Abstract
Biochemical recurrence after radical prostatectomy (RP) is associated with risk factors including high baseline levels of prostate-specific antigen (PSA), extraprostatic extension, positive surgical margins (R1), and Gleason score ≥8. The 5-year biochemical progression rate for patients with these characteristics has been estimated to be as high as 50–70 %. Two treatment approaches for the postoperative management of these patients are adjuvant radiation therapy in men with an undetectable PSA or observation followed by early salvage radiation therapy (SRT) in men with persisting or rising PSA after initial postoperative undetectable values.
Three randomized phase III trials demonstrated a nearly 20 % absolute benefit for biochemical progression free survival after adjuvant radiation therapy (ART) (60–64 Gy) compared to a “wait-and-see” policy. The greatest benefit has been revealed in patients with positive margins and pT3 tumors.
SRT can be offered to patients with rising PSA after RP. Of these patients, 30–70 % will experience a decrease in their PSA to an undetectable range, and in about 40–50 % of these patients, the PSA will remain stable after 5 years.
At the present time, there are no published randomized trials to compare ART versus SRT.
The purpose of this chapter is to review the rationale, results, and possible side effects for the two treatment approaches ART and SRT.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Radical Prostatectomy
- Gleason Score
- Adjuvant Radiation Therapy
- Clinical Target Volume
- Radiation Therapy Oncology Group
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
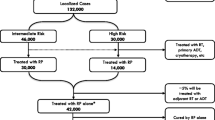
For patients with localized prostate cancer, radical prostatectomy (RP) and external-beam radiation therapy (RT) enable a 10-year overall survival of 83 % and 89 %, respectively [46]. Following RP, serum prostate-specific antigen (PSA) should become undetectable within 4–6 weeks, as half-life is approximately 2–3 days [89]. Persistent PSA levels indicate residual prostatic tissue, either malignant or benign (e.g. benign prostatic hyperplasia).
A PSA increase of ≥0.2 ng/ml is a common definition of progression of disease following RP [38, 105]. Vital tumor tissue has been found in biopsies form the urethrovesical anastomosis in 35–55 % of all patients with rising PSA after RP without clinical correlates suggestive of recurrent tumor [80]. In these cases, PSA levels predate clinically evident disease and do correlate well with disease progression.
After RP, approximately 15–25 % of the patients experience recurrence [90]. Numerous models are available to predict the probability of relapse [28, 79, 99]. With adverse risk factors such as high baseline levels of PSA, extraprostatic extension, positive surgical margins (R1), and Gleason score ≥8, the 10-year biochemical recurrence rate may grow to 75 % [16, 38, 107]. However, biochemical recurrence is a common event even in patients with favorable prognostic factors. The rate of biochemical progression after 7 years for patients with organ confined tumors (pT2) and positive surgical margins is about 25 % [93].
The optimal management of patients with clinical and pathologic features of increased risk for developing a biochemical recurrence remains controversial. Two treatment approaches for the postoperative management of these patients are adjuvant radiation therapy (ART) in men with an undetectable PSA or observation followed by early salvage radiation therapy (SRT) in men with persisting or rising PSA after initial postoperative undetectable values.
The purpose of this chapter is to review the rationale, results, and possible side effects for the different treatment approaches ART and SRT.
2 Adjuvant Radiation Therapy
2.1 Randomized Clinical Trials
Three randomized prospective trials (SWOG 8794, EORTC 22911, and ARO 96–02) demonstrated an approximately 20 % absolute benefit for biochemical progression-free survival (bNED) after adjuvant radiation therapy compared with a “wait-and-see” policy, mostly for pT3 cN0 or pN0 tumors (Table 1). The greatest benefit (30 % bNED after 5 years) has been demonstrated in patients with pT3 tumors and positive margins [11, 96, 102, 108]. In the meantime, 10-year follow-up data of the EORTC trial and the ARO trial were reported and confirmed these results [12, 106].
In the prospective study of the South Western Oncology Group (SWOG), overall survival was improved from 13.5 years without to 15.2 years with adjuvant radiation therapy [97].
Notably, central pathological review on the EORTC-trial showed that only surgical margin status had an effect on the outcome, such that the treatment benefit in patients with negative margins did not remain significant. The hazard ratio in the group with negative surgical margins was 0.87 (p = 0.601), compared to 0.38 (p < 0.0001) in the group with positive surgical margins according to the review pathology. Excluding the patients with a PSA of >0.2 ng/ml after prostatectomy, the hazard ratio for postoperative irradiation was 1.11 (p = 0.740) and 0.29 (p < 0.0001) for the patients with negative and positive margins, respectively [102]. This benefit was also seen in the real adjuvant situation, when the PSA was undetectable before the start of radiation therapy [106, 108]. In the trial of the German Cancer Society 159 patients were randomized into the observation and 148 into the ART arm (60 Gy in 30 fractions over 6 weeks). After a median follow-up of nearly 10 years, there was a significant benefit from ART for bNED: 56 % vs. 35 % (p < 0.0001). In the subgroup of pT3 R1 tumors, this benefit increased from 21 to 30 % [106].
The three randomized studies have used different definitions of biochemical progression: SWOG: PSA >0.4 ng/ml, EORTC: PSA >0.2 ng/ml, ARO: PSA >0.05 ng/ml. Consequently, biochemical recurrences (as an increase of the PSA out of the undetectable range) were detected earlier in the EORTC and the ARO study. This led to apparently worse results in bNED of the ARO study after 5 years, but long term results are quite similar between the three trials (Table 1).
In the ARO study, a pathology review was performed on 85 % of RP specimens of patients to investigate the influence of pathology review on the analysis. There was fair concordance between pathology review and local pathologists for seminal vesicle invasion (pT3c: 91 %; k = 0.76), surgical margin status (84 %; k = 0.65), and for extraprostatic extension (pT3a/b: 75 %; k = 0.74). Agreement was much less for Gleason score (47 %; k = 0.42), whereby the review pathology resulted in a shift to Gleason score 7. In contrast to the analysis of progression-free survival with local pathology, the multivariate analysis including review pathology revealed positive surgical margins and Gleason score >6 as significant prognostic factors [14].
It is well known that the location, the extent, and the number of positive surgical margins after radical prostatectomy are predictors of biochemical progression after radical prostatectomy. The investigators of the Cleveland Clinic/Ohio found in their retrospective series of 7160 patients treated with radical prostatectomy 1540 patients with positive margins. The 7-year progression-free probability was 60 % in those patients, resulting in a hazard ratio for biochemical recurrence of 2.3 compared with negative margins. There was also an increased risk of biochemical recurrence in patients with multiple vs. solitary positive surgical margins (HR 1.4) and extensive vs. focal positive surgical margins (adjusted HR 1.3) [93]. From the data of the randomized trials mentioned above, these patients with positive margins and pT3-tumors do stand to profit mostly from ART.
In the EORTC trial, when the data of patients with pT2 tumors and positive surgical margins were analyzed, there was a significant benefit of 10-year biochemical progression-free survival rate in the irradiated group (71.4 % versus 46.8 % in the wait-and-see group) [12]. However, these data come from a subgroup analysis and biochemical progression-free survival was not the primary endpoint of this study. The possible benefit of radiotherapy must be weighed out carefully in consideration of potential late effects as erectile dysfunction (ED).
2.2 Definition of Clinical Target Volume (CTV)
In the EORTC and SWOG trials, radiation was based on 2D treatment planning, where the prostatic fossa was targeted by using large treatment portals. Obviously, precise definition of target volumes was not essential, which is in great contrast to modern radiation treatment techniques such as IMRT. Compared to 2D based planning, IMRT provides significant normal tissue sparing, but also demands exact definition of target volume.
Consideration of the local failure patterns in the post-RP setting is essential for optimal definition of CTV. The most common sites of local relapse proven by biopsy are the vesicourethral anastomosis (VUA) (66 %), followed by the bladder neck (16 %) and retrotrigone area (13 %) [27]. Recently, endorectal magnetic resonance imaging (MRI) was used to detect local relapse patterns following RP in order to further define the optimal CTV [56]. Based on the results of this study, the authors recommended a cylindrical-shaped CTV centered 5 mm posterior and 3 mm inferior to the VUA, concordant also with the previously mentioned pathologic studies.
