Abstract
The optimal management of patients with adverse clinical and pathologic features concerning the risk of a biochemical recurrence after radical prostatectomy is still under discussion. The two treatment approaches for patients with undetectable PSA are immediate adjuvant radiotherapy or observation followed by early salvage radiation therapy in case of PSA increase out of the undetectable range. The purpose of this chapter is to review the rationale, results, and possible side effects of adjuvant radiotherapy with main focus on the three randomized phase III trials: Southwest Oncology Group (SWOG) 8794, the European Organization for Research and Treatment of Cancer (EORTC 22911), and the German Cancer Society (ARO 96-96/AUOAP 09/95). All three trials demonstrated a benefit in terms of bNED (biochemically no evidence of disease) after adjuvant radiotherapy compared to a “wait-and-see” policy. The greatest benefit was achieved in patients with positive margins and pT3 tumors. The rate of side effects was comparably low. It remains unknown if early salvage radiation therapy initiated after PSA failure is equivalent to adjuvant radiotherapy. At the present time, there are no published randomized trials to compare adjuvant radiotherapy versus salvage radiation therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Radical Prostatectomy
- Gleason Score
- Positive Surgical Margin
- Biochemical Recurrence
- Pelvic Lymph Node Dissection
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
For patients with low-risk prostate cancer/localized disease and/or higher age active surveillance or watchful waiting are suitable options regarding side effects and quality of life (Kyrdalen et al. 2013; McVey et al. 2010; Cooperberg et al. 2011; Budaus et al. 2012). Alternatively, and for more advanced stages, radical prostatectomy (RP) and radiation therapy are the two major first-line therapeutic options. There are multiple established risk factors for recurrence of prostate cancer after RP such as infiltration of the seminal vesicles, advanced tumor stage, positive surgical margins, a high Gleason score, and a high pre-RP PSA level (Chun et al. 2006; Salomon et al. 2003; Swindle et al. 2005; Pfitzenmaier et al. 2008; Pinto et al. 2006). However, recurrences do even occur with a favorable pattern of risk parameters; their overall absolute rates in terms of biochemical relapse are 15–30 % (Cooperberg et al. 2005; Stephenson et al. 2007, 2012; Bianco et al. 2005), while with adverse features, figures greater than 60 % have been reported (Kawamorita et al. 2009; Swanson et al. 2007).
Post-RP PSA should fall below detection threshold within 4–6 weeks (biochemically no evidence of disease; bNED), as its serum half-life is only 2–3 days (Lotan and Roehrborn 2002). Measurable PSA levels after RP indicate residual prostatic tissue, either malignant or benign (BPH). In the former case, persisting PSA levels predate clinically evident disease and do correlate well with disease progression.
A PSA value of ≥0.2 ng/ml is a widely accepted threshold to state biochemical relapse if confirmed in a second measurement, while minimum detectable concentrations are approximately 1 pg/ml or less (Chikkaveeraiah et al. 2011; Triroj et al. 2011), (Stephenson et al. 2007; Wiegel et al. 2009b; Freedland et al. 2005; Heidenreich et al. 2011; Wenz et al. 2010).
Vital tumor tissue is histopathologically proven by biopsies form the vesicourethral anastomosis in up to 53 % of all patients with rising PSA after RP without clinical correlates suggestive of recurrent tumor (Shekarriz et al. 1999).
Rising PSA values serve as a surrogate marker of recurrence after primary therapy, as they precede metastatic progression and tumor-specific death by several years (Stephenson et al. 2006). However, patients with (slowly) rising PSA values do not coercively develop distant metastases. Although there is no fixed relation between PSA level and risk of metastasis, bone scintigrams at a PSA <7 ng/ml are mostly negative, while at >20 ng/ml they are quite likely to be positive (Gomez et al. 2004; Mottet et al. 2011).
The optimal management of patients with adverse clinical and pathologic features concerning the risk of a biochemical recurrence after RP continues to be a source of controversy. The two treatment approaches for the postoperative management of these patients are immediate adjuvant radiation therapy in men with an undetectable PSA or observation followed by early salvage radiation therapy in case of PSA persistence or increase after initially postoperative undetectable values.
The purpose of this chapter is to review the rationale, results, and possible side effects of adjuvant radiotherapy with main focus on the three phase III randomized trials SWOG 8794, EORTC 22911 and ARO 96-02/AUO AP 09/95.
2 Adjuvant Radiotherapy
Adjuvant radiotherapy (ART) implies that the patient has achieved an undetectable post-RP PSA level and, despite this apparent success, is irradiated. Evidently, a dilemma results from the unavoidable overtreatment by ART, which must be justified by clinical advantage.About 40–50 % percentage of the patients are presumably overtreated, which is the percentage of bNED 5 years after RP alone (King 2012; Briganti et al. 2012). Furthermore, in patients with tumor spread beyond the pelvis, ART is useless and thus 30 % of ART patients are expected to develop progression or die despite treatment (King 2012; Richaud et al. 2010). Such concern probably causes low ART application rates (Tyldesley et al. 2012; Showalter et al. 2012; Hoffman et al. 2011). On the other hand, ART might be superior to (delayed) salvage radiation therapy for those patients who are at higher risk of post-RP recurrence and who could profit from early initiation of radiotherapy.
3 Randomized Clinical Trials
Definitive evidence that adjuvant radiotherapy improves the outcome of men with pathologically advanced prostate cancer is available from three phase III randomized trials: Southwest Oncology Group (SWOG) 8794, the European Organization for Research and Treatment of Cancer (EORTC) 22911, and the German Cancer Society (ARO 96-02/AUO AP 09/95).
All three trials demonstrated a benefit in terms of bNED after adjuvant radiation therapy (60–64 Gy) compared to a “wait-and-see” policy, mostly for pT3 cN0 or pN0 tumors (Table 1).
3.1 Southwest Oncology Group (SWOG) 8794
The SWOG 8794 was a randomized multi-institutional prospective trial of ART with 60–64 Gy versus observation alone for locally advanced prostate cancer following RP. Between 1988 and 1997, the study enrolled 425 patients with pathological stage T2 or T3 tumors who met at least one of the following pathological criteria: extracapsular extension, positive surgical margin, or seminal vesicle invasion. Pelvic lymph node dissection was obligatory, an undetectable PSA level before study entry was not mandatory. Thirty-three percentage of men in both arms had a serum PSA level >0.2 ng/ml at the time of randomization. A total 8 % of patients received pre-RP androgen deprivation therapy (ADT) (Thompson et al. 2006, 2009; van der Kwast et al. 2007).
