was based on 2D treatment planning, where the prostatic fossa was targeted by using large treatment portals. Obviously, precise definition of target volumes was not essential, which is in great contrast to modern radiation treatment techniques such as IMRT. Compared to 2D-based planning, IMRT provides significant normal tissue sparing but also demands exact definition of target volume.
Consideration of the local failure patterns in the post-RP setting is essential for optimal definition of CTV. The most common sites of local relapse proven by biopsy are the vesicourethral anastomosis (VUA) (66%) followed by the bladder neck (16%) and retrotrigone area (13%) (Connolly et al. 1996). Recently, endorectal magnetic resonance imaging (MRI) was used to detect local relapse patterns following RP in order to further define the optimal CTV (Miralbell et al. 2007). Based on the results of this study, the authors recommended a cylindrical-shaped CTV centered 5 mm posterior and 3 mm inferior to the VUA, concordant with the previously mentioned pathologic studies.
To address any uncertainties in definition of CTV, the Radiation Therapy Oncology Group (RTOG) (Michalski et al. 2010), the EORTC Radiation Oncology Group (Poortmans et al. 2007), and other cooperative groups (Wiltshire et al. 2007) have created consensus guidelines for delineation of target volumes for postprostatectomy patients. In the RTOG recommendations, the CTV should extend superiorly from the level of the caudal vas deferens remnant (or 3–4 cm superior to the pubic symphysis, whichever is higher) and inferiorly 8–12 mm inferior to VUA. The VUA is defined as the retropubic region that can be visualized one slice below the most inferior urine-containing image of the bladder (often best seen on a sagittal reconstruction). Below the superior border of the pubic symphysis, the anterior border is at the posterior aspect of the pubis and extends posteriorly to the rectum. At this level, the lateral border extends to the levator ani muscles. Above the pubic symphysis, the anterior border should encompass the posterior 1–2 cm of the bladder wall and should extend posteriorly to the mesorectal fascia.
14.2.3 Use of Image Guidance to Improve Postprostatectomy Prostatic Fossa Localization
In recent years, several innovative methods have been developed to improve localization of the prostatic fossa and minimize daily internal setup error. Techniques currently utilized in most practices include daily portal imaging with implanted gold fiducial markers (Schiffner et al. 2007), daily cone-beam or kilovoltage imaging (Nath et al. 2010), and the use of electromagnetic transponders (Canter et al. 2010). Such image-guidance techniques allow for a minimal (7–10 mm) expansion from a CTV to a planning target volume, thereby providing further normal tissue sparing by minimizing RT dose to the rectum and bladder (Showalter et al. 2008).
14.2.4 Adjuvant RT of Pelvic Lymph Nodes?
The three randomized trials included only patients with cN0 or pN0 disease. The effect of adjuvant RT in node-positive prostate cancer has not yet been prospectively assessed. However, there are interesting retrospective data raising the question whether men with nodal involvement confirmed during prostatectomy could benefit from adjuvant RT. A recent retrospective study reported a significant positive impact of RT in combination with hormonal therapy in patients with nodal metastases treated with RP and pelvic lymph node dissection (Da Pozzo et al. 2009). However, this study was limited by a potential patient selection bias mainly due to its retrospective and unmatched design. In fact, patients treated with adjuvant RT were those affected by more aggressive disease. For this reason, no effect of adjuvant RT on cancer-specific survival was demonstrated on univariate survival analyses. There was significant gain in predictive accuracy when adjuvant RT was included in multivariable models predicting biochemical recurrence-free and cancer-specific survival (gain: 3.3% and 3%, respectively; all P < 0.001).
In a huge retrospective series, Briganti et al. assessed the effect of adjuvant RT in node-positive prostate cancer including two homogeneous matched patient cohorts exposed to either adjuvant RT plus HT or adjuvant HT alone after surgery. In this series from Milan and Jacksonville, a total of 703 patients were treated, with a median follow-up of 95 months. Patients were matched for age at surgery, pathologic T stage and Gleason score, number of nodes removed, surgical margin status, and length of follow-up. The overall survival advantage was 19% in favor of adjuvant radiation therapy plus hormonal treatment compared with hormonal treatment alone. Similarly, higher survival rates associated with the combination of HT plus RT were found when patients were stratified according to the extent of nodal invasion (namely, ≤2 vs. >2 positive nodes; all P ≤ 0.006) (Briganti et al. 2011). Because of the retrospective nature of this series with no standardized definition of target volumes, radiation dose, and duration of hormonal treatment, these results should be interpreted with caution. However, it provides support for this treatment in selected cases, whereas it should be validated in prospective clinical trials.
14.2.5 Additional Use of Hormone Therapy to ART
It is now clearly established that the standard nonoperative management for patients with locally advanced prostate adenocarcinoma includes long-term ADT. Two previous cooperative group trials have demonstrated an overall survival advantage for high-risk patients with an intact prostate treated with 2–3 years of ADT (Bolla et al. 2009; Horwitz et al. 2008). It remains unknown if there is a benefit to the addition of adjuvant ADT for men with high-risk, node-negative prostate adenocarcinoma initially treated with RP and pelvic lymph node dissection. The primary rationale for use of ADT post-RP is to (1) improve local control by eradicating disease in a hypoxic scar that may be radioresistant, (2) address micrometastatic disease which may have spread to the lymph nodes or distant sites, and (3) alter PSA kinetics in patients who will eventually relapse (Hanlon et al. 2004. Kaminski et al. 2003; Rossi et al. 2011).
Previous studies have indicated a potential benefit for men at high risk of recurrence treated with combination therapy. A secondary analysis of patients’ status post RP enrolled on Radiation Therapy Oncology Group (RTOG) 85-31 (Corn et al. 1999), a phase III trial comparing standard external beam RT plus immediate ADT versus RT alone for patients with non bulky prostate cancer, found a biochemical control advantage for patients who received combination therapy as compared to men treated with RT alone. With a median follow-up of 5 years, the progression-free survival for men treated with combination therapy was estimated to be 65% as compared to 42% for men treated with RT alone (P = 0.002). Similar results were seen in a retrospective study performed at Stanford University (King et al. 2004). A subsequent RTOG study (P-0011) was designed to determine the benefit of combination therapy for man with unfavorable prognostic factors and an undetectable PSA treated with ART. This trial was unfortunately closed due to poor accrual (Elshaikh et al. 2011).
