Abstract
Habitat selection is an important phenomenon that may greatly affect individual fitness. Using an artificial stream, we examined the relationship between the percentage of prey captured, reactive distance, dominance, and water velocity for juvenile Chinook Salmon (Oncorhynchus tshawytscha) from the Chena River, Alaska, and tested the fitness-based microhabitat selection model of Grossman et al. (Ecol Freshw Fish 11:2–10, 2002). Recent declines in the abundance of Chinook accentuate our need for habitat selection studies on this species. We conducted three experiments: two with single fish (1st N = 27, fish SL 58–84 mm, 2nd N = 14, fish SL 49–56 mm) and one with pairs of dominant and subordinate fish (N = 10 pairs, 64–96 mm, mean difference in SL = 7 mm). We placed individual or pairs of fish in an artificial flume and recorded reactive distance and the percent prey capture with individual dead brine shrimp (Artemia spp.) as prey. Prey were presented at 10 cm/s velocity intervals ranging from 10 to 60 cm/s; velocities found in the natural habitat. Mean reactive distance in single fish experiments (henceforth SFE) averaged 33 and 29 cm respectively, and was not related to velocity. We detected a negative, curvilinear relationship between velocity and percent prey capture. Holding velocities for juvenile Chinook were significantly lower than prey capture velocities. The Grossman et al. (Ecol Freshw Fish 11:2–10, 2002) model yielded an optimal focal-point velocity prediction of 35 cm/s for juvenile Chinook, however focal-point velocities occupied by juveniles in the Chena River averaged 12 cm/s. Predicted optimal velocities were present in the Chena River; hence, this discrepancy suggests that other factors such as distraction from drifting debris or predation risk influenced habitat selection. There were no differences in reactive distances or holding velocity/capture velocity relationships for dominant and subordinate fish; however, dominants captured significantly more prey than subordinates. Being subordinate resulted in a decrease of 61% in mean percent prey capture (the difference between what was captured by the fish alone versus the difference with a dominant), whereas the mean cost to fish with dominant rank was a 21% decline between the percentage captured alone versus that with a subordinate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Chinook salmon (Oncorhynchus tshawytscha) is native to rivers in the Pacific drainages of the western United States from Alaska to Northern California. The general life-history pattern for Chinook Salmon (henceforth Chinook) includes: 1) a freshwater juvenile period ranging from six to eighteen months, 2) an outmigration to the ocean where they mature for 2–6 years as adults, 3) followed by a return to their natal stream to spawn and die. As juveniles, Chinook inhabit boreal and cool-temperate lotic systems, and primarily forage on drifting invertebrates (Quinn 2005). Chinook demonstrate high levels of local adaptation (Quinn 2005), however, many populations, including the Chena River population (Alaska Department of Fish and Game Chinook Research Team 2013), are experiencing declines (Muir and Coley 1996; Tolimieri and Levin 2004; Shaffer et al. 2009; Walters et al. 2013). Chena River Chinook are negatively affected by high flows during the juvenile period which subsequently produce low numbers of returning adults (Neuswanger et al. 2015). Because our knowledge of the physical and biological factors affecting habitat selection in juvenile Chinook is incomplete, such studies should increase our understanding of the biology of this ecologically and commercially important species.
Most research on habitat selection in lotic fishes is based on correlative studies relating fish abundances, or the spatial distribution of individuals, to physical characteristics within sampling units, typically reaches (Grossman 2014). Descriptive studies indicate that juvenile Chinook utilize pools more frequently than riffles and runs, and are over-represented in habitats with pebble, cobble, or sand substrata (Everest and Chapman 1972; Bravender and Shirvell 1990; Holecek et al. 2009; Tabor et al. 2011). Typically, woody debris is utilized as cover (Holecek et al. 2009; Tabor et al. 2011). Juvenile Chinook may shift microhabitat use seasonally, moving to deeper water from spring to winter and shifting to microhabitats with faster water velocities in summer [e.g., 2.1–6.1 cm/s in Washington (Allen 2000) and 0–25 cm/s in Idaho (Holecek et al. 2009)]. Laboratory studies demonstrate juvenile Chinook are more strongly associated with cover at both lower temperatures (i.e., 2 °C) and velocities (Taylor 1988). Juvenile Chinook also display diel shifts in microhabitat use, presumably to reduce predation risk; during daylight hours, juveniles utilize microhabitats with cover, whereas at night they shift to more open areas with smaller substrata (Tabor et al. 2011). Finally, juvenile Chinook also exhibit ontogenetic microhabitat shifts, and occupy positions with greater depth as they increase in size (Holecek et al. 2009; Tabor et al. 2011). Ultimately, however, descriptive, correlation-based studies cannot elucidate the mechanisms producing microhabitat selection in juvenile Chinook.
In contrast to studies based on the physical characteristics of habitat alone, fitness-based (i.e., net-energy gain) approaches link habitat selection to a correlate of individual fitness such as prey-capture success, mortality, or growth (Fausch 1984; Hughes and Dill 1990; Hill and Grossman 1993; Hayes et al. 2007; Grossman 2014). Although fitness-based approaches are more difficult logistically than descriptive studies of physical habitat characteristics, they have been quite successful at predicting microhabitat use for fishes, even though trade-offs sometimes exist between energy-gain and predation risk (Gilliam and Fraser 1987; Grossman 2014; Piccolo et al. 2014). A great advantage of fitness-based habitat selection models is that they operate under mechanistic principles that directly address the potential causal factors (e.g., energy gain and expenditure) responsible for habitat selection by individuals. Consequently, they greatly increase our understanding of the basic principles behind habitat selection in organisms (Fausch 1984; Hughes and Dill 1990; Grossman 2014). Although several fitness-based models have been proposed in the literature, few have been rigorously tested in the field in varying environments and with varying species (Fausch 2014; Grossman 2014; Piccolo et al. 2014).
