Abstract
Considering that emerging infectious diseases are one of the major drivers of global amphibian decline, controlling the spread of infections are even more challenging. Amphibian skin disease chytridiomycosis, which is caused by two species of fungi belonging to the Batrachochytrium genus, has been detected in at least 700 amphibian species causing mass mortalities in all continents where amphibians occur. Most Alytes species, including the Betic midwife toad (A. dickhilleni), are highly susceptible to B. dendrobatidis (Bd) with lethal consequences. The presence of Bd infection in A. dickhilleni was confirmed ten years ago in just three localities across the entire distribution range of this threatened species. Here we report the extraordinary Bd expansion through the entire distribution range of A. dickhilleni and analyse if former infected populations acted as the source of transmission events to current infected populations. Currently, Bd infection is broadly distributed across the entire distribution range of the species and the increase of infection prevalence reached 30–50% during a decade. The populations where the infection was detected a decade ago could be identified as likely sources of infection for some locations where the pathogen is now present. The introduction of infected amphibian hosts into previously naïve A. dickhilleni breeding sites, and other anthropogenic processes, are seeming to be the most plausible way of Bd range expansion, motivating mass mortalities, population declines and extirpation events of this threatened amphibian species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The control of infectious diseases has always been one of the key challenges facing human society. With the recognition that infectious diseases can pose conservation threats, though, the objective of controlling the spread of infections has become even more challenging (Daszak et al. 2000). Identifying the routes of transmission in humans and livestock, while still difficult, is not as complex a task as understanding transmission chains in wild host populations. For example, applying contact networks used in the medical and veterinary fields of endeavour is impossible for much of wildlife where sampling is sparse and host movement is difficult, if not impossible, to track persistently (see White et al 2017 for a review). Instead wildlife disease epidemiology has had to often rely on episodic or retrospective spatiotemporal studies without individual-based data to understand how infectious agents of wildlife propagate across landscapes. At various geographic scales, recent studies that have adopted this strategy have illustrated how the movement of many important infectious diseases of wildlife often involves anthropogenic movement of hosts or parasites, or human modifications of habitat that increase the risk of transmission (Price et al. 2016; Lawson et al. 2018; Yon et al. 2019). For the control of the spread of infectious disease, this is a crucial distinction to make from transmission attributable to the movement of infected hosts during migrations or dispersal events.
The panzootic amphibian disease chytridiomycosis, caused by the fungal pathogens of the genus Batrachochytrium, has been responsible for declines of at least 500 species of amphibians, and infecting hundreds more (Scheele et al. 2019; Fisher and Garner 2020). Although some non-amphibian hosts have been identified (Garmyn et al. 2012; McMahon et al. 2013; Liew et al. 2017; Oficialdegui et al. 2019) most of the studies of transmission dynamics within amphibian populations have described a pre-eminent role for among-amphibian transmission events (Mitchell et al. 2008; Rachowicz and Briggs 2007; Briggs et al. 2010; Searle et al. 2011). At a global scale, however, intercontinental and international transmission is largely attributed to human activities, in particular global trade in live amphibians (Fisher and Garner 2007; Picco and Collins 2008; Nguyen et al. 2017; O’Hanlon et al. 2018). At a landscape scale, some studies are indicative of a significant role for amphibian dispersal in expanding geographic ranges of batrachochytrids (Lips et al. 2008, 2006; Vredenburg et al. 2010; Rodriguez-Brenes et al. 2016). However, recent, retrospective analyses have identified several anthropogenic factors that may have facilitated range expansion by Bd into various California landscapes (Yap et al. 2016; Chaukulkar et al. 2018; Vredenburg et al. 2019).
In Europe, published evidence of mass mortalities and, in two cases, population declines have been reported affecting three of the five species in the nominate genus, Alytes (Bosch et al. 2001, 2013; Walker et al. 2008, 2010). As a result, the species in the family Alytidae have been identified as the taxonomic group most at risk of declines due to chytridiomycosis (e.g. Baláž et al 2014). One of these species, the Betic midwife toad, Alytes dickhilleni, endemic to the Betic mountain range of the south-eastern Iberian Peninsula, was reported to be affected by chytridiomycosis at only three breeding ponds (Bosch & González-Miras 2012; Bosch et al. 2013; Dias et al. 2014). Despite the presence of infections with Bd detected in 2009, small range size and significant degradation of habitat, the 2009 IUCN red list assessment of the Betic midwife only rates the species as Vulnerable. Given the ability of infection with batrachochytrids to spread rapidly across landscapes, we resampled A. dickhilleni to determine if the risk of chytridiomycosis remained or had increased since we sampled ten years previously. Our objectives were fourfold: (i) to determine if infection had persisted at locations we had previously detected infection; (ii) to evaluate the joint effects of life history and locality-associated factors on infection intensity; (iii) to ascertain if infection with Bd has spread into new locations, and, in the event range expansion by Bd was detected; (iv) to determine if populations previously identified as infected acted as the source of transmission events to previously uninfected populations.