In ongoing EORTC trial 22043, patients with Gleason score 5–10, undetectable PSA, and pathologic stage pT2R1 or pT3a–b will be randomized within 3 months after radical prostatectomy between postoperative irradiation alone and postoperative irradiation and short-term adjuvant androgen deprivation for 6 months. The primary trial end point is 5-year biochemical progression-free survival.
14.3 Salvage Radiation Therapy
As an alternative, salvage radiation therapy should be considered for men presenting with persistent PSA after prostatectomy or showing an increase of PSA levels after initially postoperative undetectable values (Stephenson et al. 2007; Wiegel et al. 2009b; Neuhof et al. 2007; Trock et al. 2008; Bottke et al. 2009; Swanson et al. 2011) (Table 14.2).
It remains uncertain whether a PSA increase after RP indicates isolated local disease, distant metastatic progression, or both (Shekarriz et al. 1999). Therefore, the best treatment for recurrent prostate cancer in patients with increasing or persisting PSA without clinical evidence of disease still remains controversial. On the other hand, only RT can offer the chance of cure to patients with truly localized malignant disease after RP.
There are indicators for a higher likelihood of local recurrence, e.g., slow PSA rise (PSA doubling time ≥12 months), more than 1 year between RP and the demonstration of PSA in the serum, Gleason score <7, and negative surgical margins (Pisansky et al. 2000). On the other hand, there are also indicators suggesting metastatic disease such as short PSA doubling time (<12 months) or Gleason score at RP from 8 to 10 (Pazona et al. 2005; Ward et al. 2004). Some authors tried to define combinations of risk factors. For example, patients with a combination of PSA < 1 ng/ml before RT, pre-RP Gleason score < 7, and a long PSA doubling time after progression have a high risk of local disease (Stephenson et al. 2004). Recently, a predictive model for the outcome of RT for PSA progression after RP has been established (Stephenson et al. 2007). Assuming a local nature of the underlying disease, salvage radiotherapy (SRT) of the prostatic bed has widely been used to treat patients in the absence of biopsy-proven local recurrence. An established standard is conformal radiotherapy to the prostatic fossa with a dose of about 66 Gy, aiming to irradiate the presumed local recurrence and hence to reduce the risk of a “second wave of metastasis” leading to clinical progression of disease (Coen et al. 2002; Heidenreich et al. 2011; Wenz et al. 2010). In the light of these well-known problems in detecting local recurrence in the prostatic bed, radiotherapy to the prostatic fossa is one of the rare therapies in which most radiation oncologists irradiate without a histologic proof of tumor recurrence.
14.3.1 Role of Investigations in Case of Persisting/Rising PSA
A local recurrence is more likely to be confirmed with biopsy when abnormal tissue in the post-radical prostatectomy bed is detected with either digital rectal exam (DRE) or imaging (Stephenson et al. 2004). Imaging modalities that can detect post-radical prostatectomy recurrence and potentially guide biopsy include TRUS, MRI, and nuclear medicine methods; these modalities can also aid in monitoring disease progression or planning salvage radiation therapy.
TRUS is the most available and most commonly performed imaging technique used in post-radical prostatectomy patients with suspected recurrence. The main role of TRUS is in detecting sites of suspected recurrence and directing biopsies. The sensitivity of TRUS-guided biopsies (66–75%) has been shown to be greater than that of DRE-guided biopsies (29–50%) in the post-radical prostatectomy patient (Scattoni et al. 2003; Deliveliotis et al. 2007). The sensitivity of TRUS-guided biopsies increases with higher PSA levels at the time of recurrence (Shekarriz et al. 1999), obviously related to larger tumor volume. A recent study showed that only 25% of patients with PSA < 1 ng/ml had biopsy-proven recurrence compared with 53% of patients with PSA levels >2 ng/ml (Deliveliotis et al. 2007). More recent advances in TRUS of post-radical prostatectomy patients include the use of color and power Doppler to detect areas with increased vascularity. Both techniques have been shown to improve sensitivity and specificity (Tamsel et al. 2006).
The advantages of MRI over TRUS are its superior soft-tissue resolution and its ability to cover the entire postprostatectomy fossa and reveal recurrences that are located beyond the region routinely imaged on ultrasound. The combination of an external and an endorectal coil improves the ability to detect local recurrence of prostate cancer (Huch Boni et al. 1996). The anatomic detail and wide coverage of the pelvis by MRI facilitates its increasing use in directing salvage radiation therapy when a recurrence is demonstrated (Miralbell et al. 2007). Additionally, pelvic lymphadenopathy and osseous metastases, the most common early metastatic sites from prostate cancer, are routinely evaluated on MRI.
The reported sensitivity and the specificity of MRI for depicting local recurrences by experienced investigators in 82 patients who underwent prostatectomy are 87% and 78%, respectively. PSA levels at MR imaging in patients with clinically proved recurrences ranged from undetectable to 10 ng/ml (mean, 2.18 ng/ml) (Sella et al. 2004).
Advancements in MRI technique, including magnetic resonance spectroscopy and DCE-MRI, have not yet been systematically evaluated for detection of post-radical prostatectomy recurrence.
A variety of nuclear medicine techniques are currently being evaluated in post-radical prostatectomy patients with a PSA relapse. These studies include evaluation for local recurrence and for metastatic disease in the pelvis with combined PET/CT, utilizing various tracers. Older studies using the radiotracer 18F-FDG, which is commonly used in cancer imaging, showed a low sensitivity and specificity (Hofer et al. 1999). With the clinical introduction of newer image reconstruction algorithms, however, newer generations of PET scanners with higher spatial resolution, and the use of combined PET/CT, this has changed. Although 18F-FDG continues to be a suboptimal radiotracer for the detection of local recurrence, disease can be detected in selected patients, with the probability of detection depending on PSA level and PSA doubling time (Schoder et al. 2005). New radiotracers, including 11C or 18F choline, 11C or 18F acetate, or anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid, appear more promising for the detection of both local and metastatic recurrent prostate cancer (Cimitan et al. 2006; Scattoni et al. 2007).