To address any uncertainties in the definition of CTV, the Radiation Therapy Oncology Group (RTOG) [53], the EORTC Radiation Oncology Group [73] and other cooperative groups [84, 111] have created consensus guidelines for delineation of target volumes for postprostatectomy patients. In the RTOG recommendations, the CTV extends superiorly from the level of the caudal vas deferens remnant (or 3–4 cm superior to the pubic symphysis, whichever is higher) and inferiorly 8–12 mm inferior to VUA. The VUA is defined as the retropubic region that can be visualized one slice below the most inferior urine-containing image of the bladder. Below the superior border of the pubic symphysis, the anterior border is at the posterior aspect of the pubis and extends posteriorly to the rectum. At this level, the lateral border extends to the levator ani muscles. Above the pubic symphysis, the anterior border should encompass the posterior 1–2 cm of the bladder wall and should extend posteriorly to the mesorectal fascia.
2.3 Use of Image-Guidance to Improve Postprostatectomy Prostatic Fossa Localization
In recent years, several innovative methods have been developed to improve localization of the prostatic fossa and minimize daily internal set-up error. Techniques currently utilized in most practices include daily portal imaging with implanted gold fiducial markers [77], daily cone beam or kilovoltage imaging [59], and the use of electromagnetic transponders [21]. Such image-guidance techniques allow for a minimal (7–10 mm) expansion from a CTV to a planning target volume, thereby providing further normal tissue sparing by minimizing RT dose to the rectum and bladder [83].
2.4 Adjuvant RT of Pelvic Lymph Nodes?
The three randomized trials included only patients with cN0 or pN0-disease. The effect of adjuvant RT in node-positive prostate cancer has not yet been prospectively assessed. A retrospective study by Da Pozzo et al. reported a significant positive impact of RT in combination with hormonal therapy (HT) in patients with nodal metastases treated with RP and pelvic lymph node dissection [31]. However, this study was limited by a potential patient selection bias mainly due to its retrospective and unmatched design. In fact, patients treated with adjuvant RT were those affected by more aggressive disease. Therefore, no effect of adjuvant RT on cancer-specific survival was demonstrated on univariate survival analyses. There was significant gain in predictive accuracy when adjuvant RT was included in multivariable models predicting biochemical recurrence-free and cancer-specific survival (gain: 3.3 % and 3 %, respectively; all p < 0.001).
In a large retrospective series, Briganti et al. assessed the effect of adjuvant RT in node-positive prostate cancer including two homogeneous matched patient cohorts exposed to either adjuvant RT plus HT or adjuvant HT alone after surgery. In this series from Milan and Jacksonville, a total of 703 patients were assessed at a median follow-up of 95 months. Patients were matched for age at surgery, pathologic T stage and Gleason score, number of nodes removed, surgical margin status, and length of follow-up. The overall survival advantage was 19 % in favor of adjuvant radiation therapy plus hormonal treatment compared with hormonal treatment alone. Similarly, higher survival rates associated with the combination of HT plus RT were found when patients were stratified according to the extent of nodal invasion (namely ≤2 versus >2 positive nodes; all p ≤ 0.006) [15].
In 2014 the same working group has published an analysis of 1107 patients with node-positive prostate cancer. After surgery with elective lymph node dissection, the men received either adjuvant HT alone (intended but not confirmed lifelong, n = 721) or HT plus ART (66.6–70.2 Gy to the prostate bed and 45–50.4 Gy to the pelvic lymph nodes, n = 386). The median follow-up was 7.1 years. Based on the pathologic T stage, Gleason score, number of positive lymph nodes, and surgical margin status, five risk groups were defined. In the intermediate-risk group, there was an overall survival advantage from combined therapy of 6 % and 18 %, after 5 and 8 years, respectively. In the high-risk group, the figures were 6 % and 20 %, respectively, in favor of ART plus HT compared with HT alone. In multivariate analysis, two groups had a significant benefit from additional ART, namely: (1) patients with ≤2 positive lymph node, Gleason score 7–10, pT3b/pT4 stage, or positive surgical margins; and (2) patients with 3–4 positive lymph nodes irrespective of other features [1]. Because of the retrospective nature of this series with no standardized definition of target volumes, radiation dose, and duration of HT, these results should be interpreted with caution. However, they provide support for this treatment in selected cases, but should be validated in prospective clinical trials.
2.5 Additional Use of Hormone Therapy to ART
It is now clearly established that the standard nonoperative management for patients with locally advanced prostate adenocarcinoma includes long-term hormone therapy. Two cooperative group trials, RTOG 96–02 and EORTC 22961, have demonstrated an overall survival advantage if these patients, and specifically those with additional high-risk factors like Gleason score 8–10, are treated for 2–3 years with hormone therapy [10, 41]. It remains unknown whether men with high-risk, node-negative prostate adenocarcinoma initially treated with RP and pelvic lymph node dissection benefit from additional adjuvant hormone therapy. The primary rationale for the use of hormone therapy post-RP is to: (1) improve local control by eradicating disease in a hypoxic scar that may be radioresistant; (2) address micrometastatic disease which may have spread to the lymph nodes or distant sites; and (3) alter PSA kinetics in patients who will eventually relapse [37, 44, 74].
Previous studies have indicated a potential benefit from combination therapy for men at high risk of recurrence. A secondary analysis of Radiation Therapy Oncology Group (RTOG) 85–31, a phase III trial comparing standard external beam RT plus immediate ADT versus RT alone for patients with nonbulky prostate cancer, found improved biochemical control in patients who received combination therapy as compared to men treated with RT alone [29]. With a median follow-up of 5 years, the progression-free survival for men treated with combination therapy was estimated to be 65 % as compared to 42 % for men treated with RT alone (p = 0.002). Similar results were seen in a retrospective study performed at Stanford University [48].
Two further randomized trials into HT-RT combination therapy, RTOG-P-0011 and EORTC 22043, closed prematurely because of poor recruitment.
The ongoing RADICALS trial will address the question of duration of hormone therapy combined with ART [68].
3 Salvage Radiation Therapy
Salvage radiation therapy (SRT) should be considered for men presenting with persistent PSA after prostatectomy or showing an increase of PSA levels after initial postoperative undetectable values [5, 9, 18, 20, 70, 85, 92, 93, 104].
It remains uncertain whether a PSA increase after RP indicates isolated local disease, distant metastatic progression, or both [80]. Therefore, the best treatment for recurrent prostate cancer in patients with increasing or persisting PSA without clinical evidence of disease still remains controversial. On the other hand, only RT can offer the chance of cure to patients with truly localized malignant disease after RP.
There are indicators for a higher likelihood of local recurrence, e.g. slow PSA rise (PSA doubling time ≥12 months), more than 1 year between RP and the detection of PSA in the serum, Gleason score <7, and negative surgical margins [72]. On the other hand, there are also indicators suggesting metastatic disease such as short PSA doubling time (<12 months) or Gleason score at RP from 8 to 10 [70, 104]. Some authors tried to define combinations of risk factors. For example, patients with a PSA <1 ng/ml before RT, and pre-RP Gleason score <7, and a long PSA doubling time after progression have a high risk of local disease [92]. A predictive model for the outcome of RT for PSA progression after RP has been proposed and validated [58, 91]. Assuming a local nature of the underlying disease, SRT of the prostatic bed has widely been used to treat patients in the absence of biopsy-proven local recurrence. An established standard is conformal radiotherapy to the prostatic fossa with a dose of about 66 Gy, aiming to irradiate the presumed local recurrence and hence to reduce the risk of a “second wave of metastasis” leading to clinical progression of disease [26, 38, 105]. In the light of the well-known problems in detecting local recurrence in the prostatic bed, radiotherapy to the prostatic fossa is one of the rare therapies in which most radiation oncologists irradiate without a histologic proof of tumor recurrence.
