Abstract
Type I interferons (IFNs) play a central role in the immune defense against viral infections. Type I IFN signaling is activated by pattern recognition receptors upon sensing of viral nucleic acids and induces antiviral programs through modulation of innate and adaptive immune responses. Type I interferonopathies comprise a heterogenous group of genetically determined diseases that are characterized by inappropriate activation of type I IFN. While their phenotypic spectrum is broad, ranging from severe neurological impairment to mild cutaneous disease, systemic autoinflammation, and autoimmunity are commonly shared signs of type I interferonopathies. Although the mechanisms underlying various disease phenotypes associated with inappropriate type I IFN activation have yet to be fully elucidated, our current understanding of the molecular pathogenesis of type I interferonopathies has provided a set of candidate molecules that can be interrogated in search of targeted therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type I interferons (IFNs), IFN-α and IFN-β, constitute the major effector cytokines of the host immune response against viruses and other intracellular pathogens. Type I IFNs, which can be produced by almost every cell, are normally not secreted constitutively but induced by pattern recognition receptors of the innate immune system that recognize danger signals such as viral nucleic acids. Induction of type I IFN signaling results in the transcriptional activation of numerous interferon-stimulated genes (ISGs) which comprise a complex cross-regulatory network of pathways to restrict viral spread, to eliminate infected cells, and to provide protection with minimum damage to the host [1, 2]. However, if inappropriately activated, type I IFNs can be detrimental to the host by promoting autoinflammatory responses and a break of immune tolerance leading to autoimmunity. This is exemplified by the well-recognized role of type I IFN in the pathogenesis of systemic lupus erythematosus (SLE) [3].
The genetic and molecular dissection of rare Mendelian disorders associated with inappropriate type I IFN activation has provided unique insight into disease mechanisms that initiate and sustain autoinflammation and autoimmunity. Indeed, functional analysis of the genes causing type I interferonopathies has revealed pathways that protect the organism against inappropriate immune activation caused by self nucleic acids, while maintaining a prompt and efficient immune response to foreign nucleic acids derived from invading pathogens. Moreover, these findings have also contributed to our understanding of pathomechanisms underlying certain forms of complex SLE.
Nucleic acid sensing and type I interferon activation
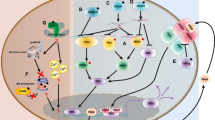
Detection of viral infection by the host organism is primarily achieved through recognition of viral nucleic acids. In dendritic cells, RNA and DNA of endocytosed viral particles or apoptotic virally infected cells are recognized by toll-like receptors (TLRs) such as TLR7 and TLR9 [4]. Endosomal TLR signaling is initiated by engagement of the adaptor proteins myeloid differentiation primary-response protein 88 (MYD88) or TIR domain-containing adaptor protein inducing IFN-β (TRIF). This stimulates downstream signaling pathways that lead to the activation of nuclear factor-κB (NF-κB) and interferon-regulatory factors (IRFs) IFR3 and IRF7 resulting in the induction of pro-inflammatory cytokines and IFN-α [4].
Sensing of cytosolic nucleic acids is mediated by a growing number of pattern recognition factors [5]. The best-studied cytosolic RNA sensors include the ubiquitously expressed RIG-I-like helicases retinoic acid-inducible gene 1 (RIG-I) and melanoma differentiation-associated protein 5 (MDA5) which activate NF-kB and IRFs via recruitment to the mitochondrial antiviral signaling (MAVS) adapter protein [5]. While MDA5 binds long double-stranded (dsRNA), RIG-I ligands are rather short and characterized by a 5′-triphosphate or a 5′-diphosphate moiety with blunt-end base pairing at the 5′-end [6–8]. RIG-I can also recognize viral DNA via the RNA polymerase III pathway, which transcribes DNA into 5′-triphosphate RNA [9, 10]. The central mechanism of cytosolic DNA sensing involves the nucleotidyl transferase cyclic GMP-AMP synthase (cGAS), which catalyzes the synthesis of the second messenger cyclic GMP-AMP (cGAMP) following binding of cGAS to dsDNA or single-stranded DNA (ssDNA) [11, 12]. cGAMP then binds to the adapter molecule stimulator of interferon genes (STING), which forms homodimers and activates IRF3 through TANK-binding kinase 1 (TBK1) resulting in the transcriptional activation of the IFNB gene [13].
