Abstract
To our knowledge, little attention has been paid to evaluating ZnO nanoparticles (ZNPs) roles in plants grown under salinity stress. In this study, seeds of lupine (Lupinus termis) plants were grown in plastic pots and exposed to 0 (control) and 150 (S) mM NaCl with or without priming with different concentrations of ZnO [20 mg L−1 (ZNPs1), 40 mg L−1 (ZNPs2), and 60 mg L−1 (ZNPs3)] for 20 days. Salinized plants showed a reduction in plant growth parameters (root length, shoot length, fresh weight, and dry weight) and in the contents of photosynthetic pigments (chlorophyll a and b, and carotenoids) and Zn, as well as in the activity of catalase (CAT) against control plants. On the other side, salinity stress boosted the contents of organic solutes (soluble sugar, soluble protein, total free amino acids, and proline), total phenols, malondialdehyde (MDA), ascorbic acid and Na, as well as the activities of superoxide dismutase (SOD), peroxidase (POD), and ascorbate peroxidase (APX) in stressed plants over control plants. However, seed-priming with ZNPs mostly stimulated growth of stressed plants, which was accompanied by reinforcement in the levels of photosynthetic pigments, organic solutes, total phenols, ascorbic acid and Zn, as well as in the activities of SOD, CAT, POD, and APX enzymes over stressed plants alone. On the contrary, priming with ZNPs caused a decrement in the contents of MDA and Na in stressed plants relative to salinized plants alone. It is worthy to mention that, this improvement in salt tolerance of plants primed with ZNPs was more obvious in plants primed with ZNPs3 and grown both in unstressed and stressed regimes. Thus, our findings suggest that seed-priming with ZNPs, especially 60 mg L−1 ZnO is an effective strategy that can be used to enhance salt tolerance of lupine plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is a prevalent abiotic stressor that severely affects crop yield in some specific geographical regions, especially in arid and semiarid regions such as Egypt. Egypt is a country that has a history of 5000 years of experience in irrigation (Mohamed and others 2007). However, a major proportion of governmental funds is invested in addressing serious salinity issues, because 33 % of the cultivated lands are already salinized throughout the country. Effective remediation of the salinity stress hence, is a major initiative to be taken to secure sustainable crop yield (Mohamed and others 2007).
Multimodal applications of engineered nanoparticles (NPs) and nanoconjugates have been in high demand over the last few decades in the textile, cosmetic, electronic, agriculture, or pharmaceutical industries (Gerber and Lang 2006; Nel and others 2006; Choudhury and others 2011, 2012; Siddiqui and others 2015). In particular, applications of NPs as pesticides and fertilizers in crop fields are in high demand among farmers and profit-finding industries. Nanotechnology based on new generations NPs might offer enhanced food productivity by lowering or minimizing pathogenic invasions (Choudhury and others 2011). These nanoproducts allow small quantities of fertilizers/pesticides to be used effectively over a given period of time, while their design allows for sustained release and resistance from severe environmental hardness, compared to the conventional chemicals which are prone to frequent leaching, evaporation, photolytic–hydrolytic damages, and microbial degradation (Kah 2015).
Zinc oxide (ZnO) NPs (ZNPs), one of the most frequently used nanoproducts, are used in food packaging and drugs due to their superior antimicrobial efficacy (Brayner and others 2006; Jones and others 2008). ZnO NPs are also being frequently used in sun-protective lotions, wall paints, ceramic manufactures, or sporting goods (Fan and Lu 2005; Singh and Nanda 2014). The increased popularity of using Zn in fertilizers and pesticides is also commissioned due to its natural demand as a micronutrient in the body (Prasad and others 2012). Moreover, Zn is also an important cofactor in essential biocatalytic enzymes including oxidoreductases, transferases, hydrolases, ligases, and isomerases (Auld 2001). Previous literature has already reported both positive and negative effects of ZNPs on the physio-biochemical attributes of plants using different model systems (Mahajan and others 2011; Prasad and others 2012; de la Rosa and others 2013; Patra and others 2013; Raliya and Tarafdar 2013; Sedghi and others 2013; Ramesh and others 2014). However, the impact of nanoparticles on plants vary with age, species, and the features of the nanoparticles (Burman and others 2013).
Lupine (Lupinus termis) is grown in Egypt or in the general Mediterranean region for its edible seeds (Khodary 2004). Lupine is one of the most vital plants from nutritional and medical points of view (Khodary 2004).
