Abstract
Radical prostatectomy (RP) is now the most common definitive treatment for high-risk prostate cancer. Unfortunately, many men will have residual microscopic disease after surgery alone. Despite level 1 evidence supporting the use of adjuvant radiation therapy (ART), <10% of men with adverse pathology (positive margins or T3 disease) receive ART in the USA. Early salvage radiation therapy (eSRT) at the time of biochemical recurrence has been proposed as an alternative strategy despite the lack of published randomized trials to support this approach. Multiple randomized trials are ongoing or recently completed to compare ART to eSRT, but given the long natural history of prostate cancer, long-term oncologic outcomes from these trials will not be reported for several years. In this review, we discuss the shifting trends in the diagnosis of high-risk prostate cancer given a decline in PSA screening, use of RP for high-risk disease, and compare and contrast the retrospective and randomized evidence regarding ART and SRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2013, the American Urological Association (AUA) and American Society of Radiation Oncology (ASTRO) issued a joint recommendation regarding a clinical framework for the use of radiotherapy (RT) after radical prostatectomy (RP). The panel stated, “Physicians should offer adjuvant radiotherapy [ART] to patients with adverse pathologic findings at prostatectomy including seminal vesicle invasion, positive surgical margins, or extraprostatic extension because of demonstrated reductions in biochemical recurrence, local recurrence, and clinical progression” (Grade A recommendation) [1, 2]. Additionally, for men with a rising prostate specific antigen (PSA) or local recurrence after prostatectomy, it was recommended that physicians should offer salvage RT (SRT). The recommendations to offer ART or SRT were to be “in the context of a thoughtful discussion of possible short- and long-term side effects of radiotherapy as well as the potential benefits of preventing recurrence”. Additionally, AUA/ASTRO, along with American Society of Clinical Oncology (ASCO) [3] and European Association of Urology (EUA) [4], state that the benefits of SRT are greatest when administered at lower PSA levels.
Despite these recommendations, current practice patterns suggest that less than 10% of patients with adverse pathology receive ART [5,6,7]. Furthermore, and perhaps more concerning, for men who reach an undetectable PSA post-operatively (≤0.1) and then develop a rising PSA (≥0.1), studies suggest only approximately 30% receive SRT [7].
With decreased PSA screening and current trends of increased radical prostatectomy use in high-risk patients, the number of physicians and patients facing the decision between ART and early SRT will all but certainly grow. In this review, we discuss the available evidence for both adjuvant and salvage RT. Current ongoing randomized trials and potential future directions are highlighted. We conclude with our interpretation of the current most prudent strategy for the use of post-operative RT.
Radical Prostatectomy Trends: a Growing Need for Post-Operative Radiotherapy
A confluence of several factors has increased the percentage of patients with high-risk pathologic features following RP, thus expanding the eligible population for post-operative RT. First, in 2012, the United States Preventive Services Task Force (USPTSF) discouraged the use of PSA screening for prostate cancer [8], which has been effective in reducing the PSA screening rate in the USA [9]. As PSA screening rates have declined, recent data suggest that patients may be increasingly diagnosed with higher-risk disease [10].
In addition to the decline of PSA screening, practice patterns are changing. Historically, RP was primarily used in low-risk localized prostate cancer patients and RT was used in high-risk patients given the knowledge of the high failure rate after RP in patients with T3 disease. RP has become the most common definitive treatment modality for localized prostate cancer, and use of RP for those with high-risk disease has dramatically increased. In fact, recent data suggests that RP has now surpassed definitive RT as the most common modality for high-risk prostate cancer [11]. The driving force behind this pattern of care shift towards RP for high-risk disease is unclear. It is possible that recent advances in multiparametric magnetic resonance imaging, with its improved delineation of the prostatic and periprostatic tissues, have increased surgeons’ willingness and ability to pursue surgical resection [12]. Still others have suggested market forces may be, at least in part, at work given the association of higher socioeconomic status with receipt of RP [13], along with the increased dissemination of robotic surgical platforms [14].
Nevertheless, based on current estimates, approximately 30,000 men per year in the USA will have preoperative high-risk disease, of which 50% will undergo RP. There will also be thousands more with intermediate-risk disease that will get upstaged at the time of RP to have adverse pathologic features (Fig. 1). Unfortunately, based on nomograms from centers of excellence, such as Memorial Sloan Kettering, a large percentage of men (40–90%) with high-risk features, such as Gleason 8–10, high pretreatment PSA, T3 disease, or positive margins will have biochemical recurrence (BCR) following RP [17]. Even modern RP outcomes at specialized, high volume centers demonstrate significant rates of BCR in high-risk men, with some series exceeding 50% at 5 years [18, 19]. Therefore, we estimate that there will be approximately 15,000 to 25,000 men per year who develop recurrent disease following RP, which would make this entity about as common as pancreatic cancer, rectal cancer, esophageal cancer, multiple myeloma, or all brain tumors in men combined. Given the burden of this problem, it therefore comes as no surprise that several large trials have investigated the role of ART in those with adverse pathologic features following RP.
