Abstract
Soil salinization represents one of the major limiting factors of future increase in crop production through the expansion or maintaining of cultivation area in the future. High salt levels in soils or irrigation water represent major environmental concerns for agriculture in semiarid and arid zones. Recent advances in research provide great opportunities to develop effective strategies to improve crop salt tolerance and yield in different environments affected by the soil salinity. It was clearly demonstrated that plants employ both the common adaptative responses and the specific reactions to salt stress. The review of research results presented here may be helpful to understand the physiological, metabolic, developmental, and other reactions of crop plants to salinity, resulting in the decrease of biomass production and yield. In addition, the chapter provides an overview of modern studies on how to mitigate salt stress effects on photosynthetic apparatus and productivity of crop plants with the help of phytohormones, glycine betaine, proline, polyamines, paclobutrazol, trace elements, and nanoparticles. To understand well these effects and to discover new ways to improve productivity in salinity stress conditions, it is necessary to utilize efficiently possibilities of promising techniques and approaches focused on improvement of photosynthetic traits and photosynthetic capacity, which determines yield under salt stress conditions.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
The progressive soil degradation, especially soil salinization, will represent one of the major obstacles to increase the crop production through the expansion of cultivation area in the future (Munns and Gilliham 2015). In many regions of the world, where precipitation is insufficient to leach soluble salts from the root zone, high salt levels in soils or irrigation water represent major environmental concerns for agriculture, the severity of which can increase in conditions of climate change (Lachhab et al. 2013).
Salinity of soil is one of the main abiotic stresses limiting the growth of crops (Munns and Tester 2008). The high salt concentration in the root zone can be natural or induced by agricultural activities such as irrigation with low-quality water or the use of certain fertilizers (Bartels and Nelson 1994). Nearly 400 million hectares of land is affected by salinization, 80% of which are of natural origin and 20% of anthropogenic origin (FAO 2015).
Globally, no less than 10 million hectares of agricultural land is abandoned annually (Berthomieu et al. 1988) due to the acclimatization over time of small quantities of salts contained in the irrigation water. About 15% of the cultivated land has an excess of salt (Berthomieu et al. 1988), and large quantities of water are of very poor quality (Aissaoui and Reffas 2007). Globally, salinity affects more than 6% of the land; in case of irrigated lands, it is over 40% (Chaves et al. 2009).
In salt zones covering 16 million hectares (Hamdy 1999), plants are often subjected to strong sunlight and low rainfall. In these areas, salinity is not only related to climatic conditions but also to the often poorly controlled use of irrigation. Therefore, the high evaporation demand and low infiltration due to precipitation lead to the accumulation of salt on the soil surface (Gucci et al. 1997). Soils in affected areas contain high concentrations of soluble salts, mainly NaCl, but also Na2SO4, CaSO4, and KCl (Hachicha 2007).
The presence of salts in the soil causes a limitation and decline in yield in many regions, in which the salt concentration of the soil solution exceeds 100 mM, inhibiting the growth of plants (Shahbaz and Ashref 2013). Salt accumulation in soils induces changes in plant physiology and metabolism. It affects germination, seedling growth, vegetative phase, flowering, and fruiting leading to decreased yields and quality of production (Zid and Grignon 1991; Vicente et al. 2004; Parida and Das 2005).
Salt tolerance has been broadly studied in numerous plant species and varieties and in halophytes to understand the mechanisms developed for their adaptation (Abdelly 2006; Messedi et al. 2004; Slama et al. 2017; Ben Hamed et al. 2013; Flowers and Colmer 2015). Salt tolerance is a complex trait that involves a set of mechanisms in plants (Lachhab et al. 2013). Different studies have shown that cultivation of specific plant species or varieties may improve productivity of marginal areas affected by salinity. This can be important, especially in conditions where complementary irrigations are often carried out with water containing high concentrations of soluble ions. In many cases, this has been done without taking into account the tolerance of the different varieties, resulting in poor crop production. Therefore, the breeding for better salt tolerance in crops has become a critical requirement for the future of agriculture in arid and semiarid regions (Owens 2001).
The identification of salt-tolerant genetic resources would certainly contribute to crop improvements, subsequently supporting the agricultural production of salinized areas or areas irrigated with brackish water. In this respect, the knowledge on different adaptative mechanisms, morphological, physiological, biochemical, and other strategies, with which different plants cope with the challenges of the salt stress is critical for success in crop improvement. Although the topic of salt tolerance is very broad and complex, this chapter will be specifically focused on the mechanisms contributing to the protection of the photosynthetic processes against detrimental effects of the salt stress.
4.2 Origin of Soil Salinization
Salts are composed of the different mineral elements of the soil, and some of them represent the essential nutrients needed for plant life. Their concentrations can become very high due to natural processes and/or poor management. In any part of the world, when evaporation exceeds precipitation, salts tend to be accumulated in soils, leading to increased concentrations (Hachicha 2007). Also, salinity increases due to poor drainage and poor water use in irrigation.
Salty soils are rich in soluble salts that affect plant growth and productivity (Mermoud 2006; Hachicha et al. 2017). Generally, in soils where drainage is weak or absent due to their impermeability, salts accumulate in the superficial horizons.
Salinity is a common phenomenon in arid and semiarid regions. Although identification of the natural processes that lead to salinization is essential for understanding the status of salt in any habitat, identification of the origin of salinity and its progression in the wild is important to cope with this constraint. Soluble salts can have three major sources.
4.2.1 Marine Origin
The salts of marine origin are transported and deposited in the continents in three ways. Cyclic salts, brought to the continents in the form of salty spray, are solubilized by rainwater, then redistributed, and transported to the oceans by drainage. Infiltration salts, brought to coastal habitats by seawater infiltration, are the main local source. Fossil salts, precipitated for a long time in certain localities, are led to the rhizosphere or to the surface of the earth by capillarity.
4.2.2 Lithogenic Sources
Some sedimentary rocks contain high levels of chloride and sulfate. The extent of salinization of groundwater and soils depends on the rate of alteration of these sedimentary rocks, which, in turn, varies considerably by region and climate zone. Generally, these rocks release more sulfate than chloride. The weathering of these rocks also releases significant amounts of carbonates, calcium, and magnesium. It was documented that these rocks may represent important sources for the local formation of saline lands (Siadat et al. 1997; FAO 2000).
4.2.3 Anthropogenic Sources
During the last centuries, large quantities of soluble salts accumulated in soils due to human activity. In some areas, poor-quality irrigation with intense evaporation in arid and semiarid regions and the use of inappropriate cultivation practices have led to the accumulation of large quantities of salt in the upper layers of the soil ground (Qadir et al. 2008). Following the development of the industry and the extensive use of fuels, another source of soluble salts has been added. The use of urban water leads to an increase in salinity. Similarly, the reuse of wastewater causes considerable salinization of groundwater (Shakir et al. 2017).
Saline soils are characterized by the predominance of Ca and Mg ions, as well as Na. The presence of salts leads to an increase in the osmotic pressure of the circulating solution, making it difficult for the plant roots to absorb water and nutritive elements and may cause the plasmolysis of the root cells. The excessive presence of certain anions or cations may affect the absorption and development of some plant species, while other ions (Cl, B, and Na) may also be toxic.
In Table 4.1 is the situation presented by FAO about the total area of saline soil in the world (FAO 2008).
4.3 Effects of High Salinity on Plants
Accumulation of electrolytic solutes in water and soil in higher concentrations is stressful to many plants. As stated before, saline soils occur in different regions of the world, although the majority of their occurrence is in semiarid and arid regions. There, as a result of insufficient downward percolation and lack of salt leaching, the soluble minerals can accumulate in the root zone. In hot and dry areas, the meager rainfall is accompanied by high temperature and low air humidity, leading to excessive evaporation. Moreover, in many places, such as river valleys, these conditions are associated with the presence of salt-bearing sediments, and shallow, brackish groundwater, resulting in gradual conversion of rich and fertile soils into barren saline soils (Hillel and Vlek 2005).
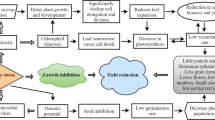
Presence of high salt concentrations in soil or water leads to osmotic stress, which is one of the most severe stress factors worldwide, with particular importance for numerous regions located in semiarid and arid climate zones. Figure 4.1 briefly illustrates the different ways by which the salinity affects plant functions. In addition to physical (osmotic) effects of soluble particles limiting water uptake by roots, the salinity effects are associated also with toxicity of individual ions and nutritional imbalance or interactions of these factors (Ashraf and Harris 2004).
Overview of salt stress effects on plants (Adapted from Evelin et al. 2009)
4.3.1 Effect of Salinity on Germination and Emergence
Salt stress can limit plant growth, by changing the balance between availability and needs. The scarcity of rains in many regions of the world accentuates salinization of irrigated land and makes them unsuitable for cultivation and abandoned (Yan et al. 2015). Salinity is a limiting factor in agriculture (Qadir et al. 2014). Generally salinity causes a decrease in soil hydraulic conductivity and root aeration and an increase in resistance to root penetration. Moreover, roots meet greater difficulty in suction of water and absorption of nutritional elements.
The salinity results in most of plants in reduction of growth and development (Munns and Tester 2008). A large number of alterations occur in plants (e.g., osmotic adjustments and various reactions) which affect the various organs from the roots to the stems and leaves and which may actually prevent plant growth. This adverse effect is translated by physiological, morphological, molecular, and biochemical changes which negatively affect plant growth and production (Qadir et al. 2014).
Dynamics of germination depends on genetic predispositions and health status, but it is strongly influenced by environmental conditions, including soil water availability (Gutterman 1993). Abdelly (2006) showed that most plants are more sensitive to salinity during their germination and emergence, when the harmful osmotic or toxic effects of salts are very direct and strong.
Germination of seeds represents often the critical step in the establishment of crop canopy, and thus, it can determinate successful agricultural production. Indeed, under salt stress, a late development favors the accumulation of toxic ions that can kill plants before the end of their development cycle (Munns 2002). Salt tolerance can therefore be evaluated by the precocity of germination.
The response to salt of plant species depends on several variables, starting with the species itself and specific genotypes, but also on salt concentration, growing conditions, and developmental stage of the plant. The decrease of the soil osmotic potential prevents the imbibition of the seed following a decrease in enzymatic activities and a strong absorption of Na+ compared to K+, leading to embryonic toxicity and delay in metabolic processes (Pires et al. 2017). Percentage of germination under salt stress depends on the level and duration of salinity applied (Fig. 4.2). In addition to final percentage, the salinity influences also the germination dynamics and vitality of the seedlings (Wang et al. 2011).
Relationship between rate of germination and time after sowing at different salinity levels (Modified from Ibrahim 2016)
4.3.1.1 Plant Responses on the Different Levels of the Salt Stress
The salinity of soils and waters is induced by the presence of too high concentrations of ions, especially Na+ and Cl-. Salinity has three effects, reduces water potential, affects ionic homeostasis, and induces toxicity. At the same time, salt stress affects by different ways different levels of plant organization (Fig. 4.3) and various plant organs (Fig. 4.4).
Changes in plant growth under salinity effects (Modified from de Oliveira et al. 2013)
Excess salt induces osmotic and ionic stress (Yan et al. 2015). The fast response to salt stress is the slowdown of leaf expansion that ceases at high concentrations (Wang and Nil 2000) and affects negatively plant growth (Hernández et al. 1995). The reduction in growth has been shown to be correlated with the salt concentration and the osmotic potential of soils (Flowers and Colmer 2015). The harmful effect of salt is remarkable and causes the death of plants or reduces their productivity. However, growth reduction occurs in most of plant species, but a level of tolerance or sensitivity varies widely among species. For example, for Raphanus sativus (radish) plants, 80% of the growth reduction under salt stress is attributed to loss of leaf area (Chartzoulakis and Klapaki 2000).
The action of salinity on the growth leading to both an imbalanced nutritional value of essential ions and a high uptake of toxic ions by the plant (Munns 2002) is connected with stress inducing low osmotic potential of soil solution (Munns and Tester 2008; Yan et al. 2013). Growth reduction in Poaceae can be attributed to an excessive uptake of Na+ ions (Tester and Davenport 2003, Gu et al. 2016). In tomato, salinity significantly reduces the mass of aerial parts, the number of leaves, the height of plants, and the plant root area and length (Mohammad et al. 1998).
However, in other obligatory halophytes, growth is increased with the salinity of the medium. These plants require a certain concentration of salt to express their maximum growth potential. In fact, the growth optimum is obtained at 200 mM NaCl in Salicornia rubra (Khan et al. 2010) and at 50 mM NaCl in Alhagi pseudalhagi (Fabaceae) (Kurban et al. 1999). In a non-excretory mangrove (Bruguiera parviflora), optimal growth is obtained at 100 mM NaCl, but 500 mM NaCl may be often lethal for these species (Parida et al. 2004). Optimal growth is achieved at 50% seawater in Rhizophora mucronata (Aziz and Khan 2001). The beneficial effect of salt was also observed in Sesuvium portulacastrum and Batis maritima (Messedi et al. 2004).
Growth of different organs of the same plant does not have the same degree sensitivity to salinity (Negrão et al. 2017). In fact, salt stress reduces the growth of aerial parts by decreased carbon allocation for the foliar growth in favor of root growth (Yeo et al. 1991; Huez-López et al. 2011). On the other hand, other studies (Hajlaoui et al. 2010, Lv et al. 2015) report the opposite; the roots are the most affected.
According to Munns and Rawson (1999), the effect of salinity usually results in a reduction in vegetative growth (reduction in height, number of tillers and leaves) which is a function of division and cell elongation. The growth of shoots is more sensitive to salts than the roots (Fig. 4.4).
Salinity affects the growth of plant roots (Kamiab et al. 2012; Rewald et al. 2012). It have shown that salt stress increases the PR/PA ratio. Indeed, plants maintain relatively high root growth under high saline stress; the increase in the PR/PA ratio that follows seems to be associated with an increase of their tolerance to salt.
Kafkai (1991) suggests that under salt stress, the plant spends more photosynthetic energy to maintain high water status and for production of roots for reduction of water loss. In these conditions, it seems that the arrest of leaf growth is triggered by hormones (Munns and Tester 2008) and a significant proportion of assimilates is then relocated to root growth (Fig. 4.5). This is one of the key anatomical responses to osmotic stress in many species, whose adaptative nature is evident since an increase conditions, it seems that the arrest of leaf growth is triggered by of the root mass/mass ratio of the canopy maximizes the water absorption area in decreasing the evaporation surface (Munns 2002).
The mechanisms of salt tolerance include the changes of low as well as of high complexity. The low complex mechanisms are associated with the expression of particular biochemical pathways. On the other hand, the mechanisms of high complexity modify the activities of key processes of energy metabolism, such as photosynthesis and respiration, as well as processes related to water uptake and transpiration and stomatal activity. The highly complex responses involve also the processes related to changes in the cell wall, cytoskeleton, and plasma membrane, related to water use efficiency, and securing the key cell elements (Botella et al. 1994).