Little attention has been paid to studying the impact of ZNPs application on plants grown under saline conditions. To our knowledge, this is the first report dealing with the impact of priming with ZNPs on salinized lupine. Therefore, this study was carried out to investigate whether the phytoremediation properties of ZNPs could be used on lupine plants for their healthy growth, by lowering the persistent saline stress. Growth parameters, photosynthetic pigments, organic solutes, total phenols, oxidative stress, antioxidant enzymes, and ascorbic acid in lupine seeds treated with sodium chloride (NaCl) or NaCl plus priming with different concentrations of ZNPs were assessed to evaluate the possible roles of ZNPs in mitigating the adverse impacts of salt stress.
Materials and Methods
Synthesis of ZnO Nanocrystals
ZnO nanocrystals (ZNCs) were synthesized by the direct chemical precipitation method. The preparation was started by solution A that contains 0.1 M of zinc nitrate dissolved in distilled water. Simultaneously, another solution B (0.4 M of sodium hydroxide dissolved in distilled water) was prepared. Next, solution B was added dropwise to solution A under gentle stirring and at a temperature of 60 °C. This step takes approximately 40 min. The resultant mixture was then sealed and stirred for 2 h under continuous heating (60 °C). The obtained precipitate was separated and washed several times with deionized water. Finally, the products were dried at 60 °C for about 8 h.
The shape and actual size of the ZNCs were primarily determined with high-resolution transmission electron microscopy (HRTEM). The average particle size was 21.3 nm for the prepared samples (Fig. 1).
Plant Growth Conditions
Lupine seeds, used for the present study were procured from the local seed center, Qena, Egypt. Healthy lupine seeds were surface sterilized with 70 % ethanol for 2 min, then washed three times with sterilized water. Then the seeds were divided into four groups according to the priming solution as follows:
-
1.
The 1st (control) and 2nd [salinity (S)] sets were priming with distilled water.
-
2.
The 3rd (ZNPs1) and 4th (ZNPs1 + S) sets were primed with 20 mg L−1 ZnO for 12 h.
-
3.
The 5th (ZNPs2) and 6th (ZNPs2 + S) sets were primed with 40 mg L−1 ZnO for 12 h.
-
4.
The 7th (ZNPs3) and 8th (ZNPs3 + S) sets were primed with 60 mg L−1 ZnO for 12 h.
After priming, the thoroughly washed seeds were sown (5 five seeds/pot) in plastic pots filled with 2 kg of dried soil. The pots were kept in the wire house of the experimental farm of South Valley University, Qena, Egypt located at latitude 26°11′25″N and longitude 32°44′42″E under natural conditions of temperature, light, and humidity during the growing season of 2015. The pots were arranged in a completely randomized design in a factorial arrangement with three replications. At the time of sowing, the seeds were irrigated at field capacity with 0 (control) and 150 (S) mM NaCl. The salt solution was added once and leaching was avoided by maintaining soil water below field capacity at all times. The pots were then irrigated at field capacity with normal water through the whole experimental period (20 days). Twenty days after sowing, the lupine plants were harvested for further analyses.
Growth Traits
The lengths of roots and shoots were measured using a measuring scale. Additionally, fresh weights of full-length plants were recorded, followed by drying of the samples at 80 °C in an oven, and dry weights of the plants were then measured.
Photosynthetic Pigments
According to Lichtenthaler and Wellburn (1983), the contents of photosynthetic pigments (chlorophyll a and b, and carotenoids) in fresh leaves were assessed spectrophotometrically. The pigment extract was determined versus a blank of pure 80 % acetone at 663, 644, and 452.5 nm for chlorophyll a, chlorophyll b, and carotenoid contents, respectively.
Organic Solutes (Soluble Sugar, Soluble Protein, Total Free Amino Acids, and Proline)
The anthrone sulfuric acid method described by Irigoyen and others (1992) was used to estimate soluble sugar content. The absorbance was measured spectrophotometrically at 620 nm against a blank (distilled water + anthrone reagent). The Bradford (1976) method using bovine serum albumin as a standard was used to assess soluble protein content. Total free amino acids content was measured using the method of Lee and Takanashi (1966). The absorbance was read at 570 nm against a blank (only distilled water and the same reagent). The proline content was estimated according to Bates and others (1973) and the absorbance was measured at 520 nm using toluene as a blank.
Total Phenols
Total phenol content was assayed by the Folin–Ciocalteu reagent (Skerget and others 2005). The absorbance was measured spectrophotometrically at 760 nm.
Malondialdehyde (MDA)
The thiobarbituric acid (TBA) reaction cited in Abdel Latef and Tran (2016) was used to determine MDA content in fresh leaf samples. The absorbances were read at 532, 600, and 450 nm.