Estimated cases of post-prostatectomy recurrences each year in the United States. Asterisk is the conservative estimated as it excludes low-risk patients from analysis assuming low-risk patients will not undergo immediate treatment. RP radical prostatectomy, RT radiotherapy, ADT androgen deprivation therapy. Estimates are based on the following: [5,10,11,13,15,16].
Adjuvant Radiation: the Level 1 Evidence
Three large, phase 3 randomized trials have been performed to test the hypothesis of whether or not adjuvant radiation offers an oncologic benefit in men with T3 disease or positive margins following radical prostatectomy: Southwest Oncology Group (SWOG) 8794, European Organization for Research and Treatment of Cancer (EORTC) 22,911, and the German Cancer Society ARO 96–02 (Table 1). With long-term follow-up, each trial met its primary endpoint and consistently demonstrated a reduction in the risk of BCR by approximately 50% with the use of adjuvant RT compared to observation [20–22]. Moreover, locoregional control was significantly improved in both the SWOG 8794 and EORTC 22911 trials.
The results of the two largest trials (SWOG and EORTC) are different with respect to the impact of ART on distant metastasis (DM). In the SWOG 8794 trial, ART was associated with an improvement in DM-free survival (hazard ratio (HR): 0.7, p = 0.02) and decreased the crude rate of distant metastasis (18 vs 9%). In contrast, EORTC 22911 reported no statistical difference in distant metastasis between the ART and observation (11.0 vs 11.3%, p = 0.94), and a lower overall rate of DM in the control arm than seen in SWOG 8794. Important differences should be noted between these trials. The proportion of patients with individual indications for ART (positive margins, extracapsular extension, or seminal vesicle invasion) was similar between the SWOG and EORTC trials; however, the SWOG trial had a greater proportion of men with all three factors (22 vs 12%). Furthermore, compared to the SWOG trial, patients on the EORTC study were followed more closely (every 6 months vs annually in years 4–5) and imaged more regularly (chest radiography and bone scans annually vs for symptoms only). While salvage RT was administered to men at similar rates in the observation arms of both trials (33%), it is possible that earlier, more effective salvage treatment was offered in the EORTC observation arm, and that the combination of a higher baseline risk population and infrequent monitoring led to an increased rate of metastatic disease in the SWOG observation arm. Of note, both SWOG 8794 and EORTC 22911 enrolled a substantial minority of patients with a detectable PSA (>0.2 ng/mL) prior to ART, 33 and 30%, respectively. This contrasts with the ARO 96–02 study which uniquely enrolled only men with an undetectable PSA (<0.1 ng/mL). Given its trial size (n = 315), however, the ARO study was not powered to detect a difference in distant metastasis.
Given the long natural history of prostate cancer, one must not only focus on cure but also on quality of life. Valid concerns exist regarding toxicity and the potential negative impact on quality of life when delivering multi-modality care, as ideally patients would be cured from their initial treatment. The EORTC 22911 trial recorded physician-reported outcomes and noted a small, but near-statistically significant increase in the 10-year cumulative incidence of grade 3 late toxicity with adjuvant RT compared to observation (5.3 vs 2.5%; p = 0.052). In contrast to physician reported outcomes, the SWOG 8794 investigators uniquely collected patient-reported outcomes, an important measure of quality of life [23] pertaining to urinary, bowel, sexual, and global domains for 5-year post-randomization. Moinpour et al. reported that early declines in urinary frequency as well as tenderness and urgency with bowel movements were significantly increased in the first 2 years following ART compared to observation [24]. Urinary frequency remained impaired over 5 years, but bowel function recovered by year 2 post-ART. Sexual function did not differ between ART and observation groups. Perhaps most strikingly, however, global health-related quality of life was significantly improved with ART compared to observation beginning after year 2 post-ART. This coincides with both the recovery of side effects from ART and the decreased likelihood of PSA recurrence, hormonal therapy, and distant metastasis in the ART arm.
A key take-home message is the fact that all three ART trials met their primary endpoint, and ART decreases BCR after RP for men with adverse pathology by approximately 50% compared to observation. There is potential that ART may also reduce DM based on the SWOG trial results. Additionally, ART causes a small absolute increase in toxicity in the first few years after treatment compared to observation, though long-term quality of life is minimally impacted, and if anything may be improved with ART given the reduction in recurrence. Based on this favorable level 1 evidence, one might conclude that an increased number of patients would receive ART. This has not occurred. Over the time span of these trials being conducted, followed, and reported, ART utilization in high-risk patients remained low and, if anything, declined: 9% in 2005 to 7% in 2011 [5]. Even young (<60) men with Gleason 8–10 disease with extraprostatic disease and a positive margin with no comorbidities still only received ART 33% of the time [5].
Given that a minority of patients with high-risk disease will have their disease eradicated with RP alone, and very few will be offered or receive ART, the importance of SRT becomes critical. SRT spares treatment in the patients with high-risk disease who remain disease-free following RP, and may be an alternative to ART if recurrences are local within the prostate bed where SRT is targeted.