4.3.2 Effects on the Anatomy of the Leaf
Salinity causes the increased thickness of epidermis, palisade and spongy parenchyma, and mesophyll, cell length, and cell diameter of the leaves of Atriplex, Faba, and Gossypium (Longstreth and Nobel 1979). However, it was shown that in mangroves (Bruguiera parviflora), the thickness of the epidermis and mesophyll, and the intercellular space in the leaves, is reduced (Parida et al. 2004). In wheat, area of flag leaf was significantly lowered in conditions of salt stress, significantly limiting yield (Ahmad et al. 2005). Salt causes increased vacuolation, development of the endoplasmic reticulum and mitochondria, vesicle formation and tonoplast fragmentation, and cytoplasmic degradation by mixing the matrices of cytoplasm and vacuole in the potato leaves (Mitsuya et al. 2000). It induces a reduction in intercellular spaces and number of chloroplasts in potato (Bruns and Hecht-Buchholz 1990) and stoma density in tomato (Romero-Aranda et al. 2001).
4.3.3 Plant Mineral Nutrition
In salt stress conditions, the mineral nutrition of the plant is disturbed. Indeed, the Na+ ions disrupt cation (K+, Ca2+) absorption (Haouala et al. 2007). At the root level, Na+ moves Ca2+ from cell walls (Zhu 2002). For example, in the isolated cell walls of barley roots, Na+ and Ca2+ are competing for the same site absorption, while K+ is fixed on other sites. As a result, the K+/Na+ ratio at the surface of cells depends on competition with Na+/Ca2+ (Stassart et al. 1981).
4.3.4 Effect of Salinity on Agronomic Yield
Components of crop performance, such as the number of tillers per plant, the number of ears, the number of spikelets per ear, and the grain weight, are usually influenced by the presence of salinity stress. Ahmad et al. (2005) have shown that all performance parameters are reduced under the salinity and that the higher the salinity, the higher yield decrease is observed.
The barley plants under salt stress during heading or differentiation of the ear have shown the reduced plant height and leaf area (Singh et al. 1994), as well as decrease in stem weight, stem length, and shoot dry matter. The number of spikelets per spike is decreased, as well as the number of grains. Salinity has a detrimental effect on the remobilization of reserves during the filling of the grains. Salinity decreases yield more often by reducing the number of spikes bearing the spikelets, ear weight, and 1000-seed weight (Munns and Rawson 1999). Mass and Grieve (1990) observed the changes in the final capacity of spike, associated with lower number of spikelets and grains per spike, as well as a decrease in spike length. Salt stress led to an increase of the phyllochron. In addition, the number of leaves on main stem was lower, and a vegetative period of stem meristem was shorter (Mass and Nieman 1978).
In case of rice, screening of cultivars for salt tolerance has been based mostly on growth rate and grain yield (Kafi et al. 2013). In this respect, it was shown that yield under salt stress was well correlated with panicle weight, number, height, and tiller number. The significant variability in salt resistance and yields in salt stress conditions was observed among families of rice, providing promising background for salt stress studies and breeding in this crop (Souleymane et al. 2017).
The effect of irrigation with saline water was tested by Lamsal et al. (1999) in wheat, observing decreases of grain yield and of all yield components and growth parameters, including flag leaf area, plant height, grain per spike, number of spikes per plant, grain weight per spike, 1000 grain weight, as well as total dry matter accumulation.
4.3.4.1 Photosynthetic Responses and Acclimations on Salt Stress
Photosynthesis is strongly involved in plant productivity (reduction of production of biomass, leaves) and nutrient flows in plants. Salinity affects the physiological activity of the leaf, particularly photosynthesis, which is the main cause of reduced plant productivity (Alem et al. 2002). The adaptation of photosynthesis under salt stress is presented in Fig. 4.6.
In specific environments, the resistance to drought and salinity are important traits determining the yield of main crops (Munns and Tester 2008; Munns, et al. 2010). The phenotypic effects of these two stresses are often very similar, and, therefore, the similar screening methods and tools can be used for both stresses. Important and relatively fast response to salinity is stomatal closure, associated partly with the osmotic effect of salts on the capacity of roots to absorb the soil water (Munns and Tester 2008). Insufficient uptake of the water due to salinity is denoted as “chemical drought” (Munns et al. 2010). In the early stages of the stress, the decrease of photosynthetic rate is caused by stomatal closure. The standard measurements of stomatal conductance or screening based on photosynthetic parameters are usually quite slow, and the reproducibility is often low (Munns et al. (2010). Therefore, there is still a need for fast and reliable methods of monitoring photosynthesis in salt stress conditions. Chlorophyll fluorescence measurements can be used in plant phenotyping and breeding programs to monitor different biotic and abiotic stresses including mineral deficiencies, soil salinity, and pathogenic diseases (Brestic and Zivcak 2013; Kalaji et al. 2017).
Under moderate salinity, the photosynthetic efficiency can reach the values similar to the control, but under high salinity, photosynthesis is significantly inhibited, as it was found in Desmostachya bipinnata (L.) Staph. (Asrar et al. 2017). At high concentrations of salt, the photosynthetic pigments, photochemical quenching, and electron transport rate were significantly decreased, whereas at moderate salt stress, the decrease was not observed. The content of MDA increased at high salinity, documenting excessive accumulation of ROS. Under increasing salinity treatments, a subsequent decrease of Rubisco content was also observed. The proteins of photosynthetic complexes were overexpressed (D1, AtpA, PetD) or remained unaffected (PsbO) under moderate salinity but decreased at higher concentrations, except of AtpA. Severe salt stress caused damages to PSII photochemistry and downregulation of chloroplast proteins connected with biochemical limitations in D. bipinnata (Asrar et al. 2017). The rate of photosynthesis increases for low levels of salinity and decreases in high level, without modification on stomatal conductance Bruguiera parviflora (Parida et al. 2004).
Salinity stress induces for the majority of plants a reduction in production of biomass essentially due to a decrease in photosynthesis (Kalaji et al. 2018). These changes are mostly connected with changes of carboxylation processes, but not photophosphorylation, which are most affected by salt stress. The response of plants to salt stress is heavily dependent on genotype. The salt stress has both short- and long-term effects on photosynthetic processes. The early effects can be observed after a few hours or days of exposure of the plants to salinity, and there can be a full cessation of carbon uptake for a few hours. The long-term effect appears after several days of salt treatment. The reduction of photosynthesis is caused by the accumulation of salt in the leaves (Munns and Termaat 1986). Whereas some studies indicate a reduction in photosynthesis by salinity (Chaudhuri and Choudhuri 1997; Soussi et al. 1998; Romero-Aranda et al. 2001; Kao et al. 2001), others show that carbon assimilation is not affected by salt stress or, in some cases, also enhanced by moderate salt concentrations (Rajesh et al. 1998; Kurban et al. 1999).
In the mulberry tree, the net assimilation of CO2 (PN), the stomatal conductance (gs), and the rate of transpiration (E) are reduced under salt stress, whereas the intercellular concentration of CO2 (Ci) is increased (Agastian et al. 2000). In Bruguiera parviflora, PN increases at low salinity but decreases at high salinity, whereas at low salinity, it remains comparable to that of control plants and falls at high concentrations (Parida et al. 2004).
Many studies showed, however, a major role of salt stress in limiting osmotic conductance and reducing reactive oxygen species (ROS) and main enzymes of detoxification of ROS. According to Munns and Tester (2008), the reduction in photosynthesis is related to the decrease in leaf water potential, which is responsible for the closure of stomata (Allen et al. 1985), which causes the reduction of photosynthesis and stomatal conductance (Orcutt and Nilsene 2000).
Salt stress reduces chlorophyll content and increases respiration, but has no significant effect on carotenoid levels in alfalfa (Khavari-Nejad and Chaparzadeh 1998). In Atriplex lentiformis, uptake of CO2 and the ratio of Rubisco to phosphoenolpyruvate carboxylase (PEPC) activity decreased under salt stress. Phosphoenolpyruvate carboxylase (PEPC) activity increases linearly with salinity, whereas that of ribulose bisphosphate carboxylase/oxygenase (Rubisco) is not changing significantly (Zhu and Meinzer 1999). The reduction in photosynthesis is caused also by a decrease in stomatal conductance which restricts the access of CO2 for Calvin-Benson cycle (Brugnoli and Bjorkman 1992). Stomata closure minimizes transpiration and affects light capture by chloroplasts and photosystems leading to impaired activity of these organelles (Iyengar and Reddy 1996).
In the process of photosynthesis, two key complex events occur: light reactions, in which light energy is converted into ATP and NADPH and oxygen is evolved, and light-independent, dark reactions, in which CO2 is fixed into carbohydrates by utilizing both of the products of light reactions (Allakhverdiev et al. 2002).
Pigment analysis showed that salt stress resulted in a significant decrease in chlorophyll a content, whereas the content of chlorophyll b remained unaffected. It appeared that chlorophyll b tolerated more the salinity than chlorophyll a. However, total carotenoid contents were increased. For some crops carotenoid concentration can be increased in conditions with low salinity and on the other hand under high salinity can be significantly decreased.
CO2 assimilation rate (Chaves et al. 2011; Kalaji et al. 2011; Kanwal et al. 2011; Chen et al. 2015; Hniličková 2017; Dąbrowski et al. 2017) and photosynthetic oxygen evolution can be also inhibited (Dąbrowski et al. 2016). Increases in Fo have been attributed to physical separation of the PSII from associated pigment antennae and decreases in the number of active RCs (Strasser et al. 2010). In the present study, the salt-induced increase in Fo indicates that some active RCs were inactivated by salt stress. This inactivation of RCs by salt stress was further evidenced by the increase in the ABS/RC value and the decrease in the values of RC/CSO and TRO/ABS after salinity treatment. Specific chlorophyll a fluorescence transient parameters derived from OJIP test (φEo, φPo, ψO, RC/CS, RC/ABS, PIABS, and PICS) were decreased under salt stress, while dVo/dto(Mo), Vj, and φDo were increased. The decrease of ETRmax and yield and the change of chlorophyll a fluorescence transients showed that salt stress had an important influence on photosynthesis. These results indicated that the effects of salinity stress on photosynthesis may depend on the inhibition of electron transport and the inactivation of the reaction centers, but this inhibition may occur in the electron transport pathway at the photosystem II (PSII) donor and acceptor sites (Mehta et al. 2010). Salt stress can damage active reactive centers of PSII and destroys the oxygen-evolving complex (OEC) and impairs the electron transfer capacity on the donor side of PSII (Misra et al. 2001; Mehta et al. 2010; Sun et al. 2016; Kan et al. 2017; Kalaji et al. 2018).
The major consequences of salt stress on the main photosynthetic parameters in the various crop plants are presented in Table 4.2.
According to Munns and Tester (2008), the reduction of photosynthesis is related to decreased leaf water potential, which is responsible for stomatal closure (Price and Hendry 1991; Allen et al. 1985), which causes the reduction of stomatal conductance (Orcutt and Nilsen 2000). The diffusion of CO inside the stomata then becomes limited, and its binding at the chloroplast level consequently decreases; the regeneration of RuBP (ribulose bisphosphate) becomes limited.
4.3.5 Effects of Salt Stress on Ultrastructure of Chloroplasts
The diffusion of CO2 inside the stomata then becomes limited, and its binding at the level of chloroplasts decreases (Graan and Boyer 1990); consequently the regeneration of RuBP (ribulose bisphosphate) becomes limited (Gimenez et al. 1992). Stomatic control and regulation involve cell turgor but also root signals, such as abscisic acid (ABA) (Zhang and Davies 1989). Cellular turgor occurs more or less directly in the chloroplast: directly by maintaining the volume of the chloroplast and indirectly by its effect on the stomatal opening, which controls the conductance and conditions the use of energy photochemical (ATP, NADPH) in chloroplasts (Gupta and Berkowitz 1987).
Salt causes disorganization of the thylakoid structure, increases the number and size of plastoglobules, and reduces starch ranges (Hernández et al. 1995, 1999). In mesophyll potato cells, thylakoid membranes are distended, and most are altered under severe salt stress (Mitsuya et al. 2000). Salinity reduces the number and thickness of the stack of thylakoids by grana (Bruns and Hecht-Buchholz 1990a, b). In NaCl-treated tomato plants, chloroplasts are assembled, cell membranes are deformed and wavy, and the structure of grana and thylakoids is affected (Khavari-Nejad and Mostofi 1998). Ultrastructural changes of chloroplasts under salt stress are well studied in Eucalyptus microcorys. These changes include the appearance of large starch grains, dilation of thylakoid membranes, almost complete absence of grana, and development of mesophyll cells (Keiper et al. 1998).
4.3.5.1 Effect of Salinity on Reproduction Process
Salinity reduces the growth rate of the plant and its reproductive organs (Hu et al. 2017). They studied the effect of salinity on the physiology of reproduction; they found that the number of pollen in two different types of barley cultivars was reduced from 24 to 37%. Studies by Munns and Rawson (1999) on the effect of salt accumulation in the meristem of barley on reproduction and development show that short periods of salt stress during organogenesis may have irreversible consequences on the fertility of the ear; it causes the abortion of the ovaries.
It was observed higher floret fertility contributed to higher seed set and grain yields in tolerant genotypes, whereas higher spikelet sterility led to poor seed set and lower grain yields in sensitive to salt stress genotypes. It is important to do screening at reproductive stage for morphological traits like floret fertility are thus more useful to identify plant genotypes tolerant to salinity stress (Rao et al. 2008).
Floral phenology, pollen quality, and seed set of Plantago crassifolia plants were optimal in plants grown in non-saline conditions. Same positive tendency was observed in the presence of 100 mM NaCl. But progressive reduction of pollen fertility, seed set, and seed viability has been observed by higher salt concentrations (Boscaiu et al. 2005).
4.3.5.2 Symptoms of Toxicity Connected with Ionic and Nutritional Balance in Plants
Saline solutions impose ionic and osmotic stress in plants. The effects of this stress can be observed at different levels. In sensitive plants, the growth of aerial parts and to a lesser extent that of roots is rapidly reduced. This reduction phenomenon appears to be independent of the tissue Na+ concentration but would rather be a response to the osmolarity of the culture medium (Munns 2002). The specific toxicity of Na+ ions is related to the accumulation of these ions in the leaf tissues and leads to necrosis of the aged leaves. Generally, this necrosis begins with the tip and the edges to finally invade the entire leaf. The reduction in growth is due to a reduction in leaf life, and thus there will be a reduction in growth and productivity (Munns 1993 and 2002). In saline soils, Na+ ions induce deficiency in other elements (Silberbush et al. 2005). The effects of Na+ are also the result of deficiency in other nutrients and interactions with other environmental factors, such as drought, which increase the problems of Na+ toxicity. The excess of Na+ ions inhibits the uptake of other nutrients either by competition at the sites of the root cell plasma membrane transporters or by inhibition of root growth by an osmotic effect. Thus, the absorption of water and the limitation of the nutrients essential for the growth and the development of the microorganisms of the soil can be inhibited.
