Abstract
With the incidence of emerging infectious diseases on the rise, it is becoming increasingly important to identify refuge areas that protect hosts from pathogens and therefore prevent population declines. For the chytrid fungus Batrachochytrium dendrobatidis, temperature and humidity refuge areas for amphibian hosts exist but are difficult to manipulate. Other environmental features that may affect the outcome of infection include water quality, drying regimes, abundance of alternate hosts and isolation from other hosts. We identified relationships between water bodies with these features and infection levels in the free-living hosts inhabiting them. Where significant relationships were identified, we used a series of controlled experiments to test for causation. Infection loads were negatively correlated with the salt concentration of the aquatic habitat and the degree of water level fluctuation and positively correlated with fish abundance. However, only the relationship with salt was confirmed experimentally. Free-living hosts inhabiting water bodies with mean salinities of up to 3.5 ppt had lower infection loads than those exposed to less salt. The experiment confirmed that exposure to sodium chloride concentrations >2 ppt significantly reduced host infection loads compared to no exposure (0 ppt). These results suggest that the exposure of amphibians to salt concentrations found naturally in lentic habitats may be responsible for the persistence of some susceptible species in the presence of B. dendrobatidis. By manipulating the salinity of water bodies, it may be possible to create refuges for declining amphibians, thus allowing them to be reintroduced to their former ranges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of emerging diseases in wildlife has increased over the past few decades, due largely to the anthropogenically assisted spread of pathogens (Dobson and Foufopoulos 2001). Novel diseases have the potential to rapidly alter host population size, community dynamics and ecosystem structure, and are one of the most difficult threatening processes to manage (Dobson and Foufopoulos 2001). As a result, models that compare the fundamental niche of pathogens with the availability of suitable environments are commonly used to predict areas at risk of disease spread and emergence (e.g. Costa and Peterson 2012; Peterson 2006; Pinkard et al. 2010). However, more recently these models have also been used to identify refuge areas that protect the host from the effects of the pathogen (Levinton et al. 2011; Puschendorf et al. 2011; Springer et al. 2007).
Refuge areas where pathogen pressure on the host is reduced exist because features of the environment lower pathogen virulence (transmission rates, fitness and survivorship) and/or host susceptibility (recognition and response to infection). For example, estuarine areas with oligohaline salinities act as a refuge for the eastern oyster (Crassostrea virginica) from the parasites MSX (Haplosporidium nelson) and Dermo (Perkinsus marinus), which only thrive in higher salinity waters (Levinton et al. 2011). Areas with elevations >1,200 m above sea level act as refuges for Hawaiian forest birds from avian malaria (Plasmodium relictum) because the cooler temperatures exclude the mosquito vector (Culex quinquefasciatus) (Atkinson et al. 2000). Soils with high calcium contents also act as a refuge area for California dwarf flax (Hesperolinan californicum) against the pathogenic rust fungus (Melampsora lini) because calcium assists in the host’s defensive response to pathogen invasion (Springer et al. 2007).
For some host populations that are declining due to a novel disease, refuge areas may be the only protection against population extirpation and/or species extinction. In such situations, identifying the existence of refugia needs to be a priority so they can be protected and monitored (Puschendorf et al. 2011). Identification of the underlying agents that reduce pathogen virulence or host susceptibility may also allow refuge areas to be created, extending the species distribution back into their former range, in the presence of the pathogen. Given that the eradication of a disease is unlikely, control measures for wildlife diseases will be required to continue in perpetuity (Wobeser 2002). Therefore, the most suitable control agent must be widely applicable, active over time and must have minimal impact on non-target organisms. The protection and creation of refuge areas may be one way of achieving this.
Chytridiomycosis, caused by the chytrid fungus (Batrachochytrium dendrobatidis), is an emerging infectious disease of amphibians that has been implicated in the decline or extinction of over 200 species and the alteration of amphibian community dynamics worldwide (Bosch and Rincon 2008; Parris and Beaudoin 2004; Parris and Cornelius 2004; Skerratt et al. 2007). Physiologically, B. dendrobatidis is intolerant to desiccation (Johnson et al. 2003) and temperatures above 28 °C (Piotrowski et al. 2004; Stevenson et al. 2013), while prolonged periods of low temperature can also reduce amphibian immunocompetence and increase susceptibility to disease (Carey et al. 1999). As a result, amphibian species from upland areas (McDonald and Alford 1999) or rainforests (Puschendorf et al. 2009, 2011) that declined due to B. dendrobatidis have been found to persist with infection in adjacent lowland or dry forests, respectively, and distribution models predict that areas with high temperatures and low rainfall act as refuges for susceptible species (Muths et al. 2008; Rodder et al. 2008; Ron 2005).
Refuge sites that protect susceptible amphibians from the effects of B. dendrobatidis could therefore be created by altering the microenvironmental temperature and humidity. This could be achieved by reducing canopy cover over an aquatic habitat (Becker et al. 2012; Becker and Zamudio 2011; Heard et al. 2014; Raffel et al. 2010) but could also interfere with growth rates and breeding behaviours that depend upon such cues. The identification of alternate refuge sites would therefore be beneficial. Epidemiological models and experiments show that hosts can survive infection and populations can persist with B. dendrobatidis if the rate of infection establishment and increase in infection load are suppressed (Briggs et al. 2010; Carey et al. 2006). Other environmental features that may constrain B. dendrobatidis growth, survival or transmission include water quality, drying regimes, abundance of alternate hosts and isolation from other host populations.