The Wait-and-See Approach: Salvage Radiation as an Alternative Treatment Option
PSA is a sensitive marker for prostate cancer [25]. Natural history studies suggest that PSA recurrence following RP precedes DM often by greater than 5 years [26]. Upon PSA recurrence, older studies suggest that over 40% of blind biopsies will demonstrate evidence of local disease [27, 28]. Similarly, pelvic MRI imaging can detect evidence of local disease at time of PSA recurrence in up to 25% of patients [29, 30]. Moreover, evidence from the SWOG 8794 observation arm suggests that the most common pattern of failure following RP is local-only disease [31]. Empiric data suggest that upwards of 80% of men will have an initial response to SRT and many have long-term durable biochemical control [32], a finding not surprising based on patterns of failure data. Furthermore, retrospective studies suggest that SRT reduces prostate cancer-specific mortality compared with other approaches [33, 34]. It is important to note, there has never been, nor will there likely ever be, a dedicated randomized trial comparing SRT to observation following RP.
Nomograms have been developed that demonstrate those men most likely to have durable, long-term biochemical control following SRT [35]. A contemporary update of the widely used “Stephenson nomogram” was recently published, now including nearly 2500 patients treated with SRT [36•]. One of the most prognostic factors of outcome after SRT that emerged from this study, and others, is the importance of the pre-SRT PSA. A growing body of literature has now found that early (PSA ≤ 0.5 ng/mL) or even “very early” (≤0.2 ng/mL) SRT not only has more favorable rates of biochemical control but also distant metastasis [37, 38]. In fact, those who receive SRT at a preRT PSA ≥ 0.5 can have upwards of >4 times the risk of distant metastasis compared to those with low preRT PSAs of ≤0.2 ng/mL.
As with ART, data suggests that SRT has been historically underutilized [39, 40]. This continues despite consensus recommendations supporting SRT [1, 2, 4]. In a statewide consortium of Michigan urologists between 2012 and 2014, only 30% of men received SRT for a post-prostatectomy PSA recurrence (≥0.1) [7]. And of those receiving SRT, over 40% had progressed to a PSA ≥ 0.5 and 20% progressed to a PSA ≥ 1.0 before SRT initiation. Several factors may contribute to this low rate of SRT. Retrospective studies suggest urinary incontinence can continue to improve for up to 2 years post-RP and there is concern that radiation may adversely impact post-operative healing [41]. Recent studies have examined the impact of SRT and its timing on health-related quality of life [42]. Similar to ART data, patients undergoing SRT have declines in bowel quality of life compared to those treated with RP alone without a lasting difference beyond 2 years. Moreover, patients with an interval greater than 7 months between RP and SRT had significantly improved sexual satisfaction and urinary function (p < 0.05), further encouraging a wait-and-see approach. Long-term overall quality of life were unchanged with SRT, again consistent with the ART experience. These transient quality of life concerns have merit. Unfortunately, the aforementioned data suggests a wait-and-see approach often equates to a wait-too-long approach.
Salvage Radiotherapy and Hormone Therapy
One of the primary concerns regarding the adoption of ART is the concern of overtreatment and potential side effects. However, the discussion of use of ART versus SRT may no longer be simply comparing timing of radiotherapy, as SRT is going to be increasingly accompanied by potentially years of hormone therapy given the recent reports of two phase III trials comparing SRT to SRT plus hormone therapy (Table 1).
The first trial reported was the Groupe d’Etude des Tumeurs Uro-Génitales (GETUG)-AFU-16 trial, which demonstrated that the addition of 6 months of goserelin (androgen deprivation therapy) improved biochemical control but did not significantly lower distant metastasis rates or improve overall survival [43••]. The men treated on this trial were relatively favorable with 75% of patients having a pre-SRT PSA of ≤0.5 ng/mL and none were allowed to have persistently positive PSAs. The second trial, Radiation Therapy Oncology Group (RTOG) 9601, recently reported their long-term results [44••]. With a median follow-up of 13 years, the addition of 2 years of the nonsteroidal antiandrogen, bicalutamide, improved biochemical control, distant metastasis, prostate cancer-specific survival, and overall survival. This trial included men with markedly more aggressive disease than those in GETUG-16. RTOG 9601 should be viewed as a trial of men with high-risk features, as approximately 70% had T3 disease, 75% had positive margins, men had to have at least a pre-SRT PSA of ≥0.5 ng/mL to even enroll during the first one third of the trial enrollment period, approximately 50% had pre-SRT PSAs of 0.7–4.0 ng/mL, and a significant number had persistently positive PSAs. Notably, for men with low pre-SRT PSAs <0.7 ng/mL (a post-hoc hypothesis-generating cutpoint), low Gleason scores, or negative margins, there was minimal to no benefit from the addition of hormone therapy, and there was a concerning signal for worse overall survival in men with low pre-SRT PSAs likely given the side effects of long-term hormone therapy, including worsening cardiac disease [45].