Methods
Recent sampling took place between March and June, 2019, across the species range in south-eastern Spain and followed procedures used ten years previously (Fig. 1; Bosch et al. 2013). In brief we focussed on overwintered (OW) tadpoles, although if overwintered tadpoles were not present, young-of-the-year (YoY) tadpoles were sampled, up to a maximum of 20 animals per location. Where A. dickhilleni tadpoles were not in evidence, we sampled other species that occupied the location, or at some sites included in the 2013 study we sampled another pond located in close proximity to the pond sampled in our original study (Table SI 1; Bosch et al. 2013). In addition to this targeted sampling, we included a selection of dead amphibians opportunistically collected in 2015–2016 by rangers working for the Junta de Andalucía: these animals were not used to generate descriptive epidemiological statistics, nor included in analyses cofactors associated with risk of infection, but were used for geographic profiling (see below). As previously, we swabbed oral discs and applied standard biosecurity measures to prevent cross contamination between sites and samples. Swabs were stored dry and refrigerated until DNA extraction. The exception was the carcasses collected in 2015–2016, which were preserved in ethanol until DNA extraction from tissue samples. We used quantitative PCR (Boyle et al. 2004) to detect infections with Bd and to estimate infection loads (genomic equivalents of zoospores and hereinafter referred to as GE; Blooi et al. 2013). Extractions were diluted 1:10 before amplification and run on a myGo mini qPCR machine. We included negative and positive controls (known concentrations of 10, 100, and 1000 GE) for each PCR plate. Infection loads equal to or greater than 0.1 were considered as positives.
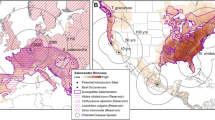
Changes in current prevalence of infection (2019) of sampling localities respect to a previous study (2009; Bosch et al. 2013). Bd positive localities are shown in shades of red, while Bd free localities are shown in white or in shades of grey. Low prevalence increase, infection prevalence increase < 20%; High prevalence increase, infection prevalence increase > 60%). The inner panels show in red Bd positive provinces in 2009 and in 2019
We calculated prevalence of infection with 95% confidence intervals for each study population and province, as well as the mean, median, standard deviation and variance to mean ratio (VMR) of infection intensity (Bd loads) of infected animals in Bd positive populations. We employed analysis of variance (ANOVA) and a Fisher's exact test to compare infection intensity (log-transformed) and prevalence of infection across different life history stages. Because metamorphs were sampled at only one location in 2019, we restricted this analysis to comparisons across the two tadpole age classes. We used linear regressions to examine the effect of the altitude of the study localities on both prevalence of infection (arcosin transformed) and infection intensity (log-transformed). We used Fisher’s exact tests to compare differences in the proportion of infected localities in 2019 to that detected in 2009 and a general linear mixed model (GLMM) with prevalence of infection of study localities (arcsin transformed) as response variable to determine the effect of sampling year and localities (nested within province and treated as a random factor). We also used a general linear model (GLM) to investigate the effect of localities, nested within natural areas, defined as parks, either national or natural, and mountain range, and provinces, on GE (log-transformed) using infected animals from 2019. We used both natural areas and provincial political boundaries because natural areas may fall into more than one province. Normality assumptions of the residuals of linear models were visually confirmed. Data were analysed using the software JMP Pro 12.0 (SAS Inc.), and maps were generated with QGIS 3.6.0 software.