The objective of this study was to identify aspects of the environment that reduce the infection prevalence and load of B. dendrobatidis in susceptible hosts. We achieved this by swabbing 300 dwarf tree frogs (Litoria fallax) across ten sites in the Lower Hunter Region of NSW Australia and identifying relationships with environmental variables collected over a period of up to 12 months. Where significant relationships were identified, we then tested for causation through a series of controlled experiments that exposed hosts to B. dendrobatidis in the presence of potential environmental inhibitors.
Materials and methods
Field methods
Study site
This study was conducted in the Lower Hunter Region of New South Wales, Australia, 140 km north of Sydney. This area has an elevation of <50 m above sea level and experiences a temperate climate with mean maximum temperatures of 25.6 °C in January, mean minimum temperatures of 8.4 °C in July and a mean annual rainfall of 1,100 mm (Australian Govt. Bureau of Meteorology 2007). Parts of the Lower Hunter have been suggested to act as an environmental refuge from B. dendrobatidis for the endangered green and golden bell frog (Litoria aurea). This species underwent a directional range contraction throughout the 1970s, disappearing from the inland portions of its range and persisting within close proximity to the coastline (Mahony 1999). Many of the remaining populations occur in sites that are highly urbanised and/or industrial, possibly because they have water solutes that inhibit B. dendrobatidis (Lane and Burgin 2008; Threlfall et al. 2008). Within the Lower Hunter Region, surveys over the past decade have identified five water bodies that were consistently occupied by L. aurea and 16 that were never occupied (unpublished data). A total of ten of these water bodies were included in this study, five with consistent occupancy and a random selection of five that were never occupied.
Study species
The eastern dwarf tree frog (Litoria fallax) is a common, primarily lentic species that co-occurs with L. aurea throughout much of its range and occupied all ten study sites selected. Litoria fallax is susceptible to infection by B. dendrobatidis (Speare and Berger 2005), but infection does not appear to result in population decline (Stockwell et al. 2008). This species was therefore selected to quantify and compare infection levels across the study sites. Although susceptibility to infection and disease may vary between L. aurea and L. fallax, it was anticipated that the impact of any environmental inhibitors allowing bell frogs to persist within this region would also be evident in the infection levels in L. fallax. This study design could be confounded if the exposure of L. fallax to environmental inhibitors affected host susceptibility or response to infection (independent of their effect on the fungus) in ways that did not occur in L. aurea. However, there is no evidence to indicate this, and the sympatry of these species suggests similar environmental tolerances.
Frog surveys and chytrid fungus sampling
Litoria fallax were captured during nocturnal visual encounter surveys conducted within a 4-week period throughout spring. Individuals were detected by searching the pond edge and emergent vegetation with a spotlight and captured by hand, where the hand was covered in a disposable plastic bag. Once captured, the plastic bag was inverted to contain the animal. The ventral surface of each animal was swabbed for B. dendrobatidis in a standardised manner using sterile fine-tipped rayon swabs (Medical Wire and Equipment Company, Corsham, UK), and each animal was marked with a visible implant elastomer (VIE, Northwest Marine Technology, Shaw Island, WA, USA) tag under the skin. The colour of the tag and the position of the injection on the body were different for animals captured from different water bodies so that any movement between them could be detected. Following elastomer injection, each animal was released back to its point of capture. Surveys continued until the desired sample size of 30 individuals were captured from each site. This sample size was chosen because it statistically detects a 10 % or greater prevalence within a population with a likelihood of 95 % (DiGiacomo and Koepsell 1986). Strict hygiene protocols were followed when moving between water bodies (Johnson et al. 2003) to prevent the spread of disease between sites.
Swabs were stored at −4 °C within 8 h of being used. Extraction and quantification of B. dendrobatidis on swabs was performed following standard protocols for a qPCR Taqman assay (Boyle et al. 2004) using a Rotor Gene 6000 real-time DNA amplification system (Corbett Life Science, San Francisco, CA, USA). Infection load was determined from amplification curves as the number of zoospore genomic equivalents (GE) when compared to known standards. Where amplification did not occur in any of the replicates, the sample was considered negative for the presence of B. dendrobatidis, provided the qPCR reaction was not inhibited. To detect inhibition within the reactions, internal positive controls were included in one replicate of each sample. Following qPCR, the number of cycles taken to cross a threshold set midway up the amplification curve was compared with that for the negative template control. If the sample crossed the threshold more than five cycles after the negative control, the sample was considered inhibited. Where inhibition was detected, a 1/100 dilution of the originally extracted DNA was prepared to dilute inhibitory agents and the reaction was repeated.
