Abstract
Type I interferonopathies are a diverse group of monogenic diseases which hallmarked the type I interferon (IFN) pathway activation. Abnormal accumulation of endogenous nucleic acids, excessive sensitivity or activity of DNA/RNA sensors, and the dysregulation of type I IFN pathway have been identified as the main contributors to excessive type I IFN signaling in this setting. Chilblain-like lesions, central nervous system calcifications, interstitial lung disease, and growth retardation are among the most common features shared by the majority of type I interferonopathies. Targeting of type I IFN signaling seems to hold a promising therapeutic role.
Authors Christina Maria Flessa and Evangelia Argiriou are equally contributed to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Interferonopathies
- Type I interferon
- Toll-like receptors
- Aicardi-Goutieres syndrome
- SAVI
- STING
- Chilblain
- Endosomal receptors
- Cytosolic receptors
Introduction
Type I interferonopathies are a heterogeneous group of autoinflammatory and autoimmune diseases characterized by distinct genetic and phenotypic features and hallmarked by activation of type I interferon (IFN) pathway [1]. IFNs have been first described in 1957 by Isaacs and Lindenmann as soluble factors that “interfere” with viral replication in host cells [2, 3]. IFNs are classified into three subgroups, known as types I, II, and III, based on their structure, chromosomal location, and receptor specificity. In particular, type I interferons consist of 13 functional α-genes and single genes for interferon-β, interferon-ε, interferon-κ, and interferon-ω [4].
In 1979, it has been first reported that circulating IFN levels are increased in patients with several autoimmune diseases including systemic lupus erythematosus (SLE), Sjogren’s syndrome and systemic sclerosis [5], a finding extensively replicated during the last decades [6,7,8,9]. An interesting early observation reported by Gresser et al. on experimental models implied a putatively harmful role of type I IFNs in developing embryos , which resulted in growth retardation, several organ damage, and necrosis [10]. Subsequently, it has been recognized a subset of patients presenting with progressive neurological problems resembling transplacental infections in the absence of an infectious agent. These abnormalities were transmitted following an autosomal recessive Mendelian pattern [11]. Of interest, serum and cerebrospinal fluid (CSF) of these patients suffering from the so-called Aicardi-Goutieres syndrome (AGS) demonstrated increased IFNα activity [12]. In 2003, the shared phenotypes between AGS, SLE and the utero-human immunodeficiency virus (HIV) infection, led Crow and colleagues to support type I IFN upregulation as a common denominator in these distinct clinical entities [13]. However, whether deregulation of type I IFN system is directly related to clinical manifestations remains to be further explored. Nevertheless, experimental and clinical evidence supports neurotoxic effects of IFNs in mice models and associations with thrombotic microangiopathy [14,15,16].
Over the past decade, it has been increasingly appreciated that certain clinical entities occurring early in life were characterized by a constellation of clinical features including chilblain-like skin lesions , lung inflammation, and intracranial calcifications together with overexpression of IFN-related genes. The latter has been shown to occur as a result of genetic aberrations of key molecules of the type I IFN pathway. Despite the fact that type I IFN activation is a common denominator in these disorders – the so-called interferonopathies – several differences in both clinical expression and underlying genetic background have been recognized. It seems that the observed variability relates to the presence of multiple biological effects of the type I IFN pathway molecules, the timing of IFN-related effects, as well as the impact of environmental stressors , such as cold or infections [1].
As mentioned above, interferonopathies are characterized by a mixture of autoinflammatory and autoimmune characteristics . Upregulation of type I IFNs results not only in the mobilization of innate immunity mechanisms, but also in chemokine expression and loss of self-tolerance, which ultimately leads to activation of autoreactive B and T cells and autoantibody production. Thus according to Crow and colleagues, type I interferonopathies can be considered “as autoinflammatory in origin with spill over into autoimmunity” [1], especially when there is a phenotypic overlap to SLE.
Nowadays, the current understanding of the mechanism contributing to the activation of innate immunity system against viral invasion, along with the identification of specific genetic aberrations leading to type I IFN activation, highlights the pivotal contribution of nucleic acid metabolism and signaling to type I IFN overexpression.
Type I IFN Pathway Signaling
Type I IFNs activate intracellular antimicrobial programs and influence the development of both innate and adaptive immune responses [17]. They are polypeptides that are secreted by infected cells, and their role can be summarized in three basic functions:
-
1.
Limitation of the spread of microbial insults and especially viral pathogens by inducing an “antiviral state” in infected and neighboring cells.
-
2.
Modulation of innate immune responses such as enhancement of antigen presentation and natural killer cell function together with restraining pro-inflammatory pathways.
-
3.
Activation of the adaptive immune system, thus promoting the development of high-affinity antigen-specific T- and B-cell responses and immunological memory [17].
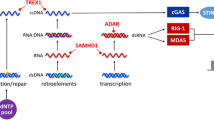
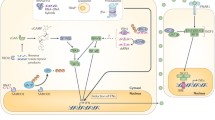
Canonical type I IFN signaling activates the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, leading to transcription of IFN-stimulated genes (ISGs) [17]. More specifically, IFN acts as a ligand and binds a heterodimeric transmembrane protein receptor called IFNα/β receptor (IFNAR) which is composed of IFNAR1 and IFNAR2 subunits [17]. Recent data indicate that the signal can be transmitted through IFNAR1 alone, through specific binding of IFNβ to IFNAR1, which is independent of IFNAR2 [18]. In the canonical type I IFN pathway, IFN binding to its receptor activates the receptor-associated protein tyrosine kinases JAK1 and tyrosine kinase 2 (TYK2), which in turn phosphorylate the cytoplasmic transcription factors signal transducer and activator of transcription 1 (STAT1) and STAT2. The latter subsequently dimerize and translocate to the nucleus, where they associate with IFN-regulatory factor 9 (IRF9). As a result, they form a complex called IFN-stimulated gene factor 3 (ISGF3), which binds to its cognate DNA sequences, the IFN-stimulated response elements (ISREs), part of the IFN-stimulated genes (ISGs), and directs transcription of ISGs [19,20,21,22] (Fig. 10.1).