Patients randomized to the ART arm began radiotherapy within 18 weeks after surgery. Treatment delivery was done utilizing 2D-based planning aimed at the prostatic fossa and paraprostatic tissues.
The primary end point in this study was metastasis-free survival. bNED was a secondary end point. A biochemical failure was defined as PSA level >0.4 ng/ml.
At the time of initial publication of the study (median follow-up 10.6 years), there was a significant benefit for patients treated with ART in terms of PSA relapse-free survival (median time to PSA relapse 10.3 vs. 3.3 years; p < 0.001) and recurrence-free survival (median time 13.8 vs. 9.9 years; p = 0.001).
In the observation arm, the use of salvage radiotherapy was not mandated by protocol. Ultimately a total of 70 men (33 %) received postoperative radiotherapy, mostly for a rising serum PSA level. The median PSA level at the time of salvage radiotherapy in these patients was 1.0 ng/ml, which would be considered ‘late’ salvage therapy by current standards.
By 5 years, twice as many men in the observation arm had received hormonal therapy versus in the ART arm (21 vs. 10 %; p < 0.001).
The initial report did not reveal advantage for ART concerning metastasis-free survival or overall survival. However, after a median follow-up of 12.5 years, a subsequent publication demonstrated a significant improvement in metastasis-free survival (12.9 years for the observation arm vs. 14.7 years for the ART arm; p = 0.016) as well as in overall survival in favor of ART (59 % for the ART arm vs. 48 %, observation arm; p = 0.023). The authors calculated that, on average, 12.2 patients had to be treated with ART to prevent one case of metastatic disease and 9.1 patients to prevent one death. It is interesting to note that the differences between the treatment groups become measurable not before 10 years, highlighting the importance of long-term follow-up in these patients.
However, the rate of observed distant metastasis was low (37 men in the observation arm and 20 men in the radiotherapy arm) and the majority of events in the analysis of metastasis-free survival and overall survival in both groups were deaths without evidence of metastatic prostate cancer (77 of 114 men in the observation arm and 73 of 93 men in the radiotherapy arm). Consequently, it has been argued that the survival benefit after ART was largely due to a lower rate of competing-cause deaths without evidence of distant metastasis, and that the impact of ART on metastatic disease and cancer-specific death was still uncertain.
3.2 European Organization for Research and Treatment of Cancer (EORTC) 22911
The EORTC 22911 was a phase III clinical trial of ART versus no immediate further treatment for patients with pN0 M0 prostate cancer with non-organ-confined disease (extracapsular extension or seminal vesicle invasion) or positive margins. All patients had ilio-obturator lymphadenectomy. A total of 1,005 men <75 years were accrued to the trial between 1992 and 2001. An undetectable PSA following prostatectomy was not mandatory for study enrollment. In total, 69.5 % of the patients had an undetectable PSA following RP. A total of 10 % of the patients received pre-RP ADT (Bolla et al. 2005, 2012).
For patients randomized to the ART arm, radiotherapy was initiated within 16 weeks following surgery, after recovery of urinary function. RT was delivered using 2D-based treatment planning to a total dose of 60 Gy over a period of 6 weeks.
The ‘revised primary end point’ of the study was PSA progression (initially it was metastasis-free survival), defined as an increase of more than 0.2 ng/ml over the lowest post-RP value measured on three occasions at least 2 weeks apart.
Overall, 301 (30 %) of men had a serum PSA level >0.2 ng/ml at the time of randomization (157 in the observation arm, 144 in the radiotherapy arm). In the observation arm, patients with biochemical or local recurrence were recommended to receive salvage radiotherapy. However, only 113 (51 %) of men with recurrent cancer after RP in the control arm received salvage radiotherapy, and 45 % of these received ‘late’ radiotherapy on the basis of clinically evident locoregional recurrence.
After a median follow-up of 5 years, biochemical progression-free survival (bPFS), clinical progression-free survival, and the cumulative rate of locoregional failures were significantly improved in the ART group (74 vs. 56 %; p < 0.0001 for bPFS). In total, 22.5 % of men in the observation arm subsequently underwent pelvic RT and 9 % eventually required hormonal treatment. Overall, the rates of distant metastasis (seen in 18 men in the observation arm and 19 in the radiotherapy arm) and deaths from prostate cancer (15 in the observation arm and 8 in the radiotherapy arm) as secondary endpoints in both arms were low and not significant different as well as in overall survival (p = 0.7).
Updated results with 10 -year follow-up data showed a continued bPFS advantage in favor of ART (61 vs. 41 %; p < 0.001) and a nonsignificant trend toward improved overall survival in the ART group (81 vs. 77 %; p > 0.1).
3.3 German Study Group (ARO 96-02/AUO AP 09/95)
The third phase III trial of ART versus a wait-and-see policy for patients with non-organ-confined prostate cancer (pathological stage pT3 pN0) with or without positive margins enrolled a total of 385 patients between 1994 and 2004. Approximately 11 % of patients received pre-RP ADT. All patients were required to have undergone a pelvic lymph node dissection. Unlike the SWOG and EORTC trials, patients were required to have an undetectable PSA following RP. Seventy-eight patients did not achieve an undetectable PSA and were excluded from treatment according to random assignment. Of the remaining 307 patients, 34 patients on the RT arm did not receive RT and five patients on the wait-and-see arm received RT. Therefore, 114 patients underwent RT and 154 patients were treated with a wait-and-see policy (Wiegel et al. 2009a, 2013b).
The primary end point of the study was bPFS. A biochemical failure was defined as a PSA increase out of the undetectable range with a consecutive confirmation.
Unlike the prior two studies, patients in this trial were treated with more modern 3D conformal RT. RT was prescribed to a dose of 60 Gy and initiated within 6–12 weeks following RP.
Over a median follow-up duration of 54 months, 67 progression events were observed in the observation arm and 38 in the radiotherapy arm, most of which were due to biochemical recurrence. Five-year progression-free survival was 54 and 72 % in the observation and radiotherapy arms, respectively (p = 0.0015). The benefit in favor of adjuvant radiotherapy was also observed when the 78 patients with persistent serum PSA elevation after radical prostatectomy were included in the analysis (p = 0.05), and persisted across all subgroups, with the exception of those with negative surgical margins. There was no benefit for metastasis-free survival or overall survival.