The relative abundance of other host species at each site was calculated as the mean number of individuals detected relative to the search effort over four monthly survey events from October to January. The abundance of individuals of each frog species around each water body was determined using nocturnal timed visual encounter surveys by teams of up to 10 people where the water body, emergent and fringing vegetation and the terrestrial habitat within 5 m of the water’s edge were thoroughly searched by spotlight without overlap. All surveys were conducted in a single evening, visiting sites in a random order, for each survey event. The relative abundance of tadpoles in each water body was determined by dip-netting, where a hand-held net was swept through the open water column in a standardised manner and the number of tadpoles captured per sweep calculated. The number of sweeps per water body was proportional to size and ranged from 10 to 40. The number of fish captured opportunistically in each sweep was also recorded and the relative abundance determined. There is no record of fish acting as hosts to B. dendrobatidis but, as they contain keratin in the epidermal layer covering their scales (Moyle and Cech 2000), they were included in the study as a potential reservoir.
Environmental variables
The salinity, pH and depth of each water body were recorded monthly for 12 months. Salinity and pH were measured from the middle of the water column at three randomly selected locations within each water body using a water quality meter (YSI Life Sciences, Morningside, Australia). The mean and maximum salt concentration and the minimum, mean and maximum pH at each site were then calculated. The depth of each water body was measured at the deepest point in each pond and the water level fluctuation was then calculated as the proportion of the maximum annual water depth averaged over the 12-month period. Water temperatures were measured hourly using two iButton data loggers (Thermochron, Baulkham Hills, Australia) placed 5 cm above the substrate and 5 cm below the surface in the deepest section of each water body. The mean and maximum temperatures over a 12-month period were then calculated.
The isolation of each water body within a 5-km radius was determined using patch isolation indices to represent the likelihood of transmission. Indices were calculated using a formula commonly used in metapopulation and patch dynamics studies (Hanski 1999):
where S i is the isolation index for water body i, α is the inverse of the mean dispersal distance of L. fallax, d ij is the distance between water bodies i and j determined using aerial photographs and corrected for any dispersal barriers, and N j is the relative abundance of L. fallax at water body j determined using standardised visual encounter surveys. Because the dispersal distances of L. fallax are unknown, they were estimated as a third of the maximum distance L. aurea are known to move (Hamer 2008), given that L. fallax are approximately one-third of their body length.
Data analysis
The prevalence of infection in the L. fallax population at each water body was calculated as the number of infected individuals divided by the number tested, and 95 % confidence intervals were generated using Bayesian methods for proportions to indicate an interval estimate for the true prevalence. The infection load data from L. fallax and the isolation indices were normalised by logarithmic transformation. The relative abundance data for frogs, tadpoles and fish were normalised with square root transformations and the water level fluctuation was normalised using arcsine. Principal component analysis was conducted on the predictor variables to account for correlations between the variables. Components were extracted if the eigenvalues were greater than 1 and were rotated using the Varimax method. The number of components was considered to be the number below which there was little variability in eigenvalues, identified by a bend in the scree plot. The resulting components were used in a multiple regression analysis to investigate their relationships with infection prevalence and mean infection load. Where significant predictors of infection levels were found, they were compared between water bodies where L. aurea were present and absent using a one-way ANOVA. Litoria fallax infection loads were also compared between water bodies where L. aurea were present and absent using an independent samples t test.
Experimental methods
Study species
The experimental component of this study was done using striped marsh frog tadpoles (Limnodynastes peronii) that were captively bred under B. dendrobatidis-free conditions. Limnodynastes peronii is a common species that is susceptible to B. dendrobatidis infection (Stockwell et al. 2010) and was used due to its availability. Tadpoles were used because they do not develop chytridiomycosis (Berger et al. 1998), allowing experiments to progress without the ethical concern of individuals becoming diseased. There is no evidence to suggest that treatments would impact on the susceptibility of L. peronii tadpoles in ways that would confound our understanding of their effect on the fungus. It was therefore anticipated that the impact of treatments on tadpole infection levels would reflect those seen in other species and life stages. All tadpoles used were at developmental stages 26–30 (Gosner 1960).
Effect of sodium chloride on infection load
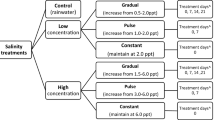
The effect of sodium chloride (NaCl) on B. dendrobatidis infection load was investigated by housing tadpoles in infected water bodies with six different NaCl concentrations (0, 1, 2, 3, 4 and 5 ppt), each with 15 replicates. One tadpole was then added to each tub and the water inoculated with 1 ml (106 zoospores) of a B. dendrobatidis suspension obtained by flooding actively growing tryptone, gelatin hydrolysate and lactose (TGhL) agar plates with sterile water (strain Gibbo River-Llesueuri-00-LB-1). An additional group of 15 tubs with water bodies at 0 ppt NaCl were inoculated with a sham suspension obtained by flooding sterile TGhL agar plates with sterile distilled water, to act as negative controls. Tubs were kept at 22 °C under 12 h light/dark regimes. Tadpoles were fed one trout pellet a week and tubs were topped up with water daily if evaporation occurred. The water from each tub was replaced with new water at the appropriate NaCl concentration after 15 and 30 days. After 45 days, the infection status of each tadpole was determined by swabbing their mouthparts ten times in a standardised manner and infection load quantified using a qPCR assay. The infection load data was not normally distributed and could not be normalised with transformation, so a nonparametric Kruskal–Wallis H test was used to compare infection severities in each salt treatment. Mann–Whitney U tests were then used post hoc to determine which groups were significantly different.