The most widely used fitness-based approach for exploring habitat selection relationships in fishes involves quantifying net-energy gain under realistic environmental conditions. This approach has been most widely applied to drift-feeding fishes, mainly salmonids (Grossman 2014) and typically involves quantifying both the costs and benefits incurred by holding position at a given focal-point velocity (Hill and Grossman 1993; Grossman 2014). However, Hill and Grossman (1993) and Grossman et al. (2002) found that inclusion of physiological costs actually reduced the predictive ability of a net-energy gain microhabitat model for Rainbow Trout (Oncorhynchus mykiss) and Rosyside Dace (Clinostomus funduloides). These authors established that net-energy gain models based on prey capture – velocity relationships yielded excellent predictions of the holding (i.e., focal-point) velocities occupied by 1) Rainbow Trout, 2) Rosyside Dace, 3) Warpaint Shiners (Luxilus coccogenis), 4) Yellowfin Shiner (Notropis lutipinnis) and 5) Tennessee Shiner (Notropis leuciodus) in a Southern Appalachian stream. Although the Grossman et al. (2002) model performed reasonably well in predicting focal-point (i.e., holding positions) of the aforementioned species in a single North Carolina stream, it requires further testing in different environments and with a more diverse array of test species. Consequently, we used the approach of Grossman et al. (2002) to quantify relationships between current velocity and biological factors potentially affecting prey capture and microhabitat selection for juvenile Chinook from the Chena River AK. Specifically, we examined relationships between current velocity and: 1) holding velocity, 2) reactive distance, 3) prey-capture velocity and percentage captured, and 4) dominance – percent prey capture. In addition, we tested the optimal focal-point (holding) velocity predicted by the Grossman et al. (2002) fitness-based model against holding velocities occupied by juvenile Chinook in the Chena River.
Materials and methods
Experimental procedures
We captured wild juvenile Chinook using minnow traps baited with frozen salmon eggs in September 2014 and June 2015 from the Chena River, Alaska (64° 54′ 8.03" N 146 ° 28' 35.50" W). Within two days of capture, specimens were shipped via airfreight to our laboratory at the University of Georgia. Neither shipping nor handling/holding produced either injury or mortality, and fish in both holding and experimental tanks appeared to behave naturally when compared to videos of Chinook in the Chena River. Mean standard length (mm SL ± SD) of Chinook at the beginning of single-fish experiment 1 was 68 ± 6 mm and mean mass (g) ± SD was 4.5 ± 1.1 (N = 28 individuals). Mean SL and mass of fish in single-fish experiment 2 were 49 ± 3 mm and 1.8 ± 0.4 g, respectively (N = 15 individuals). We held fish in tanks at water temperatures approximating capture temperatures (10 °C) for 121–210 days prior to Single Fish Experiments (henceforth SFE) and between 255 and 301 days for Dominance experiments. We fed fish an ad libitum ration of frozen brine shrimp (Artemia spp.) during the holding period, however, food was withheld on the day preceding an experiment to increase feeding motivation (Grossman et al. 2002).
We conducted experiments in an artificial stream (Fig. 1) described in detail by Hazelton and Grossman (2009). In brief, the stream was 3.5 m L x .75 m W × 1 m H, (Fig. 1) and was bisected horizontally by a plexiglass floor to create partially separated test (top half), and water-return (bottom half) chambers. The test chamber was bounded by upstream and downstream mesh screens and measured 1.5 m L × 0.75 m W × 1 m H with a water depth of 35 cm. We maintained water velocity using two, variable speed (24-V, 80-lb thrust) trolling motors: placed at the downstream end of the water-return chamber. The trolling motors propelled water forward through the water-return chamber and up into the test chamber. We placed a polyvinyl chloride collimator at the upstream most portion of the test chamber (Fig. 1) to produce semi-laminar flow. However, it was necessary to add bricks and a small structure made out of thin bamboo sticks to the tank to: 1) provide structure for fish orientation, and 2) encourage fish to hold position approximately in the middle of the tank to facilitate measurements of reactive distance. For SFE 1 we attached a 2.5 mm square plastic mesh screen to the outside of the rear wall of the test chamber to aid positional measurements of fish during a trial, however the use of Vidsync (Neuswanger et al. 2016) in later experiments rendered this screen unnecessary. We filled the stream with filtered, dechlorinated tap water and added water to the stream as necessary. Turbidities in the stream were low, tap water turbidities in Athens GA are less than 0.001 NTU’s (Athens Clarke County). Nonetheless turbidities in the experimental stream were visually similar to those in the Chena River during field observations, which displayed maximum visibilities up to 3.4 m (range 1.9–3.4 m, mean 2.3 m) in five field videos shot during June, July and August 2015, and June and August 2016. To demonstrate the comparability of turbidity conditions in the field and in the experimental stream we have posted four videos, three showing the variety of field habitat conditions in the Chena River www.youtube.com/watch?v=ivJmf0r8A1o (maximum visibility is 2.9 m for this video, measured with Vidsync), www.youtube.com/watch?v=EW55LexEPxQ, www.youtube.com/watch?v=BJokgZrAi84, and one lab experiment video at https://youtu.be/RXcn1ew3KuM .
We presented prey to juvenile Chinook via one of three, randomly selected, flexible plastic feeding tubes (6 mm diameter). Feeding tubes were attached to the upstream screen of the test chamber, at equal intervals across the width of the tank (Fig. 1). We delivered prey to fish by flushing them into the tank using water run through a feeding tube. Prey were delivered at a depth of 5 cm below the water’s surface. We used frozen adult brine shrimp (mean body length 5.7 mm, N = 50) as prey because they elicited natural foraging and capture behaviors in juvenile Chinook, and also were readily visible during observations (personal observation). It was impossible logistically to secure a source of consistent Alaskan invertebrate prey for experiments. We conducted pilot experiments to track the trajectory of the prey and to determine motor speeds necessary to produce treatment velocities of 10–60 cm/s increasing by 10 cm/s increments. There was minor variation between treatment and capture velocities in the tank; nonetheless, we used treatment velocity for statistical analyses, because values for the two variables were similar and highly correlated (R2 = 0.93). During SFE 1, we made velocity availability measurements within the tank (see below) to quantify within-tank variation in velocity (see below). To eliminate human disturbance of the fish, we made all observations from behind a black plastic blind.
