Abstract
Current research on chromium (Cr) ecotoxicity primary focuses on the adverse effects of Cr(VI). Concerns about high levels of Cr(III) in the environment are mostly driven by its possible (re)oxidation to the highly toxic hexavalent form, but trivalent chromium is considered of limited ecotoxicological relevance. However, Cr(III) can also elicit a large range of responses in aquatic and terrestrial plants including inhibition of growth and seed germination, damage to chloroplasts, reduced photosynthesis, oxidative stress, and alteration of nutrient balance, organelles and cellular function. Furthermore, most studies pay little if any attention to the complex chemistry of Cr(III) in the ecotoxicological test media used in controlled laboratory studies. In particular, Cr(III) can rapidly undergo hydrolysis that transforms soluble Cr3+ ions into Cr oxy-hydroxides—Cr(OH)3. Given the very low theoretical solubility of Cr(OH)3 (about 5 µg/L), their formation can markedly decrease the Cr(III) levels to which test organisms are actually exposed during the tests. These phenomena make comparison among studies far from straightforward and question the validity of many concentrations vs. response relationships reported for Cr(III). Although the high ecotoxicity of Cr(VI) is unquestionable, the critique presented in this chapter suggests that current consensus suffer from a general underestimation of Cr(III) ecotoxicity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
A great deal of research exists on the biological responses induced by Cr in terrestrial and aquatic plants. The vast majority of published studies is concerned with the adverse effects triggered by exposure to Cr(VI) (Cervantes et al. 2001; Shadid et al. 2017; Shanker et al. 2005) and justifies the large interest on possible techniques for remediation of Cr(VI)-contaminated sites (Beretta et al. 2018; Ao et al. 2022; Murthy et al. 2022). On the other hand, current consensus usually regards Cr(III) as being of little ecotoxicological significance and limits the risks related to the presence of Cr(III) to its potential (re)oxidation to Cr(VI) following abiotic or biologically-mediated reactions (Gorny et al. 2016; Liang et al. 2021).
The high ecotoxicity of Cr(VI) is linked to its structural analogy with phosphate and sulphate anions that facilitates intracellular uptake (Viti et al. 2014). Possible mechanisms of Cr(III) entrance into cells involve Fe(III) transporters, internalization of hydrophobic Cr(III) complexes or endocytosis of Cr-bearing particles (Beyersmann and Hartwig 2008). Inside cells, Cr(VI) is rapidly reduced to Cr(III) (Zhitkovich et al. 2005; Viti et al. 2014). The reduction of Cr(VI) results in the formation of reactive oxygen species that are associated with the severe ecotoxicological effects of Cr(VI), but also in the production of intracellular Cr(III) that reacts with cellular constituents and eventually causes DNA damage (Viti et al. 2014; Medeiros et al. 2003). Indeed, Cr(III) is the predominant or sole oxidation state of Cr inside cells (Zayed et al. 1998; Montes-Holguin et al. 2006). In higher plants, Cr(III) is also the oxidation state that is transported in sap from roots to shoots and leaves, regardless of the Cr form to which the plants are exposed (Marković et al. 2022). A similar situation likely occurs in unicellular algae. Aharchaou et al. (2017) showed that chromium had the same distribution among operationally defined subcellular fractions in cells of Chlamydomonas reinhardtii exposed to either Cr(VI) and Cr(III). Overall, a proper understanding of the possible risks linked to Cr contamination requires a solid knowledge of Cr(III) uptake and effects on living organisms.
This chapter provides a critique of the current knowledge on the ecotoxicity of Cr(III) to aquatic and terrestrial plants. It specifically focuses on studies performed under controlled laboratory conditions that allow to establish clear relationships between exposure to Cr and biological responses in the absence of confounding factors that may exist in natural soils and waters. Despite the consensus considering Cr(VI) as much more toxic than Cr(III), studies showing a higher toxicity of Cr(III) are regularly published. In this chapter, we will try to reconcile the results of such studies with the current consensus and to evaluate if studies documenting low Cr(III) ecotoxicity can suffer from unknown bias.
4.2 An Ecotoxicological Perspective of the Chemistry of Cr(III)
Cr has several oxidation states, but only Cr(III) and Cr(VI) are ecotoxicologically relevant for exposures via environmental matrices (Gorny et al. 2016). Cr(VI) occurs in the form of chromate anions that show little reactivity toward environmental particles, usually bearing a negative net charge (Warren and Haak 2001), and exhibit high mobility and long-distance transport (Gorny et al. 2016). At the opposite, Cr(III) predominantly occurs as cationic species (Rai et al. 1989; Giusti and Barakat 2005) that are easily adsorbed onto negatively charged environmental particles. Redox interconversions between the two oxidation states do occur in the environment and are mainly linked to the presence of reduced iron and sulphur for reduction of Cr(VI) to Cr(III) and of Mn oxides for oxidation of Cr(III) to Cr(VI) (Gorny et al. 2016). Bacterial activity can also facilitate both oxidation and reduction reactions. In aquatic ecosystems, particle-bound Cr(III) progressively accumulates into bed sediments via gravitational settling of suspended particulate matter and colloidal pumping (Dominik et al. 2007). In terrestrial (and aquatic) ecosystems, the formation of Cr(III) organic complexes can remobilize Cr (Löv et al. 2017; Liao et al. 2020; Zhu et al. 2022) and oxidation of Cr(III) to Cr(VI) can result in groundwater contamination even in the absence of anthropogenic inputs of chromium (Oze et al. 2007). Although sediments and soils act as large reservoirs of potentially bioavailable Cr, chromium uptake by terrestrial and (rooted) aquatic plants is linked to the presence of soluble Cr pools. It is therefore particularly important to understand the aqueous chemistry of Cr(III), especially when exposure is performed in controlled conditions by the addition of soluble Cr salts, which is common practice in ecotoxicological studies using the aqueous exposure route.
Three aspects of the aqueous chemistry of trivalent chromium are particularly relevant during ecotoxicological experiments: hydrolysis, solubility, and possible oxidation to Cr(VI). Ecotoxicological studies with Cr(III) are usually performed by amending appropriate (aqueous) test media with soluble Cr(III) salts such as CrCl3 · 6H2O, Cr(NO3)3 · 9H2O and KCr(SO4)2 · 12H2O. Following addition to aqueous media, the soluble salts dissociate into Cr(III) cations and the corresponding counter ions. At pH values above 4, free Cr3+ ions rapidly undergo hydrolysis according to the following reaction:
Because hydrolysis is accompanied by the release of protons (Eq. 4.1), addition of soluble Cr(III) salts can acidify ecotoxicological test media if their buffering capacity is exceeded by e.g., addition of large quantities of Cr(III) for experimental purposes. Following hydrolysis, the predominant Cr(III) species are expected to be CrOH2+ in the pH range 3.8–6.3, Cr(OH)3 at pH between 6.3 to 11.5 and \({\text{Cr}}({\text{OH}})_{4}^{ - }\) for pH > 11.5 (Rai et al. 1989).
The species Cr(OH)3 is characterized by a very low solubility product (Ksp = 6.7 × 10–31; Gorny et al. 2016), corresponding to a theoretical solubility limit of about 1.5 µg L−1 of chromium. This value agrees well with data reported by Rai et al. (1987) who estimated the solubility of Cr(III) at about 4 µg L−1 in a non-complexing perchlorate medium. Furthermore, Rai et al. (1987) obtained similar results in solutions amended with Cr(NO3)3 · 9H2O or CrCl3 · 6H2O. Otherwise stated, the formation of Cr(OH)3 precipitates seems independent from the initial composition of the Cr solution; an important observation considering that ecotoxicity testing can be carried out with different Cr(III) salts across different studies. Indeed, Vignati et al. (2008) and Aharchaou et al. (2018) observed a similar decrease in Cr concentrations in algal ISO medium 8692 (ISO 2012) amended with Cr nitrate, chloride or sulphate. The presence of EDTA in ISO 8692 medium did not prevent Cr(III) precipitation because Cr(III) complexation with multidentate chelators such as EDTA is sluggish compared with the kinetics of hydrolysis (Vignati et al. 2010). The solubility of Cr(III) is further decreased in the presence of iron following the formation of mixed Cr–Fe hydroxides (Sass and Rai 1987). Finally, hydrolysis takes place within microseconds (Giusti and Barakat 2005) and Cr(OH)3 formation can occur within tens of minutes (Pettine et al. 2008; Aharchaou et al. 2018). The kinetics of both processes is therefore very fast compared with the typical duration of ecotoxicity tests (hours to several days). In summary, amending aqueous ecotoxicological test media with soluble Cr(III) salts will result in the rapid formation, and possible precipitation, of insoluble Cr(OH)3 if the added Cr concentrations exceed the corresponding solubility limit of a few µg L−1. These chemical processes have two major ecotoxicological consequences: the decrease of the actual soluble (bioavailable) Cr(III) concentrations in the exposure medium during the test and the formation of a pool of nano-particulate Cr(III) in the exposure medium (Aharchaou et al. 2018). Correct interpretation of Cr(III) ecotoxicity in aqueous media must consider both phenomena and cannot be achieved without an exhaustive knowledge of Cr speciation, including its possible changes over the test duration. In particular, neglecting the decrease in soluble (bioavailable) concentration over time can lead to an underestimation of the actual ecotoxicity of Cr(III).