Effect of pond drying on infection load
The effect of pond drying on infection load was investigated by housing tadpoles in infected tubs that had undergone three different degrees of drying, each with 15 replicates. Pond models consisting of 2-L transparent plastic tubs containing a 50-mm-deep substrate of autoclaved commercial potting mix and 1 L of water inoculated with 1 ml (106 zoospores) of a B. dendrobatidis suspension were used. Pond drying was simulated in the treatment tubs by placing the tubs in front of a propeller fan. Fifteen of the treatment tubs were placed close to the fan to increase the drying rate until all free water had evaporated, the soil appeared completely dry, and core samples of potting mix lost <5 % of their dry weight when placed in an oven at 80 °C for 24 h, indicating that most of the moisture had been removed. Another 15 of the treatment tubs were placed further from the fan, and evaporation was monitored until all free water had evaporated and the soil surface appeared dry but the deeper soil appeared damp and core samples of potting mix lost 40–60 % of their dry weight. The final 15 treatment tubs were placed furthest from the fan until all free water had evaporated down to the surface of the potting mix but the soil remained wet. These treatments were referred to as “dry”, “damp” and “wet”, respectively. An additional group of 15 tubs were not placed in front of the fan and were inoculated with a sham suspension to act as negative controls.
Once all treatment tubs had dried to the desired level, water was added to within 4 cm of the container’s lip and the tubs were placed at randomised positions in a constant-temperature room at 22 °C to settle for 12 h. One tadpole was then placed into each tub. Tadpoles remained in the tubs for 35 days under 12 h light/dark regimes and were fed one trout pellet a week. Tubs were topped up with water daily if evaporation occurred, and a water change was done after 16 days. Water changes involved removing and replacing one-third of the free water in each tub. After 35 days, the tadpole’s infection status was determined by swabbing and qPCR, and groups were compared using a Kruskal–Wallis H test.
Determining if fish can carry Batrachochytrium dendrobatidis infections
The ability of B. dendrobatidis to infect the outer epidermal layer of fish was investigated using wild-caught adult mosquito fish (Gambusia holbrooki), an introduced invasive species that can occur in high densities in water bodies used by amphibians (McDowall 1980; Pyke 2008). Thirty adult female G. holbrooki and 30 L. peronii tadpoles (used as positive controls for infection) at developmental stages 26–30 (Gosner 1960) were placed individually into 2-L plastic tubs filled with 1.5 L of water. Fifteen tubs with G. holbrooki and 15 tubs with L. peronii were inoculated with 2 ml (105 zoospores) of a B. dendrobatidis suspension, and the remaining negative control tubs were inoculated with a sham suspension. The fish and tadpoles remained in the tubs for 45 days under 12 h light/dark regimes and were fed one trout pellet a week. Tubs were topped up with water daily if evaporation occurred, and two water changes were conducted after 15 and 30 days, when one-third of the free water in each tub was removed and replaced.
After 45 days in the tubs, the lateral surface of each fish was swabbed five times in a standardised manner to detect the presence of chytrid. Tadpole mouthparts were also swabbed. To determine whether B. dendrobatidis was found on the fish and tadpoles in higher numbers than environmental levels, swabs were also waived through the water column of each tub using ten standardised 10-mm-long strokes. The number of B. dendrobatidis genomic equivalents on each swab was then determined using qPCR and compared between species and the number detected in their water bodies using a Kruskal–Wallis H test. Mann–Whitney U tests were then used post hoc to determine which groups were significantly different. True infections were assumed to have occurred where a significantly higher number of genomic equivalents was detected relative to the number detected in the water body.
Results
Field sampling
Litoria fallax were infected with B. dendrobatidis at all ten sites throughout the Lower Hunter at prevalences and mean infection loads that ranged from 5.6 to 61.5 % and from 4.5 to 549.5 genomic equivalents, respectively. None of the sampled individuals moved between sites. Four other frog species were recorded in water bodies; L. aurea, Peron’s tree frog (Litoria peronii), the common eastern froglet (Crinia signifera) and the striped marsh frog (Limnodynsates peronii). Two species of tadpole (L. fallax and L. peronii) and one species of fish (G. holbrooki) were also detected.
Principal component analysis of the environmental predictor variables generated four components which together explain 87.9 % of the variation (Table 1). Principal component (PC) 1 described the pH, water temperature and relative abundance of frogs (30.6 % variation explained). PC2 described the salinity and the water level fluctuation (24.6 %). PC3 described the relative abundance of tadpoles and pond isolation (17.3 %), and PC4 described the relative abundance of fish (15.4 %). The multiple regression relating infection prevalence at each site to the four principal components describing environmental variables did not result in a model that significantly improved the predictive power of the dependent variable (F 4,5 = 3.03, P = 0.13). The multiple regression relating mean infection load at each water body did result in a predictive model (F 4,5 = 7.49, P = 0.02), with PC2 and PC4 significant (Table 2).