Geographic profiling is a technique used in criminology to prioritise large lists of suspects using the spatial locations associated with serial crimes to generate a probability surface (a geoprofile), from which a suspect’s anchor point can be identified (Rossmo 2000). More recently it has been adapted to fit a Bayesian framework for use in biological data, and applied to epidemiology (Le Comber et al. 2011; Verity et al. 2014), conservation biology (Faulkner et al. 2015, 2018; Struebig et al. 2018) and invasive species biology (Stevenson et al. 2012; Faulkner et al. 2016). Here, we used the Dirichlet Process Mixture (DPM) model of GP, introduced by Verity et al. (2014) and extended in Faulkner et al. (2016). Model details and settings are explained in Verity et al. (2014). Our model was implemented in R (R Core Team 2015) using the 2.1.0 version of the package Rgeoprofile. To identify possible sources of the 2019 Bd cases, we produced a geoprofile and used this resulting surface to infer the most likely points (sources) from which infection may have spread (Fig. 2). The model output was assessed using a hit score percentage – the proportion of the study area searched by the model before a source, in this case the 2009 and 2015–2016 cases were located. A lower hit score percentage indicates the model had to search less of the total area before finding a putative source.
Geoprofile showing the results of the geospatial analysis using the positive records in 2019 to predict the sources for these outbreaks. Locations of 2019 positive Bd cases are shown as red circles and grey circles represent locations of positive cases in 2009 and 2015–2016. Contours show the top 50% of the geoprofile, with the highest priority areas shown in yellow
Results
We sampled 324 A. dickhilleni tadpoles distributed across 27 breeding sites in the six provinces where the species occurs (Table 1 and Fig. 1: mean of 12 individuals per site). Forty-three percent (n = 140; 95% CI: 37.9–48.7) of tadpoles occupying 56% (n = 15) of breeding sites tested positive for Bd. Infection loads were on average 86.7 GE (± standard deviation, median and range = 130.9, 30.5, and 1–671 GE: see Table SI 1). Ten of the 27 breeding populations sampled in 2009 and that lacked detectable infections now contained infected tadpoles, with prevalence ranging from 60 to 100%, with one exception, and the host species was absent at another 8 of the previously sampled locations (Table SI1). Eleven out of 28 larvae of other species at 2 of the 5 locations where A. dickhilleni could no longer be found but where we sampled other species in 2019 also tested positive (Table 2). All of the carcasses collected opportunistically in 2015–2016 tested positive, in many cases with GE values in the thousands (Table 3).
Recent metamorphosed animals sampled in 2019 at one location were not infected. The prevalence of infection was higher and infection intensity was greater in OW versus YoY tadpoles (respectively, 50.60 vs. 38.20%; Fisher's exact test, P = 0.0186; 124.8 vs. 27.8 GE, F1,139 = 25.69, P < 0.000, respectively). Altitude did not significantly affect either prevalence or infection intensity (F1,25 = 0.07, P = 0.7944; F1,13 = 0.25, P = 0.6193; respectively). However, in ten years the proportion of infected localities has significantly increased (58.6 versus 10.0%, excluding Bd negative breeding sites where A. dickhilleni was not detected in 2019; Fisher's exact test, P < 0.0001; Tables 1, SI1 and 2; Fig. 1), and prevalence of infection at affected sites has also increased (F1,28 = 8.21, P = 0.0079). We detected a significant geographic component to this, as prevalence was inconsistent across provinces (F5,16 = 5.08, P = 0.0058; Fig. 1), even when the increment was similar across provinces (F5,23 = 1.26, P = 0.3159). Infection loads also differed significantly across localities (F8,125 = 5.59, P < 0.0001), but there was no geographic pattern based on natural areas (F3,125 = 1.87, P = 0.1384) or provinces (F3,125 = 1.26, P = 0.2903).
The GP model successfully identified all three 2009 Bd positive populations as likely sources for the 2019 positive cases (Fig. 2). A hitscore indicates what proportion of the area needed to be searched before the source was identified given the data, the 2019 cases. In this case, the hit scores associated with the three 2009 populations were 0.2, 7.4 and 7.5%, indicating limited searches of the study area were required before these locations were identified as possible sources by the GP model. The model also identified multiple other possible source locations which could not be attributed to the 2009 samples, highest priority areas shown in yellow (Fig. 2). In addition, we analysed the 2015–2016 mortality data (n = 11) as additional potential sources for the 2019 positive cases. Eight of these had hitscores of less than 10%, suggesting that the positive A. dickhilleni breeding site in Granada identified in 2019 was associated with the mortality events that were discovered by the ranger surveys in 2015–2016.
