Abstract
Net energy intake (NEI) models are useful for quantifying mechanisms driving habitat selection in drift-feeding stream fishes; nonetheless, their complexity has limited their application in conservation. We evaluated the validity of assumptions and the performance of multiple variants of an exemplar NEI model for juvenile Chinook Salmon (Oncorhynchus tshawytscha), Dolly Varden Char (Salvelinus malma), and Arctic Grayling (Thymallus arcticus) in interior Alaska. We tested model assumptions that: (1) drift concentration, (2) fish visual reaction area, and (3) swimming cost do not vary meaningfully within the range of focal velocities occupied by drift-feeding stream fishes and can therefore be treated as constants or ignored. We then compared the predictive success of complex and simplified model variants. Comparisons of literature and field data indicated model assumptions were: (1) plausible, (2) plausible, and (3) implausible, respectively. Simplified model variants generally performed as well or better than the complex model. Drift concentration, visual reaction field, and swimming cost are important components of drift-feeder habitat selection; however, the difficulty of accurately estimating these variables may currently limit the utility of complex NEI models. Simplified NEI models are pragmatic tools for addressing urgent conservation needs and can guide development of complex NEI models as estimation techniques improve.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
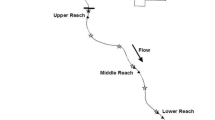
Rivers and streams are important habitats for many aquatic organisms, including the highly diverse fish assemblages of North America (Abell et al. 2008; Grossman et al. 1990; Poff et al. 2001). Many fishes in these lotic systems—including many if not most salmonids—feed on prey drifting downstream in the water column for all or a part of their lifetime and are known as drift-feeders (Quinn 2018). Drift feeding is a distinct foraging strategy whereby: (1) individuals occupy a fixed focal position facing upstream, (2) pursue and intercept prey flowing downstream, and (3) return to their initial focal position after attack. The suite of physical and biological characteristics in the immediate vicinity (~ m2) of a fish’s focal position comprise its microhabitat (Grossman and Freeman 1987; Piccolo et al. 2014; Grossman 2014).
Given that streams are heterogeneous in space and time, the ability to discern and select favorable microhabitats from the mélange of available options within the broader habitat matrix has important implications for individual fitness (Vannote et al. 1980; LaPerriere 1981; Hughes 1992). Studies of the mechanisms affecting microhabitat choice of drift-feeders have long been of interest to ecologists, because of their relevance to community and behavioral ecology, habitat and population management, and conservation (Jenkins 1969; Everest and Chapman 1972; Grossman et al. 1998). Correlative habitat selection studies comparing abundance to physical and chemical habitat characteristics are common but are generally unable to identify specific characteristics that drive habitat use (Boyce and McDonald 1999). Mechanistic models are a promising alternative to correlative studies because they quantify habitat characteristics relevant to a target species’ physiology and behavior (e.g., energy balance) and, ultimately, fitness (Grossman 2014; Rosenfeld et al. 2014; Naman et al. 2019).
Mechanistic net energy intake (NEI) models are useful tools that quantify the energetic benefits and costs associated with microhabitat use by drift-feeding stream fishes and then predict focal position selection or potential growth or abundance based on energy optimization criterion (Hayes et al. 2007, 2016; Wall et al. 2015). By quantifying the energetic benefits and costs associated with a given focal position, NEI models can identify optimal focal positions where the difference between the energetic benefits and costs is the greatest (Fig. 1). Drift-feeders are good candidates for mechanistic habitat use studies because they have been shown to preferentially select focal positions on the basis of energy optimization by occupying the stream position that affords the greatest energy intake that they can successfully defend in competitive hierarchies (Fausch 1984; Hughes 1998; Rosenfeld et al. 2014). Most NEI models assume drift-feeders maximize fitness by selecting focal positions that optimize energy intake (Fausch 1984; Hughes and Dill 1990; Hill and Grossman 1993); however, newer models have begun incorporating elements of survival (e.g., predation risk) in addition to strict energy optimization (Railsback et al. 2021).
Conceptual depiction of a cost–benefit NEI model for microhabitat use (via focal position velocity). The broken line is energetic cost; the solid line is energetic benefit. The maximum difference between cost and benefit lines is the optimal focal position velocity (denoted with an asterisk) where NEI is maximized
1.1 NEI Model Background
NEI models are grounded in optimal foraging theory, which connects habitat choice and foraging to fitness via energy optimization within the heterogeneous environmental matrix of a stream (MacArthur and Pianka 1966; Pyke et al. 1977; Schoener 1971). The many NEI models that have been developed and refined in the decades since Fausch’s (1984) original model vary in predictive goals, information requirements, complexity, and realism (Piccolo et al. 2014; Rosenfeld et al. 2014). NEI models with different predictive goals have different input requirements and different sensitivities to potential biases of those inputs. Models that predict instantaneous microhabitat selection rank stream positions by their relative energetic potential (e.g., Guensch et al. 2001; Grossman et al. 2002). Therefore, slightly biased estimates of microhabitat energetic potential—via inaccurate estimates of input variables or structural errors in how the model estimates NEI—may still produce accurate predictions of optimal focal point velocities as long as the relative ranking of microhabitats is correct. Conversely, models that predict drift-feeder growth or abundance over entire stream reaches or fish lifespans (e.g., Hayes et al. 2000, 2007; Wall et al. 2015) are dependent on accurate input variable estimates (e.g., drift abundance, swimming costs) to produce accurate estimates of absolute NEI. Therefore, NEI models that predict instantaneous microhabitat selection based on relative energetic potential may be more easily and appropriately simplified and generalized than NEI models which rely on more complete characterizations of absolute NEI to predict potential growth or carrying capacity at a given site.
NEI models also differ in terms of the variables they use to quantify energetic benefits and costs to predict habitat use. Variables associated with energetic gain include prey energy content, density of prey in the drift, and prey encounter and capture rates (e.g., Grossman et al. 2002; Jenkins and Keeley 2010; Naman et al. 2019). Energetic cost variables include metabolic cost of swimming at focal positions, maneuvering costs for prey pursuit and capture, and prey processing costs, which often are estimated via equations from bioenergetic models for different species (Hughes and Kelly 1996; Hayes et al. 2000, 2016). Finally, NEI models with both instantaneous and long-term predictive goals frequently incorporate environmental and behavioral variables hypothesized to influence fish energetics, including velocity, depth at fish focal position, fish visual reaction area, foraging time, turbidity, presence of competitors, and amount of woody material (Harvey and Railsback 2009; Wall et al. 2017; Kalb et al. 2018).
The variables included in a given NEI model are largely dependent upon the predictive goals of the model, the species or system it is to be applied to, and insights gained from previous modeling and parameter estimation efforts. Most contemporary NEI models are built on the shoulders of one or more foundational models (Piccolo et al. 2014). For instance, Dodrill et al. (2016) developed an NEI model based on a previous model adapted by Hayes et al. (2000) from one of the earliest NEI models (Hughes and Dill 1990). The development of new NEI models is an incremental process incorporating more recent information, such as variable estimates or measurements that previously were held constant or neglected. For example, Hayes et al. (2016) incorporated the effects of velocity and turbidity on prey capture success, as well as prey pursuit costs across velocity gradients, which were not included in an earlier iteration of the model (Hayes et al. 2007). In general, mechanistic drift-foraging NEI models are better predictors of drift-feeder growth than correlative models and newer, more realistic NEI models ostensibly should be better predictors of drift-feeder habitat selection than their predecessors (Grossman 2014; Naman et al. 2019). However, empirical comparisons of the performance of incrementally progressive NEI models are rare (Hughes and Dill 1990; Naman et al. 2019; Jowett et al. 2021). Model parsimony generally is desirable, and more work is needed to assess how NEI models with differing amounts of complexity and biological realism perform in comparative studies with the same data.
In all modeling applications, there is tension between ease of parameterization and use, and biological realism. Simplified NEI models contain few input variables and are relatively easy to parameterize and test. For instance, an NEI model that uses the relationship between prey capture success and velocity to predict optimal focal point velocity is easily parameterized via laboratory experiments that characterize this relationship (e.g., Hill and Grossman 1993). However, the mechanistic insight and predictive value of these simplified models may be limited because they do not incorporate all variables that potentially influence focal position selection, such as the amount of available prey in the drift, metabolic costs of swimming and pursuing prey, or fish visual reaction area. This fact necessitates evaluation of simplified models under varying conditions and with varying species.
Conversely, complex NEI models incorporate a range of biological and physical variables to more accurately characterize biological reality. Because complex models may more closely approximate the actual habitat conditions and foraging processes that determine drift-feeder focal position selection, they potentially have greater ability to explain habitat use, growth or carrying capacity than simplified models. However, the predictive ability success of complex NEI models is dependent upon our ability to estimate input variables precisely and accurately. Each variable incorporated into a complex model has both a value and an error term; if variable error terms are large, models that incorporate greater realism may actually exhibit reduced ability to predict optimal focal velocities, growth, or reach-specific abundances. Furthermore, the high spatial and temporal heterogeneity of complex variables, such as macroinvertebrate drift dynamics, further complicates our ability to incorporate these processes in NEI models in useful ways (Brittain and Eikeland 1988; Naman et al. 2016).
Species-specific data for some complex NEI model variables is limited, so researchers sometimes substitute data from different species to parameterize models. For example, Brett and Glass’ (1973) swimming cost equations for Sockeye Salmon (Oncorhynchus nerka) and Rao’s (1968) model of oxygen consumption of Rainbow Trout (Oncorhynchus mykiss) frequently are used to estimate some or all of the metabolic costs associated with drift-feeding for other salmonid species (e.g., Hayes et al. 2000; Hughes and Dill 1990; Rosenfeld and Taylor 2009) or are extrapolated beyond the range of temperatures, masses, and velocities to which the original models were fit. Species-borrowing is not inherently bad, but even closely related species can exhibit substantively different metabolic rates (Trudel and Welch 2005). Therefore, the utility of complex NEI models that incorporate greater biological realism may be limited or negated by practical constraints associated with uncertainty regarding the quality and error of parameter estimates, or a lack of empirical data.
Simplified predictive models sometimes emerge when modelers, who set out to explain a natural phenomenon with as much biological realism as is practical, observe that one or a few model parameters exert disproportionate effects on model output, and condense the model to highlight those influential parameters. Hill and Grossman (1993) attempted to build a complex NEI model to explain focal position selection of Rainbow Trout (Oncorhynchus mykiss) and Rosyside Dace (Clinostomus funduloides) as a function of standard and active metabolic rate (data from Facey and Grossman 1990), food utilization efficiency, prey capture success, and prey abundance in a North Carolina stream. This model described focal position selection in terms of focal velocity, which is the velocity at the focal position as measured from the nose of the fish. They found, however, that prey capture success contributed disproportionately to the output of the complex model, and that the point at which prey capture success declined most rapidly with increasing velocity (i.e., the minima of the third derivative of the prey capture success-velocity function) was a better predictor of focal velocities occupied by these species in the stream than the complex model.
Consequently, Grossman et al. (2002) developed and field tested a simplified NEI model for four cyprinid species based solely on the negative logistic relationship between focal velocity and prey capture success (Fig. 2). The original, more complex version of their model included energy content of prey in the drift, fish visual reaction area, and swimming costs. However, many drift-feeding species in the study system (a fifth order stream in the Southern Appalachian Mountains) occupied focal positions within a relatively small range of low velocities (~5–20 cm/s; Grossman and Freeman 1987; Facey and Grossman 1992; Hill and Grossman 1993), and previous work in the same system suggested there was little variation in energetic costs at these velocities (Facey and Grossman 1990). This observation led to the removal of swimming costs, fish visual reaction area, and energy content of prey in the drift from the full model under the assumption that they varied minimally across the low and narrow range of velocities occupied by these drift-feeders, and could be considered constant (Facey and Grossman 1990, 1992; Grossman et al. 2002).