Cytosolic nucleic acid sensing machinery resulting in type I IFN production (upper panel). Molecules implicated in the pathogenesis of type I interferonopathies are depicted in red. Lower panel: type I IFN signaling pathway following ligation of the type I IFN receptor leading to transcription of IFN-stimulated genes through the JAK/STAT pathway. ADAR1 adenosine deaminase acting on RNA 1; cGAMP cyclic GMP-AMP; cGAS cyclic GMP-AMP synthase; IFN interferon; IFNAR IFNα receptor; IKK IκB kinase; IRF3 IFN-regulatory factor 3; IRF9 IFN-regulatory factor 9; ISG IFN-stimulated gene; ISG15 interferon-stimulated gene 15; ISGF3 IFN-stimulated gene factor 3; ISRE IFN-stimulated response element; JAK Janus kinase; MAVS mitochondrial antiviral signaling; MDA5 melanoma differentiation-associated gene 5; NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells; POLA1 polymerase-α1; RIG-I retinoic acid-inducible gene I; RNAse H2, RNase H2A, RNase H2B, and RNase H2C ribonuclease H2 subunits A, B, and C; SAMHD1 deoxynucleoside triphosphate triphosphohydrolase and ribonuclease SAM domain and HD domain 1;STAT signal transducer and activator of transcription; STING stimulator of IFN genes; TBK1 TANK-binding kinase 1; TREX1 three prime (3′) repair exonuclease 1; TYK2 tyrosine kinase 2; USP18 ubiquitin-specific peptidase 18
Recent evidence point toward the presence of a STAT2-/IRF9-dependent IFNα but STAT1-independent signaling pathway [23]. In this case, STAT2 is capable of forming stable homodimers, when phosphorylated in response to IFNα, and these homodimers interact with IRF9 and can activate transcription of ISGs that carry ISRE sequences [23, 24]. Beyond the canonical pathway, IFN stimulates other pathways as well, such as STAT3 (especially in the absence of STAT1), p38 and ERK, PI-3K cascades, as well as the mTOR-Akt-S6K axis [19, 25].
Activation of Type I IFN Pathway: Role of Toll-Like Receptors (TLRs) and Cytosolic Nucleic Acid Sensors
Activation of type I IFN system has been shown to occur either by sensing of exogenous or endogenous nucleic acids that can be recognized by two distinct categories of sensors: a. endosomal Toll-like receptors (TLRs) and b. cytosolic nucleic acid sensors . It is important that the immune system can distinguish between exogenous and self-nucleic acids in order to avoid unnecessary reactions against self.
Toll-Like Receptors (TLRs)
The main TLRs identifying nucleic acids include TLR3 which recognizes dsRNA, TLR7, and TLR8 triggered by ssRNA and TLR9 that senses unmethylated CpG DNA sequences. It is of note that mutations of these molecules have not been so far associated with type I interferonopathies [26,27,28].
RNA Sensors
Three Toll-like receptors function in the endosomes to recognize pathogen-derived RNA : TLR3, TLR7, and TLR8 [29, 30]. TLR3 activates TIR-domain-containing adapter-inducing IFNβ (TRIF), whereas TLR7 and TLR8 activate MyD88. Both adaptor proteins lead to the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), whereas IFN-regulatory factor 3 (IRF3) is activated by the TRIF pathway and IRF7 by the MyD88 pathway [31].
DNA Sensors
CpG DNA was initially found to be able to induce type I IFNs through TLR9 ligation in plasmacytoid dendritic cells (pDCs) [30]. TLR9 activates MyD88, which in turn leads to the activation of NF-κB and IRF7 in pDCs [31]. Nevertheless, cytoplasmic DNA of many other cell types that do not express TLR9 can also robustly produce type I IFNs [30] and will be discussed below.
Cytosolic Sensors
RNA Sensors
Cytosolic viral RNA is identified by retinoic acid-inducible gene I (RIG-I) and its homolog melanoma differentiation-associated gene 5 (MDA5) [30]. RIG-I is the founding member of the RIG-I-like receptor (RLR) family of cytosolic RNA sensors. The other two members are MDA5 and laboratory of genetics and physiology 2 (LGP2) [32]. Downstream of RIG-1, the adaptor protein mitochondrial antiviral signaling (MAVS) induces the cytosolic kinases IκB kinase (IKK) and TANK-binding kinase 1 (TBK1) which, in turn, activate the NF-κB, IRF3, and IRF7 pathways to induce type I IFNs [33]. Before the discovery of RLRs, two other proteins were implicated in the recognition of viral RNA: a. the IFN-inducible 2′–5′-oligoadenylate synthetase (OAS), an activator of the ribonuclease RNAse L [34] which degrades viral RNA inducing type I IFN production through the RIG-I pathway [30] and b. the dsRNA-dependent protein kinase R (PKR) which suppresses translation initiation and also induces type I IFN production as a response to dsRNA in some cell types [35].