Leaves are more sensitive to Na+ ions than roots because these ions accumulate more in the aerial parts than in the roots. These can regulate the concentration of Na+ ions by their export either to the aerial parts or to the ground. The metabolic toxicity of Na+ is mainly related to its competition with K+ at sites essential for cell function. Thus, more than 50 enzymes are activated by K+ ions; Na+ ions cannot replace K+ in these functions (Bhandal and Malik 1988; Tang et al. 2015; Gu et al. 2016). For that, a high concentration of Na+ can affect the functioning or the synthesis of several enzymes. In addition, protein synthesis requires high K+ concentrations for tRNA binding on ribosomes (Blaha et al. 2000) and probably for other ribosome functions (Wyn Jones et al. 1979). The disruption of protein synthesis by the high concentration of Na+ represents the major toxic effect of Na+ ions. Osmotic stress could occur following an increase in Na+ concentration at leaf apoplasm (Oertli 1968). This result was verified by microanalyses (R-X) of Na+ concentration in apoplasm of rice leaves (Flowers et al. 1991). The presence of high concentrations of Na+ in the cells allows the plant to maintain its water potential lower than that of the soil to maintain its turgor and water absorption capacity. This leads to an increase in osmotic concentration either by absorption of solutes from the soil or by synthesis of compatible solutes. The former, usually Na+ and Cl-, are toxic, while the latter are compatible but energetically expensive for the plant.
4.4 Specific Adaptative Strategies of Plants Under Salt Stress
Several studies have shown that plants adapted to salt stress use one or more mechanisms to mitigate the effect of NaCl such as (i) the Na+ reabsorption by transfer cells or vascular parenchyma (Karray-Bouraoui 1995); (ii) the compartmentalization of ions between the organs (roots/aerial parts), tissues (epidermis/mesophyll), or cellular compartments (vacuole/cytoplasm) (Cheeseman 1988); and (iii) the dilution of Na+ by the material produced growing leaves (Tester and Davenport 2003).
The mechanism by which a plant tolerates salt is complex, and it differs from species to species (Munns and Tester 2008; Bueno et al. 2017). The main effects of salt stress and development of plant adaptation of various crop plants are presented in Table 4.3.
Salt affects seed germination through osmotic effects (Khan et al. 2000), ion toxicity, or a combination of the two (Khan and Ungar 1998; Munns and Tester 2008). Osmotic stress can result in inhibition of water uptake that is essential for enzyme activation, breakdown, and translocation of seed reserves (Ashraf and Foolad 2007; Munns and Tester 2008). Furthermore, ionic stress can inhibit critical metabolic steps in dividing and expanding cells and may be toxic at high concentrations (Munns and Tester 2008). The excess Na+ and Cl− have the potential to affect plant cell enzymes, resulting in reduced energy production and some physiological processes (Munns and Tester 2008; Morais et al. 2012). The degree of salt tolerance varies among plant species and, for a given species, also at different developmental stages (Ahmad et al. 2013; Bueno et al. 2017). Some plants have adapted to grow in high-salinity environments due to the presence of different mechanisms in them for salt tolerance, such plants are known as salt-tolerant plants or halophytes (Ahmad et al. 2013), but a large majority of plant species grown in non-saline areas are salt-sensitive (glycophytes). Glycophytic and halophytic species differ greatly in their tolerance to salt stress (Munns and Tester 2008).
With the degree of salinity of soil solution, glycophytes in general are exposed to changes in their morphophysiological (Bennaceur et al. 2001) and biochemical (Grennan 2006) behavior. So the plants react to these variations in salinity in the biotope to set off resistance mechanisms. Among these mechanisms, the osmotic adjustment plays a vital role in the resistance or the tolerance of the plant to stress (Munns 2002). The plant will have to synthesize organic solutes to adjust its water potential. A salinity adaptation strategy consists of synthesizing osmoprotective agents, mainly amino compounds and sugars, and to the accumulated in the cytoplasm and organelles (Ashraf and Foolad 2007; Chen et al. 2010; Sengupta and Majumder 2010; Yan et al. 2013). Identification and understanding of plant tolerance mechanisms to salinity therefore have a clear interest in varietal improvement. The objective of this study is to determine certain morphological and physiological criteria allowing early identification of saline-tolerant plants.
4.4.1 Morphological and Anatomical Adaptations of Plants
Native plants in saline and desert environments have developed, over time, certain traits that give them the ability to develop in these stressful conditions. These traits are often of morphological and anatomical types. However, scientific studies have been done on the development and role of these morpho-anatomical features and also studies with other adaptative reactions of plants to the salt stress.
The most obvious morphological adaptations of plants in saline desert habitats are the reduction in leaf size and the number of stomata per unit leaf area, the increase in succulence and cuticle thickness of the leaf, and the formation of a layer of wax (Gale 1975; Mass and Nieman 1978). These adaptations play a crucial role in the conservation of water for the development of the plant in saline conditions. More recently, it has been shown that there is variability among the Cenchrus ciliaris ecotypes that allows it to withstand severe salinity conditions especially during drought periods (Hameed et al. 2015).
These are the most visible adaptations found throughout the plant (Dickison 2000). At the leaf level, there are certain structures that allow the plant to secrete excess salt. The most important are the secretory trichomes (Atriplex spp.) and the salt glands found in many plants of desert flora and coastal habitats. The latter are characteristic of a few families, including Poaceae, Avicenniaceae, Acanthaceae, Frankeniaceae, Plumbaginaceae, and Tamaricaceae (Mauseth 1988; Thomson et al. 1988, Marcum and Murdoch 1994).
Desert plants have usually succulent stems characterized by a well-developed water storage tissue in the cortex and marrow (Lyshede 1917; Dickison 2000). A multilayered epidermis may have thick walls, covered with thick cuticle surmounted by wax. For example, at Anabasis sp. the epidermis is formed of 8 to 11 layers. The stem of Salicornia fruticosa consists of a single cortex and an epidermis with a single thin-walled cell base. The palisadic and parenchymal tissue of photosynthetic organs is used for water storage (Fahn 1990).
The roots of xerohalophytes reduced the cortex to shorten the distance between the epidermis and the stele (Wahid 2003). The Caspari band is larger in dry-habitat plants than in mesophytes (Wahid 2003). In saline environment plants, the endoderm and exoderm represent barriers of variable resistance to the radial flow of water and ions, from the cortex to the stele (Hose et al. 2001; Taiz and Zeiger 2002).
4.4.2 Salt Tolerance Mechanisms
The physiological characterization of plant tolerance to salinity results from processes that allow the plant to absorb water and mineral salts from substrates with low hydric potential but also to live by accepting the important presence of the sodium in its tissues; halophytes, which accumulate the most sodium (Elzam and Epstein 1969, Guerrier 1984a), are distinguished by a strong capacity development of organic compounds (Briens and Larhe 1982), these two factors allowing the maintenance of a high internal osmotic pressure which favors the water exchange between the external and cellular compartments (Guerrier 1984b).
All plants do not react in the same way to salt stress; according to their production of biomass in the presence of salt, four main trends have been discerned: The first is true halophyte, whose production of biomass is stimulated by the presence of salt. These plants (Atriplex sp., Salicornia sp., Sueda sp.) present extensive adaptations and are naturally favored by soil salinity. Optional halophytes can show a slight increase in biomass at levels low in salts: Plantago maritima and Aster tripolium. Nonresistant halophytes can support low concentrations of salts: Hordeum sp. Glycophytes or halophytes can be sensitive to the presence of salts: Phaseolus vulgaris (Cheeseman 2015).
The adaptation reaction of various glycophytes to different salt stress treatments is presented in Table 4.4.
According to Munns et al. (2006), tolerance of cereals to salinity depends on variability genetics such as some species that resist this type of abiotic stress than others. In particular, the toxic effect of salts is less pronounced in common wheat than in durum wheat. This character is conferred by the presence of Kna1, a gene responsible for the exclusion of sodium. In addition barley can grow normally under conditions considered as limiting. Indeed, in addition to the exclusion of sodium, the barley plant uses another salinity tolerance mechanism that manifests itself by the imprisonment of salts in a very specific compartment in the leaf. This not only spares their effects toxic but also counteracts the osmotic pressure of the soil (Munns and Tester 2008).
4.4.3 Ion Exclusion and Compartmentalization Mechanisms of Plant Tolerance to Salinity
According to Berthomieu (1988), the plant prevents salt from rising up to leaves. A first barrier exists at the level of the endoderm, an inner layer of the root. However, this barrier can be interrupted, especially during emergence ramifications of the root. Other mechanisms limit the passage of salt from the roots to leaves, but the genes that govern them are still largely unknown.
It is also indicated that the exclusion capacity of Na+ and/or Cl− stems is good correlated with the degree of salt tolerance. Maintaining a low concentration of Na in the leaves may be due to an exclusion mechanism that causes an accumulation of Na in the roots, avoiding excessive translocation to the stems; but it can also be linked to a high mobility of this element in the phloem. However, some physiological measures concord to suggest the existence of an active expulsion of cytoplasmic sodium apoplasm or to the vacuole, thus protecting enzymatic equipment from the cytoplasm in aerial organs (Greenway and Munns 1980).
An organism can hardly exclude the Na+ completely of its tissues. At the plants, one of the best-known salinity tolerance strategies is compartmentalization ions (Na+, Cl−) in excess in the tissues. This controlled redistribution is mainly in vacuoles (Niu et al. 1995) and possibly at the whole-plant scale, in the oldest or least sensitive organs (Cheeseman 1988a, b; Munns 1993).
To be controlled, the movement of ions through the membranes involves an active transport, energy consumer, who uses different carriers (in variable density) to the surface of cell membranes (Orcutt and Nelen 2000; Tyerman and Skerret 1999; Al-Khateeb 2006). Once vacuolated, the excess Na+ contributes to the osmotic adjustment without altering the process metabolic rate (Levitt 1980; Yeo 1983, 1998).
The best way to maintain a low cytoplasmic concentration in Na+ is to compartmentalize this ion in the vacuole. This intracellular compartmentalization can be associated with succulence, which increases the volume of vacuoles in which Na+ ions accumulate. These ions are pumped into the vacuole before being concentrated in the cytoplasm. Ion pumping is provided by Na+/H+ antiports. The difference in pH is restored by H+-ATPase and pyrophosphatases (Blumwald 2000). Anti-Na+/H+ activity was increased following the addition of Na+ in wheat roots (Garbarino and DuPont 1989), tomato (Wilson and Shannon 1995), and sunflower (Ballesteros et al. 1997). Stimulation of activity is greater in the tolerant species, Plantago maritima, than in susceptible species, Plantago media (Staal et al. 1991). In sensitive rice, however, salinity does not induce the activity of anti-Na+ /H+ antibodies in tonoplasts (Fukuda et al. 1998). Salinity also induces the activity of H+ vacuolar pumps in tolerant and sensitive plants (Hasegawa et al. 2000). To maintain the osmotic balance between the vacuole and the cytoplasm, there will be synthesis of compatible organic compounds. Generally these compatible compounds protect the biochemical reactions against high concentrations of inorganic compounds (Shomer et al. 1991). These compatible compounds are neutral and highly soluble and contain secondary metabolites such as glycine betaine, proline, and sucrose (Hu and Schmidhalter 2000). There is a close correlation between the synthesis of these organic compounds and tolerance to salinity or drought. This correlation has been noted in maize (Saneoka et al. 1995). These compounds appear to be very effective in maintaining a negative osmotic potential in the cytoplasm and in protecting proteins and ribosomes against the deleterious effects of Na+ ions. Although organic compounds give the plant some tolerance to salinity, they need to be accompanied by strong regulation of Na+ pumps.
4.4.4 Tolerance of Halophyte Plants to Salinity
Plants in salt-exposed (e.g., costal) environments have certainly acquired characteristics to adapt to soils whose chemical composition varies in time and space, depending on salinity and associated stress (Ben Hamed et al. 2013). Therefore, the adaptations of these coastal plants are complex and different. Nowadays, many authors study halophytes as plant object with developed mechanisms of salt resistance. Halophytes are plants which can be productive under stress (Ben Hamed et al. 2013; Llanes et al. 2013; Slama et al. 2015).
Salinity tolerance reflects the ability of plant halophytes to grow and complete their life cycle in environments containing soluble salts at high concentrations. Halophytes are characterized by low morphological and taxonomic diversity. At the same time, halophytes require salt for optimal growth. The high concentrations of the ions in the tissues of halophytes suggest that their metabolic process may be more tolerant to salt stress compared to the glycophytic metabolism.
4.4.5 Regulation of Salt Loads in Aerial Parts
4.4.5.1 Salt Release in Aerial Parts
Soluble substances pass from the inside of the leaves to the outside where they are accumulated. These substances are released from the leaves through the epidermis and stored in the cuticle. It is called pseudo-secretion (Klepper and Barrs 1968). The sodium is the most flexible element among osmotic cations. However, chlorine is the most flexible element among anions (Tukey et al. 1958; Tukey and Morgan 1962). The release of electrolytes is highly dependent on the plant water status. The salts released from the leaves of Atriplex sp. normally present half of the leaf contents. The phenomenon of salt release in coastal areas where mangroves and other native halophytes grow is based on the desalination process with halophyte participation.
4.4.5.2 Guttation
Salt glands are not the only structures through which salt is removed from the plant. Hydathodes are structures which can also eliminate water. Guttation is a common phenomenon in young leaves. The liquid obtained is not a direct secretion of the xylem sap. In fact, the content of nutrient ions in the xylem sap liquid is much lower compared to the sap liquid from the other plant tissues.
Guttation liquid contains mainly calcium, carbonate, sodium, and silicate. In some plant species, hydathodes can function as salt glands and participate in the selective removal of ions. The secretion of salt in this way may be important in young halophytes that develop under humid conditions.
4.4.5.3 Elimination of Organs Saturated with Salt
It is a phenomenon that can eliminate a large amount of salt in halophytes. In some species such as Juncus maritimus or Juncus gerardii, leaves fall after being loaded with unwanted ions. Some succulent plants such as Halocnemum or Salicornia get rid of part of the cortex. This part of the cortex released large amount of salt, which allows the plant to survive (Chapman 1960).
4.4.5.4 Remobilization of Salt
The substances accumulated in the aerial parts can be transported by the phloem vessels to the roots and after into the rhizosphere. This has been verified for sodium (Cooil et al. 1965). A similar process has been observed for Suaeda monoica and Salicornia europaea (Von Willert 1968). Salt recirculation also exists in non-excretory mangroves (Scholander et al. 1962; Atkinson et al. 1967).
4.4.5.5 Accumulation of Salt in Secretory Epidermic Hairs
In some species, epidermic hairs remove salt from sensitive sites of leaf mesophyll. The epidermic hairs work for a short time, but they are very effective. Salt accumulation by vesicle traffic under salt stress is widely known in semi-halophytic and halophytic species of the family Chenopodiaceae. For example, in Atriplex, salt hairs or trichomes are formed by two cells: a small basal cell and a large vesicle (Osmond et al. 1969, Mozafar and Goodin 1970). The basal cell has a high structural similarity with the cells of the salt glands. It is characterized by a dense cytoplasm rich in mitochondria, endoplasmic reticulum, and numerous small vesicles. It differs from glandular cells by the presence of chloroplasts.
4.4.5.6 Excretion of Salt
Salt excretion is the most important mechanism which supports halophyte resistance to salinity (Waisel 1972). It allows plants to eliminate excess salt and prevents excessive buildup without reaching toxic levels inside the tissues. It is typical for several halophyte species: Convolvulaceae, Frankeniaceae, Poaceae, Primulaceae, Tamaricaceae, Avicenniaceae, and Plumbaginaceae.