3.1 Role of Investigations in Case of Persisting/Rising PSA
Once biochemical failure has been diagnosed, it is essential to distinguish between local recurrence and systemic metastases in order to plan the best therapeutic approach. For this reason, there is a strong need for imaging techniques which may be able to recognize small lesions and to identify their nature (persistent or recurrent neoplastic tissue, healthy residual glandular tissue, and granulation tissue or fibrosis). These techniques should be able to detect residual or recurrent disease when the PSA serum level is very low (less than 1 ng/ml) in order to deliver the more relevant therapeutic option as early as possible.
Currently, transrectal ultrasound (TRUS) has neither good sensitivity nor good specificity in detecting early recurrent cancer [51]. Scattoni et al. showed that TRUS-guided biopsy to detect local relapse after RP has a limited sensitivity (25–54 %) when the PSA serum value is less than 1 ng/ml [76]. TRUS-guided biopsy of the postprostatectomy fossa is not recommended by EAU-guidelines in patients with PSA serum level less than 1 ng/ml [38].
Over the last few years, technological innovations have allowed the development of superimposed imaging, which links anatomic, functional, and biological information together. Magnetic resonance imaging (MRI) and positron emission computed tomography (PET/CT) have proven to be useful tools in the early diagnosis of prostate cancer recurrence.
The advantages of MRI over TRUS are its superior soft-tissue resolution and its ability to cover the entire postprostatectomy fossa and reveal recurrences that are located beyond the region routinely imaged on ultrasound. The combination of an external and an endorectal coil improves the ability to detect local recurrence of prostate cancer [42]. The anatomic detail and wide coverage of the pelvis by MRI facilitates its increasing use in directing salvage radiation therapy when a recurrence is demonstrated [56]. Additionally, as pelvic lymphadenopathy and osseous metastases are routinely evaluated with MRI, the most common early metastatic sites of prostate cancer are covered by this method.
The reported sensitivity and the specificity of MRI for depicting local recurrences by experienced investigators in 82 patients who underwent prostatectomy were 87 % and 78 %, respectively. PSA levels at MR imaging in patients with clinically proved recurrences ranged from undetectable to 10 ng/ml (mean, 2.18 ng/ml) [78].
Panebianco et al. found that a combined technique of proton magnetic resonance spectroscopic imaging (1H-MRSI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) at 3 Tesla was a valid tool to detect locoregional relapse. It was more accurate than Cholin-PET/CT in the identification of small lesions in 84 men with low biochemical progression after RP (PSA serum values ranging from 0.2 to 2 ng/ml) [67].
Various targets have been addressed by molecular imaging to improve the detection of recurrent prostate cancer. For PET imaging, mainly 11C- and 18F-labeled choline derivates have been used in the past [39, 49]. However, especially in patients with PSA values below 3 ng/ml, the detection rate is only 40–60 % [7, 23, 49]. Recently, molecular probes have been developed to target for example the gastrin-releasing peptide receptor or the prostate-specific membrane antigen (PSMA), [94, 110]. PSMA is a membrane-bound enzyme with significantly elevated expression in prostate cancer cells in comparison to benign prostatic tissue [86]. A newly developed compound (coupling 68Ga via the chelator HBED-CC to the extracellular PSMA ligand Glu-NH-CO-NH-Lys) demonstrated a high specificity for PSMA expressing tumor cells as well as high and specific uptake in a mouse model [32]. A first preliminary study in prostate cancer patients revealed a higher image contrast and detection rate with 68Ga-PSMA- than with 18F-choline-PET/CT [3]. Afshar-Oromieh et al. performed a retrospective analysis in 319 patients with different primary treatment including 226 patients with recurrent prostate cancer after radical prostatectomy. In 82.8 % of the patients at least one lesion indicative of prostate cancer was detected. A lesion-based analysis of sensitivity, specificity, negative predictive value, and positive predictive value revealed values of 76.6 %, 100 %, 91.4 %, and 100 %, respectively. Of 116 patients available for follow-up, 50 received local therapy after 68Ga-PSMA PET/CT [2]. Eiber et al. investigated the detection rate of 68Ga-PSMA PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. Median PSA level was 1.99 ng/ml. The detection rates were 96.8 %, 93.0 %, 72.7 %, and 57.9 % for PSA levels of ≥2, 1 to <2, 0.5 to <1, and 0.2 to <0.5 ng/ml, respectively. With higher Gleason score (≤7 versus ≥8), detection efficacy was significantly increased (p = 0.019) [33]. 68Ga-PSMA ligand PET/CT shows substantially higher detection efficacy than reported for other tracers. Most importantly, it reveals a high number of positive findings in the clinically important range of low PSA values (<0.5 ng/ml). However, case numbers in that PSA range are very low in all reports [2, 19, 33, 40].
3.2 Results of Salvage Radiotherapy/Prognostic Factors
So far, there are no published data available from randomized trials on SRT after RP and the question of whether or not SRT can improve survival is not answered, yet. Numerous retrospective studies focus on biochemical recurrence and there is clear evidence for an advantage from early SRT for that endpoint. However, the definition of “early” varies throughout the literature. European guidelines recommend SRT at a PSA <0.5 ng/ml, AUA/ASTRO suggest a threshold at 0.2 ng/ml, and even lower values (down to 0.05 ng/ml) have been proposed [38, 55, 98]).
Stevenson et al. reported the results of a multi-institutional cohort of 1540 patients. These patients received SRT with a median dose of 66 Gy. Median follow-up was 53 months. A six-years biochemical progression-free survival-rate of 48 % (95 % CI, 40–56 %) could be achieved when the PSA was <0.5 ng/ml compared with only 18 %, when the preradiation therapy PSA was >1.5 ng/ml. In the whole series, the 6-year progression-free survival-rate was 32 % (95 % CI, 28–35 %) [91]. The authors identified several prognostic factors that were associated with a poor response to RT including Gleason score of 8–10, pre-SRT PSA >2 ng/ml, negative surgical margins, postoperative PSA doubling time <10 months and seminal vesicle invasion. Patients without these adverse features had a 6 year progression-free survival of 69 %. Also, some subsets of patients with Gleason score 8–10 would benefit from salvage radiation therapy if the pretreatment PSA was <2.0 ng/ml, surgical margins were positive and PSA doubling time was >10 months [91].
Briganti et al. reported on a multi-institutional cohort of 472 node-negative patients who experienced biochemical recurrence after RP. All patients received SRT at a PSA <0.5 ng/ml. In univariate analysis, pT-stage, Gleason score and margin status were significant predictors of progression. In multivariable Cox regression, also the pre-SRT PSA was a significant predictor (all parameters with p < 0.04). The study aimed to develop a nomogram predictive for biochemical progression. Therefore, the pre-SRT PSA was a continuous variable with no discrete value to differentiate low versus high risk. Positive margins were a high-risk factor in that data set [17].
Lohm et al. reported the results of 151 patients receiving SRT at a PSA <0.2 ng/ml. After a median follow-up of 82 months, a biochemical progression was diagnosed in 83 patients (55 %). Multivariate analysis confirmed the impact of pre-SRT PSA level, Gleason score, and PSADT on biochemical progression-free survival and tumor stage on overall survival. The margin status was no significant risk factor at all [52]. Also, in a cohort of 409 men who had SRT at higher PSA levels (range 0.3–1.7 ng/ml), surgical margins did not reach significance (p = 0.2) in the regression model for biochemical failure [43].
Trock et al. conducted a retrospective analysis of a cohort of 675 patients undergoing RP from 1982 to 2004. Median follow-up was 9 years since RP and 6 years since SRT. They show a benefit for prostate cancer-specific survival after SRT (with or without additional hormone treatment) compared with sole androgen deprivation. Particularly, there was an advantage for patients who achieved a post-SRT PSA <0.2 ng/ml (the undetectable range in that study) and for men with a short PSA doubling time (<6 months) at recurrence. However, other established prognostic features such as pT stage or Gleason score failed statistical significance for overall survival [101].