In the meantime, an update with data of 10-year follow-up was presented at the 2013 Genitourinary Cancers Symposium in Orlando, Florida. At 10 years, freedom from biochemical failure was achieved in 56 % of the adjuvant radiotherapy arm versus 35 % of the wait-and-see arm, for an absolute difference of 21 % favoring adjuvant treatment (p = 0.00002). No significant benefit was observed for adjuvant radiotherapy regarding metastasis-free survival or overall survival, though the trial was not powered to show this.
For patients with positive surgical margins, adjuvant radiotherapy had a clear advantage: Biochemical control was achieved in 55 versus 27 % of those in the wait-and-see arm, for an absolute difference of 28 %. Baseline factors associated with greater efficacy of adjuvant radiotherapy included higher Gleason scores, higher PSA levels, and more aggressive tumors. In a multivariate analysis, adjuvant radiotherapy reduced the risk of biochemical failure by 54 %. The relative risk of biochemical failure was reduced for patients with positive surgical margins, higher PSA level, stage T3a/b, and higher Gleason scores.
3.4 Clinical Trials Overview
Patients with pT3 tumors and positive margins have been demonstrated to benefit most from ART (30 % bNED after 5 years) (Bolla et al. 2005; Thompson et al. 2006; Van der Kwast et al. 2007; Wiegel et al. 2009a). The 10-year follow-up data of all three trials confirm these results (Bolla et al. 2012; Wiegel et al. 2013b). In the prospective study of the South Western Oncology Group (SWOG), overall survival was improved from 13.5 years without to 15.2 years with ART (Thompson et al. 2009).
It is notable that the three randomized studies have used different definitions of biochemical progression: SWOG: PSA >0.4 ng/ml, EORTC: PSA >0.2 ng/ml, ARO/AUO: PSA >0.05 ng/ml.
Consequently biochemical recurrences (as an increase of PSA over the detection threshold) were detected earlier in the latter two studies, which explains the apparently worse results of the ARO study including patients with more favorable risk profile (undetectable PSA after RP) (Table 1).
In the EORTC and SWOG trials radiation was based on 2D treatment planning, where the prostatic fossa and paraprostatic tissue were targeted by using large treatment portals. Obviously, precise definition of target volumes was not essential, which is in great contrast to modern 3D conformal radiation treatment techniques such as IMRT. Compared to 2D-based planning, IMRT provides significant normal tissue sparing, but also demands exact definition of target volume.
Due to more precise techniques in treatment delivery, the Radiation Therapy Oncology Group (RTOG) (Michalski et al. 2010), the EORTC Radiation Oncology Group (Poortmans et al. 2007), and other cooperative groups (Wiltshire et al. 2007) have created consensus guidelines for delineation of target volumes for post-prostatectomy patients.
In 2011, Daly et al. reported the results of a meta-analysis of the three randomized clinical trials comparing radical prostatectomy alone to radical prostatectomy plus adjuvant radiation therapy for the treatment of men with prostate cancer and at least one of the following adverse pathologic features: extracapsular tumor extension, positive surgical margins, or seminal vesicle invasion. In total, 1,815 men were studied (385 from ARO, 1005 from EORTC, and 425 from SWOG). Analysis of oncological outcome was performed at 5- and 10-year time points. At this date, 10-year follow-up data were only available from the SWOG trial. An improved bPFS after ART could be demonstrated at 5 and 10 years with risk differences (RDs, risk difference is the risk in the treated group minus the risk in the control group) of 0.16 (95 % confidence interval [CI], 0.21–0.11) and 0.29 (95 % CI, 0.39–0.19), respectively. Furthermore, at 10 years, adjuvant radiation improved overall survival (RD: 0.11; 95 % CI, 0.20–0.02) and reduced the risk of metastatic disease (RD: 0.11; 95 % CI, 0.20–0.01) (Daly et al. 2011).
3.5 The Role of Positive Margins
Notably, central pathological review on the outcome at 5 years in the EORTC trial demonstrated positive surgical margins interacting statistically significantly with the treatment effect, to such an extent that the treatment benefit in patients with negative margins did not remain significant. The hazard ratio for the treatment benefit in the group with negative surgical margins was 0.87 (p = 0.601), compared to 0.38 (p < 0.0001) in the group with positive surgical margins according to the review pathology. Excluding the patients with a PSA of >0.2 ng/ml after prostatectomy, the hazard ratio for postoperative irradiation was 1.11 (p = 0.740) and 0.29 (p < 0.0001) for the patients with negative and positive margins, respectively (Van der Kwast et al. 2007). This benefit was also seen in the real adjuvant situation with the undetectable PSA before the start of radiation therapy (Wiegel et al. 2009a, b). After a median follow-up of nearly 5 years, there was a significant benefit from adjuvant radiation therapy for bNED: 72 versus 54 % (p < 0.03). In the subgroup of pT3 R1-tumors this benefit increased from 18 to 28 % (Wiegel et al. 2009a).
The location, the extent and the number of positive surgical margins after radical prostatectomy are significant predictors of biochemical progression after radical prostatectomy. The investigators of the Cleveland Clinic/Ohio found in their retrospective multi-institutional series of 7,160 patients treated with radical prostatectomy 1,540 patients with positive margins. The 7-year progression-free probability was 60 % in those patients, resulting in a hazard ratio for biochemical recurrence of 2.3 in the case of positive surgical margins compared with negative margins. The risk of biochemical recurrence was increased in patients with multiple versus solitary positive surgical margins (HR 1.4) and extensive versus focal positive surgical margins (adjusted HR 1.3) (Stephenson et al. 2009). Summing up the data from randomized trials and large retrospective series patients with positive margins and pT3-tumors have the largest profit from postoperative radiation therapy.
3.6 pT2 R1 Tumors
In the EORTC trial, when the data of patients with pT2 tumors and positive surgical margins were analyzed, there was a significant benefit with regard to 5-year biochemical progression-free survival rate in the irradiated group (76.4 vs. 52.2 % in the wait-and-see group) (Bolla et al. 2005). However, these data come from a subgroup analysis and biochemical progression-free survival was not the primary end point of this study. Therefore, the results must be interpreted with caution. The possible benefit of radiotherapy must be weighed out carefully in consideration of potential late effects as impaired erectile dysfunction.
4 The Impact of Pathology Review
The precise histologic assessment of RP specimens in patients with prostate cancer is of major importance for an accurate risk assessment of disease recurrence. The three histopathologic parameters of greatest prognostic importance are pathologic stage, Gleason score, and surgical margin status, where pathologic stage includes assessment for seminal vesicle invasion and extraprostatic extension. Several studies have previously evaluated interobserver variability between local pathologists and review pathologists in Gleason score in both the settings of needle biopsy and RP specimens (Allsbrook et al. 2001a, b; Glaessgen et al. 2004a, b; Oyama et al. 2005). In contrast, only five studies have evaluated interobserver variability in pathologic staging and margin status after RP (Van der Kwast et al. 2006; Ekici et al. 2003; Evans et al. 2008; Kuroiwa et al. 2010; Netto et al. 2011).