Assays of Antioxidant Enzyme Activities
Samples were extracted from fresh leaves based on the method, as cited by Ahmad and others (2016). The activity of superoxide dismutase (SOD; EC 1.15.1.1) was determined using the nitro blue tetrazolium (NBT) method described by Giannopolitis and Ries (1977). Catalase (CAT; EC 1.11.1.6) activity was assessed according to the method previously described by Aebi (1984). Peroxidase (POD; EC 1.11.1.7) activity was estimated according to the method described by Maehly and Chance (1954) and Klapheck and others (1990). Ascorbate peroxidase (APX; EC 1.11.1.11) activity was measured by the method of Chen and Asada (1992).
Ascorbic Acid
Ascorbic acid content of fresh leaves was estimated according to Mukherjee and Choudhuri (1983). The absorbance was estimated spectrophotometrically at 525 nm.
Na and Zn
The determination of Na and Zn contents was carried out in ground-dried samples and analyzed by atomic absorption spectrometry according to (Allen 1989).
Statistical Analysis
All data shown are the mean values. Data were statistically analyzed by the analysis of variance (ANOVA) with SAS software (Version 9.1; SAS Institute, Cary, NC, USA) and Duncan’s multiple range test was calculated at the 0.05 level of significance (P < 0.05). Data represented in the tables and figures are mean ± standard deviation (SD) of three independent replicates of each treatment.
Results
Effect of Priming with ZNPs on Growth Traits of Lupine Plants under Normal and Salt Stress Conditions
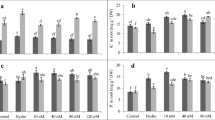
The lengths of roots and shoots and weights (fresh and dry) of lupine-treated plants were assessed to evaluate the impact of priming with different concentration of ZNPs on plant growth under salinity stress (Fig. 2a, b). Salinity pressure provoked a significant suppression in root length (51.00 %), shoot length (33.33 %), fresh weight (32.45 %), and dry weight (47.92 %) versus control. Seed-priming with ZNPs mostly induced an elevation in the formerly mentioned growth characteristics (Fig. 2a, b). This promotion influence of ZNPs was not only observed in the growth of salinized plants, but also elevated the growth in plants subjected to unstressed regimes. Interestingly, the maximum increment in growth parameters was recorded in stressed plants primed with ZNPs3 and reached in ZNPs3 + S plants to 80.07, 43.30, 36.71, and 52.00 % in root length, shoot length, fresh weight, and dry weight, respectively over S plants alone. This indicates that ZNPs3 is probably the best effective nanoparticle to boost plant growth under salt stress (Fig. 2a, b). Accordingly, the three concentrations of ZNPs can be arranged in the following order from most to least effective: ZNPs3 > ZNPs2 > ZNPs1.
Effects of salinity stress and seed-priming with ZnO nanoparticles (ZNPs) on a root length and shoot length and b fresh weight and dry weight in 20-day-old lupine plants. Bars represent standard deviation (±SD) of the means (n = 3). Different letters indicate significant differences among the treatments at P < 0.05, according to Duncan’s multiple range test. S, 150 mM NaCl; ZNPs1, 20 mg L−1 ZnO; ZNPs2, 40 mg L−1 ZnO; ZNPs3, 60 mg L−1 ZnO
Effect of Priming with ZNPs on Photosynthetic Pigments of Lupine Plants under Normal and Salt Stress Conditions
Chlorophyll a, chlorophyll b and carotenoids decreased by 41.13, 44.85, and 39.13 %, respectively in salinized plants relative to control plants (Table 1). However, seed-priming with ZNPs alone as well as in combination with NaCl mostly enhanced photosynthetic pigments in unstressed and stressed plants. An increase by 68.67, 43.28, and 85.71 % in chlorophyll a, chlorophyll b, and carotenoids, respectively was observed in the ZNPs3 + S treatment compared to the S treatment alone (Table 1).
Effect of Priming with ZNPs on Organic Solutes and Total Phenols of Lupine Plants under Normal and Salt Stress Conditions
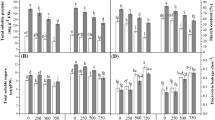
NaCl stress resulted in a marked accumulation in soluble sugar (21.63 %), soluble protein (39.18 %), total free amino acids (44.29 %), proline (60.78 %), and total phenols (58.33 %) over control plants (Fig. 3a, b). Seed-priming with ZNPs especially ZNPs3 alone as well as in combination with NaCl induced a further accumulation in organic solutes and total phenols compared to control and NaCl-stressed plants (Fig. 3a, b). An increase by 36.84, 68.40, 71.56, 32.31, and 45.65 % in soluble sugar, soluble protein, total free amino acids, proline, and total phenols, respectively was observed in stressed plants primed with ZNPs3 compared to the plants treated with NaCl alone (Fig. 3a, b).