Chang et al. determined the PSA of 164 prostatectomy patients 4 months after the administration of SRT for recurrent prostate cancer. The median follow-up was 53.4 months. If at that time the PSA was ≥0.2 ng/ml or was incompletely reduced (≥45 % of the pre-SRT PSA), then the 5-year rates of clinical recurrence were significantly increased [24].
Jackson et al. identified men with a detectable nadir (0.1–0.2 ng/ml) within 6 months after SRT as a high-risk group regarding biochemical failure, distant metastases, prostate cancer-specific death, and overall mortality. A total of 448 patients (15 % seminal vesicle invasion, 50 % extracapsular extension, 46 % positive margins, 2 % positive lymph nodes) had been followed up for median 64 months. Clinical/pathological risk factors again failed statistical significance in Cox regression analysis when the post-SRT nadir was included [43]. A lower pre-SRT PSA was significantly related with achieving an undetectable post-SRT nadir. The median pre-SRT level was 0.5 ng/ml in responders and 0.7 ng/ml in nonresponders and a PSA maximum of 18 ng/ml. In multivariable analysis, the pre-SRT PSA was a significant parameter for biochemical recurrence (p = 0.005), metastasis (p = 0.05) and borderline significant even for OS (p = 0.07). In a retrospective analysis of 306 patients, the application of SRT at a PSA <0.2 ng/ml correlated significantly with achieving a post-SRT PSA nadir <0.1 ng/ml and with improved freedom from progression (median follow-up 7.2 years). The post-SRT nadir <0.1 ng/ml correlated significantly with less recurrence and with better overall survival [5].
There is now strong evidence that achieving a post-SRT PSA nadir <0.1 ng/ml enables a better overall survival in long-term follow-up. The association of the pre-SRT PSA with post-SRT nadir indicates indirectly that selected patients may have a significant survival benefit from SRT. Moreover, patients whose post-SRT PSA declines to the undetectable range may not need additional hormonal treatment before secondary progress. As a hypothesis, this requires confirmation/validation in the framework of prospective clinical trial (Table 2).
3.3 Total Dose of Salvage Radiotherapy
With reference to the three randomized studies, a dose of 60–64 Gy for adjuvant RT is consensus in the guidelines [38, 105]. The situation is less clear for salvage RT. To avoid radiation toxicity, most SRT studies do not exceed 70 Gy. In the guidelines, total doses of “at least 66 Gy” are recommended [38, 105]. However, some recently published series demonstrated a better outcome with higher total doses [9, 47, 85]. Bernard et al. investigated 364 men with salvage radiation therapy after radical prostatectomy after a median follow-up of 6.0 years. They defined three dose groups (low, <64.8 Gy; moderate, 64.8–66.6 Gy; high, >66.6 Gy). In multivariate analysis, they found that compared with the high dose level, there was a decreased bNED for patients treated with the low dose level (HR 0.60) [9]. This was similar to the results published by Siegmann et al. from the group in Berlin and Ulm. In their retrospective series including 301 patients, 234 received 66.6 Gy while 67 patients with a PSA decrease during salvage radiation therapy were selected and irradiated up to 70.2 Gy. In the multivariate analysis the total dose was a significant predictor of reduced risk of biochemical progression (p = 0.017) [85].
The need for a higher irradiation dose remains uncertain; nevertheless it seems justified especially in patients with histologically confirmed local recurrence after radical prostatectomy.
The SAKK 09/10 trial randomized 344 patients without evidence of residual disease between 2011 and 2014 to receive SRT at 70 Gy (n = 175) or 64 Gy (n = 169). In 44 % of the patients, the RT was applied using a 3D-conformal approach and in 56 % of the patients using an IMRT technique. The primary endpoint was freedom from biochemical failure. The trial was closed for accrual after it met its accrual goal of 350 patients.
A first analysis of the trial reported acute toxicity rates and early quality of life. There was no significant difference in acute genitourinary and gastrointestinal toxicity rates between both arms. Generally, changes in health related quality of life were minor; however, there was a relevant worsening of urinary symptoms in the 70 Gy arm. There was no significant difference in acute toxicity associated with RT technique [35]. The first randomized prospective data regarding freedom from biochemical recurrence and late toxicity are awaited in 2017.
3.4 RT of Pelvic Lymph Nodes?
An important, but unsolved question is the value of an additional whole pelvic irradiation compared with prostate bed irradiation alone. Spioto from the Stanford University reported on 160 patients who underwent adjuvant or salvage radiation therapy, out of which 87 had short course total androgen suppression. A total of 114 patients were considered at high risk of lymph node involvement although cN0 (Gleason score >8, preoperative PSA level >20 ng/ml, seminal vesicle involvement); 72 underwent whole pelvic radiation therapy and 42 underwent prostate bed radiation therapy. The median follow up was >5 years. Limited- to high-risk patients, there was a superior bNED of whole pelvic radiation therapy compared with prostate bed radiation therapy (5-year rate 47 % vs. 21 %, p < 0.05) [88]. While these data have to be confirmed in a prospective trial, whole pelvic radiation therapy combined with modern delivery techniques like IMRT can be offered as a promising option for high-risk patients [38, 105].
3.5 Additional Use of Hormone Therapy to SRT
Interesting retrospective data have been reported from the Mayo Clinic and from the University of Michigan [25, 87]. They raise the question of the efficacy of an additional androgen deprivation during and after SRT. Choo and coworkers reported on a prospective pilot study with 75 patients treated with SRT + 2-year androgen deprivation. With a median follow-up from SRT of 6.5 years, all patients achieved an initially complete PSA response (<0.2 ng/ml). Relapse-free survival rate at 7 years was 78 % of the whole population [25]. A group of the University of Michigan treated all together 630 men for salvage indications after RP. In this group, 66 % had high-risk factors. The mean RT dose was 68 Gy and 24 % of all patients received concurrent androgen deprivation. The median ADT duration for these patients was 11 months. With a median follow-up of 3 years, the concurrent androgen deprivation was shown to be a significant independent predictor of progression-free survival in the high-risk group (p < 0.05) [87]. Therefore, it seems attractive to treat high-risk patients with SRT and an additional androgen deprivation. The optimal duration of this androgen deprivation therapy remains uncertain.
RTOG 96–01 is a randomized, multicenter phase III trial, designed to compare anti-androgen therapy (bicalutamide monotherapy 150 mg/d) plus SRT (n = 384) with a placebo plus SRT alone (n = 377) in men with pT3/pT2 R1 N0 M0 prostate cancer who have an elevated PSA after surgery. The primary end-point is overall survival. The results presented at the 2015 Annual Meeting of the American Society for Radiation Oncology (ASTRO) reveal that the addition of anti-androgen therapy to SRT reduces prostate cancer death and the development of metastatic prostate cancer without increasing radiation toxicity. With a median follow-up of 12.6 years, the actuarial overall survival at 10 years was 82 % for the RT plus HT arm and 78 % for the RT plus placebo arm (p = 0.036). The 12-year incidence of prostate cancer-related deaths was 2.3 % for the RT plus HT arm, compared with 7.5 % for the RT plus placebo arm. Late bladder and bowel toxicity were low and similar in both groups, whereas 70 % of men in the RT plus HT arm reported swelling of the breasts, compared with 11 % in the RT plus placebo arm [82].
The subgroup analysis on overall survival and time to metastatic prostate cancer presented at the 2016 Genitourinary Cancers Symposium indicates that patients most likely to benefit have Gleason score 7 or 8–10, pre-SRT PSA value of 0.7–4 ng/ml, and positive surgical margins RP [81].
