Abstract
The amphibian fungal disease chytridiomycosis is considered one of the greatest threats to biodiversity. This lethal skin disease is caused by chytridiomycete fungi belonging to the genus Batrachochytrium. Although sudden amphibian population declines had occurred since the 1970s in the Americas and Australia, mass mortalities were not observed until the 1990s. The fungus Batrachochytrium dendrobatidis (Bd) was identified as the cause of these declines. It is estimated that Bd has caused the rapid decline or extinction of at least 200 amphibian species, which is probably an underestimation due to the cryptic behaviour of many amphibians such as many salamanders and also the lack of monitoring. A second chytrid species, B. salamandrivorans (Bsal), has recently emerged and caused mass mortality in salamanders in Belgium, the Netherlands and Germany, affecting most salamander and newt taxa in the amphibian community and is considered a major threat to the western Palearctic amphibian biodiversity. In this chapter we review the epidemiology, host pathogen interactions and mitigation strategies of both chytrid pathogens.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Chytrids
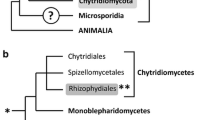
The causative agents of the disease chytridiomycosis belong to a “lower” fungal phylum: the Chytridiomycota (Longcore et al. 1999). Bd and Bsal belong to the order of the Rhizophydiales and are, together with the enigmatic fish pathogen Ichthyochytrium vulgare (Plehn 1920), to date the only known members of this phylum, which are adapted to vertebrate hosts.
Chytridiomycota are unusual among the fungi in that they produce zoospores. Asexual reproduction in Bd and Bsal occurs through the release of motile flagellated spores (zoospores) from the reproductive body or thallus in which they are produced (zoosporangium) (Fig. 14.1) (Longcore et al. 1999). Neither Bd nor Bsal have yet been observed in culture to reproduce sexually. However, as several genetic studies have identified Bd isolates with a hybrid genotype, sexual recombination and hybridization must have been important mechanisms in its evolutionary history (Farrer et al. 2011; Schloegel et al. 2012; Rosenblum et al. 2013).
In vitro, both pathogens grow well in tryptone broth and have a similar life cycle (Van Rooij et al. 2015). The zoospore first encysts by developing a cell wall and absorbing its flagellum and forms a germling with fine threadlike rhizoids. The germling matures into a zoosporangium in which the cytoplasm cleaves mitotically to form new zoospores. Discharge papillae or tubes are formed during the growth of the sporangium. At maturity , the plug blocking the papillae dissolves, and the zoospores are released into the environment to continue their life cycle (Longcore et al. 1999; Berger et al. 2005; Van Rooij et al. 2015). Bd zoosporangia are, in contrast with Bsal thalli, predominantly monocentric (a thallus containing a single sporangium) rather than colonial (a thallus containing more than one sporangium). Despite the difference in thermal preference (Bd 22 °C, Bsal 15 °C), the life cycle of both chytrids in culture is completed within 5 days (Berger et al. 2005; Martel et al. 2013). The life cycle of Bd in amphibian skin is largely similar to that observed in culture (Van Rooij et al. 2015).
2 Global Epidemiology of Batrachochytrium dendrobatidis Emergence
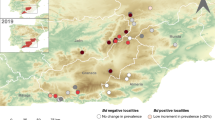
Following the discovery that Bd was a key driver of declines in amphibian species in Australia and the Americas (Berger et al. 1998; Longcore et al. 1999), attention has been focused on determining where outbreaks of chytridiomycosis have occurred and whether there were any spatiotemporal patterns that indicate the original sources of infection as well as pathways of spread. A key technological advance has been the development of a Bd-specific quantitative PCR reaction that has been widely used to screen amphibian populations for infection (Boyle et al. 2004). A global mapping project (http://www.bd-maps.net) (Olson et al. 2013) showed Bd occurred in 56 of 82 (68%) countries and, in 516 of 1240 (42%) species tested, determined using a dataset of more than 36,000 individuals (Bd-maps.net data snapshot August 2012). Across the world, broadscale distribution patterns are evident, with Bd widely detected in the Americas and detected only patchily in Australia, Africa, Asia, and Europe.
Combining data on amphibian population health alongside molecular qPCR surveys has uncovered regional epidemiological trends. In eastern Australia, prospective and retrospective sampling of amphibian populations showed that populations were Bd-negative prior to 1978 followed by an expansion north and south from an inferred centre in Southern Queensland, reaching its northern limits in the mid-1990s. Western Australia was Bd-negative until mid-1985 where upon the arrival and spread of disease was documented (Berger et al. 2009). Mesoamerica has witnessed a very rapid wave-like front of expansion from a putative origin in Mexico in the 1970s (James et al. 2006), through Guatemala to Monteverde, Costa Rica, in the 1980s, and then southwards at estimated rates of up to 50 km y−1, until the infection jumped the Panama Canal. This epidemic front of chytridiomycosis along the North-South transect of Central America was predictable to the extent that researchers were able to anticipate the arrival of Bd in uninfected regions, such as El Copé in Panama in 2004, and to then document the subsequent collapse of the amphibian community (Lips et al. 2008). North America saw steep declines across populations of the Sierra mountain yellow-legged frogs (Rana muscosa and R. sierrae), most notably in the Sequoia-Kings Canyon National Park, where regional populations were sequentially extirpated as Bd invaded lakes in 2004 (Vredenburg et al. 2010). However, historical declines across other regions of the Sierras suggest that the infection had been present since at least the 1970s, and elsewhere in the United States, retrospective surveys of museum collections have demonstrated a widespread prevalence since as early as 1888 in Illinois (Talley et al. 2015). Although the pattern of declines in Ecuador, Venezuela, Bolivia and Peru are consistent with chytridiomycosis, most of South America lacks convincing evidence for outbreaks of chytridiomycosis, and retrospective analyses of museum specimens have shown Bd to be enzootic in the Brazilian Atlantic Forests for over a century (Rodriguez et al. 2014). Surveillance in Europe showed that outbreaks occurred around the 1990s in Spain (Bosch et al. 2001; Walker et al. 2010); however, it is suspected that asymptomatic infections were widespread prior to the 1990s as outbreaks of the disease have only been witnessed at high altitudes (Walker et al. 2010). In Southeast Asia, Bd has a low prevalence, patchy distribution and outbreaks of chytridiomycosis, or cryptic population declines have not been recorded (Swei et al. 2011).
Given these patterns of declines, is it possible to determine whether there is a single original ‘source’ of the panzootic of chytridiomycosis or are we rather witnessing multiple regional and perhaps unlinked foci? Answers to this question have been sought by attempting to identify geographic regions where Bd has had a long and stable association with host species (which is possibly indicative of long-term co-evolutionary associations), as well as through phylogenomic analyses. Histological studies from Southern African museum collections (Weldon et al. 2004; Soto-Azat et al. 2010) identified Africa as a potential source of the panzootic, leading to the ‘Bd Out of Africa’ hypothesis being coined to suggest that Bd was spread around the world via the extensive trade in the African clawed frog Xenopus laevis from the 1930s onwards. However, the widespread occurrence of century-old infections from similar retrospective studies means that Brazil, the United States and Asia are now also included as possible origins (Goka et al. 2009; Rodriguez et al. 2014; Talley et al. 2015; Fong et al. 2015).
A paradigm shift in our understanding of Bd’s epidemiology occurred with the onset of high-throughput whole-genome sequencing (WGS). The phylogenetic resolution afforded by being able to align and detect single-nucleotide polymorphisms (SNPs) across the ~24 Mb genome of Bd has uncovered over 500,000 polymorphic sites. An initial phylogenetic analysis of 20 global isolates showed that Bd is composed of at least 3 deeply diverged lineages (Farrer et al. 2011). Of these, one was found to have a global distribution and showed low levels of genetic polymorphism as well as a lack of genetic structure among continents. These data are consistent with a rapid global emergence that was dated to sometime in the twentieth century, and accordingly the lineage was named the ‘Bd Global Panzootic Lineage’ (Bd GPL) . Alongside Bd GPL, two other regionally endemic lineages were identified: one was found in Switzerland (Bd CH), while the other was found widely occurring in South Africa (Bd CAPE). Subsequent genome sequencing of Bd isolates from other global regions identified a fourth endemic lineage that infects amphibians in the Brazilian Atlantic forest (Bd Brazil) (Rodriguez et al. 2014). Further genome sequencing from a broader spatial collection of isolates will undoubtedly uncover further endemic lineages, consolidating the finding that Bd is an amphibian pathogen with a broad global distribution and ancient associations with its amphibian hosts.
These phylogenomic analyses have shown that the worldwide emergence of chytridiomycosis is mostly likely explained by the rapid transmission of Bd GPL across a global scale as this lineage has now been found to co-occur in all continents alongside endemic lineages. In support of this hypothesis is that all mass mortality and extinction events thus far attributed to chytridiomycosis are associated with the presence of Bd GPL. In vivo laboratory assessments of Bd’s virulence have shown that the lineage is hypervirulent when compared against other lineages, a feature that confers “superbug” status on Bd GPL (Farrer et al. 2011). However, the origin of Bd GPL remains currently unknown, as are details on the historical timing of this lineage’s emergence across different continents. Answers to these questions await the accumulation of genome sequences from a more representative set of spatial regions and a more nuanced understanding of the molecular clock rates that govern Bd’s evolution (Rosenblum et al. 2013; Farrer et al. 2013).