Effects of salinity stress and seed-priming with ZnO nanoparticles (ZNPs) on a organic solutes (soluble sugar, soluble protein, total free amino acids, and proline) and b total phenols in 20-day-old lupine plants. Bars represent standard deviation (±SD) of the means (n = 3). Different letters indicate significant differences among the treatments at P < 0.05, according to Duncan’s multiple range test. DW, dry weight; GAE, gallic acid equivalent; S, 150 mM NaCl; ZNPs1, 20 mg L−1 ZnO; ZNPs2, 40 mg L−1 ZnO; ZNPs3, 60 mg L−1 ZnO
Effect of Priming with ZNPs on MDA of Lupine Plants under Normal and Salt Stress Conditions
Exposure of lupine plants to 150 mM NaCl dramatically accumulated MDA content by 83.76 % over control plants (Fig. 4). Priming with ZNPs1, ZNPs2, and ZNPs3 reduced MDA content by 11.96, 39.24, and 74.23 %, respectively compared to control (Fig. 4). In salinized plants, priming with ZNPs1, ZNPs2, and ZNPs3 hampered the accumulation of MDA by 11.56, 21.12, and 41.91 %, respectively against salinized plants alone (Fig. 4).
Effects of salinity stress and seed-priming with ZnO nanoparticles (ZNPs) on malondialdehyde content (MDA) in 20-day-old lupine leaves. Bars represent standard deviation (±SD) of the means (n = 3). Different letters indicate significant differences among the treatments at P < 0.05, according to Duncan’s multiple range test. FW, fresh weight; S, 150 mM NaCl; ZNPs1, 20 mg L−1 ZnO; ZNPs2, 40 mg L−1 ZnO; ZNPs3, 60 mg L−1 ZnO
Effect of Priming with ZNPs on Antioxidant Enzymes of Lupine Plants under Normal and Salt Stress Conditions
The data in Table 2 illustrated that salinity stress resulted in an increment in the activity of SOD, POD, and APX by 44.50, 79.30, and 81.25 % over control. On the other side, CAT activity decreased by 35.96 % under salinity stress in comparison to control. Seed-priming with ZNPs alone or plus 150 mM NaCl mostly stimulated the activity of all tested antioxidant enzymes and this stimulation was more obvious in ZNPs3 plants than ZNPs1 and ZNPs2 plants, respectively (Table 2). In ZNPs3 + S treatment, the stimulation in SOD, CAT, POD, and APX reached to 46.95, 100.73, 47.52, and 61.66 %, respectively over S treatment alone (Table 2).
Effect of Priming with ZNPs on Ascorbic Acid of Lupine Plants under Normal and Salt Stress Conditions
Figure 5 showed that, treatment with NaCl caused a significant increase (44.54 %) in the content of ascorbic acid over control. Ascorbic acid content of control plants primed with ZNPs1, ZNPs2, and ZNPs3 increased by 23.12, 44.54, and 71.21 % over control plants alone, respectively. Ascorbic acid content of salinized plants primed with ZNPs1, ZNPs2, and ZNPs3 was enhanced by 25.02, 52.60, and 100.03 % over salinized plants alone, respectively (Fig. 5).
Effects of salinity stress and seed-priming with ZnO nanoparticles (ZNPs) on ascorbic acid content in 20-day-old lupine leaves. Bars represent standard deviation (± SD) of the means (n = 3). Different letters indicate significant differences among the treatments at P < 0.05, according to Duncan’s multiple range test. FW, fresh weight; S, 150 mM NaCl; ZNPs1, 20 mg L−1 ZnO; ZNPs2, 40 mg L−1 ZnO; ZNPs3, 60 mg L−1 ZnO
Effect of Priming with ZNPs on Na and Zn of Lupine Plants under Normal and Salt Stress Conditions
Na content accumulated by 2.22-fold over control (Fig. 6a). However, seed-priming with ZNPs decreased the accumulation of Na content. The maximum decrement in Na content was recorded in seeds primed with ZNP3 and this reduction in Na content of ZNPs3 + S plants was 45.29 % lower than S plants alone (Fig. 6a). In contrast to the Na pattern, Zn content markedly decreased under salinity stress by 58.06 % compared to control (Fig. 6b). Seed-priming with ZNPs increased Zn content and the maximum increase was recorded in ZNPs3 plants. In ZNPs3 + S plants, the increase in Zn content reached to 2.24-fold over S plants alone (Fig. 6b).