4.4.6 Salt-Secreting Structures
4.4.6.1 Trichomes
The secretory trichomes of salt are typical for Atriplex spp. These are vesicles that emerge at the leaf surface. They consist of a large secretory cell or vesicle at the apex or vesicle from one or a few cells on a pedicel (Smaoui 1971; Dickison 2000). These cells have mitochondria, dictyosomes, ribosomes, endoplasmic reticulum, and a large flattened nucleus. Chloroplasts are found also but rudimentary or partially developed. In the secretory cell or vesicle is present a large vacuole, while the cell on the pedicle contains the several small vacuoles (Osmond et al. 1969). A symplasmic continuity exists between the mesophyll cells and the secretory cell, for the movements of the ions. The outer walls of vesicular and pedicellar cells are cutinized, while the inner walls are not (Thomson and Platt-Aloia 1979). The salts are externally released by the removal of the leaf.
4.4.6.2 Salt Glands
The glands of herbaceous plants are usually bicellular and formed of an apical and basal cell. They can be submerged, semi-sunken, extended outside the epidermis (Liphschitz and Waisel 1974; Marcum and Murdoch 1994), or lying on the leaf surface in parallel lines on the ridges (Marcum et al. 1998). In dicotyledons (Fig. 4.7), salt glands are multicellular, consisting of basal cells and secretory cells. The number of cells can vary from 6 to 40 depending on the plant genus (Fahn 1990). For example, in Tamarix spp., salt glands are composed of two basal and internal collecting cells and six external secretory cells (Mauseth 1988). The glands of Avicennia and Glaux contain several secretory cells surmounting a discoidal basal cell (Rozema and Riphagen 1977).
Salt excretion on adaxial leaf side of Aeluropus littoralis plants treated with 0, 200, 400, 600, and 800 mM NaCl observed with a magnifying glass (×4) (a). Note that leaves were more or less rolled. SEM micrographs of salt crystals observed on adaxial leaf surface from 400 mM NaCl-treated plants (b). Adaxial leaf surface of 400 mM NaCl-treated plant which was washed and observed 2 h later. The appearance of salts indicates location of salt glands (c)
At the ultrastructural level, glandular cells in dicotyledonous herbaceous plants contain some lipid bodies, a large flattened nucleus, an endoplasmic reticulum, ribosomes, several mitochondria, rudimentary plastids, and small vacuoles (Thomson 1975). The glands are covered with an elongated cuticle at the level of the excretory cell to form a collecting chamber, in which the salt accumulates before being excreted outside. There is continuity between the basal cell and the apical cell and between the basal cell and the mesophyll cells, thanks to plasmodesmata (Zeigler and Lüttge 1967).
4.4.7 Osmotic Adjustment
The one of the main physiological traits of tolerance to environmental stress is the osmotic adjustment. This one is realized, thanks to an accumulation of osmoregulatory compounds that may be ions such as K+ and Cl− or organic compounds such as soluble sugars (fructose, glucose, trehalose, raffinose, fructans) and certain amino acids (proline, glycine betaine, β-alaninebetaine, proline betaine) leading to a reduction of the osmotic potential, thus allowing the maintenance of the turgor potential (Zivcak et al. 2016). The accumulation of these compounds has been evident in several plant species subjected to salt stress. It varies in large proportions depending on the species, the stage of development, and the degree of salinity. The differences in solute accumulation (free amino acids, proline, and total soluble sugars) between control plants and plants subjected to salt stress are very important. This phenomenon allows the maintenance of many physiological functions (photosynthesis, transpiration, growth, etc.) and can intervene at all stages of plant development. It allows protection of membranes and enzymatic systems especially in organs where proline appears to play a role in maintaining cytosol-vacuole pressures and pH regulation (El Hassani et al. 2008).
4.4.8 Regulation of Growth by Phytohormones Under Salt Stress
They have been shown to have physiological responses to various stresses such as drought or salinity and have similar characteristics. They cause a whole increase in the ABA concentration in the aerial part or a reduction in concentrations in cytokinin (Itai 1999). According to Zhu (2001), the reduction of growth is an adaptative capacity necessary for survival of a plant exposed to abiotic stress. Indeed, this delay in plant development can support energy accumulation and resources to limit the effects of stress before that the imbalance between the inside and outside of the plant body does not increase until a threshold where the damage is irreversible. To illustrate this trend, in nature, growth is inversely correlated with salt stress resistance of a species or variety (Zhu 2001). In more control of growth by hormonal signals, the reduction of growth results from the expenditure of resources in adaptation strategies and cytosol-vacuole and pH regulation (Hassani et al. 2008).
4.4.9 Mechanism of Membrane Control Under Salt Stress
Adaptation to salt stress is also taking place at the level of membrane cell (plasma membrane, tonoplast). The qualitative and quantitative modification of aquaporins (transmembrane proteins) is, for example, a process capable of modifying the water conductivity of the plant and promoting restricting water movements (Yeo 1998). In terms of ion transport, the salinity resistance strategy is qualitative and quantitative. The selectivity of the ions at the input constitutes the component which is defined from the different recent membrane transporters (Na+/H+). In the diffusion facilitated as in the active transport, the membrane proteins can be very specific to certain solutes. Nevertheless, several solutes can compete for the same transport protein (Na+ and K). From a point of quantitative view, Na+ membrane permeability and activity, quantity, and sensitivity of the membrane Na+/H+ antiports evolve to adapt to sodium stress at long term (Niu et al. 1995; Tyerman and Skerrett 1999).
4.4.10 The Biological Compounds, Trace Elements Useful for Mitigation of Salt Stress Effects
4.4.10.1 Jasmonic Acid and Salicylic Acid
Generally, under stressful conditions such as salinity stress, plants employ multiple mechanisms to increase their tolerance (Borsani et al. 2003). One of the adaptative plant responses to salt stress is the production of phytohormones such as abscisic acid, salicylic acid (SA), and jasmonates that might be involved in the alleviation of salinity stress (Wang et al. 2001; Yoon et al. 2009). It was observed that SA reduced salt stress injuries via enhancing antioxidant enzyme activities. Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts have been found (Moons et al. 1997).
Plant hormones such as methyl jasmonate (MeJA) and jasmonic acid (JA) have an ameliorating effect on different plant species under salt stress (Yoon et al. 2009; Manan et al. 2016). Jasmonates play a role of cellular regulators in the response to stress factors, such as salt, drought, and heavy metal (Anjum et al. 2011; Poonam et al. 2013; Qiu et al. 2014).
Jasmonic acid (JA) and methyl jasmonate (MeJA), which is the methyl ester of JA, are natural plant growth regulators, involved in regulation of the morphological, biochemical, and physiological processes in plants. Their exogenous plant treatment under conditions of high salinity can support the development of biomass yield (Sheteawi 2007).
The several studies have shown that methyl jasmonate can diminish the inhibitory effect of NaCl on photosynthesis rate and can enhance the growth and development of plants (Hristova and Popova 2002; Javid et al. 2011). Application of JA after the stress decreased the adverse effect of high salinity on photosynthesis and growth of barley (Tsonev et al. 1998). In addition, exogenous pretreatment of JA could ameliorate salt-stressed rice seedlings, particularly in salt-sensitive cultivars, and could decrease sodium concentration remarkably (Kang et al. 2005).
4.4.10.2 Brassinosteroids
One of the promising options to mitigate the detrimental effects of salt stress is the exogenous application of plant protectants such as brassinosteroids (BRs) (Vardhini and Anjum 2015). BRs belong to plant polyhydroxysteroids, which are important for the regulation of plant growth and development.
The ability to protect the cellular structures was documented for 24-epibrassinolide (EBL), which reduced damage to membrane lipids, and hence a low MDA concentration. MDA, a product of polyunsaturated fatty acid decomposition, is used as a marker to assess the lipid peroxidation in plasmalemma or organelle membranes, which typically occurs in stress conditions (Sharma et al. 2012). The peroxidation of lipids disturbs the bilayer structure, affecting the membrane fluidity, permeability, bilayer thickness, and other membrane properties due to oxidative damage of lipids and membrane proteins. This may alter ion gradients, strongly influencing the metabolic processes. The lipid peroxidation caused by salt stress is probably one of the major reasons inhibiting plant growth.
In perennial ryegrass exposed to salt stress, it was observed that the treatment by exogenous brassinosteroids led to higher K+, Ca2+, and Mg2+ content and lower Na+/K+ ratio (Sun et al. 2015). The exogenous brassinosteroid application led also to upregulation of antioxidant enzyme (SOD, CAT, and APX) activity, keeping the level of plant hormones at a physiologically favorable level and an increase of proline and ion content (K+, Ca2+, and Mg2+). Exogenous brassinosteroids could prevent the nutritional imbalance and ion toxicity under salt stress (Wu et al. 2017). The main effects of brassinosteroid use have been presented in Table 4.5.
4.4.10.3 Amino Acids: Glycine Betaine and Proline
Amino acids glycine betaine and proline are effectively used for exogenous treatment to mitigate salt stress effects on plants (Sobahan et al. 2012; Li et al. 2014). Improvements of salt tolerance can be partly attributed to more favorable water status, as well as to activity of antioxidative enzymes in leaves, especially of peroxidase. It was found that mitigative effects of exogenous proline in salt stress conditions are more efficient than exogenous application of betaines (Hoque et al. 2007). The proline compared to the betaine can directly scavenge superoxide (O2 •) or hydrogen peroxide (H2O2) and induce an increase of antioxidant enzyme activities (Demiral 2004; Ashraf and Foolad 2007; Nawaz and Ashraf 2010). At the same time, the unequal reaction of antioxidative enzymes has been observed under different level of salt concentrations. Treatment of 5 mM proline significantly reduced POX3 activity, which resulted in modulating salinity stress compared to 200 mM concentration (Varjovi et al. 2016). Therefore, it is obvious that the effect of proline in plants exposed to salinity is specific, at least to some extent.
The role of proline and also betaine in maintaining the plant water status under salt stress is important, as the initial slowdown of plant growth after salt imposition is a result of salt osmotic effects (Munns and Tester 2008; Yang and Lu 2006). The water-retaining ability can enhance salt tolerance by preventing too high concentration of ions (Romero-Aranda et al. 2006).
In rice, it was found that exogenous application of glycine betaine and proline may suppress the Na+ uptake from the apoplast, preventing the detrimental effects of salts (Yang and Lu 2005; Sobahan et al. 2012). Proline inhibits opening of stomata, which keeps the transpiration and Na+ uptake low. Moreover, in rice treated with betaine, the cells of the root tip and root cap produced numerous vacuoles, playing a role of storage vessels for Na+ (Rahman et al. 2002). Thus, the specific functions of proline and betaine can contribute to improvements of salinity tolerance.
The use of exogenous proline can balance grain and straw yields under increased salinity levels. The production of grain and straw yields under salt stress conditions after the use of exogenous proline was kept on the significant level. Foliar application of proline and betaine decreased the sodium content and uptake by plants. Thus, it can be concluded that the exogenous application of proline and betaine may mitigate significantly the salt stress effects in crop plants (Siddique et al. 2015; Athar et al. 2015).
4.4.10.4 Polyamines
Polyamines (PAs) are abundant compounds, present in plant cells in concentrations from 10 μM to 10 mM (Roychoudhury et al. 2011). They represent low-molecular-weight, straight-chain, aliphatic amines, including the diamine putrescine (Put2+), triamine spermidine (Spd3+), and tetramine spermine (Spm4+), and are involved in various biochemical and physiological processes related to the regulation of plant growth and development (Puyang et al. 2015). Thanks to their polycationic nature at physiological pH, these compounds can interact with membrane phospholipids, proteins, nucleic acids, and constituents of cell walls, which stabilize these molecules (Roychoudhury et al. 2011).
In the last period, the role of polyamines as second messengers was investigated, especially in response to environmental stresses like osmotic stress, salinity, drought, heat, mineral nutrient deficiency, heavy metals, pH variation, UV irradiation, etc. (Liu et al. 2015). It was documented that exogenous spermidine application diminished the oxidative stress induced by salinity, leading to lower MDA, H2O2, and O2 ·− concentrations in cultivars of bluegrass. Results indicated that exogenous spermidine treatment is able to improve quality of turfgrass, thanks to promoting the tolerance to salinity by eliminating the oxidative damages and upregulating activities of antioxidative enzymes directly or through gene expression (Puyang et al. 2015).
Spermidine efficiently alleviated the inhibitory role of alkaline ions on plant growth and inhibited related oxidative stress (Zhang et al. 2015). At the same time, the exogenous spermidine treatment had positive effects on nitrogen metabolism and activity of its enzymes in tomato seedlings under salt stress (Zhang et al. 2013). Exogenous spermidine application helps tomato seedlings to overcome salinity stress by regulation of protective mechanism of plant cells, including activating of detoxification, which may protect the cellular structures from oxidative damage under salinity stress. Exogenous spermidine is also able to increase salt tolerance of Panax ginseng by upregulation of scavenging enzyme activities, which eliminates the oxidative impairment (Parvin et al. 2014).
4.4.10.5 Paclobutrazol
The enhancement of salt tolerance in plants can be achieved through exogenous application of plant growth regulators with specific effects on the content of key plant phytohormones and signal molecules (Kishor et al. 2009; Hu et al. 2017). Paclobutrazol [(2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl) pentan-3-ol] is a triazole fungicide which regulates the plant growth mostly by antagonizing the hormone gibberellin (Hajihashemi et al. 2006; Kishor et al. 2009). Paclobutrazol and other triazole compounds are synthetic plant growth regulators, which cause different physiological responses in plants, such as increasing content of chlorophylls, promoting net photosynthesis, regulating cytokinin biosynthesis, inhibiting ABA biosynthesis, reducing free-radical damage, and enhancing the peroxidase and SOD activities and proline content (Kishor and Sreenivasulu 2014; Khunpon et al. 2017).
Paclobutrazol has also morphological effects on the leaf thickness, cuticle and epidermis, palisade layer, and spongy layer. It can reduce the diameter of xylem vessels; however the phloem elements had shown an increased diameter (Tehranifar et al. 2009). Exogenous treatment has also stimulated effect on root growth and decreasing shoot growth (Banon et al. 2003; Nivedithadevi et al. 2012). Some authors suggest that the paclobutrazol minimizes the absorption of toxic ions such as Na+ and Cl−, which eliminates the negative effects of NaCl. Moreover, the experimental results supported the role of paclobutrazol in upregulating the K+ uptake (Kishor and Sreenivasulu 2014; Hu et al. 2017). We can conclude that paclobutrazol can improve important plant responses and increase crop production with a consequent benefit to saline agriculture.
4.4.10.6 Trace Elements and Nanoparticles
During the last few years, rapid advances of nanotechnology are associated with release of different types of nanoparticles. Some of them may be accumulated in soil or natural environment with negative effects on biota (Alharby et al. 2016; Yassen et al. 2017). The authors have reported the detrimental effects of nanoparticles (usually at relatively high concentration) on plant health. However, there is an evidence about the positive effects of nanoparticles, which is achieved at relatively low concentrations. This provides the scope for possible agricultural applications of nanoparticles (Siddiqui et al. 2014; Siddiqui and Al-Whaibi 2014; Askary et al. 2016).