The diagnostic accuracy of choline PET in detecting sites of prostate cancer relapse has been investigated by several authors, the overall reported sensitivity ranges between 38% and 98%. It has been demonstrated that choline PET technology’s positive detection rate improves with increasing PSA values. The routine use of choline PET/CT cannot be recommended for PSA values <1 ng/ml (Rinnab et al. 2007; Picchio et al. 2011).
14.3.2 Results of Salvage Radiotherapy/Prognostic Factors
The level of PSA at the time of salvage radiation therapy is one of the most important predictors for response. Stevenson et al. reported the results of 1,540 patients from 16 contributors. These patients received salvage radiation therapy with a median dose of 66 Gy and had a median follow-up of 53 months. A 6-year biochemical progression-free survival rate of 48% could be achieved when the PSA was <0.5 ng/ml compared with only 18% when the pre-radiation therapy PSA was >1.5 ng/ml. In the whole series, the 6-year progression-free survival rate was 32% (Stephenson et al. 2007). The authors identified several prognostic factors that were associated with a poor response to radiation therapy including Gleason score of 8–10, preradiation PSA > 2 ng/ml, negative surgical margins, postoperative PSA doubling time <10 months, and seminal vesicle invasion. Patients without these adverse features had a 6-year progression-free survival of 69%. Also, some subsets of patients with Gleason score 8–10 would benefit from salvage radiation therapy if the pretreatment PSA was <2.0 ng/ml, surgical margins were positive, and PSA doubling time was >10 months. In this situation, the 6-year bNED was 33% (Stephenson et al. 2007).
It is important to point out that achieving an undetectable PSA after salvage radiation therapy offers a second chance of cure. Wiegel et al. reported the results of a homogenously treated group of 162 patients, all pN0, treated with a median dose of 66 Gy in fractions of 1.8 Gy. In the multivariate analysis, the most important predictor for biochemical progression-free survival was the achieving of an undetectable PSA after salvage radiation therapy (Wiegel et al. 2009b). These results were confirmed by others (Neuhof et al. 2007).
14.3.3 Total Dose of Salvage Radiotherapy
There remains, however, a controversy about the best irradiation dose for those patients. In the guidelines, total doses of “at least 66 Gy” are recommended (Heidenreich et al. 2011; Wenz et al. 2010). However, some recently published series demonstrated a better outcome with higher total doses (Bernard et al. 2010; Siegmann et al. 2011; King and Kapp 2008). Bernard and coworkers from the Mayo Clinic, Jacksonville, investigated 364 men with salvage radiation therapy after radical prostatectomy after a median follow-up of 6.0 years. They used three dose groups (low: <64.8 Gy, moderate: 64.8–66.6 Gy, high: >66.6 Gy). In multivariate analysis, they found that compared with the high-dose level, there was a decreased bNED for patients treated with the low-dose level (HR 0.60) (Bernard et al. 2010). This was similar to the results published by Siegmann et al. from the group in Berlin and Ulm. In their retrospective series including 301 patients, 234 received 66.6 Gy, while 67 patients with a PSA decrease during salvage radiation therapy were selected and irradiated up to 70.2 Gy. In the multivariate analysis, the total dose was a significant predictor of reduced risk of biochemical progression (P = 0.017) (Siegmann et al. 2011).
The need for a higher irradiation dose remains uncertain, nevertheless, seems justified especially in patients with histologically confirmed local recurrence after radical prostatectomy. Some data suggest a better outcome with a total dose of more than 66 Gy in these patients (Roscigno et al. 2007).
14.3.4 RT of Pelvic Lymph Nodes?
An important, but unsolved, question is the value of an additional whole pelvic irradiation compared with prostate bed irradiation alone. Spioto from the Stanford University reported on 160 patients who underwent adjuvant or salvage radiation therapy, out of which 87 had short-course total androgen suppression. One hundred fourteen patients were considered at high risk of lymph node involvement although cN0 (Gleason Score > 8, preoperative PSA level >20 ng/ml, seminal vesicle involvement). Seventy-two underwent whole pelvic radiation therapy, and 42 underwent prostate bed radiation therapy. The median follow-up was >5 years. Limited to high-risk patients, there was a superior bNED of whole pelvic radiation therapy compared with prostate bed radiation therapy (5-year rate 47% vs. 21%, P < 0.05). Whereas these data have to be confirmed in a prospective trial, whole pelvic radiation therapy combined with modern delivery techniques like IMRT can be offered as an attractive option for high-risk patients (Heidenreich et al. 2011; Wenz et al. 2010; Spiotto et al. 2007).
14.3.5 Additional Use of Hormone Therapy to SRT
Interesting retrospective data have been reported from the Mayo Clinic and from the University of Michigan (Choo et al. 2009; Soto et al. 2011). They raise the question of the efficacy of an additional androgen deprivation during and after salvage radiation therapy. Choo and coworkers reported on 75 patients treated with salvage radiation therapy + 2-year androgen deprivation treated in a pilot prospective study. With a median follow-up from salvage radiation therapy of 6.5 years, all patients achieved an initially complete PSA response (<0.2 ng/ml). Relapse-free survival rate at 7 years was 78% of the whole population (Choo et al. 2009). A group of the University of Michigan treated all together 630 men for salvage indications after radical prostatectomy. Out of this group, 66% had high risk factors and the mean radiation therapy dose was 68 Gy. Twenty-four percent of all patients received concurrent androgen deprivation. The median ADT duration for these patients was 11 months. With a median follow-up of 3 years, the concurrent androgen deprivation was shown to be a significant independent predictor of progression-free survival in the high-risk group (P < 0.05) (Soto et al. 2011). Therefore, it seems attractive to treat high-risk patients with salvage radiation therapy and an additional androgen deprivation. The optimal duration of this androgen deprivation therapy remains uncertain.
RTOG 96-01 is a randomized, multicenter phase III trial designed to compare antiandrogen therapy (bicalutamide monotherapy 150 mg/day) plus salvage radiation therapy (n = 387) to a placebo plus salvage radiation alone (n = 383) in men with pT3 (n = 518)/pT2 R1 (n = 252) N0 M0 prostate cancer who have an elevated PSA after surgery. Median follow-up in surviving patients was 7.1 years. The primary end point is overall survival. The addition of 24 months of peripheral androgen blockade during and after RT significantly improved freedom from PSA progression (FFP) 57% vs. 40% (P < 0.0001) and reduced the incidence of metastatic prostate cancer (7.4% vs. 12.6%, P < 0.04) without adding significantly to radiation toxicity. The significance of benefit in overall survival, as well analysis of risk-stratified subsets, must await longer follow-up (Shipley et al. 2010). Therefore, there are currently no clear conclusions from these data. Possibly, high-risk patients profit from additional antiandrogen therapy.