DNA Sensors
The mechanisms through which the immune system senses DNA were not elucidated until recent years. The activated pathway in this case is the RNA polymerase III/RIG-I pathway. More specifically, double-stranded poly(dA-dT) DNA is converted by the DNA-dependent enzyme RNA polymerase III (Pol III) to an RNA species in the cytosol that bears 5′-triphosphate and forms a double-stranded RNA [36]. This form of RNA acts as a ligand for RIG-I which triggers type I IFN production through MAVS [30, 36]. The strict dependence of Pol III on AT-rich sequence as well as the DNA-induced IFN production in a sequence-independent manner pointed toward the presence of another more general cytoplasmic DNA-sensing pathway [30]. In 2008, a molecule was identified as a crucial signaling adaptor for type I IFN induction and was named stimulator of IFN genes (STING) [37]. STING [also known as transmembrane protein 173 (TMEM173)] is a predominantly endoplasmic reticulum-localized protein which when stimulated with dsDNA relocalizes to Golgi apparatus and assembles into punctate structures that contain the kinase TBK1 [38]. Subsequently, the C-terminal tail (CTT) of the carboxy-terminal domain of STING provides a scaffold to bring IRF3 in close proximity to TBK1, leading to TBK1-dependent phosphorylation and activation of IRF3, which can subsequently induce the expression of type I IFN genes [28, 38]. Upstream activators of STING include pathogen-derived nucleotides [cyclic (3′–5′) diguanylate (c-di-GMP) and cyclic (3′–5′) diadenylate (c-di-AMP)] [39, 40] as well as second messengers (cyclic GMP-AMP or cGAMP) produced by the enzyme cyclic GMP-AMP synthase (cGAS) through an enzymatic degradation of cytosolic DNA [30, 41,42,43]. Additionally, genetic variants of DNAse II – an endonuclease located in lysosomes and expressed in a variety of cells – and three prime (3′) repair exonuclease 1 (TREX1), the most abundant 3′➔5′ DNA exonuclease in cells, have been both shown to promote autoimmune responses by inability of degrading cytoplasmic DNA [30, 44, 45]. TREX1 specifically targets and digests DNA reverse-transcribed from endogenous retroelements or replication debris [30, 44]. If not degraded, these endogenous DNA substrates further activate the STING-dependent cytosolic DNA-sensing pathway, triggering type I IFN-dependent autoimmune diseases [30].
Finally, the presence of DNA in the cytosol of macrophages can activate the inflammasome, a multiprotein complex that activates the proteolytic enzyme caspase-1 and leads to the maturation of IL-1β [30]. The receptor for cytosolic DNA in the inflammasome pathway is the protein absent in melanoma 2 (AIM2) [46].
Type I Interferonopathies: Genetic Defects and Clinical Phenotypes
A growing body of evidence points toward an activated type I IFN pathway in several autoimmune diseases, possibly as a result of inappropriate immune reactions against endogenous nucleic acids. Overproduction of type I IFN can be attributed to three main events [47] including (a) increased availability of endogenous nucleic acids as a result of defective clearance or impaired silencing mechanisms , (b) enhanced activation or sensitivity of an innate immune sensor or adaptive molecule , and (c) dysregulation of type I IFN response negative feedback loops . Inappropriate expression of endogenous nucleic acids as a result of defective epigenetic silencing such as methylation has been shown to be able to induce type I IFNs in both SLE and Sjogren’s syndrome [48, 49] through TLR-dependent and TLR-independent pathways. More specifically, it has been suggested that L1 RNA can transduce the signal through both endosomal TLRs and the RIG-I and MDA5 pathways [49].
Next, we wished to present genetic, clinical, and laboratory characteristics of the main type I interferonopathies. Mutations in genes mainly affecting the recognition of DNA and RNA by cytosolic sensors as well as deregulation of type I IFN production seem to be causally linked with the so far recognized type I interferonopathies (Table 10.1, Figs. 10.1 and 10.2).
Main organ systems affected in type I interferonopathies. Indicative manifestations are displayed. (a 1) Vasculitic-like cutaneous lesions in a patient with SAVI syndrome (Source: Manoussakis et al. [101]). (a 2) Face lipodystrophy in a patient with CANDLE syndrome (Source: Torrelo A et al., J Am Acad Dermatol. 2010;62:489–95). (b) Cotton woollike spots in a patient with RVCL syndrome (Source: Stam AH et al., BRAIN. 2016;139: 2909–22). (c 1) Growth retardation in a patient suffering from CANDLE syndrome (Source: Torrelo A et al., J Am Acad Dermatol. 2010; 62: 489–95). (c 2) Skeletal dysplasias in a patient with SPENCD syndrome (Source: Lausch et al. [81]). (d) Calcifications in the left ventricle, aortic valve and ascending aorta, postmortem specimen in a patient with SMS syndrome (Source: Rutsch et al. [60]). (e) Interstitial lung disease-honeycombing-ground gland opacities in a patient with SAVI syndrome (Source: Manoussakis et al. [101]). (f) Cerebral calcifications within basal ganglia and the cerebral white matter (Source: Lausch et al. [81]). AGS Aicardi-Goutieres syndrome, CANDLE chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature, CNS central nervous system, FCL familial chilblain lupus, ISG15 deficiency interferon-stimulated gene 15 deficiency, MSK musculoskeletal system, PAN polyarteritis nodosa, RVCL retinal vasculopathy with cerebral leukodystrophy, SAVI STING-associated vasculopathy, infantile onset, SMS Singleton-Merten syndrome, SPENCD spondyloenchondrodysplasia, USP18 deficiency ubiquitin-specific peptidase 18 deficiency, XLPDR X-linked reticulate pigmentary disorder
Phenotypes Related to Aberrant Nucleic Acid Sensing
DNA Sensors
STING-Associated Vasculopathy, Infantile Onset (SAVI)
STING-associated vasculopathy with onset in infancy (SAVI) is a monogenic autoinflammatory disorder with an autosomal dominant type of inheritance , due to de novo, heterozygous gain-of-function mutations in TMEM173 gene. Of note, newly recognized mutations in exons 6 and 7 of TMEM173 have recently been reported [50]. As a result, activation of type I IFN pathway occurs, through cGAS-STING-TBK1-IRF3 pathway. In a recent experimental model, it was shown that upregulation of IFN-related genes in the context of SAVI occurs independently of IRF3 stimulation [51].
Similarly to other interferonopathies such as AGS, TREX1-related familial chilblain lupus, and CANDLE syndrome, the main clinical manifestations – which occur in early childhood – include vasculitic skin manifestations. These are mainly manifested as ulcerating acral skin lesions in fingers, toes, ears, and nose following cold exposure. SAVI patients frequently present with pulmonary involvement as well, mainly interstitial lung disease associated with fever attacks. Neurological complications have not been observed so far. Transiently increased autoantibody titers such as anti-neutrophil cytoplasmic antibody (ANCA) and anti-cardiolipin have been reported. Extensive perivascular inflammation with IgM and C3 deposition is a common finding in skin tissues from these patients [52, 53]. SAVI has been related to increased mortality rates due to pulmonary involvement in the context of infections in the lower respiratory system [54].