The interpretation of these recent trial results is yet to be fully established, but most agree that for men with higher pre-SRT PSAs ≥0.7 ng/mL that the addition of 6–24 months of hormone therapy should be the standard of care, and that for men with more favorable features and lower pre-SRT, an informed discussion should be had about the risks and benefits of adding hormone therapy given that these men did not appear to derive an improvement in distant metastasis or overall survival. So when comparing ART to SRT, oncologists and patients alike must appreciate that SRT is now frequently going to be given with hormone therapy, which has a multitude of side effects, especially considering the poor oncologic outcomes with “late” SRT.
Data Comparing ART to SRT
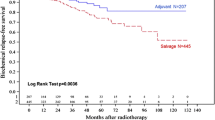
It is important to emphasize that there are no reported randomized comparisons of ART to SRT, or observation to SRT. Many retrospective comparisons have been performed [46,46,47,48,49,50,51,52,54]. Table 2 highlights select retrospective comparisons of ART and SRT. The studies selected for this table included studies with over 100 patients per group, an attempt to reasonably match ART and SRT cohorts, and at least 5 years of follow-up. Buscariollo et al. reported after performing propensity matching that ART was associated with a 6% absolute improvement in freedom from distant metastasis compared to “early” SRT as defined by receipt at a rising post-RP PSA level of ≤0.5 [48]. In contrast, Fossati et al. reported no significant difference in rates of distant metastasis when SRT was administered at post-operative PSA levels ≤0.5 when compared to ART [50]. Both of these studies demonstrated similar overall survival between ART and SRT, but none had a median follow-up greater than 10 years.
Comparisons such as those from Fossati et al. are reassuring, but limited in their practice changing utility given the inherent bias of retrospective comparisons. Fortunately, three randomized trials are ongoing or recently completed: Radiotherapy and Androgen Deprivation In Combination After Local Surgery (RADICALS; NCT00541047), Radiotherapy Adjuvant Versus Early Salvage (RAVES; NCT00860652), and GETUG-17 (NCT00667069) (Table 2) [55,55,57]. A fourth, EORTC 22043, was terminated early due to poor accrual [58]. The RADICALS-RT trial is comparing ART with SRT, with the salvage trigger defined as two consecutive PSA > 0.1 or three consecutive rises. The primary endpoint is freedom from metastasis. RADICALS-HD is also separately testing the benefit of hormonal therapy by randomizing those receiving ART or SRT to 0, 6, or 24 months of hormone therapy. The primary endpoint of RADICALS-HD is cancer-specific survival. The RAVES trial is comparing ART versus SRT, and SRT is to be delivered within 4 months of a PSA ≥ 0.2. GETUG-17 is testing the non-inferiority of SRT versus ART with the SRT trigger PSA >0.2. Both ART and SRT are delivered with 6 months of hormonal therapy in GETUG-17.
The results of the above trials comparing ART to early SRT will help establish the new standard of care, but will near certainly require long-term follow-up to provide meaningful results. RTOG 9601 did not demonstrate an improvement in overall survival until the second decade of follow-up. Furthermore, even in RTOG 9601 relatively high-risk SRT population, there was still only a 5% prostate cancer-specific mortality 12-year post-treatment after SRT and hormone therapy, and clinically meaningful improvements will be challenging to demonstrate with such a low event rate. Therefore, it is highly likely that even if biochemical recurrence is improved with ART, it is less likely that distant metastasis and survival will be impacted without long-term follow-up.
Given the poor adoption of ART after three positive phase III trials and guidelines that support its use, it will be of interest of whether results from these trials comparing ART to eSRT will impact practice patterns. Clearly, future directions must focus on how to best personalize, optimize, and increase the utilization of ART and SRT for appropriate men.
Future Directions
Patient Selection: a Risk Adapted Approach
Not all men are at equal risk to develop clinically meaningful recurrence following RP. For example, post-hoc analysis after central pathologic review on the EORTC 22911 trial suggests that men with positive margins benefit most from ART [59]. In addition, pathologic specimens are heterogeneous. Men with higher Gleason scores at the margin, as well as those with longer linear lengths of margin positivity, are at higher risk of biochemical recurrence [60]. Moreover, investigators have demonstrated that pretreatment risk status informs post-operative biochemical recurrence risk [61] and others have noted that the number of pathologic risk factors increases risk of recurrence [62]. We suggest discussing the risks and benefits of ART with all men with T3 disease or positive margins as endorsed by AUA, ASTRO, and ASCO. Furthermore, we strongly encourage ART in men who have a Memorial Sloan Kettering Cancer Center or CAPRA-S nomogram predicted likelihood of progression after RP within 5 years of >50%, as the need for salvage therapy is very high and will likely be accompanied by additional therapies (e.g., hormone therapy or even docetaxel, as this chemotherapy is being tested in this setting).