It is well known that pathology review has a significant impact on the results of randomized studies of definitive treatment of prostate cancer (Lawton et al. 2001). The RTOG trial 8531 randomized patients with locally advanced prostate cancer to either androgen suppression therapy (AST) or no AST after the administration of RT. In a subgroup of patients with pathologically reviewed biopsy specimens with Gleason score 8–10, there was a significant difference in overall survival (Lawton et al. 2001). However, comparable information is scarce concerning the postoperative treatment of prostate cancer.
The first published results came from the EORTC 22911 trial: 552 radical prostatectomy specimens (approximately 50 % of the patients) were retrospectively reviewed by a single pathologist with experience in urogenital pathology who examined all slides of the sample series (Van der Kwast et al. 2006, 2007). While there was a close concordance between local and review pathology regarding seminal vesicle invasion (94 %), less agreement was reached for extraprostatic extension (58 %) and for surgical margin status (69 %). An agreement rate cannot be given for the Gleason score, because it was not determined by the local pathologists in the EORTC trial (van der Kwast et al. 2006).
Biochemical progression was significantly delayed in all subgroups of men treated with adjuvant radiotherapy in EORTC 22911 (p ≤ 0.02 for all comparisons) (Bolla et al. 2005). However, the subsequent retrospective study involving central pathology review found that only surgical margin status was significantly associated with a benefit of adjuvant radiotherapy treatment (p < 0.01), and that the treatment benefit in patients with negative margins was not significant, irrespective of other risk factors (p = 0.6) (Van der Kwast et al. 2007). Among patients with positive surgical margins, a beneficial effect on biochemical recurrence was seen with adjuvant radiotherapy treatment in men with high Gleason score cancers and those with seminal vesicle invasion.
In the German ARO/AUO study, a prospective pathology review was performed on 85 % of RP specimen of 307 patients with undetectable PSA to investigate the influence of pathology review on the analysis. There was a fair concordance between pathology review and local pathologists for seminal vesicle invasion (91 %), surgical margin status (84 %), and for extraprostatic extension (75 %). Agreement was much less for Gleason score (47 %), whereby the review pathology resulted in a shift to Gleason score seven. In contrast to the analysis of progression-free survival with local pathology, the multivariate analysis including review pathology reveals positive surgical margins and Gleason score >6 as significant prognostic factors (Bottke et al. 2013b). The authors conclude, that phase 3 studies of postoperative treatment of prostate cancer should be accomplished in the future with a pathology review. In daily practice, a second opinion by a pathologist experienced in urogenital pathology would be desirable, in particular, for high-risk patients after RP.
This is why the PREFERE study has included pathology review as a mandatory step for study inclusion in the design of the nationwide German prostate cancer trial Evaluation of Four Treatment Modalities in Prostate Cancer with Low or “Early Intermediate” Risk (PREFERE), which has just opened (Wiegel et al. 2013a; Bottke et al. 2013a). PREFERE is a prospective randomized multicenter trial developed to compare the four possible treatment options currently recommended by the European guidelines (Heidenreich et al. 2011) for favorable risk prostate cancer (radical prostatectomy, external beam radiotherapy, permanent seed implantation, and active surveillance) (Wiegel et al. 2013a).
5 Optimal Radiation Dose
To date there is no established consensus regarding the optimal prescription dose for adjuvant radiotherapy. Petrovich et al. have indicated that even low doses in the range of 45–50 Gy are beneficial in terms of local control and disease-free survival (Petrovich et al. 1991, 2002). The findings of the three randomized studies have been obtained with a prescription dose of 60 Gy with conventional irradiation over 6 weeks.
Based on American Society of Therapeutic Radiation Oncology recommendations, a dose of 64 Gy or higher (with conventional fractionation) should be prescribed (Thompson et al. 2013). Valicenti and Gomella have demonstrated evidence of improved biochemical outcomes using higher radiation doses. Despite higher doses, in fact, treatment is generally well tolerated with minimal late severe toxicity (Valicenti and Gomella 2000).
6 Adjuvant RT of Pelvic Lymph Nodes?
The three randomized trials included only patients with cN0 or pN0-disease. The effect of adjuvant RT in node-positive prostate cancer has not yet been prospectively assessed. However, there are interesting retrospective data raising the question whether men with nodal involvement confirmed during prostatectomy could benefit from adjuvant RT. A recent retrospective study reported a significant positive impact of RT in combination with hormonal therapy in patients with nodal metastases treated with RP and pelvic lymph node dissection (Da Pozzo et al. 2009). However, this study was limited by a potential patient selection bias mainly due to its retrospective and unmatched design. In fact, patients treated with adjuvant RT were those affected by more aggressive disease. For this reason, no effect of adjuvant RT on cancer-specific survival was demonstrated on univariate survival analyses. There was significant gain in predictive accuracy when adjuvant RT was included in multivariable models predicting biochemical recurrence-free and cancer-specific survival (gain: 3.3 and 3 %, respectively; all p < 0.001).
In a huge retrospective series, Briganti et al. assessed the effect of adjuvant RT in node-positive prostate cancer including two homogeneous matched patient cohorts exposed to either adjuvant RT plus HT or adjuvant HT alone after surgery. In this series from Milan and Jacksonville a total of 703 patients were treated, with a median follow-up of 95 months. Patients were matched for age at surgery, pathologic T stage and Gleason score, number of nodes removed, surgical margin status, and length of follow-up. The overall survival advantage was 19 % in favor of adjuvant radiation therapy plus hormonal treatment compared with hormonal treatment alone. Similarly, higher survival rates associated with the combination of HT plus RT were found when patients were stratified according to the extent of nodal invasion (namely, ≤2 vs. >2 positive nodes; all p ≤0.006) (Briganti et al. 2011). Because of the retrospective nature of this series with no standardized definition of target volumes, radiation dose and duration of hormonal treatment, these results should be interpreted with caution. However, it provides support for this treatment in selected cases, whereas it should be validated in prospective clinical trials.