We acclimated juvenile Chinook by placing them in the test chamber overnight without flow. We conducted experiments between 09:00 and 18:30 h because daytime feeding is more common in juvenile Chinook (Quinn 2005). Experiments began by increasing flow to 5 cm/s for 15 min. Velocities were gradually increased until the first treatment velocity (10 cm/s) was reached. We measured treatment velocities at the midpoint of the test chamber at approximately 25% depth from the center of the chamber, using an electronic water velocity meter. Several prey were then delivered to induce feeding behavior and experiments began after the test subject attempted to capture a prey. If the test subject failed to capture one of the first five prey presented, or if we observed abnormal behavior (e.g., erratic swimming), we then decreased tank velocity and rested the test subject for 20 min, prior to retesting. If the test subject again refused prey, it was eliminated from experiments.
Once a test subject fed, we released prey at approximately 20 s intervals until seven to 10 prey were delivered at a given treatment velocity. In a few cases, we excluded prey from the presentation count if they failed to elicit a foraging response from the test fish and appeared to be outside its visual field. We used either visual (SFE 1) or video (SFE 2, Dominance experiment) observations to record the following for each test subject: 1) reactive distance (the distance between the prey and subject’s nose when first orienting towards the prey), 2) the number, location and velocity of each prey captured, and 3) fish holding position (i.e., focal-point, position consistently occupied by the test specimen Grossman and Freeman 1987) and velocity (cm/s). To prevent fatigue and reduce satiation effects, each fish was rested for 30 min at 5 cm/s velocity between each velocity treatment. We ended each velocity trial when the subject captured two or fewer prey out of the seven delivered.
After completion of an experiment, we measured velocity (±0.01 cm/s) at both holding and prey capture locations using an electronic velocity meter (Grossman et al. 2002). Reactive distance and positional measurements were made using either direct visual (SFE 1), or video (SFE 2, Dominance experiment) observations of the fish’s position (X and Y coordinates on the grid, Barrett et al. 1992). Visual measurements in SFE 1 were made in two dimensions, whereas in SFE 2 and the Dominance experiment we measured reactive distance in three dimensions using Vidsync (Neuswanger et al. 2016), which can calculate the three-dimensional position of a fish with one millimeter accuracy (www.vidsync.org). Velocity availability measurements (±0.01 cm/s) were made at three different locations within the tank: 25% chamber length, 50% chamber length, and 75% chamber length, to quantify flow variability. At each location, we measured cross-sectional velocities across the tank (19 cm, 38 cm, and 58 cm) at three different locations perpendicular to the feeding tubes and at three different depths (10 cm, 20 cm, 30 cm) for a total of nine measurements per cross section and 27 velocity availability measurements per treatment velocity. Data for each experiment were analyzed separately.
Test of the net-energy gain habitat selection model
We used the proportion of prey captured at a given treatment velocity to parameterize a curve describing capture probability P as a function of water velocity V (cm/s) with fitting constants b and c (Hill and Grossman 1993):
Grossman et al. (2002) showed that the constants b and c can predict the holding velocity that maximizes net-energy intake through an implicit relationship, which is solved iteratively for V:
Equation (1) assumes capture probability is determined primarily by water velocity, whereas eq. (2) assumes that the size of the reactive volume, prey density, and the swimming cost of holding position can be treated as constant over the range of velocities being modeled (Grossman et al. 2002).
We compared the predicted optimal (i.e., velocity maximizing net energy gain) holding velocity to holding velocities occupied by juvenile Chinook and available velocities in the Chena River during June and August 2009, July and September 2010, and June–August 2015. We used a calibrated stereo pair of underwater video cameras to observe fish and measure the velocities of neutrally buoyant tracers (natural debris or soaked Israeli cous cous) passing through the area occupied by drift-feeding juvenile Chinook and used Vidsync for measurements and analyses (Neuswanger et al. 2016). Each field velocity data point represented the mean of 20 or more tracer measurements spread throughout a microhabitat patch occupied by a group of feeding juvenile Chinook (N = 12). Microhabitat availability measurements were taken by measuring at least 40 buoyant particles located throughout the viewing pane. If the predicted optimal velocity fell within the 95% confidence interval of the field focal point velocity data (Grossman et al. 2002) the model yielded a successful prediction. We made additional microhabitat availability measurements in the Chena River in 2008 (25 June, 19 July, 4 and 17 September) and 2016 (3 June). Velocity availability measurements were taken at mid-water column depths using an electronic flow meter. These measurements were used to test whether the optimal focal point velocity predicted by the Grossman et al. (2002) model was available in the Chena River.
Effects of dominance
Dominance affects resource use in salmonids and generally is related to size and innate behavior (White and Gowan 2013). We quantified the effects of dominance on holding velocity, reactive distance, and percent prey capture by conducting an experiment with large and small juvenile Chinook. Because of constraints on collecting, we used the same fish that were used in SFEs. Fish were rested an average of 61 days (range 43–89 days) before being used in the dominance experiment. Mean SL of the 20 fish (N = 10 pairs) was 75 mm (SD ± 6 mm) and the mean weight was 6.4 g (SD ± 1.2 g). The range of lengths was 96 mm to 64 mm with a mean SL difference of 7 mm. The range of weights was 10.8 g to 4.2 g with a mean difference of 1.5 g.
The first six pairs of fish in the dominance experiment differed in SL by at least 7 mm (mean difference: 12 mm with a range of 7 mm–19 mm); the following five pairs of fish differed in SL by a minimum of 1 mm (mean 2.3 mm) with a range of 1–3 mm. We pooled data from these two groups of fish because results from both sets of fish were identical (all dominants had similar holding velocities, reactive distances and percent prey capture). We recorded which fish initiated aggressive interactions and who was displaced (forced away from their original position), if displacement occurred.
To begin an experiment, we measured fish and placed them in the tank overnight to acclimate as per SFEs. Methods generally were held constant between single fish and dominance experiments, except we released 15 prey per velocity treatment in the latter experiments to keep the number of prey released/fish constant over all experiments. We ended an experiment at a given treatment velocity when the dominant fish captured seven or less of the 15 prey for pairs with at least a 7 mm length difference or when both fish caught seven or fewer prey for experiments with length differences <7 mm. We justified these thresholds by reasoning that seven or less captures is a percentage of <50% and would represent a substantially reduced level of energy intake.
We recorded data for the dominance experiment using stereo video and VidSync. We recorded reactive distances and percent prey capture for both dominant and subordinate fish as well as position within the tank and treatment velocity. We calculated the “cost” of being subordinate as the difference in percent prey capture between dominant and subordinate fish, and the cost of being dominant as the difference between the mean percent prey capture for fish in the SFE and the Dominance experiment.