Type I IFNs bind to the interferon-α receptor (IFNAR), a cell surface receptor composed of two subunits, IFNAR1 und IFNAR2. Canonical type I IFN signaling activates the Janus kinase (JAK) - signal transducer and activator of transcription (STAT) pathway, leading to transcription of ISGs [14]. Induction of the antiviral state is mediated by autocrine and paracrine actions of type I IFN which promote apoptosis of infected cells and alert surrounding cells to the presence of a viral infection. This also includes immunomodulatory effects on the adaptive immune system leading to the maturation and proliferation of lymphocytes. Collectively, host, pathogen, and environmental factors regulate the responses of cells to the various type I IFN signaling pathways and determine whether pathogens are cleared effectively or chronic infection or autoimmune disease ensues.
Walking a fine line between non self and self
Nucleic acid sensors have evolved to recognize a feature common to all viruses—a genome composed of DNA or RNA. However, these nucleic acid sensors have only limited capacity to differentiate between non-self and self DNA or RNA, which means that a type I IFN response can in principle also be initiated by endogenous nucleic acids. Indeed, type I IFN activation induced by immune recognition of self nucleic acids is central to SLE pathogenesis [3]. Thus, virus-induced IFN-α initiates a self-perpetuating feedback loop to drive maturation and proliferation of autoreactive B cells and formation of antinuclear autoantibodies which commonly target nucleic acids [3]. These autoantibodies form immune complexes with self nucleic acids originating for example from dying cells. Following Fcγ receptor-mediated uptake of immune complexes by dendritic cells, the internalized nucleic acids subsequently activate TLR signaling to stimulate more IFN-α-production further promoting a loss of tolerance and autoimmunity [15]. Given that an antiviral immune response launched in the wrong place at the wrong time can cause damage to the host, the organism must be equipped with efficient means to overcome limitations in pattern recognition. This is achieved by confinement of certain nucleic acid species to distinct cellular compartments away from the cytosolic nucleic acid sensing machinery [4]. In addition, clearance of DNA derived from cell debris is accomplished by nucleolytic DNA degradation within the extracellular space or following phagocytosis within the lysosome [16, 17]. Finally, pattern recognition is also determined by structural properties or chemical modification of nucleic acid ligands as outlined above.
Type I interferonopathies
The term type I interferonopathy was coined by Yanick Crow in 2011 based on the concept of grouping Mendelian disorders associated with an abnormal upregulation of type I IFN [18]. Accordingly, type I interferonopathies comprise a growing number of genetically determined disorders that are caused by a dysfunction of the innate immune system. Although the underlying molecular defects affect highly diverse biological functions, the associated disease pathways converge to a common route which is inappropriate overproduction of type I IFN. This is also reflected on a phenotypic level. Thus, despite a remarkable phenotypic heterogeneity, type I interferonopathies are commonly characterized by signs of both autoinflammation and autoimmunity. Based on the currently identified molecular defects, a pathogenic type I IFN response can result from (i) abnormal accumulation of or abnormal chemical modification of endogenous nucleic acids, (ii) enhanced sensitivity or ligand-independent activation of nucleic acid sensors or of downstream components of type I IFN signaling pathways, (iii) dysregulated negative regulation of nucleic acid-induced type I IFN signaling, or (iv) defects in pathways that modulate type I IFN responses independent of nucleic acid sensing.
Aicardi-Goutières syndrome
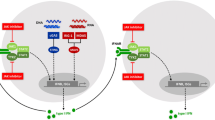
Aicardi-Goutières syndrome (AGS) is a systemic inflammatory disease with onset in early infancy [19]. In its classic form, AGS presents as a leukoencephalopathy characterized by basal ganglia calcifications, progressive cerebral atrophy as well as lymphocytosis and elevated IFN-α in cerebrospinal fluid [20]. The clinical phenotype of AGS mimics an in utero acquired viral infection. Infants typically present with a subacute onset of irritability, dystonia, seizures, and fever, leading to severe developmental delay and microcephaly. Some patients develop signs that are also observed in patients with SLE including hepatopathy, arthritis, thrombocytopenia, lymphopenia, antinuclear antibodies, as well as cold-induced cutaneous chilblain lesions [21, 22]. AGS patients typically exhibit constitutive upregulation of ISGs in peripheral blood cells which is also referred to as type I IFN signature [23]. The intrafamilial variability can be high with one sibling presenting with classic AGS and the other with only mild spasticity and normal intellectual abilities [24, 25]. AGS is a genetically heterogenous disorder caused by mutations in at least seven different genes (Fig. 1 and Table 1).