The simplified Grossman et al. (2002) NEI model has been field tested on nine species in systems ranging from the Southeastern US to Alaska. The model has successfully predicted optimal habitat selection (via focal position velocity) for seven species, displayed marginal success for interior Dolly Varden Char, and failed to predict microhabitat selection by juvenile Chinook Salmon (Grossman et al. 2002; Donofrio et al. 2018; Bozeman and Grossman 2019a, b; Sliger and Grossman 2021). Despite its success at predicting focal position velocities, the validity of the simplified Grossman et al. (2002) model assumptions has not been assessed, and the simplified version of the model has not been tested against the full, more complex version.
To our knowledge, there has not been a review, comparison, and field test of simplified and complex versions of an NEI model to assess potential differences in predictive abilities. The lack of understanding of the influence of complex variables on NEI model output—as well as the validity of simplifying assumptions—is a potential blind spot that hinders our ability to determine the utility and generality of these models. Consequently, we used full and simplified variants of the Grossman NEI models, empirical data, and data from the literature to evaluate the validity of model assumptions and compare predictive success of models with differing levels of complexity.
1.2 NEI Model Variants
Conceptually, the full NEI model explains focal position energetics for drift-feeders as a function of energy intake
where I is the net energy intake, E is the prey encounter rate, P is the proportion of prey captured that enter the visual field of the fish, and S is the swimming cost, all at microhabitat x (Grossman et al. 2002). Thus, net energy intake is a function of the number of prey that a fish encounters, pursues, and successfully captures at a specific focal position, minus the metabolic cost of maintaining that focal position.
Prey encounter rate, E, at a given microhabitat x, is expressed as
where D is the abundance of prey in the drift converted to energy density (J/m3), A is the visual reaction area of the fish (m2), and V is the velocity (m/s). The proportion of prey captured that enter the visual field of the fish (P) at a given microhabitat can be expressed as
where b and c are curve-fitting constants as estimated by nonlinear least squares regression for the relationship between P and V at microhabitat x.
Therefore, given Eqs. (1), (2), and (3), net energy intake (I) at microhabitat x is mechanistically estimated via
Equation (4) is the full NEI model.
After simplifying the full NEI model based on the assumption that D, A, and S vary minimally across the range of drift-feeder focal velocities and thus can be dropped from the equation (Facey and Grossman 1990, 1992; Grossman et al. 2002), we obtain
which is solved iteratively to predict the velocity (V) at optimal microhabitat x (i.e., optimal focal velocity) where net energy intake (I) is maximized by a drift-feeder. Equation (5) is the simplified NEI model, which is dependent only on the relationship between prey capture success and velocity (Fig. 2).
The velocity term in the simplified NEI model reflects the velocity at which the prey are traveling when captured (as driven by treatment velocities in the experimental stream flume). However, drift-feeding stream fish are known to occupy slower focal velocities and capture prey in nearby faster velocities (Hughes and Dill 1990). Therefore, we used the experimentally derived relationship between focal and foraging velocities to adjust the simplified NEI model output reflect observed differences in focal and foraging velocities; this is the adjusted NEI model (Sliger and Grossman 2021). The third derivative of the negative logistic relationship between prey capture success and velocity (Fig. 2) is the rate of increase of acceleration of prey capture success as velocity increases. We calculated the minima of the third derivative function—which is the maximum point of deceleration of the P–V curve—for each of our study species (Hill and Grossman 1993). This is the third derivative NEI model.
1.3 Study Objectives
We had two study objectives: (1) to assess the validity of the assumptions made by the simplified NEI model (Eq. 5)—that energy content of prey in the drift, fish visual reaction area, and swimming cost terms from the full model could be omitted; and (2) to compare the optimal focal velocity predictions of the full, simplified, adjusted, and third derivative NEI models. To satisfy these objectives, we used empirical field data and data from the literature to address the following questions: (1) Are energy content of prey in the drift, fish visual reaction area, and swimming cost correlated with focal position velocities occupied by juvenile Chinook Salmon (Oncorhynchus tshawytscha), Dolly Varden Char (Salvelinus malma), and Arctic Grayling (Thymallus arcticus) from interior Alaskan streams? (2) What is the range of focal position velocities occupied by drift-feeding fishes as reported in the literature? (3) Does the published literature reveal consistent correlations between commonly occupied focal velocities and energy content of prey in the drift, fish visual reaction area, and swimming cost? and (4) What is the comparative performance of the original Grossman et al. (2002) full NEI model, simplified NEI model, adjusted NEI model, and third derivative model with respect to predicting optimal focal velocities?
2 Methods
We tested for correlations between energy content of prey in the drift (D), visual reaction area (A), and swimming cost (S) and focal velocities of juvenile Chinook Salmon, Dolly Varden Char, and Arctic Grayling using field observations and laboratory experiments. We also reviewed the primary literature to summarize the range of focal velocities commonly occupied by drift-feeders and the reported relationships between D, A, S, and stream velocity, within and beyond the range of common focal velocities. Finally, we used these data to evaluate the validity of simplified NEI model assumptions and parameterize and compare output of four NEI model variants.
2.1 Study Species and Systems
We studied populations of juvenile Chinook Salmon in the Chena River, Dolly Varden Char in Panguingue Creek, and Arctic Grayling in the Richardson Clearwater River in Alaska’s Yukon River Drainage. Additional site and species information may be found in Donofrio et al. (2018), and Bozeman and Grossman (2019a, b). These three species are ecologically, economically, and culturally important in interior Alaska. Chinook Salmon populations in the Chena River have been studied and monitored for several decades, and are in decline in some parts of the state (Barton 1986; Schindler et al. 2013). Similarly, Arctic Grayling populations in the Richardson Clearwater River have been monitored for many years (Ridder 1988; Gryska 2001). Comparatively, little is known about the Dolly Varden Char population in Panguingue Creek or other interior populations of this species within its native range in the Pacific Northwest (Washington Department of Fish and Wildlife 2000; Bozeman and Grossman 2019b). Interior Dolly Varden Char are widely but patchily distributed throughout much of Alaska (Armstrong and Morrow 1980). We chose these system-species combinations because they were representative of ideal habitats for the respective study species and had water clarity permitting extensive video observations.
2.2 Field Observations
We conducted field observations during summer (June–August) of 2015 and 2016 in the Chena River (juvenile Chinook Salmon), Richardson Clearwater River (Arctic Grayling), and Panguingue Creek (Dolly Varden Char and Arctic Grayling). Mean standard length (± SD) of fish observed in the field for foraging behavior data collection was 4.7 cm (±1.0) for juvenile Chinook Salmon (N = 24), 17.6 cm (±2.8) for Dolly Varden Char (N = 32), and 42.4 cm (±4.5) for Arctic Grayling (N = 29). Field data were obtained by identifying drift-feeding individuals via streamside observation, placing paired underwater video cameras near drift-feeding positions, and recording drift-feeding activity once fish had resumed normal foraging behavior, and then capturing videoed individuals via hook and line once videography data was collected for length and mass measurements and diet content analysis. Turbidity was low in study systems (visibility >1 m, see: https://www.youtube.com/watch?v=BJokgZrAi84&t=15s), and not dissimilar to conditions in the experimental flume (see: https://www.youtube.com/watch?v=RXcn1ew3KuM).
2.2.1 Energy Density in the Drift (D)
We estimated energy density in the drift (D, J/m3) by placing fine mesh (100 μm, 47.7 × 29.2 cm opening, Chena River only), coarse mesh (243 μm, 49.5 × 29.5 cm opening), and ultra-coarse mesh (500 μm, 32 × 32 cm opening, 2016 Richardson Clearwater River only) drift nets in our study sites in habitat that contained drift-foraging fish. We measured velocity (m/s, electronic velocity meter) and water depth (straightedge, m) at net placement sites. We placed drift nets as close as possible (straight upstream or downstream) to drift-feeding fish without disturbing them (3–20 m away) for an average of 45 min (range: 10–186 min). After we removed drift nets from the stream, we split captured prey into 1 mm size classes (1–10 mm) and estimated energy content based on prey identity and published length-mass regressions (e.g., Rogers et al. 1977; Benke et al. 1999; Sabo et al. 2002). We used the length and width of the net openings (m2) along with water velocity measurements (m/s) at drift-net placement positions to measure the volume of water filtered per sampling time. We estimated prey drift concentration (items/m3) using the maximum observed value for either the fine or coarse net for each taxon to account for backwash bias (J. Neuswanger pers. comm.). Finally, we multiplied mean prey energy content (J) by prey drift concentration (items/m3) for each size class and then summed across size classes to estimate energy content of prey in the drift (D, J/m3) for use in analyses.
2.2.2 Visual Reaction Area of the Fish (A)
We used videos of juvenile Chinook Salmon in the Chena River, Dolly Varden Char in Panguingue Creek, and Arctic Grayling in the Richardson Clearwater and VidSync 3D video analysis software to estimate several metrics of fish reaction distance (VidSync.org; Neuswanger et al. 2016). We reviewed field video footage for each of our study species and recorded the distance between a drift-foraging individual and a prey item when the fish first oriented toward the prey item to initiate a discrete foraging attempt. Reaction distance measurements were linear (cm) in three-dimensional space (i.e., straight line distance from fish snout to prey item in any direction). We used the 95th percentile of fish lateral reaction distance (i.e., cross-stream plane) as the radius to calculate a circular reaction area (cm2) perpendicular to the direction of stream flow (Hughes and Dill 1990) for use in our analysis. We truncated the circular reaction area when the radius was greater than the distance from fish focal position to the surface and/or stream bottom. Reaction distance values for each individual observed (juvenile Chinook Salmon N = 24, Dolly Varden Char N = 32, Arctic Grayling N = 29) were based on an average of 103 measurable foraging attempts (range: 46–180) per individual. Mean lengths (± SD) of prey items consumed during foraging attempts were 2.3 mm (±0.4) for juvenile Chinook Salmon, 3.9 mm (±0.6) for Dolly Varden Char, and 6.0 (±0.9) for Arctic Grayling.
2.2.3 Swimming Cost (S)
We estimated the total metabolic costs of drift feeding as the sum of standard metabolic rate, swimming activity at the focal position, and foraging maneuvers to capture prey. We estimated standard metabolic rate as a function of temperature and mass using models parameterized for species closely related to our study species; Baikal Grayling (Thymallus baicalensis; Hartman and Jensen 2017) for Arctic Grayling, Bull Trout (Salvelinus confluentus; Mesa et al. 2013) for Dolly Varden Char, and an Oncorhynchus spp. model that is widely used for Chinook Salmon (Stewart et al. 1983; Stewart and Ibarra 1991). We used a mass- and swimming speed-dependent equation from Trudel and Welch (2005) parameterized for Sockeye Salmon (Brett and Glass 1973) to estimate swimming cost associated with holding a fixed focal position in the stream. Finally, we used a maneuver model (Neuswanger et al. in preparation) to estimate the metabolic cost of maneuvering to capture prey in the drift and returning to the focal position. Accounting for standard metabolic rate, swimming cost, and foraging maneuvers likely is a more accurate characterization of metabolic costs incurred by drift-feeders than steady swimming costs alone (Hughes and Kelly 1996).