Retinal Vasculopathy with Cerebral Leukodystrophy (RVCL)
Retinal vasculopathy with cerebral leukodystrophy (RVCL) previously considered to encompass distinct clinical entities, namely, cerebroretinal vasculopathy (CVR), hereditary vascular retinopathy (HVR), and hereditary endotheliopathy, retinopathy, nephropathy, and stroke (HERNS), has been shown to result from a common autosomal dominant-type heterozygous TREX1 mutation. The latter leads to C-terminal truncation of TREX1, with preservation of the N-terminal DNAse domain resulting in impaired DNA clearance.
The main characteristics of RVCL include the middle-age onset, the progressive visual loss due to retinal vasculopathy (telangiectasias, microaneurysms, and retinal capillary obliteration around the macula), and variable neurological manifestations such as dementia or migraine. Neuroimaging studies demonstrate cerebral white matter abnormalities, including small infarcts resembling pseudotumors, as well as contrast-enhancing lesions similar to multiple sclerosis white matter alterations. Raynaud’s phenomenon, micronodular cirrhosis, and glomerular dysfunction have been also reported [55, 56].
RNA Sensors
Singleton-Merten Syndrome (SMS)
Singleton-Merten syndrome (SMS) – inherited in an autosomal dominant manner – is caused by heterozygous gain-of-function mutations in interferon-induced helicase C domain-containing protein 1 (IFIH1) encoding the cytosolic pattern recognition receptors for dsRNA MDA5 or RIG-I, resulting in constitutive type I IFN activation [57,58,59,60].
Though there is extensive phenotypic variability, the core characteristics of the syndrome include aortic and valvular calcifications, dental anomalies (early-onset periodontitis and root resorption), abnormal ossification (mainly distal limbs), alveolar bone loss, skeletal abnormalities as well as osteoporosis, osteopenia, and acro-osteolysis. Psoriasis, glaucoma, muscle weakness, abnormal ligaments of joints and muscle, photosensitivity, recurrent respiratory infections and typical face features can also frequently occur [57, 59, 60]. MDA-5 mutations have been particularly linked to Jaccoud’s arthropathy, also observed in patients with systemic lupus erythematosus (SLE) [61].
DNA and RNA Sensors
Aicardi-Goutieres Syndrome (AGS)
Aicardi-Goutieres syndrome (AGS) is a hereditary neurodegenerative disorder of inflammatory etiology, characterized by early-onset progressive encephalopathy with increased levels of IFNα in the CSF. It is regarded as the prototypic disease in the context of interferonopathies [59]. Clinical manifestations occur between 3 and 7 months of age and sometimes during the first week after birth. Especially, in cases of intrauterine onset, newborns are presented with microcephaly.
The main symptoms observed are vomiting, irritability with feeding difficulties, dystonia, epileptic seizures, fever episodes, and a rather subacute onset leading gradually in motor and social skills retardation. With regard to extra-neurological symptoms, patients may present with skin manifestations, like acral chilblain lesions upon cold exposure, raised intraocular pressure and sometimes overlapping lupus-like symptoms, such as hepatosplenomegaly, thrombocytopenia, lymphopenia, and positive antinuclear antibodies (ANA) [62].
Given that mild fever attacks are frequently combined with neurological features, AGS might be misdiagnosed as congenital encephalitis or meningitis caused by TORCH (toxoplasma, other agents, rubella, cytomegalovirus, herpes simplex) or HIV infection. It is estimated that about 25% of patients will die between 1 and 17 years [63].
MRI imaging findings mainly include basal ganglia calcification, white matter abnormalities and brain atrophy, while CSF lymphocytosis and increased IFNα levels are characteristic laboratory manifestations. Similarly to lupus and other autoimmune diseases [8, 64, 65], overexpression of IFN-related genes in peripheral blood samples, known as interferon (IFN)-signature, has been detected in almost every patient at any age [62].
Aberrations of a number of genes have been found to account for AGS. These include TREX1, the three subunits of the ribonuclease H2 (RNAse H2), endonuclease complex (RNase H2A, RNase H2B and RNase H2C), the deoxynucleoside triphosphate triphosphohydrolase and ribonuclease SAM domain and HD domain 1 (SAMHD1), adenosine deaminase acting on RNA (ADAR; also known as DRADA), and the double-stranded RNA (dsRNA) cytosolic sensor IFIH1, also known as MDA5.
Biallelic loss-of-function mutations both for TREX1 and for each subunit of RNAse H2 complex result in intracellular accumulation of endogenous nucleic acid products, whose source is considered to be either chronic DNA damage, especially during DNA replication, or human genome-derived retroelements [66]. Eventually, single-stranded DNA (ssDNA) products trigger the activation of type I IFN axis through cGAS/TBK1/IRF3 pathway [67]. The latter has been also recently shown to be the downstream pathway activated by RNase H2 sensors [68].
Furthermore, biallelic loss-of-function mutations of SAMHD1 lead to chronic DNA damage, activation of type I IFN pathway as well as cerebrovascular aneurysms or stenosis, early-onset strokes and increased malignancy risk [69, 70], through a so far unexplored mechanism. It is of note that SAMHD1 and TREX1 enzymes are implicated in long interspersed nuclear element-1 (LINE-1) retroelements metabolism . Recent findings report activation of the cGAS/STING pathway as the downstream effectors for type I IFN production [71].
With regard to ADAR enzyme, both biallelic and heterozygous mutations lead to excessive recognition of endogenous double-stranded RNA (dsRNA) and to IFN release, via defective editing of short interspersed element (SINES) retroelements, which account for 10% of human genome [72, 73]. In particular, mutations of ADAR1 are implicated in expression of neurological defects, such as bilateral striatal necrosis (BSN) and spastic paraparesis (SP) as well. Additionally, they are related to dyschromatosis symmetrica hereditaria (DSH), a rare autosomal dominant disorder, detected in East Asian population, characterized by mixed hyper- and hypopigmented macules on the dorsal aspect of the hands and feet and freckle-like macules on the face [74].
