Abstract
Patients with advanced cancers are frequently diagnosed with bone metastasis, which is an incurable condition associated with pathological bone remodeling. Despite its widespread impact, understanding of the mechanisms underlying bone metastasis remains relatively limited. While traditional cancer research approaches focus on cancer cells, increasing evidence suggests a role for their surrounding microenvironment in tumorigenesis and metastasis. Therefore, model systems recapitulating physiologically relevant cell-microenvironment interactions are needed in order to study the specific underlying signaling mechanisms. Tissue-engineered, humanized in vitro models may provide an attractive alternative to conventional cell culture and rodent models, as they offer systematic control of microenvironmental aspects relevant to basic and translational studies of bone metastasis. Here, we use breast cancer as an example to review metastasis-associated changes to the bone microenvironment and current approaches to study bone metastasis. In light of their limitations, we discuss tissue-engineered model systems of bone metastasis as a promising alternative, and describe specific design parameters that should be considered when developing such models. Collectively, engineering-inspired culture approaches will be valuable to investigate the functional contribution of the microenvironment to the development, progression, and therapy response of bone metastasis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Metastasis accounts for approximately 90% of cancer-related deaths [1] and very frequently targets the skeleton [2]. In particular, patients with advanced breast and prostate cancer, but also with lung, thyroid, and kidney cancers, are often diagnosed with incurable bone metastasis [3]. The pathological bone remodeling associated with skeletal metastasis increases morbidity and mortality, and can span a wide spectrum of changes that range from excess new bone formation, as in the case of prostate cancer, to complete bone degradation, as often observed with breast cancer [4, 5]. Despite its devastating socioeconomic consequences, our understanding of the molecular, cellular, and tissue-level mechanisms that underlie bone metastasis remains relatively limited.
Traditionally, most cancer research has centered on cancer cells; however, it is now well accepted that the microenvironment in which cancer cells are located is equally important. In fact, an accumulating body of work suggests that tumors can only develop in a permissive context that may, for example, form during the process of aging or inflammation, while a healthy or embryonic microenvironment can prevent tumorigenesis [6]. Although most studies on tumor-microenvironment interactions have been performed in the context of primary tumors, the same concepts apply to secondary tumors that have spread to distant sites including the skeleton. Indeed, the “seed and soil” hypothesis has long argued that metastasis is a non-random process which specifically targets organs that provide fertile ground for tumor cells to seed [7, 8]. Nevertheless, due in part to a lack of relevant model systems, there exists relatively little knowledge about the surrounding “soil”, or microenvironment, and what makes it fertile for seeding and progression of metastases.
Historically, bone metastasis has been studied in conventional two-dimensional (2D) cell culture and mouse models. However, both approaches are limited in their ability to recapitulate conditions characteristic of human disease. More specifically, species-specific differences in mice often prevent extrapolation of results to patients, while 2D cultures lack physiologically relevant 3D cell-microenvironment interactions. Nevertheless, these contextual cues are critical regulators of the phenotypic changes that mediate metastasis, including proliferation, differentiation, and gene expression [9]. To address this challenge, cancer biologists increasingly utilize tumor spheroids and organoids. Still, these systems are not easily suited to recapitulate the unique cell-cell and cell-extracellular matrix (ECM) interactions as well as mechanical forces intrinsic to bone metastasis. Tissue-engineered, humanized in vitro models may provide an attractive alternative and advance basic and translational studies of bone metastasis.
In this chapter, we will use breast cancer as an example to introduce biological changes to the bone microenvironment associated with metastasis and review current approaches to study the underlying mechanisms. Subsequently, we will discuss tissue-engineered model systems of bone metastasis as a valuable alternative and define specific design parameters that should be considered when developing such models. Collectively, engineering-inspired culture approaches will be valuable to investigate the functional contribution of the microenvironment to the development, progression, and therapy response of bone metastasis.
2 The Microenvironment in Bone Metastasis
2.1 Bone Structure and Homeostatic Bone Remodeling
The skeleton serves to provide structural support in the body, and is constantly undergoing remodeling (~10% annually [10]) to maintain mechanical strength and integrity. Bone remodeling is a sequential process by which bone is degraded/resorbed (osteolysis) and then replaced by newly formed bone (osteogenesis). At the cellular level, osteolysis and osteogenesis are carried out by bone-degrading osteoclasts, bone-forming osteoblasts, and mechanosensing osteocytes. Through acid and protease secretion, osteoclasts primarily function to degrade bone matrix, a composite material composed of collagen type I fibrils that are reinforced by hydroxyapatite (HA) nanocrystals [11]. Osteoclasts are hematopoietic in origin and derived from macrophage/monocytes that have differentiated and fused (i.e. osteoclastogenesis) in the presence of osteoblast-derived cues (e.g. receptor activator of nuclear factor kappa-B ligand [RANKL], macrophage colony-stimulating factor [M-CSF]) [12]. Osteoblasts, on the other hand, are derived from bone marrow mesenchymal stem cells (BM-MSCs) via transforming growth factor-beta (TGF-β), bone morphogenetic protein (BMP), and WNT signaling [10]. Following behind osteoclasts, osteoblasts deposit new collagen type I matrix for mineralization, then undergo apoptosis or become lining cells or osteocytes. Generally accepted as the primary mechanosensors in bone, osteocytes form an interconnected network embedded within bone matrix, and secrete factors that regulate osteoclastogenesis (e.g. RANKL) and osteoblast differentiation (e.g. TGF-β) in response to physical forces [13]. Critical to the balance of bone resorption and formation is the local strain environment in the bone, which is modulated by external mechanical stimuli. For example, increases in mechanical loading of the bone (e.g. due to physical activity) lead to a net increase in osteogenesis whereas reductions in loading (e.g. due to bed rest) promote osteolysis [14]. In the context of breast cancer, tumor cells deregulate the above-described homeostatic signaling between bone cells and mechanical stimuli to drive their own growth and metastatic potential.
2.2 The Vicious Cycle of Bone Metastasis
Bone metastasis results when cancer cells originating from a primary tumor initiate secondary tumors in the skeleton. The metastatic process is highly selective [8]; primary tumor cells must successfully invade local tissue, intravasate into nearby blood vessels, and circulate systemically while evading the immune system, before localizing and extravasating into bone. Even then, the disseminated cells must survive a period of dormancy before reactivating to establish secondary tumor growth [15]. Survival at each of these steps is rate-limiting and requires crosstalk between a cancer cell and its microenvironment. The bone matrix exemplifies fertile “soil” for cancer cells, as it is packed with morphogens (i.e. growth factors, cytokines, chemokines) that attract tumor cells and feed their growth. For example, the sequestration of osteoblast-secreted stromal-derived factor-1 (SDF-1/CXCL12) in the bone ECM is not only important for homing of CXCR4-expressing immune, hematopoietic, and stem cells, but also plays a role in seeding and proliferation of breast cancer cells in bone [16,17,18,19].
