Abstract
This chapter evaluates the “state-of-the-art” application of sentinel lymph node (SLN) procedure in patients with thyroid carcinoma. All PubMed/Medline listed papers including the key words “sentinel lymph node biopsy” and “thyroid carcinoma” published until January 2015 are taken into consideration. Both vital blue dye and radioisotope techniques are used in thyroid cancer patients and are discussed in this chapter. The SLN identification rates ranged from 0 to 100 % for blue dye, 64 to 100 % for radioisotopes, and 98 to 100 % for the combination of both techniques, respectively.
In conclusion, there is sufficient evidence to propagate the increasing use of the SLN technique in thyroid cancer. If the SLN is shown to consistently and accurately predict regional lymph node metastasis, a controlled randomized multicenter trial evaluating the effectiveness of this technique in patients with suspected or proven PTC is warranted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sentinel Lymph Node
- Thyroid Cancer
- Sentinel Lymph Node Biopsy
- Papillary Thyroid Carcinoma
- Differentiate Thyroid Cancer
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Rationale for Sentinel Node in Thyroid Cancer
1.1 The Concept of Sentinel Lymph Node (SLN) in Thyroid Cancer
Thyroid cancer is rare, but the most common endocrine malignancy [1, 2]. Differentiated thyroid cancer (DTC) accounts for over 90 % of thyroid malignancies and arises from thyroid follicular epithelial cells. DTC includes papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) with PTC representing more than 80 % of DTC [3]. Approximately 15 to 50% of patients with PTC have clinical evidence of cervical lymph node metastases at presentation, with up to 80% having micrometastatic disease. FTC represents about 20 % of DTC, and lymph node metastases are rare and metastatic disease mainly located in the liver and lungs; moreover, the metastatic spread happens usually by blood in FTC. FTC is typically diagnosed histopathologically, and not at cytology as for PTC.
Locoregional lymph node metastases of PTC are described to be associated with a worse prognosis, and extensive resection can improve the outcome of these patients. Therefore, correct identification of SLN involvement in PTS is crucial and impacts patient treatment and survival.
The SLN concept in DTC has been developed as an alternative to elective lymph node dissection in patients with clinically node-negative disease and was considered as an accurate technique for obtaining information about cervical lymph node involvement in patients undergoing thyroidectomy [4–6]. One of the complicating aspects in neck surgery and specifically in thyroid surgery is that the lymphatic drainage pathways are quite intricate [7]. The lymphatic vessels usually accompany blood vessels and nerves in directions that are not always predictable. The intrathyroid capillaries drain the lymphatic fluid to the lymphatic vessels associated with the capsule, potentially cross-communicating with the isthmus and the opposite lobe. Usually the superior lymphatic vessels drain the isthmus and the medial superior portion of the thyroid lobes, ascending in front of the larynx and terminating in the subdigastric lymph nodes of the internal jugular chain. The media inferior lymphatic vessels descend with the inferior vein to the pretracheal nodes. The lateral collecting vessels drain superiorly to the anterior and superior nodes of the internal jugular vein. Numerous classifications have been proposed to describe the location and the anatomic boundaries of lymph node groups in the neck. The most commonly used classifications are the ones of the American Joint Committee on Cancer (AJCC) and the American Society of Head and Neck Surgery (AHNS) [8, 9].
1.2 Lymph Node Metastases in Papillary Thyroid Carcinoma (PTC)
Patients with PTC frequently have lymph node metastases at the time of initial diagnosis and less frequently during successive follow-up. Incidence of metastasis is reported to range between 15 and 50 %, but microscopic metastases have been found in even up to 80 % of patients with PTC [10]. The thyroid gland has an extensive network of draining lymphatic vessels, both intraglandular and extraglandular [5, 9, 10]. Not surprisingly, the central neck compartment (level VI) is involved in approximately 90 % of patients with metastatic PTC [10, 11]; however, lateral and mediastinal compartment disease is also common [8, 9].
The involvement of lateral lymph nodes varies between 51 and 100 % in different series, with the caudal compartments involved more frequently than the cranial compartments [12]. Supraclavicular lymph nodes are the third site involved in terms of frequency, with a reported rate ranging from 10 to 52 % [11, 13]. Contralateral lymph node involvement is not rare with an incidence of up to 18 % for PTC [13, 14]. Mediastinal lymph node involvement, mostly the anterosuperior mediastinal lymph nodes, is less frequent at 1.9–15 % [11–13].