A current RTOG trial (0534) is investigating the benefit of short-term ADT, as well as pelvic nodal irradiation, in the SRT setting. In this trial, patients will be randomized to one of three treatment arms: (1) prostatic fossa irradiation alone, (2) prostatic fossa + whole pelvic irradiation alone, or (3) prostatic fossa + whole pelvic irradiation with short-term ADT. The primary end points of this study are to determine (1) whether the addition of short-term androgen deprivation therapy to prostatic fossa irradiation improves freedom from progression for 5 years over that of prostatic fossa irradiation therapy alone and (2) whether short-term ADT and whole pelvic RT improves freedom from progression over that of short-term ADT and prostatic fossa irradiation alone for men treated with SRT. The target of accrual for this trial is 1,764 patients and, to date, nearly 40% of the target accrual goal has been met.
14.4 Radiation Therapy Techniques
Traditionally, a 4-field technique has been used. The conventional treatment volumes were typically very generous, being approximately 10 × 10 cm in the anterior-posterior fields with the inferior border at the ischial tuberosities. The lateral fields extended from the anterior aspect of the pubic symphysis and split the rectum posteriorly.
After introduction of modern 3D CRT techniques, a major controversy about the target volumes of postoperative radiation therapy started. Critical evaluation of target volume delineation by different authors and participation of experienced radiation oncologist showed that variations up to 65% maybe present even in cases of adjuvant or salvage radiation to the prostatic fossa (Michalski et al. 2009). These differences have been presented despite the presence of guidelines published on behalf of the EORTC Radiation Oncology Group two years earlier (Poortmans et al. 2007).
In 3D CRT, the target volume should include the bladder neck (pulled into the prostate bed), the periprostatic tissue and surgical clips, and the seminal vesicle bed (including any seminal vesicle remnants if present) if initially involved or as a confirmed site of recurrence. There are some anatomic landmarks that are useful in maximizing coverage of the surgical bed. Inferiorly, the vesical-urethral anastomosis should be included. This anastomosis is the most frequent area of positive prostate bed biopsies. By placing the inferior field edge at the top of the bulb of the penis (best seen on magnetic resonance imaging) and adding a margin for uncertainties, there should be adequate coverage. Laterally, the field should extend to about the medial aspect of each obturator internus muscle. Although the rectum is a landmark posteriorly, the relative position of the rectum appears to shift after the prostate is removed as well as during radiation therapy (Naya et al. 2005; Fiorino et al. 2005). For this reason, a generous margin from CTV to pTV posteriorly is recommended, such as setting an 8-mm margin with image guidance (Paskalev et al. 2005). The superior margin is more subjective. The former prostate can extend above the pubic symphysis, but it is recommended that the anterior part of the bladder be avoided at this level because this is the least likely area for extracapsular extension and involved margins. Treatment of the seminal vesicle bed, lying behind the bladder, is advised for pT3b tumors. If vascular clips were used at prostatectomy, they are likely to be seen in this region. The level of the posterior-superior clinical target volume is somewhat subjective and should be guided by the extent of disease at the prostate base and whether the seminal vesicles were involved.
For all these reasons, the recommendations of the RTOG (Michalski et al. 2009) and of the EORTC (Poortmans et al. 2007) should be considered as being very helpful in delineation of the target volume for irradiation of the prostatic fossa.
However, the definition of the target volumes remains difficult. Recently, a study assessed the interobserver agreement of prostate bed delineation after radical prostatectomy as proposed by the EORTC guidelines. Six observers delineated the prostate bed (PB) and the original seminal vesicle position (SV) of 10 patients. Contours were then compared for agreement between observers. The mean volume of 100% agreement was only 5.0 (±3.3) ml for the PB and 0.9 (±1.5) ml for the SV, whereas the mean union of all contours (±1 SD) was 41.1 (±11.8) ml and 25.3 (±13.4) ml, respectively. The overall standard deviation of the outer margins of the PB ranged from 4.6 to 7.0 mm (Ost et al. 2011b).
Given the potential for late toxicity after postoperative radiation therapy, the use of IMRT is appealing (Bastasch et al. 2002). As with 3D CRT, a generous definition of the prostate bed target volume and adequate margins to account for target motion (especially due to the variation in rectal and bladder filling) and setup uncertainties are critical. The theoretical advantages of IMRT are that dose falloff is more geometrically rapid than for 3D CRT and the better conformation of the dose to irregularly shaped targets (e.g., the superior-posterior aspect of the postoperative field). A greater sparing of the superior-anterior part of the bladder, the posterior part of the rectum, and the penile bulb can be achieved using IMRT, despite using the same target volume definition (Pinkawa et al. 2007). The comparison of a 5-field IMRT and a rotational IMRT (e.g., “Rapid Arc”) technique is displayed in Fig. 14.1.
For optimization of the margins needed for delivery of IMRT, IGRT remains a helpful tool. Ost and coworkers from Gent University demonstrated a significant reduction of acute toxicity using patient positioning with cone beam CT (Ost et al. 2011a). Sandhu et al. from the University of California used IGRT in patients undergoing postprostatectomy irradiation. Prostate bed localization was done using image guidance based on surgical clips, relative to the reference isocenter on the digitally reconstructed radiographs made during radiation therapy planning. They assumed that surgical clips are a useful surrogate for the prostate bed and measured daily shifts of the position of the surgical clips in 3 dimensions. With an average (standard deviation) prostate bed motion in anterior-posterior, superior-inferior, and left-right directions of 2.7 mm (2.1), 2.4 mm (2.1), and 1.0 mm (1.7), the majority of the patients experienced only grade 1 side effects. They recommended daily IGRT for accurate target localization (Sandhu et al. 2008). However, the most efficient approach for IGRT during the 6–8 weeks of irradiation remains controversial (Kupelian et al. 2006; Schiffner et al. 2007).