What is clear, however, is that the global trade in amphibians is a potent force in spreading Bd into naïve populations and species. This statement is especially true for the so-called ‘Typhoid Mary’ species such as X. laevis and the North American bullfrog Lithobates catesbeianus; these species carry Bd infections but rarely become diseased (Fisher et al. 2009). They are also widely traded and are often highly invasive when introduced by accident or deliberately into new environments (Lips et al. 2008). Therefore, these two species constitute ideal vectors for introducing Bd into uninfected regions of the globe (Garner et al. 2006) and are likely a major source of new Bd infections when released into naïve environments. Molecular epidemiology from whole-genome sequencing is now being used to identify the sources of regional outbreaks. The best understood example of this is the introduction of Bd onto the Balearic island of Mallorca where genome sequencing showed that Bd CAPE was found to be infecting two populations of Mallorcan midwife toads Alytes muletensis. Retrospective analyses identified a spillover event within a zoo captive assurance population of A. muletensis that had been subsequently released onto the island in order to supplement the wild populations. In this example, the co-housing of Southern African Xenopus species alongside A. muletensis led to cross-transmission and the vectoring of Bd CAPE onto the island (Walker et al. 2008; Doddington et al. 2013).
3 Global Epidemiology of Batrachochytrium salamandrivorans Emergence
The outbreak of chytridiomycosis caused by Bsal in the European low countries shares many features with the emergence of Bd. While population genomic analyses have not yet been published, the rapid development of a qPCR-based molecular diagnostic has enabled the use of global surveillance to map the distribution of the pathogen which has indicated likely sources of European infection (Blooi et al. 2013). In total, over 5000 amphibians were screened by qPCR from across four continents (Martel et al. 2014). These data showed that detections of Bsal were limited to East Asia, namely, Thailand, Japan and Vietnam. The data also point to a likely Asian origin of Bsal, and assessments of host associations have strengthened this hypothesis by demonstrating that, while Palearctic caudates from Europe and North America suffer clinical disease following infection by Bsal, Asian species tolerate or clear their infections. Molecular surveys of museum collections have shown that Bsal DNA can be detected in museum specimens of Asian Cynops ensicauda that were collected over 150 years ago and molecular dating shows that Bsal separated from Bd over 30 million years ago. Taken together, these data strongly indicate an Asian evolutionary centre of origin for Bsal infections that have spilled over to infect European salamander and newt species.
As with Bd, there has been much focus on how the transcontinental vectoring of Bsal may have occurred. It is clear now that the international trade in Asian caudate species is enormous, with, for instance, over 2.3 million individuals of Cynops orientalis imported into the United States from 2001 to 2009. Further, the infection is associated with outbreaks of disease in captive European caudates, which constitute a continuous threat to vectoring the disease to wild amphibians (Cunningham et al. 2015; Sabino-Pinto et al. 2015). Therefore, the international movement of traded species of amphibians is a key mechanism contributing to the spread of Bsal and its invasion of naïve disease-free ecosystems.
4 Pathogenesis
Whereas the pathogenesis of Bd infections is relatively well known, Bsal has only recently been discovered, and its pathogenesis is incompletely understood. This chapter will focus on the pathogenesis of Bd, while highlighting any known differences to Bsal.
4.1 Skin Colonization
The different steps in the infection process comprise attraction of the free swimming zoospore to a suitable host with subsequent attachment to and invasion into the host skin, leading to impairment of the skin function. Bd is attracted to keratin and to carbohydrate components of the mucus and epidermis (Meyer et al. 2007; Moss et al. 2008; Van Rooij et al. 2015). If the zoospores resist the defence mechanisms of the mucus (see below), adhesion to the skin surface occurs within 2–4 h after exposure (Van Rooij et al. 2012), after which the zoospores mature into thick-walled cysts. Cysts are anchored to the skin surface by fine fibrillar projections. Several adhesion proteins such as vinculin, fibronectin and fasciclin are expected to contribute to this process (Rosenblum et al. 2008, 2012). Besides, Bd is equipped with a chitin binding module (CBM18) that is hypothesized to facilitate survival on its amphibian host by limiting access for foreign chitinases by binding to the chitin of their proper cell wall. In addition, CBM18 would also allow attachment of the pathogen to non-host chitinous structures (e.g. insect or crustacean exoskeletons) allowing vectored disease spread (Abramyan and Stajich 2012; McMahon et al. 2013; Liu and Stajich 2015; Van Rooij et al. 2015).
Bd further develops endobiotically (with sporangia located inside the host cell). Colonization is established via a tubular extension or germ tube arising from the zoospore cyst that penetrates the host cell membrane and enables transfer of genetic material into the host cell. The distal end of the germ tube swells and gives rise to a new intracellular chytrid thallus. The pathogen then uses the same tactics to dig its way to deeper skin layers: older “mother” thalli develop rhizoid-like structures spreading to deeper skin layers, forming a swelling inside the host cell to finally give rise to a new “daughter” thallus (Berger et al. 2005; Van Rooij et al. 2012; Greenspan et al. 2012; Van Rooij et al. 2015). This intracellular proliferation occurs within the cells of the stratum corneum and the stratum granulosum. Immature sporangia are carried from the deeper skin layers to the skin surface by differentiating epidermal cells. At the time sporangia have developed discharge tubes and contain mature zoospores, they occur in the stratum corneum, where the zoospores are released in the environment (Longcore et al. 1999; Berger et al. 2005; Van Rooij et al. 2015).
Conversely, in explanted skin of the infection tolerant X. laevis, the pathogen develops merely epibiotically (with sporangia developing upon the skin) (Van Rooij et al. 2015). Here, the affected epidermal cells seem to be solely used as nutrient source for the growing sporangium upon the epidermis (Van Rooij et al. 2012). Due to the lack of conclusive histological evidence, it is not clear how infections manifest in this species under natural conditions (Van Rooij et al. 2015). As this “saprobic” type of development has only been observed in vitro, further in vivo evidence is needed. Skin invasion by Bsal was also shown to occur within 24 h (Martel et al. 2014), although the mechanism of Bsal invasion is not known.
Bd selectively colonizes keratinized, stratified epidermis. In anuran larvae, colonization is limited to the keratinized mouthparts, i.e. tooth rows and jaw sheaths, but absent from the body, limbs, tail, mouth and gills. Studies in Mixophyes fasciolatus and Osteopilus septentrionalis tadpoles show that during metamorphosis, colonization of the skin by Bd progresses following the distribution of keratin (Marantelli et al. 2004; McMahon and Rohr 2015). In contrast with anuran larvae, no colonization of salamander larvae by Bd has been demonstrated so far (Van Rooij et al. 2015).
Susceptibility to Bsal infection was equally shown to be life stage dependent. Whereas Bsal infects all fire salamander (Salamandra salamandra) life stages post metamorphosis, larvae seem to be refractory to infection (Van Rooij et al. 2015).
4.2 Pathophysiology
Severe chytridiomycosis causes functional disruption of the epidermal barrier. Bd possesses a large number of proteolytic enzymes, including serine-type proteases and fungalysin metallopeptidases, which can cause damage to skin integrity by disturbing the host’s intracellular junctions (Joneson et al. 2011; Brutyn et al. 2012 Rosenblum et al. 2013). Furthermore, infection due to Bd triggers a decreased expression of host genes encoding essential skin integrity components such as keratin, collagen, elastin and fibrinogen (Rosenblum et al. 2012).
Physical disruption of the epidermis directly affects the osmoregulatory function of the skin: it impairs the electrolyte transport across the skin, accompanied by a reduction in transepithelial resistance and leakage of ions, giving rise to ion imbalances, and a reduced ability of amphibians to osmoregulate or rehydrate. Blood samples from amphibians with clinical chytridiomycosis show significantly reduced plasma sodium, potassium and chloride ion concentrations and reduced overall blood plasma osmolality. The low plasma potassium concentrations (or hypokalaemia) are linked to abnormal cardiac electrical activity and cardiac arrest and are thought to be the proximate cause of death in diseased amphibians (Campbell et al. 2012, Van Rooij et al. 2015).
Besides the detrimental effect on the skin, Bd also actively suppresses host responses (Rosenblum et al. 2009; Ellison et al. 2014; Ribas et al. 2009; Woodhams et al. 2012a, b; Young et al. 2014). Bd culture supernatant inhibits lymphocyte proliferation and induces apoptosis (Fites et al. 2013, 2014). Fungal proteases, such as a subtilisin-like serine protease, inhibit antimicrobial peptide (AMP) release from dermal granular glands and/or selectively degrade AMPs (Woodhams et al. 2012a, b; Thekkiniath et al. 2013).
5 Clinical Signs and Lesions
Clinical signs of chytridiomycosis due to Bd in juvenile and adult anurans may include erythema of ventral surfaces, abnormal posture such as splayed limbs, depression, slow righting reflex, abnormal skin shedding and tetanic spasms upon handling (Berger et al. 2009). Signs generally occur in the terminal stages of disease and correlate with heavy infections, severe skin pathology and loss of plasma electrolytes due to disturbance of epidermal ion transport (Voyles et al. 2009). Therefore most of the course of infection remains subclinical, and dead frogs are often in good body condition. With Bsal, there is also lethargy and excessive skin shedding, but widespread multifocal ulceration is a marked and typical sign (Martel et al. 2013; Van Rooij et al. 2015).
Both Batrachochytrium species occur only within epidermal cells, but sporangia of Bd infect the superficial epidermal layers (including the stratum granulosum), whereas Bsal grows in all layers (Berger et al. 2005; Martel et al. 2013). Bd generally has a predilection for ventral skin areas and feet, but Bsal occurs over all the skin including the dorsum. Histopathology of Bd infection includes hyperkeratosis, disordered epidermal cell layers, spongiosis, erosions and occasional ulcerations. Hyperplasia may occur resulting in diffuse or focal thickening, whereas other regions of epidermis may be thin and eroded. Bacteria often colonize the layers of sloughing keratin and grow within “empty” sporangia. A mild inflammatory response may occur as a slight increase in mononuclear cells in the dermis and epidermis but is often negligible (Berger et al. 2009). The deeper infection by Bsal leads to more erosive and ulcerative changes without hyperkeratosis or hyperplasia (Van Rooij et al. 2015).