Carrie et al. presented at the 2015 ASCO Annual Meeting the first results of the GETUG-AFU 16. The phase III randomized trial assessed the efficacy of RT alone versus RT + HT on progression-free survival for patients with biochemical recurrence after RP. From 2006 to 2010, 743 patients were randomized to RT alone (66 Gy on prostate bed ± pelvic irradiation according to pN status and risk of initial node involvement) or RT + goserelin, for 6 months. With a median follow-up of 63.1 months, 216 cases of progression were noted (138 in RT versus 78 in RT + HT). The intent to treat analysis showed an improved 5-year PFS of 62.1 % versus 79.6 % for RT and RT + HT, respectively (p < 0.0001). The 5-year overall survival was 94.8 % for RT versus 96.2 % for RT + HT (p = 0.18). Acute toxicities occurred more frequently in RT + HT arm (89 % versus 79 %). No difference was found in grade 3 acute toxicities and late toxicities [22].
So far, the current data of both studies are published in abstract form only. Until final publication there is no reason to give an additional hormone therapy to all patients. Until now, the recommended type of hormone therapy is also unclear Nevertheless, experience shows that bicalutamide is usually better tolerated than LHRH agonists like Goserelin.
RTOG 0534 is investigating the benefit of short-term ADT as well as pelvic nodal irradiation in the SRT setting. In this trial, patients will be randomized to one of three treatment arms: (1) prostatic fossa irradiation alone; (2) prostatic fossa + whole pelvic irradiation alone; or (3) prostatic fossa + whole pelvic irradiation with short-term ADT. The primary endpoints of this study are to determine: (1) whether the addition of short-term androgen deprivation therapy to prostatic fossa irradiation improves freedom from progression for 5 years over that of prostatic fossa irradiation therapy alone; and (2) whether short-term ADT and whole pelvic RT improve freedom from progression over that of short-term ADT and prostatic fossa irradiation alone for men treated with SRT. The target of accrual for this trial was 1764 patients and, to date, the study is closed to accrual.
4 Radiation Therapy Techniques
Traditionally, a 4-field technique has been used. The conventional treatment volumes were typically very generous, being approximately 10 × 10 cm in the anterior-posterior fields with the inferior border at the ischial tuberosities. The lateral fields extended from the anterior aspect of the pubic symphysis and split the rectum posteriorly.
After the introduction of modern 3D CRT techniques, a major controversy about the target volumes of postoperative radiation therapy started. Critical evaluation of target volume delineation by different authors and participation of experienced radiation oncologist showed that variations up to 65 % maybe present even in cases of adjuvant or salvage radiation restricted to the prostatic fossa [54].
In 3D CRT, the target volume should include the bladder neck (pulled into the prostate bed), the periprostatic tissue and surgical clips, and the seminal vesicle bed (including any seminal vesicle remnants if present) if initially involved or as a confirmed site of recurrence. Some anatomic landmarks are useful in maximizing/optimizing coverage of the surgical bed: Inferiorly, the vesical–urethral anastomosis should be included. This anastomosis is the most frequent area of positive prostate bed biopsies. By placing the inferior field edge at the top of the bulb of the penis (best seen on magnetic resonance imaging) and adding a margin for uncertainties, there should be adequate coverage. Laterally, the field should extend to about the medial aspect of each obturator internus muscle. Although the rectum is a landmark posteriorly, the relative position of the rectum appears to shift after the prostate is removed as well as during radiation therapy [34, 60]. For this reason, a generous margin from CTV to PTV posteriorly is recommended, such as setting an 8-mm margin with image guidance [69]. The superior margin is more subjective. The former prostate can extend above the pubic symphysis, but it is recommended that the anterior part of the bladder be avoided at this level because this is the least likely area for extracapsular extension and involved margins. Treatment of the seminal vesicle bed, lying behind the bladder, is advised for pT3b tumors. If vascular clips were used at prostatectomy, they are likely to be seen in this region. The level of the posterior-superior clinical target volume is somewhat subjective and should be guided by the extent of disease at the prostate base and by whether or not the seminal vesicles were involved.
The recommendations of the RTOG [53] and of the EORTC [73] are very helpful in delineation of the target volume for irradiation of the prostatic fossa. However, the definition of the target volumes remains difficult. Recently, a study assessed the interobserver agreement of prostate bed delineation after radical prostatectomy as proposed by the EORTC guidelines. Six observers delineated the prostate bed (PB) and the original seminal vesicle position (SV) of ten patients. Contours were then compared for agreement between observers. The mean volume of 100 % agreement was only 5.0 (±3.3) ml for the PB and 0.9 (±1.5) ml for the SV, whereas the mean union of all contours (±1 SD) was 41.1 (±11.8) ml and 25.3 (±13.4) ml, respectively. The overall standard deviation of the outer margins of the PB ranged from 4.6 to 7.0 mm [64].
Furthermore, Croke et al. showed that none of the guidelines adequately covered the prostate bed and/or gross tumor based on preoperative MRI in a nonselect group of 20 patients. On average, 38 % of the prostate volume and 41 % of gross tumor volume on preoperative MRI were not included in the CTV. This suggests that improved target delineation could potentially improve outcomes [30].
Wang et al. recently evaluated regions of local recurrence after RP in relation to whether these would have been covered using the RTOG guidelines. They reported that the RTOG CTV contours did not appear adequate posterolaterally near the rectum/mesorectal fascia and inferiorly at the posterior urogenital diaphragm. Use of the CTV MRI should improve coverage of such regions [103].
Given the potential for late toxicity after postoperative radiation therapy, the use of IMRT is appealing [6]. As with 3D CRT, a generous definition of the prostate bed target volume and adequate margins to account for target motion (especially due to the variation in rectal and bladder filling) and setup uncertainties are critical. The theoretical advantages of IMRT over conventional 3D CRT are its geometrically steep dose falloff and improved conformity with irregularly shaped targets (e.g., the superior-posterior aspect of the postoperative field). A greater sparing of the superior-anterior part of the bladder, the posterior part of the rectum, and the penile bulb can be achieved using IMRT, despite using the same target volume definition [71]. The comparison of a 5-field IMRT and a rotational IMRT (for example “Rapid Arc”) technique is displayed in Fig. 1.
For optimization of the margins needed for delivery of IMRT, IGRT remains a helpful tool. Ost and co-workers from Gent University demonstrated a significant reduction of acute toxicity using patient positioning with cone beam CT [63]. Sandhu et al. from the University of California used IGRT in patients undergoing postprostatectomy irradiation. Prostate bed localization was done using image guidance based on surgical clips, relative to the reference isocenter on the digitally reconstructed radiographs made during radiation therapy planning. They assumed that surgical clips are a useful surrogate for the prostate bed and therefore measured daily shifts of the position of the surgical clips in 3 dimensions. With an average (standard deviation) prostate bed motion in anterior-posterior, superior-inferior, and left-right directions of 2.7 mm (2.1), 2.4 mm (2.1), and 1.0 mm (1.7), respectively, the majority of the patients experienced only grade 1 side effects. The authors recommended daily IGRT for accurate target localization [75]. However the most efficient approach for IGRT during the 6–8 weeks of irradiation remains controversial [50, 77].
5 Side Effects and Toxicity of ART/SRT
The three randomized clinical trials discussed above included prospective collection of data on gastrointestinal and genitourinary toxicity in the two cohorts (ART vs. observation). However, in the EORTC and SWOG trials, radiation was based on 2D treatment planning which did not enable normal tissue sparing to nowadays state-of-the-art. The toxicity data of both studies are therefore no longer relevant.
In contrast, modern 3D based radiation treatment techniques such as IMRT allow for minimization of dose to the rectum and bladder.
A total of 217 patients from the SWOG therapeutic trial patients were eligible and registered to a health-related quality of life (HRQL) study. Patients completed the SWOG Quality of Life Questionnaire at baseline, 6 weeks, 6 months, and annually for 5 years. Patients receiving adjuvant RT reported worse bowel function (through approximately 2 years) and worse urinary function. There were no statistically significant differences for ED. Global HRQL was initially worse for the ART arm but improved over time and was better at the end of the period than the global HRQL reported for RP alone [57].
Unlike the SWOG trial, the EORTC trial did not assess total urinary incontinence; however, in an interim analysis there was no significant difference concerning urinary incontinence between the two treatment arms [11].