2.2.4 Focal Position Velocity (V)
We quantified focal velocity using in situ stream velocity measurements at fish focal positions and field videos and VidSync. Focal velocity is the velocity at the nose of a drift-feeding stream fish. For juvenile Chinook Salmon in the Chena River, Dolly Varden Char in Panguingue Creek, and Arctic Grayling in the Richardson Clearwater, we estimated focal velocities by observing drift-feeding individuals via the cameras and releasing pre-soaked, neutrally buoyant Israeli cous-cous upstream of the individual. During video analysis, we used the cous-cous particles as velocity tracers and averaged the velocities of the six tracers nearest to the drift-feeding fish. For Arctic Grayling in Panguingue Creek, we identified drift-feeding individuals (N = 25) in the camera viewfinders, observed each individual pursue and capture at least five prey items and return to the same fixed focal position between foraging attempts, and then measured focal position velocity with a Marsh McBirney Model 201 electronic flow meter.
To evaluate the assumption that energy content of prey in the drift (D), visual reaction area (A), and swimming cost (S) could be held constant across the range of velocities occupied by drift-feeders, we regressed values of A and S against focal velocities from each species-stream combination. Because D was sampled in locations that did not necessarily correspond to stream fish focal positions, we regressed values of D with velocities taken at drift-net placement positions, which were well within the range of focal velocities occupied by drift-feeders in the same stream. We used a t-test to test the null hypothesis that the slope of the regression line does not differ significantly from zero.
2.3 Laboratory Experiments
We captured specimens for laboratory experiments from the same streams and in the same seasons as field observations and shipped them to the University of Georgia for prey capture success—velocity experiments (Fall 2014–Fall 2016). Mean standard length (± SD) of fish used in laboratory experiments was 6.2 cm (± 1.1) for juvenile Chinook Salmon (N = 43), 16.5 cm (±2.4) for Dolly Varden Char (N = 20), and 16.8 cm (±3.0) for Arctic Grayling (N = 40). A full description of laboratory experiment protocol can be found in Donofrio et al. (2018) and Bozeman and Grossman (2019a, b).
We fed individual subjects 9 prey (frozen bloodworms, 8.8 ±1.4 mm) per specimen per velocity treatment (10–70 cm/s in 10 cm increments) in an experimental stream flume and recorded the proportion of those prey captured (prey capture success, P). We also measured the velocity at the focal position occupied by the subject during the trial to assess potential differences in treatment velocity in the stream flume (V) and focal velocity. Turbidity in the stream flume was negligible (Bozeman and Grossman 2019b). We then used nonlinear least squares regression (package “nlstools” in R; Baty et al. 2015) to estimate species-specific curve-fitting constants b and c to best describe the negative logistic relationship between prey capture success (P) and treatment velocity in the stream flume (V) (Fig. 2; Eq. 3).
2.4 Literature Review
We reviewed the published literature to quantify the patterns of focal velocities occupied by drift-feeders as well as patterns in relationships between D, A, and S and stream velocity. We searched Google Scholar and Web of Science for relevant papers using combinations of the terms “microhabitat,” “stream fish,” “habitat use,” “stream velocity,” “fish metabolism,” “focal position,” “reaction area,” and “energy content of prey in the drift.” We also identified relevant papers by checking the reference sections of published NEI studies and other articles identified in the review. In our review of focal velocities, we only included sources that reported focal velocities measured in situ directly at a drift-feeder’s focal position following observations of active, undisturbed feeding. We did not include information from sources that reported average velocities at locations where fish were collected or embedded focal velocities within PCA or habitat suitability curves instead of reporting them directly.
2.5 Parameterizing and Testing NEI Model Variants
We parameterized and tested: (1) the full NEI model that includes data for D, A, and S (Eq. 4), (2) the simplified NEI model (Eq. 5), (3) the adjusted NEI focal model, and (4) the third derivative NEI model. To parameterize and run the simplified NEI model, we used nonlinear least squares regression in R package “nlstools” (Baty et al. 2015) to estimate species-specific b and c values for the relationship between prey capture success and velocity (Eq. 3). We then solved Eq. (5) iteratively to produce the optimal foraging velocity prediction of the simplified NEI model. Note that the simplified NEI model is based on the relationship between velocity and prey capture success as characterized in the experimental stream flume, where velocity refers to the speed prey were traveling at when captured. Therefore, the simplified NEI model predicts optimal foraging velocities, which may or may not be different from focal velocities, depending on the species and system. The simplified NEI model is the variant tested by Donofrio et al. (2018), and Bozeman and Grossman (2019a, b).
Because drift-feeders are known to select focal positions at slower velocities and forage for prey in nearby faster velocities (Fausch and White 1981; Fausch 1984), we used the experimentally derived relationship between foraging velocities (i.e., water velocity treatment levels in stream flume experiments) and focal velocities (generally less than foraging velocity, see Bozeman and Grossman 2019a, b) to predict the optimal focal velocity. We ran a simple linear model to characterize the relationship between focal and foraging velocities from our laboratory experiments and used model coefficients and the simplified NEI model prediction to obtain the optimal focal velocity prediction of the adjusted NEI model.
To test the third derivative model, we calculated the third derivative of our experimentally derived prey capture success-velocity relationship and identified the minima of the resulting function—the maximum point of deceleration of the curve describing the negative logistic P–V relationship—as the optimal velocity predicted by the third derivative model. Finally, we used a combination of nonlinear least squares and simple linear regression to parameterize and solve the full NEI model (Eq. 4): we related model variables D, A, and S (P already is incorporated as a function of V with curve-fitting constants b and c estimated in parameterization of the simplified NEI model) to fish focal position velocity via regression and then identified the focal position velocity at which I was maximized.
The Grossman NEI model was developed in a system where predation and competition were not important drivers of microhabitat selection (Grossman et al. 1998), and drift-feeders were assumed to select focal positions solely based on NEI maximization (Hill and Grossman 1993; Grossman et al. 2002). Accordingly, we tested NEI model variants by comparing model predictions with the velocities of focal positions occupied by fish in their respective study streams. If model predictions fell within the 95% confidence interval of field focal position velocities, we considered them successful. Predictions that fell outside of this interval were considered unsuccessful.
2.6 Statistical Analyses
All statistical analyses were performed in R statistical software (R Core Team 2018; www.R-project.org) and alpha for frequentist statistics was 0.05. Potential outliers in regression analyses were identified via a combination of Cook’s Distance and studentized and standardized residuals (R package “olsrr,” Hebbali 2020). We removed outliers with a Cook’s Distance value greater than 4× the mean of Cook’s Distance and an absolute studentized and standardized residual greater than two (Kutner et al. 2005). To limit data loss, we removed outliers identified during evaluation of the full data set, but not during subsequent evaluation of the data (i.e., new outliers were not identified after removal of outliers from the full data set). This outlier removal protocol resulted in the removal of no more than two data points in any species/system-variable combination.
3 Results
3.1 Literature Review of Drift-Feeder Focal Velocities
Our search of the literature for focal velocity measurements for drift-feeders revealed 21 peer-reviewed articles containing 50 independent reports of focal velocity from 7113 individual records encompassing a wide range of age classes, seasons, geographic locations, and seasons (Table 1). Our literature review indicated that mean focal velocity for drift-feeding stream fish species was 16.5 cm/s (±8.5 SD) (Fig. 3). More than 75% of stream fish held position at velocities below 20 cm/s, and more than 90% occupied microhabitats with velocities below 35 cm/s. The assumptions of the simplified NEI model state that D, A, and S can be considered constant across the range of focal velocities occupied by most stream fishes (Grossman et al. 2002). Consequently, we evaluate our NEI model assumptions in the context of this summary of common drift-feeder focal point velocities.
Frequency distribution histogram of published focal velocities (N = 50 data sets representing 7113 individual measurements, Table 1) for stream fishes
3.2 Energy Content of Prey in the Drift (D)
3.2.1 Empirical Analysis: Energy Content of Prey in the Drift (D)
There were no significant relationships between drift-net velocity and energy density of prey in the drift for any of the three systems observed (Fig. 4, p = 0.33 (a), 0.96 (b), 0.10 (c), respectively). Linear models described only a small proportion of the variation of D (R2 < 0.15). The relationship between drift-net velocity and energy density in the drift generally was negative for the Chena River and Richardson Clearwater; there was no relationship observed between these variables in Panguingue Creek. The three species occupied focal velocities over the lower range of drift-net velocities.
Mean drift-net velocity (cm/s) versus total energy density in the drift (J/m3) in habitats occupied by: (a) juvenile Chinook Salmon (Chena River), (b) Dolly Varden Char (Panguingue Creek), and (c) Arctic Grayling (Richardson Clearwater). Note differences in axis scales. The gray shaded areas are the focal velocities of the respective species in their respective streams
3.2.2 Literature Review, Energy Content of Prey in the Drift (D)
Our literature review revealed a generally positive relationship between velocity and drift. Multiple studies have shown that various measures of drift abundance (e.g., concentration, rate, proportion) increase across velocities of 10–80 cm/s (Elliott 1971; Townsend and Hildrew 1976; Ciborowski 1983; LaPerriere 1983; Smith and Li 1983; Brittain and Eikeland 1988; Gibbins et al. 2010). This encompasses the range of focal velocities occupied by most drift-feeders (8.0–25.0 cm/s) and argues for inclusion of drift abundance metrics in microhabitat models.
However, the drift-velocity relationship is complex and mediated by several other factors. Macroinvertebrate drift mechanics are driven by a combination of hydraulics (i.e., passive drift) and behavior (i.e., active drift), the balance of which shifts as a function of environmental conditions and species-specific traits (Naman et al. 2016). Positive relationships between drift and flow observed between streams or habitat types (pools, riffles, runs) may disappear at smaller spatial and temporal scales (e.g., within a single habitat type in a single stream) relevant to drift-feeder ecology and habitat use (LaPerriere 1983; Leung et al. 2009). Numerous studies have shown that in addition to velocity, drift processes are dependent upon many interacting factors including: season; time of day; macroinvertebrate species, body size and origin (terrestrial or aquatic); presence of predators; stream alkalinity; and substrate type (Everest and Chapman 1972; Wankowski and Thorpe 1979; Ciborowski 1983; Brittain and Eikeland 1988; Hoover and Richardson 2010).
Drift-flow relationships vary based on which metrics of flow are considered; increases in drift concentration may be positively correlated with increasing velocity, a linear measurement, and concurrently negatively correlated with increasing discharge, a volumetric measurement, via dilution (LaPerriere 1981, 1983). Heavy rainfall events that cause flows to increase at a given stream station may result in lower drift concentration per flow volume, but an overall increase in drift concentration export longitudinally downstream. Drift-feeding fishes upstream also may deplete drift concentrations immediately downstream (Hughes 1992; Hayes et al. 2007). These relationships may shift at velocity extremes; at high velocities (>40 cm/s) some macroinvertebrates may reduce drift rates and shelter in substrate and at low velocities (<10 cm/s) macroinvertebrates may increase drift rates to escape drying streams (Elliott 1971; Hoover and Richardson 2010). Finally, drift rates also may depend on previous flow conditions, with taxa responding differently to the same flow conditions based on whether flow is increasing or decreasing (Gunderson 2000; Naman et al. 2016).
3.2.3 Constant Drift Versus Velocity Assumption
In summary, the relationship between metrics of drift and flow is complicated, but D and V generally appear to be positively correlated. The observed relationship depends on which metrics of drift (e.g., concentration, abundance, rate, etc.) are compared to which metrics of flow (e.g., discharge, filtered volume, velocity, etc.), in addition to other potentially correlated factors (e.g., season, time of day, macroinvertebrate species, alkalinity, drift-feeder depletion, etc.). Sampling techniques also may affect the observed relationship between drift and flow due to phenomena such as net clogging and backwash at high velocities.