Electron microscopy revealed a zone of apparently condensed host cytoplasm, up to 2.5 μm thick, around some Bd sporangia that appeared fibrillar and excluded organelles (Berger et al. 2005). It is possible this host reaction provides protection of sporangia from antimicrobials or immune response. Keratinization appears to occur prematurely in infected cells, compared with uninfected cells in the same epidermal layer (Berger et al. 2005).
Specific internal lesions are not observed in frogs with severe chytridiomycosis, besides some terminal changes. In an Australian survey, concurrent diseases were diagnosed in 12% of frogs with severe chytridiomycosis, but most frogs had no other specific lesions (Berger et al. 2004).
6 Susceptibility to Chytridiomycosis
Considerable variation in susceptibility to chytridiomycosis has been observed between hosts at the individual, population and species levels and is attributable to both extrinsic and intrinsic host factors including immunological defences (Blaustein et al. 2005; Tobler and Schmidt 2010; Pilliod et al. 2010; Searle et al. 2011).
6.1 Extrinsic Host Factors
At the broad scale, population declines due to Bd appear strongly linked to species’ distribution and habitat use. Declines have been particularly severe among tropical high-altitude, stream-associated anurans, although in many cases, susceptibility and population declines are likely associated with environmental suitability for the fungus rather than innate variations between species (Lips et al. 2006; Fisher et al. 2009). Even among syntopic species, however, habitat use may differ which may explain some degree of variation in perceived susceptibility to chytridiomycosis (Rowley and Alford 2007).
In Central and South America, the Atelopus genus of neotropical toads appears to have been most severely affected (La Marca et al. 2005). In Australia, diverse species of Myobatrachid and Hylid frogs have become extinct or are critically endangered (Skerratt et al. 2016). Globally, Bd-associated declines and extinctions have been recorded in numerous other amphibian taxa, particularly other anurans (Fisher et al. 2009). Many salamanders may also have been significantly affected (Rovito et al. 2009; Cheng et al. 2011). There are several host species that are predominantly resistant to or tolerant of infection and may serve as reservoirs or carriers of infection. These species may have been responsible for the widespread dissemination of Bd and include, for example, Pseudacris regilla, L. catesbeianus, L. pipiens, X. laevis, Eleutherodactylus coqui, Litoria lesueurii complex, L. ewingii and Crinia signifera (Retallick et al. 2004; Weldon et al. 2004; Beard and O’Neill 2005; Ricardo 2006; Schloegel et al. 2010; Reeder et al. 2012).
Behaviour that increases exposure to the pathogen may constitute an important extrinsic host factor contributing to variation in susceptibility. This may be through types and degrees of host contact with conspecifics, contaminated water and environmental substrates containing Bd (Rowley and Alford 2007). Importantly, however, other general movement patterns, including thermoregulatory behaviours such as basking, and the use of retreat sites may also play a role by altering existing infection intensities or pathogen growth rates due to the optimal thermal range of the fungus (Rowley et al. 2007; Richards-Zawacki 2010; Puschendorf et al. 2011; Daskin et al. 2011). Behaviour may differ particularly between life stages due to aquatic and terrestrial or arboreal habitats as well as between male and female adults due to their differing seasonal utilisation of the environment (Duellman and Trueb 1994). Other factors may include the density of individuals within the population (higher densities may increase environmental zoospore load; Hudson and Dobson 1998), the age structure of the population (tadpoles may act as reservoir hosts, or dispersal patterns and habitat use differ between stages; Rachowicz and Vredenburg 2004) and the presence of and interactions with any syntopic species and vectors (Reeder et al. 2012; Rivas 1964).
6.2 Intrinsic Host Factors
Intrinsic host factors associated with signalment (life stage, age and body size) have been found to differentiate susceptibility to chytridiomycosis between individuals in laboratory experiments and field observations. Infections are limited to the keratinized mouthparts of larval anurans and are typically not fatal during this stage (Berger et al. 1998; Pessier et al. 1999; Fellers et al. 2001; Rachowicz and Vredenburg 2004), although infection prevalence and intensity has been found to increase with larval development (Smith et al. 2007). Tadpole mouthpart abnormalities may affect feeding efficiency leading to the smaller body size seen in experimentally infected animals (Parris and Beaudoin 2004). Despite interspecific variation, metamorphosis from larval to adult form and the immediate post-metamorphic phase appear to be the most susceptible periods (Berger et al. 1998), which may be associated with immune restructuring. The immune system of tadpoles, while competent, is functionally less well developed compared with that of adult anurans and undergoes substantial remodelling accompanied by immunosuppression during metamorphosis (Rollins-Smith et al. 2011). There is little evidence from experimental infections to suggest an intrinsic difference between sexes in susceptibility to chytridiomycosis (Grogan 2014), suggesting that any observed variation from field studies may be more likely associated with extrinsic factors such as differences in behaviour, and hence fungal exposure or infection development (Johnson and Hoverman 2014). Other putative intrinsic determinants of susceptibility may include concurrent infection, nutritional level and the presence of stressors (Murphy et al. 2011; Kinderman et al. 2012; Young, unpublished).
Controlling for the factors described above, experimental evidence suggests the importance of both genetic and phenotypic immunologic mechanisms in differentiating susceptibility between individuals, clutches, populations and species (Rosenblum et al. 2012; Grogan 2014). These variations in response may be associated with inherent or evolved differences in innate immunity (Savage and Zamudio 2011; Bataille et al. 2015), previous exposure history and the development of adaptive immunity (Cashins et al. 2013; McMahon et al. 2014) or phenotypic differences in pathogenesis and differing functional expression of immune responses. While predisposing immunosuppression is not necessary for epidemics to occur, apparent immunosuppression has been observed in Bd-infected individuals (detected via skin histopathology, and corroborated via gene expression and in vitro immune studies (Berger et al. 2005; Ribas et al. 2009; Rollins-Smith et al. 2011; Rosenblum et al. 2012). Thus it appears that Bd may have low inherent antigenicity perhaps due to intracellular localization, may suppress adaptive immune responses in susceptible hosts and may elicit immunopathologic responses in late infection (Ellison et al. 2015).
6.2.1 Innate Immune Response
Many components of the innate immune response to Bd infection differ between experimental groups and may be associated with differential host susceptibility. The secreted mucus and epidermal layer of amphibian skin provide a constitutive physical and chemical defence barrier against pathogen invasion and may include variable expression of lysozyme (Rollins-Smith 2009), mucosal antibodies, inducible AMPs (such as defensins, cathelicidins and histatins; Ramsey et al. 2010; Pask et al. 2013) as well as commensal symbiotic bacterial communities and their antimicrobial metabolites (Lauer et al. 2007; Harris et al. 2009a, b).
6.2.2 Antimicrobial Peptides
AMPs are small (12–50 amino acid residues), cationic and hydrophobic peptides produced in the dermal granular glands. Most of our current knowledge concerning amphibian AMPs stems from studies on Anura (Van Rooij et al. 2015). To date, approximately 40 anuran AMPs inhibiting Bd have been characterized. Both purified and natural mixtures of these AMPs effectively inhibit in vitro growth of both Bd zoospores and sporangia (Woodhams et al. 2007; Rollins-Smith 2009; Ramsey et al. 2010). However, it is not clear to which extent these peptides provide protection against chytridiomycosis in vivo (Van Rooij et al. 2015). Species with peptides active in vitro such as the mountain yellow-legged frog (Rana muscosa) may still be very susceptible to Bd (Rollins-Smith et al. 2006). Moreover, the efficacy of skin peptide defences may vary at species and population level (Tennessen et al. 2009).
6.2.3 Bacteria and Their Antifungal Metabolites
Several secondary metabolites secreted by symbiotic bacteria present on amphibian skin have been shown to inhibit Bd growth in vitro and in vivo (Van Rooij et al. 2015). In the bacteria Janthinobacterium lividum, Lysobacter gummosus and Pseudomonas fluorescens, these metabolites are 2,4-diacetylphloroglucinol (2,4-DAPG), indol-3-carboxaldehyde (I3C) and violacein (Brucker et al. 2008; Myers et al. 2012; Lam et al. 2010; Harris et al. 2009a, b). In addition, the metabolites 2,4-DAPG and I3C seem to exert a repellent action on Bd zoospores (Lam et al. 2011). Besides, coculture of skin bacterial isolates can lead to secretion of new, more potent metabolites than when grown in monoculture. As such, the inhibitory metabolite tryptophol was found to emerge from coculturing an unknown Bacillus skin bacterium and Chitinophaga arvensicola (Loudon et al. 2014). Myers et al. (2012) showed that these metabolites work synergistically with AMPs to inhibit growth of Bd, at lower minimal inhibitory concentrations (MIC) than necessary for inhibition by either metabolites or AMPs. As for AMPs, variation in infection susceptibility among populations is thought to result in part from differences in skin bacterial communities (Van Rooij et al. 2015). By comparing bacterial communities on the skin of a declining R. muscosa population and a population coexisting with Bd, researchers found a significantly higher number of individuals with culturable bacterial species displaying antifungal properties in coexisting populations than in those at decline. Alteration of this microbial community composition, for example, by environmental factors, can considerably increase susceptibility to disease (Lam et al. 2010).