Lastly, gain-of-function mutations of IFIH1, which encodes the MDA5 sensor, have also an increased affinity to dsRNA molecules and through MAVS activation result eventually in IFN overexpression [75, 76]. Similar to ADAR1 and SAMHD1 mutations, patients demonstrate especially neurological complications, such as SP [66].
Familial Chilblain Lupus (FCL)
Familial chilblain lupus (FCL) is a rare monogenic form of cutaneous lupus erythematosus, which is inherited in an autosomal dominant way. It affects patients in early childhood in a more rapidly progressive way compared to spontaneous lupus erythematosus, which is presented in middle-aged women. Patients suffer from partly ulcerating acral lesions or painful bluish-red papules located in the fingers, toes, nose and ears. These lesions are exacerbated by cold exposure and usually improve during summer. They can also be accompanied by arthralgias, affecting mainly large joints, without evidence of true arthritis, photosensitivity, or mouth ulcers.
Laboratory testing revealed slightly elevated antinuclear antibody (ANA) titers and/or lymphopenia, without any other specific serological marker. Given that nailbed alterations seem to be specific in a subset of FCL patients, capillaroscopy may be a promising modality to be implemented. Histopathological findings in affected skin tissues include perivascular inflammatory infiltrates with granular deposits of immunoglobulins and complement along the basement membrane.
Several genetic defects have been so far related to FCL. Heterozygous TREX1 mutations (implicated in AGS and impairment of susceptibility to granzyme-A-mediated cell death) as well as of SAMHD1 and STING molecules lead to FCL, through activation of the known cGAS-cGAMP-STING-TBK1-IRF3 pathway [77,78,79].
Type I IFN Deregulation
Spondyloenchondrodysplasia (SPENCD)
Spondyloenchondrodysplasia (SPENCD) is inherited in an autosomal recessive manner and it is caused by biallelic mutations in acid phosphatase 5 (ACP5), encoding tartrate-resistant acid phosphatase (TRAP) [80]. TRAP dephosphorylates and inactivates the protein osteopontin (OPN) – a bone matrix protein which upon dephosphorylation by TRAP leads a. to reduced osteoclast binding to different substrates [81], and b. dampened IFNα production by plasmacytoid dendritic cells (pDCs) through disruption of TLR-9/myeloid differentiation factor 88 (MyD88) mediated IFN regulatory factor 7 (IRF-7) activation [82]. Patients with SPENCD present constitutively activated osteopontin, which is probably responsible for increased bone resorption and immune dysregulation that leads to overproduction of type I IFN [59, 83].
Spondyloenchondrodysplasia (SPENCD) – first identified by Schorr et al. in 1976 – is a skeletal dysplasia characterized by enchondromatous radiolucent, irregular spondylar and metaphyseal lesions which represent islands of cartilage tissue within bone leading to platyspondyly. The severity of the lesions varies, but slow progression during childhood is most frequently observed, resulting in significantly short stature. It appears that SPENCD is clinically heterogeneous, as some SPENCD patients are neurologically intact, while others present with neurological dysfunction including spasticity, mental retardation and cerebral calcifications in different combinations. In addition, signs of immune dysregulation and systemic autoimmunity, such as arthritis, antinuclear antibodies and recurrent infections, are often observed in patients with SPENCD [59, 80, 84,85,86,87].
Interferon-Stimulated Gene 15 Deficiency (ISG15 Deficiency)
Deficiency of the IFNγ-inducing molecule named interferon-stimulated gene 15 (ISG15) leads to a clinical phenotype characterized by a prominent type I IFN signature in peripheral blood and increased susceptibility to mycobacterial disease possibly as a result of dampened IFNγ production. Absence of intracellular ISG15 prevents the accumulation of ubiquitin-specific peptidase 18 (USP18), a potent negative regulator of type I IFN signaling, resulting in the enhancement and amplification of type I IFN responses [88]. ISG15 deficiency is inherited in an autosomal recessive manner. Similarly to AGS and SPENCD, ISG15-deficient individuals also display marked intracranial calcification [59, 88, 89].
Ubiquitin-Specific Peptidase 18 Deficiency (USP18 Deficiency)
This disorder also known as pseudo-TORCH syndrome is caused by autosomal recessive homozygous mutations of the gene USP18 , encoding the protein ubiquitin-specific protease 18, which cleaves the ubiquitin-like ISG15 protein from its conjugated proteins [59, 90]. Given the negative regulatory role of this molecule in type I IFN activation, type I IFN overexpression occurs. The clinical spectrum resembling a congenital infection but in the absence of an infectious agent includes microcephaly, enlarged ventricles, and cerebral calcification.
Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated Temperature (CANDLE)
CANDLE – a proteasome-associated autoinflammatory syndrome (PRAAS) – has been shown to occur in the context of several defects in catalytic activity of proteasome-immunoproteasome system related to mutations of genes encoding distinct protein subunits (β5i, β7, β1i, α3) [59, 91, 92]. It is inherited in an autosomal recessive pattern and characterized by enhanced peripheral blood ISG expression and constitutive STAT1 phosphorylation [59]. The latter seems to be related to previous observations supporting that proteasome inhibition led to activation of type I IFN pathway through enhancement of MAVS activity [93]. Recurrent fevers in the first months of life, along with characteristic skin lesions, lipodystrophy, violaceous swollen eyelids, arthralgias, extremity contractures, and delayed physical development as well as systemic inflammation markers have been identified as CANDLE -related clinical manifestations [92, 94]. CANDLE has been linked to early mortality rates due to cardiomyopathy, infections, and cardiac arrhythmias [54].