The distribution of locoregional lymph node involvement is poorly correlated to the site of the primary thyroid tumour. Even when the tumour is located in the upper third of the thyroid lobes, the subdigastric lymph nodes are often involved. Tumours located in the isthmus may cause bilateral cervical metastases with major risk of nodal recurrence on the contralateral neck side. The size of the primary tumour seems to have some importance: lymph node metastases in PTC <10 mm are usually found in the paratracheal area and rarely in the jugular nodes [8, 9]. It is important to mention that the reported incidence of regional lymphatic metastases identified in PTC patients varies according to the extent of nodal dissection performed [5, 15].
1.3 Prognostic Significance of Lymph Node Metastases in PTC
Surgical treatment is considered as the most effective therapy for patients with PTC. It remains controversial whether a prophylactic lymph node dissection improves the prognosis of PTC patients and whether the lymph node status predicts patient survival in PTC [4, 7, 10–13, 15]. Locoregional lymph node metastases of PTC are characterized by a worse prognosis, and extensive resection can improve the outcome of these patients. Some authors reported that nodal involvement has little influence on long-term survival of PTC patients, which is in contrast with other reports that found the presence of cervical lymph node metastases related to a worse prognosis due to an increased prevalence of locoregional recurrences; moreover, the finding of extracapsular invasion of lymph node metastases has been reported to be an indicator for the development of distant metastases and poor outcome [16, 17].
1.4 Surgical Techniques for Staging Neck in PTC
Currently, the extent of lymph node dissection is based predominantly on the histological type, stage of the primary tumour and the preoperative knowledge of lymph node involvement [18, 19]. In the presence of gross lymph node involvement, there is no debate about the need and prognostic benefit of a neck dissection in addition to total thyroidectomy. On the other hand, the management of a clinical N0 status node is generally much more conservative [4–7, 15].
In the absence of evidence favoring of routine prophylactic neck dissection, some surgeons perform “node picking”; while others perform lymphadenectomy of the ipsilateral central compartments. A more aggressive approach of routine prophylactic neck dissection has been suggested by some surgeons for PTC clinically lymph node negative patients secondary to the known high rate of occult micrometastatic disease in up to 80% of such PTC patients and the higher rate of locoregional recurrence of such patients who did not previously undergo routine prophylactic neck dissection [4–7, 15]. However, a general dissection of the central compartment may cause complications such as a damaged recurrent laryngeal nerve or higher hypoparathyroidism rates [7] and leads to overtreatment in patients with negative lymph nodes. The SLN procedure can avoid unnecessary lymph node dissection and reduce morbidity [7], identifying PTC patients with positive lymph nodes even if non-palpable or with a negative ultrasound (US) from true negative patients.
2 Methods of Sentinel Lymph Node Biopsy in PTC
2.1 Vital Blue Dye Technique
Early attempts of thyroid cromo-lymphoscintigraphy employed chlorophyll and Lipiodol UF in DTC, demonstrating the feasibility of the “sentinel lymph node concept” by showing coloured nodes to harbour metastasis [7]. Several studies have evaluated the vital blue dye technique for identification of the SLN in PTC [20–45] (Table 12.1).
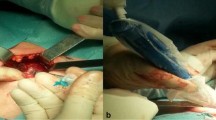
At the time of surgery, the vital blue dye is injected intratumorally or around the tumour using a tuberculin syringe (Fig. 12.1). It is important not to mobilize the thyroid gland before blue dye injection to secure intact lymphatic drainage. The blue dye can usually be seen within seconds, sometimes only after 1–2 min, passing through lymphatic vessels towards the SLN (Fig. 12.2). Blue-stained lymph nodes are then resected with extreme caution to avoid accidental removal of parathyroid glands that can also be blue coloured. After removal, the SLNs are submitted to histopathology for frozen section analysis. Disadvantages of the vital blue dye technique include the following: (1) risk of disruption and interruption of the lymphatic channels from the nodule, (2) difficulties in identifying SLN lying outside the central compartment; (3) need to identify the parathyroid glands prior to injection as they also take up the blue dye and (4) the technique is sometimes difficult and requires experience.
2.2 Lymphoscintigraphy and Intraoperative Gamma Probe Technique
To overcome some of these drawbacks, the use of preoperative lymphoscintigraphy with radiocolloids and intraoperative gamma probe detection was introduced (Table 12.2). Lymphoscintigraphy is an excellent method to visualize the lymphatic pathways and the SLN. It offers numerous important advantages compared to the vital blue dye technique: (1) preoperative injection of radiopharmaceutical eliminates risk of lymphatic disruption during operation, (2) it allows identification of SLN located outside the central compartment and (3) there is no physiological uptake in the parathyroid glands.