Nonetheless, data from our study streams show no significant relationships between drift-net velocities and drift concentrations (Fig. 4). Despite the nuance in previously reported drift-flow relationships, the consensus in the literature is that flow and drift concentration are positively related, even at the focal velocities of 8.0–25.0 cm/s occupied by most drift-feeders (Brittain and Eikeland 1988). The discrepancy between our empirical observations and the literature may be due to the complexity and subtlety of the flow-drift relationship (e.g., mediating factors of season, daylight, species, substrate, dilution, habitat type, etc.), the fact that this relationship may become homogenized at small scales of time and space relevant to the drift-feeders in our study, or methodological issues such as net backwash or net clogging. Nonetheless, the assumption of constant D over the range of focal velocities occupied by drift-feeders is plausible for models predicting instantaneous microhabitat selection within many systems although in general it may be context-specific.
3.3 Fish Visual Reaction Area (A)
3.3.1 Empirical Analysis: Fish Visual Reaction Area (A)
There were no significant relationships between focal velocity and visual reaction area for any of our study species (Fig. 5, p = 0.06 (a), 0.34 (b), and p = 0.89 (c), respectively). Linear models were poor fits to the data in each case (Fig. 5, all R2 values were <0.16). Arctic Grayling reaction areas were nearly two orders of magnitude greater than those of juvenile Chinook Salmon and one order of magnitude greater than Dolly Varden Char reaction areas.
3.3.2 Literature Review, Fish Visual Reaction Area (A)
Our literature review revealed few papers that directly measured the relationship between A and velocity or prey density, and the studies that measured these variables yielded mixed results. Most studies measured reaction distance, which is the straight line distance between a drift-feeder’s nose and the prey item at the moment the fish initiates prey pursuit. Godin and Rangeley (1989) observed decreases in reaction distance across velocities from 4 to 14 cm/s for juvenile Atlantic Salmon (Salmo salar); however, they also noted that fish oriented to prey items prior to pursuing them in faster velocities, concluding that fish minimized pursuit costs by delaying attack maneuvers at faster velocities. This implies that fish visual reaction distance remained high at fast velocities. Piccolo et al. (2008) reported declining prey detection distances across velocities ranging from 30 to 60 cm/s for juvenile Coho Salmon (Oncorhynchus kisutch) and steelhead (Oncorhynchus mykiss irideus), which is faster than most drift-feeder focal velocities (Fig. 3). O’Brien and Showalter (1993) likewise found that the prey search window decreased with increasing velocities for Arctic Grayling; however, this decrease primarily occurred at velocities greater than 32 cm/s and was offset by increased prey encounter rates at velocities up to 46 cm/s. O’Brien et al. (2001) found that increasing velocities from 25 to 40 cm/s (near the high end of typical focal velocities) resulted in decreased location distance and efficiency for Arctic Grayling, although feeding rate remained unchanged, which suggests a trade-off between increasing prey encounter rates and reaction area. It is possible that at faster velocities, drift-feeders alter foraging strategies and intercept prey predominately by moving laterally rather than hurriedly pursuing prey upstream before returning downstream to the focal position (Wankowski and Thorpe 1979).
Early models conceptualized reaction distance as a positive function of prey size, fish size, turbidity, and light conditions (Schmidt and Obrien 1982; Sweka and Hartman 2001; Hughes et al. 2003), rather than velocity. Laboratory experiments that hold prey size, prey density, light, and turbidity constant have shown that reaction distance increases slightly from 10 to 70 cm/s or remains unchanged and is not strongly correlated with fish size (Donofrio et al. 2018; Bozeman and Grossman 2019a; Sliger and Grossman 2021). Holding prey density constant in experiments is important because prey encounter rate increases with velocity, which may confound a potential relationship between velocity and reaction distance (Fausch 1984; Hughes and Dill 1990). Are fish traveling shorter distances to capture prey because reaction area is decreased at higher velocities, or because more prey is available nearer the focal position?
3.3.3 Constant Visual Reaction Area Versus Velocity Assumption
Our field data displayed no significant relationships between focal velocity and visual reaction area for our study species, which parallels results of our past laboratory experiments (Donofrio et al. 2018; Bozeman and Grossman 2019a, b; Sliger and Grossman 2021) as well as assumptions of original reaction distance models (Schmidt and Obrien 1982; Hughes and Dill 1990; Hughes et al. 2003). The relationships reported in the literature contradict these results but are confounded by correlations with other variables (i.e., declining reaction distances at velocities greater than those commonly occupied by drift-feeders or observations of fish noticing prey prior to initiating capture maneuvers). Functionally, accounting for visual fields of drift-feeders in NEI models explains—in conjunction with D and V—the amount of prey a drift-feeder encounters at its focal position, which is important for energy intake. Our data suggest visual field does not decrease with increasing velocity. The literature suggests that visual field decreases with velocity, but drift-feeders do not exhibit concurrent decreases in prey consumption. In both circumstances, A values have little effect on energy intake across focal velocities generally occupied by drift-feeders. Therefore, we suggest that the assumption of constant A across the range of velocities occupied by drift-feeders is plausible for NEI models predicting microhabitat selection based on relative energetic potential between available focal positions.
3.4 Swimming Cost (S)
3.4.1 Empirical Analysis: Swimming Cost (S)
We observed a significant positive relationship between focal velocity (cm/s) and total swimming cost (J/s) for juvenile Chinook Salmon (Fig. 6a, p = 0.01), Dolly Varden Char (Fig. 6b, p = 0.02), and Arctic Grayling (Fig. 6c, p = 0.04). Linear models fit the data poorly (R2 values: 0.18–0.27); however, residual patterns did not suggest that nonlinear functions would be better descriptors. Average swimming cost increased by 500%, 240%, and 150% across the range of relatively low focal velocities occupied by juvenile Chinook Salmon, Dolly Varden Char, and Arctic Grayling, respectively (Fig. 6). Note that total swimming costs increase from juvenile Chinook Salmon to Dolly Varden Char to Arctic Grayling such that swimming cost estimates differ by approximately one order of magnitude between species.
3.4.2 Literature Review, Swimming Cost (S)
Our literature review revealed that drift-feeder swimming costs generally are positively related to water velocity as well as fish mass and water temperature (Ware 1978; Boisclair and Tang 1993; Trudel and Welch 2005). Drift-feeder swimming costs (as estimated via equations and constants derived from oxygen consumption studies; e.g., Brett and Glass 1973) largely are exponentially related to velocity within and beyond the range of velocities occupied by drift-feeders (Rao 1968; Feldmeth and Jenkins Jr. 1973; Lee et al. 2003), though for some species and seasons this relationship is linear (Facey and Grossman 1990). Dickson and Kramer (1971) observed an asymptotic relationship between velocity and active metabolism for Rainbow Trout; however, this only occurred at velocities of 40–100 cm/s, which is greater than the range of velocities occupied by most drift-feeders (8.0–25.0 cm/s, Fig. 3) including Rainbow Trout in other natural systems (Grossman and Freeman 1987; Grossman and Ratajczak 1998).
The relationship between velocity and swimming costs is mediated by many factors, including water temperature, fish mass, turbulence, and fish swimming activity (Enders et al. 2005; Trudel and Welch 2005; Jowett et al. 2021). In cooler months, swimming costs may only increase linearly with velocity, or not at all (Facey and Grossman 1990). The effects of temperature on metabolism are greatest at low velocities (i.e., < 30 cm/s where most drift-feeders are found), and temperature becomes less important relative to velocity as velocities approach critical swimming speeds (Brett and Glass 1973). Models that estimate fish metabolism based on steady swimming at a fixed velocity within flumes with no turbulence and neglect the additional costs of foraging maneuvers and prey assimilation may dramatically underestimate actual metabolic costs incurred by drift-feeders in turbulent streams with considerable velocity heterogeneity (Facey and Grossman 1990; Hughes and Kelly 1996; Tang et al. 2000). Additionally, applications that estimate swimming costs by extrapolating models beyond the ranges of fish masses, velocities, and temperatures at which they were parameterized, or those that use parameters developed for different species, may be vulnerable to bias (Trudel and Welch 2005).
3.4.3 Constant Swimming Cost Versus Velocity Assumption
Our data (Fig. 6) and the literature clearly indicate that there is a significant positive relationship between swimming costs and the range of velocities occupied by drift-feeding fish. The literature suggests this relationship generally is exponential (e.g., Lee et al. 2003). When pooled, our data show a positive exponential relationship between velocity and swimming cost, largely due to the considerable discrepancies in species-specific swimming cost estimates; however, this relationship is linear when separated by species. Drift-feeders often select focal velocities near the low (i.e., flat) end of the exponential relationship, yet may still experience potentially meaningful increases in swimming costs even at those focal velocities. Our data and the literature suggest that the assumption of constant S over the range of velocities occupied by drift-feeding stream fishes is not valid for NEI models predicting microhabitat selection.
3.5 NEI Model Variant Predictions
We compared model output for the four NEI models to quantify their comparative ability to predict the optimal focal velocities of juvenile Chinook Salmon, Dolly Varden Char, and Arctic Grayling in natural systems. We judged model performance by comparing predicted optimal velocities with the 95% confidence interval of velocities of focal positions occupied by drift-feeders in their respective study streams.
Model performance varied between species and model variant. The 95% confidence interval of focal velocities occupied by juvenile Chinook Salmon (N = 24) in the Chena River was 9.7–13.9 cm/s. All four models overestimated optimal focal velocities of juvenile Chinook Salmon in the Chena River; the adjusted NEI model was the closest to field focal velocities (<5 cm/s from the upper CI), with the other three models producing worse predictions (Table 2). The 95% confidence interval of focal velocities of Dolly Varden Char (N = 32) in Panguingue Creek was 25.1–29.2 cm/s. The adjusted NEI model and full NEI model each missed the 95% CI of Dolly Varden Char focal velocities in Panguingue Creek by less than one cm/s, which is well within the range of measurement error. In addition, a potential competitor (Arctic Grayling) was present in Panguingue Creek at the time of our study. Dolly Varden Char optimal microhabitat was underestimated by the third derivative NEI model and overestimated by the simplified NEI model (Table 2). Finally, the 95% confidence interval for Arctic Grayling was 34.0–42.3 cm/s in the Richardson Clearwater (N = 29) and 20.8–27.2 cm/s in Panguingue Creek (N = 25). Three of the four model variants were successful for Arctic Grayling, albeit in different contexts. The simplified NEI model successfully predicted microhabitat selection of Arctic Grayling in the Richardson Clearwater, but not in Panguingue Creek, where a potential competitor (Dolly Varden Char) was present. Both the adjusted NEI model and the third derivative model successfully predicted microhabitat selection in Panguingue Creek, but not in the Richardson Clearwater (Table 2). The full NEI model prediction fell between the optimal focal velocities observed in Panguingue Creek and the Richardson Clearwater (Table 2), and thus was unsuccessful in both contexts.
4 Discussion
Investigations of the factors affecting habitat selection are essential for our understanding of how animals behave, which is a requirement for effective, science-based conservation and management. A key challenge for aquatic ecologists is identifying the fitness consequences of habitat selection. Mechanistic NEI models for drift-feeding stream fish are potentially useful tools for this task because they connect habitat use to fitness via energetics. Our evaluation of the assumptions of a simplified NEI model and comparison of complex and simplified models illuminates the mechanics of these models, highlights potential shortcomings associated with input variable estimation and parameterization, and provides important insight into how such models might be improved in the future.