7 Additional Use of Hormone Therapy to ART
It is now clearly established that the standard nonoperative management for patients with locally advanced prostate adenocarcinoma includes long-term ADT. Two previous cooperative group trials have demonstrated an overall survival advantage for high-risk patients with an intact prostate treated with 2–3 years of ADT as compared to patients treated with short-term ADT (Bolla et al. 2009; Horwitz et al. 2008). It remains unknown if there is a benefit for the addition of adjuvant ADT for men with high-risk, node negative prostate adenocarcinoma initially treated with RP and pelvic lymph node dissection. The primary rationale for use of ADT post-RP is to (1) improve local control by eradicating disease in a hypoxic scar that may be radioresistant; (2) address micrometastatic disease which may have spread to the lymph nodes or distant sites; and (3) alter PSA kinetics in patients who will eventually relapse (Hanlon et al. 2004; Kaminski et al. 2003; Rossi et al. 2011).
Previous studies have indicated a potential benefit for men at high risk of recurrence treated with combination therapy. A secondary analysis of patients status-post an RP enrolled on Radiation Therapy Oncology Group (RTOG) 85-31 (Corn et al. 1999), a phase III trial comparing standard external beam RT plus immediate ADT versus RT alone for patients with nonbulky prostate cancer, found a biochemical control advantage for patients who received combination therapy as compared to men treated with RT alone. With a median follow-up of 5 years, the progression-free survival for men treated with combination therapy was estimated to be 65 % as compared to 42 % for men treated with RT alone (p = 0.002). Similar results were seen in a retrospective study performed at Stanford University (King et al. 2004). A subsequent RTOG study (P-0011) was designed to determine the benefit of combination therapy for man with unfavorable prognostic factors and an undetectable PSA treated with ART. This trial was unfortunately closed due to poor accrual (Elshaikh et al. 2011).
Recently, Abdollah et al. evaluated the long-term survival of prostate cancer patients who have experienced biochemical recurrence after RP and ART. Patients with a short time to biochemical recurrence, a Gleason score of ≥8 and ≥2 positive lymph nodes had lower survival rates than other patients (Abdollah et al. 2013).
In ongoing EORTC trial 22043, patients with Gleason score 5–10, undetectable PSA and pathological stage pT2R1 or pT3a-b will be randomized within 3 months after radical prostatectomy between postoperative irradiation alone or postoperative irradiation and short-term adjuvant androgen deprivation for 6 months. The primary trial endpoint is 5-year biochemical progression-free survival.
Another large randomized study is underway; RADICALS aims to recruit >4,000 patients and addresses both the comparison of ART versus SRT and the question of additional hormone treatment (using a gonadotropin-releasing hormone analog) and its appropriate timing after RP (Parker et al. 2007).
8 Side Effects and Toxicity
The three randomized clinical trials included prospective collection of data on gastrointestinal or genitourinary toxicity in the two cohorts (ART vs. observation). However, it should be mentioned that in the EORTC and SWOG trials radiation was based on 2D treatment planning which did not enable significant normal tissue sparing. In contrast, modern 3D-based radiation treatment techniques such as IMRT allow for minimization of dose to the rectum and bladder.
In the SWOG 8794 study, 3.3 % of postoperative irradiated patients developed grade 3 or higher adverse events such as rectal bleeding or proctitis as compared to 0 % of patients in the observation group (p = 0.002). The incidence of urethral strictures was significantly higher in the immediate postoperative RT group (17.8 vs. 9.5 %. RR 1.9, p = 0.02). Total urinary incontinence occurred in 6.5 % of men in the RT group as compared to 2.8 % of men in the observation group (RR 2.3, p = 0.11) (Thompson et al. 2006).
In the EORTC trial, there was no significant difference in high-grade (grade 3 or higher) toxicity between both arms, ART and observation. At 5 years, the cumulative incidence of late grade 3 events was 4.3 % versus 2.6 % (p = 0.0726). Though, in the ART cohort all late grade 2 and 3 toxicity events combined were more prominent (p = 0.0005). Unlike the SWOG trial, the EORTC trial did not assess total urinary incontinence, however in an interim analysis there was no significant difference concerning urinary incontinence between the two treatment arms (Bolla et al. 2005).
In the German study, which utilized 3-D-based radiation treatment planning, the incidence of late grade 3 or higher adverse events was only 0.3 % (Wiegel et al. 2009a). One patient developed a urethral stricture in the observation arm, compared to two patients in the ART arm. Urinary incontinence was not assessed in this trial.
In the EORTC study, 100 randomized patients were evaluated concerning the continence situation. There was no difference in the number of fully continent patients after 24 months between the group receiving 60 Gy and the group under observation (Van Cangh 1998).
It may be difficult to differentiate side effects of RT from pre-existing disabilities and sequelae of RP. At least equivalent rates of severe genitourinary complications following RP alone have been reported in a SEER data base analysis of 11,522 patients (Begg et al. 2002). Formenti et al. investigated the rate and degree of incontinence and erectile dysfunction after nerve-sparing RP with or without adjuvant RT. Unfortunately, follow-up examinations only comprised a questionnaire with inherent weaknesses. No difference was found between 72 patients who underwent both RP and RT and 138 patients who underwent RP only when total doses of 45–54 Gy were applied (Formenti et al. 1996).
9 Adjuvant Versus Salvage Radiation Therapy
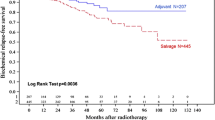
PubMed shows >250 entries between 2008 and 2012 for a search of adjuvant radiotherapy, radical prostatectomy and just under 200 entries for salvage radiotherapy. While prospective randomized trials are underway to compare ART and SRT, many retrospective/indirect analyses into that question have been conducted (Thompson et al. 2006; Bolla et al. 2005; Wiegel et al. 2009a, b; Stephenson et al. 2007; Neuhof et al. 2007; Trock et al. 2008; Loeb et al. 2008; Bernard et al. 2010; Siegmann et al. 2011; King and Kapp 2008). Some are nonrandomized retrospective series comparing ART and SRT or ART and surveillance with delayed treatment. A consistently higher improvement in local control and freedom from biochemical failure (FFBF) has been observed in adjuvant radiation therapy compared with salvage radiation therapy patients. The 5-yr FFBF rates are approximately 69–89 % after adjuvant radiation therapy. Local control is 96–100 % after adjuvant radiation therapy and 79–93 % after salvage radiation therapy (Bottke et al. 2007, 2012; Bartkowiak et al. 2013a, b). Recently, Trabulsi and colleagues studied a group of patients undergoing adjuvant radiation therapy with a matched control group undergoing salvage radiation therapy after biochemical failure. Using a multi-institutional database of 2,299 patients, 449 patients with pT3–4 N0 disease were eligible, including 211 patients receiving adjuvant radiation therapy and 238 patients receiving salvage radiation therapy. Adjuvant radiation therapy significantly reduced the risk of long-term biochemical progression after radical prostatectomy compared with salvage radiation therapy (5-yr FFBF was 73 % after adjuvant radiation therapy compared with 50 % after salvage radiation therapy; p = 0.007). Gleason score eight was a significant predictor of FFBF (Trabulsi et al. 2008). These results were confirmed by others (Budiharto et al. 2010), but Ost et al. reported a better outcome after salvage radiation therapy compared with adjuvant radiation therapy (Ost et al. 2011). For all of these reasons, the best choice for treatment (adjuvant radiation therapy vs. salvage radiation therapy) has to be discussed individually with each patient, taking into account the possible risk for overtreatment with immediate postoperative irradiation.