Statistical analysis
For both single-fish experiments, we used generalized linear models and information-theoretic model averaging to quantify the importance of the continuous, fixed effects of treatment velocity, fish size, and days in captivity, on capture probability, reaction distance, and holding velocity. These models used identity link functions for reaction distance and holding velocity and a logit link function for capture probability. For dominance trials, we added a categorical effect of dominant or subordinate classification to the models. We did not model interaction terms, because we were primarily interested in main effects, and the inclusion of interaction terms would obfuscate interpretation of model parameter effect sizes. Furthermore, the explanatory power of interaction terms could not have been directly compared to main effects using the information theoretic approach (see below) because they would not be present in an equal number of models.
For each response to each experiment, we used the R library ‘glmulti’ (R Core Team 2015; Calcagno and de Mazancourt 2010) to run the full models described above and all possible reduced versions of them. Akaike weights were calculated for the models based on the AICc criterion (Burnham and Anderson 2002), and variable weights calculated as the sum of the model weights of all models containing each variable. Similar to McGrann et al. (2014) and Burnham and Anderson (2002), we interpreted variable weights (w + ) from 0.85 to 1.0 as strong support for a relationship between a predictor and the response variable, weights from 0.7 to 0.84 as support, and weights from 0.5 to 0.69 as weak support. These weights indicate the probability that a given variable is included in the best model out of all our candidate models. Weights below 0.5 were not considered to provide evidence of a relationship. Parameter estimates and confidence intervals were obtained by averaging estimates across models in proportion to their Akaike weights using the ‘Lukacs’ method in the coef.glmulti function.
To clarify the relationship between behavior and percent prey capture, we used one-tailed, paired t-tests to test the alternative hypothesis that dominant fish captured significantly more prey than subordinate fish (null hypothesis: no difference). We also employed one tailed paired t-tests to test the alternative hypothesis that capture velocity was significantly greater than holding velocities (null hypothesis: no difference) using test velocities from 20 to 60 cm as blocks and mean values for fish holding and capture velocities as data.
Results
Holding velocity
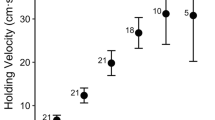
In all three experiments, treatment velocity was a strong predictor of holding velocity (Akaike weights w + ≥ 0.97), which increased slowly as treatment velocity increased (positive slopes from 0.14–0.41, Table 1, Figs. 2 and 3). The remaining relationships between predictor variables and holding velocity were weakly supported and inconsistent among experiments. For example, only the Dominance experiment displayed a weakly supported (w + = 0.62) positive relationship between fish size and holding velocity; whereas only SFE 1 exhibited a weakly supported positive relationship between holding velocity and days in captivity (w + = 0.58).
Reactive distance
Reactive distance measurements made visually in 2D and 3d were similar and in all three experiments, days in captivity was supported (w + ≥ 0.84) as a predictor of reactive distance and as fish spent more days in captivity, reactive distances increased at a rate of 1–2 mm/day (Table 1; Figs. 4 and 5). Consequently, captivity was not detrimental to the foraging capabilities of juvenile Chinook. This result also suggests fish displayed a gradual and slight increase in foraging capability with time; possibly due to increased familiarity with their prey or environment or both. Nonetheless, reactive distances did not differ substantially among experiments (Figs. 3, 4 and 5) despite major differences in captivity time (range 121–211 days for SFE 1, 15–50 for SFE 2, and 261–300 for the dominance experiment) suggesting this within-experiment trend may be artefactual. The only other supported relationship (w + = 0.80) was that reactive distance increased at a rate of 2.3 mm per 1 mm of fish size in SFE 1. Finally, we detected a weakly supported (w + = 0.52) negative relationship in this same experiment between reactive distance and increasing treatment velocity.
Percent prey capture
Treatment velocity was a strong negative predictor of percent prey capture in all three experiments (all w + values ≥ 0.98; Table 1, Figs. 6 and 7). Dominance also was very strongly linked to percent prey capture (w + = 1.0), demonstrating that dominant fish captured more prey and had greater energy intake than subordinates. As per previous results, the remaining supported relationships only were observed in individual experiments. In SFE 2, days in captivity was strongly supported (w + = 0.99) as a positive predictor of percent prey capture; whereas, in the dominance experiment, there was support (w + = 0.71) for a positive relationship between fish size and percent prey capture, independent of dominance effects.
Mean percent prey capture for dominant and subordinate fish in Experiment 3. N represents the number of prey caught by either the subordinate or dominant fish and error bars represent SD. Number of pairs at each treatment 10 cm/s-11 pairs, 20 cm/s-11 pairs, 30 cm/s-9 pairs, 40 cm/s-9 pairs, 50 cm/s-8 pairs, 60 cm/s-5 pairs. Equation for dominant fish y = −0.47× + 78.0, R2 = 0.73. Equation for subordinate fish y = −0.48× + 41.3, R2 = 0.70
Capture versus holding velocities
In all experiments Chinook held positions at velocities that were significantly lower than prey capture velocities (Figs. 2 and 3; SFE 1: t = 4.00, df = 5, p = 0.005; SFE 2: t = 3.30, df = 5, p = 0.001; Dominance Exp. 3: t = 3.14, df = 5, p-value = 0.013) with mean capture velocities ranging from 12 cm/s to 21 cm/s greater than mean holding velocities respectively.
Dominance effects
Dominant juvenile Chinook held position in the center of the tank typically behind the bamboo structure, and captured prey in the center and to one side of the tank (pers. obs.). By contrast, subordinate fish were relegated to a position behind the dominant fish, sometimes near the back of the tank and at velocities above 30 cm/s, frequently by a side wall (pers. obs.). Dominant and subordinate fish captured prey in their respective feeding lanes and aggressive interactions were not common (52 interactions in 870 min of observation). A substantial majority of interactions (73%) occurring at velocities less than 30 cm/s. Dominant fish initiated and won most aggressive interactions (83%).
Both dominant and subordinate fish displayed a significant, non-linear relationship between treatment velocity and percent prey capture (Fig. 7). The cost of being subordinate varied by treatment velocity (Fig. 7) and reductions in percent prey capture (i.e., the difference between the percentage of prey captured when alone versus with a subordinate or dominant) ranged from 48 to 88%, with a mean of 60%. Although dominant fish captured more prey than subordinates, these fish also experienced a mean decline in percent prey capture of 21% (range 6–28%) when a subordinate was present.