Our empirical analysis demonstrated no relationships between velocity and energy content of prey in the drift or fish visual reaction area for any of our study species and a positive relationship between velocity and swimming cost for all of our study species. In conjunction with our review of the literature for each of these variables, we concluded that energy content of prey in the drift and fish visual reaction area could plausibly be considered constant within the range of drift-feeder focal position velocities, but swimming cost could not. When we parameterized and tested the four model variants, we found the adjusted NEI model was the best predictor of focal velocities occupied by the drift-feeders in this study; it was successful for Arctic Grayling in Panguingue Creek and was consistently closer to the 95% CI focal velocity window for Dolly Varden Char and juvenile Chinook Salmon than the other variants. These findings have important implications for how we theorize and estimate the various components of drift-feeder energetics and habitat use.
4.1 NEI Model Variable Estimation: Challenges and Implications
Our data suggests the Grossman et al. (2002) simplifying assumption for energy content of prey in the drift (D) is plausible, because we observed no significant correlations between these D and V for any of our study species. However, it is possible we did not observe a significant relationship between these variables due to high natural variability in the drift process, biased sampling techniques, or some combination of these things. The lack of observed relationship between D and V in our empirical analysis stands in contrast to the majority of the published literature, which suggests a positive relationship between velocity and metrics of drift (see Brittain and Eikeland 1988 for a review). Drift at any focal velocity is a complex function of lateral and vertical hydrodynamics, entry point (i.e., benthos, drift from upstream, or terrestrial sources), settling rate, abundance, and depletion by drift-feeders upstream. Drift processes also are influenced by macroinvertebrate species-specific traits, whereby macroinvertebrates actively enter or exit the drift based on abundance, season, time of day, and velocity (Nakano and Murakami 2001; Stark et al. 2002; Naman et al. 2016). The amount of energy in the drift available to drift-feeders is a complex function of the interaction between the abiotic dynamics of the stream and the ecological and biological characteristics of the invertebrate species themselves; any estimate of that amount is dependent on the time, place, and techniques used to sample this phenomenon.
There are a few potential biases which may have affected our estimates of energy concentration of prey in the drift. Sampling drift concentrations 3–20 m away from drift-feeders may not be reflective of drift concentrations encountered by drift-feeders at their focal positions given that drift can be highly spatially heterogeneous (Brittain and Eikeland 1988). Backwash due to net clogging and drift-net placement in the water column (the typical method of sampling macroinvertebrate drift) may underestimate drift concentrations, especially in fast velocities, which could potentially explain the negative trends observed in our data. However, removing the five fastest velocity data points from our velocity-drift concentration analyses did not change the observed relationship between drift and velocity in any of the three study systems. In addition, it possible that we did not observe a relationship between drift and velocity, because the velocity at drift-net positions potentially did not reflect flow conditions upstream that produced drift conditions.
Our field data showed no significant relationships between fish reaction area (A) and focal velocity, which matches results from laboratory experiments on these same species (Donofrio et al. 2018; Bozeman and Grossman 2019a, b). These results stand in contrast to the negative relationships between metrics of reaction field and velocity frequently reported in the literature. One possible explanation of these differences is that our method of recording reaction distance (for both laboratory experiments and field videos), which was the basis of our reaction area estimates, may not accurately capture the visual field of drift-feeding fish. We measure reaction distance between a drift-feeder and a prey item at the moment the drift-feeder initiates movement toward the prey. However, it is possible that drift-feeders visually observe prey prior to orienting toward it, thus decoupling the moment of prey recognition from the initiation of prey pursuit (Godin and Rangeley 1989). This phenomenon would bias our reaction area estimates such that they underestimate the true size of the visual window within which drift-feeders are foraging for prey items.
It is unclear how true visual reaction areas could be detected and measured because of the difficulties associated with discerning when a fish sees a prey item versus when it initiates pursuit of that prey item. Feeding in faster currents may necessitate that drift-feeders initiate foraging maneuvers earlier than they would in slower currents despite visually observing prey items at similar distances from their focal position. Published reports of decreased reaction distances for drift-feeders with increasing velocity either reported this relationship at velocities greater than most drift-feeders occupy (O’Brien and Showalter 1993; Piccolo et al. 2008) or observed constant or increasing prey encounter rates (O’Brien et al. 2001). Additionally, drift-feeders must discriminate between similarly sized prey items and inedible debris, the latter of which can vastly outnumber consumable prey especially for small-bodied drift-feeders (Neuswanger et al. 2014). The presence of potential competitors also may influence reaction distance, whereby drift-feeders are more likely to pursue prey on sight rather than let it drift closer and risk losing it to competition. Collectively, these dynamics make it difficult to know whether fish travel shorter distances to capture prey due to decreased prey recognition ability, large quantities of inedible debris, or increased prey availability nearer their focal position.
Original reaction distance models conceptualized reaction distance as a function of fish size, prey size, and light conditions (Schmidt and Obrien 1982; Hughes and Dill 1990; Hughes et al. 2003). Fish size was not significantly correlated with reaction distance in past laboratory experiments (Donofrio et al. 2018; Bozeman and Grossman 2019a, b) despite a wide range of experimental specimen lengths (4–27 cm) including many fish within the range of sizes at which are hypothesized to influence reaction distance (<19 cm; Hughes and Dill 1990). Light intensity may influence reaction distance (Mazur and Beauchamp 2003; Hansen et al. 2013), but it is unlikely that light conditions affected our reaction distance measurements, because laboratory measurements were conducted in a well-lit facility, and field observations were conducted during the Alaskan summer (>16 h in a day of daylight). Turbidity has been shown to be positively associated with stream velocity and negatively associated with fish reaction distance and foraging success (Vogel and Beauchamp 1999; Sweka and Hartman 2001; Hansen et al. 2013), but was negligible in our laboratory experiments (stream flume <0.001 NTUs) and low in our field observations (visibility greater than 1 m). We are hopeful that advances in underwater videography (e.g., VidSync) will continue to improve our understanding of three-dimensional fish foraging areas—including how fish visual field shifts in response to fish and prey size, light, turbidity, and presence of competitors—to address shortcomings of early foraging models (Dunbrack and Dill 1984; Neuswanger et al. 2016).
Unsurprisingly, swimming costs were positively related to focal velocities for all three species; a trend also observed in our literature review (e.g., Rao 1968; Feldmeth and Jenkins Jr. 1973). Nonetheless, several studies have shown that the incorporation of swimming costs in NEI models—a parameter that is logistically difficult to quantify and highly variable—does not necessarily improve the predictive ability of NEI models (Hughes and Dill 1990; Hill and Grossman 1993). Indeed, the full NEI model did not outperform the more simplified model variants despite being the only model containing this information. It is possible that drift-feeders occupy focal positions where energetic benefits overwhelm even considerable energetic costs, which would explain why costs did not improve the predictive ability of our full NEI model that ranks focal position based on relative energetic potential. However, this does not mean costs associated with swimming and foraging are unimportant for drift-feeder energetics modeling, because NEI models that calculate absolute NEI require accurate estimates of swimming cost even when costs are small relative to benefits.
The relative importance of energetic benefits (e.g., prey capture success) and costs in determining focal velocity selection via NEI is dependent on fish size. Jowett et al. (2021) found that swimming cost was more important for predicting optimal velocities of large fish (>96 g, 20 cm) than prey capture success, but that prey capture success was more important than costs for small fish optimal velocity predictions. It is widely known that fish metabolism is dependent on mass, especially for small fish (Trudel and Welch 2005; Rosenfeld and Taylor 2009). Finally, most NEI models that include energetic costs—including our full NEI model—estimate this variable using equations that were parameterized for different species using swimming trials in laminar flow swimming chambers (e.g., Trudel and Welch 2005), or extrapolate the models beyond the ranges of fish sizes, temperatures, or velocities for which they were parameterized. This may or may not be appropriate depending on the modeled species and the severity of the extrapolation.
Ideally, we would like to be able to quantify and include each element of swimming metabolism potentially affecting and affected by focal position choice by drift-feeders. However, the complexity and logistical difficulties of accurately and precisely measuring multi-faceted metabolic costs (e.g., standard metabolism, active metabolism, anaerobic foraging burst maneuvers, digestive costs, etc.) may limit their utility to NEI models, at least those which rank focal positions based on relative NEI. Previous studies demonstrated that estimates of swimming cost that do not incorporate the effects of turbulence or the energetic demands of burst foraging maneuvers may considerably underestimate the full energetic costs of drift-feeding in streams (Hughes and Kelly 1996; Tang et al. 2000; Enders et al. 2003). Therefore, although foraging maneuvers certainly inflate swimming costs it remains to be seen whether the inclusion of the complete energetic costs associated with drift-feeding can be incorporated in NEI models with sufficient precision to increase their predictive ability (see Facey and Grossman 1990, 1992). Clearly, more work is needed to reliably and precisely estimate swimming costs and incorporate them into NEI habitat selection models, and our results illustrate the difficulty of including accurate energetic cost data in these models.
Prey capture success is the most important determinant of output of the NEI models tested in this study. Prey capture success was the only model input variable derived from laboratory experiments, and as such, likely is the most precise variable included in the models. Nonetheless, there are several potential biases associated with our protocol for estimating prey capture success that could influence the output of each of our NEI model variants.
The experimental stream flume we used to measure prey capture success differed from natural stream environments in serval important ways. The stream flume received consistent lighting during all experiments, and contained very little visual complexity, outside of a small clump of bamboo placed at the upstream end of the flume to facilitate fish orientation. We regularly cleaned the stream flume to minimize debris and turbidity, and only presented prey items to fish one at a time. Each of these departures from the natural stream environment were necessary to facilitate laboratory experiments (whose scope extended beyond simple prey capture success measurements) and keep fish healthy; however, these simplifications of the stream environment potentially result in prey capture success being overestimated at a given velocity. Clearly, this would have serious implications for model output given the importance of the prey capture success-velocity function to the formulation of the NEI models. However, this bias has not apparently been reflected in the past success of our simplified and adjusted NEI models (Grossman et al. 2002; Donofrio et al. 2018; Bozeman and Grossman 2019a, b; Sliger and Grossman 2021). Future experiments focusing purely on prey capture success (and not other processes that require flume water clarity or bright lighting, e.g., video recording for reaction distance) under more natural conditions of turbidity, turbulence, prey-like inedible debris, and variable lighting conditions may more appropriately characterize prey capture success of drift-feeders in natural systems and improve foraging models.
4.2 Implications of Simplified Versus Complex NEI Model Success
The predictive ability of the four variants of the Grossman NEI model varied among species and systems. Overall, the adjusted NEI model outperformed the other model variants by successfully predicting Arctic Grayling optimal focal velocities in Panguingue Creek, underestimating Dolly Varden Char optimal focal velocities in Panguingue Creek by less than 1 cm/s, and being the closest of the variants to the 95% confidence interval of juvenile Chinook Salmon focal velocities in the Chena River (<5 cm/s away). There was no clear-cut second-best model, with the simplified, full, and third derivative model variants performing differentially for different species. This observation indicates parameter estimates for D, A, and S did not increase the predictive ability of the full NEI model in our study.