One of the promising ways is the use of nanofertilizers. Applying the nutrients in the form of nanoparticles improves the nutrient use efficiency, with low risk of toxicity for soil microbiota and roots. Moreover, such a way of application reduces the frequency of the application and prevents the risk of overdosage. Hence, the potential of nanotechnology to support the sustainable farming is high, including developing countries (Naderi and Danesh-Shahraki 2013; Yassen et al. 2017).
The second way of application relates to exogenous use of trace elements and nanoparticles to mitigate stress effects by influencing some specific plant processes (Zhao et al. 2012; Rico et al. 2013; Rossi et al. 2016). For example, the zinc treatment led to lower MDA and H2O2 concentration in tissues in the experimental plants under salt stress, which was associated with upregulation of total APX, CAT, POD, and PPO activities under salt stress (Weisany et al. 2012). Decreasing of lipid peroxidation and proline contents under salinity by applying Fe2O3NPs has been found in the peppermint plants. The appropriate concentration of iron nanoparticles can be used for stress resistance of the peppermint (Askary et al. 2017). Fathi et al. (2017) and Soliman et al. (2015) have demonstrated the positive influence of Zn and Fe and their NPs in stress conditions. Nanoparticles were more efficient than other tested forms of these micronutrients. It can be caused by their size, shape, distribution, and other physical characteristics. Latef et al. (2017) reported that priming of seeds with ZNPs is a useful strategy to increase the salt tolerance of lupine plants. The most efficient was concentration of ZnO NPs 60 mg L−1.
It has been also shown that exogenous nanoparticles such as cerium oxide nanoparticles (CeO2-NPs) positively influence plant growth and production under normal growth conditions. Depending on soil moisture content, CeO2-NPs supported photosynthesis, which led to increase of water use efficiency (WUE), especially in water-restricted conditions (Cao et al. 2017). Under salinity, it was found that CeO2-NPs application led to improved plant growth and physiological responses of canola, improving the salt stress responses. However, the stress effects were not fully alleviated by CeO2-NPs (Rossi et al. 2016).
Adding SiO2 nanoparticles was found to be able to improve germination and seedling early growth under salinity stress (Sabaghnia and Janmohammadi 2014; Siddiqui and Al-Whaibi 2014). In similar, nano-silicon (N-Si) was shown to improve seed germination, plant growth, and photosynthesis under environmental stresses in tomato (Almutairi 2016a, b).
Also in the case of application of AgNPs, the alleviative effects in conditions of salt stress were found, including positive influence on seed germination, growth of roots, and thus the overall growth and dry mass increase in tomato seedlings under NaCl stress (Almutairi 2016a). The combined application of AgNPs and salinity increased the soluble sugars and proline contents. On the other hand, it decreased catalase activity and increased peroxidase activity compared to the respective AgNP treatments alone. AgNPs enhanced the salt tolerance in wheat, but the long-term response of AgNPs under salt stress needs further investigation.
El-Sharkawy et al. (2017) have demonstrated that application of K nanoparticles in alfalfa may be more efficient than the use of conventional fertilizers, as the nutrition can be more adequate and this way of application may prevent the negative effects of salt stress in some specific conditions.
The abovementioned results suggest that the application of different nanoparticles is a promising strategy to stimulate the plant tolerance to salt stress. According to the many researchers, engineered nanoparticles have a great chance of getting into agricultural lands (Delfani et al. 2014; Benzone et al. 2015; Liu et al. 2015; Liu and Lai 2015; Mastronardi et al. 2015; Rastogi et al. 2017). We report that a common industrial nanoparticle could in fact have a positive impact on crops. Modern nanofertilizers are expected to contribute to the improvement of crop growth, photosynthesis, and tolerance to environmental stress, which will result to better nutrient and water use efficiency and yield increase.
4.5 Conclusion
Nowadays, the advances in research aimed at salt stress effects on plants at different levels, described broadly in this chapter, provide great opportunities to develop effective strategies to improve crop tolerance and yield in different environments affected by the soil salinity. It was clearly demonstrated that plants employ both the common adaptative responses and the specific reactions to salt stress. Presented data may be helpful to understand the physiological, metabolic, developmental, and other reactions of crop plants to salinity, resulting in the decrease of biomass production and yield. In addition, the chapter provides an overview of modern studies on how to mitigate salt stress effects on photosynthetic apparatus and productivity of crop plants with the help of phytohormones, glycine betaine, proline, polyamines, paclobutrazol, trace elements, and nanoparticles. Plant production in saline agriculture can avoid or, at least, diminish the negative salt effects with use of different approaches and tools, which can have an economic impact worldwide but, especially, in most endangered developing countries.
Abbreviations
- ABA:
-
Abscisic acid
- APX:
-
Ascorbate peroxidase
- BRs:
-
Brassinosteroids
- CAT:
-
Catalase
- DW:
-
Dry weight
- EBL:
-
24-Epibrassinolide
- FW:
-
Fresh weight
- GPX:
-
Guaiacol peroxidase
- JA:
-
Jasmonic acid
- MeJA:
-
Jasmonate
- MDHAR:
-
Monodehydroascorbate reductase
- MDA:
-
Malonic dialdehyde
- NPs:
-
Nanoparticles
- Pn:
-
Photosynthetic rate
- PAs:
-
Polyamines
- RWC:
-
Relative water content
- SA:
-
Salicylic acid
- SOD:
-
Superoxide dismutase
- WUE:
-
Water use efficiency
References
Abdelly C (2006) Caractérisation des halophytes pour le dessalement des sols salins et letraitement des eaux salines. Rapport d’activités 2007. Centre de biotechnologie à la technopoledeBorj-Cedria, Tunisie, pp 28–31
Abdi N, Wasti S, Slama A, Ben Salem M, El Faleh M, Mallek-Maalej E (2016) Comparative study of salinity effect on some tunisian barley cultivars at germination and early seedling growth stages. J Plant Physiol Pathol 4(3):1–9. https://doi.org/10.4172/2329-955X.1000151
Agastian P, Kingsley SJ, Vivekanandan M (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 38:287–290
Ahmad M, Niazi BH, Zaman B, Athar M (2005) Varietals differences in agronomic performance of six wheat varieties grown under saline field environment. Int J Environ Sci Technol 2(1):49–57
Ahmad M, Zahir Zahir A, Nazli F, Akram F, Arshad MKM (2013) Effectiveness of halo-tolerant, auxin producing pseudomonas and rhizobium strains to improve osmotic stress tolerance in mung bean (Vigna radiata L.) Braz. J Microbiol 44(4):1341–1348
Aissaoui HS, Reffas S (2007) Effet de stress salin sur la productivité de populations sahariennes locales de la luzerne (Medicago sativa L.), Université Kasdi Merbah Ouargla.
Al Hassan M, Chaura J, Donat-Torres MP, Boscaiu M, Vicente O (2017) Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 9(2):1–20. https://doi.org/10.1093/aobpla/plx009
Alem C, Labhilili M, Brahmi K, Jlibene M, Nasrallah N, Filali-Maltouf A (2002) Adaptations hydrique et photosynthétique du blé dur et du blé tendre au stress salin. C R Biologies 325(11):1097–1109
Alharby HF, Metwali EMR, Fuller MP, Aldhebiani AY (2016) Impact of application of zinc oxide nanoparticles on callus induction, plant regeneration, element content and antioxidant enzyme activity in tomato (Solanum lycopersicum Mill.) under salt stress. Arch Biol Sci 68(4):723–735
Ali B, Hayat S, Fariduddin Q, Ahmad A (2008) 24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere 72:1387–1392
Al-Khateeb SA (2006) Effect of calcium/sodium ratio on growth and ion relations of alfalfa (Medicago sativa L.) seedling grown under saline condition. J Agron 5(2):175–181
Allakhverdiev SI, Nishiyama Y, Miyairi S, Yamamoto H, Inagaki N, Kanesaki Y, Murata N (2002) Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiol 130:1443–1453
Allen SG, Dobrenz AK, Scharnhorst M, Stoner JEA (1985) Heritability of NaCl tolerance in germinating alfalfa seeds. Agron J 77:99–105
Almutairi ZM (2016a) Influence of silver nano-particles on the salt resistance of tomato (Solanum lycopersicum L.) during germination. Int J Agri Biol 18(2):449–457. https://doi.org/10.17957/IJAB/15.0114
Almutairi ZM (2016b) Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics Journal 9(1):106–114
Anjum SA, Xie X, Wang L et al (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agr Res 6:2026–2032
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–6
Askari-Khorasgani O, Emadi S, Mortazaienezhad F, Pessarakli M (2017) Differential responses of three chamomile genotypes to salinity stress with respect to physiological, morphological, and phytochemical characteristics. J Plant Nutr 40(18):2619–2630
Askary M, Talebi SM, Amini F, Ali D, Bangan B (2017) Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija 63(1):65–75
Askary M, Talebi SM, Amini F, Bangan ADB (2016) Effect of NaCl and iron oxide nanoparticles on Mentha piperita essential oil composition. Environ Exp Biol 14:27–32. https://doi.org/10.22364/eeb.14.05
Asrar H, Hussain T, Midhat S, Hadi S, Gul B, Nielsen BL, Khan MA (2017) Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) staph. Environ Exp Bot 135:86–95
Athar H-U-R, Zafar ZU, Ashraf M (2015) Glycinebetaine improved photosynthesis in canola under salt stress: evaluation of chlorophyll fluorescence parameters as potential indicators. J Agro Crop Sci 201:428–442. https://doi.org/10.1111/jac.12120
Atkinson MR, Findlay CP, Hope AB, Pitman MG, Saddler HDW, West KR (1967) Salt regulation in the mangroves Rhizophora mucronata Lam. and Aegialitis annulata R. Br Aust J Biol Sci 20:589–599
Aziz I, Khan MA (2001) Experimental assessment of salinity tolerance of Ceriops tagal seedlings and saplings from the Indus delta, Pakistan. Aqua Bot 70(3):259–268
Azri W, Chambon C, Herbette S, Brunel N, Coutand C, Leplé JC, Ben Rejeb I, Ammar S, Julien JL, Roeckel-Drevet P (2009) Proteome analysis of apical and basal regions of poplar stems under gravitropic stimulation. Physiol Plant 136:193–208. https://doi.org/10.1111/j.1399-3054.2009.01230.x
Ballesteros E, Blumwald E, Donaire JP, Belver A (1997) Na+/H+ antiport activity in tonoplast vesicles isolated from sunflower roots induced by NaCl stress. Physiol Plant 99:328–334. https://doi.org/10.1111/j.1399-3054.1997.tb05420.x
Banon S, Ochoa J, Martinez JA, Fernandez JA, Franco JA, Sanchez-Blanco MJ, Alarcon JJ, Morales MA (2003) Paclobutrazol as an aid to reducing the effects of salt stress in Rhamnus alaternus plants. Acta Hortic 609:263–268
Barhoumi Z, Djebali W, Smaoui A, Chaïbi W, Abdelly C (2007) Contribution of NaCl excretion to salt resistance of Aeluropus littoralis (Willd) Parl. J Plant Physiol 164(7):842–850
Bartels D, Nelson D (1994) Approaches to improve stress tolerance using molecular genetics. Plant Cell Environ 17:659–667. https://doi.org/10.1111/j.1365
Belkheiri O, Mulas M (2013) The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ Exp Bot 86:17–28
Ben Ahmed C, Ben Rouina B, Sensoy S, Boukhriss S, Ben Abdullah F (2010) Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J Agricult Food Chem 58:416–422
Ben Amor N, Ben Hamed K, Debez A, Grignon C, Abdelly C (2005) Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci 168:889–899
Ben Hamed K, Ellouzi H, Talbi-Zribi O, Hessini K, Slama I, Ghnaya T, Munné Bosch S, Savouré A, Abdelly C (2013) Physiological response of halophytes to multiple stresses. Funct Plant Biol 40(9):883–896. https://doi.org/10.1071/FP13074
Ben Hamed KB, Castagna A, Salem E, Ranieri A, Abdelly C (2007) Sea fennel (Crithmum maritimum L.) under salinity conditions: a comparison of leaf and root antioxidant responses. Plant Growth Regul 53:185–194. https://doi.org/10.1007/s10725-007-9217-8
Ben Hamed KB, Chibani F, Abdelly C, Magne C (2014) Growth, sodium uptake and antioxidant responses of coastal plants differing in their ecological status under increasing salinity. Biologia 69(2):193–201. https://doi.org/10.2478/s11756-013-0304-1
Bennaceur M, Rahmoune C, Sdiri H, Meddhi ML, Selmi M (2001) Effet du stress salin sur la germination, la croissance et la production en grains de quelques variétés maghrébines de blé. Sècheresse 12(3):167–174
Benzarti M, Ben Rejeb K, Debez A, Messedi D, Abdelly C (2012) Photosynthetic activity and leaf antioxidative responses of Atriplex portulacoides subjected to extreme salinity. Acta Physiol Plant 34:1679–1688
Benzon HRL, Rubenecia MRU, Ultra VU, Lee SC (2015) Nano-fertilizer affects the growth, development, and chemical properties of rice. IJAAR 7(1):105–117
Berthomieu P, Conejero G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oik S, Yamada K, Cellier F, Gosti F, Simonneau T, Essah PA, Tester M, Véry AA, Sentenac H, Bhandal IS, Malik CP (1988) Potassium estimation, uptake, and its role in the physiology and metabolism of flowering plants. Int Rev Cytol 110:205–254
Bhandal IS, Malik CP (1988) Potassium estimation, uptake, and its role in the physiology and metabolism of flowering plants. Int Rev Cytol 110:205–254
Blaha G, Stelzl U, Spahn CM, Agrawal RK, Frank J, Nierhaus KH (2000) Preparation of functional ribosomal complexes and effect of buffer conditions on tRNA positions observed by cryoelectron microscopy. Methods Enzymol 317:292–309
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12(4):431–434
Borsani O, Valpuesta V, Botella MA (2003) Developing salt tolerant plants in a new century: a molecular biology approach. Plant Cell Tissue Organ 73:101–115
Boscaiu M, Estrelles E, Soriano P, Vicente O (2005) Effects of salt stress on the reproductive biology of the halophyte Plantago crassifolia. Biol Plant 49:141–143
Botella MA, Quesada MA, Kononowicz A, Bressan RA, Hasegawa PM, Valpuesta V (1994) Characterization and in situ localization of a salt induced tomato peroxidase gene. Plant Mol Biol 25:105–114