Disease pathways in nucleic acid-induced type I IFN activation. TREX1 deficiency results in cytosolic accumulation of ssDNA derived from aberrant DNA replication intermediates or reverse transcription of retroelements. Defective removal of ribonucleotides from genomic DNA due to RNase H2 deficiency or imbalances of dNTP pools due to SAMHD1 deficiency causes genome instability. The ensuing low-level DNA damage triggers a DNA damage response and enhanced formation of DNA repair metabolites. The nuclease activity of SAMHD1 may also prevent accumulation of yet unknown immunostimulatory RNA species. Similarly, editing of dsRNA by ADAR is thought to alter its immunostimulatory properties. Gain-of-function mutations in the dsRNA sensors RIG-I and MDA5 (encoded by the IFIH1 gene) or in the cGAMP-binding adaptor molecule STING lead to inadequately or constitutively increased type I IFN signaling
TREX1
TREX1 (AGS1; OMIM 225750) encodes 3′ repair exonuclease 1, a cytosolic DNase with high specificity for ssDNA [26]. TREX1 was shown to degrade ssDNA metabolites derived from granzyme A-mediated apoptosis [27], aberrant DNA replication [28] or reverse transcription of retroelements [29], remnants of ancient retroviral infection that comprise almost half of the mammalian genome. Trex1 −/− mice develop autoimmune-mediated organ inflammation initiated in nonhematopoietic cells and succumb to heart failure [30], a phenotype that is rescued upon ablation of type I IFN signaling by cross-breeding these animals with mice lacking the Irf3 or Ifnar gene [29]. TREX1 deficiency results in the cytosolic accumulation of ssDNA species that are recognized as danger signals and trigger a type I IFN response in a cGAS/TBK1/IRF3-dependent manner [31]. In the majority of cases, patients with AGS1 harbor biallelic loss-of-function mutations of TREX1. In addition, rare cases with heterozygous de novo mutations have been described [26, 32, 33].
RNASEH2A, RNASEH2B, RNASEH2C
RNASEH2A (AGS4; OMIM 610333), RNASEH2B (AGS2; OMIM 610181), and RNASEH2C (AGS3; OMIM 610329) encode the three subunits of the ribonuclease H2 (RNase H2) complex, which degrades RNA within an RNA/DNA hybrid or cleaves the phosphodiester bond 5′ of a single ribonucleotide embedded within a DNA duplex [34]. RNase H2 plays an essential role in genome integrity as it mediates removal of misincorporated ribonucleotides from genomic DNA [35, 36]. A lack of ribonucleotide excision repair renders genomic DNA susceptible to DNA strand breaks [37, 38]. Indeed, complete RNase H2 deficiency in mice is embryonal lethal due to a massive p53-dependent DNA damage response without any evidence for type I IFN activation [35, 36]. In contrast, RNase H2 mutations in patients with AGS were shown to be hypomorphic and to result in low-level DNA damage leading to a chronic DNA damage response and constitutive type I IFN activation possibly induced by DNA repair metabolites [39, 40].
SAMHD1
SAM domain and HD domain-containing protein 1 (SAMHD1; AGS5; OMIM 612952) functions as a dGTP-dependent triphosphohydrolase which converts deoxynucleoside triphosphates (dNTPs) to the constituent deoxynucleoside and inorganic triphosphate [41]. SAMHD1 restricts infection of myeloid cells with human immunodeficiency virus type 1 (HIV-1) [42, 43] by depleting the dNTP pool required for reverse transcription of the viral RNA genome [44]. SAMHD1 binds to nucleic acids and exhibits nuclease activity [45–48] and may therefore lead to accumulation of yet undefined immunostimulatory nucleic acids. SAMHD1 is regulated in a cell cycle-dependent manner by cyclin A/CDK1-dependent phosphorylation [49, 50]. SAMHD1 deficiency leads to imbalances in the intracellular dNTP pools resulting in genome instability. The ensuing chronic DNA damage triggers a DNA damage response and senescence accompanied by type I activation [50].