In 2007, a prospective randomized study was initiated to address this question as well as the potential role of concomitant androgen deprivation (Parker et al. 2007). The RADICALS (Radiotherapy and Androgen Deprivation in Combination After Local Surgery) trial is an effort to evaluate adjuvant versus salvage radiation therapy. Patients are randomized after surgery to early or delayed radiation. Delayed radiation will be given when there are either two consecutive PSA rises and a final PSA >0.1 ng/ml or three consecutive PSA rises. The planned accrual is 2,600 patients with cause-specific survival being the primary outcome. There is a second randomization regarding androgen deprivation therapy.
In the meantime, the American Society for Radiation Oncology (ASTRO) and the American Urological Association (AUA) has published “The Adjuvant and Salvage Radiotherapy After Prostatectomy: ASTRO/AUA Guideline,” a comprehensive review of 324 research articles of English-language publications within the Pubmed, Embase, and Cochrane databases, published from January 1, 1990 through December 15, 2012 (Thompson et al. 2013). According to this guideline, physicians should offer adjuvant radiotherapy to patients with adverse pathologic findings at prostatectomy (i.e., seminal vesicle invasion, positive surgical margins, extraprostatic extension) and should offer salvage radiotherapy to patients with PSA or local recurrence after RP in whom there is no evidence of distant metastatic disease. The decision to administer radiotherapy should be made by the patient and the multidisciplinary treatment team with full consideration of the patient’s history, values, preferences, quality of life, and functional status (Thompson et al. 2013).
10 Second Malignancies
One point that was not included in the above model is the risk of second malignancies. This is an issue of growing concern specifically with modern multiportal radiation techniques (Bartkowiak et al. 2012). Presumably, the risk is most prominent after first cancer therapy at a younger age. After prostate cancer treatment with definitive IMRT (n = 897) or brachytherapy (n = 413), no significantly increased rates of second cancer were observed within or out the treatment field compared with the general population extracted from the National Cancer Institute’s Surveillance, Epidemiology, and End Results dataset combined with the 2000 census data (Zelefsky et al. 2012). While the cohorts were small and follow-up was comparably short regarding the potentially long latency of radiation induced tumors, there was a positive trend toward early diagnosis, resulting from routine surveillance and increased awareness of patients after the first malignancy.
11 Conclusions
Treatment decisions after prostatectomy require risk assessment. Adjuvant radiotherapy (ART) provides improved biochemical relapse-free survival, and potentially, overall survival for patients at high-risk following prostatectomy compared to a wait-and-see policy. The long-term results of the completed randomized trials will identify subgroups of patients who profit from ART. For others, such as pN + with ≤2 involved nodes, new randomized trials are planned.
It remains unknown if early salvage radiation therapy (SRT) initiated after a PSA failure is equivalent to ART. At the present time, there are no published randomized trials to compare ART versus SRT. Until the ongoing trials hopefully settle this question ART should be regarded as an option at least in the case of positive surgical margins.
Modern radiation therapy techniques like intensity-modulated radiation therapy (IMRT) or arc radiation therapy and image-guided radiotherapy (IGRT) are going to become standards. The resulting reduction of toxicity may influence the decision about how and when to apply radiotherapy in post-RP prostate cancer patients.
References
Abdollah F, Boorjian S, Cozzarini C (2013) Survival following biochemical recurrence after radical prostatectomy and adjuvant radiotherapy in patients with prostate cancer: the impact of competing causes of mortality and patient stratification. Eur Urol 64:557–564
Allsbrook WC Jr, Mangold KA, Johnson MH et al (2001a) Interobserver reproducibility of Gleason grading of prostatic carcinoma: urologic pathologists. Hum Pathol 32:74–80
Allsbrook WC Jr, Mangold KA, Johnson MH et al (2001b) Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum Pathol 32:81–88
Bartkowiak D, Bottke D, Wiegel T (2013a) Adjuvant radiotherapy or early salvage radiotherapy in pT3R0 or pT3R1 prostate cancer. Curr Opin Urol 23:360–365
Bartkowiak D, Bottke D, Wiegel T (2013b) Radiotherapy in the management of prostate cancer after radical prostatectomy. Future Oncol 9:669–679
Bartkowiak D, Humble N, Suhr P et al (2012) Second cancer after radiotherapy, 1981–2007. Radiother Oncol 105:122–126
Begg CB, Riedel ER, Bach PB et al (2002) Variations in morbidity after radical prostatectomy. N Engl J Med 346:1138–1144
Bernard JR Jr, Buskirk SJ, Heckman MG et al (2010) Salvage radiotherapy for rising prostate-specific antigen levels after radical prostatectomy for prostate cancer: dose-response analysis. Int J Radiat Oncol Biol Phys 76:735–740
Bianco FJ Jr, Scardino PT, Eastham JA (2005) Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”). Urology 66:83–94
Bolla M, De Reijke TM, Van Tienhoven G et al (2009) Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 360:2516–2527
Bolla M, Van Poppel H, Collette L et al (2005) Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet 366:572–578
Bolla M, Van Poppel H, Tombal B et al (2012) Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380:2018–2027
Bottke D, Abrahamsson PA, Welte B et al (2007) Adjuvant radiotherapy after radical prostatectomy. Eur J Cancer Suppl 5:171–176
Bottke D, Bartkowiak D, Schrader M et al (2012) Radiotherapy after radical prostatectomy: immediate or early delayed? Strahlenther Onkol 188:1096–1101
Bottke D, Kristiansen G, Golz R et al (2013a) Central pathology review: a must for prostate cancer studies and an option for selected patients in daily practise. Eur Urol 64:202–203
Bottke D, Golz R, Störkel S et al (2013b) Phase 3 study of adjuvant radiotherapy versus wait and see in pT3 prostate cancer: Impact of pathology review on analysis. Eur Urol 64:193–198
Briganti A, Karnes RJ, Da Pozzo LF et al (2011) Combination of adjuvante hormonal and radiation therapy significantly prolongs survival of patients with pT2-4 pN + prostate cancer: results of a matched analysis. Eur Urol 59:832–840
