Abstract
Bullfrogs (Rana catesbeiana) are widely believed to be nonclinical carriers of Batrachochytrium dendrobatidis (Bd), the fungal pathogen that invades keratinized tissues of amphibians and causes the disease, chytridiomycosis. Although most research on this disease focuses on adults, larval anurans are also susceptible to infections in their keratinized mouthparts, and this allows for visual diagnosis of the disease via the degree of mouthpart depigmentation. When an unplanned outbreak of chytridiomycosis occurred in a set of captive bullfrog tadpoles in our lab, we conducted the current investigation into its effects on the nonspecific immune system (i.e., the leukocyte populations) of the tadpoles. We compared leukocyte counts from blood smears of 27 tadpoles that had contracted the disease (evidenced by severe mouthpart depigmentation and confirmed by histology) to those of 21 tadpoles that had little depigmentation (i.e., with little evidence of the disease). Tadpoles with severe depigmentation had significantly more neutrophils and less eosinophils than those with little depigmentation, while numbers of lymphocytes, basophils, and monocytes were not statistically different. That there was any effect at all on circulating leukocyte numbers is surprising since leukocytes are usually not seen migrating to sites of infection in tissue sections of amphibians infected with Bd, and since most research points to this disease having little outward effect on bullfrogs. Since monocyte numbers were unchanged, the leukocyte alterations were likely not due to a simple inflammation response. It is possible that Bd infections elicit increases in glucocorticoid hormones, which can cause increased numbers of circulating neutrophils and lower numbers of eosinophils, although this is often accompanied by a reduction in lymphocyte numbers, which we did not see. Further research is warranted to clarify if this effect is limited to this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of the myriad of diseases affecting animal populations world-wide, none may be as well-known as the amphibian disease, chytridiomycosis, which is caused by infection with the fungal pathogen, Batrachochytrium dendrobatidis (Bd) and is widely believed to be responsible for massive population declines in tropical regions of the world (reviewed in Lips et al. 2005). Moreover, there is now evidence that temperate populations are also susceptible to this pathogen (Fellers et al. 2001; Muths et al. 2003; Green and Dodd 2007; Schlaepfer et al. 2007). However, it has also recently become clear that not all amphibian species are affected by the pathogen (Kriger and Hero 2006; Longcore et al. 2007), and that antimicrobial peptides in the skin of some species can inhibit the growth of Bd (Rollins-Smith and Conlon 2005; Woodhams et al. 2007). One well-known example of a species that appears unaffected by the disease is the American bullfrog (Rana catesbeiana), which has native and introduced populations world-wide. In fact, numerous researchers have speculated that bullfrogs may be nonclinical carriers, or “resevoirs” of Bd, since the disease appears to have little effect on adults (Daszak et al. 2004; Hanselmann et al. 2004; Garner et al. 2006; Green and Dodd 2007). Furthermore, it is also thought that Bd does not negatively affect larval bullfrogs, since high numbers of infected larvae can be found in ponds with apparently stable populations (Peterson et al. 2007). In other species, the effects of Bd on anuran larvae have varied. Smith et al (2007) found no evidence of decreased growth in Heleophryne natalensis and Strongylopus hymenopus larvae infected with Bd. However, Parris and Cornelius (2004) showed that infections in larval stages cause increases in developmental instability of Bufo fowleri and Hyla chrysoselis metamorphs. Collectively though, most evidence points to few directly observable effects of Bd infections in anuran larvae, and especially bullfrogs. Missing from this body of work though, are investigations into the range of possible physiological effects of Bd on anuran larvae.
One important physiological component that should be considered with infections is the effect on animals' immune systems. In particular, the nonspecific immune system (i.e., the leukocyte or white blood cell population) of any animal is one of the primary lines of defense against invading pathogens, and is made up of five different types of white blood cells, which each perform different tasks in the immune process (Jain 1986, 1993). Thus, by assessing the relative numbers of each cell type currently in circulation (i.e., the leukocyte profile of an animal), it is possible to determine if this component of the immune system has been activated in study subjects. For example, in most animals, infections cause increases in circulating numbers of neutrophils (or heterophils in birds and reptiles), which are the primary phagocytic cells that attack (i.e., engulf) foreign particles and organisms (Turner 1988; Jain 1993; Campbell 1995; Davis et al. 2004). Similarly, monocytes are also phagocytic and can increase in circulation during infections (Latimer et al. 1988; Davis et al. 2004; Thrall et al. 2006). Eosinophils are involved in modulation of the immune response by secretions of chemical substances that promote phagocytosis (Maxwell 1987; Rothenberg and Hogan 2006), though are not typically found in higher numbers in circulation during infections. Finally, infections can also lead to a general stress response, marked by increases in stress hormones (Lindström et al. 2005), and this hormonal increase can in turn lead to reductions in circulating numbers of lymphocytes, increases in numbers of neutrophils, and even reductions in eosinophils in some animals (reviewed in Davis et al. 2008a).