The examples in Table 4.1 show that few studies provide sufficiently detailed information on Cr(III) chemistry and speciation during ecotoxicity testing. Even basic analytical verification of exposure concentrations is not common practice although the range of added Cr(III) concentrations usually includes concentrations well above the theoretical solubility limit of Cr(OH)3. At the same time, formation of poorly soluble Cr(OH)3 appears very likely in most studies, considering that the pH of most ecotoxicological test media fall in the window favouring Cr(OH)3 formation (6–11 units). In such situations, measurements of total concentrations (e.g., Yu et al. 2008; Yu and Gu 2008b, 2007; Ponce et al. 2019) may include a fraction of Cr-containing nanoparticles whose bioavailability may differ from that of soluble Cr ions. The formation of Cr-containing particles (80–140 nm) has been documented by Aharchaou et al. (2018) in ISO medium 8692 for freshwater algae. Finally, the presence of organic ligands in test medium can affect Cr(III) speciation via the formation of organic-Cr(III) complexes again with possible consequences on chromium bioavailability.
These considerations do not question the quality of the studies listed in Table 4.1 nor the ecotoxicological significance of their results. They simply highlight two major caveats in Cr(III) ecotoxicology. First, Cr(III) speciation markedly changes among test media. Comparisons among studies are therefore far from straightforward when analytical information on (total) exposure concentration is available and close to meaningless when only nominal concentrations are provided. Second, in the absence of analytical verification, relationships between exposure concentrations and biological responses may underestimate Cr(III) ecotoxicity by including both soluble and insoluble Cr species in the pool of chromium bioavailable to the test organisms.
A similar situation is observed with regard to pH, with a very limited number of studies providing information on the temporal stability (or lack thereof) of this parameter over the course of the experiments. Because of hydrolysis (Eq. 4.1), changes in the pH of test medium may occur following the release of protons. One early study warned about possible strong decreases in pH values in a simple test medium (0.05% K2HPO4, 0.05% KH2PO4, 0.05% (NH4)2SO4, 0.05% KNO3; initial pH just below 7) amended with chromium chloride (Den Dooren de Jong and Roman 1965). Thompson et al. (2002) documented that initial pH differed by about 1.5 units between BG-11 medium amended with 50 µM Cr(III) (pH = 7.27) and 300 µM Cr(III) (pH = 6.14). Most interestingly pH values were comparable at about 9.5 units at the end of the test regardless of the added Cr(III) concentration. However, the differences in pH at the beginning of the test suggest that Cr speciation and its temporal evolution probably depended on the initial concentration of added Cr(III). In practice, exposure conditions may not be fully consistent even within an individual study. As in the case of analytical verification of exposure concentrations over the test duration, monitoring of pH values during the tests should always be performed at least for the lowest and highest tested concentrations.
Information is equally scant as to the possible Cr(III) to Cr(VI) interconversions in the test medium during ecotoxicological experiments. In the absence of biologically mediated reactions, oxidation of Cr(III) to Cr(VI) could be catalyzed only by Mn oxides that are not a standard component of test media. Aharchaou et al. (2018) used ion chromatography ICP-MS to verify the possible occurrence of Cr(III) to Cr(VI) redox interconversion in ISO medium 8692 for freshwater algae (ISO 2012) and did not observe any changes in the oxidation state of chromium. Because Mn enters in the composition of ISO medium 8692 as soluble MnCl2, the general applicability of these results to other aqueous media remains to be verified. The situation is much more complicated in experiments involving the use of synthetic or, especially, natural soils where the presence of some form of organic matter and Fe and Mn oxides is the norm. Similarly, in natural waters, including pore waters and soil solutions, the behavior of Cr(III) can be modified by the presence of natural organic matter (NOM) and other colloidal carrier phases. In particular, adsorption on NOM can increase Cr(III) solubility by avoiding precipitation of Cr hydroxides (Fukushima et al. 1995; Gustafsson et al. 2014). Nonetheless, the presence of colloidal Cr2O3 has been documented in soils (Zhu et al. 2022) and polynuclear species have been detected in natural waters (Hu et al. 2016). These considerations confirm that ecotoxicological laboratory studies should start paying much more attention to Cr(III) speciation to correctly assess its actual toxicity and to facilitate extrapolation of laboratory results to real-field conditions.
4.3 Cr(III) Transport and Distribution
4.3.1 Cr(III) Uptake
Cr is not an essential nutrient for plants that, consequently, do not have Cr specific transporters (Panda and Choudhury 2005; Adhikari et al. 2020). However, plants can import both Cr(III) and Cr(VI), the general consensus being that Cr(VI) is more easily taken up than Cr(III) due to its higher transmembrane transport efficiency and solubility (Shanker et al. 2005). However, the accumulation of Cr(VI) and Cr(III) in Arabidopsis thaliana was similar (Ding et al. 2019) and Cr(III) is the main form present inside plant tissue (Zayed and Terry 2003; Markovich et al. 2022). Cr(VI) uptake occurs mainly via sulphate or phosphate transporters in some bacteria, fungi, algae and plants because of their structural similarities with chromate anions (Tang et al. 2023; Viti et al. 2014; Xu et al. 2021). Mechanisms involving Cr(III) uptake by plants are not yet completed understood. Cr(III) uptake could mainly be via the same carriers as for essential ion elements (ion channels) such as Fe, Ca, Mg or K or through the simple diffusion of cations exchanges sites in the cell wall (Ding et al. 2019; Singh et al. 2013; Ao et al. 2022). In Leersia hexandra Swartz, the antagonistic effect of Fe(III) on Cr(III) uptake by root cells suggests that Cr(III) uptake may be mediated partly by Fe(III)-phytosiderophore complex transporters (Liu et al. 2011). Cr(III) can also be transported by passive mechanism, by cation diffusion facilitators (Skeffington et al., 1976). In this study, Hordeum vulgare L. was exposed to Cr(III) and Cr(VI) in presence and absence of metabolic inhibitors and Cr uptake was measured. They demonstrated that the uptake of Cr(VI) was reduced by the inhibitors whereas Cr(III) uptake was not affected, suggesting different uptake mechanisms for the two forms. However, the passive and active uptake mechanisms are not clearly established and evidence of this process is still needed. Precautions needs to be taken as Skeffington et al. (1976) proposed that Cr(VI) was the only form of Cr inside root cells which was corrected later: in this case, Cr(III) was detected in apoplast of root cells (Zayed and Terry 2003). Cr(III) can also be retained by the cation-exchange sites of the cell walls (Marschner 1995). The complexation of Cr(III) with organic acid (e.g. carboxylic acid or amino acid) enhance root uptake of Cr(III), suggesting that organic complexation of Cr(III) would contribute to Cr(III) uptake (Srivastava et al. 1999; Panda and Choudhury 2005). Cr(III) uptake clearly occurs in plant cells and Cr(III) can cross biological membranes, although the exact mechanisms are not yet fully understood.