Once localized to the bone secondary site, tumor cells modify the microenvironment in their favor by deregulating the signals that govern homeostatic bone remodeling in a feed-forward loop that promotes bone metastatic progression. While osteogenesis appears to be essential for initial seeding of metastasis [20], osteolysis is the primary outcome at later stages of breast cancer bone metastasis. Tumor cells activate the latter process by secreting elevated levels of parathyroid hormone-related peptide (PTHrP), which stimulates the secretion of osteoblast-derived RANKL to increase osteoclast activation and bone resorption [21,22,23]. Increased bone resorption, in turn, leads to the release of matrix-bound growth factors (e.g. TGF-β, BMPs) that further enhance tumor growth, in a process known as the “vicious cycle” of bone metastasis [4, 11, 24]. This vicious cycle is additionally stimulated by elimination of functional osteoblasts [25]. Furthermore, upregulated RANKL-independent signaling mechanisms may play an important role in bone metastasis, for example, tumor cells expressing elevated levels of interleukin-8 (IL-8) and lysyl oxidase (LOX) also exhibit increased migration [26] and invasiveness [27], and have been correlated with increased osteolysis [28, 29].
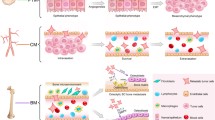
More recently, experimental evidence has suggested that tumor cells at the primary site may direct the formation of distant “pre-metastatic niches” primed for metastatic initiation even prior to their own dissemination. Through endocrine-like actions, primary tumor cells release factors that circulate systemically and transform cell behavior from afar in a manner that may ultimately direct organ-specific metastasis [30, 31]. For example, primary breast cancer in rodent models changes bone strength, structure, and mineralization, suggesting that circulating factors may play a role in this process [32]. Indeed, tumor-free mice that were injected with tumor cell-conditioned media similarly present with osteolytic lesions, confirming that systemically circulating tumor-derived factors (e.g. LOX) lead to pre-metastatic conditioning of the bone [33]. However, whether these changes to the bone ECM are critical to bone metastasis remains to be confirmed. Studies with cancer cell-derived extracellular vesicles (e.g. exosomes) strongly suggest this possibility because they have been demonstrated to direct organotropic metastasis via pre-metastatic niche development at sites such as the lungs and the liver [34, 35]. Developing models to specifically investigate the interactions between tumor cells and the bone microenvironment at each stage of the metastatic cascade will further mechanistic understanding of bone metastatic progression (Fig. 1).
3 In Vivo Models of Bone Metastasis
Various mouse models of bone metastasis have advanced our knowledge of how tumor cells interact with the bone microenvironment, but not all aspects of human disease may be mimicked with this approach. Transgenic mice reflecting certain genetic mutations found in human breast cancer have facilitated greater understanding of tumor growth and invasion, and tumor-immune interactions. For example, overexpression of the oncogenes Her2/neu, Ras, and Myc is commonly driven by the Mouse Mammary Tumor Virus (MMTV) promoter [36]. Immune-competent MMTV-driven mice develop spontaneous mammary tumors, but bone metastasis occurs rarely in such models [36]. In fact, most spontaneous breast cancer models in rodents do not metastasize to the bone, and thus other approaches are often utilized.
Inoculation of breast cancer cells through various injection routes has yielded greater rates of bone metastasis compared to transgenic models. Following orthotopic injection into the mammary fat pad, the murine breast cancer line 4T1 has limited ability to spontaneously metastasize to bone after 3–6 weeks, while its clonal subline 4T1.2 exhibits strong bone tropism [37]. The 4T1 model provides the opportunity to study the full bone metastatic cascade in mice, as well as tumor-immune studies in syngeneic BALB/c mice. However, by the time bone metastases become apparent, the tumor burden is typically high at the orthotopic site and in the lungs, leaving a small time window to study bone metastasis [36]. Intracardiac injection through the left ventricle introduces tumor cells directly into the systemic circulation, improving rates of bone metastases [38]. While this model skips key initial steps of the metastatic process, it has enabled study of factors that influence tumor cell seeding and colonization of bones, including the development of bone-tropic sub-lines of the human breast cancer cell line MDA-MB231, as well as the identification of a bone metastasis gene signature [39, 40]. Intraosseous injections, for example by the intratibial route, place tumor cells directly into the bone marrow cavity, allowing study of tumor-bone microenvironmental interactions. This approach is limited to the late stages of the metastatic cascade, but has been especially useful in studying the vicious cycle of bone metastasis [41] and the effectiveness of potential treatments such as bisphosphonates [42], denosumab [43], and even mechanical loading [14]. Collectively, these techniques have shed light on several aspects of breast cancer bone metastasis, however they remain limited by their inability to recapitulate species-specific interactions between human breast cancer cells and human bone in the presence of a functional immune system [44].
Orthotopic injection of human breast cancer cells into mice with implanted human bone tissue may overcome this issue, and confirm a role for human-specific microenvironmental aspects of bone in driving metastasis [45]. The use of patient-derived xenografts (PDX) models, in which tissue from patient primary tumors is transplanted to immunodeficient mice, has been rising because they offer improved predictive value for malignant potential compared to cancer cell lines [46]. Indeed, tumor cells derived from PDX models have displayed spontaneous metastases similar to those of patients, and as such may metastasize to bone in the host mouse [47]. Still, many of these models lack immune interaction, which could be addressed by engrafting human hematopoietic cells within the immunodeficient murine hosts (e.g. nude [48], SCID [49,50,51], NOD-scid [52]). These humanized models aim to confer partial human immunity to the hosts, however the success of these approaches has been limited by eventual takeover of the hematopoietic compartment by host immune cells and low life spans of mice [53]. Collectively, mouse models of bone metastasis have led to much advancement in our understanding of the disease. Even so, using these models to study the spatiotemporal dynamics of bone metastasis, species-specific differences, and the role of the immune system continues to be a challenge. While certainly limited in their ability to recapitulate full biologic complexity, in vitro culture platforms may address some of these challenges, as they enable the study of human cells under well-defined conditions, in a patient-specific manner, at reduced cost, and with fewer ethical issues relative to animal studies.
4 Tissue-Engineered Models to Study Bone Metastasis
4.1 Dimensionality: 2D Versus 3D
Standard 2D monolayer cultures of human cancer cells have provided valuable insights on cancer biology and informed therapeutic development. However, these 2D culture models are unable to recapitulate most of the heterogeneous interactions within the tumor microenvironment in vivo, including those involving the surrounding extracellular matrix (ECM), as well as other resident cell types, and external physical forces [9, 54]. In fact, cells cultured in 3D compared to 2D exhibit appreciably altered proliferation [55], differentiation [56], metabolism [57], and protein expression [58, 59].
3D cancer cell cultures more appropriately mimic tumors in vivo, as tissue level interactions and dimensionality influence tumor growth [60,61,62], migration [63], signaling [64, 65], and drug response [66]. For example, multicellular spheroids recapitulating certain aspects of tumor heterogeneity and transport limitations in vivo have led to improved understanding of antitumor drug resistance [67]. Tumor organoids, which are spheroids cultured from primary cells, can retain patient-specific genetic and pathological characteristics, and have helped elucidate genotype-drug interactions and niche contributions to growth, metastasis, and drug response [68,69,70]. While tumor spheroids and organoids have also been used to study the role of ECM in regulating invasive behavior of tumor cells [71], they typically lack cell-matrix interactions characteristic of bone. Furthermore, they exclude tumor-stromal cell interactions and mechanical stimuli, thus more physiologically relevant 3D models of the bone microenvironment are needed to investigate the mechanisms of bone metastasis [54].