6.2.4 Other Inducible Pathways of the Innate Immune System
Zoospores that survive to invade beyond the skin mucus layer induce host innate immune responses via antigens that are either secreted or expressed on the pathogen cell surface or released through lysosomal degradation within host cells. These antigens often contain epitopes of widely recognized pathogen-associated molecular patterns (PAMPs) that are common to many different microorganisms. These PAMPs bind to host pattern recognition receptors (PRRs; including toll-like receptors, mannose receptors, scavenger receptors, glucan receptors, C-type lectin receptors and NOD-like receptors, among others), activating the inflammatory and complement cascades and stimulating the release of cytokines (including interleukins, tumour necrosis factors, interferons, chemokines, and stress proteins among others). Gene expression studies in Bd-infected frogs have demonstrated mixed results on the expression of various PRRs and cytokines, and more research is needed to tease apart these interactions, their roles in host defence and their association with infection susceptibility (Ribas et al. 2009; Rosenblum et al. 2012; Ellison et al. 2014). Leukocyte recruitment and infiltration to the site of infection is typically lower than expected in infected susceptible amphibians (examined via haematology and histopathology; Woodhams et al. 2007; Davis et al. 2010; Peterson et al. 2013; Young et al. 2014).
6.2.5 Adaptive Immune Response
There is currently little evidence to suggest the effective activation of the adaptive immune response to chytridiomycosis in terms of a systemic or localized lymphocyte response (Pessier et al. 1999; Berger et al. 2005; Peterson et al. 2013; Young et al. 2014; Nichols et al. 2001) that may differentiate susceptible from resistant individuals. Mapping of transcriptomic changes in immunologically important tissues (skin, liver, spleen, small intestine) from frogs with chytridiomycosis demonstrated the absence of a robust adaptive immune response at various time points post exposure in various species (e.g. lymphocyte, immunoglobulin, MHC and classical complement pathway genes; Ribas et al. 2009; Rosenblum et al. 2009; Ellison et al. 2014). Attempts to immunize frogs by subcutaneous or intraperitoneal injection of formalin (R. muscosa; Stice and Briggs 2010) or heat-killed Bd (Bufo boreas; Rollins-Smith 2009) failed to elicit a protective immune response. Only in X. laevis, Bd-specific IgM, IgX (mammalian IgA-like) and IgY (mammalian IgG-like) antibodies were found in skin mucus after injection with heat-killed zoospores (Ramsey et al. 2010). Although repeated exposure to Bd did not result in increased resistance in all experiments (Cashins et al. 2013), repeated cycles of exposure to Bd with subsequent temperature treatment of infection resulted in a marginally higher survival rate, reduction in the infection load (Ramsey et al. 2010; McMahon et al. 2014), which coincided with increased lymphocyte proliferation and abundance in the spleen. As such, some species of frogs are capable of acquiring at least some degree of immunity against Bd (McMahon et al. 2014), although in general, adaptive immune responses are suppressed by Bd.
6.3 Host Defences Against Bsal
Little is known about amphibian defences against Bsal. Unlike for anurans, information about the AMP arsenal in skin secretions of urodelans is scant (Van Rooij et al. 2015). To date only a single antimicrobial peptide (the defensin CFBD) has been described from Cynops fudingensis (Fuding fire belly newt) (Meng et al. 2013). Although the antimicrobial action of CFBD against both Batrachochytrium species has not (yet) been evaluated, it is suggested that AMPs may be involved in the pronounced anti-Bd activity of salamander skin secretions (Sheafor et al. 2008; Pasmans et al. 2013). Infection trials demonstrated that host responses vary dramatically, not only within but also between urodelan species (Martel et al. 2014). Interestingly, in some species that are likely Bsal reservoirs, a proportion of the exposed individuals do not develop lethal skin disease but are capable of self-cure after initial infection, in some cases with complete fungal elimination. This process can take several months and is most likely due to either increased innate defence mechanisms or the buildup of protective acquired immunity. Other urodelan species, however, which were shown to invariably develop lethal disease, do not seem to increase resistance against Bsal, even after five cycles of exposure and thermal treatment (unpublished results).
7 Diagnosis
Laboratory tests are needed to diagnose chytridiomycosis, as tolerant species are infected without being sick, and because in susceptible hosts clinical signs of chytridiomycosis only occur in late terminal stages and are often non-specific. With heavy infections, changes that are suggestive of chytridiomycosis may include erythema of ventral surfaces, abnormal posture such as splayed limbs, slow righting reflex and abnormal skin shedding (Fig. 14.2a) (Berger et al. 2009). With Bsal, susceptible salamanders usually develop obvious ulcers (Fig. 14.2b) (Martel et al. 2014). These signs are typical in sick amphibians, and two major diseases that can present similarly are ranaviral disease and bacterial septicemia “redleg.” In tadpoles with Bd, infections cause mouthpart abnormalities including loss of dark tooth rows which can lead to emaciation (Rachowicz and Vredenburg 2004).
(a) Bd-infected frogs (Phyllobates bicolor) that develop chytridiomycosis show abnormal posture such as splayed limbs and abnormal skin shedding, with remnants of shed skin. (b) After infection with Bsal, susceptible salamanders (Salamandra salamandra) usually develop obvious skin ulcers, here coinciding with haemorrhages (© Pasmans and Martel 2015. All Rights Reserved)
Details of diagnostic methods for Bd are available in Berger et al. (2009) and the online OIE Manual of Diagnostic Tests for Aquatic Animals 2017 (Chapter 2.1.1), and for Bsal see Martel et al. (2013). A nationwide survey protocol for detecting Bd is also available (Skerratt et al. 2008).
Quantitative polymerase chain reaction (qPCR) is the gold standard for testing. As sampling for PCR involves taking skin swabs, which can be stored at room temperature, this method is non-invasive and is convenient for researchers, veterinarians and conservation managers testing wild or captive animals. For Bd, qPCR was shown to be much more sensitive (72.9%) than histology (26.5%) although was less specific (94.2% versus 99.5%) (Skerratt et al. 2011a, b). Quantitative PCR permits relative quantification of conserved Bd 18S and 28S ribosomal DNA from skin swabs down to a resolution of one genomic zoospore equivalent from as soon as 7 days post infection (Annis et al. 2004; Boyle et al. 2004; Kriger et al. 2006; Hyatt et al. 2007). Standard PCR, however, is also accurate and may be cheaper (Garland et al. 2011). A qPCR to detect Bsal is based on the 5.8S rRNA gene (Martel et al. 2013). Besides, a duplex qPCR detecting Bd and Bsal is available (Blooi et al. 2013, 2016).
Microscopy was the original diagnostic method for chytridiomycosis and is highly accurate in sick animals which have heavy burdens. Microscopy includes histology, wet preparations and immunostaining, and requires pieces of whole or shed skin. Also histological examination of all organs by a pathologist is important if ill or dead frogs are found, as part of general disease surveillance (Duffus 2009). For histology, skin from body, digits, or tadpole mouthparts can be examined with haematoxylin and eosin (H&E) staining (Berger et al. 2000, 2009). Sporangia occur in clusters within the epidermis and appear spherical or oval (5–13 μm) with a smooth eosinophilic wall. Discharge papillae are occasionally seen and project towards the skin surface. Focal hyperkeratosis and erosions are common in Bd-infected areas, whereas Bsal causes necrosis with subsequent erosive and ulcerative lesions (Fig. 14.3). To confirm suspect cases, sporangia can be highlighted using other stains, such as periodic acid-Schiff (PAS) or silver, or an immunoperoxidase stain using polyclonal antibodies with high affinity for Bd and Bsal (Berger et al. 2002; Van Ells et al. 2003; Martel et al. 2013). Wet preparations are quick to prepare using shedding stratum corneum, whole skin or excised tadpole mouthparts that are spread on a slide and cover-slipped (Berger et al. 2009). Diagnosis requires some practice, but the observation of internal septa within sporangia increases confidence in the diagnosis. As Batrachochytrium species are slow-growing compared with microorganism contaminants, culture is difficult and is not used for diagnosis (Berger et al. 2009).
Immunohistochemical staining of the skin of a Bd-infected frog, which died due to chytridiomycosis (Alytes obstetricans) (reprinted from Berger et al. 2002) (a) and a Bsal-infected fire salamander (b). Bd causes focal epidermal hyperplasia and hyperkeratosis (with abundant thalli present in the keratin), whereas Bsal causes focal necrosis with subsequent erosive and ulcerative lesions. For both chytrids, intracellular thalli (stained dark brown) abound in the lesions (© Pasmans and Martel 2015. All Rights Reserved)
8 Treatment
8.1 Treatment with Antimicrobial Compounds
The most important class of antifungal drugs used to treat chytridiomycosis in amphibians is that of the imidazole, triazole and thiazole or “azole” group. The most widely used azole antifungal in treating chytridiomycosis is itraconazole. In general, most itraconazole treatment protocols are based on a study performed by Nichols et al. (2000) describing successful treatment of chytridiomycosis by bathing Bd-infected amphibians in a 0.01% solution (100 mg/L) of itraconazole diluted in 0.6% saline, daily for 5 min during 11 days (Nichols et al. 2000; Forzan et al. 2008; Une et al. 2008; Pessier and Mendelson 2010; Georoff et al. 2013). Studies describing successful treatment of chytridiomycosis with minor adaptations to this protocol (other concentration, other diluting agent and longer exposure time or treatment period) also exist (Garner et al. 2009; Tamukai et al. 2011; Jones et al. 2012; Georoff et al. 2013). However, treatment failure and adverse side effects due to itraconazole toxicity at this concentration (and even lower concentrations) have also been reported for some amphibian species (Brannelly et al. 2012; Woodhams et al. 2012a, b). Variable outcome of itraconazole treatment in clearing Bd infections might be explained not only by the used concentration but also by the frequency of exposure to itraconazole (Woodward et al. 2014). Other antifungals belonging to the azole group used to treat chytridiomycosis are miconazole and voriconazole. Nichols et al. (2000) demonstrated that treatment by using miconazole baths at a concentration of 100 μg/ml, once daily for 5 min during 8 days was effective in clearing Bd infections. Voriconazole has been shown to have potent Bd inhibitory effects both in vitro and in vivo (Woodward et al. 2014, Martel et al. 2011), with successful clearance of Bd in experimentally and naturally infected amphibians using a treatment protocol composed of topically spraying voriconazole once daily during 7 days at a concentration of 1.25 μg/ml (Martel et al. 2011).