The effects of Bd on leukocyte populations has rarely been examined in amphibians, perhaps because histological sections of infected skin usually do not show leukocytes migrating to sites of tissue infection (Berger et al. 2005a), and it is therefore assumed that the leukocyte response is minimal (Densmore and Green 2007). In the only study to examine this topic thus far, Woodhams et al. (2007) examined circulating numbers of leukocytes in postmetamorphic Litoria chloris and did find an effect of chytrid infection, but surprisingly, it was a reduced number of neutrophils (and also eosinophils) in infected individuals, which is not consistent with the classical immune response, per se, to a pathogen infection (typified by increased numbers of phagocytic cells).
While conducting a laboratory experiment involving late-stage bullfrog tadpoles in the summer of 2007, an outbreak of chytridiomycosis occurred in our captive population. While this unplanned outbreak disrupted our original plans for the tadpoles, it fortuitously allowed us to conduct an investigation into the effects of this disease on the leukocyte populations of bullfrog tadpoles, and we present the results of this investigation here. We compared leukocyte populations of tadpoles that had contracted the disease (based on visual assessment of mouthpart depigmentation) to those that had not yet become infected. While we examined all white blood cell types, we were particularly interested in numbers of neutrophils and monocytes, which when elevated is a sign of immune activation. We also looked for evidence of increases in stress hormones in the depigmented group, which would be primarily indicated by low numbers of lymphocytes combined with high numbers of neutrophils (Davis and Maerz 2008b, a; Davis et al. 2008a).
Methods
Lab setup
As part of the initial experiment we hand-captured late-stage bullfrog tadpoles from a local pond in Clarke County, GA, in the spring of 2007, and brought them into captivity, where they were housed in 38 L aquaria in a temperature-controlled room set to 23°C. Thereafter, they were fed ReptoMin turtle sticks ad libitum, and their water was changed weekly. At the same time, bullfrog tadpoles from a separate site, where there were known cases of chytridiomycosis, had also been captured and housed separately in the same room. At some point, water or equipment from these groups was unintentionally mixed, and the tadpoles in the initial set became exposed to the disease. When we discovered the outbreak in July 2007, we ceased the initial experiment and removed the tadpoles (n = 55) to conduct the present study.
Processing tadpoles
Tadpoles were anesthetized via immersion in a solution of 5% MS-222, then they were blotted dry, weighed, and their developmental stage was recorded following Gosner (1960). Next, we examined the tadpoles for evidence of chytridiomycosis. Since Bd only attacks keratinized tissue of amphibians, the darkly pigmented keratinized mouthparts of tadpoles are where the infection is typically found, with infections usually leading to depigmentation of the keratinized toothrows around the oral disc (Fig. 1). Because of this, many investigators have relied on the degree of tadpole mouthpart depigmentation as a proxy for infection with Bd (Fellers et al. 2001; Knapp and Morgan 2006; Symonds et al. 2007). We also used this approach and visually assessed the degree of mouthpart depigmentation based on a scoring system we devised that evaluated all keratinzed parts of the mouth (i.e., toothrows and beak). In this system an observer (A.K.D.) separately scored the beak, upper, and all three lower toothrows of each tadpole on a 0 to 3 scale based on how much pigmentation was lost (three being nearly all depigmented). Then the five separate scores were summed for each individual so that each tadpole was assigned a single number that varied between 0 and 15, with 15 being the most depigmented. To be conservative, we then collapsed these into five “mouth score” groups: scores 0–2, 3–5, 6–8, 9–11, and 12–15, were groups 1 through 5, respectively. Finally, a blood sample was obtained from each tadpole via heart puncture and a standard blood film made on a clean microscope slide. Slides were air-dried and later stained with giemsa.