4.3.2 Cr(III) Translocation and Accumulation
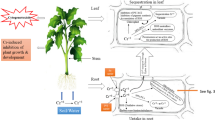
In root cells, Cr(III) ions are highly stabilized by complex formation with organic molecules, such as proteins (glutathione), carbohydrates (especially pentoses), NAD(P)H, FADH2, and probably also with organic acids, and stored and immobilized in root cell vacuoles in precipitated form (Caldelas et al. 2012; Zeng et al. 2011) or in apoplast in cell walls, which is the reason for restricted mobility of chromium in plants (Shanker et al. 2004; Mangabeira et al. 2011; Babula et al. 2008). Cr has a lower migration rate from root to shoot, than other heavy metals such as Hg, Cd and As (Shanker et al. 2005). For most terrestrial and aquatic plants, Cr distribution in different parts is in the order of roots > stem > leaves > fruits. Many studies showed that Cr(III) is accumulated mostly in roots and only a small part of Cr(III) is translocated to shoots (Paiva et al. 2009). Little translocation of Cr(III) to aerial part was reported in G. americana, with a concentration of 45 and 50 mg kg−1 in leaves and stems respectively, and most of the Cr(III) immobilized and stored in the roots, with accumulation concentration in the roots of 3841 mg kg−1 (Barbosa et al. 2007). In this case, Cr(III) is poorly translocated due to formation of Cr(III) insoluble complexes. Organic compounds, like citrate or EDTA are involved in Cr(III) translocation in xylem vessels and plant distribution. Cr(III)-citrate or Cr(III)-EDTA complexes are therefore more soluble and easily transported by the plants or immobilized and stored after translocation to leaves or fruits (Yu et al. 2008c; Juneja and Prakash 2005). Moreover, a study on Taraxacum officinale roots suggested that Cr(III) transport only occurs as Cr(III)-organic complexes with organic acids no matter if the plant is exposed to Cr(VI) or Cr(III), suggesting that Cr(VI) is reduced to Cr(III) inside the plants to be translocated and that Cr(III) is more mobile after complexation with organic compounds as suggested before (Markovich et al. 2022). In parenchyma cells, Cr(III) is accumulated in vacuoles and in the cell wall of xylem cells (Mangabeira et al. 2011; Vazquez et al. 1987). The leafy vegetables that tend to accumulate Fe (i.e., spinach, turnip leaves) appear to be the most effective in translocating Cr to the shoot. The leafy vegetables that do not accumulate relatively high concentrations of Fe in their leaves (i.e. lettuce and cabbage) are substantially less effective in translocation of Cr to the leaves (Cary et al. 1977a, b). Onion, spinach, chive and celery have a higher shoot/root concentration ratio than cabbage, peas, kale, cauliflower and lettuce after Cr(III) exposure (Zayed et al. 1998). Cr(III) is mainly retained in the roots, in epidermal cells. Depending on the chosen biological model, Cr(III) can be transported to the stem (xylem cells), leaf and fruits and could be transported as Cr(III)-organic complex (Fig. 4.1).
Plant uptake, translocation and accumulation of Cr(III) from soil and water. a Cr(III) induce effects on seed germination, shoot and root parts and Cr(III) accumulation is more important in root than shoot. b Uptake and translocation of Cr(III) at cellular level in epidermal, xylem parenchyma, and leaf cells and translocation in xylem vessels. c Accumulation of Cr(III) in algae and aquatic plants. PS: phytosiderophores, CDFs: cation diffusion facilitators, PC: phytochelatins, MT: metallothioneins MTP: metal tolerance proteins
4.4 Biological Effects
4.4.1 Effects on Plant Morphology
Studies reported a decrease of total biomass and plant growth (Davies et al. 2001; Arduini et al. 2006; Lopez-Luna et al. 2009). Cr(III) also caused inhibition of growth in Brassica oleracea after exposure to 0.5 mM (Chatterjee and Chatterjee 2000). Roots are the first organ in contact with Cr(III) and Cr(III) preferentially accumulates in plant roots. Several studies showed an inhibition of roots growth, reduction of roots lengths, volume and roots dry weight (Davies et al. 2001; Arduini et al. 2006; Barcelo et al. 1993; Lopez-Luna et al. 2009; Liu et al. 1992; Vajpayee et al. 2011). The reduction of root length is correlated to an increase of the Cr(III) concentration (Table 4.2) (Lopez-Luna et al. 2009; Liu et al. 1992; Barbosa et al. 2007). Opposite effects on root dry weigh, at low concentration of Cr(III) (0.05 mg L−1) were observed on roots of Phaseolus vulgaris. Moreover, the root dry weight increased more in presence of Cr(III) when P. vulgaris was grown in Fe-deficient conditions (Barcelo et al. 1993). Arduini et al. (2006) observed an increase of root dry weight of Miscanthus sinensis after Cr(III) treatment (50 and 100 mg L−1). Changes in root morphology can indicate Cr(III) stress, with a stimulation of root elongation below 150 mg L−1 of Cr(III) and a severe inhibition of root length observed at concentrations equal or higher than 150 mg L−1 which demonstrates that Cr(III) affects root morphology at all level of concentration (Arduini et al. 2006). In addition, Cr(III) can also affect aerial parts of plants and cause a decrease in leaf size and number (total and green leaves), growth rate, biomass and dry weight and affect the morphology of leaves (Wallace et al.1976; Chatterjee and Chatterjee 2000; Davies et al. 2001; Barbosa et al. 2007; Arduini et al. 2006; Chatterjee and Chatterjee 2000). Increase of leaf dry weight in P. vulgaris was observed after exposure to low concentration of Cr(III) (1 µM) (Barcelo et al. 1993).
Cr(III) have negative effects on roots and aerial parts of the plants. Studies reported that root growth was a more sensitive indicator than shoots for Cr(III) toxicity because Cr(III) is uptake via roots and accumulates more on roots than leaves (Chatterjee and Chatterjee 2000; Fargasova et al. 2012). Some studies also reported an opposite effect on roots and leaves of Cr(III) in specific conditions such as low concentration of Cr(III) and imbalanced nutrient supply of Fe (Barcelo et al. 1993; Arduini et al. 2006). However, it is important to consider all parameters for roots, because an increase in length could also indicate a stress reaction when the morphology of the roots is changed (Arduini et al. 2006).
4.4.2 Reproduction and Seed Germination
Cr(III) has a negative effect in the seed germination. Cr(III) exposure and accumulation in seeds delay, decrease and inhibit germination process. For Triticum aestivum, a treatment under 10 mg L−1 of Cr(III) showed no impact on the germination but treatment of 25, 50 and 100 mg L−1 led to 5–19% reduction of germination (Vajpayee et al. 2011). Cr(III) affected germination and growth of wheat (T. aestivum) and sorghum (Sorghum bicolor) after treatment of 500–1000 mg kg−1 of soil, but no effect on germination was observed for oat (Avena sativa), more resistant to Cr(III) than the other two species. This is confirmed by the EC50 of oat of 2216.84 mg kg−1 in roots, two times higher than EC50 of wheat and sorghum, 1631.14 and 1089.01 mg kg−1 respectively (Lopèz-Luna et al. 2009). Cr(III) has also been reported to interfere with structure and function of male gametophyte in kiwifruit (Actinidia deliciosa var. deliciosa) and can inhibit pollen germination and tube growth and induce alterations in pollen tube shape. Modification of callose deposition pattern and arabinogalactan protein distribution in kiwifruit pollen wall was also observed after Cr(III) exposure (Speranza et al. 2007, 2009). The reduction of α-amylase and β-amylase activities observed after Cr(III) treatment and causing a reduction of sugar supply required for the embryo development may be linked to germination reduction rate (Dua and Sawhaney 1991; Zeid 2001; Singh et al. 2013).