Except for juvenile Chinook Salmon, which likely are selecting habitat for reasons other than energy optimization (e.g., predator avoidance via strong association with shelter), our NEI models performed reasonably well and were able to yield insights into the process of microhabitat focal velocity selection. The performance of the models for Dolly Varden Char and Arctic Grayling was impressive given that model predictions fell within ~10 cm/s of the 95% CI of field focal velocities for these species in the Richardson Clearwater and Panguingue Creek despite water column velocities in our study sites ranging from negligible to at least 120 cm/s. These insights are important because many NEI models have been developed in the 40 years since their inception (Fausch 1984; Piccolo et al. 2014), but few if any studies have directly assessed the predictive ability of various forms of an NEI model, and the majority of NEI models have not undergone rigorous testing with multiple species and in multiple years and seasons.
Given that the Grossman et al. (2002) NEI model was developed for systems in which interspecific competition and predation were not strong driving factors affecting microhabitat selection (Grossman et al. 1998), it is not surprising that the model and its variants performed poorly for juvenile Chinook Salmon in the Chena River (Donofrio et al. 2018). Juvenile Chinook Salmon in the Chena River typically were observed in shallow areas near or underneath shelter (e.g., within root balls of fallen trees), which suggests that the proximity to shelter from predators may be an important component of microhabitat selection (Quinn 2018). This habitat preference is evidenced by lower focal velocities and swimming costs (by one and two orders of magnitude) for Chinook Salmon compared to Dolly Varden Char and Arctic Grayling, respectively. However, this observation is unsurprising, because juvenile Chinook Salmon in this study were very small (4.7 ± 1.0 SD SL), and focal velocity typically increases with length (Everest and Chapman 1972; Grossman and Ratajczak 1998). Larger individuals often select microhabitats nearer the center of the channel with greater focal velocities and are not as vulnerable to potential predators (Hughes and Reynolds 1994; Hughes 1998; Bozeman and Grossman 2019a).
One interesting aspect of model variant performance is that the simplified NEI model successfully predicted optimal microhabitats of Arctic Grayling in the Richardson Clearwater, whereas the adjusted NEI model (and the third derivative NEI model) successfully predicted Arctic Grayling optimal microhabitats in Panguingue Creek. We observed that these systems differ markedly in depth, velocity heterogeneity, habitat complexity, and the presence of a potential competitor (Dolly Varden Char). It is important to consider the possibility that model variants may perform differentially based on the systems in which they are applied. For instance, it is well known that drift-feeders may occupy slightly slower focal velocities adjacent to higher velocity microhabitats in which they forage for drifting prey (Everest and Chapman 1972; Fausch and White 1981; Naman et al. 2022). In systems with considerable velocity heterogeneity with potentially large differences between focal and foraging velocities (e.g., Panguingue Creek), models that predict optimal focal velocity (as discounted from foraging velocity) may outperform models that predict optimal foraging velocity. By contrast, optimal foraging velocity models may perform better in systems with less velocity heterogeneity and fewer focal and foraging velocity shears. Some NEI models address this issue by accounting for vertical or lateral velocity differentials in foraging areas (Hayes et al. 2000; Dodrill et al. 2016). Understanding how different models (or different versions of models that account for spatial velocity heterogeneity) perform in different systems is an important area of research for the development and application of future NEI models.
From a logistical point of view, it is encouraging that the simplified, adjusted, and third derivative models performed just as well or better than the full NEI model because model parsimony generally is desirable and estimates for D, A, and S are costly and difficult to obtain. However, from a NEI model development and managerial perspective, it is discouraging that our estimates of these additional variables do not improve model output given that many NEI models calculate absolute NEI, which is dependent on D, A, and S, to predict potential growth, abundance, or carrying capacity for applied management strategies. One potential explanation for the underwhelming performance by the full model is that the linear models we used to relate D, A, and S to velocity and subsequently parameterize the full model explain very little of the variation in D, A, and S due to velocity (R2 ranged from 0.00 to 0.27). This is not a particularly robust or elegant way to parameterize the full NEI model; however, this is the first attempt to parameterize and test this model, and inspection of the data suggested that nonlinear functions would not be better descriptors than linear functions.
Another potential and related reason for underperformance of some variants is bias associated with our data collection. In each application of the model variants, the full and simplified NEI model predictions were greater than the third derivative and adjusted NEI model predictions. This pattern suggests we likely are overestimating drift-feeder NEI. Two potential sources of overestimation of NEI are underestimation of swimming costs and overestimation of prey capture success (it seems less likely that drift density and visual reaction area would be biased high). Improved estimation techniques for both of these variables, as previously discussed, will provide additional insight into the dynamics of these models.
Our NEI model comparison has important implications for NEI models with different predictive goals. For NEI models that rank instantaneous optimal microhabitat selection based on relative NEI, parsimonious models that do not account for energy content of prey in the drift, visual reaction area, and swimming cost perform reasonably well. This conclusion is supported by the finding that the full model rarely outperforms the adjusted or simplified models despite incorporating more biological realism by including additional variables.
However, parsimony is inappropriate for models that predict potential growth or carrying capacity via absolute NEI; these models require accurate estimates and arrangements of energetics variables to produce reasonable results. For instance, swimming costs may be overwhelmed by energetic benefits in NEI models that predict instantaneous habitat selection via ranking of available focal positions (Hughes and Dill 1990; Hill and Grossman 1993), but even small swimming cost estimates may be highly influential in NEI model applications that predict potential growth or carrying capacity over space or time (e.g., Hayes et al. 2016; Naman et al. 2019). Likewise, temporal (diel) and spatial (within or between habitats) variation in drift may hinder our ability to detect patterns at scales relevant to modeling of instantaneous focal position selection by drift-feeders (LaPerriere 1981; Leung et al. 2009; Naman et al. 2016). Drift density may also interact with predation risk to explain focal position selection. If predation risk is high, drift-feeders may forage in faster velocities to achieve satiation in less time compared to foraging all day in slower velocities absent predation risk (Naman et al. 2022; Railsback et al. 2021). Drift dynamics certainly are critical components of drift-feeder habitat quality given that drifting macroinvertebrates, both terrestrial and aquatic, comprise most of the food for drift-feeding fishes (Elliott 1973; Quinn 2018).
4.3 Looking Forward
Variables that regulate energetic gain (prey quantity and quality, fish visual reaction field, prey capture success) and expenditure (cost of holding a fixed focal position in the stream, cost of foraging) certainly are important determinants of drift-feeder habitat selection, ecology, and fitness. This observation is evidenced by the inclusion of these variables in the vast majority of NEI models, including the earliest and latest applications (e.g., Fausch 1984; Rosenfeld and Taylor 2009; Naman et al. 2019), and is substantiated by our review of the relevant literature. More sophisticated methods of parameter estimation for energy content of prey in the drift, visual reaction area, and swimming costs will improve our understanding of the intricacies of drift-feeder microhabitat selection and may ultimately improve the power, tractability, and utility of complex NEI models that use these and other variables to estimate absolute NEI.
Our results indicate that prey capture success is the variable with the most influence on the predictions made by our NEI model variants. Future research should parameterize prey capture success-velocity functions for additional species and age classes that could be incorporated into user-friendly habitat suitability estimation software (e.g., Naman et al. 2020) or generalized across populations. Developing prey capture success-velocity functions specific to species, age classes, or even types of systems (e.g., stream size), especially through methods that guard against overestimation of prey capture success in oversimplified stream flumes, will provide important insight into model formulation and drift-feeder foraging behavior for absolute and relative NEI models alike. Understanding species-specific foraging performance also will help us predict how species may respond in the face of shifts in habitat quality or quantity, or the presence of competitors (e.g., Nakano et al. 1999).
Global climate change and other anthropogenic stressors necessitate that we develop practical conservation and management strategies to mitigate threats to freshwater biodiversity (Dauwalter et al. 2011; Williams et al. 2011; Jenkins et al. 2015). One of the most promising aspects of NEI models is their potential ability to be linked to hydrodynamic models to predict microhabitat quality and quantity at broader spatial scales (Hayes et al. 2007; McHugh et al. 2017; Railsback 2016), or incorporated into software that can readily estimate absolute NEI based on user-selected fish species, mass, water depth, velocity, and other variables (Hayes et al. 2020; Naman et al. 2020). Global climate change will affect drift-feeder habitat quality and quantity through many mechanisms, including altering metabolic rates (Trudel and Welch 2005) and availability of prey in the drift. Although simplified variants of NEI models can be linked to climate modeling based on predicted changes in flow, complex NEI models that predict absolute NEI will be necessary to capture the full suite of effects of climate change on drift-feeder populations.
In conclusion, our results demonstrate that energy content of prey in the drift and fish visual reaction area potentially can be considered constant across the range of velocities occupied by drift-feeders, but swimming cost cannot. Nonetheless, we found that simplified variants of an NEI model based on the prey capture success-velocity function performed as well or better than a more complex NEI model, which is more difficult to parameterize. In the short term, this is encouraging because we can use simplified NEI models to predict instantaneous habitat selection by drift-feeders. However, complex NEI models that predict potential growth, abundance, or carrying capacity via absolute NEI ultimately are needed for robust management and conservation applications. We support the continued improvement of complex habitat variable estimation techniques, as well as the parameterization of species-specific prey capture success-velocity functions to advance our understanding of drift-feeding foraging behavior and our ability to evaluate stream fish habitat quality and quantity in an uncertain future.