Histological examination
As a check of the visual scoring method for diagnosing chytridiomycosis and to further elucidate the infection in tissues, a random subset (n = 18) of tadpoles were preserved in formalin after processing and prepared for histological examination of keratinized mouthparts. To prepare slides, 5-μm thick sections of paraffin-embedded tissues were rehydrated through graded alcohol and stained with hematoxylin and eosin (H&E). These tissue sections of mouthparts were examined by one observer (K.K.), and the degree of lesions was subjectively scored on a 0–3 scale, based on severity of erosions and loss of denticles.
Comparison of both scoring schemes from the subset of 18 tadpoles showed that tadpoles with visual mouthpart scores of 3 or higher were always assigned the maximum lesion score histologically (Fig. 2), so, we therefore considered all individuals from the larger tadpole set with a three or more to be infected with chytridiomycosis (n = 27). Those with a visual score of 2 in the subset (n = 7) appeared to have variable histological scores, so we did not include any individuals with this visual score in our investigation. In general, tadpoles with the lowest visual scores (category 1, n = 21) also had low histological lesion scores (average of 1 for histological scoring), though of the nine tadpoles in this subset, two had evidence of light to moderate lesions in tissue sections (i.e., lesion scores 1 and 2), and two tadpoles showed heavy lesions (score 3). Thus, even though we observed very little depigmentation in the mouthparts of these two individuals, there was clear evidence of Bd infections from the tissue sections. This means that in our visual scoring scheme for the entire set of tadpoles, we cannot rule out the possibility that those with no mouthpart depigmentation (i.e., score 1) had active infections. However, since the majority of individuals in this category appeared free of infection, comparisons of the mean leukocyte parameters of tadpoles in this category versus the unquestionably infected tadpoles (score 3 or higher) are still valuable to help elucidate the effects of this disease.
Comparison of histological scoring of chytridiomycosis and visual assessment of mouthparts from 18 tadpoles (see “Methods”). Mean histological scores (which ranged from 0–3) shown with standard deviations. High visual scores indicate severe mouthpart depigmentation
Reading blood smears
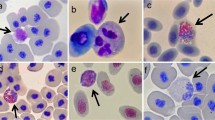
All slides were viewed with a standard light microscope under 1,000 × oil immersion, and leukocytes were counted (by A.K.D.) following established procedures in our lab (Davis et al. 2008b; Davis and Maerz 2008b, a; Davis 2009). Leukocytes were identified as neutrophils, lymphocytes, eosinophils, basophils, and monocytes, following (Thrall 2004); Hadji-Azimi et al. (1987), and Turner (1988). All leukocytes were counted until at least 100 leukocytes had been recorded, or when 150 fields of view had been examined. Only fields of view with even distributions of red blood cells were used. Fields of view in this study had an average of 30 red blood cells (±7, based on examination of 60 fields). The number of each cell type counted was then transformed into the number counted per ten fields of view for analyses (which is equivalent to ~300 red blood cells).
Data analysis
For each of the five cell types we log-transformed the number of cells per ten fields of view to approximate normal distributions. To examine the effect of Bd infection on numbers of each cell type we used a multivariate analysis of covariance (MANCOVA) design, where the log-transformed abundance of the cell type were the (five) response variables and infection was the fixed dichotomous independent variable (i.e., mouthparts severely depigmented vs little depigmentation). Since anuran white blood cell populations have recently been found to vary naturally throughout tadpole development (Davis 2009), Gosner stage was included as a continuous covariate. The analysis was conducted using Statistica 6.1 software (Statistica 2003).
Results
Histological observations
Microscopic examination of the subset of tadpoles with active infections in their mouthparts revealed erosion of the keratin layers and attenuation or complete loss of denticles (Fig. 3). Fungal elements were only present in keratin and areas lacking keratin did not have any active infection. Dermal infiltrates of lymphocytes were not correlated with the presence of Bd and were present at a low level in all tadpoles regardless of infection.
General comparisons
The mean Gosner stage of the 21 tadpoles with little mouthpart depigmentation was 34, which was not significantly different from the average stage of the 27 tadpoles with severe mouthpart depigmentation (two-sample t test, t = 0.988, df = 46, p = 0.328). There was also no difference in average mass of both groups (7.3 g; two-sample t test, t = 1.67, df = 46, p = 0.100).
Leukocyte differentials
The relative numbers of each leukocyte type for both groups of tadpoles are shown in Table 1. In general, tadpoles with severely depigmented mouthparts had relatively more abundant neutrophils than those with little depigmentation (16% versus 8% of all white blood cells). It is also interesting to compare the leukocyte profiles of the current study to those reported previously for larval (Davis 2009) and adult bullfrogs (Cathers et al. 1997). In general, there was concordance with most cells types (Table 1), though the relative number of monocytes in all tadpoles in this study (mean = 6.9%) appeared to be greater than normal (<1%).