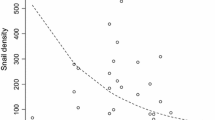
In Litoria fallax, infection load was negatively correlated with salinity (Fig. 1) and water level fluctuation (Fig. 2) and positively correlated with fish abundance (Fig. 3). Litoria aurea occured in water bodies with significantly higher salinities than those they did not inhabit (F 126,127 = 39.36, P < 0.001), but no significant difference in water level fluctuation (F 126,127 = 0.05, P = 0.82) and fish abundance (F 126,127 = 0.60, P = 0.44) occurred. Mean infection loads in L. fallax were also significantly lower in the water bodies that L. aurea also inhabit (t = 2.11, df = 122, P = 0.04). Given the significant and potentially influential effects of salt, drying and fish on infection load, these relationships were examined experimentally.
Batrachochytrium dendrobatidis infection loads, measured as genomic equivalents (no. GE), in Litoria fallax captured from water bodies with differing salinities on Kooragang Island where green and golden bell frogs Litoria aurea were present (open circles, n = 150) or absent (closed circles, n = 150)
Batrachochytrium dendrobatidis infection loads, measured as genomic equivalents (no. GE), in Litoria fallax captured from water bodies with differing proportions of water level fluctuation on Kooragang Island where green and golden bell frogs Litoria aurea were present (open circles, n = 150) or absent (closed circles, n = 150)
Batrachochytrium dendrobatidis infection loads, measured as genomic equivalents (no. GE), in Litoria fallax captured from water bodies with differing abundances of fish on Kooragang Island where green and golden bell frogs Litoria aurea were present (open circles, n = 150) or absent (closed circles, n = 150)
Experimental results
All animals in the negative control groups were negative for the presence of B. dendrobatidis. The addition of NaCl to tubs corresponded to the deaths of 2 tadpoles in the 2-ppt and 2 tadpoles in the 5-ppt treatment groups. Among the remaining tadpoles in the treatment groups, all were infected after 45 days of exposure at severities that differed significantly (χ 2 = 14.40, df = 5, P = 0.01). Tadpoles exposed to salt concentrations of 2–5 ppt had significantly lower infection loads than those in the 0-ppt control (Fig. 4). In the pond-drying experiment, all tadpoles in the treatment tubs were positive for infection. No significant effect of pond drying on infection load was detected (χ 2 = 3.11, df = 2, P = 0.21).
Batrachochytrium dendrobatidis infection loads, measured as genomic equivalents (no. GE), in Limnodynastes peronii tadpoles exposed to differing sodium chloride (NaCl) concentrations for 45 days (n = 15 per treatment). Groups with different letters above their data are significantly different (χ 2 = 14.40, df = 5, P = 0.01)
In the experiment investigating whether fish can be infected, seven of the swabs taken from fish in the treatment group and nine samples taken from their water bodies were positive for B. dendrobatidis. All 15 of the tadpoles in the treatment groups and six swabs taken from their water bodies were also positive. The median number of genomic equivalents detected on tadpole mouthparts was 10.2, and was significantly higher than in the other treatment groups (χ 2 = 26.15, df = 3, P < 0.001), where median numbers of 0.1, 0.07 and 0.2 genomic equivalents were detected on G. holbrooki, in G. holbroki water and in L. peronii water, respectively. These results indicate that the B. dendrobatidis was infective because the number of genomic equivalents detected in the tadpole mouthparts was significantly greater than the number detected in their environment. However, G. holbrooki did not appear to become infected because the levels detected on their body and in their environment were not significantly different.
Discussion
Our results show that host infection loads are lower in the presence of salt than in fresh water. Litoria fallax had significantly lower B. dendrobatidis infection loads in water bodies with mean annual salinities of up to 3.5 ppt. This apparent inhibitory effect was confirmed in the captive experiment, with exposure to 2–5 ppt resulting in significantly lower infection loads when compared to 0 ppt. Salt is a known antifungal agent that acts by interfering with osmotic gradients (Blomberg and Adler 1993). Exposure of B. dendrobatidis to sodium chloride at concentrations of 3–4 ppt slows its growth rate and zoospore motility, which in turn lowers the infection load via their effects on the generation time of the fungus and the establishment rate of secondary infections (Stockwell et al. 2012). Because the infection load of an individual determines the degree of damage to cellular function and in turn the onset of disease (McConnell 2007), a reduction in the rate at which the infection load increases over time may lower the incidence of disease. Species-specific disease thresholds appear to exist for B. dendrobatidis, with particular infection loads triggering a high probability of disease and death (Carey et al. 2006; Stockwell et al. 2010; Vredenburg et al. 2010). Therefore, if salt can maintain infection loads below these thresholds, it may delay or prevent mortality.
Although adapted to fresh water, amphibians can occupy habitats with salinity levels that vary considerably, particularly in lentic systems. The salinity of a water body fluctuates with seasonal changes in the hydroperiod. In temperate climates, salinities are generally lowest in winter and spring, when ponds are filled with the most water, and salinity increases during summer due to higher rates of evaporation. Exposure to salt at an elevated level is therefore most likely to occur immediately prior to the colder months, when B. dendrobatidis is most virulent (Piotrowski et al. 2004). For many species, this timing also corresponds to when tadpoles are metamorphosing and adults are moving away from the aquatic breeding habitat. The inhibitory impact of salt on individuals may therefore translate into fewer infected or severely infected individuals entering the colder months. Given that mortality is seasonally driven (Berger et al. 2004), exposure to salt may therefore assist hosts in surviving these periods and ensure population persistence to the next breeding season.