4.2 Cell-Matrix Interactions
4.2.1 Organic Matrix (Collagen, Decellularized Matrices, etc.)
To study tumor-matrix interactions, natural ECM-derived materials such as collagen type I and reconstituted basement membrane (i.e. Matrigel®) are frequently used due to their cytocompatibility, inclusion of cell adhesion sites, remodelability, as well as the ability to control physical matrix properties (e.g. porosity, fiber structure, stiffness) through casting conditions (e.g. temperature, concentration, pH) [72, 73]. Matrigel® and collagen type I hydrogels have also been used to direct stem cell osteogenic differentiation and mineralization [74,75,76,77,78], leading to compositional similarities to organic bone matrix. However, batch-to-batch variability and inability to control specific biological, biochemical, and biophysical characteristics of these matrices [54, 79] limit study reproducibility and thus, mechanistic understanding.
In particular, the ECM composition, structure, and mechanical properties (e.g. stiffness, or elastic modulus) encountered by cells in the bone microenvironment are not reflected or independently controllable in collagen type I or Matrigel®-based hydrogels models. For example, ECMs at common metastatic sites (bone, lung, brain) are complex in their composition and physical properties, yet most naturally-derived hydrogels comprise only one individual component that does not capture the tissue-specific integrin-ECM interactions that critically mediate breast cancer cell adhesion and motility (Fig. 2a) [80]. In addition, the stiffness of bone is orders of magnitude greater than the upper limit possible using natural ECM hydrogels [84]. As substrate mechanics are critical in regulating BM-MSC osteogenic differentiation [85, 86], tumor cell malignancy [87], as well as the progression of bone metastasis (Fig. 2b) [81, 84], the inability to capture bone ECM mechanics inherently limits the physiologic relevance of these models. Furthermore, varying the concentration of collagen gels to control bulk stiffness simultaneously alters fibrillar network structure and adhesive ligand density, which independently modulate cell behavior [88]. Inability to recapitulate biochemical and physical properties of bone matrix restricts the physiologic relevance of cell behavior in hydrogel cultures, which may be improved by using platforms that allow systematic control of such parameters.
Cell-matrix interactions. (a) Tissue-specific ECM protein density and composition influence breast cancer cell adhesion and motility [80]. (b) Osteolytic PTHrP gene expression increases with substrate modulus for bone-metastatic breast (MDA), lung (RWGT2), and prostate (PC3) cancer cell lines [81]. (c) Compared to collagen type I matrices (COL I), decellularized osteoblast-derived matrix (OBM) bone tissues induce greater alignment of prostate cancer cell lines (PC3 and LNCaP) [90]. (d) Breast cancer cells penetrate deeper into and adhere better onto mineralized, HA-containing scaffolds. Arrows = walls, asterisks = pores, scale bars = 200 μm [83]. (Figures reproduced with permission from Royal Society of Chemistry, Elsevier, and Public Library of Science)
Decellularized matrices, which preserve the natural composition and structure laid down by osteogenic cells, not only direct osteogenic differentiation of BM-MSCs [89], but have also facilitated studies of tumor cell-ECM interactions. Compared to 2D collagen matrices, decellularized matrices derived from primary human osteoblasts have been shown to enhance alignment, migration, and osteogenic gene expression of prostate cancer cells (Fig. 2c) [90] as well as bone-metastatic breast cancer cells [82]. The feasibility of long-term studies with cell-derived ECMs can be further improved by surface-anchorage, a technique that preserves structural integrity of the ECMs and prevents their detachment in response to cell-mediated traction forces [91]. BM-MSCs in such cultures deposit even more physiologically relevant ECMs under macromolecular crowding conditions [92]. This results in enhanced expansion of hematopoietic progenitor cells, indicating that tumor cells may also respond to such conditions. Still, cell-derived matrices are commonly derived from monolayer cultures. Given that cellular ECM deposition is influenced by the underlying substrate [93], these ECMs may still not fully recapitulate the in vivo ECM structure and composition that can independently affect availability of ECM binding sites, and subsequent phenotypic changes of secondary cell types [94].
Decellularized bone tissue offers compositional and structural matrix cues inherent to native bone that may be explored for studies of bone metastasis. Indeed, decellularized bone tissue alters cellular phenotypes, and can support osteogenic differentiation of progenitor cells (adipose-derived stem cells [95], embryonic stem cells [96], BM-MSCs [97, 98]) as well as studies of tumor cell-bone interactions [99, 100]. However, it is worth noting that bone tissue architecture, marrow mechanics, and mineral content can vary greatly within a single bone, let alone across samples and species, limiting reproducibility of these models [101, 102]. These changes are important, for example, as bone mineral materials properties can independently modulate tumor cell behavior [103]. This suggests that bone metastasis models of the ECM should not only recapitulate proper organic ECM composition, but also the respective mineral component.
4.2.2 Inorganic Matrix (Mineral)
Along with collagen, HA mineral platelets constitute a fundamental building block of bone matrix, however few bone metastasis models incorporate this inorganic matrix component. Inclusion of HA nanoparticles within 3D scaffolds enhances osteogenic differentiation of stem cells in bone tissue engineering approaches [104,105,106], but has also been demonstrated to affect breast cancer cell adhesion and secretion of pro-osteoclastic IL-8 (Fig. 2d) [83]. Accordingly, biomaterial substrates mineralized by incubation with Simulated Body Fluid (SBF) equally promote adhesion and proliferation of breast cancer cells [107]. However, it should be noted that the materials properties of HA itself can vary extensively depending on patient age and disease [108]. In particular, HA particle size, crystallinity, and carbonate substitution are parameters that may vary in the presence of a secondary and/or primary mammary tumor [109, 110]. Hence, synthesis schemes that allow the formation of HA crystals with defined nanoparticle properties have been developed [103, 111]. Indeed, polymeric scaffolds containing HA with differentially controlled particle size and crystallinity impact breast cancer cell adhesion, proliferation, and osteolytic factor secretion as a function of varying HA characteristics [103]. While these in vitro studies strongly suggest a regulatory role of HA materials properties in bone metastasis, the in vivo relevance of these findings will need to be confirmed. Furthermore, HA is associated with collagen type I fibrils in the body. Hence, strategies to mineralize collagen fibrils based on SBF incubation [112, 113] and mineral co-precipitation during fibrillogenesis [114] should be considered to establish platforms that will allow dissection of the individual and combined effects of bone organic and inorganic ECM components during the pathogenesis of bone metastasis.