ADAR
ADAR (AGS6; OMIM 615010, adenosine deaminase, RNA-specific) catalyzes the deamination of adenosine to inosine in dsRNA [51]. Studies in ADAR-deficient mice have shown that editing of dsRNAs by ADAR is required for self-renewal capacity of hematopoietic stem cells by suppressing apoptotic type I IFN signaling [52]. ADAR was shown to modulate the innate immune response to RNA by altering the immunoreactive properties of dsRNA molecules [53]. Patients with AGS6 were shown to carry biallelic as well as heterozygous de novo mutations [51].
IFIH1
Interferon induced with helicase C domain 1 (IFIH1, AGS7; OMIM 615846) encodes MDA5, a cytoplasmic sensor for dsRNA [54]. Functional analysis revealed that AGS7-associated mutations act as gain-of-function mutations and exhibit an increased affinity for dsRNA leading to enhanced type I IFN signaling. AGS7 causing IFIH1 mutations are inherited in an autosomal dominant manner with reduced penetrance or may arise de novo.
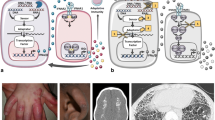
Retinal vasculopathy with cerebral leukodystrophy
Retinal vasculopathy with cerebral leukodystrophy (RVCL; OMIM 192315) is an autosomal dominant disorder with onset in early adulthood. Patients present with loss of vision, cerebrovascular disease, and dementia [55]. Some patients also develop migraine, glomerulopathy, as well as Raynaud’s disease. RVCL is inherited in a dominant fashion and caused by heterozygous frameshift mutations of TREX1 that lead to C-terminal truncation with preservation of the N-terminal DNase domain. While RCVL was originally thought to be caused by a structural vasculopathy, patients with RVCL have been shown to exhibit signs of autoimmunity as well as an IFN signature in blood suggesting an inflammatory process [56].
Familial chilblain lupus
Familial chilblain lupus is a rare monogenic form of cutaneous lupus erythematosus with onset in early childhood. Patients present with cold-induced bluish-red inflammatory lesions in acral locations such as fingers, toes, nose cheeks, and ears [57]. Some patients develop arthralgia, antinuclear antibodies, immune complexes, as well as lymphopenia. Histological findings include perivascular inflammatory infiltrates with increased mucin formation and deposits of immunoglobulins or complements along the basement membrane [57]. Constitutive type I IFN activation is evident from increased expression of myxovirus resistance protein 1 (MxA) in lesional skin as well as an upregulation of IFN-stimulated genes in peripheral blood cells [58]. Familial chilblain lupus is caused by heterozygous TREX mutations (CHBL1; OMIM 610448) [32, 59]. In addition, a heterozygous SAMHD1 mutation was reported in a single family (CHBL2; OMIM 614415) [60].
STING-associated vasculopathy, infantile-onset
STING-associated vasculopathy, infantile-onset (SAVI; OMIM 615934) is an autoinflammatory vasculopathy causing severe necrotizing skin lesions affecting the face, ears, nose, and digits [61]. Many patients develop fever episodes and inflammatory interstitial lung disease that may lead to lung fibrosis. SAVI patients were found to harbor heterozygous de novo mutations in STING, encoding an adaptor protein which mediates IFN-β activation in a cGAMP/TBK1/IRF3-dependent manner. Mutations result in a gain of function leading to constitutive activation of the IFNB promoter, even in the absence of stimulation by cGAMP [61]. Consistent with this, patients also show an IFN signature as well as increased levels of IFN-induced cytokines in blood. In addition, a single family with SAVI and lupus-like features segregating a dominant STING mutation was reported [62].