In the German study, which utilized 3D-based radiation treatment planning, the incidence of late grade 3 or higher adverse events was only 0.3 % [106]. One patient in the observation arm developed a urethral stricture, compared to two patients in the ART arm. Urinary incontinence was not assessed in this trial.
A low rate of side effects is of particular importance for a therapy without histologic confirmation. The side effects of SRT have so far been reported to be tolerable. Although in general, side effects tend to be underreported in retrospective analyses, a proportion of <3 % severe late side effects seems to be a realistic estimate. Higher rates of 10 % genitourinary grade 3 complications, namely anastomotic strictures and bladder neck contractures requiring dilatation, reported in a series of 115 patients from the Memorial Sloan-Kettering Cancer Center, need to be interpreted with caution [45]. It may be difficult to differentiate side effects of RT from preexisting disabilities and sequelae of RP. At least equivalent rates of severe genitourinary complications following RP alone have been reported in a SEER data base analysis of 11,522 patients published by the same institution [8].
A meta-analysis of 25 studies covers 3282 patients who received 60–72 (median 65) Gy, largely with older albeit 3D-planned techniques. Model calculations predict the 5 % incidence in both organ systems at 68–69 Gy [62].
In a German cohort of 306 patients, there were too few events to test for a dose–response relationship. However, with the majority of our patients receiving 66.6 Gy, a total rate of 1.3 % grade 3 complications compares favorably with previous studies on conventional 3D-SRT [5].
Goenka et al. reported on 285 patients receiving post-RP SRT. The highest doses were 70–72 Gy in conventional 3D technique (n = 40) or IMRT (n = 165). Five-year actuarial rates for grade ≥2 GI and GU toxicity were 5.2 % and 17 %, respectively [36]. Ost et al. applied salvage IMRT with 70–79 (median 76) Gy to 136 post-RP patients. Their respective figures were 8 % (GI) and 22 % (GU) [66]. Both studies report with 60 months median follow-up. A longer observation and additional studies may be necessary to judge conclusively on the potential side effects of dose escalation with IMRT. Nevertheless, compared to ART with 60–64 Gy, the rate of side effects of SRT with ≥70 Gy appears to be higher.
6 Adjuvant Versus Salvage Radiation Therapy
While prospective randomized trials are underway to compare SRT and ART, several retrospective analyses into that question have been conducted. In a first report, 75 patients receiving ART at a median dose of 60 Gy were compared with 71 patients who had SRT at 70 Gy. Although 49 % of the SRT patients and only 3 % of the ART patients received adjuvant HT, the 5‑year post-RT bNED rate was 66 versus 88 % in favor of ART (p < 0.0008) [95].
In a case–control analysis, 361 ART patients were compared with 722 non‑ART patients, who were selected to match the cases by age, pre‑RP PSA, tumor stage, Gleason score, and surgical margin status. While 10‑year bNED after ART was significantly improved over non‑ART (63 vs. 45 %), there was no difference in overall survival. In the same study, an SRT cohort of 856 patients who were treated after biochemical relapse (median PSA: 0.8 ng/ml) was followed up over a median of 5.9 years. A total of 63 % of the SRT patients achieved an undetectable PSA after SRT and the hazard ratio for local recurrence after SRT was 0.13. However, similar to ART, no improved overall survival could be shown after SRT [13].
A straight retrospective comparison with salvage (76 Gy) and adjuvant (74 Gy) IMRT patients (n = 89 in both arms) who were matched for personal and tumor characteristics resulted in a significant bNED advantage from ART calculated either from the time of RP or from the end of RT (90 vs. 65 % 3 years post‑RT and 91 vs. 84 % post-RP). However, the pre‑RT PSA was a key parameter for that difference: a subcohort (n = 38) receiving early SRT (at PSA <0.5 ng/ml) had a 3‑year post‑RT bNED rate of 86 %, quite different from the delayed SRT group, who had 46 % bNED, but very similar to ART patients. Therefore, while overall Kaplan–Meier rates of bNED calculated in either mode suggested a benefit from ART, it was concluded that ART and early SRT did not yield significantly different results. This study included tumor stages from pT2 to pT4 and approximately 30 % of the patients had received HT [65].
Recently, Trabulsi and colleagues studied a group of patients undergoing adjuvant radiation therapy with a matched control group undergoing salvage radiation therapy after biochemical failure. Using a multi-institutional database of 2299 patients, 449 patients with pT3–4N0 disease were eligible, including 211 patients receiving adjuvant radiation therapy and 238 patients receiving salvage radiation therapy. Adjuvant radiation therapy significantly reduced the risk of long-term biochemical progression after radical prostatectomy compared with salvage radiation therapy (5-years freedom from biochemical failure (FFBF) was 73 % after adjuvant radiation therapy compared with 50 % after salvage radiation therapy; p = 0.007). Gleason score ≥8 was a significant predictor of FFBF [100].
The largest retrospective case-matching study to evaluate ART versus early SRT only included pT3 N0 R0/R1 patients. HT was excluded. A total of 390 out of 500 observation-plus-early-SRT patients (median pre‑SRT PSA was 0.2 ng/ml) were propensity matched with 390 ART patients. At 2 and 5 years after surgery, bNED rates were 91 and 78 %, respectively, for ART versus 93 and 82 %, respectively, for SRT. Subgroup analyses, too, yielded no significant differences for the two approaches. The study suggests that timely administration of SRT is comparable to ART in improving BCR-free survival in the majority of pT3pN0 PCa patients [18].
When comparing ART with SRT, it must be kept in mind that a considerable number of ART patients would be relapse-free even without RT. The proportion is likely to be the same as in the observation arms of the three randomized studies which was approximately 35 % after 10 years.
Currently, four prospective randomized trials are investigating the therapeutic benefit of early SRT with or without androgen-deprivation therapy compared with adjuvant RT: Radiotherapy and Androgen Deprivation in Combination After Local Surgery (RADICALS), Radiotherapy Adjuvant Versus Early Salvage (RAVES), GETUG-17, and EORTC 22043–30031. The results of these prospective studies will certainly contribute to guiding clinical practice in terms of indication and timing of postoperative RT.
Conclusions
Adjuvant radiation therapy (ART) provides improved biochemical relapse-free survival, and, possibly, overall survival for patients with a high-risk of recurrence after prostatectomy, when compared to observation. ART seems clearly indicated for patients with combined risk factors like pT3 and positive margins or positive margins and Gleason score 7–10.
It remains unknown whether early salvage radiation therapy (SRT) initiated after a PSA failure is equivalent to ART. At the present time, there are no published randomized trials to compare ART versus SRT. To this end, the results of the ongoing randomized clinical trials RADICALS, RAVES, GETUG-17 and EORTC 22043–30041 that compare ART and SRT directly are still awaited. When SRT is indicated, it should be initiated as early as possible (with PSA <0.5 ng/ml). In this situation SRT is the only curative therapy option.
The role of AD after adjuvant or salvage RT needs further investigation. But in two trials (RTOG 96–01 and GETUG-AFU 16) the addition of HT during and after RT significantly improved survival. Patients who most likely benefit have Gleason score ≥7, pre-SRT PSA to a maximum of 4.0 ng/ml and positive surgical margins. Up to now, the recommended type of hormone therapy and the optimum duration in this situation is unknown.
Modern radiation therapy techniques like IMRT should be used, ideally with image guidance. Serious side effects are apparently low, thus confirming the suitability of this therapeutic approach.
References
Abdollah F, Karnes RJ, Suardi N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. 2014;32:3939–47.
Afshar-Oromieh A, Avtzi E, Giesel FL. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209.
Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20.
Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys. 2000;48:369–75.
Bartkowiak D, Bottke D, Thamm R, et al. The PSA-response to salvage radiotherapy after radical prostatectomy correlates with freedom from progression and overall survival. Radiother Oncol. 2016;118:131–5.
Bastasch MD, Teh BS, Mai WY, et al. Post-nerve-sparing prostatectomy, dose-escalated intensity-modulated radiotherapy: effect on erectile function. Int J Radiat Oncol Biol Phys. 2002;54:101–6.