Effects of salinity stress and seed-priming with ZnO nanoparticles (ZNPs) on a Na content and b Zn content in 20-day-old lupine. Bars represent standard deviation (± SD) of the means (n = 3). Different letters indicate significant differences among the treatments at P < 0.05, according to Duncan’s multiple range test. DW, dry weight; S, 150 mM NaCl; ZNPs1, 20 mg L−1 ZnO; ZNPs2, 40 mg L−1 ZnO; ZNPs3, 60 mg L−1 ZnO
Discussion
Zn is an essential element necessary for growth and development of plants (Pathak and others 2012). The most common impact of salt pressure on plant physiology is a depression in the growth which is necessary for the survival of a plant exposed to this pressure. In this study, salinity stress caused depression in plant growth by lessening growth attributes due to water deficit which leads to abnormal changes in plant morphology (Jiang and others 2014), osmotic stress, nutritional disorders, and physiological and biochemical imbalance (Soliman and others 2015; Ahmad and others 2016). Seed-priming with ZNPs positively affects the growth traits in NaCl-stressed lupine as Zn plays a vital role in (1) building natural auxin (IAA) and consequently activating cell division and enlargement (Ali and Mahmoud 2013), (2) maintenance of the structural integrity of biomembranes (Weisany and others 2012), (3) phospholipids accumulation (Jiang and others 2014), (4) improvement in protein synthesis (Ebrahimian and Bybordi 2011), (5) scavenging free oxygen radicals (Jiang and others 2014), (6) translocation of nutrients from the aged cells to newborn cells (Rockenfeller and Madeo 2008; Jiang and others 2014), and (7) decreasing the uptake of excess of Na+ and Cl− (Weisany and others 2012; Ibrahim and Faryal 2014; Jiang and others 2014). Our finding is compatible with the results obtained by Laware and Raskar (2014) on onion, Mukherjee and others (2014) on green pea (Pisum sativum), Rezaei and Abbasi (2014) on cotton (Gossypium hirsutum L.) and Soliman and others (2015) on moringa (Moringa peregrina). It is worth mentioning that the maximum increment in growth parameters under normal and saline regimes was noticed in plants priming with ZNPs3, which means that ZNPs3 has a superior promoting impact either in unstressed or stressed regimes and could be the subject of future studies. Prasad and others (2012) reported that treating groundnut seeds with nanoscale ZnO particles with a concentration of 1000 ppm has induced a marked increase in germination, root and shoot length, and vigor index over other concentrations of the same material. They reported that, the main reason for these influences is not known but it is likely to be due to the higher concentrations of zinc in the seed when treated with nanoscale ZnO particles.
The decrement in photosynthetic pigments of L. termis leaves under the saline condition is in full agreement with the finding of Weisany and others (2011). They stated that, the reduction of pigment content was ascribed to enhanced chloroplast structure damage, pigment-protein complex instability, and chlorophyllase activity (Singh and Dubey 1995). In this investigation, a similar increased trend of soluble sugar, soluble protein, total free amino acids, proline, and total phenols was pronounced in lupine plants exposed to NaCl stress. Increased accumulation of total soluble sugar and soluble protein in response to salt stress was reported by Ahmad and others (2016) in chickpea. Total free amino acids and proline were also reportedly boosted under salt stress in wheat (Abdel Latef 2010). Total phenol content was increased in wheat (Yasmeen and others 2013) under salinity stress. Soluble sugar acts as an important organic solute to keep the cell homeostasis (Ahmad and others 2016). Soluble protein plays a pivotal role in osmoregulation under saline conditions and can supply a storage form of nitrogen (Ahmad and others 2016). Accumulation of soluble protein content under stress may be the result of improved synthesis of specific stress-related proteins (Ahmad and others 2016). Total free amino acids and proline are important organic solutes that assist in cell osmoregulation under salinity stress (Azooz and others 2004). Phenolic compounds played a vital role in safeguarding the plants against harmful impacts induced by various pressures. Total phenols have the antioxidant feature because they are electron-donating agents (Ahmad and others 2016), thus eliminating reactive oxygen species (ROS).
Priming with ZNPs at different concentrations resulted in a significant increment in photosynthetic pigments, organic solutes and total phenols in unstressed and stressed lupine plants. This increment reached its maximum value in plants primed with ZNPs3. Zn probably keeps chlorophyll synthesis through the protection of the sulfydryl group, a function primarily associated with Zn (Cakmak 2000; Weisany and others 2011). Moreover, it shares in chlorophyll synthesis (Li and others 2006; Weisany and others 2011). Zn also plays a main role in sugar formation and enzyme structure involved in the biosynthesis of amino acids (Soliman and others 2015). These findings are in agreement with Soliman and others (2015). The manifest accumulation of organic solutes and total phenols due to priming with ZNPs3 might boost salt tolerance of cells through osmotic adjustment, consequently improve plant growth.