Net-energy gain habitat selection model
Fitting the Grossman et al. (2002) model for juvenile Chinook in the Chena River to data from SFE 1 yielded parameters of b = −5.554 and c = 0.122 for eq. (1). Using these values, eq. (2) predicted an optimal holding velocity of 35 cm/s for fish in SFE 1; SFE 2 yielded very similar parameters and an identical optimal holding velocity. The 95% CI of 28 holding velocity measurements of juvenile Chinook in the Chena River ranged from 3.7 cm/s to 20 cm/s (median: 8.5 cm/s) and did not encompass the predicted optimal holding velocity. Consequently, the model failed to predict the holding velocities of juvenile Chinook in this habitat. Local velocity availability data indicated that velocities closer to the predicted optimum (i.e., 20–40 cm/s) commonly comprised 25% or more of total velocity measurements within inches to meters of a specimen, although two fish had almost no velocities available within that range. Faster water was widely available in the main channel, where 63 velocity measurements (collected at 25, 50, and 75% of channel width on 21 transects spaced 30 m apart throughout one riffle-pool-run unit) showed velocities with a 95% CI of 33 to 160 cm/s (median: 88 cm/s). Consequently, the failure of the Grossman et al. (2002) model was not due to the fact that the optimal velocity was unavailable to Chinook in the Chena River, but because the fish clearly selected lower velocities.
Discussion
Our laboratory experiments demonstrate that juvenile Chinook frequently held position in slower areas adjacent to higher velocity microhabitats where prey were captured; a result similar to that of Everest and Chapman (1972) for Chinook in Idaho streams. In fact, multiple investigators have observed this phenomenon in various drift-feeding stream fishes (Grossman 2014). Because of the positive correlation between stream velocity and drift (Grossman 2014), holding position in lower velocity areas adjacent to high velocity microhabitats likely enables fish to reduce the energetic cost of prey capture. Video analyses of fish in the Chena River did not indicate that fish typically held position at significantly lower focal-point velocities than they captured prey (pers. obs.), but fish in the Chena typically occupied focal point velocities below 20 cm/s, and Chinook in lab experiments did not display the holding – prey capture velocity difference at those velocities either. At velocities above ~25 cm/s, percent prey capture was inversely related to treatment velocities and dominant fish had significantly greater percent prey capture than subordinate fish. Intraspecific competition resulted in energetic costs for both dominant and subordinate individuals, although these costs were much higher for subordinate fish. Finally, the Grossman et al. (2002) model failed to predict the optimal holding velocity for juvenile Chinook in the Chena River, indicating either a failure of the model or that some other factor such as difficulty distinguishing prey from debris (Neuswanger et al. 2014) or predation significantly affect optimal microhabitat selection. This last finding substantiates the need for further testing of fitness-based (e.g., net energy gain) habitat selection models in diverse habitats and with diverse species, to better understand the mechanisms affecting habitat selection in fishes.
The finding that juvenile Chinook held position in the laboratory stream at velocities that were slower than velocities at which prey were captured is supported by studies on other drift-feeders such as juvenile Rainbow Trout, Rosyside Dace, juvenile Coho Salmon (Oncorhynchus kisutch) and juvenile Chinook (Everest and Chapman 1972; Hill and Grossman 1993; Shirvell 1994; Piccolo et al. 2008). In addition, Facey and Grossman (1990) quantified energy expenditure incurred by holding position at varying velocities by juvenile Rainbow Trout and Rosyside Dace and showed these species avoided energetically costly high velocity microhabitats in a Southern Appalachian stream (Facey and Grossman 1992). Finally, holding position in lower velocity microhabitats adjacent to higher velocity microhabitats is likely a common phenomenon amongst drift-feeding fishes, especially given the positive relationship between current velocity and drift abundance (Everest and Chapman 1972; Grossman 2014; Piccolo et al. 2014).
Although there are few studies on the relationships among reactive distance, prey percent prey capture and water velocity, extant work demonstrates a pattern of decreasing percent prey capture as velocity increases to high values (Grossman et al. 2002; Piccolo et al. 2008; Grossman 2014; Piccolo et al. 2014). Most studies demonstrate an exponential decline beginning at moderate water velocities (25 to 30 cm/s); nonetheless, juvenile Chinook were able to capture prey at velocities over 60 cm/s. It is not surprising that juvenile Chinook possess such an exceptional ability to capture prey, given their habitats and migratory patterns (Quinn 2005).
Reactive distances for juvenile Chinook remained relatively constant and were not significantly affected by velocity, nor by dominance status. There are scant comparative data for reactive distances in stream fishes, however, reactive distance is affected by turbidity in Rainbow Trout and Rosyside Dace (Barrett et al. 1992; Zamor and Grossman 2007; Hazelton and Grossman 2009) and conspecific density in Rosyside Dace (Hazelton and Grossman 2009). Although turbidity may affect several of the variables measured in our study; visual comparisons indicate that laboratory turbidities did not differ substantially from those observed in the field. Further work on this topic is needed, especially given the prevalence of water quality issues that may affect fish vision (e.g., turbidity).
Dominance typically facilitates increased access to limited resources such as food or space (Grossman 1980; Fausch 1984; Quinn 2005). Dominance relationships are particularly common in salmonids (Quinn 2005), and may manifest themselves at the population level to ultimately produce intraspecific competition and density-dependent population regulation (Grant and Imre 2005; Grossman et al. 2010, 2012). Our experiment indicated a strong positive advantage to being dominant: 1) access to more profitable foraging positions, and 2) increased percent prey capture. However, being dominant also resulted in reductions in percent prey capture at a given velocity, although these reductions were much lower than those experienced by subordinate fish. The costs of dominance are not well known; but a portion of the decrease in the percentage of prey captured by dominant fish with subordinates likely is due to the fact that dominants initiated the vast majority of aggressive interactions with subordinates. Nonetheless, the relationship between dominance in laboratory settings and fitness in the wild is not well established and a variety of responses have been observed including: 1) positive relationships between dominance and fitness correlates (Wessel et al. 2006; Evans et al. 2007), and 2) a lack of relationship between these two factors (Harwood et al. 2003; Adriaenssens and Johnsson 2011. The lack of a relationship between dominance and fitness in a natural habitat must be viewed as tentative, however, because it is very difficult to examine every aspect of fitness in wild populations.