When indicated, like in patients at a high risk for lymph node involvement or confirmed pelvic lymph node involvement, the pelvic lymphatics should be irradiated (Heidenreich et al. 2011; Wenz et al. 2010). For this case, the recommendations from the RTOG, published by Lawton et al. following a consensus reached by a group of specialized uro-oncologic radiation oncologists, can be used (Lawton et al. 2009). The typical dose recommended for pelvic irradiation is 1.8 Gy per fraction up to a total dose of 45–50.4 Gy. The value of IMRT for irradiation of the pelvic lymphatics has been proven by reducing acute and late gastrointestinal and genitourinary toxicity (Lawton et al. 2009; Alongi et al. 2009).
14.5 Side Effects and Toxicity
The three randomized clinical trials discussed above included prospective collection of data on gastrointestinal or genitourinary toxicity in the two cohorts (ART vs. observation). However, it should be mentioned that in the EORTC and SWOG trials, radiation was based on 2D treatment planning which did not enable to significant normal tissue sparing. In contrast, modern 3D-based radiation treatment techniques such as IMRT allow for minimization of dose to the rectum and bladder.
In the SWOG 8794 study, 3.3% of postoperative irradiated patients developed grade 3 or higher adverse events such as rectal bleeding or proctitis as compared to 0% of patients in the observation group (P = 0.002). The incidence of urethral strictures was significantly higher in the immediate postoperative RT group (17.8% vs. 9.5%, RR 1.9, P = 0.02). Total urinary incontinence occurred in 6.5% of men in the RT group as compared to 2.8% of men in the observation group (RR 2.3, P = 0.11) (Thompson et al. 2006).
In the EORTC trial, there was no significant difference in high-grade (grade 3 or higher) toxicity between both arms, ART, and observation. At 5 years, the cumulative incidence of late grade 3 events was 4.3% versus 2.6% (P = 0.0726). Though, in the ART cohort all late grade 2 and 3 toxicity events combined were more prominent (P = 0.0005). Unlike the SWOG trial, the EORTC trial did not assess total urinary incontinence; however, in an interim analysis, there was no significant difference concerning urinary incontinence between the two treatment arms (Bolla et al. 2005).
In the German study, which utilized 3D-based radiation treatment planning, the incidence of late grade 3 or higher adverse events was only 0.3% (Wiegel et al. 2009a). One patient developed a urethral stricture in the observation arm compared to two patients in the ART arm. Urinary incontinence was not assessed in this trial.
In the EORTC study, 100 randomized patients were evaluated concerning the continence situation. There was no difference in the number of fully continent patients after 24 months between the group receiving 60 Gy and the group under observation (Van Cangh et al. 1998).
SRT with a dose of 66.6 Gy is generally associated with a low rate of severe acute and late side effects. Urinary incontinence in 0–5% of the cases, moderate proctitis in 0–10%, and mild to moderate cystitis in up to 10% may result from this procedure (Stephenson et al. 2004; Neuhof et al. 2007; Do et al. 1998). Severe late effects are rare events affecting 3–6% or fewer of the patients (Do et al. 1998). In our study, SRT was well tolerated, with only a few severe effects: Only 4 patients (2.4%) had grade 3 cystitis, and 4 of 162 patients (2.4%) had urethral strictures after SRT after radical prostatectomy (Wiegel et al. 2009b).
A low rate of side effects is of particular importance for a therapy without histologic confirmation. As literature data attest, doses up to 66 Gy given in the frame of three-dimensional RT treatment planning are rarely associated with serious long-term side effects (grade 3/4 according to the RTOG-EORTC grading system) involving the rectum and bladder. Although in general, side effects tend to be underreported in retrospective analyses, a proportion of <3% seems to be a realistic estimate. Fairly higher rates of 10% genitourinary grade 3 complications, namely anastomotic strictures and bladder neck contractures requiring dilatation, reported in a series of 115 patients from the Memorial Sloan-Kettering Cancer Center, need to be interpreted with caution (Katz et al. 2003). It may be difficult to differentiate side effects of RT from preexisting disabilities and sequelae of RP. At least equivalent rates of severe genitourinary complications following RP alone have been reported in a SEER database analysis of 11,522 patients published by the same institution (Begg et al. 2002). Formenti et al. investigated the rate and degree of incontinence and erectile dysfunction after nerve-sparing RP with or without adjuvant RT. Unfortunately, follow-up examinations only comprised a questionnaire with inherent weaknesses. No difference was found between 72 patients who underwent both RP and RT and 138 patients who underwent RP only when total doses of 45–54 Gy were applied (Formenti et al. 1996).
14.6 Adjuvant Versus Salvage Radiation Therapy
Multiple prospective and retrospective studies dealt with the clinical question whether adjuvant radiation therapy or salvage radiation therapy is preferable in terms of local control and freedom from biochemical failure (FFBF) (Thompson et al. 2006; Bolla et al. 2005; Wiegel et al. 2009a, b; Stephenson et al. 2007; Neuhof et al. 2007; Trock et al. 2008; Loeb et al. 2008; Bernard et al. 2010; Siegmann et al. 2011; King and Kapp 2008). A consistently higher improvement in local control and FFBF has been observed in adjuvant radiation therapy compared with salvage radiation therapy patients. The 5-year FFBF rates are approximately 69–89% after adjuvant radiation therapy. Local control is 96–100% after adjuvant radiation therapy and 79–93% after salvage radiation therapy (Bottke et al. 2007). Recently, Trabulsi and colleagues studied a group of patients undergoing adjuvant radiation therapy with a matched control group undergoing salvage radiation therapy after biochemical failure. Using a multi-institutional database of 2,299 patients, 449 patients with pT3–4 N0 disease were eligible, including 211 patients receiving adjuvant radiation therapy and 238 patients receiving salvage radiation therapy. Adjuvant radiation therapy significantly reduced the risk of long-term biochemical progression after radical prostatectomy compared with salvage radiation therapy (5-year FFBF was 73% after adjuvant radiation therapy compared with 50% after salvage radiation therapy; P = 0.007). Gleason score 8 was a significant predictor of FFBF (Trabulsi et al. 2008). These results were confirmed by others (Budiharto et al. 2010), but Ost et al. reported a better outcome after salvage radiation therapy compared with after adjuvant radiation therapy (Ost et al. 2011c). For all of these reasons, the best choice for treatment (adjuvant radiation therapy vs. salvage radiation therapy) has to be discussed individually with each patient, taking into account the possible risk for overtreatment with immediate postoperative irradiation.