Membrane lipid peroxidation is a sign of membrane destruction under saline stress regimes (Katsuhara and others 2005; Abdel Latef and Chaoxing 2011; Ahmad and others 2016). Lipid peroxidation is generally estimated in terms of MDA content (Abdel Latef 2011; Ranjit and others 2016). MDA is a secondary end product of polyunsaturated fatty acid oxidation and is widely used to assess the extent of lipid peroxidation as an indicator of oxidative stress (Lin and Kao 2000; Abdel Latef and Chaoxing 2014; Zheng and others 2016). The present study showed that there was high accumulation in MDA content under saline conditions, suggesting that salt stress could destroy the integrity of the cellular membrane, as well as cellular compounds, like proteins and lipids. ZNPs application, especially ZNPs3, reduced MDA content, thus ameliorating the injury normally induced by salinity stress. This is consistent with the results of Burman and others (2013) who reported that ZNPs induced defensive impacts on biomembranes versus alternations of membrane permeability and oxidative stress in chickpea seedlings.
It has been reported in many studies that exposure to salt stress could induce ROS formation causing increased activity of antioxidative enzymes as a defense system (Weisany and others 2012; Soliman and others 2015; Ahmad and others 2016). This is harmonious with our results that showed that growing lupine in saline conditions led to a marked increase in the antioxidant enzymes (except CAT), which can be considered as good evidence of ROS production. The high activity of SOD, POD and APX under salt stress is a good signal of lupine ability to adapt with ROS. Therefore, it suggested that the increase in the activities of antioxidant enzymes may be attributed to the adaptive defense system of L. termis against the harmful impact imposed by NaCl. On the other hand, the decrease in CAT might be due to the increasing rate of ROS scavenging by the other antioxidant enzymes.
Beside these antioxidant enzymes, there are metabolites that act as ROS scavengers either in conjunction with the antioxidative enzymes or independently. Nonenzymatic components of the antioxidative defense system include the major cellular redox buffers ascorbic acid and glutathione. They are involved in many cellular processes and not only have critical roles in plant tolerance and act as enzyme cofactors, but also affect plant growth and development from earlier growth stages to senescence (Hajiboland 2013). Ascorbic acid is a water soluble antioxidant that acts to prevent injury induced by ROS in plants (Gill and Tuteja 2010). It plays a main role in the detoxification of ROS due to its ability to donate electrons in a wide range of enzymatic and nonenzymatic reactions. Ascorbic acid is able to reduce H2O2 to H2O via the APX reaction (Hajiboland 2013). In this work, exposure to salt stress increased the level of ascorbic acid in lupine leaves as compared to the control. Our results are in agreement with findings of Soliman and others (2015). The results of the present study showed that seeds primed with ZNPs, especially ZNPs3, had high antioxidant enzymes and nonenzymatic activity when compared with salinized plants indicating that the further increase in enzyme activity in response to ZNPs treatment was due to extreme oxidative stress caused by NaCl and the protection against salt stress by high levels of antioxidant enzymes induced by ZNPs especially ZNPs3. Probably, Zn is able to assist the enzymes and nonenzymatic antioxidant biosynthesis (Weisany and others 2012; Rezaie and Abbasi 2014; Soliman and others 2015).
Salt-stressed L. termis accumulated lower content of Na and higher content of Zn upon priming application of ZNPs. The accumulation of less Na is a great sign of salt resistance in plants treated with ZNPs. Our results are consistent with the findings of Soliman and others (2015) who reported that foliar application of ZNPs could mitigate Na injury in M. peregrina.
Conclusions
It is inferred from the results of the current research that priming with ZNPs, especially ZNPs3 (60 mg L−1 ZnO), lessened the negative impacts of NaCl on lupine plants through enhancing photosynthetic pigments, adjusting osmoregulation, and lowering the contents of MDA and Na. Further, protection under NaCl stress was achieved via ameliorated total phenols and activities of antioxidant enzymes. Thus, application of ZNPs could be a strategy to energize the growth and economic yield in plants growing in salinized soils. Further efforts are required to gain a full understanding of how zinc oxide nanoparticles alleviate the adverse effects of salinity stress in plants.