References
Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak NE, Balderas SC, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibel TJ, Wikramanayake E, Olson D, Lopez HL, Reis RE, Lundberg JG, Perez MHS, Petry P (2008) Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience 58(5):403–414
Armstrong RH, Morrow JE (1980) Charrs. Salmonid fishes of the genus Salvelinus. Perspectives in vertebrate science. Dr W Junk, The Hague, Netherlands
Baltz DM, Vondracek B, Brown LR, Moyle PB (1987) Influence of temperature on microhabitat choice by fishes in a California Stream. Trans Am Fish Soc 116:12–20
Baltz DM, Vondracek B, Brown LR, Moyle PB (1991) Seasonal changes in microhabitat selection by rainbow trout in a small stream. Trans Am Fish Soc 120:166–176
Barton LH (1986) Population estimate of chinook salmon escapement in the Chena River in 1986 based upon mark recapture techniques. Alaska Department of Fish and Game, Division of Commercial Fisheries AYK Region Yukon Salmon Escapement Report No. 31, pp 1–38
Baty F, Ritz C, Charles S, Brutsche M, Flandrois J-P, Delignette-Muller M-L (2015) A toolbox for nonlinear regression in R: The package nlstools. J Stat Softw 66(5):1–21
Benke AC, Huryn AD, Smock LA, Wallace JB (1999) Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the Southeastern United States. J N Am Benthol Soc 18(3):308–343
Boisclair D, Tang M (1993) Empirical analysis of the influence of swimming pattern on the net energetic cost of swimming in fishes. J Fish Biol 42:169–183
Boyce MS, McDonald LL (1999) Relating populations to habitats using resource selection functions. Trends Ecol Evol 14(7):268–272. https://doi.org/10.1016/S0169-5347(99)01593-1
Bozeman BB, Grossman GD (2019a) Foraging behaviour and optimal microhabitat selection in Yukon River Basin nonanadromous Dolly Varden Charr (Salvellinus malma). Ecol Freshw Fish 28:586–601
Bozeman BB, Grossman GD (2019b) Mechanics of foraging success and optimal microhabitat selection in Alaskan Arctic grayling (Thymallus arcticus). Can J Fish Aquat Sci 76(5):815–830. https://doi.org/10.1139/cjfas-2018-0115
Brett JR, Glass NR (1973) Metabolic rates and critical swimming speeds of sockeye salmon (Oncorhynchus nerka) in relation to size and temperature. J Fish Res Board Can 30:379–387
Brittain JE, Eikeland TJ (1988) Invertebrate drift—a review. Hydrobiologia 166:77–93
Ciborowski JJH (1983) Downstream and lateral transport of nymphs of two mayfly species (Ephemeroptera). Can J Fish Aquat Sci 40:2025–2029
Dauwalter DC, Wenger SJ, Gelwicks KR, Fesenmyer KA (2011) Land use associations with distributions of declining native fishes in the upper Colorado River Basin. Trans Am Fish Soc 140(3):646–658. https://doi.org/10.1080/00028487.2011.587753
Dickson IW, Kramer RH (1971) Factors influencing scope for activity and active and standard metabolism of Rainbow Trout (Salmo gairdneri). J Fish Res Board Can 28:587–596
Dodrill MJ, Yackulic CB, Kennedy TA, Hayes JW (2016) Prey size and availability limits maximum size of rainbow trout in a large tailwater: insights from a drift-foraging bioenergetics model. Can J Fish Aquat Sci 73(5):759–772
Donofrio E, Simon T, Neuswanger JR, Grossman GD (2018) Velocity and dominance affect prey-capture and microhabitat selection in juvenile chinook (Oncorhynchus tshawytscha). Environ Biol Fish 101:609–622
Dunbrack RL, Dill LM (1984) Three-dimensional prey reaction field of the juvenile coho salmon (Oncorhynchus kisutch). Can J Fish Aquat Sci 41:1176–1182
Elliott JM (1971) The distances travelled by drifting invertebrates in a lake district stream. Oecologia 6(4):350–379
Elliott JM (1973) The food of brown and rainbow trout (Salmo trutta and S. gairdneri) in relation to the abundance of drifting invertebrates in a mountain stream. Oecologia 12(4):329–347
Enders EC, Boisclair D, Roy AG (2003) The effect of turbulence on the cost of swimming for juvenile Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 60(9):1149–1160. https://doi.org/10.1139/f03-101
Enders EC, Buffin-Bélanger T, Boisclair D, Roy AG (2005) The feeding behaviour of juvenile Atlantic salmon in relation to turbulent flow. J Fish Biol 66:242–253
Everest PH, Chapman DW (1972) Habitat selection and spatial interaction by juvenile chinook salmon and steelhead trout in two Idaho streams. J Fish Res Board Can 29(1):91–100
Facey DE, Grossman GD (1990) The metabolic cost of maintaining position for four North American stream fishes: Effects of season and velocity. Physiol Zool 63(4):757–776
Facey DE, Grossman GD (1992) The relationship between water velocity, energetic costs, and microhabitat use in four North American stream fishes. Hydrobiologia 239:1–6
Fausch KD (1984) Profitable stream positions for salmonids: relating specific growth rate to net energy gain. Can J Zool 62:441–451
Fausch KD, White RJ (1981) Competition between Brook Trout (Salvelinus fontinalis) and Brown Trout (Salmo trutta) for positions in a Michigan Stream. Can J Fish Aquat Sci 38(10):1220–1227
Feldmeth CR, Jenkins TM Jr (1973) An estimate of energy expenditure by rainbow trout (Salmo gairdneri) in a small mountain stream. J Fish Res Board Can 30:1755–1759
Gibbins CN, Vericat D, Batalla RJ (2010) Relations between invertebrate drift and flow velocity in sand-bed and riffle habitats and the limits imposed by substrate stability and benthic density. J N Am Benthol Soc 29(3):945–958
Godin J-GJ, Rangeley RW (1989) Living in the fast lane: effects of cost of locomotion on foraging behaviour in juvenile Atlantic salmon. Anim Behav 37:943–954
Grossman GD (2014) Not all drift feeders are trout: A short review of fitness-based habitat selection models for fishes. Environ Biol Fishes 97(5):465–473. https://doi.org/10.1007/s10641-013-0198-3
Grossman GD, Freeman MC (1987) Microhabitat use in a stream fish assemblage. J Zool 212:151–176
Grossman GD, Ratajczak RE (1998) Long-term patterns of microhabitat use by fish in a southern Appalachian stream from 1983 to 1992: effects of hydrologic period, season and fish length. Ecol Freshw Fish 7:108–131
Grossman GD, Dowd JF, Crawford M Jr (1990) Assemblage stability in stream fishes: a review. Environ Manag 14(5):661–671
Grossman GD, Ratajczak RE, Crawford MJ, Freeman MC (1998) Assemblage organization in stream fishes: Effects of environmental variation and interspecific interactions. Ecol Monogr 68(3):395–420
Grossman GD, Rincon PA, Farr MD, Ratajczak RE (2002) A new optimal foraging model predicts habitat use by drift-feeding stream minnows. Ecol Freshw Fish 11:2–10
Gryska AD (2001) Abundance and length and age composition of Arctic grayling in the Delta Clearwater River, 2000. Alaska Department of Fish and Game Fishery Data Series No. 01-27, Anchorage, AK
Guensch GR, Hardy TB, Addley RC (2001) Examining feeding strategies and position choice of drift-feeding salmonids using an individual-based, mechanistic foraging model. Can J Fish Aquat Sci 58:446–457
Gunderson LH (2000) Ecological resilience—in theory and application. Annu Rev Ecol Syst 31:425–439
Hansen AG, Beauchamp DA, Schoen ER (2013) Visual prey detection responses of piscivorous trout and salmon: effects of light, turbidity, and prey size. Trans Am Fish Soc 142(3):854–867
Hartman KJ, Jensen OP (2017) Anticipating climate change impacts on Mongolian salmonids: bioenergetics models for lenok and Baikal grayling. Ecol Freshw Fish 26:383–396
Harvey BC, Railsback SF (2009) Exploring the persistence of stream-dwelling trout populations under alternative real-world turbidity regimes with an individual-based model. Trans Am Fish Soc 138(2):348–360. https://doi.org/10.1577/t08-068.1
Hayes JW, Jowett IG (1994) Microhabitat models of large drift-feeding brown trout in three New Zealand rivers. N Am J Fish Manag 14(4):710–725
Hayes JW, Stark JD, Shearer KA (2000) Development and test of a whole-lifetime foraging and bioenergetics growth model for drift-feeding Brown Trout. Trans Am Fish Soc 129:315–332
Hayes JW, Hughes NF, Kelly LH (2007) Process-based modelling of invertebrate drift transport, net energy intake and reach carrying capacity for drift-feeding salmonids. Ecol Model 207(2-4):171–188. https://doi.org/10.1016/j.ecolmodel.2007.04.032
Hayes JW, Goodwin E, Shearer KA, Hay J, Kelly L (2016) Can weighted useable area predict flow requirements of drift-feeding salmonids? Comparison with a net rate of energy intake model incorporating drift–flow processes. Trans Am Fish Soc 145(3):589–609. https://doi.org/10.1080/00028487.2015.1121923
Hayes JW, Naman SM, Neuswanger JR, Goodwin E, Jowett IG (2020) Bioenergetic habitat suitability model for drift-feeding salmonids and guidance on its use in hydraulic-habitat modeling. Cawthron Report No. 3740, pp 1–56
Healy BD, Lonzarich DG (2000) Microhabitat use and behavior of overwintering juvenile coho salmon in a lake superior tributary. Trans Am Fish Soc 129:866–872
Hebbali A (2020) Olsrr: Tools for building OLS regression models. R package version 0.5.3. https://CRAN.R-project.org/package=olsrr
Heggenes J (2002) Flexible summer habitat selection by wild, allopatric brown trout in lotic environments. Trans Am Fish Soc 131(2):287–298
Hill J, Grossman GD (1993) An energetic model of microhabitat use for rainbow trout and rosyside dace. Ecology 74(3):685–698
Hillman TW, Griffith JS, Platts WS (1987) Summer and winter habitat selection by juvenile chinook salmon in a highly sedimented Idaho stream. Trans Am Fish Soc 116:185–195
Hoover TM, Richardson JS (2010) Does water velocity influence optimal escape behaviors in stream insects? Behav Ecol 21:242–249
Hughes NF (1992) Ranking of feeding positions by drift-feeding Arctic grayling (Thymallus arcticus) in dominance hierarchies. Can J Fish Aquat Sci 49:1994–1998
Hughes NF (1998) A model of habitat selection by drift-feeding stream salmonids at different scales. Ecology 79(1):281–294
Hughes NF, Dill LM (1990) Position choice by drift-feeding salmonids: Model and test for Arctic grayling (Thymallus arcticus) in subarctic mountain streams, interior Alaska. Can J Fish Aquat Sci 47:2039–2048
Hughes NF, Kelly LH (1996) A hydrodynamic model for estimating the energetic cost of swimming maneuvers from a description of their geometry and dynamics. Can J Fish Aquat Sci 53:2484–2493
Hughes NF, Reynolds JB (1994) Why do Arctic grayling (Thymallus arcticus) get bigger as you go upstream? Can J Fish Aquat Sci 51(10):2154–2163. https://doi.org/10.1139/f94-216
Hughes NF, Hayes JW, Shearer KA, Young RG (2003) Testing a model of drift-feeding using three-dimensional videography of wild brown trout, Salmo trutta, in a New Zealand river. Can J Fish Aquat Sci 60(12):1462–1476. https://doi.org/10.1139/f03-126
Jenkins TM (1969) Social structure, position choice and micro-distribution of two trout species (Salmo trutta and Salmo gairdneri) resident in mountain streams. Anim Behav 2:55–123. https://doi.org/10.1016/s0066-1856(69)80002-6
Jenkins AR, Keeley ER (2010) Bioenergetic assessment of habitat quality for stream-dwelling cutthroat trout (Oncorhynchus clarkii bouvieri) with implications for climate change and nutrient supplementation. Can J Fish Aquat Sci 67(2):371–385. https://doi.org/10.1139/f09-193
Jenkins CN, Van Houtan KS, Pimm SL, Sexton JO (2015) US protected lands mismatch biodiversity priorities. PNAS 112(16):5081–5086. https://doi.org/10.1073/pnas.1418034112
Jowett IG, Hayes JW, Neuswanger JR (2021) Salmonid bioenergetic drift-foraging: swimming costs and capture success. J Ecohydraul 1–12
Kalb BW, Huntsman BM, Caldwell CA, Bozek MA (2018) A mechanistic assessment of seasonal microhabitat selection by drift-feeding rainbow trout Oncorhynchus mykiss in a Southwestern headwater stream. Environ Biol Fishes 101:257–273