In 2007, a prospective randomized study was initiated to address this question as well as the potential role of concomitant androgen deprivation (Parker et al. 2007). The RADICALS (Radiotherapy and Androgen Deprivation in Combination After Local Surgery) trial is an effort to evaluate adjuvant versus salvage radiation therapy. Patients are randomized after surgery to early or delayed radiation. Delayed radiation will be given when there are either two consecutive PSA rises and a final PSA > 0.1 ng/ml or three consecutive PSA rises. The planned accrual is 2,600 patients with cause-specific survival being the primary outcome. There is a second randomization regarding androgen deprivation therapy.
14.7 Conclusions
Adjuvant radiation therapy (ART) provides improved biochemical relapse-free survival and, potentially, overall survival for patients at high-risk following prostatectomy compared to observation. Therefore, ART is really indicated for selected patients. However, it remains unknown if early salvage radiation therapy (SRT) initiated after a PSA failure is equivalent to ART. At the present time, there are no published randomized trials to compare ART versus SRT. When SRT is indicated, it should be initiated as early as possible (with PSA < 0.5 ng/ml). In this situation, SRT is the only curative therapy option.
Modern radiation therapy techniques like IMRT and IGRT should be used. Serious side effects are apparently low, thus confirming the suitability of this therapeutic approach. The role of AD after adjuvant or salvage RT remains poorly defined. But in the RTOG 96-01 trial, the addition of 24 months of peripheral androgen blockade during and after RT significantly improved freedom from PSA progression and reduced the incidence of metastatic prostate cancer. The analysis of risk-stratified subsets must await longer follow-up.
References
Alongi F, Fiorino C, Cozzarini C et al (2009) IMRT significantly reduces acute toxicity of whole-pelvis irradiation in patients treated with post-operative adjuvant or salvage radiotherapy after radical prostatectomy. Radiother Oncol 93:207–212
Anscher MS, Clough R, Dodge R (2000) Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys 48:369–375
Bastasch MD, Teh BS, Mai WY et al (2002) Post-nerve-sparing prostatectomy, dose-escalated intensity-modulated radiotherapy: effect on erectile function. Int J Radiat Oncol Biol Phys 54:101–106
Begg CB, Riedel ER, Bach PB et al (2002) Variations in morbidity after radical prostatectomy. N Engl J Med 346:1138–1144
Bernard JR Jr, Buskirk SJ, Heckman MG et al (2010) Salvage radiotherapy for rising prostate-specific antigen levels after radical prostatectomy for prostate cancer: dose-response analysis. Int J Radiat Oncol Biol Phys 76:735–740
Bolla M, Van Poppel H, Collette L et al (2005) Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet 366:572–578
Bolla M, De Reijke TM, Van Tienhoven G et al (2009) Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 360:2516–2527
Bolla M, Van Poppel H, Tombal B et al (2010) 10-year results of adjuvant radiotherapy after radical prostatectomy in pT3N0 prostate cancer (EORTC 22911). Int J Radiat Oncol Biol Phys 78(suppl):S29
Bottke D, Abrahamsson PA, Welte B et al (2007) Adjuvant radiotherapy after radical prostatectomy. Eur J Cancer Suppl 5:171–176
Bottke D, de Reijke TM, Bartkowiak D et al (2009) Salvage radiotherapy in patients with persisting/rising PSA after radical prostatectomy for prostate cancer. Eur J Cancer 45(suppl 1):148–157
Briganti A, Karnes RJ, Da Pozzo LF et al (2011) Combination of adjuvant hormonal and radiation therapy significantly prolongs survival of patients with pT2–4 pN + prostate cancer: results of a matched analysis. Eur Urol 59:832–840
Budiharto T, Perneel C, Haustermans K et al (2010) A multi-institutional analysis comparing adjuvant and salvage radiation therapy for high-risk prostate cancer patients with undetectable PSA after prostatectomy. Radiother Oncol 97:474–479
Buskirk SJ, Pisansky TM, Schild SE et al (2006) Salvage radiotherapy for isolated prostate specific antigen increase after radical prostatectomy: evaluation of prognostic factors and creation of a prognostic scoring system. J Urol 176:985–990
Cadeddu JA, Partin AW, DeWeese TL et al (1998) Long-term results of radiation therapy for prostate cancer recurrence following radical prostatectomy. J Urol 59:173–178
Canter D, Greenberg RE, Horwitz EM et al (2010) Implantation of electromagnetic transponders following radical prostatectomy for delivery of IMRT. Can J Urol 17:5365–5369
Chawla AK, Thakral HK, Zietman AL et al (2002) Salvage radiotherapy after radical prostatectomy for prostate adenocarcinoma: analysis of efficacy and prognostic factors. Urology 59:726–731
Choo R, Danjoux C, Gardner S et al (2009) Efficacy of salvage radiotherapy plus 2-year androgen suppression for postradical prostatectomy patients with PSA relapse. Int J Radiat Oncol Biol Phys 75:983–989
Chun FK, Graefen M, Zacharias M et al (2006) Anatomic radical retropubic prostatectomy-long-term recurrence-free survival rates for localized prostate cancer. World J Urol 24:273–280
Cimitan M, Bortolus R, Morassut S et al (2006) [18F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging 33:1387–1398
Coen JJ, Zietman AL, Thakral H et al (2002) Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol 20:3199–3205
Connolly JA, Shinohara K, Presti JC Jr et al (1996) Local recurrence after radical prostatectomy: characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology 47:225–231
Corn BW, Winter K, Pilepich MV (1999) Does androgen suppression enhance the efficacy of postoperative irradiation? A secondary analysis of RTOG 85–31. Radiation Therapy Oncology Group. Urology 54:495–502
Da Pozzo LF, Cozzarini C, Briganti A et al (2009) Long-term follow-up of patients with prostate cancer and nodal metastases treated by pelvic lymphadenectomy and radical prostatectomy: the positive impact of adjuvant radiotherapy. Eur Urol 55:1003–1011
Deliveliotis C, Manousakas T, Chrisofos M et al (2007) Diagnostic efficacy of transrectal ultrasound-guided biopsy of the prostatic fossa in patients with rising PSA following radical prostatectomy. World J Urol 25:309–313
Do T, Parker RG, Do C et al (1998) Salvage radiotherapy for biochemical and clinical failures following radical prostatectomy. Cancer J Sci Am 4:324–330