X-Linked Reticulate Pigmentary Disorder (XLPDR)
XLPDR is caused by an intronic mutation of the POLA1 gene, which encodes the catalytic subunit of DNA polymerase-α, an enzyme necessary for the synthesis of RNA-DNA primers during DNA replication. Unexpectedly, it was found that POLA1 is required for the synthesis of cytosolic RNA-DNA hybrids as well, which display an inhibitory action on type I IFN activation. In the setting of XLPDR, missplicing and disruption of POLA1 expression occur, leading to heightened type I IFN production through constitutive activation of IRF- and NF-κB-dependent genes [95] and a constellation of clinical features distinct between male and female carriers. Thus affected male individuals present with generalized hyperpigmentation intermingled with small hypomelanotic macules during early childhood, a distinctive face characterized by an upswept frontal hairline and arched eyebrows, as well as severe photophobia, recurrent respiratory infections and severe gastrointestinal disorders. In female carriers, the type and distribution of the pigmentation mimic that of incontinentia pigmenti (patchy pigmentary skin lesions along the lines of Blaschko), without any systemic manifestations [59, 96, 97].
Childhood-Onset Polyarteritis Nodosa: ADA2 Deficiency
Childhood-onset polyarteritis nodosa (PAN) is an autosomal recessive systemic vascular inflammatory disorder affecting mainly the brain and skin caused by biallelic mutations of the cat eye syndrome chromosome region, candidate 1 (CECR1). CECR1 encodes the enzyme adenosine deaminase 2 (ADA2) which deaminates adenosine to inosine. Patients with ADA2 deficiency exhibit constitutive type I IFN activation in blood, although the underlying mechanism is unclear [59, 98].
Fever, necrotizing vasculitis of the gastrointestinal tract, and renal aneurysms as well as varying degrees of immunodeficiency and autoimmunity have been described and related to this disorder. The ensuing tissue ischemia can affect any organ, including the skin, musculoskeletal system, kidneys, gastrointestinal tract, and the cardiovascular and nervous systems [59, 98].
Therapeutic Implications
The delineation of pathogenetic mechanisms , implicated in type I IFN-related diseases, has provided promising prospects for the development of new therapeutic strategies, given the relative ineffectiveness of conventional immunosuppressive treatment. Since gene expression products of TREX1, SAMHD1, RNAse H2, and ADAR are involved in metabolism of endogenous retrovirus-derived nucleic acids, it has been postulated that the use of reverse transcriptase inhibitors might be effective in controlling inflammatory activity of the disease. In fact, the successful effect of reverse transcriptase inhibitors in an experimental TREX1-deficient mouse model has been reported [99], while a pilot phase II study is in progress, in pediatric population, using as treatment modalities zidovudine, lamivudine, and abacavir [NCT02363452].
In the context of interferonopathies, the common pathogenetic mechanism, leading to ISGs upregulation, is the JAK/STAT activation through triggering of the IFNAR receptor. Given the possibility of a potential therapeutic target, in 2016, Fremond and his colleagues described three cases of children, carrying TMEM173 mutations, who were successfully treated with Janus kinase 1/2 inhibitor ruxolitinib [100]. Furthermore, another case of patient with TMEM173-related FCL was treated with JAK (1/3) inhibitor tofacitinib for a very short period of time with doubtful results [101] [102]. Moreover, JAK-inhibitor baricitinib, previously shown to be successful in a case of CANDLE syndrome with alopecia areata [103], is currently tested in an ongoing clinical trial including patients with SAVI, juvenile dermatomyositis, and proteasome-related autoinflammatory syndrome [NCT01724580].
In the same context, targeted therapies against IFNAR receptor (anifrolumab) and IFNα itself (sifalimumab) – with promising so far results in lupus populations though [104,105,106], as well as hydroxychloroquine recently shown to inhibit cGAS stimulation by dsDNA molecules [107] – seem to be additional future options for patients with interferonopathies.
Conclusions
Type I interferonopathies is a recently discovered group of genetic disorders hallmarked by activation of type I IFN pathway and a wide variety of clinical phenotypes possibly related to tissue-specific gene or protein, together with the effects of environmental triggers. Major issues to be resolved also include the underlying mechanistic relationship between type I IFN pathway, tissue damage, and clinical phenotype as well the specific molecules along the extended type I IFN pathway that need to be targeted. To conclude, further investigations to better clarify the underlying mechanisms on excessive activation of type I IFN system are required leading to novel discoveries enabling more effective treatment strategies in the future.
ADAR1 adenosine deaminase acting on RNA 1; AGS Aicardi-Goutieres syndrome; BSN bilateral striatal necrosis; CANDLE chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature; cGAS cyclic GMP-AMP synthase; CVD cerebrovascular disease; DSH dyschromatosis symmetrica hereditaria; FCL familial chilblain lupus; IFIH1 interferon-induced helicase C domain-containing protein 1; IFN type I interferon; IRF3 IFN-regulatory factor 3; IRF7 IFN-regulatory factor 7; ISG15 deficiency interferon-stimulated gene 15 deficiency; ISG15 interferon-stimulated gene 15; MAVS mitochondrial antiviral signaling; MDA5 melanoma differentiation-associated gene 5; POLA1 polymerase-α1; RIG-I retinoic acid-inducible gene I; RNase H2A, RNase H2B, and RNase H2C RNAse H2 ribonuclease H2 subunits A, B, and C; RVCL retinal vasculopathy with cerebral leukodystrophy; SAMHD1 deoxynucleoside triphosphate triphosphohydrolase and ribonuclease SAM domain and HD domain 1; SAVI STING-associated vasculopathy, infantile onset; SLE systemic lupus erythematosus; SMS Singleton-Merten syndrome; SP spastic paraparesis; SPENCD spondyloenchondrodysplasia; STING stimulator of IFN genes; TBK1 TANK-binding kinase 1; TLR Toll-like receptor; TMEM173 transmembrane protein 173; TORCH toxoplasma, other agents, rubella, cytomegalovirus, herpes simplex; TREX1 three prime (3′) repair exonuclease 1; USP18 deficiency ubiquitin-specific peptidase 18 deficiency; USP18 ubiquitin-specific peptidase 18; XLPDR X-linked reticulate pigmentary disorder
References
Rodero MP, Crow YJ. Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J Exp Med. 2016;213(12):2527–38.
Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–67.
Isaacs A, Lindenmann J, Valentine RC. Virus interference. II. Some properties of interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):268–73.
Mavragani CP, Crow MK. Activation of the type I interferon pathway in primary Sjogren's syndrome. J Autoimmun. 2010;35(3):225–31.
Hooks JJ, et al. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301(1):5–8.
Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192(12):5459–68.
Kim D, et al. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008;58(7):2163–73.
Nezos A, et al. Type I and II interferon signatures in Sjogren's syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjogren's related lymphomagenesis. J Autoimmun. 2015;63:47–58.
Vakaloglou KM, Mavragani CP. Activation of the type I interferon pathway in primary Sjogren’s syndrome: an update. Curr Opin Rheumatol. 2011;23(5):459–64.
Gresser I, et al. Interferon-induced disease in mice and rats. Ann N Y Acad Sci. 1980;350:12–20.
Aicardi J, Goutieres F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15(1):49–54.
Lebon P, et al. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J Neurol Sci. 1988;84(2–3):201–8.
Crow YJ, et al. Cree encephalitis is allelic with Aicardi-Goutieres syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J Med Genet. 2003;40(3):183–7.
Akwa Y, et al. Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161(9):5016–26.
Campbell IL, et al. Structural and functional neuropathology in transgenic mice with CNS expression of IFN-alpha. Brain Res. 1999;835(1):46–61.
Kavanagh D, et al. Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood. 2016;128(24):2824–33.
Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49.
de Weerd NA, et al. Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nat Immunol. 2013;14(9):901–7.
Mostafavi S, et al. Parsing the interferon transcriptional network and its disease associations. Cell. 2016;164(3):564–78.
Muller M, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993;366(6451):129–35.
Stark GR, Darnell JE Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–14.
Velazquez L, et al. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70(2):313–22.
Blaszczyk K, et al. STAT2/IRF9 directs a prolonged ISGF3-like transcriptional response and antiviral activity in the absence of STAT1. Biochem J. 2015;466(3):511–24.
Bluyssen HA, Levy DE. Stat2 is a transcriptional activator that requires sequence-specific contacts provided by stat1 and p48 for stable interaction with DNA. J Biol Chem. 1997;272(7):4600–5.
Uddin S, Platanias LC. Mechanisms of type-I interferon signal transduction. J Biochem Mol Biol. 2004;37(6):635–41.
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–7.
Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–61.
Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38(5):870–80.
Vidya MK, et al. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol. 2018;37(1):20–36.
Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–88.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–84.
Yoneyama M, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175(5):2851–8.
Seth RB, et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–82.
Silverman RH. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol. 2007;81(23):12720–9.
Diebold SS, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424(6946):324–8.
Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138(3):576–91.
Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–8.
Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5(214):ra20.
Du XX, Su XD. Detection of cyclic dinucleotides by STING. Methods Mol Biol. 2017;1657:59–69.
Burdette DL, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478(7370):515–8.
Xiao TS, Fitzgerald KA. The cGAS-STING pathway for DNA sensing. Mol Cell. 2013;51(2):135–9.
Sun L, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91.
Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–30.
Stetson DB, et al. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134(4):587–98.
Crow YJ. The story of DNase II: a stifled death-wish leads to self-harm. Eur J Immunol. 2010;40(9):2376–8.
Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10(3):266–72.
Picard C, Belot A. Does type-I interferon drive systemic autoimmunity? Autoimmun Rev. 2017;16(9):897–902.
Mavragani CP, et al. Defective regulation of L1 endogenous retroelements in primary Sjogren’s syndrome and systemic lupus erythematosus: role of methylating enzymes. J Autoimmun. 2018;88:75–82.
Mavragani CP, et al. Expression of long interspersed nuclear element 1 retroelements and induction of type I interferon in patients with systemic autoimmune disease. Arthritis Rheumatol. 2016;68(11):2686–96.
Melki I, et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J Allergy Clin Immunol. 2017;140(2):543–52. e5
Warner JD, et al. STING-associated vasculopathy develops independently of IRF3 in mice. J Exp Med. 2017;214:3279–92.
Jeremiah N, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124(12):5516–20.
Liu Y, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507–18.
Kim H, Sanchez GA, Goldbach-Mansky R. Insights from Mendelian interferonopathies: comparison of CANDLE, SAVI with AGS, monogenic lupus. J Mol Med (Berl). 2016;94(10):1111–27.
Richards A, et al. C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat Genet. 2007;39(9):1068–70.
Schuh E, et al. Multiple sclerosis-like lesions and type I interferon signature in a patient with RVCL. Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e55.
Feigenbaum A, et al. Singleton-Merten syndrome: an autosomal dominant disorder with variable expression. Am J Med Genet A. 2013;161A(2):360–70.
Jang MA, et al. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet. 2015;96(2):266–74.
Lee-Kirsch MA. The type I interferonopathies. Annu Rev Med. 2017;68:297–315.
Rutsch F, et al. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am J Hum Genet. 2015;96(2):275–82.
de Carvalho LM, et al. Musculoskeletal disease in MDA5-related type I interferonopathy: a Mendelian mimic of Jaccoud’s arthropathy. Arthritis Rheumatol. 2017;69(10):2081–91.
Chahwan C, Chahwan R. Aicardi-Goutieres syndrome: from patients to genes and beyond. Clin Genet. 2012;81(5):413–20.
Barth PG. The neuropathology of Aicardi-Goutieres syndrome. Eur J Paediatr Neurol. 2002;6(Suppl A):A27–31; discussion A37–9, A77–86.
Ekholm L, et al. Autoantibody specificities and type I interferon pathway activation in idiopathic inflammatory myopathies. Scand J Immunol. 2016;84(2):100–9.