In addition to varying temporally, the salinity of water bodies also varies spatially, because salinity increases as the distance to a marine environment decreases. This spatial heterogeneity may influence the distribution of species susceptible to chytridiomycosis. In this study, L. aurea occurred in water bodies with higher average salinities, which appears to echo their pattern of persistence close to the eastern Australian coastline (Mahony et al. 1999). This species is highly susceptible to chytridiomycosis (Stockwell et al. 2010) and can tolerate elevated salinity levels (Kearney et al. 2012), supporting the postulate that their current habitat acts as a refuge. Similar trends have been observed for the growling grass frog (Litoria raniformis) in Victoria, Australia and the natterjack toad (Epidalea calamita) in the United Kingdom. The chytrid prevalence and infection loads in L. raniformis show negative trends with the salinity of the wetlands they inhabit (Heard et al. 2014), while E. calamita inhabiting brackish water have a lower likelihood of being infected than those in fresh water (Bramwell 2011; Minting 2012). Given that the salinity of waterways is increasing globally due to agricultural clearing, irrigation and the use of de-icing salt on roads (Kaushal et al. 2005; Pitman 2002), salt refuges may be widespread and increasingly created unintentionally. As such, further amphibian declines may be prevented if salt refuges, whether natural or anthropogenically created, are identified and protected.
Salt refuges may also be created specifically for the management and control of chytridiomycosis. Sodium chloride is frequently used in the human and animal health industries and has been recommended for the prevention and treatment of fungal infections in captive amphibians (Wright and Whitaker 2001). However, the use of salt on free-living populations in field situations has not been attempted. Any conservation efforts that involve altering the environment require an understanding of likely outcomes and caution in implementation (Wobeser 2007). For the creation of salt refuges, this would involve confirmation that the salinity of water bodies can be modified and maintained, that the target organisms will use the modified water bodies, and that this use will result in desired outcomes for survival and no detrimental effect on target and non-target organisms. This could be achieved through the use of site- and species-specific trials, the creation of mosaic landscapes where both saline and freshwater options are available, detailed monitoring of environmental responses and the capacity to manage adaptively over time. Management options also need to be logistically feasible and widely applicable (Wobeser 2007). Within natural lentic systems, manipulations of salt levels could be achieved by the addition of naturally derived salt, promoting the intrusion of saline groundwater or altering hydroperiods. As a management tool in salt-tolerant systems, increasing the NaCl concentration of such water bodies would be both time- and cost-effective because it can be applied widely with little effort and would remain active over time.
In addition to the relationships found between infection loads and salt, this study found lower infection loads in water bodies with higher levels of water fluctuation and fewer fish. Batrachochytrium dendrobatidis zoospores can remain viable in pond water for up to 6 weeks without a host (Johnson and Speare 2003) but do not tolerate desiccation (Johnson et al. 2003). Therefore, when a pond dries out, the load of viable zoospores that are settled in the substrate may decrease as the edges of the water body recede, resulting in lower transmission rates and incidents of disease. A potential reason for higher infection loads in ponds with more fish is that they act as a reservoir for the fungus. Because the epidermal layer covering the scales in some fish species contains keratin (Moyle and Cech 2000), they may be able to carry B. dendrobatidis asymptomatically. Reservoir hosts can heighten environmental densities and transmission rates and Batrachochytrium dendrobatidis has been found to infect a number of non-amphibian hosts, including nematodes (Shapard et al. 2012), crayfish (McMahon et al. 2012) and waterfowl (Garmyn et al. 2012). Despite these apparent relationships, the current study was unable to confirm causal relationships. The observations made in the field may therefore be due to other underlying factors. For example, principal component analysis grouped salinity and drying, suggesting that the negative relationship found between infection load and drying may be due to the effect of salt. Similarly, relationships between fish and infection load may be the result of preferences for similar environmental conditions not identified in this study.
In conclusion, our study indicates that saline environments may protect free-living amphibians from the effects of chytridiomycosis, and the creation of salt refuges could be a feasible management option for many susceptible species. Our study also highlights the importance of protecting refuge areas that may be preventing species decline and extinction. Refuge areas occur naturally in many systems, protecting populations from processes such as competition (Orrock et al. 2010), predation (Hixon and Beets 1993) and fire (Segerström et al. 1994). However, with the environmental changes anticipated to occur over the next century, refuge areas will become increasingly important as natural distributions shift (Carroll et al. 2010). For free-living populations, the protection and creation of areas where populations can occur independent of the processes that threaten their persistence may be one of the most time- and cost-effective long-term conservation strategies available.