Statistical results
The overall MANCOVA that considered the effects of infection on counts of all white blood cell types simultaneously showed the expected effect of Gosner stage (F 5,41 = 4.97, p = 0.001), which is addressed in detail elsewhere (Davis 2009), but more importantly, a significant overall effect of Bd infection on leukocyte numbers (F 5,41 = 2.47, p = 0.048). To understand which cell types were most affected by Bd infection, the effects of infection on the abundance of individual cell types are presented graphically (Fig. 4), and we report results from follow-up models that considered individual cell types (with the same independent variables included). Lymphocyte numbers were unaffected by chytridiomycosis (F 1,45 = 0.013, p = 0.908). The abundance of circulating neutrophils, however, significantly increased in the severely depigmented tadpoles (F 1,45 = 6.38, p = 0.015; Fig. 4). In contrast, the numbers of circulating eosinophils tended to decrease with infection (F 1,45 = 4.36, p = 0.042; Fig. 4). Finally, there was no difference in numbers of basophils (F 1,45 = 0.012, p = 0.734) or monocytes (F 1,45 = 1.08, p = 0.304) between infection groups (Fig. 4).
Discussion
The data from this study indicate that B. dendrobatidis infection in bullfrog larvae has an effect on their non-specific immune system (i.e., the numbers of circulating white blood cells). This effect is at least partly consistent with the reaction typical of most infections, which begins with an increase in phagocytic neutrophils (Stockham and Scott 2002; Thrall et al. 2006). These cells act to rid the blood of foreign bodies, including inert particles and microbial cells (Turner 1988). However, it is not clear what these cells are targeting in the chytrid infection, since most histological investigations, including our own (Fig. 3), do not show neutrophils (or any other leukocyte) migrating to the site of zoospore infection. To answer this question, it is helpful to understand the normal course of chytridiomycosis infection in amphibian skin. Infections are typified by the presence of zoosporangia which live inside cells of the keratinized epidermal tissue of the host (mouthparts for anuran tadpoles, skin in adults) (Berger et al. 2005b). The zoosporangia initially infect cells a few layers deep but eventually these cells move outward to the skin surface. Multiple zoospores develop within zoosporangia and when mature are released through discharge tubes which open to the surface of the animal. Importantly, this process has been shown to result in a degree of host tissue damage; in heavily infected animals, many epidermal cells become swollen, or necrotic, often with degenerate nuclei (Berger et al. 2005b). While it has not been specifically examined thus far, this type of tissue damage is sure to lead to cellular debris. It is therefore possible that the increase in numbers of circulating phagocytic neutrophils we observed in infected bullfrog tadpoles is a response to this tissue damage and cellular debris, not necessarily to the foci of infection per se. Berger et al. (2005b) also report that chytrid infections can lead to opportunistic entry of bacteria through the zoosporangia discharge tubes. These too would likely be targeted by neutrophils (Wright 2001).
There is an alternative explanation for the rise in neutrophil abundance, and which might also explain the decrease in eosinophils. Infections in animals can lead to chronically elevated stress hormones (Lindström et al. 2005), either as a direct result of the infection, or perhaps in this case, by the reduced capacity of the tadpoles to consume food (since infections damage tadpole mouthparts). Stress hormones are well-known to cause alterations in the normal distributions of white blood cells, and the primary cell affected is the neutrophil, which increases when hormones increase (reviewed in Davis et al. 2008a). In most, but not all cases, this increase is accompanied by a decrease in numbers of lymphocytes. These opposing effects are thought to ‘redistribute’ cells to where they would best be served in stressful situations (neutrophils in circulation, lymphocytes migrate into tissues) (Dhabhar et al. 1996). In some animals, stress can also cause a reduction in the numbers of circulating eosinophils (Jain 1986; Davis et al. 2008a), which was indeed seen here. Importantly, eosinophils are the primary cell responsible for protections against metazoan parasites, and within amphibians, investigators have shown that experimental reductions in eosinophil numbers by administration of corticosterone leads to increased susceptibility to other biologically important pathogens such as trematodes (Belden and Kiesecker 2005). Interestingly, in the only other investigation of the effects of chytridiomycosis on amphibian white blood cells (adults in this case), a reduction in eosinophils was also found (Woodhams et al. 2007). Thus, given the proven role of eosinophils in defending against metazoan parasites, the combined evidence of this and the Woodhams et al. study strongly suggest that Bd infections could lead to increased host susceptibility to metazoan parasites.