Early Detection, Early Treatment: Use of Very Early Salvage Radiotherapy
The ART rate is <10% for men with adverse pathology, far from universal adoption. As we await the outcomes of the aforementioned ART versus SRT trials, the Urologic and Radiation Oncology communities should consider the emerging data supporting the advantages of very early SRT (pre-SRT PSA ≤ 0.2). Men with adverse pathology who do not receive ART should be followed closely (every 3 months) and counseled that the risk of distant metastasis increases substantially as their post-operative PSA rises [37]. Those who receive SRT at a preRT PSA ≥ 0.5 can be upwards of >4× risk of distant metastasis compared to those ≤0.2 [38]. Tests such as ultrasensitive PSA may lead to further improvements in early detection of micrometastatic local recurrence and improve outcomes of SRT for patients with high-risk disease features [63, 64].
Improved Radiotherapy Targeting: Novel Molecular Imaging
Patient selection for SRT may be improved with novel molecular imaging. Such tests can help identify patients with local-only recurrences, locoregional recurrences (and thus warranting pelvic RT and or hormonal therapy), or metastatic recurrences. A 2016 meta-analysis of 68Ga-prostate-specific membrane antigen PET-CT demonstrated that the test can identify lesions in >50% of patients with biochemical recurrence and PSA levels of 0.2–1.0 [65]. Moreover, investigators from Emory are recruiting patients to test the role of FACBC (anti-1-amino-3-[18F] fluorocyclobutane-1-carboxylic acid (anti-3-[18F]), a synthetic amino acid analog, to guide post-prostatectomy decision and targeting (NCT01666808).
Biomarker Selection
Numerous genomic classifiers have been developed to provide patients and clinicians an improved understanding of a patients personalized risk of harboring aggressive prostate cancer and/or cancer that has a predilection to metastasize or result in death [66]. Recently, Spratt et al. reported the first meta-analysis of individual patient level and genomic level data on the genomic classifier Decipher [67]. Decipher independently predicts a patient’s risk of distant metastases within essentially every demographic, clinicopathologic, and treatment subgroups. Decipher is utilized to stratify patients in the ongoing NRG GU-002 trial (“Phase II-III Trial of Adjuvant Radiotherapy and Androgen Deprivation Following Radical Prostatectomy With or Without Adjuvant Docetaxel”). Decipher is also being used in an ongoing, prospective randomized controlled trial, G-MINOR (Genomics in Michigan Impacting Observation or Radiation; NCT02783950). This trial randomizes patients with pT3 disease or positive surgical margins after prostatectomy to Decipher or control (CAPRA-S only) arms and is evaluating the impact of Decipher on utilization of ART and long-term oncologic outcomes in this setting.
Beyond the ability to prognosticate patients by risk of recurrence, genomic biomarkers have the potential to have predictive capacity to determine which patients specifically benefit from a treatment. The group from the University of Michigan recently developed a predictive 24-gene gene expression signature to determine who benefits most from post-operative radiation therapy, termed “PORTOS” [68]. Additionally, Feng et al. recently presented the results of applying a well-studied breast biomarker, PAM50, to prostatectomy samples [69]. Not only did they find that PAM50 could identify luminal and basal subtypes but they also discovered that patients with luminal B cancers had improved response to post-operative hormone therapy compared to luminal A or basal subtypes. These exciting findings have sparked the first ever predictive biomarker stratified trial in radiation oncology for men with prostate cancer. This trial, NRG GU-006, is a randomized phase II trial comparing men receiving SRT with or without the second generation antiandrogen, apalutamide. Patients will be stratified by PAM50 molecular subtype.
Conclusion
Level 1 evidence supports the routine consideration of ART in men at high risk of local recurrence post-prostatectomy. Results from ongoing randomized trials will help to determine the role of early SRT compared to ART. Currently, data suggest both ART and SRT are underutilized, and when SRT is delivered, it is often delayed. The use of hormone therapy with SRT is evolving, and it is unclear if patients treated with early SRT, or a <10-year life expectancy, will derive a clinically meaningful benefit of hormone therapy. Ongoing and future clinical trials (NRG GU-006) will aim to better address this question. Looking to the future, imaging and genomic biomarkers will likely not only help in patient selection of post-operative radiotherapy but also to improve radiotherapy targeting. Given the history of poor adoption of level 1 evidence for patients receiving RP [19, 70,70,72], the largest challenge will be implementation of the results from the many ongoing and recently reported clinical trials in the post-operative space.
As we await the aforementioned randomized control trial results, it is our practice to recommend a multi-disciplinary discussion of the benefits and harms of ART for all men with T3 disease and/or a positive margin. Furthermore, Gleason grade can also impact a man’s risk of recurrence, and we advocate utilizing publically available nomograms (e.g., Memorial Sloan Kettering) to personalize our recommendations of ART versus early SRT. Generally, men with T3 disease with a gross positive margin (not simply a focal positive margin) we recommend a strong consideration of ART, especially if they have a life expectancy over 10 years. Additionally, men who have a >50% risk of recurrence within 5 years of surgery we recommend ART over early SRT, as treatment will likely be more intensive with SRT (higher radiotherapy dose and often with hormonal therapy). Many of the authors also utilize genomic classifiers, such as Decipher, to further help personalize the use of ART, and recent nomograms have incorporated genomic and clinicopathologic risk to aid clinicians when genomic findings are discrepant with clinicopathologic findings [73].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, Hahn C, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol. 2013;190(2):441–9.