Topical treatment of Bsal-infected animals with a combination of polymyxin E (2000 IU/ml) and voriconazole (12.5 μg/ml) at an ambient temperature of 20 °C results in clearance of Bsal infections (Blooi et al. 2015a, b). For all treatments, post-treatment assessment for clearance of infection is necessary, and treatments may need to be repeated.
8.2 Physical Therapy
Short exposure to relatively high ambient temperatures (37 °C less than 16 h (Woodhams et al. 2003) and 30 °C, 10-day exposure (Chatfield and Richards-Zawacki 2011) and longer exposure to lower ambient temperatures (27 °C, clearance after 98 days; Berger et al. 2004) have been able to clear Bd infections from adult amphibians. Exposure to 26 °C during 5 days was able to clear Bd infections in midwife toad (Alytes obstetricans) larvae (Geiger et al. 2011). Ten-day exposure at 25 °C is able to clear salamanders from Bsal infections (Blooi et al. 2015a, b). The main disadvantages linked to temperature treatment of chytrid infections is that elevated temperature might not be endured by all amphibian species and that thermal shock might occur (especially when taking into account that the treatment is applied on sick individuals). Furthermore, strain dependent thermal preferences of Bd or Bsal may compromise thermal treatment protocols.
9 Mitigation
Mitigation of chytridiomycosis has two broad aims: (1) reducing spread and (2) reducing impacts. By the time Bd was recognized widely as the cause of global declines, it had already spread to most areas that contain suitable habitats. The faster recognition of Bsal, however, has meant that there is potential for biosecurity measures to keep countries disease-free, such as the United States (Martel et al. 2014). However, for both fungi, there is a risk that spread of different strains could lead to dangerous recombinations; hence, reducing movement across already-infected areas is recommended. Long-distance spread is most likely to have occurred due to movement of infected amphibians, particularly through the pet trade but also via accidental movement in produce and in the frog meat industry (although the latter is likely more important for viruses such as ranaviruses, since most frog products are frozen; Kolby et al. 2014). The listing of chytridiomycosis as an internationally notifiable disease by the OIE represents the first disease listed that is solely a biodiversity concern, with the aim to improve trade safety. However, although rigorous quarantine and surveillance protocols are often in place for livestock diseases, improved standards are needed for wildlife (Grogan et al. 2014). Fortunately, the US Fish and Wildlife Service announced restrictions from January 2016 on the importation and interstate movement of salamanders in the United States. In the United States, planning and surveillance for early detection of Bsal incursions and emergency responses is underway (Grant et al. 2016). Ideally, however, all amphibian trade should be restricted. A good example is Australia , where exotic frogs are rarely allowed to be imported and are restricted to biosecured facilities such as zoos or research institutes.
Within a region, risk of anthropogenic spread of chytridiomycosis to naive populations, and between infected regions, may be mitigated through containment. This is a priority for isolated populations such as those on islands or in habitats where natural spread is unlikely to or could not occur (Berger and Skerratt 2012). In moist wilderness areas with abundant wildlife, attempts to stop natural spread appear unlikely to succeed. Control of anthropogenic spread involves restricting access to sites and the use of stringent hygiene protocols on equipment (Murray et al. 2013; Phillott et al. 2010). However, the efficacy of reducing the risk of spread by focusing on humans has not been assessed. Educating the community about basic disease management and the risks of transport of potentially infected amphibians and water is important.
Much research has focused on reducing impacts of Bd, which also has high relevance to Bsal where studies have recently commenced. Although small-scale eradication of Bd has been achieved in a specific type of isolated or ephemeral habitat (Bosch et al. 2015), this approach is not broadly applicable. Hence in areas where chytridiomycosis has established, the emphasis is on ensuring the persistence of amphibian populations and species (Skerratt et al. 2016). Extinction has been prevented via establishing amphibian ex situ captive assurance colonies, but methods to ensure self-sustaining wild populations are obviously the goal (McFadden et al. 2013; Scheele et al. 2014; Skerratt et al. 2016).
Currently, reintroductions have had low success, with the continued presence of the pathogen in the environment leading to eventual mortality of reintroduced individuals. Research and trials are currently underway on potential management strategies to improve survival rates in the wild; however these are largely in the experimental phase.
As infected frog populations can thrive in naturally suboptimal habitats for the fungus, which may include warmer, drier or more saline regions (Heard et al. 2015; Puschendorf et al. 2011; Stockwell et al. 2015a, b), eradication of disease is not necessary for successful mitigation. This also shows the importance of assessing suitability for Batrachochytrium species when choosing habitats to preserve for amphibians. This may lead to conservation of areas that may not have previously been considered prime habitat (Skerratt et al. 2016).
Another angle involves altering the environmental suitability for chytridiomycosis. Physical modifications of the environment might be used on a local scale for critically threatened amphibians in situ and may render the habitat less suitable for Bd. These include drying, drainage or alteration of waterflow, provision of shallow warm-water areas, reduction in canopy cover to increase temperature or the addition of basking sites or artificial heat (Scheele et al. 2014). A number of chemical treatments have been proposed or trialled in the field, including the application of salt (Stockwell et al. 2015a, b) and agricultural fungicides (Johnson et al. 2003; Woodhams et al. 2011). Reducing transmission might also be achieved by removal of reservoir hosts or by making habitat less favourable for reservoir species (Scheele et al. 2014; Skerratt et al. 2016).
Other ideas for fighting the pathogen directly include manipulating microbial competition via bioaugmentation of the host or environment with probiotic bacteria that express antifungal metabolites (Becker et al. 2009; Muletz et al. 2012; Vredenburg et al. 2011), augmenting the numbers of Bd predators such as zooplankton (Schmeller et al. 2014) and pathogens such as mycoviruses (Skerratt et al. 2016) and the identification or engineering and release of nonvirulent strains of Bd (Woodhams et al. 2011).
Management strategies aimed at improving host immunity have long-term potential. Manipulation of the host adaptive and innate immune response (via vaccination and assisted selection for disease resistance) is a proven strategy in humans and domestic animals, with potential to reduce the impact of chytridiomycosis in the field. Although adaptive immunity is not heritable and hence immunization may be perceived as a short-term approach, it could assist in providing a population size buffer for the natural evolution of innate immunity, although the artificial maintenance of susceptible genotypes may counter this beneficial effect (Harding et al. 2005). Unfortunately, reinfection trials in the few species examined to date have not demonstrated strong acquired immunity against Bd (Cashins et al. 2013; McMahon et al. 2014).
The innate immune system is generally considered responsible for the evolution of inter-generational immunity, and disease resistance or tolerance may be upregulated within a population via assisted selection for less susceptible individuals (Venesky et al. 2012). Comparative techniques (e.g. marker-assisted selection and estimated breeding values) have been widely and successfully used for breeding of disease resistance in plant and domestic animal agriculture (Heringstad et al. 2007; Miedaner and Korzun 2012). This may present a sustainable approach for repatriating the numerous amphibian species that are now extinct in the wild and only persist in ex situ captive programs. Two main approaches that might be feasible in practice for promoting disease resistance include (1) direct selection via exposure of post-metamorphic individuals to Bd, then breeding from those with lower susceptibility, and (2) identifying molecular markers of resistance to advance selection to earlier life stages, removing the need to regularly expose individuals to infection. Some progress has been made towards this latter goal via studies of the major histocompatibility complex (MHC; Bataille et al. 2015; Savage and Zamudio 2011). The increasing longevity of some recovering wild populations suggests evolution of resistance may occur naturally (Newell et al. 2013), although in other species individual annual survival rates remain very low despite a long history of infection (i.e. 15–20 years) (Phillott et al. 2013; Brannelly et al. 2015).
Scheele et al. (2014) present immediately applicable suggestions for bolstering overall population size and recruitment to counteract disease-associated mortality. This strategy is based on the observation of species with high mortality rates persisting via high recruitment (Phillott et al. 2013, Scheele et al. 2015). Interventions may involve removal of other threatening processes from small and declining populations such as improving habitat or excluding competitors and introduced pests (Scheele et al. 2014). Increase of population size can be achieved through reintroductions and minimizing the effect of early predation by head-starting larval stages through metamorphosis in captivity (Hunter et al. 1999; Scheele et al. 2014). An alternative to establishing long-term captive assurance colonies is to rear wild-caught eggs or tadpoles in captivity to ensure higher survival rates before release. Direct translocation to ‘disease refugia’ sites could be an efficient way to create sustainable populations (Puschendorf et al. 2011; Skerratt et al. 2016).
As disease ecology varies greatly between species and habitats, management will be context-specific; hence research aimed at understanding each situation is needed to devise effective local strategies. A proactive approach is crucial, as many of the most endangered species occur in already protected areas and will not survive without intervention (Skerratt et al. 2016).