References
Abdel Latef AA (2010) Changes of antioxidative enzymes in salinity tolerance among different wheat cultivars. Cereal Res Commun 38:43–55
Abdel Latef AA (2011) Ameliorative effect of calcium chloride on growth, antioxidant enzymes, protein patterns and some metabolic activities of canola (Brassica napus L.) under seawater stress. J Plant Nutr 34:1303–1320
Abdel Latef AA, Chaoxing H (2011) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci Hortic 127:228–233
Abdel Latef AA, Chaoxing H (2014) Does the inoculation with Glomus mosseae improve salt tolerance in pepper plants? J Plant Growth Regul 33:644–653
Abdel Latef AA, Tran LSP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front Plant Sci 7:243
Aebi H (1984) Catalase in vitro. Method Enzym 105:121–126
Ahmad P, Latef AAA, Hashem A, Abd_Allah EF, Gucel S, Tran LSP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Ali EA, Mahmoud AM (2013) Effect of foliar spray by different salicylic acid and zinc concentration on seed yield components of mungbean in sandy soil. Asian J Crop Sci 5:33–40
Allen SE (1989) Chemical analysis of ecological materials, 2nd edn. Blackwell, Oxford
Auld DS (2001) Zinc coordination sphere in biochemical zinc sites. Biometals 14:271–313
Azooz MM, Shaddad MA, Abdel-Latef AA (2004) The accumulation and compartmentation of proline in relation to salt tolerance of three sorghum cultivars. Ind J Plant Physiol 9:1–8
Bates LS, Wladren PR, Tear DT (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem 72:248–254
Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet F (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6:866–870
Burman U, Saini M, Kumar Praveen (2013) Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol Environ Chem 95:605–612
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Chen G, Asada K (1992) Inactivation of ascorbate peroxidase by thoils requires hydrogen peroxide. Plant Cell Physiol 33:117–123
Choudhury SR, Ghosh M, Mandal A, Chakravorty D, Pal M, Pradhan S, Goswami A (2011) Surface-modified sulfur nanoparticles: an effective antifungal agent against Aspergillus niger and Fusarium oxysporum. Appl Microbiol Biotechnol 90:733–743
Choudhury SR, Roy S, Goswami A, Basu S (2012) Polyethylene glycol-stabilized sulphur nanoparticles: an effective antimicrobial agent against multidrug-resistant bacteria. J Antimicrob Chemother 67:1134–1137
de la Rosa G, Lopez-Moreno ML, de Haro D, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2013) Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: root development and X-ray absorption spectroscopy studies. Pure Appl Chem 85:2161–2174
Ebrahimian E, Bybordi A (2011) Exogenous silicium and zinc increase antioxidant enzyme activity and alleviate salt stress in leaves of sunflower. J Food Agri Env 9:422–427
Fan Z, Lu JG (2005) Zinc oxide nanostructures: synthesis and properties. J Nanosci Nanotechnol 5:1561–1573
Gerber C, Lang HP (2006) How the doors to the nanoworld were opened. Nat Nanotechnol 1:3–5
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Review. Plant Physiol Biochem 48:909–930
Hajiboland R (2013) Reactive oxygen species and photosynthesis. In: Ahmad P (ed) Oxidative damage to plants. Elsevier Inc, New York, pp 1–63
Ibrahim S, Faryal S (2014) Augmentation of Trigonella foenum-graecum L. (methi) growth under salinity stress and allelochemical stress through Mn + B + Zn mixture foliar spray. J Pharmac Phytochem 3:39–44
Irigoyen JJ, Emerich DW, Sanchez-Dıaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugar in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Jiang W, Sun XH, Xu HL, Mantri N, Lu HF (2014) Optimal concentration of zinc sulfate in foliar spray to alleviate salinity stress in Glycine soja. J Agr Sci Tech 16:445–460
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76
Kah M (2015) Nanopesticides and nanofertilizers: emerging contaminants or opportunities for risk mitigation? Front Chem 3:64
Katsuhara M, Otsuka T, Ezaki B (2005) Salt stress-induced lipid peroxidation is reduced by glutathione S-transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in Arabidopsis. Plant Sci 169:369
Khodary SEA (2004) Effect of NaCl salinity on improvement of nitrogen metabolism and some ions uptake in lupine plants subjected to gamma irradiation. Int J Agric Biol 1:1–4
Klapheck S, Zimmer I, Cosse H (1990) Scavenging of hydrogen peroxide in the endosperm of Ricinus communis by ascorbate peroxidase. Plant Cell Physiol 31:1005–1013
Laware SL, Raskar SV (2014) Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Int J Curr Microbiol App Sci 3:467–473