4.4.3 Effect of Cr(III) on Photosynthesis and Chloroplast Structure
As other trace elements, Cr(III) can affect plant photosynthesis and cause ultrastructural changes in the chloroplasts leading to inhibition of photosynthesis (Panda and Choudhury 2005; Panda and Patra 2000). Do Nascimiento et al. (2018) observed chloroplast damages after they exposed cocoa plants (Theobroma cacao) to a high concentration of Cr(III) (600 mg kg−1). Alteration in shape of leaf chloroplasts resulting in the structural disarrangement of thylakoids and stroma was observed in Alternanthera philoxeroides and Borreria scabiosoides under Cr(III) stress (Mangabeira et al. 2011). Cr(III) treatment reduced chlorophyll contents in celery seedlings at 1 mM (Scoccianti et al. 2006), in genipayer (Genipa americana) seedlings at 30 mg L−1 (Barbosa et al, 2007), and in cauliflower (Brassica oleracea) at 0.5 mM (Chatterjee and Chatterjee 2000). At the same concentration of Cr(III) and Cr(VI), Cr(III) was much less toxic than Cr(VI) on photosynthesis parameters of water hyacinth (Eichhornia crassipes) and might eventually increase photosynthesis and chlorophyll content (Paiva et al. 2009). One mM of Cr(III) stimulated growth and photosynthetic parameters such as photosynthetic rate and stomatal conductance on aquatic hyacinths after a 2 day treatment, but a decrease of photosynthetic rate and signs of toxicity (chlorosis) were observed for plants treated with 10 mM of Cr(III) for 4 days (Paiva et al, 2009). Similar results were shown for P. vulgaris; low (1 µM) or moderate (100 µM) concentrations of Cr(III) in irrigation solution increased chlorophyll a and b, and carotenoids content in leaves, but high Cr(III) concentration (10 mM) reduced the contents of chlorophylls and carotenoids (Zeid 2001). In mosses (Fontinalis antipyretica), Cr(III) modified chlorophyll a/b ratio. Cr(III) as Cr(NO3)3 decreased total chlorophyll content whereas Cr(III) as CrCl3 lead to chlorophyll accumulation at low concentration of Cr(III). The effect on chlorophyll seem to depend on Cr(III) form and Cr(III) as a nitrate salt seems to be more toxic (Dazy et al. 2008). Like Pb, Cd or Hg, Cr may reduce δ-aminolevulinic acid dehydratase (ALAD) activity or degrade ALAD, an important enzyme involved in chlorophyll biosynthesis, thereby affecting the δ-aminolevulinic acid (ALA) utilization resulting in the increase of ALA and reducing chlorophyll production (Stobort et al. 1985; Prasad and Prasad 1987; Vajpayee et al. 2011). In cells, Cr(III) may compete with Mg and Fe for assimilation and transport to leaves, affecting therefore pigment biosynthesis (Vernay et al. 2007). Cr(III) exposure can also increase the production of ROS (Shanker and Pathmanabhan 2004). The ROS induce damages in pigment-protein complexes located in thylakoid membranes followed by pheophytinization (two H+ ions replace the Mg2+ ion found in the center of the porphyrin ring of chlorophylls) and destruction of thylakoid membranes (Juarez et al. 2008). Cr(III) decrease the photosystem II (PSII) activity in Datura innoxia (Vernay et al. 2008). Barton et al. (2000) observed that Cr(III) at 10 µM increased the ferric chelate reductase activity in alfalfa (Medicago sativa L.) roots in iron-limited media. Cr(III) also induced chlorosis on plants (Barton et al. 2000; Schmidt et al. 1996). Chlorosis is generally correlated with Fe-deficiency in plant (Kaya and Ashraf 2019; Jin et al. 2007; Briat et al. 2015). It is possible that chlorosis is due to an inhibitory effect of Cr(III) on iron reductase involved in Fe(III) uptake (Alcántara et al. 1994). Cr(III) could also compete with iron for entry in root cells or interfere with iron uptake (Skeffington et al. 1976).
4.4.4 Gas Exchanges
Leaf gas exchange monitored by photosynthetic rate, stomatal conductance and transpiration was severely affected by Cr(III) in the first 24 h of treatment of T. cacao (Do Nascimiento et al. 2018). Severe changes in leaf gas exchange have also been reported for the macrophytes Alternanthera philoxeroides, Borreria scabiosoides, Polygonum ferrugineum, Eichhornia crassipes (Mangabeira et al. 2011), Genipa americana (Santana et al. 2012) and Eichirnia crassipes (Paiva et al. 2009) subjected to Cr(III) stress. The leaf gas exchanged and stomates opening can be linked to photosynthesis rate, as a decrease in CO2 will reduce optimal rates of photosynthesis.
4.4.5 Alteration of Organelles and Cellular Functions
Under Cr(III) stress, the shapes of chloroplast and nuclei were altered in two aquatic macrophytes Alternanthera philoxeroides (alligator grass) and Borreria scabiosoides. At 50 mg L−1 of Cr(III), disintegration of the nucleus and deformation of chloroplasts were observed leading to structural disarrangement of thykaloids and stroma (Mangabeira et al. 2011). Damage to chloroplast can affect photosynthesis and plant growth. Alteration of mitochondrial cristae and dense electron material in mitochondria was also observed for both Allium cepa and Borreria scabiosoides treated with Cr(III) (Mangabeira et al. 2011; Liu and Kottke 2003). In kiwi pollen, similar findings have been reported with an alteration of the shape of mitochondria (swelling and loss of mitochondrial cristae) and the shape of endoplasmic reticulum (Speranza et al. 2007). Cytoplasmic vacuolization was also observed in kiwi pollen after Cr(III) treatment, usually a sign of cell death (Speranza et al. 2007). The impact of Cr(III) on organelles can affect cellular function of the plant.
The presence of Cr(III) produce mitotic irregularities (i.e. anaphase bridges or mitosis lagging), chromosomal aberrations (i.e. chromosome stickiness, chromosome fragmentation) (Liu et al. 1992; Qian 2004; Kumar et al. 2015), chromatin condensation (Speranza et al. 2007) and nuclear abnormalities (nuclear bud, micro nucleus, nuclear notch) (Kumar et al. 2015). These chromosomal irregularities and DNA damage could be linked to the production of ROS (Kumar et al. 2015) or to the formation of DNA adducts with Cr(III) (Viti et al. 2014). The chromosomal aberration observed can be linked with the production of ROS, as Cr(III) induced the formation of ROS and antioxidant enzyme induced to counter oxidative stress can cause chromosomal aberration (Kumar et al. 2015). Cr(III) induces the expression of genes encoding for proteins involved in cellular stress responses. These proteins are also induced in pathogen defence, senescence process and heavy metal stress, suggesting the existence of a common ROS-mediated mechanism of gene regulation (Quaggiotti et al. 2007).
Exposure to Cr(III) induced proteasome misfunction in kiwi (A. deliciosa var. deliciosa) pollen that generated accumulation of misfolding and damaged proteins. Similar results were observed after Cr(VI) exposure, but molecular targets at proteasome level may be different (Vannini et al. 2011). The 20S proteasome α-subunit expression was decreased in presence of Cr(III) and the 26S regulatory subunit Rpn11 level was decreased after Cr(VI) exposure (Vannini et al. 2011).
4.4.6 Effects of Cr(III) on Mineral Nutrition
Like other trace elements, Cr(III) is structurally similar to other essential elements and may affect plant mineral nutrition. In rhizosphere soil, excessive Cr reduces the accumulation of essential nutriments (Fe, Cu, Mg, Zn Ca, S and P) by masking adsorption sites and forming insoluble or low-bioavailability complexes (Woke et al. 2019; de Oliveira et al. 2015, 2016; Sharma et al. 2020). There is also evidence of increased Fe availability and uptake for plants in presence of Cr(III) in soil (Cary et al. 1977a, b). Yu et al. (2018a, b) found that Cr(III) exposure decreased Mn and Zn concentration in root cells and Zn concentration in shoot cells in rice seedlings. Mn and Zn concentrations were also decreased in tomato root cells after Cr(III) exposure. A decrease of Fe and Cu concentrations was also observed in tomato roots (Moral et al. 1996). Gardea-Torresdey et al. (2005) showed in Salsola kali roots a decrease of K, P, Mg and Cu after Cr(III) treatment. In Phaseolus vulgaris L., very small quantities of Cr(III) are transported to leaves, but Cr(III) exposure induces a decrease of Fe, Zn and Mo and to a lesser extent a decrease of K, Ca and Mg in leaves (Wallace et al. 1976). Davies et al. (2001) reported that Cr(III) treatment decrease N, P and K levels in Helianthus annuus leaves, but enhance Al, Fe and Zn concentration. These effects were enhanced by the presence of mycorrhiza (Davies et al. 2001). These decreases in nutrient uptake are probably due to deterioration of root nutriment penetration under Cr(III) stress and the decline in root growth (Ao et al. 2022; Sharma et al. 2020). The decrease in nutrient uptake could indicate that Cr(III) displaces ions from physiologically important binding sites in plant cells, thus affecting signal transduction, photosynthesis or plant nutrient metabolism (Cipriani et al. 2012; Sharma et al. 2020). Also, the Cr(III) accumulation in the plant cell wall may damage the plasmodesmata, which are important for mineral nutrients transport channels, thus leading to an imbalance in mineral nutrient metabolism (Ao et al. 2022; Fujita 2015; Kitagawa et al. 2015). In presence of 1 μM of Cr(III), nitrate reductase (NR) activity was enhanced suggesting a request in ammonium (\({\text{NH}}_{4}^{ + }\)) or nitric oxide (NO) during the cellular response to Cr(III), whereas in presence of Cr(VI) (≥2 μM) NR activity decreased in T. aestivum (Panda and Patra 2000). Nitrogen is an essential macro-element and plays a role in growth and in plant development and is available as nitrate \({\text{NO}}_{3}^{ - }\). An enhanced nitrate reductase could indicate an increased demand of energy, due to a dysfunction of photosynthesis or mitochondrial respiration.