References
Abramyan J, Stajich JE (2012) Species-specific chitin binding module 18 expansion in the amphibian pathogen Batrachochytrium dendrobatidis. MBio 3:e00150–e00112
Annis SL, Dastoor FP, Ziel H et al (2004) A DNA-based assay identifies Batrachochytrium dendrobatidis in amphibians. J Wildl Dis 40:420–428
Bataille A, Cashins SD, Grogan L et al (2015) Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proc Biol Sci 22:282
Beard KH, O’Neill EM (2005) Infection of an invasive frog Eleutherodactylus coqui by the chytrid fungus Batrachochytrium dendrobatidis in Hawaii. Biol Conserv 126:591–595
Becker MH, Brucker RM, Schwantes CR et al (2009) The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl Environ Microbiol 75:6635–6638
Berger L, Skerratt L (2012) Disease strategy chytridiomycosis (infection with Batrachochytrium dendrobatidis) Version 1, 2012. Department of Sustainability, Environment, Water, Populations and Communities, Public Affairs, Commonwealth of Australia, Canberra. Available at: http://www.environment.gov.au/system/files/resources/387d3e66-3cdc-4676-8fed-759328277da4/files/chytrid-fungus-manual.pdf
Berger L, Speare R, Daszak P et al (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A 95:9031–9036
Berger L, Speare R, Kent A (2000) Diagnosis of chytridiomycosis in amphibians by histological examination. Zoos Print J 15:184–190
Berger L, Hyatt AD, Olsen V et al (2002) Production of polyclonal antibodies to Batrachochytrium dendrobatidis and their use in an immunoperoxidase test for chytridiomycosis in amphibians. Dis Aquat Org 48:213–220
Berger L, Speare R, Hines HB et al (2004) Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust Vet J 82:434–439
Berger L, Hyatt AD, Speare R et al (2005) Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Org 68:51–63
Berger L, Longcore J, Speare R, Hyatt A, Skerratt LF (2009) Fungal diseases in amphibians. In: Heatwole H, Wilkinson JW (eds) Amphibian biology, volume 8 amphibian decline: disease, parasites, maladies, and pollution. Surrey Beatty and Sons, Baulkham Hills, NSW, pp 2986–3052
Blaustein AR, Romansic JM, Scheessele EA et al (2005) Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv Biol 19:1460–1146
Blooi M, Pasmans F, Longcore JE et al (2013) Duplex real-time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J Clin Microbiol 51:4173–4177
Blooi M, Martel A, Haesebrouck F et al (2015a) Treatment of urodelans based on temperature dependent infection dynamics of Batrachochytrium salamandrivorans. Sci Rep 5:8037
Blooi M, Pasmans F, Rouffaer L et al (2015b) Succesful treatment of Batrachochytrium salamandrivorans infections in salamanders requires synergy between voriconazole, polymyxin E and temperature. Sci Rep 5:11788
Blooi M, Pasmans F, Longcore JE et al (2016) Duplex real-time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J Clin Microbiol 54:246–246
Bosch J, Martínez-Solano I, García-París M (2001) Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol Conserv 97:331–337
Bosch J, Sanchez-Tome E, Fernandez-Loras A et al (2015) Successful elimination of a lethal wildlife infectious disease in nature. Biol Lett 11(11):20150874
Boyle DG, Boyle DB, Olsen V et al (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org 60:141–148
Brannelly LA, Richards-Zawacki CL et al (2012) Clinical trials with itraconazole as a treatment for chytrid fungal infections in amphibians. Dis Aquat Org 101:95–104
Brannelly LA, Hunter DA, Skerratt LF et al (2015) Chytrid infection and post-release fitness in the reintroduction of an endangered alpine tree frog. Anim Conserv 19(2):153–162. https://doi.org/10.1111/acv.12230
Brucker RM, Harris RN, Schwantes CR et al (2008) Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J Chem Ecol 34:1422–1429
Brutyn M, D’Herde K, Dhaenens M et al (2012) Batrachochytrium dendrobatidis zoospore secretions rapidly disturb intercellular junctions in frog skin. Fungal Genet Biol 49:830–837
Campbell CR, Voyles J, Cook DI et al (2012) Frog skin epithelium: electrolyte transport and chytridiomycosis. Int J Biochem Cell Biol 44:431–434
Cashins SD, Grogan LF, McFadden M et al (2013) Prior infection does not improve survival against the amphibian disease Chytridiomycosis. PLoS One 8:e56747
Chatfield MWH, Richards-Zawacki CL (2011) Elevated temperature as a treatment for Batrachochytrium dendrobatidis infection in captive frogs. Dis Aquat Org 94:235–238
Cheng TL, Rovito SM, Wake DB et al (2011) Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci U S A 108:9502–9507
Cunningham AA, Beckmann K, Perkins M et al (2015) Surveillance emerging disease in UK amphibians. Vet Rec 176:468–468
Daskin JH, Alford RA, Puschendorf R (2011) Short-term exposure to warm microhabitats could explain amphibian persistence with Batrachochytrium dendrobatidis. PLoS One 6:e26215
Davis AK, Keel MK, Ferreira A et al (2010) Effects of chytridiomycosis on circulating white blood cell distributions of bullfrog larvae (Rana catesbeiana). Comp Clin Pathol 19:49–55
Doddington BJ, Bosch J, Oliver JA et al (2013) Context dependent amphibian host population response to an invading pathogen. Ecology 94:1795–1804
Duellman WE, Trueb L (1994) Biology of amphibians. The Johns Hopkins University Press, Baltimore
Duffus ALJ (2009) Chytrid blinders: what other disease risks to amphibians are we missing? EcoHealth 6:335–339
Ellison AR, Savage AE, DiRenzo GV et al (2014) Fighting a losing battle: vigorous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki. G3 (Bethesda) 4:1275–1289
Ellison AR et al (2015) More than skin deep: functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biol Evol 7:286–298. https://doi.org/10.1093/gbe/evu285
Farrer RA, Weinert LA, Bielby J et al (2011) Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc Natl Acad Sci U S A 108:18732–18736
Farrer RA, Henk DA, Garner TWJ et al (2013) Chromosomal copy number variation, selection and uneven rates of recombination reveal cryptic genome diversity linked to pathogenicity. PLoS Genet 9(8):e1003703
Fellers GM, Green DE, Longcore JE (2001) Oral chytridiomycosis in the mountain yellow-legged frog (Rana muscosa). Copeia 2001:945–953
Fisher MC, Garner TWJ, Walker SF (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol 63:291–310
Fites JS, Ramsey JP, Holden WM et al (2013) The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342:366–369
Fites JS, Reinert LK, Chappell TM et al (2014) Inhibition of local immune responses by the frog-killing fungus Batrachochytrium dendrobatidis. Infect Immun 82:4698–4706
Fong JJ, Cheng TL, Bataille A et al (2015) Early 1900s detection of Batrachochytrium dendrobatidis in Korean amphibians. PLoS One 10(3):e0115656
Forzan MJ, Gunn H, Scott P (2008) Chytridiomycosis in an aquarium collection of frogs: diagnosis, treatment, and control. J Zoo Wildl Med 39:406–411
Garland S, Wood J, Skerratt LF (2011) Comparison of sensitivity between real-time detection of a TaqMan assay for Batrachochytrium dendrobatidis and conventional detection. Dis Aquat Organ 94:101–105
Garner TW, Perkins MW, Govindarajulu P et al (2006) The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the north American bullfrog, Rana catesbeiana. Biol Lett 2:455–459
Garner TW, Garcia G, Carroll B et al (2009) Using itraconazole to clear Batrachochytrium dendrobatidis infection, and subsequent depigmentation of Alytes muletensis tadpoles. Dis Aquat Organ 83:257–260
Geiger CC, Kupfer E, Schar S et al (2011) Elevated temperature clears chytrid fungus infections from tadpoles of the midwife toad, Alytes obstetricans. Amphibia-Reptilia 32:276–280
Georoff TA, Moore RP, Rodriguez C et al (2013) Efficacy of treatment and long-term follow-up of Batrachochytrium dendrobatidis PCR-positive anurans following itraconazole bath treatment. J Zoo Wildl Med 44:395–403
Goka K, Yokoyama J, Une Y et al (2009) Amphibian chytridiomycosis in Japan: distribution, haplotypes and possible route of entry into Japan. Mol Ecol 18(23):4757–4774
Grant EHG, Muths E, Katz RA, et al (2016) Salamander chytrid fungus (Batrachochytrium salamandrivorans) in the United States—Developing research, monitoring, and management strategies. USGS Report https://doi.org/10.3133/ofr20151233
Greenspan SE, Longcore JE, Calhoun AJ (2012) Host invasion by Batrachochytrium dendrobatidis: fungal and epidermal ultrastructure in model anurans. Dis Aquat Org 100:201–210
Grogan LF (2014) Understanding host and environmental factors in the immunology and epidemiology of chytridiomycosis in anuran populations in Australia. PhD thesis, James Cook University
Grogan LF, Berger L, Rose K et al (2014) Surveillance for emerging biodiversity diseases of wildlife. PLoS Pathog 10:1–4
Harding KC, Hansen BJL, Goodman SJ (2005) Acquired immunity and stochasticity in epidemic intervals impede the evolution of host disease resistance. Am Nat 166:722–730
Harris RN, Lauer A, Simon MA et al (2009a) Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Dis Aquat Org 83:11–16
Harris RN, Brucker RM, Walke JB et al (2009b) Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J 3:818–824
Heard GW, Thomas CD, Hodgson JA et al (2015) Refugia and connectivity sustain amphibian metapopulations afflicted by disease. Ecol Lett 18:853–863
Heringstad B, Klemetsdal G, Steine T (2007) Selection responses for disease resistance in two selection experiments with Norwegian red cows. J Dairy Sci 90:2419–2426