Briganti A, Wiegel T, Joniau S et al (2012) Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol 62:472–487
Budaus L, Bolla M, Bossi A et al (2012) Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol 61:112–127
Budiharto T, Perneel C, Haustermans K et al (2010) A multi-institutional analysis comparing adjuvant and salvage radiation therapy for high-risk prostate cancer patients with undetectable PSA after prostatectomy. Radiother Oncol 97:474–479
Chikkaveeraiah BV, Mani V, Patel V et al (2011) Microfluidic electrochemical immunoarray for ultrasensitive detection of two cancer biomarker proteins in serum. Biosens Bioelectron 26:4477–4483
Chun FK, Graefen M, Zacharias M et al (2006) Anatomic radical retropubic prostatectomy-long-term recurrence-free survival rates for localized prostate cancer. World J Urol 24:273–280
Cooperberg MR, Carroll PR, Klotz L (2011) Active surveillance for prostate cancer: progress and promise. J Clin Oncol 29:3669–3676
Cooperberg MR, Pasta DJ, Elkin EP et al (2005) The University of California, San Francisco cancer of the prostate risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol 173:1938–1942
Corn BW, Winter K, Pilepich MV (1999) Does androgen suppression enhance the efficacy of postoperative irradiation? A secondary analysis of RTOG 85-31. Radiat Ther Oncol Group Urol 54:495–502
Da Pozzo LF, Cozzarini C, Briganti A et al (2009) Long-term follow-up of patients with prostate cancer and nodal metastases treated by pelvic lymphadenectomy and radical prostatectomy: the positive impact of adjuvant radiotherapy. Eur Urol 55:1003–1011
Daly T, Hickey BE, Lehman M et al (2011) Adjuvant radiotherapy following radical prostatectomy for prostate cancer. Cochrane Database Syst Rev 12:CD007234
Ekici S, Ayhan A, Erkan I et al (2003) The role of the pathologist in the evaluation of radical prostatectomy specimens. Scand J Urol Nephrol 37:387–391
Elshaikh MA, Ibrahim DR, Stricker H et al (2011) Adjuvant radiation treatment after prostatectomy. Where do we stand? Can J Urol 18:5592–5600
Evans AJ, Henry PC, Van der Kwast TH et al (2008) Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol 32:1503–1512
Formenti SC, Lieskovsky G, Simoneau AR et al (1996) Impact of moderate dose of postoperative radiation on urinary continence and potency in patients with prostate cancer treated with nerve sparing prostatectomy. J Urol 155:616–619
Freedland SJ, Humphreys EB, Mangold LA et al (2005) Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 294:433–439
Glaessgen A, Hamberg H, Pihl CG et al (2004a) Interobserver reproducibility of modified Gleason score in radical prostatectomy specimens. Virchows Arch 445:17–21
Glaessgen A, Hamberg H, Pihl CG et al (2004b) Interobserver reproducibility of percent Gleason grade 4/5 in prostate biopsies. J Urol 171:664–667
Gomez P, Manoharan M, Kim SS et al (2004) Radionuclide bone scintigraphy in patients with biochemical recurrence after radical prostatectomy: when is it indicated? BJU Int 94:299–302
Hanlon AL, Horwitz EM, Hanks GE et al (2004) Short-term androgen deprivation and PSA doubling time: their association and relationship to disease progression after radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 58:43–52
Heidenreich A, Bellmunt J, Bolla M et al (2011) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 59:61–71
Hoffman KE, Nguyen PL, Chen MH et al (2011) Recommendations for post-prostatectomy radiation therapy in the United States before and after the presentation of randomized trials. J Urol 185:116–120
Horwitz EM, Bae K, Hanks GE et al (2008) Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26:2497–2504
Kaminski JM, Hanlon AL, Joon DL et al (2003) Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. Int J Radiat Oncol Biol Phys 57:24–28
Kawamorita N, Saito S, Ishidoya S et al (2009) Radical prostatectomy for high-risk prostate cancer: biochemical outcome. Int J Urol 16:733–738
King CR (2012) Adjuvant versus salvage radiotherapy after prostatectomy: the apple versus the orange. Int J Radiat Oncol Biol Phys 82:1045–1046
King CR, Kapp DS (2008) Radiotherapy after prostatectomy: is the evidence for dose escalation out there? Int J Radiat Oncol Biol Phys 71:346–350
King CR, Presti JC Jr, Gill H et al (2004) Radiotherapy after radical prostatectomy: does transient androgen suppression improve outcomes? Int J Radiat Oncol Biol Phys 59:341–347
Kyrdalen AE, Dahl AA, Hernes E et al (2013) A national study of adverse effects and global quality of life among candidates for curative treatment for prostate cancer. BJU Int 111:221–232
Kuroiwa K, Shiraishi T, Ogawa O et al (2010) Discrepancy between local and central pathological review of radical prostatectomy specimens. J Urol 183:952–957
Lawton CA, Winter K, Murray K et al (2001) Updated results of the phase III radiation therapy oncology group (RTOG) trial 85-31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int J Radiat Oncol Biol Phys 49:937–946
Loeb S, Roehl KA, Viprakasit DP et al (2008) Long-term rates of undetectable PSA with initial observation and delayed salvage radiotherapy after radical prostatectomy. Eur Urol 54:88–94
Lotan Y, Roehrborn CG (2002) Clearance rates of total prostate specific antigen (PSA) after radical prostatectomy in African-Americans and Caucasians. Prostate Cancer Prostatic Dis 5:111–144
McVey GP, McPhail S, Fowler S et al (2010) Initial management of low-risk localized prostate cancer in the UK: analysis of the British association of urological surgeons cancer registry. BJU Int 106:1161–1164
Michalski JM, Lawton C, El Naqa I et al (2010) Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 76:361–368
Mottet N, Bellmunt J, Bolla M et al (2011) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 59:572–583
Netto GJ, Eisenberger M, Epstein JI (2011) Interobserver variability in histologic evaluation of radical prostatectomy between central and local pathologists: findings of TAX 3501 multinational clinical trial. Urology 77:1155–1157
Neuhof D, Hentschel T, Bischof M et al (2007) Long-term results and predictive factors of three-dimensional conformal salvage radiotherapy for biochemical relapse after prostatectomy. Int J Radiat Oncol Biol Phys 67:1411–1417
Ost P, De Troyer B, Fonteyne V et al (2011) A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 80:1316–1322
Oyama T, Allsbrook WC Jr, Kurokawa K et al (2005) A comparison of interobserver reproducibility of Gleason grading of prostatic carcinoma in Japan and the United States. Arch Pathol Lab Med 129:1004–1010