4.4.7 ROS Production, Lipid Peroxidation and Antioxidative Mechanisms
Exposure to heavy metals induces the overproduction of ROS (reactive oxygen species) including superoxide radicals (\({\text{O}}_{{2}}^{\cdot-}\)), hydroxyl radicals (OH·), oxygen singlets (\(^{{1}} {\text{O}}_{{2}}\)) and hydrogen peroxide (H2O2). Hyperaccumulation of ROS affects the growth and development of plants (Maiti et al. 2012; Xie et al. 2019). Redox active metals such as Fe, Cu, Co or Cr have the capacity to produce ROS via Haber-Weiss and Fenton reactions (Sharma et al. 2020; Bokare and Choi 2014). Plants can develop antioxidant enzyme systems for scavenging excessive accumulation of ROS under metal stress. The enzymatic antioxidants include the key enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidases (POD), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), glutathione reductase (GR), glutathione S-transferases (GST), single dehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) (Ahmad et al. 2010). Under normal condition, ROS are generated in little quantities in cellular organelles of plants (Maiti et al. 2012) and play important roles in regulating and controlling essential metabolisms, such as signal transduction for programmed cell death, seed dormancy, senescence, and growth (Pourrut et al. 2011). In many studies, Cr(III) exposure induces an increase of antioxidant enzyme activities including SOD, CAT, POD, GPX, APX, GR, MDHAR and DHAR (see references in Table 4.3). Some studies showed a downregulation of antioxidant enzyme activities like CAT and POD in Brassica oleracea (Pandey and Sharma 2003; Chatterjee and Chaterjee 2000) and GR in T. cacao (Do Nascimiento et al. 2018). CAT uses heme (iron-porphyrin) as a cofactor. Reduction in CAT activity indicates that Cr has the potential to interact with iron in metabolic pool or it may influence the presence of active form of iron (Sharma et al. 2003, 2020). The non-enzymatic antioxidant responses (i.e. ascorbic acid, glutathione (GSH), phenolic acid) are also observed in presence of Cr(III). In S. bicolor, after Cr(III) treatment, the GSH/GSSH ratio decreases only in roots but not in leaves, suggesting an increase of oxidative species in root cells (Shanker and Pathmanabhan 2004). Dehydroascorbate (root and leaf) and total ascorbate (root) levels exhibited a high degree of significant increase irrespective of speciation or concentration of Cr(III) in the medium (Shanker and Pathmanabhan 2004). Cr(III) affects the membrane potential by inducing lipid peroxidation. Malondialdehyde (MDA), a biomarker of lipid peroxidation is excessively produced due to lipid peroxidation increase after Cr(III) treatment in root and leaf (Shanker and Pathmanabhan 2004). Oxidative damages resulting from ROS towards biomolecules such as lipids, proteins and nucleic acids is well documented for plant species (Kanazawa et al. 2000; Singh et al. 2006).
4.4.8 Regulation of Phytochelatins, Metallothioneins and Metal Tolerance Proteins
To cope with Cr(III) induced stress, plants have developed different strategies involving morphological, anatomical and molecular defence mechanisms. In order to regulate the uptake and accumulation of trace elements, plants can sequester and chelate metals with metal binding ligands such as metallothioneins (MT), phytochelatins (PC) and metal tolerance proteins (MTP), produced within the plant cells to aid in heavy metal transport and sequestration. These metal chelators protect plants against high heavy metal concentrations through different mechanisms, such as chelation, sequestration (MT and PC) or efflux (MTP). MT are cysteine-rich proteins that play a crucial role in heavy metals detoxification, metal homeostasis and metabolism via binding through the thiol group (SH) in cysteine residues. MT are transcribed constitutively or induced in response to several types of stress including heavy-metal exposures (Ziller and Fraissinet-Tachet 2018). The increased expression of MT-like protein in sorghum exposed to Cr(III) can indicate a potential role of metal binding ligands in Cr(III) detoxification (Shanker et al. 2004). After chelation, Cr can be compartmentalized in the cell wall and vacuoles. In plants, the cell wall is mainly composed of cellulose, hemicelluloses and pectins (Carpita and McCann 2000; Wolf and Greiner 2012). In the cell wall of root cells, Cr(III) can bind cellulose and pectin (Wang and Lee 2011; Yamada and Shiiba 2015). In Oryza sativa tissues, expression of MT genes was increased after Cr(III) exposure suggesting a role of MT in Cr(III) chelation (Yu et al. 2019). PC are synthesized in the cytoplasm under heavy metals toxic stress (Sharma et al. 2016). Biosynthesis of PC is catalysed by phytochelatin synthase (PCS) that is constitutively expressed. However, PCS activity is increased in the presence of heavy metals (Sharma et al. 2016). PT are low-molecular-weight, cysteine-rich small polypeptides with a general structure (γ-Glu-Cys)nGly (n = 2–11) (Mirza et al. 2014). PC are one of the most important classes of metal chelators. PC-metal complexes are very stable and are formed and sequestered in vacuoles (Sharma et al. 2016). Several studies on metal detoxification via PC have suggested the important role of PC in the detoxification of heavy metals including Cr (Ao et al. 2022). MTP are described as metal efflux transporters such as Fe, Zn, Mn, Cd, Co and Ni from the cytoplasm generally to vacuoles or extracellular spaces to prevent cytoplasmic damages (Ricachenevsky et al. 2013). In O. sativa, expression of several mRNA encoding for MTP was induced after Cr(III) exposure in root and shoot (Yu et al. 2018a, b). However, few studies have investigated the detoxification response of MTP to Cr(III) exposure and the transport mechanisms of Cr(III) by MTP in plant remain unclear (Ao et al. 2022).
4.5 Conclusions
Cr(III) clearly has a variety of impacts on terrestrial and aquatic plants and therefore deserves full consideration by ecotoxicologists, stakeholders and regulators. Current consensus regards Cr(VI) as much more toxic than Cr(III) and underpins extensive research efforts to find economically viable processes based on the reduction of Cr(VI) to Cr(III) for remediation purposes. However, Cr(III) chemistry in ecotoxicological studies requires much better consideration to correctly understand the biological effects of this form of chromium. In particular, too few studies have checked the actual speciation of Cr(III) in the exposure media along with the measured biological responses. The lack of information on actual Cr(III) speciation in ecotoxicological studies can lead to an underestimation of Cr(III) toxicity and complicates both comparisons across studies and extrapolation of laboratory findings to real field situations.
The effects of Cr(III) on plants include inhibition of plant growth, seed germination process, damage to chloroplast, reduced photosynthesis, oxidative stress associated with generation of ROS, and alteration of nutrient balance, organelles and cellular function (Fig. 4.2). More knowledge is needed on Cr(III) speciation in ecotoxicological test media to establish reliable concentrations vs. responses relationships for all these effects and improve risk assessment for this important oxidation state of chromium in natural environments.