The optimal holding velocity for juvenile Chinook predicted by the Grossman et al. (2002) model (35 cm/s) was significantly faster than the focal point velocities occupied by this species in the Chena River 3.7 cm/s to 20 cm/s (median: 8.5 cm/s). It is uncertain why this disparity existed, but two plausible alternatives are possible: 1) fish were optimizing behavior with respect to some fitness currency other than net-energy intake (e.g., predator avoidance), or 2) juvenile Chinook in the Chena River were not choosing microhabitats via optimization behavior, especially behavior that involves velocity choice. A comparison of velocity use by juvenile Chinook in other systems does not clarify this discrepancy, because this species is capable of using a wide variety of velocities in lotic systems: e.g., summer focal point velocities of 2–84 cm/s in Washington populations Allen (2000), 0–50 cm/s in tributaries of Lake Ontario (Johnson 2014), and 25 cm/s or less in Idaho streams (Holecek et al. 2009).
It also is likely that predator avoidance is important for juvenile Chinook in the Chena River, especially given their strong association with large woody debris in this and other rivers (pers. obs.; Mossop and Bradford 2004). Potential predators are not uncommon in portions of the Chena River occupied by this species including: piscivorous mammals (mink Neovison vison and possibly otter Lutra canadensis), birds (common merganser Mergus merganser, common goldeneye Bucephala clangula, belted kingfisher Megaceryle alcyon), and even large grayling (Thymallus arcticus) (personal observation). Although, the Grossman et al. (2002) model was able to successfully predict field microhabitat use by four species of minnows, we have previously shown that predation is not an important phenomenon in the streams occupied by these species (Grossman et al. 1998).
Alternatively, it also is possible that the model’s predictions do not reflect all the major variables determining the optimal focal-point velocity for juvenile Chinook in the Chena River. Specifically, the assumption of eq. (1) that detection probability is entirely determined by water velocity may not be valid for this system. Evidence for this hypothesis comes from contrasting the mean reactive distance in our laboratory experiments (32.7 cm) with the field studies of Neuswanger et al. (2014), who found that juvenile Chinook in the Chena River reacted to 867 potential prey items at an average distance of 6.3 cm (99% of reactive distances less than 17.1 cm). One possible reason for this difference is a difference in prey size between the Chena River and our experiments. It appears there were more small prey consumed in the Chena River (length range 1 to 5 mm, L. Gutierrez, University of Alaska, 2011 unpublished data), although the brine shrimp used in experiments were only slightly larger (mean body size 5.7 mm) than this range. In addition, when brine shrimp were flushed into the experimental tank, they did not necessarily expand to their full length; hence, their perceived length would be smaller than the mean length, although this also may be true for prey in the Chena River. There is no doubt that juvenile Chinook consume many smaller prey in the Chena River, although it seems unlikely that differences in prey size were responsible for the large difference in reactive differences observed. It also is possible, given the close proximity of juveniles in the Chena to large woody debris, that long reactive distances are affected by the shadowing effect and complex background presented by this debris.
A final factor that may have produced the discrepancy between observed and predicted focal point velocities in the Chena River, is that natural drifting debris, such as pieces of plant matter or insect exuviae, vastly outnumber drifting prey in these small size classes. Neuswanger et al. (2014) found that 90% + of foraging attempts in the Chena River entail the pursuit and rejection of debris rather than prey. O’Brien and Showalter (1993) showed that natural stream debris reduced the distance and angle at which adult Arctic Grayling detected prey, and there is a substantial literature on the negative effects of turbidity on prey capture by drift feeding minnows as well as reactive distance of Rainbow Trout (Barrett et al. 1992; Zamor and Grossman 2007; Hazelton and Grossman 2009). Chinook may be exhibiting a similar reduction in reactive distances because of the high volume of debris, which would produce a lower optimal velocity in the field to maximize net-energy intake.
Our results demonstrate that velocity and dominance significantly affect percent prey capture and most likely, microhabitat selection of juvenile Chinook. Nevertheless, our findings could have been affected by several shortcomings, including the amount of time fish were held in captivity and captivity itself. Days in captivity had a significant effect on reactive distances for all experiments, despite the fact that fish were held for differing amounts of time for experiments (ranges for SFE 1 = 90 days; SFE 2 = 50 days; Dominance Experiment = 39 days). Although one would expect a deterioration of health with extended captivity, the significant relationships between reactive distance and days in captivity all were positive, indicating that fish were not stressed by captivity. More importantly, a days in captivity effect was not manifested in percent prey capture results in two of three experiments; the only significant effect observed was for “days in captivity” in SFE 2.
We attempted to link microhabitat selection to a fitness correlate, in contrast to descriptive studies of habitat selection. In the latter studies, physical characteristics (e.g., depth, velocity, shelter, substratum composition) at or near the fish’s position typically are compared with their availability in the environment (e.g., Grossman and Freeman 1987), or fish abundances within a reach are compared to environmental characteristics of that reach (Grossman 2014). Such studies may yield insights into the differential selection of microhabitats but unless they are linked to some aspect of fitness, such as energy gain, or survivorship, they only are suggestive of the causal factors involved in habitat selection (Nislow et al. 1999; Guensch et al. 2001). Hayes et al. (2016) reached similar conclusions in a comparative study of the ability of a physically based correlative model (weighted usable area) and a fitness based model to predict brown trout (Salmo trutta) abundance in a New Zealand stream. The physical model used by Hayes et al. (2016) typically underestimated trout abundance. One interesting result from our dominance experiment is that when a competitor was present, dominant juvenile Chinook captured prey with greater percentage at velocities above 40 cm/s than fish without a competitor present (compare Figs. 6 and 7). Although we do not know whether this resulted in greater growth for dominants versus fish in SFEs, it suggests that intraspecific competition and perhaps competitor density may influence habitat quality.
Although the Grossman et al. (2002) model did not successfully predict holding velocities occupied by juvenile Chinook in the Chena River, this finding does tell us that velocity, although clearly important to percent prey capture, likely is not the sole factor affecting microhabitat selection in this environment. This finding is informative and calls for development of new fitness-based habitat selection models as well as refinement of existing models to further our understanding of the behavioral, ecological and evolutionary processes affecting habitat selection in this and other species.