Beer AJ, Eiber M, Souvatzoglou M, et al. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 2011;12:181–91.
Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–44.
Bernard Jr JR, Buskirk SJ, Heckman MG, et al. Salvage radiotherapy for rising prostate-specific antigen levels after radical prostatectomy for prostate cancer: dose–response analysis. Int J Radiat Oncol Biol Phys. 2010;76:735–40.
Bolla M, De Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27.
Bolla M, Van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366:572–8.
Bolla M, van Poppel H, Tombal B. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018–27.
Boorjian SA, Karnes RJ, Crispen PL, et al. Radiation therapy after radical prostatectomy: impact on metastasis and survival. J Urol. 2009;182:2708–14.
Bottke D, Golz R, Störkel S, et al. Phase 3 study of adjuvant radiotherapy versus wait and see in pT3 prostate cancer: impact of pathology review on analysis. Eur Urol. 2013;64:193–8.
Briganti A, Karnes RJ, Da Pozzo LF, et al. Combination of adjuvante hormonal and radiation therapy significantly prolongs survival of patients with pT2-4 pN+ prostate cancer: results of a matched analysis. Eur Urol. 2011;59:832–40.
Briganti A, Karnes RJ, Gandaglia G, et al. Natural history of surgically treated high-risk prostate cancer. Urol Oncol. 2015;33:163.e7-13.
Briganti A, Karnes RJ, Joniau S. Prediction of outcome following early salvage radiotherapy among patients with biochemical recurrence after radical prostatectomy. Eur Urol. 2014;66:479–86.
Briganti A, Wiegel T, Joniau S, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol. 2012;62:472–87.
Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, Graefen M, Steuber T, Rosenbaum C. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69:393–6.
Buskirk SJ, Pisansky TM, Schild SE, et al. Salvage radiotherapy for isolated prostate specific antigen increase after radical prostatectomy: evaluation of prognostic factors and creation of a prognostic scoring system. J Urol. 2006;176:985–90.
Canter D, Greenberg RE, Horwitz EM, et al. Implantation of electromagnetic transponders following radical prostatectomy for delivery of IMRT. Can J Urol. 2010;17:5365–9.
Carrie C, Hasbini A, De Laroche G, et al. Interest of short hormonotherapy (HT) associated with radiotherapy (RT) as salvage treatment for biological relapse (BR) after radical prostatectomy (RP): results of the GETUG-AFU 16 phase III randomized trial. J Clin Oncol Meet Abst. 2015;33(Suppl):5006.
Castellucci P, Picchio M. 11C-choline PET/CT and PSA kinetics. Eur J Nucl Med Mol Imaging. 2013;40 Suppl 1:S36–40.
Chang JH, Park W, Park JS, et al. Significance of early prostate-specific antigen values after salvage radiotherapy in recurrent prostate cancer patients treated with surgery. Int J Urol. 2015;22:82–7.
Choo R, Danjoux C, Gardner S, et al. Efficacy of salvage radiotherapy plus 2-year androgen suppression for postradical prostatectomy patients with PSA relapse. Int J Radiat Oncol Biol Phys. 2009;75:983–9.
Coen JJ, Zietman AL, Thakral H, et al. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–205.
Connolly JA, Shinohara K, Presti Jr JC, et al. Local recurrence after radical prostatectomy: characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology. 1996;47:225–31.
Cooperberg MR, Davicioni E, Crisan A, et al. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur Urol. 2015;67:326–33.
Corn BW, Winter K, Pilepich MV. Does androgen suppression enhance the efficacy of postoperative irradiation? A secondary analysis of RTOG 85-31. Radiation Therapy Oncology Group. Urology. 1999;54:495–502.
Croke J, Malone S, Roustan Delatour N, et al. Postoperative radiotherapy in prostate cancer: the case of the missing target. Int J Radiat Oncol Biol Phys. 2012;83:1160–8.
Da Pozzo LF, Cozzarini C, Briganti A, et al. Long-term follow-up of patients with prostate cancer and nodal metastases treated by pelvic lymphadenectomy and radical prostatectomy: the positive impact of adjuvant radiotherapy. Eur Urol. 2009;55:1003–11.
Eder M, Schäfer M, Bauder-Wüst U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–97.
Eiber M, Maurer T, Souvatzoglou M. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74.
Fiorino C, Foppiano F, Franzone P, et al. Rectal and bladder motion during conformal radiotherapy after radical prostatectomy. Radiother Oncol. 2005;74:187–95.
Ghadjar P, Hayoz S, Bernhard J, et al. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate cancer after prostatectomy: first results of the randomized trial SAKK 09/10. J Clin Oncol. 2015;33:4158–66.
Goenka A, Magsanoc JM, Pei X, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol. 2011;60:1142–8.
Hanlon AL, Horwitz EM, Hanks GE, et al. Short-term androgen deprivation and PSA doubling time: their association and relationship to disease progression after radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:43–52.
Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79.
Heinisch M, Dirisamer A, Loidl W, et al. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA <5 ng/ml? Mol Imaging Biol. 2006;8:43–8.
Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, Gildehaus FJ, Stief CG, Gratzke C, Fendler WP. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;pii: S0302-2838(16)00009-9.
Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92–02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504.
Huch Boni RA, Meyenberger C, Pok Lundquist J, et al. Value of endorectal coil versus body coil MRI for diagnosis of recurrent pelvic malignancies. Abdom Imaging. 1996;21:345–52.
Jackson WC, Johnson SB, Foster B, et al. Combining prostate-specific antigen nadir and time to nadir allows for early identification of patients at highest risk for development of metastasis and death following salvage radiation therapy. Pract Radiat Oncol. 2014;4:99–107.
Kaminski JM, Hanlon AL, Joon DL, et al. Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. Int J Radiat Oncol Biol Phys. 2003;57:24–8.
Katz MS, Zelefsky MJ, Venkatraman ES, et al. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21:483–9.
Kibel AS, Ciezki JP, Klein EA, et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol. 2012;187:1259–65.
King CR, Kapp DS. Radiotherapy after prostatectomy: is the evidence for dose escalation out there? Int J Radiat Oncol Biol Phys. 2008;71:346–50.
King CR, Presti Jr JC, Gill H, et al. Radiotherapy after radical prostatectomy: does transient androgen suppression improve outcomes? Int J Radiat Oncol Biol Phys. 2004;59:341–7.
Krause BJ, Souvatzoglou M, Tuncel M, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:18–23.
Kupelian PA, Langen KM, Willoughby TR, et al. Daily variations in the position of the prostate bed in patients with prostate cancer receiving postoperative external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2006;66:593–6.
Leventis AK, Shariat SF, Slawin KM. Local recurrence after radical prostatectomy: correlation of US features with prostatic fossa biopsy findings. Radiology. 2001;219:432–9.
Lohm G, Lütcke J, Jamil B, et al. Salvage radiotherapy in patients with prostate cancer and biochemical relapse after radical prostatectomy: long-term follow-up of a single-center survey. Strahlenther Onkol. 2014;190:727–31.
Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76:361–8.
Michalski JM, Roach 3rd M, Merrick G, et al. ACR appropriateness criteria on external beam radiation therapy treatment planning for clinically localized prostate cancer expert panel on radiation oncology – prostate. Int J Radiat Oncol Biol Phys. 2009;74:667–72.
Mir MC, Li J, Klink JC, et al. Optimal definition of biochemical recurrence after radical prostatectomy depends on pathologic risk factors: identifying candidates for early salvage therapy. Eur Urol. 2014;66:204–10.
Miralbell R, Vees H, Lozano J, et al. Endorectal MRI assessment of local relapse after surgery for prostate cancer: a model to define treatment field guidelines for adjuvant radiotherapy in patients at high risk for local failure. Int J Radiat Oncol Biol Phys. 2007;67:356–61.
Moinpour CM, Hayden KA, Unger JM, et al. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26:112–20.