Kutner MH, Nachtsheim CJ, Neter J, Li W (2005) Applied linear statistical models, 5th edn. McGraw-Hill, New York
LaPerriere JD (1981) Chemical and physical influences on invertebrate drift in subarctic Alaskan streams. Dissertation, Iowa State University, Ames, IA
LaPerriere JD (1983) Alkalinity, discharge, average velocity, and invertebrate drift concentration in Subarctic Alaskan streams. J Freshwat Ecol 2(2):141–151
Lee CG, Farrell AP, Lotto A, MacNutt MJ, Hinch SG, Healey MC (2003) The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol 206:3239–3251
Leung ES, Rosenfeld JS, Bernhardt JR (2009) Habitat effects on invertebrate drift in a small trout stream: implications for prey availability to drift-feeding fish. Hydrobiologia 623:113–125
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100(916):603–609
Mazur MM, Beauchamp DA (2003) A comparison of visual prey detection among species of piscivorous salmonids: effects of light and low turbidities. Environ Biol Fishes 67:397–405
McHugh PA, Saunders WC, Bouwes N, Wall CE, Bangen S, Wheaton JM, Nahorniak M, Ruzycki JR, Tattam IA, Jordan CE (2017) Linking models across scales to assess the viability and restoration potential of a threatened population of steelhead (Oncorhynchus mykiss) in the Middle Fork John Day River, Oregon, USA. Ecol Model 355:24–38. https://doi.org/10.1016/j.ecolmodel.2017.03.022
Mesa MG, Weiland LK, Christiansen HE, Sauter ST, Beauchamp DA (2013) Development and evaluation of a bioenergetics model for bull trout. Trans Am Fish Soc 142(41):41–49
Moyle PB, Baltz DM (1985) Microhabitat use by an assemblage of California stream fishes: developing criteria for instream flow determinants. Trans Am Fish Soc 114(5):695–704
Nakano S, Murakami M (2001) Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. PNAS 98(1):166–170
Nakano S, Fausch KD, Kitano S (1999) Flexible niche partitioning via a foraging mode shift: A proposed mechanism for coexistence in stream-dwelling charrs. J Anim Ecol 68:1079–1092
Naman SM, Rosenfeld JS, Richardson JS (2016) Causes and consequences of invertebrate drift in running waters: from individuals to populations and trophic fluxes. Can J Fish Aquat Sci 73:1292–1305
Naman SM, Rosenfeld JS, Neuswanger JR, Enders EC, Eaton BC (2019) Comparing correlative and bioenergetics-based habitat suitability models for drift-feeding fishes. Freshwat Biol 64:1613–1626
Naman SM, Rosenfeld JS, Neuswanger JR, Enders EC, Hayes JW, Goodwin EO, Jowett IG, Eaton BC (2020) Bioenergetic habitat suitability curves for instream flow modeling: introducing user-friendly software and its potential applications. Fisheries 45(11):605–613
Naman SM, Rosenfeld JS, Lannan AS (2022) Diel patterns of foraging and microhabitat use by sympatric rainbow trout and bull trout: implications for adaptive differentiation and instream flow assessment. Can J Fish Aquat Sci 79(2):223–233
Neuswanger JR, Wipfli MS, Rosenberger AE, Hughes NF (2014) Mechanisms of drift-feeding behavior in juvenile Chinook salmon and the role of inedible debris in a clear-water Alaskan stream. Environ Biol Fishes 97:489–503
Neuswanger JR, Wipfli MS, Rosenberger AE, Hughes NF (2016) Measuring fish and their physical habitats: versatile 2D and 3D video techniques with user-friendly software. Can J Fish Aquat Sci 73(12):1861–1873. https://doi.org/10.1139/cjfas-2016-0010
Nielsen JL (1992) Microhabitat-specific foraging behavior, diet, and growth of juvenile coho salmon. Trans Am Fish Soc 121:617–634
O’Brien WJ, Showalter JJ (1993) Effects of current velocity and suspended debris on the drift feeding of Arctic grayling. Trans Am Fish Soc 122:609–615
O’Brien WJ, Barfield M, Sigler K (2001) The functional response of drift-feeding Arctic grayling: The effects of prey density, water velocity, and location efficiency. Can J Fish Aquat Sci 58(10):1957–1963. https://doi.org/10.1139/f01-138
Piccolo JJ, Hughes NF, Bryant MD (2008) Water velocity influences prey detection and capture by drift-feeding juvenile coho salmon (Oncorhynchus kisutch) and steelhead (Oncorhynchus mykiss irideus). Can J Fish Aquat Sci 65(2):266–275. https://doi.org/10.1139/f07-172
Piccolo JJ, Frank BM, Hayes JW (2014) Food and space revisited: The role of drift-feeding theory in predicting the distribution, growth, and abundance of stream salmonids. Environ Biol Fishes 97(5):475–488. https://doi.org/10.1007/s10641-014-0222-2
Poff NL, Angermeier PL, Cooper SD, Lake PS, Fausch KD, Winemiller KO, Mertes LAK, Oswood MW, Reynolds JB, Rahel FJ (2001) Fish diversity in streams and rivers. In: Chapin FS, Sala OE, Huber-Sannwald E (eds) Global biodiversity in a changing environment. Ecological studies, vol 152. Springer, New York, pp 315–349
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52(2):137–154
Quinn TP (2018) The behavior and ecology of Pacific salmon and trout. University of Washington Press, Seattle, WA
Railsback SF (2016) Why it is time to put PHABSIM out to pasture. Fisheries 41:720–725. https://doi.org/10.1080/03632415.2016.1245991
Railsback SF, Ayllón D, Harvey BC (2021) InSTREAM 7: Instream flow assessment and management model for stream trout. River Res Appl 37(9):1294–1302
Rao GMM (1968) Oxygen consumption of rainbow trout (Salmo gairdneri) in relation to activity and salinity. Can J Zool 46:781–786
Ridder WP (1988) Age, length, sex, and abundance of Arctic grayling in the Richardson Clearwater River and Shaw Creek, 1988. Alaska Department of Fish and Game, Division of Sport Fish Fishery Data Series No. 120
Rimmer DM, Paim U, Saunders RL (1984) Changes in the selection of microhabitat by juvenile Atlantic salmon (Salmo salar) at the summer-autumn transition in a small river. Can J Fish Aquat Sci 41:469–475
Rincón PA, Lobón-Cerviá J (1993) Microhabitat use by stream-resident brown trout: bioenergetic consequences. Trans Am Fish Soc 122:575–587
Rogers LE, Buschbom RL, Watson CR (1977) Length-weight relationships of shrub-steppe invertebrates. Ann Entomol Soc Am 70(1):51–53
Rosenfeld JS, Taylor J (2009) Prey abundance, channel structure and the allometry of growth rate potential for juvenile trout. Fish Manag Ecol 16(3):202–218. https://doi.org/10.1111/j.1365-2400.2009.00656.x
Rosenfeld JS, Bouwes N, Wall CE, Naman SM (2014) Successes, failures, and opportunities in the practical application of drift-foraging models. Environ Biol Fishes 97(5):551–574. https://doi.org/10.1007/s10641-013-0195-6
Sabo JL, Bastow JL, Power ME (2002) Length-mass relationships for adult aquatic and terrestrial invertebrates in a California watershed. J N Am Benthol Soc 21(2):336–343
Schindler DE, Kreuger C, Bisson P, Bradford M, Clark B, Conitz J, Howard K, Jones M, Murphy J, Myers K, Scheuerell MD, Volk E, Winton J (2013) Arctic-Yukon-Kuskokwim Chinook Salmon Research Action Plan: Evidence of decline of chinook salmon populations and recommendations for future research. Prepared for the AYK Sustainable Salmon Initiative, Anchorage, AK, 70 pp
Schmidt D, Obrien WJ (1982) Planktivorous feeding ecology of Arctic Grayling (Thymallus arcticus). Can J Fish Aquat Sci 39(3):475–482
Schoener TW (1971) Theory of feeding strategies. Annu Rev Ecol Evol Syst 2(1):369–404
Sliger R, Grossman GD (2021) A test of a generalized, optimal habitat selection model for drift-feeding fishes: brook charr. J Zool 315:313–318
Smith JJ, Li HW (1983) Energetic factors influencing foraging tactics of juvenile steelhead trout, Salmo gairdneri. In: Noakes DLG, Lindquist DG, Helfman GS, Ward JA (eds) Predators and prey in fishes, Developments in environmental biology of fishes, vol 2. Springer, Dordrecht, pp 173–180
Stark JD, Shearer KA, Hayes JW (2002) Are aquatic invertebrate drift densities uniform? Implications for salmonid foraging models. Internationale Vereinigung für theoretische und angewandte Limnologie 28(2):988–991
Stewart DJ, Ibarra M (1991) Predation and production by salmonine fishes in Lake Michigan, 1978-1988. Can J Fish Aquat Sci 48:909–922
Stewart DJ, Weininger D, Rottiers DV, Edsall TA (1983) An energetics model for lake trout, Salvelinus namaycush: application to the lake Michigan population. Can J Fish Aquat Sci 40(6):681–698
Sweka JA, Hartman KJ (2001) Influence of turbidity on brook trout reactive distance and foraging success. Trans Am Fish Soc 130:138–146
Tang M, Boisclair D, Ménard C, Downing JA (2000) Influence of body weight, swimming characteristics, and water temperature on the cost of swimming in brook trout (Salvelinus fontinalis). Can J Fish Aquat Sci 57:1482–1488
Townsend CR, Hildrew AG (1976) Field experiments on the drifting, colonization and continuous redistribution of stream benthos. J Anim Ecol 45(3):759–772
Trudel M, Welch DW (2005) Modeling the oxygen consumption rates in Pacific salmon and steelhead: model development. Trans Am Fish Soc 134:1542–1561
Vannote RL, Minshall WG, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Vogel JL, Beauchamp DA (1999) Effects of light, prey size, and turbidity on reaction distances of lake trout (Salvelinus namaycush) to salmonid prey. Can J Fish Aquat Sci 56:1293–1297
Wall CE, Bouwes N, Wheaton JM, Saunders WC, Bennett SN (2015) Net rate of energy intake predicts reach-level steelhead (Oncorhynchus mykiss) densities in diverse basins from a large monitoring program. Can J Fish Aquat Sci 73:1081–1091
Wall CE, Bouwes N, Wheaton JM, Bennett SN, Saunders WC, McHugh PA, Jordan CE (2017) Design and monitoring of woody structures and their benefits to juvenile steelhead (Oncorhynchus mykiss) using a net rate of energy intake model. Can J Fish Aquat Sci 74(5):727–738. https://doi.org/10.1139/cjfas-2016-0131
Wankowski JWJ, Thorpe JE (1979) Spatial distribution and feeding in Atlantic salmon, Salmo salar L. juveniles. J Fish Biol 14:239–247
Ware DM (1978) Bioenergetics of pelagic fish: theoretical change in swimming speed and ration with body size. J Fish Res Board Can 35:220–228
Washington Department of Fish and Wildlife (2000) Bull Trout and Dolly Varden Management Plan. 1–19. https://wdfw.wa.gov/sites/default/files/publications/00930/wdfw00930.pdf
Williams JE, Williams RN, Thurow RF, Elwell L, Philipp DP, Harris FA, Kershner JL, Martinez PJ, Miller D, Reeves GH, Frissell CA, Sedell JR (2011) Native Fish Conservation Areas: a vision for large-scale conservation of native fish communities. Fisheries 36(6):267–277
Acknowledgments
This research represents work conducted as part of the primary author’s PhD thesis. We would like to thank the many individuals who aided in various aspects of this research and reviewed the manuscript including: J. Neuswanger, T. Simon, S. Naman, R. Sliger, E. Donofrio, Jittery Joe’s, Creature Comforts and two anonymous referees. BBB would also like to acknowledge the many years of support and mentorship of D. Bozeman. The North Pacific Research Board (grant #1424), USDA McIntire-Stennis program (GEO-00196-MS), University of Georgia Graduate School, University of Georgia's Center for Integrative Conservation Research and Integrative Conservation program, and Daniel B. Warnell School of Forestry and Natural Resources provided financial and material support for this project.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply
About this chapter
Cite this chapter
Bozeman, B.B., Grossman, G.D. (2024). The Use of Net Energy Intake Models to Predict Microhabitat Selection by Drift-Feeding Fishes: Are Common Assumptions Warranted?. In: Lobon-Cervia, J., Budy, P., Gresswell, R. (eds) Advances in the Ecology of Stream-Dwelling Salmonids. Fish & Fisheries Series, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-031-44389-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-44389-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44388-6
Online ISBN: 978-3-031-44389-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)