4.3 Cell-Cell Interactions
4.3.1 Direct: Cell-Cell Contact in Co-cultures of Tumor and Bone Cells
While isolating tumor cell interactions with the bone ECM will be essential for studies of skeletal metastasis, direct interactions of tumor cells with osteoblasts, osteoclasts, and other cells located in the bone are equally important. To design model systems that recapitulate these interactions, a variety of existing co-culture approaches initially developed for regenerative approaches [115,116,117,118] or studies of bone biology [119, 120] could be easily adapted. Still, mimicking the bone remodeling process in vitro remains a significant challenge due to the long time frames over which bone cells mature and the need for continuous supplementation of osteogenic precursor cells to carry out bone formation following resorption by osteoclasts. Nevertheless, appropriate combination of culture substrates and cell types can recapitulate conditions observed in vivo and thus, may ultimately reveal novel insights. For example, co-culturing breast cancer cells and osteoclasts within mineralized, collagenous osteoblastic tissue upregulates osteoclast differentiation and downregulates osteoblast differentiation, both of which are features observed in osteolytic bone lesions in vivo [121]. While this specific tri-culture model is very promising and yields physiologically relevant cell behavior, it may not be easily implemented in many conventional biology labs due to the need for custom bioreactors to ensure adequate nutrient and waste transport for the 3D tissue.
To circumvent the challenge of implementing long-term tri-cultures, a majority of co-culture studies focus solely on the interactions between tumor cells and a single type of bone cell. Several studies have explored the interactions between breast cancer cells and osteoblastic cells in co-culture, demonstrating that their interaction stimulates osteoclast formation [125, 126], exhibits hallmarks of in vivo bone metastatic progression [127, 128], and upregulates expression of the metastatic gene metadherin in breast cancer cells (Fig. 3a) [122]. Biomimetic 3D bone scaffolds have been increasingly used for these co-cultures, as they can help to simulate the behavior of cancer cells in vivo [122, 129]. In addition, co-culture of metastatic breast cancer cells with osteoclast precursor cells supplemented with soluble RANKL can mimic tumor-induced osteolytic activation in culture due to increased osteoclast formation [126]. Together, these studies may further improve understanding of how breast cancer cells alter the signaling between osteoblasts and osteoclasts that is critical to the development of bone metastasis. Nevertheless, current approaches primarily focus on osteoblasts and osteoclasts and typically disregard other bone-resident cells that may play equally important roles. For example, bone marrow progenitor cells such as hematopoietic stem cells are recruited to the bone via similar signaling pathways (e.g. the SDF-1/CXCR4 pathway) as tumor cells and, in fact, directly compete with tumor cells in the bone marrow niche [130,131,132]. To fully understand the mechanisms of pre-metastatic niche development and the vicious cycle of bone metastasis, culture models that incorporate crosstalk between various different populations of bone-resident cells and tumor cells will be essential. Finally, for effective therapeutic targets to be identified, it will be critical to determine whether phenomena observed in co-cultures are dependent on direct cell-cell contact or on paracrine signaling between cells.
Cell-cell interactions. (a) Increasing ratios of MSCs co-cultured with breast cancer cells (MDA-MB-231, BrCa) in bone-mimetic scaffolds yield greater metastasis-associated gene expression of metadherin (MTDH) [122]. (b) Exosomes derived from prostate cancer cells transform BM-MSCs into pro-migratory, alpha smooth muscle actin (αSMA) expressing myofibroblasts. Scale bars = 100 μm [124]. (Figures reproduced with permission from Elsevier and Impact Journals)
4.3.2 Indirect: Membranes, Cell-Derived Factors, Soluble Cues
Non-contact co-cultures utilizing transwell inserts have enabled study of the effects of bi-directional paracrine signaling between breast cancer cells and osteoclasts [133] as well as between breast cancer cells and BM-MSCs [123] in 2D cultures. To permit more physiologically relevant communication between multiple cell types, non-contact 3D co-cultures have also been established, for example, by placing two scaffolds, each seeded with either breast cancer cells or BM-MSCs, into a single well for culture [123]. Using this method of indirect 3D co-culture, BM-MSC osteogenic differentiation is decreased in the presence of breast cancer cells. While these findings suggest that breast cancer cell-secreted factors reduce osteogenic differentiation of BM-MSCs, the opposite, namely enhanced osteogenic differentiation of BM-MSCs, has also been shown [134]. Hence, it is imperative to consider whether bidirectional paracrine signaling is necessary for the given research question. Indeed, the importance of such feedback is underscored by studies implanting engineered bone microenvironments into tumor-bearing mice, in which BM-MSC migration from implants to mammary tumors in turn affects metastatic growth and frequency [135]. Furthermore, tissue-engineered bone implants have also highlighted that BM-MSCs exposed to BMP-2, a growth factor commonly associated with both osteogenesis [136] and tumorigenesis [137], enhances bone metastatic colonization [138]. Hence, methods to isolate the signaling of specific cell-secreted biomolecules remain relevant.
Historically, the effect of tumor-derived morphogens on cell signaling including BM-MSC migration [139], gene and protein expression [140], and differentiation [134], as well as osteoblast inflammatory response [141] have been frequently isolated with conditioned media. More recently, however, it has become clear that conditioned media not only contains secreted biomolecules, but also tumor cell-shed extracellular vesicles (EVs; e.g. exosomes, microvesicles) and that these EVs may be critical for tumor initiation and progression. More specifically, EVs are membrane-enclosed vesicles that are produced by tumor cells and can be isolated from conditioned media via size-based sorting and filtration techniques [142, 143]. EVs can promote cancer progression via stably transported cargo molecules (e.g. proteins, miRNAs, DNA). Additionally, cancer cell derived-EVs can direct organ-specific metastasis [35], transform the behavior of BM-MSCs and other stromal cells toward cancer-promoting phenotypes (Fig. 3) [124, 144], and increase the metastatic potential of poorly metastatic cells [145]. However, the exact mechanisms underlying these observations are not well understood. For example, whether tumor cells within bone shed different populations of EVs relative to those located at the primary site, and how these vesicles transmit information to recipient cells remains largely unclear. Studying the biogenesis and signaling mechanisms intrinsic to EVs in physiologically relevant models of bone metastasis promises to shed some light on these phenomena.
4.4 Mechanical Forces
Considering the load-bearing nature of bone and its functional adaptation to mechanical forces, as well as the observation that mechanical cues can affect bone metastatic progression, appropriate mechanical stimuli should be considered when designing bone metastasis models. In the context of bone regeneration, various bioreactor platforms (spinner flasks [146], rotating-wall vessels [147], direct perfusion [148], direct compression [149]) have been developed to impart physical forces that promote bone tissue formation. Similar setups can also be applied to probe the functional impact of such stimuli on the pathogenesis of bone metastasis. In general, tumor growth within bones induces static compression, which can enhance metastatic phenotypes in prostate cancer cells via osteocyte-secreted factors [150]. On the other hand, external cyclic compression of tumor-bearing tibiae to mimic the effect of physical activity has been shown to inhibit secondary tumor growth and osteolysis [14]. Together, these findings indicate that physical forces modulate metastatic progression, but the underlying mechanisms may be diverse. While load-bearing physical activity imparts cyclic compressive loads on bone-resident cells in vivo, it also generates interstitial flow that in and of itself can alter cell behavior due to altered transport of nutrients and waste products as well as small scale mechanical forces (e.g. shear stress, drag forces) [152]. Indeed, introducing interstitial flow into collagen scaffolds using microfluidic approaches influences the direction of breast cancer cell migration (Fig. 4a) [151]. Additionally, flow-derived shear stresses may regulate the drug resistance of tumors as suggested by studies in which tissue-engineered bone tumors were cultured in a flow perfusion bioreactor [153]. Whether these differences were mediated by direct effects on the tumor cells, altered transport of soluble factors, or a combination of the two remains to be investigated. Similarly, direct cyclic compression of HA-containing scaffolds using a custom bioreactor with loading platen upregulates expression of genes associated with bone metastasis by breast cancer cells (Fig. 4b) [14], while the same stimuli promote osteogenic differentiation of BM-MSCs when exposed to breast cancer cell-derived soluble factors [134]. Again, whether these changes are due to direct effects on the tumor cells or altered transport phenomena has yet to be elucidated. Nevertheless, these studies collectively underscore the need to incorporate physiologically relevant mechanical stimuli into bone metastasis models. This approach will be particularly useful in co-culture models involving osteocytes, given the key role of these cells in mechanotransduction [154].