Elshaikh MA, Ibrahim DR, Stricker H et al (2011) Adjuvant radiation treatment after prostatectomy. Where do we stand? Can J Urol 18:5592–5600
Fiorino C, Foppiano F, Franzone P et al (2005) Rectal and bladder motion during conformal radiotherapy after radical prostatectomy. Radiother Oncol 74:187–195
Formenti SC, Lieskovsky G, Simoneau AR et al (1996) Impact of moderate dose of postoperative radiation on urinary continence and potency in patients with prostate cancer treated with nerve sparing prostatectomy. J Urol 155:616–619
Garg MK, Tekyi-Mensah S, Bolton S et al (1998) Impact of postprostatectomy prostate-specific antigen nadir on outcomes following salvage radiotherapy. Urology 51:998–1002
Hagan M, Zlotecki R, Medina C et al (2004) Comparison of adjuvant versus salvage radiotherapy policies for postprostatectomy radiotherapy. Int J Radiat Oncol Biol Phys 59:329–340
Hanlon AL, Horwitz EM, Hanks GE et al (2004) Short-term androgen deprivation and PSA doubling time: their association and relationship to disease progression after radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 58:43–52
Heidenreich A, Bellmunt J, Bolla M et al (2011) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 59:61–71
Hofer C, Laubenbacher C, Block T et al (1999) Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol 36:31–35
Horwitz EM, Bae K, Hanks GE et al (2008) Ten-year follow-up of radiation therapy oncology group protocol 92–02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26:2497–2504
Huch Boni RA, Meyenberger C, Pok Lundquist J et al (1996) Value of endorectal coil versus body coil MRI for diagnosis of recurrent pelvic malignancies. Abdom Imaging 21:345–352
Kaminski JM, Hanlon AL, Joon DL et al (2003) Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. Int J Radiat Oncol Biol Phys 57:24–28
Katz MS, Zelefsky MJ, Venkatraman ES et al (2003) Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol 21:483–489
King CR, Kapp DS (2008) Radiotherapy after prostatectomy: is the evidence for dose escalation out there? Int J Radiat Oncol Biol Phys 71:346–350
King CR, Presti JC Jr, Gill H et al (2004) Radiotherapy after radical prostatectomy: does transient androgen suppression improve outcomes? Int J Radiat Oncol Biol Phys 59:341–347
Kupelian PA, Langen KM, Willoughby TR et al (2006) Daily variations in the position of the prostate bed in patients with prostate cancer receiving postoperative external beam radiation therapy. Int J Radiat Oncol Biol Phys 66:593–596
Lawton CA, Michalski J, El-Naqa I et al (2009) Variation in the definition of clinical target volumes for pelvic nodal conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 74:377–382
Loeb S, Roehl KA, Viprakasit DP et al (2008) Long-term rates of undetectable PSA with initial observation and delayed salvage radiotherapy after radical prostatectomy. Eur Urol 54:88–94
Michalski JM, Roach M 3rd, Merrick G et al (2009) ACR appropriateness criteria on external beam radiation therapy treatment planning for clinically localized prostate cancer expert panel on radiation oncology – prostate. Int J Radiat Oncol Biol Phys 74:667–672
Michalski JM, Lawton C, El Naqa I et al (2010) Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 76:361–368
Miralbell R, Vees H, Lozano J et al (2007) Endorectal MRI assessment of local relapse after surgery for prostate cancer: a model to define treatment field guidelines for adjuvant radiotherapy in patients at high risk for local failure. Int J Radiat Oncol Biol Phys 67:356–361
Nath SK, Sandhu AP, Rose BS et al (2010) Toxicity analysis of postoperative image-guided intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 78:435–441
Naya Y, Okihara K, Evans RB et al (2005) Efficacy of prostatic fossa biopsy in detecting local recurrence after radical prostatectomy. Urology 66:350–355
Neuhof D, Hentschel T, Bischof M et al (2007) Long-term results and predictive factors of three-dimensional conformal salvage radiotherapy for biochemical relapse after prostatectomy. Int J Radiat Oncol Biol Phys 67:1411–1417
Ost P, De Gersem W, De Potter B et al (2011a) A comparison of the acute toxicity profile between two-dimensional and three-dimensional image-guided radiotherapy for postoperative prostate cancer. Clin Oncol (R Coll Radiol) 23:344–349
Ost P, De Meerleer G, Vercauteren T et al (2011b) Delineation of the postprostatectomy prostate bed using computed tomography: interobserver variability following the EORTC delineation guidelines. Int J Radiat Oncol Biol Phys 81:e143–e149, Epub 2011 Mar 4
Ost P, De Troyer B, Fonteyne V et al (2011c) A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 80:1316–1322
Parker C, Sydes MR, Catton C et al (2007) Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int 99:1376–1379
Paskalev K, Feigenberg S, Jacob R et al (2005) Target localization for post-prostatectomy patients using CT and ultrasound image guidance. J Appl Clin Med Phys 6:40–49
Pazona JF, Han M, Hawkins SA et al (2005) Salvage radiation therapy for prostate specific antigen progression following radical prostatectomy: 10-year outcome estimates. J Urol 174(4 Pt 1):1282–1286
Peyromaure M, Allouch M, Eschwege O et al (2003) Salvage radiotherapy for biochemical recurrence after radical prostatectomy: a study of 62 patients. Urology 62:503–507
Picchio M, Briganti A, Fanti S et al (2011) The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol 59:51–60
Pinkawa M, Siluschek J, Gagel B et al (2007) Postoperative radiotherapy for prostate cancer: evaluation of target motion and treatment techniques (intensity-modulated versus conformal radiotherapy). Strahlenther Onkol 183:23–29
Pinto F, Prayer-Galetti T, Gardiman M et al (2006) Clinical and pathological characteristics of patients presenting with biochemical progression after radical retropubic prostatectomy for pathologically organ-confined prostate cancer. Urol Int 76:202–208
Pisansky TM, Kozelsky TF, Myers RP et al (2000) Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol 163:845–850
Poortmans P, Bossi A, Vandeputte K et al (2007) Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC radiation oncology group. Radiother Oncol 84:121–127
Rinnab L, Mottaghy FM, Blumstein NM et al (2007) Evaluation of [11C]-choline positron-emission/computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int 100:786–793
Roehl KA, Han M, Ramos CG, Antenor JA et al (2004) Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol 172:910–914