Discussion
Although Scheele et al. (2019) showed that approximately a third of amphibian species affected by chytridiomycosis are still experiencing decline, their analysis identified Europe as a region relatively less impacted by chytrid-associated declines than other continents (Scheele 2019; but see Lambert et al. 2020). However, a more detailed analysis could probably yield a large list of victims in the Mediterranean countries (e.g. Ayllón and Bosch 2020) and may change the conclusions of that study about the relative incidence of Bd across different regions of the world. In this study, we revisited and more thoroughly sampled the distribution of A. dickhilleni to characterize the threat of chytridiomycosis to this species not covered by Scheele et al. (2019). Our findings are disturbing: the proportion of breeding sites where infection occurs has increased sixfold and opportunistic sampling in 2015–2016 has shown that mortality attributable to chytridiomycosis continues to affect our study species, as well as other Iberian native amphibian species not included either by Scheele et al. (2019). Alytes dickhilleni could not be found at 8 locations which we had sampled in 2009, and many locations where we could still find the species and where we did not detect infections with Bd in 2009 now harbour infected tadpoles (Table SI1). This process of invasion and intraspecific amplification of infection is likely facilitated by delayed metamorphosis exhibited by tadpoles of this and all species of the genus Alytes. In A. obstetricans and A. muletensis overwintered tadpoles that are not susceptible to lethal disease carry infections that are transmissible to hatching tadpoles. This is associated with increased prevalence and strength of infection in the reservoir tadpole stage and no evidence of mortality until near, at, or just following metamorphic climax (Bosch et al. 2001; Walker et al. 2010; Doddington et al. 2013). In this study we have shown that overwintered tadpoles are both more frequently and more heavily infected than tadpoles recruited the year of sampling. Also, as with A. obstetricans, we did not detect any pattern of infection associated with altitude, however mortality of Iberian common midwives was strongly associated with altitude and temperature metrics (Bosch et al. 2007; Walker et al. 2010).
Bd has been on the European continent for decades and has even reached endemicity in some locations (Spitzen—van der Sluijs et al. 2014; Bates et al. 2018). Long-term epidemiological studies of A. obstetricans in the Pyrenees where it is experiencing mass mortalities and population declines suggest that range expansion of Bd has not been occurring as aggressively as appears to be happening in A. dickhilleni (Walker et al. 2010; unpubl. data). In fact, ours is the first study to report a relatively rapid and significant geographic range expansion of batrachochytrids on Iberia in a highly susceptible host species. Infection of A. dickhilleni breeding sites expanded to twice as many provinces as were detected in the previous study, associated with a significant increase in prevalence in the original two. While the picture for range expansion is incomplete, we could attribute at least some of the observed range expansion of Bd to two likely sources. First, the outbreaks we originally detected a decade ago were rapidly identified as likely sources of infection for some of the locations we identified in this current study. Second, opportunistic sampling of mortality events in 2015–2016 were closely located to the emergence of infection of one of our 2019 sample locations. These two types of sources indicate that expansion of Bd across the range of A. dickhilleni is to some extent accomplished by the introduction of infected amphibian hosts into previously naïve A. dickhilleni breeding sites. The spatial scale at which this is happening also indicates that transmission amongst breeding sites is through amphibian dispersal. However, sources for many of the other, newly identified locations cannot be attributed to the pattern we described previously, so dismissing human-aided dispersal or other anthropogenic processes as contributing to Bd range expansion would be presumptuous.
It remains to be proven if risk of mortality follows a similar pattern in A. dickhilleni as in A. obstetricans at various Iberian locations but work to determine this is underway. Our previous experimental work (Bosch et al. 2013) showed clearly the typical pattern of post-metamorphic mortality associated with infection in A. dickhilleni, and the mortalities reported here included our study species, suggesting that the expanding distribution of infections in this species maps to risk of death and decline. Evidence of ongoing and increasing risk of chytridiomycosis in European amphibians has been documented since risk was first identified affecting Iberian amphibians (e.g. Bosch et al. 2001; Walker et al. 2008, 2010; Bielby et al. 2009; Rosa et al. 2013; Clare et al. 2016; Spitzen—van der Sluijs et al. 2016). Over the past 15 years Bd-related mortalities and declines of several Iberian species have been detected by the citizen science program of the Spanish Herpetological Association (http://siare.herpetologica.es/sare), a pattern not captured in the study by Scheele et al (2019). All Iberian amphibian species are in decline and although other threatening processes are involved, recent data suggests that chytridiomycosis is increasing in impact across the Peninsula (Ayllón and Bosch 2020). While we have had some success at eradicating infection at a very limited geographical scale (Bosch et al. 2015), developing a strategy for mitigating the impacts of chytridiomycosis across Iberia is still far off, and requires urgent investment by all stakeholders.