Mechanical forces. (a) Microfluidic device generating a consistent interstitial flow field via pressure gradient across cell-embedded collagen I gel. Breast cancer cell migration occurs against the flow direction [151]. (b) Direct compression of breast cancer cell-seeded scaffolds in a loading bioreactor reduces expression of osteolysis-associated gene Runx2 [14]. (Figures reproduced with permission from National Academy of Sciences and John Wiley and Sons)
5 Future Perspectives
In conclusion, 3D tissue-engineered models of cancer bone metastasis have the potential to more accurately define the functional interplay between tumor and bone-resident cells that regulates bone metastasis. However, current models remain limited in their ability to fully recapitulate in vivo complexity of microenvironmental factors, including matrix properties (organic and inorganic components, mechanical properties), bone-resident cellular compartments (osteoblasts, osteocytes, osteoclasts, adipocytes, endothelial cells, immune cells), and physical forces (interstitial flow and cyclic compression). Looking forward, thorough characterization of metastasis-associated material changes to the bone microenvironment will be critical to more appropriately model and study their functional consequences. Considering the systemic nature of cancer metastasis, integrating these models with body-on-a-chip systems that also represent other organ sites will enable examination of relative metastatic frequencies as well as mechanistic investigations. The knowledge to be gained from integrative models of bone metastasis will inform therapeutic development, and when using patient-derived cells these models could provide predictive insights for precision medicine.
References
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331:1559–1564. doi:10.1126/science.1203543
Davila D, Antoniou A, Chaudhry MA (2015) Evaluation of osseous metastasis in bone scintigraphy. Semin Nucl Med 45:3–15. doi:10.1053/j.semnuclmed.2014.07.004
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931
Guise TA (2002) The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact 2:570–572
Zheng Y, Zhou H, Dunstan CR et al (2013) The role of the bone microenvironment in skeletal metastasis. J Bone Oncol 2:47–57. doi:10.1016/j.jbo.2012.11.002
Brock A, Krause S, Ingber DE (2015) Control of cancer formation by intrinsic genetic noise and microenvironmental cues. Nat Rev Cancer 15:499–509. doi:10.1038/nrc3959
Paget S (1889) The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 133:571–573. doi:10.1016/S0140-6736(00)49915-0
Fidler IJ (2003) The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer 3:453–458. doi:10.1038/nrc1098
Infanger DW, Lynch ME, Fischbach C (2013) Engineered culture models for studies of tumor-microenvironment interactions. Annu Rev Biomed Eng 15:29–53. doi:10.1146/annurev-bioeng-071811-150028
Weilbaecher KN, Guise TA, McCauley LK (2011) Cancer to bone: a fatal attraction. Nat Rev Cancer 11:411–425. doi:10.1038/nrc3055
Bussard KM, Gay C V, Mastro AM (2008) The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev 27:41–55. doi:10.1007/s10555-007-9109-4
Takayanagi H (2007) Osteoclast differentiation and activation. Clin Calcium 17:484–492. doi:10.1038/nature01658
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238. doi:10.1002/jbmr.320
Lynch ME, Brooks D, Mohanan S et al (2013) In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J Bone Miner Res 28:2357–2367. doi:10.1002/jbmr.1966
Lawson MA, McDonald MM, Kovacic N et al (2015) Osteoclasts control re-activation of dormant myeloma cells by remodeling the endosteal niche. Nat Commun 6:1–15. doi:10.1038/ncomms9983
Smith MCP (2004) CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 64:8604–8612. doi:10.1158/0008-5472.CAN-04-1844
Lapteva N, Yang A-G, Sanders DE et al (2005) CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther 12:84–89. doi:10.1038/sj.cgt.7700770
Liang Z, Yoon Y, Votaw J et al (2005) Silencing of CXCR4 blocks breast cancer metastasis silencing of CXCR4 blocks breast cancer metastasis. Cancer Res 65:967–971
Teicher BA, Fricker SP (2010) CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 16:2927–2931. doi:10.1158/1078-0432.CCR-09-2329
Wang H, Yu C, Gao X et al (2015) The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27:193–210. doi:10.1016/j.ccell.2014.11.017
Guise TA, Yin JJ, Taylor SD et al (1996) Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 98:1544–1549. doi:10.1172/JCI118947
Yin JJ, Selander K, Chirgwin JM et al (1999) TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 103:197–206. doi:10.1172/JCI3523
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593. doi:10.1038/nrc867
Kozlow W, Guise TA (2005) Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia 10:169–180. doi:10.1007/s10911-005-5399-8
Phadke PA, Mercer RR, Harms JF, Jia Y (2006) Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res 12:1431–1440
Youngs SJ, Ali SA, Taub DD, Rees RC (1997) Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer 71:257–266
Kirschmann DA, Seftor EA, Fong SFT et al (2002) A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res 1:4478–4483
Bendre MS, Montague DC, Peery T et al (2003) Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 33:28–37. doi:10.1016/S8756-3282(03)00086-3
Bendre MS, Margulies AG, Walser B et al (2005) Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res 65:11001–11009. doi:10.1158/0008-5472.CAN-05-2630
Kaplan RN, Riba RD, Zacharoulis S et al (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827. doi:10.1038/nature04186
Kaplan RN, Rafii S, Lyden D (2006) Preparing the “soil”: the premetastatic niche. Cancer Res 66:11089–11093. doi:10.1158/0008-5472.CAN-06-2407
Thorpe MP, Valentine RJ, Moulton CJ et al (2011) Breast tumors induced by N-methyl-N-nitrosourea are damaging to bone strength, structure, and mineralization in the absence of metastasis in rats. J Bone Miner Res 26:769–776. doi:10.1002/jbmr.277
Cox TR, Rumney RMH, Schoof EM et al (2015) The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522:106–110. doi:10.1038/nature14492
Costa-Silva B, Aiello NM, Ocean AJ et al (2015) Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17:1–7. doi:10.1038/ncb3169
Hoshino A, Costa-Silva B, Shen T-L et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature:1–19. doi:10.1038/nature15756
Kretschmann KL, Welm AL (2012) Mouse models of breast cancer metastasis to bone. Cancer Metastasis Rev 31:579–583. doi:10.1007/s10555-012-9378-4
Lelekakis M, Moseley JM, Martin TJ et al (1999) A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis 17:163–170. doi:10.1023/A:1006689719505
Campbell JP, Merkel AR, Masood-Campbell SK et al (2012) Models of bone metastasis. J Vis Exp. doi:10.3791/4260