Valicenti RK, Thompson I, Albertsen P, Davis BJ, Goldenberg SL, Wolf JS, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. International Journal of Radiation Oncology Biology Physics. 2013;86(5):822–8.
Freedland SJ, Rumble RB, Finelli A, Chen RC, Slovin S, Stein MN, et al. Adjuvant and salvage radiotherapy after prostatectomy: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2014;32(34):3892–8.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent—update 2013. Eur Urol. 2014;65(1):124–37.
Sineshaw HM, Gray PJ, Efstathiou JA, Jemal A. Declining use of radiotherapy for adverse features after radical prostatectomy: results from the National Cancer Data Base. Eur Urol. 2015;68(5):768–74.
Kalbasi A, Swisher-McClure S, Mitra N, Sunderland R, Smaldone MC, Uzzo RG, et al. Low rates of adjuvant radiation in patients with nonmetastatic prostate cancer with high-risk pathologic features. Cancer. 2014;120(19):3089–96.
Morgan T, Hawken S, Ghani K, Miller D, Feng F, Linsell S, et al. Variation in the use of postoperative radiotherapy among high-risk patients following radical prostatectomy. Prostate Cancer Prostatic Dis. 2016;19(2):216–21.
Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–34.
Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416–23.
Banerji JS, Wolff EM, Massman JD, Odem-Davis K, Porter CR, Corman JM. Prostate needle biopsy outcomes in the era of the US Preventive Services Task Force recommendation against prostate specific antigen based screening. J Urol. 2016;195(1):66–73.
Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314(1):80–2.
Sciarra A, Barentsz J, Bjartell A, Eastham J, Hricak H, Panebianco V, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011;59(6):962–77.
Gray PJ, Lin CC, Cooperberg MR, Jemal A, Efstathiou JA. Temporal Trends and the Impact of Race, Insurance, and Socioeconomic Status in the Management of Localized Prostate Cancer. Eur Urol. 2017 May;71(5):729-37.
Chang SL, Kibel AS, Brooks JD, Chung BI. The impact of robotic surgery on the surgical management of prostate cancer in the USA. BJU Int. 2015;115(6):929–36.
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387.
Morgan SC, Waldron TS, Eapen L, Mayhew LA, Winquist E, Lukka H, et al. Adjuvant radiotherapy following radical prostatectomy for pathologic T3 or margin-positive prostate cancer: a systematic review and meta-analysis. Radiother Oncol. 2008;88(1):1–9.
Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98(10):715–7.
Briganti A, Karnes RJ, Gandaglia G, Spahn M, Gontero P, Tosco L et al., editors. Natural history of surgically treated high-risk prostate cancer. Urologic Oncology: Seminars and original investigations. Elsevier; 2015.
Abdollah F, Sood A, Sammon JD, Hsu L, Beyer B, Moschini M, et al. Long-term cancer control outcomes in patients with clinically high-risk prostate cancer treated with robot-assisted radical prostatectomy: results from a multi-institutional study of 1100 patients. Eur Urol. 2015;68(3):497–505.
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–62.
Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, De Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018–27.
Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96–02/AUO AP 09/95 trial. Eur Urol. 2014;66(2):243–50.
Chen RC, Chang P, Vetter RJ, Lukka H, Stokes WA, Sanda MG, et al. Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. J Natl Cancer Inst. 2014;106(7):dju132.
Moinpour CM, Hayden KA, Unger JM, Thompson Jr IM, Redman MW, Canby-Hagino ED, et al. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26(1):112–20.
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–16.
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–7.
Lightner D, Lange P, Reddy P, Moore L. Prostate specific antigen and local recurrence after radical prostatectomy. J Urol. 1990;144(4):921–6.
Leventis AK, Shariat SF, Slawin KM. Local recurrence after radical prostatectomy: correlation of US features with prostatic fossa biopsy findings 1. Radiology. 2001;219(2):432–9.
Liauw SL, Pitroda SP, Eggener SE, Stadler WM, Pelizzari CA, Vannier MW, et al. Evaluation of the prostate bed for local recurrence after radical prostatectomy using endorectal magnetic resonance imaging. International Journal of Radiation Oncology Biology Physics. 2013;85(2):378–84.
Vargas HA, Martin-Malburet AG, Takeda T, Corradi RB, Eastham J, Wibmer A et al., editors. Localizing sites of disease in patients with rising serum prostate-specific antigen up to 1ng/ml following prostatectomy: how much information can conventional imaging provide? Urologic Oncology: Seminars and Original Investigations. Elsevier; 2016.
Swanson GP, Hussey MA, Tangen CM, Chin J, Messing E, Canby-Hagino E, et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25(16):2225–9.
Stephenson AJ, Shariat SF, Zelefsky MJ, Kattan MW, Butler EB, Teh BS, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325–32.
Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, et al. Prostate cancer–specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299(23):2760–9.
Cotter SE, Chen MH, Moul JW, Lee WR, Koontz BF, Anscher MS, et al. Salvage radiation in men after prostate-specific antigen failure and the risk of death. Cancer. 2011;117(17):3925–32.
Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25(15):2035–41.
• Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34(30):3648–54. This is a large, multi-institutional nomogram for salvage radiotherapy after prostatectomy. Lower pretreatment PSA was strongly associated with improved outcomes including distant metastasis
Stish BJ, Pisansky TM, Harmsen WS, Davis BJ, Tzou KS, Choo R et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol. 2016. doi:10.1200/JCO.2016.68.3425.
Abugharib A, Jackson WC, Tumati V, Dess RT, Lee JY, Zhao SG, et al. Very Early Salvage Radiotherapy Improves Distant Metastasis-Free Survival. J Urol. 2017 Mar;197(3 Pt 1):662-8.
Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer. Cancer. 2008;112(2):307–14.
Moreira DM, Bañez LL, Presti Jr JC, Aronson WJ, Terris MK, Kane CJ, et al. Predictors of secondary treatment following biochemical recurrence after radical prostatectomy: results from the shared equal access regional cancer hospital database. BJU Int. 2010;105(1):28–33.
Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FD, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173(5):1701–5.
van Stam M-A, Aaronson NK, Pos FJ, Bosch JR, Kieffer JM, Tillier CN, et al. The effect of salvage radiotherapy and its timing on the health-related quality of life of prostate. Cancer Patients European urology. 2016;70(5):751–7.
•• Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. The Lancet Oncology. 2016;17(6):747–56. This is a large randomized trial that demonstrates the addition of 6 months of hormonal therapy (goserelin) to salvage radiation therapy in men with a recurrent, rising PSA (0.2-2.0) after radical prostatectomy decreased PSA recurrence, with no evidence improvement in metastasis or overall survival to-date.
•• Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417–28. This is a large randomized trial that demonstrates the addition of hormonal therapy (bicalutamide) for 2 years to salvage radiation therapy in men with a persistently elevated or recurrent, rising PSA (0.2–4.0) after radical prostatectomy decreases distant metastasis and improves overall survival. Men with long life expectancy (>12 years) and a high PSA (>0.7) are most likely to benefit from the addition of hormonal therapy.
Zapatero A, Guerrero A, Maldonado X, Álvarez A, González-San Segundo C, Rodriguez MAC, et al. Late radiation and cardiovascular adverse effects after androgen deprivation and high-dose radiation therapy in prostate cancer: results from the DART 01/05 randomized phase 3 trial. International Journal of Radiation Oncology Biology Physics. 2016;96(2):341–8.
Briganti A, Wiegel T, Joniau S, Cozzarini C, Bianchi M, Sun M, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol. 2012;62(3):472–87.
Budiharto T, Perneel C, Haustermans K, Junius S, Tombal B, Scalliet P, et al. A multi-institutional analysis comparing adjuvant and salvage radiation therapy for high-risk prostate cancer patients with undetectable PSA after prostatectomy. Radiother Oncol. 2010;97(3):474–9.
Buscariollo DL, Drumm M, Niemierko A, Clayman RH, Galland-Girodet S, Rodin D, et al. Long-term results of adjuvant versus early salvage post-prostatectomy radiation: a large single institutional experience. Practical Radiation Oncology. 2016;
D’Amico AV, Chen MH, Sun L, Lee WR, Mouraviev V, Robertson CN, et al. Adjuvant versus salvage radiation therapy for prostate cancer and the risk of death. BJU Int. 2010;106(11):1618–22.
Fossati N, Karnes RJ, Boorjian SA, Moschini M, Morlacco A, Bossi A, et al. Long-term Impact of Adjuvant Versus Early Salvage Radiation Therapy in pT3N0 Prostate Cancer Patients Treated with Radical Prostatectomy: Results from a Multi-institutional Series. Eur Urol. 2017 Jun;71(6):886-93.
Jereczek-Fossa BA, Zerini D, Vavassori A, Fodor C, Santoro L, Minissale A, et al. Sooner or later? Outcome analysis of 431 prostate cancer patients treated with postoperative or salvage radiotherapy. International Journal of Radiation Oncology Biology Physics. 2009;74(1):115–25.
Mishra MV, Scher ED, Andrel J, Margules AC, Hegarty SE, Trabulsi EJ, et al. Adjuvant versus salvage radiation therapy for prostate cancer patients with adverse pathologic features: comparative analysis of long-term outcomes. Am J Clin Oncol. 2015;38(1):55–60.
Ost P, De Troyer B, Fonteyne V, Oosterlinck W, De Meerleer G. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. International Journal of Radiation Oncology Biology Physics. 2011;80(5):1316–22.
Trabulsi EJ, Valicenti RK, Hanlon AL, Pisansky TM, Sandler HM, Kuban DA, et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology. 2008;72(6):1298–302.