References
Atkinson C, Dusek R, Woods K, Iko W (2000) Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J Wildl Dis 36:197–204
Australian Govt. Bureau of Meteorology (2007) Homepage. http://www.bom.gov.au
Becker CG, Zamudio KR (2011) Tropical amphibian populations experience higher disease risk in natural habitats. Proc Natl Acad Sci USA 108:9893–9898. doi:10.1073/pnas.1014497108
Becker CG, Rodriguez D, Longo AV, Talaba AL, Zamudio KR (2012) Disease risk in temperate amphibian populations is higher at closed-canopy sites. PLoS One 7:e48205. doi:10.1371/journal.pone.0048205
Berger L et al (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95:9031–9036
Berger L et al (2004) Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust Vet J 82:434–440
Blomberg A, Adler L (1993) Tolerence of fungi to NaCl. In: Jennings D (ed) Stress tolerance of fungi. Marcel Dekker, New York, pp 209–231
Bosch J, Rincon PA (2008) Chytridiomycosis-mediated expansion of Bufo bufo in a montane area of Central Spain: an indirect effect of the disease. Divers Distrib 14:637–643. doi:10.1111/j.1472-4642.2007.00461.x
Boyle DB, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org 60:141–148
Bramwell R (2011) Do salinity and pH help protect natterjack toads from chytridiomycosis, a disease caused by the amphibian fungus Batrachochytrium dendrobatidis (B.d.)? Masters thesis. Imperial College, London
Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci USA 107:9695–9700
Carey C, Cohen N, Rollins-Smith L (1999) Amphibian declines: an immunological perspective. Dev Comp Immunol 23:459–472
Carey C et al (2006) Experimental exposures of boreal toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth 3:5–21
Carroll C, Dunk JR, Moilanen A (2010) Optimizing resiliency of reserve networks to climate change: multispecies conservation planning in the Pacific Northwest USA. Glob Change Biol 16:891–904. doi:10.1111/j.1365-2486.2009.01965.x
Costa J, Peterson AT (2012) Ecological niche modeling as a tool for understanding distributions and interactions of vectors, hosts, and etiologic agents of chagas disease. In: Mylonakis E, Ausubel FM, Gilmore M, Casadevall A (eds) Recent advances on model hosts, vol 710. Springer, Berlin, pp 59–70
DiGiacomo RF, Koepsell TD (1986) Sampling for detection of infection or disease in animal populations. J Am Vet Med Assoc 189:22–23
Dobson A, Foufopoulos J (2001) Emerging infectious pathogens of wildlife. Philosop Trans Biol Sci 356:1001–1012
Garmyn A, Van Rooij P, Pasmans F, Hellebuyck T, Van Den Broeck W, Haesebrouck F, Martel A (2012) Waterfowl: potential environmental reservoirs of the chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 7:e35038
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hamer AJ (2008) Movement patterns of adult green and golden bell frogs Litoria aurea and the implications for conservation management. J Herpetol 42:397–407
Hanski I (1999) Metapopulation ecology. Oxford University Press, New York
Heard GW, Scroggie MP, Clemann N, Ramsey DSL (2014) Wetland characteristics influence disease risk for a threatened amphibian. Ecol Appl 24:650–662. doi:10.1890/13-0389.1
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101. doi:10.2307/2937124
Johnson ML, Speare R (2003) Survival of Batrachochytrium dendrobatidis in water: qarantine and disease control implications. Emerg Infect Dis 9:922–925
Johnson ML, Berger L, Philips L, Speare R (2003) Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Org 57:255–260
Kaushal SS et al (2005) Increased salinization of fresh water in the northeastern United States. Proc Natl Acad Sci USA 102:13517–13520
Kearney BD, Byrne PG, Reina RD (2012) Larval tolerance to salinity in three species of Australian anuran: an indication of saline specialisation in Litoria aurea. PLoS ONE. doi:10.1371/journal.pone.0043427
Lane A, Burgin S (2008) Comparison of frog assemblages between urban and non-urban habitats in the upper Blue Mountains of Australia. Freshwat Biol 53:2484–2493. doi:10.1111/j.1365-2427.2008.02068.x
Levinton J, Doall M, Ralston D, Starke A, Allam B (2011) Climate change, precipitation and impacts on an estuarine refuge from disease. PLoS ONE. doi:10.1371/journal.pone.0018849
Mahony M (1999) Review of the declines and disappearances within the bell frog species group (Litoria aurea species group) in Australia. In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra, pp 81–93
Mahony MJ, Clulow J, Browne RK, Pomering M (1999) Declines and disappearances of frogs: risk assessment and contingency strategies. In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra
McConnell TH (2007) The nature of disease. Lippincott, Williams and Wilkins, Baltimore
McDonald K, Alford R (1999) A review of declining frogs in Northern Queensland. In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra, pp 14–22
McDowall RM (1980) Freshwater fishes of south-eastern Australia. Reed, Terrey Hills
McMahon TA, Brannelly LA, Chatfield MWH, Johnson PTJ, Joseph MB, McKenzie VJ, Richards-Zawacki CL, Venesky MD, Rohr JR (2012) Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. Proc Natl Acad Sci 110:210–215
Minting PJ (2012) An investigation into the effects of Batrachochytrium dendrobatidis (Bd) on natterjack toad (Bufo calamita) populations in the UK. Ph.D. thesis. The University of Sussex, Brighton
Moyle PB, Cech JJJ (2000) Fishes: an introduction to ichthyology, 4th edn. Prentice Hall, Sydney