Current techniques use intranodular injection of 15–37 MBq 99mTc nanocolloid particles (particle size 20–80 nm) in a volume of 0.1–0.5 ml of saline. US-guided injection of the radionuclide is useful in small nodules located deep in the thyroid lobe (Fig. 12.3). Peritumoral injection should be avoided because of the high density of blood vessels in the thyroid gland with the risk of radiocolloid spillage outside the gland. Lymphatic drainage from the thyroid gland is visualized by dynamic images (1 frame per 15s, 64 × 64 matrix) in the anteroposterior projection for up to 10 min after injection. Longer acquisition times have been proposed (Fig. 12.4). Additional 5-min static images in anterior, lateral, and oblique views are usually obtained (256 × 256 matrix) for up to 1–3 h post injection or until adequate accumulation of the radiocolloid in the SLN is obtained. Surface localization of the SLN is marked with a water-resistant dye.
After a variable time interval (2–24 h), the patient is taken to the operating room for a total or near-total thyroidectomy. Following the removal of the thyroid tissue, a handheld collimated gamma probe is used to scan the central compartments (through the incision) and the lateral compartments (through skin surface) for “radioactive” lymph nodes (Fig. 12.5). SLN detection has been reported to be feasible up to 24 h post injection. A lesion-to-background ratio of 2:1 or greater is significant for SLN identification, although a smaller threshold level of 10 or 20 % is acceptable in breast cancer and melanoma.
The SLN(s) is (are) selectively excised, and the activity of the lymphatic bed is monitored with the handheld gamma probe to verify completeness of surgical removal (Fig. 12.6). It is important to emphasize that the thyroidectomy must precede SLN detection to avoid interference from radioactivity in the primary tumour. Finally, the SLN(s) is (are) sent for histopathology to screen for occult metastasis.
2.3 Combination of Vital Blue Dye and Lymphoscintigraphy and Intraoperative Gamma Probe Techniques
The combination of vital blue dye, lymphoscintigraphy, and intraoperative gamma probe techniques was described first in 2001 by Catarci et al. in 6 PTC patients (Table 12.3) [58]. They performed an intratumoral injection of 0.1 ml 99mTc-labelled colloidal albumin to visualize the SLN 2 h prior to surgery. At surgery, 0.1 ml per cm tumour diameter of Blue Patent V (2.5 %) was injected directly into the tumour, identified without dividing any structure in order to preserve the lymphatic drainage.
The SLN was identified by the flow and accumulation of the blue dye and the handheld gamma detection probe. SLN(s) was (were) correctly identified in all cases, suggesting that these techniques have a complementary role.
3 Summary of Available Studies
3.1 Data Collection
A PUBMED search was performed on 20 January 2015 for the MeSH headings “sentinel lymph node biopsy” and “thyroid carcinoma”. All original articles (retrospective and prospective) that examined the SLN techniques in human thyroid carcinoma were reviewed. The full text versions of the studies were obtained for further detailed evaluation.
Articles were subdivided depending on the technique used for SLN detection: group 1 included articles describing the vital blue dye technique, group 2 consisted of articles evaluating lymphoscintigraphy with radioisotopes and group 3 contained manuscripts involving both techniques for SLN detection. Extracted data included number of patients; preoperative diagnosis; postoperative diagnosis; SLN technique, in particular use of vital blue dye, isotope or both; volume and concentration; site of injection; time of injection; SLN detection rate; and metastatic SLN rate.
Reviews and letters were excluded [7, 59–63]. To avoid duplication of patient data in this chapter, multiple articles from the same authors and institutions were evaluated carefully for possible duplication. If this was thought likely, only the most recent article was included. Moreover, we excluded the studies of Saliba et al. and Maniakas et al. [64, 65] as they did not report the SLN technique.
Saliba et al. performed a retrospective chart review of 96 low-risk PTC patients who underwent a total thyroidectomy including SLN procedure. Patients with a negative SLN had a significantly lower postoperative thyroglobulin (Tg) level [64]. Maniakas et al. included 311 patients undergoing a total thyroidectomy and SLN biopsy for well-differentiated thyroid carcinoma in their retrospective chart review. Younger age (<45 years) and higher T category were found to be associated with a higher rate of positive SLNs [65].