Kang Y, Siegel PM, Shu W et al (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3:537–549. doi:10.1016/S1535-6108(03)00132-6
Garcia T, Jackson A, Bachelier R et al (2008) A convenient clinically relevant model of human breast cancer bone metastasis. Clin Exp Metastasis 25:33–42. doi:10.1007/s10585-007-9099-1
Juárez P, Guise TA (2011) TGF-β in cancer and bone: implications for treatment of bone metastases. Bone 48:23–29. doi:10.1016/j.bone.2010.08.004
Mundy GR, Yoneda T, Hiraga T (2001) Preclinical studies with zoledronic acid and other bisphosphonates: impact on the bone microenvironment. Semin Oncol 28:35–44. doi:10.1053/sonc.2001.24158
Canon JR, Roudier M, Bryant R et al (2008) Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis 25:119–129. doi:10.1007/s10585-007-9127-1
Holzapfel BM, Thibaudeau L, Hesami P et al (2013) Humanised xenograft models of bone metastasis revisited: novel insights into species-specific mechanisms of cancer cell osteotropism. Cancer Metastasis Rev 32:129–145. doi:10.1007/s10555-013-9437-5
Kuperwasser C, Dessain S, Bierbaum BE et al (2005) A mouse model of human breast cancer metastasis to human bone. Cancer Res 65:6130–6138. doi:10.1158/0008-5472.CAN-04-1408
Whittle JR, Lewis MT, Lindeman GJ, Visvader JE (2015) Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res 17:17. doi:10.1186/s13058-015-0523-1
DeRose YS, Wang G, Lin Y-C et al (2011) Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 17:1514–1520. doi:10.1038/nm.2454
Ganick DJ, Sarnwick RD, Shahidi NT, Manning DD (1980) Inability of intravenously injected monocellular suspensions of human bone marrow to establish in the nude mouse. Int Arch Allergy Immunol 62:330–333
Kyoizumi BS, Baum CM, Kaneshima H et al (1992) Implantation and maintenance of functional human bone marrow in SCID – hu Mice. Blood 79:1704–1711
Boynton E, Aubin J, Gross A et al (1996) Human osteoblasts survive and deposit new bone when human bone is implanted in SCID mouse. Bone 18:321–326. doi:10.1016/8756-3282(96)00015-4
Christianson SW, Greiner DL, Schweitzer IB et al (1996) Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell Immunol 171:186–199. doi:10.1006/cimm.1996.0193
Shultz LD, Schweitzer PA, Christianson SW et al (2010) Multiple defects in innate and adaptive immunologic function in NOD / LtSz-scid mice. J Immunol 154:180–191
Meyerrose TE, Herrbrich P, Hess DA, Nolta JA (2003) Immune-deficient mouse models for analysis of human stem cells. BioTechniques 35:1262–1272
Hutmacher DW, Horch RE, Loessner D et al (2009) Translating tissue engineering technology platforms into cancer research. J Cell Mol Med 13:1417–1427. doi:10.1111/j.1582-4934.2009.00853.x
Wang F, Weaver VM, Petersen OW et al (1998) Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A 95:14821–14826. doi:10.1073/pnas.95.25.14821
Hosseinkhani H, Hosseinkhani M, Tian F et al (2006) Osteogenic differentiation of mesenchymal stem cells in self-assembled peptide-amphiphile nanofibers. Biomaterials 27:4079–4086. doi:10.1016/j.biomaterials.2006.03.030
Rhodes NP, Srivastava JK, Smith RF, Longinotti C (2004) Metabolic and histological analysis of mesenchymal stem cells grown in 3-D hyaluronan-based scaffolds. J Mater Sci Mater Med 15:391–395. doi:10.1023/B:JMSM.0000021108.74004.7e
Fischbach C, Chen R, Matsumoto T et al (2007) Engineering tumors with 3D scaffolds. Nat Methods 4:855–860. doi:10.1038/nmeth1085
Kenny PA, Lee GY, Myers CA et al (2007) The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 1:84–96. doi:10.1016/j.molonc.2007.02.004
Weaver VM, Petersen OW, Wang F et al (1997) Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137:231–245. doi:10.1083/jcb.137.1.231
Weaver VM, Lelièvre S, Lakins JN et al (2002) β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2:205–216. doi:10.1016/S1535-6108(02)00125-3
Rizki A, Weaver VM, Lee S-Y et al (2008) A human breast cell model of preinvasive to invasive transition. Cancer Res 68:1378–1387. doi:10.1158/0008-5472.CAN-07-2225
Fraley SI, Feng Y, Krishnamurthy R et al (2010) A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12:598–604. doi:10.1038/ncb2062
Fischbach C, Kong HJ, Hsiong SX et al (2009) Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A 106:399–404. doi:10.1073/pnas.0808932106
DelNero P, Lane M, Verbridge SS et al (2015) 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials 55:110–118. doi:10.1016/j.biomaterials.2015.03.035
Pickl M, Ries CH (2009) Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 28:461–468. doi:10.1038/onc.2008.394
Sutherland RM, Eddy HA, Bareham B et al (1979) Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys 5:1225–1230. doi:10.1016/0360-3016(79)90643-6
Sato T, Stange DE, Ferrante M et al (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772. doi:10.1053/j.gastro.2011.07.050
Gao D, Vela I, Sboner A et al (2014) Organoid cultures derived from patients with advanced prostate cancer. Cell 159:176–187. doi:10.1016/j.cell.2014.08.016
Van De Wetering M, Francies HE, Francis JM et al (2015) Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161:933–945. doi:10.1016/j.cell.2015.03.053
Cheung KJ, Gabrielson E, Werb Z, Ewald AJ (2013) Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155:1639–1651. doi:10.1016/j.cell.2013.11.029
Elsdale T, Bard J (1972) Collagen substrata for studies on cell behavior. J Cell Biol 54:626–637. doi:10.1083/jcb.54.3.626
Benton G, Kleinman HK, George J, Arnaoutova I (2011) Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int J Cancer 128:1751–1757. doi:10.1002/ijc.25781
Kang B-J, Ryu H-H, Park S-S et al (2012) Effect of matrigel on the osteogenic potential of canine adipose tissue-derived mesenchymal stem cells. J Vet Med Sci 74:827–836. doi:10.1292/jvms.11-0484
Donzelli E, Salvadè A, Mimo P et al (2007) Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch Oral Biol 52:64–73. doi:10.1016/j.archoralbio.2006.07.007
Salasznyk RM, Williams WA, Boskey A et al (2004) Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol 2004:24–34. doi:10.1155/S1110724304306017
Evans ND, Gentleman E, Chen X et al (2010) Extracellular matrix-mediated osteogenic differentiation of murine embryonic stem cells. Biomaterials 31:3244–3252. doi:10.1016/j.biomaterials.2010.01.039
Shih Y-RV, Tseng K-FF, Lai H-YY et al (2011) Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res 26:730–738. doi:10.1002/jbmr.278
Pampaloni F, Reynaud EG, Stelzer EHK (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839–845. doi:10.1038/nrm2236
Barney LE, Dandley EC, Jansen LE et al (2015) A cell–ECM screening method to predict breast cancer metastasis. Integr Biol 7:198–212. doi:10.1039/C4IB00218K