References
Adriaenssens B, Johnsson JI (2011) Shy trout grow faster: exploring links between personality and fitness-related traits in the wild. Beh Ecol 22:135–143. https://doi.org/10.1093/beheco/arq185
Alaska Department of Fish and Game Chinook Salmon Research Team (2013) Chinook salmon stock assessment and research plan, 2013. Alaska Department of Fish and Game, Special Publication No. 13–01, Anchorage
Allen MA (2000) Seasonal microhabitat use by juvenile spring Chinook in the Yakima River basin, Washington. Rivers 7:314–332
Barrett JC, Grossman GD, Rosenfeld J (1992) Turbidity-induced changes in reactive distance of rainbow trout. Trans Am Fish Soc 121:437–443. https://doi.org/10.1577/1548-8659(1992)121<0437:TICIRD>2.3.CO;2
Bravender BA, Shirvell CS (1990) Microhabitat requirements and movements of juvenile Coho and Chinook at three streamflows in Kloiya Creek, B.C. Canadian Data Report of Fisheries and Aquatic Sciences-Department of Fisheries and Oceans 801:1–115
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach. 2nd ed. Springer, Secaucus
Calcagno V, de Mazancourt C (2010) Glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Software 34:1–29
Everest FH, Chapman DW (1972) Habitat selection and spatial interaction by juvenile Chinook Salmon and Steelhead Trout in two Idaho streams. J Fish Res Bd Canada 29:91–100
Facey DE, Grossman GD (1990) The metabolic cost of maintaining position for four North American stream fishes: effects of season and velocity. Physiol Zool 63:757–776. https://doi.org/10.1086/physzool.63.4.30158175
Facey DE, Grossman GD (1992) The relationship between water velocity, energetic costs, and microhabitat use in four north American stream fishes. Hydrobiologia 239:1–6. https://doi.org/10.1007/BF00027524
Fausch KD (1984) Profitable stream positions for salmonids: relating specific growth rate to net energy gain. Can J Zool 62:441–451. https://doi.org/10.1139/z84-067
Fausch KD (2014) A historical perspective on drift foraging models for stream salmonids. Environ Biol Fish 97:453–464. https://doi.org/10.1007/s10641-013-0187-6
Gilliam JF, Fraser DF (1987) Habitat selection under predation hazard: test of a model with foraging minnows. Ecology 68:1856–1862. https://doi.org/10.2307/1939877
Grant JWA, Imre I (2005) Patterns of density dependent growth in juvenile stream-dwelling salmonids. J Fish Biol 67(B): 100–110, DOI: https://doi.org/10.1111/j.0022-1112.2005.00916.x
Grossman GD (1980) Food, fights, and burrows: the adaptive significance of intraspecific aggression in the bay goby (Pisces: Gobiidae). Oecologia 45(2):261–266. https://doi.org/10.1007/BF00346467
Grossman GD (2014) Not all drift feeders are trout: a short review of fitness-based habitat selection models for fishes. Environ Biol Fish 97:465–473. https://doi.org/10.1007/s10641-013-0198-3
Grossman GD, Freeman MC (1987) Microhabitat use in a stream fish assemblage. J Zool (Lond) 212:151–176. https://doi.org/10.1111/j.1469-7998.1987.tb05121.x
Grossman GD, Ratajczak RE, Crawford MS, Freeman MC (1998) Assemblage organization in stream fishes: effects of environmental variation and interspecific interactions. Ecol Monogr 68:395–342.
Grossman GD, Rincon PA, Farr MD, Ratajczak RJ (2002) A new optimal foraging model predicts habitat use by drift-feeding stream minnows. Ecol Freshw Fish 11:2–10. https://doi.org/10.1034/j.1600-0633.2002.110102.x
Grossman GD, Ratajczak RE, Wagner CM, Petty JT (2010) Dynamics and population regulation of southern brook trout (Salvelinus fontinalis) in a southern Appalachian stream. Freshwat Biol 55:1494–1508. https://doi.org/10.1111/j.1365-2427.2009.02361.x
Grossman GD, Nuhfer A, Zorn T, Sundin G Alexander G (2012) Population regulation of brook trout (Salvelinus fontinalis) in Hunt Creek Michigan: a 50-year study. Freshwat Biol 57:1434–1448
Guensch GR, Hardy TB, Addley RC (2001) Examining feeding strategies and position choice of drift-feeding salmonids using an individual-based, mechanistic foraging model. Can J Fish Aquat Sci 58:446–457
Harwood AJ, Armstrong JD, Metcalfe NB, Griffiths SW (2003) Does dominance status correlate with growth in wild stream-dwelling Atlantic Salmon (Salmo salar)? Beh Ecol 14(6):902–908. https://doi.org/10.1093/beheco/arg080
Hayes JW, Hughes NF, Kelly LH (2007) Process-based modeling of invertebrate drift transport, net energy intake and reach carrying capacity for drift-feeding salmonids. Ecol Model 207(2-4):171–188. https://doi.org/10.1016/j.ecolmodel.2007.04.032
Hayes JW, Goodwin E, Shearer KA, Hay J, Kelly L (2016) Can weighted useable area predict flow requirements of drift-feeding salmonids? Comparison with a net rate of energy intake model incorporating drift–flow processes. Trans Am Fish Soc 145:589–609
Hazelton PD, Grossman GD (2009) The effects of turbidity and an invasive species on foraging success of Rosyside Dace (Clinostomus funduloides). Freshwat Biol 54(9):1977–1989. https://doi.org/10.1111/j.1365-2427.2009.02248.x
Hill J, Grossman GD (1993) An energetic model of microhabitat use for rainbow trout and Rosyside dace. Ecology 74:685–698. https://doi.org/10.2307/1940796
Holecek DE, Cromwell KJ, Kennedy BP (2009) Juvenile Chinook summer microhabitat availability, use, and selection in a central Idaho wilderness stream. Trans Am Fish Soc 138:633–644. https://doi.org/10.1577/T08-062.1
Hughes NF, Dill LM (1990) Position choice by drift-feeding salmonids: model and test for Arctic grayling (Thymallus arcticus) in subarctic mountain streams, interior Alaska. Can J Fish Aquat Sci 47:2039–2048. https://doi.org/10.1139/f90-228