Data availability
All relevant data are included in the manuscript.
References
Ayllón E, Bosch J (2020) El programa SARE de la Asociación Herpetológica Española: seguimiento de anfibios y reptiles en España. Spanish Ministry for the Ecological Transition and the Demographic Challenge Website. https://www.miteco.gob.es/es/red-parques-nacionales/boletin/boletin_64_anfibios_tcm30-510343.pdf. Accessed 7 January 2021
Baláž V, Vörös J, Civiš P et al (2014) Assessing risk and guidance on monitoring of Batrachochytrium dendrobatidis in Europe through Identification of taxonomic selectivity of infection. Conserv Biol 28:213–223. https://doi.org/10.1111/cobi.12128
Bates KA, Clare FC, O’Hanlon S et al (2018) Amphibian chytridiomycosis outbreak dynamics are linked with host skin bacterial community structure. Nat Commun 9:1–11. https://doi.org/10.1038/s41467-018-02967-w
Bielby J, Bovero S, Sotgiu G et al (2009) Fatal chytridiomycosis in the tyrrhenian painted frog. EcoHealth 6:27–32. https://doi.org/10.1007/s10393-009-0232-2
Blooi M, Pasmans F, Longcore JE et al (2013) Duplex real-Time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J Clin Microbiol 51:4173–4177. https://doi.org/10.1128/JCM.02313-13
Bosch J, Martínez-Solano I, García-París M (2001) Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol Conserv 97:331–337. https://doi.org/10.1016/S0006-3207(00)00132-4
Bosch J, Carrascal LM, Durán L et al (2007) Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc R Soc B Biol Sci 274:253–260. https://doi.org/10.1098/rspb.2006.3713
Bosch J, Tejedo M, Lizana M, et al (2009) Alytes dickhilleni (errata version published in 2016). The IUCN Red List of Threatened Species 2009: e.T979A86229986. https://doi.org/10.2305/IUCN.UK.2009.RLTS.T979A13099604.en. Accessed 10 July 2020
Bosch J, González-Miras E (2012) Seguimiento de Alytes dickhilleni: Informe final. Monografías SARE. Asociación Herpetológica Española, Ministerio de Agricultura, Alimentación y Medio Ambiente, Madrid
Bosch J, García-Alonso D, Fernández-Beaskoetxea S et al (2013) Evidence for the introduction of lethal chytridiomycosis affecting wild betic midwife toads (Alytes dickhilleni). EcoHealth 10:82–89. https://doi.org/10.1007/s10393-013-0828-4
Bosch J, Sanchez-Tomé E, Fernández-Loras A et al (2015) Successful elimination of a lethal wildlife infectious disease in nature. Biol Lett 11:20150874. https://doi.org/10.1098/rsbl.2015.0874
Boyle DG, Boyle DB, Olsen V et al (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ 60:141–148. https://doi.org/10.3354/dao060141
Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci U S A 107:9695–9700. https://doi.org/10.1073/pnas.0912886107
Chaukulkar S, Sulaeman H, Zink AG, Vredenburg VT (2018) Pathogen invasion and non-epizootic dynamics in Pacific newts in California over the last century. PLoS ONE 13:1–10. https://doi.org/10.1371/journal.pone.0197710
Clare FC, Halder JB, Daniel O et al (2016) Climate forcing of an emerging pathogenic fungus across a montane multi-host community. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2015.0454
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science 287:443–449. https://doi.org/10.1126/science.287.5452.443
Dias G, Beltrán JF, Tejedo M et al (2015) Limited gene flow and high genetic diversity in the threatened Betic midwife toad (Alytes dickhilleni): evolutionary and conservation implications. Conserv Genet 16:459–476. https://doi.org/10.1007/s10592-014-0672-2
Doddington BJ, Bosch J, Oliver JA et al (2013) Context-dependent amphibian host population response to an invading pathogen. Ecology 94:1795–1804. https://doi.org/10.1890/12-1270.1