3.2 Results of Studies
A total of 41 articles about SLN detection in thyroid carcinoma provided valuable information. Twenty-six studies reported on vital blue dye, 12 on SLN detection with radioisotopes and 3 on a combination of both techniques. The corresponding studies are summarized in Tables 12.1, 12.2, and 12.3 accordingly.
3.2.1 Vital Blue Dye Technique
In the 26 studies [20–45] evaluating vital dye for SLN detection, the patient number ranged between 9 [29] and 300 [43]. Further information including the preoperatively suspected diagnosis, the type of vital blue dye employed, the injected volume and injection technique are displayed in Table 12.1. The SLN was successfully visualized in a range between 0 % [29] and 100 % [20]. The SLN was positive for metastases in a range between 14 % [43] and 86 % [44].
3.2.2 Lymphoscintigraphy and Intraoperative Gamma Probe Technique
Table 12.2 summarizes the studies employing radioisotope-guided SLN technique [15, 42, 46–57] displaying in more detail the number of patients studied, the preoperative diagnosis and injection techniques. The SLN was successfully detected in a range of 64 % [51] to 100 % [15, 46–49, 56, 57] with corresponding detection rates of 64–100 % for intratumoral injection and 91–100 % for peritumoral injection, respectively. The identified SLN was positive for tumour cells in a range of 16 % [55] to 100 % [47].
3.2.3 Combination of Vital Blue Dye and Lymphoscintigraphy/Intraoperative Gamma Probe Techniques
Only three studies were reported on the combined use of vital blue dye and lymphoscintigraphy/intraoperative gamma probe techniques for SLN detection in thyroid carcinoma [34, 38, 58]. Corresponding patient numbers, preoperative diagnosis, the type of vital blue dye employed as well as injection technique and volumes are summarized in Table 12.3. In all three studies, the detection rate of the SLN was very high with 98 % [34] and 100 % [38, 58], respectively. Percentage of tumour-positive SLNs ranged between 50 % [34] and 67 % [58].
4 Current Status of Sentinel Lymph Node in Thyroid Cancer
The SLN technique is well standardized in melanoma and breast cancer, providing important information for patient treatment [7, 59]. The application of the SLN technique in DTC was first proposed 17 years ago by Kelemen et al. [20]. Since then the value of SLN in thyroid cancer including indications, benefits and limitations has been controversially discussed [59, 60]. Identification of the SLN is of particular value in PTC, as this tumour predominantly metastasizes lymphogenous, in contrast to the predominantly hematogeneous dissemination of FTC. Identification and resection of the SLN allow for the detection of microscopic metastatic disease, thus potentially reducing patient morbidity by avoiding unnecessary complete nodal dissection [59–63]. A topic of controversy is the prognostic significance of lymph node involvement in PTC and therefore whether accurate SLN detection is worthwhile and also questioning the indication of prophylactic lymphadenectomy of the central neck compartment [60–62].
SLN detection can be performed using vital blue dye, radioisotope-based lymphoscintigraphy and gamma probe detection as well as by the combined use of both techniques. Various studies reported in the literature have shown the isotopic procedure to be more precise (95–100 %) for SLN localization compared to vital blue dye (80–90 %); however, the isotopic procedure also has detractors [59–62]. In respect to feasibility and accuracy, preoperative lymphoscintigraphy and intraoperative gamma probe offer important advantages over the vital blue dye technique: (i)the injection of the radiopharmaceutical is done preoperatively, therefore eliminating disruption of the lymphatic vessels during the initial dissection; (ii) the use of the radiolabelled material permits to disclose the SLN that lies outside the central compartment; and (iii) there is no false-positive staining of the parathyroid glands. After identification, SLNs are selectively excised, and the activity of the lymphatic bed is assessed with the handheld gamma probe to verify background activity only within the resection bed after completion of the SLN biopsy procedure. Some authors have suggested that a combination of both procedures provides an even better yield [34, 38, 58].
Non-visualization of the lymphatics and of the SLN has been described for both techniques [59–63]. Potential explanations include lymphatic disruption during resection of the thyroid nodule, blockage of lymphatics by tumour or lymphatics, leading to a non-accessible site such as a retro-oesophageal or retrothyroid location. SLNs may occasionally be located in areas that are relatively inaccessible via a collar incision; this has been reported for both the radioisotope and the blue dye techniques [34, 38, 58]. Specific for the radioisotope technique is the requirement to remove the thyroid gland before identification of the SLN, because of the so-called “shine-through” effect. This is due to the close proximity of the central neck compartment lymph nodes to the thyroid nodes to the thyroid nodule where the radioisotope is injected.