Parker CC, Clarke NW, Kynaston H, Sydes MR. When should radiotherapy be used after radical prostatectomy? The RADICALS-RT Trial. British Journal of Medical and Surgical Urology. 2010;3(5):190–3.
Pearse M, Fraser-Browne C, Davis ID, Duchesne GM, Fisher R, Frydenberg M, et al. A phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: background and rationale of the radiotherapy–adjuvant versus early salvage (RAVES) trial. BJU Int. 2014;113(S2):7–12.
Richaud P, Sargos P, de Figueiredo BH, Latorzeff I, Mongiat-Artus P, Houédé N, et al. Radiothérapie postopératoire des cancers de la prostate. Cancer/Radiothérapie. 2010;14(6):500–3.
Fenton PA, Hurkmans C, Gulyban A, van der Leer J, Matzinger O, Poortmans P, et al. Quality assurance of the EORTC 22043-30041 trial in post-operative radiotherapy in prostate cancer: results of the dummy run procedure. Radiother Oncol. 2013;107(3):346–51.
Van der Kwast TH, Bolla M, Van Poppel H, Van Cangh P, Vekemans K, Da Pozzo L, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25(27):4178–86.
Cao D, Kibel AS, Gao F, Tao Y, Humphrey PA. The Gleason score of tumor at the margin in radical prostatectomy is predictive of biochemical recurrence. Am J Surg Pathol. 2010;34(7):994–1001.
Imnadze M, Sjoberg DD, Vickers AJ. Adverse pathologic features at radical prostatectomy: effect of preoperative risk on oncologic outcomes. Eur Urol. 2016;69(1):143–8.
Levis M, Guarneri A, Giaj LN, Spratt DE, Bartoncini S, Munoz F, et al. Risk stratification system and pattern of relapse in patients treated with adjuvant radiotherapy after radical prostatectomy. Tumori. 2016;2016(3):323–9.
Kang JJ, Reiter RE, Steinberg ML, King CR. Ultrasensitive prostate specific antigen after prostatectomy reliably identifies patients requiring postoperative radiotherapy. J Urol. 2015;193(5):1532–8.
Morgan T, Meng M, Cooperberg M, Cowan J, Weinberg V, Carroll P, et al. A risk-adjusted definition of biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis. 2014;17(2):174–9.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68 Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926–37.
Den RB, Yousefi K, Trabulsi EJ, Abdollah F, Choeurng V, Feng FY, et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J Clin Oncol. 2015;33(8):944–51.
Spratt DE, Yousefi K, Deheshi S, Ross AE, Den RB, J SB et al. Individual patient-level meta-analysis of the performance of the decipher genomic classifier in high-risk men after prostatectomy to predict development of metastatic disease. J Clin Oncol. 2017.
Zhao SG, Chang SL, Spratt DE, Erho N, Yu M, Ashab HA-D, et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: a matched, retrospective analysis. The Lancet Oncology. 2016;17(11):1612–20.
Feng FY, Zhao SG, Seiwon LC, Erho N, Lehrer J, Alshalalfa M et al. Luminal and basal subtyping of prostate cancer. J Clin Oncol. 2017;35:(suppl 6S; abstract 3).
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–13.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–24.
Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. The lancet oncology. 2006;7(6):472–9.
Dalela D, Santiago-Jiménez M, Yousefi K, Karnes RJ, Ross AE, Den RB, et al. Genomic classifier augments the role of pathological features in identifying optimal candidates for adjuvant radiation therapy in patients with prostate cancer: development and internal validation of a multivariable prognostic model. J Clin Oncol. doi:10.1200/jco.2016.69.9918.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Robert T. Dess declares no potential conflicts of interest.
Todd M. Morgan serves on the Advisory Boards for and received research funding from MDxHealth and Myriad Genetics and is supported by the Prostate Cancer Foundation Young Investigator Award and by the A. Alfred Taubman Medical Research Institute.
Paul L. Nguyen does consulting for Genome DX, Ferring, Medivation, and Dendreon, and received research funding from Astellas and Janssen.
Rohit Mehra is supported by the Prostate Cancer Foundation Young Investigator Award (RM).
Howard M. Sandler does consulting for Janssen, Medivation/Astellas, Sanofi, Ferring, Clovis Oncology, and Varian.
Felix Y. Feng serves on the Advisory Boards for Medivation/Astellas, GenomeDx, Nanostring, and Celgene, and reports grant funding from Varian, Medivation/Astellas, and Celgene.
Daniel E. Spratt is supported by the Prostate Cancer Foundation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Prostate Cancer
Rights and permissions
About this article
Cite this article
Dess, R.T., Morgan, T.M., Nguyen, P.L. et al. Adjuvant Versus Early Salvage Radiation Therapy Following Radical Prostatectomy for Men with Localized Prostate Cancer. Curr Urol Rep 18, 55 (2017). https://doi.org/10.1007/s11934-017-0700-0
Published:
DOI: https://doi.org/10.1007/s11934-017-0700-0