Faulkner SC, Stevenson MD, Verity R et al (2015) Using geographic profiling to locate elusive nocturnal animals: A case study with spectral tarsiers. J Zool 295:261–268. https://doi.org/10.1111/jzo.12203
Faulkner SC, Verity R, Roberts D et al (2016) Using geographic profiling to compare the value of sightings vs trap data in a biological invasion. Divers Distrib 23:104–112. https://doi.org/10.1111/ddi.12498
Faulkner SC, Stevens MCA, Romañach SS et al (2018) A spatial approach to combatting wildlife crime. Conserv Biol 32:685–693. https://doi.org/10.1111/cobi.13027
Fisher MC, Garner TWJ (2007) The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biol Rev 21:2–9. https://doi.org/10.1016/j.fbr.2007.02.002
Fisher MC, Garner TWJ (2020) Chytrid fungi and global amphibian declines. Nat Rev Microbiol 18:332–343. https://doi.org/10.1038/s41579-020-0335-x
Garmyn A, van Rooij P, Pasmans F et al (2012) Waterfowl: Potential environmental reservoirs of the chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 7:1–5. https://doi.org/10.1371/journal.pone.0035038
Lambert MR, Womack MC, Byrne AQ, et al (2020) Comment on “Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity”. Science, 367. https://doi.org/10.1126/science.aay1838
Lawson B, Robinson RA, Fernandez JRR et al (2018) Spatio-temporal dynamics and aetiology of proliferative leg skin lesions in wild British finches. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-32255-y
Liew N, Moya MJM, Wierzbicki CJ et al (2017) Chytrid fungus infection in zebrafish demonstrates that the pathogen can parasitize non-amphibian vertebrate hosts. Nat Commun 8:1–10. https://doi.org/10.1038/ncomms15048
Lips KR, Brem F, Brenes R et al (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci U S A 103:3165–3170. https://doi.org/10.1073/pnas.0506889103
Lips KR, Diffendorfer J, Mendelson JR, Sears MW (2008) Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol 6:0441–0454. https://doi.org/10.1371/journal.pbio.0060072
McMahon TA, Brannelly LA, Chatfield MWH et al (2013) Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. Proc Natl Acad Sci U S A 110:210–215. https://doi.org/10.1073/pnas.1200592110
Mitchell KM, Churcher TS, Garner TWJ, Fisher MC (2008) Persistence of the emerging pathogen Batrachochytrium dendrobatidis outside the amphibian host greatly increases the probability of host extinction. Proc R Soc B Biol Sci 275:329–334. https://doi.org/10.1098/rspb.2007.1356
Nguyen TT, Van NT, Ziegler T et al (2017) Trade in wild anurans vectors the urodelan pathogen Batrachochytrium salamandrivorans into Europe. Amphib Reptil 38:554–556. https://doi.org/10.1163/15685381-00003125
O’Hanlon SJ, Rieux A, Farrer RA et al (2018) Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360:621–627. https://doi.org/10.1126/science.aar1965
Oficialdegui FJ, Sánchez MI, Monsalve-Carcaño C et al (2019) The invasive red swamp crayfish (Procambarus clarkii) increases infection of the amphibian chytrid fungus (Batrachochytrium dendrobatidis). Biol Invasions 21:3221–3231. https://doi.org/10.1007/s10530-019-02041-6
Picco AM, Collins JP (2008) Amphibian commerce as a likely source of pathogen pollution. Conserv Biol 22:1582–1589. https://doi.org/10.1111/j.1523-1739.2008.01025.x
Price SJ, Garner TWJ, Cunningham AA et al (2016) Reconstructing the emergence of a lethal infectious disease of wildlife supports a key role for spread through translocations by humans. Proc R Soc B Biol Sci 283:20160952. https://doi.org/10.1098/rspb.2016.0952
R Core Team, 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 20 May 2020.