Muths E, Pilliod DS, Livo LJ (2008) Distribution and environmental limitations of an amphibian pathogen in the Rocky Mountains USA. Biol Conserv 141:1484–1492. doi:10.1016/j.biocon.2008.03.011
Orrock JL, Holt RD, Baskett ML (2010) Refuge-mediated apparent competition in plant–consumer interactions. Ecol Lett 13:11–20. doi:10.1111/j.1461-0248.2009.01412.x
Parris MJ, Beaudoin JG (2004) Chytridiomycosis impacts predator–prey interactions in larval amphibian communities. Oecologia 140:626–632
Parris MJ, Cornelius TO (2004) Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology 85:3385–3395
Peterson AT (2006) Ecologic niche modeling and spatial patterns of disease transmission. Emerg Infect Dis 12:1822–1826
Pinkard EA, Kriticos DJ, Wardlaw TJ, Carnegie AJ, Leriche A (2010) Estimating the spatio-temporal risk of disease epidemics using a bioclimatic niche model. Ecol Model 221:2828–2838. doi:10.1016/j.ecolmodel.2010.08.017
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15
Pitman MG (2002) Global impact of salinity and agricultural ecosystems. In: Lauchli A, Luttge U (eds) Salinity: environment–plants–molecules. Kluwer, Dordrecht, pp 3–20
Puschendorf R et al (2009) Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: proposing climatic refuges as a conservation tool. Divers Distrib 15:401–408. doi:10.1111/j.1472-4642.2008.00548.x
Puschendorf R et al (2011) Environmental refuge from disease-driven amphibian extinction (Refugio ambiental para la extinción de anfibios causada por enfermedad). Conserv Biol 25:956–964. doi:10.1111/j.1523-1739.2011.01728.x
Pyke GH (2008) Plague minnow or mosquito fish? A review of the biology and impacts of introduced species. Annu Rev Ecol Evol Syst 39:171–191
Raffel T, Michel P, Sites E, Rohr J (2010) What drives chytrid infections in newt populations? Associations with substrate, temperature, and shade. EcoHealth 7:526–536. doi:10.1007/s10393-010-0358-2
Rodder D, Veith M, Lotters S (2008) Environmental gradients explaining the prevalence and intensity of infection with the amphibian chytrid fungus: the host’s perspective. Anim Conserv 11:513–517. doi:10.1111/j.1469-1795.2008.00210.x
Ron SR (2005) Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 37:209–221
Segerström U, Bradshaw R, Hörnberg G, Bohlin E (1994) Disturbance history of a swamp forest refuge in northern Sweden. Biol Conserv 68:189–196. doi:10.1016/0006-3207(94)90350-6
Shapard EJ, Moss AS, San Francisco MJ (2012) Batrachochytrium dendrobatidis can Infect and cause mortality in the Nematode Caenorhabditis elegans. Mycopathologia 173:121–126
Skerratt LF et al (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Speare R, Berger L (2005) Chytridiomycosis status of wild amphibians in Australia. http://www.jcu.edu.au/school/phtm/PHTM/frogs/chy-au-status.htm. Accessed 14/3/12
Springer YP, Hardcastle BA, Gilbert GS (2007) Soil calcium and plant disease in serpentine ecosystems: a test of the pathogen refuge hypothesis. Oecologia 151:10–21. doi:10.1007/s00442-006-0566-1
Stevenson LA, Alford RA, Bell SC, Roznik EA, Berger L, Pike DA (2013) Variation in thermal performance of a widespread pathogen, the amphibian chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 8:e73830. doi:10.1371/journal.pone.0073830
Stockwell MP, Clulow S, Clulow J, Mahony M (2008) The impact of the amphibian chytrid fungus Batrachochytrium dendrobatidis on a green and golden bell frog Litoria aurea reintroduction program at the Hunter Wetlands Centre Australia in the Hunter Region of NSW. Aust Zool 34:379–386
Stockwell MP, Clulow J, Mahony MJ (2010) Host species determines whether infection load increases beyond disease-causing thresholds following exposure to the amphibian chytrid fungus. Anim Conserv 13(Suppl 1):62–71
Stockwell MP, Clulow J, Mahony MJ (2012) Sodium chloride inhibits the growth and infective capacity of the amphibian chytrid fungus and increases host survival rates. PLoS ONE 7:e36942. doi:10.1371/journal.pone.0036942
Threlfall CG, Jolley DF, Evershed N, Goldingay RL, Buttemer WA (2008) Do green and golden bell frogs Litoria aurea occupy habitats with fungicidal properties? Aust Zool 34:350–360
Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ (2010) Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA 107:9689–9694
Wobeser G (2002) Disease management strategies for wildlife. Rev Sci Tech Off Int Epizoot 21:159–178
Wobeser G (2007) Disease management through environmental modification. Disease in wild animals. Springer, Berlin, pp 271–290
Wright KM, Whitaker BR (2001) Pharmacotherapeutics. In: Wright KM, Whitaker BR (eds) Amphibian medicine and captive husbandry. Krieger, Malabar, pp 309–319
Acknowledgments
We would like to acknowledge the Australian Animal Health Laboratory for training in real-time PCR and providing chytrid isolates. We thank Evan Pickett, Riona Tindal, Dale Bond and Tegan Hunter for assistance with data collection. This work was funded by the Port Waratah Coal Service through the Kooragang Wetland Rehabilitation Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ross Andrew Alford.
Rights and permissions
About this article
Cite this article
Stockwell, M.P., Clulow, J. & Mahony, M.J. Evidence of a salt refuge: chytrid infection loads are suppressed in hosts exposed to salt. Oecologia 177, 901–910 (2015). https://doi.org/10.1007/s00442-014-3157-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3157-6