The “shine-through” effect is especially problematic in the central neck compartment where the lymph nodes are located in close proximity to the thyroid. The “shine-through” phenomenon is also well known from SLN biopsy in oral cancers with reduced identification rates in floor of the mouth tumours due to the location of the SLNs in close vicinity of the injection site [66, 67]. Therefore, hybrid tracers [68], more specific radioactive tracers [69], intraoperative gamma cameras [70], and intraoperative 3D imaging [71] have been investigated and a multimodality approach, including preoperative hybrid imaging (SPECT/CT), has been proposed [72]. No corresponding studies are so far available for DTC, but these new approaches may also solve the “shine-through” problem in thyroid cancer. A limitation of the radioactive technique is the fact that in the majority of countries, the intraoperative use of radioactivity requires the existence of a Nuclear Medicine Unit and/or the presence of a nuclear medicine physician at surgery.
This chapter demonstrates that there is sufficient preliminary evidence to suggest the more rigorous use of the SLN technique in thyroid cancer. The utilization of SLN biopsy for PTC patients allows one to identify lymph node metastases more readily than based upon preoperative clinical exam [59–63]. Therefore, a controlled, randomized clinical trial evaluating the efficacy of SLN biopsy for identifying lymph node metastases in PTC patients and its resultant impact on management and long-term patient outcome seems warranted.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214.
Pasieka JL. Sentinel lymph node biopsy in the management of thyroid disease. Br J Surg. 2001;88(3):321–2.
Wiseman SM, Hicks Jr WL, Chu QD, Rigual NR. Sentinel lymph node biopsy in staging of differentiated thyroid cancer: a critical review. Surg Oncol. 2002;11(3):137–42.
Delbridge L. Sentinel lymph node biopsy for thyroid cancer: why bother? ANZ J Surg. 2004;74(1–2):2.
Rubello D, Pelizzo MR, Al-Nahhas A, Salvatori M, O’Doherty MJ, Giuliano AE, et al. The role of sentinel lymph node biopsy in patients with differentiated thyroid carcinoma. Eur J Surg Oncol. 2006;32(9):917–21.
Robbins KT. Classification of neck dissection: current concepts and future considerations. Otolaryngol Clin North Am. 1998;31(4):639–55.
Robbins KT, Medina JE, Wolfe GT, Levine PA, Sessions RB, Pruet CW. Standardizing neck dissection terminology. Official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg. 1991;117(6):601–5.
Shaha AR. Management of the neck in thyroid cancer. Otolaryngol Clin North Am. 1998;31(5):823–31.
Mirallie E, Visset J, Sagan C, Hamy A, Le Bodic MF, Paineau J. Localization of cervical node metastasis of papillary thyroid carcinoma. World J Surg. 1999;23(9):970–3; discussion 3–4.
Machens A, Hinze R, Thomusch O, Dralle H. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg. 2002;26(1):22–8.
Frankenthaler RA, Sellin RV, Cangir A, Goepfert H. Lymph node metastasis from papillary-follicular thyroid carcinoma in young patients. Am J Surg. 1990;160(4):341–3.
Noguchi M, Kumaki T, Taniya T, Miyazaki I. Bilateral cervical lymph node metastases in well-differentiated thyroid cancer. Arch Surg. 1990;125(6):804–6.
Boschin IM, Toniato A, Piotto A, Ide EC, Casara D, Guolo A, et al. 99Tc Nanocolloid sentinel node procedure in thyroid carcinoma. Langenbecks Arch Surg. 2008;393(5):705–8.
Yamashita H, Noguchi S, Murakami N, Kawamoto H, Watanabe S. Extracapsular invasion of lymph node metastasis is an indicator of distant metastasis and poor prognosis in patients with thyroid papillary carcinoma. Cancer. 1997;80(12):2268–72.
Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86(4):1447–63.
Mazzaferri EL, Massoll N. Management of papillary and follicular (differentiated) thyroid cancer: new paradigms using recombinant human thyrotropin. Endocr Relat Cancer. 2002;9(4):227–47.
Watkinson JC. The British Thyroid Association guidelines for the management of thyroid cancer in adults. Nucl Med Commun. 2004;25(9):897–900.
Kelemen PR, Van Herle AJ, Giuliano AE. Sentinel lymphadenectomy in thyroid malignant neoplasms. Arch Surg. 1998;133(3):288–92.