Page JM, Merkel AR, Ruppender NS et al (2015) Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin β3 and TGF-β receptor type II. Biomaterials 64:33–44. doi:10.1016/j.biomaterials.2015.06.026
Taubenberger A V, Quent VM, Thibaudeau L et al (2013) Delineating breast cancer cell interactions with engineered bone microenvironments. J Bone Miner Res 28:1399–1411. doi:10.1002/jbmr.1875
Pathi SP, Kowalczewski C, Tadipatri R, Fischbach C (2010) A novel 3-D mineralized tumor model to study breast cancer bone metastasis. PLoS One. doi:10.1371/journal.pone.0008849
Guelcher SA, Sterling JA (2011) Contribution of bone tissue modulus to breast cancer metastasis to bone. Cancer Microenviron 4:247–259. doi:10.1007/s12307-011-0078-3
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689. doi:10.1016/j.cell.2006.06.044
Chaudhuri O, Gu L, Klumpers D et al (2015) Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. doi:10.1038/nmat4489
Paszek MJ, Zahir N, Johnson KR et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–254. doi:10.1016/j.ccr.2005.08.010
Baker BM, Trappmann B, Wang WY et al (2015) Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater 14:1262–1268. doi:10.1038/nmat4444
Datta N, Holtorf HL, Sikavitsas VI et al (2005) Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials 26:971–977. doi:10.1016/j.biomaterials.2004.04.001
Reichert JC, Quent VMC, Burke LJ et al (2010) Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials 31:7928–7936. doi:10.1016/j.biomaterials.2010.06.055
Prewitz MC, Seib FP, von Bonin M et al (2013) Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat Methods 10:788–794. doi:10.1038/nmeth.2523
Prewitz MC, Stißel A, Friedrichs J et al (2015) Extracellular matrix deposition of bone marrow stroma enhanced by macromolecular crowding. Biomaterials 73:60–69. doi:10.1016/j.biomaterials.2015.09.014
Antia M, Baneyx G, Kubow KE, Vogel V (2008) Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Discuss 139:229–249; discussion 309–325, 419–420. doi:10.1039/b718714a
Herklotz M, Prewitz MC, Bidan CM et al (2015) Availability of extracellular matrix biopolymers and differentiation state of human mesenchymal stem cells determine tissue-like growth in vitro. Biomaterials 60:121–129. doi:10.1016/j.biomaterials.2015.04.061
Fröhlich M, Grayson WL, Marolt D et al (2010) Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A 16:179–189
Marolt D, Marcos Campos I, Bhumiratana S et al (2012) Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci 109:8705–8709. doi:10.1073/pnas.1201830109
Mauney JR, Sjostorm S, Blumberg J et al (2004) Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int 74:458–468. doi:10.1007/s00223-003-0104-7
Grayson WL, Bhumiratana S, Cannizzaro C et al (2008) Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A 14:1809–1820. doi:10.1089/ten.tea.2007.0255
Villasante A, Marturano-Kruik A, Vunjak-Novakovic G (2014) Bioengineered human tumor within a bone niche. Biomaterials 35:5785–5794. doi:10.1016/j.biomaterials.2014.03.081
Holen I, Nutter F, Wilkinson JM et al (2015) Human breast cancer bone metastasis in vitro and in vivo: a novel 3D model system for studies of tumour cell-bone cell interactions. Clin Exp Metastasis 32:1–14. doi:10.1007/s10585-015-9737-y
Marcos-Campos I, Marolt D, Petridis P et al (2012) Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials 33:8329–8342. doi:10.1016/j.biomaterials.2012.08.013
Jansen LE, Birch NP, Schiffman JD et al (2015) Mechanics of intact bone marrow. J Mech Behav Biomed Mater 50:299–307. doi:10.1016/j.jmbbm.2015.06.023
Pathi SP, Lin DDW, Dorvee JR et al (2011) Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 32(22):5112. doi:10.1016/j.biomaterials.2011.03.055
Kim S-S, Sun Park M, Jeon O et al (2006) Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 27:1399–1409. doi:10.1016/j.biomaterials.2005.08.016
Shih Y-RV, Hwang Y, Phadke A et al (2014) Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc Natl Acad Sci U S A 111:990–995. doi:10.1073/pnas.1321717111
Mattei G, Ferretti C, Tirella A et al (2015) Decoupling the role of stiffness from other hydroxyapatite signalling cues in periosteal derived stem cell differentiation. Sci Rep 5:10778. doi:10.1038/srep10778
Ye M, Mohanty P, Ghosh G (2014) Biomimetic apatite-coated porous PVA scaffolds promote the growth of breast cancer cells. Mater Sci Eng C 44:310–316. doi:10.1016/j.msec.2014.08.044
Boskey A (2003) Bone mineral crystal size. Osteoporos Int 14:S16–S21. doi:10.1007/s00198-003-1468-2
Haka AS, Shafer-Peltier KE, Fitzmaurice M et al (2002) Identifying microcalcifications in benign and malignant breast lesions by probing differences in their chemical composition using Raman spectroscopy. Cancer Res 62:5375–5380
Bi X, Sterling JA, Merkel AR et al (2013) Prostate cancer metastases alter bone mineral and matrix composition independent of effects on bone architecture in mice–a quantitative study using microCT and Raman spectroscopy. Bone 56:454–460. doi:10.1016/j.bone.2013.07.006
Choi S, Coonrod S, Estroff L, Fischbach C (2015) Chemical and physical properties of carbonated hydroxyapatite affect breast cancer cell behavior. Acta Biomater 24:333–342. doi:10.1016/j.actbio.2015.06.001
Al-Munajjed AA, Plunkett NA, Gleeson JP et al (2009) Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J Biomed Mater Res - Part B Appl Biomater 90 B:584–591. doi:10.1002/jbm.b.31320
Xia Z, Yu X, Jiang X et al (2013) Fabrication and characterization of biomimetic collagen-apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater 9:7308–7319. doi:10.1016/j.actbio.2013.03.038
Zhang W, Liao SS, Cui FZ (2003) Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem Mater 15:3221–3226. doi:10.1021/cm030080g
Nakagawa K, Abukawa H, Shin MY et al (2004) Osteoclastogenesis on tissue-engineered bone. Tissue Eng 10:93–100. doi:10.1089/107632704322791736
Jones GL, Motta A, Marshall MJ et al (2009) Osteoblast: osteoclast co-cultures on silk fibroin, chitosan and PLLA films. Biomaterials 30:5376–5384. doi:10.1016/j.biomaterials.2009.07.028
Bernhardt A, Thieme S, Domaschke H et al (2010) Crosstalk of osteoblast and osteoclast precursors on mineralized collagen-towards an in vitro model for bone remodeling. J Biomed Mater Res - Part A 95:848–856. doi:10.1002/jbm.a.32856
Heinemann S, Heinemann C, Wenisch S et al (2013) Calcium phosphate phases integrated in silica/collagen nanocomposite xerogels enhance the bioactivity and ultimately manipulate the osteoblast/osteoclast ratio in a human co-culture model. Acta Biomater 9:4878–4888. doi:10.1016/j.actbio.2012.10.010
Jimi E, Nakamura I, Amano H et al (1996) Osteoclast function is activated by osteoblastic cells through a mechanism involving cell-to-cell contact. Endocrinology 137:2187–2190. doi:10.1210/endo.137.5.8612568