References
Adhikari A, Adhikari S, Ghosh S, Azahar I, Shaw AK, Roy D, Roy S, Saha S, Hossain Z (2020) Imbalance of redox homeostasis and antioxidant defense status in maize under chromium(VI) stress. Environ Exp Bot 169:103873
Aharchaou I, Py JS, Cambier S, Loizeau JL, Cornelis G, Rousselle P, Battaglia E, Vignati DAL (2018) Chromium hazard and risk assessment: new insights from a detailed speciation study in a standard test medium. Environ Toxicol Chem 37:983–992
Aharchaou I, Rosabal M, Liu F, Battaglia E, Vignati DAL, Fortin C (2017) Bioaccumulation and subcellular partitioning of Cr(III) and Cr(VI) in the freshwater green alga Chlamydomonas reinhardtii. Aquat Toxicol 182:49–57
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–175
Alcántara E, Romera FJ, Cañete M, De la Guardia MD (1994) Effects of heavy metals on both induction and function of root Fe(lll) reductase in Fe-deficient cucumber (Cucumis sativus L.) plants. J Exp Bot 45:1893–1898
Anjum SA, Ashraf U, Khan I, Tanveer M, Shahid M, Shakoor A, Wang L (2017) Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27:262–273
Ao M, Chen X, Deng T, Sun S, Tang Y, Morel JL, Qiu R, Wang S (2022) Chromium biogeochemical behaviour in soil-plant systems and remediation strategies: a critical review. J Hazard Mater 424:127233
Arduini I, Masoni A, Ercoli L (2006) Effects of high chromium applications on miscanthus during the period of maximum growth. Environ Exp Bot 58:234–243
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6:189–213
Barbosa RMT, de Almeida AAF, Mielke MS, Loguercio LL, Mangabeira PAO, Gomes FP (2007) A physiological analysis of Genipa americana L.: a potential phytoremediator tree for chromium polluted watersheds. Environ Exp Bot 61:264–271
Barceló J, Poschenrieder C, Dolores Vázquez M, Gunsé B (1993) Beneficial and toxic effects of chromium in plants: solution culture, pot and field studies. In: Vernet J-P (ed) Studies in environmental science. Elsevier, pp 147–171
Barton LL, Johnson GV, O’Nan AG, Wagener BM (2000) Inhibition of ferric chelate reductase in alfalfa roots by cobalt, nickel, chromium, and copper. J Plant Nutr 23:1833–1845
Beretta G, Mastorgio AF, Pedrali L, Saponaro S, Sezenna E (2018) Support tool for identifying in situ remediation technology for sites contaminated by hexavalent chromium. Water 10:1344
Beyersmann D, Hartwig A (2008) Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 82:493–512
Bokare AD, Choi W (2014) Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater 275:121–135
Briat J-F, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20:33–40
Caldelas C, Araus JL, Febrero A, Bort J (2012) Accumulation and toxic effects of chromium and zinc in Iris pseudacorus L. Acta Physiol Plant 34:217–1228
Carpita NC, McCann MC (2000) The cell wall. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, MD, pp 52–108
Cary EE, Allaway WH, Olson OE (1977a) Control of chromium concentrations in food plants. 2. Chemistry of chromium in soils and its availability to plants. J Agric Food Chem 25:305–309
Cary EE, Allaway WH, Olson OE (1977b) Control of chromium concentrations in food plants. 1. Absorption and translocation of chromium by plants. J Agric Food Chem 25:300–304
Cervantes C, Campos-García J, Devars S, Gutiérrez-Corona F, Loza-Tavera H, Torres-Guzmán JC, Moreno-Sánchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109:69–74
Chow YN, Lee LK, Zakaria NA, Foo KY (2018) Phytotoxic effects of trivalent chromium-enriched water irrigation in Vigna unguiculata seedling. J Clean Prod 202:101–108
Cipriani HN, Bastos ARR, de Carvalho JG, da Costa AL, Oliveira NP (2012) Chromium toxicity in hybrid eucalyptus (Eucalyptus urophylla S. T. Blake X Grandis W. Hill Ex. Maiden) cuttings. J Plant Nutr 35:1618–1638
Davies FT, Puryear JD, Newton RJ, Egilla JN, Saraiva Grossi JA (2001) Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus). J Plant Physiol 158:777–786
Dazy M, Béraud E, Cotelle S, Meux E, Masfaraud JF, Férard JF (2008) Antioxidant enzyme activities as affected by trivalent and hexavalent chromium species in Fontinalis antipyretica Hedw. Chemosphere 73:281–290
de Oliveira LM, Lessl JT, Gress J, Tisarum R, Guilherme LRG, Ma LQ (2015) Chromate and phosphate inhibited each other’s uptake and translocation in arsenic hyperaccumulator Pteris vittata L. Environ Pollut 197:240–246
de Oliveira LM, Gress J, De J, Rathinasabapathi B, Marchi G, Chen Y, Ma LQ (2016) Sulfate and chromate increased each other’s uptake and translocation in As-hyperaccumulator Pteris vittata. Chemosphere 147:36–43
den Dooren de Jong LE, Roman WB (1965) Tolerance of Chlorella vulgaris for metallic and non-metallic ions. Antonie van Leeuwenhoek 31:301–313. https://doi.org/10.1007/BF02045910
Ding G, Jin Z, Han Y, Sun P, Li G, Li W (2019) Mitigation of chromium toxicity in Arabidopsis thaliana by sulfur supplementation. Ecotoxicol Environ Saf 182:109379
do Nascimento JL, de Almeida A-AF, Barroso JP, Mangabeira PAO, Ahnert D, Sousa AGR, Silva JVS, Baligar VC (2018) Physiological, ultrastructural, biochemical and molecular responses of young cocoa plants to the toxicity of Cr (III) in soil. Ecotoxicol Environ Safety 159:272–283
Dominik J, Vignati DL, Koukal B, Pereira de Abreu MH, Kottelat R, Szalinska E, Baś B, Bobrowski A (2007) Speciation and environmental fate of chromium in rivers contaminated with tannery effluents. Eng Life Sci 7:155–169
Dua A, Sawhney SK (1991) Effect of chromium on activities of hydrolytic enzymes in germinating pea seeds. Environ Exp Bot 31:133–139
Fan WJ, Feng YX, Li YH, Lin YJ, Yu XZ (2020) Unraveling genes promoting ROS metabolism in subcellular organelles of Oryza sativa in response to trivalent and hexavalent chromium. Sci Total Environ 744:140951
Fargašová A (2012) Plants as models for chromium and nickel risk assessment. Ecotoxicology 21:1476–1483
Fujita T (2015) Plasmodesmata: function and diversity in plant intercellular communication. J Plant Res 128:3–5
Fukushima M, Nakayasu K, Tanaka SHN (1995) Chromium(III) binding abilities of humic acids. Anal Chim Acta 317:195–206
Gardea-Torresdey JL, de la Rosa G, Peralta-Videa JR, Montes M, Cruz-Jimenez G, Cano-Aguilera I (2005) Differential uptake and transport of trivalent and hexavalent chromium by tumbleweed (Salsola kali). Arch Environ Contam Toxicol 48:225–232
Giusti L, Barakat S (2005) The monitoring of Cr(III) and Cr(VI) in natural water and synthetic solutions: an assessment of the performance of the DGT and DPC methods. Wat Air Soil Pollut 161:313–334
Gorny J, Billon G, Noiriel C, Dumoulin D, Lesven L, Madé B (2016) Chromium behavior in aquatic environments : a review. Environ Rev 24:503–516
Greene JC, Miller WE, Debacon M, Long MA, Bartels CL (1988) Use of Selenastrum capricornutum to assess the toxicity potential of surface and ground water contamination caused by chromium waste. Environ Toxicol Chem 7:35–39
Gustafsson JP, Persson I, Oromieh AG, van Schaik JWJ, Sjöstedt C, Kleja DB (2014) Chromium(III) complexation to natural organic matter: mechanisms and modeling. Environ Sci Technol 48:1753–1761
Henriques FS (2010) Changes in biomass and photosynthetic parameters of tomato plants exposed to trivalent and hexavalent chromium. Biol Plant 54:583–586
Hu L, Cai Y, Jiang G (2016) Occurrence and speciation of polymeric chromium(III), monomeric chromium(III) and chromium(VI) in environmental samples. Chemosphere 156:14–20
ISO (2012) Water quality—Fresh water algal growth inhibition test with unicellular green algae (Norm 8692)
Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ (2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144:278–285
Juarez AB, Barsanti L, Passarelli V, Evangelista V, Vesentini N, Conforti V, Gualtieri P (2008) In vivo microspectroscopy monitoring of chromium effects on the photosynthetic and photoreceptive apparatus of Eudorina unicocca and Chlorella kessleri. J Environ Monit 10:1313–1318