Systemic lupus erythematosus
SLE is a prototypic autoimmune disease with a broad spectrum of clinical presentations encompassing virtually all organs. Patients commonly experience fatigue, fever, rash, and arthritis but may also develop more severe internal organ disease affecting the kidney, heart, or the central nervous system. A hallmark of SLE is the formation of antinuclear antibodies which target ubiquitous nuclear antigens including DNA. The etiology of SLE includes both genetic and environmental components and numerous genes have been linked to SLE [63]. Although the encoded genes affect diverse aspects of both innate and adaptive immune functions, there is a large body of evidence to support a central role of type I IFN in SLE pathogenesis. Indeed, SLE was the first disease suspected to be caused by inadequately increased type I IFN activity as outlined above. In agreement with this, many SLE patients exhibit enhanced levels of IFN-α level in the serum as well as an IFN signature in peripheral blood cells [49, 64]. Moreover, candidate gene studies have demonstrated that individuals carrying rare variants of TREX1 or the RNASEH2 genes have an increased risk for SLE underpinning the relevance of cell-intrinsic mechanisms of nucleic acid-induced type I IFN activation in SLE pathogenesis [40, 65, 66]. Interestingly, ribonucleotides contained in genomic DNA were shown to promote UV light-induced photodamage in DNA, a finding that could explain the high prevalence of photosensitivity in SLE patients carrying RNASEH2 variants [40]. In addition, mutations in nucleases responsible for the removal of extracellular waste such as DNase 1 or DNase 1L3 were also shown to cause SLE [67, 68]. Given that defective clearance of nucleic acid-containing immune complexes results in IFN-α production by dendritic cells [69], deficiency of the complement components C1q or C4 may also be viewed as type I interferonopathy.
Spondyloenchondrodysplasia
Spondyloenchondrodysplasia (SPENCD; OMIM 271550) is a skeletal dysplasia characterized by enchondromatous nonossifying metaphyseal and spondylar lesions [70]. Patients exhibit varying degrees of neurological impairment including spasticity, developmental delay, and basal ganglia calcification. In addition, lupus-like symptoms are commonly observed. Patients may also suffer from recurrent infections. SPENCD is inherited in an autosomal recessive manner and caused by bilallelic mutations in the ACP5 gene encoding tartrate-resistant acid phosphatase 5 [71, 72]. Loss of tartrate-resistant acid phosphatase (TRAP) activity results in decreased dephosphorylation of osteopontin, a cytokine present in bone-dissolving osteoclasts as well as in antigen-presenting macrophages and dendritic cells [71, 72]. Enhanced levels of active phosphorylated osteopontin are thought to be responsible for increased bone resorption and immune dysregulation resulting in skeletal abnormalities and overproduction of type I interferon.
Singleton-Merten syndrome
Singleton-Merten syndrome is characterized by progressive calcifications of large vessels, dental anomalies with periodontal disease and alveolar bone resorption, as well as bone disease with osteoporosis and osteolysis [73]. In addition, patients may suffer from psoriasis, early-onset glaucoma and recurrent infections. Singleton-Merton syndrome is inherited in an autosomal dominant manner and caused by heterozygous mutations in IFIH1 (SGMRT1; OMIM 182250) or RIGI (SGMRT2; OMIM 616298) encoding cytosolic pattern recognition receptors for dsRNA [74, 75]. Functional studies have shown that IFIH1 or RIGI mutations in patients act as gain-of-function mutations which results in constitutive type IFN activation.
ISG15 deficiency
Interferon-stimulated protein 15 (ISG15) deficiency (immmunodeficiency 38, with basal ganglia calficiation; OMIM 61626) is an autosomal recessive immunodeficiency which predisposes to mycobacterial disease [76]. Patients appear to be relatively resistant to viral infection. Similar to AGS patients, they also exhibit an IFN signature in blood and develop basal ganglia calcification which may reflect an inflammatory vascular process [77]. ISG15 is an ubiquitin-like protein that modifies proteins by ISGylation. Absence of ISG15 in patient cells prevents the accumulation of USP18, a potent negative regulator of type I IFN signaling, resulting in the enhancement and amplification of type I IFN responses [77].
CANDLE syndrome
Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE; OMIM 256040) is an autosomal recessive autoinflammatory syndrome with onset in infancy characterized by annular erythematous skin lesions with partial lipodystrophy, hepatomegaly, and arthralgias. Patients may also suffer from recurrent fever, joint contractions with muscle atrophy, and basal ganglia calcification, and their blood cells were shown to exhibit enhanced ISG expression as well as constitutive STAT1 phosphorylation [78]. CANDLE is caused by homozygous mutations in PSMB8 encoding proteasome subunit β type 8, which functions as the chymotrypsin-like catalytic subunit of the immunoproteasome and is involved in processing of MHC class-I restricted T cell epitopes in antigen presenting cells [79].