Dixon E, McKinnon JG, Pasieka JL. Feasibility of sentinel lymph node biopsy and lymphatic mapping in nodular thyroid neoplasms. World J Surg. 2000;24(11):1396–401.
Arch-Ferrer J, Velazquez D, Fajardo R, Gamboa-Dominguez A, Herrera MF. Accuracy of sentinel lymph node in papillary thyroid carcinoma. Surgery. 2001;130(6):907–13.
Fukui Y, Yamakawa T, Taniki T, Numoto S, Miki H, Monden Y. Sentinel lymph node biopsy in patients with papillary thyroid carcinoma. Cancer. 2001;92(11):2868–74.
Tsugawa K, Ohnishi I, Nakamura M, Miwa K, Yokoyama K, Michigishi T, et al. Intraoperative lymphatic mapping and sentinel lymph node biopsy in patients with papillary carcinoma of the thyroid gland. Biomed Pharmacother. 2002;56 Suppl 1:100s–3s.
Takami H, Sasaki K, Ikeda Y, Tajima G, Kameyama K. Detection of sentinel lymph nodes in patients with papillary thyroid cancer. Asian J Surg. 2003;26(3):145–8.
Chow TL, Lim BH, Kwok SP. Sentinel lymph node dissection in papillary thyroid carcinoma. ANZ J Surg. 2004;74(1–2):10–2.
Dzodic R, Markovic I, Inic M, Jokic N, Djurisic I, Zegarac M, et al. Sentinel lymph node biopsy may be used to support the decision to perform modified radical neck dissection in differentiated thyroid carcinoma. World J Surg. 2006;30(5):841–6.
Falvo L, Marzullo A, Palermo S, Biancafarina A, De Stefano M, Vanni B, et al. The sentinel lymph node in papillary cancer of the thyroid including histological subtype. Ann Ital Chir. 2006;77(1):13–8; discussion 8.
Peparini N, Maturo A, Di Matteo FM, Tartaglia F, Marchesi M, Campana EP. Blue-dye sentinel node mapping in thyroid carcinoma: debatable results of feasibility. Acta Chir Belg. 2006;106(5):523–7.
Abdalla HM. Feasibility of sentinel lymph node detection in nodular thyroid disease. J Egypt Natl Canc Inst. 2006;18(1):35–40.
Rubello D, Nanni C, Merante Boschin I, Toniato A, Piotto A, Rampin L, et al. Sentinel lymph node (SLN) procedure with patent V blue dye in 153 patients with papillary thyroid carcinoma (PTC): is it an accurate staging method? J Exp Clin Cancer Res. 2006;25(4):483–6.
Roh JL, Park CI. Sentinel lymph node biopsy as guidance for central neck dissection in patients with papillary thyroid carcinoma. Cancer. 2008;113(7):1527–31.
Bae JS, Park WC, Song BJ, Jung SS, Kim JS. Endoscopic thyroidectomy and sentinel lymph node biopsy via an anterior chest approach for papillary thyroid cancer. Surg Today. 2009;39(2):178–81.
Lee SK, Choi JH, Lim HI, Kim WW, Kim SM, Choe JH, et al. Sentinel lymph node biopsy in papillary thyroid cancer: comparison study of blue dye method and combined radioisotope and blue dye method in papillary thyroid cancer. Eur J Surg Oncol. 2009;35(9):974–9.
Takeyama H, Tabei I, Uchida K, Morikawa T. Sentinel node biopsy for follicular tumours of the thyroid gland. Br J Surg. 2009;96(5):490–5.
Anand SM, Gologan O, Rochon L, Tamilia M, How J, Hier MP, et al. The role of sentinel lymph node biopsy in differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135(12):1199–204.
Cunningham DK, Yao KA, Turner RR, Singer FR, Van Herle AR, Giuliano AE. Sentinel lymph node biopsy for papillary thyroid cancer: 12 years of experience at a single institution. Ann Surg Oncol. 2010;17(11):2970–5.
Huang O, Wu W, Wang O, You J, Li Q, Huang D, et al. Sentinel lymph node biopsy is unsuitable for routine practice in younger female patients with unilateral low-risk papillary thyroid carcinoma. BMC Cancer. 2011;11:386.
Ji YB, Lee KJ, Park YS, Hong SM, Paik SS, Tae K. Clinical efficacy of sentinel lymph node biopsy using methylene blue dye in clinically node-negative papillary thyroid carcinoma. Ann Surg Oncol. 2012;19(6):1868–73.