Juneja S, Prakash S (2005) The chemical form of trivalent chromium in xylem sap of maize (Zea mays L.). Chem Speciat Bioavailab 17:161–169
Kanazawa S, Sano S, Koshiba T, Ushimaru T (2000) Changes in antioxidative enzymes in cucumber cotyledons during natural senescence: comparison with those during dark-induced senescence. Physiol Plant 109:211–216
Kaya C, Ashraf M (2019) The mechanism of hydrogen sulfide mitigation of iron deficiency-induced chlorosis in strawberry (Fragaria × ananassa) plants. Protoplasma 256:371–382
Kitagawa M, Paultre D, Rademaker H (2015) Intercellular communication via plasmodesmata. New Phytol 205:970–972
Kumar D, Rajeshwari A, Jadon PS, Chaudhuri G, Mukherjee A, Chandrasekaran N, Mukherjee A (2015) Cytogenetic studies of chromium (III) oxide nanoparticles on Allium cepa root tip cells. J Environ Sci 38:150–157
Liang J, Huang X, Yan J, Li Y, Zhao Z, Liu Y, Ye J, Wei Y (2021) A review of the formation of Cr(VI) via Cr(III) oxidation in soils and groundwater. Sci Total Environ 774:145762
Liao P, Pan C, Ding W, Li W, Yuan S, Fortner JD et al (2020) Formation and transport of Cr(III)-NOM-Fe colloids upon reaction of Cr(VI) with NOM-Fe(II) colloids at Anoxic-Oxic interfaces. Environ Sci Technol 54:4256–4266
Liu D, Jiang W, Li M (1992) Effects of trivalent and hexavalent chromium on root growth and cell division of Allium cepa. Hereditas 117:23–29
Liu D, Kottke I (2003) Subcellular localization of chromium and nickel in root cells of Allium cepa by EELS and ESI. Cell Biol Toxicol 19:299–311
Liu J, Duan C-Q, Zhang X-H, Zhu Y-N, Hu C (2011) Characteristics of chromium(III) uptake in hyperaccumulator Leersia hexandra Swartz. Environ Exp Bot 74:122–126
López-Luna J, González-Chávez MC, Esparza-García FJ, Rodríguez-Vázquez R (2009) Toxicity assessment of soil amended with tannery sludge, trivalent chromium and hexavalent chromium, using wheat, oat and sorghum plants. J Hazard Mater 163:829–834
Löv Å, Sjöstedt C, Larsbo M, Persson I, Gustafsson JP, Cornelis G et al (2017) Solubility and transport of Cr(III) in a historically contaminated soil—Evidence of a rapidly reacting dimeric Cr(III) organic matter complex. Chemosphere 189:709–716
Maiti S, Ghosh N, Mandal C, Das K, Dey N, Adak MK (2012) Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz J Plant Physiol 24:203–212
Mangabeira PA, Ferreira AS, de Almeida AAF, Fernandes VF, Lucena E, Souza VL, dos Santos Júnior AJ, Oliveira AH, Grenier-Loustalot MF, Barbier F, Silva DC (2011) Compartmentalization and ultrastructural alterations induced by chromium in aquatic macrophytes. Biometals 24:1017–1026
Marković S, Levstek L, Žigon D, Ščančar J, Milačič R (2022) Speciation and bio-imaging of chromium in Taraxacum officinale using HPLC post-column ID-ICP-MS, high resolution MS and laser ablation ICP-MS techniques. Front Chem 10:863387
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, 889 p. ISBN: 9780124735439
Medeiros MG, Rodrigues AS, Batoréu MC, Laires A, Rueff J, Zhitkovich A (2003) Elevated levels of DNA–protein crosslinks and micronuclei in peripheral lymphocytes of tannery workers exposed to trivalent chromium. Mutagenesis 18:19–24
Mirza N, Mahmood Q, Maroof Shah M, Pervez A, Sultan S (2014) Plants as useful vectors to reduce environmental toxic arsenic content. Sci World J 921581
Montes-Holguin MO, Peralta-Videa JR, Meitzner G, Martinez-Martinez A, de la Rosa G, Castillo-Michel HA, Gardea-Torresdey JL (2006) Biochemical and spectroscopic studies of the response of Convolvulus arvensis L. to chromium(III) and chromium(VI) stress. Environ Toxicol Chem 25:220–226
Moral R, Gómez I, Pedreno JN, Mataix J (1996) Absorption of Cr and effects on micronutrient content in tomato plant (Lycopersicum esculentum M.). Agrochimica
Murthy MK, Khandayataray P, Samal D (2022) Chromium toxicity and its remediation by using endophytic bacteria and nanomaterials: a review. J Environ Manag 318:115620
Oze C, Bird DK, Fendorf S (2007) Genesis of hexavalent chromium from natural sources in soil and groundwater. Proc Natl Acad Sci 104:6544–6549
Paiva LB, de Oliveira JG, Azevedo RA, Ribeiro DR, da Silva MG, Vitória AP (2009) Ecophysiological responses of water hyacinth exposed to Cr3+ and Cr6+. Environ Exp Bot 65:403–409
Panda SK, Choudhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17:95–102
Panda SK, Patra HK (2000) Nitrate and ammonium ions effect on the chromium toxicity in developing wheat seedlings. Proc Natl Acad Sci India. Section B, Biol Sci 70:75–80
Pandey N, Sharma CP (2003) Chromium interference in iron nutrition and water relations of cabbage. Environ Exp Bot 49(3):195–200. https://doi.org/10.1016/S0098-8472(02)00088-6
Peralta JR, Gardea-Torresdey JL, Tiemann KJ, Gomez E, Arteaga S, Rascon E, Parsons JG (2001) Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L.). Bull Environ Contam Toxicol 66:727–734. https://doi.org/10.1007/s001280069
Pereira M, Bartolomé MC, Sánchez-Fortún S (2013) Bioadsorption and bioaccumulation of chromium trivalent in Cr(III)-tolerant microalgae: A mechanisms for chromium resistance. Chemosphere 93:1057–1063
Pettine M, Genneri F, Campanella L, Millero FJ (2008) The effect of organic compounds in the oxidation kinetics of Cr(III) by H2O2. Geochim Cosmochim Acta 72:5692–5707
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, vol 213. Springer, New York, NY, pp 113–136
Prasad DDK, Prasad ARK (1987) Effect of lead and mercury on chlorophyll synthesis in mung bean seedlings. Phytochemistry 26:881–883
Qian XW (2004) Mutagenic effects of chromium trioxide on root tip cells of Vicia faba. J Zhejiang Univ Sci 5:1570–1576
Quaggiotti S, Barcaccia G, Schiavon M, Nicolé S, Galla G, Rossignolo V, Soattin M, Malagoli M (2007) Phytoremediation of chromium using Salix species: cloning ESTs and candidate genes involved in the Cr response. Gene 402:68–80
Rai D, Sass BM, Moore DA (1987) Chromium(III) hydrolysis constants and solubility of chromium(III) hydroxide. In: Inorganic chemistry, pp 345–349
Rai D, Eary LE, Zachara JM (1989) Environmental chemistry of chromium. Sci Total Env Chromium Paradox Modern Life 86:15–23
Ricachenevsky F, Menguer P, Sperotto R, Williams L, Fett J (2013) Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Front Plant Sci 4
Santana KB, de Almeida A-AF, Souza VL, Mangabeira PAO, Da Silva D, Gomes FP, Dutruch L, Loguercio LL (2012) Physiological analyses of Genipa americana L. reveals a tree with ability as phytostabilizer and rhizofilterer of chromium ions for phytoremediation of polluted watersheds. Environ Exp Botany 80:35–42
Sass BM, Rai D (1987) Solubility of amorphous chromium(III)-iron(III) hydroxide solid solutions. Inorg Chem 26:2228–2232
Schmidt W (1996) Influence of chromium(lll) on root-associated Fe(lll) reductase in Plantago lanceolata L. J Exp Bot 47:805–810
Scoccianti V, Crinelli R, Tirillini B, Mancinelli V, Speranza A (2006) Uptake and toxicity of Cr(III) in celery seedlings. Chemosphere 64:1695–1703
Scoccianti V, Bucchini AE, Iacobucci M, Ruiz KB, Biondi S (2016) Oxidative stress and antioxidant responses to increasing concentrations of trivalent chromium in the Andean crop species Chenopodium quinoa Willd. Ecotoxicol Environ Saf 133:25–35
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Shanker AK, Pathmanabhan G (2004) Speciation dependant antioxidative response in roots and leaves of sorghum (Sorghum bicolor (L.) Moench cv CO 27) under Cr(III) and Cr(VI) stress. Plant Soil 265(1–2):141–151. https://doi.org/10.1007/s11104-005-0332-x
Shanker AK, Djanaguiraman M, Sudhagar R, Chandrashekar CN, Pathmanabhan G (2004) Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R.Wilczek. cv CO4) roots. Plant Sci 166:1035–1043
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D (2020) Chromium bioaccumulation and its impacts on plants: an overview. Plants 9:100