Rachowicz LJ, Briggs CJ (2007) Quantifying the disease transmission function: Effects of density on Batrachochytrium dendrobatidis transmission in the mountain yellow-legged frog Rana muscosa. J Anim Ecol 76:711–721. https://doi.org/10.1111/j.1365-2656.2007.01256.x
Rodríguez-Brenes S, Rodriguez D, Ibáñez R, Ryan MJ (2016) Spread of amphibian chytrid fungus across lowland populations of Túngara frogs in panamá. PLoS ONE 11:1–8. https://doi.org/10.1371/journal.pone.0155745
Rosa GM, Anza I, Moreira PL et al (2013) Evidence of chytrid-mediated population declines in common midwife toad in Serra da Estrela, Portugal. Anim Conserv 16:306–315. https://doi.org/10.1111/j.1469-1795.2012.00602.x
Rossmo DK (2000) Geographic profiling. CRC Press, Florida
Scheele BC, Pasmans F, Skerratt LF et al (2019) Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363:1459–1463. https://doi.org/10.1126/science.aav0379
Searle CL, Biga LM, Spatafora JW, Blaustein AR (2011) A dilution effect in the emerging amphibian pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci U S A 108:16322–16326. https://doi.org/10.1073/pnas.1108490108
Spitzen-van der Sluijs A, Martel A, Asselberghs J et al (2016) Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in Europe. Emerg Infect Dis 22:1286–1288. https://doi.org/10.3201/eid2207.160109
Spitzen-Van Der Sluijs A, Martel A, Hallmann CA et al (2014) Environmental determinants of recent endemism of Batrachochytrium dendrobatidis infections in amphibian assemblages in the absence of disease outbreaks. Conserv Biol 28:1302–1311. https://doi.org/10.1111/cobi.12281
Stevenson MD, Rossmo DK, Knell RJ, Le Comber SC (2012) Geographic profiling as a novel spatial tool for targeting the control of invasive species. Ecography 35:704–715. https://doi.org/10.1111/j.1600-0587.2011.07292.x
Struebig MJ, Linkie M, Deere NJ et al (2018) Addressing human-tiger conflict using socio-ecological information on tolerance and risk. Nat Commun 9:3455. https://doi.org/10.1038/s41467-018-05983-y
Verity R, Stevenson MD, Rossmo DK et al (2014) Spatial targeting of infectious disease control: Identifying multiple, unknown sources. Methods Ecol Evol 5:647–655. https://doi.org/10.1111/2041-210X.12190
Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ (2010) Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci U S A 107:9689–9694. https://doi.org/10.1073/pnas.0914111107
Vredenburg VT, McNally SVG, Sulaeman H et al (2019) Pathogen invasion history elucidates contemporary host pathogen dynamics. PLoS ONE 14:1–14. https://doi.org/10.1371/journal.pone.0219981
Walker SF, Bosch J, Gomez V et al (2010) Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecol Lett 13:372–382. https://doi.org/10.1111/j.1461-0248.2009.01434.x
Walker SF, Bosch J, James TY et al (2008) Invasive pathogens threaten species recovery programs. Curr Biol 18:853–854. https://doi.org/10.1016/j.cub.2008.07.033
White LA, Forester JD, Craft ME (2017) Using contact networks to explore mechanisms of parasite transmission in wildlife. Biol Rev 92:389–409. https://doi.org/10.1111/brv.12236
Yap TA, Gillespie L, Ellison S et al (2016) Invasion of the fungal pathogen Batrachochytrium dendrobatidis on California Islands. EcoHealth 13:145–150. https://doi.org/10.1007/s10393-015-1071-y
Yon L, Duff JP, Ågren EO et al (2019) Recent changes in infectious diseases in european wildlife. J Wildl Dis 55:3–43. https://doi.org/10.7589/2017-07-172
Acknowledgements
Funding for this study was provided by the Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible of Junta de Andalucía. Special thanks to Jesús del Río, coordinator of the conservation program of endangered amphibians of Andalusia. We also thank Ester Cerezo-Valverde for field assistance, Cristina Sausor for laboratory assistance and Trenton WJ Garner for providing insightful and detailed comments on earlier versions of the paper.
Funding
This work was funded by the Consejería de Medio Ambiente of Junta de Andalucía (Contract Number NET378406/1).
Author information
Authors and Affiliations
Contributions
JB and EGM conceived the ideas and designed methodology. EGM, BT, and JB collected the samples. BT performed DNA extraction and qPCR analyses. SCF conducted geographic profiling analysis. JB and BT ran the statistical analyses. BT, SCF, and JB wrote the first draft; all authors contributed to revisions.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
Field work was carried out under permission of Consejería de Medio Ambiente of Junta de Andalucía, Castilla La Mancha and Región de Murcia.
Consent to participate
All the authors voluntarily agreed to participate in this research study.
Consent for publication
All the authors voluntarily consented publish this research study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thumsová, B., González-Miras, E., Faulkner, S.C. et al. Rapid spread of a virulent amphibian pathogen in nature. Biol Invasions 23, 3151–3160 (2021). https://doi.org/10.1007/s10530-021-02571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02571-y