Brestic M, Zivcak M (2013) PSII fluorescence techniques in drought and high temperature stress signal measurement of crop plants: protocols and applications. In: Rout GR, Das AB (eds) Molecular stress physiology of plants. Springer, India, pp 87–113. https://doi.org/10.1007/978-81-322-0807-5_4
Briens M, Larher F (1982) Osmoregulation in halophytic higher plants: a comparative study of soluble carbohydrates, polyols, betaines and free proline. Plant Cell Environ 5:287–292
Brugnoli E, Björkman O (1992) Growth of cotton under continuous salinity stress: influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta 187(3):335–347
Bruns S, Hecht-Buchholz C (1990) Light and electron microscope studies on the leaves of several potato cultivars after application of salt at various development stages. Potato Res 33:33–41. https://doi.org/10.1007/BF02358128
Bueno M, Lendínez ML, Aparicio C, Cordovilla MP (2017) Germination and growth of Atriplex prostrata and Plantago coronopus: two strategies to survive in saline habitats. Flora 227:56–63
Cao Z, Rossi L, Stowers C, Zhang W, Lombardini L, Ma X (2017) The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ Sci Pollut Res Int 25(1):930–939. https://doi.org/10.1007/s11356-017-0501-5
Chapman VJ (1960) Salt marshes and salt deserts of the world. Leonard Hill, London
Chartzoulakis K, Klapaki G (2000) Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci Hortic 86:247–260
Chaudhuri K, Choudhuri M (1997) Effects of short-term NaCl stress on water relations and gas exchange of two jute species. Biol Plant 40:373. https://doi.org/10.1023/A:1001013913773
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chaves MM, Earl HJ, Flexas J, Loreto F, Medrano H (2011) Photosyn- thesis under water stress, flooding and salinity. In: Terrestrial photosynthesis in a changing environment. Cambridge University Press pp 49–104
Cheeseman JM (1988) Mechanisms of salinity tolerance in plants. Plant Physiol 87(3):547–550
Cheeseman JM (2015) The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol 206(2):557–570. https://doi.org/10.1111/nph.13217
Chen Q, Goldstein I, Jiang W (2010) Payoff complementarities and financial fragility: evidence from mutual fund outflows. J Financ Econ 97(2):239–262
Chen TW, Kahlen K, Stützel H (2015) Disentangling the contributions of osmotic and ionic effects of salinity on stomatal, mesophyll, biochemical and light limitation to photosynthesis. Plant Cell Environ 38:1528–1542. https://doi.org/10.1111/pce.12504
Cooil BJ, de la Fluente RK, de la Pena RS (1965) Absorption and transport of sodium and potassium in squash. Plant Physiol 40:625–633
Dąbrowski P, Baczewska AH, Pawluśkiewicz B, Paunov M, Alexantrov V, Goltsev V, Kalaji MH (2016) Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J Photochem Photobiol B 157:22–31. https://doi.org/10.1016/j.jphotobiol.2016.02.001
Dąbrowski P, Kalaji MH, Baczewska AH, Pawluśkiewicz B, Mastalerczuk G, Borawska-Jarmułowicz B, Paunov M, Goltsev V (2017) Delayed chlorophyll a fluorescence, MR 820, and gas exchange changes in perennial ryegrass under salt stress. J Lumin 183:322–333. https://doi.org/10.1016/j.jlumin.2016.11.031
De Oliveira VP, Marques EC, de Lacerda CF, Prisco JT, Gomes-Filho E (2013) Physiological and biochemical characteristics of Sorghum bicolor and Sorghum sudanense subjected to salt stress in two stages of development. Afr J Agric Res 8:660–670
Debez A, Hamed KB, Grignon C, Abdelly C (2004) Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima. Plant Soil 262:179–189
Debez A, Koyro HW, Grignon C, Abdelly C, Huchzermeyer B (2008) Relationship be-tween the photosynthetic activity and the performance of Cakile maritima after long-term salt treatment. Physiol Plant 133:373–385
Delfani M, Firouzabadi MB, Farrokhi N, Makarian H (2014) Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun Soil Sci Plant Anal 45:530–540
Demiral IT (2004) Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? J Plant Physiol 161:1089–1100
Dickison WC (2000) Integrative plant anatomy, 1st edn. Harcount Academic, San Diego
Ellouzi H, Hamed KB, Cela J, Munné-Bosch S, Abdelly C (2011) Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol Plant 142:128–143. https://doi.org/10.1111/j.1399-3054.2011.01450.x
Ellouzi H, Hamed KB, Hernández I, Cela J, Müller M, Magné C, Abdelly C, Munné-Bosch S (2014) A comparative study of the early osmotic, ionic, redox and hormonal signaling response in leaves and roots of two halophytes and a glycophyte to salinity. Planta 240(6):1299–1317. https://doi.org/10.1007/s00425-014-2154-7
El-Sharkawy MS, El-Beshsbeshy TR, Mahmoud EK, Abdelkader NI, Al-Shal RM, Missaoui AM (2017) Response of alfalfa under salt stress to the application of potassium sulfate nanoparticles. Am J Plant Sci 8:1751–1773. https://doi.org/10.4236/ajps.2017.88120
Elzam OE, Epstein E (1969) Salt relations of two grass species differing in salt tolerance. II kinetics of the absorption of K, Na and Cl by their excised roots. Agrochimica 13:196–206
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Fahn A (1990) Plant anatomy, 4th edn. Pergamon Press, New York
FAO (2000) Global network on integrated soil management for sustainable use of salt-affected soils. Country Specific Salinity Issues— Iran.FAO, Rome. Available at http://www.fao.org/ag/agl/agll/spush/degrad.asp?country¼iran
FAO (2015) FAO cereal supply and demand brief. http://www.fao.org/worldfoodsituation/csdb/en/
FAO/IIASA/ISRIC/ISS-CAS/JRC (2008) Harmonized world soil database (version 1.0). FAO, Rome
Fathi A, Zahedi M, Torabian S (2017) Effect of interaction between salinity and nanoparticles (Fe2O3 and ZnO) on physiological parameters of Zea mays L. J Plant Nutr 40(19):2745–2755. https://doi.org/10.1080/00103624.2013.863911
Flowers TJ, Colmer TD (2015) Plant salt tolerance: adaptations in halophytes. Ann Bot 115(3):327–331
Flowers TJ, Hajibagheri MA, Yeo AR (1991) Ion accumulation in the cell walls of rice plants growing under saline conditions: evidence for the Oertli hypothesis. Plant, Cell and Environ 14:319–325
Fukuda A, Yazaki Y, Ishikawa T, Koike S, Tanaka Y (1998) Na+ /H+ antiporter in tonoplast vesicles from rice roots. Plant Cell Physiol 39:196–201
Gale J (1975) The combined effect of environmental factors and salinity on plant growth. In: Book: plants in saline environ, pp 186–192. https://doi.org/10.1007/978-3-642-80929-3_12
Garbarino J, Dupont FM (1989) Rapid induction of na/h exchange activity in barley root tonoplast. Plant Physiol 89(1):1–4
García-Caparrós P, Llanderal A, Pestana M, Correia PJ, Lao MT (2017) Lavandula multifida response to salinity: growth, nutrient uptake, and physiological changes. J Plant Nutr Soil Sci 180:96–104. https://doi.org/10.1002/jpln.201600062
Gimenez C, Mitchell VJ, Lawlor DW (1992) Regulation of photosynthetic rate of two sunflower hybrids under water stress. Plant Physiol 98(2):516–524
Graan T, Boyer JS (1990) Very high CO2 partially restores photosynthesis in sunflower at low leaf water potentials. Planta 181:378–384
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31:149–190
Grennan AK (2006) Abiotic stress in rice. An “omic” approach. Plant Physiol 140(4):1139–1141
Gu MF, Li N, Long XH, Brestic M, Shao HB, Li J, Mbarki S (2016) Accumulation capacity of ions in cabbage (Brassica oleracea L.) supplied with sea water. Plant Soil Environment 62(7):314–320. https://doi.org/10.17221/771/2015-PSE
Gucci R, Lombardini L, Tattini M (1997) Analysis of water relations in leaves of two olive (Olea Europaea) cultivars differing in tolerance to salinity. Tree Physiol 17:13–21
Guerrier G (1984a) Relations entre la tolérance ou la sensibilité à la salinité lors de la germination des semences et les composantes de la nutrition en sodium. Biol Plant 26:22–28. https://doi.org/10.1007/BF02880421
Guerrier G (1984b) Selectivité de fixation du sodium au niveau des embryons et des jeunes plantes sensible or tolerant au NaCl. Can J Bot 62:1791–1792
Gupta A, Berkowitz GA (1987) Osmotic adjustment, symplast volume, and nonstomatally mediated water stress inhibition of photosynthesis in wheat. Plant Physiol 85:1040–1047
Gutterman Y (1993) Seed germination in desert plants. Adaptations of desert organisms. Springer-Verlag, Berlin
Hachicha M (2007) Les sols salés et leur mise en valeur en Tunisie. Science et changements planétaires/Sécheresse 18(1):45–50
Hachicha M, Kahlaoui B, Khamassi N, Misle E, Jouzdan O (2017) Effect of electromagnetic treatment of saline water on soil and crops Journal of the Saudi Society of Agricultural Sciences (2017) In Press, Corrected Proof, Available on line 25 March 2016
Hajihashemi S, Kiarostami K, Enteshari S, Saboora A (2006) The effects of salt stress and paclobutrazol on some physiological parameters of two salt-tolerant and salt-sensitive cultivars of wheat. Pakistan J Biol Sci 9(7):1370–1374
Hajlaoui H, El Ayeb N, Garrec JP, Denden M (2010) Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind Crop Prod 31(1):122–130
Hamdy A (1999) Active damping of vibrations in elevator cars. J Struct Control 6:53–100. https://doi.org/10.1002/stc.4300060105
Hameed A, Gulzar S, Aziz I, Hussain T, Gul B, Khan MA (2015) Effects of salinity and ascorbic acid on growth, water status and antioxidant system in a perennial halophyte. AoB Plants 7:plv004. https://doi.org/10.1093/aobpla/plv004
Haouala F, Ferjani H, Ben El Hadj S (2007) Effet de la salinité sur la répartition des cations (Na+, K+ et Ca2+) et du chlore (Cl–) dans les parties aériennes et les racines du ray-grass anglais et du chiendent. Biotechnol Agron Soc Environ 11(3):235–244
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hassani A, Dellal A, Belkhodja M, Kaid-Harche M (2008) Effect of salinity on water and some osmolytes in barley (Hordeum vulgare). Eur J Sci Res 23:61–69
He S, Schulthess AW, Mirdita V, Zhao Y, Korzun V, Bothe R, Ebmeyer E, Reif JC, Jiang Y (2016) Genomic selection in a commercial winter wheat population. Theor Appl Genet 129(3):641–651. https://doi.org/10.1007/s00122-015-2655-1
Heidari M, Sarani S (2012) Growth, biochemical components and ion content of chamomile (Matricaria chamomilla L.) under salinity stress and iron deficiency. J Saudi Soc Agric Sci 11(1):37–42
Hernández JA, Olmos E, Corpas FJ, Sevilla F, del Río LA (1995) Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci 105(2):151–167
Hernández JA, Campillo A, Jiménez A, Alarcón JJ, Sevilla E (1999) Response of antioxidant systems and leaf water relations to NaCl stress in pea plants. New Phytol 141:241–251
Hillel D, Vlek PLG (2005) The sustainability of irrigation. Adv Agron 87:55–84. https://doi.org/10.1016/S0065-2113(05)87002-6
Hniličková H, Hnilička F, Martinková J, Kraus K (2017) Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ 63:362–367
Hoque MA, Okuma E, Banu MNA, Nakamura Y, Shimoishi Y, Murata Y (2007) Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J Plant Physiol 164:553–561
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplastic barrier. J Exp Bot 52(365):2245–2264
Hossain MS, Persicke M, AI ES, Kalinowski J, Dietz KJ (2017) Metabolite profiling at the cellular and subcellular level reveals metabolites associated with salinity tolerance in sugar beet. J Exp Bot 68(21–22):5961–5976
Hristova V, Popova L (2002) Treatment with methyl jasmonate alleviates the effects of paraquat on photosynthesis in barley plants. Photosynthetica 40:567. https://doi.org/10.1023/A:1024356120016
Hu Y, Schmidhalter U (2000) A two-pinhole technique to determine distribution profiles of relative elemental growth rates in the growth zone of grass leaves. Aust J Plant Physiol 27:1187–1190
Hu Y, Yu W, Liu T, Shafi M, Song L, Du X, Huang X, Yue Y, Wu J (2017) Effects of paclobutrazol on cultivars of Chinese bayberry (Myrica rubra) under salinity stress. Photosynthetica 55(3):443–453. https://doi.org/10.1007/s11099-016-0658-z
Huez-López MA, Ulery April L, Samani Z, Picchioni G, Flynn RP (2011) Response of chile pepper (Capsicum annuum L.) to salt stress and organic and inorganic nitrogen sources: i. Growth and yield. Trop Subtrop Agroecosyst 14:137–147
Ibrahim EA (2016) Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol 192:38–46. https://doi.org/10.1016/j.jplph.2015.12.011
Itai C (1999) Role of phytohormones in plant responses to stresses. In: Lerner HR (ed) Plant responses to environmental stress. From phytohormones to genome reorganization. Marcel Dekker, New York, pp 287–301
Iyengar ERR, Reddy MP (1996) Photosynthesis in highly salt-tolerant plants. In: Pessaraki M (ed) Handbook of photosynthesis. Marcel Dekker, New York, pp 897–909
Jan SA, Bibi N, Shinwari KS, Rabbani MA, Ullah S, Qadir A, Khan N (2017) Impact of salt, drought, heat and frost stresses on morpho-biochemical and physiological properties of Brassica species: An updated review. J Rural Dev Agric 2(1):1–10
Javid MG, Sorooshzadeh A, Moradi F, Sanavy SAMM, Allahdadi I (2011) The role of phytohormones in alleviating salt stress in crop plants. Aust J Crop Sci 5:726–734
Jia J, Bai J, Gao H, Wen X, Zhang G, Cui B, Liu X (2017) In situ soil net nitrogen mineralization in coastal salt marshes (Suaeda salsa) with different flooding periods in a Chinese estuary. Ecol Indic 73:559–565
Joshi R, Sahoo KK, Tripathi AK, Kumar R, Gupta BK, Pareek A, Singla-Pareek SL (2017) Knockdown of an inflorescence meristem‐specific cytokinin oxidase–OsCKX2 in rice reduces yield penalty under salinity stress condition. Plant Cell Environ https://doi.org/10.1111/pce.12947
Kachout SS, Mansoura AB, Leclerc JC, Jaffel K, Rejeb MN, Ouerghi Z (2009) Effects of heavy metals on antioxidant activities of Atriplex hortensis and Atriplex rosea. J Appl Bot Food Qual 83(1):37–43
Kafi M, Shariat JM, Moayedi A (2013) The sensitivity of grain sorghum (Sorghum bicolor L.) developmental stages to salinity stress: an integrated approach. J Agric Sci Tech 15(4):723–736
Kafkai U (1991) Root growth under stress. Plant roots: the hidden half. Marcel Dekker, New York, USA, pp 375–391
Kalaji H, Rastogi A, Živčák M, Brestic M et al (2018) Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica. https://doi.org/10.1007/s11099-018-0766-z
Kalaji HM, Govindjee BK et al (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot 73:64–72