Moreira DM, Jayachandran J, Presti Jr JC, et al. Validation of a nomogram to predict disease progression following salvage radiotherapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2009;104:1452–6.
Nath SK, Sandhu AP, Rose BS, et al. Toxicity analysis of postoperative image-guided intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:435–41.
Naya Y, Okihara K, Evans RB, et al. Efficacy of prostatic fossa biopsy in detecting local recurrence after radical prostatectomy. Urology. 2005;66:350–5.
Neuhof D, Hentschel T, Bischof M, et al. Long-term results and predictive factors of three-dimensional conformal salvage radiotherapy for biochemical relapse after prostatectomy. Int J Radiat Oncol Biol Phys. 2007;67:1411–7.
Ohri N, Dicker AP, Trabulsi EJ, et al. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer. 2012;48:837–44.
Ost P, De Gersem W, De Potter B, et al. A comparison of the acute toxicity profile between two-dimensional and three-dimensional image-guided radiotherapy for postoperative prostate cancer. Clin Oncol (R Coll Radiol). 2011;23:344–9.
Ost P, De Meerleer G, Vercauteren T, et al. Delineation of the postprostatectomy prostate bed using computed tomography: interobserver variability following the EORTC delineation guidelines. Int J Radiat Oncol Biol Phys. 2011;81:e143–9.
Ost P, De Troyer B, Fonteyne V, et al. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1316–22.
Ost P, Lumen N, Goessaert AS, et al. High-dose salvage intensity-modulated radiotherapy with or without androgen deprivation after radical prostatectomy for rising or persisting prostate-specific antigen: 5-year results. Eur Urol. 2011;60:842–9.
Panebianco V, Sciarra A, Lisi D, et al. Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy. Eur J Radiol. 2012;81:700–8.
Parker C, Sydes MR, Catton C, et al. Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int. 2007;99:1376–9.
Paskalev K, Feigenberg S, Jacob R, et al. Target localization for post-prostatectomy patients using CT and ultrasound image guidance. J Appl Clin Med Phys. 2005;6:40–9.
Pazona JF, Han M, Hawkins SA, et al. Salvage radiation therapy for prostate specific antigen progression following radical prostatectomy: 10-year outcome estimates. J Urol. 2005;174(4 Pt 1):1282–6.
Pinkawa M, Siluschek J, Gagel B, et al. Postoperative radiotherapy for prostate cancer: evaluation of target motion and treatment techniques (intensity-modulated versus conformal radiotherapy). Strahlenther Onkol. 2007;183:23–9.
Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163:845–50.
Poortmans P, Bossi A, Vandeputte K, et al. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol. 2007;84:121–7.
Rossi Jr CJ, Joe Hsu IC, Abdel-Wahab M, et al. ACR appropriateness criteria postradical prostatectomy irradiation in prostate cancer. Am J Clin Oncol. 2011;34:92–8.
Sandhu A, Sethi R, Rice R, et al. Prostate bed localization with image-guided approach using on-board imaging: reporting acute toxicity and implications for radiation therapy planning following prostatectomy. Radiother Oncol. 2008;88:20–5.
Scattoni V, Montorsi F, Picchio M, et al. Diagnosis of local recurrence after radical prostatectomy. BJU Int. 2004;93:680–8.
Schiffner DC, Gottschalk AR, Lometti M, et al. Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;67:610–9.
Sella T, Schwartz LH, Swindle PW, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004;231:379–85.
Shariat SF, Karakiewicz PI, Roehrborn CG, et al. An updated catalog of prostate cancer predictive tools. Cancer. 2008;113:3075–99.
Shekarriz B, Upadhyay J, Wood Jr DP, et al. Vesicourethral anastomosis biopsy after radical prostatectomy: predictive value of prostate-specific antigen and pathologic stage. Urology. 1999;54:1044–8.
Shipley WU, Pugh SL, Lukka HR, et al. NRG Oncology/RTOG 9601, a phase III trial in prostate cancer patients: anti-androgen therapy (AAT) with bicalutamide during and after salvage radiation therapy following radical prostatectomy and an elevated PSA. J Clin Oncol. 2016;34:(suppl 2S; abstr 3).
Shipley WU, Seiferheld W, Lukka H, et al. Report of NRG oncology/RTOG 9601, a phase III trial in prostate cancer: anti-androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) in patients following radical prostatectomy (RP) with pT2-3pN0 disease and an elevated PSA. ASTRO meeting: Abstract LBA5; 2015.
Showalter TN, Nawaz AO, Xiao Y, et al. A cone beam CT-based study for clinical target definition using pelvic anatomy during postprostatectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:431–6.
Sidhom MA, Kneebone AB, Lehman M, et al. Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol. 2008;88:10–9.
Siegmann A, Bottke D, Faehndrich J, et al. Dose escalation for patients with decreasing PSA during radiotherapy for elevated PSA after radical prostatectomy improves biochemical progression-free survival: results of a retrospective study. Strahlenther Onkol. 2011;187:467–72.
Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Soto DE, Passarelli MN, Daignault S, et al. Concurrent androgen deprivation therapy during salvage prostate radiotherapy improves treatment outcomes in high-risk patients. Int J Radiat Oncol Biol Phys. 2011;82:1227–32.
Spiotto MT, Hancock SL, King CR. Radiotherapy after prostatectomy: improved biochemical relapse-free survival with whole pelvic compared with prostate bed only for high-risk patients. Int J Radiat Oncol Biol Phys. 2007;69:54–61.
Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–16.
Stephenson AJ, Bolla M, Briganti A, et al. Postoperative radiation therapy for pathologically advanced prostate cancer after radical prostatectomy. Eur Urol. 2012;61:443–51.
Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41.
Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–32.
Stephenson AJ, Wood DP, Kattan MW, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009;182:1357–63.
Sweat SD, Pacelli A, Murphy GP, et al. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40.
Taylor N, Kelly JF, Kuban DA, et al. Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 2003;56:755–63.
Thompson Jr IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–35.
Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–62.
Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol. 2013;190:441–9.
Tilki D, Mandel P, Schlomm T, et al. External validation of the CAPRA-S score to predict biochemical recurrence, metastasis and mortality after radical prostatectomy in a European cohort. J Urol. 2015;193:1970–5.
Trabulsi EJ, Valicenti RK, Hanlon AL, et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology. 2008;72:1298–302; discussion 1302–1294.
Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–9.
Van der Kwast TH, Bolla M, van Poppel H, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25:4178–86.
Wang J, Kudchadker R, Choi S, et al. Local recurrence map to guide target volume delineation after radical prostatectomy. Pract Radiat Oncol. 2014;4:e239–46.
Ward JF, Zincke H, Bergstralh EJ, et al. Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol. 2004;172(6 Pt 1):2244–8.
Wenz F, Martin T, Böhmer D, et al. The German S3 guideline prostate cancer: aspects for the radiation oncologist. Strahlenther Onkol. 2010;186:531–4.
Wiegel T, Bartkowiak D, Bottke D. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66:243–50.
Wiegel T, Bartkowiak D, Bottke D. Prostate-specific antigen persistence after radical prostatectomy as a predictive factor of clinical relapse-free survival and overall survival: 10-year data of the ARO 96–02 trial. Int J Radiat Oncol Biol Phys. 2015;91:288–94.
Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30.
Wiegel T, Lohm G, Bottke D, et al. Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome – results of a retrospective study. Int J Radiat Oncol Biol Phys. 2009;73:1009–16.
Wieser G, Mansi R, Grosu AL, et al. Positron emission tomography (PET) imaging of prostate cancer with a gastrin releasing peptide receptor antagonist-from mice to men. Theranostics. 2014;4:412–9.
Wiltshire KL, Brock KK, Haider MA, et al. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;69:1090–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bottke, D., Bartkowiak, D., Wiegel, T. (2017). Postoperative Irradiation: Immediate or Early Delayed?. In: Bolla, M., van Poppel, H. (eds) Management of Prostate Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-42769-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-42769-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42768-3
Online ISBN: 978-3-319-42769-0
eBook Packages: MedicineMedicine (R0)