Parker C, Sydes MR, Catton C et al (2007) Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new medical research council/national cancer institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int 99:1376–1379
Petrovich Z, Lieskovsky G, Langholz B (2002) Postoperative radiotherapy in 423 patients with pT3N0 prostate cancer. Int J Radiat Oncol Biol Phys 53:600–609
Petrovich Z, Lieskovsky G, Langholz B (1991) Radiotherapy following radical prostatectomy in patients with adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 21:949–954
Pfitzenmaier J, Pahernik S, Tremmel T et al (2008) Positive surgical margins after radical prostatectomy: do they have an impact on biochemical or clinical progression? BJU Int 102:1413–1418
Pinto F, Prayer-Galetti T, Gardiman M et al (2006) Clinical and pathological characteristics of patients presenting with biochemical progression after radical retropubic prostatectomy for pathologically organ-confined prostate cancer. Urol Int 76:202–208
Poortmans P, Bossi A, Vandeputte K et al (2007) Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC radiation oncology group. Radiother Oncol 84:121–127
Richaud P, Sargos P, Henriques de Figueiredo B et al (2010) Postoperative radiotherapy of prostate cancer. Cancer Radiother 14:500–503
Rossi CJ Jr, Joe Hsu IC, Abdel-Wahab M et al (2011) ACR appropriateness criteria postradical prostatectomy irradiation in prostate cancer. Am J Clin Oncol 34:92–98
Salomon L, Anastasiadis AG, Antiphon P et al (2003) Prognostic consequences of the location of positive surgical margins in organ-confined prostate cancer. Urol Int 70:291–296
Shekarriz B, Upadhyay J, Wood DP Jr et al (1999) Vesicourethral anastomosis biopsy after radical prostatectomy: predictive value of prostate-specific antigen and pathologic stage. Urology 54:1044–1048
Showalter TN, Ohri N, Teti KG et al (2012) Physician beliefs and practices for adjuvant and salvage radiation therapy after prostatectomy. Int J Radiat Oncol Biol Phys 82:e233–e238
Siegmann A, Bottke D, Faehndrich J et al (2011) Dose escalation for patients with decreasing PSA during radiotherapy for elevated PSA after radical prostatectomy improves biochemical progression-free survival: results of a retrospective study. Strahlenther Onkol 187:467–472
Stephenson AJ, Bolla M, Briganti A et al (2012) Postoperative radiation therapy for pathologically advanced prostate cancer after radical prostatectomy. Eur Urol 61:443–451
Stephenson AJ, Kattan MW, Eastham JA et al (2006) Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol 24:3973–3978
Stephenson AJ, Scardino PT, Kattan MW et al (2007) Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 25:2035–2041
Stephenson AJ, Wood DP, Kattan MW et al (2009) Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol 182:1357–1363
Swanson GP, Hussey MA, Tangen CM et al (2007) Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol 25:2225–2229
Swindle P, Eastham JA, Ohori M et al (2005) Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol 174:903–907
Thompson IM Jr, Tangen CM, Paradelo J et al (2006) Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 296:2329–2335
Thompson IM, Tangen CM, Paradelo J et al (2009) Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181:956–962
Thompson IM, Valicenti RK, Albertsen P et al (2013) Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol 190:441–449
Trabulsi EJ, Valicenti RK, Hanlon AL et al (2008) A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology 72:1298–1302; discussion 1302–1294
Triroj N, Jaroenapibal P, Shi H et al (2011) Microfluidic chip-based nanoelectrode array as miniaturized biochemical sensing platform for prostate-specific antigen detection. Biosens Bioelectron 26:2927–2933
Trock BJ, Han M, Freedland SJ et al (2008) Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299:2760–2769
Tyldesley S, Peacock M, Morris JW et al (2012) The need for, and utilization of prostate-bed radiotherapy after radical prostatectomy for patients with prostate cancer in British Columbia. Can Urol Assoc J 6:89–94
Valicenti RK, Gomella LG (2000) Durable efficacy of adjuvant radiation therapy for prostate cancer: will the benefit last? Semin Urol Oncol 18:115–120
Van Cangh PJ, Richard F, Lorge F et al (1998) Adjuvant radiation therapy does not cause urinary incontinence after radical prostatectomy: results of a prospective randomized study. J Urol 159:164–166
Van der Kwast TH, Collette L, Van Poppel H et al (2006) Impact of pathology review of stage and margin status of radical prostatectomy specimens (EORTC trial 22911). Virchows Arch 449:428–434
Van der Kwast TH, Bolla M, van Poppel H et al (2007) Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol 25:4178–4186
Wenz F, Martin T, Böhmer D et al (2010) The German S3 guideline prostate cancer: aspects for the radiation oncologist. Strahlenther Onkol 186:531–534
Wiegel T, Albers P, Bussar-Maatz R et al (2013a) PREFERE—the German prostatic cancer study: questions and claims surrounding study initiation in January 2013. Urol A 52:576–579
Wiegel T, Bottke D, Bartkowiak D et al (2013b) Phase III results of adjuvant radiotherapy versus wait-and-see in patients with pT3 prostate cancer following radical prostatectomy (ARO96-02/AUO AP 9/95). In: Genitourinary Cancers Symposium, Abstract 4. Presented, 14 Feb 2013
Wiegel T, Bottke D, Steiner U et al (2009a) Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol 27:2924–2930
Wiegel T, Lohm G, Bottke D et al (2009b) Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome–results of a retrospective study. Int J Radiat Oncol Biol Phys 73:1009–1016
Wiltshire KL, Brock KK, Haider MA et al (2007) Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys 69:1090–1099
Zelefsky MJ, Housman DM, Pei X et al (2012) Incidence of secondary cancer development after high-dose intensity-modulated radiotherapy and image-guided brachytherapy for the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys 83:953–959
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bottke, D., Wiegel, T. (2014). Randomized Trials for Adjuvant Radiotherapy. In: Geinitz, H., Roach III, M., van As, N. (eds) Radiotherapy in Prostate Cancer. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/174_2013_948
Download citation
DOI: https://doi.org/10.1007/174_2013_948
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37098-4
Online ISBN: 978-3-642-37099-1
eBook Packages: MedicineMedicine (R0)