In contrast to our study, Woodhams et al. (2007) observed reductions in neutrophil counts, while here, there was a general increase. These opposing results could be because larvae were examined here as opposed to later life stages in the Woodhams et al. study, or because different anuran species were examined in the two studies. In fact, this last point brings up an interesting question in itself; since chytridiomycosis does not appear to kill bullfrog tadpoles, could the immune response we observed be one of the reasons why? Comparisons of infected larvae of other amphibian species (such as those known to be susceptible to Bd) would no doubt clarify this issue.
In summary, comparison of leukocyte populations in bullfrog tadpoles with and without evidence of chytridiomycosis suggests the disease causes an alteration in the circulating complement of leukocytes in this species. This result is surprising, both because of the fact that Bd zoospores are not attacked by leukocytes, and given the otherwise benign effects chytridiomycosis has on this species. Further study is warranted to determine if this effect is limited to bullfrogs and if this response is what makes them resistant to this disease.
References
Belden LK, Kiesecker JM (2005) Glucocorticosteroid hormone treatment of larval treefrogs increases infection by Alaria sp trematode cercariae. J Parasitol 91:686–688
Berger L, Speare R, Skerratt LF (2005a) Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis Aquat Org 68:65–70
Berger L, Hyatt AD, Speare R, Longcore JE (2005b) Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Org 68:51–63
Campbell TW (1995) Avian hematology and cytology. Iowa State University Press, Ames
Cathers T, Lewbart GA, Correa M, Stevens JB (1997) Serum chemistry and hematology values for anesthetized American bullfrogs (Rana catesbeiana). J Zoo and Wildl Med 28:171–174
Daszak P, Strieby A, Cunningham AA, Longcore JE, Brown CC, Porter D (2004) Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetol J 14:201–207
Davis AK (2009) Metamorphosis-related changes in leukocyte profiles of larval bullfrogs (Rana catesbeiana). Comp Clin Path 18:181–186
Davis AK, Maerz JC (2008a) Comparison of hematological stress indicators in recently captured and captive paedomorphic mole salamanders, Ambystoma talpoideum. Copeia 2008:613–617
Davis AK, Maerz JC (2008b) Sex-related differences in hematological stress indices of breeding, paedomorphic mole salamanders. J Herpetol 42:197–201
Davis AK, Cook KC, Altizer S (2004) Leukocyte profiles of House Finches with and without mycoplasmal conjunctivitis, a recently emerged bacterial disease. Ecohealth 1:362–373
Davis AK, Maney DL, Maerz JC (2008a) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772
Davis AK, Diggs NE, Marra PP, Cooper RJ (2008b) Hematological stress indices show no effect of radio-transmitters on wintering Hermit Thrushes. J Field Ornithol 79:293–297
Densmore CL, Green DE (2007) Diseases of amphibians. Institute for Laboratory Animal Research Journal 48:235–254
Dhabhar FS, Miller AH, McEwen BS, Spencer RL (1996) Stress-induced changes in blood leukocyte distribution - role of adrenal steroid hormones. J Immunol 157:1638–1644
Fellers GM, Green DE, Longcore JE (2001) Oral chytridiomycosis in the mountain yellow-legged frog (Rana muscosa). Copeia 945–953
Garner TWJ, Perkins MW, Govindarajulu P, Seglie D, Walker S, Cunningham AA, Fisher MC (2006) The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana. Biol Lett 2:455–459
Gosner KL (1960) A simplified table for staging anuran embryos and larvae. Herpetologica 16:183–190
Green DE, Dodd CK (2007) Presence of amphibian chytrid fungus, Batrachochytrium dendrobatidis, and other amphibian pathogens at warm-water fish hatcheries in southeastern North America. Herpetological Conservation and Biology 2:43–47
Hadji-Azimi I, Coosemans V, Canicatti C (1987) Atlas of adult Xenopus laevis laevis hematology. Dev Comp Immunol 11:807–874
Hanselmann R, Rodriguez A, Lampo M, Fajardo-Ramos L, Aguirre AA, Kilpatrick AM, Rodriguez JP, Daszak P (2004) Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biol Conserv 120:115–119