Sharma DC, Sharma CP, Tripathi RD (2003) Phytotoxic lesions of chromium in maize. Chemosphere 51:63–68
Sharma R, Bhardwaj R, Handa N, Gautam V, Kohli SK, Bali S, Kaur P, Thukral AK, Arora S, Ohri P, Vig AP (2016) Responses of phytochelatins and metallothioneins in alleviation of heavy metal stress in plants: an overview. In: Ahmad P (ed) Plant metal interaction. Elsevier, pp 263–283
Singh HP, Batish DR, Kaur S, Arora K, Kohli RK (2006) α-Pinene inhibits growth and induces oxidative stress in roots. Ann Bot 98:1261–1269
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254
Skeffington RA, Shewry PR, Peterson PJ (1976) Chromium uptake and transport in barley seedlings (Hordeum vulgare L.). Planta 132:209–214
Speranza A, Ferri P, Battistelli M, Falcieri E, Crinelli R, Scoccianti V (2007) Both trivalent and hexavalent chromium strongly alter in vitro germination and ultrastructure of kiwifruit pollen. Chemosphere 66:1165–1174
Speranza A, Taddei AR, Gambellini G, Ovidi E, Scoccianti V (2009) The cell wall of kiwifruit pollen tubes is a target for chromium toxicity: alterations to morphology, callose pattern and arabinogalactan protein distribution. Plant Biol 11:179–193
Srivastava S, Nigam R, Prakash S, Srivastava MM (1999) Mobilization of trivalent chromium in presence of organic acids: a hydroponic study of wheat plant (Triticum vulgare). Bull Environ Contam Toxicol 63:524–530
Stobart AK, Griffiths WT, Ameen-Bukhari I, Sherwood RP (1985) The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiol Plant 63:293–298
Tang J, Zhang X, Zhang X, Li X, Chen J, Dong Y (2023) Transformation of chromium and its removal by Fe2O3 during the thermal disposal of municipal solid waste: a study based on density functional theory. Fuel 331:125734
Terrestrial and aquatic ecotoxicity assessment of Cr(VI) by the ReCiPe method calculation (LCIA): application on an old industrial contaminated site | SpringerLink, 2021. https://springerlink.bibliotecabuap.elogim.com/article/https://doi.org/10.1007/s11356-012-1254-9. Accessed 10 Dec 21
Thompson SL, Manning FCR, McColl SM (2002) Comparison of the toxicity of chromium III and chromium VI to cyanobacteria. Bull Environ Contam Toxicol 69:286–293
Turbak SC, Olson SB, McFeters GA (1986) Comparison of algal assay systems for detecting waterborne herbicides and metals. Water Res 20:91–96
UdDin I, Bano A, Masood S (2015) Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol Environ Saf 113:271–278
Vajpayee P, Khatoon I, Patel CB, Singh G, Gupta KC, Shanker R (2011) Adverse effects of chromium oxide nano-particles on seed germination and growth in Triticum aestivum L. J Biomed Nanotechnol 7:205–206
Vannini C, Domingo G, Marsoni M, Bracale M, Sestili S, Ficcadenti N, Speranza A, Crinelli R, Carloni E, Scoccianti V (2011) Proteomic changes and molecular effects associated with Cr(III) and Cr(VI) treatments on germinating kiwifruit pollen. Phytochemistry 72:1786–1795
Vazquez MD, Poschenrieder CH, Barcelo J (1987) Chromium VI induced structural and ultrastructural changes in bush bean plants (Phaseolus vulgaris L.). Ann Bot 59:427–438
Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68:1563–1575
Vernay P, Gauthier-Moussard C, Jean L, Bordas F, Faure O, Ledoigt G, Hitmi A (2008) Effect of chromium species on phytochemical and physiological parameters in Datura innoxia. Chemosphere 72:763–771
Vignati DAL, Beye ML, Dominik J, Klingemann AO, Filella M, Bobrowski A, Ferrari BJ (2008) Temporal decrease of trivalent chromium concentration in a standardized algal culture medium: experimental results and implications for toxicity evaluation. Bull Environ Contam Toxicol 80:305–310
Vignati DAL, Dominik J, Beye ML, Pettine M, Ferrari BJD (2010) Chromium(VI) is more toxic than chromium(III) to freshwater algae: a paradigm to revise? Ecotoxicol Environ Saf 73:743–749
Viti C, Marchi E, Decorosi F, Giovannetti L (2014) Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Microbiol Rev 38:633–659
Volland S, Lütz C, Michalke B, Lütz-Meindl U (2012) Intracellular chromium localization and cell physiological response in the unicellular alga Micrasterias. Aquat Toxicol 109:59–69. https://doi.org/10.1016/j.aquatox.2011.11.013
Wallace A, Soufi SM, Cha JW, Romney EM (1976) Some effects of chromium toxicity on bush bean plants grown in soil. Plant Soil 44:471–473
Wang SL, Lee JF (2011) Reaction mechanism of hexavalent chromium with cellulose. Chem Eng J 174:289–295
Warren LA, Haack EA (2001) Biogeochemical control on metal behaviour in freshwater environments. Earth Sci Rev 54:261–320
Woke J, Osu C, Chukwu U (2019) Dynamic impact of chromium on nutrient uptake from soil by fluted pumpkin (Telfairia occidentalis) 7:1–9
Wolf S, Greiner S (2012) Growth control by cell wall pectins. Protoplasma 249:169–175
Xu ZR, Cai ML, Chen SH, Huang XY, Zhao FJ, Wang P (2021) High-affinity sulfate transporter Sultr1; 2 is a major transporter for Cr(VI) uptake in plants. Environ Sci Technol 55:1576–1584
Yamada, M., Shiiba, S., (2015) Preparation of pectin-inorganic composite material as accumulative material of metal ions. Journal of Applied Polymer Science 132.
Yu X-Z, Gu J-D (2007) Accumulation and distribution of trivalent chromium and effects on hybrid willow (Salix matsudana Koidz x alba L.) metabolism. Arch Environ Contam Toxicol 52:503–511
Yu XZ, Gu J-D (2008a) Effect of available nitrogen on phytoavailability and bioaccumulation of hexavalent and trivalent chromium in hankow willows (Salix matsudana Koidz). Ecotoxicol Environ Saf 70:216–222
Yu XZ, Gu JD (2008b) The role of EDTA in phytoextraction of hexavalent and trivalent chromium by two willow trees. Ecotoxicology 17:143–152
Yu XZ, Gu JD, Xing LQ (2008c) Differences in uptake and translocation of hexavalent and trivalent chromium by two species of willows. Ecotoxicology 17:747–755
Yu XZ, Fan WJ, Lin YJ (2018a) Analysis of gene expression profiles for metal tolerance protein in rice seedlings exposed to both the toxic hexavalent chromium and trivalent chromium. Int Biodeterior Biodegrad 129:102–108
Yu XZ, Lu CJ, Li YH (2018b) Role of cytochrome c in modulating chromium-induced oxidative stress in Oryza sativa. Environ Sci Pollut Res 25:27639–27649
Yu XZ, Lin YJ, Zhang Q (2019) Metallothioneins enhance chromium detoxification through scavenging ROS and stimulating metal chelation in Oryza sativa. Chemosphere 220:300–313
Zayed A, Lytle CM, Qian JH, Terry N (1998) Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206:293–299
Zayed AM, Terry N (2003) Chromium in the environment: factors affecting biological remediation. Plant Soil 249:139–156
Zeid IM (2001) Responses of Phaseolus vulgaris chromium and cobalt treatments. Biol Plant 44:111–115
Zeng F, Zhou W, Qiu B, Ali S, Wu F, Zhang G (2011) Subcellular distribution and chemical forms of chromium in rice plants suffering from different levels of chromium toxicity. J Plant Nutr Soil Sci 174:249–256
Zhitkovich A (2005) Importance of chromium−DNA Adducts in mutagenicity and toxicity of chromium(VI). Chem Res Toxicol 18:3–11
Ziller A, Fraissinet-Tachet L (2018) Metallothionein diversity and distribution in the tree of life: a multifunctional protein. Metallomics 10:1549–1559
Zhu L, Hong C, Zhang J, Qiu Y (2022) Long-distance mobilization of chromium(III) in soil associated with submicron Cr2O3. J Hazard Mater 130519
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Salles, E., Normant, V., Vignati, D.A.L. (2023). A Critical Evaluation of Chromium(III) Ecotoxicity to Aquatic and Terrestrial Plants. In: Kumar, N., Walther, C., Gupta, D.K. (eds) Chromium in Plants and Environment. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-44029-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-44029-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44028-1
Online ISBN: 978-3-031-44029-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)