Kalaji MH, Goltsev V, Zuk-Golaszewska B, Zivcak M, Brestic M (2017) Chlorophyll fluorescence: understanding crop performance—basics and applications. Taylor and Francis, p 222. ISBN 9781498764490
Kamiab F, Talaie A, Javanshah A, Khezri M, Khalighi A (2012) Effect of long-term salinity on growth, chemical composition and mineral elements of pistachio (Pistacia vera cv. Badami-Zarand) rootstock seedlings. Annals Biol Res 3(12):5545–5551
Kan X, Ren J, Chen T, Cui M, Li C, Zhou R, Zhang Y, Liu H, Deng D, Yin Z (2017) Effects of salinity on photosynthesis in maize probed by prompt fluorescence, delayed fluorescence and P700 signals. Environ Exp Bot 140:56–64
Kang DJ, Seo YJ, Lee JD, Ishii R, Kim KU, Shin DH, Park SK, Jang SW, Lee IJ (2005) Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J Agron Crop Sci 191:273–282
Kanwal H, Ashraf M, Shahbaz M (2011) Assessment of salt tolerance of some newly developed and candidate wheat (Triticum Aestivum L.) cultivars using gas exchange and chlorophyll fluorescence attributes. Pak J Bot 43:2693–2699
Kao RR, Gravenor MB, McLean AR (2001) Modelling the national scrapie eradication programme in the UK. Math Biosci 174:61–76
Karray-Bouraoui N (1995) Analyse des facteurs responsables de la tolérance au stress salin chez une céréale hybride le triticale: croissance, nutrition et métabolisme respiratoire. Thèse Doc. Univ, Tunis
Kavi Kishor PB, Sreenivasulu N (2014) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 37:300–311. https://doi.org/10.1111/pce.12157
Keiper FJ, Chen DM, De Filippis LF (1998) Respiratory, photosynthetic and ultrastructural changes accompanying salt adaptation in culture of Eucalyptus microcorys. J Plant Physiol 152(4-5):564–573
Khan AM, Ungar IA (1998) Germination of the salt tolerant shrub Suaeda fruticosa from. Pakistan: salinity and temperature responses. Seed Sci Technol 26:657–667
Khan MA, Ungar IA, Showalter AM (2000) The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte Suadea fruticosa (L.) Forssk. J Arid Environ 45:73–84
Khan N, Syeed S, Masood A, Nazar R, Iqbal N (2010) Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int J Plant Biol 1(1):1–8
Khavari-Nejad RA, Chaparzadeh N (1998) The effects of NaCl and CaCl2 on photosynthesis and growth of alfalfa plants. Photosynthetica 35(3):461–466
Khavari-Nejad R, Mostofi Y (1998) Effects of NaCl on photosynthetic pigments, saccharides, and chloroplast ultrastructure in leaves of tomato cultivars. Photosynthetica 35:151. https://doi.org/10.1023/A:1006846504261
Khunpon B, Chaum S, Faiyue B, Uthaibutra J, Saengnil K (2017) Influence of paclobutrazol on growth performance, photosynthetic pigments, and antioxidant efficiency of Pathumthani 1 rice seedlings grown under salt stress. ScienceAsia 43:70–81. https://doi.org/10.2306/scienceasia1513-1874.2017.43.070
Kishor A, Srivastav M, Dubey AK, Singh AK, Sairam RK, Pandey RN, Dahuja A, Sharma RR (2009) Paclobutrazol minimises the effects of salt stress in mango (Mangifera indica L.) J Hortic Sci Biotechnol 84(4):459–465
Klepper B, Barrs HD (1968) Effects of salt secretion on psychrometric determinations of water potential of cotton leaves. Plant Physiol 43(7):1138–1140
Kurban H, Saneoka H, Nehira K, Adilla R, Premachandra GS, Fujita K (1999) Effect of salinity on growth, photosynthesis and mineral composition in leguminous plant Alhagi pseudoalhagi (Bieb.) Soil Sci Plant Nutr 45(4):851–862
Lachhab I, Louahlia S, Laamarti M, Hammani K (2013) Effet d’un stress salin sur la germination et l’activité enzymatique chez deux génotypes de Medicago sativa. IJIAS 3(2):511–516
Lamsal K, Paudyal GN, Saeed M (1999) Model for assessing impact of salinity on soil water availability and crop yield. Agr Water Manage 41:57–70
Latef AAHA, Alhmad MFA, Abdelfattah KE (2017) The possible roles of priming with zno nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J Plant Growth Regul 36(1):60–70. https://doi.org/10.1007/s00344-016-9618-x
Levitt J (1980) Responses of plant to environmental stress water, radiation, salt and other stresses. Academic Press, New York
Li M, Guo S, Xu Y, Meng Q, Li G, Yang X (2014) Glycine betaine-mediated potentiation of HSP gene expression involves calcium signaling pathways in tobacco exposed to NaCl stress. Physiol Plant 150(1):63–75
Liphschitz N, Waisel Y (1974) Existence of salt glands in various genera of Gramineae. New Phytol 73(3):507–513
Liu JH, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci 6:827. https://doi.org/10.3389/fpls.2015.00827
Liu R, Lai R (2015) Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci Total Environ 51:131–139
Llanes A, Bertazza G, Palacio G, Luna V (2013) Different sodium salts cause different solute accumulation in the halophyte Prosopis strombulifera. Plant Biol 15:118–125
Longstreth DJ, Nobel PS (1979) Salinity effects on leaf anatomy. Plant Physiol 63(4):700–703
Lv YC, Xu G, Sun JN, Brestič M, Živčák M, Shao HB (2015) Phosphorus release from the soils in the Yellow River Delta: dynamic factors and implications for eco-restoration. Plant Soil Environ 61(8):339–343. https://doi.org/10.17221/666/2014-PSE
Lyshede OB (1917) Studies on the mucilaginolls cells in lhl.': k ar ol' .YparlO()'Slislis jilipes. PIOllfa 133:255–260
Manan MM, Ibrahim NA, Aziz NA, Zulkifly HH, Al-Worafi YM, Long CM (2016) Empirical use of antibiotic therapy in the prevention of early onset sepsis in neonates: a pilot study. Arch Med Sci 12:603–613. https://doi.org/10.5114/aoms.2015.51208
Marcum KB, Anderson SJ, Engelk MC (1998) Salt gland ion secretion/A salinity tolerance mechanism among five Zoysiagrass species. Crop Sci 38:806–810
Marcum KB, Murdoch CL (1994) Salinity tolerance mechanisms of six C4 turfgrasses. J Amer Soc Hort Sci 119(4):779–784
Mass EV, Grieve CM (1990) Spike and leaf development in salt stressed wheat. Crop Sci 30:1309–1313
Mass EV, Nieman RH (1978) Physiology of plant tolerance to salinity. In: Jung GA (ed) Crop tolerance to suboptimal land conditions. Amer. Soc. Agron. Spec. Publ, USA, pp 277–299
Mastronardi E, Tsae P, Zhang X, Monreal CM, DeRosa MC (2015) Strategic role of nanotechnology in fertilizers: potential and limitations. In: Rai M, Ribeiro C, Mattoso L, Duran N (eds) Nanotechnologies in food and agriculture. Springer, Berlin
Mauseth JD (1988) Plant Anatomy. The Benjamin/Cummings Publishing Co, Inc, California
Megdiche W, Amor BN, Debez A, Hessini K, Ksouri R, Zuily-Fodil Y, Abdelly C (2007) Salt tolerance of the annual halophyte Cakile maritima as affected by the provenance and the developmental stage. Acta Physiol Plant 29:375–384
Mehta P, Jajoo A, Mathur M, Bharti S (2010) Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol Biochem 48:16–20
Menezes RV, Azevedo Neto AD, Oliveira Ribeiro M, Cova AMW (2017) Growth and contents of organic and inorganic solutes in amaranth under salt stress. Pesq Agropec Trop 47(1):22–30
Mermoud A (2006) Cours de physique du sol : Maîtrise de la salinité des sols. Ecole polytechnique fédérale de Lausanne, p 23
Messedi D, Labidi N, Grignon C, Abdelly C (2004) Limits imposed by salt to the growth of the halophyte Sesuvium portulacastrum. JPNSS 167(6):720–725
Misra AN, Srivastava A, Strasser RJ (2001) Utilization of fast chlorophyll a fluorescence technique in assessing the salt/ion sensitivity of mung bean and brassica seedlings. J Plant Physiol 158:1173–1181
Mitsuya S, Takeoka Y, Miyake H (2000) Effects of sodium chloride on foliar ultrastructure of sweet potato (Ipomoea batatas Lam.) plantlets grown under light and dark conditions in vitro. J Plant Physiol 157(6):661–667
Mohammad M, Shibli R, Ajlouni M, Nimri L (1998) Tomato root and shoot responses to salt stress under different levels of phosphorus nutrition. J Plant Nutr 21(8):1667–1680
Moles TM, Pompeiano A, Huarancca Reyes T, Scartazza A, Guglielminetti L (2016) The efficient physiological strategy of a tomato landrace in response to short-term salinity stress. Plant Physiol Biochem 109:262–272. https://doi.org/10.1016/j.plaphy.2016.10.008
Moons A, Prinsen E, Bauw G, Montagu MV (1997) Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. Plant Cell 9:2243–2259
Morais MC, Panuccio MR, Muscolo A, Freitas H (2012) Salt tolerance traits increase the invasive success of Acacia longifolia in Portuguese coastal dunes. Plant Physiol Biochem 55:60–65
Mozafar A, Goodin JR (1970) Vesiculated hairs: a mechanism for salt tolerance in Atriplex halimus L. Plant Physiol 45:62–65
Muchate NS, Nikalje GC, Rajurkar NS, Suprasanna P, Nikam TD (2016) Physiological responses of the halophyte Sesuvium portulacastrum to salt stress and their relevance for saline soil bio-reclamation. Flora 224:96–105
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Gilliham M (2015) Salinity tolerance of crops what is the cost? New Phytol 208(3):668–673
Munns R, James RA, Sirault XR, Furbank RT, Jones HG (2010) New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J Exp Bot 61(13):3499–3507. https://doi.org/10.1093/jxb/erq199
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57(5):1025–1043
Munns R, Rawson HM (1999) Effect of salinity on salt accumulation and reproductive development in the apical meristem of wheat and barley. Funct Plant Biol 26(5):459–464
Munns R, Termaat A (1986) Whole plant responses to salinity. Aust J Plant Physiol 13:143–160
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Naderi MR, Danesh-Shahraki A (2013) Nanofertilizers and their roles in sustainable agriculture. Int J Agric Crop Sci 5:2229–2232
Nawaz K, Ashraf M (2010) Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J Agri Crop Sci 196:28–37. https://doi.org/10.1111/j.1439-037X.2009.00385.x
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119(1):1–11
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742
Nivedithadevi D, Somasundaram R, Pannerselvam R (2012) Effect of abscisic acid, paclobutrazol and salicylic acid on the growth and pigment variation in Solanum trilobatum (I). Int J Drug Dev Res 4(3):236–246
Oertli JJ (1968) Extracellular salt accumulation a possible mechanism of salt injury in plants. Agrochimica 12:461–469
Orcutt DM, Nilsen ET (2000) The physiology of plants under stress: soil and biotic factors. JohnWiley and Sons, New York
Orcutt DM, Nilsene T (2000) Physiology of plants under stress. John Wiley & Sons Inc., New York, NY, USA
Orsini F, Cascone P, De Pascale S, Barbieri G, Corrado G, Rao R, Maggio A (2010) Systemin-dependent salinity tolerance in tomato: evidence of specific convergence of abiotic and biotic stress responses. Physiolo Plant 138:10–21. https://doi.org/10.1111/j.1399-3054.2009.01292.x
Osmond CB, Lüttge U, West KR, Pallaghy CK, Shacher-Hill B (1969) Ion absorption in Atriplex leaf tissue. II. Secretion of ions to epidermal bladders. Aust J Biol Sci 22:797–814
Owens S (2001) Salt of the earth. Genetic engineering may help to reclaim agricultural land lost due to salinisation. EMBO Rep 2:877–879
Özdemir F, Bor M, Demiral T, Türkan I (2004) Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul 42:203–211. https://doi.org/10.1023/B:GROW.0000026509.25995.13
Parida AK, Das AB (2005) Salt tolerance and salinity effect on plants: a review. Ecotoxicol Environ Saf 60:324–349
Parida AK, Das AB, Mittra B, Mohanty P (2004) Salt-stress induced alterations in protein profile and protease activity in the mangrove Bruguiera parviflora. Zeitschrift für Naturforschung C 59(5-6):408–414
Parvin S, Lee OR, Sathiyaraj G, Khorolragchaa A, Kim YJ, Yang DC (2014) Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Gene 537(1):70–78. https://doi.org/10.1016/j.gene.2013.12.021
Pires RMO, Leite DG, Santos HO, Souza GA, Von Pinho EVR (2017) Physiological and enzymatic alterations in sesame seeds submitted to different osmotic potentials. Genet Mol Res 16(3). https://doi.org/10.4238/gmr16039425
Pompeiano A, Di Patrizio E, Volterrani M, Scartazza A, Guglielminetti L (2016) Growth responses and physiological traits of seashore paspalum subjected to short-term salinity stress and recovery. Agric Water Manag 163:57–65
Poonam T, Tanushree B, Sukalyan C (2013) Water quality indicesimportant tools for water quality assessment: a review. Int J Adv Chem (IJAC) 1(1):15–28
Price AH, Hendry GAF (1991) Iron-catalysed oxygen radical formation and its possible contribution to drought damage in nine native grasses and three cereals. Plant Cell Environ 14:477–484. https://doi.org/10.1111/j.1365-3040.1991.tb01517.x
Puyang X, An M, Han L, Zhang X (2015) Protective effect of spermidine on salt stress induced oxidative damage in two Kentucky bluegrass (Poa pratensis L.) cultivars. Ecotoxicol Environ Saf 117:96–106. https://doi.org/10.1016/j.ecoenv.2015.03.023
Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Noble AD (2014) Economics of salt-induced land degradation and restoration. Nat Res Forum 38(4):282–295
Qadir M, Qureshi AS, Cheraghi SAM (2008) Extent and characterisation of salt-affected soils in Iran and strategies for their amelioration and management. Land Degrad Dev 19(2):214–227
Qiu Z, Guo J, Zhu A, Zhang L, Zhang M (2014) Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol Environ Saf 104:202–208
Quin L, Guo S, Ai W, Tang Y, Cheng Q, Chen G (2013) Effect of salt stress on growth and physiology in amaranth and lettuce: implications for bioregenerative life support system. Adv Space Res 51(3):476–482
Rahman MS, Miyake H, Takeoka Y (2002) Effects of exogenous glycinebetaine on growth and ultrastructure of salt-stressed rice seedlings (Oryza sativa L.) Plant Prod Sci 5:33–44
Rajesh A, Arumugam R, Venkatesalu V (1998) Growth and photosynthetic characteristics of Ceriops roxburghiana under NaCl stress. Photosynthetica 35:285. https://doi.org/10.1023/A:1006983411991
Rao PS, Mishra B, Gupta SR, Rathore A (2008) Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breed 127:256–261. https://doi.org/10.1111/j.1439-0523.2007.01455.x
Rastogi A, Zivcak M, Sytar O, Kalaji HM, He X, Mbarki S, Brestic M (2017) Impact of metal and metal oxide nanoparticles on plant: a critical review. Front Chem 5:78. https://doi.org/10.3389/fchem.2017.00078
Rewald B, Raveh E, Gendler T, Ephrath JE, Rachmilevitch S (2012) Phenotypic plasticity and water flux rates of Citrus root orders under salinity. J Exp Bot 63(7):2717–2727. https://doi.org/10.1093/jxb/err457