Jain NC (1986) Schalm's veterinary hematology. Lea & Febiger, Philadelphia
Jain NC (1993) Essentials of veterinary hematology. Blackwell Publishing, Philadelphia
Knapp RA, Morgan JAT (2006) Tadpole mouthpart depigmentation as an accurate indicator of chytridiomycosis, an emerging disease of amphibians. Copeia 188–197
Kriger KM, Hero JM (2006) Survivorship in wild frogs infected with chytridiomycosis. EcoHealth 3:171–177
Latimer KS, Tang KN, Goodwin MA, Steffens WL, Brown J (1988) Leukocyte changes associated with acute inflammation in chickens. Avian Dis 32:760–772
Lindström K, Hawley D, Davis AK, Wikelski M (2005) Stress responses and disease in three wintering house finch (Carpodacus mexicanus) populations along a latitudinal gradient. Gen Comp Endocrinol 143:231–239
Lips KR, Burrowes PA, Mendelson JR, Parra-Olea G (2005) Amphibian population declines in Latin America: a synthesis. Biotropica 37:222–226
Longcore JR, Longcore JE, Pessier AP, Halteman WA (2007) Chytridiomycosis widespread in anurans of northeastern United States. J Wildl Manage 71:435–444
Maxwell MH (1987) The avian eosinophil: a review. Worlds Poultry Science Journal 43:190–207
Muths E, Corn PS, Pessier AP, Green DE (2003) Evidence for disease-related amphibian decline in Colorado. Biol Conserv 110:357–365
Parris MJ, Cornelius TO (2004) Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology 85:3385–3395
Peterson JD, Wood MB, Hopkins WA, Unrine JM, Mendonca MT (2007) Prevalence of batrachochytrium dendrobatisis in American bullfrog and southern leopard frog larvae from wetlands on the Savannah River site, South Carolina. J Wildl Dis 43:450–460
Rollins-Smith LA, Conlon JM (2005) Antimicrobial peptide defenses against chytridiomycosis, an emerging infectious disease of amphibian populations. Dev Comp Immunol 29:589–598
Rothenberg ME, Hogan SP (2006) The eosinophil. Annu Rev Immunol 24:147–174
Schlaepfer MA, Sredl MJ, Rosen PC, Ryan MJ (2007) High prevalence of Batrachochytrium dendrobatidis in wild populations of lowland leopard frogs Rana yavapaiensis in Arizona. EcoHealth 4:421–427
Smith KG, Weldon C, Conradie W, du Preez LH (2007) Relationships among size, development, and Batrachochytrium dendrobatidis infection in African tadpoles. Dis Aquat Org 74:159–164
Statistica (2003) Statistica version 6.1, Statsoft Inc
Stockham SL, Scott MA (2002) Fundamentals of veterinary clinical pathology. Blackwell Publishing, Ames, Iowa, USA
Symonds EP, Hines HB, Bird PS, Morton JM, Mills PC (2007) Surveillance for Batrachochytrium dendrobatidis using Mixophyes (Anura: Myobatrachidae) larvae. J Wildl Dis 43:48–60
Thrall MA (2004) Hematology of amphibians. In: Thrall MA, Baker DC, Lassen ED (eds) Veterinary hematology and clinical chemistry: text and clinical case presentations. Lippincott Williams & Wilkins, Philadelphia, PA
Thrall MA, Baker DC, Lassen ED, Campbell TW, DeNicola D, Rebar A, Fettman MJ, Weiser G (2006) Veterinary hematology and clinical chemistry. Blackwell Publishing, Ames, Iowa, USA
Turner RJ (1988) Amphibians. In: Rawley AF, Ratcliffe NA (eds) Vertebrate blood cells. Cambridge University Press, Cambridge, pp 129–209
Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith LA (2007) Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim Conserv 10:409–417
Wright KM (2001) Amphibian hematology. In: Wright KM, Whitaker BR (eds) Amphibian medicine and captive husbandry. Krieger Publishing Company, Malabar, FL, pp 129–146
Acknowledgements
We thank the members of the herpetology lab for assistance with lab procedures. Kerry Holcomb and Andrew Grosse helped with tadpole collection. Statistical advice was provided by Sonia Altizer. A.K.D. was supported by the Warnell School of Forestry and Natural Resources, at the University of Georgia. All procedures in this study were approved by the University of Georgia Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davis, A.K., Keel, M.K., Ferreira, A. et al. Effects of chytridiomycosis on circulating white blood cell distributions of bullfrog larvae (Rana catesbeiana). Comp Clin Pathol 19, 49–55 (2010). https://doi.org/10.1007/s00580-009-0914-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-009-0914-8