Hudson PJ, Dobson AP (1998) In: Grenfell BT, Dobson AP (eds) Ecology of infectious diseases in natural populations. Cambridge University Press, Cambridge
Hunter D, Osborne W, Marantelli G et al (1999) Implementation of a population augmentation project for remnant populations of the southern corroboree frog (Pseudophryne corroboree). In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra
Hyatt AD, Boyle DG, Olsen V et al (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Org 73:175–192
James TY, Kauf F, Schoch CL et al (2006) Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature 443:818–822
Johnson ML, Berger L, Philips L et al (2003) Fungicidal effects of chemical disinfectans, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Org 57:255–260
Johnson PTJ, Hoverman JT (2014) Heterogeneous hosts: how variation in host size, behaviour and immunity affects parasite aggregation. J Anim Ecol 83:1103–1112
Jones ME, Paddock D, Bender L et al (2012) Treatment of chytridiomycosis with reduced-dose itraconazole. Dis Aquat Org 99:243–249
Joneson S, Stajich JE, Shiu SH et al (2011) Genomic transition to pathogenicity in Chytrid fungi. PLoS Pathog 7:e1002338
Kindermann C, Narayan EJ, Hero JM (2012) Urinary corticosterone metabolites and chytridiomycosis disease prevalence in a free-living population of male Stony Creek frogs (Litoria wilcoxii). Comp Biochem Physiol A 162:171–176
Kolby JE, Smith KM, Berger L et al (2014) First evidence of amphibian chytrid fungus (Batrachochytrium dendrobatidis) and ranavirus in Hong Kong amphibian trade. PLoS One 9:e90750
Kriger KM, Hines HB, Hyatt AD et al (2006) Techniques for detecting chytridiomycosis in wild frogs: comparing histology with real-time Taqman PCR. Dis Aquat Org 71:141–148
La Marca E, Lips KR, Lötters S et al (2005) Catastrophic population declines and extinctions in Neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 37:190–201
Lam BA, Walke JB, Vredenburg VT et al (2010) Proportion of individuals with anti-Batrachochytrium dendrobatidis skin bacteria is associated with population persistence in the frog Rana muscosa. Biol Conserv 143:529–531
Lam BA, Walton DB, Harris RN (2011) Motile zoospores of Batrachochytrium dendrobatidis move away from antifungal metabolites produced by amphibian skin bacteria. EcoHealth 8:36–45
Lauer A et al (2007) Common cutaneous bacteria from the eastern red-backed salamander can inhibit pathogenic fungi. Copeia 3:630–640
Lips KR, Brem F, Brenes R et al (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci U S A 103:3165–3170
Lips KR, Diffendorfer J, Mendelson JR et al (2008) Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol 6:e72
Liu P, Stajich JE (2015) Characterization of the carbohydrate binding module 18 gene family in the amphibian pathogen Batrachochytrium dendrobatidis. Fungal Genet Biol 77:31–39
Longcore J, Pessier A, Nichols D (1999) Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mycologia 91:219–227
Loudon AH, Holland JA, Umile TP et al (2014) Interactions between amphibians’ symbiotic bacteria cause the production of emergent anti-fungal metabolites. Front Microbiol 5:441
Marantelli G, Berger L, Speare R et al (2004) Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pac Conserv Biol 10:173–179
Martel A, Van Rooij P, Vercauteren G et al (2011) Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians. Med Mycol 49:143–149
Martel A, Spitzen-van der Sluijs A, Blooi M et al (2013) Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci U S A 110:15325–15329
Martel A, Blooi M, Adriaensen C et al (2014) Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346:630–631
McFadden M, Hobbs R, Marantelli G et al (2013) Captive management and breeding of the critically endangered southern corroboree frog (Pseudophryne corroboree) (Moore 1953) at Taronga and Melbourne zoos. Amphib Reptile Conserv 5:70–87
McMahon TA, Rohr JR (2015) Transition of chytrid dungus infection from mouthparts to hind limbs during amphibian metamorphosis. EcoHealth 12:88–193
McMahon TA, Brannelly LA, Chatfield MWH et al (2013) Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in absence of infection. Proc Natl Acad Sci U S A 110:210–215
McMahon TA, Sears BF, Venesky MD et al (2014) Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511:224–227
Meng P, Yang S, Shen C et al (2013) The first salamander defensing antimicrobial peptide. PLoS One 8:e83044
Meyer W, Seegers U, Schnapper A et al (2007) Possible antimicrobial defense by free sugars on the epidermal surface of aquatic vertebrates. Aquat Biol 1:167–175
Miedaner T, Korzun V (2012) Marker-assisted selection for disease resistance in wheat and barley breeding. Phytopathology 102:560–566
Moss AS, Reddy NS, Dortaj IM et al (2008) Chemotaxis of the amphibian pathogen Batrachochytrium dendrobatidis and its response to a variety of attractants. Mycologia 100:1–5
Muletz CR, Myers JM, Domangue RJ et al (2012) Soil bioaugmentation with amphibian cutaneous bacteria protects amphibian hosts from infection by Batrachochytrium dendrobatidis. Biol Conserv 152:119–126
Murphy PJ, St-Hilaire S, Corn PS (2011) Temperature, hydric environment, and prior pathogen exposure alter the experimental severity of chytridiomycosis in boreal toads. Dis Aquat Org 95:31–42
Murray KA, Skerratt LF, Garland S et al (2013) Whether the weather drives patterns of endemic amphibian chytridiomycosis: a pathogen proliferation approach. PLoS One 8(4):e61061
Myers JM, Ramsey JP, Blackman AL et al (2012) Synergistic inhibition of the lethal fungal pathogen Batrachochytrium dendrobatidis: the combined effect of symbiotic bacterial metabolites and antimicrobial peptides of the frog Rana muscosa. J Chem Ecol 38:958–965
Newell DA, Goldingay RL, Brooks LO (2013) Population recovery following decline in an endangered stream-breeding frog (Mixophyes fleayi) from subtropical Australia. PLoS One 8:e58559
Nichols DK, Lamirande EW, Pessier AP et al (2000) Experimental transmission and treatment of cutaneous chytridiomycosis in poison dart frogs (Dendrobates auratus and Dendrobates tinctorius). In: Proceedings of the Joint Conference of American Association of Zoo Veterinarians and International Association for Aquatic Animal Medicine, pp 42–44
Nichols DK, Lamirande EW, Pessier AP et al (2001) Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. J Wildl Dis 37:1–11
Olson DH, Aanensen DM, Ronnenberg KL et al (2013) Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS One 8:e56802
Parris MJ, Beaudoin JG (2004) Chytridiomycosis impacts predator-prey interactions in larval amphibian communities. Oecologia 140:626–632
Pask JD, Cary TL, Rollins-Smith LA (2013) Skin peptides protect juvenile leopard frogs (Rana pipiens) against chytridiomycosis. J Exp Biol 216:2908–2916
Pasmans F, Van Rooij P, Blooi M et al (2013) Resistance to chytridiomycosis in European plethodontid salamanders of the genus Speleomantes. PLoS One 8:e63639
Pessier AP, Mendelson JR (2010) A manual for control of infectious diseases in amphibian survival assurance colonies and reintroduction programs. IUCN/SSC Conservation Breeding Specialist Group
Pessier AP, Nichols DK, Longcore JE et al (1999) Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). J Vet Diagn Investig 11:194–199
Peterson JD, Steffen JE, Reinert LK et al (2013) Host stress response is important for the pathogenesis of the deadly amphibian disease, chytridiomycosis, in Litoria caerulea. PLoS One 8:e62146
Phillott AD, Speare R, Hines HB et al (2010) Minimising exposure of amphibians to pathogens during field studies. Dis Aquat Org 92:175–185
Phillott AD, Grogan LF, Cashins SD et al (2013) Chytridiomycosis and seasonal mortality of tropical stream-associated frogs 15 years after introduction of Batrachochytrium dendrobatidis. Conserv Biol 27:1058–1068
Pilliod DS, Muths E, Scherer RD et al (2010) Effects of amphibian chytrid fungus on individual survival probability in wild boreal toads. Conserv Biol 24:1259–1267
Plehn M (1920) Neue Parasiten in Haut and Kiemen von Fischen. Ichthyochytrium und Mucophilus Zentralblatt für Bakteriologie und Parasitenkunde Abteilung, vol 1, pp 275–281
Puschendorf R, Hoskin CJ, Cashins SD et al (2011) Environmental refuge from disease-driven amphibian extinction. Conserv Biol 25:956–964
Rachowicz LJ, Vredenburg VT (2004) Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Dis Aquat Org 61:75–83
Ramsey JP, Reinert LK, Harper LK et al (2010) Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the south African clawed frog, Xenopus laevis. Infect Immun 78:3981–3992
Reeder NMM, Pessier AP, Vredenburg VT (2012) A reservoir species for the emerging amphibian pathogen Batrachochytrium dendrobatidis thrives in a landscape decimated by disease. PLoS One 7:e33567
Retallick RWR, McCallum H, Speare R (2004) Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biol 2:1965–1971
Ribas L, Li MS, Doddington BJ, Robert J et al (2009) Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis. PLoS One 4:e8408
Ricardo H (2006) Distribution and ecology of chytrid in Tasmania. Honours thesis, University of Tasmania
Richards-Zawacki CL (2010) Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc R Soc Lond B Biol Sci 277:519–528