Li X, Ma H, Tian X, Jin X. Elective neck dissection in papillary thyroid carcinoma patients. Acta Chir Belg. 2012;112(1):44–50.
Larrad Jimenez A, de Quadros Borrajo P, Martin Duce A. Evaluation of the sentinel lymph node in T1-T2 papillary thyroid cancer: a preliminary study. Cir Esp. 2012;90(7):440–5. Valoracion del ganglio centinela en el cancer papilar de tiroides T1-T2. Estudio preliminar.
Hao RT, Chen J, Zhao LH, Liu C, Wang OC, Huang GL, et al. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur J Surg Oncol. 2012;38(8):718–24.
Jozaghi Y, Richardson K, Anand S, Mlynarek A, Hier MP, Forest VI, et al. Frozen section analysis and sentinel lymph node biopsy in well differentiated thyroid cancer. J Otolaryngol Head Neck Surg. 2013;42:48.
Wang JD, Deng XC, Jin XJ, Zhang C, Zhou JQ, Zhou QY, et al. [Surgical exploration of the sentinel lymph nodes in the papillary thyroid carcinoma]. Rev Laryngol Otol Rhinol (Bord). 2008;129(4–5):285–7. Exploration chirurgicale des ganglions sentinelles dans le cancer papillaire de la thyroide.
Kaczka K, Luks B, Jasion J, Pomorski L. Sentinel lymph node in thyroid tumors – own experience. Contemp Oncol (Pozn). 2013;17(2):184–9.
Rettenbacher L, Sungler P, Gmeiner D, Kassmann H, Galvan G. Detecting the sentinel lymph node in patients with differentiated thyroid carcinoma. Eur J Nucl Med. 2000;27(9):1399–401.
Stoeckli SJ, Pfaltz M, Steinert H, Schmid S. Sentinel lymph node biopsy in thyroid tumors: a pilot study. Eur Arch Otorhinolaryngol. 2003;260(7):364–8.
Pelizzo MR, Merante Boschin I, Toniato A, Piotto A, Bernante P, Paggetta C, et al. Sentinel node mapping and biopsy in thyroid cancer: a surgical perspective. Biomed Pharmacother. 2006;60(8):405–8.
Pelizzo MR, Rubello D, Boschin IM, Piotto A, Paggetta C, Toniato A, et al. Contribution of SLN investigation with 99mTc-nanocolloid in clinical staging of thyroid cancer: technical feasibility. Eur J Nucl Med Mol Imaging. 2007;34(6):934–8.
Carcoforo P, Feggi L, Trasforini G, Lanzara S, Sortini D, Zulian V, et al. Use of preoperative lymphoscintigraphy and intraoperative gamma-probe detection for identification of the sentinel lymph node in patients with papillary thyroid carcinoma. Eur J Surg Oncol. 2007;33(9):1075–80.
Lee SK, Kim SH, Hur SM, Choe JH, Kim JH, Kim JS. The efficacy of lateral neck sentinel lymph node biopsy in papillary thyroid carcinoma. World J Surg. 2011;35(12):2675–82.
Lee J, Na KY, Lee SJ, An YS, Yoon JK, Soh EY. The usefulness and accuracy of sentinel lymph node biopsy using single photon emission computed tomography/computed tomography with 99mTc phytate to detect locoregional lymph node metastases in patients with papillary thyroid carcinoma. J Korean Surg Soc. 2013;84(4):195–201.
Garcia-Burillo A, Roca Bielsa I, Gonzalez O, Zafon C, Sabate M, Castellvi J, et al. SPECT/CT sentinel lymph node identification in papillary thyroid cancer: lymphatic staging and surgical management improvement. Eur J Nucl Med Mol Imaging. 2013;40(11):1645–55.
Cabrera RN, Chone CT, Zantut-Wittmann D, Matos P, Ferreira DM, Pereira PS, et al. Value of sentinel lymph node biopsy in papillary thyroid cancer: initial results of a prospective trial. Eur Arch Otorhinolaryngol. 2015;272(4):971–9.
Carcoforo P, Portinari M, Feggi L, Panareo S, De Troia A, Zatelli MC, et al. Radio-guided selective compartment neck dissection improves staging in papillary thyroid carcinoma: a prospective study on 345 patients with a 3-year follow-up. Surgery. 2014;156(1):147–57.
Boni G, Mazzarri S, Grosso M, Manca G, Biricotti M, Ambrosini CE, et al. Sentinel node radioguided biopsy in surgical management of the medullary thyroid carcinoma A case report. Ann Ital Chir. 2014;21:85(ePub).