Therapeutic implications
Type I interferonopathies are chronic multisystem diseases causing significant and severe morbidities. The inflammatory etiology of type I interferonopathies suggests that they are amenable to immunomodulatory treatment. Moreover, timely diagnosis of children with AGS may provide a window of opportunity during which early intervention could significantly limit or even prevent irreversible neurological impairment. Based on our current knowledge on the molecular pathogenesis of type I interferonopathies, targeting IFN-α/β, the IFNAR receptor or the JAK/STAT pathway to inhibit the pathogenic type I IFN response might be therapeutically effective. Undoubtedly, these advances will also foster the development of novel compounds targeting components of the type I IFN signaling axis such as cGAS, TBK1, and STING. In addition, future studies will also address the potential therapeutic efficacy of antiretroviral agents in patients with type I interferonopathies.
Conclusions
Understanding the cellular and molecular functions of the genes causing type I interferonopathies such as AGS and familial chilblain lupus has led to the identification of a number of novel pathways of the intracellular nucleic acid metabolism that modulate innate immune responses to prevent autoinflammation and autoimmunity induced by inappropriate immune recognition of self nucleic acids. This knowledge will also help defining molecules and pathways that could potentially be targeted for specific therapeutic intervention in the future.
Abbreviations
- ADAR:
-
Adenosine deaminase, RNA-specific
- AGS:
-
Aicardi-Goutières syndrome
- CANDLE:
-
Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome
- cGAMP:
-
Cyclic GMP-AMP
- cGAS:
-
Cyclic GMP-AMP synthase
- IFIH1:
-
Interferon induced with helicase C domain 1
- IFN:
-
Interferon
- IRF:
-
Interferon-regulatory factor
- ISG:
-
Interferon-stimulated gene
- MAVS:
-
Mitochondrial antiviral signaling protein
- MDA5:
-
Melanoma differentiation-associated gene 5
- MYD88:
-
Myeloid differentiation primary-response protein 88
- NF-κB:
-
Nuclear factor-κB
- RIG-I:
-
Retinoic acid-inducible gene 1
- RNASEH2:
-
Ribonuclease H2
- RVCL:
-
Retinal vasculopathy with cerebral leukodystrophy
- SAMHD1:
-
SAM domain and HD domain-containing protein 1
- SAVI:
-
STING-associated vasculopathy, infantile-onset
- SLE:
-
Systemic lupus erythematosus
- STING:
-
Stimulator of interferon genes
- TBK1:
-
TANK-binding kinase 1
- TLR:
-
Toll-like receptor
- TREX1:
-
3′ Repair exonuclease 1
- TRIF:
-
TIR domain-containing adaptor protein inducing IFN-β
References
Stetson DB, Medzhitov R (2006) Type I interferons in host defense. Immunity 25:373–381
Taniguchi T, Takaoka A (2001) A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol 2:378–386
Marshak-Rothstein A (2006) Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 6:823–835
O’Neill LA, Golenbock D, Bowie AG (2013) The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol 13:453–460
Atianand MK, Fitzgerald KA (2013) Molecular basis of DNA recognition in the immune system. J Immunol 190:1911–1918
Kawai T, Takahashi K, Sato S et al (2005) IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6:981–988
Hornung V, Ellegast J, Kim S et al (2006) 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997
Goubau D, Schlee M, Deddouche S et al (2014) Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 514:372–375
Ablasser A, Bauernfeind F, Hartmann G et al (2009) RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol 10:1065–1072
Chiu YH, Macmillan JB, Chen ZJ (2009) RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591
Sun L, Wu J, Du F et al (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791
Wu J, Sun L, Chen X et al (2013) Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339:826–830
Xiao TS, Fitzgerald KA (2013) The cGAS-STING pathway for DNA sensing. Mol Cell 51:135–139
Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5:375–386
Lovgren T, Eloranta ML, Bave U et al (2004) Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 50:1861–1872
Napirei M, Karsunky H, Zevnik B et al (2000) Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet 25:177–181
Kawane K, Ohtani M, Miwa K et al (2006) Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443:998–1002
Crow YJ (2011) Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci 1238:91–98
Aicardi J, Goutieres F (1984) A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol 15:49–54