Rivas LR (1964) A reinterpretation of the concepts “sympatric” and “allopatric” with proposal of the additional terms “syntopic” and “allotopic”. Syst Zool 13:42–43
Rodriguez D, Becker CG, Pupin NC et al (2014) Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic Forest of Brazil. Mol Ecol 23:774–787
Rollins-Smith LA (2009) The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim Biophys Acta 1788:1593–1599
Rollins-Smith LA, Woodhams DC, Reinert LK et al (2006) Antimicrobial peptide defenses of the mountain yellow-legged frog (Rana muscosa). Dev Comp Immunol 30:831–842
Rollins-Smith LA, Ramsey JP, Pask JD et al (2011) Amphibian immune defenses against chytridiomycosis: impacts of changing environments. Integr Comp Biol 51:552–562
Rosenblum EB, Stajich JE, Maddox N et al (2008) Global gene expression profiles for life stages of the deadly amphibian pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci U S A 105:17034–17039
Rosenblum EB, Poorten TJ, Settles M et al (2009) Genome-wide transcriptional response of Silurana (Xenopus) tropicalis to infection with the deadly chytrid fungus. PLoS One 4:e6494
Rosenblum EB, Poorten TJ, Settles M et al (2012) Only skin deep: shared genetic response to the deadly chytrid fungus in susceptible frog species. Mol Ecol 21:3110–3120
Rosenblum EB, James TY, Zamudio KR et al (2013) Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc Natl Acad Sci U S A 110:9385–9390
Rovito SM, Parra-Olea G, Vasquez-Almazan CR et al (2009) Dramatic declines in neotropical salamander populations are an important part of the global amphibian crisis. Proc Natl Acad Sci U S A 106:3231–3236
Rowley JJL, Alford RA (2007) Behaviour of Australian rain forest stream frogs may affect the transmission of chytridiomycosis. Dis Aquat Org 77:1–9
Rowley JJL, Skerratt LF, Alford RA et al (2007) Retreat sites of rain forest stream frogs are not a reservoir for Batrachochytrium dendrobatidis in northern Queensland, Australia. Dis Aquat Organ 74:7–12
Sabino-Pinto JS, Bletz M, Hendrix R et al (2015) First detection of the emerging fungal pathogen in Batrachochytrium salamandrivorans in Germany. Amphibia-Reptilia 36(4):411–416. https://doi.org/10.1163/15685381-00003008
Savage AE, Zamudio KR (2011) MHC genotypes associate with resistance to a frog-killing fungus. Proc Natl Acad Sci U S A 108:16705–16710
Scheele BC, Guarino F, Osbourne W et al (2014) Decline and re-expansion of an amphibian with high prevalence of chytrid fungus. Biol Conserv 170:86–91
Scheele BC, Hunter DA, Skerratt LF et al (2015) Low impact of chytridiomycosis on frog recruitment enables persistence in refuges despite high adult mortality. Biol Conserv 182:36–43
Schloegel LM, Ferreira CM, James TY et al (2010) The north American bullfrog as a reservoir for the spread of Batrachochytrium dendrobatidis in Brazil. Anim Conserv 13:53–61
Schloegel LM, Toledo LF, Longcore JE et al (2012) Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol Ecol 21:5162–5177
Schmeller DS, Blooi M, Martel A et al (2014) Microscopic aquatic predatorsstrongly affect infection dynamics of a globally emerged pathogen. Curr Biol 24:176–180
Searle CL, Gervasi SS, Hua J et al (2011) Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conserv Biol 25:965–974
Sheafor B, Davidson EW, Parr L et al (2008) Antimicrobial peptide defenses in the salamander Ambystoma tigrinum, against emerging amphibian pathogens. J Wildl Dis 44:226–236
Skerratt L, Speare R, Berger L (2011a) Mitigating the impact of diseases affecting biodiversity—retrospective on the outbreak investigation for chytridiomycosis. Ecohealth 7:S26
Skerratt LF, Berger L, Hines HB et al (2008) Survey protocol for detecting chytridiomycosis in all Australian frog populations. Dis Aquat Org 80:85–94
Skerratt LF, Mendez D, McDonald KR et al (2011b) Validation of diagnostic tests in wildlife: the case of chytridiomycosis in wild amphibians. J Herpetol 45:444–450
Skerratt LF, Berger L, Clemann N et al (2016) Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildlife Res 43(2):105–120
Smith KG, Weldon C, Conradie W et al (2007) Relationships among size, development, and Batrachochytrium dendrobatidis infection in African tadpoles. Dis Aquat Organ 74:159–164
Soto-Azat C, Clarke BT, Poynton JC (2010) Widespread historical presence of Batrachochytrium dendrobatidis in African pipid frogs. Divers Distrib 16:126–131
Stice MJ, Briggs CJ (2010) Immunization is ineffective at preventing infection and mortality due to the amphibian chytrid fungus Batrachochytrium dendrobatidis. J Wildl Dis 46:70–77
Stockwell MP, Clulow J, Mahony MJ (2015a) Evidence of a salt refuge: chytrid infection loads are suppressed in hosts exposed to salt. Oecologia 177:901–910
Stockwell MP, Storrie LJ, Pollard CJ et al (2015b) Effects of pond salinization on survival rate of amphibian hosts infected with the chytrid fungus. Conserv Biol 29:391–399
Swei A, Rowley JJL, Rodder D et al (2011) Is chytridiomycosis an emerging infectious disease in Asia? PLoS One 6(8):e23179
Talley BL, Muletz CR, Vredenburg VT et al (2015) A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biol Conserv 182:254–261
Tamukai K, Une Y, Tominaga A et al (2011) Treatment of spontaneous chytridiomycosis in captive amphibians using itraconazole. J Vet Med Sci 73:155–159
Tennessen JA, Woodhams DC, Chaurand P et al (2009) Variations in the expresses antimicrobial peptide repertoire of northern leopard frog (Rana pipiens) populations suggest intraspecies differences in resistance to pathogens. Dev Comp Immunol 33:1247–1257
Thekkiniath JC, Zabet-Moghaddam M, San Francisco SK et al (2013) A novel subtilisin-like serine protease of Batrachochytrium dendrobatidis is induced by thyroid hormone and degrades antimicrobial peptides. Fungal Biol 117:451–461
Tobler U, Schmidt BR (2010) Within- and among-population variation in chytridiomycosis-induced mortality in the toad Alytes obstetricans. PLoS One 5:e10927
Une Y, Kadekaru S, Tamukai K et al (2008) First report of spontaneous chytridiomycosis in frogs in Asia. Dis Aquat Organ 82:157–160
Van Ells T, Stanton J, Strieby A et al (2003) Use of immunohistochemistry to diagnose chytridiomycosis in dyeing poison dart frogs (Dendrobates tinctorius). J Wildl Dis 39:742–745
Van Rooij P, Martel A, D’Herde K et al (2012) Germ tube mediated invasion of Batrachochytrium dendrobatidis in amphibian skin is host dependent. PLoS One 7:1–8
Van Rooij P, Martel A, Haesebrouck F et al (2015) Amphibian chytridiomycosis: a review with focus on fungus-host interactions. Vet Res 46:137
Venesky MD, Mendelson JR, Sears BF et al (2012) Selecting for tolerance against pathogens and herbivores to enhance success of reintroduction and translocation. Conserv Biol 26:586–592
Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF, Speare R (2009) Pathogenesis of Chytridiomycosis, a Cause of Catastrophic Amphibian Declines. Science 326: 582–585.
Vredenburg VT, Knapp RA, Tunstall TS et al (2010) Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Nat Acad Sci U S A 107:9689–9694
Vredenburg VT, Briggs CJ, Harris RN (2011) Host-pathogen dynamics of amphibian chytridiomycosis: the role of the skin microbiome in health and disease. In: Fungal diseases: an emerging threat to human, animal and plant health: workshop summary. National Academy Press, Washington
Walker S, Bosch J, James TY et al (2008) Invasive pathogens threaten species recovery programs. Curr Biol 18:853–R854
Walker SF, Bosch J, Gomez V et al (2010) Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecol Lett 13(3):372–382
Weldon C, du Preez LH, Hyatt AD et al (2004) Origin of the amphibian chytrid fungus. Emerg Infect Dis 10:2100–2105
Woodhams DC, Alford RA, Marantelli G (2003) Emerging disease of amphibians cured by elevated body temperature. Dis Aquat Org 55:65–67
Woodhams DC, Ardipradja K, Alford RA et al (2007) Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim Conserv 10:409–417
Woodhams DC, Bosch J, Briggs CJ et al (2011) Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front Zool 8(1):8
Woodhams DC, Bell SC, Kenyon N et al (2012a) Immune evasion or avoidance: fungal skin infection linked to reduced defence peptides in Australian green-eyed treefrogs, Litoria serrata. Fungal Biol 116:1203–1211
Woodhams DC, Geiger CC, Reinert LK et al (2012b) Treatment of amphibians infected with chytrid fungus: learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Dis Aquat Organ 98:11–25
Woodward A, Berger L, Skerratt LF (2014) In vitro sensitivity of the amphibian pathogen Batrachochytrium dendrobatidis to antifungal therapeutics. Res Vet Sci 97:364–366
Young S, Whitehorn P, Berger L et al (2014) Defects in host immune function in tree frogs with chronic chytridiomycosis. PLoS One 9:e107284
Acknowledgements
LB was supported by the Australian Research Council (grant FT100100375).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Martel, A., Pasmans, F., Fisher, M.C., Grogan, L.F., Skerratt, L.F., Berger, L. (2018). Chytridiomycosis. In: Seyedmousavi, S., de Hoog, G., Guillot, J., Verweij, P. (eds) Emerging and Epizootic Fungal Infections in Animals. Springer, Cham. https://doi.org/10.1007/978-3-319-72093-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-72093-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72091-3
Online ISBN: 978-3-319-72093-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)