Hikita A, Iimura T, Oshima Y et al (2015) Analyses of bone modeling and remodeling using in vitro reconstitution system with two-photon microscopy. Bone 76:5–17. doi:10.1016/j.bone.2015.02.030
Krishnan V, Vogler EA, Sosnoski DM, Mastro AM (2014) In vitro mimics of bone remodeling and the vicious cycle of cancer in bone. J Cell Physiol 229:453–462. doi:10.1002/jcp.24464
Zhu W, Wang M, Fu Y et al (2015) Engineering a biomimetic three-dimensional nanostructured bone model for breast cancer bone metastasis study. Acta Biomater 14:164–174. doi:10.1016/j.actbio.2014.12.008
Dhawan A, von Bonin M, Bray LJ et al (2016) Functional interference in the bone marrow microenvironment by disseminated breast cancer cells. Stem Cells:1–17. doi:10.1002/stem.2384
Chowdhury R, Webber JP, Gurney M et al (2015) Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 6:715–731. doi:10.18632/oncotarget.2711
Thomas RJ, Guise TA, Yin JJ (1999) Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140:4451–4458
Mancino AT, Klimberg VS, Yamamoto M et al (2001) Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. J Surg Res 100:18–24. doi:10.1006/jsre.2001.6204
Dhurjati R, Krishnan V, Shuman LA et al (2008) Metastatic breast cancer cells colonize and degrade three-dimensional osteoblastic tissue in vitro. Clin Exp Metastasis 25:741–752. doi:10.1007/s10585-008-9185-z
Krishnan V, L a S, Sosnoski DM et al (2011) Dynamic interaction between breast cancer cells and osteoblastic tissue: comparison of two- and three-dimensional cultures. J Cell Physiol 226:2150–2158. doi:10.1002/jcp.22550
Sieh S, Lubik AA, Clements JA et al (2010) Interactions between human osteoblasts and prostate cancer cells in a novel 3D in vitro model. Organogenesis 6:181–188. doi:10.4161/org.6.3.12041
Müller A, Homey B, Soto H et al (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56. doi:10.1038/35065016
Shiozawa Y, Pedersen EA, Havens AM et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121:1298–1312. doi:10.1172/JCI43414DS1
Shiozawa Y, Eber MR, Berry JE, Taichman RS (2015) Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. Bonekey Rep 4:1–7. doi:10.1038/bonekey.2015.57
Pederson L, Winding B, Foged NT et al (1999) Identification of breast cancer cell line-derived paracrine factors that stimulate osteoclast activity. Cancer Res 59:5849–5855
Lynch ME, Chiou AE, Lee MJ et al (2016) 3D mechanical loading modulates the osteogenic response of mesenchymal stem cells to tumor-derived soluble signals. Tissue Eng Part A 22:1–10. doi:10.1089/ten.tea.2016.0153
Goldstein RH, Reagan MR, Anderson K et al (2010) Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res 70:10044. doi:10.1158/0008-5472.CAN-10-1254
Wozney JM, Rosen V, Celeste a J et al (1988) Novel regulators of bone formation: molecular clones and activities. Science 242:1528–1534. doi:10.1126/science.3201241
Katsuno Y, Hanyu A, Kanda H et al (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27:6322–6333. doi:10.1038/onc.2008.232
Moreau JE, Anderson K, Mauney JR et al (2007) Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res 67:10304. doi:10.1158/0008-5472.CAN-07-2483
Gao H, Priebe W, Glod J, Banerjee D (2009) Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 27:857–865. doi:10.1002/stem.23
Wobus M, List C, Dittrich T et al (2015) Breast carcinoma cells modulate the chemoattractive activity of human bone marrow-derived mesenchymal stromal cells by interfering with CXCL12. Int J Cancer 136:44–54. doi:10.1002/ijc.28960
Kinder M, Chislock E, Bussard KM et al (2008) Metastatic breast cancer induces an osteoblast inflammatory response. Exp Cell Res 314:173–183. doi:10.1016/j.yexcr.2007.09.021
Jia S, Zocco D, Samuels ML et al (2014) Emerging technologies in extracellular vesicle-based molecular diagnostics. Expert Rev Mol Diagn 14:307–321. doi:10.1586/14737159.2014.893828
Santana SM, Antonyak MA, Cerione RA, Kirby BJ (2014) Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations. Biomed Microdevices 16:869–877. doi:10.1007/s10544-014-9891-z
Antonyak MA, Li B, Lindsey K et al (2011) Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci 108:17569–17569. doi:10.1073/pnas.1114824108
Le MTN, Hamar P, Guo C et al (2014) miR-200 – containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest 124:5109–5128. doi:10.1172/JCI75695DS1
Stiehler M, Bünger C, Baatrup A et al (2009) Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res - Part A 89:96–107. doi:10.1002/jbm.a.31967
Song K, Liu T, Cui Z et al (2008) Three-dimensional fabrication of engineered bone with human bio-derived bone scaffolds in a rotating wall vessel bioreactor. J Biomed Mater Res A 86:323–332. doi:10.1002/jbm.a.31624
Huang C, Ogawa R (2012) Effect of hydrostatic pressure on bone regeneration using human mesenchymal stem cells. Tissue Eng Part A 18:2106–2113. doi:10.1089/ten.tea.2012.0064
Matziolis G, Tuischer J, Kasper G et al (2006) Simulation of cell differentiation in fracture healing: mechanically loaded composite scaffolds in a novel bioreactor system. Tissue Eng 12:201–208. doi:10.1089/ten.2006.12.201
Sottnik JL, Dai J, Zhang H et al (2015) Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res 75:2151–2158. doi:10.1158/0008-5472.CAN-14-2493
Polacheck WJ, Charest JL, Kamm RD (2011) Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci U S A 108:11115–11120. doi:10.1073/pnas.1103581108
Knothe Tate ML, Knothe U, Niederer P (1998) Experimental elucidation of mechanical load-induced fluid flow and its potential role in bone metabolism and functional adaptation. Am J Med Sci 316:189–195. doi:10.1097/00000441-199809000-00007
Santoro M, Lamhamedi-Cherradi S-E, Menegaz BA et al (2015) Flow perfusion effects on three-dimensional culture and drug sensitivity of Ewing sarcoma. Proc Natl Acad Sci U S A 112:1506684112. doi:10.1073/pnas.1506684112
Xiong J, Piemontese M, Onal M et al (2015) Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One 10:e0138189. doi:10.1371/journal.pone.0138189
Acknowledgements
We acknowledge financial support through NCI (R01CA173083), the Alexander von Humboldt Foundation (Fellowship for Experienced Researchers to CF), and a fellowship by the Graduate Assistance in Areas of National Need training grant from the Department of Education to AEC (P200A150273).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Chiou, A.E., Fischbach, C. (2018). Tissue-Engineered Models for Studies of Bone Metastasis. In: Soker, S., Skardal, A. (eds) Tumor Organoids. Cancer Drug Discovery and Development. Humana Press, Cham. https://doi.org/10.1007/978-3-319-60511-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-60511-1_6
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-60509-8
Online ISBN: 978-3-319-60511-1
eBook Packages: MedicineMedicine (R0)