Lebon P, Badoual J, Ponsot G et al (1988) Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J Neurol Sci 84:201–208
Tolmie JL, Shillito P, Hughes-Benzie R et al (1995) The Aicardi-Goutieres syndrome (familial, early onset encephalopathy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis). J Med Genet 32:881–884
Ramantani G, Kohlhase J, Hertzberg C et al (2010) Expanding the phenotypic spectrum of lupus erythematosus in Aicardi-Goutieres syndrome. Arthritis Rheum 62:1469–1477
Rice GI, Forte GM, Szynkiewicz M et al (2013) Assessment of interferon-related biomarkers in Aicardi-Goutieres syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case–control study. Lancet Neurol 12:1159–1169
Vogt J, Agrawal S, Ibrahim Z et al (2013) Striking intrafamilial phenotypic variability in Aicardi-Goutieres syndrome associated with the recurrent Asian founder mutation in RNASEH2C. Am J Med Genet A 161A:338–342
Tüngler V, Schmidt F, Hieronimus S et al (2014) Phenotypic variability in a family with Aicardi-Goutières syndrome due to the common A177T RNASEH2B mutation. Case Rep Clin Med 3:153–156
Crow YJ, Hayward BE, Parmar R et al (2006) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet 38:917–920
Chowdhury D, Beresford PJ, Zhu P et al (2006) The exonuclease TREX1 is in the SET complex and acts in concert with NM23-H1 to degrade DNA during granzyme A-mediated cell death. Mol Cell 23:133–142
Yang YG, Lindahl T, Barnes DE (2007) Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131:873–886
Stetson DB, Ko JS, Heidmann T et al (2008) Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134:587–598
Gall A, Treuting P, Elkon KB et al (2012) Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36:120–131
Ablasser A, Hemmerling I, Schmid-Burgk JL et al (2014) TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J Immunol 192:5993–5997
Rice G, Newman WG, Dean J et al (2007) Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am J Hum Genet 80:811–815
Tungler V, Silver RM, Walkenhorst H et al (2012) Inherited or de novo mutation affecting aspartate 18 of TREX1 results in either familial chilblain lupus or Aicardi-Goutieres syndrome. Br J Dermatol 167:212–214
Crow YJ, Leitch A, Hayward BE et al (2006) Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat Genet 38:910–916
Reijns MA, Rabe B, Rigby RE et al (2012) Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell 149:1008–1022
Hiller B, Achleitner M, Glage S et al (2012) Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J Exp Med 209:1419–1426
Sparks JL, Chon H, Cerritelli SM et al (2012) RNase H2-initiated ribonucleotide excision repair. Mol Cell 47:980–986
Kim N, Huang SN, Williams JS et al (2011) Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332:1561–1564
Kind B, Muster B, Staroske W et al (2014) Altered spatio-temporal dynamics of RNase H2 complex assembly at replication and repair sites in Aicardi-Goutieres syndrome. Hum Mol Genet 23:5950–5960
Gunther C, Kind B, Reijns MA et al (2015) Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J Clin Invest 125:413–424
Goldstone DC, Ennis-Adeniran V, Hedden JJ et al (2011) HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382
Hrecka K, Hao C, Gierszewska M et al (2011) Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661
Laguette N, Sobhian B, Casartelli N et al (2011) SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657
Lahouassa H, Daddacha W, Hofmann H et al (2012) SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13:223–228
Goncalves A, Karayel E, Rice GI et al (2012) SAMHD1 is a nucleic-acid binding protein that is mislocalized due to aicardi-goutieres syndrome-associated mutations. Hum Mutat 33:1116–1122
Tungler V, Staroske W, Kind B et al (2013) Single-stranded nucleic acids promote SAMHD1 complex formation. J Mol Med (Berl) 91:759–770
Beloglazova N, Flick R, Tchigvintsev A et al (2013) Nuclease activity of the human SAMHD1 protein implicated in the Aicardi-Goutieres syndrome and HIV-1 restriction. J Biol Chem 288:8101–8110
Ryoo J, Choi J, Oh C et al (2014) The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat Med 20:936–941
Cribier A, Descours B, Valadao AL et al (2013) Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 3:1036–1043
Kretschmer S, Wolf C, Konig N et al (2014) SAMHD1 prevents autoimmunity by maintaining genome stability. Ann Rheum Dis
Rice GI, Kasher PR, Forte GM et al (2012) Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet 44:1243–1248