Rico CM, Morales MI, McCreary R, Castillo-Michel H, Barrios AC, Hong J, Tafoy A, Lee WY, Varela-Ramirez A, Peralta-Videa JR, Gardea-Torresdey JL (2013) Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environ Sci Technol 47(24):14110–14118. https://doi.org/10.1021/es4033887
Romero-Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163(8):847–855
Romero-Aranda R, Soria T, Cuartero J (2001) Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Sci 160(2):265–272
Rossi L, Zhang W, Lombardini L, Ma X (2016) The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ Pollut 219:28–36
Roychoudhury A, Basu S, Sengupta DN (2011) Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J Plant Physiol 168:317–328
Rozema J, Riphagen J (1977) Physiology and ecologic relevance of salt secretion by the salt gland of Glaux maritima L. Oecologia 29:349–357
Sabaghnia N, Janmohammadi M (2014) Effect of nano-silicon particles application on salinity tolerance in early growth of some lentil genotypes. Ann UMCS Biol 69:39–55
Saneoka H, Nagasaka C, Hahn DT, Yang WJ, Premachandra GS, Joly RJ, Rhodes D (1995) Salt tolerance of glycinebetaine-deficient and -containing maize lines. Plant Physiol 107:631–638
Scholander PF, Hammel HT, Hemmingson ED, Garey W (1962) Salt balance in mangroves. Plant Physiol 37:722–729
Sekmen AH, Turkan I, Tanyolac ZO, Ozfidan C, Dinc A (2012) Different antioxidant defense responses to salt stress during germination and vegetative stages of endemic halophyte Gypsophila oblanceolata bark. Environ Exp Bot 77:63–76
Sengupta S, Majumder AL (2010) Porteresia. coarctata (Roxb.) Tateoka, a wild rice: a potential model for studying salt-stress biology in rice. Plant Cell Environ 33:526–542
Shahbaz M, Ashraf M (2013) Improving salinity tolerance in cereals. Crit Rev Plant Sci 32(4):237–249
Shahid MA, Pervez MA, Balal RM, Mattson NS, Rashid A, Ahmad R, Ayyub CM, Abbas T (2011) Brassinosteroid (24-epibrassinolide) enhances growth and alleviates the deleterious effects induced by salt stress in pea (Pisum sativum L.) Aust J Crop Sci 5:500–510
Shakir E, Zahraw Z, Al-Obaidy AHM (2017) Environmental and health risks associated with reuse of wastewater for irrigation. Egypt J Pet 26(1):95–102
Shang Q, Song S, Zhang Z, Guo S (2006) Exogenous brassinosteroid induced salt resistance of cucumber (Cucumis sativus L.) seedlings. Sci Agric Sinica 39:1872–1877
Sharma I, Bhardwaj R, Pati PK (2012) Mitigation of adverse effects of chlorpyrifos by 24-epibrassinolide and analysis of stress markers in a rice variety Pusa Basmati-1. Ecotoxicol Environ Saf 85:72–81
Sheteawi AS (2007) Improving growth and yield of salt stressed soybean by exogenous application of jasmonic acid and ascobin. Int J Agric Biol 3:473–478
Shomer I, Frenkel H, Polinger C (1991) The existence of a diffuse electric layer at cellulose fibril surfaces and its role in the swelling mechanism of parenchyma plant cell walls. Carbohydr Polym 16:199–210
Siadat H, Bybordi M, Malakouti MJ (1997) Salt-affected soils of Iran: a country report. international symposium on sustainable management of salt-affected soils in the arid ecosystem, Cairo
Siddique AB, Islam R, Hoque A, Hasan M, Tanvir RM, Mahir UM (2015) Mitigation of salt stress by foliar application of proline in rice. Univers J Agric Res 3:81–88. https://doi.org/10.13189/ujar.2015.030303
Siddiqui MH, Al-Whaibi MH (2014) Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds mill.) Saudi J Biol Sci 21:13–17
Siddiqui MH, Al-Whaibi MH, Faisal M, Al Sahli AA (2014) Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ Toxicol Chem 33:2429–2437
Silberbush M, Ben-Asher J, Ephrath JE (2005) A model for nutrient and water flow and their uptake by plants grown in a soilless culture. Plant Soil 271(1-2):309–319
Singh SB, Singh BB, Singh M (1994) Effect of kinetin on chlorophyll, nitrogen and proline in mung bean under saline conditions. Indian J Plant Physiol 37:37–39
Slama I, Abdelly C, Bouchereau A, Flowers T, Savoure A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115:433–447. https://doi.org/10.1093/aob/mcu239
Slama I, M’Rabet R, Ksouri R, Talbi O, Debez A, Abdelly C (2017) Effects of salt treatment on growth, lipid membrane peroxidation, polyphenol content, and antioxidant activities in leaves of Sesuvium portulacastrum L. Arid Land Res Manag 31(4):404–417
Smaoui MA (1971) Differentiation des trichomes chez Atriplex halimus L. CR Acad Sci Paris Ser D 273:1268–1271
Sobahan MA, Akter N, Ohno M, Okuma E, Hirai Y, Mori IC, Nakamura Y, Murata Y (2012) Effects of exogenous proline and glycinebetaine on the salt tolerance of rice cultivars. Biosci Biotechno Biochem 76(8):1568–1570. https://doi.org/10.1271/bbb.120233
Soliman AS, El-feky SA, Darwish E (2015) Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J Hortic For 7(2):36–47
Souleymane O, Hamidou M, Salifou M, Manneh B, Danquah E, Ofori K (2017) Genetic improvement of rice (Oryza sativa) for salt tolerance: a review. Inter J Advanc Res Botany 3:22–33. https://doi.org/10.20431/2455-4316.0303004
Soussi M, Ocana A, Lluch C (1998) Effects of salt stress on growth, photosynthesis and nitrogen fixation in chickpea (Cicer arietinum L.) J Expt Bot 49:1329–1337
Staal M, Maathuis FJM, Elzenga JTM, Overbeek JHM, Prins HBA (1991) Na+/H+ antiport activity in tonoplast vesicles from roots of the salt-tolerant Plantago maritima and the salt-sensitive Plantago media. Physiol Plant 82:179–184. https://doi.org/10.1111/j.1399-3054.1991.tb00078.x
Stassart JM, Neirinckx L, De Jaegere R (1981) The interactions between monovalent cations and calcium during their adsorption on isolated cell walls and absorption by intact barley roots. Ann Bot 47(5):647–652
Stefanov M, Yotsova E, Rashkov G, Ivanova K, Markovska Y, Apostolova EL (2016) Effects of salinity on the photosynthetic apparatus of two Paulownia lines. Plant Physiol Biochem 101:54–59. https://doi.org/10.1016/j.plaphy.2016.01.017
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1797:1313–1326
Sun S, An M, Han L, Yin S (2015) Foliar application of 24-epibrassinolide improved salt stress tolerance of perennial ryegrass. Hortscience 50:1518–1523
Sun ZW, Ren LK, Fan JW, Li Q, Wang KJ, Guo MM, Wang L, Li J, Zhang GX, Yang ZY, Chen F, Li XN (2016) Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant Soil Environ 62:515–521
Taiz L, Zeiger E (2002) Plant physiology, 3rd edn. Sinauer Associates, Sunderland, MA, 690 pp
Tang X, Mu X, Shao H, Wang H, Brestic M (2015) Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit Rev Biotechnol 35(4):425–437. https://doi.org/10.3109/07388551.2014.889080
Tehranifar A, Jamalian S, Tafazoli E, Davarynejad GH, Eshghi S (2009) Interaction effects of paclobutrazol and salinity on photosynthesis and vegetative growth of strawberry plants. Acta Hortic 842:821–824. https://doi.org/10.17660/ActaHortic.2009.842.181
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91(5):503–527
Thomson WW (1975) The structure and function of salt glands. In: Poljakoff-Mayber A, Gale J (eds) Plants in saline environments. Springer, Heidelberg, pp 118–146
Thomson WW, Faraday CD, Oross JW (1988) Salt glands. In: Baker DA, Hall JL (eds) Solute transport in plant cells and tissues. Longman Scientific & Technical, Essex, UK, pp 498–537
Thomson WW, Platt-Aloia K (1979) Ultrastructural transitions associated with the development of the bladder cells of the trichomes of Atriplex. Cytobios 25:105–114
Tsonev TD, Lazova GN, Stoinova ZG, Popova LP (1998) A possible role for jasmonic acid in adaptation of barley seedling to salinity stress. J Plant Growth Regul 17:153–159
Tukey HB Jr, Tukey HB, Wittwer SH (1958) Loss of nutrients by foliar leaching as determined by radioisotopes. P Am Soc Hortic Sci 71:496–506
Tukey HB, Morgan JV (1962) The occurrence of leaching from above ground plant parts and the nature of the material leached. 16th Intern Hort Congr Brussels:153–160
Tyerman SD, Skerrett IM (1999) Root ion channels and salinity. Sci Hort 78:175–235. https://doi.org/10.1016/s0304-4238(98)00194-0
Vardhini BV, Anjum NA (2015) Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front Environ Sci 2:67
Varjovi MB, Valizadeh M, Vahed MM (2016) Effect of salt stress and exogenous application of proline on some antioxidant enzymes activity in barley cultivars seedling. Biological forum. An International Journal 8(2):34–41
Vicente O, Boscaiu M, Naranjo MÁ, Estrelles E, Bellés JM, Soriano P (2004) Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J Arid Environ 58(4):463–481
Von Willert DJ (1968) Tagesschwankungen des Ionengehalts in Salicornia europaea in Abhängigkeit vom Standort und von der Überflutung. Ber Dtsch Bot Ges Bd 81(10):442–449
Wahid A (2003) Physiological significance of morpho-anatomical features of halophytes with particular reference to Cholistan flora. Int J Agric Biol 5:207–212
Waisel Y (1972) Biology of halophytes. Academic Press, New York
Wang L, Li W, Yang H, Wu W, Ma LI, Huang T, Wang X (2016) Physiological and biochemical responses of a medicinal halophyte Limonium bicolor (Bag.) kuntze to salt-stress. Pak J Bot 48(4):1371–1377
Wang W, Wang R, Yuan Y, Du N, Guo W (2011) Effects of salt and water stress on plant biomass and photosynthetic characteristics of tamarisk (Tamarix chinensis Lour.) seedlings. Afr J Biotechnol 10:17981–11789
Wang Y, Nil N (2000) Changes in chlorophyll, ribulose biphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus Tricolor leaves during salt stress. J Hortic Sci Biotechnol 75:623–627
Wang YY, Mopper S, Hasenstein KH (2001) Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J Chem Ecol 27:327–342
Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Golezani KG (2012) Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.) Plant OMICS 5(2):60–67
Wilson C, Shannon MC (1995) Salt-induced Na+/H+ antiport in root plasma membrane of a glycophytic and halophytic species of tomato. Plant Sci 107:147–157
Wu W, Zhang Q, Ervin EH, Yang Z, Zhang X (2017) Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front Plant Sci 8:1017. https://doi.org/10.3389/fpls.2017.01017
Wyn Jones RG, Brady CJ, Speirs J (1979) Ionic and osmotic relations in plant cells. In: Laidman DL, Wyn Jones RG (eds) Recent advances in the biochemistry of cereals. Academic Press, London, pp 63–103
Yan K, Wu C, Zhang L, Chen X (2015) Contrasting photosynthesis and photoinhibition in tetraploid and its autodiploid honeysuckle (Lonicera japonica Thunb.) under salt stress. Front Plant Sci 6:227. https://doi.org/10.3389/fpls.2015.00227
Yan P, Shao HB, Shao C, Chen P, Zhao S, Brestic M, Chen X (2013) Physiological adaptive mechanisms of plant grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiologia Plantarum:2867–2878. https://doi.org/10.1007/s11738-013-1325-7
Yang X, Lu C (2006) Effects of exogenous glycinebetaine on growth, CO2 assimilation, and photochemistry of maize plants. Photosystem II. Physiol Plant 127(4):593–602
Yang X, Lu C (2005) Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol Plant 124:343–352. https://doi.org/10.1111/j.1399-3054.2005.00518.x
Yasemin S, Koksal N, Özkaya A, Yener M (2017) Growth and physiological responses of ‘Chrysanthemum paludosum’ under salinity stress. J Biol Environ Sci 11(32):59–66
Yassen A, Abdallah E, Gaballah M, Zaghloul S (2017) Role of silicon dioxide nano fertilizer in mitigating salt stress on growth, yield and chemical composition of cucumber (Cucumis sativus L.) Int J Agric Res 12:130–135. https://doi.org/10.3923/ijar.2017.130.135
Yeo A (1998) Molecular biology of salt tolerance in the context of whole-plant physiology. J Exp Bot 49(323):915–929
Yeo AR (1983) Salinity resistance: physiologies and prices. Physiol Plant 58:214–222. https://doi.org/10.1111/j.1399-3054.1983.tb04172.x
Yeo AR, Lee ΛS, Izard P, Boursier PJ, Flowers TJ (1991) Short-and long-term effects of salinity on leaf growth in rice (Oryza sativa L.) J Exp Bot 42(7):881–889
Yoon JY, Hamayun M, Lee SK, Lee IJ (2009) Methyl jasmonate alleviated salinity stress in soybean. J Crop Sci Biotechnol 12:63–68
Zhang J, Davies WJ (1989) Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant Cell Environ 12:73–81
Zhang Y, Hu XH, Shi Y, Zou ZR, Yan F, Zhao YY, Zhang H, Zhao JZ (2013) Beneficial role of exogenous spermidine on nitrogen metabolism in tomato seedlings exposed to saline–alkaline stress. J Am Soc Hortic Sci 138(1):38–49
Zhang Y, Zhang H, Zou ZR, Liu Y, Hu XH (2015) Deciphering the protective role of spermidine against saline–alkaline stress at physiological and proteomic levels in tomato. Phytochemistry 110:13–21. https://doi.org/10.1016/j.phytochem.2014.12.021
Zhao L, Peng B, Hernandez-Viezcas JA, Rico C, Sun Y, Peralta-Videa JR, Tang X, Niu G, Jin L, Varela-Ramirez A, Zhang JY, Gardea-Torresdey JL (2012) Stress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein and lipid peroxidation. ACS Nano 6:9615–9622. https://doi.org/10.1021/nn302975u
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6(2):66–71
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zhu J, Meinzer CF (1999) Efficiency of C4 photosynthesis in Atriplex lentiformis under salinity stress. Aust J Plant Physiol 26:79–86
Zid E, Grignon C (1991) Les tests de sélection précoce pour la résistance des plantes aux stress. Cas des stress salin et hydrique. L’amélioration des plantes pour l’adaptation aux milieux arides. Ed. John Libbey. Eurotext, Paris, pp 91–108
Ziegler H, Lüttge U (1967) Die Salzdrüsen von Limonium vulgare. Planta 74:1–17. https://doi.org/10.1007/BF0038516
Zivcak M, Brestic M, Sytar O (2016) Osmotic adjustment and plant adaptation to drought stress. In: Hossain MA (ed) Drought stress tolerance in plants, vol 1. Springer International Publishing, Switzerland, pp 105–143
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Mbarki, S. et al. (2018). Strategies to Mitigate the Salt Stress Effects on Photosynthetic Apparatus and Productivity of Crop Plants. In: Kumar, V., Wani, S., Suprasanna, P., Tran, LS. (eds) Salinity Responses and Tolerance in Plants, Volume 1. Springer, Cham. https://doi.org/10.1007/978-3-319-75671-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-75671-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75670-7
Online ISBN: 978-3-319-75671-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)