Lee YP, Takanashi T (1966) An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem 14:71–77
Li WYF, Wong FL, Tsai SN, Tsai SN, Phang TH, Shao GH, Lam HM (2006) Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (by)-2 cells. Plant Cell Environ 29:1122–1137
Lichtenthaler HK, Wellburn RR (1983) Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lin CC, Kao CH (2000) Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul 30:151–155
Maehly AC, Chance B (1954) The assay of catalase and peroxidase. In: Glick D (ed) Methods in biochemistry analysis, vol 1. Interscience Publishers, New York, pp 357–425
Mahajan P, Dhoke SK, Khanna AS (2011) Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J Nanotechnol 2011:1–7
Mohamed AA, Eichler-Löbermann B, Schnug E (2007) Response of crops to salinity under Egyptian conditions: a review. Landbauforsch Völkenrode 2:119–125
Mukherjee SP, Choudhari MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:116–170
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S, Rico CM, Zhaob L, Gardea-Torresdey JL (2014) Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nano level. Science 311:622–627
Pathak GC, Gupta B, Pandey N (2012) Improving reproductive efficiency of chickpea by foliar application of zinc Braz. J Plant Physiol 24:173–180
Patra P, Choudhury SR, Mandal S, Basu A, Goswami A, Gogoi R, Srivastava C, Kumar R, Gopal M (2013) Effect sulfur and ZnO nanoparticles on stress physiology and plant (Vigna radiata) nutrition. In: Giri PK (ed) Advanced nanomaterials and nanotechnology. Springer, Berlin, pp 301–309
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35:905–927
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.). Agric Res 2:48–57
Ramesh M, Palanisamy K, Babu K, Sharma NK (2014) Effects of bulk & nano-titanium dioxide and zinc oxide on physio-morphological changes in Triticum aestivum Linn. J Glob Biosci 3:415–422
Ranjit SL, Manish P, Penna S (2016) Early osmotic, antioxidant, ionic, and redox responses to salinity in leaves and roots of Indian mustard (Brassica juncea L.). Protoplasma 253:101–110
Rezaei M, Abbasi H (2014) Foliar application of nanochelate and non-nanochelate of zinc on plant resistance physiological processes in cotton (Gossipium hirsutum L.). Iran J Plant Physiol 4:1137–1144
Rockenfeller P, Madeo F (2008) Apoptotic death of ageing yeast. Exp Gerontol 43:876–881
Sedghi M, Hadi M, Toluie SG (2013) Effect of nano zinc oxide on the germination of soybean seeds under drought stress. Ann West Uni Timisoaraser Biol 2:73–78
Siddiqui MH, Al-Whaibi MH, Firoz M, Al-Khaishany MY (2015) Role of nanoparticles in plants. In: Siddiqui MH (ed) Nanotechnology and plant sciences. Springer, Basel, pp 19–35
Singh AK, Dubey RS (1995) Changes in chlorophyll a and b contents and activities of photosystems 1 and 2 in rice seedlings induced by NaCl. Photosythetica 31:489–499
Singh P, Nanda A (2014) Enhanced sun protection of nano-sized metal oxide particles over conventional metal oxide particles: an in vitro comparative study. Int J Cosmet Sci 36:273–283
Skerget M, Kotnik P, Hadolin M, Hras A, Simonic M, Knez Z (2005) Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem 89:191–198
Soliman AS, El-feky SA, Darwish E (2015) Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J Hortic Fores 7:36–47
Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K (2011) Physiological responses of soybean (Glycine max L.) to zinc application under salinity stress. Aust J Crop Sci 5:1441–1447
Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K (2012) Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics J 5:60–67
Yasmeen A, Basra SMA, Farooq M, Rehman H, Hussain N, Athar HR (2013) Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul 69:225–233
Zheng JL, Zhao LY, Shen B, Jiang LH, Zhu AY (2016) Effects of salinity on activity and expression of enzymes involved in ionic, osmotic, and antioxidant responses in Eurya emarginata. Acta Physiol Plant 38:1–9
Author contributions
Arafat Abdel Hamed Abdel Latef conceived and designed the experiments. Khaled Ebnalwaled Abdelfattah prepared ZnO nanoparticles. Arafat Abdel Hamed Abdel Latef and Mona Fawzy Abu Alhmad conducted the experiments. Mona Fawzy Abu Alhmad collected the data. Arafat Abdel Hamed Abdel Latef analyzed the data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel Latef, A.A.H., Abu Alhmad, M.F. & Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J Plant Growth Regul 36, 60–70 (2017). https://doi.org/10.1007/s00344-016-9618-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9618-x