Wang Q, Khillan J, Gadue P et al (2000) Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science 290:1765–1768
Mannion NM, Greenwood SM, Young R et al (2014) The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep 9:1482–1494
Rice GI, Del Toro DY, Jenkinson EM et al (2014) Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 46:503–509
Richards A, van den Maagdenberg AM, Jen JC et al (2007) C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat Genet 39:1068–1070
Schuh E, Ertl-Wagner B, Lohse P et al (2015) Multiple sclerosis-like lesions and type I interferon signature in a patient with RVCL. Neurol Neuroimmunol Neuroinflamm 2:e55
Lee-Kirsch MA, Gong M, Schulz H et al (2006) Familial chilblain lupus, a monogenic form of cutaneous lupus erythematosus, maps to chromosome 3p. Am J Hum Genet 79:731–737
Gunther C, Hillebrand M, Brunk J et al (2013) Systemic involvement in TREX1-associated familial chilblain lupus. J Am Acad Dermatol 69:e179–e181
Lee-Kirsch MA, Chowdhury D, Harvey S et al (2007) A mutation in TREX1 that impairs susceptibility to granzyme A-mediated cell death underlies familial chilblain lupus. J Mol Med 85:531–537
Dale RC, Gornall H, Singh-Grewal D et al (2010) Familial Aicardi-Goutieres syndrome due to SAMHD1 mutations is associated with chronic arthropathy and contractures. Am J Med Genet A 152A:938–942
Liu Y, Jesus AA, Marrero B et al (2014) Activated STING in a vascular and pulmonary syndrome. N Engl J Med 371:507–518
Jeremiah N, Neven B, Gentili M et al (2014) Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest 124:5516–5520
Harley IT, Kaufman KM, Langefeld CD et al (2009) Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet 10:285–290
Baechler EC, Batliwalla FM, Karypis G et al (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100:2610–2615
Lee-Kirsch MA, Gong M, Chowdhury D et al (2007) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet 39:1065–1067
Namjou B, Kothari PH, Kelly JA et al (2011) Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun 12:270–279
Yasutomo K, Horiuchi T, Kagami S et al (2001) Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet 28:313–314
Al-Mayouf SM, Sunker A, Abdwani R et al (2011) Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet 43:1186–1188
Manderson AP, Botto M, Walport MJ (2004) The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol 22:431–456
Renella R, Schaefer E, LeMerrer M et al (2006) Spondyloenchondrodysplasia with spasticity, cerebral calcifications, and immune dysregulation: clinical and radiographic delineation of a pleiotropic disorder. Am J Med Genet A 140:541–550
Briggs TA, Rice GI, Daly S et al (2011) Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet 43:127–131
Lausch E, Janecke A, Bros M et al (2011) Genetic deficiency of tartrate-resistant acid phosphatase associated with skeletal dysplasia, cerebral calcifications and autoimmunity. Nat Genet 43:132–137
Gay BB Jr, Kuhn JP (1976) A syndrome of widened medullary cavities of bone, aortic calcification, abnormal dentition, and muscular weakness (the Singleton-Merten syndrome). Radiology 118:389–395
Rutsch F, MacDougall M, Lu C et al (2015) A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am J Hum Genet 96:275–282
Jang MA, Kim EK, Now H et al (2015) Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet 96:266–274
Bogunovic D, Byun M, Durfee LA et al (2012) Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science 337:1684–1688
Zhang X, Bogunovic D, Payelle-Brogard B et al (2015) Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature 517:89–93
Liu Y, Ramot Y, Torrelo A et al (2012) Mutations in proteasome subunit β type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum 64:895–907
Basler M, Kirk CJ, Groettrup M (2013) The immunoproetasome iin antigen processing and other immunolgical functions. Curr Opin Immunol 25:74–80
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinsschaft (Clinical Research Group 249 to M.L.-K. and A.R.) and the Friede Springer Stiftung to M.L.-K.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the Special Issue on The Inflammasome and Autoinflammatory Diseases - Guest Editors: Seth L. Masters, Tilmann Kallinich and Seza Ozen
Rights and permissions
About this article
Cite this article
Lee-Kirsch, M.A., Wolf, C., Kretschmer, S. et al. Type I interferonopathies—an expanding disease spectrum of immunodysregulation. Semin Immunopathol 37, 349–357 (2015). https://doi.org/10.1007/s00281-015-0500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0500-x