Puccini M, Manca G, Ugolini C, Candalise V, Passaretti A, Bernardini J, et al. Interest of sentinel node biopsy in apparently intrathyroidal medullary thyroid cancer: a pilot study. J Endocrinol Invest. 2014;37(9):829–34.
Catarci M, Zaraca F, Angeloni R, Mancini B, de Filippo MG, Massa R, et al. Preoperative lymphoscintigraphy and sentinel lymph node biopsy in papillary thyroid cancer. A pilot study. J Surg Oncol. 2001;77(1):21–4; discussion 5.
Balasubramanian SP, Brignall J, Lin HY, Stephenson TJ, Wadsley J, Harrison BJ, et al. Sentinel node biopsy in papillary thyroid cancer--what is the potential? Langenbecks Arch Surg. 2014;399(2):245–51.
Gonzalez O, Zafon C, Roca I. Selective sentinel lymph node biopsy in papillary thyroid carcinoma. Endocrinol Nutr. 2013;60(3):111–4.
Barczynski M. Systematic review and meta-analysis of sentinel node biopsy in thyroid cancer (Br J Surg 2010; 98: 334–344). Br J Surg. 2011;98(3):344–5.
Raijmakers PG, Paul MA, Lips P. Sentinel node detection in patients with thyroid carcinoma: a meta-analysis. World J Surg. 2008;32(9):1961–7.
Doherty GM. Sentinel lymph node biopsy for papillary thyroid cancer: commentary on the Efficacy of lateral neck sentinel lymph node biopsy in papillary thyroid carcinoma by Se Kyung Lee et al. World J Surg. 2011;35(12):2683.
Saliba J, Payne RJ, Varshney R, Sela E, Maniakas A, Rahme E, et al. Sentinel lymph node biopsy status correlates with postoperative stimulated thyroglobulin levels in low-risk papillary thyroid cancer patients. Endocr Pract. 2014;20(5):399–404.
Maniakas A, Forest VI, Jozaghi Y, Saliba J, Hier MP, Mlynarek A, et al. Tumor classification in well-differentiated thyroid carcinoma and sentinel lymph node biopsy outcomes: a direct correlation. Thyroid. 2014;24(4):671–4.
Civantos FJ, Zitsch RP, Schuller DE, Agrawal A, Smith RB, Nason R, et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J Clin Oncol. 2010;28(8):1395–400.
Alkureishi LW, Ross GL, Shoaib T, Soutar DS, Robertson AG, Thompson R, et al. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann Surg Oncol. 2010;17(9):2459–64.
van den Berg NS, Brouwer OR, Klop WM, Karakullukcu B, Zuur CL, Tan IB, et al. Concomitant radio- and fluorescence-guided sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity using ICG-(99m)Tc-nanocolloid. Eur J Nucl Med Mol Imaging. 2012;39(7):1128–36.
Agrawal A, Civantos FJ, Brumund KT, Chepeha DB, Hall NC, Carroll WR, et al. [Tc]tilmanocept accurately detects sentinel lymph nodes and predicts node pathology status in patients with oral squamous cell carcinoma of the head and neck: results of a phase III multi-institutional trial. Ann Surg Oncol. 2015;22(11):3708–15.
Vermeeren L, Valdes Olmos RA, Klop WM, Balm AJ, van den Brekel MW. A portable gamma-camera for intraoperative detection of sentinel nodes in the head and neck region. J Nucl Med. 2010;51(5):700–3.
Heuveling DA, van Weert S, Karagozoglu KH, de Bree R. Evaluation of the use of freehand SPECT for sentinel node biopsy in early stage oral carcinoma. Oral Oncol. 2015;51(3):287–90.
Valdes Olmos RA, Vidal-Sicart S, Giammarile F, Zaknun JJ, Van Leeuwen FW, Mariani G. The GOSTT concept and hybrid mixed/virtual/augmented reality environment radioguided surgery. Q J Nucl Med Mol Imaging. 2014;58(2):207–15.
Conflict of Interest Statement
The authors declare no conflict of interest with the present chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Boschin, I.M., Rubello, D., Bluemel, C., Herrmann, K., Pelizzo, M.R. (2016). Radioguided Sentinel Lymph Node Mapping and Biopsy in Thyroid Cancer. In: Herrmann, K., Nieweg, O., Povoski, S. (eds) Radioguided Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-26051-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-26051-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26049-5
Online ISBN: 978-3-319-26051-8
eBook Packages: MedicineMedicine (R0)