Roscigno M, Cozzarini C, Scattoni V et al (2007) A reappraisal of the role of vesicourethral anastomosis biopsy in patient candidates for salvage radiation therapy after radical prostatectomy. Radiother Oncol 82:30–37
Rossi CJ Jr, Joe Hsu IC, Abdel-Wahab M et al (2011) ACR appropriateness criteria postradical prostatectomy irradiation in prostate cancer. Am J Clin Oncol 34:92–98
Salomon L, Anastasiadis AG, Antiphon P et al (2003) Prognostic consequences of the location of positive surgical margins in organ-confined prostate cancer. Urol Int 70:291–296
Sandhu A, Sethi R, Rice R et al (2008) Prostate bed localization with image-guided approach using on-board imaging: reporting acute toxicity and implications for radiation therapy planning following prostatectomy. Radiother Oncol 88:20–25
Scattoni V, Roscigno M, Raber M et al (2003) Multiple vesico-urethral biopsies following radical prostatectomy: the predictive roles of TRUS, DRE, PSA and the pathological stage. Eur Urol 44:407–414
Scattoni V, Picchio M, Suardi N et al (2007) Detection of lymph-node metastases with integrated [11C]choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvic-retroperitoneal lymphadenectomy. Eur Urol 52:423–429
Schiffner DC, Gottschalk AR, Lometti M et al (2007) Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int J Radiat Oncol Biol Phys 67:610–619
Schoder H, Herrmann K, Gonen M et al (2005) 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res 11:4761–4769
Sella T, Schwartz LH, Swindle PW et al (2004) Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology 231:379–385
Shekarriz B, Upadhyay J, Wood DP Jr et al (1999) Vesicourethral anastomosis biopsy after radical prostatectomy: predictive value of prostate-specific antigen and pathologic stage. Urology 54:1044–1048
Shipley WU, Hunt D, Lukka H et al (2010) Initial report of RTOG 9601: a phase III trial in prostate cancer: anti-androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) improves freedom from progression and reduces the incidence of metastatic disease in patients following radical prostatectomy (RP) with pT2–3, N0 disease, and elevated PSA levels. Int J Radiat Oncol Biol Phys 78(suppl):S27
Showalter TN, Nawaz AO, Xiao Y et al (2008) A cone beam CT-based study for clinical target definition using pelvic anatomy during postprostatectomy radiotherapy. Int J Radiat Oncol Biol Phys 70:431–436
Siegmann A, Bottke D, Faehndrich J et al (2011) Dose escalation for patients with decreasing PSA during radiotherapy for elevated PSA after radical prostatectomy improves biochemical progression-free survival: results of a retrospective study. Strahlenther Onkol 187:467–472
Soto DE, Passarelli MN, Daignault S et al (2011) Concurrent androgen deprivation therapy during salvage prostate radiotherapy improves treatment outcomes in high risk patients. Int J Radiat Oncol Biol Phys: Epub ahead of print
Spiotto MT, Hancock SL, King CR (2007) Radiotherapy after prostatectomy: improved biochemical relapse-free survival with whole pelvic compared with prostate bed only for high-risk patients. Int J Radiat Oncol Biol Phys 69:54–61
Stamey TA, Yang N, Hay AR et al (1987) Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 317:909–916
Stephenson AJ, Shariat SF, Zelefsky MJ et al (2004) Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 291:1325–1332
Stephenson AJ, Scardino PT, Kattan MW et al (2007) Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 25:2035–2041
Stephenson AJ, Wood DP, Kattan MW et al (2009) Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol 182:1357–1363
Swanson GP, Du F, Michalek JE et al (2011) Long-term follow-up and risk of cancer death after radiation for post-prostatectomy rising prostate-specific antigen. Int J Radiat Oncol Biol Phys 80:62–68
Swindle P, Eastham JA, Ohori M et al (2005) Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol 174:903–907
Tamsel S, Killi R, Apaydin E et al (2006) The potential value of power Doppler ultrasound imaging compared with grey-scale ultrasound findings in the diagnosis of local recurrence after radical prostatectomy. Clin Radiol 61:325–330; discussion 323–324
Taylor N, Kelly JF, Kuban DA et al (2003) Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys 56:755–763
Thompson IM Jr, Tangen CM, Paradelo J et al (2006) Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 296:2329–2335
Thompson IM, Tangen CM, Paradelo J et al (2009) Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181:956–962
Trabulsi EJ, Valicenti RK, Hanlon AL et al (2008) A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3–4N0 prostate cancer. Urology 72:1298–1302; discussion 1302–1294
Trock BJ, Han M, Freedland SJ et al (2008) Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299:2760–2769
Tsien C, Griffith K, Sandler H et al (2003) Long term results of three-dimensional conformal adjuvant and salvage radiotherapy after radical prostatectomy. Urology 62:93–98
Van Cangh PJ, Richard F, Lorge F et al (1998) Adjuvant radiation therapy does not cause urinary incontinence after radical prostatectomy: results of a prospective randomized study. J Urol 159:164–166
Van der Kwast TH, Bolla M, van Poppel H et al (2007) Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol 25:4178–4186
Ward JF, Zincke H, Bergstralh EJ et al (2004) Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol 172(6 Pt 1):2244–2248
Wenz F, Martin T, Böhmer D et al (2010) The German S3 guideline prostate cancer: aspects for the radiation oncologist. Strahlenther Onkol 186:531–534
Wiegel T, Bottke D, Steiner U et al (2009a) Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96–02/AUO AP 09/95. J Clin Oncol 27:2924–2930
Wiegel T, Lohm G, Bottke D et al (2009b) Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome–results of a retrospective study. Int J Radiat Oncol Biol Phys 73:1009–1016
Wiltshire KL, Brock KK, Haider MA et al (2007) Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys 69:1090–1099
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bottke, D., Wiegel, T. (2012). Postoperative Irradiation: Immediate or Early Delayed?. In: Bolla, M., van Poppel, H. (eds) Management of Prostate Cancer. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-27597-5_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-27597-5_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-27596-8
Online ISBN: 978-3-642-27597-5
eBook Packages: MedicineMedicine (R0)