Eloranta ML, Ronnblom L. Cause and consequences of the activated type I interferon system in SLE. J Mol Med (Berl). 2016;94(10):1103–10.
Crow YJ, Manel N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15(7):429–40.
Ablasser A, et al. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J Immunol. 2014;192(12):5993–7.
Mackenzie KJ, et al. Ribonuclease H2 mutations induce a cGAS/STING-dependent innate immune response. EMBO J. 2016;35(8):831–44.
Clifford R, et al. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood. 2014;123(7):1021–31.
Kretschmer S, et al. SAMHD1 prevents autoimmunity by maintaining genome stability. Ann Rheum Dis. 2015;74(3):e17.
Maelfait J, et al. Restriction by SAMHD1 limits cGAS/STING-dependent innate and adaptive immune responses to HIV-1. Cell Rep. 2016;16(6):1492–501.
Mannion NM, et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 2014;9(4):1482–94.
Vitali P, Scadden AD. Double-stranded RNAs containing multiple IU pairs are sufficient to suppress interferon induction and apoptosis. Nat Struct Mol Biol. 2010;17(9):1043–50.
Hayashi M, Suzuki T. Dyschromatosis symmetrica hereditaria. J Dermatol. 2013;40(5):336–43.
Funabiki M, et al. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity. 2014;40(2):199–212.
Oda H, et al. Aicardi-Goutieres syndrome is caused by IFIH1 mutations. Am J Hum Genet. 2014;95(1):121–5.
Fiehn C. Familial chilblain lupus - what can we learn from type I interferonopathies? Curr Rheumatol Rep. 2017;19(10):61.
Lee-Kirsch MA, et al. A mutation in TREX1 that impairs susceptibility to granzyme A-mediated cell death underlies familial chilblain lupus. J Mol Med (Berl). 2007;85(5):531–7.
Lee-Kirsch MA, et al. Familial chilblain lupus, a monogenic form of cutaneous lupus erythematosus, maps to chromosome 3p. Am J Hum Genet. 2006;79(4):731–7.
Briggs TA, et al. Spondyloenchondrodysplasia due to mutations in ACP5: a comprehensive survey. J Clin Immunol. 2016;36(3):220–34.
Lausch E, et al. Genetic deficiency of tartrate-resistant acid phosphatase associated with skeletal dysplasia, cerebral calcifications and autoimmunity. Nat Genet. 2011;43(2):132–7.
An J, et al. Tartrate-resistant acid phosphatase deficiency in the predisposition to systemic lupus erythematosus. Arthritis Rheumatol. 2017;69(1):131–42.
Briggs TA, et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet. 2011;43(2):127–31.
Navarro V, et al. Two further cases of spondyloenchondrodysplasia (SPENCD) with immune dysregulation. Am J Med Genet A. 2008;146A(21):2810–5.
Renella R, et al. Spondyloenchondrodysplasia with spasticity, cerebral calcifications, and immune dysregulation: clinical and radiographic delineation of a pleiotropic disorder. Am J Med Genet A. 2006;140(6):541–50.
Roifman CM, Melamed I. A novel syndrome of combined immunodeficiency, autoimmunity and spondylometaphyseal dysplasia. Clin Genet. 2003;63(6):522–9.
Schorr S, Legum C, Ochshorn M. Spondyloenchondrodysplasia. Enchondromatomosis with severe platyspondyly in two brothers. Radiology. 1976;118(1):133–9.
Zhang X, et al. Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature. 2015;517(7532):89–93.
Bogunovic D, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337(6102):1684–8.
Meuwissen ME, et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med. 2016;213(7):1163–74.
Brehm A, et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest. 2015;125(11):4196–211.
Torrelo A. CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front Immunol. 2017;8:927.
Castanier C, et al. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol. 2012;10:44.
Tufekci O, et al. CANDLE syndrome: a recently described autoinflammatory syndrome. J Pediatr Hematol Oncol. 2015;37(4):296–9.
Starokadomskyy P, et al. DNA polymerase-alpha regulates the activation of type I interferons through cytosolic RNA:DNA synthesis. Nat Immunol. 2016;17(5):495–504.
Pezzani L, et al. X-linked reticulate pigmentary disorder with systemic manifestations: a new family and review of the literature. Am J Med Genet A. 2013;161A(6):1414–20.
Zhang J, Li M, Yao Z. Updated review of genetic reticulate pigmentary disorders. Br J Dermatol. 2017;177(4):945–59.
Navon Elkan P, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370(10):921–31.
Beck-Engeser GB, Eilat D, Wabl M. An autoimmune disease prevented by anti-retroviral drugs. Retrovirology. 2011;8:91.
Fremond ML, et al. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol. 2016;138(6):1752–5.
Manoussakis MN, et al. Type I interferonopathy in a young adult. Rheumatology (Oxford). 2017;56:2241–3.
Konig N, et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis. 2017;76(2):468–72.
Jabbari A, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine. 2015;2(4):351–5.
Oon S, Wilson NJ, Wicks I. Targeted therapeutics in SLE: emerging strategies to modulate the interferon pathway. Clin Transl Immunol. 2016;5(5):e79.
Petri M, et al. Sifalimumab, a human anti-interferon-alpha monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013;65(4):1011–21.
Relle M, et al. Genetics and novel aspects of therapies in systemic lupus erythematosus. Autoimmun Rev. 2015;14(11):1005–18.
An J, et al. Cutting edge: antimalarial drugs inhibit IFN-beta production through blockade of cyclic GMP-AMP synthase-DNA interaction. J Immunol. 2015;194(9):4089–93.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Flessa, C.M., Argiriou, E., Mavragani, C.P. (2019). Type I Interferonopathies: From Pathophysiology to Clinical Expression. In: Efthimiou, P. (eds) Auto-Inflammatory Syndromes. Springer, Cham. https://doi.org/10.1007/978-3-319-96929-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-96929-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96928-2
Online ISBN: 978-3-319-96929-9
eBook Packages: MedicineMedicine (R0)