Johnson JH (2014) Habitat use by subyearling Chinook and coho salmon in Lake Ontario tributaries. J Great Lakes Res 40:149–154. https://doi.org/10.1016/j.jglr.2013.12.006
McGrann MC, Tingley MW, Thorne JH, Elliott-Fisk DL, McGrann AM (2014) Heterogeneity in avian richness-environment relationships along the Pacific Crest Trail. Avian Cons Ecol 9(2): 8. https://doi.org/10.5751/ACE-00695-090208
Mossop B, Bradford MJ (2004) Importance of large woody debris for juvenile Chinook Salmon habitat in small boreal forest streams in the upper Yukon River basin, Canada. Can J For Res 34:1955–1966. https://doi.org/10.1139/x04-066
Muir WD, Coley TC (1996) Diet of yearling Chinook and feeding success during downstream migration in the snake and Columbia Rivers. Northw Sci 70:298–305
O’Brien WJ, Showalter JJ (1993) Effects of current velocity and suspended debris on the drift feeding of Arctic Grayling. Trans Am Fish Soc 122:609–615
Neuswanger J, Wipfli MS, Rosenberger AE, Hughes NF (2014) Mechanisms of drift-feeding behavior in juvenile Chinook and the role of inedible debris in a clear-water Alaskan stream. Environ Biol Fish 97:489–503. https://doi.org/10.1007/s10641-014-0227-x
Neuswanger J, Wipfli MS, Evenson MJ, Rosenberger AE, Hughes NF (2015) Low productivity of Chinook strongly correlates with high summer stream discharge in two Alaskan rivers in the Yukon drainage. Can J Fish Aquat Sci 72:1125–1137. https://doi.org/10.1139/cjfas-2014-0498
Neuswanger J, Wipfli MS, Rosenberger AE, Hughes NF (2016) Measuring fish and their physical habitats: versatile 2-D and 3-D video techniques with user-friendly software. Can J Fish Aquat Sci (in press)
Nislow KH, Folt CL, Parrish DL (1999) Favorable foraging locations for young Atlantic Salmon: application to habitat and population restoration. Ecol Appl 9:1085–1099.
Piccolo JJ, Hughes NF, Bryant MD (2008) Water velocity influences prey detection and capture by drift-feeding juvenile Coho Salmon (Oncorhynchus kisutch) and steelhead (Oncorhynchus mykiss irideus). Can J Fish Aquat Sci 65:266–275. https://doi.org/10.1139/f07-172
Piccolo JJ, Frank BM, Hayes JW (2014) Food and space revisited: the role of drift-feeding theory in predicting the distribution, growth, and abundance of stream salmonids. Environ Biol Fish 97:475–488. https://doi.org/10.1007/s10641-014-0222-2
Quinn T (2005) The behavior and ecology of Pacific Salmon and trout. American Fisheries Society and University of Washington Press, Bethesda
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Shaffer JA, Beirne M, Ritchie T, Paradis R, Barry D, Crain P (2009) Fish habitat use response to anthropogenic induced changes of physical processes in the Elwha estuary, Washington, USA. Hydrobiologia 636:179–190. https://doi.org/10.1007/s10750-009-9947-x
Shirvell CS (1994) Effect of changes in streamflow on the microhabitat use and movements of sympatric juvenile Coho Salmon (Oncorhynchus kisutch) and Chinook (O. tshawytscha) in a natural stream. Can J Fish Aquat Sci 51(7):1644–1652. https://doi.org/10.1139/f94-165
Tabor RA, Fresh KL, Piaskowski RM, Gearns HA, Hayes DB (2011) Habitat use by juvenile Chinook in the nearshore areas of Lake Washington: effects of depth, lakeshore development, substrate, and vegetation. N Am J Fish Man 31:700–713. https://doi.org/10.1080/02755947.2011.611424
Taylor EB (1988) Water temperature and velocity as determinants of microhabitats of juvenile Chinook and Coho Salmon in a laboratory stream channel. Trans Am Fish Soc 117:22–28. https://doi.org/10.1577/1548-8659(1988)117<0022:WTAVAD>2.3.CO;2
Tolimieri N, Levin P (2004) Differences in responses of Chinook to climate shifts: implications for conservation. Environ Biol Fish 70(2):155–167. https://doi.org/10.1023/B:EBFI.0000029344.33698.34
Walters AW, Bartz KK, McClure MM (2013) Interactive effects of water diversion and climate change for juvenile Chinook in the Lemhi River basin (U.S.A.) Con Biol 27:1179–1189. https://doi.org/10.1111/cobi.12170
Wessel ML, Smoker W, Fagen R, Joyce J (2006) Variation of agonistic behavior among juvenile Chinook (Oncorhynchus tshawytscha) of hatchery, hybrid, and wild origin. Can J Fish Aquat Sci 63:438–447. https://doi.org/10.1139/f05-227
White SL, Gowan C (2013) Brook trout use individual recognition and transitive inference to determine social rank. Beh Ecol 24:63–69. https://doi.org/10.1093/beheco/ars136
Zamor RM, Grossman GD (2007) Turbidity affects foraging success of drift-feeding Rosyside dace. Trans Am Fish Soc 136:167–176. https://doi.org/10.1577/T05-316.1
Acknowledgements
This research was completed with the help of many individuals including: B. Bozeman, D. Bullock, R. Chandler, B. Grossman, B. Irwin, T. Simon, Jittery Joes, Two Story, and Michter’s single barrel. The North Pacific Research Board funded this research via grant #1424 and the Daniel B. Warnell School of Forestry and Natural Resources provided supplementary funding. This research was conducted under an AUP granted to G. Grossman by the UGA IACUC. A version of the manuscript was reviewed by B. Bozeman, R. Chandler, J. Cullen, and B. Irwin, and T. Simon and we appreciate the comments of two anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donofrio, E., Simon, T., Neuswanger, J.R. et al. Velocity and dominance affect prey capture and microhabitat selection in juvenile Chinook (Oncorhynchus tshawytscha). Environ Biol Fish 101, 609–622 (2018). https://doi.org/10.1007/s10641-018-0723-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-018-0723-5